Abstract
Most renal transplants ultimately fail secondary to chronic allograft nephropathy (CAN). Vimentin (vim) is a member of the intermediate filament family of proteins and has been shown to be important in the development of CAN. One of the pathways leading to chronic renal fibrosis after transplant is thought to be epithelial to mesenchymal transition (EMT). Even though vim expression is one of the main steps of EMT, it is unknown whether vim expression is required for EMT leading to renal fibrosis and allograft loss. To this end, the role of vim in renal fibrosis was determined via unilateral ureteral obstruction (UUO) in vim knockout mice (129 svs6 vim −/−). Following UUO, kidneys were recovered and analyzed via Western blotting, immunofluorescence, and transcriptomics. Cultured human proximal renal tubular (HK-2) cells were subjected to lentiviral-driven inhibition of vim expression and then treated with transforming growth factor (TGF)-β to undergo EMT. Immunoblotting as well as wound healing assays were used to determine development of EMT. Western blotting analyses of mice undergoing UUO reveal increased levels of vim soon after UUO. As expected, interstitial collagen deposition increased in control mice following UUO but decreased in vim −/− kidneys. Immunofluorescence analyses also revealed altered localization of β-catenin in vim −/− mice undergoing UUO without significant changes in mRNA levels. However, RNA sequencing revealed a decrease in β-catenin-dependent genes in vim −/− kidneys. Finally, vim-silenced HK-2 cell lines undergoing EMT were shown to have decreased cellular migration during wound healing. We conclude that vim inhibition decreases fibrosis following UUO by possibly altering β-catenin localization and downstream signaling.
Keywords: β-catenin, CAN, EMT, fibrosis, renal transplant, vimentin
INTRODUCTION
Kidney transplantation has improved the lives of thousands of patients with renal failure. During the last 50 years, there have been many advances in the pharmacology of prevention of organ rejection, allowing for kidney transplant recipients to live longer with functioning grafts. However, most patients will eventually suffer from a slow but steady decline in renal function leading to graft loss. Chronic allograft nephropathy (CAN) is the most common cause of late graft failure and is characterized by a constellation of immune- and nonimmune-related damage responsible for a slow but continual decline in renal function (33).
The development of interstitial fibrosis, glomerulopathy, and tubular layering in addition to chronic inflammation ultimately results in a gradual decline in glomerular filtration rate, leading to graft loss. Clinically, patients experience increasing difficulty controlling their blood pressure and develop proteinuria. There are many reasons for the development of these constellation of findings, including 1) donor issues such as donor age and cold ischemia time, 2) medication-related issues from exposure to calcineurin inhibitors, 3) recipient issues such as recipient age and comorbid conditions (e.g., diabetes or hypertension), and finally 4) immunologic issues such as repeated bouts of cellular and/or humoral rejection. Unfortunately, there is no effective treatment for CAN (33). The insidious nature of the damage, in addition to the fact that there are currently no viable markers to predict the development of CAN, is what has made it so difficult to improve long-term outcomes following transplantation. Even though we have made great strides in preventing and treating acute rejection, we have not improved long-term graft function in the last 30 years (47).
One of the hallmarks of CAN is the development of interstitial fibrosis with concomitant loss of normal renal architecture. During this process, proximal tubular epithelial cells develop mesenchymal-like morphology with subsequent release of profibrotic mediators. This epithelial to mesenchymal transition (EMT) is a common pathway in not only renal fibrosis but also wound healing and carcinogenesis (24, 51, 55). In fact, in transplanted kidneys, EMT-related morphology has been demonstrated not only during CAN but also in acute rejection (40). There have been many factors associated with the development of EMT, as well as many possible triggers, including transforming growth factor (TGF)-β stimulation, hypoxia, infection, or T cell-mediated signaling (5, 42). One of the hallmarks of EMT in renal grafts is the de novo expression of vimentin (vim) in renal tubular epithelial cells (21, 22, 31). The presence of vim during EMT in other systems has been attributed to the need for rigid epithelial-like cells to adopt more mesenchymal (pliable) phenotypes. In the setting of carcinogenesis, this may allow tumor cells to invade through tissue planes. However, in the setting of CAN there are still many questions. Specifically, is vim facilitating the release of profibrotic cytokines by allowing cells to invade into the renal parenchyma? Is vim allowing the modulation of cell-cell contacts and therefore facilitating downstream signaling of EMT-related factors? Finally, is vim itself contributing to the signaling pathways that allow for the development of EMT phenotypes and subsequent fibrosis? It is unclear from current studies if vim is necessary and/or sufficient for the development of EMT leading to renal graft fibrosis.
Vim is a member of the intermediate filament family of proteins (18). These proteins polymerize to form the basis of the cytoskeleton in fibroblasts, smooth muscle cells, and endothelial cells. These protein networks allow for the maintenance of cellular structure in addition to contributing to cell signaling and division (18, 36). In the initial characterizations, intermediate filaments were thought to be static structures providing only support for cellular and nuclear shape. However, it is clear now that vim as well as other intermediate filaments have important roles in gene expression, cell division, and differentiation [reviewed in (18, 36)]. Specifically, in epithelial cells vim expression may be sufficient for changes not only in cell shape but also cell-cell contacts, as well as the expression and organization of cell membrane-associated proteins (27). Vim may also have a significant role in signal transduction. Studies have shown that MAPKs ERK1 and ERK2 may interact with vim to prevent dephosphorylation during nerve injury (38, 39). Phosphorylated vim has been shown to sequester 14-3-3 and block its ability to interact with Raf, another component of the ERK pathway and interact with p21-activated kinase and Rho a-binding kinase (19, 30, 45, 52). Finally, vim has been shown to be the target of numerous kinases involved in signal transduction, cell motility, and differentiation (2, 23). These results suggest that vim may have multiple roles in cell signaling and organization, which may be required for the development of EMT.
We hypothesize that vim expression is a critical component of EMT. In fact, vim may be required for cells to undergo EMT, allowing for the modulation of the cytoskeleton in proximal renal tubular cells but also providing for the downstream signaling of EMT-related factors and, ultimately, renal fibrosis.
In this study, we present data on the role of vim in the development of renal fibrosis following unilateral ureteral obstruction (UUO) in mice. Although the UUO model is not a transplant model, we believe that it is a tool for determining the molecular steps in renal fibrosis, which is a common pathway for both UUO and CAN. UUO is commonly used as a model for progressive renal interstitial fibrosis because of the technical advantages of manipulating the severity and duration of obstruction but also the availability of the contralateral kidney as an internal control (11). In fact, UUO has been previously utilized to model CAN in rodents (25, 32). Following surgery, a substantial ischemic injury results in decreased renal blood flow, hydronephrosis, apoptosis, and inflammation leading to global renal ischemia and ultimately fibrosis via activation of TGF-β-directed mechanisms (14, 53) Additionally, the renin-angiotensin axis is activated, leading to renovascular hypertension and reactive oxygen species induction, as well as macrophage activation, further increasing the profibrotic milieu in kidneys following UUO (28, 29). Finally, UUO results in the development of several mediators, leading to EMT, a known intermediary step in the profibrotic pathways common to wound healing, metastasis, and renal fibrosis (24, 54). Many of these pathways leading to fibrosis have been shown to occur during CAN. Specifically, chronic and acute rejection, native recipient disease, and donor-related issues may lead to immune- and nonimmune-related pathways that ultimately result in chronic tissue hypoxia, secondary tissue edema, and vascular injury. This continued injury ultimately leads to the development of interstitial fibrosis. It is understood that these pathways (UUO and posttransplant injury), although a result of different mechanisms, lead to the activation of similar profibrotic molecular pathways [reviewed in (7)]. Therefore, the use of the UUO model in mice is an excellent window into defining the various steps leading to CAN.
Because vim knockout mice have been shown to have deficits in wound healing as well as the development of fibrosis in lungs following injury, we tested whether vim inhibition affected the development of postinjury fibrosis after UUO (15). We find that the lack of vim inhibits the development of fibrosis in mice undergoing UUO. We also show that vim inhibition alters the dynamics of β-catenin translocation and downstream function. Our findings are confirmed in human renal tubular cells undergoing EMT. Therefore, our study provides the first description of the effects of UUO on mice that lack vim and suggests a role for vim in the development of EMT leading to renal fibrosis.
MATERIALS AND METHODS
UUO.
UUO was used to induce renal fibrosis. Vim knockout mice (129 svs6 vim −/−) were initially described by Babinet et al. (13) and are a gift from Dr. Karen Ridge (Chicago, IL) (15). Mice were anesthetized using isoflurane for 5 min and were supplied with isoflurane and oxygen throughout the procedure. Following initial incision, the left ureter was exposed and ligated three times with a non-reabsorbable suture (Fig. 1A). Mice were treated with buprenorphine for analgesia (200 mg/kg body wt) after the procedure. The mice were killed 1, 2, 3, and 4 wk postprocedure via cervical dislocation, and the kidneys were snap-frozen in liquid nitrogen or fixed in 4% PFA. Animal protocol was approved by our institutional animal review board (Institutional Animal Care and Use Committee Protocol no. 15–08003; approved 10/2015).
Fig. 1.
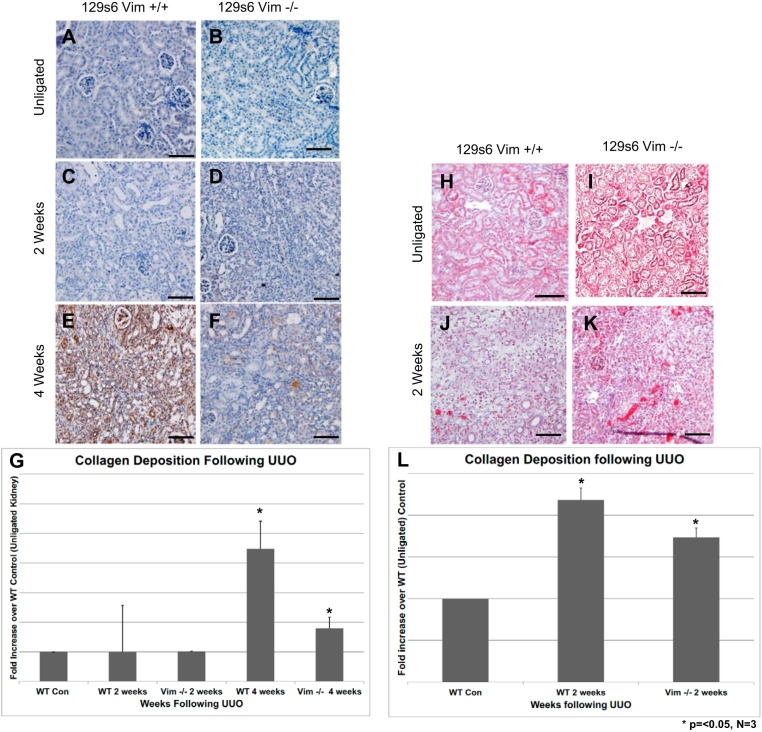
Vimentin (vim) expression is important for the development murine renal fibrosis following unilateral ureteral obstruction (UUO). Following UUO, mice kidneys were harvested and underwent immunohistochemistry (IHC) with anticollagen 11α antibody (A–F) or Masson’s trichrome staining (H–L). Four weeks post-UUO, there was a significant decrease in the intensity of anticollagen signal comparing control mice (E) and vim −/− mice (F). Unligated control kidneys show very minimal staining (A), whereas 2 wk post-UUO, there is little difference in collagen staining comparing control vs. vim −/− mice (C vs. D). Masson’s trichrome staining also showed a decrease in collagen deposition (blue staining) comparing control (K) and vim −/− mice (L) 2 wk following UUO. Unligated control kidneys display no significant evidence of collagen deposition in the renal cortex. Size bars = 50 μm. Quantification of collagen deposition following UUO was carried out utilizing the ImageJ software as described previously. Signal intensity was calculated for both IHC and trichrome staining. Samples were then normalized to unligated control kidneys. Data represent three separate experiments, looking at five sections. Collagen deposition by IHC showed a >50% decrease in collagen staining at 4 wk (G). There was a smaller but significant decrease in intensity of trichrome staining at 2 wk (M). n = 3. Error bars = standard deviations, *P < 0.05.
ShRNA production.
A pTRIPZ vector expressing nonsilencing control short hairpin (sh) RNA and shRNA under control of a doxycycline-inducible promoter was obtained (Dharmacon, Lafayette, CO). Oligonucleotides were inserted followed by a rhodamine-tagged sequence. DNA was amplified and lentivirus produced by transfection of HEK-293FT cells (Invitrogen, Carlsbad, CA) with pTRIPZ containing shRNA, PCMV-DR8.2 DVPR, and PCMV-VSV-G. Media was collected between 24 and 48 h following transfection, and lentivirus was concentrated (Amicon, Sigma-Aldrich, St. Louis, MO) and stored at −80°C. Cultured human proximal renal tubular (HK-2) cells at passage 2 were infected at 60% confluence, and the infected cells were allowed to grow to 80%–90% confluency. Both RFP and shRNA expression was induced by adding doxycycline (0.5–1 ug/ml). After 36–48 h of induction, doxycycline was removed.
Cell culture and EMT induction.
HK2 cell lines (American Type Culture Collection, Manassas, VA) were purchased and used between passages 2–5. HK-2 control cells and shRNA transfected cells were seeded at a density of 4 × 104–8 × 104 cells/cm2. On day 2, HK-2 complete culture media were replaced by DMEM/F12 medium alone and DMEM/F12 supplemented with varying concentrations of bovine pituitary extract for starvation. On day 3, medium was refreshed and supplemented with basal media containing 10 ng/ml of TGF-β. Exposure to 10 ng/ml of TGF-β continued for another 2 days by refreshing TGF-β-supplemented medium every 24 h. Cells were then subjected to wound healing assays, immunofluorescence, and Western blot protocols as described.
Wound healing assay.
HK-2 control cells and cells transfected with vim shRNA and stimulated with TGF-β were cultured in 6-well plates to 100% confluency. A pipette tip was then used to draw a straight line across the well to simulate a “wound.” Cells were then washed with PBS followed by keratinocyte serum-free media containing varying concentrations of bovine pituitary extract and EGF. Cells then were allowed to migrate for 24 h at 37°C. Images of cells were taken hourly for 24 h with a Leica DMI6000 B microscope (Wetzlar, Germany).
Tissue histology and immunofluorescence.
Immunostaining was performed on 4-μm sections of OCT-embedded kidney tissue. Tissue slides were first permeabilized for 5 min using a solution containing PBS with 1% Triton X-100, 2 mg/ml bovine serum albumin, and 1 mM NaN3. Slides were then blocked for 3 h using a blocking solution made of PBS containing 5% fetal bovine serum, 0.05% Tween 20 (vol/vol), and 1 mM Nan3. After blocking, the samples were exposed to the following primary antibodies: goat polyclonal beta-catenin (1:50, R&D Systems, Minneapolis, MN), rabbit polyclonal vim (1:50, Santa Cruz Biotechnology, Dallas, TX), and goat polyclonal E-cadherin (1:50, R&D Systems), all diluted in blocking solution. Appropriate secondary antibodies were used (1:1,500, Molecular Probes, Eugene, OR). Samples were imaged with a Leica DMI4000 B confocal microscope and analyzed using Leica Advanced Fluorescence Application Suite.
Paraffin-embedded kidney sections were stained using standard histology procedures, including hematoxylin and eosin and Masson’s trichrome staining for collagen at the Albany Med Histology Core. Additionally, immunohistochemistry was performed via incubation with a rabbit polyclonal anticollagen 11a antibody (1:200, Abcam, Cambridge, UK) followed by antirabbit horseradish peroxide (HRP) (1:1,000). Cells were imaged with an Olympus EX51 microscope (Waltham, MA).
HK-2 cells were cultured with TGF-β in 12-well plates at a seeding density of 3.8 × 104 cells/well containing glass coverslips. Cells were then washed with PBS and fixed in ice-cold ethanol for 10 min at 4°C. Cells were rinsed in PBS again for 5 min and then blocked in 5% goat serum. Following a subsequent wash, cells were incubated overnight with vim goat polyclonal antibody diluted in 5% goat serum (1:100, Santa Cruz Biotechnology). Cells were then washed with PBS/0.1% Triton X and incubated with Alexa Fluor donkey antigoat vim antibody for 2 h. Following secondary incubation, cells were washed with PBS/0.1% Triton X and then exposed to DAPI diluted in 5% goat milk (1:100) for 2 min to stain nuclei. Cell-covered coverslips were then transferred to Superfrost Microscope Slides (Fisherbrand, Fisher Scientific, Hampton, NH) to be visualized under the confocal microscope as described above.
Colocalization of β-catenin and DAPI staining in kidney samples post-UUO was obtained using the “JACoP” plugin in the ImageJ software. We determined the Mander’s colocalization coefficients between β-catenin and DAPI in both wild-type (WT) and vim −/− samples as described (16). Threshold levels were kept equivalent across all samples.
Quantification of collagen deposition.
Slides of paraffin-embedded mouse kidneys stained with anticollagen 11α antibody were imaged and then analyzed with Fiji-ImageJ software (20). “IHC toolbox” was used to select and reserve the positive color pixels, and the background color pixels were eliminated (set to 255). A statistical color model was created based on the histogram of these reserved positive color pixels as described (44). Values were then normalized to WT control (unligated) mouse kidneys. Ten sections were used in three animals at each time point. Statistical analyses were carried out as described.
qPCR.
Total RNA was extracted from fresh wet kidney tissues from vim −/− and WT control mice using TRIzol reagent (Invitrogen) 1, 2, and 4 wk following UUO. mRNAs were converted into cDNA templates using QuantiTect Reverse Transcription Kit from Qiagen (205311, Hilden, Germany) on MyCycler Thermal Cycler (579BR-0176, Bio-Rad Laboratories, Hercules, CA). Primers were designed using Integrated DNA Technologies (Coralville, IA) qPCR Primer Quest Tool and synthesized at Invitrogen. Primer set for mouse ribosome 18 S (Rn18s) was generous gift from Dr. Jourd’heuil’s laboratory. qPCR reactions were performed on Stratagene Mc3005P (Agilent Technologies, Santa Clara, CA) using PerfecCTa SYBR Green FastMix kit (95074-012, Quanta BioSciences, Beverly, MA). qPCR data were analyzed by the software Strategene MxPro (Agilent Technologies). Primer specificity and efficiency were validated by dissociation curves and amplification plots. All primers were found to have high specificity (giving single PCR product while melting point temperature Tm = 60°C) and high efficiency [threshold cycle (Ct) < 30 when there were appropriate cDNA templates and each reaction runs 40 cycles]. Primer sequences are listed below: mouse vim 5′-CACTAGCCGCAGCCTCTATTC-3′; 5′-GTCCACCGAGTCTTGAAGCA-3′; mouse β-catenin 5′-ACTTGCCACACGTGCAATTC −3′, 5′-ATGGTGCGTACAATGGCAGA-3′; mouse keratin 8 5′-TCAGGAGAAGGAGCAGATTA-3′, 5′-GGTCTCCAGCATCTTGTTC-3′; and mouse Rn18s 5′-ATGCGGCGGCGTTATTCC-3′, 5′-GCTATCAATCTGTCAATCCTGTCC-3′.
Western blotting.
Whole kidneys were homogenized in 10 mM Tris (pH 7.4) buffer with 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X, 10% glycerol, and HaltTM protease and phosphatase inhibitor cocktail (1861280, Thermo Fisher Scientific, Waltham, MA) using Minilys Homogenizer (Bertin Technologies, Rockville, MD). This SDS-deficient lysis buffer was used to minimize solubilization of membrane-associated proteins to detect total cytosolic β-catenin levels as described (48). After centrifugation at 14,000 revolutions/min for 10 min, the supernatant and the pellet were separated and diluted in 4× SDS Laemmli sample buffer (Bio-Rad Laboratories) and 2× SDS Laemmli sample buffer (Bio-Rad Laboratories), respectively. Proteins were then boiled in a VWR hot bath at 97°C for 10 min (VWR International, Radnor, PA). Proteins were separated on SDS-polyacrylamide gels and transferred to nitrocellulose paper. The membranes were incubated overnight at 4°C with the following primary antibodies: vim [a gift from Dr. Robert Goldman (26, 27)], β -catenin [Cell Signaling Technology, Danvers, MA (56)], actin [Sigma-Aldrich (57)], E-cadherin [Cell Signaling Technology (1)], and pan-keratin [Cell Signaling Technology (58)] and then probed with secondary goat antirabbit HRP-conjugated antibody (170-6515, Bio-Rad Laboratories) or goat antimouse HRP-conjugated antibody (72-1011, Bio-Rad Laboratories) for 1 h at room temperature. Antibody binding was detected with the ECL detection reagent (GE Healthcare, Little Chalfont, UK). Bands were imaged using the Fujifilm LAS-400 and quantified with Image Reader LAS-4000 (Fujifilm, Tokyo, Japan). Results are shown normalized to the protein expression in the nonligated kidney.
Statistical analysis.
Values throughout the paper are expressed as means. Mean values of groups were analyzed using GraphPad (La Jolla, CA) PRISM version 4.0 and compared by ANOVA with Neuman-Keuls post hoc analysis to determine significant differences between groups. Student’s t-test was also used as appropriate (Excel, Microsoft, Redmond, WA). Error bars represent standard error for all calculations. For all comparisons, P < 0.05 was considered statistically significant.
RESULTS
Vim expression is necessary for the development of murine renal fibrosis following UUO.
To determine whether vim expression is required for the development of fibrosis during EMT, we performed UUO on vim knockout mice (129 svs6 vim −/−) as described above. Following UUO, kidneys from vim −/− mice were removed 2 and 4 wk postsurgery. Fibrosis and scarring was determined via immunohistochemistry with anticollagen 11α antibody as well as Masson’s trichrome staining. There does not appear to be a significant difference in collagen staining 2 wk post-UUO when comparing WT control mice with vim −/− mice (Fig. 1, C and D). However, 4 wk following UUO, there is a significant decrease in collagen staining in vim −/− mice (Fig. 1, E and F). In fact, image analysis of staining intensity revealed >50% reduction in collagen staining 4 wk following UUO (Fig. 1G, *P < 0.05). Similarly, we find that 2 wk after UUO, the intensity of “blue” staining comparing Masson’s trichrome of WT versus vim −/− mice revealed diminished fibrosis in vim −/− mice when compared with controls (Fig. 1, K and L). Finally, image analysis comparing WT and vim −/− mice results in a ~30% reduction in staining 2 wk following UUO (Fig. 1M, P < 0.05). Results were derived from three separate experiments.
Vim knockout mice undergoing UUO demonstrate altered β-catenin, E-cadherin, and keratin expression.
Visualization of EMT-related proteins was determined via Western blotting of detergent-extracted kidneys following UUO (Fig. 2A). Values were then normalized to unligated control kidneys. β-catenin and vim are ubiquitous proteins in endothelial cells as well as other cell types throughout the renal parenchyma; therefore, we utilized an SDS-deficient lysis buffer to minimize solubilization of membrane-associated proteins as described previously (48). This allowed us to detect changes in cytosolic β-catenin. We compared the detection of vim, β-catenin, E-cadherin, and keratin in both unligated control (right) and ligated (left) kidneys 1, 2, and 4 wk post-UUO (Fig. 2A). We performed these experiments on WT and vim −/− mice. We find that under control conditions, vim is detected 1 wk post-UUO. However, β-catenin is detected 2 wk following UUO. Interestingly, in vim −/− mice, β-catenin was detected 1 wk following UUO (Fig. 2A). In addition, immunoblotting with β-catenin antibody resulted in multiples bands in WT mice, with only a single band in vim −/− mice, suggesting a possible alteration in the ultrastructural organization of β-catenin resulting from lack of vim expression during UUO.
Fig. 2.
Vimentin (vim) knockout mice undergoing unilateral ureteral obstruction (UUO) demonstrate altered β-catenin, E-cadherin, and keratin staining on Western blotting of whole kidneys 1, 2, and 4 wk following UUO. Whole kidneys from wild-type (WT) and vim −/− were recovered and homogenized in 10 mM Tris (pH 7.4) buffer without SDS. This SDS-deficient lysis buffer was used to minimize solubilization of membrane-associated proteins and to detect total cytosolic β-catenin levels (48). A: immunoblotting with anti-E-cadherin, -vim, -β-catenin, and -pan-keratin antibodies was performed. Vim was detected 1 wk following UUO in control mice. Nonmembrane-bound β-catenin was detected 2 wk following UUO in control mice but 1 wk after UUO in vim −/− mice. E-cadherin signaling was diminished when comparing vim −/− mice vs. control mice, whereas keratin staining increased from 1 to 2 wk in vim −/− mice and decreased in control mice. Actin staining was used as loading controls. Molecular weight is in kDa. B: Western blot staining was normalized against unligated control kidneys. Vim staining increased ~fourfold over control kidneys and peaks at 2 wk (~eightfold increase; P > 0.05). Nonmembrane-bound β-catenin increases fourfold over control kidneys 1 wk following UUO and peaks at 4 wk in vim −/− mice although β-catenin levels also increase in control kidneys over time following UUO. E-cadherin levels decrease in both WT and vim −/− kidneys 1 and 2 wk following UUO. Keratin staining increases in both vim −/− and WT mice, but keratin levels are higher in vim −/− mice. n = 4. Error bars = standard error, *P < 0.05.
E-cadherin, a cell-cell junction protein, associates with β-catenin and, under signaling from profibrotic factors, dissociates from β-catenin. This allows for translocation of β-catenin to the nucleus, leading to the expression of downstream genes. E-cadherin is found in both epithelial and endothelial cells. Consistent with published data, E-cadherin detection in ligated kidneys was significantly decreased compared with controls following UUO in WT mice (Fig. 2A). Detection of E-cadherin 1 wk following UUO in ligated and unligated control kidneys was relatively equivalent between WT and vim −/− kidneys. However, at 2 wk, E-cadherin protein concentrations in whole kidney lysates were significantly elevated when comparing ligated and unligated controls in vim −/− mice (Fig. 2A). Four weeks following UUO, E-cadherin protein levels were barely detectable in both ligated kidneys from WT and vim −/− mice.
Keratin (antibody directed against pan-murine keratins) signaling decreases over time following UUO in control mice and increases over the same period in vim −/− mice. Specifically, expression was increased at 1 wk and 2 wk following UUO in WT mice.
Vim, β-catenin, E-cadherin, and keratin 8 protein levels on Western blotting were normalized to unligated control kidneys to determine the relative fold increase or decrease following UUO (Fig. 2B). We find that vim signal increases eightfold over control 2 wk following UUO and then decreases by the fourth week. Following detergent extraction, β-catenin signal increases fourfold over control 1 wk following UUO in vim −/− kidneys (and threefold higher than WT kidneys following UUO; P < 0.05). β-catenin signaling continues to increase at 2 and 4 wk post-UUO in WT and vim −/− kidneys. Detection of E-cadherin in vim −/− mice is diminished 1 wk following UUO. However, 1 wk postsurgery, E-cadherin levels are higher in WT versus vim −/− mice (P < 0.05). This trend reverses at 2 wk (P < 0.05). Finally, although keratin 8 signaling is increased in both WT and vim −/− kidneys following UUO, the increase in keratin is more pronounced in vim −/− mice undergoing UUO (Fig. 2B; P < 0.05).
β-Catenin mRNA expression is not affected in vim −/− mice undergoing UUO.
Alterations in β-catenin levels following UUO in vim −/− mice compared with WT controls could be due to either altered expression or changes in subcellular localization and/or modifications. Thus, we investigated whether vim expression is required for β-catenin and keratin expression at the mRNA level. Data were normalized to unligated kidneys to assess the fold-increase of mRNA production when comparing UUO and nonligated control kidneys. First, our data show that WT ligated kidneys 1 wk following UUO demonstrate production of vim mRNA when compared with unligated kidneys (Fig. 3A; P < 0.05). Interestingly, contralateral WT unligated kidneys begin to produce vim mRNA 4 wk following UUO (Fig. 3A; P < 0.05). Comparatively, there is no statistical difference in β-catenin mRNA production across all our samples (WT and vim −/− ligated and unligated kidneys) 1, 2, and 4 wk following UUO (Fig. 3B). Like vim, we see an increase in keratin 8 production in WT ligated kidneys 1 wk post-UUO and a similar late increase in keratin 8 mRNA in WT unligated kidneys 4 wk following UUO (Fig. 3C; P < 0.05 where indicated).
Fig. 3.
β-catenin mRNA production is not affected in vimentin (vim) −/− mice undergoing unilateral ureteral obstruction (UUO). qPCR primers for vim, β-catenin, and keratin 8 were designed and utilized to probe wild-type (WT) and vim −/− kidneys 1, 2, and 4 wk following UUO. qPCR readouts were normalized to ribosome 18 s (Rn18s) to account for variability in loading and then expressed as fold changes of the internal control, nonligated right kidney, for each individual mouse that was analyzed. Relative mRNA expression to Rn18s shows increase in vim expression at 1 and 2 wk following UUO in WT kidneys, whereas vim expression is detected relative to Rn18s 4 wk following UUO in the unligated WT control kidney (A). β-catenin RNA expression is not statistically significant among all kidneys 1, 2, and 4 wk following UUO (B). Keratin 8 mRNA levels are also increased 1 wk post-UUO in WT ligated kidneys. At 4 wk, there is an increase of keratin 8 when compared with the other samples (C). mRNA levels were then normalized against nonligated right kidney (taken as 1). Histogram shows the mRNA levels of ligated left kidney. Vim expression was detected 1 and 2 wk following UUO and decreased sharply 4 wk post-UUO (D). There is no statistical significance in the detection of β-catenin mRNA 1, 2, and 4 wk post-UUO between WT and vim −/− mice (E). Keratin 8 mRNA levels are slightly higher in vim −/− mice 2 and 4 wk post-UUO (F). Data are representative of n = 4 independent experiments. Error bars = Standard error; *P < 0.05.
Second, the expression of mRNA was normalized to unligated controls (Fig. 3, D–F). Our data show that mRNA of vim in ligated kidneys (left) was upregulated to 4–5 times over nonligated kidneys (right) 1 wk following UUO in WT mice (Fig. 3D; P < 0.05). This upregulation of vim mRNA was slightly attenuated at 2 wk and reversed at 4 wk following UUO (Fig. 3D). Vim mRNA was not detectable in vim −/− mice.
Even though the presence of β-catenin in WT mice was detected 2 wk following UUO (Fig. 2; later than vim −/− mice), we did not detect a significant difference in β-catenin mRNA levels between WT and vim −/− mice following UUO at 1, 2, and 4 wk postsurgery (Fig. 3E).
Additionally, keratin 8 mRNA levels were similar between WT and vim −/− mice 1 wk following UUO (Fig. 3F). However, 2 wk and 4 wk following UUO, renal keratin 8 mRNA level in the ligated kidney (left) was significantly higher when compared with matched WT controls (Fig. 3F; P < 0.05).
Vim −/− mice undergoing UUO show altered cellular localization of β-catenin and E-cadherin in proximal renal tubules.
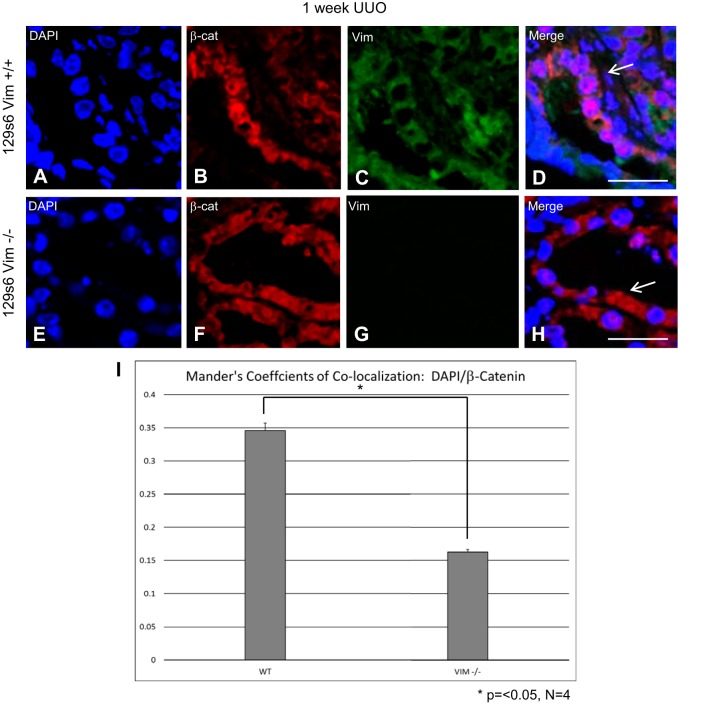
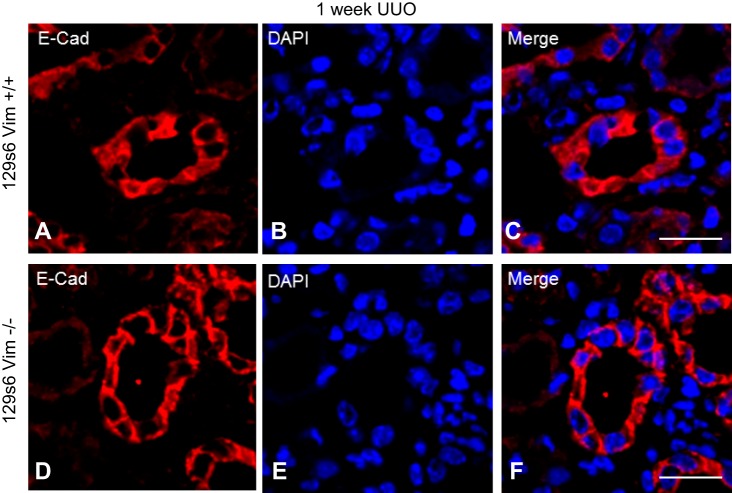
Frozen sections of ligated kidneys were subjected to confocal microscopy following staining with anti-vim, -β-catenin, and -E-cadherin antibodies as described above. One week following UUO, vim was detected in the cytoplasm of renal tubular epithelial cells in WT mice (Fig. 4C). Vim expression was not detectable in matched vim −/− mice (Fig. 4G). β-catenin in WT renal tubular cells 1 wk following UUO was more prevalent in the nuclei (Fig. 4B). Overlay images show a close association between DNA and β-catenin in the nuclei of WT kidneys following UUO (Fig. 4D, arrow). At the same time points, β-catenin was more consistently detected in the cytoplasm of proximal tubules from vim −/− mice compared with controls (Fig. 4F). Additionally, there does not appear to be a close association between nuclei and β-catenin as the bulk of β-catenin is relegated to the cytoplasm (Fig. 4H, arrow). Mander’s colocalization analyses reveal a stronger association of β-catenin with nuclei in WT vs.vim −/− kidneys (0.34 vs. 0.16; P < 0.05). One week following UUO, E-cadherin staining was similar between WT and vim −/− mice; however, overlay images show a more consistent cell-membrane staining pattern when compared with controls in vim −/− mice (Fig. 5, C and F).
Fig. 4.
Vimentin (vim) −/− mice undergoing unilateral ureteral obstruction (UUO) show altered cellular localization of β-catenin and E-cadherin in proximal renal tubules. OCT-embedded kidneys following UUO underwent immunofluorescence with antibodies directed against vim, β-catenin. DAPI was used for nuclear staining. All images were obtained on with a Leica DMI4000 B confocal microscope and analyzed using Leica Advanced Fluorescence Application Suite. Wild-type (WT) kidneys 1 wk post-UUO have an increased expression of vim in the cytoplasm of proximal renal tubular cells (C). However, β-catenin staining colocalizes with DNA in the nucleus (D, arrow). Vim −/− kidneys 1-wk post-UUO kidneys result in β-catenin signals more prominently in the cytoplasm (F, H; arrow), with no detectable vim staining (G). Mander’s colocalization coefficients calculation reveals a statically significant increase in colocalization between DAPI and β-catenin staining in WT vs. vim −/− mice (0.34 vs. 1.62). Size bars = 10 μm. *P < 0.05.
Fig. 5.
Vimentin (vim) −/− mice undergoing unilateral ureteral obstruction (UUO) display no difference in E-cadherin in proximal renal tubules following UUO. OCT-embedded kidneys following UUO underwent immunofluorescence with antibodies directed against E-cadherin and DAPI. All images were obtained on with a Leica DMI4000 B confocal microscope and analyzed using Leica advanced fluorescence application suite. E-cadherin localization 1 wk following UUO in vim −/− mice reveals a strong peripheral staining pattern along the cell membrane (D and F), whereas wild-type (WT) mice E-cadherin is seen both in at the cell membrane as well as the cytoplasm (A and C). Size bars = 10 μm.
Vim mediates TGF-β-induced EMT of human renal tubular epithelial cells.
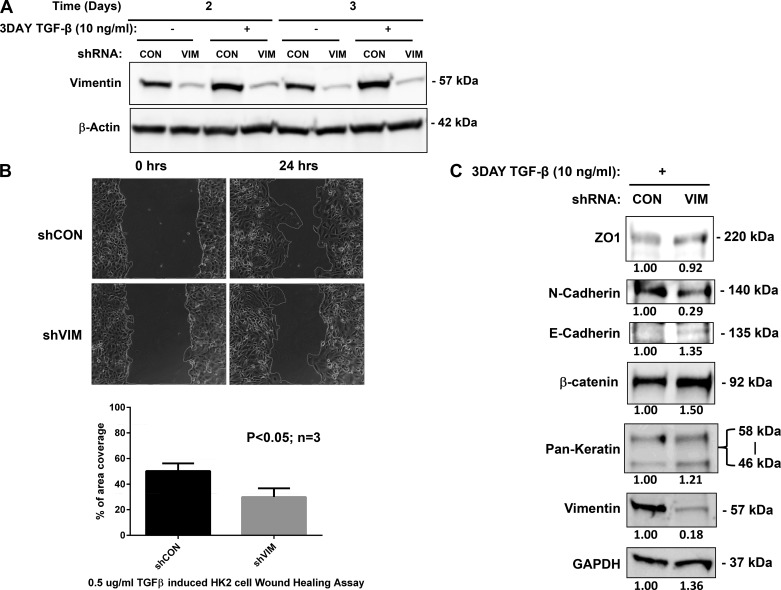
To begin to dissect the role of vim in EMT, we developed a culture-based assay using TGF-β stimulation of HK-2 cells. We used a TRIPZ doxycycline-inducible lentiviral shRNA (Dharmacon, Inc.) targeted to the vim C-terminal domain to specifically knock down vim expression in HK-2 cells (Fig. 6A). The TRIPZ lentiviral shRNA specially targeting vim (shVIM) can significantly downregulate vim expression at basal level (no TGF-β addition) compared with the negative control (data not shown). Subsequently, TGF-β-induced increase of vim expression was completely abolished by 2 and 3 days post-TGF-β addition (Fig. 6B). To determine further the effects of vim silencing on the functional consequences of TGF-β addition, we performed wound healing assays on both control and shVIM HK-2 cells (Fig. 6C). Cells with shVIM demonstrated a significantly inhibited response to TGF-β stimulation with a ~30% decrease in motility 24 h following injury. Additionally, we noticed that shVIM cells tended to translocate as “sheets” and less as isolated cells at the leading edge (Fig. 6C and Supplemental Video S1; Supplemental Material for this article is available online at the Journal website). Western blotting analyses of HK-2 cells following TGF-β induction reveal characteristic increases in β-catenin, vim, and N-cadherin. However, shVIM cell lines treated with TGF-β resulted in an increase in β-catenin and E-cadherin with a concomitant decrease in N-cadherin. Keratin expression remained equivalent in both cell lines (Fig. 6D).
Fig. 6.
Vimentin (vim) mediates transforming growth factor (TGF)-β-induced epithelial to mesenchymal transition (EMT) of human renal tubular epithelial cells (A). Sequence of shRNA to human vim. shRNA specially targeting (shVIM) downregulated vim expression efficiently in human proximal renal tubular (HK-2) cells (B). Two and 3 days following induction with TGF-β, control HK-2 cell lines express vim, whereas shVIM cell lines (VIM) do not express vim in appreciable amounts. All lanes were normalized to β-actin to ensure proper loading. Time lapse phase contrast microscopy was used to visualize wound healing in control cells (shCON) and shVIM cells following TGF-β induction (C). Quantification of cellular migration was carried out by measuring the changes in the wound area after 24 h (D). There was a ~30% reduction in migration following EMT induction in shVIM cell lines. Experiments were performed in triplicate and P values calculated. Immunoblotting using antibodies against vim, pan-keratin, and EMT markers, including zona occludens (ZO)-1, N-cadherin, E-cadherin, and β-catenin was carried out on shVIM HK-2 cells following TGF-β induction (E). Signals were normalized to GAPDH to account for variability in loading and then expressed as fold changes of the negative control, shCON. shVIM HK-2 cells demonstrate “sheet-like” motility following TGF-β stimulation. Vim-silenced HK-2 cells were treated with TGF-β and then underwent wound healing assay. Time lapse phase contrast microscopy for 24 h demonstrate sheet-like movement when compared with control cell lines. Time lapse assembled from 1-h images over 24-h period. Supplemental Material for this article is available online at the Journal website.
DISCUSSION
CAN is the most common cause of late graft failure and, even though multifactorial, results in predictable changes in renal grafts such as the development of interstitial fibrosis and tubular atrophy (33, 47). The process by which fibrosis develops is not entirely known. However, the development of EMT has been suggested to be a factor in the steps required for the development of CAN. EMT is thought to be a common pathway in not only renal fibrosis but also wound healing and carcinogenesis (24, 51, 55). One of the hallmarks of EMT in renal grafts is the de novo expression of vim in renal tubular epithelial cells (18, 21, 22, 31). Although the expression of vim may allow for the cellular plasticity required for tissue invasion or wound remodeling, there is no clear explanation for its expression in renal grafts. Therefore, it is not certain whether vim is necessary and/or sufficient for the development of EMT leading to renal graft fibrosis.
Initial reports following the development of the vim knockout mouse did not reveal significant pathological hallmarks, which tempered the interest in this model for the study of vim in disease (13). Subsequently, multiple studies have documented significant deficits in almost all organ systems. Though mice were viable, they were found to have problems with neuronal development (12, 37). Deficiencies were also documented in leukocyte migration and function (8, 34). Interestingly, mice lacking vim were shown to have impairment in wound healing as well as being resistant to the development of pulmonary fibrosis following injury (15, 17). Finally, the only studies analyzing the effects of renal function in vim −/− mice revealed alterations of expression and function of transmembrane proteins with no other significant pathology (41, 50). In this study, we provide the first evidence of the role of vim in the development of fibrosis following renal injury by performing UUO on vim knockout mice. Other studies have documented the importance of vim in wound healing and fibrosis, specifically in corneas as well as vasculature, but this is the first report of the effects of vim inhibition in a model of renal fibrosis to our knowledge (4, 43). By performing UUO on vim knockout mice, we have shown that this alone is enough to ameliorate the development of fibrosis.
Vim expression has been described as a marker for chronic renal injury following transplant, but no explanation exists as to its role in renal fibrosis. There is clearly a need for modulation in the cytoskeleton allowing for wound healing and/or metastasis, but there is no rationale for the expression of vim in tubular epithelial cells following injury. However, it is possible that the expression of vim following a fibrotic stimulus (UUO, ischemia-reperfusion injury, and/or rejection) may alter intracellular signaling pathways needed for the downstream processes leading to EMT-related fibrosis (6, 46). In addition, de novo vim expression may be required for other EMT-dependent steps such as the stabilization of collagen mRNA (9). Either way, there appears to be many pathways for vim to alter the cellular processes, leading to either the development or facilitation of downstream profibrotic signals.
Our initial experiments showed that vim −/− mice had decreased collagen deposition and decreased fibrosis following UUO (Fig. 1). These data are in accordance with previously published data on the response to fibrotic stimuli in vim −/− mice (15, 17). In addition, the use of an inhibitor of vim assembly, withaferin A, has been shown to reduce the development of fibrosis in both corneal and cardiac models following injury (3, 10). These data point to a role for vim in facilitating fibrosis, although the mechanism is not currently understood.
One of the pathways for the development of renal fibrosis is thought to be EMT, and a critical step in EMT is the release of β-catenin from E-cadherin at the cell membrane, allowing for the translocation of β-catenin to the nucleus. Although preliminary, our data hint at a role for vim in the dynamics of the E-cadherin/β-catenin complex. Specifically, vim knockout mice undergoing UUO result in altered β-catenin and E-cadherin protein concentration compared with controls (Fig. 2). However, we did not observe any increases in the mRNA levels of β-catenin, indicating that the effects of vim inhibition could be related to altered cellular localization and/or translocation (Fig. 3). Immunofluorescence data show decreased nuclear translocation of β-catenin following UUO with impaired translocation of β-catenin into the nucleus following UUO, further implying a role for vim in the modulation of cell-cell contacts leading to EMT and subsequent fibrosis (Fig. 4). Our data are supported by RNA sequencing experiments that show decreased production of β-catenin-dependent genes following UUO (Twist1, Snail 1, and MMP7, data not shown). These EMT-related factors are important for downstream activation of profibrotic factors.
One other interesting finding is the de novo expression of vim (albeit at a reduced level) in the contralateral (unligated) kidneys following UUO (Fig. 3). This finding hints at a possible systemic signaling event that may trigger EMT-like changes in the contralateral kidney. Although the trigger for this finding could be secondary to inflammation or decrease in glomerular filtration rate, with either alterations in blood flow of the contralateral kidney or systemic biochemical changes, it is possible that the development of EMT in proximal renal tubular cells results in global effects that can stimulate EMT on other organs. We will be pursuing the role of vim and signaling in subsequent studies.
Finally, our conclusions are also supported by tissue culture experiments that demonstrate that vim inhibition alters the functional consequences of EMT by affecting cellular migration following wound healing (Fig. 6). Not only is there a general inhibition of motility across the wounded area, but shVIM renal tubular cells move in a more “sheet-like” fashion when compared with control cells (Fig. 6, Supplemental Video S1) (all supplmental material for this article is available on the journal website). This may be due to either alterations in cytoskeletal dynamics or via modulation and/or stabilization of cell-cell contacts.
The work presented here comprises the first study on renal fibrosis in vim −/− mice. Our data agree with published results indicating that mice lacking vim are inhibited from developing significant fibrosis/scarring (15, 17). Mechanistically, our data hint at a role for vim expression in the dynamics of the cadherin/catenin complexes. One possible mechanism of action is through the modulation of other cytoskeletal elements. It is known that actin cytoskeletal elements can bind to and modulate the cadherin/catenin complexes. Therefore, vim expression may not need to interact directly with these complexes to alter cellular signaling dynamics; however, it may be possible that cytoskeletal crosstalk is able to modulate cell-cell dynamics leading to EMT-related fibrosis (35, 46, 49). Alternatively, vim expression alone may allow for the downstream signaling via interactions with various kinases as well as other signal transduction factors.
Although it is not precisely the same injury, UUO induces renal fibrosis in mice in a very similar fashion as is seen during EMT leading to CAN. Therefore, UUO is an excellent model to study the differing pathways leading to CAN. These commonalities between the development of fibrosis after UUO and during CAN allow us to develop hypotheses as to the role of vim in CAN. In fact, the role of vim in directly leading to CAN is currently being tested in other models. However, our data here support a role for vim in the development of EMT and therefore may be important for the steps required for the development of CAN. Much work remains to assess the role of vim in EMT/CAN, but our results with the vim −/− mice have indicated a possible new role for intermediate filaments in disease.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.W. and R.I.L.-S. conceived and designed research; Z.W., A.D., F.L.J., and R.I.L.-S. performed experiments; Z.W., A.D., F.L.J., D.J., and R.I.L.-S. analyzed data; Z.W., F.L.J., R.D.G., K.M.R., D.J., and R.I.L.-S. interpreted results of experiments; Z.W. and R.I.L.-S. prepared figures; Z.W., A.D., and R.I.L.-S. drafted manuscript; Z.W., A.D., D.J., and R.I.L.-S. edited and revised manuscript; Z.W. and R.I.L.-S. approved final version of manuscript.
Supplemental Data
ACKNOWLEDGMENTS
We acknowledge the contribution of Sanjukta Dutta in the preparation of this manuscript.
REFERENCES
- 1.Advedissian T, Proux-Gillardeaux V, Nkosi R, Peyret G, Nguyen T, Poirier F, Viguier M, Deshayes F. E-cadherin dynamics is regulated by galectin-7 at epithelial cell surface. Sci Rep 7: 17086, 2017. doi: 10.1038/s41598-017-17332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barberis L, Pasquali C, Bertschy-Meier D, Cuccurullo A, Costa C, Ambrogio C, Vilbois F, Chiarle R, Wymann M, Altruda F, Rommel C, Hirsch E. Leukocyte transmigration is modulated by chemokine-mediated PI3Kgamma-dependent phosphorylation of vimentin. Eur J Immunol 39: 1136–1146, 2009. doi: 10.1002/eji.200838884. [DOI] [PubMed] [Google Scholar]
- 3.Bargagna-Mohan P, Deokule SP, Thompson K, Wizeman J, Srinivasan C, Vooturi S, Kompella UB, Mohan R. Withaferin A effectively targets soluble vimentin in the glaucoma filtration surgical model of fibrosis. PLoS One 8: e63881, 2013. doi: 10.1371/journal.pone.0063881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bargagna-Mohan P, Paranthan RR, Hamza A, Zhan CG, Lee DM, Kim KB, Lau DL, Srinivasan C, Nakayama K, Nakayama KI, Herrmann H, Mohan R. Corneal antifibrotic switch identified in genetic and pharmacological deficiency of vimentin. J Biol Chem 287: 989–1006, 2012. doi: 10.1074/jbc.M111.297150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedi S, Vidyasagar A, Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev (Orlando) 22: 1–5, 2008. doi: 10.1016/j.trre.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beggs JE, Tian S, Jones GG, Xie J, Iadevaia V, Jenei V, Thomas G, Proud CG. The MAP kinase-interacting kinases regulate cell migration, vimentin expression and eIF4E/CYFIP1 binding. Biochem J 467: 63–76, 2015. doi: 10.1042/BJ20141066. [DOI] [PubMed] [Google Scholar]
- 7.Boor P, Floege J. Renal allograft fibrosis: biology and therapeutic targets. Am J Transplant 15: 863–886, 2015. doi: 10.1111/ajt.13180. [DOI] [PubMed] [Google Scholar]
- 8.Brown MJ, Hallam JA, Colucci-Guyon E, Shaw S. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J Immunol 166: 6640–6646, 2001. doi: 10.4049/jimmunol.166.11.6640. [DOI] [PubMed] [Google Scholar]
- 9.Challa AA, Stefanovic B. A novel role of vimentin filaments: binding and stabilization of collagen mRNAs. Mol Cell Biol 31: 3773–3789, 2011. doi: 10.1128/MCB.05263-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Challa AA, Vukmirovic M, Blackmon J, Stefanovic B. Withaferin-A reduces type I collagen expression in vitro and inhibits development of myocardial fibrosis in vivo. PLoS One 7: e42989, 2012. doi: 10.1371/journal.pone.0042989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 12.Colucci-Guyon E, Giménez Y Ribotta M, Maurice T, Babinet C, Privat A. Cerebellar defect and impaired motor coordination in mice lacking vimentin. Glia 25: 33–43, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 13.Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 79: 679–694, 1994. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 14.Docherty NG, O’Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290: F4–F13, 2006. doi: 10.1152/ajprenal.00045.2005. [DOI] [PubMed] [Google Scholar]
- 15.dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales-Nebreda L, Sennello JA, Kutuzov MA, Sitikov A, Davis JM, Lam AP, Cheresh P, Kamp D, Shumaker DK, Budinger GR, Ridge KM. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun 6: 6574, 2015. doi: 10.1038/ncomms7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol 300: C723–C742, 2011. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci 113: 2455–2462, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. Introducing intermediate filaments: from discovery to disease. J Clin Invest 119: 1763–1771, 2009. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK). Genes Cells 7: 91–97, 2002. doi: 10.1046/j.1356-9597.2001.00504.x. [DOI] [PubMed] [Google Scholar]
- 20.Hartig SM. Basic image analysis and manipulation in ImageJ. Curr Protoc Mol Biol Chapter 14: 15, 2013. doi: 10.1002/0471142727.mb1415s102. [DOI] [PubMed] [Google Scholar]
- 21.Hertig A, Anglicheau D, Verine J, Pallet N, Touzot M, Ancel PY, Mesnard L, Brousse N, Baugey E, Glotz D, Legendre C, Rondeau E, Xu-Dubois YC. Early epithelial phenotypic changes predict graft fibrosis. J Am Soc Nephrol 19: 1584–1591, 2008. doi: 10.1681/ASN.2007101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertig A, Verine J, Mougenot B, Jouanneau C, Ouali N, Sebe P, Glotz D, Ancel PY, Rondeau E, Xu-Dubois YC. Risk factors for early epithelial to mesenchymal transition in renal grafts. Am J Transplant 6: 2937–2946, 2006. doi: 10.1111/j.1600-6143.2006.01559.x. [DOI] [PubMed] [Google Scholar]
- 23.Ivaska J, Vuoriluoto K, Huovinen T, Izawa I, Inagaki M, Parker PJ. PKCɛ-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J 24: 3834–3845, 2005. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002. doi: 10.1172/JCI0215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol 20: 1223–1235, 2009. doi: 10.1681/ASN.2008050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Soler RI, Borgia JA, Kanangat S, Fhied CL, Conti DJ, Constantino D, Ata A, Chan R, Wang Z. Anti-vimentin antibodies present at the time of transplantation may predict early development of interstitial fibrosis/tubular atrophy. Transplant Proc 48: 2023–2033, 2016. doi: 10.1016/j.transproceed.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J 24: 1838–1851, 2010. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misseri R, Meldrum DR, Dinarello CA, Dagher P, Hile KL, Rink RC, Meldrum KK. TNF-alpha mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol 288: F406–F411, 2005. doi: 10.1152/ajprenal.00099.2004. [DOI] [PubMed] [Google Scholar]
- 29.Miyajima A, Kosaka T, Seta K, Asano T, Umezawa K, Hayakawa M. Novel nuclear factor kappa B activation inhibitor prevents inflammatory injury in unilateral ureteral obstruction. J Urol 169: 1559–1563, 2003. doi: 10.1097/01.ju.0000045686.21766.c1. [DOI] [PubMed] [Google Scholar]
- 30.Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene 28: 2545–2555, 2009. doi: 10.1038/onc.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muramatsu M, Miyagi M, Ishikawa Y, Aikawa A, Mizuiri S, Ohara T, Ishii T, Hasegawa A. Estimation of damaged tubular epithelium in renal allografts by determination of vimentin expression. Int J Urol 11: 954–962, 2004. doi: 10.1111/j.1442-2042.2004.00938.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi T, Morokata T, Noto T, Kubo K, Umeno H, Kinugasa F, Eikyu Y, Kozuki Y, Seki N. Effect of the inosine 5′-monophosphate dehydrogenase inhibitor BMS-566419 on renal fibrosis in unilateral ureteral obstruction in rats. Int Immunopharmacol 10: 1434–1439, 2010. doi: 10.1016/j.intimp.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 34.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol 8: 156–162, 2006. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 35.Osmanagic-Myers S, Rus S, Wolfram M, Brunner D, Goldmann WH, Bonakdar N, Fischer I, Reipert S, Zuzuarregui A, Walko G, Wiche G. Plectin reinforces vascular integrity by mediating crosstalk between the vimentin and the actin networks. J Cell Sci 128: 4138–4150, 2015. doi: 10.1242/jcs.172056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paramio JM, Jorcano JL. Beyond structure: do intermediate filaments modulate cell signalling? BioEssays 24: 836–844, 2002. doi: 10.1002/bies.10140. [DOI] [PubMed] [Google Scholar]
- 37.Pekny M. Astrocytic intermediate filaments: lessons from GFAP and vimentin knock-out mice. Prog Brain Res 132: 23–30, 2001. doi: 10.1016/S0079-6123(01)32062-9. [DOI] [PubMed] [Google Scholar]
- 38.Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 45: 715–726, 2005. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Perlson E, Michaelevski I, Kowalsman N, Ben-Yaakov K, Shaked M, Seger R, Eisenstein M, Fainzilber M. Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J Mol Biol 364: 938–944, 2006. doi: 10.1016/j.jmb.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 40.Robertson H, Ali S, McDonnell BJ, Burt AD, Kirby JA. Chronic renal allograft dysfunction: the role of T cell-mediated tubular epithelial to mesenchymal cell transition. J Am Soc Nephrol 15: 390–397, 2004. doi: 10.1097/01.ASN.0000108521.39082.E1. [DOI] [PubMed] [Google Scholar]
- 41.Runembert I, Couette S, Federici P, Colucci-Guyon E, Babinet C, Briand P, Friedlander G, Terzi F. Recovery of Na-glucose cotransport activity after renal ischemia is impaired in mice lacking vimentin. Am J Physiol Renal Physiol 287: F960–F968, 2004. doi: 10.1152/ajprenal.00064.2004. [DOI] [PubMed] [Google Scholar]
- 42.Rygiel KA, Robertson H, Willet JD, Brain JG, Burt AD, Jones DE, Kirby JA. T cell-mediated biliary epithelial-to-mesenchymal transition in liver allograft rejection. Liver Transpl 16: 567–576, 2010. doi: 10.1002/lt.22029. [DOI] [PubMed] [Google Scholar]
- 43.Schiffers PM, Henrion D, Boulanger CM, Colucci-Guyon E, Langa-Vuves F, van Essen H, Fazzi GE, Lévy BI, De Mey JG. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol 20: 611–616, 2000. doi: 10.1161/01.ATV.20.3.611. [DOI] [PubMed] [Google Scholar]
- 44.Shu J, Dolman GE, Duan J, Qiu G, Ilyas M. Statistical colour models: an automated digital image analysis method for quantification of histological biomarkers. Biomed Eng Online 15: 46, 2016. doi: 10.1186/s12938-016-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol 18: 6325–6339, 1998. doi: 10.1128/MCB.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spurny R, Gregor M, Castañón MJ, Wiche G. Plectin deficiency affects precursor formation and dynamics of vimentin networks. Exp Cell Res 314: 3570–3580, 2008. doi: 10.1016/j.yexcr.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Stegall MD, Gaston RS, Cosio FG, Matas A. Through a glass darkly: seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol 26: 20–29, 2015. doi: 10.1681/ASN.2014040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384, 2005. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 49.Sutoh Yoneyama M, Hatakeyama S, Habuchi T, Inoue T, Nakamura T, Funyu T, Wiche G, Ohyama C, Tsuboi S. Vimentin intermediate filament and plectin provide a scaffold for invadopodia, facilitating cancer cell invasion and extravasation for metastasis. Eur J Cell Biol 93: 157–169, 2014. doi: 10.1016/j.ejcb.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Terzi F, Henrion D, Colucci-Guyon E, Federici P, Babinet C, Levy BI, Briand P, Friedlander G. Reduction of renal mass is lethal in mice lacking vimentin. Role of endothelin-nitric oxide imbalance. J Clin Invest 100: 1520–1528, 1997. doi: 10.1172/JCI119675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2: 442–454, 2002. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 52.Tzivion G, Luo ZJ, Avruch J. Calyculin A-induced vimentin phosphorylation sequesters 14-3-3 and displaces other 14-3-3 partners in vivo. J Biol Chem 275: 29772–29778, 2000. doi: 10.1074/jbc.M001207200. [DOI] [PubMed] [Google Scholar]
- 53.Vaughan ED Jr, Marion D, Poppas DP, Felsen D. Pathophysiology of unilateral ureteral obstruction: studies from Charlottesville to New York. J Urol 172: 2563–2569, 2004. doi: 10.1097/01.ju.0000144286.53562.95. [DOI] [PubMed] [Google Scholar]
- 54.Vidyasagar A, Reese SR, Hafez O, Huang LJ, Swain WF, Jacobson LM, Torrealba JR, Chammas PE, Wilson NA, Djamali A. Tubular expression of heat-shock protein 27 inhibits fibrogenesis in obstructive nephropathy. Kidney Int 83: 84–92, 2013. doi: 10.1038/ki.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am J Transplant 5: 1367–1374, 2005. doi: 10.1111/j.1600-6143.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- 56. Wang D, Weng Y, Guo S, Zhang Y, Zhou T, Zhang M, Wang L, Ma J. Platelet-rich plasma inhibits RANKL-induced osteoclast differentiation through activation of Wnt pathway during bone remodeling. Int J Mol Med 41: 729–738, 2018. doi: 10.3892/ijmm.2017.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou J, Lhoták S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 111: 1814–1821, 2005. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 58.Zhou J, Zhang C, Pan J, Chen L, Qi ST. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human adamantinomatous craniopharyngioma cells and promotes tumor cell migration. Mol Med Rep 15: 4123–4131, 2017. doi: 10.3892/mmr.2017.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.