Abstract
PURPOSE
Transgenic Col1a1r/r mice develop elevated intraocular pressure (IOP) with an open angle and progressive optic nerve axon loss. The present study was undertaken to evaluate aqueous outflow facility and its age dependence in these mice.
METHODS
Homozygous B6;129S4-Col1a1tm1Jae mice and corresponding wild-type Col1a1+/+ mice from 12 to 56 weeks of age were anesthetized, and IOP was measured with a microneedle. Outflow facility was determined by a two-level, constant-pressure infusion method. Type I collagen, subunit α1 was assessed in sclera and choroid by Western blot analysis.
RESULTS
The mean IOP in 12- to 36-week-old transgenic Col1a1r/r mice was 25.1% higher than in control Col1a1+/+ mice (P < 0.01), whereas the mean outflow facility was 25.4% lower than in control mice (P < 0.01). After this period, the mean IOP in 42- to 5 6-week-old transgenic mice returned to normal levels, whereas outflow facility increased by 36.0%. Over the 12- to 56-week study period, IOP and outflow facility in the transgenic mice were inversely correlated (r2 = −0.702, P < 0.01). Collagen I α1 content was greater in 37- and 43-week-old transgenic mice than in age-matched wild-type control mice.
CONCLUSIONS
Outflow facility is reduced in transgenic Col1a1r/r mice with IOP elevation. The inverse correlation of IOP elevation to facility reduction indicates that increased resistance in the aqueous outflow pathway contributes to ocular hypertension in Col1a1r/r mice. These mice may be useful as a model for open-angle glaucoma, as well as for assessing the relationship between collagen type I metabolism and aqueous outflow.
The maturation of transgenic mouse technology and the development of methods to study aqueous dynamics in the mouse have made it possible to study the physiological and molecular processes that are central to glaucoma.1,2 In previous studies, our group has reported that a transgenic mouse strain with a targeted mutation in the gene for the α1 subunit of type I collagen develops sustained IOP elevation with an open angle and progressive optic nerve axon loss.3,4 This mutation is positioned at the consensus cleavage site for matrix metalloproteinase (MMP)-1 and inhibits hydrolysis of collagen type I, subunit α1 (Col1α1) by endogenous MMPs. With increasing age, these mice show development of marked fibrosis of the dermis and accumulation of collagen type I within several collagen-containing tissues, including sclera.5,6 In this strain, the mean IOP is higher than that of the wild-type control mice by 12 weeks of age and progresses to a plateau phase of IOP elevation from 18 to 36 weeks of age.3 The mean axonal density and total axonal number in the transgenic Col1a1r/r mice at 54 weeks of age is significantly less than in the 24-week-old transgenic mice and age-matched wild-type mice.4 It is not known, however, whether elevated IOP is associated with any change in outflow facility.
The present study was undertaken to measure total outflow facility and its relationship with IOP elevation in transgenic Col1a1r/r mice as a function of age. Our hypothesis was that the ocular hypertension in transgenic Col1a1r/r mice reflects increased resistance in the aqueous outflow pathway.
MATERIALS AND METHODS
Animals
All experiments were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Transgenic mice, designated B6;129S4-Col1a1tm1Jae (Col1a1r/r), have a targeted mutation of the gene for pro-collagen I α1 (Col1a1) that yields the following alterations in the amino acid sequence: Gln774 to Pro, Ile776 to Met, Ala777 to Pro, Val782 to Ala, and Val783 to Pro.1,2 Initial breeding pairs of these mice and the corresponding control wild-type (Col1a1+/+) mice were obtained from Jackson Laboratory (Bar Harbor, ME). Transgenic Col1a1r/r mice were backcrossed with C57BL6 mice to improve their breeding performance. Homozygous matings were further conducted to expand the number of transgenic and wild-type mice. Mice were bred and housed in clear cages covered loosely with air filters and containing white pine shavings for bedding. The environment was kept at 21°C with a 12-hour light-dark cycle. All mice were fed ad libitum. All measurements were performed between 2 and 5 PM in consideration of the mouse’s diurnal IOP rhythm7,8 and the possible diurnal variation in aqueous dynamics.
Anesthesia
The mice were anesthetized by intraperitoneal injection with a 30-gauge needle of a mixture of ketamine (100 mg/kg, Ketaset; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (9 mg/kg, TranquiVed; Vedco, Inc., St. Joseph, MO) prepared at room temperature. Each mouse was monitored carefully, to assess the state of anesthesia. When the mouse did not respond to pinching of the back skin, it was placed on the platform for IOP measurement and procedures for evaluating outflow facility.
IOP and Outflow Facility
IOP and outflow facility were measured with an infusion system, as described previously.9,10 Briefly, a microneedle made of borosilicate glass (100-µm tip diameter and 1.0-mm outer diameter; World Precision Instruments [WPI], Sarasota, FL) was mounted on a micromanip-ulator and connected to a pressure transducer (Model BLPR; WPI). A silicon tube (0.38-mm inner diameter) linking the transducer was filled with physiological saline (BSS; Alcon, Fort Worth, TX) for the measurement outflow facility (C). The system pressure detected by the transducer was recorded by computer (Chart software; ADInstruments, Colorado Springs, CO).
Determination of Outflow Facility
The measurement of outflow facility was based on total outflow volume (Vt; in nanoliters) during a specific time interval (in this study, 10 minutes) at two different levels of IOP, as described previously.9,10 After insertion of the infusion needle into the anterior chamber, the IOP was first measured. Then, the height of the open end of the tubing was adjusted to alter IOP to 25 mm Hg. After verification of IOP stability, the initial fluid level in the silicon tube was marked. After a 10-minute interval, the new fluid level was marked. The total amount of infused fluid was noted as the infused volume at 25 mm Hg (Vt=25). Next, IOP was increased to 35 mm Hg, and the 10-minute infused volume (Vt=35) was measured in the same eye. The measurements of Vt=25 and Vt=35 were used to elucidate C according to the following logic. First, total outflow is equivalent to aqueous humor production (Fa), and Fa is equivalent to the sum of conventional outflow (Fc) and uveoscleral outflow (Fu) (all expressed in nanoliters per minute), which can be written as
| (1) |
which was also calculated from Vt=25 and Vt=35 as follows,
| (2) |
and
| (3) |
Subtracting equation 2 from 3
| (4) |
By the modified Goldmann equation,11,12
| (5) |
where Cc is trabecular facility, Cu is uveoscleral facility, Pev is episcleral vein pressure, and Peo is extraocular pressure.
Thus,
| (6) |
Combining equations 4 and 6 yields
In a previous paper,9 the classic Goldman equation was used to elucidate the calculation of outflow facility: Fa = C × (IOP − Pev) + Fu•. By substitution,
| (7) |
Combining equations 4 and 7 yields
The final equation is the same regardless of whether the modified or classic Goldmann equation is used. The modified Goldmann equation as described herein, however, reflects the fact that the two-level, constant-pressure infusion method used in this study measures total outflow facility but is not specific for trabecular outflow facility.10,13
Quantification of Aqueous Humor Protein Concentration
Aqueous humor specimens were collected 1 week after measurement of invasive IOP. All procedures were performed between 2 and 4 PM in consideration of the circadian variation of mouse aqueous humor protein.14 Approximately 4 µL of aqueous humor in one eye was aspirated into a clean glass microneedle connected to a microliter syringe mounted on micromanipulators. Samples were immediately frozen on dry ice and stored at −80°C. Protein concentrations were determined with a protein quantification kit (FluoroProfile; Sigma-Aldrich, St. Louis, MO). Absorption was measured at 610 nm with a spectrofluorometer (Nanodrop 3300; Thermo Scientific, Wilmington, DE). Aqueous protein concentration was determined based on linear regression analysis of a serial dilution of bovine serum albumin standards.
Tissue Preparation and Western Blot Analysis
After aqueous humor aspiration, Col1a1r/r and Col1a1+/+ mice were killed by CO2 inhalation at 37 and 43 weeks of age, and the eyes were enucleated. The globe was cut open around the limbus after removal of conjunctiva and ocular muscles. The sclera and choroid were dissected and immediately frozen in dry ice and subsequently stored at −80°C.
For Western blot analyses, each tissue sample was homogenized in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 10 mM EDTA, 1% Triton-x 100, 1% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 5 µg/mL aprotinin, and 5 µg/mL leupeptin) on ice with a homogenizer (Polytron, Duluth, GA). The tissue homogenates were then centrifuged at 13,000 rpm at 4°C for 40 minutes. Supernatants were collected and assayed for protein concentration (DC Protein Assay; Bio-Rad, Hercules, CA). Ten micrograms of each sample was separated on 10% gels (NuPAGE; Invitrogen, Carlsbad, CA) and electrotransferred to polyvinylidene difluoride membranes. The membranes were blocked (Chemi-Blocker; Chemicon, Temecula, CA), and incubated with polyclonal goat anti-collagen type I α1 antibody (COL1A1 sc-8784, 1: 500; Santa Cruz Biotechnology, Santa Cruz, CA), an affinity-purified antibody raised against a peptide corresponding to the N terminus of human collagen type I α1 that also recognizes mouse and rat collagen type I α1. The membrane was rinsed with 0.1% Tween-20/PBS, incubated with horseradish peroxidase-conjugated donkey anti-goat IgG (1:10,000; Chemicon) and developed by chemiluminescence detection (ECL Plus; GE Healthcare, Piscataway, NJ). For internal reference, samples were also incubated with mouse anti-actin antibody (1:5000; Chemicon) and horseradish peroxidase– conjugated goat anti-mouse IgG (1:20,000; Bio-Rad). Chemiluminescence in the blot was evaluated by digital fluorescence imaging (Storm 860; GE Healthcare), and band densities were normalized with actin use as a calibrator (ImageQuant TL; GE Healthcare).
Statistical Analysis
For the IOP analysis, transgenic Col1a1r/r and wild-type Col1a1+/+ mice aged from 12 to 56 weeks were used. IOP was regularly measured starting at 12 weeks of age. Approximately 18% of the transgenic Col1a1r/r mice did not show elevated IOP during the entire 12 to 56 weeks in this cohort. These mice were excluded from analysis to focus the investigation on the mechanism of IOP elevation. All data of measurements were from one eye of each mouse.
All data are expressed as the mean ± SD. ANOVA, the Bonferroni t-test, and linear regression were used to evaluate multiple comparisons. P < 0.05 was considered to be statistically significant.
RESULTS
Intraocular Pressure
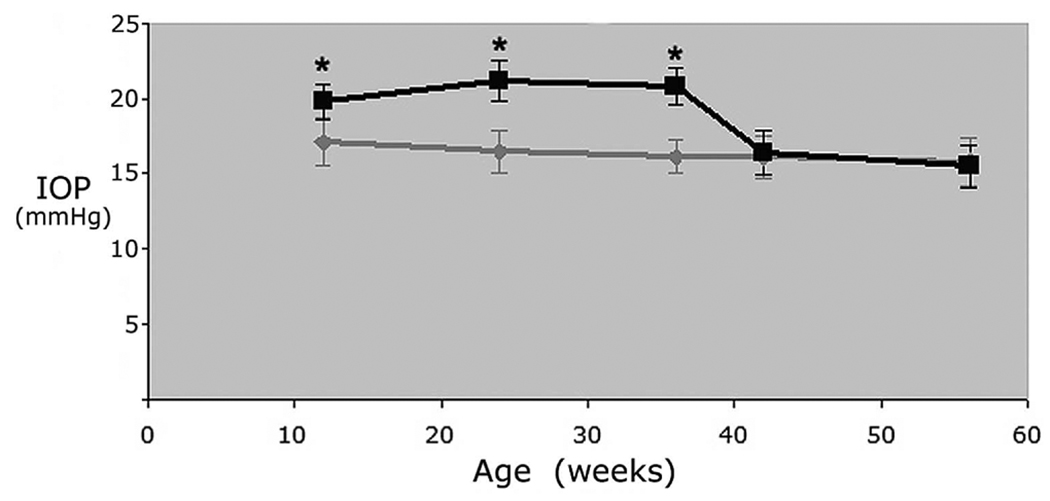
The mean IOP in the 12- to 56-week-old transgenic Col1a1r/r mice and the control wild-type Col1a1+/+ mice is shown in Figure 1. IOP in control wild-type mice was relatively stable with advancing age (P = 0.31). In the 12- to 36-week-old transgenic Col1a1r/r mice, the mean IOP (20.9 ± 1.4 mm Hg) was 25.1% higher than that in the control Col1a1+/+ mice (16.7 ± 1.5 mm Hg, P < 0.01). In the 42- to 56-week-old transgenic mice, the mean IOP was reduced to 15.7 ± 1.4 mm Hg. There was no significant difference in IOP between the transgenic and control 42- to 56-week-old mice (P = 0.56).
FIGURE 1.
Age-related changes in IOP in the transgenic Col1a1r/r mice and control Col1a1+/+ mice. Mean ± SD. *Significant difference between IOP in transgenic Col1a1r/r and control Col1a1+/+ mice (ANOVA, P < 0.05).
Outflow Facility
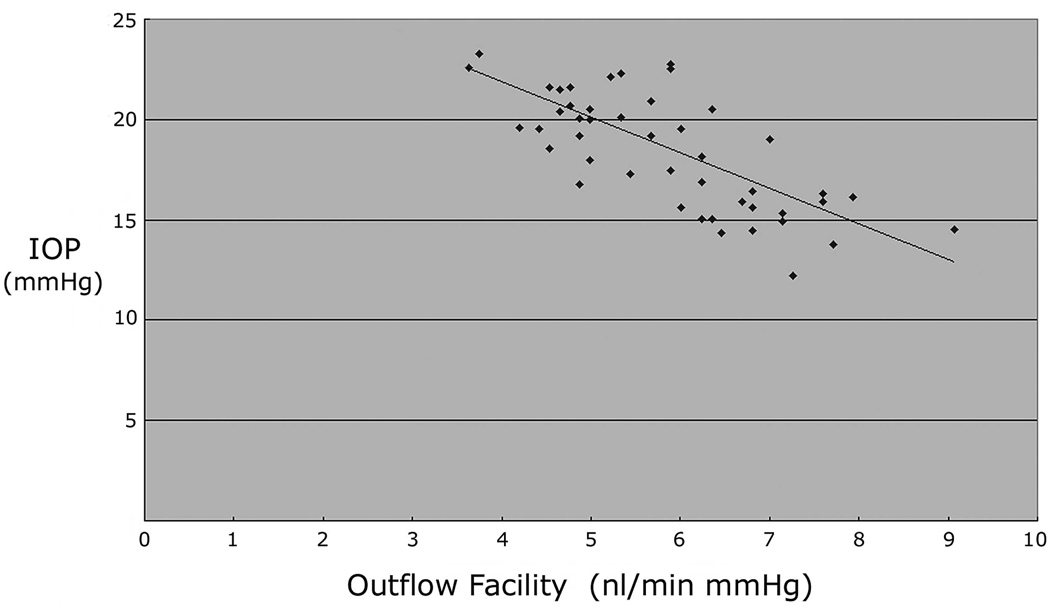
Mean outflow facility in the 12- to 56-week-old transgenic Col1a1r/r mice is shown in Table 1. Outflow facility in the 12-week-old transgenic mice was 16.9% lower than that in the age-matched control mice. The outflow facility in the 24- and 36-week-old transgenic mice was further reduced than that at 12 weeks. During the period of 12 to 36 weeks, when IOP was elevated 4.2 mm Hg in the transgenic Col1a1r/r mice, mean outflow facility (5.0 ± 0.8 nL/min per mm Hg, n = 25) was 25.4% lower than that in the control mice (6.7 ± 1.0 nL/min per mm Hg, n = 12, P < 0.01). Outflow facility in the 42- and 56-week-old transgenic mice increased, whereas IOP returned to normal. Mean outflow facility in the transgenic Col1a1r/r mice (6.8 ± 1.1 nL/min per mm Hg, n = 21) during the low-IOP period between 42 and 56 weeks was 36.0% higher than that during the high-IOP period of 12 to 36 weeks (P < 0.01). There was a significant inverse linear correlation between outflow facility and IOP in the transgenic Col1a1r/r mice (r2 = −0.702, P < 0.01, Fig. 2).
TABLE 1.
Age-Related IOP and Outflow Facility Changes in the Transgenic Col1a1r/r Mice
| Age (wk) | |||||
|---|---|---|---|---|---|
| 12 | 24 | 36 | 42 | 56 | |
| Col1a1r/r mice, n | 7 | 9 | 9 | 11 | 10 |
| Body weight, g | 28.6 ± 1.8 | 30.3 ± 3.3 | 31.7 ± 3.5 | 31.9 ± 4.1 | 33.2 ± 4.9 |
| IOP, mm Hg | 19.8 ± 1.2 | 21.2 ± 1.4 | 20.8 ± 1.2 | 16.4 ± 1.5 | 15.5 ± 1.4 |
| Outflow facility, nL/min per mm Hg* | 5.4 ± 0.9 | 4.9 ± 0.6 | 4.9 ± 0.8 | 6.8 ± 1.1 | 6.6 ± 1.1 |
For outflow facility, one-way ANOVA with Bonferroni post hoc test indicated that there were significant differences between each of weeks 24 and 36 to each of weeks 42 and 56, as well as between week 12 and week 42.
FIGURE 2.
Correlation between the outflow facility and IOP in the transgenic Col1a1r/r mice. Linear regression analysis demonstrated a significant relationship between these parameters. (r2 = −0.702, P < 0.01, n = 46).
Collagen Type I Content of Sclera and Choroid
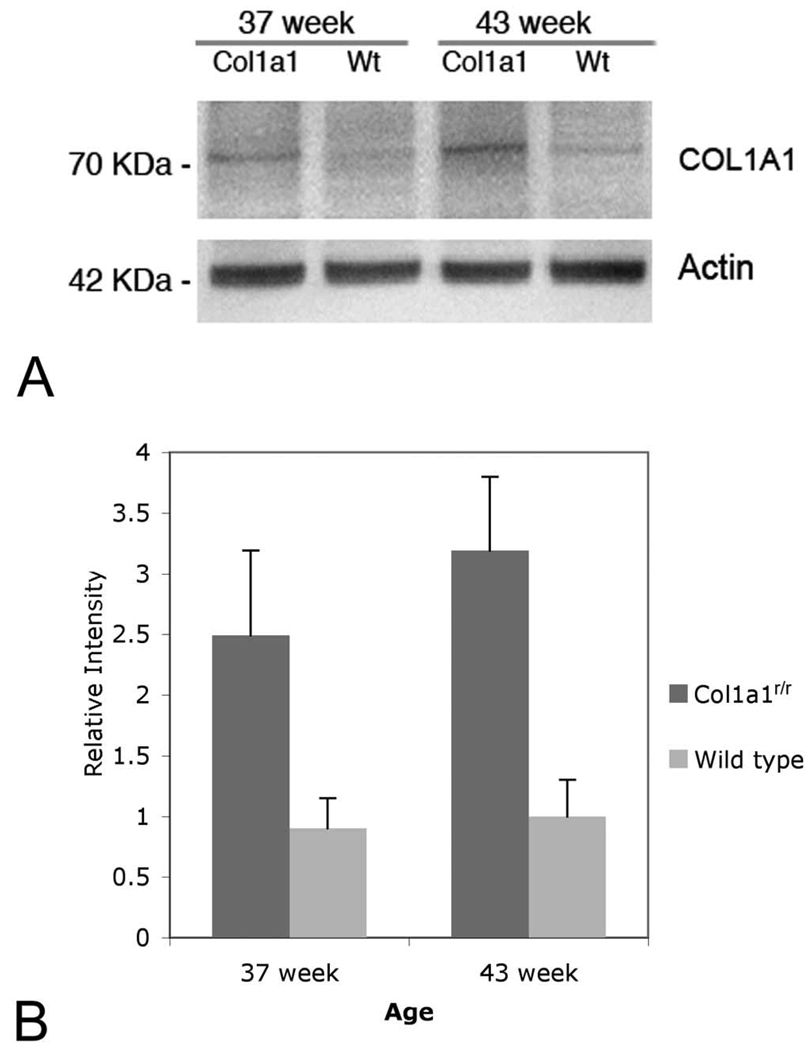
The Col1α1 protein content of the sclera and choroid was significantly increased in 37- and 43-week-old transgenic Col1a1r/r mice compared with that in the age-matched wild-type control mice (Fig. 3). The level of Col1α1 was approximately 28% higher in the 43-week-old Col1a1r/r mice than in the 37-week-old Col1a1r/r mice, but the difference is not statistically significant (P = 0.09).
FIGURE 3.
Levels of Col1α1 subunit in sclera and choroid of transgenic Col1a1r/r and age-matched wild-type mice. (A) Mature Col1a1 bands were observed at 70 kDa. (B) Relative intensity of chemiluminescence for each protein band was normalized by using actin (~42 kDa) as the calibrator. The Col1α1 in the sclera and choroid was significantly increased in the 37- and 43-week-old transgenic Col1a1r/r mice, compared with that in the age-matched wild-type control (P < 0.05). No significant difference of Col1α1 content was observed between the 37- and 43-week-old transgenic Col1a1r/r mice (P = 0.09).
Aqueous Humor Protein Concentration
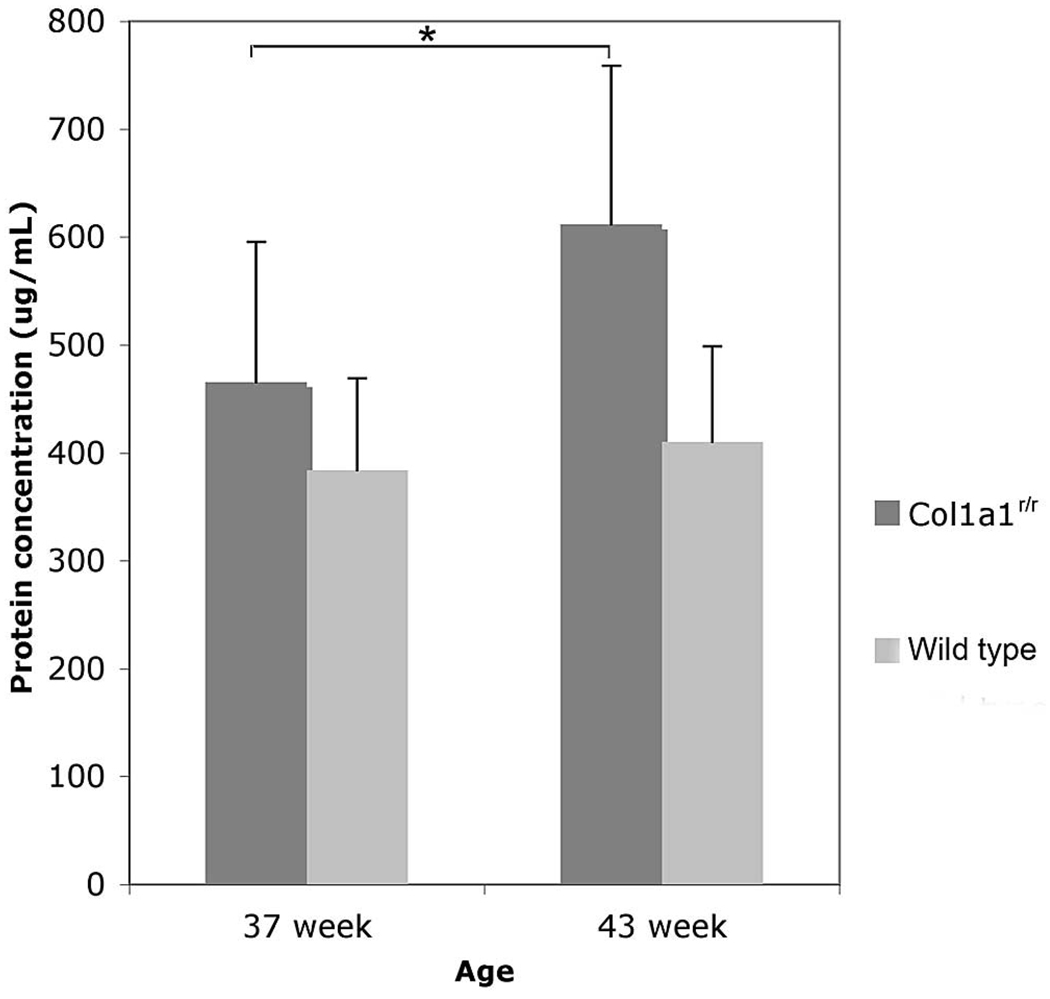
Mean aqueous humor protein concentration was stable in 37-and 43-week-old wild-type mice (n = 6; Fig. 4). In contrast, mean aqueous humor protein concentration in the 43-week-old transgenic Col1a1r/r mice (611 ± 147 µg/mL, n = 11) was 32.2% higher than in the 37-week-old Col1a1r/r mice (462 ± 130 µg/mL, n = 9, P < 0.05).
FIGURE 4.
Aqueous humor protein concentration in the transgenic Col1a1r/r and age-matched wide-type mice. Mean ± SD (*P < 0.05).
DISCUSSION
These data indicate that ocular hypertension in transgenic Col1a1r/r mice was associated with reduced outflow facility. In these mice, a 25.4% reduction in outflow facility was associated with a mean elevation in IOP of 4.2 mm Hg. Moreover, the magnitude of outflow facility reduction was inversely correlated with the magnitude of IOP elevation. The decrease in facility observed in these transgenic mice with elevated IOP was consistent with the findings of reduced outflow facility in patients with ocular hypertension.15–17
The two level, constant-pressure infusion method used in this study measures total outflow facility.10,13 Total outflow facility is a combination of trabecular facility, uveoscleral facility, and inflow facility. Although uveoscleral outflow facility is often considered to be negligible in primate eyes,18 our knowledge of the uveoscleral pathway in the mouse eye is rather limited. The hypotensive effect of latanoprost in the mouse eye was reported to be associated with a significant increase in total outflow facility,10 which is consistent with the findings in monkeys.19 Calculations based on the Goldmann equation indicate that the proportion of uveoscleral outflow is much greater in the mouse than in the human.9,10,20 Thus, it is possible that both trabecular and uveoscleral facility are affected in transgenic Col1a1r/r mice.
Aqueous outflow facility in the mouse eye is difficult to measure because the volume of outflow is small when the IOP is within the normal range. By using higher anterior chamber pressures for the facility measurements, we obtained reproducible measurements of flow. Previous investigations of facility in primate eyes have limited the maximum perfusion pressure because structural changes were observed in aqueous outflow tissues exposed to 50 mm Hg that were not seen at 15, 22, and 30 mm Hg.21,22 In addition, minor changes in the lysosomal system of monkey trabecular meshwork cells were seen at 30 mm Hg that were not observed at 15 and 22 mm Hg.22,23 It is possible that these higher pressures influence the outflow pathways, especially in Col1a1r/r mice. The accumulation of fibrillar collagen in the Col1a1r/r mice also may make outflow pathways tissues stiffer and outflow facility may no longer be inversely related to pressure elevation. Still, the IOPs used to measure outflow facility in this study are similar to those commonly encountered in human glaucomatous eyes as well as in mouse models of glaucoma. There was no observable ocular change in the Col1a1r/r mice during the measurements in the present study. Also no evidence of induced blood– aqueous barrier disruption was observed in our study on aqueous humor dynamics in mice.9 Therefore, the use of 25 and 35 mm Hg appears to be reasonable for obtaining useful measurements for the determination of outflow facility.
The gene mutation in Col1a1r/r mice is positioned at the consensus cleavage site of the α1 subunit of collagen type I for MMP-1. In our study, the effect of this mutation led to the accumulation of Col1α1 in the sclera and choroid of Col1a1r/r mice. Although not statistically significant, the greater amount of scleral Col1α1 observed in the samples from 43-week-old Col1a1r/r mice than in the 37-week-old Col1a1r/r mice is consistent with gradual age-related accumulation of this collagen. Fibrillar collagen, containing collagen types I and III, is a major component of structures with the trabecular outflow pathway, including trabecular meshwork beams, and the uveo-scleral aqueous outflow pathway including sclera, choroid, and extracellular matrix of the ciliary muscle.24–27 Hence, the accumulation of collagen type I in these tissues may explain the onset of decreased outflow facility with age in these mice.
In the present study, ocular hypertension in the Col1a1r/r mice began to decrease after 42 weeks. In a previous report,4 however, IOP in Col1a1r/r mice remained elevated at 56 weeks of age. The mice used in that study had a B6;129S4 genetic background. We obtained new founder mice of this strain from The Jackson Laboratory, but encountered poor breeding ability. To improve their breeding performance, we backcrossed these mice with C57BL6 mice. The background strain change, however, may lessen the effect of a severe phenotype. Therefore, it is possible that the C57BL6 genetic background in the presently studied Col1a1r/r mice contributed to the shorter duration of IOP elevation.
The decrease in IOP between 37 and 43 weeks of age in the present study may be related to the observed significant increase in aqueous humor protein concentration during this period. Increased aqueous humor protein has been linked to IOP reduction.28–30 However, overt inflammation is unlikely, as no anterior chamber flare or cells were observed at any time in the anterior chamber by slit lamp biomicroscopy. Hence, it is not clear whether IOP elevation induced subclinical blood– aqueous barrier breakdown that subsequently led to IOP reduction, or spontaneous IOP reduction between 37 and 43 weeks of age facilitated the increase in aqueous humor protein. Further studies on the relationship of MMP gene expression in aqueous formation and outflow pathway tissues of Col1a1r/r mice during this period may clarify the mechanism of later IOP reduction in these mice.
In conclusion, the significant correlation of IOP elevation to facility reduction suggests that the ocular hypertension in Col1a1r/r mice reflects increased resistance in the aqueous outflow pathway. These transgenic mice may be useful as an open-angle glaucoma model, as well as for assessing the relationship between collagen type I metabolism and aqueous outflow.
Acknowledgments
Supported by National Eye Institute Grant EY05990 (RNW) and National Science Foundation of China Grants 30600696 and SCTC 07QA14009 (YD)
Footnotes
Disclosure: Y. Dai, None; J.D. Lindsey, None; X. Duong-Polk, None; D. Nguyen, None; A. Hofer, None; R.N. Weinreb, None
References
- 1.Weinreb RN, Lindsey JD. The importance of models in glaucoma research. J Glaucoma. 2005;14:302–304. doi: 10.1097/01.ijg.0000169395.47921.02. [DOI] [PubMed] [Google Scholar]
- 2.Lindsey JD, Weinreb RN. Elevated intraocular pressure and transgenic applications in the mouse. J Glaucoma. 2005;14:318–320. doi: 10.1097/01.ijg.0000169411.09258.f6. [DOI] [PubMed] [Google Scholar]
- 3.Aihara M, Lindsey JD, Weinreb RN. Ocular hypertension in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci. 2003;44:1581–1585. doi: 10.1167/iovs.02-0759. [DOI] [PubMed] [Google Scholar]
- 4.Mabuchi F, Aihara M, Lindsey JD, Weinreb RN. Optic nerve damage in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci. 2004;45:1841–1845. doi: 10.1167/iovs.03-1008. [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Byrne M, Stacey A, Goldring M, Jaenisch R, Krane S. Generation of collagenase-resistant collagen by side-directed mutagenesis of murine pro alpha 1(I) collagen gene. Proc Natl Acad Sci USA. 1990;87:5888–5892. doi: 10.1073/pnas.87.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Wu H, Byrne M, Jeffrey J, Krane S, Jaenisch R. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J Cell Biol. 1995;130:227–237. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savinova OV, Sugiyama F, Martin JE, et al. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet. 2001;2:12. doi: 10.1186/1471-2156-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aihara M, Lindsey JD, Weinreb RN. Twenty-four-hour pattern of mouse intraocular pressure. Exp Eye Res. 2003;77:681–686. doi: 10.1016/j.exer.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Aihara M, Lindsey JD, Weinreb RN. Aqueous humor dynamics in mice. Invest Ophthalmol Vis Sci. 2003;44(12):5168–5173. doi: 10.1167/iovs.03-0504. [DOI] [PubMed] [Google Scholar]
- 10.Crowston J, Aihara M, Lindsey JD, Weinreb RN. Effect of latano-prost on outflow facility in the mouse. Invest Ophthalmol Vis Sci. 2004;45:2240–2245. doi: 10.1167/iovs.03-0990. [DOI] [PubMed] [Google Scholar]
- 11.Becker B, Neufeld AH. Pressure dependence of uveoscleral out-flow. J Glaucoma. 2002;11:464. doi: 10.1097/00061198-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Brubaker R. Goldmann’s equation and clinical measures of aqueous dynamics. Exp Eye Res. 2004;78:633–637. doi: 10.1016/j.exer.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Barany EH. Simultaneous measurement of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol Vis Sci. 1964;3:135–143. [PubMed] [Google Scholar]
- 14.Zhou L, Liu J. Circadian variation of mouse aqueous humor protein. Molecular Vision. 2006;12:639–643. [PubMed] [Google Scholar]
- 15.Gabelt B, Kaufman P. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24:612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Tan JC, Peters DM, Kaufman PL. Recent developments in understanding the pathophysiology of elevated intraocular pressure. Curr Opin Ophthalmol. 2006;17:168–174. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 17.Johnson TV, Fan S, Camras CB, Toris CB. Aqueous humor dynamics in exfoliation syndrome. Arch Ophthalmol. 2008;126:914–920. doi: 10.1001/archopht.126.7.914. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman PL. Letter to the editor. J Glaucoma. 2003;12:89. [Google Scholar]
- 19.Gabelt B, Kaufman P. The effect of prostaglandin F2α on trabecular outflow facility in cynomolgus monkeys. Exp Eye Res. 1990;51:87–91. doi: 10.1016/0014-4835(90)90174-s. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Davidson B, Stamer W, et al. Enhanced inflow and outflow rates despite lower IOP in Bestrophin-2 deficient mice. Invest Ophthalmol Vis Sci. 2009;50(2):765–770. doi: 10.1167/iovs.08-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grierson I, Lee WR. Pressure-induced changes in the ultrastructure of the endothelium lining Schlemm’s canal. Am J Ophthalmol. 1975;80:863–884. doi: 10.1016/0002-9394(75)90284-6. [DOI] [PubMed] [Google Scholar]
- 22.Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (1) Pressure effects within the near-physiological range (8–30 mmHg) Exp Eye Res. 1975;20:505–521. doi: 10.1016/0014-4835(75)90218-3. [DOI] [PubMed] [Google Scholar]
- 23.Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (2) Pressures outside the physiological range (0 and 50 mmHg) Exp Eye Res. 1975;20:523–530. doi: 10.1016/0014-4835(75)90219-5. [DOI] [PubMed] [Google Scholar]
- 24.Lütjen-Drecoll E, Gabelt B, Tian B, Kaufman P. Outflow of aqueous humor. J Glaucoma. 2001;10:S42–S44. doi: 10.1097/00061198-200110001-00016. [DOI] [PubMed] [Google Scholar]
- 25.Lütjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- 26.Yue BY. The extracellular matrix and its modulation in the trabecular meshwork. Surv Ophthalmol. 1996;40:379–390. doi: 10.1016/s0039-6257(96)80066-x. [DOI] [PubMed] [Google Scholar]
- 27.Weinreb RN, Lindsey J, Luo XX, Wang TH. Extracellular matrix of the human ciliary muscle. J Glaucoma. 1994;3:70–78. [PubMed] [Google Scholar]
- 28.Toris CB, Pederson JE. Aqueous humor dynamics in experimental iridocyclitis. Invest Ophthalmol Vis Sci. 1987;28:477–481. [PubMed] [Google Scholar]
- 29.Kahn AR, Brubaker RF. Aqueous humor flow and flare in patients with myotonic dystrophy. Invest Ophthalmol Vis Sci. 1993;34:3131–3139. [PubMed] [Google Scholar]
- 30.Freddo TF, Bartels SP, Barsotti MF, Kamm RD. The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci. 1990;31:125–137. [PubMed] [Google Scholar]