Abstract
Airway luminal area is the major determinant of resistance to airflow in the tracheobronchial tree. Women may have smaller central conducting airways than men; however, previous evidence is confounded by an indirect assessment of airway geometry and by subjects with prior smoking history. The purpose of this study was to examine the effect of sex on airway size in healthy nonsmokers. Using low-dose high-resolution computed tomography, we retrospectively assessed airway luminal area in healthy men (n = 51) and women (n = 73) of varying ages (19–86 yr). Subjects with a positive smoking history, cardiopulmonary disease, or a body mass index > 40 kg/m2 were excluded. Luminal areas of the trachea, right and left main bronchus, bronchus intermediate, left and right upper lobes, and the left lower lobe were analyzed at three discrete points. The luminal areas of the conducting airways were ~26%–35% smaller in women. The trachea had the largest differences in luminal area between men and women (298 ± 47 vs. 195 ± 28 mm2 or 35% smaller for men and women, respectively), whereas the left lower lobe had the smallest differences (57 ± 15 vs. 42 ± 9 mm2 or 26% smaller for men and women, respectively). When a subset of subjects was matched for height, the sex differences in airway luminal area persisted, with women being ~20%–30% smaller. With all subjects, there were modest relationships between height and airway luminal area (r = 0.73–0.53, P < 0.05). Although there was considerable overlap between sexes, the luminal areas of the large conducting airways were smaller in healthy women than in men.
NEW & NOTEWORTHY Previous evidence for sex differences in airway size has been confounded by indirect measures and/or cohorts with significant smoking histories or pathologies. We found that central airways in healthy women were significantly smaller (~26%–35%) than men. The significant sex-difference in airway size was attenuated (20%–30% smaller) but preserved in a subset of subjects matched for height. Over a range of ages, healthy women have smaller central airways than men.
Keywords: dysanapsis, exercise, work of breathing
INTRODUCTION
The pulmonary system response to aerobic exercise has been shown to be different between the sexes (39). It has been hypothesized that these sex differences in the integrative response to exercise are the result of smaller large conducting airways in women (39). The rationale is that smaller airways in women result in a greater resistive and, consequently, total work of breathing during exercise (13). The greater work of breathing further results in significantly greater oxygen consumption of the respiratory muscles (15). High respiratory muscle work has been shown to influence blood flow to the locomotor (24) and respiratory muscles (10) as well as fatigue (3, 14, 38) in healthy subjects and in patients with chronic cardiopulmonary disease (1, 33, 36, 37). Thus, if women have smaller airways than men of similar lung size, they may be especially susceptible to pulmonary constraints during exercise in healthy and in disease populations. However, previous evidence indicating women have smaller airways has been limited by the indirect nature of assessing airway geometry (i.e., dysanapsis ratio) (13, 35) by only reporting on tracheal area (19, 20, 25, 34) or by examining subjects who were older and had a significant smoking history (40).
Airflow in the respiratory system is dependent on both airway resistance and the driving pressure. Of the several factors that influence airway resistance (44), it is airway radius that is the most important. For example, under conditions of laminar flow, a halving of airway radius would increase resistance to air flow 16-fold, all else being equal. Airway size is related to lung size, the latter being significantly influenced by height; however, there is considerable variability between individuals (9, 40). The wide interindividual variability in airway size for a given lung volume has been termed “dysanapsis” (18, 35) and can, in part, explain the difference in maximal airflows between individuals of similar lung size or stature. Dynamic and/or static changes in airway area occur in many pulmonary diseases [e.g., asthma and chronic obstructive pulmonary disease (COPD)] and are at least partially responsible for pathology of the disease (4, 32).
Accordingly, the purpose of this study was to assess airway luminal area in a cohort of men and women of varying ages with no history of pulmonary disease or smoking. We hypothesized that airway size would be related to both height and biological sex. Specifically, there will be a positive relationship between airway size and height in all subjects, and men would have larger airways than women. Furthermore, we hypothesized that when men and women are matched for height, sex differences in airway luminal area would persist, with men having larger airways.
METHODS
Subjects.
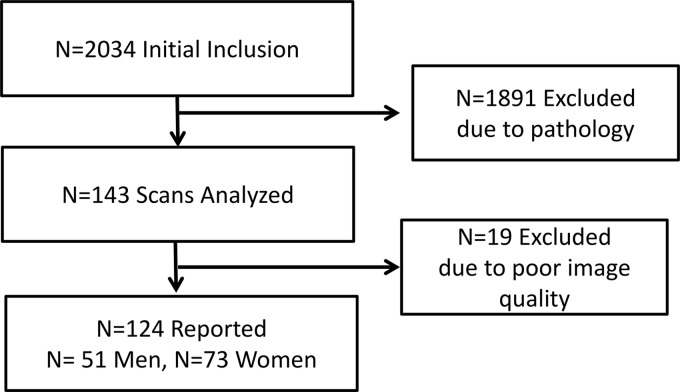
This retrospective study was approved (17–008537) by the Mayo Clinic Institutional Review Board, and the study is in accordance with the Declaration of Helsinki. The subject inclusion paradigm is displayed in Fig. 1. The initial inclusion criterion was a chest computed tomography (CT) scan for suspected pulmonary embolism in patients above the age of 18 yr. A total of n = 2,034 subjects met the initial criterion, and their medical history was screened by a physician for the exclusion criteria. Subjects were excluded if they were diagnosed with or had a history or presence of any of the following: interstitial lung disease, idiopathic pulmonary fibrosis, COPD, asthma, pulmonary embolism, tobacco use, pulmonary hypertension, systolic or diastolic heart failure, lung neoplasm or solitary nodules, lung infections or inflammation as per radiology report, scleroderma, Sjogren, unilateral or bilateral pleural effusions, obstructive sleep apnea, acute coronary syndrome, end-stage kidney disease on dialysis, end-stage liver disease, ascites, pulmonary hypertension, or any surgical intervention to the lungs. Subjects were also excluded if their body mass index was >40 kg/m2. After exclusions, n = 143 subjects met the criteria, and their images were analyzed for airway luminal area. During analysis, 19 additional (n = 11 women) subjects were eliminated due to poor quality images (e.g., unable to visualize all necessary airways). The final cohort consisted of n = 124 subjects with n = 73 women and n = 51 men.
Fig. 1.
Flow of participants for the study.
Image acquisition.
Our institution utilizes standardized CT algorithms for routine thoracic imaging. A posterior-anterior and lateral topogram is obtained at 120 kV and 35 mA. Spiral acquisitions with a pitch of 1.2 are utilized. Kilovoltage is set at 120 with a standard milliampere-second value of 140. Images are acquired at end-inspiration. Post imaging reconstructions are obtained in the axial and coronal plane using a B46 kernel. Slice thicknesses of 1.5 mm and 3 mm are reconstructed. Maximal intensity projections in the axial and coronal planes are completed with a slice thickness of 10 mm and reconstruction increment of 2.5 mm. Before image acquisition, subjects were asked to take a large inspiration and to hold their breath. As the subjects were not instructed to inhale maximally to total lung capacity, we were not able to perfectly match lung volumes (see Methodological considerations below). However, absolute lung volume was determined by image analysis and was expressed as a percent of predicted total lung capacity based on the subjects’ demographics.
Data and statistical analysis.
Images were analyzed using commercially available software (TerraRecon, AQI, Foster City, CA). The software algorithm isolates the airways from other tissue and creates a three-dimensional reconstruction. Area measurements were taken at three points for each of the following airways: trachea, right main bronchus, intermediate bronchus, right upper lobe, left main bronchus, left upper lobe, and left lower lobe. The three points corresponded to the beginning, middle, and end of the airway, with the beginning/end set as the points of the beginning of bifurcation. The average of the three measures were taken and represent the luminal area for each airway. Additionally, lengths of the trachea, right main bronchus, intermediate bronchus, and left main bronchus were assessed.
Descriptive variables were compared using Students t-tests. To compare airway luminal area between the sexes, a 2 × 7 [sex (men and women) × airway (trachea, right and left main bronchus, bronchus intermediate, left and right upper lobes, and left lower lobe)] repeated measures ANOVA was used. When significant F ratios were detected, a Tukey post hoc test was used to determine where the difference lays. Pearson product moment correlation was used to determine the relationship between height and the different airways. Significance was set at P < 0.05, and values are presented as means ± SD.
RESULTS
Subject descriptors.
Subject characteristics are presented in Table 1. As expected, men were taller, heavier, and had greater absolute lung volumes during which the CT scan was obtained (Table 1). However, there were no differences in age, body mass index, or % predicted lung volume at which the images were obtained (Table 1). When a subset of subjects was matched for height (Table 2), there were no significant differences between the sexes for any descriptive variables.
Table 1.
All subject demographics
| Men (n = 51) | Women (n = 73) | |
|---|---|---|
| Age, yr | 52 ± 18 (21–86) | 49 ± 18 (19–86) |
| Height, cm | 180 ± 7 (165–195) | 164 ± 6 (144–179)* |
| Mass, kg | 97 ± 16 (58–148) | 82 ± 18 (48–125)* |
| BMI, kg/m2 | 29.6 ± 4.4 (18.6–39.2) | 30.7 ± 6.0 (19.2–40.0) |
| Lung volume, ml | 4,117 ± 1,582 (1,789–8,406) | 2,927 ± 775 (1,303–4,752)* |
| Pred of scan TLC, % | 57 ± 20 (27–109) | 58 ± 14 (27–90) |
Values are means ± SD, and range is in parenthesis. BMI, body mass index; Pred of scan TLC, predicted relative lung volume based on the computed tomography scan, absolute measured volume, and predicted total lung capacity.
Significantly different from men. P < 0.05.
Table 2.
Height-matched subject demographics
| Men (n = 20) | Women (n = 20) | |
|---|---|---|
| Age, yr | 56 ± 20 (21–86) | 47 ± 19 (25–79) |
| Height, cm | 173 ± 4 (165–177) | 172 ± 3 (167–179) |
| Mass, kg | 90 ± 15 (58–120) | 88 ± 19 (56–125) |
| BMI, kg/m2 | 30.3 ± 4.9 (18.6–39.1) | 29.9 ± 6.0 (19.2–39.0) |
| Lung volume, ml | 3,956 ± 1,310 (1,789–6,197) | 3,228 ± 882 (1,720–4,752) |
| Pred of scan TLC, % | 58 ± 19 (28–91) | 58 ± 15 (32–90) |
Values are means ± SD, and range is in parenthesis. BMI, body mass index; Pred of scan TLC, predicted relative lung volume based on the computed tomography scan, absolute measured volume, and predicted total lung capacity.
Airway size for all subjects.
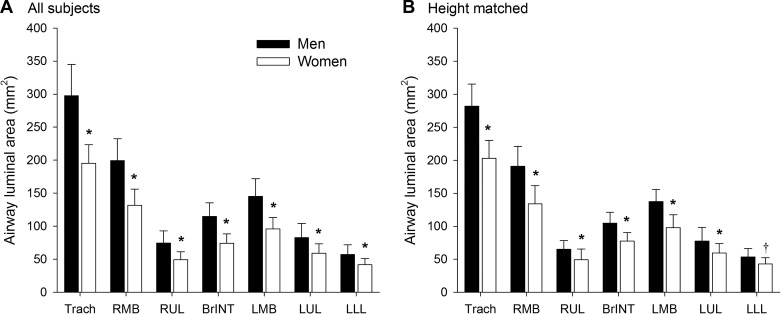
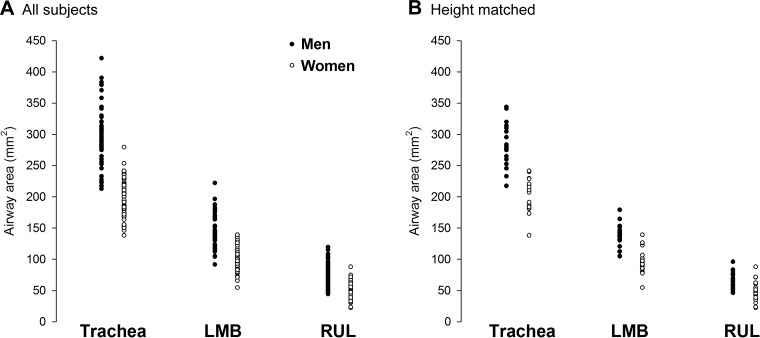
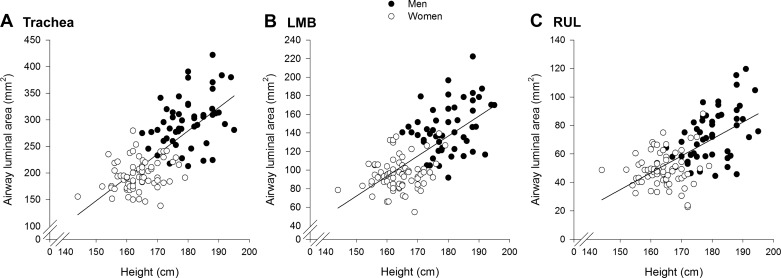
Men had significantly larger luminal area and length for all airways (Fig. 2A), with the exception of the length of the intermediate bronchus. Luminal areas in women were 26%–35% smaller than men and were 10%–14% shorter in length. However, there was considerable overlap in airway luminal area, with many women overlapping with the men and vice versa (Fig. 3A). When all subjects were pooled, there was a modest relationship between height and various airway luminal areas (Table 3, Fig. 4). However, when the relationships were determined for each sex independently, the association was weaker and/or not significant.
Fig. 2.
Airway luminal areas for all subjects (A) and those matched for height (B). Values are means ± SD. BrINT, bronchus intermediate; LLL, left lower lobe; LMB, left main bronchus; LUL, left upper lobe; RMB, right main bronchus; RUL, right upper lobe; Trach, trachea. *Significantly different from men. P < 0.001; †P < 0.1.
Fig. 3.
Individual airway luminal area for all subjects (A) and those matched for height (B). LMB, left main bronchus; RUL, right upper lobe.
Table 3.
Correlation coefficients (r) between height and different airway luminal areas
| All | Men | Women | |
|---|---|---|---|
| Trachea | 0.73* | 0.39* | 0.25* |
| RMB | 0.69* | 0.36* | 0.17 |
| RUL | 0.65* | 0.50* | 0.33* |
| BrINT | 0.70* | 0.40* | 0.20 |
| LMB | 0.69* | 0.34* | 0.20 |
| LUL | 0.56* | 0.31* | 0.16 |
| LLL | 0.54* | 0.26 | 0.20 |
BrINT, bronchus intermediate; LLL, left lower lobe; LMB, left main bronchus; LUL, left upper lobe; RMB, right main bronchus; RUL, right upper lobe.
Statistically significant. P < 0.05.
Fig. 4.
Relationship between height and the luminal area of the trachea (A), left main bronchus (B), and the right upper lobe (C). The regression line for each panel is for all subjects pooled, and the correlation coefficients can be found in Table 3. LMB, left main bronchus; RUL, right upper lobe.
Airway size for height-matched subjects.
In the height-matched subjects, sex difference in airway luminal area persisted. Specifically, all airway luminal areas, with the exception of the left lower lobe (P = 0.09), were significantly smaller in women (P < 0.05) (Fig. 2B). While the difference between the sexes was attenuated when subjects matched for height, women’s airway luminal areas were still 20%–30% smaller than men’s. The overlap in airway luminal area was more pronounced in the height-matched subjects, especially at the more distal airways (Fig. 3B).
DISCUSSION
Major findings.
The major finding from this study is that, throughout a range of ages (19–86 yr), healthy women have smaller large conducting airways than healthy men. Our work demonstrates sex differences in airway anatomy in subjects who are not confounded by indirect airway assessment, age, or smoking history. Smaller conducting airways in women are significant if we consider that smaller airways result in a greater work of breathing, which, in turn, influences the integrative response to whole body exercise in health and disease.
Sex differences in airway anatomy.
We have found that in a cohort of ostensibly healthy subjects of varying age, the large conducting airways are smaller in women. The sex differences in airway luminal area persisted, even when matching the sexes for height. In the present study, the airway sizes are of similar absolute magnitude but overall are marginally reduced compared with others who have similarly used CT images to compare the sexes in exsmokers (9, 40). The smaller absolute values for airway size in our study compared with previous work are likely due to the lower lung volume achieved during image acquisition. The previous report estimated their images to be taken at total lung capacity (40), whereas the current study had images acquired at a variety of lung volumes. Higher lung volumes increase radial traction on airways, thereby increasing luminal area in both health and obstructive disease populations (5, 16, 45). Importantly, although the absolute values differ between our studies minimally, the relative difference in airway size between the sexes is of a similar magnitude.
When we compared a subset of subjects who were matched for height, we found that the sex differences in airway luminal area persisted (Fig. 2B). The finding that height-matched women still had smaller airways demonstrates a specific effect of sex rather than body size, per se, on airway anatomy. While the magnitude of sex difference in airway anatomy was reduced when the subjects were height matched (~35% vs. ~20% smaller), the diminished luminal area would still show increased airflow resistance and result in a higher propensity to turbulent flow. The smaller airways in anthropometrically matched women are important if we consider how many aspects of pulmonary physiology scale with body size. However, in the absence of differences in fitness, men and women of similar size would be expected to have more similar maximal ventilation, and the smaller airways in women would lead to a higher relative and absolute work of breathing. The salient point is that there are both size- and sex-specific effects when considering the pulmonary system. Furthermore, sex differences in pulmonary anatomy have recently been extended to the geometry of the thorax and ribs (17). It is currently unknown how, or even if, these differences in thoracic geometry interact with our demonstrated differences in airway anatomy, but their (17) findings further implicate sexual dimorphism in the pulmonary system.
When all subjects were pooled, we found a modest relationship between height and airway luminal areas (Table 3, Fig. 4). When the correlations were determined for each sex independently, the relationship was weaker and/or not significant for some airways that we measured. The lack of a strong correlation between height and airway size is intuitive because there is not a perfect relationship between lung size and height (43). Equally, others have also shown a similar modest relationship between lung size and airway size (40). The weak, but significant, relationship between lung size and airway size provides further evidence for airway dysanapsis in healthy individuals. The exact mechanism responsible for dysanapsis is unknown but appears to occur during puberty (35).
Physiological consequences and future directions.
Airway luminal area is the major determinant of airway resistance and is particularly important when considering the implications of airway resistance on exercise tolerance in health and disease. Previously, it has been shown that women have higher resistive work of breathing during exercise (15, 23), and via indirect estimates, small airways were attributed as the causative factor (12, 13). The higher resistive and total work of breathing is especially evident during heavy to severe exercise when airflow is high and most likely turbulent in the large conducting airways. Smaller airway luminal area may also explain why the work of breathing in women begins to disproportionately rise for a given increase in ventilation at a lower absolute minute ventilation than men. Specifically, the 20%–35% smaller airways would result in predominantly turbulent flow at a lower absolute flow. We caution, however, that the presence of turbulence is also heavily influenced by airway branching and small geometric variation, which were not quantified in the current study. Given the above, it is reasonable to hypothesize that women may be especially susceptible to pulmonary system limitations to exercise, such as exercise-induced hypoxemia, blood flow redistribution [i.e., respiratory “stealing” (8)], and locomotor and diaphragm fatigue (39). However, sex differences in the integrative exercise response are less consistent. For example, women are less susceptible to diaphragm fatigue during intense exercise than men (22), and potential sex differences in exercise-induced arterial hypoxemia are not universal (11, 26, 27).
A final important physiological consideration is that while women had significantly smaller airways on average, there was considerable overlap between sexes (Fig. 3). Many women had airways that exceeded the average for men of similar height. The considerable variability with respect to airway size within and between sexes is consistent with the variability in the dysanapsis ratio (13, 35), which is an indirect measure of airway size. The within-sex variation in airway size may explain, in part, the variability in pulmonary systems influence on the integrative exercise response. For example, previous work showed that flow limitation was highly prevalent in endurance-trained women with all but one woman developing expiratory flow limitations during high-intensity exercise (23). The lone woman who did not develop expiratory flow limitations had the largest lungs and maximal expiratory flows. Thus, it could be surmised that this subject had the largest airways, and this may have prevented expiratory flow limitations despite her high ventilation during exercise. Overall, while there is growing evidence to support airway size influencing the integrative response to exercise, we caution that studies to date have only linked these measures in different groups of subjects. As such, conclusively determining the effect of smaller airways on the integrative response to exercise will require the pairing of detailed anatomical measurements (e.g., airways size) with physiological parameters (e.g., flow limitations, work of breathing) in a single cohort of healthy subjects.
A hallmark of many cardiopulmonary diseases is an increased work of breathing (6, 31), which has been implicated in the genesis of exercise intolerance and dyspnea (21, 37). As healthy women have smaller airways, they may be more prone to pulmonary disease. In line with this hypothesis, women have been shown to be more susceptible to the detrimental effects of cigarette smoke and present with increased dyspnea and exacerbations with COPD (30). In a mouse model of COPD, for a similar dose of cigarette smoke inhalation, the female mice had greater airway remodeling and structural changes associated with the disease than did male mice (41, 42). These animal findings align with cross-sectional data in humans where a similar dose of smoke has been shown to increase the risk of airflow obstruction in women (2). Yet, while there is evidence suggesting women appear to be predisposed to COPD, women have better survival for a given severity of the disease (7). The fact that women with COPD have better survival than men may be akin to the finding that women develop less diaphragm fatigue (22). Specifically, although several findings related to pulmonary mechanics would suggest that women should be increasingly susceptible to diaphragm fatigue (39), differences in muscle physiology and/or blood flow result in women being less fatigable (28). Also, the methods used to classify many pulmonary diseases (e.g., Global Initiative for Chronic Obstructive Lung Disease for COPD) may be biased toward those with relatively smaller airways as the set cutoffs are reductions in flow. Overall, the direct link between smaller airways in health and susceptibility to pulmonary disease has yet to be elucidated but may explain some of the variability in disease progression and outcomes.
Methodological considerations.
Because of the retrospective nature of the study, there are considerations that warrant discussion. First, we were not able to prospectively assess pulmonary function via spirometry or plethysmography in our subjects. Second, because of the emergency nature of the scans, the end-inspiratory lung volume was not standardized to total lung capacity. Rather, subjects were instructed to inspire and hold their breath. However, important to our central questions, there were no differences in the relative lung volume between the sexes. Lung volume also has less of an influence on the more proximal airways (which the current study assessed) compared with distal airways (29). Therefore, while caution is required when interpreting individual absolute airway diameters, our main comparison between the sexes would remain unaffected.
Conclusion.
We have demonstrated sex differences in the luminal area of large conducting airways in healthy adult men and women of all ages. Specifically, we found that women had airways that were ~30% smaller than men. Furthermore, when a subset of subjects was matched for age and height, the sex difference persisted, with women having ~20% smaller airways. Since airway area is the main determinant of airway resistance, and the larger conducting airways are the chief site of airway resistance, our findings provide data that link anatomy to observed sex differences in pulmonary physiology.
GRANTS
P. B. Dominelli was supported by a postdoctoral fellowship from the Natural Science and Engineering Council of Canada. This work was also supported by National Institutes of Health Grants R35-HL-139854 (to M. J. Joyner), F32-HL-131151 (to S. E. Baker), and T32-DK-007352–39 (to C. C. Wiggins).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.B.D., J.G.R., T.J.C., S.E.B., C.C.W., B.T.W., and M.J.J. conceived and designed research; P.B.D., J.G.R., S.E.B., C.C.W., and B.T.W. performed experiments; P.B.D. and J.G.R. analyzed data; P.B.D., J.G.R., T.J.C., S.E.B., C.C.W., B.T.W., and M.J.J. interpreted results of experiments; P.B.D., T.J.C., S.E.B., C.C.W., and M.J.J. prepared figures; P.B.D., J.G.R., T.J.C., S.E.B., C.C.W., B.T.W., and M.J.J. drafted manuscript; P.B.D., J.G.R., T.J.C., S.E.B., C.C.W., B.T.W., and M.J.J. edited and revised manuscript; P.B.D., J.G.R., T.J.C., S.E.B., C.C.W., B.T.W., and M.J.J. approved final version of manuscript.
REFERENCES
- 1.Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Dempsey JA. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol 299: R314–R324, 2010. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral AFS, Strachan DP, Burney PGJ, Jarvis DL. Female smokers are at greater risk of airflow obstruction than male smokers. UK Biobank. Am J Respir Crit Care Med 195: 1226–1235, 2017. doi: 10.1164/rccm.201608-1545OC. [DOI] [PubMed] [Google Scholar]
- 3.Babcock MA, Pegelow DF, Harms CA, Dempsey JA. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J Appl Physiol (1985) 93: 201–206, 2002. doi: 10.1152/japplphysiol.00612.2001. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ, Burney PGJ, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, Wouters EFM. Chronic obstructive pulmonary disease. Nat Rev Dis Primers 1: 15076, 2015. doi: 10.1038/nrdp.2015.76. [DOI] [PubMed] [Google Scholar]
- 5.Burger CD, Stanson AW, Daniels BK, Sheedy PF II, Shepard JW Jr. Fast-CT evaluation of the effect of lung volume on upper airway size and function in normal men. Am Rev Respir Dis 146: 335–339, 1992. doi: 10.1164/ajrccm/146.2.335. [DOI] [PubMed] [Google Scholar]
- 6.Cross TJ, Sabapathy S, Beck KC, Morris NR, Johnson BD. The resistive and elastic work of breathing during exercise in patients with chronic heart failure. Eur Respir J 39: 1449–1457, 2012. doi: 10.1183/09031936.00125011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Torres JP, Cote CG, López MV, Casanova C, Díaz O, Marin JM, Pinto-Plata V, de Oca MM, Nekach H, Dordelly LJ, Aguirre-Jaime A, Celli BR. Sex differences in mortality in patients with COPD. Eur Respir J 33: 528–535, 2009. doi: 10.1183/09031936.00096108. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol 151: 242–250, 2006. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Diaz AA, Estépar RSJ, Washko GR. Computed tomographic airway morphology in chronic obstructive pulmonary disease. Remodeling or innate anatomy? Ann Am Thorac Soc 13: 4–9, 2016. doi: 10.1513/AnnalsATS.201506-371PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominelli PB, Archiza B, Ramsook AH, Mitchell RA, Peters CM, Molgat-Seon Y, Henderson WR, Koehle MS, Boushel R, Sheel AW. Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp Physiol 102: 1535–1547, 2017. doi: 10.1113/EP086566. [DOI] [PubMed] [Google Scholar]
- 11.Dominelli PB, Foster GE, Dominelli GS, Henderson WR, Koehle MS, McKenzie DC, Sheel AW. Exercise-induced arterial hypoxaemia and the mechanics of breathing in healthy young women. J Physiol 591: 3017–3034, 2013. doi: 10.1113/jphysiol.2013.252767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominelli PB, Guenette JA, Wilkie SS, Foster GE, Sheel AW. Determinants of expiratory flow limitation in healthy women during exercise. Med Sci Sports Exerc 43: 1666–1674, 2011. doi: 10.1249/MSS.0b013e318214679d. [DOI] [PubMed] [Google Scholar]
- 13.Dominelli PB, Molgat-Seon Y, Bingham D, Swartz PM, Road JD, Foster GE, Sheel AW. Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J Appl Physiol (1985) 119: 1105–1113, 2015. doi: 10.1152/japplphysiol.00409.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominelli PB, Molgat-Seon Y, Griesdale DEG, Peters CM, Blouin JS, Sekhon M, Dominelli GS, Henderson WR, Foster GE, Romer LM, Koehle MS, Sheel AW. Exercise-induced quadriceps muscle fatigue in men and women: effects of arterial oxygen content and respiratory muscle work. J Physiol 595: 5227–5244, 2017. doi: 10.1113/JP274068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Romer LM, Sheel AW. Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol 593: 1965–1979, 2015. doi: 10.1113/jphysiol.2014.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ederle JR, Heussel CP, Hast J, Fischer B, Van Beek EJR, Ley S, Thelen M, Kauczor HU. Evaluation of changes in central airway dimensions, lung area and mean lung density at paired inspiratory/expiratory high-resolution computed tomography. Eur Radiol 13: 2454–2461, 2003. doi: 10.1007/s00330-003-1909-5. [DOI] [PubMed] [Google Scholar]
- 17.García-Martínez D, Torres-Tamayo N, Torres-Sanchez I, García-Río F, Bastir M. Morphological and functional implications of sexual dimorphism in the human skeletal thorax. Am J Phys Anthropol 161: 467–477, 2016. doi: 10.1002/ajpa.23051. [DOI] [PubMed] [Google Scholar]
- 18.Green M, Mead J, Turner JM. Variability of maximum expiratory flow-volume curves. J Appl Physiol 37: 67–74, 1974. doi: 10.1152/jappl.1974.37.1.67. [DOI] [PubMed] [Google Scholar]
- 19.Griscom NT, Wohl ME. Dimensions of the growing trachea related to age and gender. AJR Am J Roentgenol 146: 233–237, 1986. doi: 10.2214/ajr.146.2.233. [DOI] [PubMed] [Google Scholar]
- 20.Griscom NT, Wohl MEB. Dimensions of the growing trachea related to body height. Length, anteroposterior and transverse diameters, cross-sectional area, and volume in subjects younger than 20 years of age. Am Rev Respir Dis 131: 840–844, 1985. doi: 10.1164/arrd.1985.131.6.840. [DOI] [PubMed] [Google Scholar]
- 21.Guenette JA, Chin RC, Cheng S, Dominelli PB, Raghavan N, Webb KA, Neder JA, O’Donnell DE. Mechanisms of exercise intolerance in global initiative for chronic obstructive lung disease grade 1 COPD. Eur Respir J 44: 1177–1187, 2014. doi: 10.1183/09031936.00034714. [DOI] [PubMed] [Google Scholar]
- 22.Guenette JA, Romer LM, Querido JS, Chua R, Eves ND, Road JD, McKenzie DC, Sheel AW. Sex differences in exercise-induced diaphragmatic fatigue in endurance-trained athletes. J Appl Physiol (1985) 109: 35–46, 2010. doi: 10.1152/japplphysiol.01341.2009. [DOI] [PubMed] [Google Scholar]
- 23.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 581: 1309–1322, 2007. doi: 10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 82: 1573–1583, 1997. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 25.Hoffstein V. Relationship between lung volume, maximal expiratory flow, forced expiratory volume in one second, and tracheal area in normal men and women. Am Rev Respir Dis 134: 956–961, 1986. doi: 10.1164/arrd.1986.134.5.956. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins SR, Barker RC, Brutsaert TD, Gavin TP, Entin P, Olfert IM, Veisel S, Wagner PD. Pulmonary gas exchange during exercise in women: effects of exercise type and work increment. J Appl Physiol (1985) 89: 721–730, 2000. doi: 10.1152/jappl.2000.89.2.721. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins SR, Harms CA. Gender and pulmonary gas exchange during exercise. Exerc Sport Sci Rev 32: 50–56, 2004. doi: 10.1097/00003677-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol (Oxf) 210: 768–789, 2014. doi: 10.1111/apha.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kambara K, Shimizu K, Makita H, Hasegawa M, Nagai K, Konno S, Nishimura M. Effect of lung volume on airway luminal area assessed by computed tomography in chronic obstructive pulmonary disease. PLoS One 9: e90040, 2014. doi: 10.1371/journal.pone.0090040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kokturk N, Kilic H, Baha A, Lee SD, Jones PW. Sex difference in chronic obstructive lung disease. Does it matter? A concise review. COPD 13: 799–806, 2016. doi: 10.1080/15412555.2016.1199666. [DOI] [PubMed] [Google Scholar]
- 31.Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol (1985) 107: 309–314, 2009. doi: 10.1152/japplphysiol.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lumb AB. Airways disease. In Nunn’s Applied Respiratory Physiology (8th ed.). New York: Elsevier, 2017, p. 389–405.e82. doi: 10.1016/B978-0-7020-6294-0.00027-7 [DOI] [Google Scholar]
- 33.Mancini D, Donchez L, Levine S. Acute unloading of the work of breathing extends exercise duration in patients with heart failure. J Am Coll Cardiol 29: 590–596, 1997. doi: 10.1016/S0735-1097(96)00556-6. [DOI] [PubMed] [Google Scholar]
- 34.Martin TR, Castile RG, Fredberg JJ, Wohl ME, Mead J. Airway size is related to sex but not lung size in normal adults. J Appl Physiol (1985) 63: 2042–2047, 1987. doi: 10.1152/jappl.1987.63.5.2042. [DOI] [PubMed] [Google Scholar]
- 35.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121: 339–342, 1980. doi: 10.1164/arrd.1980.121.2.339. [DOI] [PubMed] [Google Scholar]
- 36.O’Donnell DE, D’Arsigny C, Raj S, Abdollah H, Webb KA. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med 160: 1804–1811, 1999. doi: 10.1164/ajrccm.160.6.9808134. [DOI] [PubMed] [Google Scholar]
- 37.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 588: 2487–2501, 2010. doi: 10.1113/jphysiol.2009.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol 571: 425–439, 2006. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheel AW, Dominelli PB, Molgat-Seon Y. Revisiting dysanapsis: sex-based differences in airways and the mechanics of breathing during exercise. Exp Physiol 101: 213–218, 2016. doi: 10.1113/EP085366. [DOI] [PubMed] [Google Scholar]
- 40.Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S, Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol (1985) 107: 1622–1628, 2009. doi: 10.1152/japplphysiol.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam A, Bates JHT, Churg A, Wright JL, Man SFP, Sin DD. Sex-related differences in pulmonary function following 6 months of cigarette exposure: implications for sexual dimorphism in mild COPD. PLoS One 11: e0164835, 2016. doi: 10.1371/journal.pone.0164835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam A, Churg A, Wright JL, Zhou S, Kirby M, Coxson HO, Lam S, Man SFP, Sin DD. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 193: 825–834, 2016. doi: 10.1164/rccm.201503-0487OC. [DOI] [PubMed] [Google Scholar]
- 43.Tan WC, Bourbeau J, Hernandez P, Chapman K, Cowie R, FitzGerald MJ, Aaron S, Marciniuk DD, Maltais F, O’Donnell DE, Goldstein R, Sin D, Chan-Yeung M, Manfreda J, Anthonisen N, Tate R, Sears M, Siersted H, Becklake M, Ernst P, Bowie D, Sweet L, Van Til L; LHCE study investigators . Canadian prediction equations of spirometric lung function for Caucasian adults 20 to 90 years of age: results from the Canadian Obstructive Lung Disease (COLD) study and the Lung Health Canadian Environment (LHCE) study. Can Respir J 18: 321–326, 2011. doi: 10.1155/2011/540396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West JB. Respiratory Physiology- The Essentials. Baltimore, MD: Lippincott Williams and Wilkins, 2008. [Google Scholar]
- 45.Yamashiro T, San José Estépar R, Matsuoka S, Bartholmai BJ, Ross JC, Diaz A, Murayama S, Silverman EK, Hatabu H, Washko GR. Intrathoracic tracheal volume and collapsibility on inspiratory and end-expiratory ct scans correlations with lung volume and pulmonary function in 85 smokers. Acad Radiol 18: 299–305, 2011. doi: 10.1016/j.acra.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]