Abstract
Background
The objective of this study was to describe patients receiving each shoulder arthroplasty procedure and to assess surgical complications, hospital admissions for surgical complications, and surgical revisions among Medicare beneficiaries undergoing shoulder arthroplasty.
Methods
Medicare patients receiving shoulder arthroplasty in the United States in 2011 were identified from Medicare administrative data and classified by surgery type: shoulder hemiarthroplasty (HA), anatomic total shoulder arthroplasty (TSA), or reverse shoulder arthroplasty (RSA). Surgical complications, hospital admissions, and revisions were identified during the year after the index arthroplasty procedure.
Results
There were 24,441 patients who met all inclusion criteria, and of those, 20.0% received HA, 42.5% received TSA, and 37.4% received RSA. Compared with RSA and TSA recipients, HA recipients tended to be older and sicker and were more likely to be Medicaid eligible. The rate of new surgical complications and related hospital admissions was greatest during the first 50 days after surgery but remained significant and stable throughout the remainder of the year. Rates of complications and related hospital admissions were greatest for HA recipients (17.4% and 6.6%, respectively), followed by RSA (14.2% and 5.1%) and TSA (9.4% and 4.0%).
Conclusions
The rate of adverse surgical outcomes after shoulder arthroplasty differed across populations that received HA, TSA, and RSA and across patients within each group by comorbidity burden. The finding that the rate of surgical complications and related hospital admissions remained meaningful during the entire year after surgery suggests that a postoperative follow-up period longer than the traditional 90 days may be warranted.
Keywords: Shoulder arthroplasty, Hemiarthroplasty, Total shoulder arthroplasty, Reverse shoulder arthroplasty, Surgical complications, Medicare
The use of shoulder arthroplasty in the United States is projected to continue to increase.11, 12, 13, 15, 22, 34 This trend is due in part to the aging population and the success of shoulder arthroplasty in minimizing pain and restoring shoulder function for patients with shoulder pain.21 Traditionally, 2 shoulder arthroplasty procedures were used to treat patients with shoulder disability, the hemiarthroplasty (HA) and anatomic total shoulder arthroplasty (TSA) procedures. Since 2003, the reverse shoulder arthroplasty (RSA) has emerged as a third surgical option for patients with complex shoulder disease, further driving the increase in shoulder arthroplasty rates.22, 27 Originally intended and approved for the treatment of rotator cuff arthropathy, the indications for RSA have rapidly expanded to include treatment for massive irreparable rotator cuff tears (RCTs), arthroplasty revision, acute and delayed proximal humeral fractures,10 and rheumatoid arthritis.16, 27
The recent addition of a unique International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) procedure code in 2011 created the opportunity to distinguish RSA from TSA. Before this, there had been limited ability to evaluate surgical complications among HA, TSA, and RSA procedures in large claims databases. Surgical complications, ranging from minor to major complications that require reoperation, have been reported to vary widely across HA, TSA, and RSA.9, 29 However, most studies have been conducted within a single clinic or within select samples in local areas that may not reflect larger populations.2, 12, 14, 17, 19, 23, 27, 28, 29, 33, 36, 37 Furthermore, previous studies were limited to only inpatient hospital data or 90-day postoperative follow-up, which may be inadequate to measure a broader array of complications. A recent publication from the Kaiser Permanente Shoulder Arthroplasty Registry assessed adverse outcomes after 4 shoulder arthroplasty procedures among Kaiser Permanente patients in California; however, study outcomes were limited to only 3 medical complications, revisions, and death.14 Our study is the first to observe a large, national sample of Medicare shoulder arthroplasty patients during the year after surgery with a focus on surgical complications. In addition, no other work to date has described and compared the rates of surgical complications, hospital admissions, and revisions for each shoulder arthroplasty procedure in the Medicare population.
Therefore, the objective of this study was to describe the patients receiving each shoulder procedure and to assess surgical complications, hospital admission for surgical complications, and surgical revisions among all Medicare beneficiaries during the year after surgery. In addition, it is well known that medical comorbidities influence the outcomes of all forms of shoulder arthroplasty1, 4, 5, 18, 26; therefore, we also analyzed the association between baseline comorbidity burden and complication rates. Our study includes an evaluation of all Medicare administrative data providing information on all health care received in the year after HA, TSA, and RSA procedures. These results will define the difference in characteristics of patients receiving each procedure, the adverse outcomes associated with each operation, and the role of comorbidity burden on complications after surgery.
Materials and methods
This study used complete Medicare administrative claims data from the years 2010-2012 for all Medicare beneficiaries diagnosed with a shoulder condition in 2011 (N = 2,525,519). Medicare is the US federal health insurance program for Americans who are 65 years of age or older and provides health coverage to >49 million Americans, or 15% of the US population.24 Most beneficiaries receive coverage because of age eligibility, although one-sixth of the Medicare population receives benefits because of disability status, such as end-stage renal failure.35 Medicare administrative data include extensive information about individual Medicare beneficiaries enrolled in traditional fee-for-service Medicare as well as claims for health care services provided to them and submitted to Medicare on their behalf. The use of comprehensive Medicare administrative data enabled patient health care utilization to be tracked across inpatient and outpatient providers.
From these data, individual patients with any record of receiving a HA, TSA, or RSA procedure in 2011 were identified using Medicare Part A inpatient claims. The index date of shoulder arthroplasty was defined for each beneficiary as the date of first shoulder arthroplasty in 2011. Additional inclusion criteria were applied to ensure complete data included the following: continuous enrollment in fee-for-service Medicare Part A and Part B from 365 days before to 365 days after the index arthroplasty and no enrollment in Medicare Part C during the study period; aged 66 years on the surgery date; and survival for the 365 days after the index arthroplasty. The minimum age criterion of 66 years was used to ensure enrollment in the Medicare system for a year before the index surgery. As this study is focusing on surgical complications for new shoulder arthroplasty, patients with a shoulder arthroplasty or upper extremity joint arthroplasty revision in the 365 days before their index arthroplasty in 2011 or a diagnosis of a mechanical complication of an internal orthopedic device implant or graft on their index shoulder arthroplasty were excluded from the study. Patients with >1 type of arthroplasty procedure indicated on the index surgery date were also excluded. The complete sample inclusion process with sample size is provided in the Appendix.
The type of arthroplasty procedure that patients received was identified using ICD-9-CM procedure codes listed on inpatient hospital records and included HA (81.81), TSA (81.80), and RSA (81.88). Inpatient hospital records and ICD-9-CM diagnosis codes were used to assess surgical indications for shoulder arthroplasty. All diagnosis positions on the index inpatient hospital record for surgery were used. Shoulder-related diagnoses were categorized into 5 groups based on a diagnosis algorithm and included osteoarthritis, fracture/dislocation, arthropathy with RCT, rheumatoid arthritis, and aseptic necrosis.12
In the year after the index shoulder arthroplasty, all claims with at least 1 diagnosis of a surgical complication or arthroplasty revision were identified. For each patient, we calculated the days from 1 day after hospital admission for the index shoulder arthroplasty to the identified complication. Using this information, short-term and 1-year postsurgical complications, hospital admissions for a surgical complication, and revisions rates were measured cumulatively and presented for the periods of 30, 60, 90, 180, and 365 days after the admission date for arthroplasty. Surgical complication groups of interest were determined by published guidance29 and clinical input from clinical research collaborators, which included the following:
-
1.
Postoperative infection or infection and inflammatory reaction due to internal prosthetic device implant and graft
-
2.
Fracture of scapula
-
3.
Mechanical complication of internal orthopedic device or other complications due to internal prosthetic device (unspecified mechanical complication of an internal orthopedic device, dislocation of prosthetic joint, mechanical loosening of prosthetic joint, mechanical loosening of prosthetic joint, broken prosthetic joint implant, periprosthetic fracture around prosthetic joint, periprosthetic osteolysis, articular bearing surface wear of prosthetic joint, and other complications due to other internal orthopedic or prosthetic device, implant, and graft)
-
4.
Nerve injury or injury to peripheral nerves of shoulder girdle and upper limb
-
5.
Hematoma
-
6.
Instability and dislocation
Hospital admission for a surgical complication was defined as an inpatient hospital record with a diagnosis of 1 of the aforementioned surgical complications or a complication requiring revision. Revision surgery was defined as a Medicare claim with a Healthcare Common Procedure Coding System or ICD-9-CM procedure code for upper extremity joint arthroplasty revision or removal of implant. All details regarding specific codes and algorithms used to define study concepts are available in the Appendix.
Demographic characteristics of the patients were measured by cross-referencing the 2011 Beneficiary Summary Files from Medicare. Specific patient-level variables included age, sex, race, and dual eligibility status. Previous shoulder-related health care utilization was used to describe the shoulder health of the arthroplasty population among procedure types. Shoulder-related utilization in the year before the index shoulder arthroplasty was analyzed using all Medicare administrative claims from the 365 days before the index shoulder arthroplasty in 2011. Measured shoulder-related utilization included arthroscopy, open reduction–internal fixation, and rotator cuff repair. General patient health at index surgery was measured using the Charlson Comorbidity Index (CCI). The CCI is a validated measure of burden of disease.6, 7, 25 Comorbidities are weighted from 1 to 6 for mortality risk and disease severity and then summed to form the total CCI score.6, 7, 25 Complication rates were stratified by CCI scores to analyze the association between baseline comorbidity burden and surgical complications after shoulder arthroplasty. Rates of complications, admission for a surgical complication, and revisions were calculated for each surgical group and for combinations of surgical group and comorbidity score. SAS software (version 9.4; SAS Institute, Cary, NC, USA) was used for data manipulation and statistical analyses.
Results
A total of 24,441 Medicare patients with shoulder arthroplasty met our inclusion criteria. Of these patients, 20.0% received HA, 42.5% received TSA, and 37.4% received RSA (Table I). After application of inclusion criteria to all shoulder arthroplasty procedures performed in the Medicare population, this sample represented approximately 85% of all shoulder arthroplasties within the US fee-for-service Medicare population in 2011. Two-thirds of all shoulder arthroplasty recipients were female. HA had the greatest proportion of female recipients. Recipients of HA and RSA were of similar age but were on average 2 years older than recipients of TSA (P < .001). A larger proportion of HA recipients (8.1%) were dually eligible for Medicare and Medicaid during the month of their shoulder arthroplasty compared with RSA (6.1%) and TSA recipients (4.3%) (P < .001). A larger proportion of RSA recipients had shoulder arthroscopy and rotator cuff repair surgery in the year preceding their index shoulder arthroplasty compared with HA and TSA recipients (P < .001). Patients undergoing HA had the highest comorbidity burden, with 21.8% of the HA recipients having a CCI score of 4 or more compared with only 20.1% of RSA and 15.5% of TSA recipients (P < .001).
Table I.
Clinical and demographic characteristics of Medicare beneficiaries undergoing shoulder arthroplasty in 2011
| Shoulder arthroplasty procedure |
|||||
|---|---|---|---|---|---|
| All | HA | RSA | TSA | P value | |
| N = 24,441 |
n = 4902 |
n = 9150 |
n = 10,389 |
||
| Patient demographics | |||||
| Mean age (y) | 76.1 | 77.0 | 77.1 | 74.8 | <.001 |
| Male | 34.9 | 26.0 | 33.0 | 40.9 | <.001 |
| Age group (y) | <.001 | ||||
| 66-69 | 19.3 | 18.6 | 13.9 | 24.2 | |
| 70-75 | 33.3 | 28.7 | 31.7 | 36.8 | |
| 76-79 | 20.1 | 19.2 | 21.9 | 18.9 | |
| 80-85 | 20.7 | 22.7 | 24.4 | 16.4 | |
| 86 + | 6.7 | 10.8 | 8.0 | 3.6 | |
| Race | <.001 | ||||
| Asian | 0.3 | 0.4 | 0.3 | 0.3 | |
| Black | 2.9 | 2.5 | 3.3 | 2.8 | |
| Hispanic | 0.7 | 0.8 | 0.9 | 0.4 | |
| Other | 1.1 | 1.2 | 0.9 | 1.1 | |
| White | 95.0 | 95.1 | 94.6 | 95.4 | |
| Fully dually eligible* (mean) | 5.7 | 8.1 | 6.1 | 4.3 | <.001 |
| Patient shoulder health | |||||
|---|---|---|---|---|---|
| Previous shoulder surgery† | <.001 | ||||
| Shoulder arthroscopy | 3.7 | 2.1 | 5.9 | 2.5 | |
| Open reduction–internal fixation | 0.9 | 1.9 | 1.1 | 0.2 | |
| Rotator cuff repair | 2.5 | 1.2 | 4.9 | 0.9 |
| Patient general health | |||||
|---|---|---|---|---|---|
| Charlson Comorbidity Index6 | <.001 | ||||
| 0 | 28.7 | 26.4 | 25.8 | 32.3 | |
| 1 | 23.3 | 22.6 | 23.0 | 23.9 | |
| 2 | 17.2 | 16.2 | 18.0 | 16.9 | |
| 3 | 12.3 | 13.0 | 13.1 | 11.3 | |
| 4+ | 18.5 | 21.8 | 20.1 | 15.5 | |
| Previous year Medicare spending‡ | $12,217.53 | $13,069.96 | $13,534.72 | $10,655.21 | <.001 |
HA, hemiarthroplasty; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
Beneficiary was fully dually eligible for Medicare and Medicaid during the month of surgery.
Shoulder-related utilization in the year before the index shoulder arthroplasty was analyzed on the basis of all Medicare administrative claims from the 365 days before the index shoulder arthroplasty in 2011.
Total payments made by Medicare for the beneficiary during the period of 365 days before the index surgery date.
Osteoarthritis, arthropathy/RCT, and fracture/dislocation were the 3 most common diagnoses among patients undergoing shoulder arthroplasty. Approximately 72% of all shoulder arthroplasty patients had a diagnosis of osteoarthritis and 35% had a diagnosis of arthropathy/RCT. Approximately 52% of patients receiving HA were diagnosed with fracture/dislocation. For patients receiving RSA, arthropathy/RCT and osteoarthritis were the two most common diagnoses, although 16% of RSA recipients had a diagnosis of fracture/dislocation. Among patients receiving TSA, 93% had a diagnosis of osteoarthritis and 15% had a diagnosis of arthropathy/RCT (Table II).
Table II.
Surgical indications for shoulder arthroplasty among Medicare beneficiaries undergoing shoulder arthroplasty in 2011
| All | HA | RSA | TSA | |
|---|---|---|---|---|
| N = 24,441 |
n = 4902 |
n = 9150 |
n = 10,389 |
|
| Diagnosis groups*,† | ||||
| Arthropathy/RCT | 34.8 | 19.9 | 65.3 | 14.9 |
| Fracture/dislocation | 17.8 | 51.7 | 16.7 | 2.7 |
| Osteoarthritis | 71.9 | 48.3 | 60.1 | 93.4 |
| Rheumatoid arthritis | 6.3 | 5.8 | 7.6 | 5.4 |
| Aseptic necrosis | 2.2 | 3.6 | 1.8 | 1.9 |
HA, hemiarthroplasty; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty; RCT, rotator cuff tear.
Numbers are presented as percentages.
Diagnoses were captured if the diagnosis code for osteoarthritis, arthropathy/RCT, fracture/dislocation, rheumatoid arthritis, or aseptic necrosis ever appeared in 1 of the 25 ICD-9 diagnosis code positions included on the index hospital stay for surgery. Patients may appear in >1 group if they had multiple diagnoses on the index surgery hospital stay; thus, columns will not sum to 100.
Shoulder-related diagnoses were categorized into 5 groups based on a diagnosis algorithm (Appendix Table S2).
Surgical complications
Across all 3 procedures, infection, mechanical complication, and instability/dislocation were the most common surgical complications occurring after shoulder arthroplasty. HA recipients had the highest rates of infection and instability/dislocation throughout the follow-up period. Initially, RSA recipients had the highest rate of mechanical complication at 30 days (1.7%), 60 days (2.8%), and 90 days (3.4%), but by 1 year, HA recipients had the highest rates of mechanical complication (8.2%). Fracture of the scapula was more common among RSA and HA patients than among TSA recipients. In the first 6 months after surgery, HA had the highest rate of scapula fracture, although by the end of the observation period, RSA had a higher scapula fracture complication rate (Table III).
Table III.
Surgical complication rates by arthroplasty procedure and time among Medicare beneficiaries undergoing shoulder arthroplasty in 2011
| 30 days |
60 days |
90 days |
180 days |
365 days |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 24,441 |
||||||||||||||||||||
| All | HA | RSA | TSA | All | HA | RSA | TSA | All | HA | RSA | TSA | All | HA | RSA | TSA | All | HA | RSA | TSA | |
| Infection | 0.6 | 1.0 | 0.5 | 0.4 | 0.9 | 1.4 | 0.8 | 0.6 | 1.1 | 1.8 | 1.0 | 0.8 | 1.5 | 2.2 | 1.5 | 1.1 | 2.4 | 3.0 | 2.4 | 2.0 |
| Fracture of scapula | 0.4 | 1.3 | 0.4 | 0.1 | 0.6 | 1.4 | 0.6 | 0.1 | 0.7 | 1.5 | 0.8 | 0.1 | 0.9 | 1.6 | 1.2 | 0.2 | 1.2 | 1.8 | 2.0 | 0.3 |
| Nerve injury | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.3 | 0.3 | 0.3 | 0.2 | 0.4 | 0.4 | 0.4 | 0.3 |
| Mechanical complication | 1.2 | 1.4 | 1.7 | 0.6 | 1.9 | 2.2 | 2.8 | 1.1 | 2.5 | 2.8 | 3.4 | 1.7 | 4.3 | 5.1 | 5.0 | 3.3 | 7.0 | 8.2 | 7.4 | 6.0 |
| Hematoma | 0.4 | 0.6 | 0.4 | 0.3 | 0.5 | 0.8 | 0.5 | 0.4 | 0.5 | 0.8 | 0.5 | 0.5 | 0.7 | 1.0 | 0.7 | 0.5 | 1.0 | 1.3 | 1.1 | 0.8 |
| Instability/dislocation | 1.7 | 3.7 | 2.0 | 0.6 | 2.3 | 4.2 | 2.8 | 1.0 | 2.5 | 4.5 | 3.1 | 1.1 | 3.1 | 5.4 | 3.6 | 1.6 | 3.7 | 6.3 | 4.2 | 2.1 |
| Any complication | 3.8 | 7.1 | 4.4 | 1.7 | 5.3 | 9.0 | 6.3 | 2.7 | 6.3 | 10.2 | 7.4 | 3.5 | 8.9 | 13.2 | 10.1 | 5.7 | 12.8 | 17.4 | 14.2 | 9.4 |
HA, hemiarthroplasty; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
Numbers are presented as percentages. Bold indicates statistically significant differences between groups (P value < .05).
Complications are presented as percentage of surgical cohort with a complication in 30, 60, 90, 180, or 365 days after shoulder arthroplasty; 4902 beneficiaries received HA, 9150 beneficiaries received RSA, and 10,389 beneficiaries received TSA.
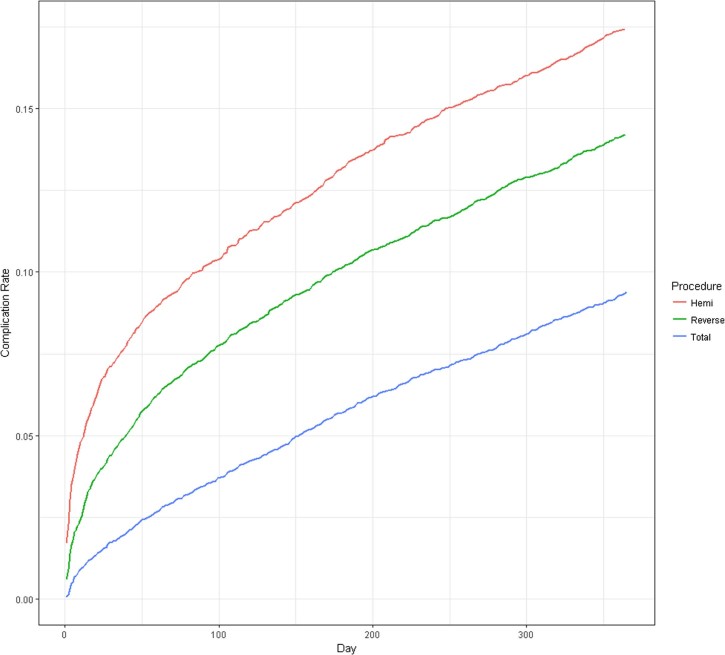
The rate of surgical complications continued to increase for all procedures after the 90-day time point. Rates of both short-term and 1-year complications were highest among patients receiving HA. Of patients receiving HA, 10.2% experienced 1 or more surgical complications within 90 days of their index shoulder arthroplasty procedure compared with 7.4% of RSA and 3.5% of TSA recipients. By 1 year, 17.4% of HA recipients experienced 1 or more surgical complications compared with 14.2% of RSA and 9.4% of TSA recipients (Fig. 1).
Figure 1.
Cumulative surgical complication rates after shoulder arthroplasty in days after index surgery for Medicare beneficiaries undergoing shoulder arthroplasty in 2011 (N = 24,441).
Surgical complications stratified by CCI score
Rates of any surgical complication occurring within 365 days of shoulder arthroplasty were stratified by CCI score to assess the relationship between comorbidity burden and complication rates for each procedure. Across all 3 arthroplasty groups, complication rates increased with increasing baseline CCI scores. Using the Cochran-Armitage 2-sided trend test,3, 8 a significant positive association was found between baseline comorbidity burden and complication rates for all procedures (Table IV).
Table IV.
One-year any surgical complication rate among Medicare beneficiaries undergoing shoulder arthroplasty in 2011 stratified by Charlson Comorbidity Index
| Charlson Comorbidity Index (CCI) |
||||||
|---|---|---|---|---|---|---|
| CCI 0 |
CCI 1 |
CCI 2 |
CCI 3 |
CCI 4 |
P value* |
|
| Surgical groups | ||||||
| HA | 14.4 | 16.9 | 17.0 | 18.6 | 21.2 | <.0001 |
| TSA | 7.2 | 9.3 | 10.0 | 11.1 | 12.2 | <.0001 |
| RSA | 13.7 | 12.5 | 14.5 | 14.9 | 16.1 | .0011 |
| All procedures | 10.7 | 12.0 | 13.1 | 14.2 | 15.9 | <.0001 |
HA, hemiarthroplasty; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
Numbers are presented as percentages.
Cochran-Armitage 2-sided trend test [3, 8].
Trends in hospital admissions for a surgical complication and surgical revisions
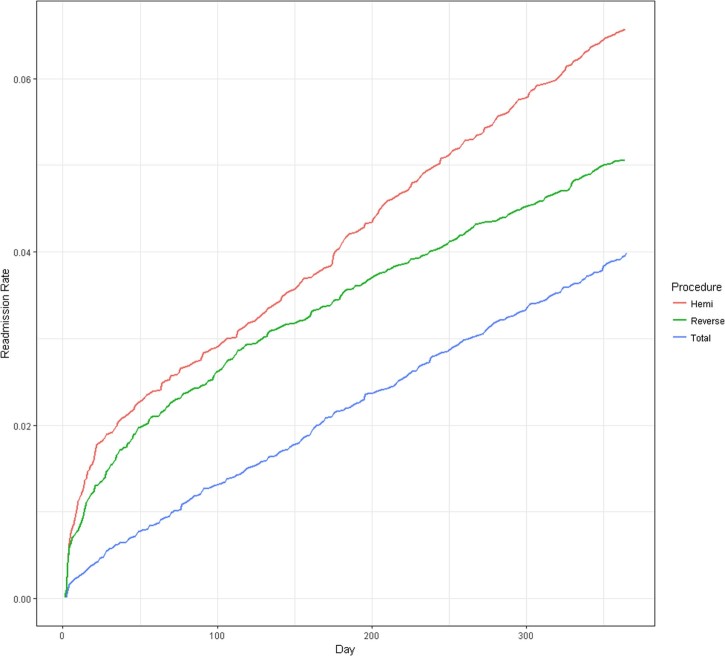
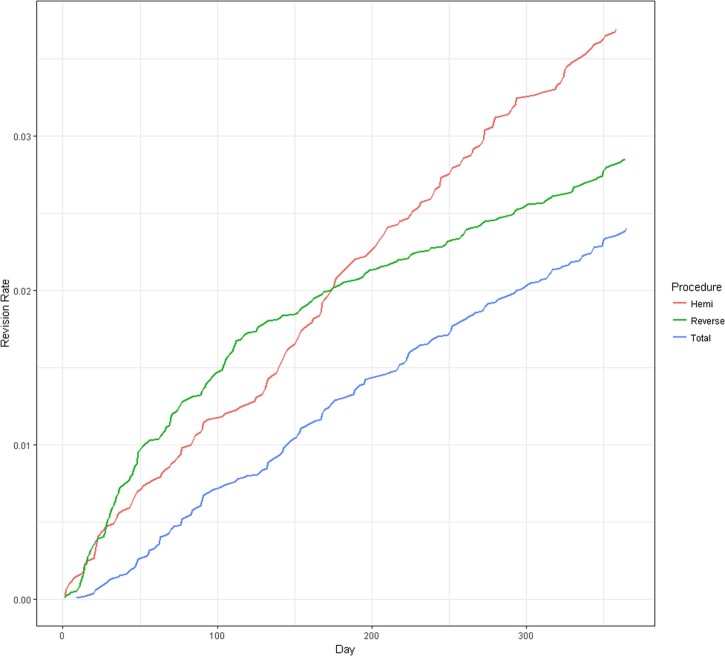
The rate of surgical complication–related hospital admissions was greatest during the first 50 days after surgery but remained significant and stable throughout the remainder of the year. Of HA recipients, 2.8% were readmitted to the hospital for a surgical complication within 90 days compared with only 2.5% of RSA and 1.2% of TSA recipients. Hospital admission for a surgical complication continued to increase for all 3 procedures after 90 days (Fig. 2). Within the first 90 days after surgery, RSA recipients had the highest rate of revision, but by the 1-year mark, HA had the highest rate of surgical revision at 3.7%. TSA recipients had the lowest rate of surgical revision (Fig. 3).
Figure 2.
Cumulative hospital admission rates for a surgical complication after shoulder arthroplasty in days after index surgery for Medicare beneficiaries undergoing shoulder arthroplasty in 2011 (N = 24,441).
Figure 3.
Cumulative surgical revision rates after shoulder arthroplasty in days after index surgery for Medicare beneficiaries undergoing shoulder arthroplasty in 2011 (N = 24,441).
Discussion
The objective of this study was to contrast the Medicare patients who underwent different shoulder arthroplasty procedures in terms of baseline characteristics and adverse surgical outcomes. This study builds on past evidence12, 17, 27, 29, 36 through assessment of a much larger, nationwide Medicare sample from multiple states and includes a complete analysis of health care utilization in the year after surgery. Our results show that Medicare patients undergoing distinct shoulder arthroplasty procedures differ in terms of socioeconomic and clinical factors. In addition, for the patients who underwent each arthroplasty procedure, different complication, hospital admission, and revision rates in the 1-year period after surgery were observed.
Infection, mechanical complication, and instability/dislocation were the 3 most common surgical complications to occur after shoulder arthroplasty. Our results showed that surgical outcomes varied by arthroplasty group, with HA having the highest rate of surgical complications, admission for a surgical complication, and revisions compared with RSA and TSA. More than half of surgical complications occurred after the 90-day postoperative window; therefore, limiting follow-up to 90 days provides low estimates of the true complication rate for each procedure. Furthermore, extended follow-up revealed that the rate of surgical complications and hospital admissions for surgical complications is similar across all 3 procedures and continues to increase at the same rate. Study follow-up must be a minimum of 1 year to fully assess the time line of surgical complications among shoulder arthroplasty groups. Moreover, the increasing rate of complications has implications for health care utilization after surgery, within and outside the 90-day postoperative window. Recent policy efforts in the United States have focused on 90-day episode-based bundled payment models; however, our study suggests that adverse outcomes continue to occur after the 90-day episode period, and policymakers should consider extending the episode window.
One-year surgical complication rates were stratified by CCI score to assess the association between baseline comorbidity burden and 1-year surgical complications.1, 4, 5, 18, 26 Across all 3 arthroplasty groups, complication rates increased with increasing baseline CCI scores. Future studies should work to elucidate the relationship between comorbidity burden and specific surgical complications. In addition, more work is needed to better understand the relationship between primary diagnosis and surgical complications. Knowledge of this trend in complications may better prepare physicians and patients for adverse surgical outcomes.
Patient groups receiving each shoulder arthroplasty procedure differed in age, socioeconomic status, diagnoses, and shoulder-specific and general health. Our results suggested that HA was associated with the highest rates of adverse outcomes after surgery, although the HA population was meaningfully different from the RSA and TSA populations; therefore, outcome comparisons across surgery types may be misleading. The HA population, composed mostly of women, had the most patients with a comorbidity burden of 4 or more and had the largest proportion of concurrently eligible Medicaid patients. In addition, half of HA patients had a diagnosis of fracture. RSA patients were on average of similar age to HA recipients but had significantly higher previous shoulder-related surgical care. In comparison to RSA and HA recipients, TSA patients were younger and healthier and had less previous shoulder surgical care.
Given the meaningful differences observed between shoulder arthroplasty groups, our findings should not be generalized to make causal statements across arthroplasty procedures. Because the characteristics of the patient populations differ greatly across the operations, further analysis is required to assess whether there are opportunities to alter the mix of HA, RSA, and TSA utilization in the Medicare population to improve patient outcomes. Furthermore, our analysis did not include information on surgeon caseloads or the facility where each procedure was performed. Surgeon acumen of each procedure may contribute to some of the variation in outcomes observed for each procedure.20 Similarly, lower rates of infection and death after shoulder arthroplasty were found for patients receiving surgery at regional high-volume hospitals.31, 32 Considering the importance of surgeon caseloads and procedure location, our findings should not be interpreted as causal statements about arthroplasty procedures.
Future studies should assess the relationship between arthroplasty procedure and surgical complications among clinically similar patients. For example, in other published reports, clinical evidence shows improved outcomes for patients with humerus fracture treated with RSA compared with HA.28, 30 Future studies should consider long-term comparative studies of HA and RSA among a representative sample of patients with humerus fracture. Such studies are needed to inform physicians about the best arthroplasty option for fracture patients and whether, in fact, RSA is superior to HA.
There are limitations associated with the nature of administrative claims data, including the lack of clinical information, such as condition severity, insight into clinical decision-making, and patient-reported outcomes; therefore, our results must be interpreted within the limits of the data. We recognize that coding limitations are inherent to administrative databases, and diagnosis codes may not reflect the entirety of a patient's shoulder complexity. Furthermore, we cannot account for bilaterality because of the limitations in the ICD-9-CM coding system. To minimize the occurrence of complications from a separate, previous joint replacement procedure, we excluded patients who had multiple procedures on the index date and those patients who had shoulder replacement in the 365 days before their index procedure in 2011. It is possible we are underestimating complication rates in that we began our follow-up period 1 day after admission for the index shoulder arthroplasty. Surgical complications occurring on the day of surgery may be omitted from this analysis; thus, our findings represent a lower bound for surgical complications. More work is needed to better understand the relationship between primary diagnosis, comorbidity burden, and surgical complication rates in addition to socioeconomic, clinical, and treatment preference differences that go far beyond what is observable in administrative claims.
However, Medicare data represent one of the most robust data sets on which to perform orthopedic research as an entire population of patients is reflected, as opposed to other claims databases, which have a varying sample that may be biased in terms of patient inclusion. Thus, the Medicare claims are ideal for certain types of questions, including data on complete large-scale surgical outcomes. Administrative claims data benefit from large, representative samples of patients and allow researchers to observe patients longitudinally throughout encounters irrespective of the location of the institution delivering care. In addition, our analysis was based on claims from 2011, which allowed us to study each procedure using distinct ICD-9-CM procedure coding, which is an advancement over previous work.29
Conclusion
These results represent the first study in which a representative sample of Medicare shoulder arthroplasty patients receiving each of the 3 shoulder procedures are observed to 1 year. Our results suggest that short-term follow-up through 90 days tells an incomplete story and that 1-year follow-up, or longer, is necessary to understand surgical complications after shoulder arthroplasty. We found that surgical complication rates were the highest for HA, with 17.4% of HA recipients experiencing 1 or more surgical complications by 1 year compared with 14.2% for RSA and 9.4% for TSA. The observed variation in the outcomes of HA, TSA, and RSA may suggest that there are opportunities to optimize the indications for these approaches to achieve more consistent outcomes for patients undergoing shoulder arthroplasty.
Disclaimer
Richard J. Hawkins and John M. Tokish report financial relationships in the form of consulting agreements and product design royalties with Arthrex, Össur, and DePuy-Mitek.
Acknowledgments
We thank Min Jee Lee for her contributions of conversation and for her involvement during the initiation of this study.
Footnotes
The University of South Carolina Institutional Review Board approved this study: research proposal Pro00039050.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jses.2017.10.002
Supplementary data
The following is the supplementary data to this article:
Tables S1-S5.
References
- 1.Aggarwal V., Tischler E., Post Z., Kane I., Orozco F., Ong A. Patients with atrial fibrillation undergoing total joint arthroplasty increase hospital burden. J Bone Joint Surg Am. 2013;95:1606–1611. doi: 10.2106/JBJS.L.00882. [DOI] [PubMed] [Google Scholar]
- 2.Antoni M., Barthoulot M., Kempf J.F., Clavert P. Revisions of total shoulder arthroplasty: clinical results and complications of various modalities. Orthop Traumatol Surg Res. 2016;102:297–303. doi: 10.1016/j.otsr.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- 4.Black E., Higgins L., Warner J. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22:1000–1009. doi: 10.1016/j.jse.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Cancienne J., Dempsey I., Holzgrefe R., Brockmeier S., Werner B. Is hepatitis C infection associated with a higher risk of complications after total shoulder arthroplasty? Clin Orthop Relat Res. 2016;474:2664–2669. doi: 10.1007/s11999-016-4979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson M., Pompei P., Ales K., MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Charlson M., Szatrowski T., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 8.Cochran W. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 9.Cuff D., Clark R., Pupello D., Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94:1996–2000. doi: 10.2106/jbjs.k.01206. [DOI] [PubMed] [Google Scholar]
- 10.Cuff D.J., Pupello D.R. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95:2050–2055. doi: 10.2106/jbjs.l.01637. [DOI] [PubMed] [Google Scholar]
- 11.Day J., Lau E., Ong K., Williams G., Ramsey M., Kurtz S. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19:1115–1120. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Day J.S., Paxton E.S., Lau E., Gordon V.A., Abboud J.A., Williams G.R. Use of reverse total shoulder arthroplasty in the Medicare population. J Shoulder Elbow Surg. 2015;24:766–772. doi: 10.1016/j.jse.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Dillon M., Chan P., Inacio M., Singh A., Yian E., Navarro R. Yearly trends in elective shoulder arthroplasty, 2005-2013. Arthritis Care Res (Hoboken) 2017;69:1574–1581. doi: 10.1002/acr.23167. [DOI] [PubMed] [Google Scholar]
- 14.Dillon M.T., Ake C.F., Burke M.F., Singh A., Yian E.H., Paxton E.W. The Kaiser Permanente shoulder arthroplasty registry: results from 6,336 primary shoulder arthroplasties. Acta Orthop. 2015;86:286–292. doi: 10.3109/17453674.2015.1024565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinnes J., Loveman E., McIntyre L., Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technol Assess. 2003;7:iii. doi: 10.3310/hta7290. 1-166. [DOI] [PubMed] [Google Scholar]
- 16.Drake G.N., O'Connor D.P., Edwards T.B. Indications for reverse total shoulder arthroplasty in rotator cuff disease. Clin Orthop Relat Res. 2010;468:1526–1533. doi: 10.1007/s11999-009-1188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehringer E.V., Mikuls T.R., Michaud K.D., Henderson W.G., O'Dell J.R. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468:717–722. doi: 10.1007/s11999-009-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Issa K., Boylan M., Naziri Q., Perfetti D., Maheshwari A., Mont M. The impact of hepatitis C on short-term outcomes of total joint arthroplasty. J Bone Joint Surg Am. 2015;97:1952–1957. doi: 10.2106/JBJS.O.00183. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J.J., Toor A.S., Shi L.L., Koh J.L. Analysis of perioperative complications in patients after total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1852–1859. doi: 10.1016/j.jse.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Katz J.N., Losina E., Barrett J., Phillips C., Mohamed N.N., Lew R. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2001;83:1622–1629. doi: 10.2106/00004623-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Khatib O., Onyekwelu I., Yu S., Zuckerman J.D. Shoulder arthroplasty in New York State, 1991 to 2010: changing patterns of utilization. J Shoulder Elbow Surg. 2015;24:E286–E291. doi: 10.1016/j.jse.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.H., Wise B.L., Zhang Y., Szabo R.M. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93:2249–2254. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 23.Mahoney A., Bosco J.A., 3rd, Zuckerman J.D. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:377–381. doi: 10.1016/j.jse.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Multack M., Noel-Miller C. AARP Public Policy Institute. 2012. Who relies on Medicare? Profile of the Medicare population. Washington, DC. [Google Scholar]
- 25.Roffman C.E., Buchanan J., Allison G.T. Charlson comorbidities index. J Physiother. 2016;62:171. doi: 10.1016/j.jphys.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Rosas S., Sabeh K.G., Buller L.T., Law T.Y., Kalandiak S.P., Levy J.C. Comorbidity effects on shoulder arthroplasty costs analysis of a nationwide private payer insurance data set. J Shoulder Elbow Surg. 2017;26:e216–e221. doi: 10.1016/j.jse.2016.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schairer W.W., Nwachukwu B.U., Lyman S., Craig E.V., Gulotta L.V. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24:91–97. doi: 10.1016/j.jse.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Schairer W.W., Nwachukwu B.U., Lyman S., Craig E.V., Gulotta L.V. Reverse shoulder arthroplasty versus hemiarthroplasty for treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2015;24:1560–1566. doi: 10.1016/j.jse.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Schairer W.W., Zhang A.L., Feeley B.T. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1349–1355. doi: 10.1016/j.jse.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Shukla D.R., McAnany S., Kim J., Overley S., Parsons B.O. Hemiarthroplasty versus reverse shoulder arthroplasty for treatment of proximal humeral fractures: a meta-analysis. J Shoulder Elbow Surg. 2016;25:330–340. doi: 10.1016/j.jse.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Singh A.Y.E., Dillon M.T., Takayanagi M., Burke M.F., Navarro R.A. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23:1187–1194. doi: 10.1016/j.jse.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Singh J.A., Kwoh C.K., Boudreau R.M., Lee G.C., Ibrahim S.A. Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk adjusted analysis of a large regional database. Arthritis Rheum. 2012;63:2531–2539. doi: 10.1002/art.30390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smucny M., Menendez M.E., Ring D., Feeley B.T., Zhang A.L. Inpatient surgical site infection after shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:747–753. doi: 10.1016/j.jse.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Trofa D., Rajaee S.S., Smith E.L. Nationwide trends in total shoulder arthroplasty and hemiarthroplasty for osteoarthritis. Am J Orthop (Belle Mead NJ) 2014;43:166–172. [PubMed] [Google Scholar]
- 35.Umans B., Nonnemaker K.L. AARP Public Policy Institute. 2009. The Medicare beneficiary population. Washington, DC. [Google Scholar]
- 36.Villacis D., Sivasundaram L., Pannell W.C., Heckmann N., Omid R., Hatch G.F., 3rd. Complication rate and implant survival for reverse shoulder arthroplasty versus total shoulder arthroplasty: results during the initial 2 years. J Shoulder Elbow Surg. 2016;25:927–935. doi: 10.1016/j.jse.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Westermann R.W., Pugely A.J., Martin C.T., Gao Y., Wolf B.R., Hettrich C.M. Reverse shoulder arthroplasty in the United States: a comparison of national volume, patient demographics, complications, and surgical indications. Iowa Orthop J. 2015;35:1–7. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S5.