Abstract
Umbrella cells, which must maintain a tight barrier, modulate their apical surface area during bladder filling by exocytosis of an abundant, subapical pool of discoidal- and/or fusiform-shaped vesicles (DFVs). Despite the importance of this trafficking event for bladder function, the pathways that promote DFV exocytosis remain to be identified. We previously showed that DFV exocytosis depends in part on a RAB11A-RAB8A-MYO5B network, but RAB27B is also reported to be associated with DFVs, and knockout mice lacking RAB27B have fewer DFVs. However, the RAB27B requirements for DFV exocytosis and the relationship between RAB27B and the other umbrella cell-expressed RABs remains unclear. Using a whole bladder preparation, we observed that filling-induced exocytosis of human growth hormone-loaded DFVs was significantly inhibited when RAB27B expression was downregulated using shRNA. RAB27A was also expressed in rat urothelium; however, RAB27A-specific shRNAs did not inhibit exocytosis, and the combination of RAB27A and RAB27B shRNAs did not significantly affect DFV exocytosis more than treatment with RAB27B shRNA alone. RAB27B and RAB11A showed a small degree of overlap when quantified using Squassh segmentation software, and expression of dominant-active or dominant-negative mutants of RAB11A or RAB8A, or expression of a RAB11A-specific shRNA, had no significant effect on the size, number, or intensity of RAB27B-positive DFVs. Likewise, treatment with RAB27B-specific shRNA had no effect on RAB11A-positive DFV parameters. We conclude that RAB27B, but not RAB27A, regulates DFV exocytosis in bladder umbrella cells in a manner that may be parallel to the previously described RAB11A-RAB8A-MYO5B pathway.
Keywords: bladder, exocytosis, Rab27B, urothelium
INTRODUCTION
To maintain its barrier function, the layer of umbrella cells that line the inner surface of the bladder must accommodate large variations in bladder volume during the micturition cycle. Whereas bladder filling stimulates the apical exocytosis of discoidal- and/or fusiform-shaped vesicles (DFVs) in these cells (32, 45, 51), voiding triggers endocytosis of added apical membrane (30). Despite their physiological importance, we have limited insights into how these mechanically triggered trafficking events are regulated at the molecular level. One likely mechanism is through the action of Rab GTPases, a family composed of 44 subfamilies in humans that play crucial roles in promoting vesicle movements, fusion, and fission during exocytosis (24). Work to date implicates three Rab GTPases in umbrella cell exocytosis: RAB11A, RAB8A, and RAB27B (10, 28, 29, 48). Whereas RAB11A and RAB8A are traditionally thought to regulate endocytic recycling pathways, DFVs are formed in the trans-Golgi network, they cannot be labeled with endocytic tracers, and they are not lysosome-related organelles (17, 23, 29, 30). RAB11A is localized to DFVs and DFV exocytosis is dependent on this GTPase (29). DFV exocytosis also requires RAB8A, and like a variety of other trafficking situations (including lumen formation and ciliogenesis) (8, 11), RAB11A may act upstream of RAB8A to promote DFV exocytosis (28). The well-known effector of RAB11A and RAB8A, MYO5B, is also implicated in DFV exocytosis (28).
The other RAB associated with DFVs is RAB27, which has A and B isoforms (10, 48). RAB27 is a so-called “secretory” RAB, a family that includes RAB3 (A–D isoforms), RAB26, and RAB37. Members of this family are required for regulated secretion of secretory vesicles/granules in a number of different cell types (13). Mouse umbrella cells express RAB27B, but not RAB27A (10, 48); but, it is unknown if this expression pattern is universal across species. Electron microscopic studies unequivocally demonstrate that RAB27B is localized to DFVs, and recent studies show that RAB27B knockout mice have fewer DFVs than controls (10, 48). However, other than these observations, there is no evidence that RAB27B regulates DFV exocytosis in umbrella cells. An additional unknown is the relationship of RAB27B to the putative RAB11A-RAB8A pathway. Based on a modest degree of colocalization between RAB27B with RAB11A or RAB8A, and the position of the vesicles, it was recently proposed that RAB11A-RAB8A act upstream of the RAB27B-dependent step(s) (48), perhaps as part of a RAB cascade. However, the data thus far are limited, and RAB27B and the RAB11A-RAB8A pathway could act in parallel pathways.
Our current studies establish that rat urothelium expresses both RAB27A and RAB27B. Whereas downregulating RAB27B expression inhibits DFV exocytosis, downregulation of RAB27A does not. Colocalization analysis confirms that there is some overlap between RAB27B-labeled DFVs and those that express RAB11A or RAB8A (~10–20%). However, upon expression of dominant-active or dominant-negative mutants of RAB11A or RAB8A, or expression of a RAB11A-specific shRNA, we do not observe a significant effect on the size, number, or intensity of RAB27B-positive DFVs. Likewise, a reduction of RAB27B expression has no effect on RAB11A-positive DFVs. We propose that RAB27B regulates exocytosis in a pathway that may be distinct from that regulated by RAB11A-RAB8A. The implication of these findings for bladder function and mechanically regulated trafficking events is discussed.
MATERIALS AND METHODS
Reagents and antibodies.
Unless specified otherwise, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Primary antibodies used in this study included the following: rabbit anti-hGH1 (National Hormone and Peptide Program, Torrance, CA) or mouse monoclonal anti-hGH1 (ab15317, Abcam, Cambridge, MA); mouse monoclonal anti-KRT20 (M7019, clone Ks20.8, Dako, Carpinteria, CA), goat polyclonal anti-laminB (sc-6216, Santa Cruz Biotechnology, Dallas, TX), mouse monoclonal anti-RAB8 (610844, clone 4, BD Biosciences, San Jose, CA), rabbit polyclonal antibodies and mouse monoclonal 8H10 antibody to RAB11A have been described (31); mouse monoclonal anti-RAB27A (MABN446, clone 16H2.1, EMD Millipore, Billerica, MA), rabbit polyclonal anti-RAB27B (HPA019849; Sigma-Aldrich); mouse monoclonal anti-MYO5A (M4812, Sigma-Aldrich), rabbit polyclonal anti-pan UPK (AUM) (kindly provided by T.T. Sun, New York University, New York); mouse monoclonal anti-UPK3A is previously described (44), and rabbit polyclonal anti-V5 (PA1-993, ThermoFisher, Grand Island, NY). Secondary antibodies included Alexa488-, Dylight549-, Cy5-, or HRP-labeled affinity-purified and minimal cross-reacting goat or donkey secondary antibodies purchased from Jackson ImmunoResearch (West Grove, PA). Rhodamine phalloidin and TO-PRO-3 were obtained from Molecular Probes-ThermoFisher.
Animals.
Urinary bladders were obtained from female Sprague-Dawley rats (250–300 g). The rats were euthanized by inhalation of 100% CO2, followed by a thoracotomy. All animal studies were performed under the approval of the University of Pittsburgh Institutional Animal Care and Use Committee.
HEK cell culture and transfection.
HEK-293T cells (ThermoFisher) were routinely cultured in Complete Medium, which was composed of DMEM high-glucose medium (GIBCO-ThermoFisher) supplemented with 10% vol/vol FBS (Hyclone-GE HealthCare, Logan, UT) and 1:100 dilution of the following supplements: nonessential amino acids, penicillin/streptomycin, and glutamine (all from GIBCO-ThermoFisher). Cells, cultured on 10-cm-diameter dishes, were incubated at 37°C in a cell culture incubator (Heraeus, ThermoFisher) gassed with a mixture of 5% vol/vol CO2-95% vol/vol air. Before transfection, cells that were 90–95% confluent were trypsinized, centrifuged at 100 g, and then resuspended in 10 ml of Complete Medium. One milliliter of this cell suspension was placed in the bottom of each well of a six-well dish. Transfection was performed using Lipofectamine 2000 reagent (ThermoFisher). For each cell well, 1.0 µg of DNA was diluted into 125 µl of Opti-MEM (GIBCO-ThermoFisher) and 2.5 µl of Lipofectamine 2000 reagent was diluted into 125 µl of Opti-MEM. The two solutions were mixed, incubated for 5 min at ambient temperature, and then added to the well. Cells were incubated for 24–72 h before experimentation.
Sample preparation and Western blot analysis.
HEK cells were cultured in six-well dishes, as described above, whereas excised bladders were cut open along the midline and the tissue pinned out loosely on a rubber mat housed in a square culture dish filled with Krebs buffer (110 mM NaCl, 25 mM NaHCO3, 5.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 11 mM glucose, 2 mM CaCl2, pH 7.4 when gassed with 5% vol/vol CO2). Before lysis, the medium covering the HEK cells or the buffer covering the split-open bladders was aspirated (except in the case where secretion of hGH1 or hGH1-V5 was assessed), the cell/tissue was rinsed with Krebs buffer. Following aspiration, the HEK cells were dissolved in lysis buffer (100 mM NaCl, 50 mM TEA pH 8.6, 5 mM EDTA, 0.2% wt/vol NaN3, 0.5% wt/vol SDS, supplemented with freshly added 0.5 mM phenyl-methylsulfonylfluoride and 1:100 dilution of a protease inhibitor cocktail; catalog no. P8340, Sigma-Aldrich). The urothelial cells were dissolved by adding 60 µl of lysis buffer to the mucosal surface and collecting the fluid with P200 Pipetman outfitted with a yellow tip. This process was repeated and the two lysates were pooled. The HEK cell lysate or urothelial cell lysate was collected into a 1.5-ml Eppendorf tube, and then shaken for 20 s at ambient temperature in a FastPrep 24 device (MP Biomedicals, San Diego, CA). Following centrifugation at 14,000 g in an Eppendorf 5415D microfuge (Hauppauge, NY) for 5 min at 4°C, the supernatant was recovered and the protein concentration determined using bicinchoninic acid assay reagents (Pierce, Rockford, IL).
Samples (typically containing 10–25 µg of protein) were mixed with an equal volume of 2X Laemmli sample buffer, and the proteins resolved by SDS-PAGE using Criterion TGX 4–15% polyacrylamide gradient gels (Bio-Rad, Hercules, CA), bathed in electrophoresis buffer (25 mM Tris, 192 mM glycine, 0.1% wt/vol SDS, pH 8.3), and exposed to 200 V constant current in a Criterion Cell electrophoresis device (Bio-Rad). Proteins in the acrylamide gels were transferred to Immobilon-P (EMD Millipore) in 100 mM CAPS, pH 11.0 buffer at 375 mA constant current for 40 min in a Mighty Small Transphor apparatus (Amersham Biosciences, Piscataway, NJ). The membrane was blocked for 1 h at ambient temperature with 5% (wt/vol) BSA in TBS-Tween (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 0.05% vol/vol Tween-20). After incubation with primary antibody (diluted in TBS-Tween containing 1% wt/vol BSA) overnight at 4°C, blots were washed with TBS-Tween (3 × 20 min on a shaker), incubated with HRP-conjugated anti-rabbit or anti-mouse antibodies (diluted in TBS-Tween containing 1% wt/vol BSA) for 60 min at ambient temperature, washed with TBS-Tween (3 × 20 min on a shaker), and immunoreactive protein species were visualized using SuperSignal West Substrate (Pierce, Rockford, IL) and exposure of the blot to Carestream Kodak BioMax MR Film (Sigma-Aldrich). The film was scanned to a 300 dpi TIFF file using a Perfection V750PRO flat-bed scanner (with transparency module; Epson, Long Beach, CA) interfaced with VueScan software (Hamrick), running on a PowerMac computer (Apple, Cupertino, CA). Alternatively, visualization and image capture was performed using a Chemidoc Touch Imaging System (Bio-Rad). Quantification of digital files was performed using ImageLab version 5.2.1 (Bio-Rad), running on an iMac computer.
RT-PCR.
The urothelium was recovered by gentle scraping as described (5), the cells were lysed, and RNA was purified using the RNAqueous Micro kit (Life Technologies). RNA was treated with DNAse I and cDNA generated using the RETROscript First Strand Synthesis kit (Life Technologies). PCR was performed using Taq polymerase (Life Technologies). The primers used are described in Table 1. Sequencing was used to confirm the identity of the PCR reaction products.
Table 1.
Primers (PCR) and target sequences for shRNAs used in analysis
| Name of Primer or shRNA | Sequence | Predicted Length of PCR Product |
|---|---|---|
| ACTB PCR primers | FWD 5′-CCCGCGAGTACAACCTTCTT-3′ REV 5′-AACACAGCCTGGATGGCTAC-3′ |
481 bp |
| RAB27A PCR primers | FWD 5′-ACCAAACCGGGTAAAGCAGAG-3′ REV 5′-CATGCTCACTCGGTGTCTCA-3′ |
715 bp |
| RAB27B PCR primers | FWD 5′-GACTGTGACTCTGTGAGGCTG-3′ REV 5′-CTGCAGTTGACTCATCCAGTT-3′ |
508 bp |
| RAB27A-shRNA-1 | 5′-gctgccaatgggacaaacata-3′ | |
| RAB27A-shRNA-2 | 5′-gcttctgttcgacctgacaaa-3′ | |
| RAB27A-shRNA-3 | 5′-ttcatcaccacagtgggca-3′ | |
| RAB27A-shRNA-4 | 5′-ccagtacactgatgggaagtt-3′ | |
| RAB27B-shRNA-a | 5′-gcataccatactttgaaacaa-3′ | |
| RAB27B-shRNA-b | 5′-ccttctggacttaatcatgaa-3′ | |
| RAB27B-shRNA-c | 5′-ccagtcaacagagtttctt-3′ | |
| RAB27B-shRNA-d | 5′-gtcaactgcaggcaaatgct-3′ | |
| RAB11A-shRNA-1 | 5′-gcctcctgtctcgatttact-3′ | |
| RAB11A-shRNA-2 | 5′-tctggaaagcaagagtacc-3′ | |
| RAB11A-shRNA-3 | 5′-tggtttgtcgttcattgag-3′ | |
| RAB11A-shRNA-4 | 5′-tgtggttcctattcatgtc-3′ | |
| Scrambled-shRNA | 5′-gtcggatttcctggtatatgt-3′ |
The shRNAs used in the urothelium are bolded. FWD, forward; REV, reverse.
Tissue fixation, detection of antigens using immunofluorescence, and image acquisition.
Excised rat bladders were cut open along the midline, rinsed with Krebs buffer, and then incubated in Krebs buffer gassed with 5% vol/vol CO2 for 30 min to allow the tissue to achieve a “relaxed” state. The tissue was subsequently fixed with 4% wt/vol paraformaldehyde in 100 mM Na-cacodylate buffer, pH 7.4 for 30 min, and then cryoprotected in 30% wt/vol sucrose (dissolved in phosphate-buffered saline, PBS) for 2 h. Bladders were embedded in cryomolds (15 × 15 × 5 mm; Fisher Scientific) filled with Optimal Cutting Temperature (OCT) solution (Tissue-Tek, Sakura Finetek, Torrance, CA), and frozen on dry ice. The blocks were stored at −70°C in tightly sealed plastic bags. Cryosections were cut using a Leica Microsystems CM1950 cryostat (4-µm sections; Buffalo Grove, IL), and collected on Superfrost Plus glass slides (ThermoFisher Scientific, Pittsburgh, PA).
In most cases, sections were washed in PBS at room temperature and then unreacted fixative quenched by incubating the tissue slices for 10 min at room temperature with Quench Buffer (75 mM NH4Cl and 20 mM glycine, pH 8.0 dissolved in PBS, containing 0.1% vol/vol Triton X-100). However, in the case of samples that were incubated with anti-RAB8 antibody, the incubation in quench buffer was limited to 7 min, and then the tissue was incubated in quench buffer containing 0.05% wt/vol SDS for 3 min. The tissue was then quickly rinsed three times with PBS, and then three times for 5 min in the same buffer. All tissues were then incubated in Block Solution (PBS containing 0.6% vol/vol fish skin gelatin, 0.05% wt/vol saponin) for 60 min at ambient temperature. Following incubation, the Block Solution was aspirated, and replaced with primary antibodies diluted in Block Solution and incubated overnight at 4°C in a humid chamber. On the second day, the slides were washed three times quickly and three times for 5 min with Block Solution, and then incubated with minimal cross-reactivity, fluorophore-labeled secondary antibodies, diluted in Block Solution, for 1 h at ambient temperature. In some cases, nuclei were counterstained with TO-PRO-3 (1:1,000; ThermoFisher Scientific), and overall tissue architecture visualized using tetramethylrhodamine isothiocyanate (TRITC)-labeled phalloidin (1:200; ThermoFisher Scientific), which labels cortical actin filaments. The labeled tissues were then rinsed three times quickly and three times 5 min with Block Solution, rinsed with PBS, and then postfixed in 4% wt/vol paraformaldehyde dissolved in 100 mM sodium cacodylate buffer, pH 7.4 for 5–10 min at ambient temperature. The slides were rinsed with PBS, excess liquid aspirated, and a drop of SlowFade Diamond Antifade (Molecular Probes-ThermoFisher) was placed on the tissue. Borosilicate coverslips (1.5-, 0.17-mm thickness, 24 × 50 mm; ThermoFisher) were placed above the drop of mounting medium, excess mounting medium was removed by aspiration, the edges of the coverslip were sealed with clear nail polish, and the slides stored at −20°C until image acquisition was performed.
Images were captured using a Leica HCX PL APO 63X, 1.3 NA glycerol objective and the appropriate laser lines of a Leica TCS SP5 CW-STED confocal microscope (in normal confocal mode). The photomultipliers were set at 900–1,200, signal and offset were optimized using the Q-LUT option, and eight-bit images collected using eight-line averages combined with six-frame averages. Cross talk between channels was prevented by use of spectral detectors coupled with sequential scanning. Furthermore, we confirmed using TetraSpeck fluorescent microspheres (0.5 and 0.2 µm; Molecular Probes-ThermoFisher) that there was limited pixel shift in images captured in the visible light spectrum. Stacks of images (1,024 × 1,024, 8-bit) were collected using a Z-step of 0.13 μm. For general microscopy, images were imported into Volocity 4-D software (Perkin Elmers, Waltham, MA), and following image reconstruction, exported as TIFF files. The contrast of the latter was corrected in Photoshop CC2015 (Adobe, San Jose, CA), and the composite images were prepared in Adobe Illustrator CC2015.
Segmentation of digital images and measures of colocalization using the Squassh protocol.
Segmentation of digital image files was performed using an implementation of the Squassh (segmentation and quantification of subcellular shapes) protocol, an open-source Java plugin for ImageJ (NIH, Bethesda, MD) and Fiji software (39). The protocol combines user-settable parameters coupled with image denoising, deconvolution, and segmentation in 2D or 3D to detect objects, regardless of size and shape, and report parameters including intensity of objects, object number, size of objects, length of objects, and degree of colocalization based on overlap of object volumes. We used Fiji (version 2.0.0, downloaded from https://fiji.sc) and its automated update feature to download MosaicSuite (version July 2016) for ImageJ and Fiji (http://mosaic.mpi-cbg.de/?q=downloads/imageJ), which contains the Squassh protocol. Confocal image stacks (each composed of 5 images) were collected using identical gain and offset for each marker imaged and saved in Leica (.lif) format. The image files were subsequently converted to two-channel TIFF image files using the .lif extractor ImageJ macro (which must be loaded in Fiji using the Plugins → Macro → Install command and then subsequently implemented using the Plugins → Macro → Run command). If data for only single channels were needed, we used the Image → Color → Split Channels command, saving the resulting single-colored image as a TIFF file. If a masked region of the image was needed, then drawing tools were used to select those regions that would be masked and the Edit → Clear outside command was used (a black background was maintained using the Edit → Options → Colors → Background → Black command before the Clear outside command). The resulting image was then saved as a TIFF file.
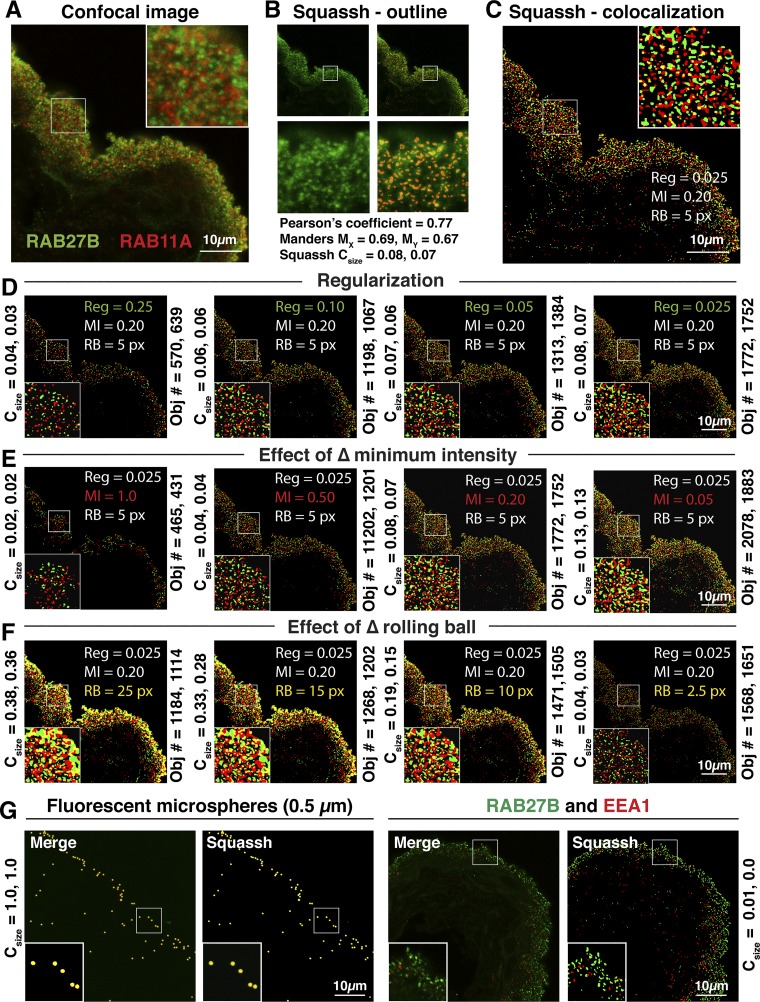
A series of trials was performed to determine the optimal settings for segmentation of DFVs. Parameters defined included the following: regularization, minimal intensity threshold, and background subtraction (using a rolling ball algorithm). This process was performed iteratively, changing one parameter at a time, until images were obtained in which visibly identifiable DFVs were automatically selected above background fluorescence and segmented from closely apposed neighbors (see Fig. 1, A–F). This was assessed by comparing sample images before processing with those images in which the outlines of segmented objects are superimposed on the original image, or in which the identified segmented objects are displayed (Fig. 1, B–C, and Supplemental Video S1; Supplemental Material for this article is available online at the Journal website). We used images of RAB27B with EEA1 as negative controls and fluorescent microspheres as positive controls (Fig. 1G).
Fig. 1.
Segmentation of discoidal- and/or fusiform-shaped vesicle (DFV) immunofluorescense using Squassh software. A: example confocal image showing the distribution of RAB27B and RAB11A in the urothelium. B and C: the image in A was processed using the following Squassh (segmentation and quantification of subcellular shapes) software parameters determined to be optimal to detect the vast majority of RAB-positive DFVs, well segmented from one another, in a large number of image files: regularization = 0.025, minimum intensity = 0.20, and background correction using a rolling ball = 5 px. In B the segmented objects in the RAB27B (green) channel are outlined (see panels at bottom for magnified view of boxed region in panel at top). Note that the outlined vesicles identified by the software are surrounded by low levels of nonvesicular fluorescence. Values for pixel-based measures of colocalization including Pearson’s correlation coefficient and Mander’s coefficients (both corrected using the automated Coste’s threshold routine) are calculated for the image in A using the JACoP plugin for ImageJ. These values indicate a higher degree of colocalization than is apparent from optical inspection of the image and are likely affected by the aforementioned nonvesicular fluorescence. In C the segmented RAB27B (green) and RAB11A (red) objects are depicted as a maximum projection, and regions of colocalization (i.e., those that have a 50% or greater overlap of their volumes) are shown in yellow. D–F: a series of trials showing the effects of changing the values for regularization, minimal intensity threshold, and background subtraction (i.e., rolling ball). The results of changing these parameters on the coefficient for colocalization (Csize) and the number of detected objects is found to the left and right of each panel, respectively. The parameter with the largest effect on segmentation was the value set for the rolling ball: too high, there was limited separation of DFVs from one another, and set too low, there was limited colocalization. The regularization or minimal intensity parameters affected the number of objects detected, with smaller values resulting in more objects detected. In addition, smaller values for these parameters increased the degree of colocalization, but at the expense of enlarging the objects and dramatically increasing the computer time needed to perform the analysis. G: we used images of RAB27B with EEA1 as negative controls and 0.5-µm fluorescent microspheres as positive controls for our analysis. The long axis of DFVs is ~0.5 µm. Values for Csize were determined using the parameters described for panels B and C.
The following scheme was ultimately used to process all image files in this manuscript. The Squassh protocol was initiated (Plugins → Mosaic → Segmentation → Squassh). After choosing the location of the image files on the computer (Apple PowerMac; multiple files are batch processed automatically by the software), the following parameters were input. Under “Background subtraction” a rolling ball = 5 was selected. For “Segmentation parameters,” the following options were chosen: Regularization (ch1/ch2) = 0.025; Minimal Intensity Threshold (ch1/ch2) = 0.20; PSF model standard deviation in XY = 1.44 pixels and in Z = 2.54 pixels (calculated using the following inputs: Emission = 519, Excitation = 496, Numerical aperture = 1.3, Refractive index of glycerol = 1.45, Pinhole = 1, Lateral pixel = 60.1 nm, Axial pixel = 125.9 nm); “Exclude Z-edge and Subpixal segmentation” = unchecked; Local intensity estimation = automatic; Noise model = Poisson; Region filter = none selected. For “Colocalization (two-channel images)” no options were selected. For “Visualization and output,” Intermediate steps, Save object characteristics, and Outlines overlay were checked, and in response to “set condition names and number of images per condition” we changed channel names as appropriate, and under condition we input the number of like image stacks in the batch. At the end of the analysis, the software generates a series of image files, as well as summaries of colocalization and object data parameters, in the following data files: stitch_ImageColoc.csv and stitch_ImageData.csv. These data files were opened in Microsoft Excel (Redmond, WA) using the import.csv option, and summary statistics were collated.
The coefficient for colocalization, Csize, describes the fraction of total volume occupied by objects in one channel that overlaps at least 50% with objects in the second channel. It is calculated as follows:
where X is the set of all objects detected in channel 1, and Y is the set of all objects detected in channel 2. The notation XY+ means the objects in channel one that share at least a 50% overlap of volume with objects in channel two. S0 is the size of object 0 in pixels, Σ0∈XS000 is the total size of colocalizing regions, and Σ0∈XS0 is the total number of pixels occupied by all objects in that channel. Csize(XY+/X) and Csize(YX+/Y) may be identical or not, as one channel may have a broader distribution than the other one in the sampled region of the tissue.
Preparation of adenoviruses, identification of shRNAs, and in situ transduction of umbrella cells.
Adenoviruses expressing hGH1, GFP, GFP-tagged DN-Rab11aS25N, GFP-tagged DA-Rab11aS20V, RFP-tagged DA-Rab8aQ67L, and GFP-tagged DN-Rab8aT22N were described previously (28, 29). To prepare hGH1-V5, cDNA encoding the entire coding region of hGH1, including signal sequence, fused in frame with the V5 epitope (GKPIPNPLLGLDST) was synthesized by Integrated DNA Technologies (Coralville, IA) as a double-stranded gBlock gene fragment. Restriction sites for BamHI and EcoRI were added at the 5′- and 3′-ends of the construct, respectively, which were used to subclone the cDNA into the pAd-Lox plasmid. Adenoviruses were prepared by the Viral Core Facility at the University of Pittsburgh.
To generate shRNAs, the following scheme was used. The iRNAi software (Nucleobytes.com) was used to search the rat RAB27A cDNA (Pubmed accession no. NM_017317.2), rat RAB27B cDNA (Pubmed accession no. NM_053459.1), or rat RAB11A cDNA (Pubmed accession no. NM_031152.2) for optimal targets (typically 21-bp in length). When possible, we sought sequences that were also found to be targets in mouse and humans in the Mission Library validated shRNA sequence library (www.sigmaaldrich.com). Four shRNA sequences were selected for each cDNA (Table 1). The top and the bottom strands were individually synthesized (Integrated DNA Technologies) and annealed by mixing the two strands in equal amounts, heating to 94°C, and cooling gradually to room temperature. The annealed shRNA sequences were then ligated into the pAd-Loss vector (25). The ability of the different shRNA sequences to silence the Rab of interest was determined by cotransfecting HEK293-T cells with the pAD-Loss vectors and the corresponding cDNA: rat RAB27A, which was obtained from OpenBiosystems and subcloned into pEGFP-C3; RAB27B cDNA, which was amplified from cDNA prepared from rat urothelial lysate. RAB27B was then subcloned into pcDNA3.1; RAB11A cDNA was obtained from OpenBiosystems. Seventy-two hours later, cells were lysed, the proteins were resolved by SDS-PAGE, and RAB27A/B or RAB11A were detected by Western blotting using the techniques described above. One of each shRNA was selected for further analysis: RAB27A-shRNA-2, RAB27B-shRNA-a, and RAB11A-shRNA-1. Adenoviruses were prepared by the Viral Core Facility at the University of Pittsburgh.
In situ transduction was performed as described previously (29). Briefly, rats were sedated with 2% isoflurane and a 22-g Jelco IV catheter (Smith Medicals, Southington, CT), trimmed to 2-cm length, was introduced into the bladder via the urethra. The bladder was rinsed with PBS and filled with 400 μl of 0.1% wt/vol dodecyl-β-d-maltoside dissolved in PBS. The urethra was clamped, and after 5 min unclamped to allow the detergent to void. The latter step was facilitated by applying slight pressure to the lower abdomen. The bladder was filled with 400 μl PBS containing adenoviruses expressing the constructs described above (2.0 × 108 infectious virus particles, typically in a volume of 2–10 µl for each virus). The amount of scrambled shRNA-expressing virus particles used was equal to the amount of specific shRNA virus particles used. The bladder was then clamped. After 30 min, the clamp was removed and the virus solution was allowed to void. The bladder was rinsed with PBS, anesthesia was discontinued, and the rats were allowed to revive. The rats were euthanized 2–4 days posttransduction.
Measurement of hGH1-V5 secretion in ex vivo bladder preparation.
Rat bladders were transduced in situ with adenoviruses expressing hGH1-V5 in combination with scrambled shRNA or RAB-specific shRNA as described above. Thirty-six hours postinfection, the animals were euthanized by inhalation of CO2, and the bladder along with the urethra were excised. A 5-ml syringe, filled with Krebs buffer warmed to 37°C, was attached to the Luer port of a 22-g Jelco catheter, which was trimmed to 2 cm in length. The catheter was gently inserted into the urethra, and the bladder was washed by slowly injecting 400 µl of Krebs buffer (gassed with 5% vol/vol CO2-95% vol/vol O2) through the catheter, allowing the buffer to flow out of the bladder from the space between the catheter and urethral orifice. The rate of flow was adjusted so that the bladder did not appear to fill during the washing protocol. The catheter was then secured in place by placing a ligature around the urethra using silk suture material (Softsilk 6-0 suture, Covidien-Medtronic, Minneapolis, MN). The bladder was then incubated, undisturbed, for 30 min, submerged in a Petri dish, filled with Krebs buffer, and placed on a heating plate to maintain temperature at 37°C.
Subsequently, a syringe was inserted in the clamp of a model PhD Ultra syringe pump (Harvard Apparatus, Holliston, MA) and the bladder was slowly filled with 500 µl of Krebs buffer at a rate of 15 µl/min. The bladder was then allowed to incubate for an additional 30 min at 37°C before a 22-gage needle, attached to a 5-ml syringe, was inserted through the bladder wall into the bladder lumen and all of the contents aspirated and then transferred to an Eppendorf tube, which was placed on ice. The bladder was then filled with 250 µl of Krebs buffer (at a rate of 50 µl/min), the bladder contents were aspirated with a needle and then pooled with the original lumen contents. The bladder was subsequently cut open along the midline, pinned out on a rubber mat, and rinsed with Krebs buffer, which was then removed by aspiration. A urothelial lysate was prepared by adding 65 µl of lysis buffer to the mucosal surface of the bladder and collecting the lysate with a yellow tip attached to a P200 Gilson Pipetman. This process was repeated one time, and the two lysates combined. The lysates were then shaken 20 s at ambient temperature in a FastPrep-24 device to shear DNA.
The hGH1-V5 that was secreted (i.e., present in the bladder contents) or remaining cell associated (i.e., present in urothelial cell lysate) was recovered by immunoprecipitation using the following protocol. The pooled bladder contents containing secreted hGH1-V5 were mixed with 30 µl of Lysis buffer, 30 µl of 2.5% vol/vol TX-100, and 15 µl of anti-V5 agarose beads (Sigma-Aldrich). The cell-associated fraction of hGH1-V5 was measured by taking one-quarter of urothelial lysate (typically 30 µl) and mixing it with 500 µl Krebs buffer, 30 µl of 2.5% vol/vol TX-100, and 15 µl of anti-V5 agarose beads. The reactions were incubated overnight at 4°C on a Labquake rotator (Barnstead/Thermolyne, Dubuque, IA), the anti-V5 beads were recovered by a centrifugation for 1 min at 14,000 g in a microfuge, washed with Krebs buffer, and then recovered again by centrifugation. The washed beads were resuspended in 15 µl of 2× Laemmili sample buffer, heated at 99°C for 5 min, and processed for Western blotting as described above.
Following image capture using the Chemidoc Touch Imaging System, the fraction of hGH1 secreted was quantified as follows. The optical density (OD) of hGH1-V5 was determined by drawing rectangles using the ImageLab Software (Bio-Rad) volume tool. Rectangles of the same size, but in regions of the gel lacking signal, were drawn to correct for background intensity. The background corrected OD signal of the bands corresponding to the bladder tissue lysate were multiplied by 4 to correct for the amount of lysate used in the immunoprecipitation. The ODs of total tissue-associated hGH1-V5 and secreted hGH1-V5 were summed up, constituting the total hGH1-V5 expressed in a given bladder. The amount of secreted hGH1-V5 was calculated as the percentage of hGH1-V5 secreted with respect to the total amount expressed using the following equation:
This served to normalize the amount of secretion to the level of hGH1-V5 expressed in the umbrella cell layer, and was important to mitigate experiment-to-experiment variations in hGH1-V5 expression and detection of this construct.
Statistical analysis.
Data are reported as means ± SE. Statistically significant differences between means were determined using a two-tailed Student’s t-test; P < 0.05 was considered statistically significant. One-way ANOVA, with Bonferonni’s correction, was used when making multiple comparisons.
RESULTS
Both RAB27A and RAB27B are expressed in rat urothelium.
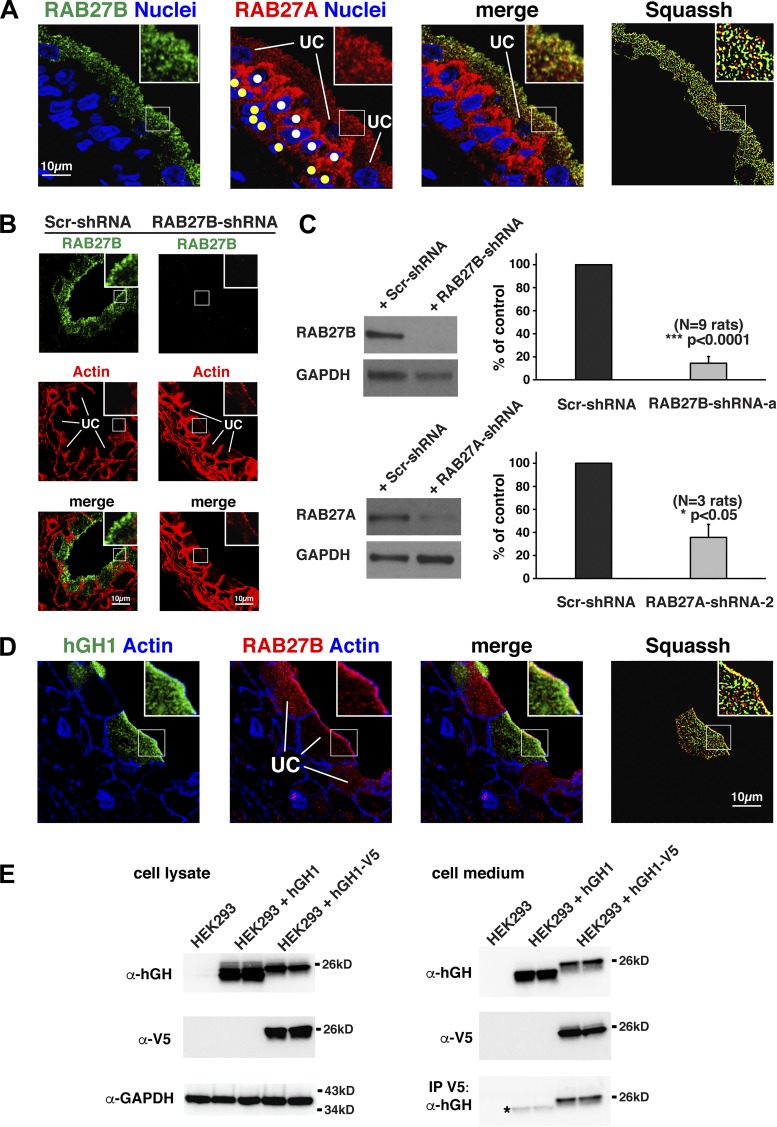
In the mouse, RAB27B is reported to be expressed in the umbrella cell layer where it is associated with DFVs, whereas RAB27A is reported to be absent (10, 48). However, in rat urothelium we observed expression of both RAB27 isoforms. This was initially established using reverse-transcriptase PCR (RT-PCR), which revealed that message for both RAB27A and RAB27B were present in rat urothelial cDNA (Fig. 2A). In addition, we probed Western blots of urothelial lysates with nominally isoform-selective antibodies against RAB27A or RAB27B, both of which recognized ~25 kDa proteins (Fig. 2A). We confirmed the specificity of these antibodies by probing Western blots of lysates prepared from HEK cells expressing GFP-RAB27A or GFP-RAB27B. The RAB27A antibodies reacted with GFP-RAB27A, but not GFP-RAB27B, and the RAB27B-selective antibodies we used reacted with GFP-RAB27B, but much less so with GFP-RAB27A (Fig. 2A).
Fig. 2.
Expression of RAB27A and RAB27B in rat urothelium. A: panels at left: RT-PCR was used to demonstrate expression of RAB27A and RAB27B in rat urothelial cDNA, and Western blotting was used to confirm RAB27A/B protein expression in urothelial lysates; panels at right: GFP-RAB27A or GFP-RAB27B were expressed in HEK cells, and the specificity of the RAB27A- and RAB27B-specific antibodies confirmed by Western blot analysis. B: distribution of RAB27B, nuclei (labeled with lamin-B), and actin (labeled with tetramethylrhodamine isothiocyanate-phalloidin) in rat urothelium. The position of the umbrella cells (UC), intermediate cells (white-colored circles), and basal cells (yellow-colored circles) are indicated. Images are projections of a confocal Z series. C: distribution of RAB27B, actin, and KRT20 in rat urothelium. Images are projections of a confocal Z series. D: distribution of RAB27B and UPK3A in rat urothelium. Images are projections of a confocal Z series. The image to the right shows the Z stack after processing with Squassh segmentation software. The segmented objects (RAB27B = green and UPK3A = red) are shown at maximum intensity, and yellow areas indicate regions of colocalization (≥50% overlap of volumes). E: distribution of MYO5A and RAB27B in rat urothelium. The results of Squassh segmentation are shown in the panel at far right.
We next immunolocalized RAB27B in rat urothelium. The three layers of this stratified epithelium were identified by costaining with rhodamine-labeled phalloidin, which labels the cortical actin cytoskeleton. These layers include a superficial layer populated by large umbrella cells (~40 µm in diameter in these samples), one to two of smaller intermediate cells (~10–15 µm in diameter), and a single layer of basal cells (~10 µm in diameter), which rest on the basement membrane (Fig. 2B). We note that the urothelium, and the umbrella cell layer in particular, is highly deformable and can change its shape depending on the degree of filling or stretch applied before fixation, thus the variation in tissue architecture we sometimes observe. Within individual umbrella cells, RAB27B was concentrated in the apical cytoplasm (Fig. 2B). Less RAB27B was expressed in the underlying intermediate and basal cell layers.
Just below the subapical membrane actin cytoskeleton is a so-called “trajectorial network” of cytokeratins (including KRT20), which encase DFVs and which may modulate their traffic toward the apical pole of the cell (46). We previously observed that whereas RAB11A-positive vesicles were mostly encased within the cytokeratin network, a population of RAB8A-positive vesicles were found past this network, and thus closer to the apical surface (28). Like the distribution of the latter, we observed that a population of RAB27B-positive vesicles also extended past this network (Fig. 2C), consistent with a recent report performed in mouse tissues (48). Because these RAB27B vesicles are very close to the apical membrane, they may be primed to undergo rapid fusion in response to stretch. We emphasize, however, that the localization of RAB27B-positive vesicles varied across the urothelium and these vesicles were also found deeper in the cytoplasm, including in and below the cytokeratin network.
The major cargoes of DFVs include the uroplakins (UPKs), which assemble into 16-nm-asymmetric unit membrane particles sometimes after exiting the endoplasmic reticulum (49). We observed some coincidence between the RAB27B and uroplakin signals when merged images were examined by eye (Fig. 2D). This is consistent with a report by Chen et al. (10), who demonstrated that RAB27B is localized to uroplakin-rich DFVs by immunoelectron microscopy. To quantify the degree of overlap in fluorescent signals, we previously used pixel-based methods of colocalization, but these can lead to overestimates of the degree of overlap as tissue sections are prone to higher levels of background staining than that observed in tissue culture cells, and RABs have both membrane-bound and cytosolic pools. For these studies, we used an automated segmentation software to detect individual vesicles in 3D and then to subsequently measure their degree of colocalization (assessed by measuring the overlap of volumes, see materials and methods for Squassh segmentation analysis). Using these techniques, we observed that ~12% of the RAB27B-positive vesicular structures colocalized with UPK-positive ones (Table 2), which is visibly similar to the degree of colocalization recently reported by Wankel et al. (48) (but not quantified). This degree of colocalization is relatively modest, likely reflecting the stringent conditions of colocalization we employed and reports that UPKs (assembled into plaques) are also found in endocytic structures (1, 20, 40).
Table 2.
Degree of colocalization measured for the indicated pair of markers using Squassh segmentation software
| Colocalization Pair (XY+/X or YX+/Y) | Csize | n |
|---|---|---|
| RAB27BUPK/RAB27B | 0.12 ± 0.00 | 3 |
| UPKRAB27B/UPK | 0.05 ± 0.01 | 3 |
| RAB27BRAB27A/RAB27B | 0.40 ± 0.02 | 4 |
| RAB27ARAB27B/RAB27A | 0.45 ± 0.03 | 4 |
| RAB27BMYO5A/RAB27B | 0.16 ± 0.01 | 3 |
| MYO5ARAB27B/MYO5A | 0.12 ± 0.01 | 3 |
| RAB27BHGH1/RAB27B | 0.40 ± 0.06 | 4 |
| HGH1RAB27B/HGH1 | 0.30 ± 0.02 | 4 |
| RAB27BRAB11A/RAB27B | 0.096 ± 0.02 | 4 |
| RAB11ARAB27B/RAB11A | 0.06 ± 0.00 | 4 |
| RAB27BRAB8A/RAB27B | 0.22 ± 0.05 | 4 |
| RAB8RAB27B/Rab8A | 0.23 ± 0.03 | 4 |
| RAB27BEEA1/RAB27B | 0.01 ± 0.00 | 4 |
| EEA1RAB27B/EEA1 | 0.03 ± 0.00 | 4 |
| Fluorescent spheres | 1.0 ± 0.0 | 1 |
Values are means ± SE; n, no. of rats, with ≥3 random images analyzed from each animal.
We also measured the degree of overlap between RAB27B and its well-known bind partner MYO5A (14), an unconventional myosin motor that promotes RAB27B-dependent vesicle motility. We previously demonstrated that message for MYO5A (and MYO5B) is expressed in the urothelium (28), and in this study we observed that MYO5A was associated with vesicular elements distributed within the umbrella and underlying cell layers (Fig. 2E). Upon masking the non-umbrella cell-associated signal, we observed that RAB27B exhibited a modest degree of overlap with MYO5A (~16%; Table 2), which is consistent with recent observations (48).
Next, we assessed the distribution of RAB27A, and then quantified the degree of overlap between the RAB27A and RAB27B signals. Whereas the majority of RAB27B staining was concentrated in umbrella cells, and not in the underlying cell layers, the opposite was true for RAB27A: the RAB27A signal was concentrated in vesicular elements that populated the cytoplasm of intermediate and basal cells (marked with yellow and white circles, respectively, in Fig. 3A). Within umbrella cells, the distribution of RAB27A was similar to RAB27B. In either case, RAB27A/B was associated with small vesicular elements that accumulated under the apical surface. On masking out the non-umbrella cell signal, we estimated that ~40% of the umbrella cell RAB27B vesicle pool colocalized with RAB27A (Table 2).
Fig. 3.
Localization of RAB27A, characterization of RAB27A and RAB27B shRNAs, and confirmation of hGH1-V5 secretion. A: distribution of RAB27B, nuclei (labeled with TO-PRO-3), and RAB27A in rat urothelium. The position of the umbrella cells (UC), intermediate cells (white-colored circles), and basal cells (yellow-colored circles) are indicated. Images are projections of a confocal Z series. The image to the right shows the Z stack after processing with Squassh segmentation software. The segmented objects (RAB27B = green and RAB27A = red) are shown at maximum intensity, and yellow areas indicate regions of colocalization (≥50% overlap of volumes). B: rat bladders were transduced with scrambled (Scr)-shRNA or RAB27B-shRNA-a, and the distribution of RAB27B and actin were assessed in fixed urothelial tissues. The position of umbrella cells (UC) are indicated. Images are projections of a confocal Z series. C: panels at left: rat bladders were transduced with the indicated shRNA, and lysates probed with antibodies specific for RAB27A, RAB27B, or GAPDH (loading control); panels at right: quantification of the amount of RAB27B (top) or RAB27A (bottom) expression in bladders transduced with RAB27B-shRNA-a, RAB27A-shRNA-2, or scrambled (Scr)-shRNA. D: rat bladders were transduced with adenovirus encoding hGH1, and the distribution of hGH1, RAB27B, and actin assessed in fixed urothelium. Images are projections of a confocal Z series. The image to the right shows the masked image after processing with Squassh segmentation software. The segmented objects (hGH1 = green and RAB27B = red) are shown at maximum intensity, and yellow areas indicate regions of colocalization (≥50% overlap of volumes). E: HEK293 cells were transfected with hGH1 or hGH1-V5. The cells were incubated overnight at 37°C, and the presence of hGH1 or the V5 epitope tag in the cell lysate or cell medium (secreted) was detected by Western blot analysis. The hGH1-V5 was immunoprecipitated from the medium using anti-V5 antibodies and detected using antibodies against hGH1. The asterisk shows background binding of hGH1 under the washing protocol used in our assay.
RAB27B, but not RAB27A, regulates DFV exocytosis.
As RAB27B was associated with at least a subset of DFVs, we next sought to determine if RAB27B regulated DFV exocytosis. We first identified a series of RAB27B-specific shRNAs, one of which was effective at downregulating RAB27B expressed in HEK cells (RAB27B-shRNA-a). This shRNA was expressed in the urothelium using adenoviral transduction (38), a method we have employed to express proteins and shRNAs in the urothelium (28–30, 34, 36). This technique has high transduction efficiency (75–95%), only the umbrella cell layer is targeted, and transduction does not perturb the barrier function or differentiation of the urothelium. Using these methods, we observed that RAB27B-shRNA-a reduced endogenous RAB27B expression as assessed by immunofluorescence (Fig. 3B) or by Western blotting (Fig. 3C). In the latter case, expression was inhibited by ~90%.
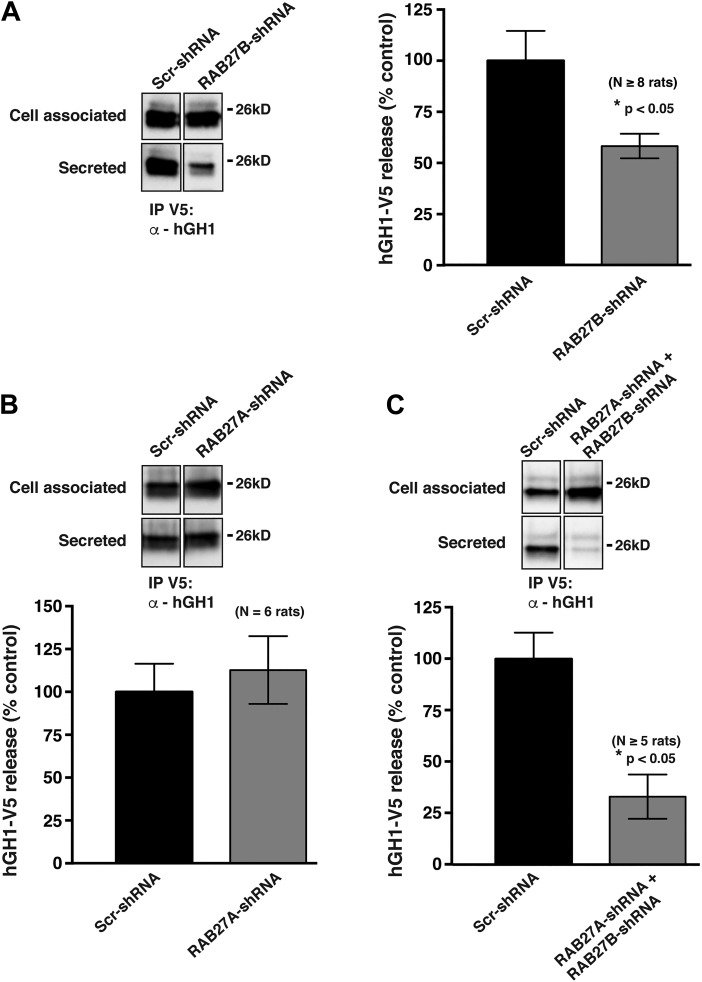
To measure exocytosis, we took advantage of previous findings that when human growth hormone (hGH1) is expressed in the urothelium of transgenic mice, or in the urothelium of rats transduced with an adenovirus encoding hGH1, it is packaged into DFVs and released (exocytosed) into the bladder lumen in response to bladder filling (26, 29). No release is observed in the absence of stretch, indicating that there is little constitutive secretion of hGH1 in these cells (36). We confirmed that RAB27B colocalized with a subset of hGH1-labeled DFVs, particularly with those near the apical membrane (Fig. 3D and Table 2). To facilitate the ease by which exocytosed hGH1 is recovered from the extracellular fluid, we generated a construct in which a V5 epitope tag was fused in frame to the C terminus of hGH1 (hGH1-V5). This allowed for the recovery of hGH1-V5 released into extracellular fluid by way of immunoprecipitation (Fig. 3E). Finally, we used an ex vivo whole bladder preparation, which when transduced can be slowly filled with buffer and hGH1-V5 recovered from the urinary space (secreted) or from the cell-associated pool. Using these techniques, we observed that RAB27B-shRNA caused an ~45% reduction in the amount of hGH1-V5 released from the urothelium into the urinary space of transduced bladders (Fig. 4A).
Fig. 4.
Inhibition of hGH1-V5 release by transduction with adenovirus encoding RAB27B-shRNA-a. Rat bladders were transduced with adenoviruses encoding hGH1-V5 and either scrambled (Scr)-shRNA, RAB27B-shRNA-a alone (A), RAB27A-shRNA-2 alone (B), or both RAB27B-shRNA-a and RAB27A-shRNA-2 (C). Bladders were excised, filled with buffer, and the amount of hGH1-V5 released in the bladder lumen (secreted) or remaining cell associated was immunoprecipitated and detected by Western blot analysis using an anti-hGH1 antibody. Each experiment included samples from rat bladders transduced with scrambled (Scr)-shRNA and either RAB27A-specific shRNAs and/or RAB27B-specific shRNA. The samples were processed side-by-side and Western blots developed simultaneously for each experiment. The total amount of secreted hGH1-V5 and its corresponding cell-associated fraction (1/4th of the total) are shown for each condition.
We also identified a RAB27A-specific shRNA (RAB27A-shRNA-2), which significantly decreased RAB27A expression in HEK cells and in rat urothelium (Fig. 3C and data not shown). However, unlike the RAB27B-specific shRNA, the RAB27A-specific one had no significant effect on the release of hGH1-V5 in our exocytosis assay (Fig. 4B). We also tested the effect of simultaneously treating umbrella cells with both RAB27A- and RAB27B-specific shRNAs (Fig. 4C). Although the combined shRNAs resulted in what appeared to be a greater degree of inhibition, this effect was not significantly different than treatment with RAB27B-shRNA alone. Taken together, these results indicate that whereas a fraction of urothelial-associated RAB27A is expressed in the umbrella cell layer, only depletion of RAB27B significantly impairs exocytosis.
Neither RAB11A nor RAB8A regulate the size, number, or distribution of RAB27B-positive DFVs.
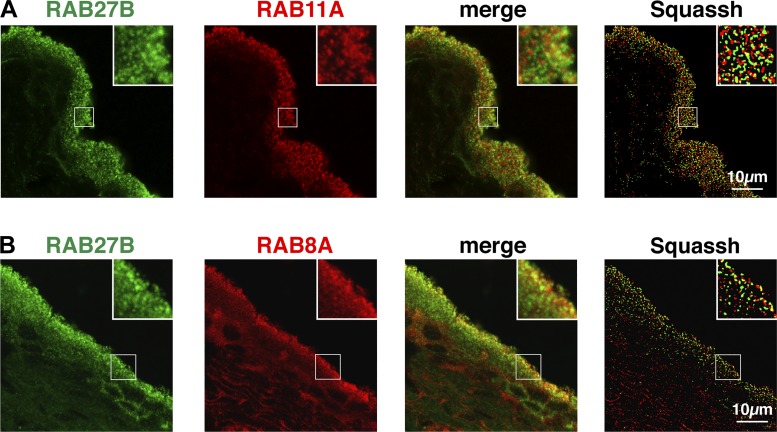
Based in large part on the limited, visual overlap of their distributions in mouse tissue, it was proposed that RAB11A and RAB8A act upstream of RAB27B (48). We too observed a small degree of overlap between the signal for RAB27B and the signals for RAB11A or RAB8A in rat tissues (Fig. 5 and Table 2).
Fig. 5.
Distribution of RAB27B, RAB11A, and RAB8A in rat urothelium. A and B: localization of RAB27B and either RAB11A (A) or RAB8A (B). Images are projections of confocal Z series. The image to the right shows the Z stack after processing with Squassh segmentation software. The segmented objects (RAB27B = green and RAB11A/RAB8A = red) are shown at maximum intensity, and yellow areas indicate regions of colocalization (≥ 50% overlap of volumes).
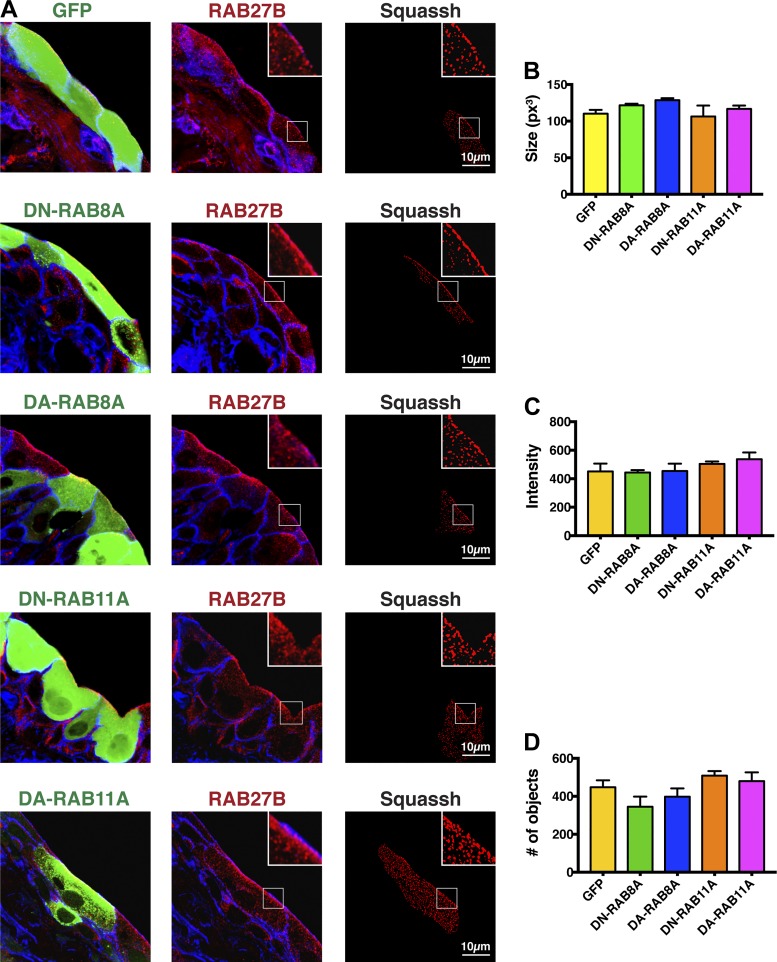
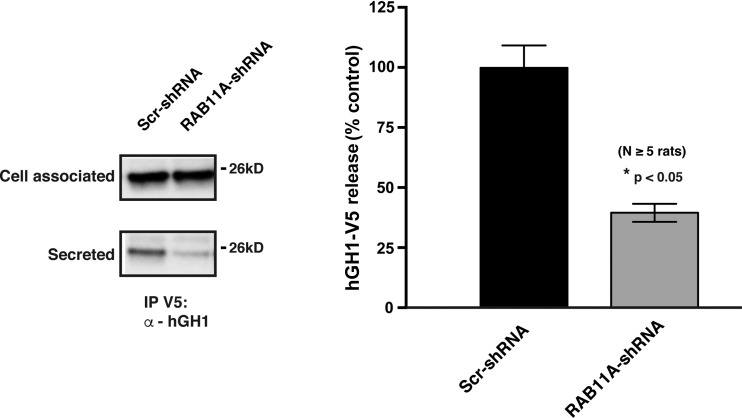
We posited that if RAB11A-RAB8A acts upstream of RAB27B, then expression of GDP-locked, dominant-negative mutants of RAB11A or RAB8A should affect the distribution, size, number, and/or intensity of RAB27B-positive vesicles. In contrast, expression of GTP-locked, dominant-active mutants may have the opposite effect. However, when compared with expression of GFP alone (control), expression of either dominant-negative RAB8A or dominant-negative RAB11A had no obvious effect on the distribution of RAB27B in umbrella cells: RAB27B retained its vesicular appearance and the vesicles accumulated under the apical surface (Fig. 6A). Similarly, expression of dominant-active RAB8A or dominant-active RAB11A also had no obvious impact on the distribution of RAB27B. To further confirm these qualitative observations, we quantified the average size of RAB27B-labeled vesicles, the average fluorescent intensity of these vesicles, and the number of these vesicles in the confocal Z stack. However, none of these parameters were significantly different from GFP-transduced controls, or from each other (Fig. 6, B–D). Similarly, downregulating RAB11A expression using a RAB11A-specific shRNA did not affect the size, average intensity, or number of RAB27B vesicles (Fig. 7, A and B). We note that downregulating RAB11A-specific shRNA also caused a significant decrease in hGH1-V5 secretion (Fig. 8). Unfortunately, we could not reliably detect whether the loss of both RAB11A and RAB27B caused an additive effect as the umbrella cell layer was morphologically impaired by this treatment. We also asked whether RAB27B governs any qualitative or quantitative aspect of RAB11A-labeled vesicles. When compared with umbrella cells transduced with scrambled shRNA, those transduced with RAB27B-shRNA exhibited no significant change in the distribution of RAB11A, or the size, intensity, or number of RAB11A-positive vesicles (Fig. 7, C and D). Taken together, our results appear to argue against RAB8A or RAB11A acting upstream of RAB27B, or that RAB27B acts upstream of RAB11A.
Fig. 6.
Effect of expressing mutants of RAB8A and RAB11A on the distribution, size, intensity, and number of RAB27B vesicles. Bladders were transduced with adenoviruses encoding GFP alone (control), or GFP-tagged dominant-negative (DN) RAB8A, or RFP-tagged dominant-active (DA) RAB8A, GFP-tagged DN-RAB11A, or GFP-tagged DA-RAB11A. A: confocal analysis. The column at left shows expression of the indicated protein (green), the column at middle shows the distribution of RAB27B in the tissue (red), and the column at right shows the Z stack after cell masking and Squassh segmentation analysis of the RAB27B channel. Summary statistics for the size (B), intensity (C), and number (D) of RAB27B vesicles in cells expressing the indicated protein. None of the values are significantly different from GFP controls or from one another (assessed using ANOVA).
Fig. 7.
Effect of depleting RAB11A or RAB27B on the distribution, size, intensity, and number of DFVs. Bladders were transduced with adenoviruses encoding scrambled (Scr)-shRNA, RAB11A-shRNA-1, or RAB27B-shRNA-a. A: confocal images showing the distribution of RAB27B and RAB11A in transduced urothelial tissue. The nonmucosal surfaces of the urothelium were labeled using an antibody to CLD4. B: summary statistics for the size, intensity, and number of RAB27B-positive vesicles in cells transduced with the indicated shRNA. None of the values are significantly different from scrambled (Scr)-shRNA controls (assessed using t-tests). C: confocal images showing the distribution of RAB27B and RAB11A in transduced urothelial tissues. The cortical actin cytoskeleton of the urothelium was labeled using rhodamine phalloidin. D: summary statistics for the size, intensity, and number of RAB11A-positive vesicles in cells transduced with the indicated shRNA. None of the values are significantly different from scrambled (Scr)-shRNA controls (assessed using t-tests).
Fig. 8.
Inhibition of hGH1-V5 release by transduction with adenovirus encoding RAB11A-shRNA-1. Rat bladders were transduced with adenoviruses encoding hGH1-V5 and either Scr-shRNA or RAB11A-shRNA-1. The amount of secreted hGH1-V5 is shown.
DISCUSSION
Exocytosis plays important roles in umbrella cell function and bladder physiology. During bladder filling, it allows the umbrella cell to increase its surface area, thus maintaining the urothelial barrier in the face of increasing fluid volume (27). Exocytosis also regulates the sensory and transducer functions of the urothelium by regulating the surface content of receptors and channels at the apical surface of the umbrella cells, the release of mediators such as ATP, and by governing tension in the apical membrane (27, 35, 47). While receptors and channels allow the urothelium to sense its extracellular milieu, the release of neurotransmitters including ATP allows the urothelium to communicate these changes to underlying afferent nerve processes, interstitial cells, and the detrusor (2). Regulation of tension determines how the urothelium will respond to wall tension as the bladder fills and empties (35). Thus understanding how exocytosis in umbrella cells is modulated has broad implications for our understanding of bladder function. Including our present studies, we now know that RAB8A, RAB11A, RAB27A, and RAB27B are expressed in the urothelium and that all of these are associated in part with DFVs, an abundant population of vesicles that fills the apical cytoplasm of umbrella cells. Moreover, we previously demonstrated that RAB8A and RAB11A, acting in conjunction with MYO5B, regulate DFV exocytosis (28, 29).
Despite the undisputable evidence that RAB27B is associated with DFVs (10, 48), an open question is what is the function of RAB27B, if any, in DFV exocytosis. Qualitatively, the umbrella cells of RAB27B knockout (KO) mice appear to have fewer DFVs than control mice (48). However, if DFV exocytosis depends on RAB27B, then one might expect that the umbrella cells from RAB27B KO mice would accumulate more DFVs in their cytoplasm, not fewer. It was hypothesized by Wankel et al. (48) that crinophagy, an autophagy pathway that degrades excess secretory vesicles, accounts for this loss in vesicle number. However, in our studies we did not note a reproducible loss of hGH1-labeled DFVs in rat bladders treated with RAB27B shRNA (as assessed by Western blot analysis). These dissimilar findings may reflect the different animal species employed in these two studies, or differences associated with the complete loss of RAB27B expression in KO mice vs. downregulation of RAB27B expression by shRNA in rat bladders.
By using methods that allow us to label DFVs and measure exocytosis in whole bladders, we now show that umbrella cell exocytosis is dependent, at least in part, on RAB27B. We also observe that a pool of RAB27A is expressed in the umbrella cell layer (of the rat urothelium), where it has a similar distribution to RAB27B. This is different from mice, which apparently do not express RAB27A in the urothelium (10, 48). However, and unlike RAB27B, we find no significant effect of downregulating umbrella cell RAB27A in our assays of exocytosis. Although there is a trend toward increased inhibition when umbrella cells are transduced with both RAB27A-shRNA and RAB27B-shRNA, the effect is not significantly different than treatment with RAB27B-shRNA alone. The function of RAB27A in the urothelium remains to be described, although its expression in the intermediate and basal cells makes it likely that it is regulating exocytosis in these cell layers.
As we have now identified three RABs that regulate DFV exocytosis, what is the relationship of RAB27B to the RAB11A-RAB8A network? It was recently proposed that RAB11A and RAB8A are acting upstream of RAB27B (48). Indeed, such a relationship between these RABs has been described in MDCK cells undergoing de novo lumen formation during cystogenesis (16). However, in our studies we cannot find a clear link between RAB27B and the RAB11A-RAB8A module. Expression of either dominant-active or dominant-negative mutants of RAB11A or RAB8A, or downregulating RAB11A expression in the umbrella cell layer, has no obvious impact on the distribution or size, intensity, or number of RAB27B-labeled DFVs. This lack of effect is unlikely to be the result of insufficient time of expression of these mutants, as we performed these assays after several days of transduction, sufficient time to significantly impair stretch-regulated exocytosis in umbrella cells. Moreover, RAB27B DFVs are unlikely to have a long half-life as they are exocytosed during each filling of the bladder, and on voiding the majority of apical membrane is internalized and degraded in lysosomes (30, 45). We also observe that downregulating RAB27B expression using shRNAs does not impact the distribution or size, intensity, or number of RAB11A-labeled DFVs. Taken together, these results argue against a simple relationship between RAB11A-RAB8A and RAB27B.
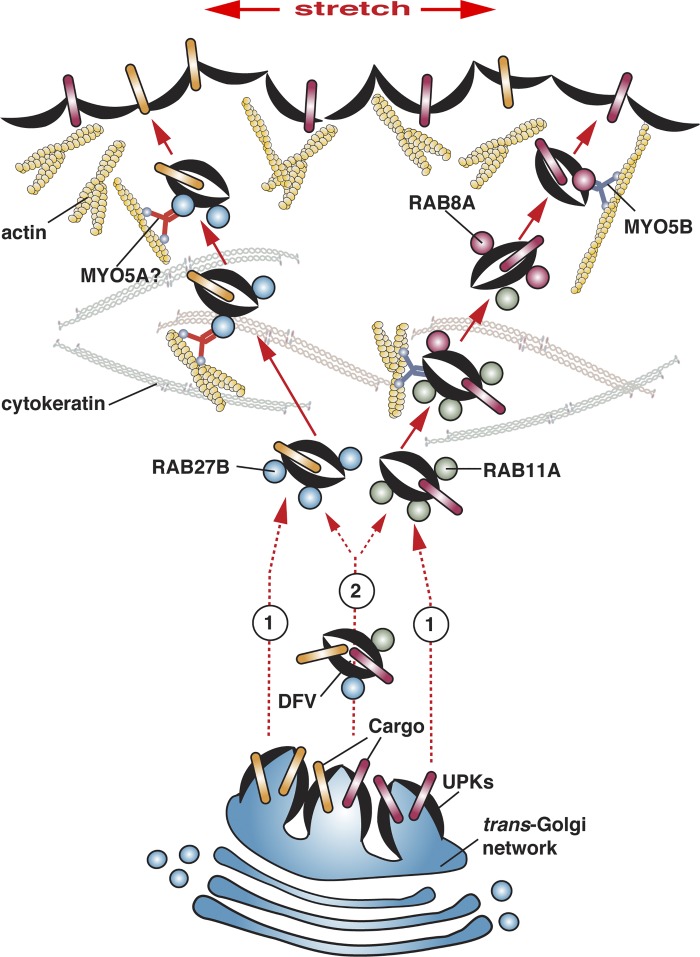
Instead, we hypothesize that umbrella cells may have at least two apically directed pathways: one regulated by RAB11A-RAB8A and the other pathway regulated by RAB27B (Fig. 9). The site of biogenesis of these distinct populations of DFVs is unknown but may occur as early as the trans-Golgi network (Fig. 9, option 1). In epithelial cells, there is ample precedence for having multiple apically directed pathways, a mechanism that has presumably evolved to allow the cell to differentially modulate the surface expression of functionally distinct classes of apical proteins (3, 4). The cargoes and associated regulatory molecules that are associated with these putative distinct populations of DFVs remain to be described; however, the presence of an asymmetric unit membrane (a feature of membranes containing UPKs assembled into 16-nm particles) in most if not all DFVs indicates that UPKs may be present as cargoes in all cases (19, 20). One possibility is that the exocytosis of mediators such as ATP may be differentially regulated than the exocytosis of membrane proteins such as UPKs or channels and receptors; however, nothing is known in this regard. Interestingly, we have described two differentially regulated pathways for exocytosis in umbrella cells (5, 37, 51): one is called the “early stage,” and it requires the activity of stretch-sensitive ion channels and Ca2+; the other is called the “late stage,” and is characterized by its requirements for ADAM17-dependent HB-EGF cleavage, EGF receptor transactivation, MAPK activation, and protein synthesis. Perhaps, each of these distinct exocytic pathways requires a different subset of RABs.
Fig. 9.
Model for RAB-dependent DFV exocytosis in bladder umbrella cells. See text for explanation.
If the umbrella cell has different populations of DFVs, then why is there any colocalization of RAB27B with RAB11A or RAB8A? One possibility, is that all three GTPases are recruited during an early step in the formation and/or maturation of DFVs, but then are subsequently sorted from one another as distinct RAB11A-RAB8A DFVs and RAB27B DFVs are generated (Fig. 9, option 2). However, it is also plausible that during DFV formation there is a significant degree of cargo and possibly Rab mis-sorting. This may be true of other cell types as well, as a large number of Rabs are associated with purified secretory granules (7, 9, 43).
In addition to cargoes (and RABs), it is expected that each population of DFVs will have a trafficking machinery that is specialized for the vesicle population at hand. In this regard, we know that the RAB11A-RAB8A cascade depends on MYO5B, which likely promotes transit across the subapical actin barrier (28). RAB27B DFVs will also likely require a myosin motor to pass the actin barrier, and the most likely candidate is MYO5A, which is a well-known effector of RAB27B and which we and Wankel et al. (48) recently showed is expressed in the urothelium and shows some overlap with RAB27B (Fig. 9) (28). It is worth noting that RAB8A, and possibly RAB11A, can also bind to MYO5A (33, 42), which could explain the broader distribution of MYO5A vs. RAB27B in the umbrella cell layer. Interestingly, Wankel et al. (48) also described a number of SNAREs and RAB27B effector molecules that are expressed in the umbrella cell layer. If these molecules are associated with all classes of DFVs or just a subset, how they modulate apical exocytosis will require additional experimentation.
Finally, understanding stretch-induced exocytosis in epithelial cells requires that we gain a better understanding of not only the RABs involved, but also their associated GTPase-activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs), both of which are likely to be central to the mechanotransduction cascades that couple stretch to exocytosis. At present, there is limited, if any, information about the RAB-specific GAPs and GEFs that function in umbrella cells. GAPs have been described for RAB3A, RAB8A, RAB11A, and RAB27B (22, 41, 50), including the recently described RAB11A GAP TBC1D9B (15). Moreover, GEFs are known for RAB3A, RAB8A, and RAB27B (12, 18, 21), but no GEF for vertebrate RAB11A has been described. We do know that RAB3IP (aliases Rabin8/Rabin3), a GEF for RAB8A (18), is expressed in umbrella cells (28). Moreover, expression of wild-type RAB3IP or a dominant-negative variant of this protein that lacks GEF activity (Rabin8-L196A/F201A) stimulates, but does not inhibit, stretch-induced exocytosis in umbrella cells (28). Interestingly, RAB3IP was originally described as a protein that interacts with RAB3A (6); however, it is unknown if RAB3 is expressed in the urothelium, and RAB3IP apparently does not act as a GEF for RAB3A (18). Future studies are needed to identify GAPs and GEFs in umbrella cells, with the ultimate goal of understanding how mechanical stimuli modulate membrane trafficking events such as DFV exocytosis, and how exocytosis in the urothelium governs bladder function.
GRANTS
This work was supported National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK104287 and P30-DK079307 (to G. Apodaca), and by the Kidney Imaging Core of the Pittsburgh Center for Kidney Research (P30-DK079307).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.I.G., M.G.D., D.R.C., W.G.R., P.K., and G.A. conceived and designed research; L.I.G., M.G.D., D.R.C., W.G.R., and P.K. performed experiments; L.I.G., M.G.D., D.R.C., W.G.R., P.K., and G.A. analyzed data; L.I.G., M.G.D., P.K., and G.A. interpreted results of experiments; L.I.G., M.G.D., D.R.C., W.G.R., and G.A. prepared figures; L.I.G., M.G.D., P.K., and G.A. edited and revised manuscript; L.I.G., M.G.D., D.R.C., W.G.R., and P.K. approved final version of manuscript; G.A. drafted manuscript.
Supplemental Data
ACKNOWLEDGMENTS
The authors thank T. T. Sun (New York University) for the kind gift of anti-AUM antibodies.
Present address of L. I. Gallo: Instituto de Fisiología, Biología Molecular y Neurociencias, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
REFERENCES
- 1.Amano O, Kataoka S, Yamamoto TY. Turnover of asymmetric unit membranes in the transitional epithelial superficial cells of the rat urinary bladder. Anat Rec 229: 9–15, 1991. doi: 10.1002/ar.1092290103. [DOI] [PubMed] [Google Scholar]
- 2.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007. doi: 10.1038/sj.ki.5002439. [DOI] [PubMed] [Google Scholar]
- 3.Apodaca G, Gallo LI. Epithelial polarity. In Colloquim Series on Building Blocks of the Cell: Cell Structure and Function, edited by Nabi IR. Williston, VT: Morgan & Claypool Life Sciences, 2013. doi: 10.4199/C00077ED1V01Y201303BBC002. [DOI] [Google Scholar]
- 4.Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol 14: 1235–1243, 2012. doi: 10.1038/ncb2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balestreire EM, Apodaca G. Apical epidermal growth factor receptor signaling: regulation of stretch-dependent exocytosis in bladder umbrella cells. Mol Biol Cell 18: 1312–1323, 2007. doi: 10.1091/mbc.E06-09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brondyk WH, McKiernan CJ, Fortner KA, Stabila P, Holz RW, Macara IG. Interaction cloning of Rabin3, a novel protein that associates with the Ras-like GTPase Rab3A. Mol Cell Biol 15: 1137–1143, 1995. doi: 10.1128/MCB.15.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner Y, Couté Y, Iezzi M, Foti M, Fukuda M, Hochstrasser DF, Wollheim CB, Sanchez JC. Proteomics analysis of insulin secretory granules. Mol Cell Proteomics 6: 1007–1017, 2007. doi: 10.1074/mcp.M600443-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12: 1035–1045, 2010. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey TM, Meade JL, Hewitt EW. Organelle proteomics: identification of the exocytic machinery associated with the natural killer cell secretory lysosome. Mol Cell Proteomics 6: 767–780, 2007. doi: 10.1074/mcp.M600365-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Guo X, Deng F-M, Liang F-X, Sun W, Ren M, Izumi T, Sabatini DD, Sun T-T, Kreibich G. Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc Natl Acad Sci USA 100: 14012–14017, 2003. doi: 10.1073/pnas.2436350100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng S, Knödler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peränen J, Guo W. A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem 287: 15602–15609, 2012. doi: 10.1074/jbc.M111.333245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueiredo AC, Wasmeier C, Tarafder AK, Ramalho JS, Baron RA, Seabra MC. Rab3GEP is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes. J Biol Chem 283: 23209–23216, 2008. doi: 10.1074/jbc.M804134200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci 65: 2801–2813, 2008. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem 277: 12432–12436, 2002. doi: 10.1074/jbc.C200005200. [DOI] [PubMed] [Google Scholar]
- 15.Gallo LI, Liao Y, Ruiz WG, Clayton DR, Li M, Liu YJ, Jiang Y, Fukuda M, Apodaca G, Yin XM. TBC1D9B functions as a GTPase-activating protein for Rab11a in polarized MDCK cells. Mol Biol Cell 25: 3779–3797, 2014. doi: 10.1091/mbc.E13-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gálvez-Santisteban M, Rodríguez-fraticelli AE, Bryant DM, Vergarajauregui S, Yasuda T, Bañón-Rodríguez I, Bernascone I, Datta A, Spivak N, Young K, Slim CL, Brakeman PR, Fukuda M, Mostov KE, Martín-Belmonte F. Synaptotagmin-like proteins control the formation of a single apical membrane domain in epithelial cells. Nat Cell Biol 14: 838–849, 2012. doi: 10.1038/ncb2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Tu L, Gumper I, Plesken H, Novak EK, Chintala S, Swank RT, Pastores G, Torres P, Izumi T, Sun T-T, Sabatini DD, Kreibich G. Involvement of vps33a in the fusion of uroplakin-degrading multivesicular bodies with lysosomes. Traffic 10: 1350–1361, 2009. doi: 10.1111/j.1600-0854.2009.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattula K, Furuhjelm J, Arffman A, Peränen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 13: 3268–3280, 2002. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hicks RM. The mammalian urinary bladder: an accommodating organ. Biol Rev Camb Philos Soc 50: 215–246, 1975. doi: 10.1111/j.1469-185X.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 20.Hicks RM, Ketterer B, Warren RC. The ultrastructure and chemistry of the luminal plasma membrane of the mammalian urinary bladder: a structure with low permeability to water and ions. Philos Trans R Soc Lond B Biol Sci 268: 23–38, 1974. doi: 10.1098/rstb.1974.0013. [DOI] [PubMed] [Google Scholar]
- 21.Horgan CP, Hanscom SR, McCaffrey MW. GRAB is a binding partner for the Rab11a and Rab11b GTPases. Biochem Biophys Res Commun 441: 214–219, 2013. doi: 10.1016/j.bbrc.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Hou Y, Chen X, Tolmachova T, Ernst SA, Williams JA. EPI64B acts as a GTPase-activating protein for Rab27B in pancreatic acinar cells. J Biol Chem 288: 19548–19557, 2013. doi: 10.1074/jbc.M113.472134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudoklin S, Zupancic D, Romih R. Maturation of the Golgi apparatus in urothelial cells. Cell Tissue Res 336: 453–463, 2009. doi: 10.1007/s00441-009-0779-9. [DOI] [PubMed] [Google Scholar]
- 24.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91: 119–149, 2011. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasahara H, Aoki H. Gene silencing using adenoviral RNAi vector in vascular smooth muscle cells and cardiomyocytes. Methods Mol Med 112: 155–172, 2005. doi: 10.1007/978-1-59259-879-3_9. [DOI] [PubMed] [Google Scholar]
- 26.Kerr DE, Liang F, Bondioli KR, Zhao H, Kreibich G, Wall RJ, Sun TT. The bladder as a bioreactor: urothelium production and secretion of growth hormone into urine. Nat Biotechnol 16: 75–79, 1998. doi: 10.1038/nbt0198-75. [DOI] [PubMed] [Google Scholar]
- 27.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297: F1477–F1501, 2009. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khandelwal P, Prakasam HS, Clayton DR, Ruiz WG, Gallo LI, van Roekel D, Lukianov S, Peränen J, Goldenring JR, Apodaca G. A Rab11a-Rab8a-Myo5B network promotes stretch-regulated exocytosis in bladder umbrella cells. Mol Biol Cell 24: 1007–1019, 2013. doi: 10.1091/mbc.E12-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khandelwal P, Ruiz WG, Balestreire-Hawryluk E, Weisz OA, Goldenring JR, Apodaca G. Rab11a-dependent exocytosis of discoidal/fusiform vesicles in bladder umbrella cells. Proc Natl Acad Sci USA 105: 15773–15778, 2008. doi: 10.1073/pnas.0805636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khandelwal P, Ruiz WG, Apodaca G. Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J 29: 1961–1975, 2010. doi: 10.1038/emboj.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lapierre LA, Dorn MC, Zimmerman CF, Navarre J, Burnette JO, Goldenring JR. Rab11b resides in a vesicular compartment distinct from Rab11a in parietal cells and other epithelial cells. Exp Cell Res 290: 322–331, 2003. doi: 10.1016/S0014-4827(03)00340-9. [DOI] [PubMed] [Google Scholar]
- 32.Lewis SA, de Moura JL. Incorporation of cytoplasmic vesicles into apical membrane of mammalian urinary bladder epithelium. Nature 297: 685–688, 1982. doi: 10.1038/297685a0. [DOI] [PubMed] [Google Scholar]
- 33.Lindsay AJ, Jollivet F, Horgan CP, Khan AR, Raposo G, McCaffrey MW, Goud B. Identification and characterization of multiple novel Rab-myosin Va interactions. Mol Biol Cell 24: 3420–3434, 2013. doi: 10.1091/mbc.E13-05-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montalbetti N, Rued AC, Clayton DR, Ruiz WG, Bastacky SI, Prakasam HS, Eaton AF, Kullmann FA, Apodaca G, Carattino MD. Increased urothelial paracellular transport promotes cystitis. Am J Physiol Renal Physiol 309: F1070–F1081, 2015. doi: 10.1152/ajprenal.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moulton DE, Sulzer V, Apodaca G, Byrne HM, Waters SL. Mathematical modelling of stretch-induced membrane traffic in bladder umbrella cells. J Theor Biol 409: 115–132, 2016. doi: 10.1016/j.jtbi.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prakasam HS, Gallo LI, Li H, Ruiz WG, Hallows KR, Apodaca G. A1 adenosine receptor-stimulated exocytosis in bladder umbrella cells requires phosphorylation of ADAM17 Ser-811 and EGF receptor transactivation. Mol Biol Cell 25: 3798–3812, 2014. doi: 10.1091/mbc.E14-03-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakasam HS, Herrington H, Roppolo JR, Jackson EK, Apodaca G. Modulation of bladder function by luminal adenosine turnover and A1 receptor activation. Am J Physiol Renal Physiol 303: F279–F292, 2012. doi: 10.1152/ajprenal.00566.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramesh N, Memarzadeh B, Ge Y, Frey D, VanRoey M, Rojas V, Yu DC. Identification of pretreatment agents to enhance adenovirus infection of bladder epithelium. Mol Ther 10: 697–705, 2004. doi: 10.1016/j.ymthe.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Rizk A, Paul G, Incardona P, Bugarski M, Mansouri M, Niemann A, Ziegler U, Berger P, Sbalzarini IF. Segmentation and quantification of subcellular structures in fluorescence microscopy images using Squassh. Nat Protoc 9: 586–596, 2014. doi: 10.1038/nprot.2014.037. [DOI] [PubMed] [Google Scholar]
- 40.Romih R, Jezernik K. Endocytosis during postnatal differentiation in superficial cells of the mouse urinary bladder epithelium. Cell Biol Int 18: 663–668, 1994. doi: 10.1006/cbir.1994.1093. [DOI] [PubMed] [Google Scholar]
- 41.Sakane A, Manabe S, Ishizaki H, Tanaka-Okamoto M, Kiyokage E, Toida K, Yoshida T, Miyoshi J, Kamiya H, Takai Y, Sasaki T. Rab3 GTPase-activating protein regulates synaptic transmission and plasticity through the inactivation of Rab3. Proc Natl Acad Sci USA 103: 10029–10034, 2006. doi: 10.1073/pnas.0600304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Chiu TT, Foley KP, Bilan PJ, Klip A. Myosin Va mediates Rab8A-regulated GLUT4 vesicle exocytosis in insulin-stimulated muscle cells. Mol Biol Cell 25: 1159–1170, 2014. doi: 10.1091/mbc.E13-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell 127: 831–846, 2006. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 44.Truschel ST, Ruiz WG, Shulman T, Pilewski J, Sun T-T, Zeidel ML, Apodaca G. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem 274: 15020–15029, 1999. doi: 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- 45.Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13: 830–846, 2002. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veranic P, Jezernik K. Trajectorial organisation of cytokeratins within the subapical region of umbrella cells. Cell Motil Cytoskeleton 53: 317–325, 2002. doi: 10.1002/cm.10077. [DOI] [PubMed] [Google Scholar]
- 47.Wang ECY, Lee J-M, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115: 2412–2422, 2005. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wankel B, Ouyang J, Guo X, Hadjiolova K, Miller J, Liao Y, Tham DK, Romih R, Andrade LR, Gumper I, Simon JP, Sachdeva R, Tolmachova T, Seabra MC, Fukuda M, Schaeren-Wiemers N, Hong WJ, Sabatini DD, Wu XR, Kong X, Kreibich G, Rindler MJ, Sun TT. Sequential and compartmentalized action of Rabs, SNAREs, and MAL in the apical delivery of fusiform vesicles in urothelial umbrella cells. Mol Biol Cell 27: 1621–1634, 2016. doi: 10.1091/mbc.E15-04-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney Int 75: 1153–1165, 2009. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 178: 363–369, 2007. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu W, Khandelwal P, Apodaca G. Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells. Mol Biol Cell 20: 282–295, 2009. doi: 10.1091/mbc.E08-04-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.