Abstract
Background
Tranexamic acid (TXA) has been shown to reduce perioperative blood loss and risk of blood transfusion. Evidence establishing its efficacy in total shoulder arthroplasty (TSA) is limited. The current study evaluated the effect of TXA on perioperative blood loss and transfusion risk after TSA.
Methods
A systematic review and meta-analysis of TXA administration for TSA was performed, and 6 studies with a total of 680 patients were found. Data on change in hemoglobin, drain output, total blood loss, and transfusion were extracted. Meta-analysis was performed with stratification into reverse and anatomic TSA subgroups.
Results
TXA administration was associated with decreased change in hemoglobin (−0.63 g/dL; 95% CI, −0.87 to −0.39 g/dL; P < .00001), drain output (−112.05 mL; 95% CI, −182.29 to −41.81 mL; P < .0001), and total blood loss (−231.87 mL; 95% CI, −334.23 to −129.48 mL; P < .00001) after reverse TSA. There was a trend toward reduction in transfusion rate after reverse TSA (−4%; 95% CI, −8% to 0%; P = .06). TXA administration was associated with reduced drain output after anatomic TSA (−123.07 mL; 95% CI, −163.93 to −82.20 mL; P < 0.00001). TXA administration was not associated with decreased transfusion rate after anatomic TSA. Data to evaluate the effect of TXA on change in hemoglobin and total blood loss after anatomic TSA were insufficient.
Conclusions
Routine administration of TXA reduces perioperative blood loss and may reduce the risk of transfusion after reverse TSA. Future studies are needed to further characterize its effect on the risk of transfusion after reverse TSA and efficacy in anatomic TSA.
Keywords: Anatomic total shoulder arthroplasty, Reverse total shoulder arthroplasty, Tranexamic acid, Transfusion, Meta-analysis, Total blood loss
The risk of allogeneic blood transfusion after total shoulder arthroplasty (TSA) ranged from 4.2% to 7.7% from 1998 to 2011.10 Blood transfusion after arthroplasty surgery results in increased health care utilization costs, longer hospital stays, and an increased risk of surgical site infection.10, 19 The most widely supported risk factors for blood transfusion after TSA include increasing age6, 10, 17, 18 and preoperative anemia.3, 6, 7, 10, 13, 14, 17, 18, 20 Other potential risk factors include, female gender,6, 10, 17, 20 number of medical comorbidities,10, 17, 18 low hospital caseload,1 proximal humeral malunion,8 and reverse vs. anatomic TSA.6, 9
Tranexamic acid (TXA) is a competitive inhibitor for plasminogen by reversibly blocking the plasminogen lysine-binding site.1, 2, 4, 5, 11, 19, 20, 21 This mechanism results in stabilization of blood clots from prevention of fibrin degradation.1, 2, 4, 5, 11, 19, 20, 21 This antifibrinolytic agent has become widely used across multiple subspecialties to decrease blood loss and to reduce the need for intraoperative and postoperative transfusions.
TXA has been shown to reduce perioperative blood loss and allogeneic blood transfusion rates after total hip (THA) and knee (TKA) arthroplasty.2, 21 Few studies, however, have evaluated its efficacy for TSA. The purpose of the study was to determine whether the current literature supports the use of TXA to reduce perioperative blood loss and transfusion rates after anatomic and reverse TSA. We hypothesize that use of TXA during shoulder arthroplasty will demonstrate similar results to those that have been reported with use during THA and TKA.
Materials and methods
Systematic review and meta-analysis
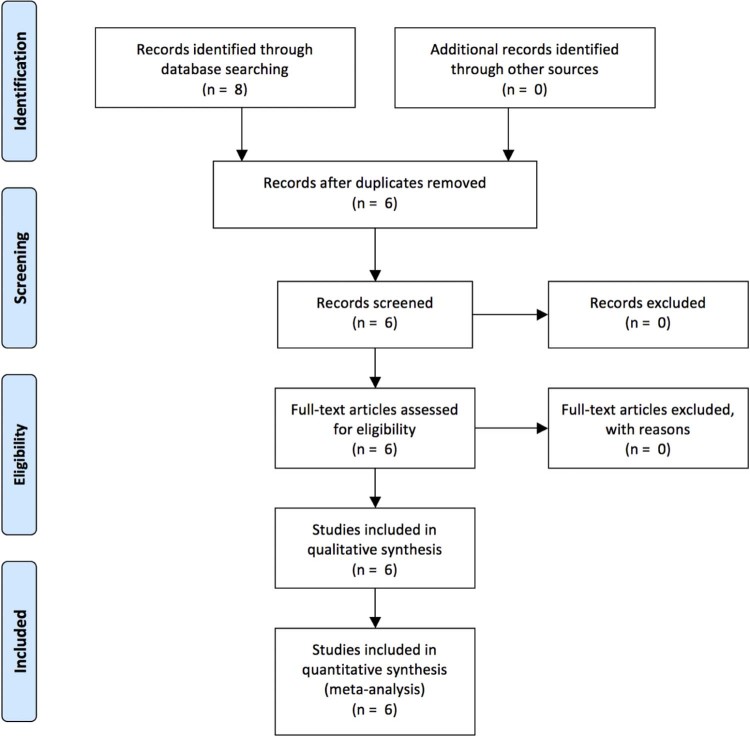
To obtain articles relating to TXA use in shoulder arthroplasty, the PubMed, Ovid MEDLINE, and CINAHL databases were queried with the terms “shoulder arthroplasty” and “tranexamic acid.” The final analysis included 6 articles1, 4, 5, 12, 15, 22 with a total of 680 patients. This search was performed by 2 independent reviewers in January 2017, and after review of the limited number of titles as well as abstracts, we arrived at our final manuscripts evaluated for further analysis. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram is shown in Fig. 1. The inclusion criteria were studies of patients undergoing anatomic or reverse TSA, administration of topical or intavenous TXA, and studies assessing outcomes measures, including blood loss, need for transfusion, drain output, postoperative change in hemoglobin or hematocrit, or both, and rate of complications. Studies were excluded if they were not clinical trials, or if the topic focused on basic science laboratory investigations, cadeveric or biomechanical models, or did not include patients who underwent shoulder arthroplasty.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
A meta-analysis was performed using RevMan 5.3 software (Cochrane Collaboration, Copenhagen, Denmark).16 Changes in hemoglobin concentration, drain output, total blood loss, and transfusion rate were evaluated. Mean differences of continuous variables were assessed. When reported, means and standard deviations were extracted from included studies. When these statistics were not available, the median was used as an approximation for the mean and the range divided by four was used as an approximation of the standard deviation as recommended by Hozo et al.8 For transfusion events, risk differences were tested. A fixed effects model was used when heterogeneity was statistically insignificant. A random effects model was used when effect size heterogeneity was statistically significant. Two-sided statistical significance of 0.05 was used.
Results
Systematic review
Descriptions of the studies included in the systematic review and meta-analysis are given in Table I. Of the 6 studies, 3 were retrospective analyses of a practice change from no TXA use to routine TXA administration.1, 4, 12 In an analysis of 168 patients undergoing reverse and anatomic TSA, Abildgaard et al1 (level of evidence [LOE] 3) found that 1 routine preoperative 1 g intravenous infusion of TXA was associated with a reduction in total blood loss, hemoglobin drop, hematocrit drop, and drain output. In an analysis of 194 and 48 patients respectively, Friedman et al4 and Kim et al12 (LOE 3) found similar results with 20 mg/kg infusions and 2 g TXA dosing regimens, respectively. Both studies were underpowered to detect a change in the transfusion rate.
Table I.
Description of the studies evaluating the efficacy of tranexamic acid for shoulder arthroplasty
| Variable | Abildgaard et al1 | Friedman et al4 | Kim et al12 | Pauzenberger et al15 | Gillespie et al5 | Vara et al22 |
|---|---|---|---|---|---|---|
| Design | Retrospective | Retrospective | Retrospective | Prospective, randomized | Prospective, randomized | Prospective, randomized |
| Total patients, No. | 171 | 194 | 48 | 54 | 111 | 102 |
| Control group, No. | 94 (42 TSA, 52 rTSA) | 88 (43 TSA, 45 rTSA) | 24 rTSA | 27 (11 TSA, 16 rTSA) | 55 (22 TSA, 33 rTSA) | 49 rTSA |
| Intervention group, No. | 77 (35 TSA, 42 rTSA) | 106 (54 TSA, 52 rTSA) | 24 rTSA | 27 (15 TSA, 12 rTSA) | 56 (22 TSA, 34 rTSA) | 53 rTSA |
| Route of administration | Intravenous | Intravenous | Intravenous | Intravenous | Topical | Intravenous |
| Dose | 1 g, 1 infusion | 20 mg/kg, 1 infusion | 500 mg, 1 infusion | 2g, 2 infusions | 2g in 100 mL NS for 5 min | 20 mg/kg, 2 infusions |
| Hematologic outcomes | ||||||
| Hemoglobin drop | ✔ | ✔ | ✔ | ✔ | ||
| Drain output | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Total blood loss | ✔ | ✔ | ✔ | |||
| Transfusion | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
TSA, anatomic total shoulder arthroplasty; rTSA, reverse total shoulder arthroplasty; NS, normal saline.
The remaining studies included in the current systematic review and meta-analysis were randomized, placebo controlled trials (LOE 1).5, 15, 22 In a study consisting of 111 patients, Gillespie et al5 found that 2 g topical TXA reduced the hemoglobin drop after reverse TSA but not anatomic TSA. No patient in the placebo or intervention arm required a transfusion. In a study including only reverse TSAs (102 subjects), Vara et al22 demonstrated the efficacy of two 10 mg/kg intravenous TXA infusions in reducing the postoperative hemoglobin drop, drain output, and total blood loss.22 The transfusion rate in the placebo group was 14.3% compared with 5.7% in the TXA group. Although fewer patients in the TXA arm required a transfusion, the effect was not statistically significant. Pauzenberger et al15 found that TXA reduced drain output and total blood loss in mixed groups of 54 patients undergoing anatomic and reverse TSA. No patient in either arm required a transfusion.
Meta-analysis
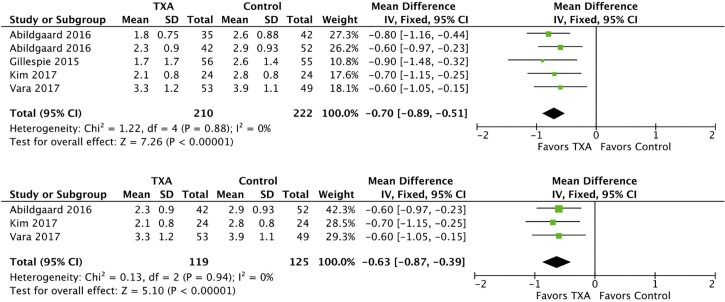
A meta-analysis of the studies listed in Table I was performed for change in hemoglobin, drain output, total blood loss, and risk of transfusion. As shown in Fig. 2, TXA administration was associated with a significantly decreased postoperative change in hemoglobin in both the combined and reverse TSA groups. A fixed effects model was used because the heterogeneity was not statistically significant for the combined (I2 = 0%, P = .88) and reverse TSA analyses (I2 = 0%, P = .94). In the combined group consisting of 432 patients, TXA reduced the postoperative hemoglobin drop by 0.70 g/dL (95% CI, −0.89 to −0.51; P < .00001). In the reverse TSA subgroup consisting of 244 patients, the reduction in the hemoglobin drop was 0.63 g/dL (95% CI, −0.87 to −0.39; P < .00001). There were too few exclusively anatomic TSA cohorts to evaluate the change in postoperative hemoglobin drop for this subgroup.
Figure 2.
Forest plots of change in hemoglobin for combined (above) and reverse total shoulder arthroplasty (below). The squares indicate the mean difference and are proportional to the weights used in the meta-analysis. The horizontal lines represent the 95% confidence interval (CI). The diamond indicates the weighted mean difference, and the lateral tips of the diamond indicate the associated 95% CIs. The vertical line indicates no effect. TXA, tranexamic acid; SD, standard deviation; IV, inverse variance.
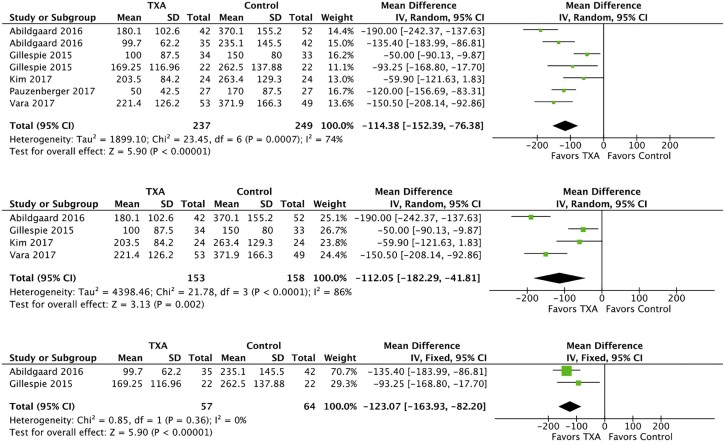
TXA administration was associated with reduced drain output in both reverse and anatomic TSA subgroups as shown in Fig. 3. In the combined group of 486 patients there was significant effect size heterogeneity (I2 = 74%, P = .0007), and a random effects model was used. The mean reduction in drain output was 114.38 mL (95% CI, −152.39 to −76.38 mL). In the reverse TSA subgroup consisting of 311 patients, the effect size heterogeneity was statistically significant with (I2 = 86%, P < .0001), and a random effects model was used. The reduction in drain output for reverse TSA was 112.05 mL (95% CI, −182.29 to −41.81 mL; P = .002]. For the anatomic TSA subgroup (121 patients), the effect size heterogeneity was not statistically significant (I2 = 0%, P = .36), and a fixed effects model was used. The reduction in drain output was 123.07 mL (95% CI, −163.93 to −82.20; P < .00001).
Figure 3.
Forest plots of drain output for combined (top), reverse total shoulder arthroplasty (middle), and anatomic total shoulder arthroplasty (bottom). The squares indicate the mean difference and are proportional to the weights used in the meta-analysis. The horizontal lines represent the 95% confidence interval (CI). The diamond indicates the weighted mean difference, and the lateral tips of the diamond indicate the associated 95% CIs. The vertical line indicates no effect. TXA, tranexamic acid; SD, standard deviation; IV, inverse variance.
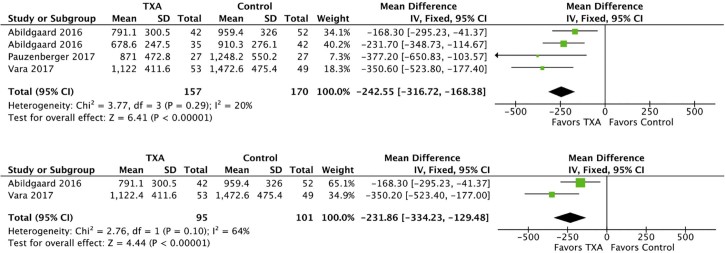
TXA administration was associated with reduced total blood loss in combined and reverse TSA cohorts as shown in Fig. 4. In both combined and reverse TSA analyses consisting of 327 patients, effect size heterogeneity was not statistically significant and fixed effects models were used. The reduction in total blood loss in the combined group was 242.55 mL (95% CI, −316.72 to −168.38; P < .00001). In the reverse TSA subgroup, the reduction in total blood loss was 231.86 mL (95% CI, −334.23 to −129.48; P < .00001). There were too few anatomic TSA cohorts reporting total blood loss for subgroup analysis.
Figure 4.
Forest plots of total blood loss for combined (above) and reverse total shoulder arthroplasty (below). The squares indicate the mean difference and are proportional to the weights used in the meta-analysis. The horizontal lines represent the 95% confidence interval (CI). The diamond indicates the weighted mean difference, and the lateral tips of the diamond indicate the associated 95% CIs. The vertical line indicates no effect. TXA, tranexamic acid; SD, standard deviation; IV, inverse variance.
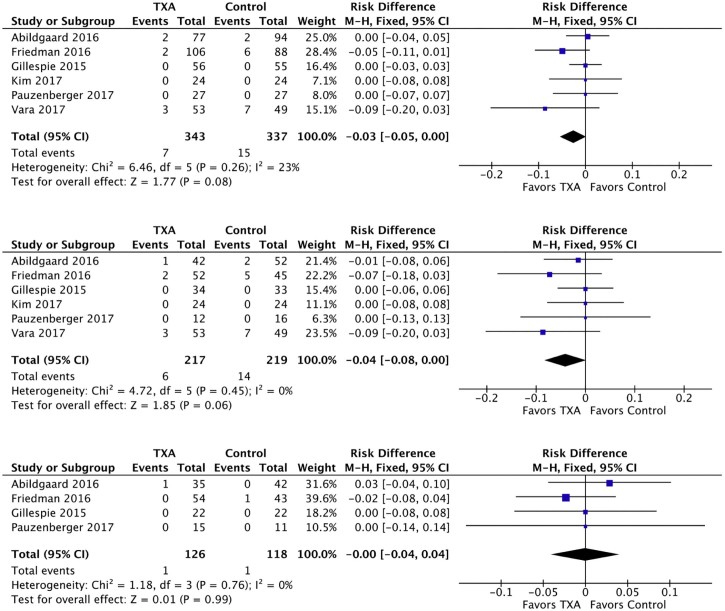
There was a trend toward the reduction of the risk of transfusion in the combined and reverse TSA groups but not the anatomic TSA subgroup (Fig. 5). Effect size heterogeneity was not statistically significant in any of the 3 groups, and fixed effects models were used. In the combined group (680 patients), there was a trend toward the reduction in transfusion risk (−3%; 95% CI, −5% to 0%; P = .08). In the reverse TSA subgroup (436 patients), the risk difference was 4% (95% CI, −8% to 0%; P = .06). Subgroup analysis of 244 anatomic TSA patients demonstrated no trend toward the reduction in the risk of transfusion after anatomic TSA (95% CI, −4% to 4%; P = .99).
Figure 5.
Forest plots of risk of transfusion for combined (top), reverse total shoulder arthroplasty (middle), and anatomic total shoulder arthroplasty (bottom). The squares indicate the mean difference and are proportional to the weights used in the meta-analysis. The horizontal lines represent the 95% confidence interval (CI). The diamond indicates the weighted mean difference, and the lateral tips of the diamond indicate the associated 95% CIs. The vertical line indicates no effect. TXA, tranexamic acid; SD, standard deviation; IV, inverse variance.
Discussion
TXA administration was associated with a significant reduction in postoperative change in hemoglobin concentration, drain output, and total blood loss after reverse TSA. Although not statistically significant, there was a trend (−4%; 95% CI, −8% to 0%; P = .06) toward the reduction in risk of blood transfusion after reverse TSA. In the setting of anatomic TSA, TXA demonstrated a significant reduction in the drain output. The few number of anatomic TSA cohorts, however, limited the ability of the current meta-analysis to evaluate the efficacy of TXA in reducing the postoperative hemoglobin drop, total blood loss, and transfusion rate in this subgroup of patients.
The results of the current study fit with the THA and TKA literature. TXA showed total blood loss reduction of 318 mL for THA and 320 mL for TKA.23 The current meta-analysis found a 232 mL reduction in total blood loss after reverse TSA. Though this is a significant reduction in total blood loss, it is less in absolute terms than the reduction in blood loss seen after THA and TKA. This may stem from a number of factors, including differences in administration and dosing regimens, baseline patient factors, and measurement techniques between the THA/TKA studies and the TSA studies. Another factor may be that TXA reduces blood loss by a similar proportion, rather than absolute amount, across a range blood loss amounts.11 Administration of TXA has resulted in the reduction in the risk of transfusion after THA and TKA by 50% and 47%.23 The current meta-analysis found a trend toward reduced transfusion risk after reverse TSA, but future studies are needed to confirm this trend.
A strength of the current study is the analysis of reverse and anatomic TSA as separate subgroups. Reverse TSA is a more technically demanding procedure and often done for more significant deformity, resulting in increased operative time and increased blood loss. Because of this, reverse versus anatomic TSA has been cited as a risk factor for transfusion after shoulder arthroplasty.6, 9 In addition, because of the change in anatomy associated with reverse TSA, there is commonly more “dead space” between the acromion and proximal humerus, which can result in more frequent hematoma accumulation. This theory is one hypothesis why reverse TSA may be more susceptible to increased blood loss.
Limitations of the current study stem from the few number randomized controlled trials and heterogeneity of TXA regimens. Distinct anatomic and reverse TSA cohorts in future randomized controlled trials are needed to discern the relative efficacy of TXA in anatomic and reverse TSA.
Furthermore, heterogeneity of TXA regimens introduces uncertainty into the assessment of overall efficacy of TXA for TSA. For example, 2 studies used 1 intravenous infusion, although with different dosages,1, 4 1 study used a 2-infusion regimen,22 and another study used topical administration.5 Future studies are needed to evaluate the relative efficacy of these different TXA regimens. In addition, standardization of TXA regimens and dosing may aid future meta-analyses. Given the inconsistent dosing and routes of administration in the studies that met inclusion criteria in our analysis, we cannot make recommendations on method of use for TXA (intravenous, topical, or both) or a standardized optimal dose.
Another limitation our study is that we considered only the efficacy of TXA and did not evaluate its safety. Well-powered meta-analyses in the THA and TKA literature have not found an increase in the rate of venous thromboembolism with TXA use.23 With only 6 studies and the low rate of venous thromboembolism after TSA, our meta-analysis would be underpowered to evaluate the safety of TXA in the setting of TSA. Future studies will be needed to evaluate the efficacy of TXA in the setting of TSA.
Conclusions
Routine administration of TXA reduces perioperative blood loss and may reduce the risk of transfusion after reverse TSA. Future studies are needed to further characterize its effect on the risk of transfusion after reverse TSA and its efficacy in anatomic TSA.
Disclaimer
Michael Khazzam receives research support from Wright Medical/Tornier unrelated to the subject of this article. The other authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
References
- 1.Abildgaard J.T., McLemore R., Hattrup S.J. Tranexamic acid decreases blood loss in total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25:1643–1648. doi: 10.1016/j.jse.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Alshryda S., Sarda P., Sukeik M., Nargol A., Blenkinsopp J., Mason J.M. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93:1577–1585. doi: 10.1302/0301-620x.93b12.26989. [DOI] [PubMed] [Google Scholar]
- 3.Anthony C.A., Westermann R.W., Gao Y., Pugely A.J., Wolf B.R., Hettrich C.M. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthop Relat Res. 2015;473:2099–2105. doi: 10.1007/s11999-014-4107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman R.J., Gordon E., Butler R.B., Mock L., Dumas B. Tranexamic acid decreases blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25:614–618. doi: 10.1016/j.jse.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Gillespie R., Shishani Y., Joseph S., Streit J.J., Gobezie R. Neer award 2015: a randomized, prospective evaluation on the effectiveness of tranexamic acid in reducing blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:1679–1684. doi: 10.1016/j.jse.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Gruson K.I., Accousti K.J., Parsons B.O., Pillai G., Flatow E.L. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18:225–230. doi: 10.1016/j.jse.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Hardy J.C., Hung M., Snow B.J., Martin C.L., Tashjian R.Z., Burks R.T. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:233–239. doi: 10.1016/j.jse.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang J.J., Toor A.S., Shi L.L., Koh J.L. Analysis of perioperative complications in patients after total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1852–1859. doi: 10.1016/j.jse.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Kandil A., Griffin J.W., Novicoff W.M., Brockmeier S.F. Blood transfusion after total shoulder arthroplasty: which patients are at high risk? Int J Shoulder Surg. 2016;10:72–77. doi: 10.4103/0973-6042.180719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ker K., Prieto-Merino D., Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100:1271–1279. doi: 10.1002/bjs.9193. [DOI] [PubMed] [Google Scholar]
- 12.Kim S.H., Jung W.I., Kim Y.J., Hwang D.H., Choi Y.E. Effect of tranexamic acid on hematologic values and blood loss in reverse total shoulder arthroplasty. Biomed Res Int. 2017;2017:9590803. doi: 10.1155/2017/9590803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millett P.J., Porramatikul M., Chen N., Zurakowski D., Warner J.J. Analysis of transfusion predictors in shoulder arthroplasty. J Bone Joint Surg Am. 2006;88:1223–1230. doi: 10.2106/jbjs.e.00706. [DOI] [PubMed] [Google Scholar]
- 14.Padegimas E.M., Clyde C.T., Zmistowski B.M., Restrepo C., Williams G.R., Namdari S. Risk factors for blood transfusion after shoulder arthroplasty. Bone Joint J. 2016;98-b:224–228. doi: 10.1302/0301-620x.98b2.36068. [DOI] [PubMed] [Google Scholar]
- 15.Pauzenberger L., Domej M.A., Heuberer P.R., Hexel M., Grieb A., Laky B. The effect of intravenous tranexamic acid on blood loss and early post-operative pain in total shoulder arthroplasty. Bone Joint J. 2017;99-b:1073–1079. doi: 10.1302/0301-620x.99b8.bjj-2016-1205.r1. [DOI] [PubMed] [Google Scholar]
- 16.2014. Review Manager (RevMan) Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. [Google Scholar]
- 17.Ryan D.J., Yoshihara H., Yoneoka D., Zuckerman J.D. Blood transfusion in primary total shoulder arthroplasty: incidence, trends, and risk factors in the United States from 2000 to 2009. J Shoulder Elbow Surg. 2015;24:760–765. doi: 10.1016/j.jse.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Schumer R.A., Chae J.S., Markert R.J., Sprott D., Crosby L.A. Predicting transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:91–96. doi: 10.1016/j.jse.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Smucny M., Menendez M.E., Ring D., Feeley B.T., Zhang A.L. Inpatient surgical site infection after shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:747–753. doi: 10.1016/j.jse.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Sperling J.W., Duncan S.F., Cofield R.H., Schleck C.D., Harmsen W.S. Incidence and risk factors for blood transfusion in shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14:599–601. doi: 10.1016/j.jse.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Sukeik M., Alshryda S., Haddad F.S., Mason J.M. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93:39–46. doi: 10.1302/0301-620x.93b1.24984. [DOI] [PubMed] [Google Scholar]
- 22.Vara A.D., Koueiter D.M., Pinkas D.E., Gowda A., Wiater B.P., Wiater J.M. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg. 2017;26:1383–1389. doi: 10.1016/j.jse.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Wei Z., Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25:151–162. doi: 10.1111/tme.12212. [DOI] [PubMed] [Google Scholar]