Abstract
We hypothesize that noninvasive photoacoustic imaging can accurately measure cerebral venous oxyhemoglobin saturation (So2) in a neonatal model of hypoxia-ischemia. In neonatal piglets, which have a skull thickness comparable to that of human neonates, we compared the photoacoustic measurement of sagittal sinus So2 against that measured directly by blood sampling over a wide range of conditions. Systemic hypoxia was produced by decreasing inspired oxygen stepwise (i.e., 100, 21, 19, 17, 15, 14, 13, 12, 11, and 10%) with and without unilateral or bilateral ligation of the common carotid arteries to enhance hypoxia-ischemia. Transcranial photoacoustic sensing enabled us to detect changes in sagittal sinus O2 saturation throughout the tested range of 5–80% without physiologically relevant bias. Despite lower cortical perfusion and higher oxygen extraction in groups with carotid occlusion at equivalent inspired oxygen, photoacoustic measurements successfully provided a robust linear correlation that approached the line of identity with direct blood sample measurements. Receiver-operating characteristic analysis for discriminating So2 <30% showed an area under the curve of 0.84 for the pooled group data, and 0.87, 0.91, and 0.92 for hypoxia alone, hypoxia plus unilateral occlusion, and hypoxia plus bilateral occlusion subgroups, respectively. The detection precision in this critical range was confirmed with sensitivity (87.0%), specificity (86.5%), accuracy (86.8%), positive predictive value (90.5%), and negative predictive value (81.8%) in the combined dataset. These results validate the capability of photoacoustic sensing technology to accurately monitor sagittal sinus So2 noninvasively over a wide range and support its use for early detection of neonatal hypoxia-ischemia.
NEW & NOTEWORTHY We present data to validate the noninvasive photoacoustic measurement of sagittal sinus oxyhemoglobin saturation. In particular, this paper demonstrates the robustness of this methodology during a wide range of hemodynamic and physiological changes induced by the stepwise decrease of fractional inspired oxygen to produce hypoxia and by unilateral and bilateral ligation of the common carotid arteries preceding hypoxia to produce hypoxia-ischemia. This technique may be useful for diagnosing risk of neonatal hypoxic-ischemic encephalopathy.
Keywords: hypoxic-ischemic encephalopathy, neonate, oxygen saturation, photoacoustics, sagittal sinus
INTRODUCTION
Perinatal hypoxic-ischemic encephalopathy (HIE) remains a significant cause of developmental brain injury despite advances in obstetric and neonatal medicine (10). Magnetic resonance imaging is the definitive diagnostic tool to detect HIE in the neonate, but it is generally not performed until 7–10 days after birth (12). Several biophotonic modalities have been proposed to allow cost-effective and continuous monitoring of the perinatal brain; near-infrared (NIR) spectroscopy (NIRS) and diffused optical tomography have shown the capability to noninvasively monitor the cerebral oxygenation level and hemodynamic changes within the neonatal brain in real time. However, both modalities have spatial resolution at the centimeter scale, which makes their measurements vulnerable to interferential off-axis blood contents (11, 23). The accuracy of the commonly used NIRS technique for assessing tissue oxygenation is also limited by the admixture of the arterialized blood compartment, which varies with the state of vasodilation. It would be desirable to have an effective diagnostic tool with greater specificity for cerebral venous oxyhemoglobin saturation (So2), without the arterial admixture, that could detect brain hypoxia during labor and postnatally and thereby inform rapid decisions during labor and management of neonates in the intensive care unit.
Photoacoustic measurements combine the benefits of ultrasound and optical modalities. When a laser pulse is transmitted to tissue, it penetrates deeply according to the distribution of the scattering coefficient and is absorbed by localized chromophores with unique absorption coefficients. The conversion of absorbed light energy to instantaneous heat generates thermal elastic expansion, which leads to the generation of pressure waves that are detectable by an ultrasound transducer (24). Because of its hybrid nature, photoacoustic monitoring combines highly attractive features attributed to both light and sound, including rich contrast and high versatility, in sensing diverse biological targets, excellent spatial resolution not compromised by light scattering, and relatively low cost of implementation. Multispectral measurements may be used to extract quantified information on the distribution of tissue chromophores based on their optical absorption spectrum. Hemoglobin is known to be the most prominent endogenous absorber in the tissue-penetrable NIR range, and multispectral photoacoustic sensing in the NIR range can measure the oxy- and deoxyhemoglobin content by differentiating its multispectral absorbance. NIR light can penetrate the pediatric human skull effectively. Moreover, the human newborn has open fontanelles that permit direct monitoring over the superior sagittal sinus (SSS). Previous studies have shown that photoacoustic monitoring can accurately measure cerebral blood oxygenation at the ovine SSS, in low-birth-weight infants, and in adults with traumatic brain injury (4, 16, 17, 17a), although the amount of validation data were limited. Here, we performed a systematic analysis of the relationship between transcranial photoacoustic-derived So2 in the SSS and that measured directly in SSS blood samples over a wide range of So2. Validation was carried out in neonatal piglets, which have a skull thickness and brain development comparable to those of term human neonates (2, 3). Our hypothesis is that photoacoustics technology can accurately measure SSS So2 and can be used to discriminate severe cerebral venous hypoxia in neonatal piglets subjected to graded systemic hypoxia and graded hypoxia with carotid artery occlusion which has been used as a model of neonatal hypoxia-ischemia (1, 20).
MATERIALS AND METHODS
Animal preparation.
All procedures on piglets received prior approval by the Johns Hopkins University Animal Care and Use Committee and followed the National Institutes of Health guidelines for animal experimentation. Male and female piglets (3–7 days old, 1.5–2.2 kg) were initially anesthetized with isoflurane by nose cone. An endotracheal tube was secured in the trachea via a tracheostomy, and the lungs were mechanically ventilated with end-tidal CO2 monitoring. Note that we did not make any attempt to study equal numbers of male and female piglets because we are not aware of any plausible biological reason for differences between the directly measured and the photoacoustic estimates in SSS So2 based on sex. Of the 15 piglets in this experiment, only 1, which was from the global hypoxia-ischemia (HYP) group, was female. Catheters were inserted in a femoral artery to monitor arterial blood pressure and blood gases and in a femoral vein for administration of anesthetics, saline, and dextrose. Normothermia was maintained with a warming blanket and heat lamp. A catheter inserted in the SSS was used to obtain samples of cortical venous blood. The skull was thinned over an anterior-posterior length of 5 mm and a width of 3 mm over the midline just rostral to the bregma. The catheter was inserted 1 cm in the posterior direction in the SSS (7, 21). In piglets in the unilateral (UCAO) and bilateral (BCAO) carotid artery occlusion groups, a ligature was placed around one or both common carotid arteries for later occlusion.
Cortical perfusion was monitored with a laser Doppler flow (LDF) probe. A 1-mm drill hole was made over the frontal cortex, the dura was punctured, and a 1-mm fiberoptic LDF probe was placed on the cortical surface. In the case of UCAO, the probe was ipsilateral to the carotid occlusion. The LDF recording provides a measure of the percent change in microcirculatory red cell flux in the cerebral cortex within about a 1-mm3 volume. A total of nine piglets were used for LDF measurements with equal numbers (i.e., n = 3) for each of the three groups, i.e., HYP, UCAO, and BCAO.
A solution of 5% dextrose and 0.45% saline was infused at a maintenance rate of 4 ml·h−1·kg−1. After the surgery was complete, piglets received intravenous loading doses of 50 µg/kg fentanyl and 10 mg/kg propofol. The isoflurane was discontinued, and anesthesia was maintained with 50 µg·kg−1·h−1 fentanyl and 5 mg·kg−1·h−1 propofol infusions. Piglets that were severely anemic (Hb <6 g/dl) or hypotensive [mean arterial pressure (MAP) <40 mmHg] at baseline were excluded.
Photoacoustic sensing configuration.
For photoacoustic recording, the clinical ultrasound linear array transducer was connected to a research package (SonixDAQ; Ultrasonix Medical). Photoacoustic signals were induced by pulsed laser light that was generated by a second-harmonic (532-nm) Nd:YAG laser pumping an optical parametric oscillator (OPO) system (Phocus Inline; Opotek). The tunable range was 690–900 nm, and the maximum pulse repetition frequency was 20 Hz. Light was delivered in the photoacoustic probe through bifurcated fiber optic bundles, each with a 40-mm-long and 0.88-mm-wide aperture. The ultrasound transducer was placed between the bifurcated fiber optic bundle outlets, which have a focus at a depth of 20 mm from the transducer. The photoacoustic probe was located on the center of the piglet's head along the coronal plane to monitor the photoacoustic signal changes originating from the SSS. We collected the photoacoustic signals from 700 to 900 nm at 10-nm intervals, producing a photoacoustic spectrum with 21 wavelengths. The acquisition time per wavelength was ~5 s, including 3.2 s for 64 excitations at 20 Hz for frame averaging and ~1.8 s for OPO transition to the next wavelength. This led to ~105 s of total acquisition time at each wavelength in the photoacoustic spectrum being required for a single SSS So2 estimation at each fractional inspired O2 ( ) level. The distance between the photoacoustic probe and the piglet's head was maintained at 20 mm with transparent acoustic gel coupling for uniform delivery of pulsed laser, which is essential to localize the SSS region with the regulated absorbance profile in the lateral direction as shown in Fig. 1. The energy density on the skin surface was 3.5 mJ/cm2, which is far below the maximum permissible exposure of 20 mJ/cm2, based on the ANSI Z136.1 standard, Safe Use of Lasers (19). In addition, 64 subsequent frames were averaged at each data acquisition sequence to mitigate the fluctuation of Nd:YAG laser pulsing and improve the signal-to-noise ratio. Figure 1 presents the experimental configuration and a representative photoacoustic cross-sectional image of a piglet's head in a coronal plane at 790 nm. The SSS was ~5 mm below the surface of the skin. Branches of superior cerebral veins also were evident on the brain surface. We localized our region of interest (ROI) for the photoacoustic image of the SSS, indicated by the 2 × 2 mm2 white box at each wavelength, to comprise the photoacoustic spectrum. The head of the piglet was fixed with customized stereotaxic equipment so that no significant disorientation of the photoacoustic image was observed.
Fig. 1.
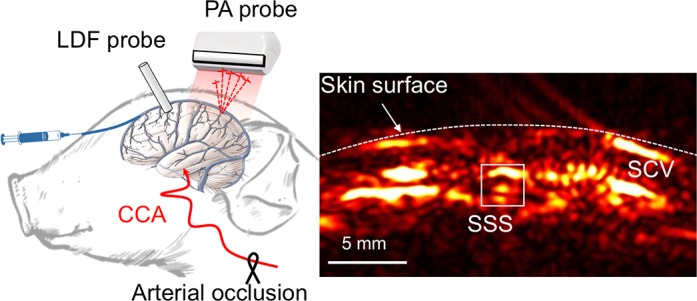
Photoacoustic sensing in a neonatal piglet model. A: piglet model preparation and representative cross-sectional photoacoustic (PA) image at 790 nm. B: schematic of protocol timeline [not all step changes in fractional inspired O2 () are shown]. CCA, common carotid artery; SSS, superior sagittal sinus; SCV, superior cerebral veins; LDF, laser Doppler flowmetry.
Photoacoustic estimation of cerebral So2.
The spectral photoacoustic transmittances across two postmortem piglet head samples were measured at each 10-nm increment across the 700- to 900-nm spectra. The photoacoustic intensity was measured from India ink-filled tubing in a water tank with and without the head samples, and their intensity ratio (transmittance) was calculated. As expected, the shorter wavelength introduced additional photoacoustic attenuation because of more light scattering and absorbance. When compared with the photoacoustic transmittance averaged across a longer wavelength range (850–900 nm), 700 nm wavelength yielded −9.06 ± 10.95% less transmittance, whereas a longer wavelength range did not present any significant spectral attenuation (1.51 ± 2.49% at 800 nm, 0.73 ± 1.73% at 850 nm, and 0.30 ± 5.52% at 900 nm). The wavelength range between these wavelengths has presented the gradual change. The inverse value of the mean fractional attenuation ratio was multiplied by the raw photoacoustic signal for calibration. The photoacoustic-derived So2 at the SSS was estimated by using least-mean-square error estimation between adjusted multispectral photoacoustic data and the known spectrophotometric absorbance of hemoglobin as a reference for differing oxygen concentrations. The photoacoustic spectrum was first obtained by averaging pixel intensity in the ROI indicated in Fig. 1. The photoacoustic spectrum was subtracted from the reference spectrum at varying So2 ranging from 0% to 100% at 0.1% intervals, which were extrapolated by using the spectrophotometric measurement at 0% and 100% (18). From the mean squares errors, the So2 testing level giving the least error was selected as the estimated So2 value. We used the following equation:
| (1) |
where and are the spectrums derived by photoacoustic sensing and spectrophotometry on Hb, respectively; λ is the wavelength between 700 and 900 nm in 10-nm intervals; and Nλ is the number of wavelengths used, i.e., 21. Note that each spectrum was normalized by the photoacoustic intensity at 750 nm, which was chosen based on a pilot study.
Experimental protocol.
Figure 1 shows the experimental setup and the quality of the signal being measured. The following three groups were studied: global hypoxia alone (HYP), UCAO followed by global hypoxia, and BCAO followed by global hypoxia (n = 5/group for a total of 15 piglets). First, was increased from the 0.3 level used during surgery to 1.0. After a 10-min equilibration period, a baseline photoacoustic image was acquired at each of the 21 wavelengths over the ensuing 2 min, after which arterial and SSS blood samples were drawn for measurement of pH, Po2, Pco2, Hb concentration, and So2 (ABL Flex 825; Radiometer, Copenhagen, Denmark). MAP was also measured. We then sequentially decreased the from 1.0 to 0.20, 0.18, 0.16, 0.15, 0.14, 0.13, 0.12, 0.11, 0.10, and 0.08. After each incremental change in , a 5-min stabilization period was performed before the next photoacoustic spectrum scanning commenced. Arterial and SSS blood samples were drawn at the end of each acquisition period. Therefore, the measurements at each level took ~8 min (i.e., 5 min stabilization + ~2 min photoacoustic spectrum scanning + 1 min of arterial and SSS blood sampling). In some experiments, data were not obtained at the two lowest levels because of low MAP or inability to draw an SSS sample. At each step, the cerebral O2 extraction was calculated from the difference between the arterial and SSS O2 content.
Statistical analysis.
Data are presented as means ± SD. Cerebral O2 extraction data were analyzed by repeated-measures analysis of variance (ANOVA), and post hoc comparisons between groups were made with Tukey’s test. Other physiological data were placed in the following three bins based on the level of SSS So2: <15%, 15–30%, and >30%. Comparisons of physiological data among the three binned datasets were done by one-way ANOVA and Tukey’s post hoc comparisons for each of the three groups of piglets. Relationships between the So2 estimated by photoacoustic sensing and the So2 measured directly in SSS were examined by linear regression analysis. Data pooled from the 5 piglets in each group and from the 15 piglets in the entire study were examined by linear regression analysis.
Detection precision of critical SSS So2.
Clinical observations with fetal pulse oximetry indicate that arterial So2 in labor ranges from 35 to 65% and that acidosis (scalp blood pH <7.2) develops when the arterial So2 drops below 30% for >10 min (2, 3, 8, 9, 22). An arterial So2 of 30% would correspond to an SSS So2 of ~20% assuming an O2 extraction fraction of 0.33 (5). However, cerebral vasodilation and cerebral O2 uptake become limited at this severe level of hypoxia, thereby rendering variability in the critical level of SSS So2 for the transition to an ischemic state. Here, we performed a sensitivity and specificity analysis of photoacoustic-derived SSS So2 to detect the directly measured SSS So2 below 30%, since 30% probably represents an upper limit for the cerebral ischemic threshold that results in a decrease in O2 uptake. In this evaluation, true and false positive (TP and FP) were defined as the number of cases correctly and incorrectly identified with photoacoustic sensing when the measured SSS So2 fell below 30%. True and false negatives (TN and FN) were defined oppositely: the number of correct and incorrect cases identified, respectively, when the SSS So2 level was above 30%. Expected clinical outcomes were calculated based on the values of sensitivity: TP/(TP + FN), specificity: TN/(TN + FP), accuracy: (TP + TN)/(TP + FP + FN + TN), positive predictive value (PPV): TP/(TP + FP), and negative predictive value (NPV): TN/(TN + FN).
RESULTS
Physiological parameters.
Table 1 shows the physiological parameters during hypoxia as functions of SSS So2 measured directly from blood samples. The arterial pH measurements revealed little change until SSS So2 fell below 15%. Arterial Pco2 was in the range of 40–50 mmHg during hypoxia, although two piglets in the HYP group had arterial Pco2 values >50 mmHg during hypoxia. Arterial Hb concentration and heart rate were stable in most piglets during hypoxia. As expected, MAP was elevated during normoxia in UCAO and BCAO groups, presumably as a result of baroreceptor unloading. These groups exhibited a decrease in MAP at SSS So2 <15%, whereas MAP in the HYP group did not change significantly with changes in SSS So2.
Table 1.
Physiological parameters
| Measured Oxygen Saturation at SSS |
||||
|---|---|---|---|---|
| Physiological Parameters | Group | 30–100% | 15–30% | 0–15% |
| Arterial pH | HYP | 7.28 ± 0.11 | 7.27 ± 0.8 | 7.18 ± 0.15 |
| UCAO | 7.37 ± 0.07 | 7.27 ± 0.2 | 7.22 ± 0.13 | |
| BCAO | 7.34 ± 0.11 | 7.38 ± 0.1 | 7.22 ± 0.08 | |
| Arterial Pco2, mmHg | HYP | 50.5 ± 8.4 | 49.8 ± 13.1 | 53.4 ± 14.6 |
| UCAO | 44.5 ± 5.8 | 43.5 ± 6.5 | 39.8 ± 6.9 | |
| BCAO | 47.0 ± 5.1 | 48.4 ± 3.9 | 45.9 ± 6.7 | |
| Hb concentration, g/dl | HYP | 7.6 ± 1.7 | 8.0 ± 1.2 | 7.6 ± 0.9 |
| UCAO | 8.4 ± 1.1 | 7.7 ± 1.5 | 8.7 ± 1.1 | |
| BCAO | 7.9 ± 1.2 | 8.1 ± 1.2 | 7.8 ± 0.8 | |
| MAP, mmHg | HYP | 74.4 ± 10.2 | 68.5 ± 7.6 | 67.1 ± 4.2 |
| UCAO | 86.0 ± 10.1 | 75.6 ± 13.6 | 60.1 ± 23.0 | |
| BCAO | 94.7 ± 19.6 | 94.0 ± 15.0 | 65.3 ± 27.4 | |
Values are means ± SD. SSS, superior sagittal sinus. Shown are arterial pH, arterial Pco2, arterial hemoglobin concentration, and mean arterial pressure (MAP) as functions of superior sagittal sinus oxygen saturation in groups with hypoxia alone (HYP), unilateral carotid artery occlusion (UCAO) followed by hypoxia, and bilateral carotid artery occlusion (BCAO) followed by hypoxia.
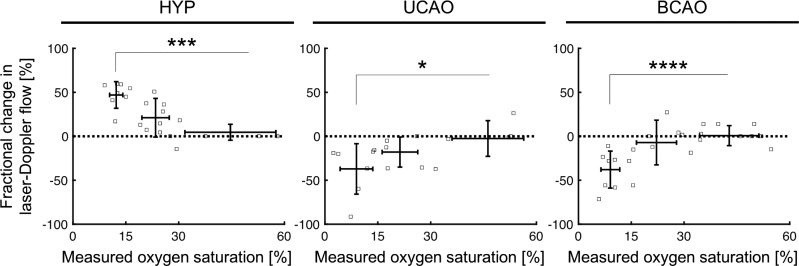
Because of technical difficulties with the LDF measurements in some piglets, LDF data are reported from three of the five piglets in each group. LDF showed progressive increases in cortical perfusion in the HYP group as SSS So2 decreased, whereas perfusion ipsilateral to the UCAO failed to increase in the 15–30% SSS So2 range and significantly decreased at SSS So2 <15% (Fig. 2). The relative changes in perfusion in the BCAO group were similar to those in the UCAO group. The HYP group showed a significantly greater increase in LDF than in the BCAO group at 15–30% SSS So2 (P = 0.0237), and was clearly differentiated from the UCAO and BCAO groups at 0–15% of SSS So2 (P = 0.0001). In accordance with the limited LDF response, O2 extraction was greater in the UCAO (P = 0.02) and BCAO (P = 0.0007) groups compared with the HYP group (ANOVA and Tukey post hoc test). For example, at an of 0.21 O2 extraction was increased from 4.20 ± 0.46 ml O2/dl in the HYP group to 6.24 ± 0.62 ml O2/dl in the UCAO group and to 6.60 ± 0.61 ml O2/dl in the BCAO group, and it remained greater at an of 0.12 in the UCAO (3.30 ± 0.81 ml O2/dl) and BCAO (4.30 ± 0.76 ml O2/dl) groups compared with the HYP group (1.93 ± 0.51 ml O2/dl).
Fig. 2.
Percent change in laser Doppler flow as a function of the measured sagittal sinus O2 saturation in the hypoxia-only group (HYP), hypoxia plus unilateral carotid artery occlusion group (UCAO), and hypoxia plus bilateral carotid artery occlusion group (BCAO). *P < 0.05, ***P ≤ 0.001, and ****P ≤ 0.0001 from 30 to 100% oxygen saturation by unpaired t-test. HYP group was statistically significantly different from the UCAO and BCAO groups in the range of 15–30% measured O2 saturation (P = 0.0036 and 0.0237, respectively) and also in the range of 0–15% O2 saturation (P = 0.0001).
Correlation of photoacoustic-estimated and measured So2.
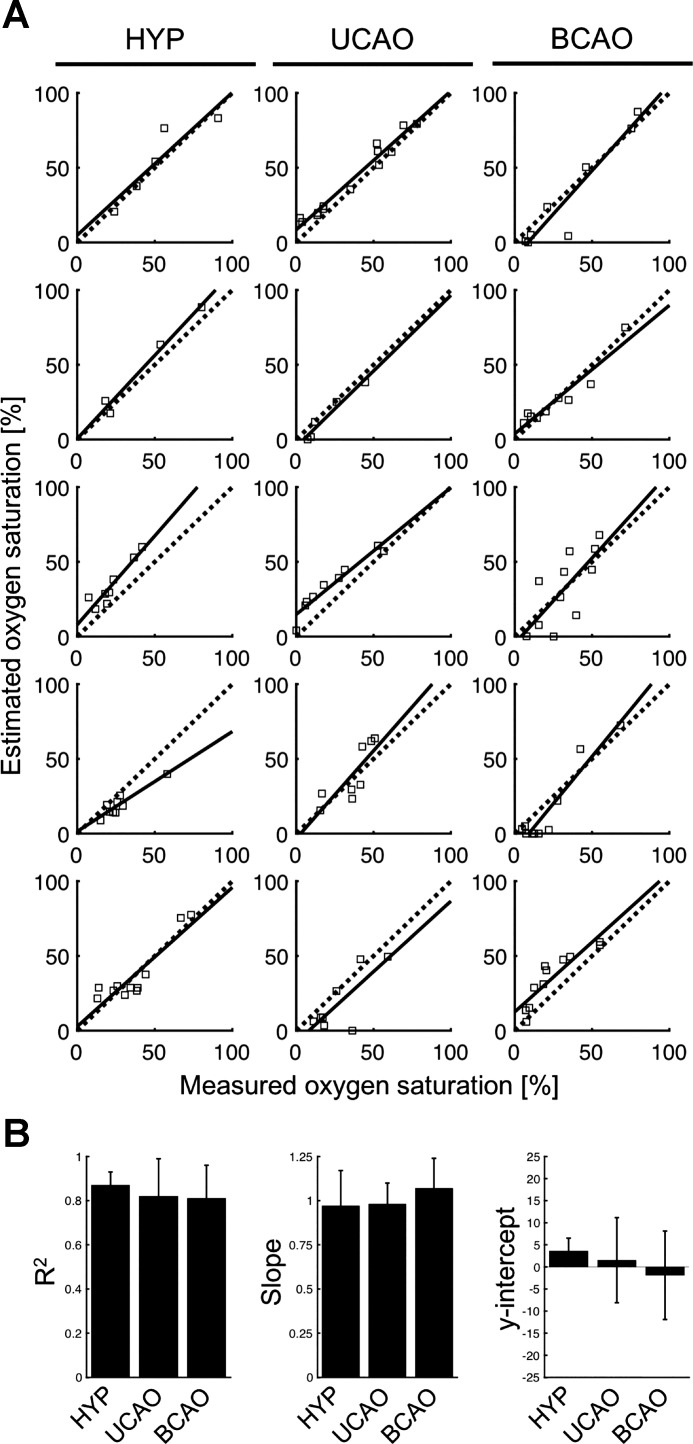
Figure 3A shows the relationship of the SSS So2 estimated by photoacoustic sensing to that measured directly in the blood sample for each of the five piglets in each of the three groups. In general, the estimated So2 correlated well with the measured So2 over the challenge levels in each group. A summary of the mean ± SD for R2, slope, and y-intercept for the individual piglets is shown in Fig. 3B. In the HYP, UCAO, and BCAO groups, R2 values were 0.87 ± 0.06, 0.82 ± 0.17, and 0.81 ± 0.15; slopes were 0.97 ± 0.20, 0.98 ± 0.12, and 1.07 ± 0.17; and y-intercepts were 3.56 ± 2.96, 1.52 ± 9.64, and −1.89 ± 10.01, respectively. These results imply that trends in SSS So2 can be tracked reliably in individual subjects.
Fig. 3.
Correlation between estimated and measured O2 saturation for each piglet in the hypoxia-only group (HYP), hypoxia plus unilateral carotid artery occlusion group (UCAO), and hypoxia plus bilateral carotid artery occlusion group (BCAO). A: linear regression lines (solid lines) are compared with lines of identity (broken lines). B: mean and SD of R2, slope, and y-intercept in each experimental group.
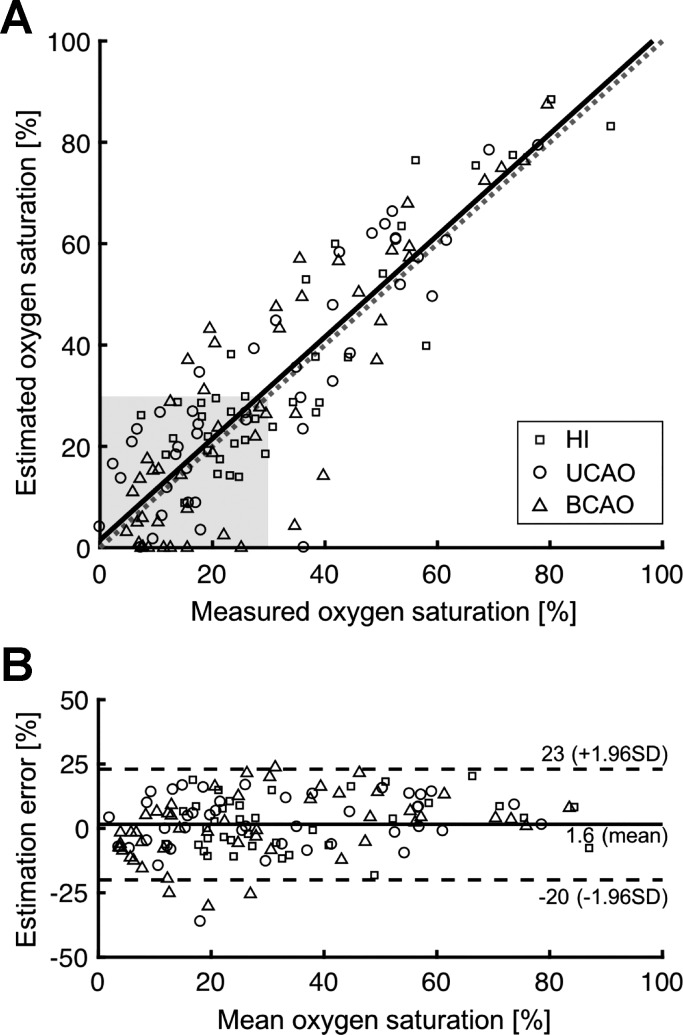
To determine whether a systematic bias existed in the photoacoustic-derived So2, we performed a Bland-Altman analysis on the pooled data. As shown in Fig. 4A, the linear regression between photoacoustic-estimated So2 and So2 directly measured in the 15 piglets had a slope of 1.00 and a y-intercept of 1.49% with good consistency (R2 = 0.77). Figure 4B shows the mean estimated error and the 95% confidence interval of the mean error between the two measurements of So2. No physiologically meaningful bias was observed at low or high So2. Importantly, the estimated So2 demonstrated well-limited error in the critical hypoxic range: 2.93 ± 9.22, 3.18 ± 10.52, and −1.77 ± 10.13 for 0 to 10%, 10 to 20%, and 20 to 30% of the measured So2 range, respectively.
Fig. 4.
Correlation between photoacoustic-estimated and measured O2 saturation in the superior sagittal sinus pooled from all groups. A: the linear regression line (solid line), with slope = 1.00 and y-intercept = 1.49, is close to the line of identity (broken line). Shaded rectangle indicates the critical oxyhemoglobin saturation (So2) range (<30%). B: Bland-Altman estimation analysis plot. HI, hypoxia-ischemia; UCAO, unilateral carotid artery occlusion; BCAO, bilateral carotid artery occlusion.
Bland-Altman analysis was also performed for each group. Photoacoustic-estimated SSS So2 remained highly correlated despite progressive differences in O2 extraction and physiological conditions among groups: HYP R2 = 0.74, slope = 0.96, y-intercept = 3.08; UCAO R2 = 0.77, slope = 0.96, y-intercept = 3.62; BCAO R2 = 0.76, slope = 1.06, y-intercept = −1.09. Again, no significant bias was apparent in the estimated So2 in any individual group: Bland-Altman estimation analysis for the HYP, UCAO, and BCAO groups indicated 1.8, 2.5, and 0.6% of mean estimation error with the ranges (±1.96 SD) of −18 – 21, −19 – 24, and −24–25%, respectively.
Receiver-operating characteristic curve.
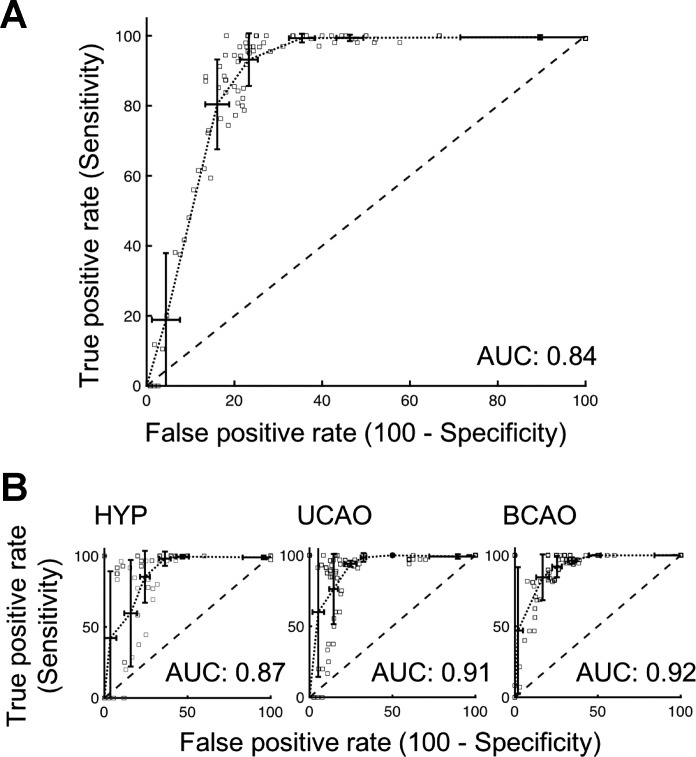
Figure 5A shows the receiver-operating characteristic (ROC) curves for pooled data from all three experimental groups. The area under the curve (AUC) value for the ROC curve was 0.84 for the combined group. Also, the AUC values for individual ROC curves of HYP, UCAO, and BCAO groups were 0.87, 0.91, and 0.92, which reflect strong and consistent correlation of the photoacoustic-estimated and measured So2 (Fig. 5B).
Fig. 5.
Receiver operating characteristic (ROC) analysis of estimated O2 saturation in predicting measured O2 saturation at a threshold <30%. A: pooled dataset of the three experimental groups. The area under the curve (AUC) for the pooled ROC curve was 0.84. B: individual datasets for the hypoxia-only group (HYP), hypoxia plus unilateral carotid artery occlusion group (UCAO), and hypoxia plus bilateral carotid artery occlusion group (BCAO).
Detection precision in critical SSS So2 range.
The accuracy of photoacoustic-estimated SSS So2 below 30% of the measured So2 was evaluated. From the combined data of all groups, the photoacoustic-based sensing revealed significant accuracy for identifying the clinically important threshold of 30%: sensitivity = 87.01%, specificity = 86.54%, accuracy = 86.82%, PPV = 90.54%, and NPV = 81.82%. In addition, the extended evaluation for each group showed comparable accuracy (Table 2).
Table 2.
Detection precision in critical O2 saturation range of <30% in HYP, UCAO, and BCAO
| Group | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|
| HYP | 83.3 | 92.3 | 86.5 | 95.2 | 75.0 |
| UCAO | 87.5 | 89.5 | 88.4 | 91.3 | 85.0 |
| BCAO | 89.7 | 80.0 | 85.7 | 86.7 | 84.2 |
| Pooled | 87.1 | 86.5 | 86.8 | 90.5 | 81.8 |
Analysis results using the pooled dataset and individual datasets of the experimental groups. HYP, hypoxia-only group; UCAO, hypoxia plus unilateral carotid artery occlusion group; BCAO, hypoxia plus bilateral carotid artery occlusion group; PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
The data provided here support the efficacy of in vivo photoacoustic sensing to detect SSS So2 in neonatal piglet models of graded hypoxia-ischemia produced by global hypoxia and unilateral/bilateral carotid artery occlusion. We found a high positive correlation between the photoacoustic-estimated SSS So2 and directly measured SSS So2. Of interest is the observation that a linear regression line provided a good fit in individual piglets—in most cases, the slope was close to one and the y-intercept close to zero (Fig. 3A). Even in cases with some deviation from the line of identity, a reasonable linear fit was obtained. This linearity indicates that the technique would be amenable for tracking trends in brain oxygenation in an individual patient. If such a system could be miniaturized, it could provide a valuable monitoring tool for the obstetrician to track changes in fetal brain oxygenation during labor or as a bedside brain monitor for the neonate in the intensive care unit.
The correlation between SSS So2 measured directly and by photoacoustics remained robust even in the presence of flow limitation during hypoxia and increased O2 extraction produced by unilateral and bilateral cerebral artery ligation. The decrease in MAP in the most severe hypoxia range was likely a contributing factor to the decrease in LDF, in addition to the carotid occlusion. Despite these differences in physiological conditions, all groups successfully produced a similar correlation with the measured SSS So2 (Figs. 3B and 4). Bland-Altman analysis did not reveal a significant bias at low or high oxygenation states. The 95% confidence limits of the error between the photoacoustic-estimated and directly measured SSS So2 were ± 21.5% for the pooled data. The magnitude of this error indicates that most of the single-point measurements are reasonably reliable. In clinical application, decisions would likely be made on the time average of multiple scans, which would presumably reduce the effect of outliers and further minimize the error. It should be noted that the blood sample measurement also has a contributing error in this analysis. Furthermore, we analyzed a ROI that included tissue surrounding the SSS. Improvements in ultrasound detection sensitivity could allow utilization of smaller ROI. Another consideration is that we applied a correction factor to the measured ultrasound intensity for each incident wavelength based on the differences in incident light transmittance through a piglet skull for each wavelength. Increased light transmittance through open fontanelles in human neonates would likely increase the signal-to-noise ratio.
Most importantly, from a clinical perspective, the precision in detecting a critical SSS So2 <30% was confirmed with high sensitivity, specificity, accuracy, PPV, and NPV in the pooled dataset and for the three individual groups (Table 2). These results strongly suggest that the photoacoustic sensing technology has the potential to offer noninvasive early detection of evolving perinatal HIE in the intensive care unit.
Our photoacoustic-derived estimation of SSS So2 was made using 21 wavelengths, which may be redundant compared with previously proposed methods (13, 15) and standard NIRS. We chose to use many wavelengths to ensure sufficient information for obtaining a good spectral fit. However, clinical implementation may be possible with fewer wavelengths, which would reduce the data acquisition time. With our current laboratory system that utilizes a tunable laser system, a data acquisition sequence required 105 s of scanning for 21 wavelengths. Our ultimate goal is to enable an efficient transcranial SSS So2 estimation using two optimal wavelengths securing imaging depth and spectral information, which would significantly reduce the total amount of time for SSS So2 estimation down to 10 s. The capability of dual-wavelength So2 estimation has been shown in various clinical translations of photoacoustic imaging (24). In addition, the temporal resolution can be further improved by increasing laser pulse repetition rate, reducing the number of frames averaged, and using higher-pulse energy within the ANSI safety limit. For example, because a signal-to-noise ratio is proportional to the square root of the number of frames averaged (i.e., ), the sensing speed can be doubled with the preserved SSS So2 estimation accuracy when using times higher laser energy density (3.5 → 5.0 mJ/cm2) with the halved number of frames averaged (64 → 32). A more rapid acquisition sequence would facilitate capture of dynamic changes in SSS So2, as would occur during cyclic uterine contractions.
In conclusion, photoacoustic techniques can be used to noninvasively monitor oxygenated Hb saturation in the cerebral SSS in vivo in neonatal piglets under varying conditions of hypoxia and ischemia. The photoacoustic measurements strongly correlate with direct blood sample measurements. This proof of concept indicates that photoacoustic imaging has the potential to be a powerful new tool that will enhance assessment of neonatal HIE and improve the early identification of affected neonates for therapy such as targeted hypothermia, which must be initiated within 6 h of birth. In future studies, we will continue to investigate the effective transcranial photoacoustic SSS So2 measurement using a prototype system developed for clinical use. In addition, the proposed method potentially can be extended to older children, or even adults. Photoacoustic imaging of the adult brain has been a significant challenge because of the skull strongly absorbing and scattering light coming in as well as attenuating and distorting the ultrasound going out. However, we still have the ability to increase the current laser energy density (3.5 mJ/cm2) up to the ANSI safety limit (20 mJ/cm2), which will proportionally improve photoacoustic intensity. Also, more advanced signal processing could be developed for better sensitivity to demonstrate the feasibility of photoacoustic sensing through a whole adult human skull as shown by others (14). The clinical translation toward HIE detection in obstetric and neonatal medicine would involve the photoacoustic spectral calibration used in this study and comparisons with the currently available oximetric methods, NIRS and diffused optical tomography.
GRANTS
Financial support was provided by NIH Brain Initiative Grant R24-MH-106083 and NIH Grant 1R01-HL-139543. In addition, internal support was provided by a Johns Hopkins University Discovery Award and by a Department of Anesthesiology and Critical Care Medicine Stimulating and Advancing ACCM Research award. Finally, this publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research, which is funded in part by Grant Number UL1-TR-001079 from the National Center for Advancing Translational Sciences, a component of the NIH, and NIH Roadmap for Medical Research. J.K. was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant 2018R1A6A3A03011551).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K., S.A., E.K., H.K.Z., R.C.K., and E.M.G. performed experiments; J.K. and R.C.K. analyzed data; J.K. and R.C.K. interpreted results of experiments; J.K. prepared figures; J.K. drafted manuscript; J.K., E.M.B., H.K.Z., R.C.K., and E.M.G. edited and revised manuscript; E.M.B., R.C.K., and E.M.G. conceived and designed research; E.M.B., R.C.K., and E.M.G. approved final version of manuscript.
REFERENCES
- 1.Alonso-Alconada D, Broad KD, Bainbridge A, Chandrasekaran M, Faulkner SD, Kerenyi Á, Hassell J, Rocha-Ferreira E, Hristova M, Fleiss B, Bennett K, Kelen D, Cady E, Gressens P, Golay X, Robertson NJ. Brain cell death is reduced with cooling by 3.5°C to 5°C but increased with cooling by 8.5°C in a piglet asphyxia model. Stroke 46: 275–278, 2015. doi: 10.1161/STROKEAHA.114.007330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbonne B, Langer B, Goffinet F, Audibert F, Tardif D, Le Goueff F, Laville M, Maillard F; The French Study Group on Fetal Pulse Oximetry . Multicenter study on the clinical value of fetal pulse oximetry. II. Compared predictive values of pulse oximetry and fetal blood analysis. Am J Obstet Gynecol 177: 593–598, 1997. doi: 10.1016/S0002-9378(97)70151-0. [DOI] [PubMed] [Google Scholar]
- 3.Garite TJ, Dildy GA, McNamara H, Nageotte MP, Boehm FH, Dellinger EH, Knuppel RA, Porreco RP, Miller HS, Sunderji S, Varner MW, Swedlow DB. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol 183: 1049–1058, 2000. doi: 10.1067/mob.2000.110632. [DOI] [PubMed] [Google Scholar]
- 4.Hariri A, Tavakoli E, Adabi S, Gelovani J, Avanaki MRN. Functional photoacoustic tomography for neonatal brain imaging: developments and challenges. Int Soc Optics Photonics 10064: 100642Z, 2017. doi: 10.1117/12.2254861 [DOI] [Google Scholar]
- 5.Jones MD Jr, Rosenberg AA, Simmons MA, Molteni RA, Koehler RC, Traystman RJ. Oxygen delivery to the brain before and after birth. Science 216: 324–325, 1982. doi: 10.1126/science.6801768. [DOI] [PubMed] [Google Scholar]
- 7.Koehler RC, Jones MD Jr, Traystman RJ. Cerebral circulatory response to carbon monoxide and hypoxic hypoxia in the lamb. Am J Physiol Heart Circ Physiol 243: H27–H32, 1982. doi: 10.1152/ajpheart.1982.243.1.H27. [DOI] [PubMed] [Google Scholar]
- 8.Kubli FW, Hon EH, Khazin AF, Takemura H. Observations on heart rate and pH in the human fetus during labor. Am J Obstet Gynecol 104: 1190–1206, 1969. doi: 10.1016/S0002-9378(16)34294-6. [DOI] [PubMed] [Google Scholar]
- 9.Kühnert M, Seelbach-Göebel B, Butterwegge M. Predictive agreement between the fetal arterial oxygen saturation and fetal scalp pH: results of the German multicenter study. Am J Obstet Gynecol 178: 330–335, 1998. doi: 10.1016/S0002-9378(98)80021-5. [DOI] [PubMed] [Google Scholar]
- 10.Lawn JE, Bahl R, Bergstrom S, Bhutta ZA, Darmstadt GL, Ellis M, English M, Kurinczuk JJ, Lee ACC, Merialdi M, Mohamed M, Osrin D, Pattinson R, Paul V, Ramji S, Saugstad OD, Sibley L, Singhal N, Wall SN, Woods D, Wyatt J, Chan KY, Rudan I. Setting research priorities to reduce almost one million deaths from birth asphyxia by 2015. PLoS Med 8: e1000389, 2011. doi: 10.1371/journal.pmed.1000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CW, Cooper RJ, Austin T. Diffuse optical tomography to investigate the newborn brain. Pediatr Res 82: 376–386, 2017. doi: 10.1038/pr.2017.107. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Mirsky DM, Beslow LA, Amlie-Lefond C, Danehy AR, Lehman L, Stence NV, Vossough A, Wintermark M, Rivkin MJ; International Paediatric Stroke Study Neuroimaging Consortium and the Paediatric Stroke Neuroimaging Consortium . Pathways for neuroimaging of neonatal stroke. Pediatr Neurol 69: 37–48, 2017. doi: 10.1016/j.pediatrneurol.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Mitcham T, Taghavi H, Long J, Wood C, Fuentes D, Stefan W, Ward J, Bouchard R. Photoacoustic-based sO2 estimation through excised bovine prostate tissue with interstitial light delivery. Photoacoustics 7: 47–56, 2017. doi: 10.1016/j.pacs.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie L, Cai X, Maslov K, Garcia-Uribe A, Anastasio MA, Wang LV. Photoacoustic tomography through a whole adult human skull with a photon recycler. J Biomed Opt 17: 110506, 2012. doi: 10.1117/1.JBO.17.11.110506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perekatova V, Subochev P, Kleshnin M, Turchin I. Optimal wavelengths for optoacoustic measurements of blood oxygen saturation in biological tissues. Biomed Opt Express 7: 3979–3995, 2016. doi: 10.1364/BOE.7.003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrov IY, Petrov Y, Prough DS, Cicenaite I, Deyo DJ, Esenaliev RO. Optoacoustic monitoring of cerebral venous blood oxygenation though intact scalp in large animals. Opt Express 20: 4159–4167, 2012. doi: 10.1364/OE.20.004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrov IY, Petrov Y, Prough DS, Richardson CJ, Fonseca RA, Robertson CS, Asokan CV, Agbor A, Esenaliev RO. Transmission (forward) mode, transcranial, noninvasive optoacoustic measurements for brain monitoring, imaging, and sensing. Int Soc Optics Photonics 10064: 97084P, 2016. doi: 10.1117/12.221891. [DOI] [Google Scholar]
- 17a.Petrova IY, Petrov YY, Esenaliev RO, Deyo DJ, Cicenaite I, Prough DS. Noninvasive monitoring of cerebral blood oxygenation in ovine superior sagittal sinus with novel multi-wavelength optoacoustic system. Opt Express 17: 7285–7294, 2009. doi: 10.1364/OE.17.007285. [DOI] [PubMed] [Google Scholar]
- 18.Prahl S. Optical Absorption of Hemoglobin. Portland, OR: Oregon Medical Laser Center, 1999. [Google Scholar]
- 19.Pritzker RN, Rohrer TE. Laser safety: standards and guidelines. In: Handbook of Lasers in Dermatology. London: Springer, 2014, p. 11–28. [Google Scholar]
- 20.Robertson NJ, Faulkner S, Fleiss B, Bainbridge A, Andorka C, Price D, Powell E, Lecky-Thompson L, Thei L, Chandrasekaran M, Hristova M, Cady EB, Gressens P, Golay X, Raivich G. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 136: 90–105, 2013. doi: 10.1093/brain/aws285. [DOI] [PubMed] [Google Scholar]
- 21.Schleien CL, Dean JM, Koehler RC, Michael JR, Chantarojanasiri T, Traystman R, Rogers MC. Effect of epinephrine on cerebral and myocardial perfusion in an infant animal preparation of cardiopulmonary resuscitation. Circulation 73: 809–817, 1986. doi: 10.1161/01.CIR.73.4.809. [DOI] [PubMed] [Google Scholar]
- 22.Seelbach-Göbel B, Heupel M, Kühnert M, Butterwegge M. The prediction of fetal acidosis by means of intrapartum fetal pulse oximetry. Am J Obstet Gynecol 180: 73–81, 1999. doi: 10.1016/S0002-9378(99)70153-5. [DOI] [PubMed] [Google Scholar]
- 23.Semyachkina-Glushkovskaya OV, Kurths J, Pavlov AN, Borisova EG, Abdurashitov AS, Zhu D, Li P, Luo Q, Tuchin VV. Silent Vascular Catastrophes in the Brain in Term Newborns: Strategies for Optical Imaging. IEEE J Sel Top Quantum Electron 22: 88–101, 2016. doi: 10.1109/JSTQE.2016.2523982. [DOI] [Google Scholar]
- 24.Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335: 1458–1462, 2012. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]