Abstract
Obstructive sleep apnea (OSA) is a disorder characterized by collapse of the velopharynx and/or oropharynx during sleep when drive to the upper airway is reduced. Here, we explore an indirect approach for activation of upper airway muscles that might affect airway dynamics, namely, unilateral electrical stimulation of the afferent fibers of the sciatic nerve, in an anesthetized rabbit model. A nerve cuff electrode was placed around the sciatic and hypoglossal nerves to deliver stimulus while airflow, air pressure, and alae nasi electromyogram (EMG) were monitored both before and after sciatic transection. Sciatic nerve stimulation increased respiratory effort, rate, and alae nasi EMG, which persisted for seconds after stimulation; however, upper airway resistance was unchanged. Hypoglossal stimulation reduced resistance without altering drive. Although sciatic nerve stimulation is not ideal for treating OSA, it remains a target for altering respiratory drive.
NEW & NOTEWORTHY Previously, sciatic nerve stimulation has been shown to activate upper airway and chest wall muscles. The supposition that resistance through the upper airway would be reduced with this afferent reflex was disproven. Findings were in contrast with the effect of hypoglossal nerve stimulation, which was shown to decrease resistance without changing muscle activation or ventilatory drive.
Keywords: respiratory dynamics, sciatic nerve stimulation
INTRODUCTION
Obstructive sleep apnea (OSA) is a disorder characterized by pauses in breathing resulting from collapse of the velopharynx and/or oropharynx during sleep. OSA causes hypoxemia, which the body counteracts by waking the individual to take a breath. This fragmented sleep pattern can manifest as daytime sleepiness, headache, decreased ability to concentrate, hypertension, metabolic disorders, cardiovascular morbidity, and psychological changes.
Treatments for OSA range in effectiveness and invasiveness. Oral devices that advance the mandible or reposition the tongue during sleep can be effective in those with mild to moderate OSA (19). Continuous positive airway pressure restores upper airway patency in those with severe OSA (15). These therapies are often poorly tolerated, reducing their efficacy (8). For example, the average continuous positive airway pressure use has been reported to be only 4.4 h per night (20). Surgical options include uvulopalatopharyngoplasty, maxillomandibular advancement, and tracheostomy, but these carry the typical risks and uncertain outcome associated with anatomically directed surgery (2, 26).
Stimulation of cranial nerve (CN) XII (the hypoglossal nerve) is a US Food and Drug Administration-approved therapy (Inspire Systems, Maple Grove, MN) that improves upper airway patency and OSA symptoms (21). Therapy is clinically determined as most effective for those with “appropriate” anatomy and depends on electrode placement on the nerve so as to prevent retrusion or mixed activation of lingual muscles. In general, this therapy has uncovered how little we understand about how muscles functionally act on upper airway size and shape and whether there are alternative targets that would also prevent prolapse to prevent OSA actively (7).
Here, we explore an indirect approach for activating upper airway muscles, namely, electrical stimulation of the afferent fibers of the sciatic nerve. Stimulation of the hind leg muscle-stretch afferents in cat increases respiratory rate and rhythm, blood pressure, and heart rate (18, 23). In the dog, stimulation of the proximal cut end had similar effects proportional to the frequency of stimulation (17, 23, 29). A neuronal tract tracer (biocytin) injected into the iliac segment of the sciatic nerve suggests the central circuit for these brainstem effects might involve the retrotrapezoid nucleus and dorsal lateral medulla (1, 14). The paratrigeminal nucleus (Pa5) showed fluorescently labeled fibers after tracer injection into the contralateral sciatic nerve. These anatomic tracings were originally envisioned as a pathway through which exercise could be coordinated with ventilatory drive, including activation of upper airway muscles that occurs with exercise. Electrophysiologic studies indicate that such premotor respiratory neurons can control inspiratory drive and tonic CN XII activity, thus closing a conceptual loop between sciatic nerve brainstem projections and a potential to change airflow through activation of upper airway muscles (10, 22).
Unlike hypoglossal nerve stimulation, sciatic nerve stimulation might produce a coordinated activation of several CNs (CN V, VII, X, IX, XI, and XII) and attendant muscle groups and influence airway patency (4, 12). In both prior studies of sciatic nerve stimulation, activation of upper airway muscles occurred without a significant change in minute ventilation, independent of changes in oxygen or carbon dioxide levels. Also, in both there was persistence of CN XII activity after the stimulus ended, consistent with a central neural network activation (12).
We recently developed a rabbit model that we believe can test OSA neurotherapies as it is bigger than rodents and its upper airway more like that of humans (3). We used an anesthetized model to determine if stimulation could reduce upper airway resistance, and compared the effects of this to stimulation of the hypoglossal nerve. We confirm that sciatic nerve stimulation before and after distal sectioning of the nerve can increase respiratory drive and upper airway muscle activation but does not alter upper airway resistance, in contrast to hypoglossal nerve stimulation, which opens the airway without effects on respiratory drive.
METHODS
All surgical and experimental procedures were approved by the Louis Stokes Cleveland VA Medical Center Institutional Animal Care and Use Committee. Five New Zealand White male rabbits (Charles River, Wilmington, MA) exhibiting signs of OSA (3) and weighing 2.9 ± 0.3 kg were used in this study. Rabbits were kept in a humidity- and temperature-controlled facility with a 12:12 h light-dark cycle where they had ad libitum access to food and water. Male rabbits were chosen because they do not have a prominent dewlap, thus making them a better model for humans.
Surgery and electrode placement.
Analgesia was initiated with a subcutaneous injection of buprenorphine (20 μg/kg). Sedation was achieved with intravenous propofol (up to 14 mg/kg) via the marginal ear vein. Once sedated, the rabbit was intubated. Anesthesia was achieved with isoflurane (1%–5%) delivered via an endotracheal tube. Marcaine (4 mg/kg) was administered as a local analgesic at each incision site before incision. Vitals were recorded throughout the experiment. The rabbit remained on a warm water circulating heating pad to maintain a body temperature. Prior to starting stimulation, the rabbit was slowly transitioned to urethane (1.5 g/kg) via intravenous injection.
A 3-cm vertical incision was made in the ventral midline. The dissection was directed lateral to the laryngotracheal complex and medial to the vascular sheath. The greater horn of the hyoid was identified. A suture was placed around the body of the hyoid in the midline to facilitate hyoid advancement. A 16-gauge polyethylene catheter with multiple side holes was inserted directly through a tracheotomy between the third and fourth tracheal rings, positioned toward the distal trachea. During the recording process, it was repositioned to face the subglottic region. The catheter was placed below the glottis and secured at the tracheal entrance with a suture to limit air leakage.
Approximately 20 mm of distal sciatic nerve proximal to its terminal branch into the tibial and common peroneal nerves was exposed by dissecting along the muscle planes of the biceps femoris of the upper hind limb. A nerve cuff electrode (Microprobes for Life Science, Gaithersburg, MD) was placed around the nerve. The cuff contained four cathodes distributed evenly around the circumference of the cuff and one circumferential anode. The cuff was 2 mm in inner diameter.
Stainless steel fine wires were inserted into the diaphragm to collect an electromyogram (EMG). Fine-wire EMG electrodes were also placed transcutaneously into the alae nasi muscle just lateral to the nostrils and into the genioglossal muscle. Button surface electrodes were placed on the right front, left front, and left rear dorsal paw to collect electrocardiogram.
Nerve stimulation.
The effect of sciatic nerve stimulation on respiration was quantified in the following fashion. Prior to any stimulation phase, the nerve was not manipulated for 30 min. A current-controlled stimulus was applied to the sciatic nerve using a Grass S48 stimulator (Natus Neurology, Middleton, WI) connected via a Natus photoelectric isolation unit (PSIU6). To find an operating level, a 500-μs pulse width was applied, and a pulse amplitude that elicited a strong plantar flexion or dorsiflexion was titrated. The pulse width was then swept between 50 and 500 μs at the titrated pulse amplitude. The sciatic nerve was then transected distal to the cuff, the animal was allowed to rest ~30 min, and stimulation was repeated.
Data collection.
A neutral head position was adopted, and a mask was placed over the nose of the rabbit. A pneumotach flow transducer (TSD160A, Biopac Systems Inc., Goleta, CA) was connected to the mask and differential amplifier (DA100C, Biopac). Differential pressure between a port on the mask and the trachea was collected. Measures of bulk flow and pressure were visualized and quantified to represent flow resistance along the upper airway from the nose to the larynx. To quantify respiratory effort, EMG activity in the alae nasi and/or diaphragm was collected. Signals were recorded with a Biopac MP150 using AcqKnowledge 4.4 software (Biopac). Data were converted to MATLAB (MathWorks, Natick, MA) for post hoc analysis. Respiratory rate and tidal volume were calculated from the recorded data.
Prior to nerve stimulation, the physiologic range (minimum and maximum) of airflow and pressure were determined by using hyoid advancement (lift) and depression. Hyoid advancement, which maximizes flow and minimizes peak-to-peak pressure, was achieved by gently pulling upward on the suture that was connected to the hyoid bone. Hyoid depression, in the opposite direction, was achieved by placing a 20-g weight on the anterior pharyngeal wall. Brief maneuvers did not alter respiratory rate depth or EMG activity.
Statistical analysis.
To quantify the effect of an event (hyoid advancement/depression, sciatic nerve stimulation), the peak-to-peak airflow and pressure were calculated for five respiratory cycles before and during the event. An ANOVA was used to assess the effect of stimulus, nerve integrity (intact, transected), and experimental time on the peak-to-peak flow and pressure, the respiration rate, and the tidal volume. The effect of an event was quantified as the percent change in these metrics before the event compared with during the event. A paired t-test was also used to assess the effect of nerve transection on these metrics. Differences with P < 0.05 were considered significant.
RESULTS
Raw data.
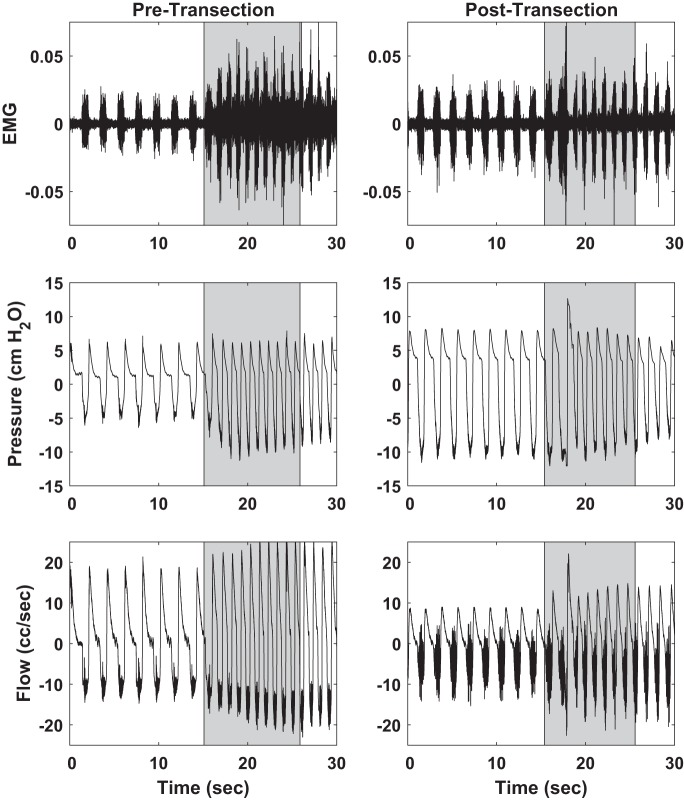
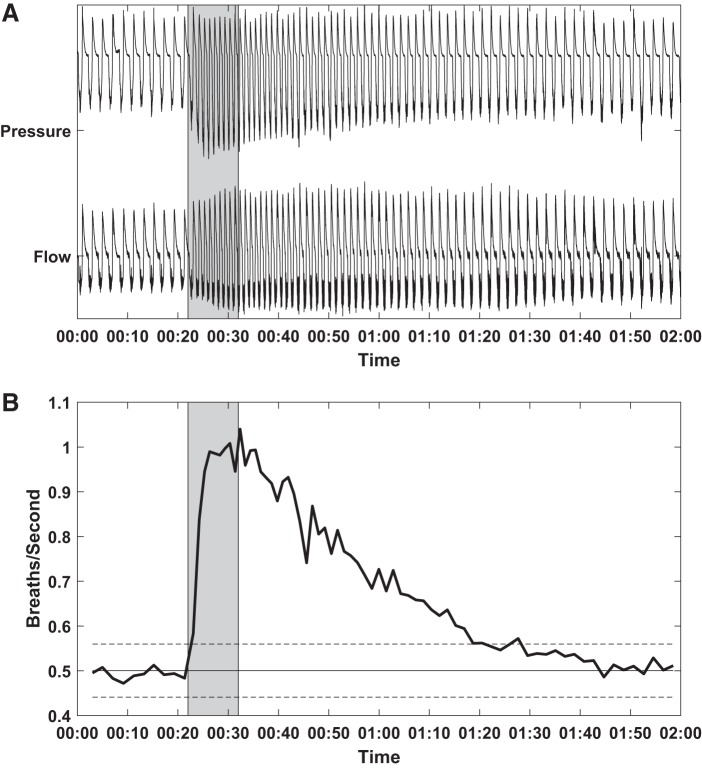
Prior to transection, sciatic nerve stimulation increased respiratory effort, as evidenced by an increase in respiration rate, alae nasi EMG, peak-to-peak pressure, and peak-to-peak flow (Fig. 1). A similar effect was observed following distal sciatic nerve transection, albeit reduced. In both cases, the effects persisted for several seconds, at times as many as 60 s, following the cessation of stimulation, consistent with the observations of (4) (Fig. 2). Persistent effects were not observed in these same animals during hypoglossal nerve stimulation (3).
Fig. 1.
Effect of stimulation (shaded regions) on alae nasi electromyogram (EMG; top), respiratory pressure (middle), and respiratory flow (bottom) both before distal sciatic nerve transection (left) and after transection (right). 250 μs, 0.25 mA, 35 Hz stimulus.
Fig. 2.
Pressure and flow (A) and instantaneous respiratory rate (B). Time shown in minutes and seconds. Stimulation (shaded region) was performed and continued to effect respiratory dynamics for as long as 60 s after the offset of stimulation. B: solid horizontal line is the average breaths per second during the baseline period before stimulation. Dashed horizontal lines indicated ± 2 standard deviations (95% confidence interval). Before transection, 250 μs, 0.25 mA, 35 Hz stimulus.
When flow was plotted against pressure and normalized to baseline, there was a noticeable change in respiratory dynamics (Fig. 3). The inspiration phase shifted rightward and upward, resulting in greater maximum pressure and flow during sciatic nerve stimulation, consistent with Fig. 1. However, the slope of the curve decreased, resulting in less flow at lower pressures. It was not until pressure exceeded 50% of the maximum baseline pressure that flow exceeded baseline flow. This was opposite to the effect observed during hypoglossal nerve stimulation, which increased the slope of the curve without the appearance of a maximal flow plateau (3).
Fig. 3.
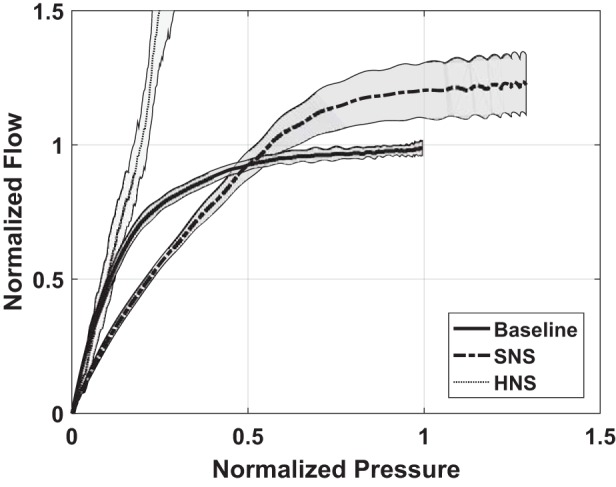
Average airflow during inspiration as a function of the airway pressure before and during sciatic nerve stimulation (SNS). Measures were normalized by the airflow and pressure occurring at the maximum pressure and flow that occurred during baseline observations. Shaded regions are 1 standard deviation from the mean. For comparison, also shown is the outcome associated with hypoglossal nerve stimulation (HNS) in the same animal model (3). Sciatic nerve was not transected.
Below a threshold value, there was a negligible effect on the flow and pressure. As the stimulus increased, the effect of the stimulus on respiratory effort increased, as evidenced by an increase in peak-to-peak flow and peak-to-peak pressure (Fig. 4). The effect of stimulus typically became asymptotic at higher stimulus levels within the range tested. The outcomes followed an asymmetric sigmoidal recruitment curve, which is characteristic of axon recruitment within a population (11, 16).
Fig. 4.
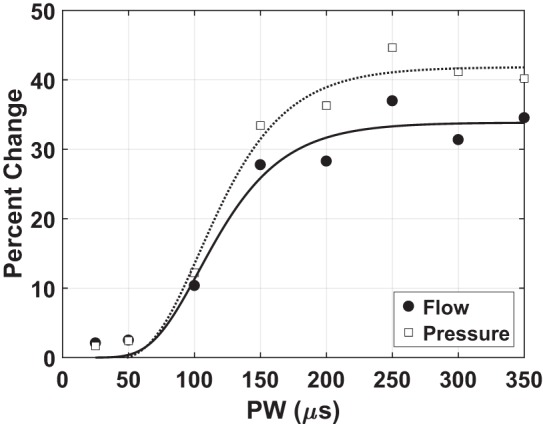
Stimulus pulse width (PW; horizontal axis) and percent change from baseline (vertical axis) in either flow (solid circle) or pressure (open square). Both peak-to-peak flow and peak-to-peak pressure increased during periods of stimulation following distal sciatic nerve transection; however, the changes were proportionate, i.e., not significantly different. Furthermore, the percent change in these metrics increased with increasing stimulus. 0.5 mA, 35 Hz stimulus.
Statistical analyses.
An ANOVA was performed to determine if sciatic nerve transection affected pressure, flow, alae nasi EMG, respiration rate, and tidal volume. The baseline (prestimulus) pressure and flow were not significantly affected by transection [P = 0.126, degrees of freedom (DoF) = 218, F = 2.36; P = 0.622, DoF = 234, F = 0.24, respectively]. However, baseline alae nasi EMG, respiration rate, and tidal volume were significantly affected by transection (P < 0.001, DoF = 83, F = 26.81; P = 0.014, DoF = 225, F = 6.14; P = 0.015, DoF = 225, F = 5.98, respectively). Similarly, the pressure and flow recorded during stimulation were not significantly affected by transection (P = 0.114, DoF = 218, F = 2.52; P = 0.700, DoF = 234, F = 0.15, respectively), whereas the alae nasi EMG, respiration rate, and tidal volume recorded during stimulation were significantly affected by transection (P < 0.001, DoF = 83, F = 28.28; P = 0.004, DoF = 225, F = 8.51; P = 0.016, DoF = 225, F = 5.88, respectively). Because there was a significant difference in three of the metrics, the pretransection and posttransection data could not be pooled immediately. Furthermore, it was not uncommon to have to make minor adjustments to the mask and/or angle of the head. These adjustments did not occur during the period before stimulation that was analyzed and compared against the data acquired during stimulation. However, such adjustments could introduce variability in the baseline recordings throughout the experiment. Therefore, to enable pooling and block against small changes in baseline values, the data were analyzed as percent increase (or decrease) between baseline data and data acquired during stimulation. Sciatic nerve transection did not significantly affect the percent increase in pressure (P = 0.828, DoF = 218, F = 0.05), flow (P = 0.128, DoF = 234, F = 2.34), alae nasi EMG (P = 0.188, DoF = 83, F = 1.76), respiration rate (P = 0.825, DoF = 225, F = 0.05), or tidal volume (P = 0.506, DoF = 225, F = 0.44). Thus, data before and after transection were pooled.
Paired t-tests on the pooled data were conducted to assess the effect of sciatic nerve stimulation on pressure, flow, alae nasi EMG, respiration rate, and tidal volume. Stimulation was found to increase each of these metrics significantly (Table 1). Furthermore, one-sample t-tests were performed on the percent increase (or decrease) in each of these metrics to determine if the change was significantly different from zero. The one-sample t-tests further supported that stimulation significantly increased each metric (P < 0.001 for all metrics).
Table 1.
Effect of sciatic nerve stimulation on various metrics with pre- and posttransection data pooled
| Metric | Baseline | Stimulation | Percent Increase, % | P Value |
|---|---|---|---|---|
| Alae nasi EMG, mV × s | 65.0 ± 71.8 | 75.2 ± 86.2 | 17.8 ± 22.4 | <0.001 |
| Respiration rate, breaths/min | 48.8 ± 25.7 | 56.2 ± 27.4 | 14.8 ± 24.3 | <0.001 |
| Pressure, cmH2O | 3.6 ± 5.5 | 3.9 ± 5.9 | 10.7 ± 18.7 | <0.001 |
| Flow, ml/min | 32.7 ± 14.4 | 35.3 ± 14.8 | 8.9 ± 11.9 | <0.001 |
| Tidal volume, ml | 101.3 ± 35.7 | 106.0 ± 37.1 | 2.6 ± 5.8 | <0.001 |
Value are presented as mean ± SD.
DISCUSSION
Although the literature suggests a potential role for sciatic nerve stimulation to affect upper airway resistance, no prior study has assessed this possibility. The hypothesis is, however, rejected that the mechanical properties change in a direction of benefit for a collapsed airway despite evidence for activation of upper airway muscles and in contrast to hypoglossal nerve stimulation.
We chose the rabbit because of a favorable anatomy, Mice and rats have central apneas but do not commonly exhibit obstructive apneas (6). Also, the rodent, dog, and cat have a pharyngeal airway anatomy that is comparatively rigid. The hyoid bone in these species is firmly attached by cartilage to the styloid process and thyroid cartilage, making the airway less collapsible (6). These animal studies investigate OSA through exposure to hypoxia rather than natural collapse (5, 6, 9). We used an anesthetized preparation in the supine position in which the pharyngeal oral and nasal airway is subjected to gravitational collapse that would make the identification of improvements in resistance more apparent.
Similar to prior studies, sciatic nerve stimulation increased respiratory rate, alae nasi EMG, and tidal volume, but we also could demonstrate an increase in flow and pressure with increasing stimulus strength (12, 17, 23, 30). Also, similar to Cherniack et al. (4), we found that respiration rate remained elevated for up to 60 s following the cessation of stimulation. Although these other studies used transected sciatic nerve, most likely to minimize mechanical feedback originating with contracting muscles or any metabolic increase they might demand, we found that nerve transection did not significantly alter the percent change in our metrics associated with stimulation. This provides further evidence that the changes in respiration caused by sciatic nerve stimulation are associated with activation of sensory afferents. A number of afferent pathways could travel to the brain and converge on the motor outflow and coordination to the upper airway muscles, but pain may be an unlikely dominant mechanism. First, the literature suggests an effect in decerebrate animals (24). Second, the animals in this study were anesthetized to the point of nonresponsiveness to painful stimuli; there was no withdrawal from strong paw pinch, which would be expected to activate nociceptors. Third, the stimulus level was rather low, in that before nerve sectioning there was little induced muscle contraction, suggesting that few large-diameter motor fibers were being recruited. These would be expected to be recruited before any small diameter, unmyelinated c-fibers. Furthermore, the stimulus level, often at or below 0.5 mA, was one that we know elicits no or minimal sensory response and certainly not pain in awake humans using similar nerve cuff electrodes (27, 28).
There was an overall increase in respiratory inspiratory and expiratory flows when stimulating the sciatic afferents in the rabbit that persisted for a time. The increase resulted from an increase in diaphragm contractive force and frequency. Figure 1 illustrated a clear increase in respiratory rate. As illustrated in Fig. 3, the flow at any given driving pressure is affected by sciatic nerve stimulation. We show this example to illustrate that at low driving pressure, flow could be slightly less than normal, indicating a narrower airway at the start of inspiration. However, as driving pressure increased, flow limitation was achieved earlier during baseline studies than with stimulation. For this one example, the argument could be that the airway is stiffer at these high driving pressures. We caution, however, that the effects at low flow were not seen in all animals, and overall there was a proportional change in flow as driving pressure increased, so that, in aggregate, there were no predictable changes in upper airway resistance. In summary, there was not an improvement in airway patency associated with sciatic nerve stimulation, but it does not suggest that there was a reduction in airway patency. In contrast, we found that the flow at the low driving pressures was elevated without any detectable flow limitation when applying hypoglossal nerve stimulation (3).
An increase in diaphragmatic drive with sciatic nerve stimulation is consistent with literature demonstrating anatomic afferent pathways to the brainstem central command center, in part mediated by the retrotrapezoid nucleus. This network increases respiratory drive independently from chemosensitivity (14). Our findings are consistent with those of (4), indicating that respiratory drive is distributed to upper airway muscles and not limited to phrenically mediated diaphragm activity. The increase in tidal volume was much smaller than the increase in flow, upper airway pressure, and alae nasi EMG, which is also consistent with prior observations (4).
The focus, however, is on whether sciatic afferent activation also decreases upper airway resistance, the functional outcome of drive. It appears that it does not. This study was not designed to answer a negative result on upper airway resistance but does invite discussion about the assumptions that any upper airway muscle activation would be functional in regard to reversing upper airway resistance. For upper airway muscle activation to not reduce resistance by affecting size or stiffness, the activation would be entirely offset by increased drive to the chest wall muscles (25). There are other reflexes mediated by the vagal nerve that activate upper airway muscles. Esophageal distention will suppress diaphragm activation yet activate the genioglossus (13). However, if this study is any precedent, an effect on upper airway resistance would have to be studied directly. In contrast, unilateral hypoglossal nerve stimulation does reduce resistance but does not increase respiratory drive (3). Any future OSA drug should have this effect.
In Fig. 1, we noted that the increase in alae nasi EMG, pressure, and flow were not as dramatic following sciatic transection. Because we elected to transect rather than temporarily block the nerve, the experiment could only progress from intact to transected nerve. Therefore, nerve transection always occurred later in the experiment. The experimental duration could mean that the effectiveness of anesthesia changed over time. Furthermore, although we attempted to use care not to manipulate the nerve cuff, the act of transecting the sciatic nerve could have slightly altered the position of the electrodes within the cuff relative to the population of axons we were recruiting.
In conclusion, these experiments utilize a newly developed rabbit model to test potential OSA therapy. Stimulation of sciatic afferents alters breathing patterns in supine, anesthetized rabbits. This activation of breathing is a centrally mediated phenomenon that can last several seconds after the offset of stimulation. However, this activation does not result in a fall in upper airway resistance despite an increase in upper airway muscle activation. Although the use of sciatic nerve stimulation is probably not ideal for treating OSA, it does provide a potential target for altering respiratory dynamics.
ENDNOTE
Data will be made available through the Stimulating Peripheral Activity to Relieve Conditions Material Sharing Policy.
GRANTS
This material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service under a Small Projects in Rehabilitation Research Grant 1I21RX002041 (to K. P. Strohl). It was also supported by the National Institutes of Health Stimulating Peripheral Activity to Relieve Conditions under Grant 1U18EB021792 (to K. P. Strohl).
DISCLAIMERS
M. Schiefer is an investigator at the Louis Stokes Cleveland Veterans Affairs Medical Center (VAMC) and an instructor in the Department of Biomedical Engineering at Case Western Reserve University. K.P. Strohl is a clinician and investigator at the Louis Stokes Cleveland VAMC and a Professor of Medicine at Case Western Reserve University. J. Gamble is a research assistant at the Louis Stokes Cleveland VAMC and a registered veterinary technician at Case Western Reserve University.
The contents do not represent the views of the US Department of Veterans Affairs or the United States government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.P.S. conceived and designed research; M.S. and J.G. performed experiments; M.S. analyzed data; M.S. and K.P.S. interpreted results of experiments; M.S. prepared figures; M.S. drafted manuscript; M.S., J.G., and K.P.S. edited and revised manuscript; M.S. and K.P.S. approved final version of manuscript.
REFERENCES
- 1.Alioto OE, Lindsey CJ, Koepp J, Caous CA. Sensory sciatic nerve afferent inputs to the dorsal lateral medulla in the rat. Auton Neurosci 140: 80–87, 2008. doi: 10.1016/j.autneu.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 2.AlRumaih HS, Baba NZ, AlShehri A, AlHelal A, Al-Humaidan A. Obstructive sleep apnea management: an overview of the literature. J Prosthodont 27: 260–265, 2018. doi: 10.1111/jopr.12530. [DOI] [PubMed] [Google Scholar]
- 3.Benderro GF, Gamble J, Schiefer MA, Baskin JZ, Hernandez Y, Strohl KP. Hypoglossal nerve stimulation in a pre-clinical anesthetized rabbit model relevant to OSA. Respir Physiol Neurobiol 250: 31–38, 2018. doi: 10.1016/j.resp.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherniack NS, Haxhiu MA, Mitra J, Strohl K, Van Lunteren E. Responses of upper airway, intercostal and diaphragm muscle activity to stimulation of oesophageal afferents in dogs. J Physiol 349: 15–25, 1984. doi: 10.1113/jphysiol.1984.sp015139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra S, Polotsky VY, Jun JC. Sleep apnea research in animals. Past, present, and future. Am J Respir Cell Mol Biol 54: 299–305, 2016. doi: 10.1165/rcmb.2015-0218TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis EM, O’Donnell CP. Rodent models of sleep apnea. Respir Physiol Neurobiol 188: 355–361, 2013. doi: 10.1016/j.resp.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker M, Yamauchi M, Strohl KP. Keep the airway open and let the brain sleep. Am J Respir Crit Care Med 190: 1207–1209, 2014. doi: 10.1164/rccm.201410-1939ED. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drager LF, Polotsky VY, O’Donnell CP, Cravo SL, Lorenzi-Filho G, Machado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol 309: H1101–H1111, 2015. doi: 10.1152/ajpheart.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol 147: 159–176, 2005. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Grill WM, Mortimer JT. Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Trans Rehabil Eng 6: 364–373, 1998. doi: 10.1109/86.736150. [DOI] [PubMed] [Google Scholar]
- 12.Haxhiu MA, van Lunteren E, Mitra J, Cherniack NS, Strohl KP. Comparison of the responses of the diaphragm and upper airway muscles to central stimulation of the sciatic nerve. Respir Physiol 58: 65–76, 1984. doi: 10.1016/0034-5687(84)90045-8. [DOI] [PubMed] [Google Scholar]
- 13.Haxhiu MA, Strohl KP, Norcia MP, van Lunteren E, Deal ECJ Jr, Cherniack NS. A role for the ventral surface of the medulla in regulation of nasal resistance. Am J Physiol Regu Integr Comp Physiol 253: R494–R500, 1987. doi: 10.1152/ajpregu.1987.253.3.R494. [DOI] [PubMed] [Google Scholar]
- 14.Kanbar R, Stornetta RL, Guyenet PG. Sciatic nerve stimulation activates the retrotrapezoid nucleus in anesthetized rats. J Neurophysiol 116: 2081–2092, 2016. doi: 10.1152/jn.00543.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, Boehlecke B, Brown TM, Coleman J Jr, Friedman L, Kapen S, Kapur VK, Kramer M, Lee-Chiong T, Owens J, Pancer JP, Swick TJ, Wise MS; American Academy of Sleep Medicine . Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 29: 375–380, 2006. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 16.Mahnam A, Hashemi SMR, Grill WM. Computational evaluation of methods for measuring the spatial extent of neural activation. J Neurosci Methods 173: 153–164, 2008. doi: 10.1016/j.jneumeth.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizumura K, Kumazawa T. Reflex respiratory response induced py chemical stimulation of muscle afferents. Brain Res 109: 402–406, 1976. doi: 10.1016/0006-8993(76)90543-6. [DOI] [PubMed] [Google Scholar]
- 18.Quest JA, Gebber GL. Modulation of baroreceptor reflexes by somatic afferent nerve stimulation. Am J Physiol 222: 1251–1259, 1972. doi: 10.1152/ajplegacy.1972.222.5.1251. [DOI] [PubMed] [Google Scholar]
- 19.Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 11: 773–827, 2015. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg 45: 43, 2016. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safiruddin F, Vanderveken OM, de Vries N, Maurer JT, Lee K, Ni Q, Strohl KP. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J, 20145. doi: 10.1183/09031936.00059414. [DOI] [PubMed] [Google Scholar]
- 22.Sawczuk A, Mosier KM. Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med 12: 18–37, 2001. doi: 10.1177/10454411010120010101. [DOI] [PubMed] [Google Scholar]
- 23.Senapati JM. Effect of stimulation of muscle afferents on ventilation of dogs. J Appl Physiol 21: 242–246, 1966. doi: 10.1152/jappl.1966.21.1.242. [DOI] [PubMed] [Google Scholar]
- 24.St John WM. Influence of reticular mechanisms upon hypoglossal, trigeminal and phrenic activities. Respir Physiol 66: 27–40, 1986. doi: 10.1016/0034-5687(86)90136-2. [DOI] [PubMed] [Google Scholar]
- 25.Strohl KP, Cherniack NS, Gothe B. Physiologic basis of therapy for sleep apnea. Am Rev Respir Dis 134: 791–802, 1986. doi: 10.1164/arrd.1986.134.4.791. [DOI] [PubMed] [Google Scholar]
- 26.Stuck BA, Ravesloot MJL, Eschenhagen T, de Vet HCW, Sommer JU. Uvulopalatopharyngoplasty with or without tonsillectomy in the treatment of adult obstructive sleep apnea - a systematic review. Sleep Med 50: 152–165, 2018. doi: 10.1016/j.sleep.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler DJ. Stability and selectivity of a chronic, multi-contact cuff electrode for sensory stimulation in human amputees. J Neural Eng 12: 026002, 2015. doi: 10.1088/1741-2560/12/2/026002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides long-term stable natural touch perception. Sci Transl Med 6: 257ra138, 2014. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tibes U. Reflex inputs to the cardiovascular and respiratory centers from dynamically working canine muscles. Some evidence for involvement of group III or IV nerve fibers. Circ Res 41: 332–341, 1977. doi: 10.1161/01.RES.41.3.332. [DOI] [PubMed] [Google Scholar]
- 30.Ward ME, Vanelli G, Hashefi M, Hussain SNA. Ventilatory effects of the interaction between phrenic and limb muscle afferents. Respir Physiol 88: 63–76, 1992. doi: 10.1016/0034-5687(92)90029-V. [DOI] [PubMed] [Google Scholar]