Abstract
The widespread nature of diabetes affects all organ systems of an individual including the bone marrow. Long-term damage to the cellular and extracellular components of the bone marrow leads to a rapid decline in the bone marrow-hematopoietic stem/progenitor cells (HS/PCs) compartment. This review will highlight the importance of bone marrow microenvironment in maintaining bone marrow HS/PC populations and the contribution of these key populations in microvascular repair during the natural history of diabetes. The autonomic nervous system can initiate and propagate bone marrow dysfunction in diabetes. Systemic pharmacological strategies designed to protect the bone marrow-HS/PC population from diabetes induced-oxidative stress and advanced glycation end product accumulation represent a new approach to target diabetic retinopathy progression. Protecting HS/PCs ensures their participation in vascular repair and reduces the risk of vasogdegeneration occurring in the retina.
Keywords: Diabetic retinopathy, bone marrow microenvironment, hematopoietic stem cells
1. INTRODUCTION
Based on an epidemiology study published in 2014, diabetes affects 382 million people worldwide [1]. Moreover, the prevalence of diabetes continues to increase globally: about 592 million people are estimated to be diagnosed with diabetes by 2035 [1]. Diabetic retinopathy (DR) is the most prevalent complication of diabetes and affects about 93 million individuals worldwide. DR accounts for 4.8% of the number of cases of blindness (37 million) worldwide [2-4]. The number of individuals with vision-threatening DR, such as severe non-proliferative DR (NPDR) and PDR, is estimated to rise to 191 million by 2030. The number of individuals with diabetic macular edema (DME) is expected to increase to 56.3 million by that same year [5, 6]. Importantly, the presence of DR indicates microcirculatory dysfunction in other organ systems [7]. Despite better control of the modifiable risk factors (glucose, blood pressure and lipids) and better screening programs, DR remains a global health issue. A weakness of current therapies for DR (laser photocoagulation, injection of corticosteroids or anti-VEGF antibodies, or vitreoretinal surgery) is that these approaches do not correct the underlying pathology and carry significant side effects.
The pathogenesis of DR suggests that it is a progressive vasodegenerative condition associated first with the loss of contractile pericytes followed by a widespread death of endothelial cells. Loss of this cellular support system culminates in under perfused areas of ischemia, depriving retina of a vital nutrient supply which triggers the remaining endothelium to release “vessel building” cytokines and growth factors such as vascular endothelial growth factor (VEGF). In addition, the retina faces a unique challenge in diabetes due to the combination of high metabolic demand and minimal vascular supply. This limits the retina’s ability to adapt to the metabolic stress of diabetes. The retina can lose as much as 38% of resident endothelial cells within 20 months of experimental diabetes [8].
DR typically progresses because hyperglycemia and dyslipidemia are not adequately controlled [9, 10]. While all microvascular beds are affected by diabetes, the ones resulting in the most profound impact on the diabetic individual are the kidney, retina, and large nerves. The resultant nephropathy, retinopathy, and neuropathy share common etiologies and pathogenic mechanisms including altered metabolic and functional/hemodynamic factors and disturbed interactions between environmental, hormonal and genetic factors [11].
In this review, we are focusing on understanding the contribution of the bone marrow in the pathogenesis of DR and we provide an argument that the bone marrow should be considered a new target tissue for treatment of DR.
2. BONE MARROW AND HEMATOPOIETIC STEM/PROGENITOR CELLS (HS/PCs)
The bone marrow provides the primary conducive environment to harbor HS/PCs in adults and children. The bone marrow can selectively give rise to a variety of cell types, including lymphoid and myeloid cells, platelets, and red blood cells [12, 13]. About a million mature blood cells are produced per second in the normal adult human bone marrow. While the majority of HSCs are in G0 phase, it is interesting to note that under physiologic conditions, there exists a perfect balance between responding to the enormous demand for hematopoietic cells and the preservation of an adequate pool of HSCs. This balance between regulated self-renewal, expansion, and differentiation is of critical importance as too little self-renewal can jeopardize the ability of the bone marrow to sustain hematopoiesis throughout the lifetime of the individual while excessive differentiation can result in aberrant phenotypes such as leukemogenesis [14]. Normally, HSCs give rise to 10% myeloid and 90% lymphoid donor type cells; however, this balance may shift towards more myeloid and less lymphoid cell types with a disease state such as diabetes or in aging, creating myeoloidosis [15].
Diabetes leads to changes in cellular metabolism that result in overproduction of reactive oxygen species, genetic instability, and disruption of homeostatic pathways. These metabolic changes, not unexpectedly, adversely affect the bone marrow and lead to the myeoloidosis [16]. The bone marrow provides specialized niches for specific stem cell types [13]. The spindle-shaped N-cadherin expressing osteoblast cells (SNO) lining the endosteal bone surface act as the endosteal niche for the HSCs while the bone marrow sinusoids together with HSCs constitute the vascular niche in the bone marrow (Figure 1). The HSCs near the endosteum are the longest-lived cells possessing unlimited self-renewal potential and are called long-term repopulating HSCs (LTR-HSCs). The well-orchestrated dynamics of the processes of self-renewal and release governs the supply of HSCs present in the circulation (15). LTR-HSCs respond to stimuli or injury and travel to the sinusoid; at this point, they lose their unlimited self-renewal capability and become committed short-term repopulating HSCs (STR-HSCs). Typically, 60% of STR-HSCs are found near sinusoidal niche, while only 20% of HSCs (i.e. LTR-HSCs) are at the endosteal surface. These two bone marrow stem cell niches have different oxygen levels further distinguishing their specialization.
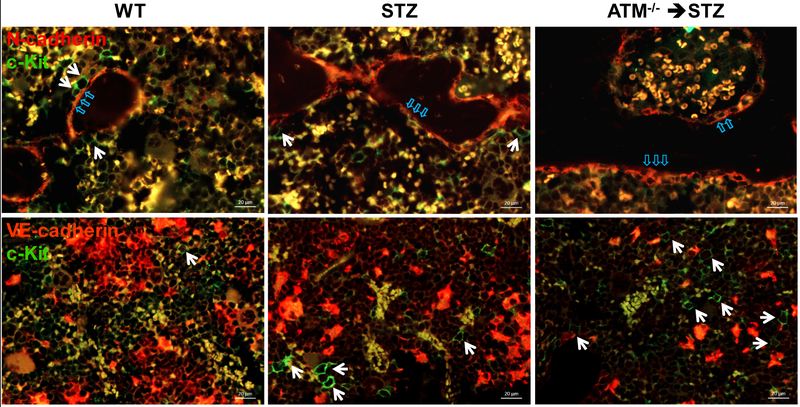
Figure 1: Localization of c-Kit+ cells in mouse bone marrow.
Upper panel: immunofluorescence staining of N-cadherin (red) and c-Kit (green) in demineralized mouse femurs. Some c-Kit+ cells (white arrow) localized to endosteal niche (blue arrow) are defined as long-term repopulating (LTR)-hematopoietic stem cells (HSCs); Lower panel: mouse femurs stained for VE-cadherin (red) and c-Kit (green). c-Kit+ cells (white arrow) located at vascular niche are defined as short-term repopulating (STR)-HSCs. Representative images showing a reduced number of LTR-HSCs and increased STR-HSCs/LTR-HSCs in the STZ-induced diabetic bone marrow. ATM−/− intensified diabetes-mediated defects of LTR- and STR-HSCs imbalance in the bone marrow. Abbreviations: WT, wild type; STZ, streptozotocin; ATM, ataxia telangiectasia mutated.
2.1. KEY MOLECULAR TARGETS AFFECTING BONE MARROW FUNCTION
We reported that ataxia telangiectasia mutated (ATM) is critical for maintaining long-term populating HSCs in the bone marrow [17]. ATM helps correct DNA damage by recruiting DNA repair proteins to sites of DNA damage. The ATM is necessary for the survival of LTR-HSCs and the loss of ATM results in an increase in more committed STR-HSCs. This imbalance may eventually contribute to the development of diabetic retinopathy. Forkhead box-O (FoxO) also plays a critical role in maintaining HSC population. Conditional deletion of FoxO isoforms FoxO1, FoxO3, and FoxO4 from bone marrow induces HSC imbalance (less LTR-HSC and more STR-HSC) results in an increase in oxidative stress [18-20], whereas overexpression of FoxO decreases oxidative stress and apoptosis [21]. The functional interaction between FoxO3 and ATM halts DNA damage due to activation of DNA damage-inducible gene 45 (Gadd45).
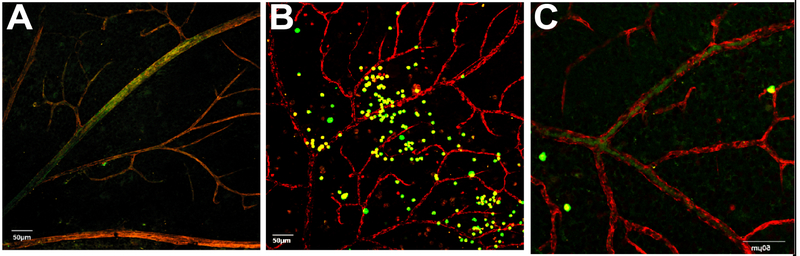
In addition to hyperglycemia-induced bone marrow damage, we also reported that dyslipidemia critically affects bone marrow function. Our published studies demonstrate that diabetes-induced derailment of cholesterol and sphingolipid metabolism leads to shift in LTR/STR-HSCs, as well as myeloid/lymphoid cell balance. As we previously demonstrated, the central enzyme of sphingolipid metabolism, acid sphingomyelinase (ASM) is highly upregulated in bone marrow niche in diabetes. As shown in Figure 2, when injected into the vitreous, control HSC migrate into the areas of retinal vascular damage, home to the vasculature and aid in the repair process. Diabetic HSCs with a high level of ASM expression level lose the ability to migrate and thus remain in the vitreous and do not participate in vascular repair. Inhibition of ASM in HSCs greatly improved HSC migration, homing to retinal vasculature and repair potential. Interestingly, when injected into the control eyes, normal control HSCs migrate out of the vitreous and return to the bone marrow niche [22]. Diabetic HSCs with high ASM do not migrate and remain in the vitreous. Inhibition of ASM in diabetic HSC restores their migration ability.
Figure 2: Inhibition of acid sphingomyelinase in diabetic CACs corrects their dysfunction.
Diabetic animals received intravitreal injections of control (A), diabetic (B), or diabetic CACs treated with siRNA for ASM (C). CACs (green) were isolated from gfp+ mice, retinal vasculature was stained with anti-collagen IV antibody (red), and localization (yellow) indicates vascular association. Diabetic CACs show reduced localization with vasculature (middle), while ASM inhibition improved vascular association of diabetic CACs (right). Abbreviations: ASM, acid sphingomyelinase; BM, bone marrow; CAC, circulating angiogenic cell.
3. WHY IS BONE MARROW PATHOLOGY LINKED TO THE PATHOGENESIS OF DIABETIC RETINOPATHY?
For the last decade, it has become increasingly evident that bone marrow dysfunction plays a key role in the pathogenesis of DR. Diabetes accelerates bone marrow HSCs aging which not only promotes myeloid-biased HSCs but also induces a rapid decline in LTR-HSCs from the bone marrow (8). Diabetes impairs the bone marrow architecture, function, and numbers of reparative cells. Diabetes also impacts bone marrow - derived immune cells. Typical features of diabetic bone marrow include microangiopathy, neuropathy, stem cell rarefaction, and extensive fat deposition, along with increased apoptosis leading to reduced numbers of reparative cells such as CD34+ cells [23-26]. Neuropathy results in reparative cells being trapped in the bone marrow unable to enter the circulation for homing to regions, such as the retina in need of repair [27-29]. There is a normal circadian regulation of release of reparative cells in healthy subjects. This release of reparative cells occurs during the rest phase. The traffic of both reparative cells and immature immune cells from the bone marrow occurs in response to activation of β-adrenergic receptors expressed on hematopoietic stem cells, osteoblasts, and mesenchymal stem cells. Previously, we showed that there are changes in the level of innervation of the bone marrow in diabetes. We showed that very early in the natural history of diabetes, the peripheral nerves innervating the bone marrow respond to the abnormal diabetic milieu and oxidative injury by compensatory neurogenesis [27]; whereas later on there is a dramatic reduction in the number of sympathetic nerves within the bone marrow by immunohistochemistry (IHC) [30].
3.1. DIABETIC MOBILOPATHY
Loss of innervation results in a ‘mobilopathy’ in which bone marrow cells are trapped in the bone marrow and are not released into the systemic circulation. This mobilopathy occurs in both diabetic rodents and humans. Possible mechanism responsible for the development of this mobilopathy include: (i) an aberrant response to beta-adrenergic stimulation, (ii) failure of nestinpositive mesenchymal stem cells to downregulate the CXCL12 expression [31, 32], (iii) defective hypoxia-inducible growth factor signaling [33], (iv) tissue-specific alteration in dipeptidyl peptidase-4 (DPP-4) [34], (v) decrease in the levels of cytokines such as SDF-1 and osetoprotegerin, (vi) increase in oxidative stress, DNA damage and apoptosis [26] and (vii) diabetes-induced increase in cell and plasma membrane rigidity [22, 35].
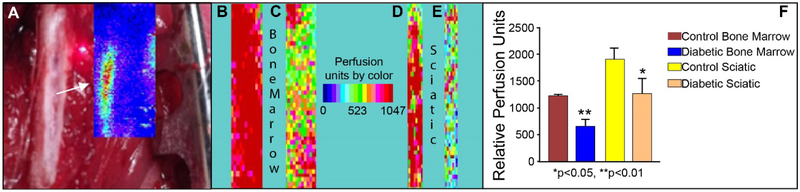
Clinical evidence of the mobilopathy is provided by retrospective analysis of bone marrow transplant subjects. These studies suggest that individuals with T1D and T2D have reduced mobilization of CD34+ cells in response to stimulation by granulocyte colony-stimulating factor (GM-CSF) [36]. We interpret this finding as presence of bone marrow neuropathyin these individuals and showed in a type 2 model that denervation is associated with reduced blood flow in the bone marrow (Figure 3).
Figure 3: BBZ/Wor T2D rats exhibit reduced blood flow to the bone marrow.
A significant reduction in blood flux is observed in T2D rats compared to controls in both the BM and sciatic nerve. (A) Background showing exposed femur under retraction. Target area was imaged using a laser Doppler (LDPI) for relative blood flux and color charted (arrow and inset). Examples of LDPI of bone marrow (B & C) and sciatic nerve (D & E) in a control and T2D rat respectively. Warm (red) colors represent regions of relative high flux whereas cool colors (blue) represent areas of low flux. F: Quantification of LDPI imaging is shown in control (n = 4) and diabetic (n = 4) rats.
Long-standing diabetes results in an imbalance of bone marrow lymphocyte and monocyte populations and a selective decline of HSCs. The increase in bone marrow-myelodiosis is reflected in an increase in the monocytic population in peripheral blood which is seen not only in rodent models [37, 38] but also in humans [28]. While diabetes promotes the production of myeloid-biased HSCs, it also induces a rapid decline in LTR-HSCs from the bone marrow endosteal niche with an increase in more committed STR-HSCs near the sinusoidal niche [39].
Since the diabetic microvasculature is in constant demand for new cells to ‘replace’ dying endothelium and to keep the vasculature healthy following an ischemic insult [10], an increase in STR-HSCs provides a ready source of vascular supporting cells; however, these cells can become exhausted. Not only are the numbers of these cells reduced, but also the cells exhibit dysfunctional behavior with reduced migratory function.
3.2. PHENOTYPIC CHARACTERIZATION OF BONE MARROW CELLS
Previously, we showed that healthy peripheral blood derived (non-diabetic) CD34+cells (human vascular progenitors) home to areas of injury in the diabetic retina and in the ischemia/reperfusion (I/R) retinal injury model [40]. CD34+ cells represent a population of progenitor cells that: i) are hematopoietic in origin and express CD45+, CD14+, CD145lo, VE-CAD+/−, CD105+, vWF+/−, CD133+, CXCR4+, VEGFR1+, and VEGFR2+ among other surface receptors; ii) consistently contribute to revascularization by providing paracrine support to the resident vasculature; iii) are easily isolated from peripheral blood, bone marrow and umbilical cord blood and iv) are currently in clinical trial for advanced DR (ClinicalTrials.gov Identifier: NCT01736059) [41]. However, the initial results have shown only modest improvement in visual outcomes [41], likely due to the defective function of the autologous diabetic CD34+ cells being injected.
A weakness of this research is that multiple mechanisms have been identified for this dysfunction, thus targeting a single pathway results only in the partial and transient improvement of CD34+cell function. A further weakness in most studies, including our own, is the use of only a single marker to isolate/identify these cells. The reason being is that the number of human cells available to study is reduced by each marker used for selection and the starting number of these cells (isolated from peripheral blood of diabetic individuals) is often severely limited. The “single marker isolation” results in a very heterogeneous population of cells for studies. Furthermore, there is disagreement about what additional markers should be used for characterization, for example, VEGFR2 or CD133 or CXCR4, making it difficult to compare results between different human studies that have used multiple markers [42-44]. In essence, CD34+cells represent an incompletely characterized population [45]. A further weakness is that murine stem/progenitors are characterized by different surface markers than humans, making it difficult to compare between species [46].
4. THE BONE MARROW- BRAIN CONNECTION IN THE PATHOGENESIS OF DIABETIC RETINOPATHY
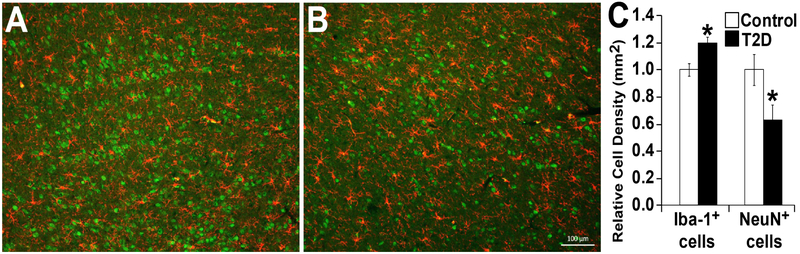
An imbalance in the sympathetic nervous system, with the loss of parasympathetic drive and increased sympathetic activation, occurs in diabetes. Ultimately these neuronal changes transition to neuronal loss and in the bone marrow can directly impact diabetic retinopathy [16, 17, 27, 28, 30, 37, 38, 47-50]. In chronic diabetes bone marrow denervation occurs and precedes the onset of diabetic retinopathy in rodent models of diabetes [28, 30, 37, 49]. The altered neuronal input to bone marrow stromal cells (due to diabetes-induced peripheral neuropathy) results in the altered production of growth factors/cytokines. Hematopoiesis shifts away from the generation of reparative vascular progenitor cells towards the production of excessive numbers of deleterious pro-inflammatory (CCR2+1 monocytes that enter the systemic circulation. Importantly, monocytosis is not limited to rodent models but is also observed in T1D human subjects. Sympathetic hyperactivation results in hypoxia of these autonomic centers which results in CCL2 expression [51]. Bone marrow -derived CCR2+ cells home to these regions (responding to the local CCL2 gradient) where they become activated. Activated bone marrow-derived macrophages in these regions stimulate resident microglia (Figure 4). CCR2+ monocytes also home to the retina to promote diabetic retinopathy pathology [28, 48]. As discussed above, diabetic dyslipidemia with increased activity of ASM in diabetic bone marrow affects the balance between pro-inflammatory macrophages and reparative progenitor cells. Use of chimeric mice with fluorescently labeled bone marrow cells allows bone marrow-derived cells to be easily detected in peripheral tissues. Inhibition of ASM only in the bone marrow niche using ASM−/− BMT was sufficient to prevent retinal microglia activation, as well as to inhibit diabetes-induced inflammation and retinal vascular damage (Figure 5).
Figure 4: Increased density of lba-1+ microglia and a decreased density of NeuN+ neurons in the hypothalamus of type 2 diabetic (T2D) rats.
Brain sections of hypothalamic region of control (A), T2D (B) rats showing lba-1 staining in red and NeuN in green. (C) Bar chart showing relative quantitation. Scale bar =100 μm.
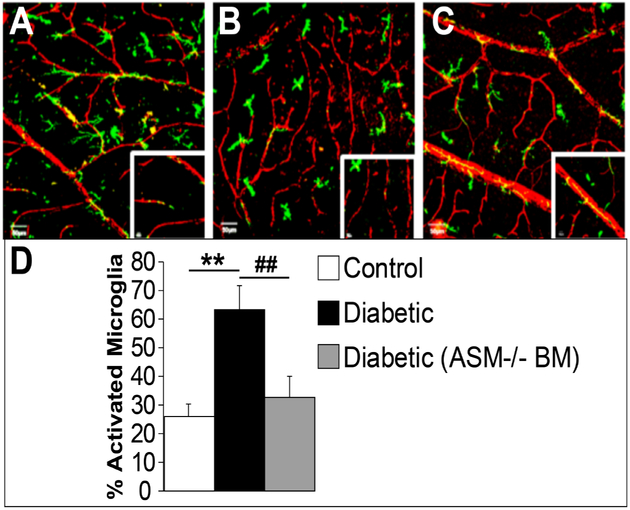
Figure 5: BM-ASM inhibition prevents activation of microglia in diabetic retinas.
Confocal images of retinal flat mounts from bone marrow chimeras with GFP (green), or GFP-ASM−/− bone marrow donors and wild type recipient mice. The retinas are stained with collagen IV for the retinal vasculature (red). (A) Control retina showing resting microglia with the typical ramified and branching shape. (B) Diabetic animals show activated microglia with more compact cells with fewer ramifications. (C) Diabetic animal showing that BM-ASM inhibition prevents activation of microglia. (D) Bar chart showing the quantification of the percentage of activated microglia in the three conditions. Scale bar is 50 μM, n=8
There is sympathetic drive/norepinephrine (NE) spillover and a decrease in parasympathetic drive [52-54] to the peripheral organs [55, 56], resulting in end-organ damage (15); vascular/endothelial dysfunction [57], and hormonal imbalance (17) in diabetes. Thus it can be reasoned that changes in autonomic nervous system (ANS) activation can have a role in the pathogenesis of diabetic complications including diabetic retinopathy [28, 48]. Previously, others and we have shown that early on in the natural history of diabetes there is an increase in neurotransmission in key regions of the hypothalamus. The excitatory neurotransmitter, glutamate is increased in diabetes [58]. Increased orexin (ORX) immunoreactivity occurs in diabetes and localizes to neurons of the lateral and posterior hypothalamus, primary sites of the brain wherein ORX acts to stimulate food intake and energy homeostasis. Activation of ORX neurons in the hypothalamus increases sympathoadrenal pathways to release epinephrine [59, 60]. Intracerebroventricular injections of ORX increased arterial pressure, renal sympathetic neuronal activity, and levels of vasopressin, epinephrine, and plasma glucose. Hypothalamic ORX immunoreactive projections to the rostral medulla can stimulate the cardiovascular center [61] and activate preganglionic sympathetic neurons in spinal cord. Both oxytocin and vasopressin are excitatory neurotransmitters found in descending projections to sympathetic premotor neurons [59]; levels of both are increased in diabetes (21). Oxytocin and vasopressin localize with the catecholamine synthesis marker tyrosine hydroxylase in PVN neurons [62-64], and the expression of these excitatory neurotransmitters and neuropeptides has been shown to be increased in diabetes [65, 66].
Not only are excitatory neurotransmitters increased, but there are reductions in inhibitory neurotransmitters such as a gamma amino butyric acid (GABA) and somatostatin (SST) [67], further serving to promote an increase in ANS activation. SST is released from a subclass of GABAergic inhibitory neurons that can be part of a local circuit or be projecting [68, 69]. Studies have shown that a deficiency or hypofunction of GABAergic mechanisms in the hypothalamus may be related to the pathogenesis of a variety of clinical conditions characterized by altered sympathetic nerve activity, such as hypertension [68, 70], and heart failure [71] and diabetes. Hambley et al. [72], reported significant reductions in endogenous hypothalamic GABA (inhibitory) concentrations and in the density of GABAa receptors in spontaneously hypertensive/metabolic syndrome rats compared to control [71-73]. Complementary glutamatergic mechanisms are involved in NMDA-mediated sympathetic outflow in the hypothalamus in heart failure due to NMDA receptor upregulation [74].
Autonomic centers innervate the bone marrow and tracing studies show direct connections between the brain SST neurons and the bone marrow. The bone marrow neuropathy is accompanied by altered blood flow to the bone marrow that contributes to the ongoing pathology. Using the BBZ/Wor rat model of type 2 diabetes, we show that somatostatin levels fall in the hypothalamus compared to controls. We also observe that these autonomic nuclei simultaneously exhibit increased regional inflammation. Based on published work, the source of this inflammation includes extravasation of circulating cells [75].
Thus, early in diabetes, there is an over-activation of the ANS whereas later there is denervation. IHC studies of BBZDR/Wor transgenic rats with T2D demonstrate loss of somatostatin-immunoreactive neurons in the hypothalamus (Figure 6), and co-localization of somatostatin with neurons labeled following bone marrow injections of Pseudorabies virus (Figure 7). These data indicate a marked adverse effect of diabetes on hypothalamic somatostatin expression and show that somatostatin-expressing neurons project to the bone marrow whereby aberrant signaling and ultimately their loss could produce bone marrow pathology and contribute to development of DR. While somatostatin neurons represent one population of neurons that are lost early in the natural history of diabetes, previously we showed tyrosine hydroxylase expressing neurons are also reduced and that loss of adrenergic nerves influences hematopoiesis and vascular repair mechanisms [30, 37, 38, 49, 76, 77]
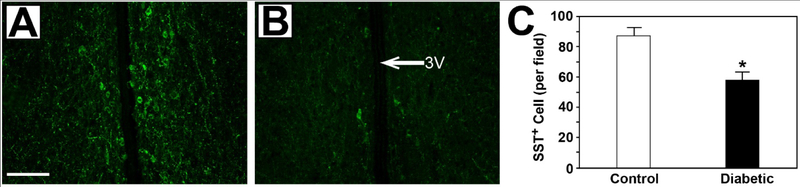
Figure 6: Decrease in SST+ cells in the hypothalamus in type 2 diabetic rats.
Brain sections from age-matched nondiabetic lean BBZ rats (A) show robust cellular SST staining compared to that observed in diabetic BBZ/Wor rats with 4 months of diabetes (B). 3V = third ventricle. Scale bar = 50μm. (C) Quantitation shows a statistically significant decrease in the number of SST+ cells with diabetes (n=4, *P<0.05).
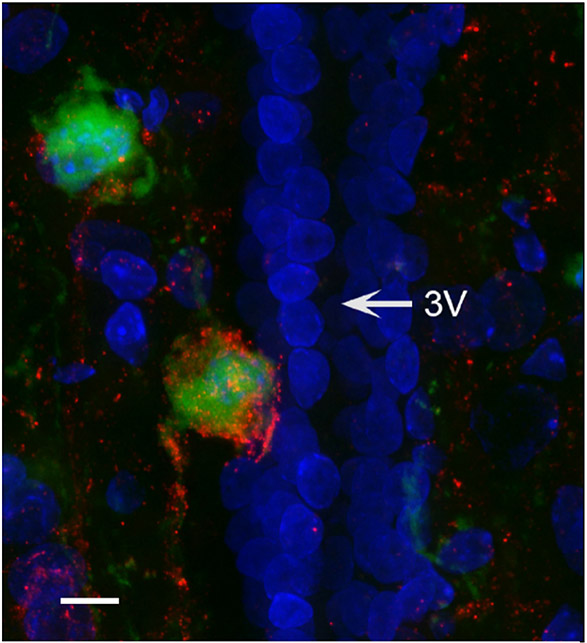
Figure 7: Somatostatin+ nerves project from the hypothalamus to the bone marrow.
Confocal (60X) z-projection of hypothalamic periventricular region from a rat that received femoral bone marrow injection of PRV-152 (green) also immune-labeled for SST (red). 3V = third ventricle. Scale bar = 24μm.
There are several possible mechanisms responsible for sympathoexcitation seen in early diabetes [78]. Poor passage of leukocytes through the microcirculation in diabetes leads to increases in vascular resistance, with reduced blood perfusion and oxygen delivery producing sympathoexcitation by inadequate brain perfusion at the level of the microvasculature. The idea that inadequate brainstem perfusion leads to excessive sympathetic nerve activity is not new and was postulated by Cushing in the early 1900s. Moreover, within the rostral ventrolateral medulla (RVLM), a region generating activity destined for the sympathetic preganglionic motor neurons, there are neurons which are intrinsically sensitive to hypoxia [79]. The latter provides a transduction mechanism by which the low perfusion of the brainstem results in sympathoexcitation to ensure adequate perfusion (33). Thus, the brain is well prepared to ‘self-protect’ at multiple levels of the neural axis in response to the potential threat of low oxygen.
Central sympathetic activity has a direct impact on inflammatory cytokines. For example, sympathetic tone is positively correlated with interleukin 6 (IL-6) plasma levels (24). Central inhibition of the ANS decreases TNF serum levels (24, 25). Similarly, stress responses that modulate ANS activity impact the inflammation (26). We have shown previously that peripheral immune challenges produce activation of sympathetic associated brain nuclei (27). In turn, neuroinflammation in these ANS regions can cause an auto-perpetuating cycle of excitation of autonomic neurons to sustain bone marrow pathology [28] (Figure 8).
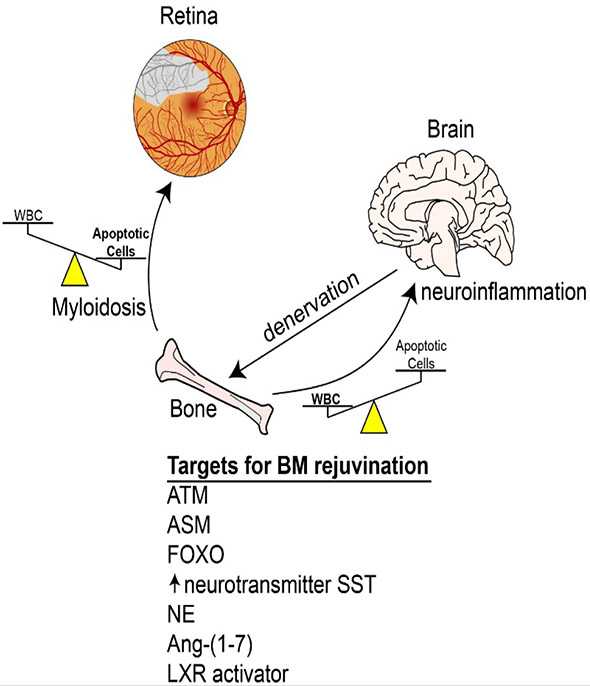
Figure 8: The bone marrow is a critical target organ of diabetes and its dysfunction can adversely affect diabetic retinopathy.
The release of bone marrow cells and hematopoiesis is under the regulation of the autonomic nervous system (ANS) and in late stage diabetes denervation of the bone marrow results in a shift in hematopoiesis with loss of generation of reparative cells but an increased production of pro-inflammatory cells. Therapeutic targets and novel strategies may include modulation of bone marrow ATM, ASM, and FOXO, systemic administration of Ang 1-7 or exogenous replacement of SST using a somatostatin analogue that crosses the BBB to influence the brain. Preservation of bone marrow function may represent a new opportunity for treatment and management of diabetic retinopathy.
VASCULAR WALL DERIVED PROGENITOR CELLS
Endothelial cell colony forming cells (ECFCs) represent a second [16, 40, 80, 81] population of cells possessing vascular reparative activity [82, 83]. ECFCs, also called “outgrowth endothelial cells” [83] have the high proliferative capacity and the ability to maintain an endothelial phenotype throughout ex vivo long-term expansion [84]. These cells are CD34+but CD45− and can be further distinguished by being CD14−, CD115−, CD146hi, VE-CAD+/+, CD105+ vWF+, CD133−. We and others have shown that ECFCs are capable of i) integrating into pre-existing retinal vessels, ii) de novo retinal capillary formation in several in vitro models and iii) de novo capillary formation in in vivo retinal models [85-87]. Furthermore, ECFCs injected into the systemic circulation of SCID mice are able to correct ischemia and lodge and survive in nine different vascular beds for up to 7 months after intravenous tail vein injection, without inducing thrombosis or infarcts [88]. Following acute vascular injury, the ability of ECFCs to repair and regenerate can be augmented by the addition of healthy CD34+CD45+ cells [89].
Previously, we examined CD34+cells and ECFCs circulating in the blood of individuals with microvascular complications (MVC) [90]. Circulating mononuclear cells from diabetic and nondiabetic individuals were used to isolate CD34+cells and grow ECFCs in culture. ECFCs could only be obtained from 15% of diabetic individuals tested, and these represented individuals without DR or other microvascular complications, whereas ECFCs could be obtained from 100% of the control individuals. This severely restricted ECFC studies to individuals without MVC and this represented a limitation of using these cells for autologous cell therapy. Similarly, depletion of ECFCs from the blood and blood vessels of individuals with the peripheral vascular disease has been reported [91].
Similarly, CD34+ cells from diabetic individuals with MVC are dysfunctional [92-103]. Since the major function of CD34+ cells is to provide paracrine production of growth/trophic factors, we examined conditioned medium (CM) derived from CD34+ cells of diabetic individuals (diabetic-CM). The levels of key stem cell survival factors (stem cell factor, hepatocyte growth factor, and thrombopoietin) were found to be markedly lower, while the expression of pro-inflammatory factors, such as IL-1β and tumor necrosis factor (TNF-α) levels were markedly higher in diabetic-CM than in CM-derived from nondiabetic individuals (nondiabetic-CM). Hypoxia did not upregulate HIF-1α in CD34+ cells of diabetic origin but did in CD34+cells isolated from healthy controls. Both migration and proliferation of nondiabetic (healthy) CD34+ cells toward diabetic-CM were lower compared to nondiabetic-CM. We also demonstrated attenuation of pressure-induced constriction, potentiation of bradykinin relaxation, and generation of cGMP and cAMP in arterioles were observed with nondiabetic-CM, but not with diabetic-CM. In contrast, diabetic-CM failed to induce endothelial tube formation from vascular tissue. Thus, the major findings of this study [90], are that the use of either CD34+ cells or ECFCs as a cell therapy is severely limited because CD34+ cells are dysfunctional (impaired autocrine/paracrine function and reduced sensitivity to hypoxia) and the vascular wall ECFCs are depleted and essentially unobtainable from individuals in need of cell therapy [90, 104-106]. Advanced age also limits the function of CD34+ cells and isolation and expansion of ECFCs [107]. Thus, our published studies and those of others emphasize i) the limitation of using autologous peripheral blood derived cells from diabetic individuals with MVC, ii) the need for alternative autologous cells for improved cell therapy, for example iPSC derived cells, iii) prevention of the loss of this critical vascular wall reparative population, the ECFCs and/or iv) the need to correct diabetes –induce bone marrow dysfunction.
BONE MARROW CELLS FOR THERAPY
Several studies over past years suggest that cell-based therapies hold a countless promise for revascularizing endothelial loss during diabetes. Park et. al. has eloquently and comprehensively described the current literature regarding cell therapy for retinal diseases [108]. Thus, this section will only highlight as few aspects of cell therapy for DR. There are a variety of sources for cell therapy such as circulating angiogenic cells, endothelial colony forming cells, mesenchymal stromal cells (MSCs), embryonic stem cells, and inducible pluripotent stem cells (iPSCs).
Our research over past several years suggests that diabetes leads to a dysfunction of the CD34+ cell. The CD34+ cells obtained from diabetic individuals exhibit a decrease in migration, proliferation, and incorporation in blood vessels. Our studies indicate that nitric oxide is a critical determinant of CD34+ plasticity. We successfully used a variety of pharmacological agents such as transforming growth factor (TGF-β1) morpholino [109], angiotensin 1-7 (Ang 1-7) [80] to correct low levels of NO and restore the functional ability of diabetic CD34+ cells.
The MSCs play an important role in the treatment of microvascular complications due to their multipotency and paracrine mechanisms. Allogeneic mesenchymal stem cells secrete neurotrophic factors, growth factors, adipokine, polarizing M2 macrophages, and inhibiting inflammation [23, 24, 110]. MSCs derived from adipocytes (i.e. adipose derived stem cells) resemble retinal pericytes. ASC help in the treatment of injured retina either by paracrine repair [111].
Some studies attempted to generate iPSCs cells from cord blood CD34+ cells. The iPSCs cells were generated using a treatment with VEGF [112]. The injection of these iPSCs cells into the vitreous of NOD/SCID mice lead to an improvement of ischemic injury. Injected iPSC cells also aligned near the luminal side of blood vessels, indicating their potential as a vessel-building cell [113].
PHARMACOLOGICAL APPROACHES
The bone marrow as a whole is relatively hypoxic with a pO2 of 32 mm of Hg. The oxygen level in different parts of the bone marrow varies considerably. The areas near the sinusoids are more hypoxic due to poorer perfusion (pO2, 9.5 mm Hg), while the areas nearer to the endosteum are more perfused (pO2, 13.5 mm Hg) [114]. Typically, the relative hypoxia in the bone marrow helps to maintain the long-term repopulation of HSCs, however, the oxidative stress from diabetes and aging induces DNA damage, compromising the repopulating ability of HSCs. Considering the hypoxic nature and the reduced level of perfusion of the bone marrow, it is a challenging region for drug delivery [115].
Lowering blood glucose exhibits some level of protection in bone marrow niche function and helps with the mobilization of HSCs [33]. The DPP-4 inhibitor, a commonly prescribed pharmacological agent for lowering blood glucose in diabetic individuals affects bone marrow function by regulating the SDF-1. DPP-4 inhibition in clinical studies lead to mobilization of stem cells and promoted vascular recovery in an animal model of ischemia [34].
As mentioned earlier, LTR-HSCs preferentially reside in endosteal regions of the bone marrow within vicinity to osteoblasts [116]. Studies performed using hematopoietic chimeras suggest a significant contribution of hemangioblast of bone marrow origin in the repair of and maintenance of retinal vasculature. About 10% of endothelial cells in neovessels that developed in response to an injury are bone marrow-derived [117]. However, under the chronic metabolic stress of diabetes, there is a decline of LTR-HSCs from the well conserved osteoblastic niche leaving primarily the local endothelium to undertake the repair of damaged vasculature endangering retina to site threatening proliferative DR [26]. Drug targeting to osteoblast cells is of intense interest in order to control HSC quiescence or egress. LTR-HSC’s rapid mobilization can be induced by targeting α9β1/α4β1 integrins through a single dose of the small molecule BOP (N-(benzene sulfonyl)-l-prolyl-l-O-(1-pyrrolidinylcarbonyl) tyrosine) [118]. Transgenic mice with increased osteoblast in bone marrow show an increase in HSCs near the endosteal niche. The granulocyte colony stimulating (G-CSF) causes HSC egress via transient ablation of osteoblast cells from bone marrow [119]. Moreover targeted deletion of Dicer-1 (necessary for the maturation and processing of all microRNAs) in osteoprogenitors results in an altered hematopoiesis with the distorted generation of selected lineages [120]. These studies support the assertion that endosteal region and osteoblast provide a unique environment necessary for HSC self-renewal, quiescence, and survival, providing an ideal target for therapeutic delivery. One of the striking findings of studies involving osteoblast depletion is the concurrent loss of bone marrow macrophages, suggesting a vital role of bone marrow macrophages in maintaining endosteal HSC niche. Liposome-mediated drug delivery to macrophages efficiently targets bone marrow, however, once liposomes are ingested by the macrophages, the phospholipid bilayers of the liposomes are disrupted under the influence of lysosomal phospholipases leading liposomal ‘suicide’ causing a non-specific delivery of drug molecules [121]. Some studies have also targeted E-selectin which is constitutively expressed on bone marrow endothelium using E-selectin thioaptamer conjugated to porous silicon particle [122].
Bone-targeting molecules such as bisphosphonates and oligopeptides can aid in the delivery of drug molecules to the bone marrow. Recent studies indicate that oligopeptide (AspSerSer)6 binds efficiently to slowly crystallized area in osteoblast cells and amorphous calcium phosphates. In fact, a recent study using (AspSerSer)6 oligopeptide linked to 1,2 Dioleoyl-3-Trimethylammonium-Propane (DOTAP)-based cationic liposome encapsulated with osteogenic siRNA markedly promoted bone formation and bone mass [123]. Boosting antioxidant pentose pathway with treatment with benfotiamine has shown to be protective in restoring the depletion of stem cells. In addition, control of dyslipidemia-induced damage by normalizing BM cholesterol metabolism through activation of LXR, or normalizing sphingolipid metabolism through inhibition of ASM in diabetes could provide an effective way of restoring the balance between BM-derived pro-inflammatory and reparative cells, as well as improving progenitor cell function. As mentioned earlier, ASM inhibition reduced diabetes-induced retinal vascular damage. Several tricyclic antidepressants (TCA), including amitriptyline, clomipramine, and desipramine, have shown promise at inhibiting ASM [124, 125]. The single cell model explanation for this occurrence is that TCAs accumulate in the lysosome and interfere with ASM binding, resulting in it being inactivated via proteolytic degradation [125]. Norepinephrine reuptake inhibitors might also present a viable strategy to combat BM dysfunction due to the high probability that neuropathy will also affect the diabetic BM [44].
CONCLUSION
The bone marrow is a critical target organ of diabetes and its dysfunction adversely affects the outcome of diabetic microvascular complications. Diabetes leads to selective depletion of most primitive HSCs from the bone marrow. This not only compromises the source of a reparative stem cell but also creates a state of oxidative stress due to enhanced production of short-term repopulating HSCs. Strategies used in promoting bone marrow niche function holds a significant potential for bone marrow rejuvenation, however, targeted delivery of pharmacological agents to bone marrow is a challenge due to relative hypoxia of the bone marrow and difficulty of delivering drugs to the bone marrow. Osteoblast-targeted drug delivery is a viable option for delivering drug molecules to endosteal niche and experimental studies are promising, in future, these therapies would be available for patient use.
The release of bone marrow cells and hematopoiesis is under the regulation of the ANS, which responds early on in diabetes with hyperactivation in an attempt to respond to diabetes-induced changes in perfusion of key autonomic centers. Later in the natural history of diabetes, denervation of the bone marrow occurs and results in loss of a generation of key reparative cells while fostering the generation of increased numbers of pro-inflammatory cells. Therapeutic targets and novel strategies directed at the preservation of bone marrow function may represent a new solution to treatment and management of diabetic retinopathy.
Acknowledgments
Support
AB: (i) Ralph and Grace Showalter Trust Fund (ii) International Retinal Research Foundation. AB and MG: an unrestricted award from Research to Prevent Blindness (RPB) foundation to Department of Ophthalmology, Indiana University. MG; NIH grants: R01EY0126001, R01EY007739, R01HL110170, R01DK090730. JB; NIH RO1EY016077. MG and JB; MEAS Grant MICL02163.
Footnotes
Disclosures
The authors declare that they have no conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, this work. All authors have materially participated in the research and/or article preparation of this article. All authors have approved the final article. All animal experiments comply with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
References
- [1].Forouhi NG, Wareham NJ, Epidemiology of diabetes, Medicine (Abingdon), 42 (2014) 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA, Group JDRC, Diabetic retinopathy: seeing beyond glucose-induced microvascular disease, Diabetes, 55 (2006) 2401–2411. [DOI] [PubMed] [Google Scholar]
- [3].Klein BE, Overview of epidemiologic studies of diabetic retinopathy, Ophthalmic Epidemiol, 14 (2007) 179–183. [DOI] [PubMed] [Google Scholar]
- [4].Cheung N, Mitchell P, Wong TY, Diabetic retinopathy, Lancet, 376 (2010) 124–136. [DOI] [PubMed] [Google Scholar]
- [5].International Diabetes Federation. IDF Diabetes Atlas, 7 ed, 7 ed., International Diabetes Federation, Place Published, 2015. [Google Scholar]
- [6].Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, T.Y. Wong G Meta-Analysis for Eye Disease Study, Global prevalence and major risk factors of diabetic retinopathy, Diabetes Care, 35 (2012) 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tooke JE, Microcirculation and diabetes, Br Med Bull, 45 (1989) 206–223. [DOI] [PubMed] [Google Scholar]
- [8].Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, Poulaki V, Semkova I, Kociok N, TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations, Mol Vis, 15 (2009) 1418–1428. [PMC free article] [PubMed] [Google Scholar]
- [9].Kilpatrick ES, Rigby AS, Atkin SL, The effect of glucose variability on the risk of microvascular complications in type 1 diabetes, Diabetes Care, 29 (2006) 1486–1490. [DOI] [PubMed] [Google Scholar]
- [10].Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S, Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice, Basic Res Cardiol, 105 (2010) 703–712. [DOI] [PubMed] [Google Scholar]
- [11].Marcovecchio ML, Tossavainen PH, Dunger DB, Prevention and treatment of microvascular disease in childhood type 1 diabetes, Br Med Bull, 94 (2010) 145–164. [DOI] [PubMed] [Google Scholar]
- [12].Calvi LM, Link DC, Cellular complexity of the bone marrow hematopoietic stem cell niche, Calcif Tissue Int, 94 (2014) 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Morrison SJ, Scadden DT, The bone marrow niche for haematopoietic stem cells, Nature, 505 (2014) 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Attar EC, Scadden DT, Regulation of hematopoietic stem cell growth, Leukemia, 18 (2004) 1760–1768. [DOI] [PubMed] [Google Scholar]
- [15].Muller-Sieburg C, Sieburg HB, Stem cell aging: survival of the laziest?, Cell Cycle, 7 (2008) 3798–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hazra S, Jarajapu YP, Stepps V, Caballero S, Thinschmidt JS, Sautina L, Bengtsson N, Licalzi S, Dominguez J, Kern TS, Segal MS, Ash JD, Saban DR, Bartelmez SH, Grant MB, Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model, Diabetologia, 56 (2013) 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bhatwadekar AD, Duan Y, Chakravarthy H, Korah M, Caballero S, Busik JV, Grant MB, Ataxia Telangiectasia Mutated Dysregulation Results in Diabetic Retinopathy, Stem Cells, 34 (2016) 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG, FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress, Cell, 128 (2007) 325–339. [DOI] [PubMed] [Google Scholar]
- [19].Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N, FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress, J Biol Chem, 277 (2002) 26729–26732. [DOI] [PubMed] [Google Scholar]
- [20].Coffer PJ, Burgering BM, Stressed marrow: FoxOs stem tumour growth, Nat Cell Biol, 9 (2007) 251–253. [DOI] [PubMed] [Google Scholar]
- [21].Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM, Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress, Nature, 419 (2002) 316–321. [DOI] [PubMed] [Google Scholar]
- [22].Chakravarthy H, Navitskaya S, O’Reilly S, Gallimore J, Mize H, Beli E, Wang Q, Kady N, Huang C, Blanchard GJ, Grant MB, Busik JV, Role of Acid Sphingomyelinase in Shifting the Balance Between Proinflammatory and Reparative Bone Marrow Cells in Diabetic Retinopathy, Stem Cells, 34 (2016) 972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mangialardi G, Madeddu P, Bone Marrow-Derived Stem Cells: a Mixed Blessing in the Multifaceted World of Diabetic Complications, Current diabetes reports, 16 (2016) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fadini GP, Ferraro F, Quaini F, Asahara T, Madeddu P, Concise review: diabetes, the bone marrow niche, and impaired vascular regeneration, Stem Cells Transi Med, 3 (2014) 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mangialardi G, Oikawa A, Reni C, Madeddu P, Bone marrow microenvironment: a newly recognized target for diabetes-induced cellular damage, Endocrine, metabolic & immune disorders drug targets, 12 (2012) 159–167. [DOI] [PubMed] [Google Scholar]
- [26].Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G, Kraenkel N, Prezioso L, Emanueli C, Madeddu P, Diabetes mellitus induces bone marrow microangiopathy, Arterioscler Thromb Vasc Biol, 30 (2010) 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dominguez JM 2nd, Yorek MA, Grant MB, Combination therapies prevent the neuropathic, proinflammatory characteristics of bone marrow in streptozotocin-induced diabetic rats, Diabetes, 64 (2015) 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hu P, Thinschmidt JS, Yan Y, Hazra S, Bhatwadekar A, Caballero S, Salazar T, Miyan JA, Li W, Derbenev A, Zsombok A, Tikhonenko M, Dominguez JM 2nd, McGorray SP, Saban DR, Boulton ME, Busik JV, Raizada MK, Chan-Ling T, Grant MB, CNS inflammation and bone marrow neuropathy in type 1 diabetes, Am J Pathol, 183 (2013) 1608–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yellowless Douglas J, Bhatwadekar A, Li Calzi S, Shaw LC, Carnegie D, Caballero S, Li Q, Stitt AW, Raizada MK, Grant MB, Bone marrow-CNS connections: implications in the pathogenesis of diabetic retinopathy, Prog Retin Eye Res, 31 (2012) 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Li Calzi S, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB, Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock, J Exp Med, 206 (2009) 2897–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, Mangoni M, Rizzoli V, Sykes SM, Lin CP, Frenette PS, Quaini F, Scadden DT, Diabetes impairs hematopoietic stem cell mobilization by altering niche function, Sci Transl Med, 3 (2011) 104ra101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].DiPersio JF, Diabetic stem-cell “mobilopathy”, N Engl J Med, 365 (2011) 2536–2538. [DOI] [PubMed] [Google Scholar]
- [33].Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A, Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats, Diabetologia, 49 (2006) 3075–3084. [DOI] [PubMed] [Google Scholar]
- [34].Fadini GP, Albiero M, Seeger F, Poncina N, Menegazzo L, Angelini A, Castellani C, Thiene G, Agostini C, Cappellari R, Boscaro E, Zeiher A, Dimmeler S, Avogaro A, Stem cell compartmentalization in diabetes and high cardiovascular risk reveals the role of DPP-4 in diabetic stem cell mobilopathy, Basic Res Cardiol, 108 (2013) 313. [DOI] [PubMed] [Google Scholar]
- [35].Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, Beem E, Shaw LC, Li Calzi S, Harrison JK, Tran-Son-Tay R, Grant MB, Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes, Diabetes, 55 (2006) 102–109. [PubMed] [Google Scholar]
- [36].Thomas J, Liu F, Link DC, Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor, Current opinion in hematology, 9 (2002) 183–189. [DOI] [PubMed] [Google Scholar]
- [37].Wang Q, Navitskaya S, Chakravarthy H, Huang C, Kady N, Lydic TA, Chen YE, Yin KJ, Powell FL, Martin PM, Grant MB, Busik JV, Dual Anti-Inflammatory and Anti-Angiogenic Action of miR-15a in Diabetic Retinopathy, EBioMedicine, 11 (2016) 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chakravarthy H, Beli E, Navitskaya S, O’Reilly S, Wang Q, Kady N, Huang C, Grant MB, Busik JV, Imbalances in Mobilization and Activation of Pro-Inflammatory and Vascular Reparative Bone Marrow-Derived Cells in Diabetic Retinopathy, PLoS One, 11 (2016) e0146829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S, Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice, Basic Res Cardiol, (2010). [DOI] [PubMed] [Google Scholar]
- [40].Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, Grant MB, Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells, Diabetes, 56 (2007) 960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, Jonnal R, Zawadzki RJ, Werner JS, Nolta J, Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings, Invest Ophthalmol Vis Sci, 56 (2015) 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fadini GP, A reappraisal of the role of circulating (progenitor) cells in the pathobiology of diabetic complications, Diabetologia, 57 (2014) 4–15. [DOI] [PubMed] [Google Scholar]
- [43].Fadini GP, Avogaro A, It is all in the blood: the multifaceted contribution of circulating progenitor cells in diabetic complications, Exp Diabetes Res, 2012 (2012) 742976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fadini GP, Ferraro F, Quaini F, Asahara T, Madeddu P, Concise review: diabetes, the bone marrow niche, and impaired vascular regeneration, Stem Cells Transl Med, 3 (2014) 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fadini GP, Losordo D, Dimmeler S, Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use, Circ Res, 110 (2012) 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jarajapu YP, Grant MB, The promise of cell-based therapies for diabetic complications: challenges and solutions, Circ Res, 106 (2010) 854–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Thinschmidt JS, Colon-Perez LM, Febo M, Caballero S, King MA, White FA, Grant MB, Depressed basal hypothalamic neuronal activity in type-1 diabetic mice is correlated with proinflammatory secretion of HMBG1, Neurosci Lett, 615 (2016) 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hu P, Thinschmidt JS, Caballero S, Adamson S, Cole L, Chan-Ling T, Grant MB, Loss of survival factors and activation of inflammatory cascades in brain sympathetic centers in type 1 diabetic mice, Am J Physiol Endocrinol Metab, 308 (2015) E688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tikhonenko M, Lydic TA, Opreanu M, Li Calzi S, Bozack S, McSorley KM, Sochacki AL, Faber MS, Hazra S, Duclos S, Guberski D, Reid GE, Grant MB, Busik JV, N-3 polyunsaturated Fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function, PLoS One, 8 (2013) e55177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yellowlees Douglas J, Bhatwadekar AD, Li Calzi S, Shaw LC, Carnegie D, Caballero S, Li Q, Stitt AW, Raizada MK, Grant MB, Bone marrow-CNS connections: implications in the pathogenesis of diabetic retinopathy, Prog Retin Eye Res, 31 (2012) 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tausendschon M, Dehne N, Brune B, Hypoxia causes epigenetic gene regulation in macrophages by attenuating Jumonji histone demethylase activity, Cytokine, 53 (2011) 256–262. [DOI] [PubMed] [Google Scholar]
- [52].Grant M, Russell B, Fitzgerald C, Merimee TJ, Insulin-like growth factors in vitreous. Studies in control and diabetic subjects with neovascularization, Diabetes, 35 (1986) 416–420. [DOI] [PubMed] [Google Scholar]
- [53].Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G, Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover, Hypertension, 11 (1988) 3–20. [DOI] [PubMed] [Google Scholar]
- [54].Guyenet PG, The sympathetic control of blood pressure, Nat Rev Neurosci, 7 (2006) 335–346. [DOI] [PubMed] [Google Scholar]
- [55].Zubcevic J, Santisteban MM, Pitts T, Baekey DM, Perez PD, Bolser DC, Febo M, Raizada MK, Functional neural-bone marrow pathways: implications in hypertension and cardiovascular disease, Hypertension, 63 (2014) e129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Holmes AR, Shepherd MG, Proline-induced germ-tube formation in Candida albicans: role of proline uptake and nitrogen metabolism, J Gen Microbiol, 133 (1987) 3219–3228. [DOI] [PubMed] [Google Scholar]
- [57].Saraswat VA, Naik SR, Endoscopy is required in all patients with dyspepsia. For the proposition, Trop Gastroenterol, 9 (1988) 211–214. [PubMed] [Google Scholar]
- [58].Santiago AR, Gaspar JM, Baptista FI, Cristovao AJ, Santos PF, Kamphuis W, Ambrosio AF, Diabetes changes the levels of ionotropic glutamate receptors in the rat retina, Mol Vis, 15 (2009) 1620–1630. [PMC free article] [PubMed] [Google Scholar]
- [59].Yi CX, Serlie MJ, Ackermans MT, Foppen E, Buijs RM, Sauerwein HP, Fliers E, Kalsbeek A, A major role for perifornical orexin neurons in the control of glucose metabolism in rats, Diabetes, 58 (2009) 1998–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bailey TW, Dimicco JA, Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats, Am J Physiol Regul Integr Comp Physiol, 280 (2001) R8–15. [DOI] [PubMed] [Google Scholar]
- [61].Matsumura K, Tsuchihashi T, Abe I, Central orexin-A augments sympathoadrenal outflow in conscious rabbits, Hypertension, 37 (2001) 1382–1387. [DOI] [PubMed] [Google Scholar]
- [62].Panayotacopoulou MT, Raadsheer FC, Swaab DF, Colocalization of tyrosine hydroxylase with oxytocin or vasopressin in neurons of the human paraventricular and supraoptic nucleus, Brain Res Dev Brain Res, 83 (1994) 59–66. [DOI] [PubMed] [Google Scholar]
- [63].Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG, Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2, J Comp Neurol, 465 (2003) 593–603. [DOI] [PubMed] [Google Scholar]
- [64].Panayotacopoulou MT, Malidelis YI, Fliers E, Bouras C, Ravid R, Swaab DF, Increased expression of tyrosine hydroxylase immunoreactivity in paraventricular and supraoptic neurons in illnesses with prolonged osmotic or nonosmotic stimulation of vasopressin release, Neuroendocrinology, 76 (2002) 254–266. [DOI] [PubMed] [Google Scholar]
- [65].Dheen ST, Tay SS, Wong WC, Arginine vasopressin- and oxytocin-like immunoreactive neurons in the hypothalamic paraventricular and supraoptic nuclei of streptozotocin-induced diabetic rats, Arch Histol Cytol, 57 (1994) 461–472. [DOI] [PubMed] [Google Scholar]
- [66].Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, Heilbronn R, Mietzsch M, Weger S, Huang XF, Enriquez RF, Baldock PA, Zhang L, Sainsbury A, Herzog H, Lin S, Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN, Cell Metab, 17 (2013) 236–248. [DOI] [PubMed] [Google Scholar]
- [67].Jiang Y, Gao H, Krantz AM, Derbenev AV, Zsombok A, Reduced GABAergic inhibition of kidney-related PVN neurons in streptozotocin-treated type 1 diabetic mouse, J Neurophysiol, 110 (2013) 2192–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Haywood JR, Mifflin SW, Craig T, Calderon A, Hensler JG, Hinojosa-Laborde C, gamma-Aminobutyric acid (GABA)--A function and binding in the paraventricular nucleus of the hypothalamus in chronic renal-wrap hypertension, Hypertension, 37 (2001) 614–618. [DOI] [PubMed] [Google Scholar]
- [69].Hyer SL, Sharp PS, Brooks RA, Burrin JM, Kohner EM, Serum IGF-1 concentration in diabetic retinopathy, Diabet Med, 5 (1988) 356–360. [DOI] [PubMed] [Google Scholar]
- [70].Herman JP, Eyigor O, Ziegler DR, Jennes L, Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat, J Comp Neurol, 422 (2000) 352–362. [PubMed] [Google Scholar]
- [71].Zhang K, Li YF, Patel KP, Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure, Am J Physiol Regul Integr Comp Physiol, 282 (2002) R1006–1015. [DOI] [PubMed] [Google Scholar]
- [72].Hambley JW, Johnston GA, Shaw J, Alterations in a hypothalamic GABA system in the spontaneously hypertensive rat, Neurochem Int, 6 (1984) 813–821. [DOI] [PubMed] [Google Scholar]
- [73].Takenaka K, Sasaki S, Uchida A, Fujita H, Nakamura K, Ichida T, Itoh H, Nakata T, Takeda K, Nakagawa M, GABAB-ergic stimulation in hypothalamic pressor area induces larger sympathetic and cardiovascular depression in spontaneously hypertensive rats, Am J Hypertens, 9 (1996) 964–972. [DOI] [PubMed] [Google Scholar]
- [74].Li YF, Cornish KG, Patel KP, Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure, Circ Res, 93 (2003) 990–997. [DOI] [PubMed] [Google Scholar]
- [75].Sinosich MJ, Dodd J, Hudson CN, Tyler JR, Seppala M, Grudzinskas JG, Saunders DM, The influence of pergonal on in vitro production of placental protein 5 (PP5) by ovarian tumour cells, Tumour Biol, 6 (1985) 233–242. [PubMed] [Google Scholar]
- [76].Bhatwadekar AD, Yan Y, Qi X, Thinschmidt JS, Neu MB, Li Calzi S, Shaw LC, Dominiguez JM, Busik JV, Lee C, Boulton ME, Grant MB, Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow, Diabetes, 62 (2013) 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Opreanu M, Tikhonenko M, Bozack S, Lydic TA, Reid GE, McSorley KM, Sochacki A, Perez FI, Esselman WJ, Kern T, Kolesnick R, Grant MB, Busik JV, The unconventional role of acid sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models, Diabetes, 60 (2011) 2370–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fukuda S, Yasu T, Kobayashi N, Ikeda N, Schmid-Schonbein GW, Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat, Circ Res, 95 (2004) 100–108. [DOI] [PubMed] [Google Scholar]
- [79].Wang G, Zhou P, Repucci MA, Golanov EV, Reis DJ, Specific actions of cyanide on membrane potential and voltage-gated ion currents in rostral ventrolateral medulla neurons in rat brainstem slices, Neurosci Lett, 309 (2001) 125–129. [DOI] [PubMed] [Google Scholar]
- [80].Jarajapu YP, Bhatwadekar AD, Caballero S, Hazra S, Shenoy V, Medina R, Kent D, Stitt AW, Thut C, Finney EM, Raizada MK, Grant MB, Activation of the ACE2/angiotensin-(1-7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors, Diabetes, 62 (2013) 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jarajapu YP, Caballero S, Verma A, Nakagawa T, Lo MC, Li Q, Grant MB, Blockade of NADPH oxidase restores vasoreparative function in diabetic CD34+ cells, Invest Ophthalmol Vis Sci, 52 (2011) 5093–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yoder MC, Ingram DA, The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process?, Biochimica et biophysica acta, 1796 (2009) 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA, Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals, Blood, 109 (2007) 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC, Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells, Blood, 105 (2005) 2783–2786. [DOI] [PubMed] [Google Scholar]
- [85].Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC, Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood, Blood, 104 (2004) 2752–2760. [DOI] [PubMed] [Google Scholar]
- [86].Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, Stitt AW, Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities, BMC medical genomics, 3 (2010) 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Medina RJ, O’Neill CL, O’Doherty TM, Chambers SE, Guduric-Fuchs J, Neisen J, Waugh DJ, Simpson DA, Stitt AW, Ex vivo expansion of human outgrowth endothelial cells leads to IL-8-mediated replicative senescence and impaired vasoreparative function, Stem Cells, 31 (2013) 1657–1668. [DOI] [PubMed] [Google Scholar]
- [88].Milbauer LC, Enenstein JA, Roney M, Solovey A, Bodempudi V, Nichols TC, Hebbel RP, Blood outgrowth endothelial cell migration and trapping in vivo: a window into gene therapy, Translational research : the journal of laboratory and clinical medicine, 153 (2009) 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS, Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases, Circulation, 112 (2005) 1618–1627. [DOI] [PubMed] [Google Scholar]
- [90].Jarajapu YP, Hazra S, Segal M, Li Calzi S, Jadhao C, Qian K, Mitter SK, Raizada MK, Boulton ME, Grant MB, Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms, PLoS One, 9 (2014) e93965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Prasain N, Lee MR, Vemula S, Meador JL, Yoshimoto M, Ferkowicz MJ, Fett A, Gupta M, Rapp BM, Saadatzadeh MR, Ginsberg M, Elemento O, Lee Y, Voytik-Harbin SL, Chung HM, Hong KS, Reid E, O’Neill CL, Medina RJ, Stitt AW, Murphy MP, Rafii S, Broxmeyer HE, Yoder MC, Differentiation of human pluripotent stem cells to cells similar to cord- blood endothelial colony-forming cells, Nat Biotechnol, 32 (2014) 1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lu CL, Leu JG, Liu WC, Zheng CM, Lin YF, Shyu JF, Wu CC, Lu KC, Endothelial Progenitor Cells Predict Long-Term Mortality in Hemodialysis Patients, Int J Med Sci, 13 (2016) 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zeng H, Jiang Y, Tang H, Ren Z, Zeng G, Yang Z, Abnormal phosphorylation of Tie2/Akt/eNOS signaling pathway and decreased number or function of circulating endothelial progenitor cells in prehypertensive premenopausal women with diabetes mellitus, BMC Endocr Disord, 16 (2016) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Aragona CO, Imbalzano E, Mamone F, Cairo V, Lo Gullo A, D’Ascola A, Sardo MA, Scuruchi M, Basile G, Saitta A, Mandraffino G, Endothelial Progenitor Cells for Diagnosis and Prognosis in Cardiovascular Disease, Stem Cells Int, 2016 (2016) 8043792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wu H, Li R, Wei ZH, Zhang XL, Chen JZ, Dai Q, Xie J, Xu B, Diabetes-Induced Oxidative Stress in Endothelial Progenitor Cells May Be Sustained by a Positive Feedback Loop Involving High Mobility Group Box-1, Oxid Med Cell Longev, 2016 (2016) 1943918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Qin Y, He YH, Hou N, Zhang GS, Cai Y, Zhang GP, Xiao Q, He LS, Li SJ, Yi Q, Luo JD, Sonic hedgehog improves ischemia-induced neovascularization by enhancing endothelial progenitor cell function in type 1 diabetes, Mol Cell Endocrinol, 423 (2016) 30–39. [DOI] [PubMed] [Google Scholar]
- [97].Fadini GP, Bonora BM, Cappellari R, Menegazzo L, Vedovato M, Iori E, Marescotti MC, Albiero M, Avogaro A, Acute Effects of Linagliptin on Progenitor Cells, Monocyte Phenotypes, and Soluble Mediators in Type 2 Diabetes, J Clin Endocrinol Metab, 101 (2016) 748–756. [DOI] [PubMed] [Google Scholar]
- [98].Sukmawati D, Tanaka R, Ito-Hirano R, Fujimura S, Hayashi A, Itoh S, Mizuno H, Daida H, The role of Notch signaling in diabetic endothelial progenitor cells dysfunction, J Diabetes Complications, 30 (2016) 12–20. [DOI] [PubMed] [Google Scholar]
- [99].Bitterli L, Afan S, Buhler S, DiSanto S, Zwahlen M, Schmidlin K, Yang Z, Baumgartner I, Diehm N, Kalka C, Endothelial progenitor cells as a biological marker of peripheral artery disease, Vasc Med, 21 (2016) 3–11. [DOI] [PubMed] [Google Scholar]
- [100].Waclawovsky G, Umpierre D, Figueira FR, ES DEL, Alegretti AP, Schneider L, Matte US, Rodrigues TC, Schaan BD, Exercise on Progenitor Cells in Healthy Subjects and Patients with Type 1 Diabetes, Med Sci Sports Exerc, 48 (2016) 190–199. [DOI] [PubMed] [Google Scholar]
- [101].Saad MI, Abdelkhalek TM, Saleh MM, Kamel MA, Youssef M, Tawfik SH, Dominguez H, Insights into the molecular mechanisms of diabetes-induced endothelial dysfunction: focus on oxidative stress and endothelial progenitor cells, Endocrine, 50 (2015) 537–567. [DOI] [PubMed] [Google Scholar]
- [102].Aso Y, Jojima T, Iijima T, Suzuki K, Terasawa T, Fukushima M, Momobayashi A, Hara K, Takebayashi K, Kasai K, Inukai T, Sitagliptin, a dipeptidyl peptidase-4 inhibitor, increases the number of circulating CD34(+)CXCR4(+) cells in patients with type 2 diabetes, Endocrine, 50 (2015) 659–664. [DOI] [PubMed] [Google Scholar]
- [103].Maiorino MI, Casciano O, Volpe ED, Bellastella G, Giugliano D, Esposito K, Reducing glucose variability with continuous subcutaneous insulin infusion increases endothelial progenitor cells in type 1 diabetes: an observational study, Endocrine, (2015). [DOI] [PubMed] [Google Scholar]
- [104].Alvarado-Moreno JA, Hernandez-Lopez R, Chavez-Gonzalez A, Yoder MC, Rangel-Corona R, Isordia-Salas I, Hernandez-Juarez J, Cerbulo-Vazquez A, Gonzalez-Jimenez MA, Majluf-Cruz A, Endothelial colony-forming cells: Biological and functional abnormalities in patients with recurrent, unprovoked venous thromboembolic disease, Thromb Res, 137 (2016) 157–168. [DOI] [PubMed] [Google Scholar]
- [105].Basile DP, Yoder MC, Renal endothelial dysfunction in acute kidney ischemia reperfusion injury, Cardiovasc Hematol Disord Drug Targets, 14 (2014) 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yoder MC, Endothelial progenitor cell: a blood cell by many other names may serve similar functions, J Mol Med (Berl), 91 (2013) 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Shelley WC, Leapley AC, Huang L, Critser PJ, Zeng P, Prater D, Ingram DA, Tarantal AF, Yoder MC, Changes in the frequency and in vivo vessel-forming ability of rhesus monkey circulating endothelial colony-forming cells across the lifespan (birth to aged), Pediatr Res, 71 (2012) 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Park SS, Moisseiev E, Bauer G, Anderson JD, Grant MB, Zam A, Zawadzki RJ, Werner JS, Nolta JA, Advances in bone marrow stem cell therapy for retinal dysfunction, Prog Retin Eye Res, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bhatwadekar AD, Guerin EP, Jarajapu YP, Caballero S, Sheridan C, Kent D, Kennedy L, Lansang MC, Ruscetti FW, Pepine CJ, Higgins PJ, Bartelmez SH, Grant MB, Transient inhibition of transforming growth factor-beta1 in human diabetic CD34+ cells enhances vascular reparative functions, Diabetes, 59 (2010) 2010–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Albiero M, Ponncina N, Ciciliot S, Cappellari R, Menegazzo L, Ferraro F, Bolego C, Cignarella A, Avogaro A, Fadini GP, Bone Marrow Macrophages Contribute to Diabetic Stem Cell Mobilopathy by Producing Oncostatin M, Diabetes, 64 (2015) 2957–2968. [DOI] [PubMed] [Google Scholar]
- [111].Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, Evans-Molina C, Maturi R, Harris A, Kern TS, March KL, Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy, PLoS One, 9 (2014) e84671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Park TS, Zimmerlin L, Zambidis ET, Efficient and simultaneous generation of hematopoietic and vascular progenitors from human induced pluripotent stem cells, Cytometry A, 83 (2013) 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Park TS, Bhutto I, Zimmerlin L, Huo JS, Nagaria P, Miller D, Rufaihah AJ, Talbot C, Aguilar J, Grebe R, Merges C, Reijo-Pera R, Feldman RA, Rassool F, Cooke J, Lutty G, Zambidis ET, Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature, Circulation, 129 (2014) 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Cote D, Vinogradov SA, Scadden DT, Lin CP, Direct measurement of local oxygen concentration in the bone marrow of live animals, Nature, 508 (2014) 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Lilly AJ, Johnson WE, Bunce CM, The haematopoietic stem cell niche: new insights into the mechanisms regulating haematopoietic stem cell behaviour, Stem Cells Int, 2011 (2011) 274564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lawal RA, Calvi LM, The niche as a target for hematopoietic manipulation and regeneration, Tissue Eng Part B Rev, 17 (2011) 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang C, Seifert RA, Bowen-Pope DF, Kregel KC, Dunnwald M, Schatteman GC, Diabetes and aging alter bone marrow contributions to tissue maintenance, Int J Physiol Pathophysiol Pharmacol, 2 (2009) 20–28. [PMC free article] [PubMed] [Google Scholar]
- [118].Cao B, Zhang Z, Grassinger J, Williams B, Heazlewood CK, Churches QI, James SA, Li S, Papayannopoulou T, Nilsson SK, Therapeutic targeting and rapid mobilization of endosteal HSC using a small molecule integrin antagonist, Nat Commun, 7 (2016) 11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Levesque JP, Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs, Blood, 116 (2010) 4815–4828. [DOI] [PubMed] [Google Scholar]
- [120].Alemdehy MF, van Boxtel NG, de Looper HW, van den Berge IJ, Sanders MA, Cupedo T, Touw IP, Erkeland SJ, Dicer1 deletion in myeloid-committed progenitors causes neutrophil dysplasia and blocks macrophage/dendritic cell development in mice, Blood, 119 (2012) 4723–4730. [DOI] [PubMed] [Google Scholar]
- [121].Van Rooijen N, Sanders A, Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications, J Immunol Methods, 174 (1994) 83–93. [DOI] [PubMed] [Google Scholar]
- [122].Mann AP, Tanaka T, Somasunderam A, Liu X, Gorenstein DG, Ferrari M, E-selectin-targeted porous silicon particle for nanoparticle delivery to the bone marrow, Adv Mater, 23 (2011) H278–282. [DOI] [PubMed] [Google Scholar]
- [123].Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L, He Y, Yang Z, Pan X, Chow H, To K, Li Y, Li D, Wang X, Wang Y, Lee K, Hou Z, Dong N, Li G, Leung K, Hung L, He F, Zhang L, Qin L, A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy, Nat Med, 18 (2012) 307–314. [DOI] [PubMed] [Google Scholar]
- [124].Kim Y, Sun H, ASM-3 acid sphingomyelinase functions as a positive regulator of the DAF-2/AGE-1 signaling pathway and serves as a novel anti-aging target, PLoS One, 7 (2012) e45890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Beckmann N, Sharma D, Gulbins E, Becker KA, Edelmann B, Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons, Front Physiol, 5 (2014) 331. [DOI] [PMC free article] [PubMed] [Google Scholar]