Abstract
Manganese (Mn) toxicity arises from nutritional problems, community and occupational exposures, and genetic risks. Mn blood levels are controlled by hepatobiliary clearance. The goals of this study were to determine the cellular distribution of Mn transporters in polarized hepatocytes, to establish an in vitro assay for hepatocyte Mn efflux, and to examine possible roles the Mn transporters would play in metal import and export. For these experiments, hepatocytoma WIF-B cells were grown for 12–14 days to achieve maximal polarity. Immunoblots showed that Mn transporters ZIP8, ZnT10, ferroportin (Fpn), and ZIP14 were present. Indirect immunofluorescence microscopy localized Fpn and ZIP14 to WIF-B cell basolateral domains whereas ZnT10 and ZIP8 associated with intracellular vesicular compartments. ZIP8-positive structures were distributed uniformly throughout the cytoplasm, but ZnT10-positive vesicles were adjacent to apical bile compartments. WIF-B cells were sensitive to Mn toxicity, showing decreased viability after 16 h exposure to >250 μM MnCl2. However, the hepatocytes were resistant to 4-h exposures of up to 500 μM MnCl2 despite 50-fold increased Mn content. Washout experiments showed time-dependent efflux with 80% Mn released after a 4 h chase period. Hepcidin reduced levels of Fpn in WIF-B cells, clearing Fpn from the cell surface, but Mn efflux was unaffected. The secretory inhibitor, brefeldin A, did block release of Mn from WIF-B cells, suggesting vesicle fusion may be involved in export. These results point to a possible role of ZnT10 to import Mn into vesicles that subsequently fuse with the apical membrane and empty their contents into bile.
NEW & NOTEWORTHY Polarized WIF-B hepatocytes express manganese (Mn) transporters ZIP8, ZnT10, ferroportin (Fpn), and ZIP14. Fpn and ZIP14 localize to basolateral domains. ZnT10-positive vesicles were adjacent to apical bile compartments, and ZIP8-positive vesicles were distributed uniformly throughout the cytoplasm. WIF-B hepatocyte Mn export was resistant to hepcidin but inhibited by brefeldin A, pointing to an efflux mechanism involving ZnT10-mediated uptake of Mn into vesicles that subsequently fuse with and empty their contents across the apical bile canalicular membrane.
Keywords: hepatotoxicity, manganese, Mn transporters, WIF-B cells
INTRODUCTION
Manganese (Mn) is an essential element necessary for proper skeletal development, immunity, hemostasis, and brain function (3, 6, 68). However, excess Mn impairs neurotransmitter systems, damages the basal ganglia, and is associated with learning disabilities, hyperactivity, and attention deficit disorder in children (19, 30, 49, 64, 79, 88). Exposure to high Mn because of contaminated drinking water (10, 62, 84), occupational hazards (8, 20), environmental emissions (28, 54), hepatic encephalopathy (46, 75), parenteral nutrition support (9, 23, 57, 71), and the use of soy infant formulas (27, 36, 37) has been reported and can lead to a parkinsonian-like condition called manganism (53).
After intestinal absorption, delivery of Mn to the liver via portal circulation provides first-pass clearance of excess metal into bile, creating a protective barrier against the neurotoxic effects of high circulating Mn. While Mn deficiency is rare, accumulation of excess Mn is more prevalent and occurs despite the body’s natural clearance mechanisms. Several recent studies have identified that perturbations in Mn acquisition, accumulation, and deposition result from disruptions in human genes thought to be involved Mn transport and homeostasis, including SLC39A14 (ZIP14), SLC39A8 (ZIP8), and SLC30A10 (ZnT10) (7, 11, 17, 31, 35, 47, 48, 51, 63, 65–67, 87). Although whether human patients with ferroportin (Fpn) disease are affected has yet to be examined, studies in flatiron mice with defects in Slc40a1 (Fpn) indicate that mutations in this importer can impair Mn transport.
Individuals carrying mutations in ZnT10 and ZIP14 display hypermanganesemia, while patients with defects in ZIP8 suffer from hypomanganesemia (18). Defects in ZIP14 cause high blood Mn with Mn loading in the brain but not in the liver, indicating that a function in liver Mn uptake must be impaired (81, 82). Patients with defects in ZnT10 also display hypermanganesemia but additionally suffer from chronic liver disease because of hepatic Mn accumulation, and thus Mn excretion must be diminished in these individuals, whereas uptake is unaffected (56, 65, 66, 76, 81, 83). In concordance with this idea, ZnT10-knockout mice display liver Mn loading (31). Children with ZIP8 deficiency have hypomanganesemia with developmental and intellectual impairment, hypotonia, and short stature (63). These symptoms reflect problems associated with Mn deficiency despite adequate intake of the nutrient. In studies of ZIP8-knockout mice and mice overexpressing this transporter, Rader and colleagues (47) reported that the transporter is localized to the bile canalicular membrane. While knockout mice had decreased activity of hepatic Mn-dependent enzymes and increased levels of Mn in bile, overexpression of ZIP8 raised tissue and blood Mn levels. Based on their evidence, this group proposes that the transporter reclaims excreted Mn from bile (47). Finally, flatiron (ffe) mice have an Fpn deficiency and features that recapitulate human “Fpn disease” (73). Our laboratory reported that blood, liver, and bile Mn levels are reduced in flatiron mice (73). Both 54Mn and 59Fe uptake after intragastric gavage are significantly decreased in this genetic model, leading us to conclude that Fpn exports both metals from intestinal enterocytes into portal circulation (73). Since liver is a major site of Fpn expression, a possible role for the transporter in Mn export from hepatocytes was raised by these studies.
While accumulating evidence from human patients and mouse genetic models is beginning to reveal the possible transport pathways involved in hepatobiliary clearance of Mn, in vitro models that recapitulate this process have yet to be established. To better understand the molecular mechanisms involved in polarized Mn trafficking in hepatocytes, we chose to study hepatocytoma WIF-B cells as a well-established model for functional investigations of biliary transport (15, 32, 33, 74, 80). Polarized WIF-B cells have been employed to study hepatic basolateral-to-apical transport and have proven useful for indirect immunofluorescence microscopy experiments. For example, the function of the Wilson’s disease transporter, ATP7b, and its intracellular trafficking and regulation by copper was first established in WIF-B cells (13, 61). Previous work also linked WIF-B cell Golgi transporters to Mn metabolism (44). WIF-B cells are a chimeric cell line established with rat hepatic Fao cells by fusion with human fibroblasts (74). The goals of this study were to determine the expression and cellular distribution of Mn transporters in WIF-B cells, to establish an in vitro assay for hepatocyte Mn efflux, and to identify the roles that Mn transporters may have in WIF-B cell Mn uptake and efflux.
MATERIALS AND METHODS
Cell culture.
WIF-B cells were obtained from Dr. Pamela Tuma (The Catholic University of America, Washington, DC) and were grown at 37°C in F12 Coon’s media supplemented with 5% fetal bovine serum, 1% penicillin-streptomycin, 1.1 µM amphotericin B, 10 µM hypoxanthine, 40 nM aminopterin, and 1.6 µM thymidine in a humidified 7% CO2 incubator at pH 7.0 (15, 32, 74). Unless otherwise indicated, cells were seeded onto glass coverslips (2 × 104 cells/well) or 10-cm2 dishes (6 × 105 cells/plate) and grown for 12–14 days. To confirm functional integrity of bile canalicular compartments in polarized cells, 0.1 μg/ml fluorescein diacetate was added for 20 min at 37°C and imaged immediately using a Zeiss Axioscope microscope (488 nm) with ×20 objective to confirm accumulation of dye in bile compartments (BCs) (33, 69).
PCR.
TRIzol (Invitrogen, Carlsbad, CA) was added directly to cells, and lysates were stored at −80°C until use. RNA was isolated using Direct-zol RNA MiniPrep (Zymo Research, Irvine, CA) and reverse transcription was carried out using the SuperScript III System (Invitrogen) according to manufacturer's instructions. Reverse transcription reaction product (2 µl) was included in 50-µl PCR assays using primers designed to amplify unique rat and human sequences of interest (Table 1). 36B4 was amplified for each reaction as a positive control. Product size and integrity were confirmed by agarose gel electrophoresis.
Table 1.
Primer sequence for manganese transporter expression studies in WIF-B cells
| Gene Symbol | Gene Name | Primer Direction | Primer Sequence (5′-3′) | Product (bp) |
|---|---|---|---|---|
| SLC40A1 | Ferroportin | F R |
CTAGTGTCATGACCAGGGCG CACATCCGATCTCCCCAAGT |
133 |
| Slc40a1 | F R |
CAGCTTTGCTGTTCTTTGCCT CCCCTTGTTTGTTCGGATGC |
154 | |
| SLC39A8 | ZIP8 | F R |
CCGGGAGGAGCATATGTACG CATTGAAGTGGCAGCGTAGC |
240 |
| Slc39a8 | F R |
TCCTTTTATCTCAGGCTCCGC AGTTAGCGAGAAGCTCGGTG |
133 | |
| SLC39A14 | ZIP14 | F R |
AAGGCCCTACTCAACCACCT CGACTGCTCGCTGAAATTGTG |
135 |
| Slc39a14 | F R |
GAGAAAGCGTTTTTCAGCCGT TCCGTGATGGTGCTCGTTTT |
225 | |
| SLC30A10 | ZnT10 | F R |
TCGCGTTATAAAAAGCGGTGG ACGTCTTGCCAGAGTAGCG |
180 |
| Slc30a10 | F R |
CCCTGAGACATGAAGACCCG TCATGCACACTGCTAACCCC |
205 | |
| RPLP0 | 36B4 | F R |
CCTCGTGGAAGTGACATCGT CTGTCTTCCCTGGGCATCAC |
76 |
| Rplp0 | F R |
AGCCAAGGTCGAAGCAAAGG TAAGCAGGCTGACTTGGTGTG |
97 |
F, forward; R, reverse.
Western blot analysis.
WIF-B cells were mixed with Laemmli sample buffer directly and heated for 10 min at 72°C. For some antigens (ZIP8 and ZIP14), samples were left unheated. Equal sample volumes were loaded onto 4–20% denaturing gels and separated by SDS-PAGE, transferred to nitrocellulose using a Bio-Rad Transblot Turbo blotting system, and immunoblots were blocked with 5% nonfat milk in Tris-buffered saline (TBS) containing 1% Tween-20 (TW20). Blots were incubated with primary antibodies described above in TBS-TW20 overnight at 4°C, rinsed in TBS-TW20, and placed in secondary IRDye 800CW donkey anti-rabbit or anti-mouse IgG (LI-COR Biosciences, Lincoln, NE, 1:5,000 dilution) or IRDye 680RD donkey anti-rabbit or anti-mouse IgG (LI-COR Biosciences, 1:5,000 dilution) and then rinsed in TBS-TW20. GAPDH was probed as a loading control and detected using mouse anti-GAPDH (Sigma-Aldrich, St. Louis, MO, 1:2000). Images were acquired on LI-COR Odyssey Clx Near-Infrared Western Blot System, and band intensity was analyzed using iS Image Studio.
Indirect immunofluorescence of WIF-B cells.
WIF-B cells were rinsed in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde in PBS for 20 min, followed by quenching with 10 mM glycine in PBS. Cells were permeabilized for 5 min in 0.2% Triton-X100-PBS then blocked for 30 min with 1% bovine serum albumin (BSA) in PBS. Cells were then incubated for 1 h with the following primary antibodies: rabbit anti-ZnT10 (Sigma-Aldrich, 1:50–1:100), rabbit anti-ZIP14 (Sigma-Aldrich, 1:50–1:100), rabbit anti-ZIP8 (Sigma-Aldrich, 1:50–1:100), rabbit anti-Fpn (Alpha Diagnostics International, San Antonio, TX, 1:100), mouse anti-Rab7 (Abcam, Cambridge, MA, 1:100), mouse anti-MRP2 (Abcam, 1:100), and rabbit anti-NaK-ATPase (Abcam, 1:100). Cells were then incubated for 30 min with secondary antibodies AlexaFluor568-labeled goat anti-rabbit (Molecular Probes, Eugene, OR, 1:500) or AlexaFluor488-labeled goat anti-mouse (Invitrogen, 1:500). Coverslips were mounted as described above. WIF-B cells were imaged using a Yokogawa CSU-X1 spinning disk confocal system with a Nikon Ti-E inverted microscope using a ×60 or ×100 Plan Apo objective lens with a Zyla cMOS camera using 561 and 488 lasers. NIS elements software was used for acquisition parameters, shutters, filter positions, and focus control. Immunofluorescence (IF) experiments were typically carried out using at least two wells and were repeated on several occasions.
Cell viability.
Cell viability was determined using the TOX-1 assay (Sigma-Aldrich). Cells were first exposed to MnCl2 at concentrations indicated in the figure legends for 4 or 16 h then incubated for 4 h with 3-[4,5-dimethylthiazol-2yl]-2,5diphenyltetrazolium bromide (MTT) (0.5 mg/ml), and the resulting formazan crystals were dissolved in isopropyl alcohol containing 0.1 N HCl. Absorbance was measured at 570 nm using a BioTek Synergy 2 plate reader, and cell viability was normalized to control (untreated) cells.
Mn uptake and efflux assay.
WIF-B cells were exposed to MnCl2 at concentrations and incubation times indicated in the figure legends, then washed twice and incubated in Mn-free medium for chase periods of 30, 60, or 240 min. Cells were then washed with ice-cold PBS to remove nonspecifically bound Mn, trypsinized, and cell pellets were collected and weights recorded. Cell pellets were analyzed for Mn content by inductively coupled mass spectrometry (ICP-MS) (Trace Metal Analysis Laboratory, Dartmouth College, Hanover, NH), and values were normalized to weights of cell pellets. In some experiments, WIF-B cells were first treated with 1.4 μM hepcidin or 10 μM brefeldin A (BFA) before and during Mn exposure as detailed in the figure legends.
Transfection of HEK293T cells.
HEK293T cells were grown as previously described (85). Expression vectors were purchased from GeneCopoeia (Rockville, MD). HEK293T cells grown in 6-well or 12-well plates were transfected with empty control vector (pReceiver), NH2-terminal 3xFLAG-ZnT10 (pReceiver-FLAG-ZnT10), or COOH-terminal ZnT10-mCherry (pReceiver-ZnT10-mCherry) using Lipofectamine 2000 (Invitrogen), according to manufacturer’s instructions. After 24–48 h, HEK293T cells were washed with PBS and fixed for 20 min in 4% paraformaldehyde in PBS followed by quenching with 10 mM glycine in PBS. Cells were permeabilized with 0.25% Triton X-100 in PBS for 5 min, and after blocking with 1% BSA in PBS for 30 min, cells were incubated with rabbit anti-FLAG antibody (Sigma-Aldrich; 1:100). After washing with 0.2% BSA in PBS, cells were incubated in Alexa Fluor568-labeled donkey anti-rabbit (Molecular Probes, 1:500). Cells were washed in 0.2% BSA in PBS, with a final wash in PBS. Coverslips were mounted onto glass slides using SlowFade Gold Antifade Mountant with DAPI (Invitrogen). FLAG-ZnT10 and ZnT10-mCherry expression in HEK292T cells was observed using a Zeiss Axio Observer Fluorescence microscope with Apotome using ×63 oil objective and Zen 2.3 software.
Statistical analysis.
One-way ANOVA and Student’s t-test was used for statistical comparisons with GraphPad Prism software. Results shown are means ± SE, and differences were considered significant at P < 0.05. ImageJ software and the JACoP plugin were used to determine Pearson’s coefficient.
RESULTS
Characteristics and expression of Mn transporters in WIF-B cells.
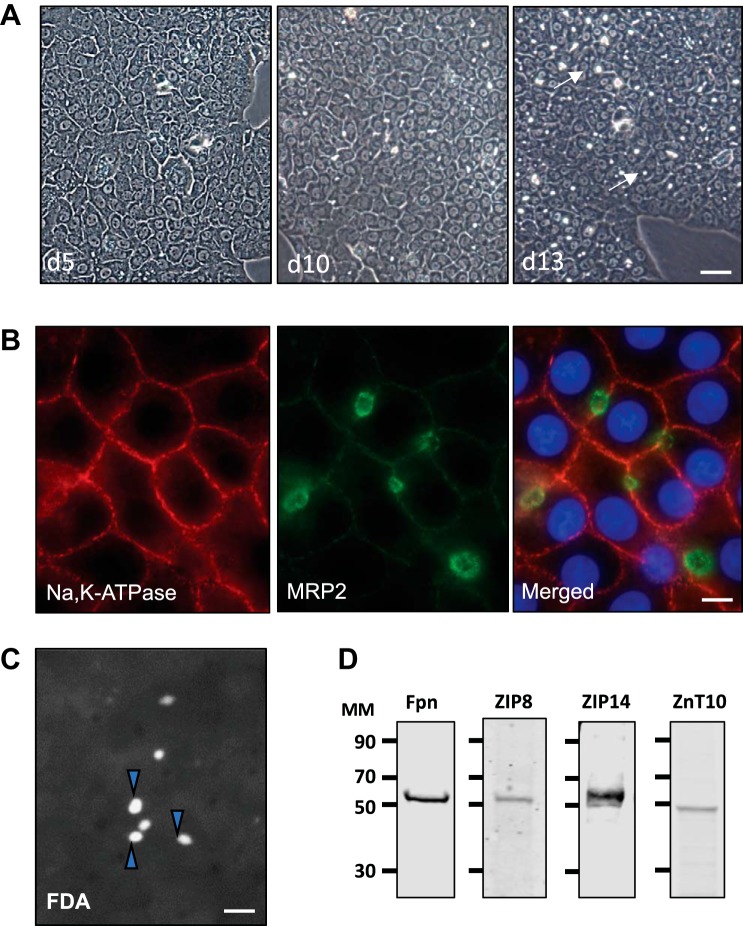
We sought to establish the characteristics and expression of known Mn transporters in polarized WIF-B cells to further study hepatic basolateral to apical transport of Mn. These cells are known to differentiate in culture to form multiple bile “cysts” at the apical surface when canalicular membrane domains from neighboring hepatocytes contact and form tight junctions. These BCs can be visualized as phase bright structures, which become more numerous with days in culture (Fig. 1A). Indirect immunofluorescence microscopy further established the polarity of WIF-B cells after 13 days in culture since the basolateral membrane is well defined by the presence of the NaK-ATPase, whereas the apical surface is enriched with MRP2, a bile canalicular marker (Fig. 1B). The functional integrity of WIF-B cell BCs was further confirmed using fluorescein diacetate, which is taken up, metabolized and delivered into the BCs. The accumulation of fluorescein in the bile cysts is a visual representation of basolateral-to-apical secretion (Fig. 1C).
Fig. 1.
WIF-B cell polarization and manganese (Mn) transporter levels. A: WIF-B cells were seeded at a density of 1 × 106 cells on 10-cm2 dishes, and cultures were observed by phase contrast microscopy after the indicated days in culture. Bile canalicular compartments (BCs) (phase bright structures) are indicated by arrows (×10 objective; bar = 40 µm). Representative micrographs of culture conditions observed throughout the time frame of all experiments presented in the paper are shown. B: indirect immunofluorescence (IF) microscopy of WIF-B cells after 13 days in culture shows immunoreactivity for Na,K-ATPase on the basolateral membrane and apical staining for the bile canalicular marker MRP2; nuclei indicated by DAPI (×63 oil objective; bar = 10 µm). IF studies were carried out with cells examined from duplicate wells, and each experiment was performed on at least three separate occasions with the same observations. C: functional integrity of WIF-B cell BCs after 12 days in culture was determined upon incubation with 0.1 µg/ml fluorescein diacetate (FDA) for 20 min at 37°C. After washing, live cell images were captured within 10 min (×20 objective; bar = 20 µm). FDA experiments were carried out with triplicate wells and on three separate occasions with similar results. Arrowheads indicate BCs that have accumulated fluorescein. D: Western blot analysis of cell lysates prepared from WIF-B cells grown for 14 days in culture. Immunoreactivity was detected for ferroportin (Fpn), ZIP14, ZIP8, and ZnT10. d5, day 5; d10; day 10; d13, day 13; MM, molecular mass (kDa).
While the polarized cells present known morphological and functional features of hepatocytes, WIF-B cells are multiploid and can therefore express genes of rat and/or human origin. To first establish the expression and species origin of Mn transporters in WIF-B cells, PCR amplification was carried out using species-selective primers to discriminate the expression of either rat or human gene products (Table 1). This analysis showed the presence of rat Fpn, human ZIP8, rat ZnT10, and both rat and human ZIP14 transcripts in polarized WIF-B cells. To confirm protein expression, WIF-B cell lysates were collected 2 to 14 days after plating and immunoblotted for the respective Mn transporter. Fpn, ZIP14, ZIP8, and ZnT10 were all maximally expressed by day 12 in culture (Fig. 1C). The experiments described below employed WIF-B cells from 12 to 14 days in culture to ensure that they had achieved maximal polarity and expressed appropriate levels of each Mn transporter.
Mn toxicity and transport in WIF-B cells.
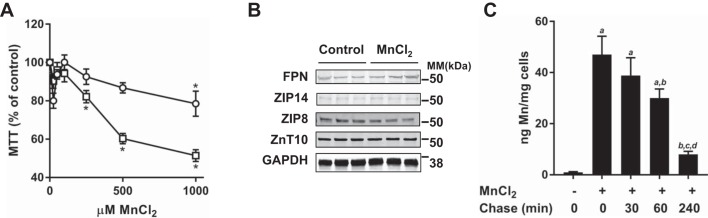
Mn neurotoxicity has been a major focus of study (4, 5, 14, 19, 21, 36, 39, 41, 78), but the metal’s hepatotoxicity is less well characterized. To study the influence of Mn on WIF-B cell viability, cells were exposed to 0–1,000 µM MnCl2 for 4 or 16 h, and viability was assessed using the MTT assay (Fig. 2). Cells treated for 16 h had significantly reduced viability at levels of MnCl2 above 250 µM. However, WIF-B cells were remarkably resistant to brief 4-h exposures, with decreased viability observed only at the highest level (1,000 µM). At the maximal tolerable dose tested (500 µM Mn), the morphological and functional features associated with the polarized WIF-B cells were similar to untreated control cells after 4 h of treatment. Immunoblotting confirmed that levels of Fpn, ZIP14, ZIP8, and ZnT10 were unchanged under these conditions (Fig. 2B).
Fig. 2.
Manganese (Mn) hepatotoxicity and WIF-B cell uptake and efflux. A: WIF-B cells were treated for 4 h (open circles) or 16 h (open squares) with indicated concentrations of MnCl2. Cell viability was determined using (3-[4,5-dimethylthiazol-2yl]-2,5diphenyltetrazolium bromide (MTT) and normalized to control (untreated) cells. Means ± SE (n = 5–6) are shown; *P > 0.05 (Student’s t-test). MTT assays were performed in duplicate on at least three separate occasions. B: Western blot analysis for Mn transporter levels in WIF-B cells treated with 500 μM MnCl2 for 4 h. A representative blot is shown with lysates collected from three individual wells; experiment was repeated on two separate occasions. GAPDH served as a loading control. C: WIF-B cell Mn uptake was carried out with a 4-h incubation at 500 μM MnCl2, and after washing, cellular efflux was followed after the indicated chase periods in Mn-free media. Cell-associated Mn levels were determined by inductively coupled mass spectrometry (ICP-MS). Means ± SE (n = 6) are shown: a, different from untreated control; b, different from Mn control; c, different from 30 min chase period; d, different from 60 min chase period; P < 0.0001 (one-way ANOVA using Tukey’s multiple comparisons test). Cellular Mn content after 240 min of chase was not significantly different from that of untreated control (P = 0.1667). The unit ng Mn/mg cells refers to the amount of Mn detected per mg weight of the cell pellet. MM, molecular mass.
The resistance of WIF-B cells to Mn toxicity presented the opportunity to test whether the metal was taken up by the hepatocytes. ICP-MS analysis confirmed that WIF-B hepatocyte Mn levels under basal conditions (1 ng/mg) are similar to those observed in normal human liver (86). Mn levels increased ~50-fold after incubation with 500 µM Mn for 4 h (Fig. 2C). Taking advantage of hepatocyte loading with Mn, we next studied cellular efflux of the metal by incubating cells in complete medium without the addition of Mn. WIF-B cell Mn levels decreased during chase periods of 30, 60, and 240 min. Greater than 80% of Mn taken up by WIF-B cells was released within 4 h, and the cells remained viable under these conditions. These results confirm efflux of Mn taken up by WIF-B cells in vitro.
Fpn is localized to the basolateral membrane of WIF-B hepatocytes.
Recently, the function of Fpn in Mn export has been defined in vivo and in vitro (38, 72, 89). In situ hybridization studies have shown that Fpn is highly expressed in liver hepatocytes, especially those cells near the portal vein where iron-rich blood circulates from the intestine (90). Immunostaining has been reported at the plasma membrane (1), but there are only scattered reports of immunohistochemical detection of Fpn in liver tissue sections (2, 77, 91). When exogenously expressed in polarized MDCK cells, the iron exporter is exclusively found on the basolateral surface (52). Using confocal imaging, we observed that Fpn colocalizes with the basolateral membrane marker NaK-ATPase in WIF-B cells (Pearson’s coefficient = 0.511; Fig. 3A). Fpn is also detected in intracellular vesicles close to the plasma membrane. Although colocalization with MRP2 was tested, no overlap was observed (data not shown).
Fig. 3.
Ferroportin (Fpn) distribution and function in WIF-B cells. A: WIF-B cells were grown on coverslips for 14 days for maximal bile canalicular compartment (BC) density, followed by indirect double immunofluorescence (IF) staining for Fpn and Na,K-ATPase (inset; Pearson’s = 0.511). Images were collected with ×60 oil immersion objective; bar = 10 µm. IF studies were performed with slides prepared in duplicate wells on three separate dates. B: WIF-B cells were incubated with increasing concentrations of hepcidin (0, 0.35, 0.70, or 1.4 μM) for 4 h, lysed, and immunoblotted to detect Fpn levels. GAPDH was used as a loading control. Similar experiments were repeated on three separate occasions using 1.4 μM hepcidin. Graph shows that relative to untreated controls, WIF-B cell Fpn decreased by half with 1.4 μM hepcidin treatment for 4 h (normalized mean values ± SE; n = 3). *P < 0.05 (Student’s t-test). C: after 4 h treatment, 1.4 μM hepcidin induced internalization of Fpn to juxtanuclear vesicles (×63 oil immersion objective; bar = 10 µm). Experiments included duplicate wells and were performed on two separate occasions. D: WIF-B cells were treated with 1.4 uM hepcidin before and during exposure to 500 μM MnCl2 for 4 h, then efflux was followed as described in Fig. 2. Values are means ± SE (n = 3); P < 0.05 (Student’s t-test): a, different from untreated control; b, different from Mn control; c, different from 60 min chase period. The unit ng Mn/mg cells refers to the amount of Mn detected per mg weight of the cell pellet.
To directly test the possible role of Fpn in WIF-B cell Mn efflux, we took advantage of the action of hepcidin to downregulate the transporter. Hepcidin binds to Fpn to induce its internalization and degradation (59). To confirm if hepcidin acts on WIF-B hepatocytes, cells were incubated with increasing amounts of the peptide, and lysates were immunoblotted to quantify protein levels (Fig. 3B). WIF-B hepatocyte Fpn levels decreased in a dose-dependent manner; incubation with 1.4 µM hepcidin reduced levels by 50% within 4 h (Fig. 3B). Immunofluorescence microscopy confirmed the downregulation of Fpn from the basolateral surface, and residual protein was observed in juxtanuclear and cytoplasmic vesicles (Fig. 3C). WIF-B cells treated with hepcidin before Mn loading and during chase times did not show reduced Mn efflux (Fig. 3D). In addition, no effect on Mn loading was observed. Thus, Mn export in WIF-B cells is hepcidin-resistant and unlikely to involve Fpn.
ZnT10 is localized to apical vesicles adjacent to the bile canalicular membrane in WIF-B hepatocytes.
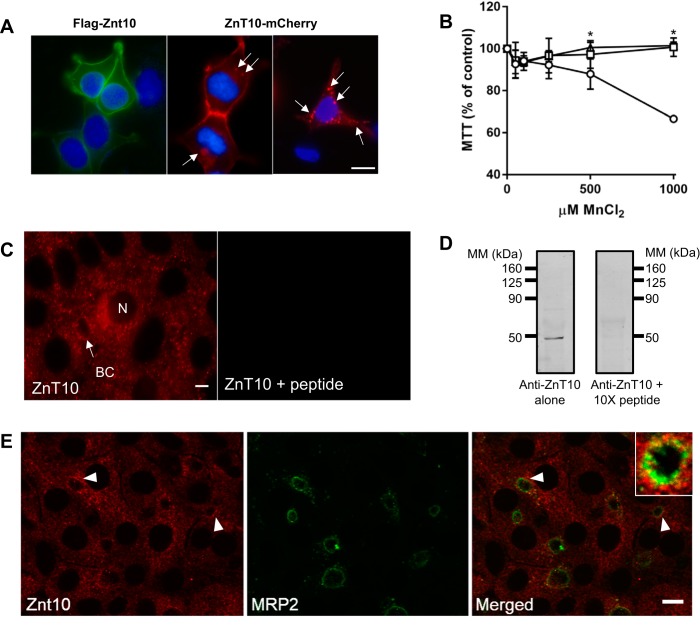
Patients with defects in ZnT10 display hypermanganesemia but additionally suffer from chronic liver disease because of hepatic Mn accumulation (56, 65, 66, 76, 81, 83). These observations have led to the conclusion that ZnT10 plays a key role in hepatobiliary secretion of Mn. There is one report identifying ZnT10 at the apical side of hepatocytes, but only a single bile duct with rather diffuse diaminobenzidine staining in that particular liver section was shown (65). Slc30a10 knockout mice display liver Mn loading, but there has been difficulty localizing the transporter in mouse tissues (31, 48). Limited studies in HepG2 and transfected HepG2 cells suggest it is localized to bile canalicular membrane (48). Otherwise, ZnT10 localization has been characterized primarily using exogenous overexpression with either NH2- or COOH-terminal tags to identify the membrane protein in nonpolarized cells. There are disparate conclusions about ZnT10s subcellular localization and function from these in vitro studies (45, 60, 92, 93). In our hands, N-terminally tagged FLAG-ZnT10 localizes primarily to the plasma membrane when exogenously expressed in HEK293T cells, whereas C-terminally tagged ZnT10-mCherry is found in intracellular vesicles as well as at the cell surface (Fig. 4A). Exogenous expression of both forms of ZnT10 protects HEK293T cells against Mn-induced cell death (Fig. 4B). The reported differences in function and cellular distribution most likely reflect altered subcellular localization because of the epitope tag; regardless of its subcellular distribution, the transporter appears to function in Mn detoxification, and it may do so by exporting the metal across the plasma membrane and/or taking it up into intracellular vesicles.
Fig. 4.
Exogenous expression of epitope-tagged ZnT10 in HEK293T cells and localization of endogenous ZnT10 in WIF-B cells. A: HEK293T cells were transiently transfected to express N-terminally FLAG-tagged or C-terminal mCherry ZnT10. Images were taken using ×100 magnification and ZEISS Apotome optical sectioning. Bar = 10 μm. Transfections were performed in triplicate and on at least three separate occasions. B: (3-[4,5-dimethylthiazol-2yl]-2,5diphenyltetrazolium bromide (MTT) assays were carried out in transfected HEK293T cells; normalized cell viability is shown for control (open circles), FLAG-ZnT10 (open squares), and ZnT10-mCherry (open triangles). MTT assays were carried out in triplicate and on three different days. Values are means ± SE (n = 6); *P < 0.05 (Student’s t-test). C: endogenous staining of ZnT10 in WIF-B cells was displaced in presence of 10-fold molar excess of antigenic peptide. Images were taken with ×63 magnification. Bar = 10 μm. Blocking experiment was performed with duplicate wells studied on two separate occasions. D: Western blot analysis confirms specificity of anti-ZnT10 antibody. A 10-fold molar excess (×10) of the antigenic peptide was added to compete for antibody binding. Western blots incubated with anti-ZnT10 antibody in the presence or absence of ×10 peptide (relative to antibody) to block immunoreactivity are shown. Tic marks indicate molecular mass (MM) markers; lanes were loaded on the same gel with equivalent volumes of WIF-B cell lysates; blots were cut after transfer and incubated under these separate conditions. E: strong, punctate ZnT10 staining was observed near MRP2-positive structures (arrows; inset, Pearson’s = 0.365). Images collected with ×60 oil immersion objective; bar = 10 µm. Immunofluorescence (IF) studies examining colocalization of ZnT10 and MRP2 were carried out with duplicate wells and on three separate occasions.
To study endogenous distribution of ZnT10 in polarized WIF-B cells, we used a commercially available antibody. Because previous reports suggested this reagent did not detect the rodent transporter (31, 48), we first characterized its specificity against the ZnT10 peptide. Both IF (Fig. 4C) and Western blot analysis (Fig. 4D) show that the peptide successfully blocked antibody binding. Western blot analysis also showed that this antibody detected exogenously expressed epitope-tagged ZnT10 at the appropriately shifted molecular mass, further confirming its specificity (data not shown). IF staining indicates that that the antibody detects ZnT10 localized to intracellular vesicles surrounding the bile canalicular membrane (Fig. 4, C and E). Some staining of juxtanuclear vesicles can also be observed. To confirm the apical distribution of the ZnT10 vesicles, WIF-B cells were costained for the bile canalicular marker MRP2 (Fig. 4E). These results show the close proximity of the ZnT10-positive vesicles with MRP2-positive structures (Pearson’s coefficient = 0.365).
The subcellular localization of ZnT10 to intracellular vesicles is reminiscent of the distribution of ZnT2, another membrane of the ZnT family that is responsible for the secretion into milk from mammary gland epithelial (34). The trafficking of this transporter suggests it functions to translocate vesicle cargo to the milk duct by a vesicular fusion mechanism (43). We hypothesized that ZnT10 might play a role in Mn transport to the bile duct by a similar secretory mechanism. To test this idea, we used the inhibitor BFA which is known to block secretion (24). WIF-B hepatocytes were incubated with 10 μM BFA during the last 20 min of the 4-h Mn-loading period and was present during the 60-min chase period. The presence of BFA blocked Mn export from WIF-B cells compared with control cells during this time period. In the absence of BFA, 43% of WIF-B cell Mn was released, similar to the results presented in Fig. 2. The ability of BFA to block Mn export suggests that vesicle fusion might be involved in this process. BFA did not affect MRP2-positive BCs and did not affect the general distribution of ZnT10 in proximal vesicles (Fig. 5, right).
Fig. 5.
Manganese (Mn) efflux is brefeldin A (BFA)-sensitive. Mn efflux was determined as described for Fig. 2. WIF-B cells were treated with 10 μM BFA for 20 min before and throughout a 60-min chase period. Values are means ± SE (n = 3); experiment was repeated twice with similar results. *P < 0.05 (Student’s t-test). Indirect immunofluorescence (IF) microscopy shows MRP2 and Znt10 staining in WIF-B cells treated with or without BFA. Arrows indicate apical structures, and bile canalicular compartment (BC) is shown in inset. IF studies were carried out with duplicate wells and on three separate occasions. ns, not significant.
Cellular localization of ZIP14 and ZIP8 in WIF-B cells.
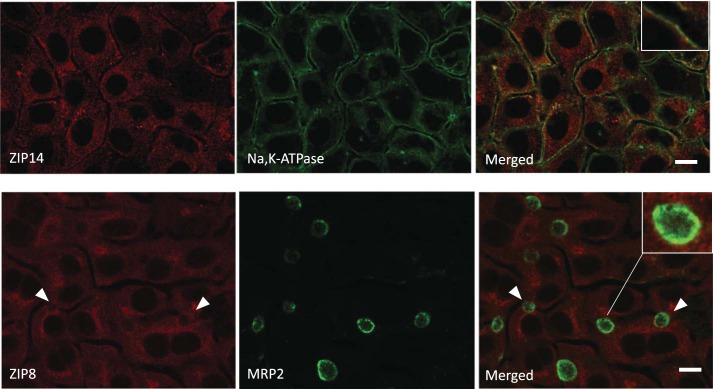
While the studies described above focused on the Mn exporters Fpn and ZnT10, WIF-B hepatocytes also express the Mn importers ZIP14 and ZIP8 (Fig. 1). These transporters have been implicated in metal uptake across the basolateral (58) and apical (26, 29, 47) surfaces of hepatocytes, respectively. We studied their distribution in polarized WIF-B cells using IF microscopy, counterstaining with basolateral and apical markers (Fig. 6). The results of these experiments show that ZIP14 colocalizes with NaK-ATPase in the basolateral membrane (Pearson’s coefficient = 0.456). Some vesicular staining close to the basolateral membrane is also observed for ZIP14. However, ZIP8 staining was entirely associated with vesicles, with punctate ZIP8-positive structures scattered throughout the cytoplasm. While some ZIP8 staining could be found near MRP2-positive structures (see inset), proximity to the apical marker was not striking (Pearson’s coefficient = 0.078).
Fig. 6.
ZIP14 and ZIP8 distribution in WIF-B cells. WIF-B cells were grown on coverslips for 14 days for maximal bile canalicular compartment (BC) density and indirect double immunofluorescence (IF) performed to image Na,K-ATPase and ZIP14 or ZIP8 and MRP2. ZIP14 colocalized with Na,K-ATPase (inset; Pearson’s = 0.456). ZIP8 staining did not associate with MRP2 structures (arrows; inset, Pearson’s = 0.078). IF results were obtained on three separate occasions with duplicate wells prepared on each day.
DISCUSSION
Mn toxicity arises from nutritional problems (9, 23, 57, 71), community (10, 28, 54, 62, 84) and occupational exposures (8, 20), environmental emissions (28, 54), and genetic risks (18, 56, 63, 65, 66, 76, 81–83). Mn intoxication, or manganism, is associated with increased levels of the metal in the brain and leads to neurological dysfunction similar to Parkinson’s disease. Because Mn levels are controlled by hepatobiliary clearance from the blood, the liver plays a key role to protect the brain from excess metal (68). The roles of different transporters in Mn homeostasis have been revealed through recent genetic studies. Defects in ZIP14 increase blood and brain Mn whereas liver is not affected (81, 82). Studies in knockout mice confirm that hepatic uptake is disrupted by loss of ZIP14 function, and therefore its clearance is reduced, promoting Mn accumulation in the brain and other tissues (7, 35). Although ZnT10 mutations are also associated hypermanganesemia, patients with these genetic defects suffer from liver disease with hepatic Mn accumulation consistent with a proposed function in Mn export into bile (56, 65, 66, 76, 81, 83). On the other hand, ZIP8 deficiency confers hypomanganesemia (63), and it has been proposed to function in reabsorption of Mn from bile to prevent deficiency (47). Our group has studied flatiron (ffe) mice with Fpn deficiency and found that blood, liver and bile Mn levels are reduced in this genetic model of “Fpn disease” (73). Mn homeostasis is perturbed by loss of intestinal Mn uptake, so it remains unclear whether reduced levels in liver and bile reflects lower blood Mn because of impaired absorption or additional loss of hepatic function. Since liver is a major site of Fpn expression, a speculative role for the transporter in Mn export from hepatocytes was raised by these findings.
To better understand how these transporters might function in hepatobiliary clearance of Mn, the present study determined the localization of endogenous Fpn, ZIP14, ZIP8, and ZnT10 in polarized WIF-B hepatocytes. WIF-B cells polarize to form both sinusoidal (basolateral) and bile canalicular (apical) membrane domains with a majority of cells in culture forming bile-canalicular structures (BCs). These cells have been used previously to study apical transport of Cu as well as Golgi transporters involved in Mn metabolism (13, 44, 61). The WIF-B cell line has proven to be an excellent biological tool for identifying protein and vesicular trafficking as well as hepatobiliary efflux parameters (15, 32, 33, 74, 80).
We identified WIF-B cell apical and basolateral membranes using indirect IF microscopy to detect the domain-specific markers MRP2 and Na,K-ATPase, respectively. Fluorescence microscopy confirmed the functional integrity of the BCs using fluorescein diacetate to mark hepatobiliary clearance. PCR and Western blot analysis revealed that all four transporters of interest (Fpn, ZIP14, ZIP8, and ZnT10) were expressed in WIF-B cells. Thus, we studied WIF-B cells with functional BCs that expressed all four transporters during days 12–14 in culture.
Cell viability studies revealed that WIF-B cells are remarkably resilient to Mn intoxication. Our studies showed that these hepatocytes withstand a 4 h exposure to high levels of MnCl2 (500 µM) without a significant loss of viability. ICP-MS analysis showed that levels of cellular Mn were increased 50-fold under these conditions. After washing, time-dependent efflux of Mn from the exposed cells was observed over the next 4 h. Thus, because of the survival of WIF-B cells under these high Mn conditions, export of Mn could be measured.
As noted above, recent investigations have aimed to identify the roles of Fpn, ZIP14, ZIP8, and ZnT10 in liver Mn import and export. ZIP8 and ZIP14 have been proposed to take up Mn at the apical and basolateral surfaces of hepatocytes, respectively (7, 35, 40, 47). ZnT10 and Fpn, on the other hand, have been implicated in Mn efflux at the apical and basolateral regions, respectively (47, 48, 73). Using indirect IF microscopy, we found that ZIP14 and Fpn staining closely overlapped with NaK-ATPase staining at the basolateral surface of WIF-B cells. The localization of ZIP14 at the basolateral surface of these cells is consistent with a role in Mn import (7, 31, 35). The fact that ZIP8 appears to distribute to cytoplasmic vesicular structures (see below) suggests that ZIP14 is the primary transporter responsible for Mn uptake we observe for WIF-B cells, although the activity of other transporters, including divalent metal transporter-1, cannot be excluded. A summary model of our findings is presented in Fig. 7.
Fig. 7.
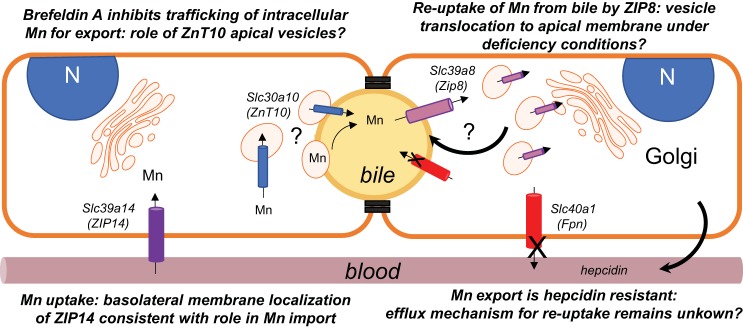
Summary model of polarized WIF-B hepatocyte manganese (Mn) transport and trafficking. Two adjacent WIF-B cells are depicted with polarity maintained by tight junctions to form basolateral (sinusoidal; orange) and apical (bile canalicular, yellow) membrane surfaces. Four transporters that are implicated in Mn transport were examined in this polarized hepatocyte model: ZIP14 (purple), ZnT10 (blue), ZIP8 (pink), and ferroportin (Fpn) (red). ZIP14 is observed to associate with the basolateral (sinusoidal) surface, consistent with its proposed role in Mn uptake. The exporter Fpn is also localized to the basolateral membrane, but Mn efflux from WIF-B cells was resistant to hepcidin despite downregulation of Fpn. This observation suggests other pathways may play a role in exporting the metal from hepatocytes. Such an Mn release pathway would be necessary for the proposed reabsorption of Mn by ZIP8 under deficiency conditions. Our study shows that ZIP8 is not localized to the apical membrane but instead localizes to vesicles distributed throughout the cytoplasm. These vesicles could place this transporter in the bile canalicular membrane upon trafficking induced by Mn deficiency. Like ZIP8, ZnT10 is also localized to intracellular vesicles, but these are directly adjacent to the bile canalicular (apical membrane); the transporter does not appear to be integrally associated with the bile canalicular surface. We observe that the secretory inhibitor brefeldin A reduces Mn export, suggesting that the ZnT10-positive apical vesicles may contain Mn and subsequently fuse with the bile canalicular membrane to empty the contents into bile. Alternatively, fusion of these vesicles with the bile canalicular membrane may place ZnT10 into the membrane, thereby accommodating Mn export by the exporter.
Whether or not Fpn might be involved hepatic Mn efflux is an open question since its function in hepatocytes has yet to be fully characterized. Therefore, we explored its possible role in WIF-B cell transport. These experiments took advantage of the fact that treatment with the peptide hormone hepcidin reduced Fpn protein levels by 50% and redistributed remaining transporters from the basolateral cell surface to cytoplasmic vesicles. These observations are consistent with the known regulatory effects of hepcidin on Fpn (59). As expected, treatment of WIF-B cells with hepcidin did not influence the uptake of Mn, but we found that Mn efflux from WIF-B cells was not altered by hormone treatment. These data argue against a role for Fpn in hepatocyte Mn export under the conditions of our experiments and suggest that other pathways contribute to WIF-B cell efflux observed after high Mn exposure.
In contrast to ZIP14 and Fpn, we found that ZIP8 and ZnT10 were distributed in punctate vesicular structures in the cytoplasm of WIF-B cells. While there was a notable distribution of ZnT10-containing vesicles proximal to the bile canalicular marker MRP2, ZIP8-containing structures were scattered throughout the cytoplasm in a nonpolarized fashion. An important note is that WIF-B cells are a fusion of human fibroblasts with rat hepatoma cells (74), and our PCR analysis discerned that only human ZIP8 mRNA was expressed. It is possible that unique structural features define species-specific transporter localization, but rat and human proteins display close homology, with 88% identity. In mouse liver, ZIP8 has been characterized by immunostaining of tissue sections and appears to colocalize with MDR1, another bile canalicular membrane marker, but immunostaining of both proteins also appears to be broadly distributed throughout hepatocytes (47). Functionally, ZIP8 is thought to reclaim Mn secreted into bile to protect against deficiency (47), but how its activity might be regulated remains unknown. In Hela cells, ZIP8 mRNA levels are enhanced by Mn exposure (17). Whether or not protein levels are posttranslationally regulated has yet to be tested; we did not observe a change in protein levels of ZIP8 or other transporters when WIF-B cells were exposed to high Mn for short periods of time. It is possible that intracellular transporters are redistributed to the apical membrane to mediate polarized uptake of Mn from bile when required. In preliminary experiments, we used the Mn-selective chelator CDTA (70) to examine this possibility by indirect IF microscopy but did not observe relocalization of vesicular ZIP8 to MRP2-positive domains; further experiments are necessary to more fully deplete cellular Mn in order to test this idea. Regulation of ZIP8 is important to understand because genetic deficiency produces neurological problems that could arise from mitochondrial disorders (17); glycosylation defects are also observed in affected patients, and there have been encouraging results indicating that loss of ZIP8 activity might be compensated by high dose Mn therapy (63).
An important observation from our study is that ZnT10-containing vesicles were juxtaposed to MRP2-staining apical membrane domains in a polarized fashion. In nonpolarized cells, ZnT10 localization has been characterized using exogenous overexpression with either N- or C-terminal tags to identify the membrane protein. There is some controversy over ZnT10s subcellular localization and function from these in vitro studies (45, 60, 92, 93). Our data indicate that C-terminal-tagged ZnT10 can localize to both membrane and vesicular compartments in HEK293T cells, and overexpression of both N- and C-terminal tagged forms resulted in protection against excess Mn. Thus, it is possible in polarized cells that ZnT10 imports Mn into vesicles that subsequently fuse with the apical membrane. One study has reported ZnT10 staining associated with the apical membrane of HepG2 cells (65). However, unlike WIF-B cells that spontaneously polarize to form BCs, HepG2 cells do not differentiate as robustly and must be induced to polarize and form BCs (80). There is also some debate concerning the ability of commercially available antibodies to detect ZnT10 in rodent tissues (31). We have carefully validated the antibody used for our analysis using blocking peptides and Western blot analysis as well as exogenous expression to confirm its specificity.
In conclusion, the vesicular ZnT10 staining pattern prompted us to examine whether Mn efflux was sensitive to BFA. Efflux experiments showed that export of Mn was attenuated by the presence of this secretory inhibitor while the integrity of the BC remained unperturbed. There was no discernable change in apical polarity of ZnT10-positive vesicles, nor was the transporter incorporated into the bile canalicular membrane. Previous studies have suggested that SPCA1 (ATP2C) plays an important role in Mn transport. Leitch et al. (44) have suggested endosomes containing secretory pathway Ca2+, Mn2+-ATPase may serve to sequester Mn as it enters from the sinusoidal/basolateral domain for detoxification. Others (55) have shown its role in uptake of Mn into the Golgi, where it is predominantly localized. The yeast ortholog Pmr1 removes Mn from cytosol into the secretory pathway, suggesting exocytosis of the metal is a primary pathway of detoxification (22, 25, 42, 50). These Golgi transporters are necessary to provide Mn for enzymes involved in protein glycosylation. It is possible that ZnT10 takes up excess Mn from the cytoplasm into more specialized vesicles that fuse to and release their contents into bile. A similar pathway has been envisioned for biliary copper excretion (12, 16). It is interesting to note that in the mammary glands, the family member ZnT2 is thought to package zinc into secretory vesicles that transfer this metal into the milk duct (34, 43). Our studies support the idea that hepatobiliary efflux of Mn involves multiple metal transporters that function at precise cellular locations to mediate vectorial delivery of the metal from the sinusoidal basolateral surface to the bile canalicular apical membrane of hepatocytes. Based on the observed resistance of WIF-B cell Mn efflux to hepcidin, it is unlikely that Fpn is involved in detoxification. However, sensitivity to BFA suggests that efflux may primarily involve a secretory mechanism. How the apical ZnT10-containing vesicle pool contributes to this process remains to be determined.
GRANTS
This work was supported by grants from the National Institute of Environmental Health Sciences Grants R01-ES-014638 (to M. Wessling-Resnick) and R01-ES-014638-11S1 (to K. J. Thompson). Inductively coupled mass spectrometry analysis was supported in part by funding from the Harvard T. H. Chan School of Public Health National Institute of Environmental Health Sciences Center for Environmental Health (ES-000002). Spinning disk confocal microscopy experiments were supported by the Sabri Ülker Center for Nutrient, Genetic, and Metabolic Research at the Harvard T. H. Chan School of Public Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.J.T. and M.W.-R. conceived and designed research; K.J.T., J.H., A.B., and J.C.S. performed experiments; K.J.T., J.H., A.B., and J.C.S. analyzed data; K.J.T., J.H., A.B., J.C.S., and M.W.-R. interpreted results of experiments; K.J.T., J.H., and M.W.-R. prepared figures; K.J.T. and M.W.-R. drafted manuscript; K.J.T. and M.W.-R. edited and revised manuscript; K.J.T., J.H., A.B., J.C.S., and M.W.-R. approved final version of manuscript.
REFERENCES
- 1.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275: 19906–19912, 2000. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 2.Adams PC, Barbin YP, Khan ZA, Chakrabarti S. Expression of ferroportin in hemochromatosis liver. Blood Cells Mol Dis 31: 256–261, 2003. doi: 10.1016/S1079-9796(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 3.Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med 26: 353–362, 2005. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aschner M. Manganese: brain transport and emerging research needs. Environ Health Perspect 108, Suppl 3: 429–432, 2000. doi: 10.1289/ehp.00108s3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aschner M, Aschner JL. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev 15: 333–340, 1991. doi: 10.1016/S0149-7634(05)80026-0. [DOI] [PubMed] [Google Scholar]
- 6.Aschner M, Erikson K. Manganese. Adv Nutr 8: 520–521, 2017. doi: 10.3945/an.117.015305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aydemir TB, Kim MH, Kim J, Colon-Perez LM, Banan G, Mareci TH, Febo M, Cousins RJ. Metal transporter Zip14 (Slc39a14) deletion in mice increases manganese deposition and produces neurotoxic signatures and diminished motor activity. J Neurosci 37: 5996–6006, 2017. doi: 10.1523/JNEUROSCI.0285-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias). Neurotoxicology 5: 13–35, 1984. [PubMed] [Google Scholar]
- 9.Bertinet DB, Tinivella M, Balzola FA, de Francesco A, Davini O, Rizzo L, Massarenti P, Leonardi MA, Balzola F. Brain manganese deposition and blood levels in patients undergoing home parenteral nutrition. J Parenter Enteral Nutr 24: 223–227, 2000. doi: 10.1177/0148607100024004223. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect 115: 122–127, 2007. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boycott KM, Beaulieu CL, Kernohan KD, Gebril OH, Mhanni A, Chudley AE, Redl D, Qin W, Hampson S, Küry S, Tetreault M, Puffenberger EG, Scott JN, Bezieau S, Reis A, Uebe S, Schumacher J, Hegele RA, McLeod DR, Gálvez-Peralta M, Majewski J, Ramaekers VT, Nebert DW, Innes AM, Parboosingh JS, Abou Jamra R; Care4Rare Canada Consortium . Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am J Hum Genet 97: 886–893, 2015. doi: 10.1016/j.ajhg.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer JL. Bile formation and secretion. Compr Physiol 3: 1035–1078, 2013. doi: 10.1002/cphy.c120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braiterman LT, Murthy A, Jayakanthan S, Nyasae L, Tzeng E, Gromadzka G, Woolf TB, Lutsenko S, Hubbard AL. Distinct phenotype of a Wilson disease mutation reveals a novel trafficking determinant in the copper transporter ATP7B. Proc Natl Acad Sci USA 111: E1364–E1373, 2014. doi: 10.1073/pnas.1314161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carl GF, Blackwell LK, Barnett FC, Thompson LA, Kissinger CJ, Olin KL, Critchfield JW, Keen CL, Gallagher BB. Manganese and epilepsy: brain glutamine synthetase and liver arginase activities in genetically epilepsy prone and chronically seizured rats. Epilepsia 34: 441–446, 1993. doi: 10.1111/j.1528-1157.1993.tb02584.x. [DOI] [PubMed] [Google Scholar]
- 15.Cassio D, Hamon-Benais C, Guérin M, Lecoq O. Hybrid cell lines constitute a potential reservoir of polarized cells: isolation and study of highly differentiated hepatoma-derived hybrid cells able to form functional bile canaliculi in vitro. J Cell Biol 115: 1397–1408, 1991. doi: 10.1083/jcb.115.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF. ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology 130: 493–506, 2006. doi: 10.1053/j.gastro.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 17.Choi EK, Nguyen TT, Gupta N, Iwase S, Seo YA. Functional analysis of SLC39A8 mutations and their implications for manganese deficiency and mitochondrial disorders. Sci Rep 8: 3163, 2018. doi: 10.1038/s41598-018-21464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clayton PT. Inherited disorders of transition metal metabolism: an update. J Inherit Metab Dis 40: 519–529, 2017. doi: 10.1007/s10545-017-0030-x. [DOI] [PubMed] [Google Scholar]
- 19.Crinella F, Cordova E, Ericson J. Manganese, aggression, and attention-deficit hyperactivity disorder. Neurotoxicology 19: 468–469, 1998. [Google Scholar]
- 20.Donaldson J. The physiopathologic significance of manganese in brain: its relation to schizophrenia and neurodegenerative disorders. Neurotoxicology 8: 451–462, 1987. [PubMed] [Google Scholar]
- 21.Dorman DC, Struve MF, Vitarella D, Byerly FL, Goetz J, Miller R. Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J Appl Toxicol 20: 179–187, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 22.Dürr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell 9: 1149–1162, 1998. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fell JM, Reynolds AP, Meadows N, Khan K, Long SG, Quaghebeur G, Taylor WJ, Milla PJ. Manganese toxicity in children receiving long-term parenteral nutrition. Lancet 347: 1218–1221, 1996. doi: 10.1016/S0140-6736(96)90735-7. [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem 263: 18545–18552, 1988. [PubMed] [Google Scholar]
- 25.García-Rodríguez N, Manzano-López J, Muñoz-Bravo M, Fernández-García E, Muñiz M, Wellinger RE. Manganese redistribution by calcium-stimulated vesicle trafficking bypasses the need for P-type ATPase function. J Biol Chem 290: 9335–9347, 2015. doi: 10.1074/jbc.M114.616334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol 73: 1413–1423, 2008. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow’s milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol 27: 615–627, 2005. [Erratum in Neurotoxicol Teratol 34: 220, 2012] doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Haynes EN, Heckel P, Ryan P, Roda S, Leung YK, Sebastian K, Succop P. Environmental manganese exposure in residents living near a ferromanganese refinery in southeast Ohio: a pilot study. Neurotoxicology 31: 468–474, 2010. doi: 10.1016/j.neuro.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol 70: 171–180, 2006. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 30.Hong JS, Hung CR, Seth PK, Mason G, Bondy SC. Effect of manganese treatment on the levels of neurotransmitters, hormones, and neuropeptides: modulation by stress. Environ Res 34: 242–249, 1984. doi: 10.1016/0013-9351(84)90092-6. [DOI] [PubMed] [Google Scholar]
- 31.Hutchens S, Liu C, Jursa T, Shawlot W, Chaffee BK, Yin W, Gore AC, Aschner M, Smith DR, Mukhopadhyay S. Deficiency in the manganese efflux transporter SLC30A10 induces severe hypothyroidism in mice. J Biol Chem 292: 9760–9773, 2017. doi: 10.1074/jbc.M117.783605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ihrke G, Martin GV, Shanks MR, Schrader M, Schroer TA, Hubbard AL. Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. J Cell Biol 141: 115–133, 1998. doi: 10.1083/jcb.141.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ihrke G, Neufeld EB, Meads T, Shanks MR, Cassio D, Laurent M, Schroer TA, Pagano RE, Hubbard AL. WIF-B cells: an in vitro model for studies of hepatocyte polarity. J Cell Biol 123: 1761–1775, 1993. doi: 10.1083/jcb.123.6.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itsumura N, Inamo Y, Okazaki F, Teranishi F, Narita H, Kambe T, Kodama H. Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant. PLoS One 8: e64045, 2013. doi: 10.1371/journal.pone.0064045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkitkasemwong S, Akinyode A, Paulus E, Weiskirchen R, Hojyo S, Fukada T, Giraldo G, Schrier J, Garcia A, Janus C, Giasson B, Knutson MD. SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc Natl Acad Sci USA 115: E1769–E1778, 2018. [Erratum in Proc Natl Acad Sci USA 115: E4730, 2018] doi: 10.1073/pnas.1720739115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kern CH, Smith DR. Preweaning Mn exposure leads to prolonged astrocyte activation and lasting effects on the dopaminergic system in adult male rats. Synapse 65: 532–544, 2011. doi: 10.1002/syn.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse 64: 363–378, 2010. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Buckett PD, Wessling-Resnick M. Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS One 8: e64944, 2013. doi: 10.1371/journal.pone.0064944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, Li Y, Buckett PD, Böhlke M, Thompson KJ, Takahashi M, Maher TJ, Wessling-Resnick M. Iron-responsive olfactory uptake of manganese improves motor function deficits associated with iron deficiency. PLoS One 7: e33533, 2012. doi: 10.1371/journal.pone.0033533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MH, Aydemir TB, Kim J, Cousins RJ. Hepatic ZIP14-mediated zinc transport is required for adaptation to endoplasmic reticulum stress. Proc Natl Acad Sci USA 114: E5805–E5814, 2017. doi: 10.1073/pnas.1704012114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, Kim JM, Kim JW, Yoo CI, Lee CR, Lee JH, Kim HK, Yang SO, Chung HK, Lee DS, Jeon B. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: what does it mean? Mov Disord 17: 568–575, 2002. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- 42.Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol 15: 1382–1388, 1995. doi: 10.1128/MCB.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S, Rivera OC, Kelleher SL. Zinc transporter 2 interacts with vacuolar ATPase and is required for polarization, vesicle acidification, and secretion in mammary epithelial cells. J Biol Chem 292: 21598–21613, 2017. doi: 10.1074/jbc.M117.794461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leitch S, Feng M, Muend S, Braiterman LT, Hubbard AL, Rao R. Vesicular distribution of secretory pathway Ca2+-ATPase isoform 1 and a role in manganese detoxification in liver-derived polarized cells. Biometals 24: 159–170, 2011. doi: 10.1007/s10534-010-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leyva-Illades D, Chen P, Zogzas CE, Hutchens S, Mercado JM, Swaim CD, Morrisett RA, Bowman AB, Aschner M, Mukhopadhyay S. SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J Neurosci 34: 14079–14095, 2014. doi: 10.1523/JNEUROSCI.2329-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Qiang JW, Ju S. Brain MR imaging changes in patients with hepatic schistosomiasis japonicum without liver dysfunction. Neurotoxicology 35: 101–105, 2013. doi: 10.1016/j.neuro.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Lin W, Vann DR, Doulias PT, Wang T, Landesberg G, Li X, Ricciotti E, Scalia R, He M, Hand NJ, Rader DJ. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J Clin Invest 127: 2407–2417, 2017. doi: 10.1172/JCI90896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu C, Hutchens S, Jursa T, Shawlot W, Polishchuk EV, Polishchuk RS, Dray BK, Gore AC, Aschner M, Smith DR, Mukhopadhyay S. Hypothyroidism induced by loss of the manganese efflux transporter SLC30A10 may be explained by reduced thyroxine production. J Biol Chem 292: 16605–16615, 2017. doi: 10.1074/jbc.M117.804989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucchini R, Bergamaschi E, Smargiassi A, Festa D, Apostoli P. Motor function, olfactory threshold, and hematological indices in manganese-exposed ferroalloy workers. Environ Res 73: 175–180, 1997. doi: 10.1006/enrs.1997.3702. [DOI] [PubMed] [Google Scholar]
- 50.Mandal D, Woolf TB, Rao R. Manganese selectivity of pmr1, the yeast secretory pathway ion pump, is defined by residue gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J Biol Chem 275: 23933–23938, 2000. doi: 10.1074/jbc.M002619200. [DOI] [PubMed] [Google Scholar]
- 51.Marti-Sanchez L, Ortigoza-Escobar JD, Darling A, Villaronga M, Baide H, Molero-Luis M, Batllori M, Vanegas MI, Muchart J, Aquino L, Artuch R, Macaya A, Kurian MA, Dueñas P. Hypermanganesemia due to mutations in SLC39A14: further insights into Mn deposition in the central nervous system. Orphanet J Rare Dis 13: 28, 2018. doi: 10.1186/s13023-018-0758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5: 299–309, 2000. doi: 10.1016/S1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 53.Mena I. The role of manganese in human disease. Ann Clin Lab Sci 4: 487–491, 1974. [PubMed] [Google Scholar]
- 54.Mergler D, Baldwin M, Bélanger S, Larribe F, Beuter A, Bowler R, Panisset M, Edwards R, de Geoffroy A, Sassine MP, Hudnell K. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology 20: 327–342, 1999. [PubMed] [Google Scholar]
- 55.Mukhopadhyay S, Linstedt AD. Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proc Natl Acad Sci USA 108: 858–863, 2011. doi: 10.1073/pnas.1013642108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukhtiar K, Ibrahim S, Tuschl K, Mills P. Hypermanganesemia with dystonia, polycythemia and cirrhosis (HMDPC) due to mutation in the SLC30A10 gene. Brain Dev 38: 862–865, 2016. doi: 10.1016/j.braindev.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, Osame M. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J Neurol Sci 162: 102–105, 1999. doi: 10.1016/S0022-510X(98)00289-5. [DOI] [PubMed] [Google Scholar]
- 58.Nam H, Wang CY, Zhang L, Zhang W, Hojyo S, Fukada T, Knutson MD. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica 98: 1049–1057, 2013. doi: 10.3324/haematol.2012.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 60.Nishito Y, Tsuji N, Fujishiro H, Takeda TA, Yamazaki T, Teranishi F, Okazaki F, Matsunaga A, Tuschl K, Rao R, Kono S, Miyajima H, Narita H, Himeno S, Kambe T. Direct comparison of manganese detoxification/efflux proteins and molecular characterization of ZnT10 protein as a manganese transporter. J Biol Chem 291: 14773–14787, 2016. doi: 10.1074/jbc.M116.728014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nyasae LK, Schell MJ, Hubbard AL. Copper directs ATP7B to the apical domain of hepatic cells via basolateral endosomes. Traffic 15: 1344–1365, 2014. doi: 10.1111/tra.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oulhote Y, Mergler D, Barbeau B, Bellinger DC, Bouffard T, Brodeur ME, Saint-Amour D, Legrand M, Sauvé S, Bouchard MF. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect 122: 1343–1350, 2014. doi: 10.1289/ehp.1307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park JH, Hogrebe M, Grüneberg M, DuChesne I, von der Heiden AL, Reunert J, Schlingmann KP, Boycott KM, Beaulieu CL, Mhanni AA, Innes AM, Hörtnagel K, Biskup S, Gleixner EM, Kurlemann G, Fiedler B, Omran H, Rutsch F, Wada Y, Tsiakas K, Santer R, Nebert DW, Rust S, Marquardt T. SLC39A8 deficiency: a disorder of manganese transport and glycosylation. Am J Hum Genet 97: 894–903, 2015. doi: 10.1016/j.ajhg.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pihl RO, Parkes M. Hair element content in learning disabled children. Science 198: 204–206, 1977. doi: 10.1126/science.905825. [DOI] [PubMed] [Google Scholar]
- 65.Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, Severijnen LA, Di Toro Mammarella L, Mignarri A, Monti L, Sanna A, Lu P, Punzo F, Cossu G, Willemsen R, Rasi F, Oostra BA, van de Warrenburg BP, Bonifati V. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet 90: 467–477, 2012. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quadri M, Kamate M, Sharma S, Olgiati S, Graafland J, Breedveld GJ, Kori I, Hattiholi V, Jain P, Aneja S, Kumar A, Gulati P, Goel M, Talukdar B, Bonifati V. Manganese transport disorder: novel SLC30A10 mutations and early phenotypes. Mov Disord 30: 996–1001, 2015. doi: 10.1002/mds.26202. [DOI] [PubMed] [Google Scholar]
- 67.Riley LG, Cowley MJ, Gayevskiy V, Roscioli T, Thorburn DR, Prelog K, Bahlo M, Sue CM, Balasubramaniam S, Christodoulou J. A SLC39A8 variant causes manganese deficiency, and glycosylation and mitochondrial disorders. J Inherit Metab Dis 40: 261–269, 2017. doi: 10.1007/s10545-016-0010-6. [DOI] [PubMed] [Google Scholar]
- 68.Roth JA. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biol Res 39: 45–57, 2006. doi: 10.4067/S0716-97602006000100006. [DOI] [PubMed] [Google Scholar]
- 69.Rotman B, Papermaster BW. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci USA 55: 134–141, 1966. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sánchez DJ, Gómez M, Domingo JL, Llobet JM, Corbella J. Relative efficacy of chelating agents on excretion and tissue distribution of manganese in mice. J Appl Toxicol 15: 285–288, 1995. doi: 10.1002/jat.2550150409. [DOI] [PubMed] [Google Scholar]
- 71.Santos D, Batoreu C, Mateus L, Marreilha Dos Santos AP, Aschner M. Manganese in human parenteral nutrition: considerations for toxicity and biomonitoring. Neurotoxicology 43: 36–45, 2014. doi: 10.1016/j.neuro.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seo YA, Li Y, Wessling-Resnick M. Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 38: 67–73, 2013. doi: 10.1016/j.neuro.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seo YA, Wessling-Resnick M. Ferroportin deficiency impairs manganese metabolism in flatiron mice. FASEB J 29: 2726–2733, 2015. doi: 10.1096/fj.14-262592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shanks MR, Cassio D, Lecoq O, Hubbard AL. An improved polarized rat hepatoma hybrid cell line. Generation and comparison with its hepatoma relatives and hepatocytes in vivo. J Cell Sci 107: 813–825, 1994. [DOI] [PubMed] [Google Scholar]
- 75.Spahr L, Butterworth RF, Fontaine S, Bui L, Therrien G, Milette PC, Lebrun LH, Zayed J, Leblanc A, Pomier-Layrargues G. Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology 24: 1116–1120, 1996. doi: 10.1002/hep.510240523. [DOI] [PubMed] [Google Scholar]
- 76.Stamelou M, Tuschl K, Chong WK, Burroughs AK, Mills PB, Bhatia KP, Clayton PT. Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: a new treatable disorder. Mov Disord 27: 1317–1322, 2012. doi: 10.1002/mds.25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Starzyński RR, Canonne-Hergaux F, Lenartowicz M, Krzeptowski W, Willemetz A, Styś A, Bierła J, Pietrzak P, Dziaman T, Lipiński P. Ferroportin expression in haem oxygenase 1-deficient mice. Biochem J 449: 69–78, 2013. doi: 10.1042/BJ20121139. [DOI] [PubMed] [Google Scholar]
- 78.Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J 21: 223–230, 2007. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tran TT, Chowanadisai W, Lönnerdal B, Le L, Parker M, Chicz-Demet A, Crinella FM. Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. Neurotoxicology 23: 645–651, 2002. doi: 10.1016/S0161-813X(02)00068-2. [DOI] [PubMed] [Google Scholar]
- 80.Treyer A, Müsch A. Hepatocyte polarity. Compr Physiol 3: 243–287, 2013. doi: 10.1002/cphy.c120009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tuschl K, Clayton PT, Gospe SM Jr, Gulab S, Ibrahim S, Singhi P, Aulakh R, Ribeiro RT, Barsottini OG, Zaki MS, Del Rosario ML, Dyack S, Price V, Rideout A, Gordon K, Wevers RA, Chong WK, Mills PB. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet 90: 457–466, 2012. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tuschl K, Meyer E, Valdivia LE, Zhao N, Dadswell C, Abdul-Sada A, Hung CY, Simpson MA, Chong WK, Jacques TS, Woltjer RL, Eaton S, Gregory A, Sanford L, Kara E, Houlden H, Cuno SM, Prokisch H, Valletta L, Tiranti V, Younis R, Maher ER, Spencer J, Straatman-Iwanowska A, Gissen P, Selim LA, Pintos-Morell G, Coroleu-Lletget W, Mohammad SS, Yoganathan S, Dale RC, Thomas M, Rihel J, Bodamer OA, Enns CA, Hayflick SJ, Clayton PT, Mills PB, Kurian MA, Wilson SW. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat Commun 7: 11601, 2016. doi: 10.1038/ncomms11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wahlberg K, Kippler M, Alhamdow A, Rahman SM, Smith DR, Vahter M, Lucchini RG, Broberg K. Common polymorphisms in the solute carrier SLC30A10 are associated with blood manganese and neurological function. Toxicol Sci 149: 473–483, 2016. doi: 10.1093/toxsci/kfv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 114: 124–129, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wetli HA, Buckett PD, Wessling-Resnick M. Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chem Biol 13: 965–972, 2006. doi: 10.1016/j.chembiol.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Worwood M, Taylor DM, Hunt AH. Copper and manganese concentrations in biliary cirrhosis of liver. BMJ 3: 344–346, 1968. doi: 10.1136/bmj.3.5614.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xin Y, Gao H, Wang J, Qiang Y, Imam MU, Li Y, Wang J, Zhang R, Zhang H, Yu Y, Wang H, Luo H, Shi C, Xu Y, Hojyo S, Fukada T, Min J, Wang F. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov 3: 17025, 2017. doi: 10.1038/celldisc.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol 70: 273–278, 1986. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- 89.Yin Z, Jiang H, Lee ES, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem 112: 1190–1198, 2010. doi: 10.1111/j.1471-4159.2009.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang AS, Xiong S, Tsukamoto H, Enns CA. Localization of iron metabolism-related mRNAs in rat liver indicate that HFE is expressed predominantly in hepatocytes. Blood 103: 1509–1514, 2004. doi: 10.1182/blood-2003-07-2378. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Z, Zhang F, Guo X, An P, Tao Y, Wang F. Ferroportin1 in hepatocytes and macrophages is required for the efficient mobilization of body iron stores in mice. Hepatology 56: 961–971, 2012. doi: 10.1002/hep.25746. [DOI] [PubMed] [Google Scholar]
- 92.Zhao Y, Feresin RG, Falcon-Perez JM, Salazar G. Differential targeting of SLC30A10/ZnT10 heterodimers to endolysosomal compartments modulates EGF-induced MEK/ERK1/2 activity. Traffic 17: 267–288, 2016. doi: 10.1111/tra.12371. [DOI] [PubMed] [Google Scholar]
- 93.Zogzas CE, Aschner M, Mukhopadhyay S. Structural elements in the transmembrane and cytoplasmic domains of the metal transporter SLC30A10 are required for its manganese efflux activity. J Biol Chem 291: 15940–15957, 2016. doi: 10.1074/jbc.M116.726935. [DOI] [PMC free article] [PubMed] [Google Scholar]