Abstract
Epidemiological and clinical research studies have provided ample evidence demonstrating that consumption of sugar-sweetened beverages increases risk factors involved in the development of obesity, Type 2 diabetes, and cardiovascular disease (CVD). Our previous study demonstrated that when compared with aspartame (Asp), 2 wk of high-fructose corn syrup (HFCS)-sweetened beverages provided at 25% of daily energy requirement was associated with increased body weight, postprandial (pp) triglycerides (TG), and fasting and pp CVD risk factors in young adults. The fatty acid ethanolamide, anandamide (AEA), and the monoacylglycerol, 2-arachidonoyl-sn-glycerol (2-AG), are two primary endocannabinoids (ECs) that play a role in regulating food intake, increasing adipose storage, and regulating lipid metabolism. Therefore, we measured plasma concentrations of ECs and their analogs, oleoylethanolamide (OEA), docosahexaenoyl ethanolamide (DHEA), and docosahexaenoyl glycerol (DHG), in participants from our previous study who consumed HFCS- or Asp-sweetened beverages to determine associations with weight gain and CVD risk factors. Two-week exposure to either HFCS- or Asp-sweetened beverages resulted in significant differences in the changes in fasting levels of OEA and DHEA between groups after the testing period. Subjects who consumed Asp, but not HFCS, displayed a reduction in AEA, OEA, and DHEA after the testing period. In contrast, there were significant positive relationships between AEA, OEA, and DHEA vs. ppTG, ppApoCIII, and ppApoE in those consuming HFCS, but not in those consuming Asp. Our findings reveal previously unknown associations between circulating ECs and EC-related molecules with markers of lipid metabolism and CVD risk after HFCS consumption.
Keywords: anandamide, ApoCIII, ApoE, high-fructose corn syrup, oleoylethanolamide, triglycerides
INTRODUCTION
High consumption of sugar-sweetened beverages (SSB) is a leading contributing factor to the obesity epidemic, Type 2 diabetes, and cardiovascular disease (CVD) (9, 20, 24, 25, 38). Consumption of fructose-containing beverages is associated with increases in body weight and CVD lipid markers, and decreases in insulin sensitivity (1, 36, 40). Sugar-sweetened beverages in the United States commonly contain high-fructose corn syrup (HFCS) with fructose content ranging from 47 to 65% (43). We previously reported that subjects consuming 0, 10, 17.5, and 25% Ereq as HFCS-sweetened beverages exhibited a dose-dependent increase in body weight in 2 wk (39), and large dose-dependent increases in postprandial (pp) triglycerides (TG), fasting and pp low-density lipoprotein, pp apoliprotein B, apolipoprotein CIII (ppApoCIII), and uric acid. Therefore, fructose-containing beverages may contribute to increased metabolic risk via both weight gain and upregulation of hepatic lipid production (38); however, specific roles for the endocannabinoid system in these processes are largely unknown. Nonetheless, a small number of studies suggest that endocannabinoids (ECs)—which are signaling molecules known to regulate both food intake (4, 10, 11, 17, 29) and lipid metabolism (8, 30, 32, 37)—may play a role in metabolic dysregulation induced by fructose-containing beverages (17, 23).
Two primary ECs, the fatty acid ethanolamide anandamide (AEA) and the monoacylglycerol 2-arachidonoyl-sn-glycerol (2-AG), act through cannabinoid type 1 receptors (CB1Rs) to stimulate palatable food intake (4, 5, 13, 14, 22). This is in contrast to oleoylethanolamide (OEA), a related fatty acid ethanolamide analog of AEA that does not interact with the CB receptors and plays a role in suppressing food intake (11, 12, 18, 19, 34). Other less-studied analogs of ECs, including the fatty acid ethanolamide, docosahexaenoyl ethanolamide (DHEA), and the monoacylglycerol, docosahexaenoyl glycerol (DHG), may stimulate glucose uptake in vitro (21) and have anti-inflammatory properties (15, 33); however, a comprehensive understanding of their physiological roles is lacking.
Our primary objective of this study was to determine whether 2 wk of HFCS-sweetened beverage consumption impacts plasma concentrations of ECs or their analogs. We hypothesized that 2 wk of HFCS-sweetened beverage consumption would be associated with increases in plasma levels of appetite-stimulating AEA and 2-AG, and decreased levels of appetite-suppressing OEA. A secondary objective was to determine whether changes in AEA, 2-AG, or their analogs are associated with changes in body weight and lipids/lipoproteins in subjects consuming HFCS for 2 wk.
METHODS
Study participants.
Participants in this study are a subgroup from a National Institutes of Health (NIH)-funded investigation in which a total of 187 participants assigned to eight experimental groups were studied, as previously described (39). The current article reports the results from 49 subjects consuming beverages containing either 0% (n = 21) or 25% (n = 28) daily energy requirement (Ereq) from high fructose corn syrup (HFCS). The study was conducted in accordance with an experimental protocol that was approved by the University of California, Davis, Institutional Review Board, and participants provided written informed consent.
Participants, who were recruited through an internet listing (www.craigslist.com) and local postings of flyers, underwent telephone and in-person screenings with medical history and completed blood count and serum biochemistry panel to assess eligibility. Inclusion criteria included age 18–40 yr, body mass index (BMI) 18–35 kg/m2 with self-report of stable body weight during the prior 6 mo. Exclusion criteria included diabetes (fasting glucose >125 mg/dl), evidence of renal or hepatic disease, fasting plasma triglyceride >400 mg/dl, hypertension (>140/90 mmHg), hemoglobin <8.5 g/dl, and surgery for weight loss. Individuals who smoked habitually ingested more than two alcoholic beverages per day, exercised >3.5 h/wk at a level more vigorous than walking, or used thyroid, lipid-lowering, glucose-lowering, antihypertensive, antidepressant, or weight loss medications were also excluded. Assignment to experimental groups was not randomized; by design, the experimental groups were matched for sex, BMI, and concentrations of fasting TG, cholesterol, high-density lipoprotein cholesterol, and insulin in plasma collected during the in-person interviews.
For the 5 wk before the start of the study, subjects who were scheduled for participation were asked to limit daily consumption of sugar-containing beverages to no more than one 237-ml serving of fruit juice and discontinue consumption of any vitamin, mineral, herbal, or dietary supplements, including fish oil. A total of 55 subjects were enrolled in experimental groups consuming either 0% [aspartame (Asp)] or 25% Ereq-HFCS. Two subjects withdrew from the study before the start of intervention, and four additional subjects withdrew for various reasons previously reported (39). A total of 51 subjects completed the study with 23 subjects in the Asp group and 28 in the HFCS group. Because of the unavailability of samples for two of the Asp subjects, results reported here include a total of 21 subjects in this group.
Study design.
This was a parallel-arm, double-blinded diet intervention study with three phases: 1) a 3.5-day inpatient baseline period during which subjects resided at the University of California, Davis, Clinical and Translational Science Center’s Clinical Research Center (CCRC), consumed a standardized baseline diet, and participated in experimental procedures; 2) a 12-day outpatient in intervention period during which subjects consumed their assigned sweetened beverages providing 0% (Asp-sweetened) or 25% Ereq-HFCS along with their usual ad libitum diets; and 3) a 3.5-day inpatient intervention period during which subjects resided at the CCRC and consumed standardized diets that included the sweetened beverages, and all experimental procedures were repeated.
In-patient diets.
During days 2 and 3 of the baseline and intervention inpatient periods, subjects consumed energy-balanced meals consisting of conventional foods. Daily Ereq were calculated by the Mifflin equation (28), with adjustment of 1.3 for activity on the days of the 24-h serial blood collections and adjustment of 1.5 for the other days. The baseline diet contained 55% Ereq mainly as low-fiber complex carbohydrate (i.e., white bread, white rice, regular pasta), 30% from fat, and 15% from protein. The meals during the inpatient intervention period included that assigned study beverages and were as identical as possible to baseline meals, except for the substitution of the sugar-sweetened beverage in place of isocaloric amounts of complex carbohydrate. The intervention meals contained 19–20 g fiber/2,000 kcal of fiber, and the baseline meals contained 22 g fiber/2,000 kcal. The timing of inpatient meals and the energy distribution were as follows: breakfast, 0900 (25%), lunch, 1300 (35%), and dinner, 1800 (40%).
Study beverages and outpatient diet.
HFCS-containing beverages were sweetened with HFCS-55 (Isosweet 5500, 55% fructose, 45% glucose: Skidmore Sales and Distributing), flavored with an unsweetened drink mix (Kool-Aid; Kraft). A fruit-flavored aspartame drink mix (Market Pantry) was used to prepare the 0% Ereq-HFCS beverages. Participants were blinded to their beverage assignment, as were all CCRC and study personnel who interacted with participants or analyzed samples. Voluntary feedback from participants indicated that they were able to distinguish between beverages containing aspartame (Asp) or HFCS. The amount (grams) of beverage provided was standardized among the two groups and based on energy requirements [calculated with the Mifflin equation (28), plus 1.5 activity adjustment]. During the 12-day out-patient phase of the study, participants were instructed to drink one serving of the study beverage with each meal, to consume their usual diet, and not to consume other sugar-sweetened beverages, including fruit juice. To monitor compliance of beverage consumption (35, 41), the study beverages contained a biomarker (riboflavin) that was measured fluorimetrically in urine samples collected. Subjects were informed about the biomarker but were not provided information regarding its identity. Fasting urinary riboflavin concentrations following days 9 and 13 of unmonitored beverage consumption were not different from those following 1 day of monitored beverage consumption at the CCRC, suggesting good and comparable compliance in all groups (39).
Fasting blood collection and lipid analysis.
Fasting blood samples reported here were collected at 0800 and stored at −80°C for the measurement of TG, apolipoprotein C III (ApoCIII), apolipoprotein E (ApoE), and EC-related outcomes. EC-related outcomes included monoacylglycerols (MAGs) [docosahexaenoyl glycerol (DHG) and 2-arachidonoyl-sn-glycerol (2-AG)] and fatty acid ethanolamides (FAEs) [anandamide (AEA), oleoylethanolamide (OEA), and docosahexaenoyl ethanolamide (DHEA)]. Lipid extraction and analysis of MAGs and FAEs were performed as previously described (4). Plasma (0.5 ml) was added to 1.0 ml of methanol solution containing the internal standards: [2H5]- 2-AG, [2H4]-AEA, and [2H4]-OEA (Cayman Chemical, Ann Arbor, MI). Lipids were extracted with chloroform (2 ml) and washed with 0.9% saline (0.5 ml). Organic phases were collected and separated by open-bed silica gel column chromatography. Eluate was gently dried under N2 stream (99.998% pure) and resuspended in 0.1 ml of methanol:chloroform (9:1), with 1 μl injection for ultraperformance liquid chromatography/tandem mass spectrometry analysis.
Lipids were analyzed using a Waters Acquity I-Class ultra-performance liquid chromatography system coupled to a Waters TQS-micro triple quadrupole mass spectrometer. Lipids were separated using an Acquity UPLC BEH C18 column (50 × 2.1 mm; ID 1.7 μm), eluted by a gradient of methanol (0.25% acetic acid, 5 mM ammonium acetate) in water (0.25% acetic acid, 5 mM ammonium acetate) (from 80 to 100% methanol in 2.5 min, 100% in 2.5–3.0 min, 100–80% in 3.0–3.1 min) at a flow rate of 0.4 ml/min. Column temperature was kept at 40°C, and samples were maintained in the sample manager at 10°C. Argon was used as collision gas (99.998% pure). 2-AG, AEA, OEA, DHG, DHEA, [2H5] 2-AG, [2H4] AEA, and [2H4] OEA were identified in the positive ionization mode based on their retention times and MS2 properties. Lipids were quantified using a stable isotope dilution method detecting protonated adducts of the molecular ions [M+H]+ in the multiple-reaction monitoring mode. Extracted ion chromatograms were used to quantify 2-AG (m/z = 379.2 > 287.26), AEA (m/z = 348.3 > 62.04), OEA (m/z = 326.3 > 62.08), DHG (m/z = 403.3 > 311.19), DHEA (m/z = 372.3 > 91.02), and [2H5] 2-AG (m/z = 384.2 > 93.4), [2H4] AEA (m/z = 352.3 > 66.11), and [2H4] OEA (m/z = 330.3 > 66.05), which were used as internal standards (Cayman Chemical, Ann Arbor, MI; [2H5] 2-AG as internal standard for 2-AG and DHG, [2H4] AEA (m/z = 352.3 > 66.11) as internal standard for AEA and DHEA, and [2H4] OEA as internal standard for OEA).
Postprandial lipid measurements.
Postprandial measures of total TG, ApoCIII, and ApoE were collected at time points 2200, 2300, and 2400 because this was the period during which peak ppTG concentrations were observed in our previous study (40). These three late-night pp plasma samples were pooled together, and multiple aliquots of each pooled sample were stored at −80°C. Lipid and lipoprotein concentrations were measured with a Polychem Chemistry Analyzer (PolyMedCo) with reagents from MedTest DX.
Statistical analyses.
Baseline anthropometric and clinical characteristics were compared between 0% Ereq (Asp) and 25% HFCS groups using a Studentʼs t-test. The percent change (%Δ) of these measures from 0 wk to 2 wk of intervention was compared using a general linear model (SAS 9.4), with %Δ outcome value at week 2 as the categorical variable, adjusting for sex and change in BMI, as well as sex × group interactions. Secondary analyses of absolute values at 0 wk and 2 wk were analyzed by repeated-measures ANCOVA, testing for an interaction between beverage group and time. By group, univariate linear regressions were conducted to determine potential relationships between changes in EC-related compounds and fasting or pp-lipid measures. Data presented in Table 1 are means ± SDs; all other data are expressed as means ± SE.
Table 1.
Baseline characteristics
| Aspartame | HFCS | ||
|---|---|---|---|
| (n = 21) | (n = 28) | P | |
| BMI | 25.3 ± 3.0 | 24.9 ± 4.0 | 0.68 |
| Age | 25.9 ± 6.3 | 26.8 ± 6.6 | 0.61 |
| Waist circumference | 76.1 ± 5.9 | 77.7 ± 10.1 | 0.72 |
| LDL, mg/dl | 84.1 ± 22.8 | 91.4 ± 27.3 | 0.30 |
| HDL, mg/dl | 39.4 ± 7.8 | 45.6 ± 13.7 | 0.08 |
| Fasting glucose, mg/dl | 90.5 ± 6.5 | 90.6 ± 6.3 | 0.87 |
| Fasting insulin, μU/ml | 13.0 ± 5.5 | 13.0 ± 5.2 | 0.84 |
| Fasting TG, mg/dl | 103.3 ± 54.4 | 107.8 ± 50.2 | 0.77 |
| Postprandial TG, mg/dl | 97.0 ± 68.4 | 108.1 ± 55.7 | 0.43 |
| Fasting ApoE, mg/dl | 3.4 ± 1.0 | 3.4 ± 0.8 | 0.91 |
| Postprandial ApoE, mg/dl | 3.0 ± 1.1 | 3.0 ± 0.9 | 0.90 |
| Fasting ApoCIII, mg/dl | 7.5 ± 3.1 | 8.2 ± 2.7 | 0.30 |
| Postprandial ApoCIII, mg/dl | 6.8 ± 3.1 | 7.4 ± 2.5 | 0.44 |
Values are expressed as means ± SD. ApoE, apolipoprotein E; ApoCIII, apolipoprotein C III; BMI, body mass index; HDL, high-density lipoprotein; HFCS, high-fructose corn syrup; LDL, low-density lipoprotein; TG, triglycerides.
RESULTS
Baseline characteristics and postintervention lipid markers of CVD.
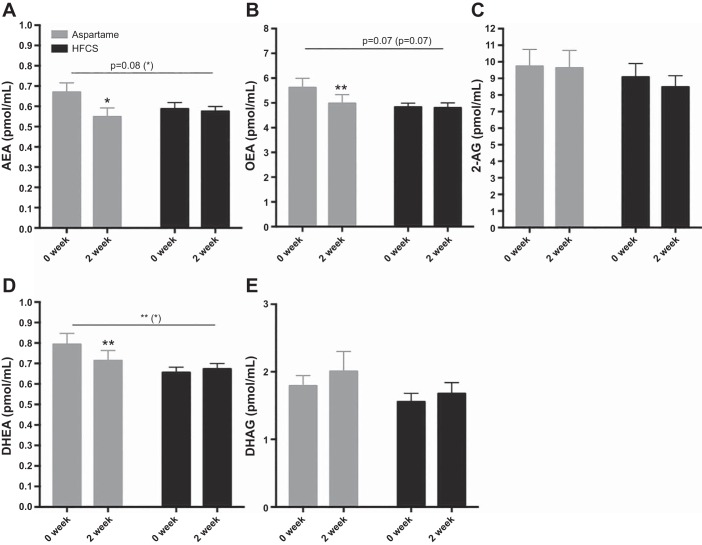
Baseline anthropometric and metabolic outcomes were not significantly different between groups (Table 1). Among the ECs at baseline, plasma concentrations of AEA, 2-AG, and the related DHG did not differ; however, OEA and DHEA were significantly higher in the aspartame group (Table 2). This difference was driven by two outliers in the Asp group. Sensitivity analyses and reanalyses of EC-related compounds with removal of these two outliers revealed that baseline differences between groups were not significant (Table 2), and %Δ at intervention was only moderately affected (Figs. 1 and 2). Therefore, these outliers were included in analyses; however, we included significance values with and without these outliers in all figures. Body weight, ppApoE, and fasting and ppTG and ApoCIII were all significantly increased in subjects consuming HFCS for 2 wk when compared with Asp controls (Table 3).
Table 2.
Baseline endocannabinoid and EC-related compounds
| Aspartame | Aspartame with Outliers Excluded | HFCS | |||
|---|---|---|---|---|---|
| (n = 21) | (n = 19) | (n = 28) | P* | P* with Outliers Excluded | |
| AEA, pmol/ml | 0.67 ± 0.04 | 0.66 ± 0.04 | 0.59 ± 0.04 | 0.14 | 0.14 |
| OEA, pmol/ml | 5.62 ± 0.3 | 5.14 ± 0.20 | 4.83 ± 0.24 | 0.04* | 0.22 |
| 2-AG, pmol/ml | 9.73 ± 1.0 | 9.36 ± 1.0 | 9.09 ± 0.8 | 0.6 | 0.62 |
| DHEA, pmol/ml | 0.79 ± 0.04 | 0.73 ± 0.03 | 0.66 ± 0.04 | 0.02* | 0.09 |
| DHG, pmol/ml | 1.79 ± 0.15 | 1.79 ± 0.17 | 1.56 ± 0.13 | 0.34 | 0.24 |
Values are expressed as means ± SE. AEA, anandamide; DHEA, docosahexaenoyl ethanolamide; DHG, docosahexaenoyl glycerol; HFCS, high-fructose corn syrup; OEA, oleoylethanolamide; 2AG, 2-arachidonoyl-sn-glycerol.
Significantly different between groups at P < 0.05 by unpaired Studentʼs t-test.
Fig. 1.
Baseline and 2-wk endocannabinoid (EC) and EC-related compound concentrations in aspartame (Asp)- or high-fructose corn syrup (HFCS)-sweetened beverage groups. Between-group comparisons were conducted by repeated-measures analysis, and within-group differences from baseline were conducted by Studentʼs t-test. *P < 0.05, **P < 0.01. P values in parentheses reflect significance after removal of two outliers in Asp group.
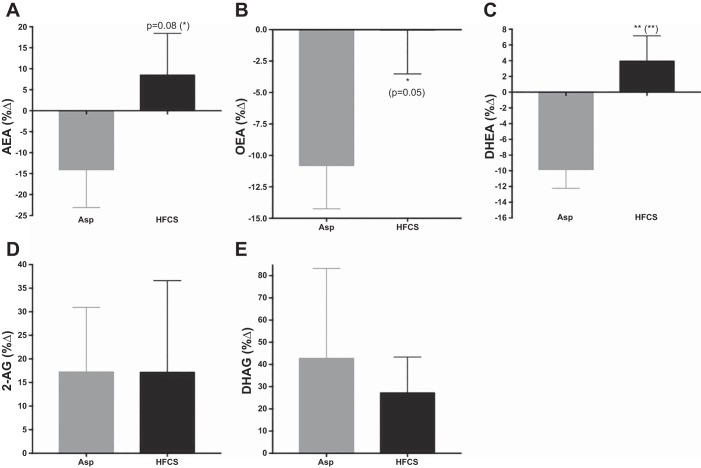
Fig. 2.
The percent change of endocannabinoids and their analogs in aspartame (Asp) and high-fructose corn syrup (HFCS) groups from 0-wk to 2-wk intervention. ANCOVA with adjustment for change in body mass index (BMI). *P < 0.05, **P < 0.01. P values in parentheses reflect significance after removal of two outliers in Asp group.
Table 3.
Absolute change in body weight, triglycerides, and lipoproteins
| Aspartame | HFCS | P | |
|---|---|---|---|
| Δ Body weight, kg | 0.01 ± 0.03 | 0.81 ± 0.026 | 0.047* |
| Δ Fasting TG, mg/dl (38) | −2.5 ± 4.3 | 11.1 ± 3.7 | 0.02* |
| Δ Postprandial TG, mg/dl (38) | −0.51 ± 5.0 | 36.9 ± 4.3 | <0.0001* |
| Δ Fasting ApoE, mg/dl | −0.002 ± 0.1 | 0.2 ± 0.08 | 0.11 |
| Δ Postprandial ApoE, mg/dl | −0.07 ± 0.1 | 0.54 ± 0.1 | 0.0004* |
| Δ Fasting ApoCIII, mg/dl (38) | −0.04 ± 0.2 | 0.66 ± 0.2 | 0.02* |
| Δ Postprandial ApoCIII, mg/dl (38) | −0.15 ± 0.2 | 1.1 ± 0.19 | <0.0001* |
Values are expressed as means ± SE.
Significantly different between groups at P < 0.05 by ANCOVA adjusted for change in body mass index. ApoE, apolipoprotein E; ApoCIII, apolipoprotein C III; HFCS, high-fructose corn syrup; TG, triglycerides.
Changes in circulating ECs and their analogs after 2 wk of SSB.
Significant beverage × time interactions were found for OEA (P = 0.03) and DHEA (P = 0.008), and a trending interaction for AEA (P = 0.08) (Fig. 1). Fig. 2 presents these differences between groups as %Δ from baseline by ANCOVA analyses. There were no differences between groups in the %Δ in 2-AG (P = 0.83) or DHG (P = 0.74). Including an adjustment for sex revealed a near-significant effect of sex on %Δ in AEA (P = 0.06); however, there were no significant sex × beverage interactions on EC-related outcomes. Paired t-tests were conducted for within-beverage group comparisons of values at baseline vs. week 2 of intervention. Consumption of HFCS-sweetened beverage for 2 wk did not result in changes in the plasma levels of the ECs and their analogs. Participants consuming Asp, however, exhibited significant reductions in the fatty acid ethanolamides, AEA (P = 0.01), OEA (P = 0.008), and DHEA (P = 0.001) (Fig. 1).
Differential associations between Asp vs. HFCS beverages in their relationships of AEA, OEA, and DHEA with CVD lipid markers.
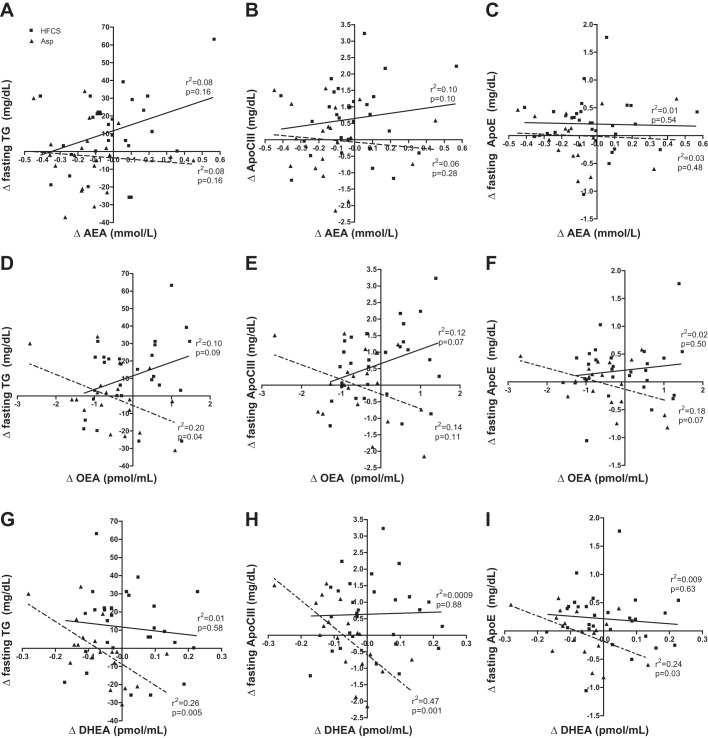
Absolute changes in ECs and their analogs did not correlate with change in body weight or BMI in either group, with the exception of OEA, which showed a weak relationship with change in body weight in the HFCS group (r2 = 0.11, P = 0.02) (data not shown). Linear regression analyses revealed differences between beverage groups in the relationships between changes in lipid and EC-related outcomes. There was no relationship between AEA and fasting lipid outcomes in either group (Fig. 3, A–C). Negative relationships were found between Δ OEA and Δ fasting TG (r2 = 0.20, P = 0.04) in the Asp group; however, the HFCS group trended toward a positive relationship (r2 = 0.10, P = 0.09) (Fig. 3D). The relationship between Δ OEA and Δ fasting ApoCIII did not reach significance in either beverage group (Asp r2 = 0.14, P = 0.11; HFCS r2 = 0.18, P = 0.07) (Fig. 3E). In the Asp group, a trend was observed between Δ OEA and Δ fasting ApoE (r2 = 0.18, P = 0.07) (Fig. 3F), but no relationship was present within the HFCS group. The strongest relationships under fasting conditions were observed in the Asp group between Δ DHEA and Δ TG (r2 = 0.26, P = 0.005), Δ ApoCIII (r2 = 0.47, P = 0.001), and Δ ApoE (r2 = 0.24, P = 0.03) (Fig. 3, G–I). Change in DHEA did not correlate to any of the lipid outcomes in the HFCS group under fasting conditions.
Fig. 3.
Linear regressions by beverage group comparing changes in anandamide (AEA), oleoylethanolamide (OEA), and docosahexaenoyl ethanolamide (DHEA) vs. changes in fasting triglycerides (TG), apolipoprotein C III (ApoCIII), and apolipoprotein E (ApoE). A–C: comparisons with Δ AEA; D–F: comparisons with Δ OEA; G–I: comparisons with Δ DHEA. Solid line with ■ denotes high-fructose corn syrup (HFCS), while dotted line with ▲ denotes aspartame (Asp).
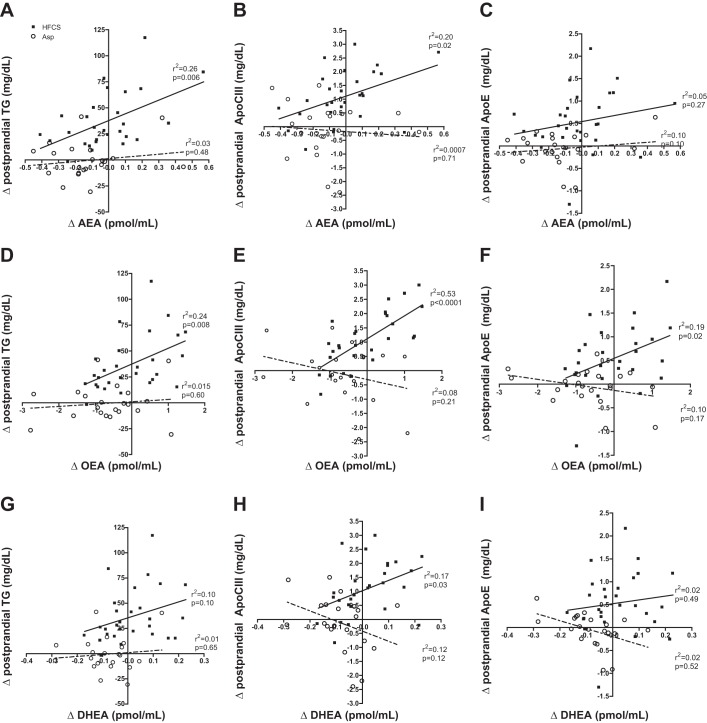
Under pp conditions, relationships between lipids and ECs were only present in those consuming HFCS. In the HFCS group, Δ AEA correlated positively with Δ ppTG (r2 = 0.26, 0.006) and Δ ppApoCIII (r2 = 0.29, P = 0.020) (Fig. 4, A and B). Changes in OEA were positively related to Δ ppTG, Δ ppApoCIII and Δ ppApoE in those consuming HFCS, with the strongest relationship being with the change in Δ ppApoCIII (r2 = 0.53, P < 0.0001) (Fig. 4, D–F). This differed from changes in DHEA, which only showed a weak relationship with changes in Δ ppApoCIII (r2 = 0.17, P = 0.03) and no relationship with either Δ ppTG or Δ ppApoE (Fig. 4, G–H). There were no associations between pp lipids and the lipid-derived EC analogs in subjects consuming Asp beverage (Fig. 4).
Fig. 4.
Linear regressions by beverage group comparing changes in anandamide (AEA), oleoylethanolamide (OEA), and docosahexaenoyl ethanolamide (DHEA) vs. changes in postprandial triglycerides (TG), apolipoprotein C III (ApoCIII), and apolipoprotein E (ApoE). A–C: comparisons with Δ AEA; D–F: comparisons with Δ OEA; G–I, comparisons with Δ DHEA. Solid line with ■ denotes high-fructose corn syrup (HFCS), while dotted line with ○ denotes Asp.
DISCUSSION
This is the first study in humans to demonstrate an association between the EC system and increased CVD risk factors in response to HFCS consumption. We hypothesized that 2-wk consumption of HFCS-sweetened beverage, when compared with Asp-sweetened beverage, would be associated with increased plasma levels of appetite-stimulating AEA and 2-AG, and decreased appetite-suppressing OEA. Contrary to our hypotheses, HFCS beverage in normal-weight adults was not associated with any significant changes in ECs and their analogs; however, subjects consuming Asp beverage displayed decreases in levels of the fatty acid ethanolamides AEA, OEA, and DHEA. Furthermore, plasma levels of AEA, OEA, and DHEA were positively associated with changes in ppTG, ppApoCIII, and ppApoE in participants consuming HFCS, but not in those consuming Asp beverage. These findings demonstrate an association between ECs and their analogs with markers of lipid metabolism and CVD in response to sugar-sweetened beverage consumption.
Despite significant increases in body weight following the HFCS beverage intervention, ECs and their analogs were not strongly associated with weight gain, with the exception of a weak relationship between Δ OEA and body weight. This effect is possibly due to the short-term intervention resulting in modest weight gain rather than longer-term interventions resulting in more clinically significant weight gain. Nonetheless, the weak positive correlation is in line with findings of higher plasma OEA concentrations in obese compared with lean individuals (2, 26). Furthermore, Matias et al. (26) demonstrated that salivary OEA and AEA correlated with BMI, body weight, and waist circumference in obese individuals.
In rodents, high-fat diet-induced obesity is associated with greater expression of hepatic CB1 receptors through which ECs may stimulate hepatic de novo lipogenesis (31), and high-fructose or -sucrose diets result in greater hypothalamic synthesis of ECs and CB1 receptor activity (17, 23). No associations were found when comparing plasma levels of the ECs with TG, ApoCIII, and ApoE in the fasted state in subjects consuming HFCS. In the pp state, however, significant positive relationships were found between AEA and OEA vs. TG and ApoCIII, and OEA vs. ApoE. This finding may suggest that ECs and related molecules did not affect TG and ApoCIII production, but rather HFCS-induced increases in ppTG, ppApoCIII, and to a lesser extent ppApoE, affected plasma levels of AEA, OEA, and DHEA. This result may be a threshold effect, however, because increases in ECs and their analogs were observed mainly in the subjects who exhibited higher increases in ppTG, ppApoCIII, and ppApoE. HFCS-induced increases in TG, ApoCIII, and ApoE were approximately twice as high in the pp state than the fasting state; thus, a positive relationship between ECs and TG/lipoproteins was apparent only in the pp state.
Consumption of Asp-sweetened beverages was associated with reduced fasting concentrations of plasma AEA, OEA, and DHEA. Whether reductions in appetite-stimulating AEA in the absence of changes in body weight are a result of the presence of Asp, or in contrast, the absence of SSB, requires further study. Understanding this relationship could have implications for interventions aimed at reducing food intake and body weight. Indeed, Asp beverage consumption has been shown to lower caloric intake and reduce desire for highly palatable foods (3), and reductions in salivary AEA were found following a 3-mo weight loss intervention (16, 26).
Reductions in DHEA, AEA, and OEA in Asp-consuming subjects may also reflect a decrease in inflammatory responses (27). DHEA, AEA, and possibly OEA have been implicated in anti-inflammatory responses (7, 42, 44), and share common fatty acid ethanolamide biosynthetic and degradative pathways (12, 18, 19, 34). The EC, 2-AG, is a monoacylglycerol (30) that is also synthesized from AA (similar to AEA) and plays a role in inflammation, but our results suggest that only fatty acid ethanolamides are associated with Asp consumption. Further studies are needed to better understand the biological relevance of the Asp-associated reduction in fatty acid ethanolamides in the context of both appetite regulation and anti-inflammatory responses.
To our knowledge, this is the first study to examine the effects of HFCS beverage consumption on circulating ECs, and importantly, in healthy, nonobese individuals. Nonetheless, this study has several limitations. Participants consumed ad libitum diets during the 12-day out-patient period; thus, we did not control for precise quantities of sugars consumed. Nonetheless, our study participants were instructed to abstain from consuming outside beverages containing added sugar but were not instructed to abstain from naturally occurring sugars, such as those found in fruits, which also contain antioxidants and polyphenols. Unlike natural sources of sugar, added sugars consumed as sweetened beverages provide little to no nutritional value. Therefore, we did not feel it necessary to restrict participants from consuming natural, nutritional food items and did not have prior evidence to suggest that this would impact the outcomes of this study. Similarly, participants were instructed to cease intake of fish oil supplements 5 wk before and during the study, as these supplements have been shown to alter levels of DHEA, docosahexaenoic acid, and ECs (6). Although participants were not prohibited from consuming fish during the study, it is unlikely that fish intake was greater in one group over the other, as there is no prior evidence to suggest that Asp or HFCS influences the desire to eat foods high in omega fatty acids. Another limitation to our study is that ECs and their analogs were measured only in fasting plasma. Future studies that include postprandial EC measures will provide valuable insight into the heterogeneous functions (e.g., regulation of appetite signaling and lipid metabolism) of AEA, OEA, and DHEA in response to a HFCS beverage. In addition, saliva measures of ECs would better assess whether or not increases in ECs can explain taste-related links between SSB, hedonic feeding behavior, and weight gain in humans.
Conclusion.
This is the first study to demonstrate the effects of Asp- and HFCS-sweetened beverage consumption on circulating ECs in humans. The unexpected absence of an effect of HFCS on the EC system in this study should be further investigated under longer-term exposure to HFCS and in response to a meal. On the contrary, observed effects of Asp on circulating ECs raise questions regarding the potential effects of artificial sweeteners on food-reward pathways and should be further explored. Lastly, our study shows differential effects of beverage type on circulating EC compounds in relationship to lipid risk factors of CVD in the fasted and postprandial states. Future studies are needed to further understand the possible implications that this may have on metabolic functions.
GRANTS
This study was supported by the following National Institutes of Health grants: National Institute of Diabetes Digestive and Kidney Disorders DK-114978 (principal investigator (PI): N. V. DiPatrizio); National Institute on Drug Abuse DA034009 (PI: N. V. DiPatrizio); National Heart, Lung and Blood Institute HL-09133 (PI: P. J. Havel) and HL-107256 (PI: P. J. Havel); National Center for Research Resources, and NIH Roadmap for Medical Research RR024146 (PI: L. Berglund). Candice Price and Kimber Stanhope were supported by the Building Interdisciplinary Research Careers in Women's Health award (K12 HD-051958; PI: Gold) funded by the National Institute of Child Health and Human Development, Office of Research on Women's Health, Office of Dietary Supplements, and the National Institute of Aging. The authors also thank the University of California Sugar Stress Environment and Weight Initiative for pilot funding.
REFERENCES
- 1.Aeberli I, Zimmermann MB, Molinari L, Lehmann R, l’Allemand D, Spinas GA, Berneis K. Fructose intake is a predictor of LDL particle size in overweight schoolchildren. Am J Clin Nutr 86: 1174–1178, 2007. doi: 10.1093/ajcn/86.4.1174. [DOI] [PubMed] [Google Scholar]
- 2.Annuzzi G, Piscitelli F, Di Marino L, Patti L, Giacco R, Costabile G, Bozzetto L, Riccardi G, Verde R, Petrosino S, Rivellese AA, Di Marzo V. Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients. Lipids Health Dis 9: 43, 2010. doi: 10.1186/1476-511X-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P, Williamson DA. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 55: 37–43, 2010. doi: 10.1016/j.appet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argueta DA, DiPatrizio NV. Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol Behav 171: 32–39, 2017. doi: 10.1016/j.physbeh.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 132: 104–106, 1997. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 6.Balvers MG, Verhoeckx KC, Bijlsma S, Rubingh CM, Meijerink J, Wortelboer HM, Witkamp RF. Fish oil and inflammatory status alter the n-3 to n-6 balance of the endocannabinoid and oxylipin metabolomes in mouse plasma and tissues. Metabolomics 8: 1130–1147, 2012. doi: 10.1007/s11306-012-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balvers MG, Verhoeckx KC, Plastina P, Wortelboer HM, Meijerink J, Witkamp RF. Docosahexaenoic acid and eicosapentaenoic acid are converted by 3T3-L1 adipocytes to N-acyl ethanolamines with anti-inflammatory properties. Biochim Biophys Acta 1801: 1107–1114, 2010. doi: 10.1016/j.bbalip.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112: 423–431, 2003. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 125: 1735–1741, 2012. doi: 10.1161/CIRCULATIONAHA.111.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci USA 108: 12904–12908, 2011. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiPatrizio NV, Joslin A, Jung KM, Piomelli D. Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. FASEB J 27: 2513–2520, 2013. doi: 10.1096/fj.13-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiPatrizio NV, Piomelli D. Intestinal lipid-derived signals that sense dietary fat. J Clin Invest 125: 891–898, 2015. doi: 10.1172/JCI76302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci 28: 9702–9709, 2008. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiPatrizio NV, Simansky KJ. Inhibiting parabrachial fatty acid amide hydrolase activity selectively increases the intake of palatable food via cannabinoid CB1 receptors. Am J Physiol Regul Integr Comp Physiol 295: R1409–R1414, 2008. doi: 10.1152/ajpregu.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dotsey E, Ushach I, Pone E, Nakajima R, Jasinskas A, Argueta DA, Dillon A, DiPatrizio N, Davies H, Zlotnik A, Crompton PD, Felgner PL. Transient cannabinoid receptor 2 blockade during immunization heightens intensity and breadth of antigen-specific antibody responses in young and aged mice. Sci Rep 7: 42584, 2017. doi: 10.1038/srep42584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 54: 2838–2843, 2005. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlanson-Albertsson C, Lindqvist A. Fructose affects enzymes involved in the synthesis and degradation of hypothalamic endocannabinoids. Regul Pept 161: 87–91, 2010. doi: 10.1016/j.regpep.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. Oleoylethanolamide, an endogenous PPAR-α agonist, lowers body weight, and hyperlipidemia in obese rats. Neuropharmacology 48: 1147–1153, 2005. doi: 10.1016/j.neuropharm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Hansen HS, Vana V. Non-endocannabinoid N-acylethanolamines and 2-monoacylglycerols in the intestine. Br J Pharmacol 2018. doi: 10.1111/bph.14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RJ, Sánchez-Lozada LG, Andrews P, Lanaspa MA. Perspective: a hstorical and scientific perspective of sugar and its relation with obesity and diabetes. Adv Nutr 8: 412–422, 2017. doi: 10.3945/an.116.014654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Carlson ME, Watkins BA. Docosahexaenoyl ethanolamide improves glucose uptake and alters endocannabinoid system gene expression in proliferating and differentiating C2C12 myoblasts. Front Physiol 5: 100, 2014. doi: 10.3389/fphys.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau BK, Cota D, Cristino L, Borgland SL. Endocannabinoid modulation of homeostatic and non-homeostatic feeding circuits. Neuropharmacology 124: 38–51, 2017. doi: 10.1016/j.neuropharm.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept 150: 26–32, 2008. doi: 10.1016/j.regpep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Malik VS, Hu FB. Sweeteners and risk of obesity and Type 2 diabetes: the role of sugar-sweetened beverages. Curr Diab Rep 12: 195–203, 2012. doi: 10.1007/s11892-012-0259-6. [DOI] [PubMed] [Google Scholar]
- 25.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 84: 274–288, 2006. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matias I, Gatta-Cherifi B, Tabarin A, Clark S, Leste-Lasserre T, Marsicano G, Piazza PV, Cota D. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS One 7: e42399, 2012. doi: 10.1371/journal.pone.0042399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijerink J, Poland M, Balvers MG, Plastina P, Lute C, Dwarkasing J, van Norren K, Witkamp RF. Inhibition of COX-2-mediated eicosanoid production plays a major role in the anti-inflammatory effects of the endocannabinoid N-docosahexaenoylethanolamine (DHEA) in macrophages. Br J Pharmacol 172: 24–37, 2015. doi: 10.1111/bph.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51: 241–247, 1990. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 29.Monteleone AM, Di Marzo V, Monteleone P, Dalle Grave R, Aveta T, Ghoch ME, Piscitelli F, Volpe U, Calugi S, Maj M. Responses of peripheral endocannabinoids and endocannabinoid-related compounds to hedonic eating in obesity. Eur J Nutr 55: 1799–1805, 2016. doi: 10.1007/s00394-016-1153-9. [DOI] [PubMed] [Google Scholar]
- 30.Naughton SS, Mathai ML, Hryciw DH, McAinch AJ. Fatty acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int J Endocrinol 2013: 361895, 2013. doi: 10.1155/2013/361895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115: 1298–1305, 2005. doi: 10.1172/JCI200523057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagano C, Rossato M, Vettor R. Endocannabinoids, adipose tissue and lipid metabolism. J Neuroendocrinol 20, Suppl 1: 124–129, 2008. doi: 10.1111/j.1365-2826.2008.01690.x. [DOI] [PubMed] [Google Scholar]
- 33.Park T, Chen H, Kevala K, Lee JW, Kim HY. N-Docosahexaenoylethanolamine ameliorates LPS-induced neuroinflammation via cAMP/PKA-dependent signaling. J Neuroinflammation 13: 284, 2016. doi: 10.1186/s12974-016-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piomelli D. A fatty gut feeling. Trends Endocrinol Metab 24: 332–341, 2013. doi: 10.1016/j.tem.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramanujam VM, Anderson KE, Grady JJ, Nayeem F, Lu LJ. Riboflavin as an oral tracer for monitoring compliance in clinical research. Open Biomark J 2011: 1–7, 2011. doi: 10.2174/1875318301104010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz JM, Noworolski SM, Wen MJ, Dyachenko A, Prior JL, Weinberg ME, Herraiz LA, Tai VW, Bergeron N, Bersot TP, Rao MN, Schambelan M, Mulligan K. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J Clin Endocrinol Metab 100: 2434–2442, 2015. doi: 10.1210/jc.2014-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon V, Cota D. Mechanisms in endocrinology: Endocannabinoids and metabolism: past, present and future. Eur J Endocrinol 176: R309–R324, 2017. doi: 10.1530/EJE-16-1044. [DOI] [PubMed] [Google Scholar]
- 38.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci 53: 52–67, 2016. doi: 10.3109/10408363.2015.1084990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, Chen GX, Keim NL, Havel PJ. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr 101: 1144–1154, 2015. doi: 10.3945/ajcn.114.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119: 1322–1334, 2009. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Switzer BR, Stark AH, Atwood JR, Ritenbaugh C, Travis RG, Wu HM. Development of a urinary riboflavin adherence marker for a wheat bran fiber community intervention trial. Cancer Epidemiol Biomarkers Prev 6: 439–442, 1997. [PubMed] [Google Scholar]
- 42.Turcotte C, Chouinard F, Lefebvre JS, Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol 97: 1049–1070, 2015. doi: 10.1189/jlb.3RU0115-021R. [DOI] [PubMed] [Google Scholar]
- 43.Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity (Silver Spring) 19: 868–874, 2011. doi: 10.1038/oby.2010.255. [DOI] [PubMed] [Google Scholar]
- 44.Yang L, Guo H, Li Y, Meng X, Yan L, Dan Zhang, Wu S, Zhou H, Peng L, Xie Q, Jin X. Oleoylethanolamide exerts anti-inflammatory effects on LPS-induced THP-1 cells by enhancing PPARα signaling and inhibiting the NF-κB and ERK1/2/AP-1/STAT3 pathways. Sci Rep 6: 34611, 2016. doi: 10.1038/srep34611. [DOI] [PMC free article] [PubMed] [Google Scholar]