Abstract
Objectives:
To examine growth, sedation needs and participation in developmental activities before and after tracheostomy among infants with severe bronchopulmonary dysplasia.
Methods:
Retrospective analysis of infants born at <32 weeks’ gestation or birth weights <1500g with severe BPD who underwent tracheostomy placement between January 1, 2010 and December 31, 2016 in a quaternary referral newborn and infant intensive care unit. Changes in growth parameters and frequency/type of participation in physical therapy sessions performed during the 4-weeks before tracheostomy and 4-weeks after the first tracheostomy tube change were compared.
Results:
A total of 72 patient were included in the study. Average weekly gain in weight, length, and head circumference were significantly higher during the 4-week period after compared to before tracheostomy. The most significant change occurred for linear growth (pre vs. post: 0.71 ± 0.40cm vs. 0.97±0.48cm, p<0.001). Median Z score improved for weight (pre −1.42 (−3,10, −0.33) vs. post −0.91 (−2.7, 0.27), p<0.001), length (pre −3.07 (−4.39, −1.31) vs. post −1.95 (−3.83, −0.93), p<0.001) and weight-to-length ratio (pre 1.66 (0.58, 2.55) vs. post 1.32 (0.17, 2.2), p=0.02). Participation in developmental therapies significantly improved post tracheostomy (pre vs. post: 5.2±2.9 vs. 8.7±4.3 sessions performed over 4 weeks, p<0.0001). Physical therapy sessions more often promoted developmental skill acquisition after tracheostomy compared to facilitating physiologic stability before tracheostomy. Daily sedation requirements decreased post tracheostomy.
Conclusions:
Tracheostomy was associated with improved proportional growth and increased participation in activities promoting developmental skill acquisition and reduced daily sedation requirements in preterm infants with severe BPD.
Keywords: tracheostomy, bronchopulmonary dysplasia, very low birth weight infants, growth and development
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is the most common chronic complication associated with preterm birth and is a strong predictor of childhood growth failure and neurodevelopmental developmental delay1–5. Infants with severe BPD often require prolonged and intensive pulmonary care. Among those with the most severe lung disease, this may include tracheostomy and long-term invasive mechanical ventilation (MV)6–8. Recently, both the Children’s Hospital Neonatal Consortium and the BPD Collaborative reported tracheostomy rates of 12% in infants with severe BPD cared for in tertiary neonatal centers9,10.
Decisions about whether and when to place a tracheostomy are difficult for both families and clinicians. Tracheostomy has traditionally been viewed as a negative neonatal outcome owing in part to it being associated with increased risk for death or neurodevelopmental impairment (NDI) in preterm infants11,12. However, causality is difficult to show as previous data have demonstrated that BPD itself is an independent risk factor for NDI in extremely low birth weight infants13 and a protracted course of mechanical ventilation is associated with increased mortality and neurodevelopmental disability in this population14,15. In addition, earlier tracheostomy placement among infants who are likely to undergo tracheostomy may confer benefit16. Tracheostomy enables long-term, stable delivery of adequate positive airway pressure that may reduce work of breathing and promote growth. Additional benefits may be minimization of agitation and airway injury caused by conventional endotracheal tubes and greater ease of movement and safer participation in developmental therapy. Currently, there are no data describing the association between tracheostomy placement and change in developmental or nutritional status in preterm infants with severe BPD. The purpose of this study is to address these knowledge gaps by evaluating short-term changes in growth, respiratory support needs, tolerance of developmental care, and daily sedation requirements before and after tracheostomy in infants with severe BPD. We hypothesize that the study infants would demonstrate improved growth and participation in developmental activities post tracheostomy.
METHODS
Subjects
Data on all preterm infants with severe BPD (as per the NIH consensus definition)17 cared for in our Newborn and Infant Intensive Care Unit by the Newborn and Infant Chronic Lung Disease (NeoCLD) Program are captured in a prospective database maintained by the program. We queried this database to identify all infants with gestational age ≤ 32 weeks or birth weight ≤ 1500g who underwent tracheostomy placement for management of severe lung disease between 1/1/2010 and 12/31/2016. Patients were excluded if they underwent tracheostomy prior to transfer to our institution; died within 1 weeks of tracheostomy placement; born with severe congenital anomalies or complex heart disease (not including ASD, VSD or PDA). The Institutional Review Board at the Children’s Hospital of Philadelphia approved this retrospective study with a waiver of informed consent.
Outcomes and Data Collection
Study data were obtained from the NeoCLD database or abstracted directly from the medical record. We compared the following study outcomes recorded during the 4 week period before tracheostomy placement to those recorded during 4 week period after the first tracheostomy tube change (performed 7 days after surgery): (1) growth velocity, defined as the average weekly change in weight, length, and head circumference; and (2) the proportion of attempted developmental therapy sessions that were able to performed (i.e. the infant tolerated at least minimal handling) and the specific activities performed during the sessions. We did not include the one-week period immediately after tracheostomy placement in our analysis as patients are typically heavily sedated and occasionally paralyzed during this time at our institution. In addition, we compared respiratory severity scores (RSS; mean airway pressure x FiO2 recorded at 8–10 a.m. )18 7 days before tracheostomy placement, on the day of surgery, 7 days after surgery, and 4 weeks after the first tracheostomy change. As part of our assessment of pre- and post-operative growth, we also calculated weight and length Z scores for each infant at birth, 4 weeks before tracheostomy, on the day of surgery, 4 weeks after the first tracheostomy tube change, and at the time of NICU discharge. Weight-for-length Z scores and total daily caloric intake (kcal/kg/day) were recorded for these same time points except the day of birth. Normative values for the weight-for-length are not available for preterm infants at birth.
All developmental therapy at our institution is performed by Physical or Occupational Therapists who specialize in the care of neonates with chronic illness. For the present analysis, we recorded the number of developmental therapy sessions that were attempted (i.e. the infant was deemed clinically stable enough to initiate at least minimal handling), the proportion of attempted sessions that were partially or fully completed, the proportion limited in scope due to clinical instability that developed during the session, and the developmental activities performed during each session. Each infant’s ability to tolerate developmental therapy is continuously assessed throughout the session by the treating therapist. In our unit, intolerance of developmental therapy is defined as a significant increase in work of breathing (new onset or worsening sustained tachypnea or retractions, hypoxia, or need for sustained increase in inspired oxygen or positive airway pressure), heart rate instability16, adverse changes in behavioral state organization (e.g. decreased alertness and interaction or increased irritability), and/or persistent signs of motoric stress (e.g. splayed fingers, yawning, arching). The activities performed during each therapy session were categorized into two main categories: 1) activities promoting physiologic stability, such as a) supporting care giver tasks (e.g. assisting with containment, obtaining vital signs, diaper change, airway suctioning and/or repositioning); b) facilitation of state control (e.g. providing pacifier, rocking and/or patting to assist the infant’s ability to achieve calm alert or sleep states); and c) massage and passive range of motion exercises; 2) activities promoting developmental skill acquisition, such as a) supine or side-lying play with reaching, hands to midline and exploration tasks; b) postural skill acquisition including supported sitting or prone play; c) postural re-education tasks aimed at postural control for dynamic play and functional activities; and d) auditory/visual skills such as localization of sound and voices, visual regard and tracking.
Finally, we conducted a post-hoc analysis evaluating daily sedation requirements at baseline (24 hours prior to tracheostomy) and 4 weeks after the first tracheostomy tube change among infants with complete pharmacy records. Opioid and benzodiazepine medications were converted to daily intravenous equivalent doses of morphine and midazolam, respectively, to allow for comparison.
Statistical Analyses
Demographic and clinical data were summarized with standard descriptive statistics. Paired Student-t tests were used to compare pre- and post-operative change in growth velocity, participation in developmental therapy, RSS, and sedation needs. Wilcoxon signed-rank tests were used to compare anthropometric z-scores. A statistically significant difference was defined as a p-value < 0.05. Stata 13.1 statistical software (StataCorp LP, College Station, TX, USA) was used to analyze study data.
RESULTS
Characteristics of the Study Population
A total of 318 premature infants diagnosed with severe BPD were admitted to our program during the study period, of which 91 (29%) underwent tracheostomy. We excluded 19 patients, leaving 72 infants for inclusion in the study cohort. Reasons for exclusion included receiving tracheostomy primarily for upper airway obstruction, not severe BPD (1 infant), born at >32 weeks’ gestation and birth weight>1500 grams (3 infants), tracheostomy placement prior to transfer to our institution (12 infants, 3 of whom died prior to discharge from our hospital), died within 1 week of tracheostomy at our institution (2 infants) and born with severe congenital heart disease requiring multiple cardiac surgeries and cauterizations prior to tracheostomy placement (1 infant, this infant died several months post tracheostomy). Demographic and clinical data for the 72 evaluated infants are summarized in table 1.
Table 1.
Demographics and comorbidities in infants with severe BPD requiring tracheostomy placement
| Demographics (n=72) | |
| Male gender, number (%) | 40 (55.6) |
| Gestational age at birth, week, mean (SD) | 26.1±1.9 |
| Birth weight, g, mean (SD) | 732.4±246.1 |
| Postmenstrual age at admission, week, median (Q1, Q3) | 40.1 (35.3, 44.9) |
| Respiratory support on admission | |
| Non-invasive support, n (%) | 24 (33.3) |
| Invasive support, n (%) | 48 (66.7) |
| Postmenstrual age at tracheostomy, week, median (Q1, Q3) | 51.8 (46.7, 55.7) |
| Postnatal age at tracheostomy, day, median (Q1, Q3) | 183 (145, 212) |
| Weight at tracheostomy, g, median (Q1, Q3) | 4595 (3796, 5775) |
| Apgar scores, median (Q1, Q3) | |
| 1 minute | 4 (2, 6) |
| 5 minute | 7 (5, 8) |
| Race, n (%) | |
| Black | 27(37.5) |
| White | 25 (34.7) |
| Other | 20 (27.8) |
| Antenatal steroids, n (%) | 52 (72.2) |
| Neonatal comorbidities, n (%) | |
| BPD-associated pulmonary hypertension | 54 (75.0) |
| Intraventricular hemorrhage (grade 3 or 4) | 11(15.3) |
| Patent ductus arteriosus with treatment (medical and/or surgical) | 44 (61.1) |
| Culture proven sepsis | 21 (29.2) |
| Necrotizing enterocolitis (stage 2 or more) | 7(9.7) |
| Severe retinopathy of prematurity (stage 3 or more) | 58 (80.6) |
The study cohort included 72 infants who had tracheostomy placement for long-term ventilation due to sBPD. 19 infants with sBPD and tracheostomy were excluded: 1 received tracheostomy mainly for airway stenosis, 12 transferred to our institution with tracheostomy already in place (3 died prior to discharge), and 3 received tracheostomy at our institution but did not survive.
Growth
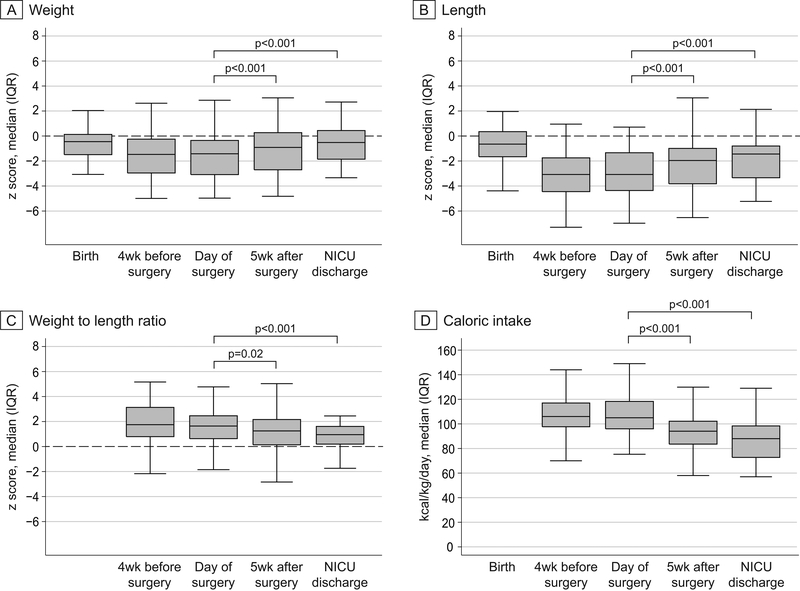
The majority of the patients in this cohort were born appropriate for gestational age or following modest in-utero growth restriction (median (IQR) Z score for weight: −0.45 (−1.5, 0.15); length: −0.64 (−1.65, 0.36)). Only a small proportion had z-scores less than −2 (15% for weight and 14% for length) and few had z-scores less than −3 (4% for weight and 3% for length) at birth. However, the frequency and severity of growth restriction was considerably higher when assessed 4 weeks before tracheostomy placement. At that time point, the proportion of infants with Z scores less than −2 increased to 38% for weight and 64% for length. Similar findings were observed at the time of tracheostomy placement. Poor linear growth was particularly prominent as reflected by the median (IQR) weight-for-length Z score of 1.77 (0.79, 3.16) and 50% of the infants having length Z score less than −3 prior to tracheostomy placement (Table 2, 3). Significant, stable improvements in weight and length growth were recorded by 4 weeks after the first tracheostomy tube change and sustained through NICU discharge (Figure 1). The largest interval improvement was observed for length, with median (IQR) z score increasing from −3.07 (−4.39, −1.31) to −1.95 (−3.83, −0.93). However, 24% of infants still had length Z score less than −3 at the time of NICU discharge (Table 3).
Table 2.
Comparison of growth and developmental activities before and after tracheostomy
| Before trach | After trach | p value | |
|---|---|---|---|
| Growth | |||
| Growth velocity* | |||
| Weight (g) | 178.74±90.34 | 216.75±95.54 | 0.004 |
| Length (cm) | 0.71 ± 0.40 | 0.97±0.48 | <0.001 |
| Head circumference (cm) | 0.41±0.23 | 0.51±0.25 | 0.01 |
| Weight-for-length z score | 1.55±1.70 | 1.17±1.47 | 0.02 |
| Caloric intake (kcal/kg/day) | 106.89±17.01 | 93.84±16.52 | <0.001 |
| Developmental therapy sessions over 4 weeks | |||
| Attempted sessions (n) | 10.0±5.4 | 14.2±6.5 | <0.0001 |
| Performed sessions (n) | 5.2±2.9 | 8.7±4.3 | <0.0001 |
Data presented as mean ± standard deviation.
Growth velocity was calculated as average increase in weight, length and head circumference over a 4-week period before and after tracheostomy.
Table 3.
Changes of weight and length Z score from birth, before and after tracheostomy through initial NICU discharge
| Birth | 4 weeks Before trach | Time of trach | 5 weeks Post trach | Discharge | ||
|---|---|---|---|---|---|---|
| WT | Median(Q1, Q3) | −0.45(−1.5, 0.15) | −1.47(−2.97, −0.23) | −1.42(−3.10, −0.33) | −0.91*(−2.7, 0.27) | −0.52*(−1.87, 0.47) |
| >−2, n (%) | 61 (84.7) | 45(62.5) | 42 (58.3) | 49 (68.1) | 58(80.6) | |
| −2 to −3, n (%) | 8 (11.1) | 11 (15.3) | 11 (15.3) | 12 (16.7) | 12 (16.7) | |
| <−3, n (%) | 3 (4.2) | 16 (22.2) | 19 (26.4) | 11 (15.2) | 2 (2.8) | |
| LT | Median (Q1, Q3) | −0.64(−1.65, 0.36) | −3.07(−4.48, −1.63) | −3.07(−4.39, −1.31) | −1.95*(−3.83, −0.93) | −1.43*(−3.37, −0.76) |
| >−2, n (%) | 62 (86.1) | 26 (36.1) | 25 (34.7) | 38 (52.7) | 47 (65.3) | |
| −2 to −3, n (%) | 8 (11.1) | 10 (13.9) | 9 (12.5) | 11(15.3) | 8 (11.1) | |
| <−3, n (%) | 2 (2.8) | 36 (50) | 38 (52.8) | 23 (31.9) | 17 (23.6) | |
WT- weight, LT- length
p<0.001 when compared to data at the time of trach.
Figure 1. Change in weight, length and caloric intake over time.
Box plots showing z score for weight, length, weight to length ratio and caloric intake recorded at the indicated time points. Caloric intake data and z score value for weight-for-length at birth were not available and not collected.
When the growth velocity was compared over the 4-week period before tracheostomy and 4 weeks after the first tracheostomy tube change, the average weekly increase in weight, length and head circumference were significantly greater post compared to prior to tracheostomy placement (Table 2). Of note, this improvement in growth was not associated with higher caloric intake. On the contrary, the study infants received significantly less calories per day post tracheostomy to achieve the observed improvement in growth (106.89±17.01 kcal/kg/d vs. 93.84±16.52 kcal/kg/d, p<0.001, Figure 1 and table 2).
Developmental Activity
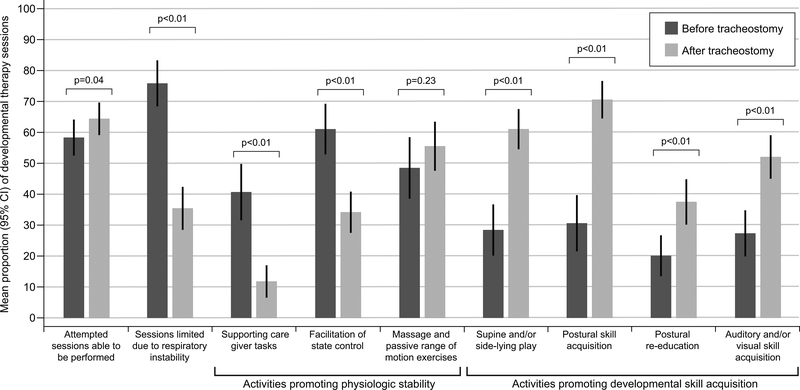
More developmental therapy sessions were attempted and performed during the 4-week period following the first tracheostomy change as compared to the 4 weeks prior to the procedure (Table 2). In addition, fewer sessions post tracheostomy were limited by decompensation in respiratory status (35% post vs. 76% pre-tracheostomy, p<0.0001) or required the therapist to facilitate state control (34% post vs. 61% pre-trachesotomy, p<0.0001). There was a positive shift in the focus of intervention sessions from supporting patient care tasks and state control before the tracheostomy toward positioning, posture and motor skill acquisition within the context of play and development of auditory and visual skills post-tracheostomy (Figure 2).
Figure 2. Developmental activity before and after tracheostomy.
Frequency participation and type of physical therapy sessions performed during the four-week period before tracheostomy placement and after the first tracheostomy change were collected and compared. Supporting care giving tasks include obtaining vital signs, diaper change, suctioning and repositioning through containment, pacing etc. Facilitation of state control includes providing pacifier, rocking, patting etc. to assist with calming. Supine and/or side-lying play include reaching, hands to midline, exploration tasks. Postural skills include facilitation of improved posture in supported sitting and/or prone position. Postural re-education includes more active postural training such as head control, righting etc.
Respiratory Support
Ventilator settings at the time of tracheostomy placement were a mean MAP 14.8±4.7, PIP 26.6±7.3, PEEP 10.5±3.5 and FiO2 0.48±0.09. There was no significant change in ventilator support and the mean RSSs were not different after tracheostomy placement as compared to before surgery (Mean RSSs 7 days before, the day of and 7 days after tracheostomy placement were 6.8±3.6, 7.0±3.2 and 6.0±2.3) (Table 4).
Table 4.
Respiratory support around the time of tracheostomy
| 7-day before | Day of trach | 1-day after | 7-day after | |
|---|---|---|---|---|
| RSS | 6.8±3.6 | 7.0±3.2 | 5.4±2.5 | 6.0±2.3 |
| MAP (cmH2O) | 14.7±5.0 | 14.8±4.7 | 14.4±4.0 | 15±4.2 |
| FiO2 | 0.45±0.15 | 0.46±0.17 | 0.38±0.14 | 0.39±0.11 |
| PIP (cmH2O) | 25.1±7.0 | 26.6±7.3 | 26.8±6.5 | 26.9±6.0 |
| PEEP (cmH2O) | 10.7±3.9 | 10.5±3.5 | 10.5±3.3 | 10.9±3.1 |
| IMV rate (breath/min) | 23.8±9.2 | 28.5±8.4 | 30.8±8.6 | 29.3±10.3 |
| Ti (sec) | 0.5±0.1 | 0.5±0.1 | 0.5±0.1 | 0.5±0.2 |
Data presented as mean ± standard deviation. Ventilator settings were collected between 8–10 in the morning.
RSS: respiratory severity score; MAP: mean airway pressure; FiO2: fraction of inspired oxygen; PIP: peak inspiratory pressure; PEEP: positive end expiratory pressure; IMV: intermittent mandatory ventilation; Ti: inspiratory time
Tracheostomy Complications
The most common post-operative complication was skin breakdown and/or soft tissue infection at the tracheostomy site (20%). Two patients had significant increases in supplemental oxygen requirements in the immediate post-operative period prompting initiation of inhaled nitric oxide and dexamethasone. One patient received a red blood cell transfusion on post-operative day 1 for a decrease in hemoglobin concentration from 10.6 g/dL to 7.8 g/dL without apparent clinical bleeding. Three patients who underwent tracheostomy at our institution died prior to discharge and were excluded from the analysis. Of these, 2 had care withdrawn due to the severity of their illness and anticipated poor long-term outcome. One died of sudden cardiac arrest 4 days post-surgery. In this patient, there was no increase in ventilator settings post- surgery; tracheostomy obstruction or accidental decannulation were excluded by chest x-ray and emergency bedside bronchoscopy.
Sedation Medications
Fifty-seven patients (79% of the full study cohort) had complete sedation medication data available for evaluation. Each received scheduled (not “as needed”) sedation medications during the 24-hour period prior to tracheostomy; 51 (89%) were treated with both opiates and benzodiazepines. In the immediate post-operative period, sedation medications were escalated in all patients per our standard practice to prevent accidental decannulation of the tracheostomy and to avoid injury to the fresh surgical site. Despite this dose increase, 89% of patients (n=51) required less opioid or benzodiazepine 4 weeks after the first tracheostomy change compared to their pre-operative baseline. Over half of the infants (n=33) experienced a ≥50% reduction in daily opioid or benzodiazepine dosage, with 43% (n=22) receiving ≥50% less of both classes of medication. The mean daily opioid dose, expressed as intravenous morphine equivalents, decreased from 2.0 ± 3.1 mg/kg/day to 1.2 ± 2.0 mg/kg/day (p< 0.009) and the mean daily benzodiazepine dose, expressed as intravenous midazolam equivalents, decreased from 2.3 ± 2.3 mg/kg/day to 1.8 ± 2.8 mg/kg/day (p< 0.02).
DISCUSSION
The present study compared respiratory, growth, developmental activity, and daily sedation requirements before and after tracheostomy placement in a quaternary referral population of preterm infants with established, severe BPD. Most infants in this cohort were outborn and transferred to our institution following prolonged high level respiratory support. In this high-risk population, of whom approximately one-third undergo tracheostomy at out institution, tracheostomy placement was associated with improvement in growth (with decreased caloric need), greater tolerance of developmental therapy and a reduction in sedation medication exposure. To our knowledge, this study is the first to evaluate the change in these clinically relevant parameters before and after tracheostomy in very preterm infants with severe BPD.
Many parents and clinicians view tracheostomy placement in preterm infants as a negative clinical outcome. Worries include that it increases caregiver burden and is associated with increased risk of death and neurologic impairment. However, for some babies with severe lung and/or airway disease, tracheostomy may be the only means to facilitate a safe, timely transition to home. DeMauro et al., using data from the US Neonatal Research Network, found that tracheostomy placement in extremely preterm infants was associated with increased risk-adjusted rates of cognitive and motor delay16. Notably, however, tracheostomy placement prior to approximately 4 months of age (<120 days) compared to after this time point, was associated with a 50% reduction in the odds of death or neurodevelopmental impairment16. The investigators suggested this observation may be due, at least in part, to an earlier transition to developmentally enriching care. The results of the present study provide some of the first empirical data to support this hypothesis and suggest further possible mechanisms why earlier tracheostomy placement may confer benefit among preterm infants who ultimately undergo this procedure.
Compared to the first weeks and months of life, preterm infants typically exhibit more frequent and longer calm and active awake states once reaching term corrected gestation19. This transition may help facilitate motor and cognitive skill acquisition through increased interaction with caregivers and the surrounding environment. Unfortunately, infants with the most severe forms of BPD are often too clinically unstable to tolerate caregiver handling. This may further exacerbate neurocognitive delay as physical therapy during the neonatal period is associated with improved outcomes among high-risk infants20,21. We observed that developmental specialists attempted and successfully performed a greater number of therapy sessions per week during the month after compared to before tracheostomy. Moreover, fewer sessions were limited in scope because of clinical instability and a greater proportion focused on developmental skill acquisition than promotion of physiologic stability. The majority of infants also experienced a substantial reduction in sedative exposure within 5 weeks post-tracheostomy. Finally, in our experience, tracheostomy improves parents’ ability to hold and play with their infant with less interruption due to clinical instability and with less assistance from healthcare practitioners.
In addition to delayed participation in developmentally appropriate activities, infants with severe BPD are at high risk for growth failure22. While most clinicians focus on weight gain, less attention is paid to linear growth. Unfortunately, infants who do not achieve appropriate linear growth are at increased risk of adverse neurodevelopmental and reduced postnatal gains in lung function23,24. Our data are consistent with previous data showing that poor linear growth is particularly common in infants with severe BPD5. Although most infants in our cohort were born appropriate for gestational age, nearly all were growth restricted, particularly with profound deficits in linear growth, before tracheostomy placement. Post-tracheostomy, we observed significant improvements in weight, length and head growth. This change was most notable for linear growth, with most infants demonstrating improvement in weight-to-length ratio and length Z scores post-tracheostomy. These changes in growth occurred despite no overall change in pre- and post-operative respiratory severity scores and a decrease in caloric intake. We speculate the improved growth following tracheostomy may result from decreased stress and agitation (and thus less work of breathing and energy expenditure) following exchange of a less secure oral/nasal endotracheal tube for a more stable and comfortable tracheostomy.
In this cohort, tracheostomy placement was clinically well tolerated in the majority of infants. The most common post-operative complication was skin breakdown and/or soft tissue infection at the tracheostomy site, occurring in less than one-quarter of infants. Overall mortality prior to hospital discharge was low and no deaths were directly attributed to a tracheostomy complication. Our results are similar to that of Mandy et al25 who reported no clinical deterioration post tracheostomy. Together, these findings suggest that tracheostomy placement among infants with severe lung disease can be safely performed. However, our results reflect outcomes at a high-level, high-volume center where the surgeons, anesthesiologists, and an ICU team have considerable experience caring for infants with severe BPD. Intra- and post- operative cardiopulmonary instability can be life-threatening in infants with unstable lung disease, especially those with severe pulmonary hypertension. Therefore, tracheostomy placement in such patients would be best performed in centers with experience in the peri-operative care of infants with severe BPD and also with established home ventilation programs.
Although our study was not designed to examine the optimal timing for tracheostomy placement, our data, along with data from DeMauro et al, raise the question whether tracheostomy should be placed earlier in some patients with severe BPD who are likely to require the procedure16. Further research is needed to address this knowledge gap. At present, there are no standard criteria for determining if and when to place a tracheostomy in these babies. At our institution, infants with severe BPD are considered for tracheostomy if they are beyond 40 weeks PMA and anticipated to require high-level respiratory support (either invasive mechanical ventilation or high level non-invasive support) for a prolonged period of time. We typically gauge the adequacy of ongoing respiratory support by evaluating growth (both weight gain and linear growth), the infant’s ability to participate in developmentally appropriate physical activities, and stability of BPD-associated pulmonary hypertension, if present. If weaning of respiratory support consistently impacts one or more of these parameters in a negative way, we begin discussions with the family regarding whether a tracheostomy is an appropriate and safe option for their baby. Tracheostomy will not be placed until risks and benefits of tracheostomy (including medical and social) for each individual patient are carefully evaluated and patient reach relative clinical stability that risk of clinical deterioration with the procedure is considered low.
Our study has several limitations. In particular, is its retrospective nature and reliance on data obtained through chart review. In addition, we cannot assess whether the observed changes in developmental therapies and growth before and after tracheostomy resulted, at least in part, from temporal change (e.g. gradual improvement over time) in these outcomes. Finally, these results only reflect data from a single center. Confirmation in a larger, multi-center cohort is needed. Nonetheless, our study provides short-term outcome data for a relatively large, contemporary cohort of infants who underwent tracheostomy placement for severe BPD.
In conclusion, our findings suggest that in infants with severe BPD who require prolonged high level respiratory support beyond term corrected gestation, tracheostomy placement is safe and may have beneficial effects on growth and short-term development. When clinicians and parents are trying to decide whether or not to place tracheostomy, these potential beneficial effects should be taken into consideration along with other benefits and balanced against the risks and caregiver burden of tracheostomy. Further studies are needed to determine the best timing of tracheostomy placement in this population.
Acknowledgement:
We appreciate Fen Xu, MD, Department of Neonatology, Bao’an Maternity and Child Health Hospital of Shenzhen Affiliated to Jinan University, Guangdong, China for her help analyzing some initial data and Brenda Waber, RD, Newborn/Infant Intensive Care Unit, Children’s Hospital of Philadelphia for her advice in assessing growth parameter Z scores.
Source of support:EAJ was supported by a grant from the National Heart, Lung, and Blood Institute (K23HL136843).
Footnotes
Primary study institution: Children’s Hospital of Philadelphia
Reference:
- 1.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–1955. [DOI] [PubMed] [Google Scholar]
- 2.Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(5):1305–1311. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dani C, Poggi C. Nutrition and bronchopulmonary dysplasia. J Matern Fetal Neonatal Med. 2012;25 Suppl 3:37–40. [DOI] [PubMed] [Google Scholar]
- 5.Natarajan G, Johnson YR, Brozanski B, et al. Postnatal weight gain in preterm infants with severe bronchopulmonary dysplasia. Am J Perinatol. 2014;31(3):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360. [DOI] [PubMed] [Google Scholar]
- 7.Overman AE, Liu M, Kurachek SC, et al. Tracheostomy for infants requiring prolonged mechanical ventilation: 10 years’ experience. Pediatrics. 2013;131(5):e1491–1496. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan G, Pappas A, Shankaran S, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. 2012;88(7):509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover TR, Brozanski BS, Barry J, et al. High surgical burden for infants with severe chronic lung disease (sCLD). J Pediatr Surg. 2014;49(8):1202–1205. [DOI] [PubMed] [Google Scholar]
- 10.Guaman MC, Gien J, Baker CD, Zhang H, Austin ED, Collaco JM. Point Prevalence, Clinical Characteristics, and Treatment Variation for Infants with Severe Bronchopulmonary Dysplasia. Am J Perinatol. 2015;32(10):960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sisk EA, Kim TB, Schumacher R, et al. Tracheotomy in very low birth weight neonates: indications and outcomes. Laryngoscope. 2006;116(6):928–933. [DOI] [PubMed] [Google Scholar]
- 12.Singer LT, Kercsmar C, Legris G, Orlowski JP, Hill BP, Doershuk C. Developmental sequelae of long-term infant tracheostomy. Dev Med Child Neurol. 1989;31(2):224–230. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt B, Asztalos EV, Roberts RS, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289(9):1124–1129. [DOI] [PubMed] [Google Scholar]
- 14.Walsh MC, Morris BH, Wrage LA, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146(6):798–804. [DOI] [PubMed] [Google Scholar]
- 15.Estournet-Mathiaud B Tracheostomy in chronic lung disease: care and follow-up. Pediatr Pulmonol. 2001;Suppl 23:135–136. [PubMed] [Google Scholar]
- 16.DeMauro SB, D’Agostino JA, Bann C, et al. Developmental outcomes of very preterm infants with tracheostomies. J Pediatr. 2014;164(6):1303–1310 e1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. [DOI] [PubMed] [Google Scholar]
- 18.Iyer NP, Mhanna MJ. Non-invasively derived respiratory severity score and oxygenation index in ventilated newborn infants. Pediatr Pulmonol. 2013;48(4):364–369. [DOI] [PubMed] [Google Scholar]
- 19.Mirmiran M, Maas YG, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003;7(4):321–334. [DOI] [PubMed] [Google Scholar]
- 20.Piek JP, Dawson L, Smith LM, Gasson N. The role of early fine and gross motor development on later motor and cognitive ability. Hum Mov Sci. 2008;27(5):668–681. [DOI] [PubMed] [Google Scholar]
- 21.Blauw-Hospers CH, Hadders-Algra M. A systematic review of the effects of early intervention on motor development. Dev Med Child Neurol. 2005;47(6):421–432. [DOI] [PubMed] [Google Scholar]
- 22.Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104(2 Pt 1):280–289. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Solis M, Perez-Fernandez V, Bosch-Gimenez V, Quesada JJ, Garcia-Marcos L. Lung function gain in preterm infants with and without bronchopulmonary dysplasia. Pediatr Pulmonol. 2016;51(9):936–942. [DOI] [PubMed] [Google Scholar]
- 24.Ramel SE, Demerath EW, Gray HL, Younge N, Boys C, Georgieff MK. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology. 2012;102(1):19–24. [DOI] [PubMed] [Google Scholar]
- 25.Mandy G, Malkar M, Welty SE, et al. Tracheostomy placement in infants with bronchopulmonary dysplasia: safety and outcomes. Pediatr Pulmonol. 2013;48(3):245–249. [DOI] [PubMed] [Google Scholar]