Abstract
The objective of the present study was to 1) analyze the ascending aortic proteome within a mouse model of Marfan syndrome (MFS; Fbn1C1041G/+) at early and late stages of aneurysm and 2) subsequently test a novel hypothesis formulated on the basis of this unbiased proteomic screen that links changes in integrin composition to transforming growth factor (TGF)-β-dependent activation of the rapamycin-independent component of mammalian target of rapamycin (Rictor) signaling pathway. Ingenuity Pathway Analysis of over 1,000 proteins quantified from the in vivo MFS mouse aorta by data-independent acquisition mass spectrometry revealed a predicted upstream regulator, Rictor, that was selectively activated in aged MFS mice. We validated this pattern of Rictor activation in vivo by Western blot analysis for phosphorylation on Thr1135 in a separate cohort of mice and showed in vitro that TGF-β activates Rictor in an integrin-linked kinase-dependent manner in cultured aortic vascular smooth muscle cells. Expression of β3-integrin was upregulated in the aged MFS aorta relative to young MFS mice and wild-type mice. We showed that β3-integrin expression and activation modulated TGF-β-induced Rictor phosphorylation in vitro, and this signaling effect was associated with an altered vascular smooth muscle cell proliferative-migratory and metabolic in vitro phenotype that parallels the in vivo aneurysm phenotype in MFS. These results reveal that Rictor is a novel, context-dependent, noncanonical TGF-β signaling effector with potential pathogenic implications in aortic aneurysm.
NEW & NOTEWORTHY We present the most comprehensive quantitative analysis of the ascending aortic aneurysm proteome in Marfan syndrome to date resulting in novel and potentially wide-reaching findings that expression and signaling by β3-integrin constitute a modulator of transforming growth factor-β-induced rapamycin-independent component of mammalian target of rapamycin (Rictor) signaling and physiology in aortic vascular smooth muscle cells.
Keywords: aortic aneurysm, data-independent acquisition mass spectrometry, integrin signaling, Marfan syndrome, Rictor, transforming growth factor-β
INTRODUCTION
Aneurysm, or dilation of the proximal thoracic aorta, is a highly penetrant and life-threatening phenotype in individuals with Marfan syndrome (MFS), a connective tissue disorder caused by mutations in the gene encoding fibrillin-1 (Fbn1). Fibrillin-1 is a cysteine-rich glycoprotein that assembles into microfibrils in the extracellular space, providing structural integrity as well as modulating cell-cell and cell-matrix signaling through interactions with signaling cytokines, cell surface proteins, and other extracellular matrix (ECM) proteins (12). Functionally, fibrillin-1 serves as a scaffold for deposition of elastin and participates in the structural assembly of elastic fibers, which in their normal state are mediators of tissue compliance particularly in the skin, lungs, and vasculature (47). Fibrillin-1 is also known to interact with the extracellular domains of selected integrins (e.g., α5β1 and αvβ3) via an arginine-glycine-asparagine (RGD) binding domain and thus may participate in matrix sensing and mechanosignaling by endothelial and vascular smooth muscle cells (VSMCs) (3). Finally, and importantly for the pathobiology in MFS, fibrillin-1 interacts with the large latent complex of transforming growth factor (TGF)-β, serving to both sequester the ligand in its inactive state and/or concentrate this potent cytokine near sites of intended action (22).
Although the centrality of aberrant TGF-β signaling in MFS has been established (18, 36), a comprehensive understanding of the exact mechanisms whereby MFS-causing mutations in Fbn1 result in the multiple abnormalities observed within the cells and ECM of aneurysm remains elusive. To add to the complexity, TGF-β signaling is highly regulated by internal and external cellular context (31). Signaling downstream of TGF-β receptors can follow the canonical SMAD-mediated route and/or can branch to several noncanonical pathways, which participate in the flexibility required to obtain context dependency, including the ERK and p38 MAPK, JNK, and NF-κβ signaling pathways (50). Although noncanonical TGF-β signaling is central in aneurysm pathogenesis (18), the full extent of signaling “options” exercised by TGF-β in the MFS aorta has yet to be defined.
Discovery proteomics presents an opportunity to more comprehensively define the effects of altered Fbn1 and subsequent TGF-β signaling via unbiased survey of the molecular perturbations associated with aneurysm remodeling. A handful of existing studies have used mass spectrometry (MS)-based protein profiling to analyze thoracic aortic aneurysms in humans (25, 32, 40, 42), although many specifically excluded (42) or failed to include (25) patients with MFS from the cohort analyzed. The one study specific to MFS used a relatively low sensitivity approach (e.g., two-dimensional gel electrophoresis) and thus generated a limited scope of molecules profiled compared with that possible with more modern MS-based proteomic technologies (40). Furthermore, these human studies are limited in that disease tissue was sampled at end stage during aortic resection surgery, which precludes the definition of early molecular pathogenesis and the comparison of early versus late stage molecular disease profiles. Mouse models of MFS with Fbn1 mutations have been fundamental in understanding the molecular pathology of the disease and enable sampling of aortic tissue at both early and late stages of aneurysm development. However, procuring adequate amounts of protein for proteomic analysis in a manner amenable to downstream MS analysis has been challenging. Recent developments using high-pressure protein extraction coupled to emerging data-independent acquisition MS (DIA-MS) for label-free proteomic quantification now enable the extraction and quantitative analysis of sample-limited material such as the mouse ascending aorta (45).
The Fbn1C1041G/+ mouse expresses a heterozygous knockin allele of a common class of human MFS-causing FBN1 mutations (i.e., cysteine substitution in an EGF-like domain) and is an established model that faithfully recapitulates several of the human phenotypes, including aortic wall degeneration and aneurysm development (8, 9, 23). Like most human forms of MFS, aortic root aneurysm progresses in the Fbn1C1041G/+ mouse from an early, relatively mild pathology into more profound elastin fiber fragmentation, disordered VSMC layers, vessel dilation, increased stiffness, and reduced contractility at later ages (e.g., 6–12 mo) (10, 37). To generate a molecular signature of early aneurysm and its progression in the aorta during MFS, in the present study, we used DIA-MS to compare the proteome of aortas from Fbn1C1041G/+ mice with healthy, wild-type (WT) counterparts at both early (10 wk) and late (12 mo) stage disease.
MATERIALS AND METHODS
DIA-MS library, raw files, and OpenSWATH-processing intermediate files have been uploaded to the PeptideAtlas SWATH data repository with data set ID no. PASS01251 and the data set tag MouseThoracicAorta.
Preparation of Aortic Tissues
All animal procedures were performed under an approved Johns Hopkins University or Cedars-Sinai Medical Center Institutional Animal Care and Use Committee protocol. Male Fbn1C1041G/+ (MFS) mice were maintained on a C57BL/6 genetic background with >20 backcrossings to ensure genetic purity. WT littermates were used as controls for each age group (10 wk and 12 mo). After euthanasia with halothane, mice were perfused through the left ventricle with 10-ml cold PBS to flush the aorta of blood. The ascending aorta and root were dissected, further cleaned of blood and fatty tissues, and snap frozen in liquid nitrogen. Frozen aortas were placed in micropestle tubes (Pressure BioSciences, Easton, MA) with 30 μl of 8 M urea-2 M thiourea lysis buffer, and proteins were extracted by high-pressure barocycling at room temperature, ramping pressure to 45 psi, holding for 50 s, returning to atmospheric pressure for 10 s, and repeating for 60 cycles. Protein concentration was assayed using the Pierce 660-nm assay. For individual proteomic analysis, 5 µg of protein lysate were aliquoted for digestion and MS analysis. For library building, an additional 3 µg of lysate from each mouse were pooled. Aliquoted samples were diluted to 4 M urea in 50 mM ammonium bicarbonate with 50 mM DTT added as a reducing agent for 30 min at 37°C. Samples were further diluted to 2 M urea in 50 mM ammonium bicarbonate with 50 mM iodoacetamide added to alkylate reduced thiol groups and incubated 30 min in the dark at room temperature. Reduced and alkylated samples were pH adjusted to 7–8, and 1 µg of a trypsin/Lys-C mixture was added for overnight digestion at 37°C with agitation. After digestion, peptides were desalted on Waters HLB microelution plates, with peptides eluted from resin in 50% acetonitrile-50% of 0.1% formic acid in water. Peptides were dried and stored at −20°C before MS analysis. Immediately before MS, samples were resuspended in 7 µl of 0.1% formic acid.
Data-Independent Acquisition Mass Spectrometry
For use in targeted peptide ion library building, 1 µg of pooled aortic digests was injected in 16 serial runs of 120-min liquid chromatography coupled to MS (LC-MS) on a SCIEX 6600 TripleTOF (SCIEX, Framingham, MA) operating in DIA mode. Each serial injection was acquired with 32 precursor isolation windows; however, with each injection the windows were staggered from the previous injection, in an effort to maximize coverage of the peptides within the pooled sample. Larger acquisition windows also facilitated longer MS1 and MS2 dwell times (250 and 101 ms, respectively) to facilitate downstream analysis. The serial acquisition window setup is provided as Supplemental Document S1 (Supplemental Material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website). One microgram of pooled sample was loaded on an Eksigent 415 HPLC system equipped with a Ekspert nanoLC 400 autosampler. Peptides were separated on a ChipLC trap-elute system equipped with a 15-cm, 75 µm-inner diameter column of C18 resin (300-Å diameter) at a flow rate of 500 nl/min using a linear AB gradient of 3–35% solvent B (0.1% formic acid in acetonitrile) for 120 min, 35–85% solvent B for 2 min, holding at 85% solvent B for 5 min, and then reequilibrating at 3% solvent B for 7 min. Source gas 1 was set to 6, source gas 2 was set to 0, curtain gas was set to 30, source temperature was set to 100°C, and source voltage was set to 2,400 V.
For quantitative analysis of experimental samples, 3.5 µg of aortic lysate were analyzed in DIA mode with a 64-variable width window setup (Supplemental Document S1). Peptides derived from aortic lysate (3.5 µg/specimen) was injected onto an Eksigent 415 HPLC system equipped with an Ekspert nanoLC 400 autosampler and separated on a linear gradient as described above for the pooled sample. Mass spectra were obtained on a 5600 TripleTOF operating in DIA mode, with 64 variable width precursor isolation windows. Dwell times in MS1 and MS2 were 250 and 45 ms, respectively, for a total cycle time of 3.2 s. Collision energy was optimized for an ion mass-to-charge ratio centered on the isolation window, with collision energy spread ranging from 5 to 15. Source gas 1 was set to 3, source gas 2 was set to 0, curtain gas was set to 25, source temperature was set to 100°C, and source voltage was set to 2,400 V.
Peptide library generation.
Pooled acquisitions as well as the 13 individual test specimen acquisitions were processed using the Signal Extraction module of the DIA-Umpire software tool (DIAu-SE) as previously described by Tsou et al. (48). Pseudospectra generated by the DIAu-SE step were then processed for library generation as previously described (39). Mouse protein sequences were defined in a FASTA database of Swiss-Prot-reviewed, canonical mouse genome downloaded September 2013 with Contaminant Repository for Affinity Purification (CRAPome) (33), Biognosys indexed retention time (iRT) peptides (Biognosys, Schlieren, Switzerland) (6), and randomized decoy sequences appended.
Quantitation of individual specimen DIA-MS files.
Raw intensity data for peptide fragments were extracted from DIA files using the open-source OpenSWATH workflow against the sample-specific peptide assay library (described above) as previously described in detail by Parker et al. (39), with retention time normalization performed with the aid of the Biognosys spiked-in in synthetic retention time standard peptides. Briefly, peptide assay peak groups were extracted from raw DIA files and scored against an equal number of decoy peak groups based on a composite of 11 data quality subscores. Target peptides with a false discovery rate (FDR) of identification <1% were included for downstream analysis.
Skyline analysis of mouse integrin proteins.
Since β3-integrin (ITβ3) was absent from the original peptide library, DIA-MS files from individual specimens were reanalyzed against integrin peptides from a previously published peptide library of mouse smooth muscle cell lysate, which enriched smooth muscle proteins including additional integrins (39). Analysis of ITβ3 was performed using manual integration supplemented with mProphet target-decoy modeling in Skyline (28) allowing us to differentiate whether ITβ3 was detectable and/or quantifiable in the DIA data set. Peptides from β1-integrin (ITβ1), α5-integrin (ITα5), and αv-integrin (ITαv) were also analyzed using this approach as a means to cross reference the reproducibility of quantitation. Peptide areas were normalized to “MS2 signal” of each file (see below) and summed to generate an estimate of protein abundance. Independent t-tests were used to compare integrin expression between MFS and WT mice within age categories. Raw and processed data from this separate Skyline analysis of ITβ3 are provided in Supplemental Master Data File S1.
Data normalization, protein-level rollup, and statistics.
The total ion current associated with the MS2 signal across the chromatogram excluding the last 15 min (truncated to avoid including signal from contaminants/noise) was calculated for normalization. This MS2 signal of each file, akin to a total protein load stain on a Western blot gel, was used to adjust the transition intensity of each peptide in a corresponding file. Normalized transition-level data were then processed using mapDIA software (46) to remove noisy/interference transitions from peptide peak groups and perform pairwise comparisons between groups. Although young MFS versus young WT, aged MFS versus aged WT, aged WT versus young WT, and aged MFS versus aged MFS comparisons were made, given the small sample size, we limited our analysis and interpretation to the comparison of MFS with WT within each age group. All comparison results are provided in Supplemental Master Data File S1. After quality control modeling and best peptide fragment selection by mapDIA, accepted fragments from the young and aged data sets were summed to generate peptide-level data, and these peptide values were further summed to generate protein-level data. Raw files from each level of processing (fragment, peptide, and protein) are provided in Supplemental Master Data File S1.
Biological interpretation of the data was facilitated by analysis with the Ingenuity Pathway Analysis (IPA) suite (Qiagen, Hilden, Germany), Database for Annotation, Visualization, and Integrated Discovery (DAVID) Gene Ontology software (19, 20), Cytoscape custom network generation (44), and the R programming space (14) for the generation of graphs and data heat maps. The mapDIA tool generates a q value to indicate FDR rather than a simple P value (46). For the initial survey of biological pathways and functions, we conservatively considered a protein significantly altered between any two groups only if it had a log2 fold change (log2FC) of >0.8 (~1.5-fold change) and a q value/FDR of <0.01. In subsequent followup analyses where a more focused question was being addressed, we allowed a lower fold change (>0.4 log2FC) but maintained the requirement of FDR < 0.01.
Immunofluorescence Analysis
Mice were anesthetized with CO2, and whole hearts and attached aortas were dissected from aged WT (n = 3) and MFS (m = 4) mice after intraventricular perfusion with 2% paraformaldehyde in PBS buffer. Hearts were frozen in optimal cutting temperature blocks on dry ice and then sectioned to 8-µm thickness on a cryostat and mounted to positively charged glass tissue slides. Sections were dried for a minimum of 3 h at room temperature. Antigens were retrieved with a 10-min incubation of 10% formic acid (Sigma-Aldrich, St. Louis, MO) in water and permeabilized in PBS with 0.1% Triton X-100 for 10 min. Sections were blocked in 3% goat serum for 30 min and incubated with primary antibodies at a 1:100 dilution in 3% goat serum (rat anti-vitronectin, catalog no. MAB3875, R&D Systems; and rabbit anti-fibronectin, catalog no. F3648, Sigma-Aldrich) overnight at 4°C. Sections were washed three times in PBS + 0.1% Triton X-100, incubated with the appropriate Alexa fluor 488- or 594-conjugated secondary antibodies (Invitrogen/ThermoFisher Scientific, Waltham, MA) for 1 h at room temperature, and then washed again as before. Slides were mounted in Vectashield hard-set mounting medium with DAPI to stain cell nuclei (Vector Laboratories, Burlingame, CA). Slides were imaged on a Keyence digital microscopy system (Keyence, Osaka, Japan) using ×20 magnification and identical settings for all images of the same target, including the secondary antibody-only stained controls. Quantification was performed using ImageJ software. A region of interest was drawn around the border of each aorta, excluding regions of the myocardium. The secondary antibody-only negative control was used to set a thresholding value for positive signal of each analyte, and this same value was used across all samples of a given analysis. Quantification was reported as mean thresholded area within the designated region of interest of each aorta.
In Vitro Smooth Muscle Cell Culture
Mouse aortic smooth muscle cells were obtained from the American Type Culture Collection (CRL-2797, Manassas, VA) and maintained in DMEM supplemented with 5% FBS and antibiotic-antimycotic (Gibco). For TGF-β stimulation experiments, cells were seeded onto six-well plates, grown to 85–90% confluency, and starved overnight with serum-free DMEM. Vehicle or integrin-linked kinase (ILK) inhibitor (3.5 µM, Calbiochem Cpd 22, EMD Millipore, Darmstadt, Germany) were added to the media and incubated for 3 h, and 10 ng/ml of recombinant TGF-β1 (Sino Biological, Beijing, China) or vehicle were then added for an additional 1 h. After stimulation, cells were rinsed with ice-cold Ca2+/Mg2+-free PBS and lysed with 150 µl of 8 M urea-0.1% Triton X-100 supplemented with protease and phosphatase inhibitor. Protein concentration was determined by a Pierce bicinchoninic acid assay (ThermoFisher Scientific).
ITβ3-overexpressing (ITβ3-OE) and green fluorescent protein (GFP; mEmerald) control cells were established using self-inactivating retroviral particles. ITβ3-Emerald cDNA and mEmerald from mEmerald-ITβ3-N-18 (produced by Michael Davidson, Addgene plasmid no. 54130) were cloned into the pBMN.i.Blast plasmid for the production of nonreplicative Moloney’s murine leukemia virus particles (17). Particles were produced by transfecting a 10-cm2 plate of Phoenix-GP cells (courtesy of Dr. Garry Nolan) with 3 µg of the packaging vector and 3 µg of envelope glycoprotein of the vesicular stomatitis virus (pCI-VSVg). Plasmids were combined with 125 µl FCS-free DMEM and 30 µg polyethyleneimine (linear, molecular weight: 25,000, no. 23966-2, Polysciences) and incubated for 20 min at room temperature, and the reaction was added to the Phoenix-GP cells. Media (DMEM with 10% FCS, penicillin-streptomycin, and l-glutamine) were replaced 24 h posttransfection, and viral supernatant was collected at 48 and 72 h. The media were then filtered through a 0.45-µm polytetrafluoroethylene filter (no. 4422, Pall). Virus-containing media were used to infect mouse aortic smooth muscle cells in a six-well plate format. Briefly, viral supernatants were mixed with Polybrene (no. 107689, Sigma-Aldrich, final concentration: 5 µg/ml), added to cells seeded at 1 × 105 cells/well, and spun at 1,500 g and 32°C for 80 min in a hanging bucket-rotors centrifuge. The transduced cells were selected with blasticidin-HCl (A1113902, ThermoFisher Scientific) at 10 µg/ml.
Western Blot Analysis
A total of 10–30 μg of tissue homogenate or cell lysates were mixed with 4× loading buffer (Bio-Rad, Hercules, CA) supplemented with 100 mM DTT and boiled for 10 min. Samples were loaded into 4–12% Tris-glycine minigels (Bio-Rad), and proteins were separated by electrophoresis in SDS-running buffer. Proteins were transferred to PVDF membranes for 8 min on a Turbo transfer system (Bio-Rad). Membranes were washed in PBS and then stained with DirectBlue 71 total protein stain (Sigma-Aldrich, protocol to dissolve dye in diluent of 40% acetic acid and 10% ethanol, stain 5 min, and rinse with diluent to reduce background) and imaged on a GE LAS 4000 imaging system. Sections of the membrane were cut to facilitate blotting for multiple targets at different molecular weights. DirectBlue staining was then reversed with 150 mM NaOH in double-distilled water-50% ethanol, and membranes were blocked with 5% BSA in Tris-buffered saline + 0.1% Tween 20 (TBST). Primary antibodies against phospho-rapamycin-independent component of mammalian target of rapamycin (Rictor) Thr1135 (D30A3, Cell Signaling Technology), phospho-Akt Ser473 (no. 4060, Cell Signaling Technology), phospho-SMAD2 (clone A5S, no. 04-953, EMD Millipore), ITβ3 (no. 4702, Cell Signaling Technology), or ITβ1 (AF2405-SP, R&D Systems) were added at a 1:1,000 (vol/vol) ratio in 5% BSA and TBST and incubated at 4°C for 18–48 h. Blots were washed with TBST, and the appropriate horseradish peroxidase-conjugated secondary antibody was added at a 1:3,000 (vol/vol) ratio and incubated 1 h at room temperature. Blots were washed and imaged on a GE LAS 4000 imaging platform (GE Healthcare, Pittsburgh, PA). Membranes were then stripped with ThermoFisher Western blot stripping buffer (ThermoFischer Scientific) and reprobed for total Rictor (A300-459A-M, Bethyl Laboratories), total Akt (no. 9272, Cell Signaling Technology), or β-tubulin (no. 2146, Cell Signaling Technology) as described above. Protein quantity was normalized to β-tubulin, and independent two-tailed t-tests (P < 0.05) were used to compare experimental differences.
Cell Migration/Wound Closure Assay
Immortalized VSMCs overexpressing either GFP (WT, n = 3) or ITβ3 (ITβ3-OE, n = 3) were generated and cultured as described above in In Vitro Smooth Muscle Cell Culture. Cells were plated on immobilized vitronectin (2 µg/ml, incubated 1 h and then removed, Promega, Madison, WI)-coated six-well plates at a density of 106 cells/well in complete DMEM (5% FBS). After 4 h, media were changed to starvation conditions (DMEM, 0% serum), and cells were incubated for 18 h. A linear scratch was made along the surface of each well, denuding the VSMCs and inducing a “wound,” or area free of cell monolayer. TGF-β1 (10 ng/ml) or vehicle was added to wells, and baseline images were captured to define the boundaries of the scratch. For inhibition conditions, 100 µM of the ITβ3 inhibitor SB-273005 were added concomitantly with TGF-β (n = 3) or vehicle (n = 3). After 16 h, the scratch area was imaged again. Differences between groups were measured by a paired-samples t-test with the significance cutoff set to P < 0.05.
Images of the scratch area at time 0 and after 16 h of incubation were captured with a ×20 phase-contrast lens in a BZ-X710 All-in-One fluorescence microscope. Multiple images were stitched together and analyzed using the microscope manufacturer’s software, BZ-X Analyzer (version 1.3.1.1). After stitching, a 10-mm length of scratched segment in each well was selected (by cropping) and analyzed using the software’s Hybrid Cell Count feature. A condition file highlighting acellular space (obtained by adjusting phase contrast threshold to highlight cells, excluding nonadherent cells by circularity or small size, and then inverting that mask) was used to determine the wounded area at baseline and after 16 h. The proliferative-migratory capacity of the VSMCs was quantified as the inverse of the cell-free area at 16 h relative to baseline (e.g., percent wound closure).
Mitochondrial Metabolism Functional Assay
Immortalized WT and ITβ3-OE VSMCs (n = 5 per condition) were plated on XF24 V7 plates coated with immobilized vitronectin as described above for the cell migration assay. After 18 h of serum starvation, cells were treated with 10 ng/ml recombinant TGF-β1 and incubated for 24 h, at which point an additional pulse of TGF-β1 was added. Forty-eight hours after the first TGF-β1 treatment, cells were refreshed with bicarbonate-free DMEM containing 25 mM glucose, 1 mM sodium pyruvate, and 2 mM glutamine and equilibrated for 1 h at 37°C in a non-CO2 incubator. Oxygen consumption was monitored after sequential injection of oligomycin (1 µM), FCCP (1 µM), and antimycin-rotenone (1 µM-1 µM). To normalize respiration rates, cells were subsequently lysed in radioimmunoprecipitation assay buffer [50 mM Tris·HCl (pH 8.0), 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, and protease inhibitor cocktail; cOmplete, Roche]. Protein concentration was determined using the bicinchoninic acid assay. Differences in basal mitochondrial respiration and spare mitochondrial respiratory capacity between WT and ITβ3-OE VSMCs were determined by independent t-test (significance cutoff, P < 0.05).
RESULTS
Quantitative Comparison of MFS and WT Aortic Proteomes
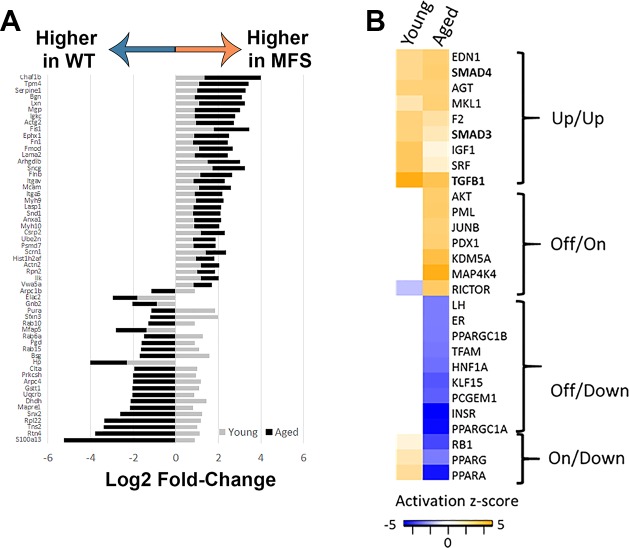
Our first step was to identify molecular signatures and associated functional pathways characteristic of Fbn1C1041G/+ aneurysm by performing a proteomic analysis of the MFS mouse aorta at an early (10 wk old) and late (12 mo old) stage of disease progression. In total, 1,079 proteins were quantified from 4,971 nonredundant (e.g., proteotypic) peptide sequences after best-peptide fragment filtering by mapDIA. Among these identifications, 984 and 905 proteins had enough high-quality transitions to enable statistical group comparisons between MFS and WT in young and aged mice, respectively. A summary of fragment, peptide, and protein data, as well as a table of all mapDIA comparisons with corresponding log2FC and FDRs, is provided in Supplemental Master Data File S1. Among these proteins, the well-characterized TGF-β-driven proteins plasminogen activator inhibitor (PAI)-1 and connective tissue growth factor (CTGF) were substantially higher in the aorta of MFS mice relative to WT mice in both young (log2FC MFS vs. WT of 1.0 for both PAI-1 and CTGF) and aged (log2FC MFS vs. WT of 2.3 for PAI1 and 0.93 for CTGF) groups, although the differences for CTGF can only be considered as trends as they were not statistically significant for either age (FDR for CTGF comparisons were 0.02 and 0.1 in young and aged mice, respectively). In addition to these sentinel “proof-of-principle” proteins, widespread differences in protein expression were observed between MFS and WT mice at both ages. Specifically, 425 proteins demonstrated altered abundance in MFS mice relative to WT mice at either age, with 71 changes specific to young mice, 297 changes specific to older mice, and 57 proteins changed in MFS mice at both ages. The 57 proteins significant at both ages represented diverse cellular functions, including cytoskeletal organization, ECM regulation, cell signaling, and many others (see Supplemental Master Data File S1). Interestingly, 20 of 57 proteins differentially abundant in both young and aged MFS mice had a reversed direction of expression in MFS mice relative to WT mice with age (Fig. 1A). This subset of proteins represented functional pathways such as intracellular membrane trafficking, microtubule organization, endoplasmic reticulum regulation, cell metabolism, and cell signaling/gene transcription.
Fig. 1.
Proteomic comparison of Marfan syndrome (MFS) and wild-type (WT) mouse aortic homogenate from young and aged donors. A: proteins significantly altered in both young and aged MFS aorta. For this initial screening analysis, proteins were considered significantly differentially abundant between MFS and WT groups if they demonstrated a log2 fold change of |0.8| and a false discovery rate of <1%. Gene names encoding each of the observed proteins are presented on the y-axis of the bar graph. The log2 fold change data for MFS versus WT groups are presented on the x-axis. A table of these proteins and their detailed descriptions is provided in Supplemental Master Data File S1. B: comparative Ingenuity Pathway Analysis of the significant protein expression differences between MFS and WT mice at young and aged time points revealed four general patterns of upstream regulator activation: consistently upregulated at both ages (up/up), uniquely activated in the aged MFS mice (off/on), uniquely inhibited in the aged MFS mice (off/down), and active in young MFS mice and subsequently inhibited in aged MFS mice (on/down). A table of upstream regulators and their calculated z-scores is provided in Supplemental Master Data File S1. For inclusion in the table, activation z-score for at least one comparison had to be >2.0, although in some cases only one comparison reached this threshold, and the other comparison shows data from a less confident prediction of activation/inhibition.
Functional Analysis of the MFS Aortic Proteome
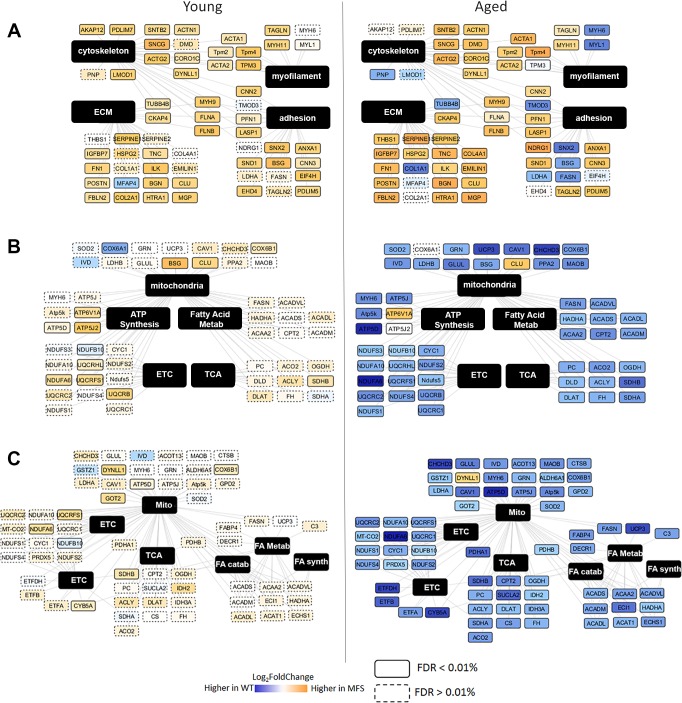
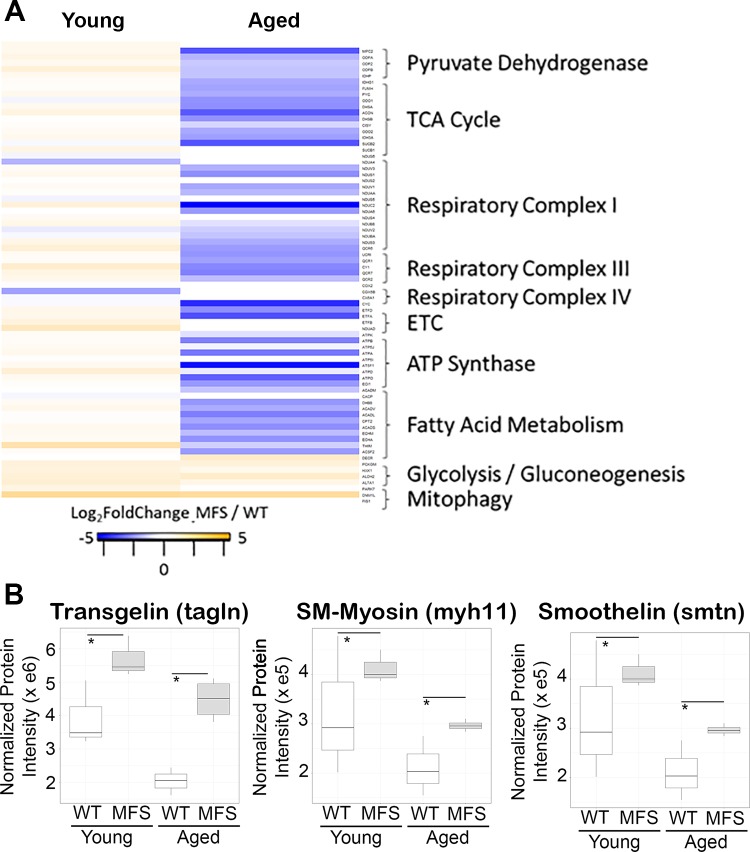
To elucidate the functional pathways and networks perturbed in MFS aneurysm, we conducted IPA of all significantly altered proteins. In both young and aged mice, the top canonical pathways enriched included integrin signaling (activation z-score 3.0 and 1.6 in young and aged MFS vs. WT comparisons, respectively) and actin cytoskeletal remodeling (activation z-score 3.5 and 1.45 in young and aged MFS vs. WT comparisons, respectively), suggestive of altered matrix sensing and/or mechanosensing machinery and corresponding changes in structural cellular physiology. Interestingly, the retinoic acid receptor/liver X receptor (RXR/LXR) pathway was predicted to be suppressed only in aged mice (activation z-score −2.7 in aged MFS vs. WT), which suggests unique changes in vascular metabolic potential in the aged MFS mouse aorta. In addition to these canonical pathways, several upstream regulators were predicted to be more active in both young and old MFS aortas relative to WT aotas (Fig. 1B). Among these, the most dominant was TGF-β signaling, with predicted activation in both young and old MFS aortas. Along with TGF-β, a number of related upstream regulators, including angiotensinogen (the precursor to angiotensin II), were also predicted to be more active in MFS mice (labeled as “up/up” in Fig. 1B). Functional ontology cluster analysis indicated that the proteins regulated by these upstream factors are involved in cytoskeletal remodeling, cellular adhesion assembly and signaling, and ECM composition (Fig. 2A). There were several other upstream regulators whose predicted activation state reversed direction [peroxisome proliferator-activated receptor (PPAR)-α, PPAR-γ, retinoblastoma-associated protein (RB1), and Rictor] or that were uniquely activated [MAP4K, lysine-specific demethylase 5A (KDM5A), protein PML (PML), transcription factor Jun-B (JUNB), pancreas/duodenum homeobox protein-1 (PDX1), and Akt] or inhibited [insulin receptor (INSR), PPAR-γ coactivator (PGC)-1α, luteinizing hormone (LH), estrogen receptor (ER), Krüppel-like factor 15 (KLF15), transcription factor A, mitochondrial (TFAM), PGC-1β, prostate-specific transcript 1 (PCGEM1), and hepatocyte nuclear factor 1β (HNF-1β)] in aged MFS mice relative to WT mice. These “reversed” upstream regulators drive proteins that are overwhelmingly enriched for mitochondrial cell compartment localization, including proteins involved in several mitochondrial metabolic pathways (Fig. 2, B and C). Closer examination of mitochondrial protein expression in the MFS and WT aorta demonstrated a clear reduction among the enzymes involved in nearly all components of the respiratory chain (pyruvate shuttling, tricarboxylic acid cycle, respiratory chain complexes, electron transport, and ATP synthase; Fig. 3A). These proteins were not found to be reduced in the young MFS aorta, nor were they reduced in the aged WT aged aorta relative to the young WT aorta (Supplemental Master Data File S1); thus, the repression of mitochondrial protein expression is not explained simply by general vascular aging. Furthermore, although a reduction in cellular content of tissue homogenates could explain reduced mitochondrial density, we observed increases in smooth muscle cell markers such as smooth muscle myosin (Myh11), smoothelin (Smtn), and transgelin [smooth muscle protein-22α (SM22α); Tagln] in aged MFS mice relative to WT mice (Fig. 3B), which suggests that cellularity does not explain reduced mitochondrial protein expression in the aged MFS aorta. Interestingly, a handful of glycolytic enzymes and the gluconeogenesis protein phosphoenolpyruvate carboxykinase (Pck2) were upregulated in aged MFS mice, as were parkin 7 (Park7) and mitochondrial fission protein 1 (Fis1), two proteins known to be involved in mitochondrial fission and degradation by mitophagy.
Fig. 2.
Functional ontology analysis of modules of proteins regulated by selected sets of upstream regulators with similar patterns of predicted behavior. A: Cytoscape map of proteins predicted to be regulated by factors that indicated uniformly increased activation in both young and aged Marfan syndrome (MFS) mice relative to wild-type (WT) mice. B: Cytoscape map of proteins predicted to be regulated by factors that indicated unique activation in only aged MFS mice relative to WT mice. C: Cytoscape map of proteins predicted to be regulated by factors that indicated unique inhibition in only aged MFS mice relative to WT mice. For all Cytoscape maps (A–C), protein nodes are clustered according to their association with one or more of the top Gene Ontology functional clusters identified from Database for Annotation, Visualization, and Integrated Discovery (DAVID) bioinformatics analyses. Each protein node is colored according to the magnitude of the log2 fold change difference of expression between MFS and WT groups (calculated by mapDIA software). Orange indicates higher expression in MFS mice, blue indicates higher expression in WT mice, and white indicates very little difference in expression between MFS and WT mice. Node borders are solid or dashed on the basis of significance testing results in mapDIA [solid borders, false discovery rate (FDR) < 1%; dashed borders, FDR > 1%]. Nodes are labeled with gene names. Original log2 fold change data for each node can be found in Supplemental Master Data File S1. Catab, catabolism; ECM, extracellular matrix; ETC, electron transport chain; FA, fatty acid; Metab, metabolism; Mito, mitochondria; Synth, synthesis; TCA, tricarboxylic acid.
Fig. 3.
Expression of mitochondrial proteins and selected vascular smooth muscle cell markers. A: comparison of several subcategories of mitochondrion-annotated proteins, identified using Database for Annotation, Visualization, and Integrated Discovery (DAVID) Gene Ontology tool, detected in Marfan syndrome (MFS) and wild-type (WT) aortas revealed a distinct suppression of mitochondrial protein expression in aged MFS mice, with upregulation of glycolytic/gluconeogenesis and mitophagy/fission-related proteins at both ages. Protein data from the heat map are provided as a tab in Supplemental Master Data File S1. B: markers of vascular smooth muscle cells transgelin (tagln), smooth muscle myosin (SM-Myosin; myh11), and smoothelin (Smtn) were elevated in both young and aged MFS mice relative to WT mice, suggesting that mitochondrial dysregulation is unlikely to be a consequence of reduced cellular content in mixed tissue homogenates used for proteomic analysis. Protein values are the sum of all observed fragments and peptides observed for that protein, after normalization of fragment intensities to total protein load in each sample. ETC, electron transport chain; TCA, tricarboxylic acid. *mapDIA false discovery rate of <1% MFS vs. WT groups.
In Vivo and In Vitro Validation of Rictor as a Downstream TGF-β Target Affected in MFS
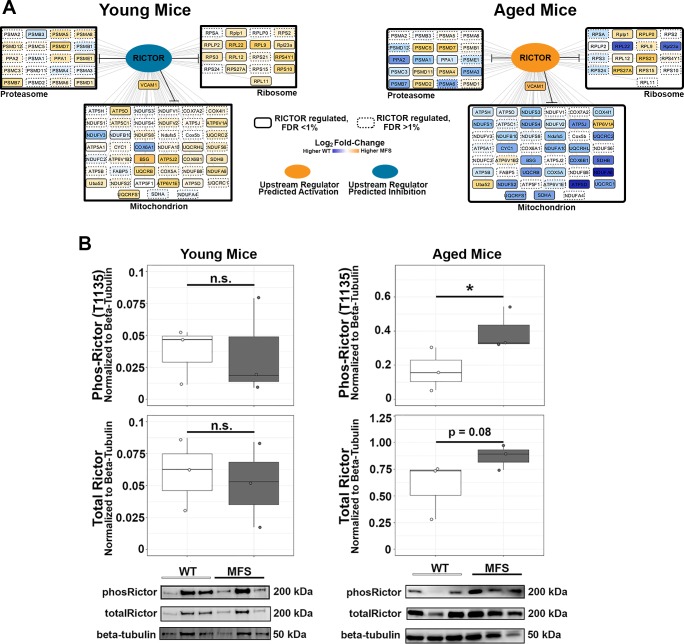
From the in silico upstream regulator prediction, we focused on the intriguing behavior of Rictor (the definitive component of the mammalian target of rapamycin 2 complex), which was predicted to be more active in aged MFS mice but inactive or even slightly inhibited in young MFS mice relative to WT mice (Fig. 4A). This observation was validated by Western blot analysis in an orthogonal cohort of mice, and, consistent with the in silico prediction, total and phosphorylated (Thr1135) Rictor levels were increased in aortic tissue lysate from aged but not young MFS mice relative to WT control mice (Fig. 4B).
Fig. 4.
Validation of rapamycin-independent component of mammalian target of rapamycin (Rictor) as a transforming growth factor-β-dependent upstream regulator active uniquely in the aged Marfan syndrome (MFS) aorta. A: representative plot of the Rictor-regulated proteins measured in MFS and wild-type (WT) aortas at young (left) and aged (right) time points. Nodes are colored on the basis of the observed log2 fold change for that protein in the MFS versus WT comparison of young (left) or aged (right) mice, respectively. B: Western blots of an orthogonal cohort of young (n = 3 each, MFS and WT) and aged (n = 3 each, MFS and WT) mice confirmed the unique activation of Rictor (phosphorylation of Thr1135) in aged MFS mice. Total and phosphorylated (phos) Rictor expression was normalized to β-tubulin expression to account for loading differences between samples. FDR, false discovery rate; n.s., not significant. *Independent-samples t-test, P < 0.05.
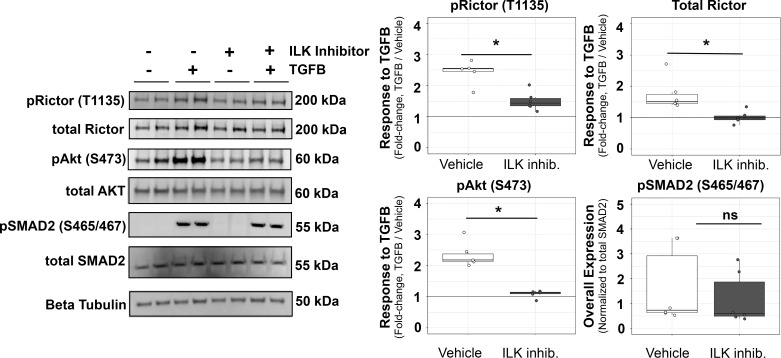
In breast cancer (43) and renal cells (24), TGF-β activates Rictor signaling in an ILK-dependent manner, but it is unknown whether this is also true in aortic VSMCs. In cultured VSMCs, we found that 1-h TGF-β incubation resulted in the phosphorylation of Rictor and Akt, and this effect was inhibited by pretreatment with a pharmacological inhibitor of ILK (Cpd 22, 3.5 μM; Fig. 5). Interestingly, canonical TGF-β signaling, as measured by phosphorylation of SMAD2, was not affected by ILK inhibition. There was a trend for a slight difference in the stability of total Rictor in the presence of TGF-β, as blots for total Rictor protein showed slightly, but not significantly, higher expression levels relative to vehicle (data not shown), although ILK inhibitor did not affect total Rictor levels or total levels of Akt protein as would be expected for this short incubation and stimulation protocol. Thus, we showed that TGF-β activates Rictor signaling within VSMCs in vitro. Data from the proteomic analysis of the in vivo aorta suggested consistently activated TGF-β signaling (Fig. 1B) and demonstrated significantly increased ILK expression in both young and old MFS aortas compared with WT aortas (log2FC MFS vs. WT of 1.17 and 0.85 in young and aged mice, respectively; Supplemental Fig. S1). Taken together, these observations suggest that an additional factor may mediate the accessibility of Rictor for activation by TGF-β.
Fig. 5.
Transforming growth factor (TGF)-β induction of rapamycin-independent component of mammalian target of rapamycin (Rictor) phosphorylation within in vitro cultured smooth muscle cells is dependent on integrin-linked kinase (ILK). Quantification of Western blot data demonstrating the phosphorylation (p) of Rictor (Thr1135), its downstream target Akt (Ser473), and the canonical TGF-β signaling target SMAD2 (p465/467) after 1-h TGF-β stimulation (10 ng/ml, 1 h) in the presence and absence of an ILK inhibitor (Cpd 22, 3.5 μM, 3-h preincubation before TGF-β1 stimulation). The enhanced chemiluminescence signal of phosphorylated proteins was normalized to the total protein signal for each target. Total Rictor was normalized to β-tubulin. Representative blots are shown at left. Inhib., inhibitor; ns, not significant. Groups were compared using an independent-samples t-test. *P < 0.05.
Altered Pattern of Integrin Expression in the Aged MFS Aorta
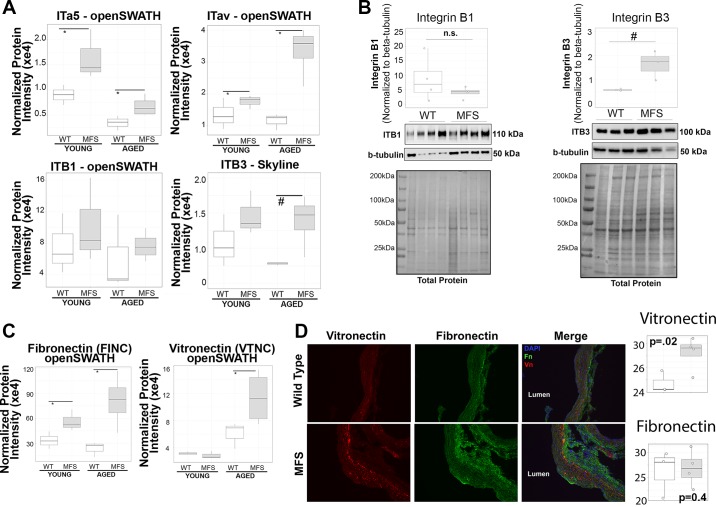
Given 1) the demonstrated dependence of TGF-β-induced Rictor activation on ILK activity in VSMCs, 2) our proteomics data suggestion of uniformly elevated TGF-β activation and observed ILK expression in vivo, and 3) the well-characterized interaction between ILK and the cytoplasmic tails of β-integrins (16), we hypothesized that differences in integrin expression profile may modulate Rictor-dependent signaling by TGF-β in aortic VSMCs. Analysis of smooth muscle α5β1-integrin (ITα5β1) and αvβ3-integrin (ITαvβ3) from peptides quantified in the original library (α5, αv, and β1) or in a targeted reanalysis against an expanded library to capture ITβ3 indicated a particularly notable upregulation of ITαvβ3 in aged mice (Fig. 6A). Interestingly, whereas ITα5 was upregulated in both young and aged MFS mice, its cosubunit ITβ1 (ITα5β1) was not altered in MFS mice at either age. Western blot quantification (Fig. 6B) on an orthogonal group of mice supported the MS-based observation of higher ITβ3, but not ITβ1, in aged MFS mice. Our proteomic analysis also identified fibronectin, a classic ligand for ITα5β1, as consistently upregulated in both young and aged MFS mice, whereas vitronectin, an ECM ligand classically associated with ITαvβ3, was uniquely upregulated in aged, and not in young, MFS mice (Fig. 6C). Immunofluorescence staining (Fig. 6D) for fibronectin appeared to be higher in the adventitial regions relative to the middle of the aortic wall, whereas vitronectin staining was not only more localized to the aortic media but also noticeably more intense in the MFS aorta (n = 4) relative to WT aorta (n = 3). Quantification of median fluorescence intensity of vitronectin supported this observation and corroborated the overall conclusion that the vitronectin-ITαvβ3 ligand-receptor pair appears uniquely upregulated in aged MFS mice relative to WT mice. Interestingly, the immunofluorescence data did not recapitulate the MS differences observed for fibronectin expression, which may be due to differences in quantitative sensitivity between these methods or spatial distribution of fibronectin captured by immunofluorescence analysis and not by bulk homogenization of the fully intact ascending aortas in MS. The full panel of images from vitronectin and fibronectin staining as well as negative control images are presented in Supplemental Master Data File S1. Taken together, these data led us to the hypothesis that the combination of increased vitronectin expression along with increased ITαvβ3 expression is correlated with the potentiation of Rictor signaling during aneurysm progression in the MFS aorta.
Fig. 6.
Identification of β3-integrin (ITβ3) as a potential mediator of rapamycin-independent component of mammalian target of rapamycin (Rictor) activation in aged Marfan syndrome (MFS) mice. A: protein-level data for α5-integrin (ITα5), β1-integrin (ITβ1), and αv-integrin (ITαv) identified from the original proteomic analysis along with a targeted reanalysis of data-independent acquisition mass spectrometry files to identify and quantify ITβ3. *mapDIA false discovery rate (FDR) of <1% from the original proteomic analysis; #independent-samples two-tailed t-test, P < 0.05 for Skyline quantification. B: Western blot confirmation of ITβ1 [n = 4, wild-type (WT) and MFS] and ITβ3 (n = 3, WT and MFS) expression in the aorta of aged mice, with integrin signals normalized to β-tubulin to account for loading differences. #Independent-samples two-tailed t-test, P < 0.05; n.s., not significant. C: quantification of fibronectin and vitronectin expression from original proteomics analysis. Statistics were performed in mapDIA software. *FDR < 1%. D: immunofluorescence analysis of vitronectin and fibronectin expression and localization in ×20 magnification images of the aorta of aged WT (n = 3) and MFS (n = 4) mice [blue, DAPI cell nuclei; red, vitronectin (Vn); green, fibronectin (Fn)]. Quantification of mean intensity for each marker is shown at the right. Independent-samples t-test P values are given on each comparison panel.
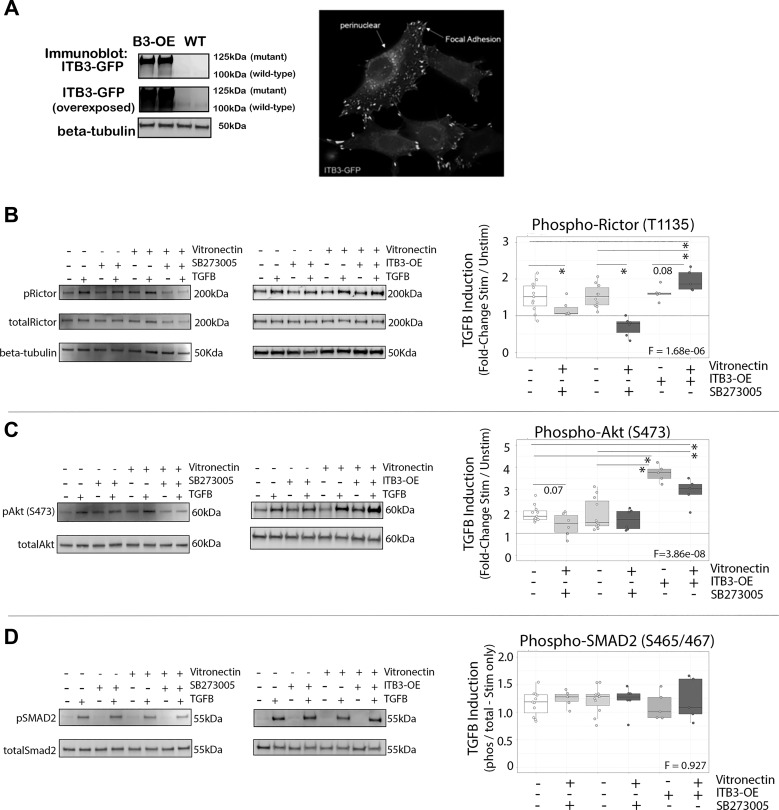
In Vitro Analysis of Vitronectin and ITβ3 as Modulators of TGF-β-Induced Rictor Signaling and VSMC Physiology
To test the relative influences of vitronectin and ITβ3 expression on TGF-β-induced Rictor signaling, we generated a line of VSMCs stably overexpressing ITβ3 (ITβ3-OE; Fig. 7A) and plated both WT and ITβ3-OE VSMCs on either uncoated or vitronectin-coated culture dishes in the presence or absence of the ITβ3 inhibitor SB-273005. In WT VSMCs, ITβ3 inhibition abrogated TGF-β-induced Rictor and downstream target Akt phosphorylation (Fig. 7, B and C). Conversely, when incubated overnight on immobilized vitronectin, ITβ3 potentiated TGF-β-induced Rictor activation in VSMCs. Interestingly, whereas phosphorylation of Akt was enhanced in ITβ3-OE VSMCs on both vitronectin or plastic, the net fold change appeared slightly smaller in the vitronectin condition. This observation was likely due to the presence of slightly higher baseline Akt phosphorylation in ITβ3-OE cells plated on vitronectin. Finally, canonical TGF-β signaling, as measured by phospho-SMAD2, was not affected by vitronectin or ITβ3 overexpression (Fig. 7D). Thus, the overall Rictor phosphorylation within VSMCs in response to TGF-β stimulation was accentuated by ITβ3-OE and blocked with ITβ3 inhibition, whereas canonical TGF-β signaling appeared unaffected.
Fig. 7.
Roles of vitronectin and β3-integrin (ITβ3) in mediating transforming growth factor (TGF)-β-induced rapamycin-independent component of mammalian target of rapamycin (Rictor) activation. A: confirmation of functional ITβ3 overexpression (ITβ3-OE) in vascular smooth muscle cells (VSMCs). Blots at the top and middle demonstrate ITβ3-green fluorescent protein (GFP) fusion protein expression (125 kDa) as well as light native ITβ3 expression (100 kDa) that was only detectable with higher exposure of blot membrane. The blot at the bottom shows β-tubulin loading control. The image on the right shows GFP-ITβ3 expression localized to the perinuclear region and focal adhesions as expected. B–D: Western blot analysis of in vitro effect of TGF-β1 stimulation on Rictor (B), Akt (C), and SMAD2 (D) phosphorylation in VSMCs (n = 5 per group) with or without the ITβ3 inhibitor SB-273005 (100 nM) or ITβ3-OE plated on regular plastic or vitronectin-coated cell culture dishes. Representative blots are shown for the ITβ3 inhibitor (left) and ITβ3-OE (right) data separately. Quantitative data for phospho-Rictor (pRictor) and pAkt are presented as the fold changes of TGF-β-stimulated (Stim) versus unstimulated (Unstim) intensity for each condition after normalization to total Rictor or total Akt protein. Data for pSMAD2 are presented as the ratio of pSMAD2 to total SMAD2 for the TGF-β-stimulated condition only. Phos, phosphorylated; WT, wild type. Groups were compared via one-way ANOVA followed by two-tailed, independent-samples pairwise post hoc t-tests. F-test P values are shown on each plot. *Post hoc comparison P < 0.05.
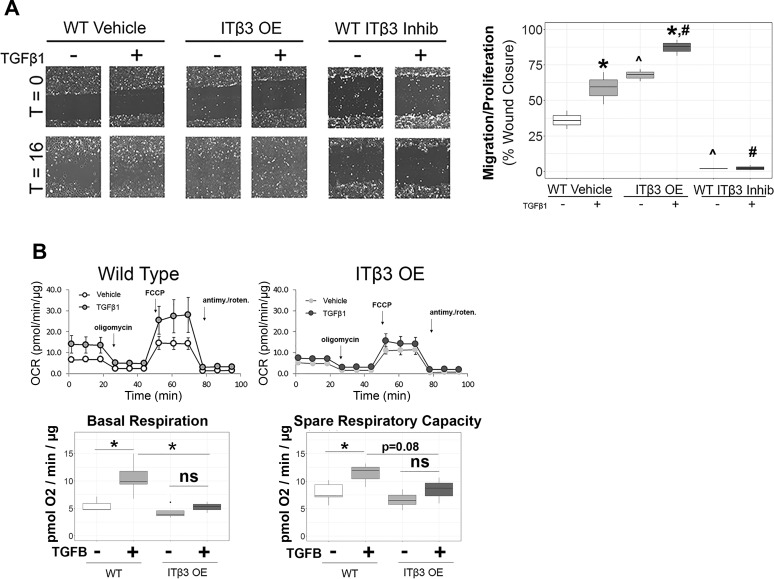
Finally, we determined whether VSMC physiology was affected by the observed Rictor-modulating ECM-integrin interactions. Consistent with the observed signaling events, overexpression of ITβ3 substantially potentiated, whereas inhibition of ITβ3 attenuated, the proliferative and migratory capacity of VTNC-seeded VSMCs, as measured by scratch assay, both under basal conditions and in response to 16 h of TGF-β (Fig. 8A), suggesting that ITβ3 potentiates a cellular growth and migration phenotype. In addition to migration and proliferation, we also observed a mitochondrial phenotype after ITβ3 overexpression, demonstrating that 48 h of TGF-β stimulation potentiated both basal mitochondrial respiration and spare respiratory capacity in WT VTNC-seeded VSMCs but not ITβ3-overexpressing VSMCs (Fig. 8B). These data support substantial alterations in the response of mitochondrial metabolism to TGF-β in ITβ3-overexpressing VSMCs relative to WT VSMCs, which is consistent with a role for ITβ3 in shunting TGF-β signaling to alternative effector pathways such as Rictor.
Fig. 8.
Effect of β3-integrin (ITβ3) and vitronectin on metabolic and migration-proliferation vascular smooth muscle cell (VSMC) physiology. A: assessment of in vitro proliferation and migration by scratch assay of VSMCs under wild-type (WT), ITβ3-overexpressing (ITβ3-OE), and ITβ3 inhibition (Inhib) conditions (n = 3 per group). Quantification is reported as the percent cell-occupied area in scratch at 16 h relative to the cell-occupied area at 0-h transforming growth factor (TGF)-β stimulation. T, time. B: quantification of mitochondrial respiration in vitro in WT (line graph on the top left and box plots on the left side of each panel below) and ITβ3-OE (line graph on the top right and box plots on the right side of each panel below) VSMCs after 48 h of serum starvation in the presence or absence of TGF-β1 (5 ng/ml). The overall oxygen consumption rate (OCR) was measured using the Seahorse assay. Antimy./roten., antimycin-rotenone; ns, not significant. Groups were compared using two-tailed, independent-samples t-test. *P < 0.05; ^P < 0.05 relative to the WT-unstimulated condition; #P < 0.05 relative to the WT-TGF-β-stimulated condition.
DISCUSSION
In the present study, we used discovery-scale protein expression screening to generate a novel series of hypotheses regarding ITβ3 influences on TGF-β signaling during aortic aneurysm in MFS. We show that in vitro overexpression of ITβ3 potentiates TGF-β-induced Rictor activation and alters the physiology of aortic VSMCs in vitro to that consistent with changes observed during aneurysm in vivo. Furthermore, informed by a novel observation of profound reductions in mitochondrial protein expression in aged MFS aortas, we demonstrate, for the first time in vitro, that overexpression of ITβ3 modulates the metabolic response of VSMCs to TGF-β stimulation.
These data represent the most complete proteomic data set of the MFS mouse ascending aorta generated to date, and the inclusion of aortic specimens from both young and aged mice enabled the visualization of proteomic changes that precede substantial aneurysm pathology as well as those that may occur as a consequence of aortic remodeling. There were greater proteomic differences between aged MFS and WT mice relative to their younger counterparts; indeed, nearly half of the identified proteins demonstrated differences. Thus, unsurprisingly, as aneurysm progresses, the molecular phenotype of the aorta becomes substantially altered. There is a common molecular phenotype that is altered at both stages, as indicated by the 57 proteins we found to be affected in both young and aged MFS mice compared with WT mice. These may represent seminal elements of MFS-derived aneurysm pathogenesis. From our IPA, it is clear that altered cytoskeletal dynamics, ECM components, and cell adherence signaling are the dominant functional themes organizing proteins altered in both young and aged MFS aortas. These proteomic observations are corroborated, in part, by a recent report that found VSMC phenotype changes in human MFS tissues and their subsequently cultured cells, characterized by increased actin stress fibers, cellular stiffness, and focal adhesion formation (11). These authors demonstrated that many of these changes are dependent on the altered TGF-β signaling generated by Fbn1 mutation, which is also consistent with our observation of TGF-β as the most prominent upstream regulator of the differentially abundant proteome between MFS and WT mice.
Comparison of the predicted upstream regulators of MFS proteome differences between young and aged mice suggested several regulatory pathways that changed in activation state with age/advanced disease. These included regulators of mitochondrial biogenesis (PPARs, PGC-1α, and INSR) and, interestingly, Rictor signaling. We focused on Rictor as a particularly interesting regulator to emerge from this analysis as it has been shown to negatively regulate mitochondrial metabolism (5) and is a known noncanonical pathway downstream of TGF-β receptor activation in other disease models (24, 43). We verified the informatically predicted activation states of Rictor in vivo by Western blot analysis of aortas from a separate cohort of young and aged mice, showing an increase in Rictor expression and phosphorylation, uniquely in aged MFS mice. Furthermore, our in vitro experiments confirmed that TGF-β is indeed an activator of Rictor in VSMCs with corresponding downstream Akt (Ser473) phosphorylation subsequent to TGF-β stimulation. These data indicate that Rictor is a novel, noncanonical TGF-β effector whose activation is context dependent in MFS mice in that it emerges only later in disease despite constant TGF-β stimulation. We show in vitro that TGF-β-induced Rictor activation is modulated by the expression of additional factors such as ITβ3 and vitronectin, whose expression in vivo is associated with Rictor activation state.
Presently, there is evidence that ITαvβ3 potentiates TGF-β signaling (1, 13, 41), and both angiotensin II (15) and TGF-β (35), as well as overall increases in tissue stiffness (38), have been shown to promote increased ITαvβ3 expression and/or its association with TGF-β receptors. Consistent with these data, we observed a particularly strong increase in expression of ITαv and ITβ3 in aged MFS mice relative to WT mice. Aging in MFS was also uniquely associated with an increase in vitronectin, a major ligand for ITαvβ3. Furthermore, we show that in vitro overexpression of ITαvβ3 promotes, whereas inhibition of ITαvβ3 blocks, increased TGF-β-induced Rictor phosphorylation in cultured VSMCs. In cancer cell lines, ITαvβ3 is associated with malignancy and a migratory-proliferative phenotype, which is consistent with some reports of VSMC physiology in aneurysm (11, 26). We demonstrate here that in vitro ITβ3 overexpression promotes, and ITβ3 inhibitor treatment blocks, the migration and proliferation of VSMC cells in the scratch wound healing assay. Although the key drivers of ITβ3 overexpression and the full spectrum of molecular perturbations downstream of ITβ3 are yet unclear, our data support a novel hypothesis that changes in ITβ3 during MFS progression may modulate the accessibility of Rictor to TGF-β activation, possibly contributing to pathogenic VSMC physiology such as migration and proliferation.
Another remarkable observation was a profound reduction in mitochondrial protein expression in MFS mice that was unique to aged mice with more advanced aortic pathology. This observation in our knockin mouse model of MFS was recently corroborated in a model of aneurysm caused by fibulin-4 deficiency (49). Furthermore, the same authors have shown reduced mitochondrial respiration in VSMCs cultured from human patients with MFS or Loeys-Dietz syndrome. Consistent with our proteomic data demonstrating a predicted downregulation of PGC-1α in aged mice, these authors also found a reduced PGC-1α activity in aneurysm samples and went on to show TGF-β-mediated regulation of PGC-1α in vitro (49). In other models of cardiovascular disease, this altered metabolic phenotype mirrors that seen in atherosclerotic lesions (29), failing myocardium (2), and some tumor cells as they progress to more malignant phenotypes (27). Chan and colleagues (4) have recently shown that an increase in tissue/ECM stiffness corresponds to a decrease in mitochondrial metabolism and a shift from oxidative to glycolytic metabolic pathways in pulmonary VSMCs (e.g., the so-called “Warburg shift”) during the pathogenesis of pulmonary hypertension. Multiple reports have demonstrated a marked increase in stiffness (e.g., reduced compliance) in the MFS aorta relative to the WT aorta (10, 30). As mentioned above, tissue stiffness is a strong driver of ITβ3 expression (38), and this observation coupled with that of Chan and colleagues collectively represents a plausible mechanism linking ECM stiffness and ITβ3-modulated TGF-β signaling to reduced oxidative/mitochondrial metabolism in MFS VMSCs during aneurysm. We observed notable shifts in mitochondrial metabolism in vitro for WT VSMCs in response to TGF-β, an effect that was blocked in the presence of ITβ3 overexpression, which is consistent with a role for this integrin in shunting TGF-β signaling toward the Rictor pathways, with at least one consequence being altered TGF-β effects on mitochondrial metabolism. Although it remains to be seen whether mitochondrial changes in aged MFS mice are central to late-stage pathogenesis or are a compensatory/corollary consequence of more critical drivers, several recent reports have highlighted the importance of optimal mitochondrial function, and the balance between mitogenesis and mitophagy, during cardiac and vascular health and disease (7). We observed increased expression of two mediators of mitophagy and mitochondrial fission, PARK7 and FIS1, in the MFS aorta relative to the WT aorta. Changes in ECM composition have recently been linked to defective mitochondrial function (21, 49), the effects of which, in some models, appear to be attenuated by losartan (34), an angiotensin II type 1 receptor inhibitor that potently attenuates aneurysm in the MFS mouse model (16). Taken together, the novel in vivo and in vitro data presented here demonstrate that the role of mitochondrial (dys)function in aneurysm progression is an important area for future study.
There are a number of caveats and limitations regarding these findings. First, these data were generated from male animals. The fact that two-thirds of ascending aneurysm cases occur in human male patients supports the value of these data despite conclusions being limited to a single sex; however, it will still be necessary to perform similar comparisons in female and mixed-sex cohorts to maximize generalizability. Second, these experiments were conducted on a small sample size, and additional evidence for preclinical value of these findings is warranted in larger animal cohorts. Although the overall number of proteins quantified is relatively small (1,079 proteins), it does represent the largest proteomic data set to date that has been collected on the ascending thoracic aorta in MFS. Sample input limitations make protein extraction particularly challenging for these miniscule specimens. Ongoing improvements in sample preparation and MS sensitivity will improve the depth of proteome coverage possible from small sample inputs. Despite the restrained proteome coverage and small sample size, we identified both known and novel molecular signatures in MFS mice and have validated a handful of these with orthogonal experiments in the present study.
In summary, label-free quantitative proteomic analysis of MFS versus WT ascending aortas at two different stages in aneurysm progression generated novel insights regarding molecular pathogenesis and identified Rictor signaling and ITβ3 as potential new targets for therapeutic intervention in aortic aneurysm that warrant further experimental testing. We labeled our observed Rictor activation as “context dependent,” given that its presumed activation by TGF-β is dependent on factors associated with aneurysm progression, including an apparent link to the expression-level ITβ3 in aortic VSMCs. We validated this link in vitro, although the dependency of Rictor activation on ITβ3 in vivo requires further study, as our in vivo data only demonstrate correlation of these two events and not causation. Our observations in vitro linking matrix sensing by a specific integrin to the modulation of TGF-β-induced Rictor may have broad-reaching implications, as there are multiple pathologies where changes in the physical properties of the ECM and tissue microenvironment (i.e., increased stiffness or matrix sensing) coincide with TGF-β signaling and shifts in cellular metabolism and pathological progression. These include wound healing, cardiac and renal fibrosis, atherosclerosis, and tumor metastasis. Ongoing work to validate these connections beyond the aorta/VSMC model are warranted to clarify the generalizability of these observations. It is important to note that these experiments were not designed to confidently ascertain causation or the centrality of any of the molecular systems and pathways identified as altered in MFS to pathogenesis. Instead, these data are meant to serve as a starting point for ongoing research efforts by highlighting new components in thoracic aortic aneurysm that can fill in functional gaps between the known genetic drivers of thoracic aneurysm and the full pathogenesis of the disease. Among these, ECM-dependent integrin signaling, Rictor, and mitochondrial dynamics warrant further study to clarify their role and therapeutic potential in aneurysm progression. Additionally, although we focused here on one important integrin species, ITαvβ3, there may be other integrins or important integrin cross-talk elements that add to the complexity of aortic physiology during aneurysm development. Furthermore, several other interesting upstream regulators and molecular correlates of aneurysm were identified in this large-scale proteomic analysis, including other metabolic regulators (PPARs, PGC-1α, and INSR) and other effectors of MAPK signaling (MAP4K4), exemplifying the depth of potentially informative content gleaned from this discovery-scale proteomic analysis of the mouse aorta.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute K99 Pathway to Independence Grant 1-K99-HL-128787-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.P., A.S., E.M., N.W., A.O., V.V., K.M., R.G., H.C.D., and J.E.V.E. conceived and designed research; S.J.P., A.S., N.W., A.O., V.V., and K.M. performed experiments; S.J.P., A.S., A.O., V.V., and K.M. analyzed data; S.J.P., A.S., E.M., N.W., V.V., K.M., R.G., H.C.D., and J.E.V.E. interpreted results of experiments; S.J.P. and K.M. prepared figures; S.J.P. drafted manuscript; S.J.P., A.S., E.M., N.W., A.O., V.V., K.M., R.G., H.C.D., and J.E.V.E. edited and revised manuscript; S.J.P., A.S., E.M., N.W., A.O., V.V., K.M., R.G., H.C.D., and J.E.V.E. approved final version of manuscript.
Supplemental Data
ACKNOWLEDGMENTS
We thank Dr. Ronald Holewinski for assistance with mass spectrometry acquisitions.
REFERENCES
- 1.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J Immunol 175: 7708–7718, 2005. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 2.Azevedo PS, Minicucci MF, Santos PP, Paiva SA, Zornoff LA. Energy metabolism in cardiac remodeling and heart failure. Cardiol Rev 21: 135–140, 2013. doi: 10.1097/CRD.0b013e318274956d. [DOI] [PubMed] [Google Scholar]
- 3.Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth CA, Humphries MJ, Kielty CM. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by α5β1 and αvβ3 integrins. J Biol Chem 278: 34605–34616, 2003. doi: 10.1074/jbc.M303159200. [DOI] [PubMed] [Google Scholar]
- 4.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J III, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN. mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci USA 110: 12526–12534, 2013. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruderer R, Bernhardt OM, Gandhi T, Reiter L. High-precision iRT prediction in the targeted analysis of data-independent acquisition and its impact on identification and quantitation. Proteomics 16: 2246–2256, 2016. doi: 10.1002/pmic.201500488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiong M, Cartes-Saavedra B, Norambuena-Soto I, Mondaca-Ruff D, Morales PE, García-Miguel M, Mellado R. Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Front Cell Dev Biol 2: 72, 2014. doi: 10.3389/fcell.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung AW, Au Yeung K, Cortes SF, Sandor GG, Judge DP, Dietz HC, van Breemen C. Endothelial dysfunction and compromised eNOS/Akt signaling in the thoracic aorta during the progression of Marfan syndrome. Br J Pharmacol 150: 1075–1083, 2007. doi: 10.1038/sj.bjp.0707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung AW, Au Yeung K, Sandor GG, Judge DP, Dietz HC, van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res 101: 512–522, 2007. doi: 10.1161/CIRCRESAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 10.Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res 102: e73–e85, 2008. doi: 10.1161/CIRCRESAHA.108.174367. [DOI] [PubMed] [Google Scholar]
- 11.Crosas-Molist E, Meirelles T, López-Luque J, Serra-Peinado C, Selva J, Caja L, Gorbenko Del Blanco D, Uriarte JJ, Bertran E, Mendizábal Y, Hernández V, García-Calero C, Busnadiego O, Condom E, Toral D, Castellà M, Forteza A, Navajas D, Sarri E, Rodríguez-Pascual F, Dietz HC, Fabregat I, Egea G. Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome. Arterioscler Thromb Vasc Biol 35: 960–972, 2015. doi: 10.1161/ATVBAHA.114.304412. [DOI] [PubMed] [Google Scholar]
- 12.Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum Mol Genet 4, Suppl 1: 1799–1809, 1995. doi: 10.1093/hmg/4.suppl_1.1799. [DOI] [PubMed] [Google Scholar]
- 13.Galliher AJ, Schiemann WP. β3 Integrin and Src facilitate transforming growth factor-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res 8: R42, 2006. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatto L, Christoforou A. Using R and Bioconductor for proteomics data analysis. Biochim Biophys Acta 1844, Part A: 42–51, 2014. doi: 10.1016/j.bbapap.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Graf K, Neuss M, Stawowy P, Hsueh WA, Fleck E, Law RE. Angiotensin II and αvβ3 integrin expression in rat neonatal cardiac fibroblasts. Hypertension 35: 978–984, 2000. doi: 10.1161/01.HYP.35.4.978. [DOI] [PubMed] [Google Scholar]
- 16.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121, 2006. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilton BJ, Wolkowicz R. An assay to monitor HIV-1 protease activity for the identification of novel inhibitors in T-cells. PLoS One 5: e10940, 2010. doi: 10.1371/journal.pone.0010940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332: 358–361, 2011. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 21.Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, Bernardi P, Bonaldo P. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet 35: 367–371, 2003. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 22.Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor β-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem 278: 2750–2757, 2003. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- 23.Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 114: 172–181, 2004. doi: 10.1172/JCI200420641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Ren J, Liu X, Jiang L, He W, Yuan W, Yang J, Dai C. Rictor/mTORC2 signaling mediates TGFβ1-induced fibroblast activation and kidney fibrosis. Kidney Int 88: 515–527, 2015. doi: 10.1038/ki.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao M, Liu Z, Bao J, Zhao Z, Hu J, Feng X, Feng R, Lu Q, Mei Z, Liu Y, Wu Q, Jing Z. A proteomic study of the aortic media in human thoracic aortic dissection: implication for oxidative stress. J Thorac Cardiovasc Surg 136: 65–72.e3, 2008. doi: 10.1016/j.jtcvs.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 473: 308–316, 2011. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett 356, Part A: 156–164, 2015. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26: 966–968, 2010. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 100: 460–473, 2007. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 30.Marque V, Kieffer P, Gayraud B, Lartaud-Idjouadiene I, Ramirez F, Atkinson J. Aortic wall mechanics and composition in a transgenic mouse model of Marfan syndrome. Arterioscler Thromb Vasc Biol 21: 1184–1189, 2001. doi: 10.1161/hq0701.092136. [DOI] [PubMed] [Google Scholar]
- 31.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol 13: 616–630, 2012. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto K, Maniwa T, Tanaka T, Satoh K, Okunishi H, Oda T. Proteomic analysis of calcified abdominal and thoracic aortic aneurysms. Int J Mol Med 30: 417–429, 2012. doi: 10.3892/ijmm.2012.985. [DOI] [PubMed] [Google Scholar]
- 33.Mellacheruvu D, Wright Z, Couzens AL, Lambert JP, St-Denis NA, Li T, Miteva YV, Hauri S, Sardiu ME, Low TY, Halim VA, Bagshaw RD, Hubner NC, Al-Hakim A, Bouchard A, Faubert D, Fermin D, Dunham WH, Goudreault M, Lin ZY, Badillo BG, Pawson T, Durocher D, Coulombe B, Aebersold R, Superti-Furga G, Colinge J, Heck AJ, Choi H, Gstaiger M, Mohammed S, Cristea IM, Bennett KL, Washburn MP, Raught B, Ewing RM, Gingras AC, Nesvizhskii AI. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nat Methods 10: 730–736, 2013. doi: 10.1038/nmeth.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Momota R, Narasaki M, Komiyama T, Naito I, Ninomiya Y, Ohtsuka A. Drosophila type XV/XVIII collagen mutants manifest integrin mediated mitochondrial dysfunction, which is improved by cyclosporin A and losartan. Int J Biochem Cell Biol 45: 1003–1011, 2013. doi: 10.1016/j.biocel.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Mori S, Kodaira M, Ito A, Okazaki M, Kawaguchi N, Hamada Y, Takada Y, Matsuura N. Enhanced expression of integrin αvβ3 induced by TGF-β is required for the enhancing effect of fibroblast growth factor 1 (FGF1) in TGF-β-induced epithelial-mesenchymal transition (EMT) in mammary epithelial cells. PLoS One 10: e0137486, 2015. doi: 10.1371/journal.pone.0137486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411, 2003. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 37.Okamura H, Emrich F, Trojan J, Chiu P, Dalal AR, Arakawa M, Sato T, Penov K, Koyano T, Pedroza A, Connolly AJ, Rabinovitch M, Alvira C, Fischbein MP. Long-term miR-29b suppression reduces aneurysm formation in a Marfan mouse model. Physiol Rep 5: e13257, 2017. doi: 10.14814/phy2.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page JM, Merkel AR, Ruppender NS, Guo R, Dadwal UC, Cannonier S, Basu S, Guelcher SA, Sterling JA. Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin β3 and TGF-β receptor type II. Biomaterials 64: 33–44, 2015. doi: 10.1016/j.biomaterials.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker SJ, Venkatraman V, Van Eyk JE. Effect of peptide assay library size and composition in targeted data-independent acquisition-MS analyses. Proteomics 16: 2221–2237, 2016. doi: 10.1002/pmic.201600007. [DOI] [PubMed] [Google Scholar]
- 40.Pilop C, Aregger F, Gorman RC, Brunisholz R, Gerrits B, Schaffner T, Gorman JH III, Matyas G, Carrel T, Frey BM. Proteomic analysis in aortic media of patients with Marfan syndrome reveals increased activity of calpain 2 in aortic aneurysms. Circulation 120: 983–991, 2009. doi: 10.1161/CIRCULATIONAHA.108.843516. [DOI] [PubMed] [Google Scholar]
- 41.Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA. αvβ3 Integrin interacts with the transforming growth factor β (TGFβ) type II receptor to potentiate the proliferative effects of TGFβ1 in living human lung fibroblasts. J Biol Chem 279: 37726–37733, 2004. doi: 10.1074/jbc.M403010200. [DOI] [PubMed] [Google Scholar]
- 42.Serhatli M, Baysal K, Acilan C, Tuncer E, Bekpinar S, Baykal AT. Proteomic study of the microdissected aortic media in human thoracic aortic aneurysms. J Proteome Res 13: 5071–5080, 2014. doi: 10.1021/pr5006586. [DOI] [PubMed] [Google Scholar]
- 43.Serrano I, McDonald PC, Lock FE, Dedhar S. Role of the integrin-linked kinase (ILK)/Rictor complex in TGFβ-1-induced epithelial-mesenchymal transition (EMT). Oncogene 32: 50–60, 2013. doi: 10.1038/onc.2012.30. [DOI] [PubMed] [Google Scholar]
- 44.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao S, Guo T, Gross V, Lazarev A, Koh CC, Gillessen S, Joerger M, Jochum W, Aebersold R. Reproducible tissue homogenization and protein extraction for quantitative proteomics using micropestle-assisted pressure-cycling technology. J Proteome Res 15: 1821–1829, 2016. doi: 10.1021/acs.jproteome.5b01136. [DOI] [PubMed] [Google Scholar]
- 46.Teo G, Kim S, Tsou CC, Collins B, Gingras AC, Nesvizhskii AI, Choi H. mapDIA: preprocessing and statistical analysis of quantitative proteomics data from data independent acquisition mass spectrometry. J Proteomics 129: 108–120, 2015. doi: 10.1016/j.jprot.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trask TM, Trask BC, Ritty TM, Abrams WR, Rosenbloom J, Mecham RP. Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J Biol Chem 275: 24400–24406, 2000. doi: 10.1074/jbc.M003665200. [DOI] [PubMed] [Google Scholar]
- 48.Tsou CC, Avtonomov D, Larsen B, Tucholska M, Choi H, Gingras AC, Nesvizhskii AI. DIA-Umpire: comprehensive computational framework for data-independent acquisition proteomics. Nat Methods 12: 258–264, 2015. doi: 10.1038/nmeth.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Pluijm I, Burger J, van Heijningen PM, IJpma A, van Vliet N, Milanese C, Schoonderwoerd K, Sluiter W, Ringuette LJ, Dekkers DH, Que I, Kaijzel EL, Te Riet L, MacFarlane E, Das D, van der Linden R, Vermeij M, Demmers JA, Mastroberardino PG, Davis EC, Yanagisawa H, Dietz H, Kanaar R, Essers J. Decreased mitochondrial respiration in aneurysmal aortas of fibulin-4 mutant mice is linked to PGC1A regulation. Cardiovasc Res. In press. doi: 10.1093/cvr/cvy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res 19: 128–139, 2009. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.