Abstract
Background
Pure squamous cell carcinoma (SCC) of the urinary tract is rare in the UK and has a poor prognosis compared with transitional cell carcinoma (TCC). Cisplatin based chemotherapy has been shown to be effective in TCC.
Methods
Patients with T3-T4, pelvic relapsed, nodal or metastatic SCC of the urinary tract were recruited into an open-label, single-arm, non-randomised, phase 2 trial evaluating the activity and safety of cisplatin, methotrexate and vinblastine (CMV) chemotherapy. CMV was given as three 21-day cycles of methotrexate 30mg/m2 (day 1 & 8), vinblastine 4mg/m2 (day 1 & 8) and cisplatin 100mg/m2 (day 2).
Results
38 patients were recruited. Overall response was 39% (95% CI 24%, 55%)–13% CR and 26% PR. Median OS was 7.8 months (95% CI 3.4, 12.6) with 39% 1-year survival. Toxicity was acceptable.
Conclusion
CMV is well tolerated and active in patients with pure SCC of the urinary tract.
Introduction
Pure squamous cell carcinoma (SCC) of the bladder is rare in the UK accounting for 6–7% of all bladder cancer. It has a poor prognosis compared with transitional cell carcinoma (TCC) with a reported 5-year overall survival rate of 2–24%. Squamous cancer invariably invades the bladder wall at presentation and usually causes death by local spread [1–3]. The poor prognosis of these cancers might be related to more advanced primary tumour stage and larger tumour size at presentation compared with transitional cell cancer, although their radiosensitivity, stage for stage, is equivalent [3, 4]. TCC of the urothelium is a chemosensitive disease and cisplatin-based combination chemotherapy has been shown to be active in clinical trials and has become a standard of care for this disease in both the neoadjuvant and metastatic setting [5, 6]. SCC of the bladder generally presents as a locally advanced tumour with distant metastases at the time of presentation being rare. Thus, there might be a greater potential for improving the results in these tumours. However, most studies of chemotherapy in bladder cancer have been restricted to TCC and so the BA08 trial was initiated to investigate cisplatin-based chemotherapy in patients with SCC of the bladder.
Material and methods
In this open-label, single-arm, non-randomised, phase 2 trial, we evaluated the activity and safety of cisplatin, methotrexate and vinblastine (CMV) chemotherapy in patients with pure squamous cell carcinoma of the urinary tract. The protocol of this Medical Research Council (MRC) BA08 trial was developed in the early 1990s when the UK did not have a centralised ethics approval process (i.e. multi-centre research ethics committees (MRECs)) and before the establishment of the UK Medicines and Healthcare Products Regulatory Agency (MHRA) and EudraCT registration (n.b. it has been registered post completion on http://www.isrctn.com/ISRCTN84356042) however according to regulatory requirements at the time local research ethics committees within each of the recruiting hospitals approved the protocol i.e. Beatson Oncology Centre, Christie Hospital, Clatterbridge Hospital, Middlesex Hospital, Western General, Weston Park, Guys Hospital, Newcastle General, Queen Elizabeth, Norwegian Radium Hospital, Walsgrave Hospital, North Staffordshire Hospital, Oncology centre Warsaw. All clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki.". Although the database was frozen and an abstract accepted and presented at ASCO 2001 it was never written up, this is the first publication of results in a peer reviewed journal. At the time of the trial CMV was the standard of care for TCC. The protocol (S1 File) was reviewed in each recruiting institution by their local research ethics committees according to the national standards at the time (please see in acknowledgements the names of all recruiting hospitals where approval was granted), and informed consent for participation in the study was given by all patients prior to inclusion.
Eligibility criteria
Eligibility inclusion criteria included patients with pure squamous cell carcinoma of the urinary tract where the following groups were included: i) those with an initial presentation with T3-T4 disease; ii) those with pelvis relapse after radiotherapy or surgery; or iii) those with nodal or metastatic disease. Patients had to have at least one site of disease assessable for response (i.e. an indicator lesion of the primary bladder tumour, other primary tumours in the urinary tract, pelvic relapse and/or nodal/metastases) by clinical examination (e.g. bimanual examination, cystoscopy and TUR biopsy) or imaging (CT scan, MRI and Chest X-rays). Exclusion criteria included patients with transitional cell carcinoma with squamous metaplasia, or other mixed tumours and patient with co-existing illness (e.g. cardiac failure) which may compromise administration of CMV chemotherapy. A full list of inclusion and exclusion criteria is given in S1 Text.
Treatment
At UK secondary care NHS Trusts all patients received three 21-day cycles of CMV chemotherapy (cisplatin 100mg/m2 given on day 2, methotrexate at 30mg/m2 and vinblastine at 4mg/m2, both given intravenously, on days 1 and 8) from their treating clinician. Folinic acid was given 24h after each methotrexate injection at a dose of 15mg orally or intravenously 6-hourly × 4. Cisplatin was given following a period of intravenous hydration in which at least 1 litre of normal saline was given and was not administered until urine output was measured as equal to or exceeding 100ml h-1 for 4h. The administration of cisplatin was followed by 2 litre further hydration with normal saline, with supplementary potassium chloride and magnesium sulphate.
Endpoints
The primary endpoint of the study was overall response at 9 weeks (or post-chemotherapy if chemotherapy was stopped) after the commencement of the treatment (i.e. end of 3 cycles). The detail on the definition of response is given in S2 Text and at the time of the trial was the standard assessment of response used in these patients. In summary: the disappearance of all known malignant disease was considered a complete response; an at least a 50% reduction in measured lesions (with no new or progression of existing lesion) was considered a partial response; a less than 50% reduction in measured lesions (with no lesion measured at registration has showing more than a 25% increase) was considered No Change (i.e. stable disease); and a 25% or more increase in the size of one or more lesions, or the appearance of new lesions, was considered progressive disease. Secondary endpoints included treatment compliance, safety and overall survival (OS—time-to-event). Data were collected on paper Case Report Forms (CRFs) by NHS staff at the treating hospital and sent to the MRC Clinical Trials Unit for entry on the clinical trial database (COMPACT).
Design and statistical considerations
This trial was conducted using a 3-stage design. In the first stage 16 previously untreated patients were entered and assessed. If no responses were observed the trial would close. If at least one response was observed in these 16 patients then the trial would continue to a second stage to recruit a further 16 previously untreated patients (to a total of 32 patients). If there were fewer than 3 responses in these 32 patients then the trial would close. If the true response rate were 20% the probability of incorrectly rejecting the treatment at the first or second stage would be approximately 5%. If the trial was not stopped at either of these stages, trial entry would continue until a maximum of 45 patients had been entered which would result a standard error of about 7% in the final estimate of the response rate (i.e. a margin of error of approximately 14%). There was no treatment blinding.
The primary endpoint of 9-week response is presented as % and 95% CI [calculated using the normal approximation interval Wald method]. In addition to the primary analysis of 9-week response (post-chemo) in all patients, the responses in those patients with pure SCC will also be calculated. The proportion of patients completing a total of 0,1,2 and 3 cycles of CMV are presented as %s, with reasons listed for non-completion, and median [with 1st quartile [Q1] and 3rd quartile [Q3], values) doses of each drug in each cycle. Patient toxicities during treatment and also long term are listed. Overall survival is calculated as the time from registration to death from any cause. Patients still alive are censored at the time of last follow up. Overall survival will be presented as a Kaplan-Meier curve with median overall survival (plus 95% Hall-Wellner confidence band) and 1-year rate. Data analysis will be carried out in the SAS statistical package (intention-to-treat) with the Kaplan-Meier curve produced in STATA.
Results
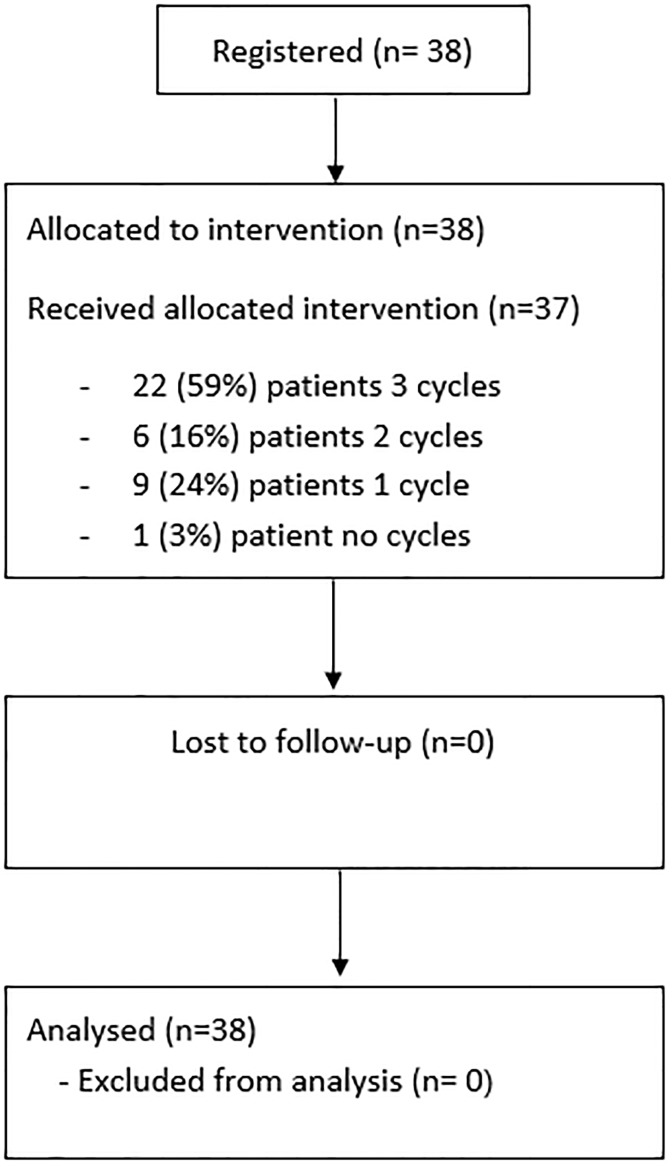
Between October 1993 and February 1999, 38 patients were recruited from 13 centres within the UK, Norway and Poland. The study progressed through all 3 stages of the design and all 38 patients were included in the analysis. The CONSORT diagram is given in Fig 1 and patient characteristics are reported in Table 1.
Fig 1. CONSORT diagram.
Table 1. Patient characteristics.
| Patient Characteristics | Number (%) | |
|---|---|---|
| Age | <= 50 | 12 (32%) |
| 51–60 | 10 (26%) | |
| 61–70 | 9 (24%) | |
| >= 71 | 7 (18%) | |
| Median (Q1, Q3) | 57 (47, 70) | |
| Sex | Male | 21 (55%) |
| Female | 17 (45%) | |
| WHO performance Status | 0: Normal activity | 15 (40%) |
| 1: Restricted | 14 (37%) | |
| 2: Ambulatory | 8 (21%) | |
| 3: Limited self-care | 1 (3%) | |
| Primary site of tumour | Bladder | 36 (95%) |
| Other site | 2 (5%) | |
| Previous treatment to primary | None | 26 (68%) |
| Surgery | 8 (21%) | |
| Radiotherapy | 4 (11%) | |
| Other (e.g. chemo) | 0 (0)% | |
| Tumour category | T2 | 2 (5%) |
| T3 | 25 (66%) | |
| T4 | 9 (24%) | |
| TX | 2 (5%) | |
| Lymph nodes involvement | N0 | 23 (60%) |
| N1 | 4 (10%) | |
| N2 | 6 (16%) | |
| N3 | 1 (3%) | |
| N4 | 1 (3%) | |
| NX | 3 (8%) | |
| Distant metastases | M0 | 32 (84%) |
| M1 | 5 (13%) | |
| MX | 1 (3%) | |
| Tumour present | Yes | 38 (100%) |
| Tumour type | Squamous cell | 36 (95%) |
| TCC with squamous | 1 (3%) | |
| Other | 1 (3%) | |
| Tumour grade | G2 | 5 (13%) |
| G3 | 33 (86%) | |
| Muscle present | No | 4 (11%) |
| Yes | 34 (89%) | |
| Muscle (or prostate) invaded | Yes | 33 (87%) |
| Equivocal | 1 (3%) | |
| Not assessable | 4 (11%) | |
| Total | 38 |
For the primary endpoint of 9-week response in all 38 patients, 5 (13%) had a complete response (CR) and 10 (26%) had a partial response (PR), corresponding to an overall response rate of 39% (95% CI 24%, 55%). For the 36 pure SCC patients it was 36% (95% CI 20%,52%), summarised in Table 2.
Table 2. Overall outcome.
| Outcome | Results | |
|---|---|---|
| Primary endpoint—all patients | ALL patients (n = 38) | SCC patients (n = 36) |
| Post-chemotherapy overall response (%, 95% CI) |
||
| Complete response | 5 (13%) | 4 (11%) |
| Partial response | 10 (26%) | 9 (25%) |
| No change | 9 (24%) | 9 (25%) |
| Progression | 6 (16%) | 6 (17%) |
| Not assessable | 8 (21%) | 8 (22%) |
| ORR = 15/38 | ORR = 13/36 | |
| 39% (95% CI 24%, 55%) | 36% (95% CI 20%,52%) |
Median post-chemotherapy assessment was at 8.9 weeks (Q1, 5.3, Q3 11)
22 out of 38 patients (58%) completed a total of 3 cycles of CMV treatment, with 6 patients (16%) receiving 2 cycles, 9 patients (24%) 1 cycle and 1 patient (3%) no cycles (Table 3). Reasons for not receiving 3 cycles included renal function (n = 4), haematological toxicity (n = 4, 2 with progression), not fit/well (n = 2, 1 with progression), death (n = 2, 1 patient had previous experienced renal toxicity and the other sepsis), sepsis (n = 1), admission to hospice (n = 1, had experienced renal toxicity, vomiting/fainting and progression) and progression for 2 patients. Reasons for delays/reductions of dose in a cycle included protocol violation (n = 8), chest infection (n = 1), renal toxicity (n = 8), haematological toxicity (n = 8) and other reasons (1 femoral embolus, 1 dehydration, 1 mucositis, 1 admin error, 1 tinnitus/headache, 1 abdominal pain). 7 patients reported long term toxicity (i.e. post treatment) due to neuropathy (n = 4, 1 with tinnitus), diarrhoea (n = 1), haematological toxicity (n = 1) and RT telangiectasia (n = 1). These toxicities recorded were similar to those reported in previous transitional cell carcinoma trials using CMV.
Table 3. Treatment received.
| Day | Cycle 1 | Cycle 2 | Cycle 3 | ||
|---|---|---|---|---|---|
| Cisplatin (C) | 2 | No. pts with cycle | 37 | 28 | 22 |
| (100mg/m2) | No. pts received C | 371 | 27 | 22 | |
| Median mg/m2 | 100.0 | 98.4 | 98.6 | ||
| (Q1, Q3) | (98.9,100.0) | (50.0, 100.0) | (50.0, 100.0) | ||
| Methotrexate (M) | 1 | No. pts with cycle | 37 | 28 | 22 |
| (30mg/m2) | No. pts received M | 36 | 27 | 22 | |
| Median mg/m2 | 30.0 | 29.8 | 29.9 | ||
| (Q1, Q3) | (29.8, 30.3) | (27.8, 30.2) | (29.4, 30.1) | ||
| Methotrexate (M) | 8 | No. pts with cycle | 37 | 28 | 22 |
| (30mg/m2) | No. pts received M | 263 | 223 | 17 | |
| Median mg/m2 | 30.0 | 29.4 | 29.8 | ||
| (Q1, Q3) | (29.4, 30.3) | (27.8, 30.2) | (28.9, 30.1) | ||
| Vinblastine (V) | 1 | No. pts with cycle | 37 | 28 | 22 |
| (4mg/m2) | No. pts received V | 37 | 27 | 22 | |
| Median mg/m2 | 4.0 | 4.0 | 4.0 | ||
| (Q1, Q3) | (3.9, 4.0) | (3.7, 4.1) | (3.9, 4.0) | ||
| Vinblastine (V) | 8 | No. pts with cycle | 37 | 28 | 22 |
| (4mg/m2) | No. pts received V | 25 | 233 | 17 | |
| Median mg/m2 | 4.0 | 4.0 | 4.0 | ||
| (Q1, Q3) | (3.9, 4.0) | (3.7, 4.0) | (3.8, 4.0) |
1 missing dose data on 1 patient
3 missing dose data on 3 patients
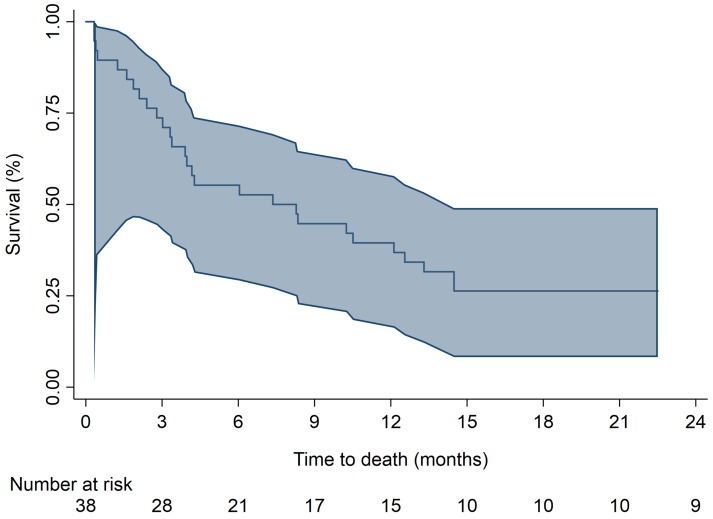
A total of 25 out of 38 patients (66%) received further treatment in their follow-up. 16/38 patients received only RT (either radical and/or palliative), 1/38 received RT and surgery, 4/38 received surgery only, and a total of 4/38 received further chemotherapy (2 only chemotherapy, 2 chemotherapy and surgery). Including all patients, a total of 30 out of 38 have died, 23 deaths (77%) were due to squamous cell carcinoma, 1 (3%) was treatment related and 6 (20%) were due to other reasons. The median survival was 7.8 months (95% CI 3.4, 12.6) with 1-year survival of 39% (95% CI 24.2%, 54.4%) (Fig 2).
Fig 2. Kaplan-Meier curve of overall survival.
Discussion
Treatment with CMV in SCC appears to be active and safe. If the true response rate were 20% the probability of incorrectly rejecting the treatment at the first or second stage would be approximately 5%. However, at each stage of the design there was sufficiently high activity to continue through all three stages of the design and give an overall response rate of the same magnitude as observed in TCC [6].
There are very few clinical trials involving just SCC of the bladder patients, indeed this being one of the largest ever conducted. Although this trial was conducted over 15 years ago the results are still relevant as little has changed in this patient population with standard of care still being cisplatin based chemotherapy, in fact the UK NICE bladder guidance in 2015 clearly states that “There was insufficient high-quality evidence on which to make specific recommendations” for SCC [7]. Although only a single arm phase II the results are generalisable due to the number of centres involved. In a SCC series, it has been suggested there is an association with an enhanced expression of EGFR and p53 [8] and that markers of apoptosis pathways (e.g. COX-2) might play an important role in its prognosis [9]. With the onset of new targeted treatments (e.g. immunotherapy) there is a need to investigate the impact of new treatment strategies in patients with this cancer. With the development of international partnerships to undertake international clinical trials of rare cancers, such as the International Rare Cancer Initiative (IRCI: a joint initiative between Cancer Research UK, the UK National Institute of Health Research Clinical Research Network [Cancer], the National Cancer Institute (NCI), the European Organisation for Research and Treatment of Cancer (EORTC), the Institut National Du Cancer (INCa), Clinical Oncology Society of Australia (COSA), Japan Clinical Oncology Group (JCOG) and Canadian Cancer Trials Group (CCTG)) there is an opportunity undertake future randomised trials in this cancer. The activity observed in this trial will be used as the control arm rate to improve on with new treatment strategies.
Conclusions
Treatment with CMV in SCC was well tolerated and was found to be active, appearing to be of a similar magnitude as in TCC. The greatest challenge was recruitment but with more recent opportunities for international collaborations for trials of rare cancers further randomised trials using more modern targeted agents should be considered [10].
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The BA08 trial was developed on behalf of the MRC Working Party in Bladder Cancer (now the UK National Cancer Research Institute Bladder and Renal Cancer Clinical Studies Group). The trial was sponsored by the MRC at the MRC Clinical Trials Unit (formally the MRC Cancer Trials office). We thank all the participants, the doctors, UK National Institute for Health Research Clinical Research Network Cancer research nurses, and other members of the multidisciplinary teams and research teams who supported this trial at participating centres (Beatson Oncology Centre—13 patients, Christie Hospital—5 patients, Clatterbridge Hospital—4 patients, Middlesex Hospital—4 patients, Western General—2 patients, Weston Park—2 patients, Guys Hospital—2 patients, Newcastle General—1 patient, Queen Elizabeth—1 patient, Norwegian Radium Hospital—1 patient, Walsgrave Hospital—1 patient, North Staffordshire Hospital—1 patient, Oncology centre, Warsaw—1 patient).
Data Availability
Data are available on request to bona fide, qualified researchers in line with MRC CTU at UCL’s Standard Operating Procedure for Data Release and Data Sharing, which is consistent with the approaches set out by MRC and CRUK (Sydes et al. Sharing data from clinical trials: the rationale for a controlled access approach. Trials 2015;16:104. doi: 10.1186/s13063-015-0604-6). MRC CTU at UCL is currently reviewing its SOP and with MRC Head Office, it is looking to assess putting some trials into an open access archive, probably data.gov.uk, with access management via Wellcome Trust’s portal for Clinical Study Data Request (CSDR -- the full CSDR system is not affordable for academic researchers). We will encourage that this trial is one of the studies that is used in this instance. Access to data can be requested via ictm.enquiries@ucl.ac.uk.
Funding Statement
The trial was funded by the UK Medical Research Council (www.mrc.ac.uk) which funded staff at the MRC Clinical Trial Unit. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Johnson DE, Schoenwald MC, Ayala EG, Miller LS. Squamous cell carcinoma of bladder. J. Urol. 1976;115: 542–544. [DOI] [PubMed] [Google Scholar]
- 2.Jones MA, Bloom HJG, Williams G, Trott PA, Wallace DM. The management of squamous cell carcinoma of the bladder. B.J.Urol. 1980: 52: 511–514. [DOI] [PubMed] [Google Scholar]
- 3.Costella AJ, Tipltaft RC, England HR, Blandy JP. Squamous cell carcinoma of the bladder. Urology. 1984; 23: 234–236. [PubMed] [Google Scholar]
- 4.Quilty PM, Duncan W. Radiotherapy for squamous cell carcinoma of the urinary bladder. Int.J.Rad.Oncol.Biol.Phys. 1986; 12:861–865. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MKB. International Phase III Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer-Long Term Results of the BA06 30894 Trial. JCO. 2011; 29: 2171–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mead GM, Russell M, Clark P, Harland SJ, Harper PG, et al. A randomized trial comparing methotrexate and vinblastine (MV) with cisplatin, methotrexate and vinblastine (CMV) in advanced transitional cell carcinoma: results and a report on prognostic factors in a Medical Research Council study. MRC Advanced Bladder Cancer Working Party. Br J Cancer. 1998; 78(8):1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bladder cancer: diagnosis and management. NICE guideline Published: 25 February 2015. http://www.nice.org.uk/guidance/ng2
- 8.Guo CC, Gomez E, Tamboli P, Bondaruk JE, Kamat A, Bassett R, et al. Squamous cell carcinoma of the urinary bladder: a clinicopathologic and immunohistochemical study of 16 cases. Hum Pathol. 2009; 40(10):1448–52. 10.1016/j.humpath.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 9.Youssef R, Kapur P, Shariat SF, Arendt T, Kabbani W, Mosbah A, et al. Prognostic value of apoptotic markers in squamous cell carcinoma of the urinary bladder. BJU Int. 2012; 110(7):961–6. 10.1111/j.1464-410X.2012.10949.x [DOI] [PubMed] [Google Scholar]
- 10.Bogaerts J, Sydes MR, Keat N, McConnell A, Benson A, Ho A, et al. Clinical trial designs for rare diseases: studies developed and discussed by the International Rare Cancers Initiative. Eur J Cancer. 2015; February 51(3):271–81. 10.1016/j.ejca.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
Data are available on request to bona fide, qualified researchers in line with MRC CTU at UCL’s Standard Operating Procedure for Data Release and Data Sharing, which is consistent with the approaches set out by MRC and CRUK (Sydes et al. Sharing data from clinical trials: the rationale for a controlled access approach. Trials 2015;16:104. doi: 10.1186/s13063-015-0604-6). MRC CTU at UCL is currently reviewing its SOP and with MRC Head Office, it is looking to assess putting some trials into an open access archive, probably data.gov.uk, with access management via Wellcome Trust’s portal for Clinical Study Data Request (CSDR -- the full CSDR system is not affordable for academic researchers). We will encourage that this trial is one of the studies that is used in this instance. Access to data can be requested via ictm.enquiries@ucl.ac.uk.