Abstract
The gut microbiome of insects directly or indirectly affects the metabolism, immune status, sensory perception and feeding behavior of its host. Here, we examine the hypothesis that in the oriental fruit fly (Bactrocera dorsalis, Diptera: Tephritidae), the presence or absence of gut symbionts affects foraging behavior and nutrient ingestion. We offered protein-starved flies, symbiotic or aposymbiotic, a choice between diets containing all amino acids or only the non-essential ones. The different diets were presented in a foraging arena as drops that varied in their size and density, creating an imbalanced foraging environment. Suppressing the microbiome resulted in significant changes of the foraging behavior of both male and female flies. Aposymbiotic flies responded faster to the diets offered in experimental arenas, spent more time feeding, ingested more drops of food, and were constrained to feed on time-consuming patches (containing small drops of food), when these offered the full complement of amino acids. We discuss these results in the context of previous studies on the effect of the gut microbiome on host behavior, and suggest that these be extended to the life history dimension.
Introduction
Animals forage for nutritional resources in order to satisfy their requirements for growth and reproduction [1,2]. This behavior is constrained by spatial and temporal factors (biotic and abiotic), and modulated to a large extent by the phenotypic plasticity and metabolic state of each organism [3]. Evidence from numerous studies suggests that insects (and other arthropods) are capable of tailoring their foraging activity and ingestion of nutrients in a manner that corresponds to their specific requirements [4] (and references therein).
In insects, responses to environmental stimuli are modulated by substrate specific chemoreceptors, whose sensitivity is modulated by the nutritional status of the individual [5–7]. Thus, for example, in the vinegar fly Drosophila melanogaster (Diptera: Drosophilidae), flies deprived of amino acids exhibit an enhanced response to amino acids missing from their diets [8]. Similarly, tephritid fruit fly sensory responses and foraging activity are affected by nutritional status [9–11].
The microbiome resident in the gut of arthropod (and vertebrate) hosts adds another layer of complexity to the modulation of behavior in general and foraging behavior in particular [12–14]. This effect has been demonstrated along the various steps of the nexus connecting the gut and its microbiome to behavior, through metabolism, the immune and nervous systems, and sensory receptors. Thus, in D. melanogaster, the microbiota has multiple impacts on metabolism such as immune homeostasis, lipid and carbohydrate storage and vitamin sequestration [15–17]. These effects are extended to responses to food and ultimately affect foraging activity. In Tenebrio molitor (Coleoptera: Tenebrionidae) mealworms, individuals whose immune system is activated by a pathogen consume significantly more proteinaceous food than healthy individuals [18]. Conversely, stinkbug (Megacopta punctatissima, Hemiptera: Plataspidae) nymphs that acquire symbionts after hatching exhibit lower activity levels than symbiont free nymphs [19]. Recently, Wong et al. [20] demonstrated that the microbiome of D. melanogaster influences the olfactory sensitivity and foraging behavior of hosts in a manner that apparently benefits the bacteria specifically. Remarkably, there is evidence that the nutritional status of the host interacts with the microbiome to control foraging behavior [21]. In D. melanogaster, the absence of specific amino acids will trigger specific appetites for the missing nutrient. However, the presence of bacteria (that presumably could provide the missing amino acid), overrides such preferences [22].
Tephritid fruit flies (Diptera: Tephritidae) harbour communities of bacteria dominated by species of Enterobacteriacae [23]. These microbes have been shown to be involved in Nitrogen fixation [24,25], reproductive success [26,27], temporal host range expansion [28], protection from pathogens [29] and detoxification [30].
Adult tephritid flies require a mixed diet consisting of carbohydrate and protein, or at least protein precursors [31]. These nutrients are acquired by active foraging during daylight hours [32]. Sugars are acquired from nectar, honeydew and fruit juices, while nitrogenous compounds are taken up by feeding on bird feces, or in some cases bacteria on the phylloplane [33]. The presence of gut bacteria in adult flies contributes to their nutrition, specifically by brokering intractable sources of Nitrogen into essential amino acids. Thus, Bactrocera oleae (Diptera: Tephritidae) females which were experimentally deprived of gut bacteria were unable to produce eggs when they only had access to non-essential amino acids [31,34]. Foraging for food is risky, as active flies are exposed to predators and incur a considerable investment of time and energy.
The oriental fruitfly Bactrocera dorsalis (Diptera: Tephritidae) is one of the most invasive, multivoltine and polyphagous members of the Tephritidae family. This fly causes considerable loss of cultivated crops in most western and eastern parts of Asia and attacks over 350 host species worldwide [24,35]. Studies based on Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis (PCR-DGGE) fingerprinting and High throughput pyrosequencing of the 16S rRNA gene have highlighted the prevalence of microbial communities inhabiting the gut [24,36–38] and reproductive organs of this insect [39], which play critical roles in host physiology and reproduction [39,40]. Pyrosequencing analysis of the B. dorsalis microbiome reveals 172 Operational Taxonomic Units assigned to 6 phyla (with Firmicutes as the most abundant in adult stages), 5 families, and 13 genera [24,41]. The predominant bacterial family in most of the previous studies was Enterobacteriaceae from which many cultivable species were identified, such as Klebsiella oxytoca, Enterobacter cloacae, Morganella sp., Moraxella proteus, Providencia rettgerii and Citrobacter freundii. Other bacterial species like Pseudomonas aeruginosa (Pseudomonadaceae), Enterococcus phoeniculicola (Enterococcaceae), Lactobacillus lactis (Streptococcaceae) and the genus Listeria (Listeriaceae) were also identified from B. dorsalis [27,37,38].
In the present study we examine the hypothesis that in the Oriental fruit fly, the presence or absence of gut symbionts will affect foraging behavior and nutrient ingestion. We offered protein-starved flies, symbiotic or aposymbiotic, a choice between diets containing all amino acids or only the non-essential ones. The different diets were presented in a foraging arena as drops that varied in their size and density, creating an imbalanced foraging environment. We predicted that symbiotic flies would consistently choose the diet that was most profitable in terms of foraging time. Conversely, we predicted that flies lacking symbionts would be constrained to forage on diets containing all amino acids, while incurring costs of increased exposure and foraging time.
Materials and methods
Fly rearing and handling
Bactrocera dorsalis larvae were collected from infested fruits from the experimental orange orchard of Huazhong Agricultural University (30°4’N and 114°3’ E). The larvae were carefully removed after peeling the orange fruits and allowed to develop in a wheat-bran based larval artificial diet. The third instar larvae were allowed to pupate in sterile sand under laboratory conditions and the resulting adults were kept under rearing since 2014 [24]. At each generation, flies were reared as described by Nash and Chapman [42] with slight modifications. Briefly, 100 adult males and females were housed in 5L cages at equal proportions. These cages were maintained under controlled environment: 12:12 light-dark photoperiod; temperature 26±3°C, and 57±5% relative humidity. Water was provisioned ad libitum and the adult diet consisted of Tryptone (25 g/L), Yeast extract (90 g/L), Sucrose (120 g/L), Agar powder (7.5 g/L), Methyl-p-hydroxybenzoate (4 g/L), Cholesterol (2.3 g/L), Choline chlorite (1.8 g/L), Ascorbic acid (5.5 g/L) dissolved in 1 L of distilled Water and steamed at 55°C for 20 min. The larval diet consisted of the same ingredients of adult diet (as described above) to which we added 250 g/L of wheat bran.
Symbiotic and aposymbiotic flies
Symbiotic and aposymbiotic flies were produced from the laboratory established colony. Until day 4 post-emergence, they were fed on sugar diet (Su) consisting of 60% sucrose and minerals, and then were divided in two groups of 30 flies each: the symbiotic flies (15 males and 15 females) were fed sugar diet (without antibiotics) till day 7 whereas the aposymbiotic ones were fed the same sugar meal but inoculated with antibiotics (3mcg/mL Norfloxacin and 5mcg/mL Ceftazedime) till day 7 [31]. The diets were provisioned in 9 cm petri dishes presented in sterilized cotton wool. The antibiotics were selected after in vitro susceptibility test to 7 bacterial isolates and their in vivo capacity to significantly clear the gut of B. dorsalis within four feeding days (Table 1). The 3mcg/mL and 5mcg/mL, respectively, represent the minimum inhibitory concentrations (MIC) of the selected antibiotics.
Table 1. Antibiotics susceptibility testing by disc diffusion of gut bacteria isolated from the oriental fruit fly Bactrocera dorsalis.
| No. | Antimicrobial Agent | Disc potency (μg/piece) |
Enterococcus faecalis DBS-LAZ-13/17 (MG231268) |
Klebsiella oxytoca FQH (MF144436) | Bacillus cereus L90 (KC428751) | Enterobacter cloacae ATCC13047 (NR_102794.2) | Pantoea dispersa DSM30073(NR_116797) | Proteus mirabilis NCTC11938 (NR_043997) |
Staphylococcus sciuri IESE:STI (KU962123) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ampicillin | 10 | R | I | S | I | R | R | R | |
| 2 | Ceftazidime | 30 | S | S | S | S | S | S | S | * |
| 3 | Chloramphenicol | 30 | S | S | I | R | S | I | I | |
| 4 | Ciprofloxacin | 5 | I | S | S | S | R | S | S | |
| 5 | Clindamycin | 2 | R | I | R | R | R | R | R | |
| 6 | Erythromycin | 15 | R | R | R | R | R | R | R | *** |
| 7 | Gentamicin | 10 | S | S | R | S | S | S | S | ** |
| 8 | Kanamycin | 30 | S | S | R | S | S | S | S | ** |
| 9 | Minocyclin | 30 | S | S | R | R | I | I | R | |
| 10 | Norfloxacin | 10 | S | S | S | S | S | S | S | * |
| 11 | Ofloxacin | 5 | S | S | R | S | S | S | S | * |
| 12 | Penicillins | 10 | I | R | R | R | R | R | R | *** |
| 13 | Piperacillin | 100 | S | S | S | I | R | S | R | |
| 14 | PolymyxinB | 300 | S | S | R | S | S | S | S | ** |
| 15 | Tetracyclin | 30 | S | S | R | R | R | R | I | *** |
Our isolated bacteria sequences share 100% similarity with species from GenBank whose strains and accession numbers are provided.
R = Resistant, S = susceptible, I = intermediate. R, S and I were determined after the in vitro susceptibility test.
* = Antibiotics potent to all gut bacterial isolates
** = Antibiotics potent to all bacterial isolates except Bacillus cereus
*** = Non potent antibiotics
To confirm the aposymbiotic status of the flies, 15 flies from each group were separately removed, individually anaesthetized by keeping them at -20°C for 20 min and then dissected. Their individual guts were aseptically homogenized and the gut homogenates were serially diluted up to 10−7 dilution in deionized distilled water and 100 μl of each dilution was spread onto LB-Agar plates (pH 7.2–7.4) and incubated at 30°C for 48–78h. Then, the Colony Forming Units (CFU) resulting from the bacterial colonies on each plate were averaged and analyzed within and between samples. The estimation level of the cultivated microbial communities in antibiotic treated flies gave 1.694x102±26.1 CFUs g-1.gut-1 (mean ± SE of 15 individual flies) representing just 0.054% of the total bacterial communities of the normal flies which was 3.127x106±8.24x102 CFUs g-1.gut-1 (Independent T-Test, F = 26.809; t = 14.517; df = 1, 28; P < 0.0001). After this estimation, the antibiotic treated flies were confirmed aposymbiotic and were used as such in subsequent experiment. All the flies were starved for 24h before experiments.
Preparation of experimental diets
Three different diets were prepared. A full diet (F) containing all amino acids (essential and non-essential), sucrose, and minerals (Table 2), required for an optimal maintenance and reproductive development of adult flies [31]; a non-essential amino acid diet (NE) containing exclusively non-essential amino acids, sucrose and minerals. A Sugar diet (Su) was provided before the experiments. The diet ingredients and preparation procedures were done as described by Ben-Yosef et al. [31].
Table 2. Nutrient composition of the experimental diets.
| Ingredients | Components | Contents (mg) | |
|---|---|---|---|
| F | NE | ||
|
Essential amino acids |
L-arginine | 50.45 | |
| L-histidine | 21.54 | ||
| L-isoleucine | 26.64 | ||
| L-leucine | 51.02 | ||
| L-lysine | 27.78 | ||
| L-methionine | 13.04 | ||
| L-phenylalanine | 33.44 | ||
| L-threonine | 25.51 | ||
| L-tryptophan | 13.60 | ||
| L-valine | 37.41 | ||
|
Non-essential amino acids |
L-alanine | 36.85 | 36.85 |
| L-aspartic acid | 53.28 | 53.28 | |
| L-aspartic acid | 19.27 | 19.27 | |
| L-glutamic acid | 185.36 | 185.36 | |
| Glycine | 42.51 | 42.51 | |
| L-proline | 58.95 | 58.95 | |
| L-serine | 36.85 | 36.85 | |
| L-tyrosine | 22.67 | 22.67 | |
| FeSO4 | 2.50 | 2.50 | |
| MnSO4 | 0.63 | 0.63 | |
| ZnCl2 | 0.63 | 0.63 | |
| Minerals and salts | CuSO4 | 0.31 | 0.31 |
| MgSO4 | 20.00 | 20.00 | |
| KH2PO4 | 84.65 | 84.65 | |
| Ca(H2PO4)2 | 10.00 | 10.00 | |
| KCl | 117.00 | 117.00 | |
| NaCl | 45.00 | 45.00 | |
| White sugar | 10000.0 | 10000.0 | |
| DDW | 50000.00 | 50000.00 | |
F: Full diet; NE: Non-essential amino acid diets; DDW: Deionized distilled water.
Experimental procedures
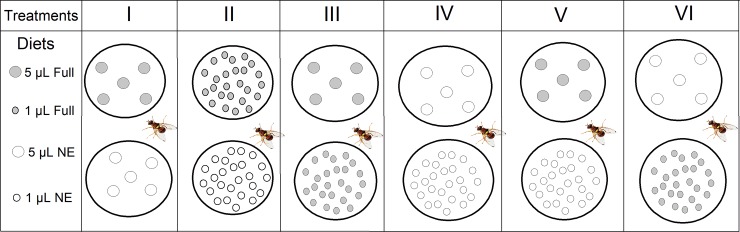
Following the seven day preparatory period during which flies were fed only sugar (as described above), an individual fly from each treatment was transferred to a 20 x 20 cm cage and allowed to acclimatize for 20 minutes before introducing a pair of petri dishes containing combinations of two different diet types (Full or NE) at different densities (Fig 1). To create different foraging environments, 25 drops of 1 μL volume (very small so as to force the flies to seek out many drops in order to become satiated), or 5 drops of 5 μL volume were pipetted onto each dish. Six treatment groups were set up representing six different foraging environments (Fig 1). Each of the six treatments was replicated 15 times (15 flies) and each replicate consisted of observing the protein starved individual male or female (symbiotic and aposymbiotic) for 1 hour. To motivate foraging behavior and allow the flies to recover from antibiotic effects, all the flies were starved for 24 hours before experimental trials. For each replicate (single fly in foraging environment), the response to the food drops (landing on a drop), Data on latency (duration from diet exposure to the initial landing), the number of flies that landed on a food drop (responses), the choice of diet made, the number of drops consumed (ingestion), and the time spent feeding were recorded and analyzed within and between treatments. Only drops that disappeared completely as a result of the fly feeding on it were considered in the estimation of the ingestion. In the rare cases when a food drop was partially consumed (n = 12 out of n = 360 feeding observations in symbiotic and aposymbiotic, male and female flies), the feeding event was discarded from the analyses.
Fig 1. Experimental arenas of the effect of diet quality and density on foraging decisions by the oriental fruit fly Bactrocera dorsalis.
Full diets contain all amino acids, and NE diets contain only the non-essential amino acids.
Statistical analyses
All data were tested for homogeneity of variances using Levene’s tests. To determine the important factors that shape the foraging behavior of experimental flies, variables of overall response (the number of flies that visited a food drop per treatment) and latency were analyzed (each one separately) using the ordinary least squares regression model (SPSS 20.0 software) with sex, symbiotic status, treatment and the interaction between symbiotic status and treatment as effects. Pearson Chi-Square test was used to determine the significance of responses based on the effects (treatments, sex and symbiotic status). Colony Forming Units of antibiotics treated and untreated samples were analyzed using an independent T-Test. The One-Way Analysis of Variance (ANOVA) was performed to analyze data on landing, the number of drops consumed, and the time spent and switching using SPSS 20.0 software (Statsoft Inc, Carey, NJ, USA). Tukey’s HSD Test (HSD) test was used for mean separations within and between each treatment. Differences among measured parameters were considered significant when the p values were less than 0.05 after comparison with HSD test. All results were expressed as the means with standard errors (SE), except data on the overall responses. OriginPro software version 8.5.1 was used to draw curves and graphs. All data collected and details on statististic models used can be found in S1 Table.
Results
Responses to the experimental arenas
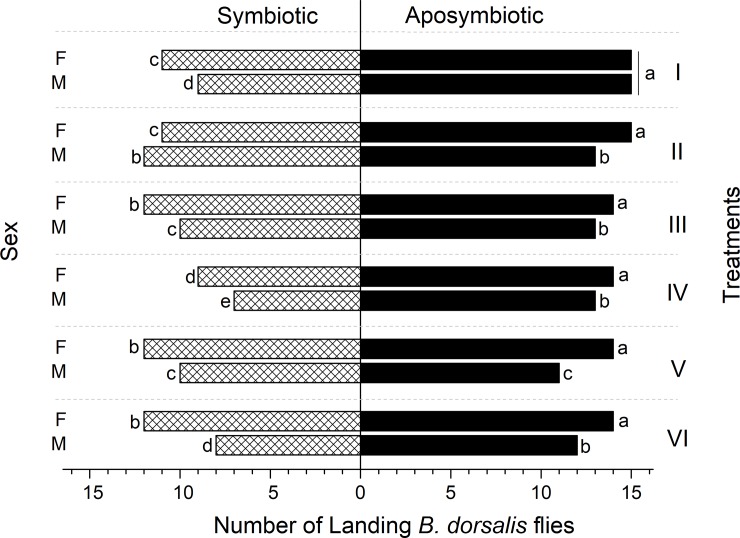
Overall, of the 15 aposymbiotic flies in each treatment, on marginal average 14.83±0.37 females and 14.67±0.75 males, responded to the diets presented, while in symbiotic groups, only 10.83±1.34 females and 8.67±1.97 males responded out of 15 flies on average (Fig 2).
Fig 2. Response parameters of Bactrocera dorsalis females to experimental diets.
Each response datum consisted of the overall number of flies which landed in different treatments regardless the diet quality or drop size.
In general, most of the aposymbiotic flies landed. The overall response (number of responding flies per treatment) of symbiotic and aposymbiotic flies to the different treatments was significantly affected by symbiotic status (Ordinary Least Squares Regression Model, F = 15.834; df = 3; r2 = 0.839; t = 6.048; P < 0.001), and by sex (F = 15.83; df = 3; r2 = 0.839; t = -2.946; P = 0.043) (Fig 3). Pearson Chi-Square test revealed significant associations between the symbiotic status, sex and the responses (χ2 = 4.756, df = 1, P = 0.029), while no significant interaction exist between the treatments and the responses (χ2 = 8.621, df = 5, P = 0.125).
Fig 3. Response parameters of Bactrocera dorsalis males to experimental diets as affected by the symbiotic status, sex and treatments.
Each latency datum is presented as a Mean±SE of the overall latency of responded flies in each treatment.
The treatment itself did not affect the overall response of flies (F = 15.834; df = 3; r2 = 0.839; t = -1.498; P = 0.197), but significantly influenced the latency to respond by either reducing or delaying the time elapsed to the first landing decision (F = 11.834; df = 3; r2 = 0.796; t = 0.216; P < 0.0001) (Fig 3). The latency to respond in the experimental chambers was similarly affected by symbiotic status (F = 11.538; df = 3; r2 = 0.796; t = -4.929; P < 0.0001) and sex (F = 11.538; df = 3; r2 = 0.796; t = 3.24; P = 0.04) (Fig 3). In general, aposymbiotic flies responded faster in the experimental chambers than the symbiotic ones, and females in aposymbiotic groups landed on food drops faster than the males (Fig 3).
Switching behavior
Shifting from one diet to another was common, and showed a clear trend. Switching from Full to NE diets was not affected by sex (Linear Regression, F = 0.857, df = 1, P = 0.373), but was significantly affected by the symbiotic status (Linear Regression, F = 30.857, df = 1, P < 0.0001) while the sex and symbiotic status did not affect the switching of flies from NE to Full diets, respectively (Linear Regression, F = 1.829, df = 1, P = 0.201 and F = 1.829, df = 1, P = 0.433, respectively) (Table 3). No aposymbiotic flies which landed on the Full diet shifted to the NE diet and those which initially landed on the NE diet recorded a faster shifting latency (time to move from an initial patch to another) toward the Full diet in comparison with the symbiotic females and males (F = 10.857; df = 3,12; P = 0.001, and F = 10.857, df = 3,12; P = 0.012, respectively) (Table 3). However, 1 symbiotic female and male among those that initially landed on the Full diet shifted to the NE diet, but within a long shifting latency (F = 15.62, df = 3, 12; P = 0.001 and F = 15.62, P = 0.008, in females and males, respectively) (Table 3).
Table 3. Switching behavior of B. dorsalis as influenced by diet quality.
The analysis involved only treatments with different diet types regardless the drop size. Each value is presented as a Mean ± SE of the four treatments, each consisting of 15 replications.
| Initial landing | Shifting to | Symbiotic | Aposymbiotic | ||
|---|---|---|---|---|---|
| Females | Males | Females | Males | ||
| Full diet | NE diets | 1.25±0.48a | 1.75±0.25a | 0.00b | 0.00b |
| Latency (min.) | 28.25±1.8A | 19±2.74B | 0.00C | 0.00C | |
| NE diet | Full diets | 2±0.41a | 2.25±0.25a | 1.25±0.48a | 2.25±0.63a |
| Latency (min.) | 15.75±1.11A | 12±0.08A | 5.75±1.65B | 5±1.29B | |
Means of shifting flies and shifting latency followed by different letters (small and capital letters, respectively) within rows are statistically different after Tukey HSD Test at P = 0.05.
Ingestion
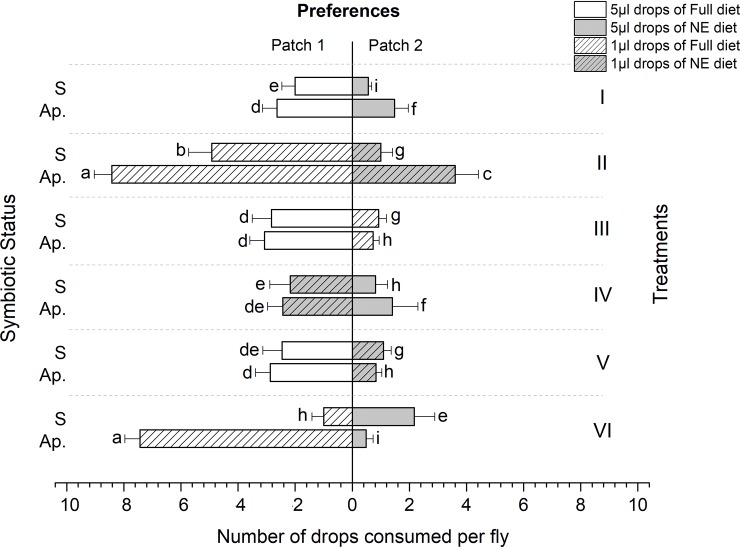
Overall, the number of drops consumed depended significantly on drop size, diet (full or NE), treatment (foraging environment), sex and symbiotic status of the flies observed (ANOVA; F = 45.86, df = 5, P < 0.0001, R2 = 0.96).
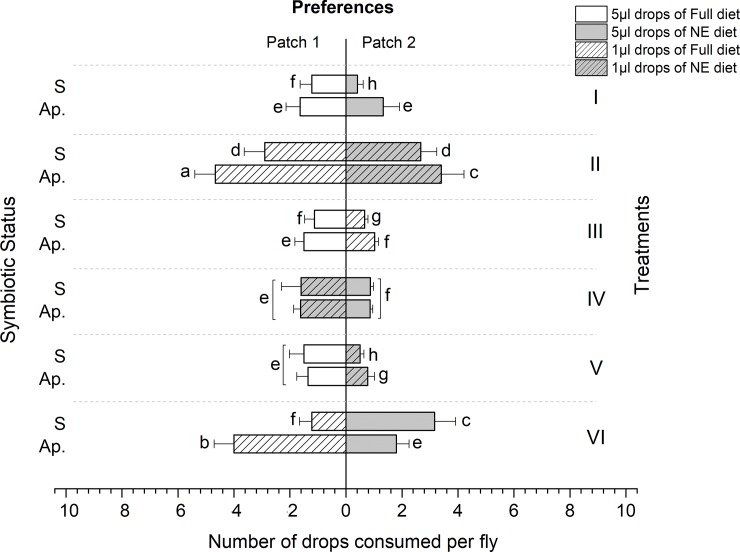
In general, aposymbiotic flies consumed significantly more of food drops than the symbiotic ones irrespective of diet quality and drop size (ANOVA, F = 39.543, df = 1, P < 0.0001, and F = 12.167, df = 1, P = 0.001 in females and males, respectively) (Treatments I, II, V & VI, Figs 4 and 5).
Fig 4. Number of nutritional drops consumed by symbiotic (S) and aposymbiotic (Ap.) Bactrocera dorsalis females exposed to two diet patches (full and non-essential amino acids’ diets).
Each bar represents the Mean±SE of consumed drops by each fly.
Fig 5. Number of nutritional drops consumed by symbiotic (S) and aposymbiotic (Ap.) Bactrocera dorsalis males exposed to two diet patches (full and non-essential amino acids’ diets).
Each bar represents the Mean±SE of consumed drops by each fly.
Ingestion of Full diets (high or low volume) from all treatment groups was significantly higher in all tested flies, males and females (F = 64.12, df = 5, P < 0.0001, R2 = 0.94, and F = 11.72, df = 5, P < 0.0001, R2 = 0.83, respectively) (Figs 4 and 5). Nevertheless, compared to males, females displayed a significant preference toward diets with high reward (full diet, large drops) in unbalanced nutritional environments (F = 41.56, df = 5, P < 0.0001, r2 = 0.87) (Figs 4 and 5).
Aposymbiotic flies of both sexes preferentially chose to feed on the Full diet in most treatments, regardless of drop size (except aposymbiotic males in treatment I, who consumed the large Full and NE drops offered to the same extent) (Fig 5). Most importantly, symbiotic condition significantly affected fly feeding behavior in treatment VI. Here, flies were offered many low volume drops of the Full diet, together with few high volume drops of the NE diet. Both male and female aposymbiotic flies were compelled to consume numerous drops of the low volume, Full diet drops, but aposymbiotic male flies ingested higher overall amount of NE diet drops (Fig 5). Interestingly, the actual volume of NE diet (presented in the large drops) consumed by aposymbiotic males was higher than the volume of the Full diet ingested (Fig 5, Treatment VI) (F = 14.22, df = 5, P < 0.0001, R2 = 0.49, and F = 5.01, df = 5, P < 0.0001, R2 = 0.38, for males and females, respectively) (Figs 4 and 5).
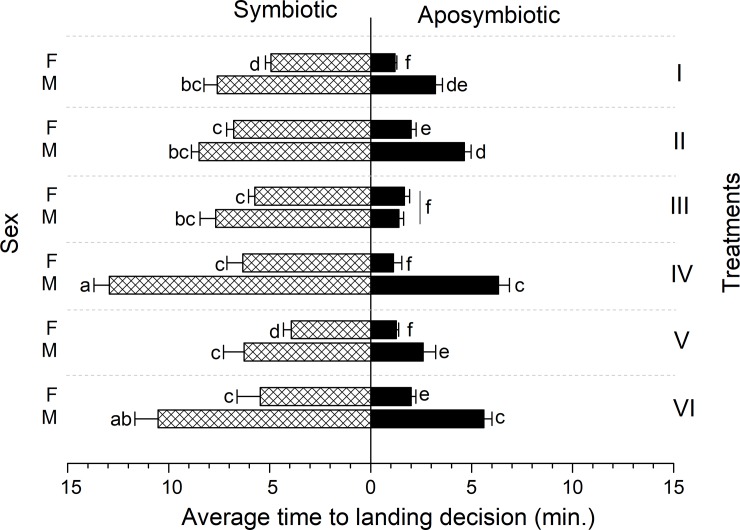
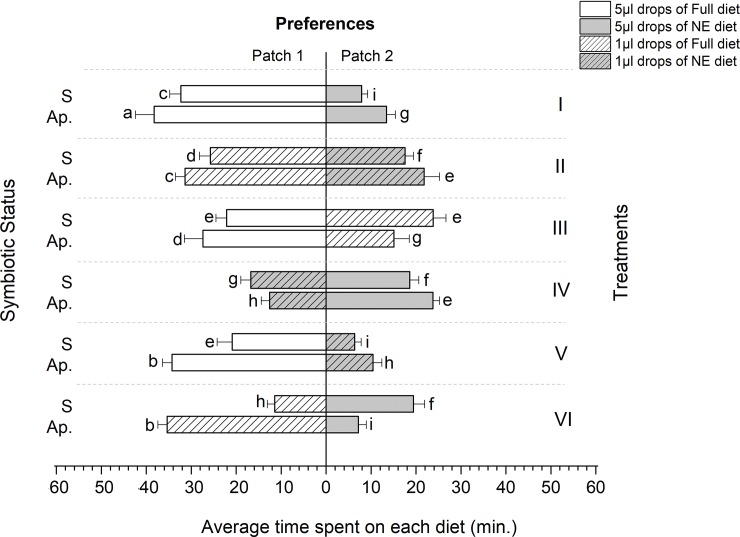
Allocation of time to feeding
With the exception of symbiotic females and males in treatment VI, all the experimental flies spent more time foraging on Full diets (Figs 6 and 7). However, the longest time spent on Full diets was recorded in aposymbiotic flies regardless of drop size. Overall, aposymbiotic females spent on average 46.27±2.15 minutes on Full diets compared to 28.43±2.49 minutes by symbiotic females (F = 94.52, df = 5, P = 0.023, R2 = 0.96) (Figs 6 and 7). Similarly, aposymbiotic males spent 38.27±4.15 minutes on Full diets, compared to 19.43±2.49 minutes for symbiotic males (F = 33.14, df = 5, P = 0.041, R2 = 0.92) (Figs 6 and 7).
Fig 6. Feeding duration of Bactrocera dorsalis females under different foraging environments (Treatments).
S: Symbiotic and Ap.: Aposymbiotic. Mean values followed by different letters are statistically different after Tukey HSD Test at P = 0.05.
Fig 7. Feeding duration of Bactrocera dorsalis males under different foraging environments (Treatments).
S: Symbiotic and Ap.: Aposymbiotic. Mean values followed by different letters are statistically different after Tukey HSD Test at P = 0.05.
A comparison based on drop size also revealed a significantly longer time spent on high volume drops by aposymbiotic flies, except in treatment VI (Figs 6 and 7). Aposymbiotic flies feeding on patches containing NE diets recorded the shortest time spent, with an average time of 5.43±1.95 minutes per patch visited by females compared to 46.27±2.15 minutes when the flies fed on patches containing Full diets. Similarly, aposymbiotic males spent 7.9±1.33 minutes feeding on patches containing NE diets compared to 38.27±4.15 minutes when they fed on patches containing Full diets (Figs 6 and 7).
Discussion
Foraging entails decision making, whereby each individual must consider trade-offs between energetic and nutrient gain, and the time and risk associated with this activity [2]. Furthermore, when organisms need to ingest nutrients from various food sources, behavioral mechanisms that optimize intake come into play [4]. Gut bacteria have been implicated in this decision making process, both in invertebrates [20,22] and vertebrates [12,43].
In our experiments, when the symbiotic flies were presented a combination of different patch volumes (Treatments III, IV, V, and VI, Fig 1), they preferentially consumed larger drops (Treatments III, V and VI) but when they chose to feed on small drops, they generally consumed a higher number compared to larger drops irrespective of the diet quality presumably to become satiated (Treatment IV). This selective behavior had direct effects on the time spent feeding on a given patch. Our result confirms the abundant previous evidence whereby insects adopt a variety of mechanisms which allow them to optimally regulate the amount of food they ingest when they are confronted with an imbalanced foraging environment, to rapidly reach their nutritional target and limit their exposure [44–46].
The suppression of the microbiome using antibiotics resulted in significant changes of the foraging behavior of both male and female flies. The flies starved for 24 hours prior to bioassays to improve appetitive motivation and allow them to recover from any unintended effects of the antibiotic that could potentially influence behavior. Previous work on medfly [47] and olive fly [28,31] determined that the behavioral changes of the flies stem from the absence of bacteria and not the presence of the antibiotic in the flies system. Accordingly, the overall behavioral changes observed in our experiments can be attributed to the absence of gut bacteria. Aposymbiotic flies responded faster to the diets offered in experimental arenas, spent more time feeding, ingested more drops of food, and were constrained to feed on time consuming patches (containing small drops of food), when these were offered the full complement of amino acids (treatment VI). These findings join a number of recent studies [15–17] in understanding the effect of gut bacteria on different stages of the nexus linking the gut to behavior, and significantly, extend this nexus to patterns of active foraging.
Aposymbiotic flies responded at a higher rate and with shorter latency to the experimental foraging arenas, compared to symbiotic flies (Figs 2 and 3). This suggests that the absence of bacteria in the gut affected the motivational state of these flies, presumably by lowering the response threshold to visual and olfactory stimuli associated with food. In insects, response thresholds to external chemical and visual stimuli are modulated by physiological status [6], which in turn is affected by the presence and composition of the gut microbiome [15,16]. Our results join previous studies in suggesting that the presence or absence of intestinal bacteria can affect behavioral thresholds [7,20,48].
The flies in our experiments, both symbiotic and aposymbiotic, were maintained on a Nitrogen free diet prior to their introduction into the foraging arenas. Previous work on tephritids has established that the gut microbiome is capable of transforming non-essential amino acids (and other intractable sources of Nitrogen), into the building blocks necessary for reproduction and development [26,31,34] (MA and CYN, unpublished data). Accordingly, we predicted that, when presented with a choice between a diet containing only the non-essential amino acids and a diet containing all amino acids, although both flies (symbiotic and aposymbiotic) would behave in a manner consistent with optimal foraging theory, aposymbiotic flies would be constrained to forage preferentially on Full diets, at the cost of significantly extending the amount of time spent foraging. Indeed, the results of our experiment support these predictions. Symbiotic flies significantly spent less time feeding than aposymbiotic flies, and achieved this (energetic and risk averse) saving by feeding on large drops, irrespective of the diet they contained. Conversely, aposymbiotic flies spent more time ingesting food drops, and were compelled to seek drops containing the full diet, even when this choice entailed ingesting a large number of small drops when large (but essential amino acid deficient) drops were available, as in treatment VI (Figs 6 and 7).
We suggest that the next dimension to be explored in this context is a life history one. In monophagous species obligatory gut symbionts enable exploitation of otherwise toxic host plants during the larval stage [28,49], or facultatively enable expansion of the native host range [50]. Empirically, the microbiome of polyphagous tephritids is more varied than that of monophagous tephritid species [23,28,51]. Thus the ability of the microbiome to contribute to the larval phase may come at a cost during the adult phase, when a varied microbiome may be advantageous. In this study we examined the foraging behavior of adult oriental fruit flies, a polyphagous species, with a varied microbiome [24,37,38].
Conclusion
The results of our study support the emergent paradigm of the effect of gut bacteria on their hosts, affecting gustatory thresholds, feeding behavior and ultimately (as shown here), to patterns of foraging in imbalanced nutritional environments. In future studies, we plan to add a life history dimension to these observations and examine the performance of monophagous flies in similar experimental foraging environments.
Supporting information
(XLSX)
Data Availability
All relevant data are uploaded along with this manuscript.
Funding Statement
This study was funded by the Joint programme of the Israel Science Foundation and the Science Foundation of China (2482/16), National Natural Science Foundation of China (31661143045), International Atomic Energy Agency (CRP No. 17153 and No. 18269), Agricultural public welfare industry research supported by Ministry of Agriculture of People’s Republic of China (201503137) and the Fundamental Research Funds for the Central Universities (2662015PY148).
References
- 1.Slansky Jr F (1993) Nutritional ecology: The Fundamental Quest for Nutrients. In Caterpillars: Ecological and Evolutionary Constraints on Foraging: 29–91. (Ed. by Stamp, N. E. & Casey, T. M.).
- 2.Stephens DW, Krebs JR (1986) Foraging theory: Princeton University Press. [Google Scholar]
- 3.Raubenheimer D, Simpson SJ, Mayntz D (2009) Nutrition, ecology and nutritional ecology: toward an integrated framework. Functional Ecology 23: 4–16. 10.1111/j.1365-2435.2008.01522.x [DOI] [Google Scholar]
- 4.Simpson SJ, Clissold FJ, Lihoreau M, Ponton F, Wilder SM, et al. (2015) Recent advances in the integrative nutrition of arthropods. Annual Review of Entomology 60: 293–311. 10.1146/annurev-ento-010814-020917 [DOI] [PubMed] [Google Scholar]
- 5.Chapman RF (2003) Contact chemoreception infeeding by phytophagous insects. Annual Review of Entomology 48: 455–484. 10.1146/annurev.ento.48.091801.112629 [DOI] [PubMed] [Google Scholar]
- 6.Gadenne C, Barrozo RB, Anton S (2016) Plasticity in insect olfaction: to smell or not to smell? Annual Review of Entomology 61: 317–333. 10.1146/annurev-ento-010715-023523 [DOI] [PubMed] [Google Scholar]
- 7.Sengupta P (2013) The belly rules the nose: feeding state-dependent modulation of peripheral chemosensory responses. Current Opinion in Neurobiology 23: 68–75. 10.1016/j.conb.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toshima N, Tanimura T, (2012) Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. Journal of Experimental Biology 215: 2827–2832. 10.1242/jeb.069146 [DOI] [PubMed] [Google Scholar]
- 9.Jin S, Zhou X, Gu F, Zhong G, Yi X (2017) Olfactory plasticity: variation in the expression of chemosensory receptors in Bactrocera dorsalis in different physiological states. Frontiers in Physiology 8: 672 10.3389/fphys.2017.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prokopy RJ, Drew RAI, Sabine BNE, Lloyd AC, Hamacek E (1991) Effect of physiological and experiential state of Bactrocera tryoni flies on intra-tree foraging behavior for food (Bacteria) and host fruit. Oecologia 87: 394–400. 10.1007/BF00634597 [DOI] [PubMed] [Google Scholar]
- 11.Reyes H, Malo EA, Toledo J, Cruz-Esteban S, Rojas JC (2017) Physiological state influences the antennal response of Anastrepha obliqua to male and host volatiles. Physiological Entomology 42: 17–25. 10.1111/phen.12157 [DOI] [Google Scholar]
- 12.Alcock J, Maley CC, Aktipis CA (2014) Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. Bioessays 36: 940–949. 10.1002/bies.201400071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisthen HL, Theis KR (2016) Animal—microbe interactions and the evolution of nervous systems. Philosophical Transactions of the Royal Society B-Biological Sciences 371: 20150052 10.1098/rstb.2015.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong ACN, Luo Y, Jing X, Franzenburg S, Bost A, et al. (2015) The host as the driver of the microbiota in the gut and external environment of Drosophila melanogaster. Applied and Environmental Microbiology 81: 6232–6240. 10.1128/AEM.01442-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newell PD, Douglas AE (2014) Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Applied and Environmental Microbiology 80: 788–796. 10.1128/AEM.02742-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridley EV, Wong ACN, Westmiller S, Douglas AE (2012) Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PloS One 7: e36765 10.1371/journal.pone.0036765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong ACN, Dobson AJ, Douglas AE (2014) Gut microbiota dictates the metabolic response of Drosophila to diet. Journal of Experimental Biology 217: 1894–1901. 10.1242/jeb.101725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catalan TP, Barcelo M, Niemeyer HM, Kalergis AM, Bozinovic F (2011) Pathogen- and diet-dependent foraging, nutritional and immune ecology in mealworms. Evolutionary Ecology Research 13: 711–723 [Google Scholar]
- 19.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T (2008) Symbiont acquisition alters behaviour of stinkbug nymphs. Biology Letters 4: 45–48. 10.1098/rsbl.2007.0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong AC-N, Wang Q-P, Morimoto J, Senior AM, Lihoreau M, et al. (2017) Gut microbiota modifies olfactory-guided microbial preferences and foraging decisions in Drosophila. Current Biology 27: 2397–2404.e2394. 10.1016/j.cub.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 21.Yuval B (2017) Symbiosis: gut bacteria manipulate host behaviour. Current Biology 27: 746–747. 10.1016/j.cub.2017.06.050 [DOI] [PubMed] [Google Scholar]
- 22.Leitao-Goncalves R, Carvalho-Santos Z, Francisco AP, Fioreze GT, Anjos M, Baltazar C, et al. (2017) Commensal bacteria and essential amino acids control food choice behavior and reproduction. PloS Biology 15: e2000862 10.1371/journal.pbio.2000862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behar A, Ben-Yosef M, Lauzon CR, Yuval B, Jurkevich E (2009) Structure and function of the bacterial community associated with the Mediterranean fruit fly. Insect Symbiosis 3: 251–271 [Google Scholar]
- 24.Andongma AA, Wan L, Dong YC, li P, Desneux N, White JA, et al. (2015) Pyrosequencing reveals a shift in symbiotic bacteria populations across life stages of Bactrocera dorsalis. Scientific Reports 5: 9470 10.1038/srep09470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behar A, Yuval B, Jurkevitch E (2005) Enterobacteria-mediated nitrogen fixation in natural populations of the fruit fly Ceratitis capitata. Molecular Ecology 14: 2637–2643. 10.1111/j.1365-294X.2005.02615.x [DOI] [PubMed] [Google Scholar]
- 26.Ben-Yosef M, Jurkevitch E, Yuval B (2008) Effect of bacteria on nutritional status and reproductive success of the Mediterranean fruit fly Ceratitis capitata. Physiological Entomology 33: 145–154. 10.1111/j.1365-3032.2008.00617.x [DOI] [Google Scholar]
- 27.Khaeso K, Andongma AA, Akami M, Souliyanonh B, Zhu J, Krutmuang P, et al. (2017) Assessing the effects of gut bacteria manipulation on the development of the oriental fruit fly, Bactrocera dorsalis (Diptera; Tephritidae). Symbiosis 74: 97–105. 10.1007/s13199-017-0493-4 [DOI] [Google Scholar]
- 28.Ben-Yosef M, Pasternak Z, Jurkevitch E, Yuval B (2015) Symbiotic bacteria enable olive fly larvae to overcome host defences. Royal Society Open Science 2: 150170 10.1098/rsos.150170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behar A, Jurkevitch E, Yuval B (2008) Bringing back the fruit into fruit fly–bacteria interactions. Molecular Ecology 17: 1375–1386. 10.1111/j.1365-294X.2008.03674.x [DOI] [PubMed] [Google Scholar]
- 30.Guo Z, Lu Y, Yang F, Zeng L, Liang G, et al. (2017) Transmission modes of a pesticide-degrading symbiont of the oriental fruit fly Bactrocera dorsalis (Hendel). Applied Microbiology and Biotechnology 101: 8543–8556. 10.1007/s00253-017-8551-7 [DOI] [PubMed] [Google Scholar]
- 31.Ben-Yosef M, Pasternak Z, Jurkevitch E, Yuval B (2014) Symbiotic bacteria enable olive flies (Bactrocera oleae) to exploit intractable sources of nitrogen. Journal of Evolutionary Biology 27: 2695–2705. 10.1111/jeb.12527 [DOI] [PubMed] [Google Scholar]
- 32.Hoskins AJ, Arnould JPY (2013) Temporal allocation of foraging effort in female Australian fur seals (Arctocephalus pusillus doriferus). PloS One 8: e79484–e79484. 10.1371/journal.pone.0079484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drew R, Yuval B (2000) The evolution of fruit fly feeding behavior. Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior: 731–749.
- 34.Ben-Yosef M, Aharon Y, Jurkevitch E, Yuval B (2010) Give us the tools and we will do the job: symbiotic bacteria affect olive fly fitness in a diet-dependent fashion. Proceedings of the Royal Society B-Biological Sciences 277: 1545–1552. 10.1098/rspb.2009.2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Y-C, Wang Z-J, Clarke AR, Pereira R, Desneux N, et al. (2013) Pupal diapause development and termination is driven by low temperature chilling in Bactrocera minax. Journal of Pest Science 86: 429–436. 10.1007/s10340-013-0493-y [DOI] [Google Scholar]
- 36.Wang H, Jin L, Peng T, Zhang H, Chen Q, et al. (2014) Identification of cultivable bacteria in the intestinal tract of Bactrocera dorsalis from three different populations and determination of their attractive potential. Pest Management Science 70: 80–87. 10.1002/ps.3528 [DOI] [PubMed] [Google Scholar]
- 37.Gujjar NR, Govindan S, Verghese A, Subramaniam S, More R (2017) Diversity of the cultivable gut bacterial communities associated with the fruit flies Bactrocera dorsalis and Bactrocera cucurbitae (Diptera: Tephritidae). Phytoparasitica 45: 453–460. 10.1007/s12600-017-0604-z [DOI] [Google Scholar]
- 38.Pramanik MK, Khan M, Miah AB (2014) Isolation and identification of mid-gut bacterial community of Bactrocera dorsalis (Hendel)(Diptera: Tephritidae). Research Journal of Microbiology 9: 278 10.3923/jm.2014.278.286 [DOI] [Google Scholar]
- 39.Shi Z, Wang L, Zhang H (2012) Low diversity bacterial community and the trapping activity of metabolites from cultivable bacteria species in the female reproductive system of the oriental fruit fly, Bactrocera dorsalis Hendel (Diptera: Tephritidae). International Journal of Molecular Sciences 13: 6266–6278. 10.3390/ijms13056266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Jin L, Zhang H (2011) Comparison of the diversity of the bacterial communities in the intestinal tract of adult Bactrocera dorsalis from three different populations. Journal of Applied Microbiology 110: 1390–1401. 10.1111/j.1365-2672.2011.05001.x [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Martinez-Sañudo I, Mazzon L, Prabhakar C, Girolami V, Deng Y, et al. (2016) Bacterial communities associated with invasive populations of Bactrocera dorsalis (Diptera: Tephritidae) in China. Bulletin of Entomological Research 106: 718–728. 10.1017/S0007485316000390 [DOI] [PubMed] [Google Scholar]
- 42.Nash WJ, Chapman T (2014) Effect of dietary components on larval life history characteristics in the Medfly (Ceratitis capitata: Diptera, Tephritidae). PLoS One 9: e86029 10.1371/journal.pone.0086029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayer EA, Tillisch K, Gupta A (2015) Gut/brain axis and the microbiota. Journal of Clinical Investigation 125: 926–938. 10.1172/JCI76304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Browne LB (1975) Regulatory mechanisms in insect feeding Advances in Insect Physiology: Elsevier; pp. 1–116. 10.1016/S0065-2806(08)60162-9 [DOI] [Google Scholar]
- 45.Chapman RF, de Boer G (1995) Regulatory mechanisms in insect feeding: Springer Science & Business Media. [Google Scholar]
- 46.Roeder KA, Behmer ST (2014) Lifetime consequences of food protein‐carbohydrate content for an insect herbivore. Functional Ecology 28: 1135–1143. 10.1111/1365-2435.12262 [DOI] [Google Scholar]
- 47.Ben Ami E, Yuval B, Jurkevitch E (2010) Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. Isme Journal 4: 28–37. 10.1038/ismej.2009.82 [DOI] [PubMed] [Google Scholar]
- 48.Peng Y, Nielsen JE, Cunningham JP, McGraw EA (2008) Wolbachia infection alters olfactory-cued locomotion in Drosophila spp. Applied and Environmental Microbiology 74: 3943–3948. 10.1128/AEM.02607-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berasategui A, Salem H, Paetz C, Santoro M, Gershenzon J, Kaltenpoth M, et al. (2017) Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Molecular Ecology 26: 4099–4110. 10.1111/mec.14186 [DOI] [PubMed] [Google Scholar]
- 50.Tsuchida T, Koga R, Fukatsu T (2004) Host plant specialization governed by facultative symbiont. Science 303: 1989–1989. 10.1126/science.1094611 [DOI] [PubMed] [Google Scholar]
- 51.Mazzon L, Martinez-Sañudo I, Simonato M, Squartini A, Savio C, Girolami V (2010) Phylogenetic relationships between flies of the Tephritinae subfamily (Diptera, Tephritidae) and their symbiotic bacteria. Molecular Phylogenetics and Evolution 56: 312–326. 10.1016/j.ympev.2010.02.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are uploaded along with this manuscript.