Abstract
Background
The subscapularis peel (SP) and the lesser tuberosity osteotomy (LTO) are 2 common exposure techniques for total shoulder arthroplasty. Although some biomechanical studies have suggested a higher resistance to failure with the LTO, clinical studies have demonstrated no difference in repair failure or tendon healing. We hypothesized that there would be no difference in biomechanically tested repair strength between our SP technique and the previously tested LTO technique.
Methods
Eleven cadaver shoulders were separated into 2 groups: 6 SPs and 5 LTOs. After initial loading for 3000 cycles, the specimens were incrementally loaded to 450 ± 50 N or catastrophic failure. Repair gapping was measured after cyclical loading, and fatigue life was analyzed after incremental loading.
Results
There was no significant difference in mean repair gapping between the SP (2.40 ± 0.36 mm; mean ± standard deviation) and the LTO groups (3.10 ± 2.93 mm; P = .57). There was also no difference in the mean number of cycles to failure (6894 ± 956 vs. 6018 ± 1179; P = .14) and mean load to failure (400 ± 79 N vs. 340 ± 91 N; P = .21) between the SP and LTO techniques. However, there was more variability in bead gapping in the LTO group (P < .01).
Conclusion
No significant differences were found in repair gapping, fatigue failure, and load to failure in comparing the SP and LTO repairs. However, the SP repair demonstrated significantly less variability in repair gapping. These findings suggest that initial fixation biomechanical properties between the 2 constructs are similar in vitro.
Keywords: Lesser tuberosity osteotomy, Subscapularis peel, Total shoulder arthroplasty, Biomechanics, Subscapularis takedown, Shoulder arthritis
Total shoulder arthroplasty (TSA) is most commonly performed through a deltopectoral approach requiring mobilization of the subscapularis for effective exposure. The majority of shoulder surgeons employ 1 of 3 techniques to take down the subscapularis: tenotomy, subscapularis peel (SP), or lesser tuberosity osteotomy (LTO). With the discovery of postoperative subscapularis dysfunction in patients undergoing subscapularis tenotomy, some surgeons have shifted to tendon-preserving procedures.11
Multiple biomechanical comparison studies have compared the SP and LTO. Van Thiel et al16 found no difference in elongation of repair or maximal load to failure between the LTO and SP. Van den Berghe et al15 evaluated the same techniques and found that the LTO provided the highest resistance to failure and provided optimal restoration of subscapularis length. Ponce et al12 compared the repairs and found the LTO to have less repair gapping and higher resistance to failure. Together, these biomechanical studies support an argument that the LTO provides a stronger repair than the SP.
Variation exists in how the SP and LTO mobilizations are repaired. The LTO is most commonly repaired with transosseous suture fixation around the osteotomy site or with horizontal mattress suture over a lateral buttress plate.2, 6, 8, 15, 16 The SP can be repaired over a buttress plate similar to the LTO.8 Alternatively, it can be repaired with transosseus fixation done either medial1, 15, 16 or lateral2, 12 to the lesser tuberosity. Although Burkhart et al3 demonstrated that rotator cuff repairs fail by a cutout mechanism, previous biomechanical studies have compared the SP only with a medial transosseous repair, which offers a small bone bridge compared with the lateral repair.7, 15
The purpose of this study was to use a biomechanical model to compare the mechanical integrity of the SP repaired with transosseus fixation lateral to the lesser tuberosity, which offers a wide, thick bone bridge, vs. the previously studied LTO.6, 7, 8, 9, 15 We hypothesized that there would be no significant difference in repair strength between the SP and LTO techniques with biomechanical testing.
Materials and methods
Eleven age-, gender-, and body mass index–matched fresh frozen cadavers were obtained for the study. Six shoulders were selected to undergo SP, and 5 shoulders were selected to undergo LTO. Characteristics of the cadaver specimens are described in Table I.
Table I.
Demographic data of cadaveric specimens
| Randomization group |
|||
|---|---|---|---|
| Variable | SP | LTO | P value |
| (n = 6) | (n = 5) | ||
| Mean | Mean | ||
| Age, y (range) | 57.3 (33-74) | 62.2 (47-74) | .56 |
| Female | 2 | 1 | |
| Male | 4 | 4 | .89 |
| Body mass index, kg/m2 (SD) | 21.2 (5.8) | 21.0 (6.3) | .96 |
SP, subscapularis peel; LTO, lesser tuberosity osteotomy; SD, standard deviation.
The specimens were thawed, and the superficial cutaneous and muscular shells were gently dissected from the shoulders while maintaining the integrity of the subscapularis. The humeral shafts were osteotomized approximately 9 cm distal to the greater tuberosity, and the remainder of the muscle attachments of the humerus were removed. The specimens then underwent either SP or LTO. The specimens were not instrumented.
Lesser tuberosity osteotomy
The LTO was performed using the technique described by Gerber et al.5, 6 Bone flecks measuring 25-30 mm in length, 8-20 mm in width, and 5-7 mm in thickness were cut. The osteotomies were started with a thin oscillating saw and completed with a 10-mm curved osteotome. The osteotomy was repaired with No. 5 FiberWire (Arthrex, Inc., Naples, FL, USA) using a horizontal mattress backpack technique described in a previous study.5, 6 The sutures were tied over a 2-hole, one-third tubular plate (Synthes, Inc., West Chester, PA, USA), similar to the suture button and miniplate used in other studies (Fig. 1).6, 7, 8, 9, 15
Figure 1.
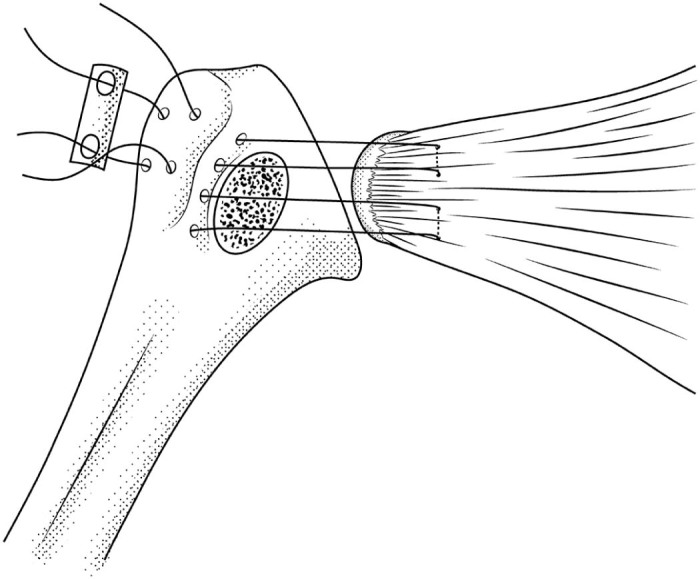
Sketch of the lesser tuberosity osteotomy repair technique featuring the horizontal mattress backpack suture repair with a one-third tubular plate.
Subscapularis peel
The SP was performed using a No. 15 scalpel beginning in the bicipital groove and progressing medially. The resultant outline of the tendinous counterpart of the subscapular footprint was readily apparent and served as a guide for repair. The SP was also repaired with No. 5 FiberWire. The repair began by drilling of 4 holes lateral and 2 holes medial to the lesser tuberosity using a 2-mm drill bit. The suture was passed into the bicipital groove and out of the corresponding medial holes. The sutures were then passed through the tendon just medial to the footprint in a modified Mason-Allen fashion and then tied with a minimum of 4 square knots (Fig. 2).
Figure 2.
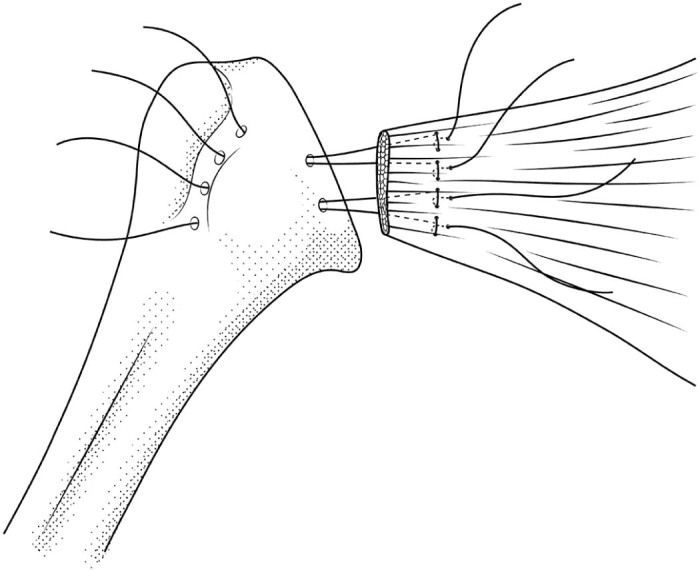
Sketch of subscapularis peel repair featuring medial and lateral drill holes around the lesser tuberosity and a modified Mason-Allen suture pattern in the tendon.
After each repair was completed, 3 pairs of 4-mm beads were evenly spaced and sutured to either side of the repair to measure repair gapping in the testing phase (Figure 3, Figure 4). When necessary, bead fixation was supplemented with Loctite adhesive (Loctite Super Glue Ultra Gel Control; Henkel Corporation, Westlake, OH, USA).
Figure 3.

Photograph illustration of lesser tuberosity osteotomy repair and material testing system setup.
Figure 4.

Photograph illustration of subscapularis peel repair and material testing system setup.
Mechanical testing
The humeral shaft of each specimen was potted in a rigid fixture using automotive cement (Bondo Lightweight Body Filler; 3M, St. Paul, MN, USA). The specimens were then affixed to a material testing machine (Instron 8501; Instron, Norwood, MA, USA). The subscapularis muscle was clamped medial to the musculotendinous junction in a custom serrated aluminum cryogenic grip with dry ice chambers to ensure attachment to the fixture.
Similar to previous research, we believe tendon repair failure occurs during daily use, making the mechanical integrity of the constructs under cyclical loads relevant.3, 12 A 25 N preload was applied for 3 minutes to eliminate crimp. The specimens were then cyclically loaded at 75 ± 50 N for 3000 cycles at 1 Hz. This loading program simulated in vivo load forces experienced by subscapularis repair constructs during initial postoperative rehabilitation.12 After the initial 3000 cycles at 75 ± 50 N, specimens underwent incremental cyclical loading. We increased the undulation point by 25 N every 300 cycles until catastrophic failure or up to a final load of 450 ± 50 N, which corresponded to a total of 7500 cycles. Throughout testing, the muscle, tendon, and repair sites were kept moist with 2 manual sprays of 37°C normal saline at 3-minute intervals.
Repair gapping during the cyclical loading phase of the test was measured manually with a digital caliper (General Tools Fraction Plus 6-inch 3-mode Digital Caliper; General Tools & Instruments LLC, Secaucus, NJ, USA). After an initial 3-minute tare load period, we measured inter-bead distance for each of the 3 bead pairs in each specimen. Each pair was measured 3 times. The average of each pair was used to calculate mean pretest bead distances for each specimen. At the end of the cyclical loading, the specimens were subjected again to a 3-minute 25 N tare load. Post-test bead distances were then measured using the digital caliper in the same fashion as in the pretest measurements.
Images of the specimens were taken from a fixed point using a digital camera before initial cyclical loading. Each image was used to assess the angle of the humerus relative to the direction of subscapularis loading (test angle) at the start of testing. The test angle was measured from the superior border of the subscapularis and lateral humerus.
Statistical analysis
Demographic differences between the specimens, including the distribution of laterality (right vs. left arm), donor age, gender, and body mass index, were examined using 2-tailed t-tests for continuous data and χ2 tests for categorical data.
Construct gapping after initial cyclical loading was analyzed using 2 separate mixed analysis of variance (ANOVA) models. The first model assessed bead distance, the distance between beads on either side of the constructs before and after initial cyclical loading. The donors were introduced as a random effect; technique (LTO vs. SP), inter-bead distances before and after initial cyclical loading, and their interaction served as fixed effects. The second model was employed to assess repair gapping, the difference between inter-bead distances before and after initial cyclical loading. Donors again served as the random effect; surgical technique was the fixed effect. In addition, the variability of the repair bead gapping among subjects was compared using a 2-sided F test.
For the failure phase of the study, we used a parametric survival model as well as a mixed ANOVA model. The survival model was created to test fatigue survival and resistance to incremental loading of the constructs. The number of fatigue cycles at different loads was converted to 75 N cycle equivalents using the Palmgren-Miner fatigue rule.10, 14 The number of 75 N–equivalent fatigue cycles served as the “time to event” parameter in the model, and the data were right-censored using a “fail or survive” criterion at the end of the fatigue regimen. The survival probabilities were modeled using a Weibull distribution, which is standard for analyzing fatigue data. The mixed ANOVA model was constructed to allow comparison to previous literature.15 Failure load, cycles to failure, and 75 N–equivalent cycles to failure were analyzed with the subject as the random effect and the technique (SP vs. LTO) as the fixed effect. In this model, a final load of 450 N and a total number of cycles of 7500 were assigned for the specimens that did not fail catastrophically by the end of the testing.
Statistical analyses were performed using JMP (version 10; SAS Institute, Cary, NC, USA). Significance was set as P < .05.
Results
There were no significant demographic differences between the 2 groups (Table I). Regarding bead distance, we found that cyclical loading caused a significant increase in the distance for both groups, indicating success of our biomechanical setup (P = .02). The technique and the interaction between technique and bead measurement time were not significant (P = .49 and P = .72, respectively). The analysis of bead gapping demonstrated that mean bead gapping was not statistically different between the SP group (2.4 ± 0.4 mm; mean ± standard deviation) and the LTO group (3.1 ± 2.9 mm; P = .57. However, the repair gapping values were more variable for the LTO than for the SP group (P < .01; Fig. 5).
Figure 5.
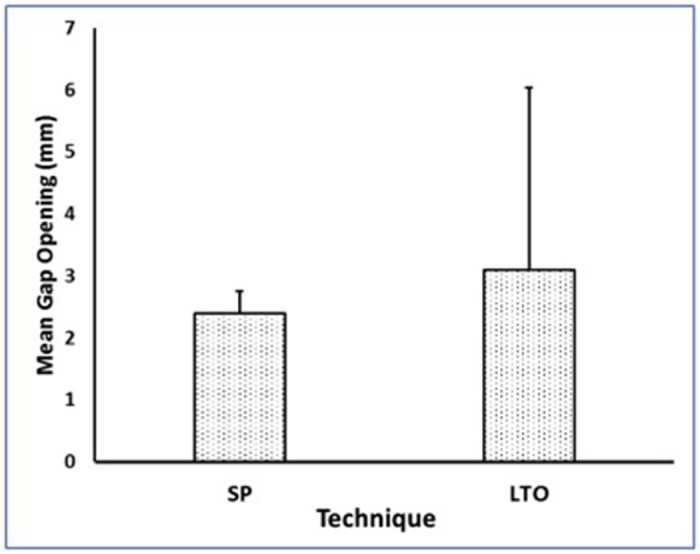
Mean repair gapping after cyclical loading. Although mean gapping did not differ, there was a wider range of repair gapping for the lesser tuberosity osteotomy (LTO) group. The error bars show standard deviations. SP, subscapularis peel.
Using the parametric survival model, we found no significant effect of surgical technique on the survival likelihood of the constructs (P = .18). In evaluating failure loading, no differences were found between the SP and LTO groups in terms of mean load to failure (400 ± 79 N vs. 340 ± 91 N; P = .21), mean number of cycles to failure (6894 ± 956 vs. 6018 ± 1179; P = .14), or total number of 75 N–equivalent cycles to failure (16,393 ± 4872 vs. 11,840 ± 5905; P = .17). There was no difference in location of failure between the SP and LTO groups, with a majority of failures occurring through the muscle. One patient in the LTO group had a catastrophic failure through the bone.
The mean testing angle at the onset of cyclical loading in the SP group was 101.5° ± 6.0° vs. 104.2° ± 19.4° in the LTO group (P = .98). Thus, the directions of force on the constructs in each group were not significantly different. Test angle, initial cyclical loading, and failure loading results are summarized in Table II.
Table II.
Biomechanical subscapularis repair testing
| Techniques |
|||
|---|---|---|---|
| SP (n = 6) | LTO (n = 5) | ||
| Study interest | Mean (SD) | Mean (SD) | P value |
| Testing angle, degrees | 101.5 (6.0) | 104.2 (19.4) | .98 |
| Pretest inter-bead distance, mm | 37.1 (4.4) | 36.0 (4.8) | * |
| Post-test inter-bead distance, mm | 39.5 (4.7) | 39.1 (4.5) | |
| Measurement time | .02 | ||
| Technique effect | .49 | ||
| Technique/measurement time interaction | .72 | ||
| Mean gap difference, mm | 2.4 (0.4) | 3.1 (2.9) | .57 |
| Probability of failure at 10,000 cycles | 14% (4%-47%)† | 36% (13%-75%)† | .18 |
| Cycles to failure | 6894 (956) | 6018 (1179) | .14 |
| Load at failure, N | 400 (79) | 340 (91) | .21 |
| 75 N–equivalent cycles | 16,393 (4872) | 11,840 (5905) | .17 |
SP, subscapularis peel; LTO, lesser tuberosity osteotomy; SD, standard deviation.
Bold values are statistically significant.
The P values of the 3-factor mixed analysis of variance are noted below.
Values in parentheses refer to 95% confidence intervals rather than SD.
Discussion
The ideal subscapularis takedown in performance of TSA is resistant to failure. However, the surgeon's preference often drives the choice of technique.4 Our results demonstrated no difference between the SP and LTO techniques in terms of repair gapping, cycles to failure, and load to failure at the time of initial fixation.
Recent clinical studies have not demonstrated a superior outcome for the LTO compared with the SP in terms of construct survival or tendon healing. Lapner et al8 performed a level 1, double-blinded comparison of subscapularis strength and shoulder function after TSA performed with the LTO described by Gerber et al6 vs. SP with a similar repair over a buttress. They found no difference in subscapularis strength or shoulder function at any time point up to 2 years after TSA. Buckley et al2 conducted a retrospective cohort study comparing internal rotation strength and functional outcome assessment between the LTO and SP after TSA. They found no difference in internal rotation strength and no clinical difference in functional outcome. Overall, there is a discrepancy between results of biomechanical studies and clinical data, suggesting that the survival rates and healing rates of LTO and SP repairs are similar.
In previous biomechanical comparisons of SP and LTO techniques, there have been mixed results. In 2005, Ponce et al12 evaluated the LTO, SP, and subscapularis tenotomy in 27 cadaveric specimens. They found significantly less cyclical displacement and greater maximum load to failure in the LTO group compared with the SP. Van Thiel et al16 evaluated the SP and LTO and found no difference in elongation of the repairs and maximal load to failure. Van den Berghe et al15 evaluated the same techniques and found that the LTO provided the most resistance to failure and optimal restoration of subscapularis length. The previous 2 studies described performing the SP repair at the humeral head osteotomy site and the anatomic neck, as opposed to the bicipital groove. This is a deviation from Rockwood's recommendation that the SP be repaired laterally to provide a more robust construct.13 Our SP repair incorporated drill holes on either side of the lesser tuberosity, which permits a more lateral repair with a wider bone bridge than a repair through the anatomic neck. Our results differed from the previous studies in that we found no difference in construct stability between the SP and LTO despite using multiple analyses to find possible differences. The contrasting findings between our studies may be attributable to our different modes of SP repair and the increased stability offered by our lateral repair.
The SP takedown has many merits. It allows a simple, wide exposure and a facile, reproducible repair. The difference in variability in repair gapping in the SP and LTO groups may reflect the reproducibility of the SP. Furthermore, the SP does not violate the subscapularis tendon, facilitating potential future revisions. In performing an LTO, there is also risk of medial calcar fractures that is not present in the SP. Finally, in cases of internal rotation contracture, the SP permits repositioning of the subscapularis insertion, unlike the tenotomy and LTO.4 These additional merits make the SP an attractive alternative to the LTO.
There are several potential limitations to our study. One limitation is the cadaveric nature of the study. Although biomechanical studies in cadavers allow in vitro analysis of repair strength at the time of initial fixation, the in vivo contribution of bone and soft tissue healing to construct stability could not be evaluated. Another potential limitation was our small sample size. However, our sample size was comparable to that of previous studies that found significant differences. Also, whereas a linear force was applied to simulate subscapularis contractions, the subscapularis tendon may be subjected to multiaxial forces, depending on shoulder position in vivo. Last, our specimens included a mix of paired and unpaired cadaver shoulders. We accounted for this by matching our specimens by age, body mass index, and gender as well as by introducing the donors as random effects in our ANOVA analyses. Strengths of this study include that we simulated the cyclical loading mechanism of tendon repair failure and that we did not instrument the humeri, eliminating the contribution of the implant to the repair constructs and facilitating direct comparison.
Conclusion
No significant differences were found in repair gapping, fatigue failure, and load to failure in comparing the SP and LTO repairs. However, the SP repair demonstrated significantly less variability in repair gapping. These findings suggest that initial fixation biomechanical properties between the 2 constructs are similar in vitro. Given the additional merits of the SP technique, this study supports further consideration of the technique.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Henry Ford Hospital Investigation Review Board approved this study.
References
- 1.Ahmad C.S., Wing D., Gardner T.R., Levine W.N., Bigliani L.U. Biomechanical evaluation of subscapularis repair used during shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16:S59–S64. doi: 10.1016/j.jse.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Buckley T., Miller R., Nicandri G., Lewis R., Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23:1309–1317. doi: 10.1016/j.jse.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart S.S., Johnson T.C., Wirth M.A., Athanasiou K.A. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13:172–176. doi: 10.1016/s0749-8063(97)90151-1. [DOI] [PubMed] [Google Scholar]
- 4.Defranco M.J., Higgins L.D., Warner J.J. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18:707–717. doi: 10.5435/00124635-201012000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Gerber C., Pennington S.D., Yian E.H., Pfirrmann C.A., Werner C.M., Zumstein M.A. Lesser tuberosity osteotomy for total shoulder arthroplasty. Surgical technique. J Bone Joint Surg Am. 2006;88:170–177. doi: 10.2106/JBJS.F.00407. [DOI] [PubMed] [Google Scholar]
- 6.Gerber C., Yian E.H., Pfirrmann C.A., Zumstein M.A., Werner C.M. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87:1739–1745. doi: 10.2106/JBJS.D.02788. [DOI] [PubMed] [Google Scholar]
- 7.Jackson J.D., Cil A., Smith J., Steinmann S.P. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:1085–1090. doi: 10.1016/j.jse.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Lapner P.L., Sabri E., Rakhra K., Bell K., Athwal G.S. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94:2239–2246. doi: 10.2106/JBJS.K.01365. [DOI] [PubMed] [Google Scholar]
- 9.Lapner P.L., Sabri E., Rakhra K., Bell K., Athwal G.S. Healing rates and subscapularis fatty infiltration after lesser tuberosity osteotomy versus subscapularis peel for exposure during shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:396–402. doi: 10.1016/j.jse.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Lindsey D.P., Kim M.J., Hannibal M., Alamin T.F. The monotonic and fatigue properties of osteoporotic thoracic vertebral bodies. Spine. 2005;30:645–649. doi: 10.1097/01.brs.0000155411.69149.49. [DOI] [PubMed] [Google Scholar]
- 11.Miller S.L., Hazrati Y., Klepps S., Chiang A., Flatow E.L. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12:29–34. doi: 10.1067/mse.2003.128195. [DOI] [PubMed] [Google Scholar]
- 12.Ponce B.A., Ahluwalia R.S., Mazzocca A.D., Gobezie R.G., Warner J.J., Millett P.J. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87:1–8. doi: 10.2106/JBJS.E.00441. [DOI] [PubMed] [Google Scholar]
- 13.Rockwood C.A., Jr The technique of total shoulder arthroplasty. Instr Course Lect. 1990;39:437–447. [PubMed] [Google Scholar]
- 14.Suresh S. Cambridge University Press; New York: 1998. Fatigue of materials. [Google Scholar]
- 15.Van den Berghe G.R., Nguyen B., Patil S., D'Lima D.D., Mahar A., Pedowitz R. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17:156–161. doi: 10.1016/j.jse.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Van Thiel G.S., Wang V.M., Wang F.C., Nho S.J., Piasecki D.P., Bach B.R., Jr Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:657–663. doi: 10.1016/j.jse.2010.01.014. [DOI] [PubMed] [Google Scholar]


