Abstract
Studies have shown that individuals with autism spectrum disorder (ASD) tend to perform significantly below typically developing individuals on standardized measures of attention, even when controlling for IQ. The current study sought to examine within ASD whether anatomical correlates of attention performance differed between those with average-to-above-average IQ (AIQ Group) compared to those with low-average to borderline ability (LIQ group) as well as in comparison to typically-developing controls (TDC). Using automated volumetric analyses, we examined regional volume of classic attention areas including the superior frontal gyrus, anterior cingulate cortex, and the precuneus in ASD AIQ (n = 38) and LIQ (n = 18) individuals along with 30 typically-developing controls (TDC). Auditory attention performance was assessed using subtests of the Test of Memory and Learning (TOMAL) compared among the groups and then correlated with regional brain volumes. Analyses revealed group differences in attention. The three groups did not differ significantly on any auditory attention-related brain volumes, however, trends toward significant size-attention function interactions were observed. Negative correlations were found between the volume of the precuneus and auditory attention performance for the AIQ ASD group, indicating larger volume related to poorer performance. Implications for general attention functioning and dysfunctional neural connectivity in ASD are discussed.
Keywords: auditory attention, attention, autism spectrum disorders, neurodevelopmental disorders, magnetic resonance imaging, volumetric findings
Attentional abilities in autism spectrum disorder (ASD) have been widely researched where problems with attention in children with ASD are often endorsed as a prominent deficit by both parents and teachers (Lecavalier, 2006). Such impairments in ASD may include difficulty disengaging attention (Townsend, Harris, & Courchesne, 1996) and impairments in the ability to shift mental focus of attention rapidly and accurately (Akshoomoff & Courchesne, 1992; Courchesne et al., 1994; Pascualvaca, Fantie, Papageorgiou, & Mirsky, 1998). Studies of attention in ASD have also focused on the role of attention in understanding social deficits and have revealed impaired joint attention (Bruinsma, Koegel, & Koegel, 2004; Mundy, Sullivan, & Mastergeorge, 2009; Toth, Munson, Meltzoff, & Dawson, 2006), attention to faces (Elsabbagh et al., 2013; Parish-Morris et al., 2013), attention to emotional tone (Ploog, Scharf, Nelson, & Brooks, 2013), and self-monitoring impairments associated with executive functioning tasks (Hill & Russell, 2002; Russell & Jarrold, 1999). Attentional strengths have also been reported in autism, including evidence for normal or even better than average selective attention abilities (Allen & Courchesne, 2001; Johnson et al., 2007), as well as ability to sustain attention in certain contexts (Garretson, Fein, & Waterhouse, 1990; Siegel et al.,1992; Siegel, Nuechterlein, Abel, Wu, & Buchsbaum, 1995).
Measurement of Attentional Processes in Relation to Attention Theory
Several traditional neuropsychological tests have been used to assess attention and working memory in both clinical and research contexts. Two of the most common are digit span and letter span (Lezak, Howieson, Bigler & Tranel, 2012). Both of these measures are simple tasks that begin with either listening to or viewing a string of single numbers or letters that must be repeated in the same order (i.e., focused attention) or reverse order (i.e., working memory). Typically, the task begins with two or three single numbers or letters, and then another number or letter is added with each successful trial.
Operationally, this task is thought to reflect the attentional processes needed to intentionally process stimuli (Robertson, Manly, Andrade, Baddeley, & Yiend, 1997). Neurobiologically, attention often has been conceptualized within three functionally independent attentional networks that operate distinct cognitive processes but also integrate across networks for effective cognitive processing. A variety of different terms have been used to describe these attention networks, including the “alerting,” “orienting,” and “executive control” networks (Posner & Petersen, 1990; Petersen & Posner, 2012). While an outline of the exact profile of attention in ASD is still ongoing (Allen & Courchesne, 2001; Chita-Tegmark, 2016; Courchesne et al., 1994; Goldstein, Johnson, & Minshew, 2001; Dawson et al., 2004; Murza, Schwartz, Hahs-Vaughn, & Nye, 2016; Swettenham et al., 1998), specific deficits in all three networks of attention (see Posner & Petersen, 1990) have been found. Specifically, impairments in the alerting network have been related to potential differences in autonomic reactivity in ASD (Anderson & Colombo, 2009; Anderson, Colombo, & Unruh, 2013; Neuhaus, Bernier, & Beauchaine, 2016) and insensitivity to novel information (Greenaway & Plaisted, 2005; Keehn & Joseph, 2008). Additionally, dysfunction in orienting networks has been found in individuals with ASD when presented with visuospatial information (Keehn, Lincoln, Muller, & Townsend, 2010) as well as in social contexts (Dawson et al., 2004). Finally, executive dysfunction has been extensively studied in ASD, with evidence of impaired set shifting (see Chen et al., 2016; Westwood, Stahl, Mandy, & Tchanturia, 2016; Yerys et al., 2009) as well as shifting attention (Richard & Lajiness-O’Neill, 2015) and poor inhibitory control in later childhood and adolescence (Luna, Doll, Hegedus, Minshew, & Sweeney, 2007; Padmanabhan et al., 2015; Sanderson & Allen, 2013; Solomon, Ozonoff, Cummings, & Carter, 2008). Indeed, attentional atypicalities and their neuroanatomical correlates have been associated with increased autism symptomatology (Belmonte, Gomot, & Baron-Cohen, 2010; Gomot, Belmonte, Bullmore, Bernard, & Baron-Cohen, 2008; Joseph, Keehn, Connolly, Wolfe, & Horowitz, 2009; Keehn & Joseph, 2008; Keehn et al., 2010). Some researchers postulate that primary deficits in attention and inhibitory controls may account for the executive dysfunctions that underlie many behaviors in ASD (Burack, 1994; Schmitz et al., 2006) and may lead to the later development of higher-level social and communication deficits (Belmonte & Yurgelun-Todd, 2003; Gold & Gold, 1975).
Studies of brain function in autism have shown that functional connectivity abnormalities are most prevalent within the association cortex, particularly connections between and within the brain’s default mode network and brain attentional networks such as the salience network (Anderson et al., 2011; Nielsen et al., 2013; Odriozola et al., 2016; Uddin and Menon, 2009). Further, connections within the salience network, which processes attention and orientation to novel information and participates in set switching behaviors, show the highest accuracy in predicting whether an individual may be classified as ASD (Elton, Di Martino, Hazlett, & Gao, 2016; Uddin et al., 2013). In longitudinal studies evaluating prognosis, connectivity involving the salience and frontoparietal executive control networks predicted outcomes at more than one year post scan in social symptomatology and adaptive behavior (Plitt, Barnes, Wallace, Kenworthy, & Martin, 2015). These studies of brain function highlight that brain connections involving attentional networks are among the most impaired in autism, serve as key prognostic indicators of function, and may comprise a core pathophysiological substrate for diverse autistic symptoms.
Clinically, attention deficit hyperactivity disorder (ADHD) has a high comorbidity with ASD, with estimates ranging from 53–78% of children with ASD who either have symptoms or carry a diagnosis of ADHD (Lee & Ousley, 2006; Ponde, Novaes, & Losapio, 2010; Simonoff et al., 2008; Yoshida & Uchiyama, 2004). In fact, some researchers believe that the attentional impairments seen in ASD may reflect shared underlying etiological processes between ASD and ADHD (Taylor et al., 2013). Indeed, Musser et al. (2014) have shown ASD disorders shares a familial transmission with ADHD.
Posner and Petersen (1990) originally reviewed cerebral blood flow studies indicating activation in the lateral superior frontal area is involved with both tasks of language and spatial imagery and activation in the anterior cingulate is sensitive to target detection, language, and visual location. Posner, Walker, Friedrich, and Rafal (1987) assert participants with parietal lesions demonstrate slowed orientation to a visual cue when monitoring a stream of auditory information, implicating the parietal lobe in auditory attention tasks. Further, positron emission topography studies in auditory discrimination tasks find a relation between metabolic rates in the middle prefrontal cortex and accuracy suggesting the middle prefrontal area is of import in sustained attention (Cohen et al., 1988). These classic studies suggest a relation between laboratory tasks requiring auditory attention and the above mentioned neuroanatomical correlates (i.e., superior frontal, anterior cingulate, parietal lobe, and the middle prefrontal area) in the study of attention function. These networks are dynamic and interactive without necessarily precise boundaries (Farrant & Uddin, 2016; Uddin, Supekar, & Menon, 2010).
Brain Regions and Networks Implicated in Attentional Processes
The first task necessary for attention is alerting (Posner & Petersen, 1990; Petersen & Posner, 2012). Alerting originates from reticular activation that engages the diffuse thalamic projection system and sensory systems with other salient activations that direct attention (Brown, Basheer, McKenna, Strecker, & McCarley, 2012; Halassa et al., 2014; Jones, 2009). The so-called salience network (SN) involves the activity of the anterior cingulate cortex (ACC) and anterior insular cortex (Corbetta, Patel, & Shulman, 2008; Posner & Petersen, 1990; Seeley et al., 2007).
Another aspect of attentional systems is the default mode network or DMN (Broyd et al., 2009; Buckner, Andrews-Hanna, & Schacter, 2008; Raichle et al., 2001) which directs attention to internal stimuli and is deactivated during tasks involving externally focused attention (Fox et al., 2005). Synergism between the SN and DMN characterizes engagement and disengagement of directed attention (Anderson, Ferguson, Lopez-Larson, & Yurgelun -Todd, 2011), and is specifically abnormal in autism (Kennedy, Redcay, & Courchesne, 2006).
Orienting tasks are responsible for the selection of information from sensory input (Keehn, Muller, & Townsend, 2013) and are thought to be controlled by temporal and parietal areas as well as by the thalamus and cerebellum (Akshoomoff, Courchesne, & Townsend, 1997; Corbetta & Shulman, 2002; Mesulam, 1990; Pelisson, Goffart, & Guillaume, 2003; Posner & Petersen, 1990). It is now well established that there are extensive functional connectivity networks involved in alerting and orienting (Salman and Tsai, 2016; Sang et al., 2012).
The executive control tasks of attention include inhibition, set shifting, working memory, and cognitive flexibility (Keehn et al., 2013). Prefrontal, medial frontal, and subcortical regions such as the basal ganglia and cerebellum are all thought to participate in this network (Heyder, Suchan, & Daum, 2004), as well as the precuneus (Cavanna & Trimble, 2006; Teixeira et al., 2014). Finally, sustained attention is associated with the prefrontal cortex (Jagtap & Diwadkar, 2016; Stuss, Shallice, Alexander, & Picton, 1995).
Attention-Related Structural and Functional Patterns and Autism
Using structural magnetic resonance imaging (MRI) techniques, Zielinski et al. (2012) demonstrated differences in both the SN and DMN in individuals with autism in comparison to typically developing controls (TDC), while Lim et al. (2013) identified the cerebellar regions, caudate/thalamus, anterior cingulate, inferior frontal cortex, middle and superior temporal and right inferior parietal regions as distinct from those participants with ADHD and healthy control subjects. A functional MRI study of sustained attention in autism conducted by Christakou et al. (2013) found that individuals with autism had significantly reduced activation relative to healthy controls (but similar activation to an ADHD sample) in the bilateral striato-thalamic regions, left dorsolateral prefrontal cortex (DLPFC), and superior parietal cortex. However, ASD individuals displayed increased precuneus activation, which was negatively correlated with DLPFC activation and became more pronounced with increased attentional demands (see also, Gadgil, Peterson, Tregellas, Hepburn, & Rojas, 2013). Overall, the literature suggests distinct attentional patterns for individuals with autism likely differ from those with typical development.
Current study
While the cognitive and neuropsychological study of attentional deficits in autism is not new, few studies have specifically examined neuroanatomical volumetric correlates, especially with traditional neuropsychological assessment methods. In the current investigation, we sought to examine the relation between key anatomical regions of interest (ROIs), described previously and known to be involved in attention. Emotional processing relevant to directed attention is not reported herein; limbic regions were not an ROI target of this investigation. The focus of this investigation was on cortical areas involved in the SN and DMN such as the superior frontal region, anterior cingulate, and the cerebellum. Total gray matter and total brain volume were also examined.
The Test of Memory and Learning (TOMAL, Reynolds & Bigler, 1994) is a standardized clinical measure of memory that includes digit and letter span tasks. Examination of memory function between ASD and TDC (see Southwick et al., 2011; Trontel et al., 2015) demonstrated differences in memory systematically relating to differences in intellectual functioning. As a group, children with ASD are more likely to have lower intellectual scores when compared to TDC children (Charman et al., 2011; Mouga et al. 2016), making it challenging to match on IQ. Therefore, the current investigation separated the ASD subjects into subgroups of those with an average IQ (AIQ; defined as verbal IQ (VIQ) ≥ 85) and those with low IQ (LIQ; defined as VIQ ≤ 84) (see Boucher, Mayes, & Bigham, 2012). The VIQ index was chosen, as the digit span and letter span subtests are both verbally mediated tasks. In this way, the main effect of ASD on measures of attention may still be examined and having two groups that differ in IQ permits better demographic pairing with the control sample, none of whom had verbal IQ scores <85.
Using the digit span and letter span subtests from the TOMAL (Reynolds & Bigler, 1994), AIQ and LIQ ASD groups were compared to TDC subjects examining focused attention and working memory. Strauss, Sherman, and Spreen (2006) acknowledge that current neuropsychological tasks of attention measure a combination of attentional and executive functions, noting no “pure” tasks measuring only attention exist. Digit and letter span tests measure attentional capacity involving auditory attention and short-term retention capacity (Lezak et al., 2012) and seem to be a good fit for the study of auditory attention. The automated FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) method was used to segment and compute volumes of ROI structures associated with attention including the superior frontal gyrus, anterior cingulate cortex, precuneus, and cerebellum. We hypothesized a main effect of ASD associated with lower performance on standardized measures of attention compared to TDC subjects. We further hypothesized that within the ASD groups, the AIQ group would exhibit higher digit and letter span scores when compared to the LIQ ASD group. Finally, we hypothesized that the ROI volume-by-attention-performance relation would be different between ASD and TDC subjects. Although we tested the above stated hypotheses, since we used standard clinical and not experimental measures designed to manipulate level of attention and processing, the nature of the current investigation is very much a descriptive study of how TOMAL measures of attention appear in ASD.
Method
Ascertainment
Subjects were recruited predominantly from community sources, including parent support groups, youth groups, schools, and from clinic social skills groups. The subjects in this study are a subset of individuals in a longitudinal investigation of late brain development from three years of age through early adulthood, with the initial findings of TOMAL performance previously reported by Southwick et al. (2011). Testing was performed by trained psychometricians as part of a research study on brain structure in autism. In brief, the investigation included the administration of a full neuropsychological battery that included measures of intellectual function, memory and learning, language, visuospatial processing, executive function and attention, sensorimotor, academic, developmental and social perception, and personality testing. The order of the testing was consistent but for some participants was broken up over two testing sessions. The subset of individuals presented in this investigation was selected from the larger sample based on those participants who are of the age within the reference norms of the TOMAL (5–19 years of age), have complete TOMAL data from the initial assessment, and have complete neuroimaging data. Additionally, only those participants with MRI scans that were completed within three months of the TOMAL testing were included in the current investigation (M = 2.1 months, SD = 0.26). All facets of this investigation were undertaken with the understanding and written consent of each subject or legal guardian, with the approval of the University of Utah and Brigham Young University Institutional Review Boards, where testing was performed, and in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association.
Subject Groups
All subjects were males, 5–19 years of age. The ASD group had a total of 56 subjects (38 AIQ, 18 LIQ) and the TDC group a total of 30 subjects. Specific characteristics are summarized in Table 1.
Table 1:
Subject Characteristics
| TDC N=30 | AIQ N=38 | LIQ N=18 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | F | p | |
| Age (years) | 12.03 | 4.15 | 5.31–19.41 | 13.20 | 4.10 | 5.03–19.67 | 10.38 | 4.06 | 5.72–18.35 | 2.92 | 0.06 |
| Head Cir (cm) | 55.37 | 2.16 | 51.80–60.50 | 55.75 | 2.67 | 50.70–60.50 | 54.47 | 1.83 | 51.50–57.90 | 1.84 | 0.17 |
| TICV (cm3) | 1679.04 | 178.60 | 1400–2170 | 1674.43 | 163.98 | 1270–2060 | 1667.87 | 148.50 | 1380–2010 | 0.03 | 0.98 |
| Handed | 64.78 | 45.68 | −80–100 | 73.24 | 45.93 | −100–100 | 49.00 | 68.52 | −93.33–100 | 1.36 | 0.26 |
| FIQ | 116.25 | 14.85 | 93–152 | 106.68 | 11.98 | 85.137 | 79.94 | 8.52 | 61–99 | 45.96abc | 0.00 |
| PIQ | 116.47 | 15.60 | 90–155 | 107.18 | 12.14 | 83–131 | 92.33 | 18.68 | 66–138 | 14.74abc | 0.00 |
| VIQ | 112.17 | 14.86 | 87–140 | 106.84 | 14.82 | 85–145 | 71.78 | 7.39 | 55–83 | 54.75bc | 0.00 |
Note. FIQ = Full Scale IQ, PIQ = Performance IQ, and VIQ = Verbal IQ; TDC = Typical Developing Control, AIQ = Average IQ, and LIQ = Low IQ
= TDC is significantly greater than AIQ at p < .05.
= TDC is significantly greater than LIQ at p < .05.
= AIQ is significantly greater than LIQ at p < .05.
Idiopathic autism sample.
Autism was diagnosed rigorously. For individuals in the autism group, the subject’s mother was interviewed using the Autism Diagnostic Interview–Revised (ADI-R; Lord, Rutter, & LeCouteur, 1994), a semi-structured, investigator-based interview with good reliability and validity. Subjects with autism were also directly assessed using the Autism Diagnostic Observation Schedule–Generic (ADOS-G; Lord et al., 2000); a semi-structured play and interview session designed to elicit social, communication, and stereotyped repetitive behaviors characteristic of autism. The scores are summarized in Table 3. All subjects in the autism group met ADI–R, ADOS–G, and the Diagnostic and Statistical Manual of Mental Disorders –Fourth Edition (DSM–IV; American Psychiatric Association, 1994) criteria for autistic disorder. History, physical exam, fragile X gene testing, and karyotype were performed on all subjects to exclude medical causes of autism.
Table 3:
Autism Severity Determined Using the ADOS
| Typically Developing | Autism | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIQ | LIQ | |||||||||||
| Module | n | Mean | SD | Range | n | Mean | SD | Range | n | Mean | SD | Range |
| ADOS S+C: Module 1 | 0 | 0 | 2 | 14.00 | 1.41 | 13–15 | ||||||
| ADOS S+C: Module 2 | 1 | 0 | 0 | 0–0 | 4 | 15.75 | 3.30 | 12–20 | 5 | 19.20 | 3.35 | 15–23 |
| ADOS S+C: Module 3 | 18 | 1.72 | 1.60 | 0–5 | 20 | 14.75 | 3.13 | 10–21 | 9 | 16.33 | 2.45 | 14–20 |
| ADOS S+C: Module 4 | 10 | 1.1 | 1.45 | 0–4 | 13 | 12.46 | 3.69 | 6–19 | 2 | 17.50 | 0.71 | 17–18 |
Note. ADOS S+C = Autism Diagnostic Observation Schedule: Social and Communication Total. The ADOS consists of four modules, and the individual being evaluated is given just one module, depending on the level of expressive language and chronological age. Each module has different cutoff scores and should not be considered equivalent. One TDC participant was not administered the ADOS and one AIQ participant received an older version of the ADOS (version 1988), which is not reported above. TDC = Typical Developing Control, AIQ = Average IQ, and LIQ = Low IQ.
Eighteen subjects in the autism sample were on prescribed medications (nine subjects were on selective serotonin repute inhibitors, two subjects were on tricyclic antidepressants, four subjects were on antipsychotic medications, one subjects was on a proton pump inhibitor). No subjects had a history of seizures, severe head injury, bipolar disorder, schizophrenia, or drug or alcohol abuse at the time of participation. While the primary diagnosis was ASD, within the ASD sample there were several secondary co-occurring diagnoses: Seven participants were diagnosed with anxiety disorders, three subjects were diagnosed with depression, and five were diagnosed with attention deficit hyperactivity disorder.
Using the verbal IQ score (see Boucher et al., 2012), the ASD group was divided into two groups, an average to above-average IQ (AIQ, VIQ ≥85) group and a low average intellectual (LIQ, VIQ≤84) group.
Typically developing sample.
Typically developing control participants had no developmental, neurological, or clinical history of major developmental, learning, cognitive, neurological, or neuropsychiatric disorders, with the exception of one subject with a history of seizure. One subject was prescribed an antihistamine medication. Control subjects likewise completed an assessment with the ADOS-G and were assessed rigorously for ASD to ensure none met any criterion, including no first-degree relatives with ASD. Level of intellectual functioning was not stratified in the control subjects.
Intelligence
Different versions of intellectual tests were used over the 10 years of subject recruitment. Intelligence (IQ) findings were reported (for descriptive purposes) including summary measures from one of the following: Wechsler Intelligence Scale for Children–Third Edition (WISC–III; Wechsler, 1991); Wechsler Adult Intelligence Scale– Third Edition (WAIS-III; Wechsler, 1997); Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999; VIQ and PIQ), or Differential Ability Scales (DAS; Elliott, 1990). Intelligence (IQ) was not used as a covariate because IQ was used as an independent selection variable for AIQ and LIQ designation (see Dennis et al. 2009).
Handedness and Head Circumference
Handedness was measured using the Edinburgh Handedness Inventory (Oldfield, 1971). A score of +100 signifies complete right handedness and −100 indicates complete left handedness. Standard occipitofrontal head circumference was obtained on all subjects as previously described (see Bigler et al., 2003). Head circumference was initially done at the time of recruitment as a quick proxy for brain volume (Tate, Bigler, McMahon, & Lainhart, 2007). Head size is a factor of consideration in autism and head circumference values should be reported in autism research (Lainhart & Lange, 2011). Therefore, total intracranial volume (TICV), a more precise estimate of maximal brain volume relative to head circumference, was controlled for during analysis.
Attention
Attention metrics were based on TOMAL subtests (Reynolds & Bigler, 1994). Details of this memory battery in autism have been previously published in Trontel et al. (2013) and Southwick et al. (2011). For the current investigation, four subtests were used, including: digits forward, digits backward, letters forward, and letters backward, which operationally are considered measures of working memory/attention. In these tasks, the examiner reads aloud a series of letters or numbers and the examinee is asked to repeat the series. Scaled scores of each of those subtests were then combined to form an overall attention score (see Table 2).
Table 2:
Results of Auditory Attention Performance on TOMAL by Group
| TDC N=30 | AIQ N=38 | LIQ N=18 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | F | ρη2 | |
| Digits Forward | 9.81 | 3.40 | 3–16 | 7.40 | 3.38 | 1–15 | 4.60 | 2.41 | 1–9 | 12.88***abc | .26 |
| Raw score | 21.23 | 26.86 | 3–94 | 14.11 | 13.68 | 1–62 | 10.17 | 7.37 | 2–27 | ||
| Digits Backward | 10.78 | 2.36 | 7–17 | 8.51 | 2.72 | 5–18 | 7.00 | 2.65 | 4–12 | 11.52***ab | .24 |
| Raw score | 35.77 | 18.61 | 8–74 | 31.82 | 20.58 | 0–77 | 12.44 | 10.61 | 0–43 | ||
| Letters Forward | 9.07 | 2.95 | 4–16 | 7.46 | 3.00 | 1–13 | 4.27 | 2.49 | 2–9 | 13.32***bc | .27 |
| Raw score | 86.90 | 31.32 | 22–126 | 64.18 | 35.03 | 6–124 | 38.28 | 31.42 | 8–95 | ||
| Letters Backward | 10.63 | 2.31 | 6–16 | 8.26 | 2.63 | 2–15 | 6.47 | 3.29 | 1–13 | 12.84***ab | .26 |
| Raw score | 28.42 | 18.99 | 9–85 | 20.71 | 16.14 | 0–75 | 13.00 | 12.93 | 0–55 | ||
| Total Attention | 40.30 | 9.12 | 23–60 | 31.63 | 9.47 | 16–58 | 22.33 | 8.54 | 14–42 | 19.04***abc | .34 |
Note. Subtest scores are scaled scores unless noted oterwise. Total Attention = total of all subtest scaled scores. Post-hoc analysis completed by Tukey. TDC = Typical Developing Control, AIQ = Average IQ, and LIQ = Low IQ.
p < .001
= TDC is significantly greater than AIQ at p < .05.
= TDC is significantly greater than LIQ at p < .05.
= AIQ is significantly greater than LIQ at p < .05.
Neuroimaging
Volumetric measurements were based on magnetic resonance images acquired on a Siemens Trio 3.0 Tesla scanner at the University of Utah. An 8-channel, receive-only radio frequency (RF) head coil was used to acquire sagittal 3D magnetization prepared rapid gradient echo (MPRAGE) spin-lattice relaxation time (T1)-weighted images (inversion time = 1100 ms, echo time = 2.93 ms, repetition time = 1800 ms, flip angle = 12°, field of view = 56 mm, slice thickness = 1.0 mm, 160 slices). No subjects in the current study underwent sedation for scanning. No complications were encountered in the scanning process and no participants were lost due to extensive quality assurance measures. Investigators utilized practice in mock scanners or ran multiple sequences when movement artifact was observed to ensure high quality scans (Alexander, Lee, Lezar, & Field, 2007). Additional neuroimaging details have been published previously (see Prigge, Bigler, et al., 2013; Prigge, Lange, et al., 2013).
Volumetric image analysis.
All analyses were performed with FreeSurfer image analysis suite, Version 5.1 (Dale, Fischl, & Sereno, 1999). The established FreeSurfer image pipeline was followed wherein individual Digital Imaging and Communications in Medicine (DICOM) image files were conformed and saved in native MGZ format. All analyses were performed on identical nodes at the Fulton Supercomputing Lab at Brigham Young University. Skull stripping, normalization, segmentation and classification were all performed as part of the normal FreeSurfer pipeline. The FreeSurfer Query, Design, Estimate, Contrast (QDEC) function was used for quality inspection of the classified images. Within the QDEC function a ROI may be identified by plotting all volumes for that ROI across all participants. This method permits identification of outliers where the segmentation/ classification can then undergo visual inspection. All participant scans were visually inspected in this manner before being included in the study. No operator-controlled editing was performed.
Volume calculations of the following FreeSurfer identified ROIs were selected: superior frontal gyrus, anterior cingulate gyrus, precuneus, cerebellum, total gray matter, and total intracranial volume (TICV). Total intracranial volume and age were used as covariates.
Statistical Analysis
Given the descriptive nature of this investigation, group means were calculated and compared for autism and control subjects for ROIs using multi-variate analysis of variance (MANOVA), and p-values were Bonferroni corrected for multiplicity. TOMAL composite, index, and subtest scores were compared using MANOVA. TOMAL scores were then correlated with neuroanatomically defined ROIs controlling for TICV and age for each group.
Results
Sample Characteristics
As shown in Table 1, no significant differences were found between groups on demographic variables (age, head circumference, TICV, handedness index, or education). However, there was a significant group difference for IQ, which was expected. Post hoc comparisons revealed that all three groups were significantly different on PIQ, F(83, 2) = 14.74, p < .001 and FIQ, F(83, 2) =45.96, p<.001 with the TDC group having the highest scores, followed by the AIQ and then LIQ. However, VIQ was not significantly different for the TDC group when compared with AIQ but VIQ for both TDC and AIQ was significantly higher when compared with LIQ (both p<.001).
Attention Performance
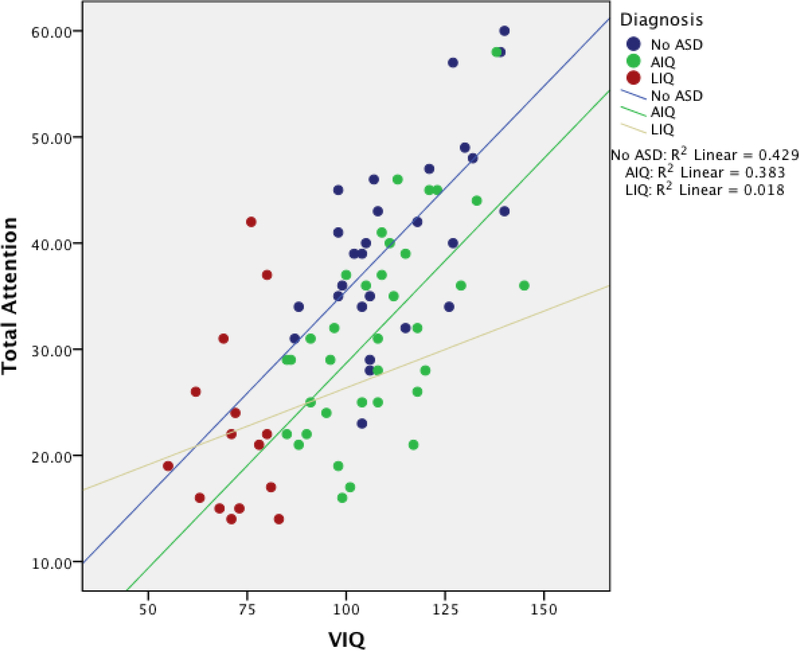
Results for the attention subtest and overall attention scores are reported in Table 2. Post hoc analyses revealed a variety of group differences. Significant differences involving all three groups occurred only for the Total Attention score and the Digits Forward subtest (p < .01), with TDCs exhibiting the highest scores, AIQ with the next highest and LIQ with the lowest scores. For the Letters Forward subtest, TDC did not differ significantly from AIQ, while LIQ scored significantly lower when compared with both AIQ and TDC (p < .01). On Digits Backward and Letters Backward, TDC performed significantly better than both ASD groups (p < .01), while no differences were found between the AIQ and LIQ groups. Effect sizes (partial-eta squared; Cohen, 1988) for all comparisons were small to moderate (see Table 2). Table 5 shows the relations between IQ and attention performance for each group. Strong (p < .01) relations were found between verbal IQ and attention performance for the TDC and AIQ groups. No significant relation between attention and VIQ was found for the LIQ group.
Table 5:
Comparisons of Structure Volume by Group Controlling for TICV and Age
| TDC | AIQ | LIQ | ||||||
|---|---|---|---|---|---|---|---|---|
| Structure Volume | Mean | SD | Mean | SD | Mean | SD | F | p |
| Superior Frontal | 59.67 | 6.69 | 59.76 | 7.51 | 61.77 | 8.33 | .77 | .47 |
| Anterior Cingulate | 11.27 | 2.08 | 11.13 | 1.92 | 11.15 | 2.64 | .06 | .94 |
| Precuneus | 24.91 | 2.90 | 25.18 | 3.58 | 27.59 | 4.13 | 3.04 | .05 |
| Cerebellum | 124.66 | 14.23 | 125.55 | 11.48 | 123.99 | 15.04 | .05 | .95 |
| Total Gray Matter | 808.34 | 65.13 | 801.79 | 73.36 | 824.24 | 82.91 | .20 | .82 |
| Total Brain | 1345.67 | 123.87 | 1337.96 | 130.06 | 1332.74 | 115.19 | .92 | .40 |
Note. Volumes are presented in cm3. Controlling for Age and TICV = total intracranial volume. TDC = Typical Developing Control, AIQ = Average IQ, and LIQ = Low IQ.
Volumetric Differences in Attention ROI
Volumetric findings are summarized in Table 5. MANOVAs comparing the two ASD groups with TDC controlling for age and TICV revealed no significant volume differences for total brain volume, total gray matter, or any of the ROIs, except for the precuneus (p = .05). However, the precuneus-group relationship did not survive correction for family-wise error.
Relation Between ROI Volume and TOMAL Performance
As shown in Table 5, performance on Total Attention was negatively correlated only with the precuneus in the AIQ ASD group, indicating that larger precuneus volume related to reduced attention performance for that group (which did not remain significant following Bonferroni correction). No relation existed between Total Attention and any of the ROIs for TDC. While not statistically significant, examination of Table 5 reveals a negative relationship between all cerebrum ROIs and Total Attention performance for the AIQ and LIQ groups (i.e., larger volume related to reduced attention performance for both ASD groups), but a positive relationship between cerebellum volume and Total Attention performance for both ASD groups.
Discussion
Main Findings
This investigation revealed a variety of significant group differences, generally consistent with our hypotheses that ASD individuals, regardless of their AIQ or LIQ classification, performed worse on TOMAL-based attention tasks than did their TDC counterparts. Furthermore, AIQ and LIQ subjects differed as expected; AIQ subjects performed better (although not significantly for the working memory subtests) than LIQ subjects on all four of the subtest scores (letters forward, letters backward, digits forward, digits backward), as well as on the overall attention score. As shown in Table 5, the only volumetric difference for the attention ROIs examined was the precuneus, with increasing volume from TDC to AIQ to LIQ, which did not survive correction for multiple comparisons. The only significant neuroanatomical relation in attention performance was a negative correlation between the overall attention score and the precuneus for the AIQ group (Table 5), which also did not survive family-wise error statistical correction. Verbal IQ performance demonstrated a significant positive relation with all attention variables for the TDC and AIQ groups. However, no relation between VIQ and attention performances was found for the LIQ group (see Figure 1).
Figure 1:
Relation between verbal IQ and total attention by group.
In TDC there is an expected positive relation between various cognitive tasks with the assumption that there is a size-function relation with brain structures as well (Bigler et al., 2007). These kinds of relations are not necessarily maintained is those with ASD, and this was most evident in the LIQ ASD group.
Focused Attention in Autism
Performance on focused attention tasks varied with individuals in the AIQ group performing similarly to TDC on one subtest of attention (i.e., letters forward), suggestive of preserved focused attention in ASD (Bennetto, Pennington, & Rogers, 1996; Boucher et al., 2012), but inconsistent on a second focused attention task (i.e., digits forward). Therefore, focused attention may differ for numbers and letters within ASD and performance is likely influenced by general cognitive factors. Motivation may be an additional potential explanation for this finding (Allen & Courchesne, 2001), but unfortunately there are no metrics within the TOMAL that provide a measure of motivation. Surpassing a minimal string-length of digits is thought to reflect appropriate mental effort (Lezak, Howieson, Bigler, & Tranel, 2012; Whearty, Allen, Lee, & Strauss, 2015). Both the LIQ and AIQ groups on average met minimal digit string-length to suggest task engagement and appropriate effort during testing. Regardless, no consistent differences were found between all three groups for focused attention, which may suggest that focused attention is variable as it relates to intelligence level in ASD.
Attention and Verbal IQ in Autism
Rather than simply controlling for VIQ, the current study attempted to capture additional potential variance in attention performance related to general cognitive ability reflected in the IQ score. By incorporating IQ status as a group variable for ASD individuals, those who scored lowest on the IQ variable could be examined separately from those ASD participants who had IQ scores similar to the TDC group. Attention measures are noted to be embedded in, and thus a necessary component of, intellectual estimates (Miller et al., 2011). Therefore, it would be expected that a positive correlation between IQ and attention would be observed. Unsurprisingly, a strong positive relationship existed between attention and VIQ for both the TDC and AIQ groups. This relationship was similar between the TDC and AIQ groups, likely because the attention measures in this study are presented orally and verbal IQ is the only measure that did not differ significantly between the TDC and AIQ groups. Indeed, the two groups had an almost identical VIQ performance range. Further, the AIQ group performed within one standard deviation on all four attention subtests compared with the TOMAL normative standard, while the LIQ group performed one standard deviation below on the attention subtests. However, in distinct contrast, no significant relation was found for attention and VIQ in the LIQ group.
The finding that intellectual test score performance cannot account for variance in attentional ability across all individuals within the ASD sample (i.e., no significant relation found for VIQ and attentional performance in the LIQ group) is consistent with findings demonstrating varying levels of overlap with cognitive impairment and heterogeneity of other testing outcomes in ASD, such as functional impairment or ASD symptom burden (Kanne et al., 2011; Weitlauf, Gotham, Vehorn, & Warren, 2014). However, this is in opposition to findings of individuals with solely low levels of intellectual ability where increments of intellectual disability are predictive of important functional impairments (see Murray, McKenzie, & Murray, 2013). It is unclear how cognitive functioning relates to a DSM-5 diagnosis of ASD and ASD classification, but when considering functional impairment modifiers, incorporating some level of cognitive impairment may be necessary (Weitlauf et al., 2014) and calls for some standardized operationalization or method. Given variation in the methodological uses of IQ (e.g., IQ matching, covariation, IQ stratification) in the research literature concerning ASD, a standardized method for use of IQ in research, in addition to diagnosis, also needs careful consideration for ASD populations (e.g., Dennis et al., 2009).
Attention and Executive Function in Autism
The results of the current study suggest that focused attention may be relatively preserved in individuals with ASD who have, at a minimum, average intellectual abilities, while more complex tasks are impaired regardless of intellectual ability. Digits and letters backward are often thought to reflect both focused attention as well as working memory aggregating into an executive control task of attention (Keehn et al., 2013). This executive task requires cognitive flexibility, inhibition, and mental manipulation. Despite differences in severity, the AIQ and LIQ groups perform in a manner that is distinct from typically developing controls as the complexity of the task increased. This pattern of responses is consistent with Barendse et al.’s (2013) review of the working memory literature in ASD that found greater deficits in working memory with heavier demands and concluded that working memory is a core deficit in ASD. Similarly, our results are also consistent with the research suggesting executive control tasks that require manipulation and process of online information are difficult for individuals with ASD (Boucher et al., 2012).
While high functioning individuals with ASD perform similarly to TDC on tasks of basic attention, they perform in a manner distinctly similar to low functioning ASD on complex executive tasks, performance that is consistent with behavioral phenotypic theories of ASD (Burack, 1994; Goldstein et al., 2001; Nyden, Hjelmquist, & Gillberg, 2000; Schmitz et al., 2006). This type of performance may lead to the later development of higher-level social and communication deficits (Belmonte & Yurgelun-Todd, 2003; Gold & Gold, 1975).
Volumetric Differences in Attention-Related ROIs
Despite disparate attention performance between the ASD and TDC groups, there were no gross volumetric differences in structures typically thought to be involved in attention processes. The finding of “normal” ROI structural volumes in ASD in the current study are consistent with several volumetric studies (Bigler et al., 2003; Piven, Bailey, Ranson, & Arndt, 1998; Trontel, et al., 2013) that have not found unique differences in gross brain morphology in ASD and suggest that the neuropathology of ASD is not consistently expressed at the level of gross morphology. Further, contrary to our hypotheses, none of the classic attention-related ROI volumes such as the superior frontal gyrus, anterior cingulate cortex, precuneus, or cerebellum were significantly related to attention performance for any of the groups. While the relations between the precuneus volume and attention performance reached significance for the AIQ group, this finding did not withstand correction for multiple comparisons. Despite the null result, this finding is interesting in that the principal extraparietal cortico-cortical connections of the precuneus are with the frontal lobes (Cavanna & Trimble, 2006), which are heavily recruited during tasks of attention (Stuss, 2006). The precuneus has been implicated in orientation of attention (Kincade, Abrams, Astafiev, Shulman, & Corbetta, 2005; Simon et al., 2002), shifting of attention between objects (Nagahama et al., 1999), and the recall of verbally presented information (Schmidt et al., 2002). The precuneus therefore remains an integral part of focus attention for individuals with autism. It may be the connectivity of the precuneus with other brain regions, which was not addressed by the design of this structural MRI study, that is central to attentional factors in ASD.
An interesting qualitative difference arose for both ASD groups despite null volumetric-performance relation findings. Attention performance was negatively correlated (although not significantly) with all structures with the exception of the cerebellum. In other words, larger volumes in the ASD group were related to poorer attention performance in our sample. Could this be related to speculation that “overgrowth” in brain structures occurs in autism (Campbell, Chang, & Chawarska, 2014; Sacco, Gabriele, & Persico, 2015)? Supporting this concept, Zielinski et al. (2012) reported ‘overgrowth’ of the posterior DMN, particularly within the precuneus, in ASD versus TDC. Overgrowth may reflect aberrant brain development and faulty pruning (Ziats, Edmonson, & Rennert, 2015), where ‘larger’ does not fit the size-function rule of brain development (Bigler, 2015). In contrast, the TDC group had a positive relation (also not significant) between structure volume and attention performance for all attention-related ROIs. Similar size to function findings within largely this same cohort of ASD subjects has been found for facial memory (Trontel et al., 2013).
The next logical step built on these attentional findings following the examination of morphological differences is examining connectivity differences. Increasing the complexity of neuroimaging methods for a research trajectory is prudent for interpreting the basic explanation of complex findings. However, Kana, Uddin, Kenet, Chugani, and Muller (2014) discuss the importance of multimodal neuroimaging approaches for a more comprehensive understanding of brain network abnormalities in ASD, such as effective connectivity (i.e., the causal influence of one brain area on another). This novel approach may serve as a biomarker for ASD (Kana et al., 2014) and revolutionize the understanding of the differences in cognitive processes such as the distinct performance on attention/working memory between TDC and ASD individuals.
Limitations
The two autism groups (i.e., AIQ and LIQ) were differentiated using a cutoff of one standard deviation below average verbal intelligence from typically developing norms. While this differentiation proved fruitful in delineating attention differences between the groups, it was arbitrary and not necessarily a clinically derived criterion for differentiating within autism. It did permit more closely matching the AIQ ASD group with TDC, with some differences in attentional performance still noted in the ASD group. More clinically meaningful phenotypes may allow better delineation of cognitive factors associated with impaired attention function in ASD. The LIQ group had the fewest subjects, which limited statistical power to demonstrate an effect. Furthermore, it should also be noted that automated image analysis methods used in this investigation have limitations and it remains possible that more refined structural image analysis methods, such as an examination of white matter structure and connections, may prove successful in defining brain structure-function relations (Hanson et al., 2012). Including additional structures in the analyses could also further elucidate structure-function relations, such as including the caudate commonly implicated in comorbid disorders such as ADHD (Bigler et al., 2007). Finally, the authors are aware of the recently highlighted concern about rigor and reproducibility in studies (https://www.nih.gov/research-training/rigor-reproducibility), and we are in the process of collecting additional data to test replication of the current findings in a much larger longitudinal sample that will allow longitudinal covariance analysis in the future. This study also helps to alleviate the “file drawer problem” which contributes to the publication of positive findings, distorting the impression that findings seem more robust than they really are (Rosenthal, 1979). The use of a practical attention measure (i.e., TOMAL subtests) allows for clinical interpretation of applied outcomes from this study. That said, the findings lacked robustness to survive statistical correction. That in itself should not mean the outcomes lack significance for the field or for clinicians working with this population. For example, negative findings can inform new ways of interpreting brain behavior relationships. Children with poor memory performance on paper and pencil tasks were thought to also have related brain atrophy until disproven and published in the literature (Trontel et al., 2015). Therefore, it is imperative that studies demonstrating methodological rigor remain accessible to the public.
Conclusion
Generally, lower performance on measures of verbal intellectual functioning was associated with worse (oral) attention performance within the ASD sample (i.e., AIQ versus LIQ), compared to TDC. AIQ individuals with autism appear to perform similarly to TDC on some brief, focused attention tasks and AIQ and LIQ ASD groups appear to perform similarly on more complex working memory tasks. Neuroanatomical structure-size did not differ between any groups. Although the precuneus volume was found to be negatively related to general attention performance in AIQ ASD individuals, these relations were not robust enough to withstand correction for multiple comparisons and unlikely to solely represent a major explanation for reduced attention/working memory performance in ASD.
Table 4:
Correlations Between VIQ and Auditory Attention Performance
| VIQ | |||
|---|---|---|---|
| TDC | AIQ | LIQ | |
| Digits Forward | .53** | .48** | .26 |
| Digits Backward | .64** | .53** | .02 |
| Letters Forward | .45* | .53** | .13 |
| Letters Backward | .57** | .42* | −.04 |
| Total Attention | .66** | .62** | .13 |
Note. VIQ = Verbal IQ, TDC = Typical Developing Control, AIQ = Average IQ, and LIQ = Low IQ.
= p < .05
= p < .01
Table 6:
Correlations Between Brain Volumes and TOMAL Performance Controlling for TICV
| Total Attention | |||
|---|---|---|---|
| Structure | TDC | AIQ | LIQ |
| Superior Frontal | .09 | −.20 | −.31 |
| Anterior Cingulate | .24 | −.16 | −.43 |
| Precuneus | .05 | −.35* | −.25 |
| Cerebellum | .12 | .01 | .18 |
Note. TICV = Total Intracranial Volume, TDC = Typical Developing Control, AIQ = Average IQ, and LIQ = Low IQ.
= p < .05
= p < .01, controlling for TICV. No correlations remained significant following Bonferroni correction.
Acknowledgments
The project described was supported by Grant Numbers RO1 MH080826 (JEL, EDB, ALA, NL), RO1 MH084795 (JEL, PTF, NL), and KO8 MH092697 (JSA) from the National Institute Of Mental Health; Grant Numbers T32 HD07489 (BGT) and P30 HD003352–45 (Waisman Center Core Grant) from the Eunice Kennedy Shriver NICHD, The Hartwell Foundation (BGT), and the Primary Children’s Foundation Early Career Development Award (BAZ). Support from the Poelman Foundation to Brigham Young University for autism research is gratefully acknowledged. The authors report no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute of Child Health & Development, or the National Institutes of Health. We thank former members of the Utah Autism CPEA for their assistance during the early stages of this project. We sincerely thank the children, adolescents, and adults with autism and the individuals with typical development, who participated in this study, and their families. Although Dr. Bigler is the co-author of the TOMAL, he receives no royalties and reports no conflict of interest. The assistance of Tracy J. Abildskov with image analysis and Jo Ann Petrie, Ph.D. with manuscript preparation is gratefully acknowledged.
References
- Akshoomoff NA, & Courchesne E (1992). A new role for the cerebellum in cognitive operations. Behavioral Neuroscience, 106(5), 731–738. [DOI] [PubMed] [Google Scholar]
- Akshoomoff NA, Courchesne E, & Townsend J (1997). Attention coordination and anticipatory control. International Review of Neurobiology, 41, 575–598. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, & Field AS (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4(3), 316–329. doi: 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G, & Courchesne E (2001). Attention function and dysfunction in autism. Frontiers in Bioscience, 6, D105–119. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- Anderson CJ, & Colombo J (2009). Larger tonic pupil size in young children with autism spectrum disorder. Developmental Psychobiology, 51(2), 207–211. doi: 10.1002/dev.20352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CJ, Colombo J, & Unruh KE (2013). Pupil and salivary indicators of autonomic dysfunction in autism spectrum disorder. Developmental Psychobiology, 55(5), 465–482. doi: 10.1002/dev.21051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, & Yurgelun-Todd D (2011). Connectivity gradients between the default mode and attention control networks. Brain Connect, 1(2), 147–157. doi: 10.1089/brain.2011.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, . . . Lainhart JE (2011). Functional connectivity magnetic resonance imaging classification of autism. Brain, 134(12), 3742–3754. doi: 10.1093/brain/awr263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse EM, Hendriks MP, Jansen JF, Backes WH, Hofman PA, Thoonen G, . . . Aldenkamp AP (2013). Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. Journal of Neurodevelopmental Disorders, 5(1), 14. doi: 10.1186/1866-1955-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Gomot M, & Baron-Cohen S (2010). Visual attention in autism families: ‘unaffected’ sibs share atypical frontal activation. Journal of Child Psychology and Psychiatry, 51(3), 259–276. doi: 10.1111/j.1469-7610.2009.02153.x [DOI] [PubMed] [Google Scholar]
- Belmonte MK, & Yurgelun-Todd DA (2003). Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Research Cognitive Brain Research, 17(3), 651–664. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, & Rogers SJ (1996). Intact and impaired memory functions in autism. Child Development, 67(4), 1816–1835. [PubMed] [Google Scholar]
- Bigler ED (2015). Structural Image Analysis of the Brain in Neuropsychology Using Magnetic Resonance Imaging (MRI) Techniques. Neuropsychology Review, 25(3), 224–249. doi: 10.1007/s11065-015-9290-0 [DOI] [PubMed] [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, . . . Lainhart JE (2007). Superior temporal gyrus, language function, and autism. Developmental Neuropsychology, 31(2), 217–238. doi: 10.1080/87565640701190841 [DOI] [PubMed] [Google Scholar]
- Bigler ED, Tate DF, Neeley ES, Wolfson LJ, Miller MJ, Rice SA, . . . Lainhart JE (2003). Temporal lobe, autism, and macrocephaly. AJNR American Journal of Neuroradiology, 24(10), 2066–2076. [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Mayes A, & Bigham S (2012). Memory in autistic spectrum disorder. Psychological Bulletin, 138(3), 458–496. doi: 10.1037/a0026869 [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, & McCarley RW (2012). Control of sleep and wakefulness. Physiological Reviews, 92(3), 1087–1187. doi: 10.1152/physrev.00032.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, & Sonuga-Barke EJ (2009). Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience and Biobehavioral Reviews, 33(3), 279–296. doi: 10.1016/j.neubiorev.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Bruinsma Y, Koegel RL, & Koegel LK (2004). Joint attention and children with autism: a review of the literature. Mental Retardation and Developmental Disabilities Research Reviews, 10(3), 169–175. doi: 10.1002/mrdd.20036 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Burack JA (1994). Selective attention deficits in persons with autism: preliminary evidence of an inefficient attentional lens. Journal of Abnormal Psychology, 103(3), 535–543. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Chang J, & Chawarska K (2014). Early generalized overgrowth in autism spectrum disorder: prevalence rates, gender effects, and clinical outcomes. Journal of the American Academy of Child and Adolescent Psychiatry, 53(10), 1063–1073.e1065. doi: 10.1016/j.jaac.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, & Trimble MR (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564–583. doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, & Baird G (2011). IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychological Medicine, 41(3), 619–627. doi: 10.1017/S0033291710000991 [DOI] [PubMed] [Google Scholar]
- Chen SF, Chien YL, Wu CT, Shang CY, Wu YY, & Gau SS (2016). Deficits in executive functions among youths with autism spectrum disorders: An age-stratified analysis. Psychological Medicine, 46(8), 1625–1638. doi: 10.1017/s0033291715002238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chita-Tegmark M (2016). Social attention in ASD: A review and meta-analysis of eye-tracking studies. Research in Developmental Disabilities, 48, 79–93. doi: 10.1016/j.ridd.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Christakou A, Murphy CM, Chantiluke K, Cubillo AI, Smith AB, Giampietro V, . . . Rubia K (2013). Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with autism. Molecular Psychiatry, 18(2), 236–244. doi: 10.1038/mp.2011.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cohen RM, Semple WE, Gross M, Holcomb HH, Dowling MS, & Nordahl TE (1988). Functional localization of sustained attention: Comparison to sensory stimulation in the absence of instruction. Cognitive and Behavioral Neurology, 1(1), 3–20. [Google Scholar]
- Corbetta M, Patel G, & Shulman GL (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58(3), 306–324. doi: 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. doi: 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, . . . Lau L. (1994). Impairment in shifting attention in autistic and cerebellar patients. Behavioral Neuroscience, 108(5), 848–865. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, & Liaw J (2004). Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Developmental Psychology, 40(2), 271–283. doi: 10.1037/0012-1649.40.2.271 [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, & Fletcher JM (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society: JINS, 15(3), 331–343. doi: 10.1017/S1355617709090481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD (1990) DAS Administration and Scoring Manual San Antonio TX: The Psychological Corporation [Google Scholar]
- Elsabbagh M, Gliga T, Pickles A, Hudry K, Charman T, Johnson MH, & Team Basis (2013). The development of face orienting mechanisms in infants at-risk for autism. Behavioural Brain Research, 251, 147–154. doi: 10.1016/j.bbr.2012.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Di Martino A, Hazlett HC, & Gao W (2016). Neural Connectivity Evidence for a Categorical-Dimensional Hybrid Model of Autism Spectrum Disorder. Biological Psychiatry, 80(2), 120–128. doi: 10.1016/j.biopsych.2015.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant K, & Uddin LQ (2016). Atypical developmental of dorsal and ventral attention networks in autism. Developmental Science, 19(4), 550–563. doi: 10.1111/desc.12359 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. doi:0504136102 [pii] 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil M, Peterson E, Tregellas J, Hepburn S, & Rojas DC (2013). Differences in global and local level information processing in autism: an fMRI investigation. Psychiatry Research, 213(2), 115–121. doi: 10.1016/j.pscychresns.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garretson HB, Fein D, & Waterhouse L (1990). Sustained attention in children with autism. Journal of Autism and Developmental Disorders, 20(1), 101–114. [DOI] [PubMed] [Google Scholar]
- Gold MS, & Gold JR (1975). Autism and attention: theoretical considerations and a pilot study using set reaction time. Child Psychiatry and Human Development, 6(2), 68–80. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Johnson CR, & Minshew NJ (2001). Attentional processes in autism. Journal of Autism and Developmental Disorders, 31(4), 433–440. [DOI] [PubMed] [Google Scholar]
- Gomot M, Belmonte MK, Bullmore ET, Bernard FA, & Baron-Cohen S (2008). Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain, 131(Pt 9), 2479–2488. doi: 10.1093/brain/awn172 [DOI] [PubMed] [Google Scholar]
- Greenaway R, & Plaisted K (2005). Top-down attentional modulation in autistic spectrum disorders is stimulus-specific. Psychological Science, 16(12), 987–994. doi: 10.1111/j.1467-9280.2005.01648.x [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, . . . Pollak SD. (2012). Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. Journal of Neuroscience, 32(23), 7917–7925. doi: 10.1523/jneurosci.0307-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, . . . Wilson MA (2014). State-dependent architecture of thalamic reticular subnetworks. Cell, 158(4), 808–821. doi: 10.1016/j.cell.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyder K, Suchan B, & Daum I (2004). Cortico-subcortical contributions to executive control. Acta Psychologica (Amsterdam), 115(2–3), 271–289. doi: 10.1016/j.actpsy.2003.12.010 [DOI] [PubMed] [Google Scholar]
- Hill EL, & Russell J (2002). Action memory and self-monitoring in children with autism: Self versus other. Infant and Child Development, 11(2), 11. doi: 10.1002/icd.303 [DOI] [Google Scholar]
- Jagtap P, & Diwadkar VA (2016). Effective connectivity of ascending and descending frontalthalamic pathways during sustained attention: Complex brain network interactions in adolescence. Human Brain Mapping, 37(7), 2557–2570. doi: 10.1002/hbm.23196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Daibhis A, . . . Bellgrove MA (2007). Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia, 45(10), 2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG (2009). Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Annals of the New York Academy of Sciences, 1157, 10–23. doi: 10.1111/j.1749-6632.2009.04534.x [DOI] [PubMed] [Google Scholar]
- Joseph RM, Keehn B, Connolly C, Wolfe JM, & Horowitz TS (2009). Why is visual search superior in autism spectrum disorder? Developmental Science, 12(6), 1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x [DOI] [PubMed] [Google Scholar]
- Kana RK, Uddin LQ, Kenet T, Chugani D, & Muller RA (2014). Brain connectivity in autism. Frontiers in Human Neuroscience, 8, 349. doi: 10.3389/fnhum.2014.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, & Saulnier CA (2011). The role of adaptive behavior in autism spectrum disorders: Implications for functional outcome. Journal of Autism and Developmental Disorders, 41, 1007–1018. [DOI] [PubMed] [Google Scholar]
- Keehn B, & Joseph RM (2008). Impaired prioritization of novel onset stimuli in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 49(12), 1296–1303. doi: 10.1111/j.1469-7610.2008.01937.x [DOI] [PubMed] [Google Scholar]
- Keehn B, Lincoln AJ, Muller RA, & Townsend J (2010). Attentional networks in children and adolescents with autism spectrum disorder. Journal of Child Psychology and Psychiatry, 51(11), 1251–1259. doi: 10.1111/j.1469-7610.2010.02257.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Muller RA, & Townsend J (2013). Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews, 37(2), 164–183. doi: 10.1016/j.neubiorev.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, & Courchesne E (2006). Failing to deactivate: resting functional abnormalities in autism. Proceedings of the National Academy of Science of the United States of America, 103(21), 8275–8280. doi: 10.1073/pnas.0600674103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, & Corbetta M (2005). An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. Journal of Neuroscience, 25(18), 4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, & Lange N (2011). Increased neuron number and head size in autism. JAMA, 306(18), 2031–2032. doi: 10.1001/jama.2011.1633 [DOI] [PubMed] [Google Scholar]
- Lecavalier L (2006). Behavioral and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders, 36(8), 1101–1114. doi: 10.1007/s10803-006-0147-5 [DOI] [PubMed] [Google Scholar]
- Lee DO, & Ousley OY (2006). Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. Journal of Child and Adolescent Psychopharmacology, 16(6), 737–746. doi: 10.1089/cap.2006.16.737 [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, & Tranel D (2012). Neuropsychological Assessment (5th ed.) (5th ed.). New York: Oxford University Press. [Google Scholar]
- Lim L, Marquand A, Cubillo AA, Smith AB, Chantiluke K, Simmons A, . . . Rubia K (2013). Disorder-specific predictive classification of adolescents with attention deficit hyperactivity disorder (ADHD) relative to autism using structural magnetic resonance imaging. PLoS One, 8(5), e63660. doi: 10.1371/journal.pone.0063660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, . . . Rutter M (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, & Sweeney JA (2007). Maturation of executive function in autism. Biological Psychiatry, 61(4), 474–481. doi: 10.1016/j.biopsych.2006.02.030 [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1990). Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology, 28(5), 597–613. doi: 10.1002/ana.410280502 [DOI] [PubMed] [Google Scholar]
- Miller JB, Millis SR, Rapport LJ, Bashem JR, Hanks RA, Axelrod BN (2011). Detection of insufficient effort using the advanced clinical solutions for the Wechsler Memory Scale, fourth edition. Clinical Neuropsychology, 25(1), 160–72. doi: 10.1080/13854046.2010.533197 [DOI] [PubMed] [Google Scholar]
- Mouga S, Café C, Almeida J, Marques C, Duque F, & Oliveira G (2016). Intellectual profiles in the autism spectrum and other neurodevelopmental disorders. Journal of Autism and Developmental Disorders, 46(9), 2940–55. doi: 10.1007/s10803-016-2838-x. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sullivan L, & Mastergeorge AM (2009). A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Research, 2(1), 2–21. doi: 10.1002/aur.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A, McKenzie K, & Murray G (2013). To what extent does g impact on conceptual, practical and social adaptive functioning in clinically referred children? Journal of Intellectual Disability Research. doi: 10.1111/jir.12092 [DOI] [PubMed] [Google Scholar]
- Murza KA, Schwartz JB, Hahs-Vaughn DL, & Nye C (2016). Joint attention interventions for children with autism spectrum disorder: a systematic review and meta-analysis. International Journal of Language and Communication Disorders, 51(3), 236–251. doi: 10.1111/1460-6984.12212 [DOI] [PubMed] [Google Scholar]
- Musser ED, Hawkey E, Kachan-Liu SS, Lees P, Roullet JB, Goddard K, . . . Nigg JT (2014). Shared familial transmission of autism spectrum and attention-deficit/hyperactivity disorders. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, . . . Shibasaki H (1999). Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. NeuroImage, 10(2), 193–199. doi: 10.1006/nimg.1999.0451 [DOI] [PubMed] [Google Scholar]
- Neuhaus E, Bernier RA, & Beauchaine TP (2016). Children with Autism Show Altered Autonomic Adaptation to Novel and Familiar Social Partners. Autism Research, 9(5), 579–591. doi: 10.1002/aur.1543 [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Zielinski BA, Fletcher PT, Alexander AL, Lange N, Bigler ED, . . . Anderson JS (2013). Multisite functional connectivity MRI classification of autism: ABIDE results. Frontiers in Human Neuroscience 7, 599. doi: 10.3389/fnhum.2013.00599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyden A, Hjelmquist E, & Gillberg C (2000). Autism spectrum and attention-deficit disorders in girls. Some neuropsychological aspects. European Child and Adolescent Psychiatry, 9(3), 180–185. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Odriozola P, Uddin LQ, Lynch CJ, Kochalka J, Chen T, & Menon V (2016). Insula response and connectivity during social and non-social attention in children with autism. Social Cognitive and Affective Neuroscience, 11(3), 433–444. doi: 10.1093/scan/nsv126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Garver K, O’Hearn K, Nawarawong N, Liu R, Minshew N, . . . Luna B (2015). Developmental changes in brain function underlying inhibitory control in autism spectrum disorders. Autism Research, 8(2), 123–135. doi: 10.1002/aur.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish-Morris J, Chevallier C, Tonge N, Letzen J, Pandey J, & Schultz RT (2013). Visual attention to dynamic faces and objects is linked to face processing skills: a combined study of children with autism and controls. Frontiers in Psychology, 4, 185. doi: 10.3389/fpsyg.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascualvaca DM, Fantie BD, Papageorgiou M, & Mirsky AF (1998). Attentional capacities in children with autism: is there a general deficit in shifting focus? Journal of Autism and Developmental Disorders, 28(6), 467–478. [DOI] [PubMed] [Google Scholar]
- Pelisson D, Goffart L, & Guillaume A (2003). Control of saccadic eye movements and combined eye/head gaze shifts by the medio-posterior cerebellum. Progress in Brain Research, 142, 69–89. doi: 10.1016/S0079-6123(03)42007-4 [DOI] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35, 73–89. doi: 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, & Arndt S (1998). No difference in hippocampus volume detected on magnetic resonance imaging in autistic individuals. Journal of Autism and Developmental Disorders, 28(2), 105–110. [DOI] [PubMed] [Google Scholar]
- Plitt M, Barnes KA, Wallace GL, Kenworthy L, & Martin A (2015). Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proceedings of the National Academy of Sciences of the United States of America, 112(48), E6699–6706. doi: 10.1073/pnas.1510098112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploog BO, Scharf A, Nelson D, & Brooks PJ (2013). Use of computer-assisted technologies (CAT) to enhance social, communicative, and language development in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(2), 301–322. doi: 10.1007/s10803-012-1571-3 [DOI] [PubMed] [Google Scholar]
- Ponde MP, Novaes CM, & Losapio MF (2010). Frequency of symptoms of attention deficit and hyperactivity disorder in autistic children. Arquivos de Neuropsiquiatria, 68(1), 103–106. [DOI] [PubMed] [Google Scholar]
- Posner M, & Petersen SE (1990). The Attention System of the Human Brain. Annual Review of Neuroscience, 13, 17. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FA, & Rafal RD (1987). How do the parietal lobes direct covert attention? Neuropsychologia, 25(1a), 135–145. [DOI] [PubMed] [Google Scholar]
- Prigge MB, Lange N, Bigler ED, Merkley TL, Neeley ES, Abildskov TJ, . . . Lainhart (2013). Corpus Callosum Area in Children and Adults with Autism. Research in Autism Spectrum Disorders, 7(2), 221–234. doi: 10.1016/j.rasd.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MD, Bigler ED, Fletcher PT, Zielinski BA, Ravichandran C, Anderson J, . . . Lainhart J (2013). Longitudinal Heschl’s gyrus growth during childhood and adolescence in typical development and autism. Autism Research, 6(2), 78–90. doi: 10.1002/aur.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. doi: 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AE, & Lajiness-O’Neill R (2015). Visual attention shifting in autism spectrum disorders. Journal of Clinical and Experimental Neuropsychology, 37(7), 671–687. doi: 10.1080/13803395.2015.1042838 [DOI] [PubMed] [Google Scholar]
- Reynolds CR, & Bigler ED (1994). Test of Memory and Learning: Examiner’s Manual: Pro-ed.
- Robertson IH, Manly T, Andrade J, Baddeley BT, & Yiend J (1997). ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia, 35(6), 747–758. [DOI] [PubMed] [Google Scholar]
- Rosenthal R (1979). “The file drawer problem and tolerance for null results.” Psychological Bulletin, 86(3), 638. [Google Scholar]
- Russell J, & Jarrold C (1999). Memory for actions in children with autism: self versus other. Cognitive Neuropsychiatry, 4(4), 303–331. doi: 10.1080/135468099395855 [DOI] [PubMed] [Google Scholar]
- Sacco R, Gabriele S, & Persico AM (2015). Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatry Research, 234(2), 239–251. doi: 10.1016/j.pscychresns.2015.08.016 [DOI] [PubMed] [Google Scholar]
- Salman MS, & Tsai P (2016). The Role of the Pediatric Cerebellum in Motor Functions, Cognition, and Behavior: A Clinical Perspective. Neuroimaging Clinics of North America, 26(3), 317–329. doi: 10.1016/j.nic.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C, & Allen ML (2013). The specificity of inhibitory impairments in autism and their relation to ADHD-type symptoms. Journal of Autism and Developmental Disorders, 43(5), 1065–1079. doi: 10.1007/s10803-012-1650-5 [DOI] [PubMed] [Google Scholar]
- Sang L, Qin W, Liu Y, Han W, Zhang Y, Jiang T, & Yu C (2012). Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage, 61(4), 1213–1225. doi: 10.1016/j.neuroimage.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Krause BJ, Mottaghy FM, Halsband U, Herzog H, Tellmann L, & Muller-Gartner HW (2002). Brain systems engaged in encoding and retrieval of word-pair associates independent of their imagery content or presentation modalities. Neuropsychologia, 40(4), 457–470. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, & Murphy DG (2006). Neural correlates of executive function in autistic spectrum disorders. Biological Psychiatry, 59(1), 7–16. doi: 10.1016/j.biopsych.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, . . . Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. doi: 10.1523/jneurosci.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel BV Jr., Asarnow R, Tanguay P, Call JD, Abel L, Ho A, . . . Buchsbaum MS (1992). Regional cerebral glucose metabolism and attention in adults with a history of childhood autism. Journal of Neuropsychiatry and Clinical Neurosciences, 4(4), 406–414. [DOI] [PubMed] [Google Scholar]
- Siegel BV Jr., Nuechterlein KH, Abel L, Wu JC, & Buchsbaum MS (1995). Glucose metabolic correlates of continuous performance test performance in adults with a history of infantile autism, schizophrenics, and controls. Schizophrenia Research, 17(1), 85–94. [DOI] [PubMed] [Google Scholar]
- Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, & Boussaoud D (2002). Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. Journal of Neurophysiology, 88(4), 2047–2057. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47(8), 921–929. doi: 10.1097/CHI.0b013e318179964f [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Cummings N, & Carter CS (2008). Cognitive control in autism spectrum disorders. International Journal of Developmental Neuroscience, 26(2), 239–247. doi: 10.1016/j.ijdevneu.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick JS, Bigler ED, Froehlich A, Dubray MB, Alexander AL, Lange N, & Lainhart JE (2011). Memory functioning in children and adolescents with autism. Neuropsychology, 25(6), 702–710. doi: 10.1037/a0024935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EM, & Spreen O (2006). A compendium of neuropsychological tests: Administration, norms, and commentary: American Chemical Society.
- Stuss DT (2006). Frontal lobes and attention: processes and networks, fractionation and integration. Journal of International Neuropsychological Society, 12(2), 261–271. doi: 10.1017/S1355617706060358 [DOI] [PubMed] [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, & Picton TW (1995). A multidisciplinary approach to anterior attentional functions. Annals of the New York Academy of Sciences, 769, 191–211. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, . . . Wheelwright S (1998). The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology and Psychiatry, 39(5), 747–753. [PubMed] [Google Scholar]
- Tate DF, Bigler ED, McMahon W, & Lainhart J (2007). The relative contributions of brain, cerebrospinal fluid-filled structures and non-neural tissue volumes to occipital-frontal head circumference in subjects with autism. Neuropediatrics, 38(1), 18–24. doi: 10.1055/s-2007-981450 [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Charman T, Robinson EB, Plomin R, Happe F, Asherson P, & Ronald A (2013). Developmental associations between traits of autism spectrum disorder and attention deficit hyperactivity disorder: a genetically informative, longitudinal twin study. Psychological Medicine, 43(8), 1735–1746. doi: 10.1017/S003329171200253X [DOI] [PubMed] [Google Scholar]
- Teixeira S, Machado S, Velasques B, Sanfim A, Minc D, Peressutti C, . . . Silva JG (2014). Integrative parietal cortex processes: neurological and psychiatric aspects. Journal of the Neurological Sciences, 338(1–2), 12–22. doi: 10.1016/j.jns.2013.12.025 [DOI] [PubMed] [Google Scholar]
- Toth K, Munson J, Meltzoff AN, & Dawson G (2006). Early predictors of communication development in young children with autism spectrum disorder: joint attention, imitation, and toy play. Journal of Autism and Developmental Disorders, 36(8), 993–1005. doi: 10.1007/s10803-006-0137-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Harris NS, & Courchesne E (1996). Visual attention abnormalities in autism: delayed orienting to location. Journal of the International Neuropsychological Society, 2(6), 541–550. [DOI] [PubMed] [Google Scholar]
- Trontel HG, Duffield TC, Bigler ED, Froehlich A, Prigge BA, Nielsen J, . . . Lainhart J (2013). Fusiform Correlates of Facial Memory in Autism. Behavioral Sciences, 3(3), 23. doi: 10.3390/bs3030348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trontel HG, Duffield TC, Bigler ED, Abildskov TJ, Froehlich A, Prigge MB, . . . Lainhart JE (2015). Mesial temporal lobe and memory function in autism spectrum disorder: an exploration of volumetric findings. Journal of Clinical and Experimental Neuropsychology, 37(2), 178–192. doi: 10.1080/13803395.2014.997677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, & Menon V (2009). The anterior insula in autism: under-connected and under-examined. Neuroscience and Biobehavioral Reviews, 33(8), 1198–1203. doi: 10.1016/j.neubiorev.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, . . . Menon V (2013). Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry, 70(8), 869–879. doi: 10.1001/jamapsychiatry.2013.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, & Menon V (2010). Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Frontiers in Systems Neuroscience, 4, 21. doi: 10.3389/fnsys.2010.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1991). The Wechsler intelligence scale for children—third edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (1997). WAIS‐III administration and scoring manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harcourt Brace & Company; New York, NY. [Google Scholar]
- Weitlauf AS, Gotham KO, Vehorn AC, & Warren ZE (2014). Brief Report: DSM-5 “Levels of Support:” A Comment on Discrepant Conceptualizations of Severity in ASD. Journal of Autism and Developmental Disorders, 44(2), 471–476. http://doi.org.liboff.ohsu.edu/10.1007/s10803-013-1882-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood H, Stahl D, Mandy W, & Tchanturia K (2016). The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin Card Sorting Test: a systematic review and meta-analysis. Psychological Medicine, 46(9), 1809–1827. doi: 10.1017/s0033291716000581 [DOI] [PubMed] [Google Scholar]
- Whearty KM, Allen DN, Lee BG, & Strauss GP (2015). The evaluation of insufficient cognitive effort in schizophrenia in light of low IQ scores. Journal of Psychiatry Research, 68, 397–404. doi: 10.1016/j.jpsychires.2015.04.018 [DOI] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, & Kenworthy LE (2009). Set-shifting in children with autism spectrum disorders: reversal shifting deficits on the Intradimensional/Extradimensional Shift Test correlate with repetitive behaviors. Autism, 13(5), 523–538. doi: 10.1177/1362361309335716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, & Uchiyama T (2004). The clinical necessity for assessing Attention Deficit/Hyperactivity Disorder (AD/HD) symptoms in children with high-functioning Pervasive Developmental Disorder (PDD). European Child and Adolescent Psychiatry, 13(5), 307–314. doi: 10.1007/s00787-004-0391-1 [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Anderson JS, Froehlich AL, Prigge MB, Nielsen JA, Cooperrider JR, . . . Lainhart JE (2012). scMRI reveals large-scale brain network abnormalities in autism. PLoS One, 7(11), e49172. doi: 10.1371/journal.pone.0049172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziats MN, Edmonson C, & Rennert OM (2015). The autistic brain in the context of normal neurodevelopment. Frontiers in Neuroanatomy, 9, 115. doi: 10.3389/fnana.2015.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]