Abstract
Many physiological functions have a circadian rhythm, including blood pressure (BP). BP is highest during the active phase, whereas during the rest period, BP dips 10–20%. Patients that do not experience this dip at night are termed “nondippers.” Nondipping hypertension is associated with increased risk of cardiovascular disease. The mechanisms underlying nondipping hypertension are not understood. Without the circadian clock gene Per1, C57BL/6J mice develop nondipping hypertension on a high-salt diet plus mineralocorticoid treatment (HS/DOCP). Our laboratory has shown that PER1 regulates expression of several genes related to sodium (Na) transport in the kidney, including epithelial Na channel (ENaC) and Na chloride cotransporter (NCC). Urinary Na excretion also demonstrates a circadian pattern with a peak during active periods. We hypothesized that PER1 contributes to circadian regulation of BP via a renal Na-handling-dependent mechanism. Na-handling genes from the distal nephron were inappropriately regulated in KO mice on HS/DOCP. Additionally, the night/day ratio of Na urinary excretion by Per1 KO mice is decreased compared with WT (4 × vs. 7×, P < 0.001, n = 6 per group). Distal nephron-specific Per1 KO mice also show an inappropriate increase in expression of Na transporter genes αENaC and NCC. These results support the hypothesis that PER1 mediates control of circadian BP rhythms via the regulation of distal nephron Na transport genes. These findings have implications for the understanding of the etiology of nondipping hypertension and the subsequent development of novel therapies for this dangerous pathophysiological condition.
Keywords: aldosterone, blood pressure, circadian clock, kidney, mineralocorticoid
INTRODUCTION
Physiological functions that have a circadian rhythm include body temperature, hormone release, sodium (Na) excretion, and blood pressure (BP) (30). Disruption of these rhythms is associated with adverse health outcomes. The molecular circadian clock functions as a master regulator of gene expression. Nearly 50% of all genes have a circadian rhythm of expression (48). At the core of the molecular circadian clock are the proteins BMAL1, CLOCK, CRYPTOCHROME (CRY), and PERIOD (PER). These proteins act as transcription factors, regulating the transcription of genes controlled by the clock. Briefly, BMAL1 and CLOCK form a heterodimer and bind to regulatory E-box sites to activate transcription of target genes, including Per and Cry. PER and CRY act in a negative feedback loop, inhibiting their own transcription by blocking BMAL1/CLOCK (43).
Every core clock gene knockout (KO) mouse tested to date exhibits a blood pressure (BP) phenotype (29). BP exhibits circadian oscillations with an active period peak and a dip in blood pressure during sleep. In healthy individuals, BP dips 10–20% at night. Mice are nocturnal; thus, their BP dips during the daytime. Absence of this BP dip is termed “nondipping” and is associated with increased cardiovascular risk and kidney disease (5, 11, 15, 18). Often, this nondipping phenotype is salt-sensitive, which leads to a greater risk of cardiovascular mortality (26). There is a high prevalence of salt-sensitive hypertension among African-Americans, which contributes to health disparities in this population (6, 24, 27).
Clinical studies show a relationship between renal Na handling and abnormal circadian BP rhythms (16, 45). Many functions of the kidney, including Na excretion and glomerular filtration rate, have a circadian rhythm (38). We and others have linked the molecular circadian clock to Na handling in the kidney (9, 25, 31, 40, 49). Our laboratory was the first to show that the Period 1 homolog (Per1) is an aldosterone target gene (8). A high-salt diet (HS) plus the long-acting aldosterone analog desoxycorticosterone pivalate (DOCP) led to BP rhythm disruption in male C57BL/6J global Per1 KO mice compared with wild-type (WT) mice (39). Male Per1 KO mice had a significantly blunted dip during their resting phase (daytime) indicative of salt-sensitive nondipping hypertension (23). A high-salt diet plus DOCP (HS/DOCP) treatment model represents low-renin/high-aldosterone hypertension that involves the kidney and mimics common forms of essential hypertension found in humans (7, 10). High plasma aldosterone and low renin levels are predictive of hypertension, especially among African-Americans (4, 12, 21, 23). Female Per1 KO mice retained the proper circadian rhythm of BP, even with HS/DOCP treatment (37) and were not included in the present study.
The goal of the present study was to characterize the role of renal Na handling in the Per1 KO mice that exhibit salt- and mineralocorticoid-induced nondipping hypertension. In response to HS/DOCP, Per1 KO mice did not properly regulate expression of Na-handling genes related to the epithelial Na channel (ENaC) and Na chloride cotransporter (NCC). Additionally, the KO mice had a significant reduction in the night/day ratio of urinary Na excretion, which may contribute to the nondipping phenotype seen in these mice. To explore the role of PER1 transcriptional regulation in the distal nephron, C57BL/6J distal nephron-specific KO mice were generated. NCC and the α-subunit of ENaC were inappropriately upregulated in the KO mice at baseline. These results suggest that PER1 transcriptional regulation of Na-handling genes in the distal nephron is critical for maintaining normal circadian rhythms of BP.
METHODS
Animals.
All experiments involving animals were approved by the University of Florida and the North Florida/South Georgia Veterans Administration Institutional Animal Care and Use Committees in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.” Male C57BL/6J Per1 KO and WT mice of ages 16–20 wk were treated as described by Solocinski et al. (39).
Kidney distal nephron-specific C57BL/6J Per1 KO mice were created using a Ksp-cadherin Cre recombinase (35). Homozygous floxed Per1 mice were generated by the KOMP Repository (University of California, Davis) and then successfully crossed with Ksp-cadherin-Cre mice (Jackson Laboratory) at the University of Florida. These Cre+ distal nephron-specific Per1 KO mice and WT Cre- littermates still contained the neomycin resistance cassette. Genomic DNA from dissected inner medulla (containing thin limbs, vasa recta, and inner medullary collecting duct) was prepared and used as a template for PCR analysis to show successful recombination and subsequent deletion of exons 2–8.
RNA isolation and real-time quantitative RT-PCR.
Kidneys were dissected into the cortex and medulla. Total RNA was isolated using TRIzol (Invitrogen). RNA was treated with DNaseI (Ambion). The resulting RNA samples were used along with the high-capacity cDNA reverse transcription kit (Applied Biosystems) to create cDNA. Applied Biosystems TaqMan probes were used for gene expression analysis as described by Solocinski et al. (40).
Semiquantitative RT-PCR.
Semiquantitative PCR was performed on kidney cDNA as previously described (9). Primers sequences used for KS-WNK1 (primers recognize exon 4a, specific to KS-WNK1, and exon 5) and L-WNK1 (primers recognize exon 1 and 2, which are not present in KS-WNK1) have been published by Vidal-Petiot et al. (46). GAPDH primer sequences are reported by Gumz et al. (9).
Metabolic cage.
After giving mice time to acclimate to metabolic cages, they were given a high-salt diet of 4% NaCl for 3 days. The mice were administered a 75 μg/g body wt im injection of DOCP. Urine was collected every 12 h for 3 days, and the kidneys were harvested at noon after metabolic collection was complete. Serum and urine Na concentrations were determined using flame photometry.
RESULTS
Expression of Na regulation genes in the distal nephron is altered in Per1 KO.
In a previous study, we showed that Per1 KO mice lost their BP dipping pattern in response to HS/DOCP (39). To determine whether aberrant Na-handling gene expression was associated with salt-sensitive nondipping hypertension, real-time quantitative PCR (qPCR) was performed to measure the expression levels of genes in the cortex and the medulla. PER1 is a circadian transcription factor that belongs to the bHLH-PER‐ARNT‐SIM (PAS) heterodimeric protein family (14). Since PER1 regulates gene expression, qPCR provides a measurement of PER1 activity. Mice were placed on an HS diet (4% NaCl) and given an injection of DOCP. After 3 days, the mice were euthanized and the kidneys were harvested at noon—the midpoint of the mouse inactive phase—because this is the time at which the difference in BP between male Per1 KO and WT mice was the greatest (39). The kidneys were dissected into the cortex and medulla.
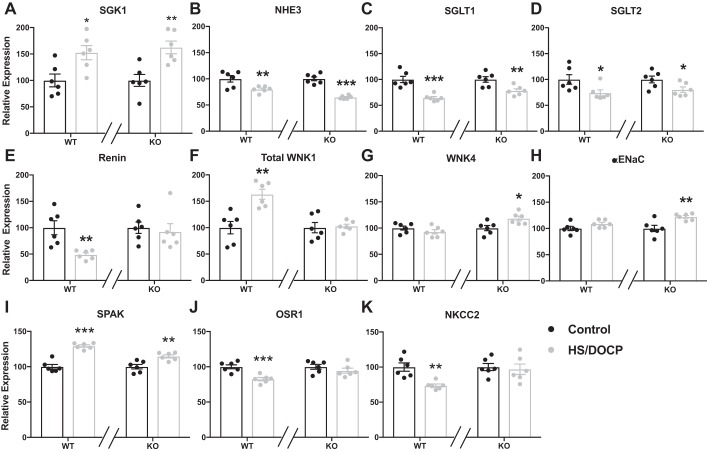
Figure 1 shows expression data for various Na-handling genes in the kidney. Expression of the serum and glucocorticoid-regulated kinase (SGK), which has been shown to regulate ENaC (44), was increased in response to HS/DOCP in both the WT and KO mice. Genes expressed primarily in the proximal tubule are shown in Fig. 1, B–D. Both WT and KO mice downregulate these genes in the same manner in response to HS/DOCP. In contrast, there were significant differences in expression of distal nephron Na transport genes between WT and KO mice in response to HS/DOCP (Fig. 1, E–K). Renin was downregulated in the WT on HS/DOCP compared with the control diet, whereas the KO failed to downregulate renin. The sodium transporter NKCC2 was downregulated in WT mice in response to HS/DOCP, but not in the Per1 KO mice. Expression of WNK4 and the α-subunit of ENaC was upregulated in the KO mice in response to HS/DOCP, but this effect was absent in WT mice. Expression of the WNK effector OSR1 decreased in WT but not in Per1 KO mice on HS/DOCP. Expression of SPAK did not show a difference between WT and KO mice in response to HS/DOCP.
Fig. 1.
Na-handling genes (A–K) in the distal nephron are inappropriately regulated in salt-sensitive nondipping hypertension. Kidneys from wild-type (WT) and Per1 knockout (KO) mice on normal diet (black) or high-salt diet plus mineralocorticoid treatment (HS/DOCP; gray) were collected at noon. Quantitative PCR was performed to evaluate various Na-handling genes. Expression was relativized to control diet for both WT and KO. Relative expression of these genes in the kidney cortex is shown. Significance was determined by Studentʼs t-test. *P < 0.05, **P < 0.01, ***P < 0.001 control diet vs HS/DOCP treatment, n = 6 mice per group per diet.
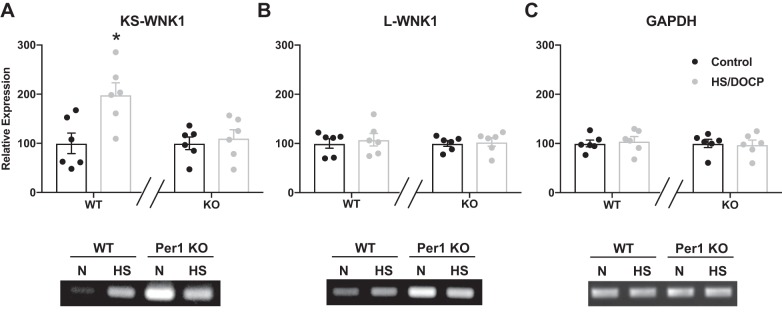
WT mice on HS/DOCP had an upregulation of total WNK1, whereas Per1 KO mice did not (Fig. 1F). Multiple isoforms of WNK1 are expressed in the kidney, and standard qPCR probes were not designed to distinguish between them. To determine whether this upregulation in response to HS/DOCP represents an increase in the full-length long-WNK1 (L-WNK1) or kidney-specific WNK1 (KS-WNK1) expression, a semiquantitative PCR was performed with primers specific to each isoform (Fig. 2, A–C) (46). KS-WNK1 was significantly upregulated in WT mice on HS/DOCP compared with control, whereas Per1 KO mice did not show a difference between control and HS/DOCP. There were no significant differences between expression of L-WNK1 or GAPDH in response to HS/DOCP. It is important to note that Per1 KO mice start with a higher expression of both KS-WNK1 and L-WNK1 at baseline compared with WT [gel image (Fig. 2)].
Fig. 2.
Kidney-specific WNK1 is upregulated in WT mice on HS/DOCP but not in Per1 KO mice. Semiquantitative RT-PCR of KS-WNK1 (A) L-WNK1 (B), and GAPDH (C) was performed on WT and Per1 KO medulla samples. ImageJ was used to quantify PCR bands. Expression was relativized to control diet for both WT and KO. Relative expression of all samples is shown in the bar graphs with representative gel images on the bottom. Similar results were seen in cortex samples. Significance was determined by Studentʼs t-test. *P < 0.05 control diet vs HS/DOCP treatment, n = 6 mice per group per diet.
Per1 KO mice have altered night/day ratio of Na excretion.
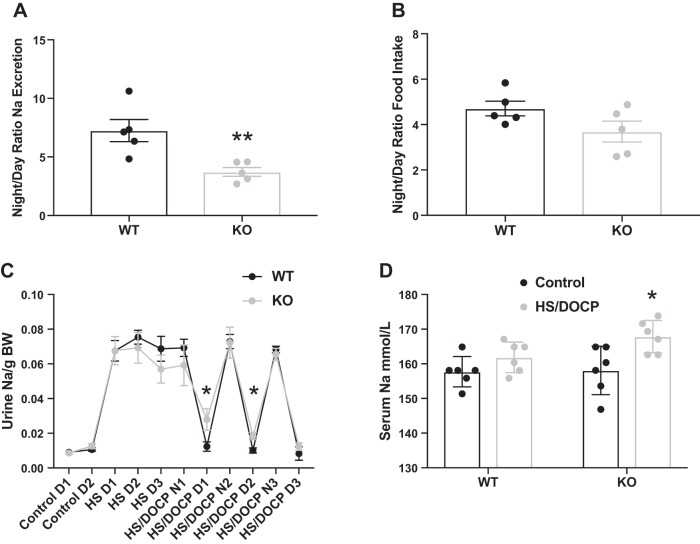
Since Per1 KO mice inappropriately upregulate Na-handling genes on HS/DOCP, these mice may also have altered Na excretion. After acclimation to metabolic cages, mice were placed on a HS diet and given an injection of DOCP. Urine was collected every 12 h immediately after administration of the DOCP injection, so these mice were not in balance, and the differences in urinary excretion are representative of their short-term response to DOCP. Urinary Na was quantified using flame photometry and µEq Na/g body wt of individual animals was determined for the night and day urine collections. On a HS/DOCP diet, WT mice had a night/day Na excretion ratio of 7×, while the KO mice had a night/day ratio of only 4× (Fig. 3A; P < 0.001). This reduction of the night/day ratio in Na excretion of the KO mice is similar to the reduction in the night/day ratio of BP previously reported (39). There was no significant difference in the night/day ratio of food intake between KO and WT mice (Fig. 3B). As seen in Fig. 3C, within the first 24 h of the DOCP injection (HS/DOCP N1 and D1), the Per1 KO mice have a blunted night/day ratio of Na excretion. After 48 h, there is still a significant reduction in the night/day Na excretion levels of the KO mice. Per1 KO mice had increased serum [Na] on HS/DOCP compared with control diet, whereas the WT mice maintained the same serum [Na] (Fig. 3D).
Fig. 3.
Per1 KO mice exhibit a reduced night/day ratio of urinary Na excretion. A: 12-h urine collections were made from WT (black) and Per1 KO (gray) mice with HS/DOCP treatment placed in metabolic cages for 72 h. Average Na excretion at night (active phase, 6 AM–6 PM) was divided by the average Na excretion during the day (resting phase, 6 PM–6 AM). Significance was determined by Studentʼs t-test. **P < 0.001 vs. WT, n = 5 mice per group. B: night/day ratio of food intake was not significantly different. C: urine Na excretion measurements every 24 (control and HS) or every 12 h (HS/DOCP). For the HS/DOCP measurements, night (N) is total urine from 6 PM–6 AM and day (D) is total urine from 6 AM–6 PM. Significance was determined by Studentʼs t-test. *P < 0.05 vs. WT, n = 5 or 6 mice per group. D: serum Na levels in WT and Per1 KO mice on control diet (black) or after 72 h on HS/DOCP treatment (gray). Significance was determined by Studentʼs t-test. *P < 0.05 vs. control diet; n = 6 mice per group.
Distal nephron-specific Per1 KO overexpress Na transport genes.
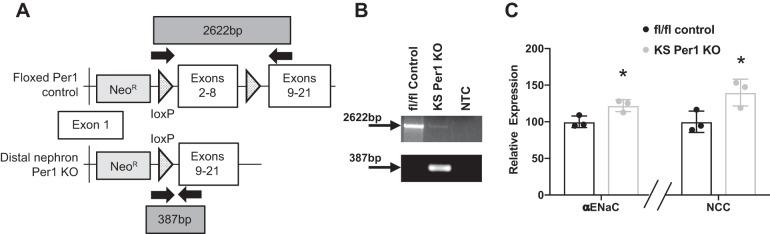
Considering that the results from the qPCR analysis suggested that PER1 is regulating gene expression in the distal part of the nephron, the first distal nephron-specific Per1 KO was generated (Fig. 4A). Gene expression of NCC and αENaC was measured in Ksp-cadherin Cre+ homozygous floxed Per1 KO mice compared with Cre-homozygous floxed controls on a normal-salt diet. Kidneys were harvested at noon, consistent with the experiments discussed above. As expected, NCC and αENaC were inappropriately upregulated in the distal nephron Per1 KO mice when compared with the floxed control mice (P < 0.05) (Fig. 4C). These results support our hypothesis that PER1 maintains a circadian rhythm of BP, at least in part, through the regulation of renal Na-handling gene expression.
Fig. 4.
Distal nephron-specific Per1 KO mice have increased Na transporter expression. A: generation of distal nephron-specific Per1 KO mice. Floxed Per1 schematic shows placement of loxP sites flanking exons 2–8 and location of the neomycin cassette, which all mice still contained. Solid arrows denote genotyping primers to detect deletion of exons 2–8. Ksp-cadherin genotyping was performed according to protocol in Ref. 17. B: PCR was performed on inner medulla genomic DNA of control and distal nephron-Per1 KO mice to confirm recombination in distal nephron Per1 KO mice. C: relative gene expression of α-epithelial Na channel (αENaC) and the sodium chloride cotransporter (NCC) in the cortex of fl/fl control (black) and distal nephron-specific Per1 KO (gray) mice on a normal diet. Significance was determined by Studentʼs t-test. *P < 0.05 vs. fl/fl control; n = 3 male mice per group.
DISCUSSION
Previously, we established that Per1 KO mice on a HS/DOCP diet are a model of salt-sensitive nondipping hypertension (39) and demonstrated that PER1 plays a critical role in the regulation of renal Na transport genes (28, 31, 40). The primary finding of the present study is that Per1 KO mice are unable to properly regulate Na-handling genes in response to HS/DOCP, and this effect is associated with a decreased night/day ratio in urinary Na excretion. As mice are nocturnal, more Na should be excreted at night during their active phase compared with the day, their inactive phase. Additionally, we report here the first construction of a distal nephron-specific Per1 KO mouse strain. These mice displayed upregulation of NCC and αENaC relative to control mice. The results of this study support the hypothesis that PER1 maintains a circadian pattern of BP, in part, via a renal Na-handling-dependent mechanism.
Gene expression profiling showed that distal nephron Na transport genes were expressed at an inappropriately high level in Per1 KO mice on HS/DOCP (Fig. 1). Both the WT and KO mice upregulated SPAK transcription in response to HS/DOCP, but it is unknown whether the proportion of the full-length SPAK and the kinase-deficient kidney-specific SPAK (20) is the same between WT and KO. Total WNK1 expression levels were increased in WT mice in response to HS/DOCP, but not in Per1 KO mice. L-WNK1 has been shown to increase the activity of NCC and ENaC (22, 47). KS-WNK1 inhibits the activity of L-WNK1, which would, in turn, decrease the activity of Na transporters and Na reabsorption (1, 42). In fact, transcription of KS-WNK1, and not the full-length L-WNK1, was increased in WT mice in response to HS/DOCP, but not in Per1 KO mice (Fig. 2). The lack of response to HS/DOCP in Per1 KO mice KS-WNK1 expression may be explained by the fact that the Per1 KO mice have a higher level of KS-WNK1 expression at baseline (Fig. 2A). It may not be possible for KS-WNK1 expression to increase even further when the Per1 KO mice are given a HS/DOCP treatment. It is not surprising that the Per1 KO mice have altered levels of KS-WNK1, as our laboratory has previously shown PER1 is involved in the regulation of WNK transcription in cultured DCT cells (31).
NCC, ENaC, and the WNKs are involved in the pathogenesis of salt-sensitive hypertension (32, 33, 36). NCC has also been shown to be activated in nondipping hypertension (13). On the basis of the gene expression data from Per1 KO mice, it was expected that these mice inappropriately regulated Na. Indeed, the Per1 KO mice had a significant reduction in their night/day ratio of urinary Na excretion compared with WT mice on HS/DOCP (Fig. 3). Nondipping hypertension in humans has been associated with increased Na excretion during the resting phase (34). After 72 h on a HS/DOCP treatment, the Per1 KO mice had a significantly elevated serum Na level compared with WT mice (Fig. 3D). Additionally, preliminary data with distal nephron-specific Per1 KO mice also demonstrated a significant increase in the transcription of NCC and ENaC (Fig. 4C). The data presented here suggest that renal Na transport genes are critical to the PER1-dependent salt-sensitive nondipping phenotype. Indeed, work by others shows a relationship between Na handling by the kidney and abnormal BP circadian rhythms (16, 45).
Previously, our laboratory demonstrated that 129/sv mice with Per1 KO had lower BP and excreted more Na than WT 129/sv control mice (28, 41). Additionally, these mice retained a circadian pattern of BP. Variations in BP and sodium handling among mouse strains have been previously reported (2, 10, 19). These strain-specific differences are most likely the reason why phenotypic differences are seen between the C57BL/6J and 129/sv Per1 KO mice (reviewed in Ref. 3). Strain differences emphasize the role of genetic background in the regulation of physiological functions.
Because PER1 is a transcription factor, the current study was designed to investigate the impact of Per1 KO on transcription of Na-handling genes in response to HS/DOCP. A limitation of this study is that only transcription products were measured, which does not always correlate to protein levels or activity. The direct impact of Per1 KO on protein products is currently being investigated in the distal nephron-specific Per1 KO mice.
In summary, here, we show for the first time that C57BL/6J male Per1 KO mice are unable to properly regulate expression of Na-handling genes, leading to circadian dysregulation of Na excretion, which, in turn, may contribute to the nondipping phenotype seen in these mice. Additionally, we produced the first distal nephron-specific Per1 KO mice, which seem to also improperly regulate Na-handling genes. Together, the data presented here suggest that PER1-dependent transcriptional regulation of Na-handling genes in the distal nephron of the kidney is important for the maintenance of 24-h BP patterns.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK109570 (to M.L. Gumz); American Heart Association Grant 16GRNT31220009 (to M.L. Gumz); NIH grants T32HL083810 (to K. Solocinski) and T32DK104721 (to L.G. Douma).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.G.D., B.D.C., C.S.W., and M.L.G. conceived and designed research; L.G.D., M.R.H., K.S., S.H.M., A.H.M., K.-Y.C., I.J.L., and M.L.G. performed experiments; L.G.D., M.R.H., and M.L.G. analyzed data; L.G.D. and M.L.G. interpreted results of experiments; L.G.D., M.R.H., and M.L.G. prepared figures; L.G.D. drafted manuscript; L.G.D., M.R.H., K.S., I.J.L., B.D.C., and M.L.G. edited and revised manuscript; L.G.D., M.R.H., K.S., S.H.M., A.H.M., K.-Y.C., I.J.L., B.D.C., C.S.W., and M.L.G. approved final version of manuscript.
REFERENCES
- 1.Cheng C-J, Baum M, Huang C-L. Kidney-specific WNK1 regulates sodium reabsorption and potassium secretion in mouse cortical collecting duct. Am J Physiol Renal Physiol 304: F397–F402, 2013. doi: 10.1152/ajprenal.00589.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu P-L, Gigliotti JC, Cechova S, Bodonyi-Kovacs G, Chan F, Ralph DL, Howell N, Kalantari K, Klibanov AL, Carey RM, McDonough AA, Le TH. Renal collectrin protects against salt-sensitive hypertension and is downregulated by angiotensin II. J Am Soc Nephrol 28: 1826–1837, 2017. doi: 10.1681/ASN.2016060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med S0891-5849(17)31228-5, 2017. doi: 10.1016/j.freeradbiomed.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durukan M, Guray U, Aksu T, Guray Y, Demirkan B, Korkmaz S. Low plasma renin activity and high aldosterone/renin ratio are associated with untreated isolated systolic hypertension. Blood Press 21: 320–325, 2012. doi: 10.3109/08037051.2012.686167. [DOI] [PubMed] [Google Scholar]
- 5.Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens 23: 645–653, 2009. doi: 10.1038/jhh.2009.9. [DOI] [PubMed] [Google Scholar]
- 6.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Hall WD, Jones WE, Kountz DS, Lea JP, Nasser S, Nesbitt SD, Saunders E, Scisney-Matlock M, Jamerson KA, International Society on Hypertension in Blacks . Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 56: 780–800, 2010. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
- 7.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285: F664–F673, 2003. doi: 10.1152/ajprenal.00353.2002. [DOI] [PubMed] [Google Scholar]
- 9.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartner A, Cordasic N, Klanke B, Veelken R, Hilgers KF. Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol Dial Transplant 18: 1999–2004, 2003. doi: 10.1093/ndt/gfg299. [DOI] [PubMed] [Google Scholar]
- 11.Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int 27: 1629–1651, 2010. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- 12.Huan Y, Deloach S, Keith SW, Goodfriend TL, Falkner B. Aldosterone and aldosterone: renin ratio associations with insulin resistance and blood pressure in African Americans. J Am Soc Hypertens 6: 56–65, 2012. doi: 10.1016/j.jash.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Ivy JR, Oosthuyzen W, Peltz TS, Howarth AR, Hunter RW, Dhaun N, Al-Dujaili EAS, Webb DJ, Dear JW, Flatman PW, Bailey MA. Glucocorticoids induce nondipping blood pressure by activating the thiazide-sensitive cotransporter. Hypertension 67: 1029–1037, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones S. An overview of the basic helix-loop-helix proteins. Genome Biol 5: 226, 2004. doi: 10.1186/gb-2004-5-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kario K, Shimada K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clin Exp Hypertens 26: 177–189, 2004. doi: 10.1081/CEH-120028556. [DOI] [PubMed] [Google Scholar]
- 16.Kimura G, Dohi Y, Fukuda M. Salt sensitivity and circadian rhythm of blood pressure: the keys to connect CKD with cardiovascular events. Hypertens Res 33: 515–520, 2010. doi: 10.1038/hr.2010.47. [DOI] [PubMed] [Google Scholar]
- 17.Lee H-W, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009. doi: 10.1152/ajprenal.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Takahashi H, Morita Y, Maruyama S, Mizuno M, Yuzawa Y, Watanabe M, Toriyama T, Kawahara H, Matsuo S. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant 18: 563–569, 2003. doi: 10.1093/ndt/18.3.563. [DOI] [PubMed] [Google Scholar]
- 19.Mattson DL. Comparison of arterial blood pressure in different strains of mice [Online]. Am J Hypertens 14: 405–408, 2001. doi: 10.1016/S0895-7061(00)01285-1. [DOI] [PubMed] [Google Scholar]
- 20.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang C-L, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011. doi: 10.1016/j.cmet.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meneton P, Galan P, Bertrais S, Heudes D, Hercberg S, Ménard J. High plasma aldosterone and low renin predict blood pressure increase and hypertension in middle-aged Caucasian populations. J Hum Hypertens 22: 550–558, 2008. doi: 10.1038/jhh.2008.27. [DOI] [PubMed] [Google Scholar]
- 22.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 23.Muntner P, Abdalla M, Correa A, Griswold M, Hall JE, Jones DW, Mensah GA, Sims M, Shimbo D, Spruill TM, Tucker KL, Appel LJ. Hypertension in Blacks: unanswered questions and future directions for the JHS (Jackson Heart Study). Hypertension 69: 761–769, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: Results from the coronary artery risk development in young adults (CARDIA) study. Am J Hypertens 28: 640–648, 2015. doi: 10.1093/ajh/hpu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol 23: 1019–1026, 2012. doi: 10.1681/ASN.2011080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohashi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin-angiotensin system in the kidney. Hypertens Res 40: 413–422, 2017. doi: 10.1038/hr.2016.166. [DOI] [PubMed] [Google Scholar]
- 27.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension 33: 1099–1104, 1999. doi: 10.1161/01.HYP.33.5.1099. [DOI] [PubMed] [Google Scholar]
- 28.Richards J, Cheng K-Y, All S, Skopis G, Jeffers L, Lynch IJ, Wingo CS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am J Physiol Renal Physiol 305: F1697–F1704, 2013. doi: 10.1152/ajprenal.00472.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards J, Diaz AN, Gumz ML. Clock genes in hypertension: novel insights from rodent models. Blood Press Monit 19: 249–254, 2014. doi: 10.1097/MBP.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J 26: 3602–3613, 2012. doi: 10.1096/fj.12-203554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards J, Ko B, All S, Cheng K-Y, Hoover RS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem 289: 11791–11806, 2014. doi: 10.1074/jbc.M113.531095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123: 657–665, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossier BC. Epithelial sodium channel (ENaC) and the control of blood pressure. Curr Opin Pharmacol 15: 33–46, 2014. doi: 10.1016/j.coph.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension 48: 527–533, 2006. doi: 10.1161/01.HYP.0000240268.37379.7c. [DOI] [PubMed] [Google Scholar]
- 35.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002. doi: 10.1097/01.ASN.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- 36.Sohara E, Uchida S. Kelch-like 3/Cullin 3 ubiquitin ligase complex and WNK signaling in salt-sensitive hypertension and electrolyte disorder. Nephrol Dial Transplant 31: 1417–1424, 2016. doi: 10.1093/ndt/gfv259. [DOI] [PubMed] [Google Scholar]
- 37.Solocinski K, Cheng K-Y, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Sex-dependent regulation of blood pressure by the circadian clock protein Per1 (Abstract). FASEB J 31: 1026.18, 2017. [Google Scholar]
- 38.Solocinski K, Gumz ML. The circadian clock in the regulation of renal rhythms. J Biol Rhythms 30: 470–486, 2015. doi: 10.1177/0748730415610879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solocinski K, Holzworth M, Wen X, Cheng K-Y, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol (Oxf) 220: 72–82, 2017. doi: 10.1111/apha.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solocinski K, Richards J, All S, Cheng K-Y, Khundmiri SJ, Gumz ML. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Renal Physiol 309: F933–F942, 2015. doi: 10.1152/ajprenal.00197.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stow LR, Richards J, Cheng K-Y, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59: 1151–1156, 2012. doi: 10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanya AR, Yang C-L, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol 290: F619–F624, 2006. doi: 10.1152/ajprenal.00280.2005. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18: 164–179, 2017. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas SV, Kathpalia PP, Rajagopal M, Charlton C, Zhang J, Eaton DC, Helms MN, Pao AC. Epithelial sodium channel regulation by cell surface-associated serum- and glucocorticoid-regulated kinase 1. J Biol Chem 286: 32074–32085, 2011. doi: 10.1074/jbc.M111.278283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uzu T, Kazembe FS, Ishikawa K, Nakamura S, Inenaga T, Kimura G. High sodium sensitivity implicates nocturnal hypertension in essential hypertension. Hypertension 28: 139–142, 1996. doi: 10.1161/01.HYP.28.1.139. [DOI] [PubMed] [Google Scholar]
- 46.Vidal-Petiot E, Cheval L, Faugeroux J, Malard T, Doucet A, Jeunemaitre X, Hadchouel J. A new methodology for quantification of alternatively spliced exons reveals a highly tissue-specific expression pattern of WNK1 isoforms. PLoS One 7: e37751, 2012. doi: 10.1371/journal.pone.0037751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu B-E, Stippec S, Chu P-Y, Lazrak A, Li X-J, Lee B-H, English JM, Ortega B, Huang C-L, Cobb MH. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA 102: 10315–10320, 2005. doi: 10.1073/pnas.0504422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111: 16219–16224, 2014. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA 106: 16523–16528, 2009. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]