Abstract
Purpose of Review
To synthesize the critical role of obesity-associated inflammation, dietary factors, and nutrition in determining breast cancer risk.
Recent Findings
Obesity-associated inflammation is strongly linked to breast cancer risk and progression, largely via two processes: inflammatory pathways and dysregulated metabolism. Cytokine production in excess adipose tissues creates a chronic inflammatory microenvironment, which favors tumor development. Lifestyle factors, including diet, have long been recognized as important determinants of breast cancer risk and mortality.
Summary
Obesity increases the risk of developing breast cancer in both pre- and postmenopausal women and also negatively affects breast cancer recurrence and survival. Poor dietary habits characterized by the high intake of refined starches, sugar, and both saturated and trans-saturated fats, as well as the low intake of omega-3 fatty acids, natural antioxidants, and fiber, modulate inflammation and, thereby, appear to be linked to increased risk of breast cancer and mortality.
Keywords: Obesity, Nutrition, Dietary patterns, Inflammation, Breast cancer risk, Survival
Introduction
Breast cancer is a leading cause of cancer mortality worldwide and the most common form of cancer affecting women [1]. It also is a highly heterogeneous disease, characterized by a broad range of prognoses, depending upon the presence versus absence of specific receptors on the tumor cells. Patients are thus characterized by the presence/absence of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2), and patients whose tumor cells have none of these are said to have triple-negative breast cancer (TNBC) [2]. The American Cancer Society has estimated that there will be roughly 316,000 breast cancer cases in 2017 (Table 1), placing emphasis on the need for cancer prevention and new cancer treatment strategies [1]. While only 5–10% of all cancers are caused by genetic predispositions, 90–95% of tumor pathogenesis can be explained by environmental factors or unhealthy lifestyles, including alcohol consumption, unhealthy eating patterns, and obesity [3]. Several well-established links have been identified between environmental factors and breast cancer risk, including reproductive factors, like the patient’s age at menarche, age at first birth, parity, whether they have breastfed, and age at menopause. Such reproductive factors are generally considered nonmodifiable risk factors, which thereby cannot be controlled or changed by public measures.
Table 1.
| Estimated breast cancer cases 2017 | 316,120 |
| Breast cancer deaths 2017 | 40,610 |
| Obesity in women | 38.3% |
| Non-Hispanic blacks | 48.1% |
| Hispanics | 42.5% |
| Non-Hispanic whites | 34.5% |
| Non-Hispanic Asians | 11.7% |
In contrast, lifestyle choices such as unbalanced diet, lack of physical activity, high body mass index (BMI), and high alcohol and/or tobacco consumption are deemed modifiable [4]. It is widely acknowledged that diet acts as carcinogenesis risk modifiers, impacting both cancer initiation and progression [5]. This review synthesizes the critical impact of obesity-associated inflammation, dietary factors, and nutrition on breast cancer risk. Finally, dietary guidelines are presented, as well as their implications with respect to both the prevention and the treatment of breast cancer.
Obesity and Breast Cancer
The Role of Obesity in Breast Cancer Risk
The prevalence of obesity has increased significantly in the USA and globally over the last three decades, with more than one third of adult US men and women categorized as obese, representing a significant public health concern [6]. Obesity is a risk factor for several major illnesses, including cardiovascular disease, diabetes, and cancer, affecting both one’s risk of developing disease and potential death [7]. Obesity results when there is a sustained energy imbalance, with caloric intake (e.g., through food and beverages) exceeding caloric expenditure (e.g., physical activity) [8]. The body mass index (BMI) has traditionally been used to classify individuals’ weight relative to others, categorizing them as underweight, of normal weight, overweight, or obese, with a BMI between 25 and 29.9 kg/m2 implying that someone is overweight and a BMI greater than or equal to 30 kg/m2 implying obesity [9]. Obesity is the result of a complex interplay of biological, genetic, environmental, cultural and psychosocial factors that influence appetite, satiety, and food storage in the form of body fat. In addition, socioeconomic factors and lower educational levels that generally promote the consumption of less expensive, high-caloric foods with low nutritional value and disparities in access to healthy food sources may account for the association between unhealthy nutritional habits and obesity [10].
Obesity is an important, but under-recognized contributor that is quickly overtaking tobacco as the leading preventable cause of cancer [11]. An estimated 84,000 cancer diagnoses each year are linked to obesity, and being overweight or obese accounts for 15 to 20% of cancer-related deaths [7, 12, 13]. Obesity is also a critical risk factor for breast cancer in both pre- and postmenopausal women [14, 15], though the association is statistically stronger in postmenopause [16, 17]. In a study by Eliassen et al. [18], while there was a 12% increased risk for breast cancer among overweight, postmenopausal women, this increased risk rose to 25% in obese, postmenopausal women. Evidence links obesity not only to the elevated risk of breast cancer, but also to breast cancer recurrence [19] and mortality [20]. While some studies suggest that obesity in pre-menopausal women has no effect or actually reduces the risk of breast cancer [16, 21, 22], obesity is associated with worse outcomes for both pre- and postmenopausal breast cancer patients, including a 30% higher risk of death relative to breast cancer patients with a normal BMI [16, 20]. These different impacts of obesity on breast cancer risk might be best explained by menopausal status. In postmenopausal women, in whom ovarian estrogen production is absent, the increased incidence of breast cancer in women with a high BMI might be associated with the relatively high plasma levels of estradiol from adipose tissue. Estrogen synthesis in adipose tissues increases with both obesity and aging and is correlated with the increased expression of aromatase, an enzyme that contributes to estrogen biosynthesis [23]. Thus, estrogen formation in adipose tissue and aromatase expression in the breast adipose may be important links to breast cancer risk [24]. In contrast, the inverse relationship between a high BMI and breast cancer risk in premenopausal women may be due to reduced progesterone serum levels in obese women [21].
Breast cancer survivors, defined as those with breast cancer who complete treatment, are also at increased risk for comorbid conditions, likely due to obesity, including hypertension, cardiovascular disease, and diabetes, placing breast cancer survivors at greater risk for CVD-related mortality [25, 26]. Apart from mortality, obesity in breast cancer can interfere with the effective delivery of systemic cancer therapy and can contribute to treatment-related morbidity [27]. In addition, obesity in breast cancer is a risk factor for surgical complications like lymphedema, wound infections, poor wound healing, and unfavorable cosmetic outcomes postbreast cancer reconstruction [28, 29].
Although there is strong evidence linking high BMI to breast cancer risk, differences across different races/ethnicities, tumor subtypes, reproductive characteristics, receptor statuses, body weight changes throughout one’s lifespan, and lifestyle determinants may confound this relationship [30].
Obesity, Inflammation, and Breast Cancer Risk
Although the link between obesity and the onset of breast cancer is well documented, the biological and molecular mechanisms that promote tumor growth and progression are not fully understood, due to the complex and multifactorial causation of obesity. Possible mechanistic pathways underlying the association between obesity and breast cancer risk and survival involve chronic inflammation, steroid hormones, up-regulation of insulin-like growth factors, hyperinsulinemia or insulin resistance, and altered immune function [14, 31]. Dysregulation of theses pathways may influence cellular processes that promote tumor initiation and progression [32]. Chronic inflammation plays a critical role in the carcinogenesis process, and inflammatory components are important constituents of the tumor microenvironment [33–35]. For instance, higher C-reactive protein (CRP), interleukin 1β (IL-1β), and interleukin 6 (IL-6) have been shown to increase the risk of breast cancer and to be associated with shortened disease-free and overall survival [36–40].
Adipocytes are endocrine cells, which produce a variety of hormones, growth factors, and cytokines, also referred to as adipokines [32]. Adipose tissues promote low-grade chronic inflammation, characterized by increased circulating adipocytokines, including CRP, IL-1β, IL-6, tumor necrosis factor-alpha (TNF-α), leptin, and restin [32]. Leptin is a protein produced by fatty tissue and is involved in suppressing appetite and regulating fat storage in the body. Restin is an adipose-derived hormone and has been controversially discussed as a contributing factor to insulin resistance and diabetes mellitus [41]. Overall, these immune mediators are associated with intracellular transcription factors that are involved in each step of carcinogenesis, including apoptosis, migration, cell proliferation, inflammation, angiogenesis, and metastasis [42]. Hypoxia is another characteristic of adipose tissues that may develop, partially due to the physical limitations of blood vessels accommodating large, lipid-engorged adipocytes [43]. Furthermore, weight gain causes a shift in macrophage sub-types to a pro-inflammatory macrophage polarization M1, resulting in chronic inflammation through the release of TNF-α, IL-1β, and IL-6, all of which can support vessel growth in the hypoxic environment of obese adipose tissues [44, 45].
Moreover, adipocytes and visceral adipose tissues produce leptin and adiponectin, which are hormones that have the capacity to modulate immune function, inflammatory cytokines, angiogenesis, insulin resistance, and other biological processes that are associated with cancer initiation and progression [46]. High levels of leptin attract monocytes and promote monocyte differentiation to macrophages that produce TNF-α, fibroblast growth factor, and epidermal growth factor, which promote angio-genesis in developing tumors [47].
Inflammation in turn, dysregulates the endocrine functions of adipocytes, as well as the secretion of adipokines [48]. Systemic inflammation and the dysregulated adipokine concentrations are hypothesized to be responsible for obesity-associated insulin resistance [49], and type 2 diabetes mellitus [50], and to be a risk factor for breast cancer development [51]. For instance, TNF-α has been proposed as a link between obesity and insulin resistance by promoting serine phosphorylation of insulin receptor substrate (IRS-1), thereby blocking insulin signaling [52]. IL-6 increases insulin resistance by inhibiting lipoprotein lipase and thus the glucose uptake by the adipocytes [53]. In particular, higher breast cancer incidence and mortality have been noted in individuals with hyperinsulinemia, caused by insulin resistance in the liver, skeletal muscle, and adipose tissue and with type 2 diabetes mellitus [54].
Stress-Induced Obesity in Breast Cancer Risk
Stress and stressful life events and the negative emotions they generate elevate cortisol production [55]. Cortisol, the primary stress hormone, exerts multiple effects on neurochemistry, neurobiology, and behaviors, which ultimately result in metabolic alterations [56]. Cortisol also dysregulates the immune response, by disturbing the sensitive interplay between the central nervous system (CNS), the endocrine system, and the immune system [57], inducing low-grade inflammation and suppressing the function of immuno-protective cells, which can lead to severe health consequences [58].
Chronic stress and stressful life events have been associated with weight gain and adiposity [59, 60]. Data obtained from longitudinal studies suggest that chronic stress and stressful life events induce the development of a condition called metabolic syndrome [61, 62], a cluster of clinical abnormalities that include increased blood pressure, high blood sugar levels, excess body fat around the waist, and abnormal serum cholesterol and/or triglyceride levels [63]. Stress also influences food choices [64]. Higher levels of cortisol stimulate the increased intake of calorie-dense foods, and insulin secretion rises as cortisol increases [56]. In a recent study by Kiecolt-Glaser et al. [65], daily stressors impacted the metabolic responses to high-fat meals, revealing a molecular pathway that links stress and obesity. Additionally, chronic stress and elevated cortisol levels are risk factors for depression [66], which is known to promote obesity [67]. Meanwhile, diet can impact mood, as well as pro-inflammatory responses to stressors [68].
While there is mounting evidence linking stress to obesity and obesity to breast cancer risk, few clinical studies have been published that have investigated potential underlying mechanisms through which obesity may influence pathogenesis of breast cancer. Stress-induced obesity could be a critical factor affecting the risk of breast cancer. One pre-clinical model revealed that stress from social isolation, combined with a high-fat diet, increased the likelihood of obesity, insulin resistance, and breast cancer risk [69]. These results have important clinical implications, as they provide a mechanistic understanding of how stress might alter the phenotype and function of abdominal and mammary adipocytes, and how these changes may contribute increased breast cancer risk and mortality, both in stressed women and animal stress models.
In summary, stress can considerably affect the relationship between obesity and breast cancer risk, primarily via inflammatory pathways. Future research should continue to examine the relationship between stress, obesity, and breast cancer so as to develop preventative measures and interventions for at-risk populations.
Dietary Factors and Nutrition in Breast Cancer Risk
Diet can be characterized as the sum of all food that is consumed. Dietary habits correspond to the habitual and cultural decisions that an individual makes when choosing which foods to eat [5]. Among different modifiable risk factors that are known to affect breast cancer risk and mortality, diet is considered one of the most important, and most modifiable. In their most recent report, the World Cancer Research Fund International (WCRF)/American Institute for Cancer Research (AICR) estimated that, encompassing the 13 most common cancers, 29% of cases could have been prevented by a healthy lifestyle—specifically, by not smoking, being physically active, maintaining a healthy weight, and eating a balanced diet [70].
Foods contain both compounds that are protective and others that may cause harm and have an oncogenic effect [71]. We can consider the impact of individual nutrients (e.g., carbohydrates, saturated fat), important dietary markers (e.g., glycemic index, insulin load), and overall dietary patterns (e.g., high-fat versus plant-based diets) on one’s risk of developing breast cancer.
Foods with Tumor-Promoting Potential
Carbohydrates
High circulating levels of insulin and insulin-like growth factor 1 (IGF-1) are associated with type-2 diabetes mellitus and are hypothesized to play a critical role in tumor growth and progression in breast cancer breast cancer, as well as potentially increasing one’s risk of ever having it [72]. Numerous dietary factors can affect circulating insulin and IGF-I. The quality and quantity of consumed carbohydrates, expressed as a glycemic index (GI) and glycemic load (GL), are the most important determinants of postprandial glucose levels, and thus, circulating insulin levels [72]. A diet with a high GI or high GL increases postprandial serum glucose levels and insulin concentrations in the blood [73], which might affect breast cancer risk through the stimulation of insulin receptors in breast tissues and/or via the increased bioactivity of IGF-1, which in turn stimulates cell proliferation [74]. Robust evidence from cohort and meta-analytic studies have demonstrated a significant association between a diet high in GI or GL and the risk of breast cancer [75–77]. Moreover, in one study, high insulin and high serum glucose levels were linked to worse outcomes in patients with breast cancer [78].
Red and Processed Meats
Red meat consumption is associated with an increased risk of total, cardiovascular, and cancer mortality [79]. In the UK Women’s Cohort study, women with high meat consumption had a greater risk of breast cancer [80]. Similar findings have been reported for the Nashville Breast Health Study [81] and the NIH-AARP Diet and Health Study among both, pre- and postmenopausal women [82]. Red and processed meats consist of heterocyclic amines, n-nitroso compounds, and polycyclic aromatic hydrocarbons, all of which are potential human carcinogens [17, 83]. Meat also contains animal fats and saturated fats, which are associated with the increased risk of breast cancer, in particular of the ER+/ER− and HER2-subtypes [84–86].
Trans-fatty Acids
Trans-fatty acids usually are found in industrially processed sweet and salty foods, like chocolate bars, candies, cookies, industrial bread, and packed snacks. Potential harmful effects from trans-fatty acids arise from alterations in metabolic and signaling pathways, higher circulating levels of lipids, systemic inflammation, endothelial dysfunction, and potentially increased visceral adiposity, body weight, and insulin resistance [87]. Data suggest that trans-fatty acids may be linked to an increased risk of breast cancer [88, 89].
Ethanol
Ethanol is considered carcinogenic to humans [90], being a risk factor for several cancer types, as demonstrated oral cavity and pharynx, esophagus, colorectum, liver, larynx, and female breast [90–93]. More specifically, alcohol intake is associated with breast cancer in both pre- and postmenopausal women, and the risk of breast cancer is stronger among women who started drinking prior to their first full-time pregnancy [92], highlighting that the timing of exposure to alcohol drinking may affect the risk of breast cancer development. The same study confirmed the aforementioned association between alcohol intake and both hormone receptor-positive and hormone receptor-negative breast tumors. Although red wine contains a number of potential protective ingredients, like anti-oxidative polyphenols, its benefits with respect to malignant diseases remain controversial [94].
Omega-6 fatty acids
Omega-6 (n-6) polyunsaturated fatty acids (PUFAs) are pro-inflammatory polyunsaturated fatty acids and commonly occur not only in poultry, eggs, corn, and most vegetable oils, but also in processed and fast foods. They are considered to be the counterpart of omega-3 (n-3) polyunsaturated fatty acids (PUFA), which are anti-inflammatory. Omega-6 and Omega-3 PUFAs should be consumed in a 1:1 ratio [95]. Adequate intake of PUFAs is essential as they play a key role in metabolism, inflammation, cell signaling, and regulation of gene expression [96]. A high-fat diet and the Western dietary pattern are characterized by high amounts of n-6 PUFAs and low amounts of n-3 PUFAs. Conversely, a low-fat diet (e.g., traditional Japanese diet) is low in n-6 PUFAS and high in n-3 PUFAs [97]. Evidence indicates that an increased ratio of n-6/n-3 PUFAs increases inflammation and the risk of chronic inflammatory diseases, including obesity, nonalcoholic fatty liver disease, and cardiovascular disease [95]. Preclinical studies indicate that n-6 PUFAs have a tumor-enhancing effect [98]. In a recent Japanese cohort study, incorporating 38,200 women, n-6 PUFA intake was positively associated with breast cancer risk, with this association strongest for ER+/PR+ tumors [99].
Foods with Tumor-Preventive Potential
Omega-3 Fatty Acids
Omega-3 (n-3) polyunsaturated fatty acids (PUFAs) have anti-inflammatory properties and are thought to be critical in breast cancer prevention [100]. n-3 PUFAs can be found in foods like fish, fish oil, marine sources, flaxseed, nuts, and eggs [99]. n-3 PUFAs intake also can boost mood [101], modulate the magnitude of inflammatory responses to stressors [102], and reduce the risks of cardiovascular disease, cancer [103, 104], and all-cause mortality [105]. Insights into mechanisms that may contribute to the beneficial health effects of n-3 PUFAs can be gleaned by reviewing recent work by Kiecolt-Glaser et al. [106], showing that n-3 PUFAs supplementation positively impacted cell aging, thereby highlighting how inflammation, oxidative stress, and immune cell aging—which appear to be important mechanisms prior to disease onset—that may be ameliorated through nutritional interventions. Among women, n-3 PUFA intake has been found to be inversely associated with breast cancer risk [99, 100, 107, 108] and breast cancer mortality [103, 104]. Mechanisms underlying the beneficial effects of higher consumption of marine n-3 fatty acids in terms of lowering breast cancer risk might be attributed to the growth-inhibitory, pro-apoptotic, or anti-angiogenic effects of n-3 fatty acids that can be found in fish [100, 103].
Fruits and vegetables
Fruits and vegetables contain numerous components with anti-inflammatory and detoxifying actions that are known to have favorable effects on inflammatory and metabolic processes, as well as on endothelial function [109, 110]. Fruit and vegetable intake is associated with a reduced risk of cancer and reduced all-cause mortality [111]. A study by Narita et al. [112] demonstrated an inverse association between adult fiber intake and breast cancer risk. Furthermore, regular consumption of fruits and vegetables was found to facilitate weight management in obese breast cancer survivors [113], suggesting a favorable role of a Mediterranean diet to counter obesity as a risk factor for cancer and poor survival outcomes. The tumor-preventative components of fruits include antioxidant vitamins and dietary fiber, both of which protect DNA from oxidative damage and, thus, might be protective against breast cancer [114].
Vitamins and Minerals
Blood concentrations of carotenoid and vitamin C are reliable biomarkers of vegetable and fruit intake. Carotenoids and vitamin C are thought to be associated with reduced cancer risk due to their anti-oxidative capacity and their ability to inhibit cell proliferation and maintaining DNA methylation and hormonal metabolism [115]. Results from case-control studies indicate that higher concentrations of total carotenoid and vitamin C are associated with lower breast cancer risk, though this association may be statistically stronger for ER− than ER+ breast cancer tumors [116, 117]. Vitamin D is important in many physiological processes, is predominantly obtained through UVB radiation, and has been ascribed an important role in calcium homeostasis, bone health, and anti-cancer activity [118]. Some research has been published documenting a protective role of vitamin D against breast cancer [119, 120]. Moreover, higher levels of vitamin D may be associated with improved survival [121].
Tree nuts
Tree nuts contain high amounts of polyphenols and phytochemicals that have antioxidant, endothelium-protective, and anti-inflammatory properties, which might help to prevent tumor pathogenesis and progression [122]. In one cohort study, it was observed that the consumption of nuts was potentially associated with the reduced risk of both cancer and cancer-related mortality, although the effect sizes for this study were small [123].
Dietary Pattern
Instead of focusing on single nutrients, the assessment of dietary pattern might be a more illuminating approach to identifying the link between nutrition and cancer [109]. As opposed to single-nutrient analyses, dietary patterns allow for the simultaneous assessment of both favorable and unfavorable food components, as well as their inter-dependencies [124]. Dietary patterns are likely to vary between populations, because of geographic characteristics and cultural differences in food habits, preferences, and availability [125]. Several studies investigating dietary patterns and breast cancer risk have documented an inverse association with a prudent/low fat dietary pattern, which includes the high intake of vegetables, fruits, whole grains, and fish [110, 111, 124, 126], and a positive association with the less healthy Western diet, which is generally characterized by large quantities of red meat, refined grains, potatoes, and fat [127].
The Mediterranean diet is an example of an a priori-defined dietary food pattern, one that is characterized by the high intake of virgin olive oil, vegetables, fruits, plant proteins, fish and other seafood, whole grains, nuts, and low-fat dairy, accompanied by moderate alcohol intake and low red meat consumption [3]. Beneficial effects of a Mediterranean diet have been noted with regards to decreasing the risk of breast cancer and breast cancer recurrence, while improving overall survival [109, 128–130]. The health-promoting effects of a Mediterranean diet might be explained by its high content of calcium, vitamins, and protein [3].
Dietary Influences on Inflammation
Experimental studies have revealed how various nutritional components can influence key pathways to inflammation, including sympathetic activity, oxidative stress, transcription nuclear factor kappa B (NF-kB) activation, and pro-inflammatory cytokine production [131]. This, in turn, increases one’s risk of the developing breast cancer [71, 132]. Red and processed meat, butter, saturated and trans-fats, alcohol, nitrosamines, diets low in omega-3 fatty acids, sugar, and Western dietary patterns are associated with increased levels of CRP and pro-inflammatory cytokines and have been identified as ingredients that may increase the risk of different types of cancer, including breast [91, 133]. The unfavorable effects of red meat and processed products might be related to components that induce pro-inflammatory and pro-oxidative metabolic processes—like nitrosamines, iron, or saturated fatty acids [134].
Compelling evidence has been provided by one preclinical animal study, in which it demonstrated that a high-fat, high-calorie intake resulted in mammary adipose tissue inflammation. In the same study, inflammation was associated with increased macrophage infiltration, elevated plasma levels of monocyte chemoattractant protein-1 (MCP-1), and increased leptin and pro-inflammatory cytokine concentrations, suggesting a critical role of obesity in creating a microenvironment favorable to angiogenesis during breast cancer progression [135]. Clinical data obtained from the Nurses Health Study clearly demonstrated a link between trans-fatty acid consumption and greater inflammation. Women who ate a “Westernized” diet, again determined by the high consumption of red and processed meats, sweets, desserts, French fries, and refrained grains, had higher CRP and IL-6 than those women who adhered to a more prudent diet, characterized by the high intake of fruits, vegetables, fish, and whole grains [136].
A pro-inflammatory diet has also been shown to increase the risk of breast cancer. The dietary inflammatory index (DII) [137] provides a tool by which to evaluate diets on a continuum, from maximally anti-inflammatory to maximally pro-inflammatory. Women, who consumed diets characterized by a high inflammatory potential, as indicated by higher DII scores, appeared to be at increased risk of breast cancer, relative to women who consumed more anti-inflammatory diets. This effect seems to be especially strong among postmenopausal women [132, 138]. Further evidence supports the assumption that dietary inflammatory potential influences breast cancer risk differentially, depending on cancer phenotype. A recent study has demonstrated that a history of pro-inflammatory diets or sustained intake of highly pro-inflammatory diets may be associated with developing the ER-, PR-, and HER2 subtypes of breast cancer [131].
Importantly, foods that are consumed not only impact the intake of particular bioactive components, but also may alter metabolism and potentially influence the sites of action of essential and nonessential nutrients. In addition, genetic polymorphisms are increasingly acknowledged as another factor that can modify the responses to dietary components, as they can influence absorption, metabolism, and/or sites of action [32]. Similarly, variations in DNA methylation patterns and other epigenetic events can be affected by dietary intake [139]. For instance, alcohol may affect DNA methylation and can change the expression of oncogenes and tumor suppressor genes. In contrast, whole grains are rich in antioxidants, which have shown to prevent oxidative damage to DNA and mutation during tumor initiation [5].
In contrast, fiber, n-3 PUFAs, vitamin C/E, fruits and vegetables, a Mediterranean diet pattern, and a low-glycemic index diet are associated with lower levels of chronic inflammation [68, 140]. Adherence to a Mediterranean diet has been demonstrated to have anti-inflammatory dietary effects. Authors of a recent meta-analysis reported that a Mediterranean diet both decreased inflammation and improved endothelial function [109].
Dietary Factors in Breast Cancer Outcomes and Recurrence
Malnutrition in Breast Cancer
Both the tumor itself and its treatment can threaten the nutritional status of cancer patients in multiple ways, including effects on the patient’s sense of taste and smell, appetite, and functional ability to eat (e.g., chew and swallow), thereby placing them at risk for malnutrition (i.e., the inadequacy of key nutrients).
Cancer patients also are at risk of developing a systemic inflammation syndrome, which can vary in severity, but may impact all relevant metabolic pathways. This includes (1) protein metabolism, resulting in lost fat and muscle mass and increased production of acute phase proteins; (2) carbohydrate metabolism, resulting in insulin resistance and impaired glucose tolerance; and (3) lipid metabolism, resulting in increased lipid oxidation [141].
One particular concern is that malnutrition is an emerging occurrence among overweight/obese cancer patients, stemming from an imbalance between the energy intake, energy expenditures, and the quality of the nutrient intake. Excessive fat mass and/or adipocytes, especially in the form of central obesity, are linked to an increased inflammatory response that likely contributes to the state of malnutrition [142]. In turn, malnutrition is associated with poor tolerance to cancer treatment, and other poor health outcomes, like excessive or inappropriate weight loss, loss of muscles, reduced immune competence with a resultant increase in infections, lower quality of life, and a greater risk of premature deaths [143].
Nutritional Support and Adherence to Healthy Lifestyle During Cancer Treatment
Adhering to a healthy lifestyle during cancer treatment can help an individual to cope with debilitating treatment-related symptoms, maintain their physical function, and sustain their psychosocial well-being, all the while reducing their risk for other chronic diseases, including diabetes and cardiovascular disease [144]. Strategies to improve the cancer-related symptoms include interventions to increase and optimize nutritional intake and, thereby, limit systemic inflammation, as well as encourage them to engage in exercise and other physical activities [145]. Convincing evidence demonstrates how nutritional support in breast cancer patients undergoing radiotherapy improves their dietary intake and weight, as well as quality of life [146–148]. In breast cancer survivors and among patients with advanced cancer and cachexia, omega-3 fatty acid supplements may be of benefit, improving appetite, weight, and quality of life, while decreasing fatigue and inflammation [149, 150].
Diet may modify biomarkers of cancer progression in patients who have been treated for cancer [151]. In observational studies, a prudent dietary pattern, rich in plant foods has been associated with reduced mortality after diagnosis of breast cancer [152, 153]. In the Life After Epidemiology Study, which involved 1900 early-stage breast cancer patients, a Western dietary pattern—characterized by high consumption of meat and refined grains—was linked to an increased risk of overall mortality [154]. These findings suggest that women diagnosed with early-breast cancer might improve their overall prognosis and survival by adopting a more healthful dietary pattern.
Targets for Prevention and Intervention
A healthy lifestyle—one which incorporates a good diet, weight management, and physical activity—is critical for both the prevention of breast cancer and reduction of breast cancer recurrence [155]. Adolescence has been hypothesized to be a stage of life that is particularly susceptible to breast cancer risk factors in women. In the second phase of the Nurses’ Health study (NHSII) [156, 157], red meat and fat intake during adolescence were both positively linked to the risk of breast cancer among pre-menopausal women. In contrast, no significant associations were identified between dietary carbohydrate and fiber intake during adolescence and breast cancer risk [158]. This being said, an adolescent dietary pattern associated with inflammation, characterized by the high intake of sugar-sweetened and diet soft drinks, refined grains, red and processed meats, and margarine, and the low intake of green leafy vegetables, cruciferous vegetables and coffee, might increase the incidence of pre-menopausal breast cancer [159]. Thus, an overall healthy diet during adolescence, one that is similar to the prudent dietary pattern, could contribute to reducing breast cancer risk.
Weight gain after breast cancer treatment confers a poor prognosis and may increase recurrence rates. Adopting a healthy lifestyle, that is eating a healthy diet, engaging in physical activity, and maintaining a healthy weight following cancer treatment, has been shown to improve the quality of life and overall outcome of cancer survivors [155, 160].
However, changing dietary behaviors that have been established over a lifetime is challenging. Although the time when cancer initially is diagnosed is frequently considered a “teachable moment”, and many cancer patients actively seek guidance on effective strategies to improve treatment-related outcomes [161], cancer patients are no more likely to maintain a healthy diet than healthy individuals of a similar age, gender, ethnicity, and socioeconomic background [162]. Thus, changing dietary behaviors requires tailored support over time to maintain behavioral changes [163].
Weight loss interventions and dietary education after breast cancer diagnosis can help patients to improve their diet quality, achieve desired weight loss, increase their cardiorespiratory fitness, and improve their overall quality of life, fatigue, body image, and lower incidence of comorbidities and favor changes in biomarkers related to breast cancer risk and prognosis (e.g., blood glucose, blood lipids, CRP, DNA methylation) [113, 155, 160, 164–167]. In addition, physical activity during and after treatment can help patients to manage treatment side effects, reduce cancer-related fatigue, improve quality of life and functional capacity, promote alterations in bio-markers, and potentially enhance the overall prognosis and survival of breast cancer patients and survivors [168, 169]. On the other hand, in one study, adhering to a strict dietary intervention resulted in decreased sleep quality in breast cancer patients, indicating that changing dietary behaviors can be both challenging and stressful [160].
While weight loss in postmenopausal women has been found to significantly lower serum estrogens, free testosterone levels, and breast cancer risk [18, 170], there is currently a lack of evidence demonstrating that weight loss actually improves survival among breast cancer patients who are either overweight or obese [171].
Overall, these findings support the ability of dietary and lifestyle interventions to improve nutrition among breast cancer survivors and may help guide the incorporation of weight loss and dietary counseling into breast cancer treatment and care. However, this work is still in its infancy and more studies are clearly needed to determine the scale of the impact.
Dietary Guidelines
Cancer prevention and health promoting guidelines provided by the World Cancer Research Fund/American Institute for Cancer Research (WCRF) [172] focus on specific lifestyle recommendations, including (1) achieving and maintaining a healthy weight throughout life, (2) adopting a physically active lifestyle, (3) consuming a healthy diet that emphasizes plant-based foods, and (4) limiting alcohol and tobacco consumption (Fig. 1). Dietary guidelines for cancer prevention are formulated on the basis of nutrition-related factors that are known to be either convincingly or probably causally related to cancer risk [173]. According to the recommendations given by the WCRF [172], a healthy diet for cancer prevention is characterized by a diet that (1) allows someone to be as lean as possible without being underweight; (2) is rich in vegetables, fruits, whole grains, and pulses; (3) contains only a small quantity of red meat; (4) contains no processed meat; (5) has limited salt intake; (6) minimizes sugary drinks; (7) limits calorie-rich foods; and (8) restricts the consumption of alcohol and tobacco.
Fig. 1.
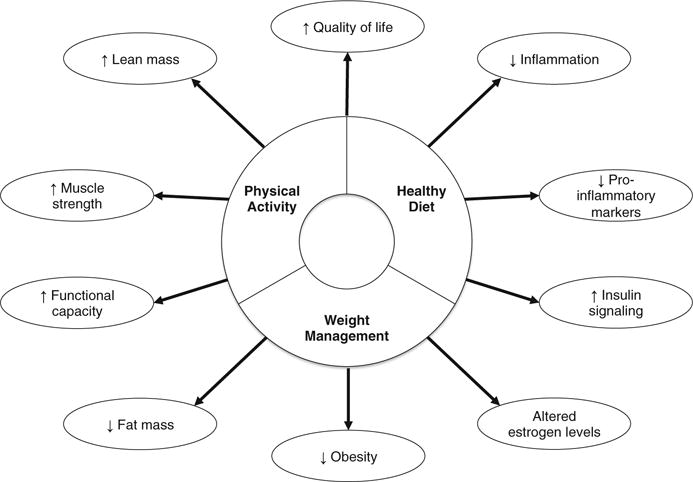
Illustrative figure demonstrating how healthy diet, weight management, and physical activity can reduce breast cancer risk
Thus, breast cancer risk reduction may be possible for women who are willing to adhere to a diet with anti-inflammatory properties, primarily composed of mostly fruits, vegetables, fish, and olive/sunflower oil, while avoiding many Western-style foods [108, 174].
Breast cancer patients who have completed all treatment of the cancer itself are encouraged to follow dietary recommendations for primary cancer prevention and to pursue and maintain a healthy body weight (BMI 20–24.9 kg/m2), preserving their lean body mass and avoiding increases in fat mass [172]. Following the recommended 150 min of moderate-to-vigorous exercise or 75 min of vigorous exercise per week, combined with two to three weekly sessions of strength training, can help to reduce breast cancer recurrence and improve survival [175].
In summary, personalized, patient-centered, and comprehensive nutritional interventions and weight management/lifestyle programs should be offered to all breast cancer patients, both during and after cancer-targeted treatment.
Conclusions
Obesity portends breast cancer risk through inflammatory pathways and dysregulated metabolism. Breast cancer pathogenesis is a prolonged process, and dietary habits can contribute to this, influencing both patients’ risk of developing breast cancer and outcomes postdiagnosis. Dietary interventions are an effective means of secondary/tertiary prevention and may be a component of adjunctive therapy. Efforts should be made to increase public awareness about the evidence linking obesity and breast cancer risk. Despite current awareness about the critical role that both obesity and nutrition have in determining breast cancer outcomes, there remains inadequate translation of research findings into clinical practices. Efforts also should be directed towards advocating for policy and systems changes to address societal factors that contribute to obesity and enhancing public access to weight management services, both for the general population and cancer patients specifically. Future research must address variables such as race/ethnicity, tumor subtype, receptor status, reproductive characteristics, and lifestyle factors that influence the relationship between excess body weight and breast cancer risk.
Acknowledgments
Funding Information Annina Seiler received funding from the Swiss National Science Foundation (SNSF) (P2FRP1-168479).
Footnotes
This article is part of the Topical Collection on Psycho-Oncology and Supportive Care
Compliance with Ethical Standards
Conflict of Interest The authors do not have any conflict of interests to declare
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.American Cancer Society. Cancer facts & figures. 2017:2017. [Google Scholar]
- 2.Kuchenbaecker KB, Neuhausen SL, Robson M, Barrowdale D, McGuffog L, Mulligan AM, et al. Associations of common breast cancer susceptibility alleles with risk of breast cancer subtypes in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2014;16:3416. doi: 10.1186/s13058-014-0492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. 2017;9(10) doi: 10.3390/nu9101063. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudolph A, Chang-Claude J, Schmidt MK. Gene–environment interaction and risk of breast cancer. Br J Cancer. 2016;114:125. doi: 10.1038/bjc.2015.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theodoratou E, Timofeeva M, Li X, Meng X, Ioannidis JPA. Nature, nurture, and cancer risks: genetic and nutritional contributions to cancer. Annu Rev Nutr. 2017;37(1):293–320. doi: 10.1146/annurev-nutr-071715-051004. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention (CDC) Adult obesity facts. Atlanta: CDC; 2017. [Google Scholar]
- 7.National Institutes of Health (NIH) Overweight and obesity. Bethesda: NIH; 2017. [Google Scholar]
- 8.Jarolimova J, Tagoni J, Stern TA. Obesity: its epidemiology, co-morbidities, and management. Prim Care Companion CNS Disord. 2013;15(5):PCC.12f01475. doi: 10.4088/PCC.12f01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) Obesity. 2017 [Google Scholar]
- 10.Dobbins M, Decorby K, Choi BC. The association between obesity and cancer risk: a meta-analysis of observational studies from 1985 to 2011. ISRN Prev Med. 2013;2013:680536. doi: 10.5402/2013/680536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ligibel JA, Alfano CM, Courneya KS, Wendy D-W, Burger Robert A, Chlebowski Rowan T, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568–74. doi: 10.1200/JCO.2014.58.4680. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–78. doi: 10.1056/NEJMoa055643. . [DOI] [PubMed] [Google Scholar]
- 13.Patel AV, Hildebrand JS, Gapstur SM. Body mass index and all-cause mortality in a large prospective cohort of white and black U.S. adults. PLoS One. 2014;9(10):e109153. doi: 10.1371/journal.pone.0109153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonnell DP, Park S, Goulet MT, Jasper J, Wardell SE, Chang CY, et al. Obesity, cholesterol metabolism, and breast cancer pathogenesis. Cancer Res. 2014;74(18):4976–82. doi: 10.1158/0008-5472.CAN-14-1756. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. . [DOI] [PubMed] [Google Scholar]
- 16.Matthews SB, Thompson HJ. The obesity-breast cancer conundrum: an analysis of the issues. Int J Mol Sci. 2016;17(6):989. doi: 10.3390/ijms17060989. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go Y, Chung M, Park Y. Dietary patterns for women with triple-negative breast cancer and dense breasts. Nutr Cancer. 2016;68(8):1281–8. doi: 10.1080/01635581.2016.1225102. . [DOI] [PubMed] [Google Scholar]
- 18.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193–201. doi: 10.1001/jama.296.2.193. . [DOI] [PubMed] [Google Scholar]
- 19.Biganzoli E, Desmedt C, Fornili M, de Azambuja E, Cornez N, Ries F, et al. Recurrence dynamics of breast cancer according to baseline body mass index. Eur J Cancer. 2017;87:10–20. doi: 10.1016/j.ejca.2017.10.007. . [DOI] [PubMed] [Google Scholar]
- 20.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–14. doi: 10.1093/annonc/mdu042. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowsett M, Folkerd E. Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: a new hypothesis. Breast Cancer Res Treat. 2015;149(1):1–4. doi: 10.1007/s10549-014-3211-4. . [DOI] [PubMed] [Google Scholar]
- 22.Nichols HB, Anderson C, White AJ, Milne GL, Sandler DP. Oxidative stress and breast cancer risk in premenopausal women. Epidemiology. 2017;28(5):667–74. doi: 10.1097/EDE.0000000000000685. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleary MP, Grossmann ME. Obesity and breast cancer: the estro-gen connection. Endocrinology. 2009;150(6):2537–42. doi: 10.1210/en.2009-0070. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, et al. Estrogen—the good, the bad, and the unexpected. Endocr Rev. 2005;26(3):322–30. doi: 10.1210/er.2004-0020. . [DOI] [PubMed] [Google Scholar]
- 25.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27(1):6–13. doi: 10.1097/EDE.0000000000000394. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haque R, Prout M, Geiger AM, Kamineni A, Thwin SS, Avila C, et al. Comorbidities and cardiovascular disease risk in older breast cancer survivors. Am J Manag Care. 2014;20(1):86–92. [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiler G. Systemic treatment in premenopausal patients with breast cancer. Breast Care. 2015;10(5):305–6. doi: 10.1159/000441614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16(1):48–54. doi: 10.1111/j.1524-4741.2009.00855.x. . [DOI] [PubMed] [Google Scholar]
- 29.McCarthy CM, Mehrara BJ, Riedel E, Davidge K, Hinson A, Disa JJ, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121(6):1886–92. doi: 10.1097/PRS.0b013e31817151c4. . [DOI] [PubMed] [Google Scholar]
- 30.Kruk J. Overweight, obesity, oxidative stress and the risk of breast cancer. Asian Pac J Cancer Prev. 2014;15(22):9579–86. doi: 10.7314/APJCP.2014.15.22.9579. . [DOI] [PubMed] [Google Scholar]
- 31.Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S, et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8(1):864. doi: 10.1038/s41467-017-00910-z. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64(1):45–57. doi: 10.1146/annurev-med-121211-091527. . [DOI] [PubMed] [Google Scholar]
- 33.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvi-ronment. Nat Rev Cancer. 2015;15(9):563–72. doi: 10.1038/nrc3978. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microen-vironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asegaonkar SB, Asegaonkar BN, Takalkar UV, Advani S, Thorat AP. C-reactive protein and breast cancer: new insights from old molecule. Int J Breast Cancer. 2015;2015:145647. doi: 10.1155/2015/145647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimm C, Kantelhardt E, Heinze G, Polterauer S, Zeillinger R, Kolbl H, et al. The prognostic value of four interleukin-1 gene polymorphisms in Caucasian women with breast cancer: a multi-center study. BMC Cancer. 2009;9(1):78. doi: 10.1186/1471-2407-9-78. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cyto-kines in breast cancer development and progression. J Interf Cytokine Res. 2015;35(1):1–16. doi: 10.1089/jir.2014.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobias DK, Akinkuolie AO, Chandler PD, Lawler PR, Manson JE, Buring JE, et al. Markers of inflammation and incident breast cancer risk in the women’s health study. Am J Epidemiol. 2017 doi: 10.1093/aje/kwx250. . [DOI] [PMC free article] [PubMed]
- 40.Wang J, Lee IM, Tworoger SS, Buring JE, Ridker PM, Rosner B, et al. Plasma C-reactive protein and risk of breast cancer in two prospective studies and a meta-analysis. Cancer Epidemiol Biomark Prev. 2015;24(8):1199–206. doi: 10.1158/1055-9965.EPI-15-0187. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro AM, Macedo-de la Concha LE, Pantoja-Meléndez CA. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev Méd Hosp Gen Méx. 2017;80(2):101–5. doi: 10.1016/j.hgmx.2016.06.011. . [DOI] [Google Scholar]
- 42.Dias JA, Fredrikson GN, Ericson U, Gullberg B, Hedblad B, Engstrom G, et al. Low-grade inflammation, oxidative stress and risk of invasive post-menopausal breast cancer—a nested case-control study from the Malmo diet and cancer cohort. PLoS One. 2016;11(7):e0158959. doi: 10.1371/journal.pone.0158959. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes. 2009;33(1):54–66. doi: 10.1038/ijo.2008.229. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249(1):218–38. doi: 10.1111/j.1600-065X.2012.01151.x. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–46. doi: 10.2337/db08-0872. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaysse C, Lomo J, Garred O, Fjeldheim F, Lofteroed T, Schlichting E, et al. Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer. 2017;3(1):19. doi: 10.1038/s41523-017-0015-9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009;20(3):193–201. doi: 10.1016/j.cytogfr.2009.05.007. . [DOI] [PubMed] [Google Scholar]
- 48.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3(12):716–24. doi: 10.1038/ncprheum0674. . [DOI] [PubMed] [Google Scholar]
- 49.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–7. doi: 10.1172/JCI57132. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29(7):1585–90. doi: 10.2337/dc06-0057. . [DOI] [PubMed] [Google Scholar]
- 51.Tabassum I, Mahmood H, Faheem M. Type 2 diabetes mellitus as a risk factor for female breast cancer in the population of Northern Pakistan. Asian Pac J Cancer Prev. 2016;17(7):3255–8. [PubMed] [Google Scholar]
- 52.Nieto-Vazquez I, Fernandez-Veledo S, Kramer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008;114(3):183–94. doi: 10.1080/13813450802181047. . [DOI] [PubMed] [Google Scholar]
- 53.Greenberg AS, Nordan RP, McIntosh J, Calvo JC, Scow RO, Jablons D. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res. 1992;52(15):4113–6. [PubMed] [Google Scholar]
- 54.Ferroni P, Riondino S, Buonomo O, Palmirotta R, Guadagni F, Roselli M. Type 2 diabetes and breast cancer: the interplay between impaired glucose metabolism and oxidant stress. Oxidative Med Cell Longev. 2015;2015:183928. doi: 10.1155/2015/183928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24(8):444–8. doi: 10.1016/S1471-4906(03)00173-X. . [DOI] [PubMed] [Google Scholar]
- 56.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21(3):159–65. doi: 10.1016/j.tem.2009.10.004. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–51. doi: 10.1038/nri1571. . [DOI] [PubMed] [Google Scholar]
- 58.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58(2–3):193–210. doi: 10.1007/s12026-014-8517-0. . [DOI] [PubMed] [Google Scholar]
- 59.Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. Am J Epidemiol. 2009;170(2):181–92. doi: 10.1093/aje/kwp104. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 2013;73(9):827–35. doi: 10.1016/j.biopsych.2013.01.032. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bergmann N, Gyntelberg F, Faber J. The appraisal of chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr Connect. 2014;3(2):R55–80. doi: 10.1530/EC-14-0031. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Troxel WM, Matthews KA, Gallo LC, Kuller LH. Marital quality and occurrence of the metabolic syndrome in women. Arch Intern Med. 2005;165(9):1022–7. doi: 10.1001/archinte.165.9.1022. . [DOI] [PubMed] [Google Scholar]
- 63.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–7. doi: 10.1242/dmm.001180. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62(6):853–65. doi: 10.1097/00006842-200011000-00016. . [DOI] [PubMed] [Google Scholar]
- 65.Kiecolt-Glaser JK, Habash DL, Fagundes CP, Andridge R, Peng J, Malarkey WB, et al. Daily stressors, past depression, and metabolic responses to high-fat meals: a novel path to obesity. Biol Psychiatry. 2015;77(7):653–60. doi: 10.1016/j.biopsych.2014.05.018. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. doi: 10.1037/a0035302. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–9. doi: 10.1001/archgenpsychiatry.2010.2. . [DOI] [PubMed] [Google Scholar]
- 68.Kiecolt-Glaser JK. Stress, food, and inflammation: psychoneuro-immunology and nutrition at the cutting edge. Psychosom Med. 2010;72(4):365–9. doi: 10.1097/PSY.0b013e3181dbf489. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macon M, Sumis A, Yu W, Hilakivi-Clarke L. Abstract LB-316: social isolation alters abdominal and mammary adipocytes to potentially increase mammary tumorigenesis in mice. Cancer Res. 2016;76(14 Supplement):LB-316. [Google Scholar]
- 70.World Cancer Research Fund International/American Institute for Cancer Research. Cancer preventability estimates. Washington DC: AICR; 2017. [Google Scholar]
- 71.Grosso G, Bella F, Godos J, Sciacca S, Del Rio D, Ray S, et al. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev. 2017;75(6):405–19. doi: 10.1093/nutrit/nux012. . [DOI] [PubMed] [Google Scholar]
- 72.Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(3–4):277–89. doi: 10.1007/s10911-013-9303-7. . [DOI] [PubMed] [Google Scholar]
- 73.Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, et al. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76(1):266s–73s. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- 74.Rose DP, Vona-Davis L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr Relat Cancer. 2012;19(6):R225–41. doi: 10.1530/ERC-12-0203. . [DOI] [PubMed] [Google Scholar]
- 75.Mullie P, Koechlin A, Boniol M, Autier P, Boyle P. Relation between breast cancer and high glycemic index or glycemic load: a meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2016;56(1):152–9. doi: 10.1080/10408398.2012.718723. . [DOI] [PubMed] [Google Scholar]
- 76.Dong JY, Qin LQ. Dietary glycemic index, glycemic load, and risk of breast cancer: meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;126(2):287–94. doi: 10.1007/s10549-011-1343-3. . [DOI] [PubMed] [Google Scholar]
- 77.Sieri S, Agnoli C, Pala V, Grioni S, Brighenti F, Pellegrini N, et al. Dietary glycemic index, glycemic load, and cancer risk: results from the EPIC-Italy study. Sci Rep. 2017;7(1):9757. doi: 10.1038/s41598-017-09498-2. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Champ CE, Volek JS, Siglin J, Jin L, Simone NL. Weight gain, metabolic syndrome, and breast cancer recurrence: are dietary recommendations supported by the data? Int J Breast Cancer. 2012;2012:506868. doi: 10.1155/2012/506868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JAE, Stampfer MJ, et al. Red meat consumption and mortality: results from two prospective cohort studies. Arch Intern Med. 2012;172(7):555–63. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor EF, Burley VJ, Greenwood DC, Cade JE. Meat consumption and risk of breast cancer in the UK women’s cohort study. Br J Cancer. 2007;96(7):1139–46. doi: 10.1038/sj.bjc.6603689. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu Z, Deming SL, Fair AM, Shrubsole MJ, Wujcik DM, Shu X-O, et al. Well-done meat intake and meat-derived mutagen exposures in relation to breast cancer risk: the Nashville Breast Health Study. Breast Cancer Res Treat. 2011;129(3):919–28. doi: 10.1007/s10549-011-1538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inoue-Choi M, Sinha R, Gierach GL, Ward MH. Red and processed meat, nitrite, and heme iron intakes and postmenopausal breast cancer risk in the NIH-AARP diet and health study. Int J Cancer. 2016;138(7):1609–18. doi: 10.1002/ijc.29901. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee H-J, Wu K, Cox DG, Hunter D, Hankinson SE, Willett WC, Sinha R, Cho E. Polymorphisms in xenobiotic metabolizing genes, intakes of heterocyclic amines and red meat, and postmenopausal breast cancer. Nutr Cancer. 2013;65(8) doi: 10.1080/01635581.2013.824991. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sieri S, Chiodini P, Agnoli C, Pala V, Berrino F, Trichopoulou A, et al. Dietary fat intake and development of specific breast cancer subtypes. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju068. . [DOI] [PubMed] [Google Scholar]
- 85.Xia H, Ma S, Wang S, Sun G. Meta-analysis of saturated fatty acid intake and breast cancer risk. Medicine. 2015;94(52):e2391. doi: 10.1097/MD.0000000000002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Playdon MC, Ziegler RG, Sampson JN, Stolzenberg-Solomon R, Thompson HJ, Irwin ML, et al. Nutritional metabolomics and breast cancer risk in a prospective study. Am J Clin Nutr. 2017;106(2):637–49. doi: 10.3945/ajcn.116.150912. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol. 2009;5(6):335–44. doi: 10.1038/nrendo.2009.79. . [DOI] [PubMed] [Google Scholar]
- 88.Chajes V, Thiebaut AC, Rotival M, Gauthier E, Maillard V, Boutron-Ruault MC, et al. Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3N-EPIC study. Am J Epidemiol. 2008;167(11):1312–20. doi: 10.1093/aje/kwn069. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J, John EM, Horn-Ross PL, Ingles SA. Dietary fat, cooking fat, and breast cancer risk in a multiethnic population. Nutr Cancer. 2008;60(4):492–504. doi: 10.1080/01635580801956485. . [DOI] [PubMed] [Google Scholar]
- 90.Lachenmeier DW, Przybylski MC, Rehm J. Comparative risk assessment of carcinogens in alcoholic beverages using the margin of exposure approach. Int J Cancer. 2012;131(6):E995–1003. doi: 10.1002/ijc.27553. . [DOI] [PubMed] [Google Scholar]
- 91.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112(3):580–93. doi: 10.1038/bjc.2014.579. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romieu I, Scoccianti C, Chajes V, de Batlle J, Biessy C, Dossus L, et al. Alcohol intake and breast cancer in the European prospective investigation into cancer and nutrition. Int J Cancer. 2015;137(8):1921–30. doi: 10.1002/ijc.29469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lowry SJ, Kapphahn K, Chlebowski R, Li CI. Alcohol use and breast cancer survival among participants in the Women’s Health Initiative. Cancer Epidemiol Biomark Prev. 2016;25(8):1268–73. doi: 10.1158/1055-9965.EPI-16-0151. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lachenmeier DW, Rehm J. Moderate red wine drinking does not help cut women’s breast cancer risk. J Women’s Health. 2012;21(4):469–70. doi: 10.1089/jwh.2012.3503. [DOI] [PubMed] [Google Scholar]
- 95.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azrad M, Turgeon C, Demark-Wahnefried W. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Front Oncol. 2013;3:224. doi: 10.3389/fonc.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bagga D, Capone S, Wang HJ, Heber D, Lill M, Chap L, et al. Dietary modulation of omega-3/omega-6 polyunsaturated fatty acid ratios in patients with breast cancer. J Natl Cancer Inst. 1997;89(15):1123–31. doi: 10.1093/jnci/89.15.1123. . [DOI] [PubMed] [Google Scholar]
- 98.Fay MP, Freedman LS, Clifford CK, Midthune DN. Effect of different types and amounts of fat on the development of mammary tumors in rodents: a review. Cancer Res. 1997;57(18):3979–88. [PubMed] [Google Scholar]
- 99.Kiyabu GY, Inoue M, Saito E, Abe SK, Sawada N, Ishihara J, et al. Fish, n-3 polyunsaturated fatty acids and n-6 polyunsaturated fatty acids intake and breast cancer risk: the Japan Public Health Center-based prospective study. Int J Cancer. 2015;137(12):2915–26. doi: 10.1002/ijc.29672. . [DOI] [PubMed] [Google Scholar]
- 100.Abdelmagid SA, MacKinnon JL, Janssen SM, Ma DW. Role of n-3 polyunsaturated fatty acids and exercise in breast cancer prevention: identifying common targets. Nutr Metab Insights. 2016;9:71–84. doi: 10.4137/NMI.S39043. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68(2):140–7. doi: 10.1016/j.biopsych.2010.03.018. . [DOI] [PubMed] [Google Scholar]
- 102.Michaeli B, Berger MM, Revelly JP, Tappy L, Chiolero R. Effects of fish oil on the neuro-endocrine responses to an endotoxin challenge in healthy volunteers. Clin Nutr. 2007;26(1):70–7. doi: 10.1016/j.clnu.2006.06.001. . [DOI] [PubMed] [Google Scholar]
- 103.Ju-Sheng Zheng, Hu Xiao-Jie, Zhao Yi-Min, Yang Jing, Li Duo. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346 doi: 10.1136/bmj.f3706. [DOI] [PubMed] [Google Scholar]
- 104.Zhihui W, Weihua Y, Zupei W, Jinlin H. Fish consumption and risk of breast cancer: meta-analysis of 27 observational studies. Nutr Hosp. 2016;33(3):282. doi: 10.20960/nh.282. . [DOI] [PubMed] [Google Scholar]
- 105.Chen G-C, Yang J, Eggersdorfer M, Zhang W, Qin L-Q. N-3 long-chain polyunsaturated fatty acids and risk of all-cause mortality among general populations: a meta-analysis. Sci Rep. 2016;6:28165. doi: 10.1038/srep28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, et al. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav Immun. 2013;28:16–24. doi: 10.1016/j.bbi.2012.09.004. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hirko KA, Chai B, Spiegelman D, Campos H, Farvid MS, Hankinson SE, et al. Erythrocyte membrane fatty acids and breast cancer risk: a prospective analysis in the Nurses’ Health Study II. Int J Cancer. 2017 doi: 10.1002/ijc.31133. . [DOI] [PMC free article] [PubMed]
- 108.Khankari NK, Bradshaw PT, Steck SE, He K, Olshan AF, Shen J, et al. Polyunsaturated fatty acid interactions and breast cancer incidence: a population-based case-control study on Long Island, New York. Ann Epidemiol. 2015;25(12):929–35. doi: 10.1016/j.annepidem.2015.09.003. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24(9):929–39. doi: 10.1016/j.numecd.2014.03.003. . [DOI] [PubMed] [Google Scholar]
- 110.He J, Gu Y, Zhang S. Consumption of vegetables and fruits and breast cancer survival: a systematic review and meta-analysis. Sci Rep. 2017;7(1):599. doi: 10.1038/s41598-017-00635-5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–56. doi: 10.1093/ije/dyw319. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Narita S, Inoue M, Saito E, Abe SK, Sawada N, Ishihara J, et al. Dietary fiber intake and risk of breast cancer defined by estrogen and progesterone receptor status: the Japan Public Health Center-based prospective study. Cancer Causes Control. 2017;28(6):569–78. doi: 10.1007/s10552-017-0881-3. . [DOI] [PubMed] [Google Scholar]
- 113.Anderson C, Harrigan M, George SM, Ferrucci LM, Sanft T, Irwin ML, et al. Changes in diet quality in a randomized weight loss trial in breast cancer survivors: the lifestyle, exercise, and nutrition (LEAN) study. NPJ Breast Cancer. 2016;2(1):16026. doi: 10.1038/npjbcancer.2016.26. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang CX, Ho SC, Chen YM, Fu JH, Cheng SZ, Lin FY. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int J Cancer. 2009;125(1):181–8. doi: 10.1002/ijc.24358. . [DOI] [PubMed] [Google Scholar]
- 115.Griffiths K, Aggarwal BB, Singh RB, Buttar HS, Wilson D, De Meester F. Food antioxidants and their anti-inflammatory properties: a potential role in cardiovascular diseases and cancer prevention. Diseases. 2016;4(3) doi: 10.3390/diseases4030028. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bakker MF, Peeters PH, Klaasen VM, Bueno-de-Mesquita HB, Jansen EH, Ros MM, et al. Plasma carotenoids, vitamin C, tocoph-erols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2016;103(2):454–64. doi: 10.3945/ajcn.114.101659. [DOI] [PubMed] [Google Scholar]
- 117.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. 2012;104(24):1905–16. doi: 10.1093/jnci/djs461. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. . [DOI] [PubMed] [Google Scholar]
- 119.Egnell M, Fassier P, Lecuyer L, Zelek L, Vasson MP, Hercberg S, Latino-Martel P, Galan P, Deschasaux M, Touvier M. B-vitamin intake from diet and supplements and breast cancer risk in middle-aged women: results from the prospective NutriNet-Sante cohort. Nutrients. 2017;9(5) doi: 10.3390/nu9050488. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shao T, Klein P, Grossbard ML. Vitamin D and breast cancer. Oncologist. 2012;17(1):36–45. doi: 10.1634/theoncologist.2011-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hines SL, Jorn HK, Thompson KM, Larson JM. Breast cancer survivors and vitamin D: a review. Nutrition. 2010;26(3):255–62. doi: 10.1016/j.nut.2009.08.020. . [DOI] [PubMed] [Google Scholar]
- 122.Hardman WE. Walnuts have potential for cancer prevention and treatment in mice. J Nutr. 2014;144(4):555S–60S. doi: 10.3945/jn.113.188466. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gonzalez CA, Salas-Salvado J. The potential of nuts in the prevention of cancer. Br J Nutr. 2006;96(Suppl 2):S87–94. doi: 10.1017/BJN20061868. . [DOI] [PubMed] [Google Scholar]
- 124.Chlebowski RT, Aragaki AK, Anderson GL, Thomson CA, Manson JE, Simon MS, et al. Low-fat dietary pattern and breast cancer mortality in the Women’s Health Initiative randomized controlled trial. J Clin Oncol. 2017;35(25):2919–26. doi: 10.1200/JCO.2016.72.0326. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. . [DOI] [PubMed] [Google Scholar]
- 126.Zuniga KE, Mackenzie MJ, Roberts SA, Raine LB, Hillman CH, Kramer AF, et al. Relationship between fruit and vegetable intake and interference control in breast cancer survivors. Eur J Nutr. 2016;55(4):1555–62. doi: 10.1007/s00394-015-0973-3. . [DOI] [PubMed] [Google Scholar]
- 127.Zang J, Shen M, Du S, Chen T, Zou S. The association between dairy intake and breast cancer in western and Asian populations: a systematic review and meta-analysis. J Breast Cancer. 2015;18(4):313–22. doi: 10.4048/jbc.2015.18.4.313. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoffmann G, Schwingshackl L. Mediterranean diet supplemented with extra virgin olive oil reduces the incidence of invasive breast cancer in a randomised controlled trial. Evid Based Med. 2016;21(2):72. doi: 10.1136/ebmed-2015-110366. . [DOI] [PubMed] [Google Scholar]
- 129.Mourouti N, Panagiotakos DB. The beneficial effect of a Mediterranean diet supplemented with extra virgin olive oil in the primary prevention of breast cancer among women at high cardiovascular risk in the PREDIMED trial. Evid Based Nurs. 2016;19(3):71. doi: 10.1136/ebnurs-2016-102303. . [DOI] [PubMed] [Google Scholar]
- 130.Skouroliakou M, Grosomanidis D, Massara P, Kostara C, Papandreou P, Ntountaniotis D, et al. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized Mediterranean dietary intervention study. Eur J Nutr. 2017 doi: 10.1007/s00394-017-1489-9. . [DOI] [PubMed]
- 131.Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Johnson KC, et al. Patterns of change over time and history of the inflammatory potential of diet and risk of breast cancer among postmenopausal women. Breast Cancer Res Treat. 2016;159(1):139–49. doi: 10.1007/s10549-016-3925-6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shivappa N, Sandin S, Lof M, Hebert JR, Adami HO, Weiderpass E. Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br J Cancer. 2015;113(7):1099–103. doi: 10.1038/bjc.2015.304. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–600. doi: 10.1016/S1470-2045(15. . [DOI] [PubMed] [Google Scholar]
- 134.Kotepui M. Diet and risk of breast cancer. Contemp Oncol. 2016;20(1):13–9. doi: 10.5114/wo.2014.40560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cowen S, McLaughlin SL, Hobbs G, Coad J, Martin KH, Olfert IM, et al. High-fat, high-calorie diet enhances mammary carcinogenesis and local inflammation in MMTV-PyMT mouse model of breast cancer. Cancers (Basel) 2015;7(3):1125–42. doi: 10.3390/cancers7030828. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80(4):1029–35. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 137.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. doi: 10.1017/S1368980013002115. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Caan B, et al. Association between dietary inflammatory potential and breast cancer incidence and death: results from the Women’s Health Initiative. Br J Cancer. 2016;114(11):1277–85. doi: 10.1038/bjc.2016.98. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Milner JA. Molecular targets for bioactive food components. J Nutr. 2004;134(9):2492s–8s. doi: 10.1093/jn/134.9.2492S. [DOI] [PubMed] [Google Scholar]
- 140.Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA, et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009;101(Suppl 1):S1–45. doi: 10.1017/S0007114509377867. [DOI] [PubMed] [Google Scholar]
- 141.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi: 10.1016/j.clnu.2016.07.015. . [DOI] [PubMed] [Google Scholar]
- 142.Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. doi: 10.1016/j.clnu.2016.09.004. . [DOI] [PubMed] [Google Scholar]
- 143.Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–96. doi: 10.1016/j.clnu.2017.06.017. . [DOI] [PubMed] [Google Scholar]
- 144.Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50(2):167–78. doi: 10.3109/0284186X.2010.529822. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7(4):17–29. doi: 10.4251/wjgo.v7.i4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blackburn GL, Wang KA. Dietary fat reduction and breast cancer outcome: results from the Women’s Intervention Nutrition Study (WINS) Am J Clin Nutr. 2007;86(3):s878–81. doi: 10.1093/ajcn/86.3.878S. [DOI] [PubMed] [Google Scholar]
- 147.Parry BM, Milne JM, Yadegarfar G, Rainsbury RM. Dramatic dietary fat reduction is feasible for breast cancer patients: results of the randomised study, WINS (UK)—stage 1. Eur J Surg Oncol. 2011;37(10):848–55. doi: 10.1016/j.ejso.2011.07.010. . [DOI] [PubMed] [Google Scholar]
- 148.Halfdanarson TR, Thordardottir E, West CP, Jatoi A. Does dietary counseling improve quality of life in cancer patients? A systematic review and meta-analysis. J Support Oncol. 2008;6(5):234–7. [PubMed] [Google Scholar]
- 149.Colomer R, Moreno-Nogueira JM, Garcia-Luna PP, Garcia-Peris P, Garcia-de-Lorenzo A, Zarazaga A, et al. N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr. 2007;97(5):823–31. doi: 10.1017/S000711450765795X. . [DOI] [PubMed] [Google Scholar]
- 150.Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, Meeske K, et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J Clin Oncol. 2012;30(12):1280–7. doi: 10.1200/JCO.2011.36.4109. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shapira N. The potential contribution of dietary factors to breast cancer prevention. Eur J Cancer Prev. 2017;26(5):385–95. doi: 10.1097/CEJ.0000000000000406. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.George SM, Ballard-Barbash R, Shikany JM, Caan BJ, Freudenheim JL, Kroenke CH, et al. Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the Women’s Health Initiative. Cancer Epidemiol Biomark Prev. 2014;23(4):575–83. doi: 10.1158/1055-9965.EPI-13-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vrieling A, Buck K, Seibold P, Heinz J, Obi N, Flesch-Janys D, et al. Dietary patterns and survival in German postmenopausal breast cancer survivors. Br J Cancer. 2013;108(1):188–92. doi: 10.1038/bjc.2012.521. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kwan ML, Weltzien E, Kushi LH, Castillo A, Slattery ML, Caan BJ. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J Clin Oncol. 2009;27(6):919–26. doi: 10.1200/JCO.2008.19.4035. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Finocchiaro C, Ossola M, Monge T, Fadda M, Brossa L, Caudera V, et al. Effect of specific educational program on dietary change and weight loss in breast-cancer survivors. Clin Nutr. 2016;35(4):864–70. doi: 10.1016/j.clnu.2015.05.018. . [DOI] [PubMed] [Google Scholar]
- 156.Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Adolescent meat intake and breast cancer risk. Int J Cancer. 2015;136(8):1909–20. doi: 10.1002/ijc.29218. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bertrand KA, Burian RA, Eliassen AH, Willett WC, Tamimi RM. Adolescent intake of animal fat and red meat in relation to pre-menopausal mammographic density. Breast Cancer Res Treat. 2016;155(2):385–93. doi: 10.1007/s10549-016-3679-1. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yaghjyan L, Ghita GL, Rosner B, Farvid M, Bertrand KA, Tamimi RM. Adolescent fiber intake and mammographic breast density in premenopausal women. Breast Cancer Res. 2016;18(1):85. doi: 10.1186/s13058-016-0747-8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Harris HR, Willett WC, Vaidya RL, Michels KB. An adolescent and early adulthood dietary pattern associated with inflammation and the incidence of breast cancer. Cancer Res. 2017;77(5):1179–87. doi: 10.1158/0008-5472.CAN-16-2273. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arikawa AY, Kaufman BC, Raatz SK, Kurzer MS. Effects of a parallel-arm randomized controlled weight loss pilot study on biological and psychosocial parameters of overweight and obese breast cancer survivors. Pilot Feasibility Stud. 2017;4:17. doi: 10.1186/s40814-017-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88(3):674–84. doi: 10.1002/(SICI)1097-0142(20000201)88:3<674::AID-CNCR26>3.0.CO;2-R. . [DOI] [PubMed] [Google Scholar]
- 162.Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: results from a national survey. Cancer. 2015;121(23):4212–21. doi: 10.1002/cncr.29488. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Klassen AC, Smith KC, Shuster M, Coa KI, Caulfield LE, Helzlsouer KJ, Peairs KS, Shockney LD, Stoney D, Hannum S. We’re just not prepared for eating over our whole life”: a mixed methods approach to understanding dietary behaviors among longer term cancer survivors. Integr Cancer Ther. 2017:1534735417731515. doi: 10.1177/1534735417731515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Braakhuis A, Campion P, Bishop K. The effects of dietary nutrition education on weight and health biomarkers in breast cancer survivors. Med Sci (Basel) 2017;5(2) doi: 10.3390/medsci5020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Christifano DN, Fazzino TL, Sullivan DK, Befort CA. Diet quality of breast cancer survivors after a six-month weight management intervention: improvements and association with weight loss. Nutr Cancer. 2016;68(8):1301–8. doi: 10.1080/01635581.2016.1224368. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Delgado-Cruzata L, Zhang W, McDonald JA, Tsai WY, Valdovinos C, Falci L, et al. Dietary modifications, weight loss, and changes in metabolic markers affect global DNA methylation in Hispanic, African American, and Afro-Caribbean breast cancer survivors. J Nutr. 2015;145(4):783–90. doi: 10.3945/jn.114.202853. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating bio-markers in women treated for breast cancer: the lifestyle, exercise, and nutrition (LEAN) study. J Clin Oncol. 2016;34(7):669–76. doi: 10.1200/JCO.2015.61.6375. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hamer J, Warner E. Lifestyle modifications for patients with breast cancer to improve prognosis and optimize overall health. CMAJ. 2017;189(7):E268–e274. doi: 10.1503/cmaj.160464. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. doi: 10.3322/caac.20140. . [DOI] [PubMed] [Google Scholar]
- 170.Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30(19):2314–26. doi: 10.1200/JCO.2011.37.9792. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jackson SE, Heinrich M, Beeken RJ, Wardle J. Weight loss and mortality in overweight and obese cancer survivors: a systematic review. PLoS One. 2017;12(1):e0169173. doi: 10.1371/journal.pone.0169173. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.WCRF. World Cancer Fund Research International. Continuous update project (Cup) 2017 [Google Scholar]
- 173.Norat T, Scoccianti C, Boutron-Ruault MC, Anderson A, Berrino F, Cecchini M, et al. European code against cancer 4th edition: diet and cancer. Cancer Epidemiol. 2015;39(Suppl 1):S56–66. doi: 10.1016/j.canep.2014.12.016. . [DOI] [PubMed] [Google Scholar]
- 174.Cottet V, Touvier M, Fournier A, Touillaud MS, Lafay L, Clavel-Chapelon F, et al. Postmenopausal breast cancer risk and dietary patterns in the E3N-EPIC prospective cohort study. Am J Epidemiol. 2009;170(10):1257–67. doi: 10.1093/aje/kwp257. . [DOI] [PubMed] [Google Scholar]
- 175.National Comprehensive Cancer Network (NCCN) Breast Cancer. NCCN; 2017. NCCN clinical practice guidelines in oncology. [Google Scholar]


