Abstract
The blood-brain barrier (BBB) prevents neurotoxic plasma components, blood cells, and pathogens from entering the brain. At the same time, the BBB regulates transport of molecules into and out of the central nervous system (CNS), which maintains tightly controlled chemical composition of the neuronal milieu that is required for proper neuronal functioning. In this review, we first examine molecular and cellular mechanisms underlying the establishment of the BBB. Then, we focus on BBB transport physiology, endothelial and pericyte transporters, and perivascular and paravascular transport. Next, we discuss rare human monogenic neurological disorders with the primary genetic defect in BBB-associated cells demonstrating the link between BBB breakdown and neurodegeneration. Then, we review the effects of genes underlying inheritance and/or increased susceptibility for Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, and amyotrophic lateral sclerosis (ALS) on BBB in relation to other pathologies and neurological deficits. We next examine how BBB dysfunction relates to neurological deficits and other pathologies in the majority of sporadic AD, PD, and ALS cases, multiple sclerosis, other neurodegenerative disorders, and acute CNS disorders such as stroke, traumatic brain injury, spinal cord injury, and epilepsy. Lastly, we discuss BBB-based therapeutic opportunities. We conclude with lessons learned and future directions, with emphasis on technological advances to investigate the BBB functions in the living human brain, and at the molecular and cellular level, and address key unanswered questions.
I. INTRODUCTION
The blood-brain barrier (BBB) prevents neurotoxic plasma components, blood cells, and pathogens from entering the brain (420). At the same time, the BBB regulates transport of molecules into and out of the central nervous system (CNS), which maintains tightly controlled chemical composition of the neuronal milieu that is required for proper neuronal functioning (682, 693). In disease states, BBB breakdown and dysfunction leads to leakages of harmful blood components into the CNS, cellular infiltration, and aberrant transport and clearance of molecules (420, 682, 693), which is associated with cerebral blood flow (CBF) reductions and dysregulation (269–271, 318), contributing to neurological deficits.
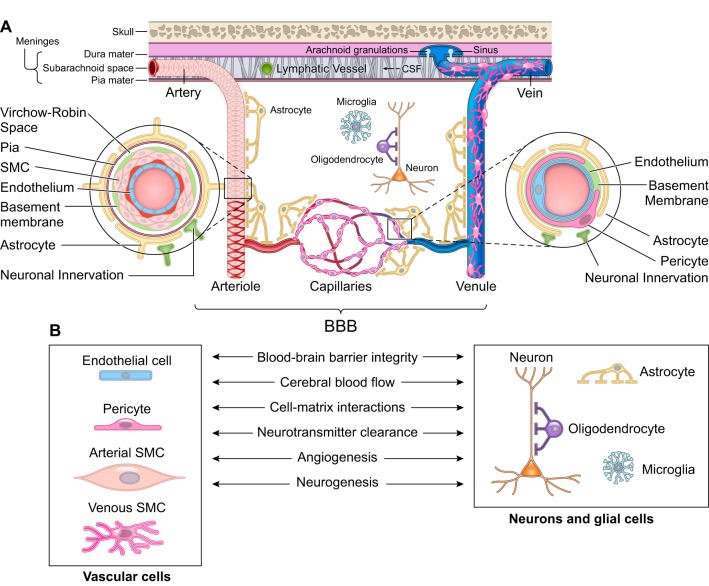
The pattern of cerebral blood vessels follows the major brain circuits tasked with sensation, memory, and motion suggesting that the cerebrovascular system plays an important role in normal CNS functioning (271, 318, 682). Under physiological conditions, the human brain receives 20% of the cardiac output and uses 20% of the body’s oxygen and glucose (270). Energy substrates are consumed by the brain “on the fly” from blood via transport across the BBB, as the brain lacks a reservoir to store fuel for use when needed (271). In the mammalian brain, cerebral arteries, arterioles, and capillaries supply CNS circuits with blood in response to neuronal stimuli by increasing the rate of CBF and oxygen delivery, a mechanism known as neurovascular coupling (271, 319). Different cell types of the neurovascular unit (NVU) including vascular cells [e.g., endothelium and mural cells including pericytes and smooth muscle cells (SMCs)], glia (e.g., astrocytes, microglia), and neurons contribute to regulation of BBB permeability, neurovascular coupling, cell-matrix interactions, neurotransmitter turnover, and angiogenesis and neurogenesis (270, 271, 692, 693) (FIGURE 1).
FIGURE 1.
The neurovascular unit. A: the neurovascular unit comprises vascular cells including endothelial cells and mural cells such as pericytes on brain capillaries, venules, and precapillary arterioles; vascular smooth muscle cells (SMC) on arterioles, small arteries, and veins; glial cells such as astrocytes, microglia, and olidogendrocytes; and neurons. Molecular expression patterns in endothelial and mural cells vary at different levels of vascular tree creating arterio-capillary-venous heterogeneity (zonation). At the level of penetrating arteries (left inset), endothelial cells form the inner layer of the vessel wall. The basement membrane separates endothelium from 1 to 3 layers of SMCs that are enveloped by pia. The Virchow-Robin space is between the pia and the glia limitans formed by astrocytic endfeet. At the arteriolar level, SMCs were reduced to a single layer, whereas the endothelial layer displays a continuity with the endothelium of penetrating arteries and capillaries. At the capillary level (right inset), pericytes and endothelial cells share a basement membrane and exhibit different types of cellular connections. Both the arteriolar and capillary vessel wall is covered by astrocytic endfeet. SMCs, pericytes, and astrocytes all have neuronal innervation. The blood-brain barrier (BBB) is centrally positioned within the neurovascular unit and is formed by a monolayer of tightly sealed endothelial cells extending along the vascular tree and expressing low paracellular and transcellular permeability at the level of brain capillaries and along arteriovenous axis. B: different cells of the neurovascular unit regulate BBB integrity, cerebral blood flow, extracellular matrix interactions, and neurotransmitter clearance and participate in angiogenesis and neurogenesis.
The BBB is centrally positioned within the NVU and is formed by a monolayer of tightly-sealed endothelial cells along the vascular tree expressing low paracellular and transcellular permeability (682, 693). In the human brain, total length of cerebral blood vessels is ~400 miles with capillaries contributing to 85% of the vessel length and providing ~12 m2 of the endothelial surface area available for transport exchanges (2, 460, 693). At the capillary level, BBB integrity is maintained by pericytes (28, 59, 130, 570). Pericytes, SMCs, and endothelial cells express thousands of transcripts encoding different transporters, receptors, active efflux pumps, ion channels, and regulatory molecules (28, 72, 86, 129, 242, 358, 359, 610, 675, 678), whose expression pattern varies by zonation along the arterio-capillary-venous axis and cell type (610).
Here, we first examine molecular and cellular mechanisms underlying the establishment of the BBB. Then, we focus on BBB transport physiology including the BBB molecular junctions, endothelial and pericyte transporters, and perivascular and paravascular transport. Next, we discuss rare human monogenic neurological disorders with the primary genetic defect in vascular cells of the BBB, and the link to neurodegeneration. Then, we review the effects of genes underlying inheritance and/or increased susceptibility for Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) on BBB in relation to other pathologies and neurological deficits. We next examine how BBB breakdown and dysfunction relates to neurological deficits and other pathologies in the majority of sporadic AD, PD, and ALS cases with no clear etiology or genetic inheritance, multiple sclerosis (MS), other neurodegenerative disorders, and acute CNS disorders such as stroke, traumatic brain injury, spinal cord injury, and epilepsy. Lastly, we discuss BBB-based therapeutic opportunities and targets. We conclude with lessons learned and future directions, with emphasis on technological advances to investigate the BBB functions in the living human brain, to provide molecular definitions of different vascular cell types, and address key unanswered questions.
II. ESTABLISHMENT OF THE BLOOD-BRAIN BARRIER
The classic tissue transplantation chick-quail experiment (559) suggested nearly 40 yr ago that the BBB characteristics are not intrinsic to brain endothelium, but rather acquired and shaped in the neural environment during early CNS development. To date, evidence obtained from developmental studies clearly depicts an orchestrated interaction between the neural and vascular systems for proper establishment of the BBB and wiring of the brain (22, 93, 158).
A. BBB Formation
Like any other organ, the brain is vascularized from surrounding vascular plexus during embryogenesis (138). In the developing mouse CNS, at embryonic day E10, the angioblasts of the perineural vascular plexus (PNVP; later turns into the leptomeningeal arteries and veins) sense the vascular endothelial growth factor (VEGF) signals from the neuroectoderm and invade the neural tube (480). This results in formation of nascent ‟leakyˮ blood vessels via sprouting angiogenesis (628, 682) followed by remodeling and patterning (326). The barriergenesis in mice occurs between days E10 and E15 (682), as shown by the emerging multiple features of the BBB such as 1) formation of a continuous endothelial cell membrane sealed by highly specialized intercellular junctional structures to restrict paracellular flow, including upregulation of tight junction (TJ) proteins zonula occludens-1 (ZO-1) and occludin (130); 2) elimination of endothelial fenestrae and pinocytosis to restrict transcellular flow (62); 3) establishment of highly selective transport systems eliminating toxic substances yet allowing exchanges of nutrients and metabolites between circulation and brain (62, 130); and 4) specialization of perivascular structures such as basement membrane and coverage of endothelial capillary wall by pericytes to fortify the barrier (28, 59, 130).
Both cerebral angiogenesis and BBB formation require precise cues from various growth factors, guidance molecules and other factors such as microRNA, as well as fine tuning of intracellular signaling pathways and gene expression (22, 628, 682, 689). Signaling pathways and factors that contribute to BBB formation in the developing CNS at different stages based on murine timeline (585) are illustrated in TABLE 1. In general, the VEGF-VEGFR2-neuropilin1 signaling drives the angiogenesis and vascular patterning (628); Wnt signaling pathway including Wnt ligands, Norrins, Frizzled receptors, Lrp5/6 coreceptors, Gpr124 and Reck cofactors, and β-catenin are critical for both vascular development and barriergenesis (138, 332, 558, 636, 682, 689); pericyte-derived angiopoietin acts on endothelial Tie2 receptor and maintains BBB function (26, 570, 648); and neural guidance molecules such as netrins and semaphorins also shape the growing cerebrovasculature and BBB functions (22, 475, 596, 628). Brain endothelial specific or enriched transporters are often indispensable for BBB barriergenesis, as for example, the glucose transporter-1 (GLUT1) (649), sodium-dependent lysophosphatidylcholine (LPC) symporter, the major facilitator superfamily (MFS) domain-containing protein 2a (MFSD2A) (62), and monocarboxylic acid transporter MCT8 (611). Deficiency or mutations of the genes encoding these BBB transporters often result in human genetic disorders associated with defects in the vasculature and BBB functions leading to cognitive impairment and/or neurological symptoms associated with different subtypes of microcephaly (218, 649) and Allan-Herndon-Dudley syndrome (611), respectively, as discussed below.
Table 1.
Establishment of the blood-brain barrier: signaling pathways and molecules
| Murine Timeline | Pathway | Cell to Cell Interaction | BBB Targets | Reference Nos. |
|---|---|---|---|---|
| Angiogenesis and vascular remodeling | ||||
| E10 to postnatal | VEGF-VEGFR/neuropilin1 | Neural epithelial-angioblasts, tip cells, stalk cells | Ras, PI3K, Src, p38MAPK (intracellular signaling) | 243 |
| E10 to postnatal | TGF-β- neuropilin1 | Neural epithelial-angioblasts, tip cells, stalk cells | SMAD, CDC42 (intracellular signaling) | 603 |
| E10 to postnatal | Notch-DLL4 | Tip cells, stalk cells | NICD, Jagged (intracellular signaling) | 65 |
| E10 to postnatal | Wnt, Norrin, Frizzled, Grp124, Reck, LRP5/LRP6 | Neural progenitors-endothelial | β-Catenin (nuclear translocation) | 609 |
| Barriergenesis and BBB maturation | ||||
| E10 to E16 | Wnt, Norrin, Frizzled, Grp124, Reck, β-catenin, LRP5/LRP6 | Neuron-endothelial | Claudin3 (tight junction) | 350 |
| Plvap (transcellular flow) | ||||
| Laminin, collagen IV | 98 | |||
| Glut1 (transporters) | 542 | |||
| DR6, Troy (receptor) | 557 | |||
| E10 to postnatal | PDGF-BB-Pdgfrβ | Endothelial-pericyte | PI3K, Src, p38MAPK (intracellular signaling) | 553 |
| E10 to postnatal | TGF-β-ALK5 | Endothelial-pericyte | SMAD (intracellular signaling) | 621 |
| E10 to postnatal | Angiopoietin-Tie2 | Pericyte-endothelial | PI3K, Rho, NFκB (intracellular signaling) | 26 |
| E10 to postnatal | Semaphorin-plexin/ neuropilin1 | Neural progenitors-endothelial | VE-cadherin (intercellular junctions) | 338 |
| E10 to postnatal | Netrin-DCC/UNC5 | Endothelial cell autonomous | JAM-A, occluding, claudin-5, ZO-1, α-catenin (intercellular junctions) | 459, 633 |
| E17 to postnatal | SHH, PTCH, SMO, Gli | Astrocyte-endothelial | Occludin, JAM-A, VE-cadherin, claudin-3 and claudin-5 (intercellular junctions) | 12 |
| IL-8, ICAM-1, CCL2 (inflammatory mediator) | ||||
| E17 to postnatal | SSeCKS | Astrocyte-endothelial | ZO-1 | 339 |
| E15 to postnatal | Transporters | Endothelial cell autonomous | Glut1 (transporters) | 622 |
| Mfsd2a (transporters) | 61 | |||
| E15 to postnatal | Junctions | Endothelial-endothelial | Claudin-5 (tight junction) | 425 |
| Lsr (tricellular junction) | 535 | |||
| BBB maintenance | ||||
| Postnatal to adult | apoE- LRP1-CypA-MMP-9 | Astrocyte-pericyte-endothelial | MMP-9 | 59 |
| Postnatal to adult | Interferon-λ-IFNLR1 | Peripheral-BBB | ZO-1, claudin-5 (tight junction) | 337 |
| Postnatal to adult | PDGF-BB-Pdgfrβ | Endothelial-pericyte | PI3K, Src, p38MAPK (intracellular signaling) | 423 |
1. VEGF
VEGF is expressed by the cells of neuroectoderm in the ventricular layer, attracting the angioblasts and tip cells of the invading capillaries to penetrate and vascularize the neural tube (480, 628). Both VEGFR2 receptor, also known as kinase insert domain receptor (KDR), and co-receptor neuropilin1 are required for the proper downstream signaling within the endothelial cells (158). Postnatally, VEGF expression persists in glial cells, further supporting vascular remodeling (387). However, aberrant activation of astrocytes and subsequent production of VEGF-A is associated with BBB breakdown in CNS inflammatory disease (24).
2. Wnt
Canonical Wnt signaling governs the cerebral vascular development and BBB formation. In the developing brain, Wnt ligands activate Frizzled receptors and Lrp5/6 complexes on the endothelium; while in the retina, Norrin independently activates Frizzled and LRP5/6 receptors with the assistance of TSPAN12, a member of the phylogenetically ancient tetraspanin family, regulating angiogenesis and barrier function (634). Gpr124, an orphan G protein-coupled receptor (19, 126, 332, 690), and GPI-anchored membrane protein Reck (reversion inducing cysteine rich protein with kazal motifs) act as specific co-activators of Wnt/β-catenin signaling contributing to CNS angiogenesis and barriergenesis (111, 479, 609). Endothelial specific knockout of Gpr124 exacerbates BBB impairment in mouse models of ischemic stroke and glioblastoma (102), indicating Gpr124 is required for the maintenance of BBB integrity under pathological conditions. Activated β-catenin can improve intercellular junctions by stabilizing VE-cadherin, and translocate into nucleus to induce target gene expression, including upregulation of GLUT1 (558), death receptors DR6 and TROY (576) and TJ component claudin-3, as well as downregulation of plamalemma vesicle-associated protein (PLVAP) to inhibit fenestration (361), which is all critical for the BBB formation.
3. Sonic hedgehog
Hedgehog signaling plays a key role in the establishment and maintenance of cell polarity, which is critical for both epithelial and endothelial barriers (13, 81). Astrocytes are the major source of sonic hedgehog (SHH) in brain, which acts on endothelial patched homolog 1 (PTC-1) receptor to release the inhibition of Smoothened on Gli transcription factors, resulting in expression of SHH-regulated genes, including occludin, junctional adhesion molecule-A (JAM-A), VE-cadherin, claudin-3, and claudin-5 (13). SHH also suppresses expression of inflammatory mediators in the brain endothelial cells, including interleukin-8 (IL-8) and chemokine ligand 2 (CCL2) and intercellular adhesion molecule-1 (ICAM-1), thereby limiting infiltration of peripheral inflammatory cells through the BBB (13).
4. Platelet derived growth factor-BB–platelet derived growth factor receptor β signaling
Pericyte-endothelial interaction plays a key role in the establishment and maintenance of the BBB (27, 570, 648, 682). During angiogenesis, pericytes are recruited by platelet-derived growth factor BB (PDGF-BB) signals from the tip cells of the endothelial sprouts and PDGF-BB retained on the extracellular matrix (27), which activates multiple intracellular signaling pathways through PDGFR-β receptor on pericytes (570, 648). Pericytes provide an important source of the basement membrane proteins at the BBB, including laminins (667) and vitronectin (242), and support endothelial tube formation and stabilization (27). Pericyte-deficient mouse models caused by aberrant PDGF-BB/PDGFR-β signaling (365, 419, 436, 570, 575) exhibit impaired BBB formation (28, 59, 130) and maintenance (59, 436). The interaction between pericytes and endothelial cells involves other signaling pathways, including but not limited to endothelial to pericyte transforming growth factor-β (TGF-β)-activin receptor-like kinases signaling, pericyte to endothelial angiopoietin-Tie-2 signaling, which have been examined elsewhere (570). Future studies are still required to determine the exact contribution of each pericyte-endothelial signaling pathway to the formation and maintenance of the BBB.
5. Axon guidance molecules
As the nervous system and the vascular system develop closely, they not only share certain mechanisms in patterning but also common molecular pathways (22, 93, 127, 297, 349). In fact, the major axon guidance molecules have been found to be important for cerebrovascular development, which interestingly also applies to BBB formation. Sema3A, a member of semaphorin protein family, is anti-angiogenic factor acting independently of VEGF, but also increases vascular permeability by destabilizing VE-cadherin through NRP-1 and PlexinA1 receptors (349). Neuropilin, a common co-receptor and regulator of many morphogens and growth factors, is required for guidance of tip cells during spouting angiogenesis (127), by balancing the pro-angiogenic and anti-angiogenic signals between major pathways including VEGF, semaphorins and Wnt signaling; its upregulation in vessels contributes to BBB breakdown during brain injury and inflammation (564, 679). Brain endothelial cells express roundabout guidance receptor 4 (Robo4), which senses slit guidance ligand 2 (Slit2) and maintains blood-retinal barrier (BRB) integrity by inhibiting VEGF signaling and pathological angiogenesis and endothelial hyperpermeability (297). Recent studies using different ablation approaches for retinal pericytes have shown that pericytes are required for establishment of the BRB; however, whether they are required for maintenance of the BRB in adult is still inconclusive based on these reports (449, 462).
6. BBB specific components
The BBB characteristics include specific junctional proteins, transporters, receptors, and basement membrane components, as a consequence of neural environment-induced transcriptional changes (682). However, these components can also play a key role in the formation of the BBB. For example, mice lacking TJ protein claudin-5 have a BBB permeable to small molecules that are <800 Da (438), whereas Lsr−/− embryos lacking lipolysis-stimulated lipoprotein receptor (LSR), a component of tricellular junctions, exhibit a BBB open to molecules that are ~10 kDa (551). The Slc2a1+/− mice with haploid deficiency in glucose transporter GLUT1 in brain endothelial cells develop microvascular reductions with BBB breakdown including loss of TJ and basement membrane proteins (649), whereas knockout of murine Mfsd2a gene results in not only diminished brain uptake of docosahexaenoic acid (DHA) in the form of lysophosphatidylcholine, but also leads to dysregulated caveolae-mediated transcellular trafficking across the BBB causing BBB breakdown (21, 62, 684). Neurological consequences of these BBB genetic defects are discussed below.
B. BBB Maturation and Maintenance
The BBB continues to mature under the influence of neural environment after day E15 in mice and over a brief period after birth (682). Astrocytes join the NVU during the maturation stage and provide additional support, including the formation of perivascular astrocytic endfeet around capillaries and the glial limitans that ensheathes the penetrating arterioles (682). Astrocytes also strengthen the basement membrane by producing laminin α1 and α2, which are important for stabilizing pericytes (667). In addition, astrocytes secrete retinoic acid and SHH, which transcriptionally regulates gene expression in endothelial cells and enhances the formation of intercellular junction functions (13). Endothelial-pericyte PDGF-BB-PDGFRβ signaling pathway, pericyte-endothelial TGF-β and Ang-1-Tie-2 signaling pathways, as well as astrocyte-endothelial SHH pathway, angiotensin II-AT1 receptor and Wnt-Frizzled signaling pathways, continue to influence the BBB maturation.
The close interactions between the NVU cells are critical for the maintenance of the BBB. For example, astrocytes secrete apolipoprotein E (APOE) to signal pericytes via low-density lipoprotein receptor-related protein-1 (LRP1), which suppress the activation of cyclophilin A (CypA)-matrix metalloproteinase 9 (MMP-9) BBB-degrading pathway, while in the absence of murine APOE mouse pericytes produce active MMP-9 causing BBB breakdown (60). Additionally, in addition to regulating neuroinflammation (360), astrocytes regulate endothelial barrier function; for example, the BBB is compromised in mice lacking src-suppressed C-kinase substrate (SSeCKS), a responder of systemic inflammation in astrocytes (350). Since the BBB breakdown is associated with many CNS diseases as discussed below, future studies should focus on druggable pathways that are critical for maintaining the BBB integrity, particularly using cell-specific inducible mouse models.
1. Neuronal activity-induced vascular plasticity
Neuronal activity during embryonic development and in the early days of postnatal development generates a relatively hypoxic environment that is permissive for vascular development and maturation (22). Additionally, the neural-vascular interactions and functional neurovascular coupling develop to meet the energy needs of the growing brain and support brain activities (105, 318, 319, 341, 605). Deprivation of sensory input in the mouse barrel cortex by either surgical deafferentation or genetic inhibition of neurotransmitter release results in postnatal reduction in vascular density (340), suggesting that neuronal activity remains an important regulator of vascular plasticity after birth. However, chronic stimulation by repetitive sounds, whisker deflection or motor activity, or chemically induced seizures, led to a near arrest of angiogenesis in barrel, auditory, and motor cortices in neonatal mice, which can be prevented with the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME) and in mice with neuronal and inducible NOS deficiency, suggesting that excessive nitric oxide (NO) released from hyperactive interneurons and glia inhibited vessel growth (645). Abnormal neuronal activities are also detrimental to a mature BBB. For example, the widespread BBB breakdown has been well documented in a mouse model of status epilepticus (209). Additionally, BBB impairment also occurs early in the mouse model of chronic sleep deprivation (241) and the rat model of social isolation (524).
2. MicroRNAs and exosomal regulation
MicroRNAs (miRNA) are small noncoding RNAs capable of silencing gene expression via the RNA-induced silencing complex (RISC), which plays a role in regulating gene expression during CNS development, remodeling, and disease (592). miRNAs are packaged into exosomes that signal nearby cells, or over longer distances through circulating body fluids. Recent findings of a long-range crosstalk between periphery and CNS via miRNA-containing exosomes position the BBB in the center of this communication (685) under physiological (635, 660) and pathophysiological (378, 493, 498, 587) conditions. For instance, embryonic neurons in zebrafish larvae produce miR-132 in exosomes, which regulates BBB integrity by controlling expression of endothelial VE-cadherin, an adherens junction (AJ) protein at the BBB (660). Exosomes secreted by hematopoietic cells during inflammation cross the BBB and carry genetic information from periphery to Purkinje neurons in the cerebellum through miRNAs including miR-574-3p (498). miR-29b targets aquaporin-4 (AQP4) expression in the astrocytic endfeet (635), miR-125a-5p suppresses tumor necrosis factor (TNF)-α-induced ICAM-1 upregulation in brain endothelial cells (493), whereas miR-210 partly mediates the BBB damage in a rat model of neonatal hypoxic-ischemic brain injury through downregulation of TJ protein occludin and AJ protein β-catenin (378). Due to the ability of crossing BBB and regulating gene expression in the brain, the role of exosomes in controlling cerebrovascular integrity in health and disease, as well as the potential of translation into a therapeutic approach, calls for more in-depth studies (685).
3. Apicobasal polarity of BBB endothelium
The apicobasal polarity of the BBB endothelium is more remarkable than those in other organs in terms of polarized distributions of lipids, glycoproteins, receptors, and transporters between apical and basal membranes (654). It is initiated during angiogenesis by the partitioning defective (PAR) protein complex, including PAR3, PAR6, and CDC42, and is tightly regulated by VEGF and Wnt signaling pathways (245, 279, 704). PAR3 interaction with VE-cadherin and JAM proteins triggers the reorganization of intercellular junction proteins including ZO1, claudin-5, and CD99, resulting in the formation of TJs on the apical side of AJs, thereby limiting paracellular flow (245, 654). On the other hand, lumen formation and expansion are achieved by redistribution of surface receptors including CD34 and glycoproteins such as podocalyxin, and reorganization of cytoskeleton networks that requires β1-integrin, non-muscle myosin II, activation of RhoA pathway, and F-actin formation (245, 539). Brain endothelial cells also express the Crumbs protein complex that stabilizes the intercellular junctions (654).
4. Vascular cell lineages
In vivo lineage tracing studies using mouse genetics have demonstrated the versatility of vascular cells in cell fate during development. During embryonic heart development, pericytes can upregulate Notch3 and become coronary artery SMCs (623), while endothelial cells are capable of differentiating into cardiac pericytes and SMCs (107). This is consistent with in vitro findings based on direct differentiation of embryonic stem cells or induced pluripotent stem cells (iPSCs). For example, mesenchymal angioblasts, previously known as endothelial progenitors, can also differentiate into both pericytes with PDGF-BB and EGF2 and SMCs with TGF-β3 and sphingosylphosphorylcholine (333). Whether a similar vascular lineage tree exists in cerebral vasculature and BBB is still unknown and requires further characterization with markers or Cre drivers that are specific to brain endothelium and pericytes.
5. Immunoregulation of the BBB
The BBB is tempered by the body’s immune system. This can happen as early as in embryonic development. For example, maternal gut microbiota influences the establishment of fetal BBB during gestation, by upregulating expression of TJ proteins such as claudin-5 (77). Systemic inflammation, infection, autoimmune disease, injuries, and neurodegenerative diseases often increase the BBB permeability, allowing inflammatory mediators such as cytokines and chemokines, peripheral leukocytes such as monocytes, macrophages, and CD4+ T cells to enter the parenchyma and accelerate disease progression (484). A handful of viruses, including poliovirus, adenovirus, Epstein-Barr virus (EBV), and West Nile virus, can directly infect human brain endothelial cells by targeting junctional proteins such as JAM-A or transporters such as GLUT1 as entry receptors to get access to the CNS (320). Viral infections in general downregulate TJ proteins and promote chemokine production and vascular cell adhesion molecule 1 (VCAM-1) expression in brain endothelial cells, thereby weakening the BBB and facilitating entry into the brain (5, 212, 399, 613). Host immune response on the other hand not only limits the spreading of the viruses, but also attenuates BBB damage (131). For example, production of interferon-γ after West Nile infection can seal the BBB possibly by stabilizing TJs (348).
III. BBB TRANSPORT PHYSIOLOGY
In contrast to leaky capillary endothelium in peripheral organs (391), the BBB endothelium is sealed by TJs (492, 590, 682) and has low rate of bulk-flow transcytosis (538, 682). Brain endothelial molecular junctions, transporters, receptors, and channels have been initially discovered by physiological experiments and ultrastructural studies (133, 692), which was followed by transcriptomic approaches of endothelial and vascular mural cells. These include suppressive subtractive hybridization (86, 358, 359), microarrays (28, 72, 129), and RNA-seq analysis (242, 675, 678).
Earlier studies of BBB transcriptome have been carried out on isolated rat brain capillaries containing endothelial cells and pericytes together (358, 359). More recent studies used endothelial-specific Tie2-eGFP (enhanced green fluorescent protein) transgenic mice to investigate transcriptomes of eGFP-positive brain capillary endothelial cells purified by fluorescence-activated cell sorting (FACS) (129); GFP-positive brain endothelial cells purified by FACS from Tie2-GFP and Pdgfrß-positive pericytes purified by immunopanning (678); and microvascular fragments isolated from brains of pericyte-deficient Pdgfb, Pdgfrß, and Pdgfbret/ret mice and controls (28, 72).
RNA-seq analysis of mural cell transcriptome has been recently performed in pericytes purified by FACS for two pericyte markers, PDGFRβ and NG2 (neural/glial antigen 2) that have been expressed in Pdgfrß-eGFP; chondroitin sulfate proteoglycan-4 (Cspg4)-DsRed mice (242). Single cell RNA-seq analysis of endothelial and pericyte clusters from the mouse brain has been also reported (610, 675). The recent Nature paper presents a landmark molecular study of cell types and zonation in the brain vasculature using a clustering approach to identify genes and protein classes that are enriched along the arterial to capillary to venous axis (610). Briefly, this study is the first to report that at the BBB endothelium transcription factors predominated at arteries and arterioles, transporters predominated capillaries and veins, and ribosomal proteins indicative of protein synthesis were spread along the arteriovenous axis (610), yielding important insights to biological functions and endothelial specialization along the brain vasculature tree. In contrast to the BBB endothelium’s gradual phenotypic change along the arteriovenous axis, mural cells formed two distinct groups comprising pericyte and venous SMCs, and arterial and arteriole SMCs (610). Although this study did not examine neurons (610), an earlier single cell RNA-seq study did investigate all NVU cell types albeit with limited sequencing depth for vascular transcriptomes due to relatively low abundance of vascular clusters compared with neurons and glial cells (675). Thus a more comprehensive single cell RNA-seq study of the entire NVU is needed, in addition to regional transcriptional analysis of the NVU.
Next, we discuss BBB junctional molecules, endothelial and pericyte transport systems, and transport of molecules across brain extracellular spaces (ECS) and by perivascular and paravascular transport.
A. BBB Junctional Molecules
1. Adherens junctions
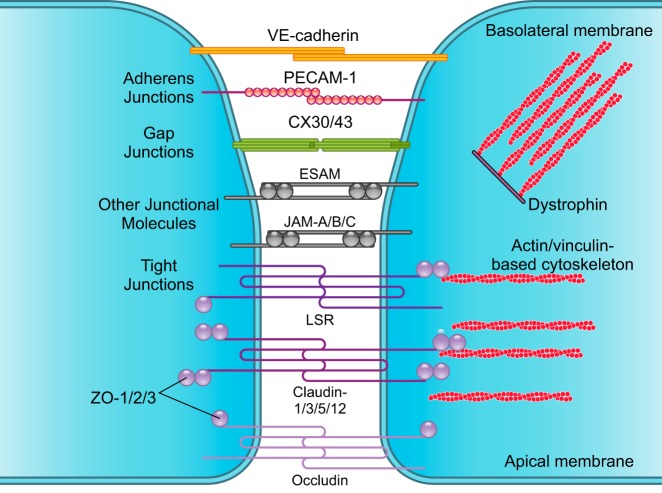
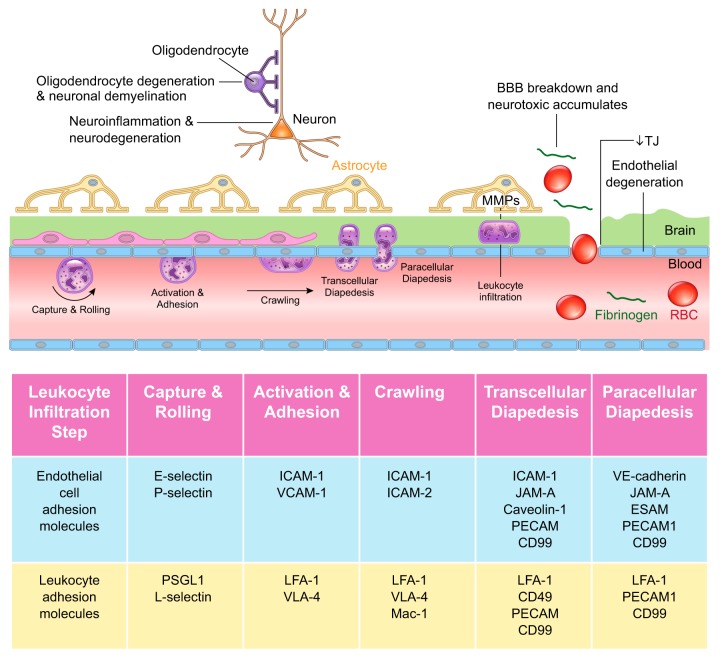
Closest to the basolateral membrane, AJ proteins, VE-cadherin, and platelet endothelial cell adhesion molecule-1 (PECAM-1) form homophilic endothelial-to-endothelial contacts roughly 20 nm wide (139, 590, 625) (FIGURE 2). AJs are connected to cytoskeleton, modulate receptor signaling (203), and regulate transendothelial migration of lymphocytes (601), monocytes (12, 203), and neutrophils (642). Tyrosine phosphorylation of VE-cadherin is required for brain transendothelial infiltration of leukocytes (601, 642).
FIGURE 2.
Brain endothelial connections. Several types of junctional molecules maintain the endothelial tight structural lining. Closest to the basolateral membrane, adherens junctions consist of vascular endothelial (VE)-cadherin and platelet endothelial cell adhesion molecule-1 (PECAM-1). Gap junctions including connexin-30 (CX30) and CX43 form hemichannels between endothelial cells. Other types of junctional molecules contribute to the tight lining including the endothelial cell adhesion molecule (ESAM) and junctional adhesion molecule (JAM)-A, -B, and -C. Closest to the apical membrane, tight junctions consist of lipolysis-stimulated lipoprotein (LSR)/angulin-1; claudin-1, -3, -5, and -12; and occludin, which limits paracellular diffusion of solutes and ions across endothelial monolayer. Zonula occludens (ZO)-1, -2, and -3 attach to claudins and occludin and bind to actin and vinculin-based cytoskeletal filaments. Dystrophin functions as a scaffold to recruit actin and vinculin, which maintains the endothelial cytoskeletal network.
2. Gap junctions
Gap junctions including connexin-37 (CX37), CX40, and CX43 form hemichannels between endothelial cells (367, 427, 597), albeit with species-dependent differences in distribution, enabling endothelial intercellular communications (682). Furthermore, brain endothelial gap junctions also function to maintain tight junction integrity (427).
3. Other junctional molecules
These include the endothelial cell adhesion molecule (ESAM) and structurally similar JAM-A, -B, and -C that modulate junctional tightness similar as AJs, and regulate transendothelial migration of leukocytes (197, 342).
4. Tight junctions
Closest to the apical membrane, TJ proteins claudin-1, -3, -5, and -12, and occludin limit paracellular diffusion of solutes and ions across the BBB (438, 590) (FIGURE 2). Loss of claudins is associated with BBB breakdown in human neurodegenerative disorders (155, 569) and acute CNS diseases (692), as well as in animal models of these diseases (420), as discussed below. Claudins can be therapeutically targeted to seal the BBB, as shown by increasing claudin-1 expression at the BBB in murine experimental autoimmune encephalomyelitis (EAE) model of MS (472). Ocln knockout mice develop male infertility, but TJs in both epithelial and CNS endothelial cells appear ultrastructurally normal and maintain normal transendothelial electrical resistance, suggesting that TJs form a functional barrier in the absence of occludin (515). Interestingly, Ocln knockout mice develop brain calcifications (515), similar as humans with OCLN mutations (448), as discussed below.
TJ proteins are connected to the actin and vinculin-based cytoskeletal filaments via scaffolding proteins of the membrane-associated guanylate kinase family ZO-1, -2, and -3 (595, 608) (FIGURE 2). ZO-1 deficiency leads to BBB breakdown in many neurodegenerative and acute CNS disorders (693). BBB also expresses the TJ protein LSR, also known as angulin-1, that has been previously identified at peripheral tricellular junctions (551). Additionally, dystrophin complex operates as a scaffold protein to recruit actin and vinculin filaments, which maintains the endothelial cytoskeletal network (603). Dystrophin knockout mice exhibit a notable brain microvascular phenotype with disrupted endothelial TJs, swollen perivascular glial endfeet, and degenerating microvessels (433).
5. Pericyte-endothelial junctions
Pericytes share a common basement membrane with brain capillary endothelial cells (570). Direct peg-and-socket contacts between pericytes and endothelial cells are formed by N-cadherin (200, 686). Gap junction CX43 hemichannels (112, 251, 347) enable intercellular communications between pericytes and endothelial cells (570, 648, 682).
6. Astrocyte junctions
Astrocytes express gap junction proteins CX30 and CX43 (167, 377, 665). Astrocyte-specific CX43 knockout (73) and/or CX43 and CX30 double knockout (167, 377) weakens the BBB leading to astrocytic edema and loss of astrocyte endfeet perivascular polarity (167, 377), and heightened leukocyte infiltration (73).
7. The basement membrane
Endothelial cells interact with the extracellular matrix (ECM) proteins in the capillary basement membrane including collagen, perlecan, and laminin via α- and β-integrin receptors, which form transmembrane heterodimers that functionally link the ECM with the cell cytoskeleton (590). Integrins mediate cell signaling by activating ECM ligands, growth factors, and growth factor receptors, which regulates multiple endothelial cell functions including survival, migration, differentiation, adhesion, and polarity (35). Integrin dysfunction leads to BBB abnormalities, as illustrated for example by β1-integrin endothelial knockout mice that develop aberrant VE-cadherin signaling, loss of claudin-5, and immature BBB (663). Conditional deletion of astrocytic laminin γ1 and acute knockdown of laminin α2 lead to breakdown of the basement membrane, loss of astrocyte endfeet polarity, reduced BBB TJs expression, and BBB disruption (667). Similarly, Lama2 knockout mice lacking laminin α2 have pronounced BBB disruption associated with reduced pericyte coverage and loss of TJ and AJ proteins (403). Thus aberrant astrocyte-capillary connections compromise BBB integrity and exacerbate microvascular dysfunction.
B. BBB Transport Systems
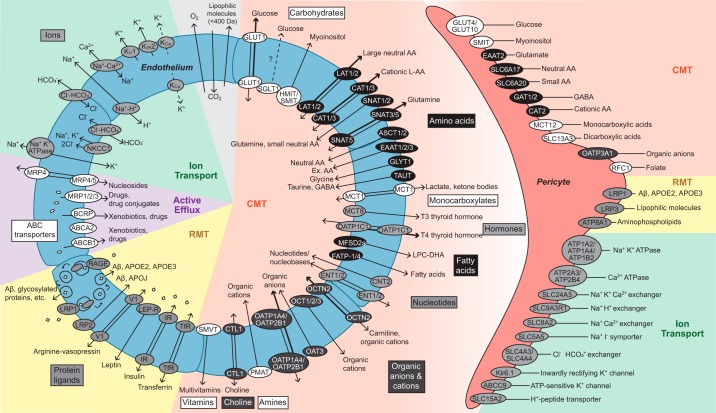
The major BBB transporters, receptors, and channels in endothelial cells and pericytes have been validated by transcriptomic studies and/or protein analysis in the rodent brain (28, 72, 86, 129, 242, 358, 359, 675, 678) (FIGURE 3). With the exception of gases (e.g., oxygen and carbon dioxide) and small lipophilic molecules (<400 Da) that freely diffuse across the endothelium (459), brain endothelial transport systems regulate molecular exchanges between blood-and-brain and brain-and-blood (2, 128, 460, 682, 692, 693). Given the close proximity and highly interactive, cooperative signaling between brain vascular pericytes and endothelial cells, it is relevant and timely to discuss current knowledge of BBB pericyte transporters from several recent studies (242, 610). While astrocytes also influence BBB integrity and transporters at astrocytic endfeet are relevant to the BBB since astrocytic endfeet surround brain vessels (13, 667, 682), currently the transcriptome or proteome specifically enriched only in astrocytic endfeet has not been examined.
FIGURE 3.
Major blood-brain barrier transport systems. Endothelium: these include solute carrier-mediated transport (CMT), receptor-mediated transport (RMT), active efflux, and ion transport. CMT systems mediate transport of carbohydrates, amino acids, monocarboxylates, hormones, fatty acids, nucleotides, organic anions and cations, amines, choline, and vitamins with precise substrate specificity and directionality, as indicated. RMT systems transport proteins including transferrin, insulin, leptin, arginine vasopressin, amyloid-β (Aβ), glycosylated proteins, and apolipoproteins E (APOE) and J (APOJ). Active efflux includes ATP-binding cassette (ABC) transporters which transport xenobiotics, drugs, drug conjugates, and nucleosides from endothelium to blood, as indicated. Ion transport underlies the movement of Na+, K+, Cl−, HCO3−, H+, and Ca2+ into and out of the endothelium via ATPases, uniporters, exchangers, and symporters, as indicated. Pericytes: presently, details about pericyte transporters’ cellular polarity and precise direction(s) of transport remain elusive. CMT systems transport carbohydrates, amino acids, carboxylates, organic anions and cations, and folate. RMT system transports Aβ, APOE, lipophilic molecules, and aminophospholipids. Ion transport of Na+, K+, Cl−, HCO3−, H+, I−, and Ca2+ occurs via ATPases, uniporters, exchangers, and symporters, as indicated. All BBB transporters indicated here are validated with RNA-sequencing and/or proteomic analysis in the rodent brain. See the main text for a more detailed discussion.
1. Endothelial solute carrier-mediated transport
Carrier-mediated transport (CMT) enables solutes such as carbohydrates, amino acids (AA), monocarboxylic acids, hormones, fatty acids, nucleotides, inorganic ions, amines, choline, and vitamins to cross the BBB via substrate-specific transporters (2, 128, 460, 682, 692, 693).
a) carbohydrate transporters.
The GLUT1 uniporter transports glucose, the key energy metabolite for the CNS, down the concentration gradient (128, 460). GLUT1 has a single binding site that can be accessed by glucose (and other hexoses) from either side of the luminal and/or abluminal endothelial membrane extracellularly or intracellularly (141). Since glucose concentration is lower in the brain interstitial fluid (ISF) compared with plasma, GLUT1 favors blood-to-brain transport of circulating glucose (141, 390, 649).
GLUT1 is expressed in endothelial cells, but not in neurons (141, 649). The importance of glucose transport across the BBB is best illustrated by the fact that Slc2a1 transcript encoding GLUT1 is one of the most abundant transcripts in brain endothelium (129). Mutations in human SLC2A gene have profound effects on brain function as discussed below.
Early immunogold electron microscopic studies have shown greater density of GLUT1 transporters on the abluminal endothelial membrane compared with the luminal membrane (171, 542). Crystallization of human GLUT1 in the inward open conformation (141) and crystallization of bacterial GLUT1 homologue have contributed to our understanding of how GLUT1 mediates glucose transport across the cell membrane (490, 565). Briefly, the U-shaped intracellular helical bundle of GLUT1 is formed by three helixes and functions as a latch to secure GLUT1 in the outward open conformation making the sugar binding site accessible extracellularly (141). After binding to the extracellular binding site, glucose enters GLUT1, which leads to a conformational change causing extracellular transmembrane domains 1, 4, and 7 to function as a latch to secure the inward open conformation of GLUT1 enabling release of glucose intracellularly (141).
Endothelial cells also express sodium glucose cotransporter 1 (SGLT1) (159) that is found in neurons (671), but its physiological role in glucose transport across the BBB remains elusive. Myo-inositol is transported via sodium/myoinositol transporter (SMIT) and H+/myo-inositol symporter (HMIT) by facilitated diffusion (682).
b) amino acid transporters.
All essential AA are transported into the brain across the BBB via large neutral endothelial AA transporter 1 and 2 (LAT1/2) that transport bidirectionally large neutral AA such as tryptophan and tyrosine (450), and the cationic AA transporter 1 and 3 (CAT1/3) that transport cationic AA such as lysine and arginine (390, 560, 692). The concentration of essential AA is lower in brain ISF compared with plasma, which favors blood-to-brain transport (693).
Glutamine levels are higher in brain ISF (240). Glutamine is transported into endothelium via sodium-coupled neutral AA transporter 1, 2, 3, and 5 (SNAT1/2/3/5) and then hydrolyzed in endothelium to glutamate via glutaminase, and removed into circulation (351).
On the abluminal endothelial membrane, sodium-dependent transporters for excitatory AA (EAAT1/2/3) transport glutamate and aspartate out of the brain (451, 692), which limits their excitotoxic effects on neurons. Sodium-dependent AA transporters ASCT1/2 and GLYT1 at the abluminal membrane remove nonessential AA alanine, serine and cysteine, and glycine, respectively, from brain-to-blood (240). Transporters of neutral and excitatory AA, glycine, taurine, and GABA are enriched abluminally and with high-affinity transport from brain-to-endothelium in a sodium-dependent fashion, and then, these AA are transported across the luminal membrane of the BBB into the blood via low-affinity transporters mediating AA clearance of nitrogen-rich and acidic AA into the circulation (351, 453).
c) monocarboxylate transporters.
Lactate released from skeletal muscles during exercise, and ketone bodies derived from liver from metabolism of fatty acids are transported from blood into the brain across the BBB by monocarboxylate transporter-1 (MCT1) (540), and then utilized as alternative energy metabolites by the brain (487).
d) hormone transporters.
Hormone endothelial transporters include MCT8 transporter for T3 (triiodothyronine) thyroid hormone and the organic anion transporting polypeptide 1c1 (OATP1C1) transporter for T4 (thyroxine) thyroid hormone (396, 502). The effects of mutations in SLC16A2 gene encoding MCT8 on brain function are discussed below.
e) fatty acid transporters.
Essential fatty acids are important for brain development and postnatal neural functions. Brain endothelium expresses luminal transporters for fatty acids, including fatty acid transport protein 1 and 4 (FATP-1/4) (413), and the MFSD2A (430). Previously an orphan MFS transporter, MFSD2A, was identified as a BBB transporter for LPC esterified DHA supplying the brain with the essential circulating omega-3 fatty acid (430). In the brain, MFSD2a is exclusively expressed in brain endothelium and is required for proper BBB development and functional integrity (62). The effects of endothelial MFSD2A mutations on brain functions are discussed below.
f) nucleotide transporters.
Nucleotides and nucleobases, e.g., cytosine, guanine, and adenine found in RNA and DNA, thymine found in DNA, and uracil found in RNA, are all transported across the BBB via sodium-independent concentrative nucleoside transporter-2 (CNT2) and the sodium-independent equilibrative nucleoside transporter-1 and 2 (ENT1/2) (97, 460). They supply brain with key substrates for DNA and RNA synthesis.
g) organic anion and cation transporters.
Organic anions are transported via organic anion transporter-3 (OAT3) and organic anion transporting polypeptide 1a4 (OATP1A4) (588) and 2b1 (OATP2B1) (193). OATP1A4 is a known BBB transporter of statin (588). Organic cation/carnitine transporter-2 (OCTN2) transports carnitine, an essential cofactor for fatty acid oxidation in mitochondria (192). Additionally, organic cations are transported via organic cation transporters 1–3 (OCT1/2/3). OCT1/2 also transport N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin causing PD-like motor impairment (363).
h) other transporters.
Amines, choline, and vitamins are also transported across the BBB. Specifically, plasma membrane monoamine transporter (PMAT) transports organic cations from brain-to-blood, choline transporter like protein type 1 (CTL1) transports choline bidirectionally across the BBB, and sodium-dependent multivitamin transporter (SMVT) transports multivitamins from blood-to-brain (2, 128, 460, 682).
2. Endothelial receptor-mediated transport
Most circulating proteins and large macromolecules (e.g., fibrinogen, immunoglobulins, albumin, thrombin, plasminogen, growth factors) are not transported across the BBB. However, some proteins and peptides use receptor-mediated transport (RMT) to traverse the BBB and enter into the brain. In general, the transport rate of circulating peptides is slower than nutrient transport across the BBB (695).
a) transferrin and insulin receptors.
Transferrin receptor (TfR) (78, 290, 461), insulin receptor (IR) (283, 458), and leptin receptor (LEP-R) (41, 206, 699) mediate blood-to-brain transport of transferrin (iron-protein carrier), insulin, and leptin across the BBB, respectively. TfR and IR have been utilized for CNS drug delivery including therapeutic antibodies and enzymes via Trojan horses’ technology (459), as discussed below. Additionally, the V1 vasopressinergic receptor mediates bidirectional arginine vasopressin transport across the endothelium (698, 701).
b) lipoprotein receptors and rage.
LRP1 and LRP2 are expressed in brain endothelium and colocalize mainly on the abluminal side of the BBB in humans and rodents (137, 404, 535, 607, 682). LRP1 binds Alzheimer’s Aβ toxin and mediates its brain-to-blood clearance (58, 137, 535, 561). Specifically, LRP1 facilitates clathrin-dependent, receptor-mediated endothelial endocytosis of Aβ at the abluminal membrane of the BBB, which requires phosphatidylinositol binding clathrin assembly protein (PICALM) (683). PICALM guides transendothelial trafficking of endocytotic Aβ-containing vesicles to Rab5, and then to Rab11 small GTPase leading to exocytosis of Aβ across the luminal membrane of the BBB into the blood (683). LRP1 also binds APOE2 and APOE3 as well as APOE2-Aβ and APOE3-Aβ complexes at the abluminal side of the BBB, mediating their efflux from brain-to-blood (135). LRP1 levels at the BBB are diminished in AD and AD models contributing to Aβ accumulation in the brain (137, 152, 410, 535). Additionally, APOJ or clusterin (CLU) binds to LRP2 (or megalin) at the BBB, which mediates Aβ42 transport from brain to blood (58, 700). Therapeutic strategies based on LRP1-mediated Aβ clearance are discussed below.
In contrast to lipoprotein receptors, the receptor for advanced glycation end products (RAGE) is expressed mainly at the luminal membrane of the BBB (134). Its expression at the BBB is increased in AD and AD models (134, 152, 410). Under pathological conditions, RAGE mediates reentry or influx of circulating Aβ across the BBB into the brain, which is associated with neuroinflammatory response, CBF reductions, and BBB breakdown (134, 136) Therapeutic strategies based on pharmacological blockade of RAGE at the BBB have advanced to phase 3 clinical trial in AD patients, as discussed below.
3. Endothelial active efflux
ATP-binding cassette (ABC) transporters utilize ATP as an energy source and are primarily expressed at the luminal side of the BBB endothelium (2, 488, 682). They function to prevent brain accumulation of drugs, xenobiotics, drug conjugates, and nucleosides via active efflux from endothelium to blood. Examples include ABCB1 (also known as P-glycoprotein, P-gp), ABCA2, breast cancer resistance protein (BCRP), and multidrug resistance-associated proteins 1–5 (MRP1/2/3/4/5). ABCB1 contributes to Alzheimer’s Aβ toxin clearance from brain-to-blood (115, 632). Diminished expression and/or dysfunction of ABCB1 were found in neurodegenerative disorders including AD and PD, as discussed below.
4. Endothelial ion transport
The BBB has a major role in controlling concentration of ions in the CNS, which is important for proper CNS functioning (582, 692).
a) sodium pump.
The abluminal sodium pump (Na+-K+-ATPase) is a key regulator of sodium (Na+) influx into the brain and potassium (K+) efflux from the brain, which keeps high concentration of Na+ and low levels of K+ in brain ISF (188, 624). This, in turn, is critical for regulating electrophysiological activity of neuronal cells including the resting membrane and action potentials, and for maintaining Na+ concentration gradient at the BBB (extracellular > intracellular), which drives Na+-dependent transport processes.
b) other ion transporters.
The luminal Na+ K+ Cl− (chloride) cotransporter (NKCC1) mediates entry of Na+, K+, and 2Cl− from blood-to-endothelium (181, 447). The bicarbonate (HCO3−)-Cl− exchanger mediates entry of intracellular Cl− and the extracellular release of HCO3− (582). The luminal Na+-H+ (hydrogen) exchanger transports H+ protons from the endothelium-to-blood in exchange for intracellular influx of Na+ and is a key regulator of intracellular endothelial pH (673).
c) calcium transporters.
The Na+-Ca2+ (calcium) exchanger cotransporter mediates Ca2+ efflux from endothelium into brain ISF, which maintains low intracellular Ca2+ levels in the microvascular endothelium (318). The abluminal transient receptor potential (TRP) channels, also known as nonselective Ca2+-conducting cation channels, are expressed both in arterial endothelium (553) and brain microvascular endothelial cell lines (38, 249). TRP channels regulate Ca2+ influx into brain endothelium that releases soluble factors such as NO, prostaglandins, and endothelial-derived hyperpolarizing factor initiating endothelium-dependent vasodilation (553).
d) potassium channels.
Capillary endothelial cells express voltage-gated K+ channel KV1 and the inward rectifier K+ channel KIR2 (318, 374, 408, 666). During physiological conditions, capillary endothelial K+ channels mediate outward K+ currents causing endothelial cell hyperpolarization that propagates vasodilatory signals upstream to arterioles contributing to blood flow regulation (374, 375).
5. Pericyte transporters
Recent transcriptomic studies suggest that pericytes express multiple transporters, receptors, and ion channels (28, 72, 129, 242, 675, 678). Some of these are discussed below.
a) solute carrier-mediated transport.
Pericytes express carbohydrate transporters such as insulin-regulated glucose transporter GLUT4 (242), facilitative glucose transporter GLUT10 (242), and the sodium/myo-inositol cotransporter SMIT (28).
Several AA transporters have been recently identified in pericytes, including the high-affinity excitatory AA transporter EAAT2 (28), sodium-dependent neutral AA transporter SLC6A17 (242), sodium- and chloride-dependent transporter SLC6A20 for small AA including glycine and proline (28, 129, 242), GABA transporter-1 and 2 (GAT1; GAT2) (28), and the cationic AA transporter CAT2 (28, 242). These transporters likely contribute to the removal of excitatory and nitrogen-rich AA from the brain to prevent excitotoxicity, similar to endothelial transporters.
Pericytes also express the monocarboxylic acid transporter-12 (MCT12) that mediates creatine transport (28, 242) and sodium-dependent SLC13A3 for dicarboxylic acids (242). Organic anions are transported via the organic anion transporter OATP3A1 (28, 129, 242). Additionally, pericytes express the vitamin transporter reduced folate carrier-1 (RFC1) (28, 72, 129, 242, 675).
The precise cellular mechanisms and function of pericyte CMT systems, and whether or not some are part of serial BBB transport mechanisms supplying brain with energy metabolites and nutrients as opposed to cell-autonomous role, remain largely unexplored.
b) receptor-mediated transport.
Pericytes express lipoprotein receptor LRP1 (129, 513), which mediates cellular uptake of Aβ followed by its intracellular degradation and clearance (513, 647). In the case of excessive Aβ load, accumulation of Aβ can lead to pericyte cell death (513, 647). Additionally, LRP1 on pericytes regulates cerebrovascular integrity in an APOE-dependent fashion (60). Studies in transgenic mice expressing human APOE isoforms have shown that astrocyte-secreted APOE2 and APOE3 bind to LRP1 on pericytes in vivo, which inhibits the proinflammatory CypA-MMP-9 pathway preventing degradation of BBB TJ and basement membrane proteins (60). On the other hand, APOE4 has a low affinity for LRP1, which activates CypA-MMP-9 pathway causing BBB breakdown (60). Activation of CypA-MMP-9 pathway associated with BBB breakdown has been also shown in human APOE4 carriers by cerebrospinal fluid (CSF) analysis (229) and post mortem brain tissue analysis (230, 267). Additionally, pericytes express LRP-3 (242) which internalizes and transports lipophilic molecules.
c) ion transport.
Transcriptomic studies suggest that pericytes express Na+-K+-ATPase α- and β-subunits (28, 129, 242), as well as Ca2+-ATPases (28, 129, 242). They also express the Na+-K+-Ca2+ exchanger SLC24A3 (28), the Na+-H+ exchanger SLC9A3R1 (72, 242), Cl−-HCO3− exchanger SLC4A4 (28) and SLC4A3 (242), and the Na+-Ca2+ exchanger SLC8A2 (242). Furthermore, pericytes express the ATP-sensitive K+ channel ATP-binding cassette subfamily C member 9 (ABCC9), the H+-peptide transporter SLC15A2 (28), the Na+-I− symporter SLC5A5 (242), and inwardly rectifying potassium (KIR) channel Kir6.1 (28, 72, 129, 242).
Functionally, adenosine binding to pericyte α1-adrenergic receptors activates ATP-sensitive K+ channels causing pericyte hyperpolarization and relaxation (232). Increases in intracellular Ca2+ in response to large increases in extracellular K+ concentrations activate voltage-gated Ca2+ channels in pericytes, which leads to pericyte depolarization and contraction (232). These findings coupled by recent physiological experiments on pericyte contractility support that pericytes play an active role in regulating CBF (65, 173, 228, 318, 319, 470).
C. Other Vascular-Mediated Transport
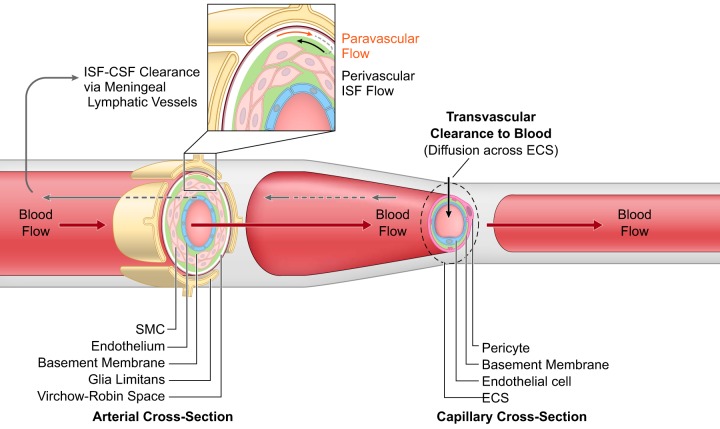
Besides the major role of transvascular transport in clearance of solutes across the BBB by CMT, RMT, major facilitators, and active efflux transporters (514, 682, 693), solutes diffuse across brain ECS and are cleared along the basement membranes of the arterial vessel walls by the perivascular ISF flow, which travels in the reverse direction of the blood flow (37, 514, 581) (FIGURE 4). Early studies in rabbits using radiolabeled albumin and in rats using India ink, albumin-labeled with colloidal gold, and Evans blue have suggested that perivascular ISF flow carries solutes and macromolecules to the subarachnoid space and CSF compartment for drainage into deep cervical lymph (75, 273). More recent studies in mice using fluorescent solutes have shown that brain has its own lymphatic vascular system in the dura matter, which drains ISF and macromolecules into the deep cervical lymph nodes (32, 160, 162, 376). These findings suggest that brain communicates directly with the peripheral immune system via meningeal lymphatic vessels. Recent magnetic resonance imaging (MRI) studies in the living human brain and marmosets utilized a combination of gadolinium-based contrast agent (Gadovist) and blood-pool contrast agent (Vasovist) to demonstrate existence of the meningeal lymphatic system (4).
FIGURE 4.
Perivascular and paravascular transport. Perivascular interstitial fluid (ISF) flows in the reverse direction of blood flow in the arterial vessel walls ultimately reaching cerebrospinal fluid (CSF)-filled subarachnoid spaces where ISF-CSF drains into the meningeal lymphatic vessels and cervical lymph nodes. Paravascular transport of solutes from subarachnoid spaces flows through Virchow-Robin spaces formed between pia membrane and glia limitans and is suggested to flow in the same direction as blood flow. At the capillary level, solutes diffuse across extracellular spaces (ECS) and undergo transvascular clearance to blood via transport systems as illustrated in FIGURE 3, and discussed in the text.
In addition to potential roles in regulating brain immune responses, the lymphatic system could also play a role in removing metabolic waste products and proteinaceous toxic accumulates from brain. For example, transport studies using radiolabeled and unlabeled Alzheimer’s Aβ peptide have shown that under physiological conditions, the perivascular ISF flow contributes to 15–20% of Aβ clearance from the mouse brain (58, 535, 659), whereas 80–85% is removed by transvascular BBB transport. Since transvascular Aβ clearance fails early in AD and AD models due to diminished expression of Aβ efflux transporters LRP1 (expressed at the abluminal endothelial membrane) and P-gp (expressed at the luminal endothelial membrane) at the BBB (420), the question persists whether perivascular Aβ clearance system is disrupted in disease due to damaged blood vessels contributing to Aβ accumulation in the arterial blood vessels and within the dura, as suggested by amyloid accumulation in the dura of Creutzfeldt-Jakob disease patients by a recent post mortem study (328). Can perivascular lymphatic system be explored therapeutically to drain Aβ from brain remains an open question.
Early studies have suggested that solutes injected into the subarachnoid space can use paravascular transport from the subarachnoid space to enter the brain through Virchow-Robin spaces in the same direction to the flow of blood (496). This concept has been explored by recent studies. For example, studies using injection of fluorescent tracers into cisterna magna of mice have suggested that paravascular circulation occurs via CSF convective flow through the ECS from the para-arterial to the paravenous spaces, which is regulated by AQP4 water channels on astrocytes, and therefore the system was renamed as the ‟glymphaticˮ system (276, 293). Other recent studies (252, 255, 547, 555), however, did not support the proposed glymphatic mechanism, nor the convective, pressure-driven fluid flow of CSF from para-arterial to paravenous spaces throughout the parenchymal ECS (31, 30, 255, 296, 547, 255). A recent report in AQP4 knockout rodents has shown that loss of AQP4 does not affect transport of fluorescent solutes from subarachnoid space to brain in rats and mice, suggesting that water production by astrocyte end feet does not control transport of solutes across brain ECS (547).
Nearly half a century ago, physiologists proposed that CSF acts as a sink for brain-derived molecules (133, 253). This concept is supported by recent findings showing that under physiological conditions brain-derived molecules secreted into ISF are present at higher concentrations in the ISF than in the CSF (32, 37, 160, 162, 514, 581).
IV. GENETIC CONTRIBUTIONS TO BBB DYSFUNCTION
A. Human Monogenic Neurological Diseases
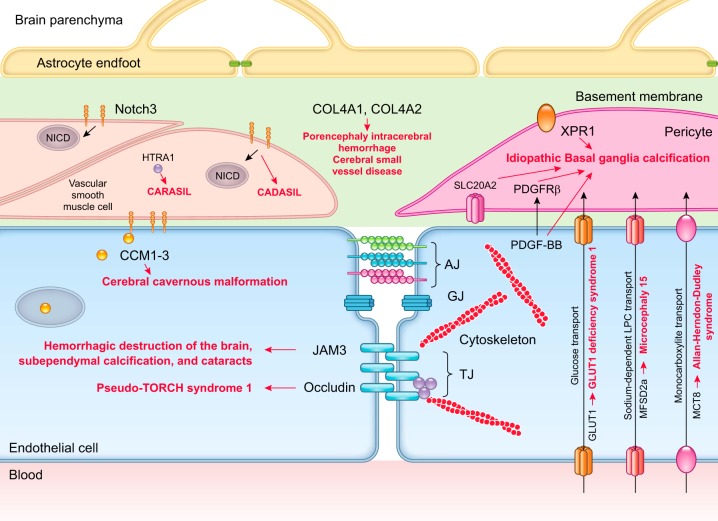
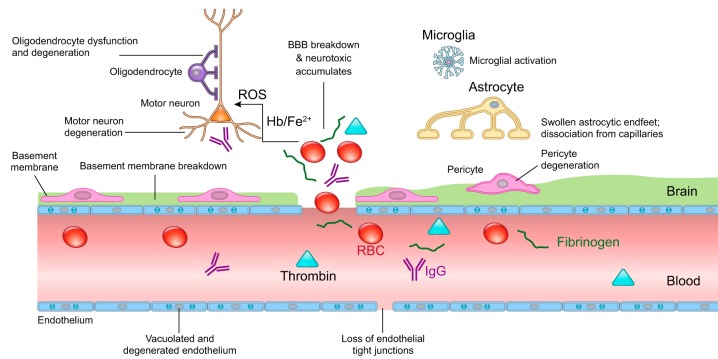
BBB disruption has been reported in several inherited human monogenic neurological diseases with genetic mutations affecting individual cell types within the BBB causing specific defects in BBB development and maintenance (682). Although rare, these neurological diseases provide direct evidence in humans for vascular and BBB contribution to neurodegeneration by demonstrating rapid disease progression from vascular defects to neurological deficits (FIGURE 5).
FIGURE 5.
Human monogenic diseases of the blood-brain barrier. Endothelium: monogenic diseases affecting transporters include glucose transporter 1 (GLUT1) causing GLUT1 deficiency syndrome, major facilitator superfamily domain-containing protein 2a (MFSD2a) causing microcephaly 15, and monocarboxylate transporter-8 (MCT8) causing Allan-Herndon-Dudley syndrome. Cerebral cavernous malformations (CCM) are caused by mutations in endothelial proteins CCM1–3. Monogenic diseases affecting tight junctions include occludin that causes Pseudo-TORCH syndrome 1 and junctional adhesion molecule 3 (JAM3) that causes brain hemorrhagic destruction, subependymal calcification and congenital cataracts. Basement membrane: mutations affecting collagen type IV alpha 1 chain (COL4A1) and collagen type IV alpha 2 chain (COL4A2) lead to porencephaly, intracerebral hemorrhage, and cerebral small vessel disease. Pericytes: mutations in platelet-derived growth factor-BB (PDGF-BB), PDGF receptor-β (PDGFRB), solute carrier family 20 member 2 (SLC20A2), and xenotropic and polytropic retrovirus receptor 1 (XPR1) lead to idiopathic basal ganglia calcification. Vascular smooth muscle cells: mutations in notch homolog protein 3 (NOTCH3) cause cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), and mutations in HtrA serine peptidase-1 (HTRA1) protein cause cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL).
1. Genetic mutations in endothelial cells
a) slc2a1.
Loss-of-function mutations in human SLC2A1 gene, which encodes the GLUT1 glucose endothelial transporter at the BBB, result in GLUT1-deficiency syndrome in humans manifesting with early-onset seizures, microcephaly, and developmental delay (630). Glut1+/− mice phenocopy human pathology, and develop initially within 2–3 wk of age BBB breakdown with loss of tight junctions and diminished glucose uptake by the brain, which is followed by microvascular regression, reduced brain perfusion and development of secondary neurodegenerative changes, microcephaly, and loss of cortical and hippocampal neurons (649).
b) mfsd2a.
Inactivating mutations in MFSD2A gene, encoding the sodium-dependent endothelial transporter that transports essential omega-3 fatty acids into the brain (430) and regulates caveolae-mediated transcellular trafficking across the BBB (21, 62, 684), lead to development of microcephaly syndrome in humans associated with loss of neurons and intellectual disability, and in case of some mutations lethal microcephaly (8, 218). As in humans, murine Mfsd2a is expressed in the brain exclusively in endothelium of the BBB (62, 430). Mfsd2a-deficient mice exhibit impaired brain uptake of lysophosphatidylcholine-long fatty acyl chains and develop BBB breakdown, which is accompanied by neuronal loss in hippocampus and cerebellum causing microcephaly and behavioral deficits (62, 63, 430, 684).
c) slc16a2.
Another disorder associated with a BBB transporter dysfunction is Allan-Herndon-Dudley syndrome caused by inactivating mutations in SLS16A2 gene encoding MCT8 transporter for T3 thyroid hormone (156, 185). These mutations lead to severe impairment in neuronal development and functional deficits causing psychomotor retardation and intellectual disability due to deficient transport of T3 from blood to brain associated with altered serum thyroid parameters (92).
d) krit1, ccm2 and pdcd10.
Mutations in endothelial KRIT1, CCM2, and PDCD10 genes encoding cerebral cavernous malformation (CCM) proteins 1, 2, and 3 lead to formation of enlarged and irregular small vessels in the brain including thin-walled capillaries and leaky vascular lesions of venous origin (178). CCMs in humans are typically located in the cortical white matter, and due to fragile vessels lead to intracerebral hemorrhages, focal neurological deficits, gait ataxia, seizures, and ischemic strokes (178). The CCM proteins form a complex that is critical for maintaining proper endothelial cell junctions as well as polarization and also inhibit endothelial-to-mesenchymal transition, which when activated contributes to the onset and progression of CCM (380). Besides familial form, the sporadic CCMs occur in people. Together, familial and sporadic CCMs are estimated to affect 1 in 200 people (179).
e) col4a1 and col4a2.
Mutations in COL4A1 and COL4A2 genes encoding the basement membrane collagen proteins COL4A1 and COL4A2 lead to cerebral small vessel disease in humans associated with lacunar ischemic strokes, intracerebral hemorrhages, and white matter hyperintensities (210). COL4A1 and COL4A2 are major proteins of the basement membranes, and their transcripts are found in vascular endothelial cells and in many other cell types (210). The exact contribution of endothelial cells versus other BBB-associated cell types to generation of mutant COL4A1 and COL4A2 proteins and small vessel disease is not clear at present. Col4a1 knockout mice develop also fragile vessels that are prone to hemorrhage upon hemodynamic stress and mild trauma (337).
f) ocln.
Mutations in OCLN gene in humans encoding the TJ endothelial protein occludin lead to development of severe neurological syndrome known as pseudo-TORCH 1 that is characterized with bands of gray matter calcification on neuroimaging, severe microcephaly with simplified gyration and polymicrogyria, early-onset seizures, and developmental delay (448).
g) jam3.
Homozygous mutations in JAM-3 gene in humans encoding endothelial junctional molecule JAM-C also result in a compromised BBB, often brain hemorrhages, subependymal calcification, seizures, and congenital cataracts (7). JAM-C-deficient mice develop hemorrhages and hydrocephalus (657).
2. Genetic mutations in mural cells
a) pdgfb or pdgfrb.
Loss-of-function mutations in endothelial PDGFB or pericyte PDGFRB genes can cause primary familial brain calcification also known as idiopathic basal ganglia calcification (IBGC) or Fahr’s disease, which is characterized by early onset of deep brain calcification, SMCs, and pericyte loss and neurological symptoms including motor and cognitive impairments (309, 434). Mice carrying inherited deficiencies in the PDGF-B/PDGFRβ signaling pathway, e.g., Pdgfbret/ret mice with deletion of the retention motif of PDGF-B casing diminished PDGF-BB bioavailability, PdgfbF7/F7 mice with seven mutations on the COOH terminus of PDGFRβ receptor disrupting the downstream signaling transduction, and Pdgfrß+/− mice with a single Pdgfrß allele and reduced expression of PDGFRβ in pericytes, all develop early pericyte loss and BBB impairment (28, 59, 130). In the case of Pdgfrß deficiency, BBB breakdown and vascular phenotype precede neuronal degeneration and loss (59, 319, 650), whereas pericyte-deficient Pdgfbret/ret mice develop deep brain calcification at a later age resembling IBGC (309).
b) notch3.
Mutations in NOTCH3 gene, which is specifically expressed in vascular mural cells including SMCs and pericytes, result in subcortical ischemic attacks or strokes between 35 and 55 yr of age before progressing to dementia (100). This disease named cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an autosomal dominant stroke syndrome with a prevalence of 2–4/100,000 individuals (100). Animal models carrying Notch3 mutations closely recapitulate human pathology and disease progression, reckoning that vascular changes or defects including BBB impairment and/or microcirculatory insufficiency can lead to secondary neuronal dysfunction and/or white matter damage. For example, mice carrying the CADASIL Notch3R169C mutation show accumulation of NOTCH ectodomain in pericytes, pericyte degeneration, and BBB breakdown as early as at 7 mo of age (202), while Notch3 null mice exhibit BBB disruption shown by leakages of circulating tracers in the brain and perivascular deposits of fibrin (244), suggesting that normal NOTCH function is required for the proper maintenance of the BBB.
c) htra1.
Biallelic mutations in high-temperature requirement serine peptidase A1 (HTRA1) gene located on chromosome 10q25 result in subcortical lacunar infarcts and subsequent vascular dementia (591). This rare familial disease is called cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) and was first described four decades ago in Japanese families with a high degree of consanguinity (381). Interestingly, HTRA1 is highly expressed in vascular mural cells, particularly SMCs (371), and its encoded protein HTRA1 plays a key role in facilitating TGF-β signaling through processing latent TGF-β binding protein 1 (LTBP-1) (55). An attenuation of TGF-β signaling caused by a lack of HTRA1-mediated LTBP-1 processing has been proposed as a mechanism underlying CARASIL pathogenesis. Dysregulation of TGF-β activity results in microvascular dysfunction leading to white matter lesions (577). In addition, TGF-β1 was shown to reduce mural cell proliferation and elevate MMPs expression and proinflammatory cytokines, which may altogether disrupt BBB function (508). Experimental studies in HTRA1-deficient animals have recently started (55), but much more needs to be done in regards to understanding the mechanisms underlying neurovascular dysfunction and BBB breakdown.
B. Chronic Neurodegenerative Diseases
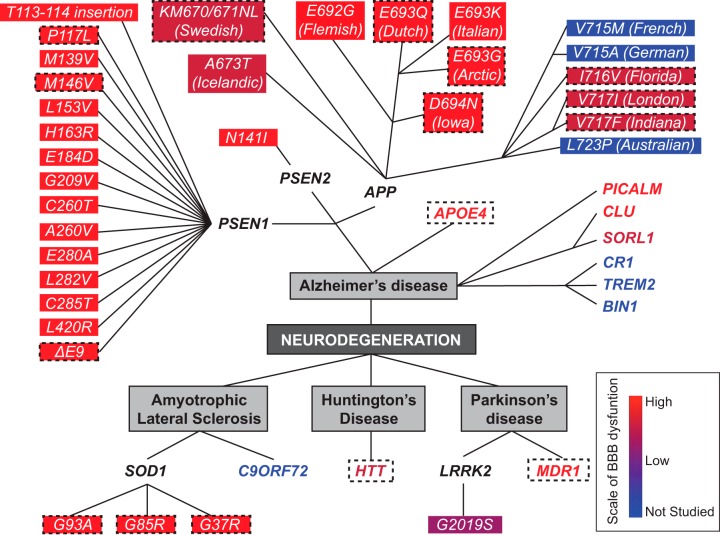
Several genetic mutations that carry inheritance or increase risk for neurodegenerative diseases such as AD, PD, HD, and ALS can lead to BBB breakdown and cerebrovascular pathology in humans and animal models (FIGURE 6).
FIGURE 6.
Effects of genetic mutations carrying inheritance or increasing risk for neurodegenerative disorders on blood-brain barrier. Alzheimer’s disease (AD): APP: amyloid precursor protein (APP) vasculotropic mutations E692G (Flemish), E693Q (Dutch), E693K (Italian), E693G (Arctic), and D694N (Iowa) lead to prominent cerebral amyloid angiopathy (CAA) causing extensive cerebrovascular pathology and blood-brain barrier (BBB) breakdown in humans (orange red). Dotted boxes denote validation of BBB breakdown in transgenic rodents carrying the respective human vasculotropic mutations. APP NH2-terminal KM670/671NL (Swedish) mutations and A673 (Icelandic) mutations lead to a moderate CAA and BBB breakdown in humans (berry red). Dotted box denotes validation of BBB breakdown in transgenic animals expressing Swedish mutation. Cerebrovascular function in human carriers of APP COOH-terminal V715M (French), V715A (German), I716V (Florida), V717I (London), V717F (Indiana), and L723P (Australian) mutations has not been examined (blue; not studied). However, the BBB breakdown has been shown in transgenic models carrying Florida, London, and Indiana mutations (berry red). PSEN1: BBB breakdown and cerebrovascular dysfunction have been reported in humans carrying different PSEN1 mutations including T113–114 insertion, P117L, M139V, M146V, L153V, H163R, E184D, G209V, C260V, E280A, L282V, C285T, L420R, and ΔE9 deletion (orange red). Dotted boxes denote validation of BBB breakdown in transgenic animal models carrying the respective human PSEN1 mutations. PSEN2: the most common PSEN2 mutation N141I in humans is associated with BBB breakdown (orange red). APOE4: apolipoprotein E (APOE4), the major genetic risk factor for sporadic AD, leads to BBB breakdown in humans and transgenic models expressing human APOE4 gene (orange red, dotted box). Others: phosphatidylinositol binding clathrin assembly protein (PICALM) and clusterin (CLU) regulates clearance of amyloid-β peptide across the BBB (orange red), while sortilin-related receptor-1 (SORL1) expressed in brain endothelial cells regulates PDGF-BB and LRP1 signaling at the BBB (berry red), as shown in animal studies. Complement receptor 1 (CR1), triggering receptor expressed on myeloid cells-2 (TREM2), and bridging integrator 1 (BIN1) have not been studied for their cerebrovascular effects. Amyotrophic lateral sclerosis (ALS): transgenic rodents expressing human superoxide dismutase-1 (SOD1) G93A, G85R, and G37R mutations develop an early and pronounced BBB and blood-spinal cord barrier (BSCB) breakdown (dotted box), also confirmed in humans with familial ALS (orange red). Vascular pathology has not been studied in C9ORF72 mutation carriers (blue; not studied). Huntington’s disease: mutation in huntingtin protein causing the disease (i.e., HTT CAG repeat expansions) leads to BBB pathology in humans and animal models (orange red + dotted box). Parkinson’s disease (PD): leucine-rich repeat kinase-2 (LRRK2) mutation leads to familial PD and LRRK2 G2019S leads to a moderate BBB breakdown in humans (purple). Mutations in multi-drug resistance gene (MDR1) lead to BBB dysfunction in humans and animal models (orange red). The color scale: BBB breakdown is pronounced (orange red), moderate (berry red), modest (purple), or not studied (blue). See the main text for more detailed discussion.
1. Alzheimer’s disease
Autosomal-dominant AD (ADAD) is an inherited form of AD caused by mutations in amyloid precursor protein (APP) and presenilin-1 and 2 (PSEN1; PSEN2) genes (49, 177, 303, 304, 580). ADAD accounts for ~1% of all AD cases and exhibits early age of onset (<65 yr of age) (17). Several APP and PSEN1 mutations lead to BBB breakdown and cerebrovascular pathology, as discussed below and illustrated in FIGURE 6.
The large majority of AD cases, however, are sporadic, late-onset without clear etiology or inheritance. Nevertheless, several genes are associated with increased or lower risk for sporadic late-onset AD. Apolipoprotein E4 (APOE4) is the major genetic risk factor for sporadic AD (258, 368, 612, 681). APOE4 leads to BBB breakdown, vascular pathology, and diminished clearance of Aβ across the BBB (694), as discussed below.
Genome-wide association studies (GWAS) have identified multiple loci associated with AD including to name a few, variants in PICALM, CLU, ATP-binding cassette transporter A7 (ABCA7), sortilin related receptor-1 (SORL1), complement receptor 1 (CR1), triggering receptor expressed on myeloid cells 2 (TREM2), and bridging integrator 1 (BIN1) genes (219, 236, 254, 300, 343, 344, 528, 543). Below, we examine variants that affect BBB transport and clearance functions associated with PICALM, CLU, and SORL1 genes (FIGURE 6).
a) app.
Approximately 40 APP mutations have been identified causing ADAD (52). APP mutations can lead to cerebrovascular pathology including BBB breakdown and cerebral amyloid angiopathy (CAA), as shown in humans (49, 211, 356, 674) and transgenic animal models expressing human APP mutations (56, 57, 137, 321, 322, 331, 336, 405, 420, 464, 467, 512, 513, 579, 606). CAA is caused by Aβ deposition in the vascular wall of small brain arteries and capillaries and develops as a result of an imbalance between Aβ production and clearance, particularly faulty transvascular and perivascular clearance of Aβ from the brain (318, 420, 581, 640). CAA is a major cause of SMC vascular degeneration that is associated with BBB breakdown at the arterial and/or arteriolar level, lobar microbleeds, infarcts, white matter changes, and cognitive impairment worsening AD pathology (514, 640).
Individuals with ‟vasculotropicˮ APP mutations within Aβ21–23 residues including Dutch (E693Q), Arctic (E693G), Flemish (A692G), Iowa (D694N), and Italian (E693K) mutation (49, 211, 335, 356, 526, 674) develop prominent CAA (238, 640) causing extensive cerebrovascular pathology. For example, the Dutch mutation leads to recurrent hemorrhages due to damage of the arterial vessel wall by the CAA, known as hereditary cerebral hemorrhage with amyloidosis (HCHWA-D), which is often fatal by mid-life (302, 356). Patients with HCHWA-D rarely develop parenchymal amyloid plaques and neurofibrillary tangles (428). CBF reductions have also been reported in humans carrying vasculotropic APP mutations (49). Experimental studies have shown that the vasculotropic Aβ mutant peptides are poorly cleared from brain across the BBB into circulation due to their low affinity for the BBB clearance receptors including LRP1 compared with wild-type Aβ peptides (137, 416); therefore, Aβ mutant peptides accumulate rapidly along the vessel walls.
In contrast to vasculotropic mutations, APP NH2-terminal mutations such as Swedish (KM670/671NL) mutation and APP COOH-terminal mutations including A713T, A714I, A714A, V715M (French), V715A (German), I716V (Florida), I716T, V717I (London), V717F (Indiana), V717G, V717L, and L723P (Australian) mutations lead aberrant and increased Aβ production by affecting β-secretase and γ-secretase processing activities of APP, respectively (303, 304, 356, 580). Compared with vasculotropic mutations, APP NH2- and COOH-terminal mutations are associated with less pronounced CAA and cerebrovascular pathology (356). The NH2-terminal APP A673T (Icelandic) mutation, however, makes APP a less favorable substrate for β-secretase, resulting in decreased Aβ production (299). Interestingly, despite sparse parenchymal amyloid deposition, the Icelandic mutation leads to mild CAA and vascular pathology including microinfarcts (311) and ischemic stroke (468), suggesting vascular vulnerability.
Similar to humans, transgenic mice expressing different human APP mutations exhibit pronounced vascular pathology including severe BBB permeability changes (322, 467, 579, 606), microbleeds (56, 321, 322, 336, 579), BBB leakages of blood-derived molecules (109, 331, 336, 467, 513), impaired Aβ clearance at the BBB (57, 137, 464, 512), endothelial cell degeneration (336, 513), loss of VSMCs (336, 464) and pericytes (336, 464, 513), and CAA (57, 151, 186, 257, 405, 583).
Some studies in transgenic murine models have examined the temporal sequence of appearance of different pathologies, revealing that progressive BBB breakdown develops early in APPSw/0 mice beginning at 1–3 mo of age (467, 513, 606) before Aβ deposition, CAA, and behavioral memory recognition deficits that are observed beginning at 10–12 mo of age (151, 336, 606, 638). These studies suggest that CAA is not the only cause of BBB breakdown in APP transgenic models. Although the precise mechanism of early BBB breakdown in APP mice is currently unclear, oligomeric Aβ toxic species and/or direct APP-mediated vasculotoxicity could play a role in CAA-independent early BBB breakdown and vascular pathology.
b) psen1.
To date, 228 PSEN1 mutations have been identified causing ADAD (49, 303, 304, 440, 566, 580). PSEN1 is the catalytical component of γ-secretase (304). PSEN1 mutations increase the faster release of long Aβ peptide species due to altered carboxypeptidase-like γ-secretase activity that increases the proportion of Aβ42, Aβ43, and even longer Aβ peptide species (Aβ45, Aβ46) (104, 172, 489, 572). Moreover, the ratio of Aβ42:Aβ40 is altered in most, but not all, PSEN1 mutations carriers (572), and the significance of the altered ratio is not well understood. PSEN1 mutations lead to faster soluble-to-fibrillar conversion of Aβ42 promoting amyloid deposition in the brain (303, 566, 580). Some PSEN1 mutations lead to very early onset ADAD (<35 yr of age) (177, 304).
Human PSEN1 mutation carriers have notable BBB breakdown and cerebrovascular dysfunction including cerebellar amyloid angiopathy and CAA (143, 183, 274, 280, 354, 389, 440, 500, 544, 573, 639, 668); disrupted meningeal, subpial, and cortical arterioles (274, 389, 440); degeneration of pericytes and SMCs (573); cerebral perivascular amyloid deposits (25, 274, 289, 354, 573, 668); and diminished FDG transport across the BBB in an early asymptomatic stage (53, 61). Particularly, cerebrovascular pathology with BBB breakdown was shown by neuropathological studies in patients with PSEN1 T113–114 insertion (544), P117L (573, 639), M139V (183, 389), M146V (389), L153V (289), H163R (280), E184D (668), G209V (389), A260V (389), C260T (274), E280A (354), L282V (143), C285T (274), and L420R (440) missense mutations, and Δe9 deletion (280).
Similar to humans, mice expressing human PSEN1M146V mutations driven by the neuronal Thy1 promoter develop BBB breakdown with microhemorrhages and basement membrane degeneration in the absence of Aβ pathology or CAA (191). Additionally, PSEN1−/− mice exhibit severe microbleeds and endothelial degeneration in the neocortex at embryonic day 18.5 (641), indicating that PSEN1 loss of function induces BBB damage. Interestingly, hemorrhages and vascular abnormalities in PSEN1−/− mice can be corrected by neuron-specific PSEN1 expression (641), suggesting that impaired vascular-neuronal cross-talk contributes to vascular pathophysiology (191, 641).
c) psen2.
PSEN2 mutations account for only ~5% of all ADAD cases (394). The mutations N141I (Volga German pedigree) and M239V represent ~75% of all PSEN2 mutations (91). Similar to PSEN1, PSEN2 mutations may also cause increased production of long Aβ peptide species (481, 523), although this needs more investigation. PSEN2 N141I mutation carriers have severe CAA and hemorrhagic strokes, but sparse parenchymal amyloid and neurofibrillary tangles (442). Thus far, few studies have investigated cerebrovascular dysfunction in individuals with other PSEN2 mutations.
d) apoe4.
APOE4 is the major genetic risk factor for sporadic, late-onset AD (199, 368, 580, 612). One and two APOE4 alleles increase risk for AD by ~3.8- and ~12-fold, respectively, compared with APOE3/APOE3 genotype. The effect of one APOE4 allele on AD risk is stronger in females than in males. One copy of APOE2 allele decreases risk by ~0.6-fold relative to APOE3/APOE3 genotype. Additionally, APOE4 increases risk for CAA.
APOE exerts toxic effects on the cerebrovascular system (694) and neurons (383, 384) and influences Aβ clearance (60, 64, 98, 135, 256, 266, 278, 294, 315, 693), amyloid deposition (39, 256, 258, 266, 278, 314, 518, 612), and tau-related neurodegeneration (533) in an allele-dependent manner APOE4 > APOE3 > APOE2. Human APOE4 carriers compared with non-carriers develop accelerated BBB breakdown and pericyte degeneration (229, 230, 267, 516, 691, 702), early neurovascular dysfunction (494, 531, 584), impaired cerebrovascular reactivity (227, 568), and diminished regional BBB uptake of glucose (457, 486). Cerebrovascular effects of APOE4 are associated with AD, stroke, and brain hemorrhage (314, 506, 549, 614, 693).
Studies in animal models support that APOE4 compared with APOE3 and APOE2 diminishes Aβ clearance across the BBB (58, 135), which has been confirmed in transgenic APOE4 mice (98). Transgenic mice expressing human APOE4 gene, but not APOE3 and APOE2, develop an early BBB breakdown (60, 437), cerebral microhemorrhages (85), and loss of endothelial GLUT1 expression (9) that is followed by secondary neurodegenerative changes (60). Transgenic mice lacking Apoe also develop an early BBB breakdown (60, 99, 147, 187, 226, 233, 406, 426, 437, 554), indicating that APOE is essential for maintaining BBB integrity. Apoe−/− mice also develop secondary neurodegenerative changes after BBB breakdown in the absence of Aβ pathology (60).
APOE in the brain is primarily synthesized by astrocytes and microglia, as indicated by a recent single cell RNA-seq study in the mouse (610). APOE3 promotes enzyme-mediated degradation of Aβ in microglia more efficiently than APOE4 (295), suggesting the APOE and microglia play a role in the innate immune response in AD (368). Nevertheless, the role of APOE secreted by other cell types such as pericytes (82, 96, 646) and SMCs (661) should be further explored.
e) picalm.
The association of PICALM polymorphisms with late-onset AD has been reported by the majority of GWAS studies (94, 119, 236, 265, 298, 344, 528). Although most of the PICALM single nucleotide variants (SNPs) associated with late-onset AD are located outside of the coding regions (683), the risk alleles of rs3851179 (236) and rs10792832 (344) lead to lower expression of PICALM isoform 2 in the frontal and temporal cortex in the human brain on the expression quantitative trait loci (eQTLs) (586), indicating that lower PICALM levels may increase the risk for AD.
The NH2 terminus of PICALM contains an epsin NH2-terminal homology domain for phosphatidylinositol-4,5-bisphosphate binding, which allows PICALM to sense membrane curvature (284) and regulate the size of clathrin-coated vesicles (409). PICALM controls receptor internalization and subsequent intracellular trafficking (683), via R-SNARE-mediated fusion of clathrin-coated vesicles with endosomes (411). These functions are central to its role in the clearance of both tau through autophagy (423) and Aβ via transvascular transport across the BBB (683).
PICALM is enriched in the endothelium of human cerebral vessels including capillaries, but is downregulated in AD patients (683). As shown in the Picalm+/−; APPSwe mice, diminished PICALM levels at the BBB accelerate amyloid pathology and behavioral deficits, which can be ameliorated by endothelial re-expression of Picalm using an adeno-associated virus (AAV) (683). Endothelial cells derived from human iPSCs carrying homozygous protective rs3851179A alleles exhibited increased PICALM expression and improved transvascular clearance of Aβ across human BBB in vitro, when compared with isogenic cells from iPSCs carrying homozygous risk rs3851179G alleles (683). Overexpression of PICALM in the primary rat cortical neurons attenuated the toxicity of soluble Aβ oligomers (598). However, reducing or overexpressing PICALM levels in hippocampal neurons of APPSwe; PSEN1L166P mice with AAV8-mediated delivery of PICALM shRNA or cDNA, respectively, indicated that PICALM might regulate Aβ production (658). Moreover, PICALM-guided APP intracellular trafficking to autophagosome for degradation (589) limits Aβ production working in harmony with Aβ transvascular BBB clearance to keep low levels of Aβ in the brain.
f) clu.
Several GWAS studies have identified CLU as a significant genetic risk factor for sporadic AD (94, 119, 236, 343). The functional impact of CLU polymorphisms is currently elusive (304). CLU gene encodes clusterin or APOJ that besides its functions in lipid transport, membrane recycling, cell adhesion, and apoptosis (90, 444, 600, 677) affects transvascular Aβ clearance by promoting Aβ42 efflux across the BBB (580). In the brain, astrocytes primarily secrete clusterin which acts as a chaperone molecule that binds soluble Aβ (444). LRP2 also known as glycoprotein 330 (gp330) or megalin mediates transport of Aβ-clusterin complexes across the BBB (58, 700).
g) sorl1.
SORL1 was identified through GWAS as a risk factor for sporadic AD (495, 503). SORL1, a vacuolar protein sorting-10 (Vps10) domain-containing protein, binds PDGF-BB (205, 247) and LRP1 ligands (205). Proper interactions with PDGF-BB and LRP1 ligands are necessary for functional downstream signaling of PDGFRβ and LRP1, respectively. SORL1 mutations may impact PDGFRβ signaling in mural cells causing pericyte dysfunction and/or degeneration that is reported in AD, and may also impair LRP1-mediated transvascular clearance, a key mechanism by which Aβ40 and 42 peptides are cleared from brain-to-blood (696).
h) other genes.
AD risk genes affect various biological functions, including vascular, immune, metabolic, trafficking, transcription and adhesion, or a combination. Some additional highly replicated risk genes include CR1, BIN1, and TREM2 (94, 119, 219, 300, 344, 543). CR1 is predominantly expressed by erythrocytes (343, 344) and Aβ-C3b-CR1-mediated interactions sequester Aβ42 to promote clearance to periphery (180, 338), suggesting that CR1 variants can increase free blood Aβ levels (343), which in turn can promote RAGE-mediated Aβ re-entry across the BBB into the brain. BIN1 is broadly expressed in brain cell types (344), but the effect of BIN1 variants on cerebrovascular function has not been explored. Microglial TREM2 variants (300, 344) are partial loss of function with impaired transport of APOE-containing lipoproteins (633). TREM2 (219, 300, 543) and two novel rare coding variants, PLCG2 and ABI3, implicate innate immunity in AD pathophysiology (543), but whether these variants can influence vascular-inflammatory cross-talk contributing to AD pathophysiology remains unclear.
2. Parkinson’s disease
PD is the second most common neurodegenerative disease after AD. It is characterized by filamentous and oligomeric α-synuclein (α-syn) accumulation, and degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) leading to motor impairments (357, 616). About 10–15% of PD cases have familial genetic etiology (616).
a) lrrk2.
Leucine-rich repeat kinase 2 (LRRK2) missense mutations cause late-onset PD (>50 yr) (259). The most common LRRK2 mutation, G2019S (gain of function), is associated with 2% of sporadic cases and 6% of familial cases (357). Recently, increased monocyte attachment was observed in the presence of endothelial cells expressing LRRK2 G2019S (259), suggesting endothelial dysfunction. Beyond this study, additional measures of vascular integrity and function have not been explored in LRRK2 mutation carriers.
b) mdr1.
MDR1 mutations are associated with familial and sporadic PD (6, 189). Highly expressed at the BBB endothelium, MDR1 encodes for ABCB1 (P-gp) that has reduced expression in vivo in PD patients, and is proposed to contribute to PD progression (327).
3. Huntington’s disease
a) htt.
HD etiology is entirely due to autosomal-dominant HTT mutations with abnormal CAG repeat expansion in exon 1, producing mutant huntingtin protein that aggregates and leads to neurodegeneration (379). HD manifests with motor, cognitive, psychiatric, and metabolic abnormalities. Although not often associated with HD pathophysiology, BBB dysfunction is evident in HD (155, 263, 364). Specifically, HD patients exhibit increased BBB permeability in the caudate nucleus as shown by dynamic contrast-enhanced (DCE)-MRI analysis that associates with disease burden, and increased gray matter cerebral blood volume (155). Histological analysis of BBB breakdown shows perivascular capillary fibrin(ogen) deposition, diminished endothelial tight junction proteins claudin-5 and occludin (155), and reduced pericyte coverage (263) in HD brains. The ratio of capillaries to arterioles is increased in the HD putamen (155), cortex, and substantia nigra (155, 364), suggestive of microvascular angiogenesis. Furthermore, mutant huntingtin aggregates accumulate in brain endothelial cells, perivascular macrophages, SMCs, and vascular basal lamina in HD (155), and in genetically unrelated neural allografts within the brains of patients with advanced HD (114), suggesting that cerebral vasculature may contribute spreading of mutant huntingtin. Similarly, HD R6/2 transgenic mice with 160 CAG repeats exhibit increased BBB permeability (155), increased microvascular density (263), and loss of TJs that precedes disease onset (148).
4. Amyotrophic lateral sclerosis
ALS is characterized by excessive motor neuron loss in the spinal cord, motor cortex, and brain stem that causes progressive paralysis, muscle atrophy, and death ~3–5 yr following symptomatic onset (157, 313). Approximately 10% of ALS cases are familial with autosomal dominant inheritance of mutations reported in at least 15 genes, including to name a few SOD1, TARDBP, FUS, ANG, and OPTN (11, 18, 174, 465), and an expanded hexanucleotide repeat of GGGGCC in a noncoding region of the C9ORF72 gene (140, 497).
A few post mortem studies revealed BBB disruption with red blood cells (RBCs) extravasation, pericyte reductions, and reduced levels of TJ proteins in the spinal cord of ALS patients with both familial and sporadic form of disease (243, 652). Next, we discuss experimental studies showing BBB breakdown in transgenic rodents carrying human SOD1 mutations.
a) sod1.
Mutations in SOD1, which encodes the antioxidant enzyme superoxide dismutase 1, account for 2% of all ALS cases (11). Mutated SOD1 can form intracellular aggregates in a trimeric form that interfere with motor neuron survival (485). Motor neuron injury in SOD1 mutations results from toxic gain-of-function rather than loss of dismutase activity (275).
Studies in SOD1 transgenic mice expressing dismutase-active (G93A and G37R) and dismutase-inactive (G85R) mutations have suggested that BSCB breakdown occurs before motor neuron injury (194, 415, 651, 687, 688). For instance, SOD1G93A mice develop ultrastructural capillary alterations, extravasation of RBCs, and deposition of fibrin(ogen) and hemosiderin deposits in the brain stem and spinal cord early in the disease (194, 651, 687). BBB disruption including diminished levels of TJ proteins, immunoglobulin G (IgG) and hemosiderin perivascular deposits, and GLUT1 reduction has been shown to precede neuroinflammation, motor neuron loss, and motor impairment in different types of SOD1 mutants (415, 687, 688). Early BBB breakdown was also shown in SOD1G93A rats at a presymptomatic stage (431) and has been confirmed using different contrast-enhanced MRI techniques (20, 51). One study, however, failed to detect any T1-enhancement after gadolinium injection in SOD1G93A mice, suggesting no change in BBB and BSCB integrity (166), in contrast to several independent previous studies discussed above showing disrupted BSCB and BBB in SOD1G93A mice (20, 50, 51, 194, 196, 415, 431, 432, 687, 688). Whether or not BBB breakdown is detectable in ALS patients carrying familial SOD1 mutations, as shown in SOD1 mutant models, has not been explored.
b) c9orf72.
The GGGGCC repeat expansion suppresses the production of C9ORF72 protein by inhibiting transcription (140, 153, 225, 369, 497, 520). The role of the C9ORF72 protein in C9ORF72 ALS disease remains, however, unclear, as is whether or not GGGGCC repeat plays any role in vascular integrity.
V. BBB BREAKDOWN AND DYSFUNCTION IN NEUROLOGICAL DISORDERS
Next, we examine sporadic forms of common neurodegenerative disorders that are not clearly linked to genetic mutations but are associated with BBB breakdown, dysfunction, and vascular pathology. We also discuss briefly BBB damage in acute neurological disorders.
A. Alzheimer’s Disease
In addition to the classic hallmark pathology, Aβ plaques, hyperphosphorylated tau neurofibrillary tangles, and neuron loss, increasing evidence supports that early cerebrovascular dysfunction contributes to AD pathophysiology and cognitive impairment (29, 285, 418, 421, 429, 569, 571, 593, 682, 693). Some studies have suggested that during preclinical stages, vascular dysfunction is among the first detectable biomarker changes reported before symptomatic onset and before changes in other standard AD biomarkers, including amyloid deposition and CSF Aβ42, phosphorylated tau (pTau), and total tau (285, 421). Moreover, neuropathological studies indicate that early cerebrovascular disorder increases risk for dementia including AD (29) and is associated with AD and other neurodegenerative disorders independently of mixed dementias including vascular dementia (593). Vascular risk factors including hypertension (169, 272), diabetes (43, 110), hyperlipidemia (74), and cardiovascular disease (594), as well as environment (e.g., pollution) (87–89, 207) and lifestyle (e.g., obesity, sedentary lifestyle) (281, 643) all affect cerebrovascular and BBB dysfunction (429, 571), and may influence onset and progression of AD.
Several AD animal models derived from ADAD human mutations including APP and PSEN1, and APOE4 transgenic mice develop an early BBB breakdown before AD pathology, as discussed above and recently reviewed (420). Additionally, tau transgenic models (TetO-TauP301L) also exhibit BBB leakage, microbleeds, IgG deposits, and leukocyte infiltration detected before tau pathology and with no evidence of Aβ pathology (66), supporting that cerebrovascular dysfunction contributes to AD progression.
FIGURE 7 illustrates BBB breakdown and dysfunction during different stages of AD progression based on clinical findings, neuroimaging, and neuropathological and biomarkers biofluid studies, as discussed next.
FIGURE 7.
Blood-brain barrier breakdown and dysfunction in sporadic Alzheimer’s disease. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) has shown blood-brain barrier (BBB) breakdown in the hippocampus in individuals with mild cognitive impairment (MCI) and in different gray and white matter regions in early Alzheimer’s disease (AD), before brain atrophy and dementia occur. Microbleeds reflecting loss of cerebrovascular integrity and BBB breakdown have been shown by T2*-MRI and susceptibility-weighted imaging (SWI)-MRI during MCI stage, which progresses and augments through early stages of AD. Fluorodeoxyglucose positron emission tomography (FDG-PET) has indicated diminished BBB GLUT1 transporter activity mediating glucose uptake by the brain before brain atrophy, dementia, or amyloid-β (Aβ) pathology. Similarly, diminished active efflux ABCB1 (P-gp) BBB transporter activity was shown by verapamil-PET in early AD. Early BBB breakdown and vascular dysfunction in MCI and AD has been confirmed by some studies by elevated levels of vascular biomarkers in cerebrospinal fluid (CSF) and blood before Aβ and tau pathology, and dementia. Neuropathological (NP) analysis of mild and advanced AD cases confirmed accumulation of perivascular blood-derived deposits including, to name a few, fibrin(ogen), thrombin, red blood cells (RBC)-derived iron-containing products that all are potentially toxic for the neural tissue. In addition, pericyte degeneration, endothelial degeneration, and brain infiltration with circulating macrophages and neutrophils were associated with BBB breakdown of AD cases on NP analysis. Diminished expression levels of BBB GLUT1 and ABCB1 (P-gp) transporters have been shown by post mortem NP analysis of AD cases, as well as downregulation of Aβ BBB clearance receptors LRP1 and ABCB1, suggesting impaired Aβ clearance. Furthermore, APOE4 carriers develop accelerated BBB breakdown associated with activation of proinflammatory cyclophilin A (CypA)-matrix metalloproteinase-9 (MMP-9) pathway at the BBB, which degrades endothelial tight junction and basement membrane proteins enhancing BBB damage. How changes in BBB permeability as measured by advanced neuroimaging techniques in the living human brain relate to disrupted structural and functional connectivity as measured by diffusion-tensor imaging (DTI)-MRI and functional MRI (fMRI), and amyloid-PET and tau-PET findings remains unclear at present.
1. Neuroimaging findings
a) dce-mri.
Recent neuroimaging studies in individuals with mild cognitive impairment (MCI) and early AD reveal BBB breakdown in the hippocampus, including its CA1 and dentate gyrus subfields (421), and several gray and white matter regions (220–222), respectively, before brain atrophy and dementia. These studies used advanced DCE-MRI to quantify the regional BBB permeability constant, Ktrans, to the contrast tracer gadolinium relative to each individual’s arterial input tracer function (421). These studies used Patlak analysis (46, 47, 466), which allows detection of subtle changes in BBB permeability (46, 248, 418, 421). Earlier studies using longer resolution time and semiquantitative analysis indicated a possible trend of increased BBB permeability in the hippocampus in MCI compared with controls (631), and suggested that accumulation of contrast agent in brains of individuals with probable AD likely occurs via blood-to-brain-to-CSF pathway (556), consistent with increased blood vessel permeability.
b) t2* and swi mri.
Microbleeds are RBC-derived iron-containing perivascular hemosiderin deposits that can be visualized as small hypointense regions on T2*- and/or susceptibility-weighted imaging (SWI) MRI sequences (620). CNS microbleeds reflect loss of cerebrovascular integrity and are found in 25% of individuals with MCI (669) and 45–78% of individuals with early AD without dementia (83, 208, 246, 452, 476, 529, 604, 702). This broad range of detection could likely be attributed to the magnet field strength, with clinical strength 1.5 and 3T magnets likely underestimating the incidence of microbleeds compared with the research-grade 7T magnet (83, 246, 452, 530, 604, 669). Although microbleeds are believed to result from CAA (514), many studies investigating microbleeds in AD did not simultaneously conduct amyloid PET imaging making it difficult to directly compare the degree of CAA to the incidence of microbleeds (83, 208, 246, 452, 476, 529, 604, 702). Importantly, microbleeds develop in the absence of Aβ pathology and are associated with cognitive impairment and dementia, as shown in patients with small vessel disease of the brain that is estimated to contribute to 50% of all dementias wordwide including AD (224, 550, 637).
c) fdg-pet and verapamil-pet.
18F-fluoro-2-deoxyglucose (2-DG) (also known as FDG) is a radioactive glucose 2-DG analog that does not enter the glycolytic or Krebs cycle metabolic pathways in the brain (124, 397, 504, 552). FDG-positron emission topography (PET) is clinically used as a surrogate for glucose uptake by the brain. As previously reviewed, FDG-PET reflects mainly the transport activity of GLUT1 BBB transporter (420, 569). Individuals with MCI and early AD have diminished regional brain uptake of 2-DG, as measured by FDG-PET, before MCI-AD conversion, brain atrophy, or neurodegenerative changes (268, 345, 424, 425, 517). This is consistent with AD post mortem studies showing diminished expression of GLUT1 in brain capillaries (261, 301, 422, 541). Experimental studies also support diminished transport of 14C-labeled glucose across the BBB in haploinsufficient Slc2a1+/− mice lacking ~50% copies of the GLUT1 BBB transporter (649).
Studies using [11C]verapamil, an ABCB1 (P-gp) ligand and PET, have shown increased uptake of verapamil in certain brain regions in individuals with early AD, suggesting diminished ABCB1-mediated active efflux of xenobiotics and drugs at the BBB (33, 142), and possibly faulty Aβ BBB clearance based on experimental studies (115, 400, 632).
2. Neuropathological studies
a) bbb breakdown.
Post mortem studies show vascular capillary leakages of blood-derived proteins in the prefrontal and entorhinal cortex and hippocampus of AD patients including perivascular accumulation of fibrin(ogen), thrombin, albumin, immunoglobulin G (IgG), and iron-containing proteins such as hemosiderin (120, 125, 230, 267, 412, 509, 527, 691). These blood-derived proteins are often found colocalized with Aβ (267, 509, 527) and are more pronounced in APOE4 carriers compared with noncarriers (230, 267, 516, 691).
b) pericyte degeneration.
Ultrastructural studies using electron microscopy reveal accumulations of osmiophilic materials in the capillary mural cells in AD cortex suggestive of pericyte loss (40, 170). Pericyte loss has been confirmed by decreased levels of pericyte marker PDGFRβ in the precuneus (412) and loss of pericytes in the subcortical white matter (419). Similarly, PDGFRβ immunostaining revealed significantly reduced pericyte coverage of brain capillaries as well as reduced pericyte numbers in AD cortex and hippocampus compared with control brains (527), which is accelerated by APOE4 gene (230).
c) endothelial degeneration.
Further evidence of BBB breakdown in AD is repeatedly observed in numerous post mortem human studies showing reduced capillary length and microvascular degeneration with diminished TJ protein expression, capillary basement membrane alterations, and brain endothelial degeneration (36, 40, 230, 516, 527, 656).
d) cell extravasation.
RBC extravasation (125) and brain infiltration by peripheral macrophages (175, 267) and neutrophils (676) are reported in AD post mortem studies, suggesting that the brain’s innate immune system is activated, which can contribute to pathophysiological changes.
e) dysregulated molecular transport.
The levels of endothelial-specific GLUT1 transporter at the BBB are greatly reduced in AD (261, 301, 422, 541). AD brain microvessels also exhibit reduced levels of LRP1, a major Aβ clearance receptor at the BBB (137, 152, 230, 410, 535). Besides oxidative stress and Aβ causing faulty LRP1 folding leading to its proteosomal degradation (137), reduced GLUT1 levels may also inhibit LRP1 transcriptionally via sterol regulatory element binding protein 2 (SREBP2) (649). In contrast to LRP1, expression of RAGE, a major Aβ influx receptor, is increased in AD brain endothelium and mural cells (134, 152, 410), likely contributing to circulating Aβ influx, neuroinflammation, and reduced CBF (134, 136).
Brain pericytes and endothelial cells in AD APOE4 carriers exhibit increased activity of BBB-degrading CypA-MMP-9 pathway compared with noncarriers (230). CypA mRNA is also increased in AD brains (118).
f) angiogenesis.
Increased levels of several angiogenic factors in response to reduced CBF and regional hypoxic changes have been reported in AD brains (213). Despite a pronounced pro-angiogenic response, AD brains fail to mount an adequate angiogenic response to renew lost capillary networks likely due to the loss of the homeobox gene MEOX2 from endothelium (656), and ongoing pericyte degeneration (570).
3. Biomarkers biofluid studies
The most common biofluid marker of BBB breakdown is the albumin quotient (Qalb), which is the ratio of CSF albumin levels to serum albumin levels. Qalb is elevated in individuals with preclinical AD (229), MCI (421), AD without vascular risk factors (287, 545, 546), and AD with vascular risk factors (67, 68, 74, 629). Given that brain macrophages, microglia, astrocytes, neurons, and neuron-glial antigen 2-positive cells can take up albumin (76, 286, 355), additional measures of BBB permeability such as MRI (e.g., DCE, T2*, SWI) should be used alongside Qalb to accurately assess the degree of BBB breakdown.
B. Parkinson’s Disease
Idiopathic PD has unknown etiology. It has been suggested that vascular risk factors accelerate the onset and severity of motor and cognitive impairments during early stages of PD (386), and that cerebrovascular dysfunction can influence development and progression of PD, as discussed next. FIGURE 8 illustrates BBB disruption in PD based on neuroimaging as well as neuropathological and biomarkers biofluid studies.
FIGURE 8.
Blood-brain barrier breakdown and dysfunction in sporadic Parkinson’s disease. Vascular dysfunction occurs throughout the basal ganglia of Parkinson’s disease (PD) patients, consisting of blood-brain barrier (BBB) breakdown and dysfunction, as shown by dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), T2*-MRI and susceptibility-weighted imaging (SWI)-MRI demonstrating microbleeds, and diminished active efflux of xenobiotics and other potential toxins, as indicated by verapamil-PET. Aberrant angiogenesis with increased number of endothelial cells, decreased tight junction (TJ) and adherens junction (AJ) proteins, and capillary basement membrane changes have been shown both in humans with PD and animal models. BBB breakdown can lead to neurotoxic accumulates of fibrin(ogen), thrombin, and plasmin(ogen), and red blood cell (RBC) extravasation, release of hemoglobin (Hb) and iron (Fe2+) causing reactive oxygen species (ROS), which all can injure dopaminergic neurons. Concurrently, localized to the substantia nigra pars compacta, Lewy bodies form from filamentous and oligomeric α-synuclein (α-syn) that accumulate within dopaminergic neurons. Recent studies suggested that α-syn can cross the BBB and contribute to α-syn pool in the brain, and is also cleared from brain across the BBB via LRP1-mediated transcytosis.
1. Neuroimaging findings
a) dce-mri.
Using gadolinium contrast agent, DCE-MRI, and Patlak quantification analysis of BBB permeability (46, 47, 421), recent studies have demonstrated BBB breakdown in the basal ganglia in PD patients compared with controls (10).
b) t2* and swi mri.
Cerebral microbleeds reflecting BBB breakdown and deposition of RBC-derived hemosiderin deposits have been detected throughout deep gray matter, cortical, and white matter regions in PD patients using T2*- and SWI-MRI (231, 288). Interestingly, they are more prevalent in PD patients with dementia compared with those without dementia and controls, and positively correlate with white matter lesions (231, 288).
c) verapamil-pet.
Diminished activity of a major BBB active efflux transporter ABCB1 (P-gp) has been shown in the mid-brain regains of PD patients by accumulation of [11C]verapamil using PET technique (327).
2. Neuropathological studies
a) bbb breakdown.
Throughout the basal ganglia, histological analysis of PD patients reveals BBB breakdown in the striatum as shown by capillary leakages and accumulation of perivascular fibrin(ogen) (214) and IgG (473) deposits, hemosiderin (214, 373), and RBCs extravasation (214). In accordance with human studies, compromised BBB integrity is also observed in rodent models of PD induced by systemic administration of MPTP (108, 680) or intrastriatal administration of 6-hydroxydopamine (6-OHDA) (95), including leakage of fluoresceinisothiocyanate (FITC)-labeled albumin (95, 108, 680), horseradish peroxidase (95), and Evan’s blue (108) in the substantia nigra and striatum. MPTP models also show leukocyte infiltration (113).
b) endothelial degeneration.
In the PD, there is a substantial loss of endothelial cells in the basal ganglia, reduced levels of TJ proteins, and alteration of capillary basement membrane (473). Reduced expression of TJ proteins has also been shown in MPTP PD models (108).
c) bbb molecular transport.
Recent studies reveal that α-syn crosses the BBB bidirectionally, which could signify an important contributory event in PD pathogenesis (469, 563). Specifically, α-syn injected intraventricularly and intravenously in rodents undergoes brain-to-blood efflux and blood-to-brain influx, respectively (469, 563). Administered systemically, α-syn oligomers, ribbons, and fibrils crossed the BBB into the brain, where α-syn amplification and strain-specific pathology and neurotoxic phenotypes are observed (469). P-gp did not affect α-syn efflux; however, α-syn inhibited Aβ efflux suggesting that endothelial LRP1 is a potential efflux transporter for α-syn (563). If LRP1 is similarly downregulated in PD as in AD (137, 561), this could result in impaired α-syn BBB clearance and accumulation of α-syn in brain. Furthermore, α-syn influx is increased following lipopolysaccharide (LPS)-induced BBB breakdown (563), suggesting that the high levels of α-syn produced peripherally can enter the brain in the presence of BBB breakdown, which may also contribute to development of PD pathology.
d) angiogenesis.
Aberrant angiogenesis is frequently observed in PD patients, as shown in the substantia nigra pars compacta, locus coeruleus, and putamen (144, 627). Similarly, increased angiogenesis is observed throughout the basal ganglia of 6-OHDA-treated rats (44, 501). Of note, BBB disruption caused by a single intra-nigral injection of VEGF in rats induces dopaminergic neuron loss in the substantia nigra (501), suggesting that BBB disruption is capable of causing neurodegeneration. Aberrant angiogenesis occurs early in PD raising the question: is it driven by local tissue hypoxia, diminished local CBF, and/or increased metabolic demand?
3. Biomarkers biofluid studies
Increased Qalb (287, 288, 362, 474) and CSF-to-serum IgG ratio (474) are observed early in PD patients before dementia.
Additionally, plasma exosomal α-syn levels are markedly higher in PD patients and correlate with disease severity (532). However, the functional role of the exosome-mediated α-syn clearance and whether it reflects increased CNS α-syn production in PD patients is presently unclear.
CSF angiogenesis biomarkers are altered in PD compared with controls including increased levels of VEGF, placental growth factor (PlGF), and soluble VEGF receptor-2 (sVEGFR-2) and decreased angiopoietin-2 (Ang2) (288). These biomarkers are further altered as patients progress into more advanced PD stages with dementia (288, 474).
4. Deep brain stimulation
Deep brain stimulation (DBS) is commonly used to improve PD-related motor symptoms, and its effectiveness is credited to microvascular improvements (250, 473). PD patients that underwent DBS exhibit increased capillary length and density, increased TJ and AJ protein expression, and reduced perivascular IgG leakage at post mortem tissue analysis (473). Additionally, resting CBF increases in the premotor cortex following DBS treatment, which correlated with improved gait velocity and cadence (250). Altogether, these data suggest that DBS may restore disrupted BBB integrity in PD, which in turn could be critical for control of motor symptoms.
C. Amyotrophic Lateral Sclerosis
Ninety percent of ALS cases are sporadic with no clear genetic linkage (11, 313). Below, we discuss studies showing BBB breakdown in ALS patients. Based on these studies, and experimental studies in transgenic rodents carrying different SOD1 mutations, as discussed above, we propose a model how BBB and BSCB disruption can contribute to development of ALS pathology (FIGURE 9).
FIGURE 9.
Blood-brain barrier breakdown and dysfunction in amyotrophic lateral sclerosis. Blood-brain barrier (BBB) breakdown with loss of tight junction proteins and pericyte and endothelial cell degeneration leads to red blood cells (RBCs) extravasation and perivascular accumulation of plasma-derived proteins such as fibrin(ogen), thrombin, and IgG that is found in the spinal cord and motor cortex both in humans with sporadic and familial forms of ALS, as well as in rodents expressing different human SOD1 mutations. The RBC extravasation leads to the release of neurotoxic hemoglobin (Hb), and free iron (Fe2+) causing generation of reactive oxygen species (ROS), which is toxic to motor neurons. Serum proteins such as fibrinogen can activate microglia enhancing non-autonomous motor neuron cell death. Astrocytic endfeet become swollen and dissociate from capillaries, and the perivascular space becomes enlarged and basement membrane breaks down. The effects of BBB breakdown on oligodendrocyte precursor cells that proliferate and mature oligodendrocytes that degenerate remain elusive at this time.
1. Neuroimaging studies
a) t2* mri.
A few MRI studies showed hypointensities indicative of microbleeds in the brains of ALS patients (277, 339, 445), whereas another study failed to detect microhemorrhages in sporadic ALS (615).
2. Neuropathological studies
a) bbb breakdown.
Several independent post mortem studies of brain stem and spinal cord tissue from patients with sporadic and familial ALS found that there is a significant reduction in tight junction proteins, accumulation of blood-derived proteins (e.g., thrombin, IgG, hemoglobin, hemosiderin), capillary basement membrane changes, astrocytic end-feet detachment from endothelium, reduced microvascular density, and enlarged perivascular spaces suggestive of BBB and BSCB breakdown (195, 243, 339, 415, 652, 662). These findings support earlier observations showing that the BBB and BSCB are damaged in a subset of ALS patients as demonstrated by accumulation of plasma-derived IgG in the spinal cord and motor cortex in 40% (154) to 90% (163) of studied cases, and accumulation of peripheral blood cells in 70% (164) to 100% (599) of studied cases.
b) pericyte degeneration.
Immunohistochemical analysis for pericyte biomarkers on the spinal cord capillaries from patients with sporadic and familial ALS found pericyte degeneration and loss that is associated with BSCB breakdown and fibrinogen and IgG perivascular deposits (652). This was confirmed recently in 25 sporadic ALS patients having a prominent reduction in pericyte coverage in the ventral horn (662).
3. Biofluid studies
A subset of ALS patients shows increased Qalb ratio and elevated CSF levels of several blood-derived proteins (79, 652). For example, increased Qalb values were found in 40% of all studied ALS cases (i.e., 55/138 from 4 independent studies) (23, 79, 407, 525), suggesting that at least a subset of ALS develop vascular pathology.
D. Multiple Sclerosis
MS is an autoimmune and neurodegenerative disease (184) in which the myelin sheath surrounding axons is attacked by immune cells, including leukocytes, T cells, B cells, and peripheral macrophages that enter the brain through a disrupted BBB (456). Experimental findings in MS models of chronic and relapsing EAE have shown that a key pathophysiological event is encephalitogenic leukocyte CNS infiltration across the BBB that occurs via sequential steps of capture, rolling, activation, adhesion, crawling, and diapedesis in postcapillary venules, capillaries, and mid-capillaries (161, 182, 215, 216, 456, 617), as illustrated in FIGURE 10.
FIGURE 10.
Blood-brain barrier breakdown and dysfunction in multiple sclerosis. Hallmark features of multiple sclerosis (MS) include vascular dysfunction, neuroinflammation, and oligodendrocyte degeneration causing neuronal demyelination and loss. An early blood-brain barrier (BBB) breakdown, neurotoxic fibrin(ogen) accumulation, reduced tight junction (TJ) protein expression, and endothelial degeneration are features of both human MS and animal MS models. Leukocytes infiltrate across the BBB in a multistep sequential process involving capture, rolling, activation, adhesion, crawling, and trans- or paracellular diapedesis. This process requires crosstalk between leukocytes and endothelial cells via precise molecular interactions, as illustrated in the diagram. See the main text for details. MMPs, matrix metalloproteinases; RBC, red blood cell.
Mechanistically, leukocyte capture and rolling are mediated by interactions between leukocyte P-selectin glycoprotein ligand 1 (PSGL1) and endothelial E- and P-selectin (521). Both leukocytes and the endothelium become activated, causing upregulation of cell surface receptors mediating leukocyte-endothelial adhesion (215, 216, 537). Adhesion occurs via interactions between leukocyte integrins lymphocyte function-associated antigen-1 (LFA-1) and very late antigen-4 (VLA-4) with endothelial ICAM-1 and VCAM-1, respectively (215, 216). Crawling is predominantly mediated by upregulated endothelial ICAM-1 and ICAM-2 (557). Endothelial ICAM-1 expression is essential for transcellular diapedesis, for example, ICAM-1- and ICAM-2-deficient endothelial cells prevent CD4+ T cell crawling and diapedesis (1). Additional essential protein interactions for transendothelial diapedesis include endothelial JAM-A, caveolin-1, PECAM1, and CD99 and leukocyte LFA-1, proteins α4-integrin (CD49), PECAM1, and CD99 (165, 215, 655). Paracellular diapedesis is regulated by endothelial VE-cadherin, JAM-A, ESAM, PECAM1, and CD99 and leukocyte LFA-1, PECAM1, and CD99 (161, 215, 617) (FIGURE 10). Comprehensive mechanistic details and discussion of leukocyte infiltration have been reviewed extensively elsewhere (161, 215, 456, 617).
A substantial body of evidence is increasingly showing BBB disruption as an early feature of MS in both human patients as well as animal models, as discussed next.
1. Neuroimaging studies
Neuroimaging studies using gadolinium enhancement have established that BBB disruption is an early feature of MS pathogenesis (14). Gadolinium enhancement is associated with active MS lesions and is considered as one of the key diagnostic criteria for an acute MS lesion. Furthermore, MRI changes suggestive of BBB disruption were also found in normally appearing white matter before enhancing lesions (176), and in nonenhancing areas (626), suggesting BBB dysfunction likely precedes the onset of symptoms and other MRI disease-related changes. The BBB changes appear to extend to the initial stages of disease, as suggested by findings of global BBB disruption at the onset of optic neuritis in patients who go on to develop MS (122).
More recently, an increase in BBB permeability has been shown in MS patients by DCE-MRI in the cortex and adjacent white matter (392), corpus callosum and internal capsule (121–123, 421, 574), as well as thalamic gray matter (123). Interestingly, increased BBB leakage is prominent around new developing MS lesions centralized around small, inflamed veins (190). Recent studies using radioactive MMP PET found that increased MMP activity localizes around active lesions that associate with leukocyte infiltration (201).
Increased BBB permeability has been also detected early in the EAE model in marmosets using high-field MRI indicating a 3.5-fold increase in BBB permeability 4 wk before the onset of MS lesions (382).
2. Neuropathological studies
a) bbb breakdown.
Seminal characterization of the MS lesion by Dawson in 1916 recognized that myelin breakdown invariably originated around parenchymal blood vessels (483). This is supported by evidence of fibrinogen perivascular deposition in the developing lesions (317, 626). Post mortem findings of perivascular immune infiltrates have established that BBB disruption is an early feature of MS pathogenesis (14). BBB dysfunction has also been confirmed by decreased levels of TJ proteins (i.e., occludin, ZO-1, and claudin-5) and basement membrane abnormalities (317). These abnormalities are seen across the brain in both relapsing-remitting and progressive stages of MS (352) being most common in active lesions. Reduced levels of TJ proteins were associated with fibrinogen leak and perivascular astrogliosis (14).
Longitudinal studies in spontaneous relapsing-remitting-EAE mice have shown that BBB disruption occurs before immune cell infiltration in focal lesions (15). A recent study also confirmed that BSCB disruption occurs following a caudo-rostral progression over time using both Evans blue dye injection and fibrin(ogen) immunostaining in EAE mice (182).
3. Biofluid studies
Elevated Qalb values (355), increased MMP-9 activity in CSF and serum (122, 168), and increased CSF leukocyte count (122), all indicative of BBB breakdown, have been shown in MS patients at different stages of the disease.
E. Other Chronic Neurodegenerative Disorders
1. HIV-1-associated dementia
Human immunodeficiency virus (HIV)-1 prognosis greatly improved with the era of anti-retroviral therapy; however, many HIV-1-positive individuals today still develop HIV-1-associated dementia (HAD) (292, 511). BBB disruption plays a major role in HAD development by allowing HIV-1-infected monocyte-macrophages to traverse the BBB and enter the brain (562). Post mortem brain tissue studies in HAD or HIV encephalitis report microvascular degeneration and BBB breakdown including reduced pericyte coverage (439), reduced and disrupted TJs (471, 664), capillary basement membrane changes (471), perivascular macrophage infiltration (471), and reduced brain endothelial ABCB1 (P-gp) expression (346). MRI studies reveal that small-vessel cerebral ischemic disease is also prominent in HAD (401). Whether treatments directed at the BBB can delay or prevent HAD development to improve the lives of individuals with HIV-1 is currently not known.
2. Chronic traumatic encephalopathy
Chronic traumatic encephalopathy (CTE) is characterized by accumulation of TAR DNA-binding protein-43 (TDP-43) and neurofibrillary tangles of hyperphosphorylated tau beginning perivascularly (54), and is reported in football (454, 455), wrestling (455), boxing (325), and soccer (366) players, as well as in veterans (237). Post mortem CTE studies reveal cerebral edema, perivascular hemosiderin-burdened macrophages, mural cell mineralization, enlarged perivascular spaces, and leukocyte infiltration (149, 325, 454, 455), supporting that repeated microvascular injury, ischemia, and axonal injury triggers a neurodegenerative cascade. To date, the majority of CTE studies have analyzed post mortem brain tissue. Future MRI studies would help to determine how the degree of regional BBB breakdown relates to structural and functional changes in brain connectivity and onset of CTE symptoms.
F. Acute Neurological Disorders
1. Stroke
BBB dysfunction is a prominent pathological feature of both ischemic and hemorrhagic stroke and is typically associated with poor outcome (307, 312, 482). Comprising 85% of all strokes, ischemic stroke exhibits extravasation of blood-borne cells, chemicals, and fluid into brain parenchyma across the impaired BBB as a result of increased paracellular and transcellular permeability and endothelial degeneration (305). Water and ion homeostasis of the brain is also disrupted, leading to cerebral edema (505). Leukocyte infiltration further exacerbates inflammatory responses and worsens brain injury (264). During and after ischemic stroke, BBB disruption facilitates injury progression and increases the risk of hemorrhage, predicting poor patient outcome and limiting the use of thrombolytic treatment with recombinant tissue-type plasminogen activator (tPA) (308, 370).
In preclinical models of ischemic stroke, opening of BBB has been shown to be a biphasic phenomenon (482). The first phase is controlled by subtle endothelial cytoskeleton alterations 30–60 min after ischemia/reperfusion injury, followed later by enzymatic cleavage of TJ proteins after inflammatory cell recruitment (324, 534, 703). Hemorrhagic stroke transformation is sometimes a consequence of thrombolytic tPA therapy for ischemia, and dysfunctional BBB is the primary initiator of the pathology (306, 312).
The existence of stroke comorbid conditions, such as hypertension and hyperglycemia, induces anatomical and functional changes to the brain microvasculature and often exacerbates BBB breakdown after stroke. Below we discuss clinical trials that are using DCE-MRI to visualize BBB permeability changes following acute ischemic stroke (619). Having received much less attention than warranted, BBB research should be better prioritized, with an emphasis on BBB-related mechanisms of neurovascular injury and developing therapeutic strategies to improve BBB integrity after ischemic stroke, as for instance with 3K3A-activated protein C (3K3A-APC) (217).
2. Traumatic brain injury
Loss of vascular integrity has a key role in mediating tissue damage after traumatic brain injury (TBI) (48, 223, 402, 536). Disruption of the walls of microvessels in the BBB activates the coagulation cascade. Intravascular coagulation leads to ischemia in the areas surrounding the impact site resulting in severely decreased blood flow; this is known as the “no-reflow” phenomenon. Since the integrity of the BBB is compromised after injury, blood-borne factors such as fibrin(ogen), thrombin, and albumin, among others, can now enter the brain.
Similar to the pathophysiology of stroke, BBB alterations after TBI occur in two phases based on preclinical models, first occurring within hours of tissue damage and the second 3 days after injury (48, 223). Early opening of BBB is caused by the shear injury of cerebral blood vessels causing physical damage to BBB integrity, and is later followed by the activation of inflammatory cells (48). However, there is still controversy especially about the cascades leading to secondary BBB breakdown in TBI due to scattered methodology as extensively discussed elsewhere (548).
3. Spinal cord injury
BSCB disruption following spinal cord injury (SCI) allows white blood cells to infiltrate the injured parenchyma and contribute to secondary injury (3, 239, 334, 692). On the basis of experimental rodent studies, BSCB disruption occurs within 5 min after spinal cord trauma (385), lasts for up to 28 days after the initial injury, and spreads along the entire length of the cord (441, 478, 644). The BSCB can remain compromised even at 56 days after SCI as assessed using DCE-MRI (117). The extended time course of barrier breakdown has been confirmed by MRI analyses (117, 507), but the time course for reestablishment of BSCB function is less clear, with results varying widely among studies (385, 441, 478). Some reports suggest that SCI generates a biphasic opening of the barrier. The first peak of abnormal leakage occurs within several hours after injury, whereas the second peak is evident between 3 and 7 days post-injury (644).
4. Epilepsy
Epilepsy is a family of neurological disorders with recurrent seizures that affects more than 50 million individuals worldwide (653). Increased microvascular density, loss of tight junctions, and IgG leakage are observed in hippocampal resections from humans with temporal lobe epilepsy (499), and are also observed in rodent epileptic models (145, 499). BBB dysfunction positively correlates with seizure frequency and is independent of neuronal loss (234, 499). BBB dysfunction is localized to epileptic regions, suggesting that the BBB plays a contributory role to epileptic disorders. The connection between epilepsy and BBB breakdown is known to be bidirectional (316). BBB breakdown caused by TBI and underlying inflammatory reactions can trigger epilepsy (204, 618). On the other hand, changes in BBB permeability may lead to progression of epilepsy making it more difficult to treat (621, 622). Multiple anti-inflammatory treatment strategies have demonstrated the beneficial effect of maintaining BBB integrity in this condition (393). Several past and current clinical trials are related to BBB function in epilepsy, as discussed below.
VI. BBB-BASED THERAPEUTIC OPPORTUNITIES
The BBB poses two major challenges for the development of therapeutics for CNS disorders. First, with its barrier function and highly effective active efflux systems, the BBB rejects most of the small molecule drugs and large therapeutic molecules including growth factors and antibodies (42, 567, 682). Second, focal BBB breakdown in the disease leads to perivascular accumulation of blood-derived toxic products and macromolecules, immune and/or inflammatory responses, vascular regression, and local CBF reductions (318, 693). These focal vascular changes limit CNS distribution of neurotherapeutics in disease-affected regions by disrupting diffusional transport across brain ECS and/or by blocking normal ISF flow dynamics (519, 569).
Selecting drug analogs with better permeability may traverse the BBB. A successful example is l-3,4-dihydroxyphenylalanine (l-DOPA) for PD over dopamine, as it crosses the BBB via LAT1 neutral amino acid transporter (459). On the other hand, approaches to circumvent the BBB have been utilized clinically for treatment of some CNS disorders. These include intracerebroventricular administration of Cerliponase for Batten’s disease (395), intrathecal administration of antisense oligo Spinraza targeting the survival motor neuron 1 gene (SMN1) for infantile spinal muscular atrophy (150), intrathecal Ziconited peptide for chronic pain (398), and intranasal administration of insulin, leptin, and oxytocin (103).
Here, we discuss recent approaches developed to protect the BBB and eliminate secondary vascular-mediated CNS changes, and/or to effectively traverse the BBB to improve CNS drug delivery (TABLE 2).
Table 2.
Circumventing, protecting, and traversing the blood-brain barrier
| Approach | Therapeutics | Mechanism | Disease | Animal Model | Clinical Trials | Reference Nos. |
|---|---|---|---|---|---|---|
| Circumventing BBB for CNS drug delivery | ||||||
| Alternative routes of administration | Cerliponase | Intracerebroventricular | Batten’s disease | Multiple species and models | FDA approved | 382 |
| Spinraza | Intrathecal | Infantile SMA | FDA approved | 144 | ||
| Ziconited peptide | Chronic pain | FDA approved | 385 | |||
| Insulin | Intranasal | Cognitive impairment | Phase II/III | 99 | ||
| Leptin | Obesity | Phase I | 99 | |||
| Oxytocin | Autism | Phase II | 99 | |||
| Protecting damaged BBB | ||||||
| BBB sealing | APC and its analogs | β-Arrestin-mediated PAR1-biased signaling | Stroke | Rodent stroke models (arterial occlusion, embolic stroke) | Phase II | 208 |
| ALS | SOD1 mutant models | NA | 208 | |||
| Glucocorticoids | Upregulation of intercellular junctional proteins, suppression of MMPs and inflammation | Niemann-Pick disease, type C | NPC1 | NA | 500 | |
| Eliminating consequences of BBB breakdown | Ancrod | Depleting fibrin(ogen) | AD | TgCRND8 | NA | 452 |
| MS | EAE | 126 | ||||
| Deferoxamine | Iron chelation | ALS | SOD1 (G93A) | NA | 624 | |
| Glutathione monoethyl ester | Antioxidant | |||||
| APC and its analogs | PI3K/Akt-mediated neuroprotection, endothelial protection | Stroke | MCAO, dMCAO, embolic stroke | Phase II | 208 | |
| ALS | SOD1 mutants | NA | 208 | |||
| Enhancing clearance function | LRP1 minigene | Improve efflux | AD | Tg2576 | NA | 624 |
| RAGE inhibitor (Azeliragon) | Reduce influx | Phase III | 128 | |||
| Allopregnanolone | Promoting Aβ and cholesterol clearance | 3xTgAD | Phase I | 79 | ||
| Cell therapy | Mesenchymal stem cells transplantation | Improve BBB functions | CNS injuries | Rodent experimental models | NA | 250, 448, 559 |
| Pericytes transplantation | ALS | SOD1 | 110 | |||
| Other BBB-targeted clinical trials | DCE-MRI | Identifying and tracking sites of BBB permeability | Ischemic stroke | Rodent experimental models | Observational trials | 300 |
| MS | 13 | |||||
| Epilepsy | Phase I | 139, 482 | ||||
| P-gp inhibitor | Prevent anti-epileptic drug resistance | Epilepsy | Phase II | 649 | ||
| Anti-VLA-4 humanized monoclonal antibody (Natalizumab) | Block CNS leukocyte infiltration | Relapsing remitting MS | Phase IV | 510 | ||
| Anti-CD52 humanized monoclonal antibody (Alemtuzumab) | FDA approved | 662 | ||||
| Traversing BBB for CNS drug delivery | ||||||
| Direct opening of the BBB | Focused ultrasound | Doxorubicin delivery | Brain tumor | Multiple species and models | Phase I | 82 |
| To promote therapeutic delivery | AD | Phase I | 461 | |||
| PD | Phase I | 461 | ||||
| Colloidal carriers | Nanoparticles | Entrap within or covalently bind to drugs | A broad spectrum of CNS diseases | Multiple species and models | Phase 1 | 503 |
| Exosome | NA | |||||
| CMT | l-DOPA | LAT-1 large amino acid transporter | PD | MPTP | FDA approved | 654 |
| RMT | Bispecific antibodies | Anti-TfR-BACE1 | AD | Tg2576 | 33 | |
| Anti-TfR-Aβ | PS2APP | 422 | ||||
| Molecular Trojan horses | l-Iduronidase fused with anti-TfR | Mycopolysaccharoidosis I | Rhesus monkey | Phase II | 68 | |
| Iduronate 2 sulfatase fused with anti-IR | Mycopolysaccharoidosis II | Phase I | 68 | |||
| Viral vectors and variants | Gene delivery | Brain tropic AAV9 variants | PD | TgSNCA-A53T mouse | NA | 140 |
AD, Alzheimer's disease; PD, Parkinson's disease; ALS, amyotrophic lateral sclerosis; FDA, United States Food and Drug Administration; NA, not applicable.
A. Protecting Damaged BBB
1. Sealing broken BBB
A few pharmacological agents have been reported to restore BBB function in animal models of acute neurological disorders (217, 697) and neurodegeneration (420). APC, for example, exerts pleotropic beneficial activities including protection of the BBB integrity, anti-inflammatory effects, direct neuroprotection, and pro-neurogenic and pro-angiogenic effects, as shown in rodent models of stroke, TBI, and ALS (217). APC cleaves protease-activated receptor 1 (PAR1) in brain endothelium and subsequently activates β-arrestin-2-dependent biased signaling pathway, which targets phosphatidylinositol 3-kinase (PI3K) for cytoprotection and Rac1 GTPase for sealing the BBB (217). 3K3A-APC, a recombinant variant of APC with reduced anticoagulant activity, has advanced from bench to bedside and has completed phase 2 clinical trial for stroke (NCT02222714). 3K3A-APC also holds promise for TBI, ALS, and possibly other neurodegenerative disorders (217).
Treatment with glucocorticoids also offers BBB protection (446). Progesterone and allopregnanolone can reduce neuroinflammation and improve barrier function by downregulating expression of metalloproteinases as shown in a mouse model of ischemic stroke (282). Allopregnanolone is beneficial for type C Niemann-Pick disease, AD, and MS based on preclinical studies in animal models (443). Interferons produced during viral infections, e.g., West Nile virus, protect the BBB integrity to virions by restricting their entry into brain parenchyma (131, 348).
Inhibition of CypA-MMP-9 BBB degrading pathway by cyclosporine, a CypA inhibitor, restored BBB integrity and reversed secondary neurodegenerative changes in transgenic humanized APOE4 mice (60). Activation of CypA-MMP-9 pathway associated with BBB breakdown has also been shown in human APOE4 carriers compared with non-carriers, as indicated by CSF (229) and post mortem BBB tissue (230) analyses. Whether a nonimmunosuppressive cyclosporine analog Debio 025, which is used in humans in phase III trial for hepatitis C (NCT01318694), can also protect cerebrovascular integrity and improve cognitive impairment in human APOE4 carriers at risk for AD remains worth exploring.
2. Eliminating consequences of BBB breakdown
When the BBB is open, plasma proteins enter the neuroglial space and often exert toxic effects on various cell types in the CNS (682, 693). Therefore, neutralizing toxic accumulates represents another valuable therapeutic approach for neurodegenerative diseases associated with neurovascular dysfunction and BBB pathology. For example, fibrinogen and its polymeric form fibrin can activate integrin receptors on glial cells and neurons to inhibit regeneration (45, 522), or receptors on microglia and bone-derived macrophages to exacerbate neuroinflammation and induce antigen-presenting genes (45, 132, 510). Depleting fibrinogen and/or preventing its accumulation in the brain, as for example with ancrod, a defibrinogenating agent, attenuated both neuroinflammation and vascular pathology in AD mice (467) and in an MS model (132). On the other hand, BBB damage causing extravasation of RBCs that leads to iron accumulation and oxidant stress can be successfully controlled by the iron chelation therapy and/or antioxidant treatment, as shown in SOD1G93A mutant mice with ALS-like disease (651).
3. Enhancing clearance function
The BBB is a major clearance site for many brain-produced potentially toxic substances, which is particularly important in maintaining brain Aβ homeostasis (682, 693). Targeting the BBB clearance machinery in AD is an emerging therapeutic approach to shift the balance between Aβ production and clearance. For example, LRP1 minigene delivery to the BBB by viral vectors facilitates Aβ clearance and attenuates Aβ pathology (649). On the other hand, blocking RAGE at the BBB (134) effectively reduces Aβ reentry into the brain, inhibits neuroinflammation, and improves CBF in a mouse model of AD (134, 136). On the basis of a better understanding of RAGE biology, a small molecule RAGE inhibitor has advanced to phase 3 clinical trial in AD patients (NCT02080364). The PICALM-dependent transcytotic machinery at the BBB can also be targeted therapeutically by gene therapy to enhance Aβ clearance across the BBB (682). Additionally, allopregnanolone promotes Aβ and cholesterol clearance in preclinical studies in animal models (80) and is currently in phase 1 clinical trials for MCI and early AD (NCT02221622).
α-Syn, a key protein found in Lewy bodies of both Lewy body dementia and PD, is also transported in and out of the brain and is cleared across the BBB (682) by LRP1-mediated transcytosis (563); thus enhancing its clearance through the BBB could be beneficial for these neurological conditions.
4. Cell therapy
Preclinical transplantation studies in animal models have demonstrated the potential of cell therapy for treating neurodegenerative diseases and acute CNS injuries, with some showing benefits for cerebral vasculature and BBB. For example, transplantation of allogeneic mesenchymal stem cells and neural stem cells, in addition to offering neuroprotection, can stabilize BBB and promote BBB integrity as shown in stroke models (260, 463, 578). Pericytes derived from adipose tissues extended the survival of ALS mice carrying SOD1G93A mutation after transplantation in the spinal cord, and protected iPSC-derived motor neurons from an ALS patient (116). Whether treatments with iPSC-derived pericytes can be beneficial to other neurological or neurodegenerative diseases associated with pericyte dysfunction and/or degeneration and BBB breakdown, such as AD, HIV-1 infection, and others (570), remains to be explored. In addition, transplantation of mesenchymal cells or endothelial progenitors that are capable of differentiating in vivo into vascular cells such as pericytes has shown promising results as vascular regeneration therapy (353) and repair BBB damage treatment after stroke (198). Genetic engineering and editing offers great opportunities to improve the safety of cell therapy, as shown by removing the immune barrier by reprogramming the polymorphic MHC locus to increase the transplant acceptance (310), which should also be considered for BBB cell therapy with endothelial cells and pericytes.
5. Other BBB-targeted clinical trials
Numerous clinical trials for BBB protection have been used in various acute and chronic neurological conditions, as discussed above. Briefly, relating to BBB integrity, trials aim to visualize BBB permeability using DCE-MRI in ischemic stroke (NCT00715533; NCT02077582), MS (NCT01836055), and epileptic regions (NCT02531880; NCT00419874). Additional trials relating to BBB function aim to prevent anti-epileptic drug resistance with ABCB1 (P-gp) inhibitors in epilepsy (NCT02144792; NCT01126307; NCT01663545; NCT00605254), and block leukocyte infiltration across the BBB with anti-VLA-4 antibodies (NCT00859482) or anti-CD52 antibodies (NCT03193086) in relapsing remitting MS.
B. Traversing BBB for CNS Drug Delivery
Traversing BBB remains a major challenge for neurotherapeutics. Strategies have been developed to breach the barrier, including 1) direct opening of the BBB with intravenous bolus injection of hypertonic sugar solution (491) or focused ultrasound (FUS) scanning with microbubbles (84, 106); 2) encapsulation in nanoparticles made from biocompatible and/or biodegradable polymers and liposomes that can penetrate the BBB (414, 670); 3) utilizing CMT systems for better penetrability of drug analogs (459); 4) engineering therapeutic peptides, oligonucleotides (e.g., antisense oligos and antagomir), and monoclonal antibodies that target RMT systems at the BBB (78, 459, 460), or specific transport system such as system L amino acid transporter in case of anti-CD98hc/BACE1 antibodies (705); and 5) viral vector-mediated gene delivery to CNS (101, 146).
1. Opening the BBB
Temporal opening of the BBB can be achieved with transient uprise of osmotic pressure (491) or physically with FUS (84), which is supposed to grant a short therapeutic window for CNS drug delivery. However, the impact of BBB opening on the diseased brain has yet to be carefully examined, especially the long-term effects. Particularly, clinical safety issues associated with osmotic challenge limit its current use to rare and specific situations such as certain brain tumors. In addition, due to the preclinical success of FUS with microbubble for noninvasive, transient, and targeted delivery of therapeutics through the BBB (84) and use in brain tumors (NCT02343991; NCT01473485), this technology is rapidly moving towards clinical testing for early AD (NCT02986932) and PD (NCT02347254; NCT02252380) (477). It is noteworthy, however, that FUS with microbubble may induce brain inflammatory responses that are comparable with ischemic injury or mild TBI (329). The exact mechanism of action in neurodegenerative disorders and long-term side effects remain unclear at present.
2. Colloidal carrier-based drug delivery systems
Colloidal carriers such as nanoparticles, polymers, liposomes, and micelles have become perhaps the most versatile approach for delivering compounds and macromolecules into inaccessible regions behind tissue barriers, particularly the brain (372, 519). Nanoparticles can derive from synthetic polymers or natural biomaterials such as albumin and polysaccharides, with targeted drug entrapped within or covalently attached. To cross the tissue barriers, nanoparticles are either negatively charged on the surface (519), or capable of binding to receptors on the cell surface (670), or densely packed around a metal core (291). Polybutyl cyanoacrylate-, polylactide-co-glycolide-, or chitosan-based nanoparticles have been tested for delivery in mouse models of AD. In addition, exosomes have also been tested in preclinical models for delivering proteins and small RNA across the BBB (16, 235, 323). Due to promising outcomes from animal studies, colloidal carriers are now moving into clinical trials in humans (71, 330), e.g., spherical nanoparticle conjugated RNAi (NU-0129) for glioblastoma (NCT03020017), and polymeric nanoparticle (BIND-014) for brain metastasis (NCT01792479).
3. Utilizing CMT systems
l-DOPA is an example of structural drug analogs that cross the BBB utilizing the large LAT1 neutral amino-acid transporter in contrast to dopamine that does not cross the BBB (459). l-DOPA is used to increase dopamine concentrations in the brain in PD and dopamine-responsive dystonia patients, and after crossing the BBB is converted in the brain into dopamine by aromatic l-amino acid decarboxylase.
4. Engineering therapeutics for RMT systems
RMT is part of the highly specialized transport system at the BBB, allowing the exchange of certain macromolecules between circulating blood and brain ISF (682). A properly functioning RMT is highly selective due to specific interaction between ligands and their preferred receptors, as well as the spatial distribution of the receptors (luminal vs. abluminal), which ensures exclusive entry of essential peptides and proteins into the brain and effective clearance of toxic waste products from brain to blood. Targeting the RMT systems offers a tremendous opportunity for CNS drug delivery, especially at the receptor level by selecting or even engineering therapeutic ligands. In fact, approaches such as molecular Trojan horses and bispecific or brain shuttle monoclonal antibodies targeting the BBB RMT systems have shown great promises for BBB penetrance in preclinical animal models (459), including anti-TfR-BACE1 antibody in Alzheimer’s Tg2576 mice (34) and nonhuman primates (672) or anti-TfR-Aβ antibody in PS2APP mouse model (435).
Molecular Trojan horses are therapeutic peptides or proteins fused to antibodies that target BBB RMT receptors for delivery across the BBB (459). Currently, molecular Trojan horse-based drugs are advancing in clinical trials for Hunter syndrome, l-iduronidase fused with monoclonal antibody against transferrin receptor (AGT-181), which is in phase 2 trial for mycopolysacharoidosis I disease (70), and iduronate 2 sulfatase fused with monoclonal antibody against insulin receptor (AGT-182) that is in phase 1 trial for mycopolysaccharoidosis II disease (69).
5. Viral vector-mediated gene delivery to CNS
Adeno-associated virus serotype 9 (AAV9) is perhaps the best known vector for gene therapy that potentially passes the BBB and infects brain cells, based on studies in neonatal and adult mice (388). However, the tropism repertoire of wild-type AAV9 is limited and favors peripheral organs rather than CNS. A few recent studies have attempted to engineer viral capsid proteins to achieve brain enrichment, resulting in novel variants that can deliver genes to different cell types in the brain and spinal cord. For example, AAV-PHP.B (146) and AAV-PHP.eB (101) offer a more than 40-fold increase in delivery efficiency to the whole CNS. AAV-PHP.A exhibits selective astrocyte tropism (146), while AV-PHP.S is more specific for dorsal root ganglion neurons as well as and cardiac and enteric neurons (101). These tools offer great opportunities for application in neurodegenerative diseases.
VII. LESSONS LEARNED AND FUTURE DIRECTIONS
Recent research has greatly advanced our understanding of BBB functions at the molecular and cellular level and has raised awareness about the role of BBB dysfunction in the pathogenesis of different CNS diseases. At the same time, these advances have uncovered gaps in our knowledge of vascular health and have provided us with the roadmap to ask new questions that should be addressed by the future studies.
The most critical evidence for the importance of vascular health for brain functions comes from human genetic studies and the corresponding studies in transgenic animal models showing that several genes mutated in monogenic neurological diseases have their principal site of expression in vascular cells (682). The many examples discussed in this review provide a direct link between BBB dysfunction and neurodegeneration. But, more than that, these genetic examples raise a question: What is the role of altered expression of BBB transporters, receptors, tight junction proteins, active efflux systems, and ion channels in common, sporadic neurodegenerative diseases such as AD and others? To name one of the many examples discussed in this review, late-onset, sporadic AD is characterized with an early loss of BBB transporters including GLUT1 transporter for glucose (268, 424, 425, 345, 517), which we know leads to severe neurological phenotype in the GLUT1-deficiency syndrome (630, 649). This begs a question whether changes in the BBB molecular makeup like one we see with the GLUT1 transporter in AD are innocent bystanders in the disease process or may have a major influence on the course of the disease, as in monogenic disorders, by synergistically promoting AD pathophysiology?
We also learned that several genetic mutations underlying inheritance or increasing risk for chronic neurodegenerative diseases are associated with BBB breakdown and cerebrovascular pathology in humans and animal models. However, the exact role of BBB dysfunction in the disease process in humans with genetic risk for neurodegenerative disorders remains still elusive. For example, APOE4 carriers at risk for sporadic AD compared with non-carriers develop BBB breakdown (229, 230, 267, 516, 691, 702) and early cerebrovascular dysfunction (227, 457, 486, 494, 531, 568, 584), and in animal models, APOE4 compared with APOE3 and APOE2 leads to impaired clearance of Aβ across the BBB (58, 98, 135) and an early BBB breakdown and transporters’ dysregulation (9, 60, 85, 437). These vascular changes precede neuronal dysfunction and neurodegeneration, but can be reversed by sealing the BBB (60). Similarly, sealing BBB reversed the course of motor neuron disorder in SOD1G93A mutant ALS mice (651). Would similar BBB-directed therapeutic approaches work in humans?
Additionally, laboratory studies tell us that BBB disruption develops early in APP transgenic models carrying human ADAD mutations (467, 513, 606) before Aβ accumulation, CAA, and behavioral deficits (151, 336, 606, 638), introducing a question: What are the molecular and cellular changes in BBB endothelial and mural cells that lead to such dysfunction? Are these changes caused by oligomeric Aβ species before amyloid deposition or direct APP vasculotoxicity, and/or could these changes be due to associated pathophysiology leading to early brain hypoperfusion and hypoxic changes (271)? Furthermore, PSEN1 mutant and knockout transgenic models that are not crossed with APP models exhibit significant vascular pathology such as BBB breakdown, hemorrhages, and loss of pericytes (191, 641), suggesting that vascular dysfunction caused by PSEN1 mutations can develop independent of amyloid pathology. Although, impact of vascular pathology to overall CNS pathology in human PSEN1 ADAD mutation carriers is not clear at present, the time courses and the exact contributions to cognitive impairment of BBB and cerebrovascular pathology (25, 53, 61, 143, 183, 274, 280, 289, 354, 389, 440, 544, 573, 639, 668) versus accelerated Aβ production remains to be determined by future studies.
Findings like these examined in this review pose a set of new questions and tasks, which require accurate and in-depth understanding of molecular definitions of the principal blood vascular and vessel-associated cell types, and the differences between endothelial and mural cells at the level of brain capillaries and along the arteriovenous axis. While we learned about organotypic differences between brain endothelial cells and peripheral endothelial cells, several questions persist about vascular mural cells, such as 1) Are pericytes heterogeneous or homogeneous cell group? 2) Does organotypicity extend to pericytes? 3) How different at the molecular level are pericytes from SMCs? 4) Does abundance and variety of molecular transporters in pericytes, as shown in rodents, imply that pericytes contribute to BBB transport functions? Some of the answers can be obtained by using large-scale analysis of vascular single cell transcriptomes and proteomes in BBB cell-specific transgenic models, and by preforming cryo-EM structural analysis of the BBB. We can also learn about the role of BBB transporters, receptors, active efflux mechanisms, and ion channels by selectively knocking down these molecules from endothelial cells and pericytes, which for the majority of key BBB molecules has yet to be done.
While our understanding of the BBB at the molecular and cellular level will continue to grow based on findings in rodent models, the question remains to what extent these findings are translatable to human BBB. To address this issue, perhaps we should attempt to develop molecular and cellular atlas for human BBB, and molecularly define vascular cell types in cerebral vasculature in humans using comparable single cell RNA-seq and proteomic analysis, as in animal models. Interestingly, comparison of 114 endothelial proteins between mice and humans revealed some striking differences in expression; for example, the active efflux ABCG2 transporter exhibited greater expression in humans, whereas LAT1 and MCT1 CMTs, TfR RMT, and ABCB1 and MRP4 active efflux transporters exhibited greater expression in mice (602). Another study compared protein expression between species (i.e., mice, rats, marmosets, cynomolgus monkeys, and humans) reporting that humans are most similar to marmosets with less than twofold differences in all measured proteins, whereas rats and humans exhibited the most differences (262). Elucidating species-specific similarities and differences in endothelial and pericyte expression profiles remains to be fully determined at the transcriptome and proteome levels.
In terms of studying BBB function in humans, recent neuroimaging studies made important advances to allow us to measure regional BBB integrity and quantify subtle changes in BBB permeability in CNS regions as small as hippocampal CA1, CA3, and dentate gyrus subfields, which has not been possible before (421). We expect that imaging methods will continue to improve our ability to detect BBB changes in humans, particularly with the use 7T human research magnet, and determine how they relate to blood flow changes, changes in structural and functional brain connectivity, and cognitive and motor deficits in different neurodegenerative disorders, as well as cell-specific biomarkers of the vascular injury and NVU in biofluids. This should remain an exciting area for future research, particularly in asymptomatic individuals at genetic risk for the disease such as, to name a few, APOE4 carriers for AD, PSEN1 mutation carriers for ADAD, and HTT mutation carriers for HD or CADASIL patients.
In general, at present we have limited imaging probes to study function of brain endothelial transporters in the living human brain, and no probes at all for pericyte transporters. Besides FDG-PET and verapamil-PET that have been used to test functions of BBB GLUT1 transporter and activity of ABCB1 (P-gp) efflux transporter in AD and PD (420, 569), respectively, we should also develop new probes such as those that have been used in animal models, like anti-VCAM-1 iron oxide microparticles to detect endothelial activation and/or inflammation by MRI (417), and probes to image health and function of mural vascular cells that are often affected early in the disease process.
The systems biology approach used to study establishment of the BBB in the developing CNS has provided invaluable insights into key molecular and cellular events governing BBB formation, maturation, and maintenance. These studies not only revealed key molecular mechanisms establishing the BBB, but also tell us how deficiency in certain pathways can lead to BBB disruption and secondary CNS injury and neurodegeneration, teaching us about potential BBB-directed therapeutic approaches in the adult and aging brain affected by disease. Although it remains challenging to understand how the systems biology work will translate to human brain and formation of the BBB in humans, use of stem cell technology, particularly iPSC-derived models of the BBB carrying disease associated risk genes (683), and combined three-dimensional models of the BBB and iPSC-derived neurons, will help us to overcome this hurdle.
Last but not the least, although nearly all of the neurodegenerative diseases are still incurable, technological advances have brought new hopes by facilitating the development of therapeutics that are more likely to circumvent, protect, and traverse the BBB. These include the colloidal carrier-based drug delivery systems, engineering new therapeutics for RMTs, and/or viral vector-mediated gene delivery to CNS, that hold promise for CNS delivery of neuropharmaceuticals currently blocked by the BBB.
Finally, based on the current state of our knowledge, it is probably time to think about BBB not only as an impermeable cellular membrane which protects brain from peripheral influences and should be breached for therapeutic CNS drug delivery, but also as an enormous source of understudied molecular and cellular targets in the disease state, which if explored could change the way we think about brain diseases and could lead to development of important new BBB-based approaches to treat them.
GRANTS
The work of B. V. Zlokovic is supported by the National Institutes of Health Grants R01AG023084, R01NS090904, R01NS034467, R01AG039452, 1R01NS100459, 5P50AG005142, and 5P01AG052350, in addition to the Cure Alzheimer’s Fund, Alzheimer’s Association, and the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease reference no. 16 CVD 05.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
M. D. Sweeney and Z. Zhao contributed equally to this work and are co-first authors.
We apologize to those authors whose original work we were not able to cite.
Address for reprint requests and other correspondence: B. V. Zlokovic, Zilkha Neurogenetic Institute, 1501 San Pablo St., Los Angeles, CA 90089 (e-mail: zlokovic@usc.edu).
REFERENCES
- 1.Abadier M, Haghayegh Jahromi N, Cardoso Alves L, Boscacci R, Vestweber D, Barnum S, Deutsch U, Engelhardt B, Lyck R. Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood-brain barrier. Eur J Immunol 45: 1043–1058, 2015. doi: 10.1002/eji.201445125. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 37: 13–25, 2010. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7: 41–53, 2006. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 4.Absinta M, Ha S-K, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife 6: e29738, 2017. doi: 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afonso PV, Ozden S, Cumont M-C, Seilhean D, Cartier L, Rezaie P, Mason S, Lambert S, Huerre M, Gessain A, Couraud P-O, Pique C, Ceccaldi P-E, Romero IA. Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog 4: e1000205, 2008. doi: 10.1371/journal.ppat.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed SSSJ, Husain RSA, Kumar S, Ramakrishnan V. Association between MDR1 gene polymorphisms and Parkinson’s disease in Asian and Caucasian populations: a meta-analysis. J Neurol Sci 368: 255–262, 2016. doi: 10.1016/j.jns.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 7.Akawi NA, Canpolat FE, White SM, Quilis-Esquerra J, Morales Sanchez M, Gamundi MJ, Mochida GH, Walsh CA, Ali BR, Al-Gazali L. Delineation of the clinical, molecular and cellular aspects of novel JAM3 mutations underlying the autosomal recessive hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. Hum Mutat 34: 498–505, 2013. doi: 10.1002/humu.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alakbarzade V, Hameed A, Quek DQY, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, Weedon MN, Rich P, Patton MA, Warner TT, Silver DL, Crosby AH. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet 47: 814–817, 2015. doi: 10.1038/ng.3313. [DOI] [PubMed] [Google Scholar]
- 9.Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E ε4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab 35: 86–94, 2015. doi: 10.1038/jcbfm.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Bachari S. MRI Assessment of Neurovascular Changes in Idiopathic Parkinson’s disease (Thesis). Manchester, UK: Univ. of Manchester, 2016. [Google Scholar]
- 11.Al-Chalabi A, van den Berg LH, Veldink J. Gene discovery in amyotrophic lateral sclerosis: implications for clinical management. Nat Rev Neurol 13: 96–104, 2017. doi: 10.1038/nrneurol.2016.182. [DOI] [PubMed] [Google Scholar]
- 12.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol 148: 203–216, 2000. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334: 1727–1731, 2011. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta 1812: 252–264, 2011. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez JI, Saint-Laurent O, Godschalk A, Terouz S, Briels C, Larouche S, Bourbonnière L, Larochelle C, Prat A. Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis 74: 14–24, 2015. doi: 10.1016/j.nbd.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29: 341–345, 2011. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 17.Alzheimer’s Association Alzheimer’s disease facts and figures. J Alzheimers Assoc 2017: 13, 2017. [Google Scholar]
- 18.Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol 7: 603–615, 2011. doi: 10.1038/nrneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 19.Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, Macdonald LE, Thurston G, Daly C, Lin HC, Economides AN, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gale NW. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc Natl Acad Sci USA 108: 2807–2812, 2011. doi: 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andjus PR, Bataveljić D, Vanhoutte G, Mitrecic D, Pizzolante F, Djogo N, Nicaise C, Gankam Kengne F, Gangitano C, Michetti F, van der Linden A, Pochet R, Bacić G. In vivo morphological changes in animal models of amyotrophic lateral sclerosis and Alzheimer’s-like disease: MRI approach. Anat Rec (Hoboken) 292: 1882–1892, 2009. doi: 10.1002/ar.20995. [DOI] [PubMed] [Google Scholar]
- 21.Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 94: 581–594.e5, 2017. doi: 10.1016/j.neuron.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreone BJ, Lacoste B, Gu C. Neuronal and vascular interactions. Annu Rev Neurosci 38: 25–46, 2015. doi: 10.1146/annurev-neuro-071714-033835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apostolski S, Nikolić J, Bugarski-Prokopljević C, Miletić V, Pavlović S, Filipović S. Serum and CSF immunological findings in ALS. Acta Neurol Scand 83: 96–98, 1991. doi: 10.1111/j.1600-0404.1991.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 24.Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122: 2454–2468, 2012. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong RA. Spatial correlations between beta-amyloid (Abeta) deposits and blood vessels in familial Alzheimer’s disease. Folia Neuropathol 46: 241–248, 2008. [PubMed] [Google Scholar]
- 26.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 97: 512–523, 2005. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 27.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature 468: 557–561, 2010. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 29.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 15: 934–943, 2016. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asgari M, de Zélicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep 6: 38635, 2016. doi: 10.1038/srep38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asgari N, Berg CT, Mørch MT, Khorooshi R, Owens T. Cerebrospinal fluid aquaporin-4-immunoglobulin G disrupts blood brain barrier. Ann Clin Transl Neurol 2: 857–863, 2015. doi: 10.1002/acn3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212: 991–999, 2015. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Assema DME, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, Comans EFI, Hoetjes NJ, Tolboom N, Langer O, Müller M, Scheltens P, Lammertsma AA, van Berckel BNM. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain 135: 181–189, 2012. doi: 10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- 34.Atwal JK, Chen Y, Chiu C, Mortensen DL, Meilandt WJ, Liu Y, Heise CE, Hoyte K, Luk W, Lu Y, Peng K, Wu P, Rouge L, Zhang Y, Lazarus RA, Scearce-Levie K, Wang W, Wu Y, Tessier-Lavigne M, Watts RJ. A therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivo. Sci Transl Med 3: 84ra43, 2011. doi: 10.1126/scitranslmed.3002254. [DOI] [PubMed] [Google Scholar]
- 35.Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 71: 1018–1039, 2011. doi: 10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey TL, Rivara CB, Rocher AB, Hof PR. The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol Res 26: 573–578, 2004. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- 37.Bakker ENTP, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AWJ, Weller RO, Carare RO. Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell Mol Neurobiol 36: 181–194, 2016. doi: 10.1007/s10571-015-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balbuena P, Li W, Rzigalinski BA, Ehrich M. Malathion/oxon and lead acetate increase gene expression and protein levels of transient receptor potential canonical channel subunits TRPC1 and TRPC4 in rat endothelial cells of the blood-brain barrier. Int J Toxicol 31: 238–249, 2012. doi: 10.1177/1091581812442688. [DOI] [PubMed] [Google Scholar]
- 39.Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 96: 15233–15238, 1999. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer’s disease: a study in Golgi technique and electron microscopy. J Neurol Sci 322: 117–121, 2012. doi: 10.1016/j.jns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Banks WA. Leptin transport across the blood-brain barrier: implications for the cause and treatment of obesity. Curr Pharm Des 7: 125–133, 2001. doi: 10.2174/1381612013398310. [DOI] [PubMed] [Google Scholar]
- 42.Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 15: 275–292, 2016. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 43.Barbagallo M, Dominguez LJ. Type 2 diabetes mellitus and Alzheimer’s disease. World J Diabetes 5: 889–893, 2014. doi: 10.4239/wjd.v5.i6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barcia C, Emborg ME, Hirsch EC, Herrero M-T. Blood vessels and parkinsonism. Front Biosci 9: 277–282, 2004. doi: 10.2741/1145. [DOI] [PubMed] [Google Scholar]
- 45.Bardehle S, Rafalski VA, Akassoglou K. Breaking boundaries-coagulation and fibrinolysis at the neurovascular interface. Front Cell Neurosci 9: 354, 2015. doi: 10.3389/fncel.2015.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnes SR, Ng TSC, Montagne A, Law M, Zlokovic BV, Jacobs RE. Optimal acquisition and modeling parameters for accurate assessment of low Ktrans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn Reson Med 75: 1967–1977, 2016. doi: 10.1002/mrm.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes SR, Ng TSC, Santa-Maria N, Montagne A, Zlokovic BV, Jacobs RE. ROCKETSHIP: a flexible and modular software tool for the planning, processing and analysis of dynamic MRI studies. BMC Med Imaging 15: 19, 2015. doi: 10.1186/s12880-015-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Başkaya MK, Rao AM, Doğan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett 226: 33–36, 1997. doi: 10.1016/S0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- 49.Basun H, Bogdanovic N, Ingelsson M, Almkvist O, Näslund J, Axelman K, Bird TD, Nochlin D, Schellenberg GD, Wahlund L-O, Lannfelt L. Clinical and neuropathological features of the arctic APP gene mutation causing early-onset Alzheimer disease. Arch Neurol 65: 499–505, 2008. doi: 10.1001/archneur.65.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bataveljić D, Nikolić L, Milosević M, Todorović N, Andjus PR. Changes in the astrocytic aquaporin-4 and inwardly rectifying potassium channel expression in the brain of the amyotrophic lateral sclerosis SOD1(G93A) rat model. Glia 60: 1991–2003, 2012. doi: 10.1002/glia.22414. [DOI] [PubMed] [Google Scholar]
- 51.Bataveljić D, Stamenković S, Bačić G, Andjus PR. Imaging cellular markers of neuroinflammation in the brain of the rat model of amyotrophic lateral sclerosis. Acta Physiol Hung 98: 27–31, 2011. doi: 10.1556/APhysiol.98.2011.1.4. [DOI] [PubMed] [Google Scholar]
- 52.Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, Salloway S, Sperling RA, Windisch M, Xiong C. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther 3: 1, 2011. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC; Dominantly Inherited Alzheimer Network . Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367: 795–804, 2012. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baugh CM, Robbins CA, Stern RA, McKee AC. Current understanding of chronic traumatic encephalopathy. Curr Treat Options Neurol 16: 306, 2014. doi: 10.1007/s11940-014-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beaufort N, Scharrer E, Kremmer E, Lux V, Ehrmann M, Huber R, Houlden H, Werring D, Haffner C, Dichgans M. Cerebral small vessel disease-related protease HtrA1 processes latent TGF-β binding protein 1 and facilitates TGF-β signaling. Proc Natl Acad Sci USA 111: 16496–16501, 2014. doi: 10.1073/pnas.1418087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beckmann N, Gérard C, Abramowski D, Cannet C, Staufenbiel M. Noninvasive magnetic resonance imaging detection of cerebral amyloid angiopathy-related microvascular alterations using superparamagnetic iron oxide particles in APP transgenic mouse models of Alzheimer’s disease: application to passive Abeta immunotherapy. J Neurosci 31: 1023–1031, 2011. doi: 10.1523/JNEUROSCI.4936-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, Miano JM, Zlokovic BV. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol 11: 143–153, 2009. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27: 909–918, 2007. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68: 409–427, 2010. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485: 512–516, 2012. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benzinger TLS, Blazey T, Jack CR Jr, Koeppe RA, Su Y, Xiong C, Raichle ME, Snyder AZ, Ances BM, Bateman RJ, Cairns NJ, Fagan AM, Goate A, Marcus DS, Aisen PS, Christensen JJ, Ercole L, Hornbeck RC, Farrar AM, Aldea P, Jasielec MS, Owen CJ, Xie X, Mayeux R, Brickman A, McDade E, Klunk W, Mathis CA, Ringman J, Thompson PM, Ghetti B, Saykin AJ, Sperling RA, Johnson KA, Salloway S, Correia S, Schofield PR, Masters CL, Rowe C, Villemagne VL, Martins R, Ourselin S, Rossor MN, Fox NC, Cash DM, Weiner MW, Holtzman DM, Buckles VD, Moulder K, Morris JC. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci USA 110: E4502–E4509, 2013. doi: 10.1073/pnas.1317918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509: 507–511, 2014. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Betsholtz C. Physiology: double function at the blood-brain barrier. Nature 509: 432–433, 2014. doi: 10.1038/nature13339. [DOI] [PubMed] [Google Scholar]
- 64.Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Wang C, Huang Y. C-terminal-truncated apolipoprotein (apo) E4 inefficiently clears amyloid-beta (Abeta) and acts in concert with Abeta to elicit neuronal and behavioral deficits in mice. Proc Natl Acad Sci USA 108: 4236–4241, 2011. doi: 10.1073/pnas.1018381108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Biesecker KR, Srienc AI, Shimoda AM, Agarwal A, Bergles DE, Kofuji P, Newman EA. Glial Cell Calcium Signaling Mediates Capillary Regulation of Blood Flow in the Retina. J Neurosci 36: 9435–9445, 2016. doi: 10.1523/JNEUROSCI.1782-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blair LJ, Frauen HD, Zhang B, Nordhues BA, Bijan S, Lin Y-C, Zamudio F, Hernandez LD, Sabbagh JJ, Selenica M-LB, Dickey CA. Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy. Acta Neuropathol Commun 3: 8, 2015. doi: 10.1186/s40478-015-0186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blennow K, Wallin A, Fredman P, Karlsson I, Gottfries CG, Svennerholm L. Blood-brain barrier disturbance in patients with Alzheimer’s disease is related to vascular factors. Acta Neurol Scand 81: 323–326, 1990. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 68.Blennow K, Wallin A, Uhlemann C, Gottfries CG. White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol Scand 83: 187–193, 1991. doi: 10.1111/j.1600-0404.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 69.Boado RJ, Hui EK-W, Lu JZ, Pardridge WM. Re-engineering iduronate 2-sulfatase for penetration of the blood-brain barrier via transport on the insulin receptor. Mol Genet Metab 111: S27, 2014. doi: 10.1016/j.ymgme.2013.12.043. [DOI] [Google Scholar]
- 70.Boado RJ, Pardridge WM. Brain and Organ Uptake in the Rhesus Monkey in Vivo of Recombinant Iduronidase Compared to an Insulin Receptor Antibody-Iduronidase Fusion Protein. Mol Pharm 14: 1271–1277, 2017. doi: 10.1021/acs.molpharmaceut.6b01166. [DOI] [PubMed] [Google Scholar]
- 71.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res 33: 2373–2387, 2016. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 72.Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellström M, Lindahl P, Betsholtz C. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J 20: 1703–1705, 2006. doi: 10.1096/fj.05-4944fje. [DOI] [PubMed] [Google Scholar]
- 73.Boulay A-C, Mazeraud A, Cisternino S, Saubaméa B, Mailly P, Jourdren L, Blugeon C, Mignon V, Smirnova M, Cavallo A, Ezan P, Avé P, Dingli F, Loew D, Vieira P, Chrétien F, Cohen-Salmon M. Immune quiescence of the brain is set by astroglial connexin 43. J Neurosci 35: 4427–4439, 2015. doi: 10.1523/JNEUROSCI.2575-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bowman GL, Kaye JA, Quinn JF. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr Gerontol Geriatr Res 2012: 184042, 2012. doi: 10.1155/2012/184042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol Renal Physiol 240: F329–F336, 1981. [DOI] [PubMed] [Google Scholar]
- 76.Braganza O, Bedner P, Hüttmann K, von Staden E, Friedman A, Seifert G, Steinhäuser C. Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling. Epilepsia 53: 1898–1906, 2012. doi: 10.1111/j.1528-1167.2012.03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice [Correction in Sci Transl Med 6: 266er7, 2014.]. Sci Transl Med 6: 263ra158, 2014. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bray N. Biologics: transferrin’ bispecific antibodies across the blood-brain barrier. Nat Rev Drug Discov 14: 14–15, 2015. doi: 10.1038/nrd4522. [DOI] [PubMed] [Google Scholar]
- 79.Brettschneider J, Petzold A, Süssmuth SD, Ludolph AC, Tumani H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66: 852–856, 2006. doi: 10.1212/01.wnl.0000203120.85850.54. [DOI] [PubMed] [Google Scholar]
- 80.Brinton R, Swanson H, Irwin R. Allopregnanolone promotes cholesterol and amyloid-beta clearance mechanisms: assessment of a regenerative therapeutic for Alzheimer’s disease. Alzheimers Dement 12: 1024–1025, 2016. doi: 10.1016/j.jalz.2016.06.2115. [DOI] [Google Scholar]
- 81.Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol 14: 416–429, 2013. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 82.Bruinsma IB, Wilhelmus MMM, Kox M, Veerhuis R, de Waal RMW, Verbeek MM. Apolipoprotein E protects cultured pericytes and astrocytes from D-Abeta(1-40)-mediated cell death. Brain Res 1315: 169–180, 2010. doi: 10.1016/j.brainres.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 83.Brundel M, Heringa SM, de Bresser J, Koek HL, Zwanenburg JJM, Jaap Kappelle L, Luijten PR, Biessels GJ. High prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer’s disease. J Alzheimers Dis 31: 259–263, 2012. doi: 10.3233/JAD-2012-120364. [DOI] [PubMed] [Google Scholar]
- 84.Burgess A, Hynynen K. Microbubble-assisted ultrasound for drug delivery in the brain and central nervous system. In: Therapeutic Ultrasound, edited by Escoffre J-M, Bouakaz A. New York: Springer International, 2016, p. 293–308. doi: 10.1007/978-3-319-22536-4_16. [DOI] [PubMed] [Google Scholar]
- 85.Cacciottolo M, Christensen A, Moser A, Liu J, Pike CJ, Smith C, LaDu MJ, Sullivan PM, Morgan TE, Dolzhenko E, Charidimou A, Wahlund L-O, Wiberg MK, Shams S, Chiang GC-Y, Finch CE; Alzheimer’s Disease Neuroimaging Initiative . The APOE4 allele shows opposite sex bias in microbleeds and Alzheimer’s disease of humans and mice. Neurobiol Aging 37: 47–57, 2016. doi: 10.1016/j.neurobiolaging.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Calabria AR, Shusta EV. A genomic comparison of in vivo and in vitro brain microvascular endothelial cells. J Cereb Blood Flow Metab 28: 135–148, 2008. doi: 10.1038/sj.jcbfm.9600518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Calderón-Garcidueñas L, Mora-Tiscareño A, Franco-Lira M, Zhu H, Lu Z, Solorio E, Torres-Jardón R, D’Angiulli A. Decreases in Short Term Memory, IQ, and Altered Brain Metabolic Ratios in Urban Apolipoprotein ε4 Children Exposed to Air Pollution. J Alzheimers Dis 45: 757–770, 2015. doi: 10.3233/JAD-142685. [DOI] [PubMed] [Google Scholar]
- 88.Calderón-Garcidueñas L, Villarreal-Ríos R. Living close to heavy traffic roads, air pollution, and dementia. Lancet 389: 675–677, 2017. doi: 10.1016/S0140-6736(16)32596-X. [DOI] [PubMed] [Google Scholar]
- 89.Calderón-Garcidueñas L, Vojdani A, Blaurock-Busch E, Busch Y, Friedle A, Franco-Lira M, Sarathi-Mukherjee P, Martínez-Aguirre X, Park S-B, Torres-Jardón R, D’Angiulli A. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J Alzheimers Dis 43: 1039–1058, 2015. doi: 10.3233/JAD-141365. [DOI] [PubMed] [Google Scholar]
- 90.Calero M, Tokuda T, Rostagno A, Kumar A, Zlokovic B, Frangione B, Ghiso J. Functional and structural properties of lipid-associated apolipoprotein J (clusterin). Biochem J 344: 375–383, 1999. doi: 10.1042/bj3440375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Canevelli M, Piscopo P, Talarico G, Vanacore N, Blasimme A, Crestini A, Tosto G, Troili F, Lenzi GL, Confaloni A, Bruno G. Familial Alzheimer’s disease sustained by presenilin 2 mutations: systematic review of literature and genotype-phenotype correlation. Neurosci Biobehav Rev 42: 170–179, 2014. doi: 10.1016/j.neubiorev.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 92.Capri Y, Friesema ECH, Kersseboom S, Touraine R, Monnier A, Eymard-Pierre E, Des Portes V, De Michele G, Brady AF, Boespflug-Tanguy O, Visser TJ, Vaurs-Barriere C. Relevance of different cellular models in determining the effects of mutations on SLC16A2/MCT8 thyroid hormone transporter function and genotype-phenotype correlation. Hum Mutat 34: 1018–1025, 2013. doi: 10.1002/humu.22331. [DOI] [PubMed] [Google Scholar]
- 93.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature 436: 193–200, 2005. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 94.Carrasquillo MM, Belbin O, Hunter TA, Ma L, Bisceglio GD, Zou F, Crook JE, Pankratz VS, Dickson DW, Graff-Radford NR, Petersen RC, Morgan K, Younkin SG. Replication of CLU, CR1, and PICALM associations with Alzheimer disease. Arch Neurol 67: 961–964, 2010. doi: 10.1001/archneurol.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci 22: 1158–1168, 2005. doi: 10.1111/j.1460-9568.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- 96.Casey CS, Atagi Y, Yamazaki Y, Shinohara M, Tachibana M, Fu Y, Bu G, Kanekiyo T. Apolipoprotein E Inhibits Cerebrovascular Pericyte Mobility through a RhoA Protein-mediated Pathway. J Biol Chem 290: 14208–14217, 2015. doi: 10.1074/jbc.M114.625251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cass CE, Young JD, Baldwin SA. Recent advances in the molecular biology of nucleoside transporters of mammalian cells. Biochem Cell Biol 76: 761–770, 1998. doi: 10.1139/o98-095. [DOI] [PubMed] [Google Scholar]
- 98.Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med 3: 89ra57, 2011. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Castillo-Gomez E, Kästner A, Steiner J, Schneider A, Hettling B, Poggi G, Ostehr K, Uhr M, Asif AR, Matzke M, Schmidt U, Pfander V, Hammer C, Schulz TF, Binder L, Stöcker W, Weber F, Ehrenreich H. The brain as immunoprecipitator of serum autoantibodies against N-Methyl-d-aspartate receptor subunit NR1. Ann Neurol 79: 144–151, 2016. doi: 10.1002/ana.24545. [DOI] [PubMed] [Google Scholar]
- 100.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser M-G. Cadasil. Lancet Neurol 8: 643–653, 2009. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 101.Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu W-L, Sánchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20: 1172–1179, 2017. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang J, Mancuso MR, Maier C, Liang X, Yuki K, Yang L, Kwong JW, Wang J, Rao V, Vallon M, Kosinski C, Zhang JJH, Mah AT, Xu L, Li L, Gholamin S, Reyes TF, Li R, Kuhnert F, Han X, Yuan J, Chiou S-H, Brettman AD, Daly L, Corney DC, Cheshier SH, Shortliffe LD, Wu X, Snyder M, Chan P, Giffard RG, Chang HY, Andreasson K, Kuo CJ. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat Med 23: 450–460, 2017. doi: 10.1038/nm.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chapman CD, Frey WH II, Craft S, Danielyan L, Hallschmid M, Schiöth HB, Benedict C. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res 30: 2475–2484, 2013. doi: 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chávez-Gutiérrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, Wiltfang J, Serneels L, Karran E, Gijsen H, Schymkowitz J, Rousseau F, Broersen K, De Strooper B. The mechanism of γ-secretase dysfunction in familial Alzheimer disease. EMBO J 31: 2261–2274, 2012. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EMC. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc 3: e000787, 2014. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen H, Konofagou EE. The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J Cereb Blood Flow Metab 34: 1197–1204, 2014. doi: 10.1038/jcbfm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Q, Zhang H, Liu Y, Adams S, Eilken H, Stehling M, Corada M, Dejana E, Zhou B, Adams RH. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat Commun 7: 12422, 2016. doi: 10.1038/ncomms12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen X, Lan X, Roche I, Liu R, Geiger JD. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J Neurochem 107: 1147–1157, 2008. doi: 10.1111/j.1471-4159.2008.05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Z-L, Revenko AS, Singh P, MacLeod AR, Norris EH, Strickland S. Depletion of coagulation factor XII ameliorates brain pathology and cognitive impairment in Alzheimer disease mice. Blood 129: 2547–2556, 2017. doi: 10.1182/blood-2016-11-753202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 42: 484–491, 2012. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 111.Cho C, Smallwood PM, Nathans J. Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation. Neuron 95: 1056–1073.e5, 2017. doi: 10.1016/j.neuron.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choudhary M, Naczki C, Chen W, Barlow KD, Case LD, Metheny-Barlow LJ. Tumor-induced loss of mural Connexin 43 gap junction activity promotes endothelial proliferation. BMC Cancer 15: 427, 2015. doi: 10.1186/s12885-015-1420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chung YC, Kim Y-S, Bok E, Yune TY, Maeng S, Jin BK. MMP-3 contributes to nigrostriatal dopaminergic neuronal loss, BBB damage, and neuroinflammation in an MPTP mouse model of Parkinson’s disease. Mediators Inflamm 2013: 370526, 2013. doi: 10.1155/2013/370526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cicchetti F, Lacroix S, Cisbani G, Vallières N, Saint-Pierre M, St-Amour I, Tolouei R, Skepper JN, Hauser RA, Mantovani D, Barker RA, Freeman TB. Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann Neurol 76: 31–42, 2014. doi: 10.1002/ana.24174. [DOI] [PubMed] [Google Scholar]
- 115.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest 115: 3285–3290, 2005. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coatti GC, Frangini M, Valadares MC, Gomes JP, Lima NO, Cavaçana N, Assoni AF, Pelatti MV, Birbrair A, de Lima ACP, Singer JM, Rocha FMM, Da Silva GL, Mantovani MS, Macedo-Souza LI, Ferrari MFR, Zatz M. Pericytes extend survival of ALS SOD1 mice and induce the expression of antioxidant enzymes in the murine model and in IPSCs derived neuronal cells from an ALS patient. Stem Cell Rev 13: 686–698, 2017. doi: 10.1007/s12015-017-9752-2. [DOI] [PubMed] [Google Scholar]
- 117.Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu S-J, Narayana PA. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed 22: 332–341, 2009. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Conejero-Goldberg C, Gomar JJ, Bobes-Bascaran T, Hyde TM, Kleinman JE, Herman MM, Chen S, Davies P, Goldberg TE. APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms. Mol Psychiatry 19: 1243–1250, 2014. doi: 10.1038/mp.2013.194. [DOI] [PubMed] [Google Scholar]
- 119.Corneveaux JJ, Myers AJ, Allen AN, Pruzin JJ, Ramirez M, Engel A, Nalls MA, Chen K, Lee W, Chewning K, Villa SE, Meechoovet HB, Gerber JD, Frost D, Benson HL, O’Reilly S, Chibnik LB, Shulman JM, Singleton AB, Craig DW, Van Keuren-Jensen KR, Dunckley T, Bennett DA, De Jager PL, Heward C, Hardy J, Reiman EM, Huentelman MJ. Association of CR1, CLU and PICALM with Alzheimer’s disease in a cohort of clinically characterized and neuropathologically verified individuals. Hum Mol Genet 19: 3295–3301, 2010. doi: 10.1093/hmg/ddq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, Bhuvanendran S, Fenz KM, Strickland S. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease. Neuron 66: 695–709, 2010. doi: 10.1016/j.neuron.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cramer SP, Larsson HBW. Accurate determination of blood-brain barrier permeability using dynamic contrast-enhanced T1-weighted MRI: a simulation and in vivo study on healthy subjects and multiple sclerosis patients. J Cereb Blood Flow Metab 34: 1655–1665, 2014. doi: 10.1038/jcbfm.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cramer SP, Modvig S, Simonsen HJ, Frederiksen JL, Larsson HBW. Permeability of the blood-brain barrier predicts conversion from optic neuritis to multiple sclerosis. Brain 138: 2571–2583, 2015. doi: 10.1093/brain/awv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cramer SP, Simonsen H, Frederiksen JL, Rostrup E, Larsson HBW. Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. Neuroimage Clin 4: 182–189, 2014. doi: 10.1016/j.nicl.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Crane RK, Sols A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem 210: 597–606, 1954. [PubMed] [Google Scholar]
- 125.Cullen KM, Kócsi Z, Stone J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab 25: 1656–1667, 2005. doi: 10.1038/sj.jcbfm.9600155. [DOI] [PubMed] [Google Scholar]
- 126.Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, Logsdon D, Hsiao E, Stein EV, Cuttitta F, Haines DC, Nagashima K, Tessarollo L, St Croix B. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci USA 108: 5759–5764, 2011. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. [Correction in Proc Natl Acad Sci USA 106: 6422, 2009.] Proc Natl Acad Sci USA 106: 641–646, 2009. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol 7: a020412, 2015. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One 5: e13741, 2010. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468: 562–566, 2010. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daniels BP, Klein RS. Knocking on Closed Doors: Host Interferons Dynamically Regulate Blood-Brain Barrier Function during Viral Infections of the Central Nervous System. PLoS Pathog 11: e1005096, 2015. doi: 10.1371/journal.ppat.1005096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, Gonias Murray S, Ling JB, Lassmann H, Degen JL, Ellisman MH, Akassoglou K. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3: 1227, 2012. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davson H. Review lecture. The blood-brain barrier. J Physiol 255: 1–28, 1976. doi: 10.1113/jphysiol.1976.sp011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 9: 907–913, 2003. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 135.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 118: 4002–4013, 2008. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest 122: 1377–1392, 2012. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 43: 333–344, 2004. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 138.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res 107: 943–952, 2010. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 139.Dejana E, Vestweber D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog Mol Biol Transl Sci 116: 119–144, 2013. doi: 10.1016/B978-0-12-394311-8.00006-6. [DOI] [PubMed] [Google Scholar]
- 140.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung G-YR, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72: 245–256, 2011. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Deng D, Xu C, Sun P, Wu J, Yan C, Hu M, Yan N. Crystal structure of the human glucose transporter GLUT1. Nature 510: 121–125, 2014. doi: 10.1038/nature13306. [DOI] [PubMed] [Google Scholar]
- 142.Deo AK, Borson S, Link JM, Domino K, Eary JF, Ke B, Richards TL, Mankoff DA, Minoshima S, O’Sullivan F, Eyal S, Hsiao P, Maravilla K, Unadkat JD. Activity of P-Glycoprotein, a β-Amyloid Transporter at the Blood-Brain Barrier, Is Compromised in Patients with Mild Alzheimer Disease. J Nucl Med 55: 1106–1111, 2014. doi: 10.2967/jnumed.113.130161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dermaut B, Kumar-Singh S, De Jonghe C, Cruts M, Löfgren A, Lübke U, Cras P, Dom R, De Deyn PP, Martin JJ, Van Broeckhoven C. Cerebral amyloid angiopathy is a pathogenic lesion in Alzheimer’s disease due to a novel presenilin 1 mutation. Brain 124: 2383–2392, 2001. doi: 10.1093/brain/124.12.2383. [DOI] [PubMed] [Google Scholar]
- 144.Desai Bradaric B, Patel A, Schneider JA, Carvey PM, Hendey B. Evidence for angiogenesis in Parkinson’s disease, incidental Lewy body disease, and progressive supranuclear palsy. J Neural Transm (Vienna) 119: 59–71, 2012. doi: 10.1007/s00702-011-0684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Deshpande T, Li T, Herde MK, Becker A, Vatter H, Schwarz MK, Henneberger C, Steinhäuser C, Bedner P. Subcellular reorganization and altered phosphorylation of the astrocytic gap junction protein connexin43 in human and experimental temporal lobe epilepsy. Glia 65: 1809–1820, 2017. doi: 10.1002/glia.23196. [DOI] [PubMed] [Google Scholar]
- 146.Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu W-L, Yang B, Huber N, Pasca SP, Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol 34: 204–209, 2016. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Di Cataldo V, Géloën A, Langlois J-B, Chauveau F, Thézé B, Hubert V, Wiart M, Chirico EN, Rieusset J, Vidal H, Pialoux V, Canet-Soulas E. Exercise Does Not Protect against Peripheral and Central Effects of a High Cholesterol Diet Given Ad libitum in Old ApoE-/- Mice. Front Physiol 7: 453, 2016. doi: 10.3389/fphys.2016.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Di Pardo A, Carrizzo A, Damato A, Castaldo S, Amico E, Capocci L, Ambrosio M, Pompeo F, De Sanctis C, Spinelli CC, Puca AA, Remondelli P, Maglione V, Vecchione C. Motor phenotype is not associated with vascular dysfunction in symptomatic Huntington’s disease transgenic R6/2 (160 CAG) mice. Sci Rep 7: 42797, 2017. doi: 10.1038/srep42797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Doherty CP, O’Keefe E, Wallace E, Loftus T, Keaney J, Kealy J, Humphries MM, Molloy MG, Meaney JF, Farrell M, Campbell M. Blood-Brain Barrier Dysfunction as a Hallmark Pathology in Chronic Traumatic Encephalopathy. J Neuropathol Exp Neurol 75: 656–662, 2016. doi: 10.1093/jnen/nlw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dolgin E. Spinal muscular atrophy approval boosts antisense drugs. Nat Biotechnol 35: 99–100, 2017. doi: 10.1038/nbt0217-99. [DOI] [PubMed] [Google Scholar]
- 151.Domnitz SB, Robbins EM, Hoang AW, Garcia-Alloza M, Hyman BT, Rebeck GW, Greenberg SM, Bacskai BJ, Frosch MP. Progression of cerebral amyloid angiopathy in transgenic mouse models of Alzheimer disease. J Neuropathol Exp Neurol 64: 588–594, 2005. doi: 10.1097/01.jnen.0000171644.00180.fc. [DOI] [PubMed] [Google Scholar]
- 152.Donahue JE, Flaherty SL, Johanson CE, Duncan JA III, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol 112: 405–415, 2006. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 153.Donnelly CJ, Zhang P-W, Pham JT, Haeusler AR, Mistry NA, Vidensky S, Daley EL, Poth EM, Hoover B, Fines DM, Maragakis N, Tienari PJ, Petrucelli L, Traynor BJ, Wang J, Rigo F, Bennett CF, Blackshaw S, Sattler R, Rothstein JD. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention [Correction in Neuron 80: 1102, 2013.]. Neuron 80: 415–428, 2013. doi: 10.1016/j.neuron.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Donnenfeld H, Kascsak RJ, Bartfeld H. Deposits of IgG and C3 in the spinal cord and motor cortex of ALS patients. J Neuroimmunol 6: 51–57, 1984. doi: 10.1016/0165-5728(84)90042-0. [DOI] [PubMed] [Google Scholar]
- 155.Drouin-Ouellet J, Sawiak SJ, Cisbani G, Lagacé M, Kuan W-L, Saint-Pierre M, Dury RJ, Alata W, St-Amour I, Mason SL, Calon F, Lacroix S, Gowland PA, Francis ST, Barker RA, Cicchetti F. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann Neurol 78: 160–177, 2015. doi: 10.1002/ana.24406. [DOI] [PubMed] [Google Scholar]
- 156.Dumitrescu AM, Liao X-H, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74: 168–175, 2004. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dunckley T, Huentelman MJ, Craig DW, Pearson JV, Szelinger S, Joshipura K, Halperin RF, Stamper C, Jensen KR, Letizia D, Hesterlee SE, Pestronk A, Levine T, Bertorini T, Graves MC, Mozaffar T, Jackson CE, Bosch P, McVey A, Dick A, Barohn R, Lomen-Hoerth C, Rosenfeld J, O’connor DT, Zhang K, Crook R, Ryberg H, Hutton M, Katz J, Simpson EP, Mitsumoto H, Bowser R, Miller RG, Appel SH, Stephan DA. Whole-genome analysis of sporadic amyotrophic lateral sclerosis. N Engl J Med 357: 775–788, 2007. doi: 10.1056/NEJMoa070174. [DOI] [PubMed] [Google Scholar]
- 158.Eichmann A, Thomas J-L. Molecular parallels between neural and vascular development. Cold Spring Harb Perspect Med 3: a006551, 2013. doi: 10.1101/cshperspect.a006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Elfeber K, Köhler A, Lutzenburg M, Osswald C, Galla H-J, Witte OW, Koepsell H. Localization of the Na+-d-glucose cotransporter SGLT1 in the blood-brain barrier. Histochem Cell Biol 121: 201–207, 2004. doi: 10.1007/s00418-004-0633-9. [DOI] [PubMed] [Google Scholar]
- 160.Engelhardt B, Carare RO, Bechmann I, Flügel A, Laman JD, Weller RO. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol 132: 317–338, 2016. doi: 10.1007/s00401-016-1606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol 33: 579–589, 2012. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 162.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol 18: 123–131, 2017. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 163.Engelhardt JI, Appel SH. IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol 47: 1210–1216, 1990. doi: 10.1001/archneur.1990.00530110068019. [DOI] [PubMed] [Google Scholar]
- 164.Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol 50: 30–36, 1993. doi: 10.1001/archneur.1993.00540010026013. [DOI] [PubMed] [Google Scholar]
- 165.Estin ML, Thompson SB, Traxinger B, Fisher MH, Friedman RS, Jacobelli J. Ena/VASP proteins regulate activated T-cell trafficking by promoting diapedesis during transendothelial migration. Proc Natl Acad Sci USA 114: E2901–E2910, 2017. doi: 10.1073/pnas.1701886114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Evans MC, Serres S, Khrapitchev AA, Stolp HB, Anthony DC, Talbot K, Turner MR, Sibson NR. T2-weighted MRI detects presymptomatic pathology in the SOD1 mouse model of ALS. J Cereb Blood Flow Metab 34: 785–793, 2014. doi: 10.1038/jcbfm.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ezan P, André P, Cisternino S, Saubaméa B, Boulay A-C, Doutremer S, Thomas M-A, Quenech’du N, Giaume C, Cohen-Salmon M. Deletion of astroglial connexins weakens the blood-brain barrier. J Cereb Blood Flow Metab 32: 1457–1467, 2012. doi: 10.1038/jcbfm.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fainardi E, Castellazzi M, Bellini T, Manfrinato MC, Baldi E, Casetta I, Paolino E, Granieri E, Dallocchio F. Cerebrospinal fluid and serum levels and intrathecal production of active matrix metalloproteinase-9 (MMP-9) as markers of disease activity in patients with multiple sclerosis. Mult Scler 12: 294–301, 2006. doi: 10.1191/135248506ms1274oa. [DOI] [PubMed] [Google Scholar]
- 169.Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, Iadecola C. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab 36: 241–252, 2016. doi: 10.1038/jcbfm.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64: 575–611, 2001. doi: 10.1016/S0301-0082(00)00068-X. [DOI] [PubMed] [Google Scholar]
- 171.Farrell CL, Pardridge WM. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci USA 88: 5779–5783, 1991. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fernandez MA, Klutkowski JA, Freret T, Wolfe MS. Alzheimer presenilin-1 mutations dramatically reduce trimming of long amyloid β-peptides (Aβ) by γ-secretase to increase 42-to-40-residue Aβ. J Biol Chem 289: 31043–31052, 2014. doi: 10.1074/jbc.M114.581165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA 107: 22290–22295, 2010. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol 7: 616–630, 2011. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 175.Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur J Clin Invest 32: 360–371, 2002. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 176.Filippi M, Rocca MA, Martino G, Horsfield MA, Comi G. Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol 43: 809–814, 1998. doi: 10.1002/ana.410430616. [DOI] [PubMed] [Google Scholar]
- 177.Filley CM, Rollins YD, Anderson CA, Arciniegas DB, Howard KL, Murrell JR, Boyer PJ, Kleinschmidt-DeMasters BK, Ghetti B. The genetics of very early onset Alzheimer disease. Cogn Behav Neurol 20: 149–156, 2007. doi: 10.1097/WNN.0b013e318145a8c8. [DOI] [PubMed] [Google Scholar]
- 178.Fischer A, Zalvide J, Faurobert E, Albiges-Rizo C, Tournier-Lasserve E. Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends Mol Med 19: 302–308, 2013. doi: 10.1016/j.molmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 179.Flemming KD, Graff-Radford J, Aakre J, Kantarci K, Lanzino G, Brown RD Jr, Mielke MM, Roberts RO, Kremers W, Knopman DS, Petersen RC, Jack CR Jr. Population-Based Prevalence of Cerebral Cavernous Malformations in Older Adults: Mayo Clinic Study of Aging. JAMA Neurol 74: 801–805, 2017. doi: 10.1001/jamaneurol.2017.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fonseca MI, Chu S, Pierce AL, Brubaker WD, Hauhart RE, Mastroeni D, Clarke EV, Rogers J, Atkinson JP, Tenner AJ. Analysis of the Putative Role of CR1 in Alzheimer’s Disease: Genetic Association, Expression and Function. PLoS One 11: e0149792, 2016. doi: 10.1371/journal.pone.0149792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Foroutan S, Brillault J, Forbush B, O’Donnell ME. Moderate-to-severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na+-K+-Cl- cotransporter. Am J Physiol Cell Physiol 289: C1492–C1501, 2005. doi: 10.1152/ajpcell.00257.2005. [DOI] [PubMed] [Google Scholar]
- 182.Fournier AP, Quenault A, Martinez de Lizarrondo S, Gauberti M, Defer G, Vivien D, Docagne F, Macrez R. Prediction of disease activity in models of multiple sclerosis by molecular magnetic resonance imaging of P-selectin. Proc Natl Acad Sci USA 114: 6116–6121, 2017. doi: 10.1073/pnas.1619424114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fox NC, Kennedy AM, Harvey RJ, Lantos PL, Roques PK, Collinge J, Hardy J, Hutton M, Stevens JM, Warrington EK, Rossor MN. Clinicopathological features of familial Alzheimer’s disease associated with the M139V mutation in the presenilin 1 gene. Pedigree but not mutation specific age at onset provides evidence for a further genetic factor. Brain 120: 491–501, 1997. doi: 10.1093/brain/120.3.491. [DOI] [PubMed] [Google Scholar]
- 184.Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol 10: 225–238, 2014. doi: 10.1038/nrneurol.2014.37. [DOI] [PubMed] [Google Scholar]
- 185.Friesema ECH, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MHA, Kuiper GGJM, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364: 1435–1437, 2004. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 186.Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci 25: 2803–2810, 2005. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fullerton SM, Shirman GA, Strittmatter WJ, Matthew WD. Impairment of the blood-nerve and blood-brain barriers in apolipoprotein e knockout mice. Exp Neurol 169: 13–22, 2001. doi: 10.1006/exnr.2001.7631. [DOI] [PubMed] [Google Scholar]
- 188.Funck VR, Ribeiro LR, Pereira LM, de Oliveira CV, Grigoletto J, Della-Pace ID, Fighera MR, Royes LFF, Furian AF, Larrick JW, Oliveira MS. Contrasting effects of Na+,K+-ATPase activation on seizure activity in acute versus chronic models. Neuroscience 298: 171–179, 2015. doi: 10.1016/j.neuroscience.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 189.Furuno T, Landi M-T, Ceroni M, Caporaso N, Bernucci I, Nappi G, Martignoni E, Schaeffeler E, Eichelbaum M, Schwab M, Zanger UM. Expression polymorphism of the blood-brain barrier component P-glycoprotein (MDR1) in relation to Parkinson’s disease. Pharmacogenetics 12: 529–534, 2002. doi: 10.1097/00008571-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 190.Gaitán MI, Shea CD, Evangelou IE, Stone RD, Fenton KM, Bielekova B, Massacesi L, Reich DS. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol 70: 22–29, 2011. doi: 10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gama Sosa MA, Gasperi RD, Rocher AB, Wang AC-J, Janssen WGM, Flores T, Perez GM, Schmeidler J, Dickstein DL, Hof PR, Elder GA. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer’s disease mutations. Am J Pathol 176: 353–368, 2010. doi: 10.2353/ajpath.2010.090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ganapathy ME, Huang W, Rajan DP, Carter AL, Sugawara M, Iseki K, Leibach FH, Ganapathy V. Beta-lactam antibiotics as substrates for OCTN2, an organic cation/carnitine transporter. J Biol Chem 275: 1699–1707, 2000. doi: 10.1074/jbc.275.3.1699. [DOI] [PubMed] [Google Scholar]
- 193.Gao B, Vavricka SR, Meier PJ, Stieger B. Differential cellular expression of organic anion transporting peptides OATP1A2 and OATP2B1 in the human retina and brain: implications for carrier-mediated transport of neuropeptides and neurosteriods in the CNS. Pflugers Arch 467: 1481–1493, 2015. doi: 10.1007/s00424-014-1596-x. [DOI] [PubMed] [Google Scholar]
- 194.Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res 1157: 126–137, 2007. doi: 10.1016/j.brainres.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 195.Garbuzova-Davis S, Hernandez-Ontiveros DG, Rodrigues MCO, Haller E, Frisina-Deyo A, Mirtyl S, Sallot S, Saporta S, Borlongan CV, Sanberg PR. Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res 1469: 114–128, 2012. doi: 10.1016/j.brainres.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 196.Garbuzova-Davis S, Saporta S, Haller E, Kolomey I, Bennett SP, Potter H, Sanberg PR. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS One 2: e1205, 2007. doi: 10.1371/journal.pone.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garrido-Urbani S, Bradfield PF, Imhof BA. Tight junction dynamics: the role of junctional adhesion molecules (JAMs). Cell Tissue Res 355: 701–715, 2014. doi: 10.1007/s00441-014-1820-1. [DOI] [PubMed] [Google Scholar]
- 198.Geng J, Wang L, Qu M, Song Y, Lin X, Chen Y, Mamtilahun M, Chen S, Zhang Z, Wang Y, Yang G-Y. Endothelial progenitor cells transplantation attenuated blood-brain barrier damage after ischemia in diabetic mice via HIF-1α. Stem Cell Res Ther 8: 163, 2017. doi: 10.1186/s13287-017-0605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O, Bullido MJ, Engelborghs S, De Deyn P, Berr C, Pasquier F, Dubois B, Tognoni G, Fiévet N, Brouwers N, Bettens K, Arosio B, Coto E, Del Zompo M, Mateo I, Epelbaum J, Frank-Garcia A, Helisalmi S, Porcellini E, Pilotto A, Forti P, Ferri R, Scarpini E, Siciliano G, Solfrizzi V, Sorbi S, Spalletta G, Valdivieso F, Vepsäläinen S, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Hanon O, Piccardi P, Annoni G, Seripa D, Galimberti D, Licastro F, Soininen H, Dartigues J-F, Kamboh MI, Van Broeckhoven C, Lambert JC, Amouyel P, Campion D. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry 16: 903–907, 2011. doi: 10.1038/mp.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn 218: 472–479, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 201.Gerwien H, Hermann S, Zhang X, Korpos E, Song J, Kopka K, Faust A, Wenning C, Gross CC, Honold L, Melzer N, Opdenakker G, Wiendl H, Schäfers M, Sorokin L. Imaging matrix metalloproteinase activity in multiple sclerosis as a specific marker of leukocyte penetration of the blood-brain barrier. Sci Transl Med 8: 364ra152, 2016. doi: 10.1126/scitranslmed.aaf8020. [DOI] [PubMed] [Google Scholar]
- 202.Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N. Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol 78: 887–900, 2015. doi: 10.1002/ana.24512. [DOI] [PubMed] [Google Scholar]
- 203.Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, Zlokovic B, Kalra VK. β-Amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol 279: C1772–C1781, 2000. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- 204.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery 75, Suppl 4: S24–S33, 2014. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gliemann J, Hermey G, Nykjaer A, Petersen CM, Jacobsen C, Andreasen PA. The mosaic receptor sorLA/LR11 binds components of the plasminogen-activating system and platelet-derived growth factor-BB similarly to LRP1 (low-density lipoprotein receptor-related protein), but mediates slow internalization of bound ligand. Biochem J 381: 203–212, 2004. doi: 10.1042/BJ20040149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Golden PL, Maccagnan TJ, Pardridge WM. Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J Clin Invest 99: 14–18, 1997. doi: 10.1172/JCI119125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.González-Maciel A, Reynoso-Robles R, Torres-Jardón R, Mukherjee PS, Calderón-Garcidueñas L. Combustion-Derived Nanoparticles in Key Brain Target Cells and Organelles in Young Urbanites: Culprit Hidden in Plain Sight in Alzheimer’s Disease Development. J Alzheimers Dis 59: 189–208, 2017. doi: 10.3233/JAD-170012. [DOI] [PubMed] [Google Scholar]
- 208.Goos JDC, Kester MI, Barkhof F, Klein M, Blankenstein MA, Scheltens P, van der Flier WM. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke 40: 3455–3460, 2009. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- 209.Gorter JA, van Vliet EA, Aronica E. Status epilepticus, blood-brain barrier disruption, inflammation, and epileptogenesis. Epilepsy Behav 49: 13–16, 2015. doi: 10.1016/j.yebeh.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 210.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E, John SWM. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med 354: 1489–1496, 2006. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 211.Grabowski TJ, Cho HS, Vonsattel JP, Rebeck GW, Greenberg SM. Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol 49: 697–705, 2001. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 212.Gralinski LE, Ashley SL, Dixon SD, Spindler KR. Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J Virol 83: 9398–9410, 2009. doi: 10.1128/JVI.00954-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Grammas P, Tripathy D, Sanchez A, Yin X, Luo J. Brain microvasculature and hypoxia-related proteins in Alzheimer’s disease. Int J Clin Exp Pathol 4: 616–627, 2011. [PMC free article] [PubMed] [Google Scholar]
- 214.Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab 35: 747–750, 2015. doi: 10.1038/jcbfm.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol 37: 24–39, 2011. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 216.Greenwood J, Wang Y, Calder VL. Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA-1, ICAM-1, VLA-4 and VCAM-1. off. Immunology 86: 408–415, 1995. [PMC free article] [PubMed] [Google Scholar]
- 217.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood 125: 2898–2907, 2015. doi: 10.1182/blood-2015-02-355974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E, Schroth J, Copeland B, Vaux KK, Cazenave-Gassiot A, Quek DQY, Wong BH, Tan BC, Wenk MR, Gunel M, Gabriel S, Chi NC, Silver DL, Gleeson JG. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet 47: 809–813, 2015. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JSK, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert J-C, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J; Alzheimer Genetic Analysis Group . TREM2 variants in Alzheimer’s disease. N Engl J Med 368: 117–127, 2013. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.van de Haar HJ, Burgmans S, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, Hofman PAM, Verhey FRJ, Backes WH. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology 281: 527–535, 2016. doi: 10.1148/radiol.2016152244. [DOI] [PubMed] [Google Scholar]
- 221.van de Haar HJ, Jansen JFA, Jeukens CRLPN, Burgmans S, van Buchem MA, Muller M, Hofman PAM, Verhey FRJ, van Osch MJP, Backes WH. Subtle blood-brain barrier leakage rate and spatial extent: considerations for dynamic contrast-enhanced MRI. Med Phys 44: 4112–4125, 2017. doi: 10.1002/mp.12328. [DOI] [PubMed] [Google Scholar]
- 222.van de Haar HJ, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, Wong SM, Hofman PAM, Burgmans S, Verhey FRJ, Backes WH. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol Aging 45: 190–196, 2016. doi: 10.1016/j.neurobiolaging.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 223.Habgood MD, Bye N, Dziegielewska KM, Ek CJ, Lane MA, Potter A, Morganti-Kossmann C, Saunders NR. Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur J Neurosci 25: 231–238, 2007. doi: 10.1111/j.1460-9568.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 224.Hachinski V; World Stroke Organization . Stroke and Potentially Preventable Dementias Proclamation: Updated World Stroke Day Proclamation. Stroke 46: 3039–3040, 2015. doi: 10.1161/STROKEAHA.115.011237. [DOI] [PubMed] [Google Scholar]
- 225.Haeusler AR, Donnelly CJ, Periz G, Simko EAJ, Shaw PG, Kim M-S, Maragakis NJ, Troncoso JC, Pandey A, Sattler R, Rothstein JD, Wang J. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 507: 195–200, 2014. doi: 10.1038/nature13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hafezi-Moghadam A, Thomas KL, Wagner DD. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am J Physiol Cell Physiol 292: C1256–C1262, 2007. doi: 10.1152/ajpcell.00563.2005. [DOI] [PubMed] [Google Scholar]
- 227.Hajjar I, Sorond F, Lipsitz LA. Apolipoprotein E, carbon dioxide vasoreactivity, and cognition in older adults: effect of hypertension. J Am Geriatr Soc 63: 276–281, 2015. doi: 10.1111/jgs.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508: 55–60, 2014. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Halliday MR, Pomara N, Sagare AP, Mack WJ, Frangione B, Zlokovic BV. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol 70: 1198–1200, 2013. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab 36: 216–227, 2016. doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ham JH, Yi H, Sunwoo MK, Hong JY, Sohn YH, Lee PH. Cerebral microbleeds in patients with Parkinson’s disease. J Neurol 261: 1628–1635, 2014. doi: 10.1007/s00415-014-7403-y. [DOI] [PubMed] [Google Scholar]
- 232.Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics 2: 5, 2010. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hammer C, Stepniak B, Schneider A, Papiol S, Tantra M, Begemann M, Sirén A-L, Pardo LA, Sperling S, Mohd Jofrry S, Gurvich A, Jensen N, Ostmeier K, Lühder F, Probst C, Martens H, Gillis M, Saher G, Assogna F, Spalletta G, Stöcker W, Schulz TF, Nave K-A, Ehrenreich H. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry 19: 1143–1149, 2014. doi: 10.1038/mp.2013.110. [DOI] [PubMed] [Google Scholar]
- 234.Han H, Mann A, Ekstein D, Eyal S. Breaking Bad: the Structure and Function of the Blood-Brain Barrier in Epilepsy. AAPS J 19: 973–988, 2017. doi: 10.1208/s12248-017-0096-2. [DOI] [PubMed] [Google Scholar]
- 235.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207: 18–30, 2015. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, Heun R, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel K-H, Klopp N, Wichmann H-E, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease [Correction in Nat Genet 45: 712, 2013.]. Nat Genet 41: 1088–1093, 2009. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hasoon J. Blast-associated traumatic brain injury in the military as a potential trigger for dementia and chronic traumatic encephalopathy. US Army Med Dep J 17: 102–105, 2017. [PubMed] [Google Scholar]
- 238.Hawkes CA, Shaw JE, Brown M, Sampson AP, McLaurin J, Carare RO. MK886 reduces cerebral amyloid angiopathy severity in TgCRND8 mice. Neurodegener Dis 13: 17–23, 2014. doi: 10.1159/000351096. [DOI] [PubMed] [Google Scholar]
- 239.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173–185, 2005. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 240.Hawkins RA, O’Kane RL, Simpson IA, Viña JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr 136, Suppl: 218S–226S, 2006. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 241.He J, Hsuchou H, He Y, Kastin AJ, Wang Y, Pan W. Sleep restriction impairs blood-brain barrier function. J Neurosci 34: 14697–14706, 2014. doi: 10.1523/JNEUROSCI.2111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.He L, Vanlandewijck M, Raschperger E, Andaloussi Mäe M, Jung B, Lebouvier T, Ando K, Hofmann J, Keller A, Betsholtz C. Analysis of the brain mural cell transcriptome. Sci Rep 6: 35108, 2016. doi: 10.1038/srep35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Henkel JS, Beers DR, Wen S, Bowser R, Appel SH. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology 72: 1614–1616, 2009. doi: 10.1212/WNL.0b013e3181a41228. [DOI] [PubMed] [Google Scholar]
- 244.Henshall TL, Keller A, He L, Johansson BR, Wallgard E, Raschperger E, Mäe MA, Jin S, Betsholtz C, Lendahl U. Notch3 is necessary for blood vessel integrity in the central nervous system. Arterioscler Thromb Vasc Biol 35: 409–420, 2015. doi: 10.1161/ATVBAHA.114.304849. [DOI] [PubMed] [Google Scholar]
- 245.Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 12: 551–564, 2011. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Heringa SM, Reijmer YD, Leemans A, Koek HL, Kappelle LJ, Biessels GJ; Utrecht Vascular Cognitive Impairment (VCI) Study Group . Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease. J Alzheimers Dis 38: 211–221, 2014. doi: 10.3233/JAD-130542. [DOI] [PubMed] [Google Scholar]
- 247.Hermey G, Sjøgaard SS, Petersen CM, Nykjaer A, Gliemann J. Tumour necrosis factor alpha-converting enzyme mediates ectodomain shedding of Vps10p-domain receptor family members. Biochem J 395: 285–293, 2006. doi: 10.1042/BJ20051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Heye AK, Thrippleton MJ, Armitage PA, Valdés Hernández MDC, Makin SD, Glatz A, Sakka E, Wardlaw JM. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. Neuroimage 125: 446–455, 2016. doi: 10.1016/j.neuroimage.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hicks K, O’Neil RG, Dubinsky WS, Brown RC. TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress. Am J Physiol Cell Physiol 298: C1583–C1593, 2010. doi: 10.1152/ajpcell.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hill KK, Campbell MC, McNeely ME, Karimi M, Ushe M, Tabbal SD, Hershey T, Flores HP, Hartlein JM, Lugar HM, Revilla FJ, Videen TO, Earhart GM, Perlmutter JS. Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson’s disease. Exp Neurol 241: 105–112, 2013. doi: 10.1016/j.expneurol.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res 93: 429–437, 2003. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- 252.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11: 26, 2014. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hochwald GM, Wald A, Malhan C. The sink action of cerebrospinal fluid volume flow. Effect on brain water content. Arch Neurol 33: 339–344, 1976. doi: 10.1001/archneur.1976.00500050025005. [DOI] [PubMed] [Google Scholar]
- 254.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert J-C, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ERLC, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Rüther E, Schürmann B, Heun R, Kölsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Gallacher J, Hüll M, Rujescu D, Giegling I, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel K-H, Klopp N, Wichmann H-E, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, van Duijn CM, Breteler MMB, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, Berr C, Campion D, Epelbaum J, Dartigues J-F, Tzourio C, Alpérovitch A, Lathrop M, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Björnsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossù P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’Donovan M, Amouyel P, Williams J; Alzheimer’s Disease Neuroimaging Initiative; CHARGE consortium; EADI1 consortium . Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 43: 429–435, 2011. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Holter KE, Kehlet B, Devor A, Sejnowski TJ, Dale AM, Omholt SW, Ottersen OP, Nagelhus EA, Mardal K-A, Pettersen KH. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci USA 114: 9894–9899, 2017. doi: 10.1073/pnas.1706942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 97: 2892–2897, 2000. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizarry MC, Hyman BT. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol 47: 739–747, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 258.Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med 2: a006312, 2012. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hongge L, Kexin G, Xiaojie M, Nian X, Jinsha H. The role of LRRK2 in the regulation of monocyte adhesion to endothelial cells. J Mol Neurosci 55: 233–239, 2015. doi: 10.1007/s12031-014-0312-9. [DOI] [PubMed] [Google Scholar]
- 260.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 29: 274–285, 2011. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Horwood N, Davies DC. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer’s disease. Virchows Arch 425: 69–72, 1994. doi: 10.1007/BF00193951. [DOI] [PubMed] [Google Scholar]
- 262.Hoshi Y, Uchida Y, Tachikawa M, Inoue T, Ohtsuki S, Terasaki T. Quantitative atlas of blood-brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J Pharm Sci 102: 3343–3355, 2013. doi: 10.1002/jps.23575. [DOI] [PubMed] [Google Scholar]
- 263.Hsiao H-Y, Chen Y-C, Huang C-H, Chen C-C, Hsu Y-H, Chen H-M, Chiu F-L, Kuo H-C, Chang C, Chern Y. Aberrant astrocytes impair vascular reactivity in Huntington disease. Ann Neurol 78: 178–192, 2015. doi: 10.1002/ana.24428. [DOI] [PubMed] [Google Scholar]
- 264.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol 66: 232–245, 2006. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 265.Huang K-L, Marcora E, Pimenova AA, Di Narzo AF, Kapoor M, Jin SC, Harari O, Bertelsen S, Fairfax BP, Czajkowski J, Chouraki V, Grenier-Boley B, Bellenguez C, Deming Y, McKenzie A, Raj T, Renton AE, Budde J, Smith A, Fitzpatrick A, Bis JC, DeStefano A, Adams HHH, Ikram MA, van der Lee S, Del-Aguila JL, Fernandez MV, Ibañez L, Sims R, Escott-Price V, Mayeux R, Haines JL, Farrer LA, Pericak-Vance MA, Lambert JC, van Duijn C, Launer L, Seshadri S, Williams J, Amouyel P, Schellenberg GD, Zhang B, Borecki I, Kauwe JSK, Cruchaga C, Hao K, Goate AM; International Genomics of Alzheimer’s Project; Alzheimer’s Disease Neuroimaging Initiative . A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat Neurosci 20: 1052–1061, 2017. doi: 10.1038/nn.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hudry E, Dashkoff J, Roe AD, Takeda S, Koffie RM, Hashimoto T, Scheel M, Spires-Jones T, Arbel-Ornath M, Betensky R, Davidson BL, Hyman BT. Gene transfer of human Apoe isoforms results in differential modulation of amyloid deposition and neurotoxicity in mouse brain. Sci Transl Med 5: 212ra161, 2013. doi: 10.1126/scitranslmed.3007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hultman K, Strickland S, Norris EH. The APOE ε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J Cereb Blood Flow Metab 33: 1251–1258, 2013. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hunt A, Schönknecht P, Henze M, Seidl U, Haberkorn U, Schröder J. Reduced cerebral glucose metabolism in patients at risk for Alzheimer’s disease. Psychiatry Res 155: 147–154, 2007. doi: 10.1016/j.pscychresns.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 269.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5: 347–360, 2004. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 270.Iadecola C. The pathobiology of vascular dementia. Neuron 80: 844–866, 2013. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Iadecola C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 96: 17–42, 2017. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S, Zeki Al Hazzouri A; American Heart Association Council on Hypertension; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council . Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension 68: e67–e94, 2016. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res 545: 103–113, 1991. doi: 10.1016/0006-8993(91)91275-6. [DOI] [PubMed] [Google Scholar]
- 274.Ikeda M, Sharma V, Sumi SM, Rogaeva EA, Poorkaj P, Sherrington R, Nee L, Tsuda T, Oda N, Watanabe M, Aoki M, Shoji M, Abe K, Itoyama Y, Hirai S, Schellenberg GD, Bird TD, St George-Hyslop PH. The clinical phenotype of two missense mutations in the presenilin I gene in Japanese patients. Ann Neurol 40: 912–917, 1996. doi: 10.1002/ana.410400614. [DOI] [PubMed] [Google Scholar]
- 275.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187: 761–772, 2009. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: 147ra111, 2012. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Imon Y, Yamaguchi S, Yamamura Y, Tsuji S, Kajima T, Ito K, Nakamura S. Low intensity areas observed on T2-weighted magnetic resonance imaging of the cerebral cortex in various neurological diseases. J Neurol Sci 134, Suppl: 27–32, 1995. doi: 10.1016/0022-510X(95)00205-G. [DOI] [PubMed] [Google Scholar]
- 278.Irizarry MC, Cheung BS, Rebeck GW, Paul SM, Bales KR, Hyman BT. Apolipoprotein E affects the amount, form, and anatomical distribution of amyloid beta-peptide deposition in homozygous APP(V717F) transgenic mice. Acta Neuropathol 100: 451–458, 2000. doi: 10.1007/s004010000263. [DOI] [PubMed] [Google Scholar]
- 279.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell 16: 222–231, 2009. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ishii K, Ii K, Hasegawa T, Shoji S, Doi A, Mori H. Increased A beta 42(43)-plaque deposition in early-onset familial Alzheimer’s disease brains with the deletion of exon 9 and the missense point mutation (H163R) in the PS-1 gene. Neurosci Lett 228: 17–20, 1997. doi: 10.1016/S0304-3940(97)00347-9. [DOI] [PubMed] [Google Scholar]
- 281.Ishii M, Iadecola C. Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochim Biophys Acta 1862: 966–974, 2016. doi: 10.1016/j.bbadis.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol 226: 183–190, 2010. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ito S, Yanai M, Yamaguchi S, Couraud P-O, Ohtsuki S. Regulation of Tight-Junction Integrity by Insulin in an In Vitro Model of Human Blood-Brain Barrier. J Pharm Sci 106: 2599–2605, 2017. doi: 10.1016/j.xphs.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 284.Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science 291: 1047–1051, 2001. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 285.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC, Weiner MW, Aisen P, Petersen R, Jack CR, Jagust W, Trojanowki JQ, Toga AW, Beckett L, Green RC, Saykin AJ, Morris J, Shaw LM, Khachaturian Z, Sorensen G, Kuller L, Raichle M, Paul S, Davies P, Fillit H, Hefti F, Holtzman D, Mesulam MM, Potter W, Snyder P, Schwartz A, Montine T, Thomas RG, Donohue M, Walter S, Gessert D, Sather T, Jiminez G, Harvey D, Bernstein M, Fox N, Thompson P, Schuff N, Borowski B, Gunter J, Senjem M, Vemuri P, Jones D, Kantarci K, Ward C, Koeppe RA, Foster N, Reiman EM, Chen K, Mathis C, Landau S, Cairns NJ, Householder E, Taylor-Reinwald L, Lee V, Korecka M, Figurski M, Crawford K, Neu S, Foroud TM, Potkin S, Shen L, Faber K, Kim S, Nho K, Thal L, Buckholtz N, Albert M, Frank R, Hsiao J, Kaye J, Quinn J, Lind B, Carter R, Dolen S, Schneider LS, Pawluczyk S, Beccera M, Teodoro L, Spann BM, Brewer J, Vanderswag H, Fleisher A, Heidebrink JL, Lord JL, Mason SS, Albers CS, Knopman D, Johnson K, Doody RS, Villanueva-Meyer J, Chowdhury M, Rountree S, Dang M, Stern Y, Honig LS, Bell KL, Ances B, Carroll M, Leon S, Mintun MA, Schneider S, Oliver A, Marson D, Griffith R, Clark D, Geldmacher D, Brockington J, Roberson E, Grossman H, Mitsis E, de Toledo-Morrell L, Shah RC, Duara R, Varon D, Greig MT, Roberts P, Albert M, Onyike C, D’Agostino D, Kielb S, Galvin JE, Cerbone B, Michel CA, Rusinek H, de Leon MJ, Glodzik L, De Santi S, Doraiswamy PM, Petrella JR, Wong TZ, Arnold SE, Karlawish JH, Wolk D, Smith CD, Jicha G, Hardy P, Sinha P, Oates E, Conrad G, Lopez OL, Oakley MA, Simpson DM, Porsteinsson AP, Goldstein BS, Martin K, Makino KM, Ismail MS, Brand C, Mulnard RA, Thai G, Mc-Adams-Ortiz C, Womack K, Mathews D, Quiceno M, Diaz-Arrastia R, King R, Weiner M, Martin-Cook K, DeVous M, Levey AI, Lah JJ, Cellar JS, Burns JM, Anderson HS, Swerdlow RH, Apostolova L, Tingus K, Woo E, Silverman DHS, Lu PH, Bartzokis G, Graff-Radford NR, Parfitt F, Kendall T, Johnson H, Farlow MR, Hake AM, Matthews BR, Herring S, Hunt C, van Dyck CH, Carson RE, MacAvoy MG, Chertkow H, Bergman H, Hosein C, Black S, Stefanovic B, Caldwell C, Hsiung G-YR, Feldman H, Mudge B, Assaly M, Kertesz A, Rogers J, Bernick C, Munic D, Kerwin D, Mesulam M-M, Lipowski K, Wu C-K, Johnson N, Sadowsky C, Martinez W, Villena T, Turner RS, Johnson K, Reynolds B, Sperling RA, Johnson KA, Marshall G, Frey M, Lane B, Rosen A, Tinklenberg J, Sabbagh MN, Belden CM, Jacobson SA, Sirrel SA, Kowall N, Killiany R, Budson AE, Norbash A, Johnson PL, Allard J, Lerner A, Ogrocki P, Hudson L, Fletcher E, Carmichael O, Olichney J, DeCarli C, Kittur S, Borrie M, Lee T-Y, Bartha R, Johnson S, Asthana S, Carlsson CM, Potkin SG, Preda A, Nguyen D, Tariot P, Reeder S, Bates V, Capote H, Rainka M, Scharre DW, Kataki M, Adeli A, Zimmerman EA, Celmins D, Brown AD, Pearlson GD, Blank K, Anderson K, Santulli RB, Kitzmiller TJ, Schwartz ES, Sink KM, Williamson JD, Garg P, Watkins F, Ott BR, Querfurth H, Tremont G, Salloway S, Malloy P, Correia S, Rosen HJ, Miller BL, Mintzer J, Spicer K, Bachman D, Finger E, Pasternak S, Rachinsky I, Drost D, Pomara N, Hernando R, Sarrael A, Schultz SK, Ponto LLB, Shim H, Smith KE, Relkin N, Chaing G, Raudin L, Smith A, Fargher K, Raj BA, Neylan T, Grafman J, Davis M, Morrison R, Hayes J, Finley S, Friedl K, Fleischman D, Arfanakis K, James O, Massoglia D, Fruehling JJ, Harding S, Peskind ER, Petrie EC, Li G, Yesavage JA, Taylor JL, Furst AJ; Alzheimer’s Disease Neuroimaging Initiative . Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 7: 11934, 2016. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 130: 535–547, 2007. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 287.Janelidze S, Hertze J, Nägga K, Nilsson K, Nilsson C, Wennström M, van Westen D, Blennow K, Zetterberg H, Hansson O; Swedish BioFINDER Study Group . Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol Aging 51: 104–112, 2017. doi: 10.1016/j.neurobiolaging.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Janelidze S, Lindqvist D, Francardo V, Hall S, Zetterberg H, Blennow K, Adler CH, Beach TG, Serrano GE, van Westen D, Londos E, Cenci MA, Hansson O. Increased CSF biomarkers of angiogenesis in Parkinson disease. Neurology 85: 1834–1842, 2015. doi: 10.1212/WNL.0000000000002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Janssen JC, Lantos PL, Fox NC, Harvey RJ, Beck J, Dickinson A, Campbell TA, Collinge J, Hanger DP, Cipolotti L, Stevens JM, Rossor MN. Autopsy-confirmed familial early-onset Alzheimer disease caused by the l153V presenilin 1 mutation. Arch Neurol 58: 953–958, 2001. doi: 10.1001/archneur.58.6.953. [DOI] [PubMed] [Google Scholar]
- 290.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature 312: 162–163, 1984. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 291.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, Stegh AH. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med 5: 209ra152, 2013. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Jessen Krut J, Mellberg T, Price RW, Hagberg L, Fuchs D, Rosengren L, Nilsson S, Zetterberg H, Gisslén M. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One 9: e88591, 2014. doi: 10.1371/journal.pone.0088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem Res 40: 2583–2599, 2015. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ji Y, Permanne B, Sigurdsson EM, Holtzman DM, Wisniewski T. Amyloid beta40/42 clearance across the blood-brain barrier following intra-ventricular injections in wild-type, apoE knock-out and human apoE3 or E4 expressing transgenic mice. J Alzheimers Dis 3: 23–30, 2001. doi: 10.3233/JAD-2001-3105. [DOI] [PubMed] [Google Scholar]
- 295.Jiang Q, Lee CYD, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron 58: 681–693, 2008. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Jin B-J, Smith AJ, Verkman AS. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol 148: 489–501, 2016. doi: 10.1085/jgp.201611684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, Lindblom P, Seth P, Frias A, Nishiya N, Ginsberg MH, Gerhardt H, Zhang K, Li DY. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med 14: 448–453, 2008. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Jones EL, Mok K, Hanney M, Harold D, Sims R, Williams J, Ballard C. Evidence that PICALM affects age at onset of Alzheimer’s dementia in Down syndrome. Neurobiol Aging 34: 2441.e1–2441.e5, 2013. doi: 10.1016/j.neurobiolaging.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488: 96–99, 2012. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 300.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368: 107–116, 2013. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J Neurochem 53: 1083–1088, 1989. doi: 10.1111/j.1471-4159.1989.tb07399.x. [DOI] [PubMed] [Google Scholar]
- 302.Kamp JA, Moursel LG, Haan J, Terwindt GM, Lesnik Oberstein SAMJ, van Duinen SG, van Roon-Mom WMC. Amyloid β in hereditary cerebral hemorrhage with amyloidosis-Dutch type. Rev Neurosci 25: 641–651, 2014. doi: 10.1515/revneuro-2014-0008. [DOI] [PubMed] [Google Scholar]
- 303.Karch CM, Cruchaga C, Goate AM. Alzheimer’s disease genetics: from the bench to the clinic. Neuron 83: 11–26, 2014. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 77: 43–51, 2015. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J 282: 4067–4079, 2015. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- 306.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11: 720–731, 2012. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Keep RF, Xiang J, Ennis SR, Andjelkovic A, Hua Y, Xi G, Hoff JT. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir Suppl (Wien) 105: 73–77, 2008. doi: 10.1007/978-3-211-09469-3_15. [DOI] [PubMed] [Google Scholar]
- 308.Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS 11: 18, 2014. doi: 10.1186/2045-8118-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Keller A, Westenberger A, Sobrido MJ, García-Murias M, Domingo A, Sears RL, Lemos RR, Ordoñez-Ugalde A, Nicolas G, da Cunha JEG, Rushing EJ, Hugelshofer M, Wurnig MC, Kaech A, Reimann R, Lohmann K, Dobričić V, Carracedo A, Petrović I, Miyasaki JM, Abakumova I, Mäe MA, Raschperger E, Zatz M, Zschiedrich K, Klepper J, Spiteri E, Prieto JM, Navas I, Preuss M, Dering C, Janković M, Paucar M, Svenningsson P, Saliminejad K, Khorshid HRK, Novaković I, Aguzzi A, Boss A, Le Ber I, Defer G, Hannequin D, Kostić VS, Campion D, Geschwind DH, Coppola G, Betsholtz C, Klein C, Oliveira JRM. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat Genet 45: 1077–1082, 2013. doi: 10.1038/ng.2723. [DOI] [PubMed] [Google Scholar]
- 310.Kelton W, Waindok AC, Pesch T, Pogson M, Ford K, Parola C, Reddy ST. Reprogramming MHC specificity by CRISPR-Cas9-assisted cassette exchange. Sci Rep 7: 45775, 2017. doi: 10.1038/srep45775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kero M, Paetau A, Polvikoski T, Tanskanen M, Sulkava R, Jansson L, Myllykangas L, Tienari PJ. Amyloid precursor protein (APP) A673T mutation in the elderly Finnish population. Neurobiol Aging 34: 1518.e1–1518.e3, 2013. doi: 10.1016/j.neurobiolaging.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 312.Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 79, Suppl 1: S52–S57, 2012. doi: 10.1212/WNL.0b013e3182697e70. [DOI] [PubMed] [Google Scholar]
- 313.Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet 377: 942–955, 2011. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 314.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron 63: 287–303, 2009. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Kim J, Jiang H, Park S, Eltorai AEM, Stewart FR, Yoon H, Basak JM, Finn MB, Holtzman DM. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-β amyloidosis. J Neurosci 31: 18007–18012, 2011. doi: 10.1523/JNEUROSCI.3773-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kim SY, Buckwalter M, Soreq H, Vezzani A, Kaufer D. Blood-brain barrier dysfunction-induced inflammatory signaling in brain pathology and epileptogenesis. Epilepsia 53, Suppl 6: 37–44, 2012. doi: 10.1111/j.1528-1167.2012.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J Pathol 201: 319–327, 2003. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- 318.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 18: 419–434, 2017. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, Boas DA, Sakadžić S, Zlokovic BV. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 20: 406–416, 2017. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Klein RS, Garber C, Howard N. Infectious immunity in the central nervous system and brain function. Nat Immunol 18: 132–141, 2017. doi: 10.1038/ni.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Klohs J, Deistung A, Schweser F, Grandjean J, Dominietto M, Waschkies C, Nitsch RM, Knuesel I, Reichenbach JR, Rudin M. Detection of cerebral microbleeds with quantitative susceptibility mapping in the ArcAbeta mouse model of cerebral amyloidosis. J Cereb Blood Flow Metab 31: 2282–2292, 2011. doi: 10.1038/jcbfm.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Klohs J, Politano IW, Deistung A, Grandjean J, Drewek A, Dominietto M, Keist R, Schweser F, Reichenbach JR, Nitsch RM, Knuesel I, Rudin M. Longitudinal Assessment of Amyloid Pathology in Transgenic ArcAβ Mice Using Multi-Parametric Magnetic Resonance Imaging. PLoS One 8: e66097, 2013. doi: 10.1371/journal.pone.0066097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Klyachko NL, Haney MJ, Zhao Y, Manickam DS, Mahajan V, Suresh P, Hingtgen SD, Mosley RL, Gendelman HE, Kabanov AV, Batrakova EV. Macrophages offer a paradigm switch for CNS delivery of therapeutic proteins. Nanomedicine (Lond) 9: 1403–1422, 2014. doi: 10.2217/nnm.13.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82: 603–617, 2014. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kokjohn TA, Maarouf CL, Daugs ID, Hunter JM, Whiteside CM, Malek-Ahmadi M, Rodriguez E, Kalback W, Jacobson SA, Sabbagh MN, Beach TG, Roher AE. Neurochemical profile of dementia pugilistica. J Neurotrauma 30: 981–997, 2013. doi: 10.1089/neu.2012.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Korn C, Augustin HG. Mechanisms of Vessel Pruning and Regression. Dev Cell 34: 5–17, 2015. doi: 10.1016/j.devcel.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 327.Kortekaas R, Leenders KL, van Oostrom JCH, Vaalburg W, Bart J, Willemsen ATM, Hendrikse NH. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57: 176–179, 2005. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 328.Kovacs GG, Lutz MI, Ricken G, Ströbel T, Höftberger R, Preusser M, Regelsberger G, Hönigschnabl S, Reiner A, Fischer P, Budka H, Hainfellner JA. Dura mater is a potential source of Aβ seeds. Acta Neuropathol 131: 911–923, 2016. doi: 10.1007/s00401-016-1565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, Bresler M, Burks SR, Frank JA. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci USA 114: E75–E84, 2017. doi: 10.1073/pnas.1614777114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev 71: 2–14, 2014. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 331.Kruyer A, Soplop N, Strickland S, Norris EH. Chronic Hypertension Leads to Neurodegeneration in the TgSwDI Mouse Model of Alzheimer’s Disease. Hypertension 66: 175–182, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Kuhnert F, Mancuso MR, Shamloo A, Wang H-T, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, Kuo CJ. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 330: 985–989, 2010. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Kumar A, D’Souza SS, Moskvin OV, Toh H, Wang B, Zhang J, Swanson S, Guo L-W, Thomson JA, Slukvin II. Specification and Diversification of Pericytes and Smooth Muscle Cells from Mesenchymoangioblasts. Cell Reports 19: 1902–1916, 2017. doi: 10.1016/j.celrep.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Kumar H, Jo M-J, Choi H, Muttigi MS, Shon S, Kim B-J, Lee S-H, Han I-B. Matrix Metalloproteinase-8 Inhibition Prevents Disruption of Blood-Spinal Cord Barrier and Attenuates Inflammation in Rat Model of Spinal Cord Injury. Mol Neurobiol 55: 2577–2590, 2018. doi: 10.1007/s12035-017-0509-3. [DOI] [PubMed] [Google Scholar]
- 335.Kumar-Singh S, Cras P, Wang R, Kros JM, van Swieten J, Lübke U, Ceuterick C, Serneels S, Vennekens K, Timmermans J-P, Van Marck E, Martin J-J, van Duijn CM, Van Broeckhoven C. Dense-core senile plaques in the Flemish variant of Alzheimer’s disease are vasocentric. Am J Pathol 161: 507–520, 2002. doi: 10.1016/S0002-9440(10)64207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven C. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am J Pathol 167: 527–543, 2005. doi: 10.1016/S0002-9440(10)62995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet 21, R1: R97–R110, 2012. doi: 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kuo YM, Kokjohn TA, Kalback W, Luehrs D, Galasko DR, Chevallier N, Koo EH, Emmerling MR, Roher AE. Amyloid-beta peptides interact with plasma proteins and erythrocytes: implications for their quantitation in plasma. Biochem Biophys Res Commun 268: 750–756, 2000. doi: 10.1006/bbrc.2000.2222. [DOI] [PubMed] [Google Scholar]
- 339.Kwan JY, Jeong SY, Van Gelderen P, Deng H-X, Quezado MM, Danielian LE, Butman JA, Chen L, Bayat E, Russell J, Siddique T, Duyn JH, Rouault TA, Floeter MK. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7: e35241, 2012. doi: 10.1371/journal.pone.0035241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lacoste B, Comin CH, Ben-Zvi A, Kaeser PS, Xu X, Costa LF, Gu C. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron 83: 1117–1130, 2014. doi: 10.1016/j.neuron.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lacoste B, Gu C. Control of cerebrovascular patterning by neural activity during postnatal development. Mech Dev 138: 43–49, 2015. doi: 10.1016/j.mod.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert RJC, Jones EY, Kiefer F, Ruga P, Imhof BA, Aurrand-Lions M. Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell 16: 4992–5003, 2005. doi: 10.1091/mbc.e05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lambert J-C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanché H, Dartigues J-F, Tzourio C, Gut I, Van Broeckhoven C, Alpérovitch A, Lathrop M, Amouyel P; European Alzheimer’s Disease Initiative Investigators . Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41: 1094–1099, 2009. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 344.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Morón FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fiévet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossù P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH Jr, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nöthen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P European Alzheimer’s Disease Initiative (EADI)Genetic and Environmental Risk in Alzheimer’s DiseaseAlzheimer’s Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology . Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 45: 1452–1458, 2013. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ; Alzheimer’s Disease Neuroimaging Initiative . Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 32: 1207–1218, 2011. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Langford D, Grigorian A, Hurford R, Adame A, Ellis RJ, Hansen L, Masliah E. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J Neuropathol Exp Neurol 63: 1038–1047, 2004. doi: 10.1093/jnen/63.10.1038. [DOI] [PubMed] [Google Scholar]
- 347.Larson DM, Wrobleski MJ, Sagar GD, Westphale EM, Beyer EC. Differential regulation of connexin43 and connexin37 in endothelial cells by cell density, growth, and TGF-beta1. Am J Physiol Cell Physiol 272: C405–C415, 1997. doi: 10.1152/ajpcell.1997.272.2.C405. [DOI] [PubMed] [Google Scholar]
- 348.Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M Jr, Klein RS, Diamond MS. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med 7: 284ra59, 2015. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Le Guelte A, Galan-Moya E-M, Dwyer J, Treps L, Kettler G, Hebda JK, Dubois S, Auffray C, Chneiweiss H, Bidere N, Gavard J. Semaphorin 3A elevates endothelial cell permeability through PP2A inactivation. J Cell Sci 125: 4137–4146, 2012. doi: 10.1242/jcs.108282. [DOI] [PubMed] [Google Scholar]
- 350.Lee S-W, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim Y-J, Kim K-W. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med 9: 900–906, 2003. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- 351.Lee WJ, Hawkins RA, Viña JR, Peterson DR. Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am J Physiol Cell Physiol 274: C1101–C1107, 1998. doi: 10.1152/ajpcell.1998.274.4.C1101. [DOI] [PubMed] [Google Scholar]
- 352.Leech S, Kirk J, Plumb J, McQuaid S. Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol Appl Neurobiol 33: 86–98, 2007. doi: 10.1111/j.1365-2990.2006.00781.x. [DOI] [PubMed] [Google Scholar]
- 353.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation 122: 517–526, 2010. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Lemere CA, Lopera F, Kosik KS, Lendon CL, Ossa J, Saido TC, Yamaguchi H, Ruiz A, Martinez A, Madrigal L, Hincapie L, Arango JC, Anthony DC, Koo EH, Goate AM, Selkoe DJ, Arango JC. The E280A presenilin 1 Alzheimer mutation produces increased A beta 42 deposition and severe cerebellar pathology. Nat Med 2: 1146–1150, 1996. doi: 10.1038/nm1096-1146. [DOI] [PubMed] [Google Scholar]
- 355.LeVine SM. Albumin and multiple sclerosis. BMC Neurol 16: 47, 2016. doi: 10.1186/s12883-016-0564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Levy E, Prelli F, Frangione B. Studies on the first described Alzheimer’s disease amyloid beta mutant, the Dutch variant. J Alzheimers Dis 9, Suppl: 329–339, 2006. doi: 10.3233/JAD-2006-9S337. [DOI] [PubMed] [Google Scholar]
- 357.Li J-Q, Tan L, Yu J-T. The role of the LRRK2 gene in Parkinsonism. Mol Neurodegener 9: 47, 2014. doi: 10.1186/1750-1326-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Li JY, Boado RJ, Pardridge WM. Blood-brain barrier genomics. J Cereb Blood Flow Metab 21: 61–68, 2001. doi: 10.1097/00004647-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 359.Li JY, Boado RJ, Pardridge WM. Rat blood-brain barrier genomics. II. J Cereb Blood Flow Metab 22: 1319–1326, 2002. doi: 10.1097/01.WCB.0000040944.89393.0f. [DOI] [PubMed] [Google Scholar]
- 360.Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46: 957–967, 2017. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 361.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol 183: 409–417, 2008. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Liguori C, Olivola E, Pierantozzi M, Cerroni R, Galati S, Saviozzi V, Mercuri NB, Stefani A. Cerebrospinal-fluid Alzheimer’s Disease Biomarkers and Blood-Brain Barrier Integrity in a Natural Population of Cognitive Intact Parkinson’s Disease Patients. CNS Neurol Disord Drug Targets 16: 339–345, 2017. doi: 10.2174/1871527316666161205123123. [DOI] [PubMed] [Google Scholar]
- 363.Lin C-J, Tai Y, Huang M-T, Tsai Y-F, Hsu H-J, Tzen K-Y, Liou H-H. Cellular localization of the organic cation transporters, OCT1 and OCT2, in brain microvessel endothelial cells and its implication for MPTP transport across the blood-brain barrier and MPTP-induced dopaminergic toxicity in rodents. J Neurochem 114: 717–727, 2010. doi: 10.1111/j.1471-4159.2010.06801.x. [DOI] [PubMed] [Google Scholar]
- 364.Lin C-Y, Hsu Y-H, Lin M-H, Yang T-H, Chen H-M, Chen Y-C, Hsiao H-Y, Chen C-C, Chern Y, Chang C. Neurovascular abnormalities in humans and mice with Huntington’s disease. Exp Neurol 250: 20–30, 2013. doi: 10.1016/j.expneurol.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 365.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277: 242–245, 1997. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 366.Ling H, Morris HR, Neal JW, Lees AJ, Hardy J, Holton JL, Revesz T, Williams DDR. Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 133: 337–352, 2017. doi: 10.1007/s00401-017-1680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol Heart Circ Physiol 268: H729–H739, 1995. [DOI] [PubMed] [Google Scholar]
- 368.Liu C-C, Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9: 106–118, 2013. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Liu EY, Russ J, Wu K, Neal D, Suh E, McNally AG, Irwin DJ, Van Deerlin VM, Lee EB. C9orf72 hypermethylation protects against repeat expansion-associated pathology in ALS/FTD. Acta Neuropathol 128: 525–541, 2014. doi: 10.1007/s00401-014-1286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J. Hydrogen Sulfide Attenuates Tissue Plasminogen Activator-Induced Cerebral Hemorrhage Following Experimental Stroke. Transl Stroke Res 7: 209–219, 2016. doi: 10.1007/s12975-016-0459-5. [DOI] [PubMed] [Google Scholar]
- 371.Liu J, Dong F, Hoh J. Loss of HtrA1-induced attenuation of TGF-β signaling in fibroblasts might not be the main mechanism of CARASIL pathogenesis. Proc Natl Acad Sci USA 112: E1693, 2015. doi: 10.1073/pnas.1500911112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev Ind Pharm 28: 1–13, 2002. doi: 10.1081/DDC-120001481. [DOI] [PubMed] [Google Scholar]
- 373.Loeffler DA, Connor JR, Juneau PL, Snyder BS, Kanaley L, DeMaggio AJ, Nguyen H, Brickman CM, LeWitt PA. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J Neurochem 65: 710–716, 1995. doi: 10.1046/j.1471-4159.1995.65020710.x. [DOI] [PubMed] [Google Scholar]
- 374.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci 20: 717–726, 2017. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Longden TA, Hill-Eubanks DC, Nelson MT. Ion channel networks in the control of cerebral blood flow. J Cereb Blood Flow Metab 36: 492–512, 2016. doi: 10.1177/0271678X15616138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341, 2015. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Lutz SE, Raine CS, Brosnan CF. Loss of astrocyte connexins 43 and 30 does not significantly alter susceptibility or severity of acute experimental autoimmune encephalomyelitis in mice. J Neuroimmunol 245: 8–14, 2012. doi: 10.1016/j.jneuroim.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Ma Q, Dasgupta C, Li Y, Huang L, Zhang L. MicroRNA-210 Suppresses Junction Proteins and Disrupts Blood-Brain Barrier Integrity in Neonatal Rat Hypoxic-Ischemic Brain Injury. Int J Mol Sci 18: E1356, 2017. doi: 10.3390/ijms18071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.MacDonald ME; The Huntington’s Disease Collaborative Research Group . A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72: 971–983, 1993. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 380.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498: 492–496, 2013. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 381.Maeda S, Nakayama H, Isaka K, Aihara Y, Nemoto S. Familial unusual encephalopathy of Binswanger’s type without hypertension. Folia Psychiatr Neurol Jpn 30: 165–177, 1976. [DOI] [PubMed] [Google Scholar]
- 382.Maggi P, Macri SMC, Gaitán MI, Leibovitch E, Wholer JE, Knight HL, Ellis M, Wu T, Silva AC, Massacesi L, Jacobson S, Westmoreland S, Reich DS. The formation of inflammatory demyelinated lesions in cerebral white matter. Ann Neurol 76: 594–608, 2014. doi: 10.1002/ana.24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron 76: 871–885, 2012. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res 50, Suppl: S183–S188, 2009. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Maikos JT, Shreiber DI. Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J Neurotrauma 24: 492–507, 2007. doi: 10.1089/neu.2006.0149. [DOI] [PubMed] [Google Scholar]
- 386.Malek N, Lawton MA, Swallow DMA, Grosset KA, Marrinan SL, Bajaj N, Barker RA, Burn DJ, Hardy J, Morris HR, Williams NM, Wood N, Ben-Shlomo Y, Grosset DG; PRoBaND Clinical Consortium . Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov Disord 31: 1518–1526, 2016. doi: 10.1002/mds.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Mancuso MR, Kuhnert F, Kuo CJ. Developmental angiogenesis of the central nervous system. Lymphat Res Biol 6: 173–180, 2008. doi: 10.1089/lrb.2008.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Manfredsson FP, Rising AC, Mandel RJ. AAV9: a potential blood-brain barrier buster. Mol Ther 17: 403–405, 2009. doi: 10.1038/mt.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Mann DM, Iwatsubo T, Cairns NJ, Lantos PL, Nochlin D, Sumi SM, Bird TD, Poorkaj P, Hardy J, Hutton M, Prihar G, Crook R, Rossor MN, Haltia M. Amyloid beta protein (Abeta) deposition in chromosome 14-linked Alzheimer’s disease: predominance of Abeta42(43). Ann Neurol 40: 149–156, 1996. doi: 10.1002/ana.410400205. [DOI] [PubMed] [Google Scholar]
- 390.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83: 183–252, 2003. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- 391.Mann GE, Zlokovic BV, Yudilevich DL. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim Biophys Acta 819: 241–248, 1985. doi: 10.1016/0005-2736(85)90179-8. [DOI] [PubMed] [Google Scholar]
- 392.Maranzano J, Rudko DA, Nakamura K, Cook S, Cadavid D, Wolansky L, Arnold DL, Narayanan S. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology 89: 714–721, 2017. doi: 10.1212/WNL.0000000000004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Marchi N, Granata T, Freri E, Ciusani E, Ragona F, Puvenna V, Teng Q, Alexopolous A, Janigro D. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One 6: e18200, 2011. doi: 10.1371/journal.pone.0018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Marín-Muñoz J, Noguera-Perea MF, Gómez-Tortosa E, López-Motos D, Antequera-Torres M, Martínez-Herrada B, Manzanares-Sánchez S, Vivancos-Moreau L, Legaz-García A, Rábano-Gutiérrez Del Arroyo A, Antúnez-Almagro C. Novel Mutation (Gly212Val) in the PS2 Gene Associated with Early-Onset Familial Alzheimer’s Disease. J Alzheimers Dis 53: 73–78, 2016. doi: 10.3233/JAD-160050. [DOI] [PubMed] [Google Scholar]
- 395.Markham A. Cerliponase Alfa: First Global Approval. Drugs 77: 1247–1249, 2017. doi: 10.1007/s40265-017-0771-8. [DOI] [PubMed] [Google Scholar]
- 396.Mayerl S, Müller J, Bauer R, Richert S, Kassmann CM, Darras VM, Buder K, Boelen A, Visser TJ, Heuer H. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest 124: 1987–1999, 2014. doi: 10.1172/JCI70324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.McDougal DB Jr, Ferrendelli JA, Yip V, Pusateri ME, Carter JG, Chi MM, Norris B, Manchester J, Lowry OH. Use of nonradioactive 2-deoxyglucose to study compartmentation of brain glucose metabolism and rapid regional changes in rate. Proc Natl Acad Sci USA 87: 1357–1361, 1990. doi: 10.1073/pnas.87.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.McDowell GC II, Pope JE. Intrathecal Ziconotide: Dosing and Administration Strategies in Patients With Refractory Chronic Pain. Neuromodulation 19: 522–532, 2016. doi: 10.1111/ner.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol 11: 318–329, 2011. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.McInerney MP, Short JL, Nicolazzo JA. Neurovascular Alterations in Alzheimer’s Disease: Transporter Expression Profiles and CNS Drug Access. AAPS J 19: 940–956, 2017. doi: 10.1208/s12248-017-0077-5. [DOI] [PubMed] [Google Scholar]
- 401.McMurtray A, Nakamoto B, Shikuma C, Valcour V. Small-vessel vascular disease in human immunodeficiency virus infection: the Hawaii aging with HIV cohort study. Cerebrovasc Dis 24: 236–241, 2007. doi: 10.1159/000104484. [DOI] [PubMed] [Google Scholar]
- 402.Meabon JS, Huber BR, Cross DJ, Richards TL, Minoshima S, Pagulayan KF, Li G, Meeker KD, Kraemer BC, Petrie EC, Raskind MA, Peskind ER, Cook DG. Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Sci Transl Med 8: 321ra6, 2016. doi: 10.1126/scitranslmed.aaa9585. [DOI] [PubMed] [Google Scholar]
- 403.Menezes MJ, McClenahan FK, Leiton CV, Aranmolate A, Shan X, Colognato H. The extracellular matrix protein laminin α2 regulates the maturation and function of the blood-brain barrier. J Neurosci 34: 15260–15280, 2014. doi: 10.1523/JNEUROSCI.3678-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Méresse S, Delbart C, Fruchart JC, Cecchelli R. Low-density lipoprotein receptor on endothelium of brain capillaries. J Neurochem 53: 340–345, 1989. doi: 10.1111/j.1471-4159.1989.tb07340.x. [DOI] [PubMed] [Google Scholar]
- 405.Merlini M, Meyer EP, Ulmann-Schuler A, Nitsch RM. Vascular β-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAβ mice. Acta Neuropathol 122: 293–311, 2011. doi: 10.1007/s00401-011-0834-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Methia N, André P, Hafezi-Moghadam A, Economopoulos M, Thomas KL, Wagner DD. ApoE deficiency compromises the blood brain barrier especially after injury. Mol Med 7: 810–815, 2001. [PMC free article] [PubMed] [Google Scholar]
- 407.Meucci G, Rossi G, Bettini R, Montanaro D, Gironelli L, Voci L, Bianchi F. Laser nephelometric evaluation of albumin, IgG and alpha 2-macroglobulin: applications to the study of alterations of the blood-brain barrier. J Neurol Sci 118: 73–78, 1993. doi: 10.1016/0022-510X(93)90248-W. [DOI] [PubMed] [Google Scholar]
- 408.Millar ID, Wang S, Brown PD, Barrand MA, Hladky SB. Kv1 and Kir2 potassium channels are expressed in rat brain endothelial cells. Pflugers Arch 456: 379–391, 2008. doi: 10.1007/s00424-007-0377-1. [DOI] [PubMed] [Google Scholar]
- 409.Miller MA. Patterning with Diffusion Barriers. Dev Cell 35: 395–396, 2015. doi: 10.1016/j.devcel.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 410.Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, Silverberg GD, Stopa EG. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res 1230: 273–280, 2008. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Miller SE, Sahlender DA, Graham SC, Höning S, Robinson MS, Peden AA, Owen DJ. The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell 147: 1118–1131, 2011. doi: 10.1016/j.cell.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Miners JS, Schulz I, Love S. Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. J Cereb Blood Flow Metab 38: 103–115, 2018. doi: 10.1177/0271678X17690761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem 117: 735–746, 2011. doi: 10.1111/j.1471-4159.2011.07245.x. [DOI] [PubMed] [Google Scholar]
- 414.Mitragotri S, Anderson DG, Chen X, Chow EK, Ho D, Kabanov AV, Karp JM, Kataoka K, Mirkin CA, Petrosko SH, Shi J, Stevens MM, Sun S, Teoh S, Venkatraman SS, Xia Y, Wang S, Gu Z, Xu C. Accelerating the Translation of Nanomaterials in Biomedicine. ACS Nano 9: 6644–6654, 2015. doi: 10.1021/acsnano.5b03569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Miyazaki K, Ohta Y, Nagai M, Morimoto N, Kurata T, Takehisa Y, Ikeda Y, Matsuura T, Abe K. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res 89: 718–728, 2011. doi: 10.1002/jnr.22594. [DOI] [PubMed] [Google Scholar]
- 416.Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic BV. Substitution at codon 22 reduces clearance of Alzheimer’s amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging 23: 405–412, 2002. doi: 10.1016/S0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 417.Montagne A, Gauberti M, Macrez R, Jullienne A, Briens A, Raynaud J-S, Louin G, Buisson A, Haelewyn B, Docagne F, Defer G, Vivien D, Maubert E. Ultra-sensitive molecular MRI of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. Neuroimage 63: 760–770, 2012. doi: 10.1016/j.neuroimage.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 418.Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol 131: 687–707, 2016. doi: 10.1007/s00401-016-1570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, Barnes SR, Daianu M, Ramanathan A, Go A, Lawson EJ, Wang Y, Mack WJ, Thompson PM, Schneider JA, Varkey J, Langen R, Mullins E, Jacobs RE, Zlokovic BV. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 24: 326–337, 2018. doi: 10.1038/nm.4482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 420.Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J Exp Med 214: 3151–3169, 2017. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85: 296–302, 2015. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging 18: 469–474, 1997. doi: 10.1016/S0197-4580(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 423.Moreau K, Fleming A, Imarisio S, Lopez Ramirez A, Mercer JL, Jimenez-Sanchez M, Bento CF, Puri C, Zavodszky E, Siddiqi F, Lavau CP, Betton M, O’Kane CJ, Wechsler DS, Rubinsztein DC. PICALM modulates autophagy activity and tau accumulation. Nat Commun 5: 4998, 2014. doi: 10.1038/ncomms5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, Tsui W, Ginestroni A, Bessi V, Fayyazz M, Caffarra P, Pupi A. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med 47: 1778–1786, 2006. [PubMed] [Google Scholar]
- 425.Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, Reiman EM, Holthoff V, Kalbe E, Sorbi S, Diehl-Schmid J, Perneczky R, Clerici F, Caselli R, Beuthien-Baumann B, Kurz A, Minoshima S, de Leon MJ. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med 49: 390–398, 2008. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Mulder M, Blokland A, van den Berg DJ, Schulten H, Bakker AH, Terwel D, Honig W, de Kloet ER, Havekes LM, Steinbusch HW, de Lange EC. Apolipoprotein E protects against neuropathology induced by a high-fat diet and maintains the integrity of the blood-brain barrier during aging. Lab Invest 81: 953–960, 2001. doi: 10.1038/labinvest.3780307. [DOI] [PubMed] [Google Scholar]
- 427.Nagasawa K, Chiba H, Fujita H, Kojima T, Saito T, Endo T, Sawada N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J Cell Physiol 208: 123–132, 2006. doi: 10.1002/jcp.20647. [DOI] [PubMed] [Google Scholar]
- 428.Natté R, Maat-Schieman ML, Haan J, Bornebroek M, Roos RA, van Duinen SG. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type is associated with cerebral amyloid angiopathy but is independent of plaques and neurofibrillary tangles. Ann Neurol 50: 765–772, 2001. doi: 10.1002/ana.10040. [DOI] [PubMed] [Google Scholar]
- 429.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta 1862: 887–900, 2016. doi: 10.1016/j.bbadis.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh ELK, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509: 503–506, 2014. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 431.Nicaise C, Mitrecic D, Demetter P, De Decker R, Authelet M, Boom A, Pochet R. Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res 1301: 152–162, 2009. doi: 10.1016/j.brainres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 432.Nicaise C, Soyfoo MS, Authelet M, De Decker R, Bataveljic D, Delporte C, Pochet R. Aquaporin-4 overexpression in rat ALS model. Anat Rec (Hoboken) 292: 207–213, 2009. doi: 10.1002/ar.20838. [DOI] [PubMed] [Google Scholar]
- 433.Nico B, Frigeri A, Nicchia GP, Corsi P, Ribatti D, Quondamatteo F, Herken R, Girolamo F, Marzullo A, Svelto M, Roncali L. Severe alterations of endothelial and glial cells in the blood-brain barrier of dystrophic mdx mice. Glia 42: 235–251, 2003. doi: 10.1002/glia.10216. [DOI] [PubMed] [Google Scholar]
- 434.Nicolas G, Pottier C, Maltête D, Coutant S, Rovelet-Lecrux A, Legallic S, Rousseau S, Vaschalde Y, Guyant-Maréchal L, Augustin J, Martinaud O, Defebvre L, Krystkowiak P, Pariente J, Clanet M, Labauge P, Ayrignac X, Lefaucheur R, Le Ber I, Frébourg T, Hannequin D, Campion D. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology 80: 181–187, 2013. doi: 10.1212/WNL.0b013e31827ccf34. [DOI] [PubMed] [Google Scholar]
- 435.Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, Loetscher H, Ghosh A, Freskgård P-O. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 81: 49–60, 2014. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 436.Nikolakopoulou AM, Zhao Z, Montagne A, Zlokovic BV. Regional early and progressive loss of brain pericytes but not vascular smooth muscle cells in adult mice with disrupted platelet-derived growth factor receptor-β signaling. PLoS One 12: e0176225, 2017. doi: 10.1371/journal.pone.0176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem 286: 17536–17542, 2011. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161: 653–660, 2003. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Niu F, Yao H, Zhang W, Sutliff RL, Buch S. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci 34: 11812–11825, 2014. doi: 10.1523/JNEUROSCI.1139-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Niwa A, Matsuo K, Shindo A, Yata K, Shiraishi T, Tomimoto H. Clinical and neuropathological findings in a patient with familial Alzheimer disease showing a mutation in the PSEN1 gene. Neuropathology 33: 199–203, 2013. doi: 10.1111/j.1440-1789.2012.01340.x. [DOI] [PubMed] [Google Scholar]
- 441.Noble LJ, Wrathall JR. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res 482: 57–66, 1989. doi: 10.1016/0006-8993(89)90542-8. [DOI] [PubMed] [Google Scholar]
- 442.Nochlin D, Bird TD, Nemens EJ, Ball MJ, Sumi SM. Amyloid angiopathy in a Volga German family with Alzheimer’s disease and a presenilin-2 mutation (N141I). Ann Neurol 43: 131–135, 1998. doi: 10.1002/ana.410430124. [DOI] [PubMed] [Google Scholar]
- 443.Noorbakhsh F, Baker GB, Power C. Allopregnanolone and neuroinflammation: a focus on multiple sclerosis. Front Cell Neurosci 8: 134, 2014. doi: 10.3389/fncel.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Clusterin: a forgotten player in Alzheimer’s disease. Brain Res Brain Res Rev 61: 89–104, 2009. doi: 10.1016/j.brainresrev.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 445.Oba H, Araki T, Ohtomo K, Monzawa S, Uchiyama G, Koizumi K, Nogata Y, Kachi K, Shiozawa Z, Kobayashi M. Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology 189: 843–846, 1993. doi: 10.1148/radiology.189.3.8234713. [DOI] [PubMed] [Google Scholar]
- 446.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 19: 1584–1596, 2013. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.O’Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab 26: 1234–1249, 2006. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- 448.O’Driscoll MC, Daly SB, Urquhart JE, Black GCM, Pilz DT, Brockmann K, McEntagart M, Abdel-Salam G, Zaki M, Wolf NI, Ladda RL, Sell S, D’Arrigo S, Squier W, Dobyns WB, Livingston JH, Crow YJ. Recessive mutations in the gene encoding the tight junction protein occludin cause band-like calcification with simplified gyration and polymicrogyria. Am J Hum Genet 87: 354–364, 2010. doi: 10.1016/j.ajhg.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Ogura S, Kurata K, Hattori Y, Takase H, Ishiguro-Oonuma T, Hwang Y, Ahn S, Park I, Ikeda W, Kusuhara S, Fukushima Y, Nara H, Sakai H, Fujiwara T, Matsushita J, Ema M, Hirashima M, Minami T, Shibuya M, Takakura N, Kim P, Miyata T, Ogura Y, Uemura A. Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight 2: e90905, 2017. doi: 10.1172/jci.insight.90905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.O’Kane RL, Hawkins RA. Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood-brain barrier. Am J Physiol Endocrinol Metab 285: E1167–E1173, 2003. doi: 10.1152/ajpendo.00193.2003. [DOI] [PubMed] [Google Scholar]
- 451.O’Kane RL, Martínez-López I, DeJoseph MR, Viña JR, Hawkins RA. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem 274: 31891–31895, 1999. doi: 10.1074/jbc.274.45.31891. [DOI] [PubMed] [Google Scholar]
- 452.Olazarán J, Ramos A, Boyano I, Alfayate E, Valentí M, Rábano A, Alvarez-Linera J. Pattern of and risk factors for brain microbleeds in neurodegenerative dementia. Am J Alzheimers Dis Other Demen 29: 263–269, 2014. doi: 10.1177/1533317513517043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Oldendorf WH, Szabo J. Amino acid assignment to one of three blood-brain barrier amino acid carriers. Am J Physiol 230: 94–98, 1976. [DOI] [PubMed] [Google Scholar]
- 454.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 57: 128–134, 2005. doi: 10.1227/01.NEU.0000163407.92769.ED. [DOI] [PubMed] [Google Scholar]
- 455.Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs 6: 130–136, 2010. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 456.Ortiz GG, Pacheco-Moisés FP, Macías-Islas MÁ, Flores-Alvarado LJ, Mireles-Ramírez MA, González-Renovato ED, Hernández-Navarro VE, Sánchez-López AL, Alatorre-Jiménez MA. Role of the blood-brain barrier in multiple sclerosis. Arch Med Res 45: 687–697, 2014. doi: 10.1016/j.arcmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 457.Ossenkoppele R, van der Flier WM, Zwan MD, Adriaanse SF, Boellaard R, Windhorst AD, Barkhof F, Lammertsma AA, Scheltens P, van Berckel BNM. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 80: 359–365, 2013. doi: 10.1212/WNL.0b013e31827f0889. [DOI] [PubMed] [Google Scholar]
- 458.Pardridge WM. Molecular biology of the blood-brain barrier. Mol Biotechnol 30: 57–70, 2005. doi: 10.1385/MB:30:1:057. [DOI] [PubMed] [Google Scholar]
- 459.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32: 1959–1972, 2012. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Pardridge WM. Blood-brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets 19: 1059–1072, 2015. doi: 10.1517/14728222.2015.1042364. [DOI] [PubMed] [Google Scholar]
- 461.Pardridge WM, Buciak JL, Friden PM. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J Pharmacol Exp Ther 259: 66–70, 1991. [PubMed] [Google Scholar]
- 462.Park DY, Lee J, Kim J, Kim K, Hong S, Han S, Kubota Y, Augustin HG, Ding L, Kim JW, Kim H, He Y, Adams RH, Koh GY. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun 8: 15296, 2017. doi: 10.1038/ncomms15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Park HJ, Shin JY, Kim HN, Oh SH, Song SK, Lee PH. Mesenchymal stem cells stabilize the blood-brain barrier through regulation of astrocytes. Stem Cell Res Ther 6: 187, 2015. doi: 10.1186/s13287-015-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Park L, Zhou P, Koizumi K, El Jamal S, Previti ML, Van Nostrand WE, Carlson G, Iadecola C. Brain and circulating levels of Aβ1-40 differentially contribute to vasomotor dysfunction in the mouse brain. Stroke 44: 198–204, 2013. doi: 10.1161/STROKEAHA.112.670976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci 7: 710–723, 2006. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 466.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5: 584–590, 1985. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 467.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J Exp Med 204: 1999–2008, 2007. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Peacock ML, Warren JT Jr, Roses AD, Fink JK. Novel polymorphism in the A4 region of the amyloid precursor protein gene in a patient without Alzheimer’s disease. Neurology 43: 1254–1256, 1993. doi: 10.1212/WNL.43.6.1254. [DOI] [PubMed] [Google Scholar]
- 469.Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522: 340–344, 2015. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 470.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704, 2006. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood 107: 4770–4780, 2006. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Pfeiffer F, Schäfer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol 122: 601–614, 2011. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Pienaar IS, Lee CH, Elson JL, McGuinness L, Gentleman SM, Kalaria RN, Dexter DT. Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson’s disease. Neurobiol Dis 74: 392–405, 2015. doi: 10.1016/j.nbd.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 474.Pisani V, Stefani A, Pierantozzi M, Natoli S, Stanzione P, Franciotta D, Pisani A. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J Neuroinflammation 9: 188, 2012. doi: 10.1186/1742-2094-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Podjaski C, Alvarez JI, Bourbonniere L, Larouche S, Terouz S, Bin JM, Lécuyer M-A, Saint-Laurent O, Larochelle C, Darlington PJ, Arbour N, Antel JP, Kennedy TE, Prat A. Netrin 1 regulates blood-brain barrier function and neuroinflammation. Brain 138: 1598–1612, 2015. doi: 10.1093/brain/awv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Poliakova T, Levin O, Arablinskiy A, Vasenina E, Zerr I. Cerebral microbleeds in early Alzheimer’s disease. J Neurol 263: 1961–1968, 2016. doi: 10.1007/s00415-016-8220-2. [DOI] [PubMed] [Google Scholar]
- 477.Poon C, McMahon D, Hynynen K. Noninvasive and targeted delivery of therapeutics to the brain using focused ultrasound. Neuropharmacology 120: 20–37, 2017. doi: 10.1016/j.neuropharm.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol 142: 258–275, 1996. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- 479.Posokhova E, Shukla A, Seaman S, Volate S, Hilton MB, Wu B, Morris H, Swing DA, Zhou M, Zudaire E, Rubin JS, St Croix B. GPR124 functions as a WNT7-specific coactivator of canonical β-catenin signaling. Cell Reports 10: 123–130, 2015. doi: 10.1016/j.celrep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 146: 873–887, 2011. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 481.Potter R, Patterson BW, Elbert DL, Ovod V, Kasten T, Sigurdson W, Mawuenyega K, Blazey T, Goate A, Chott R, Yarasheski KE, Holtzman DM, Morris JC, Benzinger TLS, Bateman RJ. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med 5: 189ra77, 2013. doi: 10.1126/scitranslmed.3005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Prakash R, Carmichael ST. Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr Opin Neurol 28: 556–564, 2015. doi: 10.1097/WCO.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Prineas JW, Parratt JDE. Oligodendrocytes and the early multiple sclerosis lesion. Ann Neurol 72: 18–31, 2012. doi: 10.1002/ana.23634. [DOI] [PubMed] [Google Scholar]
- 484.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci 20: 136–144, 2017. doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- 485.Proctor EA, Fee L, Tao Y, Redler RL, Fay JM, Zhang Y, Lv Z, Mercer IP, Deshmukh M, Lyubchenko YL, Dokholyan NV. Nonnative SOD1 trimer is toxic to motor neurons in a model of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 113: 614–619, 2016. doi: 10.1073/pnas.1516725113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Protas HD, Chen K, Langbaum JBS, Fleisher AS, Alexander GE, Lee W, Bandy D, de Leon MJ, Mosconi L, Buckley S, Truran-Sacrey D, Schuff N, Weiner MW, Caselli RJ, Reiman EM. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol 70: 320–325, 2013. doi: 10.1001/2013.jamaneurol.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Puchowicz MA, Xu K, Sun X, Ivy A, Emancipator D, LaManna JC. Diet-induced ketosis increases capillary density without altered blood flow in rat brain. Am J Physiol Endocrinol Metab 292: E1607–E1615, 2007. doi: 10.1152/ajpendo.00512.2006. [DOI] [PubMed] [Google Scholar]
- 488.Qosa H, Miller DS, Pasinelli P, Trotti D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res 1628, Pt B: 298–316, 2015. doi: 10.1016/j.brainres.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Quintero-Monzon O, Martin MM, Fernandez MA, Cappello CA, Krzysiak AJ, Osenkowski P, Wolfe MS. Dissociation between the processivity and total activity of γ-secretase: implications for the mechanism of Alzheimer’s disease-causing presenilin mutations. Biochemistry 50: 9023–9035, 2011. doi: 10.1021/bi2007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Quistgaard EM, Löw C, Moberg P, Trésaugues L, Nordlund P. Structural basis for substrate transport in the GLUT-homology family of monosaccharide transporters. Nat Struct Mol Biol 20: 766–768, 2013. doi: 10.1038/nsmb.2569. [DOI] [PubMed] [Google Scholar]
- 491.Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol 20: 217–230, 2000. doi: 10.1023/A:1007049806660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol 34: 207–217, 1967. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Reijerkerk A, Lopez-Ramirez MA, van Het Hof B, Drexhage JAR, Kamphuis WW, Kooij G, Vos JB, van der Pouw Kraan TCTM, van Zonneveld AJ, Horrevoets AJ, Prat A, Romero IA, de Vries HE. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: implications for multiple sclerosis. J Neurosci 33: 6857–6863, 2013. doi: 10.1523/JNEUROSCI.3965-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA 101: 284–289, 2004. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Reitz C, Cheng R, Rogaeva E, Lee JH, Tokuhiro S, Zou F, Bettens K, Sleegers K, Tan EK, Kimura R, Shibata N, Arai H, Kamboh MI, Prince JA, Maier W, Riemenschneider M, Owen M, Harold D, Hollingworth P, Cellini E, Sorbi S, Nacmias B, Takeda M, Pericak-Vance MA, Haines JL, Younkin S, Williams J, van Broeckhoven C, Farrer LA, St George-Hyslop PH, Mayeux R; Genetic and Environmental Risk in Alzheimer Disease 1 Consortium . Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol 68: 99–106, 2011. doi: 10.1001/archneurol.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326: 47–63, 1985. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 497.Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita V-M, Kaivorinne A-L, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ; ITALSGEN Consortium . A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72: 257–268, 2011. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Ridder K, Keller S, Dams M, Rupp A-K, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, Landfried B, Arnold B, Plate KH, Höglinger G, Sültmann H, Altevogt P, Momma S. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol 12: e1001874, 2014. doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Rigau V, Morin M, Rousset M-C, de Bock F, Lebrun A, Coubes P, Picot M-C, Baldy-Moulinier M, Bockaert J, Crespel A, Lerner-Natoli M. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain 130: 1942–1956, 2007. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- 500.Ringman JM, Monsell S, Ng DW, Zhou Y, Nguyen A, Coppola G, Van Berlo V, Mendez MF, Tung S, Weintraub S, Mesulam M-M, Bigio EH, Gitelman DR, Fisher-Hubbard AO, Albin RL, Vinters HV. Neuropathology of Autosomal Dominant Alzheimer Disease in the National Alzheimer Coordinating Center Database. J Neuropathol Exp Neurol 75: 284–290, 2016. doi: 10.1093/jnen/nlv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Rite I, Machado A, Cano J, Venero JL. Blood-brain barrier disruption induces in vivo degeneration of nigral dopaminergic neurons. J Neurochem 101: 1567–1582, 2007. doi: 10.1111/j.1471-4159.2007.04567.x. [DOI] [PubMed] [Google Scholar]
- 502.Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, Grindstaff KK, Mengesha W, Raman C, Zerangue N. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149: 6251–6261, 2008. doi: 10.1210/en.2008-0378. [DOI] [PubMed] [Google Scholar]
- 503.Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song Y-Q, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet 39: 168–177, 2007. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Rokka J, Grönroos TJ, Viljanen T, Solin O, Haaparanta-Solin M. HPLC and TLC methods for analysis of [18F]FDG and its metabolites from biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 1048: 140–149, 2017. doi: 10.1016/j.jchromb.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 505.Rosenberg GA. Ischemic brain edema. Prog Cardiovasc Dis 42: 209–216, 1999. doi: 10.1016/S0033-0620(99)70003-4. [DOI] [PubMed] [Google Scholar]
- 506.Ruitenberg A, den Heijer T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MMB. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 57: 789–794, 2005. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 507.Runge VM, Wells JW, Baldwin SA, Scheff SW, Blades DA. Evaluation of the temporal evolution of acute spinal cord injury. Invest Radiol 32: 105–110, 1997. doi: 10.1097/00004424-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 508.Rustenhoven J, Aalderink M, Scotter EL, Oldfield RL, Bergin PS, Mee EW, Graham ES, Faull RLM, Curtis MA, Park TI-H, Dragunow M. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation 13: 37, 2016. doi: 10.1186/s12974-016-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med 13, 9A: 2911–2925, 2009. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Ryu JK, Petersen MA, Murray SG, Baeten KM, Meyer-Franke A, Chan JP, Vagena E, Bedard C, Machado MR, Rios Coronado PE, Prod’homme T, Charo IF, Lassmann H, Degen JL, Zamvil SS, Akassoglou K. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun 6: 8164, 2015. doi: 10.1038/ncomms9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, Ragin A, Levine A, Miller E. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86: 334–340, 2016. doi: 10.1212/WNL.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med 13: 1029–1031, 2007. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 4: 2932, 2013. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 514.Saito S, Ihara M. Interaction between cerebrovascular disease and Alzheimer pathology. Curr Opin Psychiatry 29: 168–173, 2016. doi: 10.1097/YCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 515.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11: 4131–4142, 2000. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Salloway S, Gur T, Berzin T, Tavares R, Zipser B, Correia S, Hovanesian V, Fallon J, Kuo-Leblanc V, Glass D, Hulette C, Rosenberg C, Vitek M, Stopa E. Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J Neurol Sci 203-204: 183–187, 2002. doi: 10.1016/S0022-510X(02)00288-5. [DOI] [PubMed] [Google Scholar]
- 517.Samuraki M, Matsunari I, Chen W-P, Yajima K, Yanase D, Fujikawa A, Takeda N, Nishimura S, Matsuda H, Yamada M. Partial volume effect-corrected FDG PET and grey matter volume loss in patients with mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging 34: 1658–1669, 2007. doi: 10.1007/s00259-007-0454-x. [DOI] [PubMed] [Google Scholar]
- 518.Sanan DA, Weisgraber KH, Russell SJ, Mahley RW, Huang D, Saunders A, Schmechel D, Wisniewski T, Frangione B, Roses AD. Apolipoprotein E associates with beta amyloid peptide of Alzheimer’s disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J Clin Invest 94: 860–869, 1994. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release 235: 34–47, 2016. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 520.Sareen D, O’Rourke JG, Meera P, Muhammad AKMG, Grant S, Simpkinson M, Bell S, Carmona S, Ornelas L, Sahabian A, Gendron T, Petrucelli L, Baughn M, Ravits J, Harms MB, Rigo F, Bennett CF, Otis TS, Svendsen CN, Baloh RH. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med 5: 208ra149, 2013. doi: 10.1126/scitranslmed.3007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Sathiyanadan K, Coisne C, Enzmann G, Deutsch U, Engelhardt B. PSGL-1 and E/P-selectins are essential for T-cell rolling in inflamed CNS microvessels but dispensable for initiation of EAE. Eur J Immunol 44: 2287–2294, 2014. doi: 10.1002/eji.201344214. [DOI] [PubMed] [Google Scholar]
- 522.Schachtrup C, Lu P, Jones LL, Lee JK, Lu J, Sachs BD, Zheng B, Akassoglou K. Fibrinogen inhibits neurite outgrowth via beta 3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci USA 104: 11814–11819, 2007. doi: 10.1073/pnas.0704045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 2: 864–870, 1996. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 524.Schiavone S, Mhillaj E, Neri M, Morgese MG, Tucci P, Bove M, Valentino M, Di Giovanni G, Pomara C, Turillazzi E, Trabace L, Cuomo V. Early Loss of Blood-Brain Barrier Integrity Precedes NOX2 Elevation in the Prefrontal Cortex of an Animal Model of Psychosis. Mol Neurobiol 54: 2031–2044, 2017. doi: 10.1007/s12035-016-9791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Schliep G, Felgenhauer K. Serum-CSF protein gradients, the blood-GSF barrier and the local immune response. J Neurol 218: 77–96, 1978. doi: 10.1007/BF02402169. [DOI] [PubMed] [Google Scholar]
- 526.Sellal F, Wallon D, Martinez-Almoyna L, Marelli C, Dhar A, Oesterlé H, Rovelet-Lecrux A, Rousseau S, Kourkoulis CE, Rosand J, DiPucchio ZY, Frosch M, Gombert C, Audoin B, Miné M, Riant F, Frebourg T, Hannequin D, Campion D, Greenberg SM, Tournier-Lasserve E, Nicolas G. APP Mutations in Cerebral Amyloid Angiopathy with or without Cortical Calcifications: Report of Three Families and a Literature Review. J Alzheimers Dis 56: 37–46, 2017. doi: 10.3233/JAD-160709. [DOI] [PubMed] [Google Scholar]
- 527.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol 23: 303–310, 2013. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, Harold D, Schrijvers EMC, Ramirez-Lorca R, Debette S, Longstreth WT Jr, Janssens ACJW, Pankratz VS, Dartigues JF, Hollingworth P, Aspelund T, Hernandez I, Beiser A, Kuller LH, Koudstaal PJ, Dickson DW, Tzourio C, Abraham R, Antunez C, Du Y, Rotter JI, Aulchenko YS, Harris TB, Petersen RC, Berr C, Owen MJ, Lopez-Arrieta J, Varadarajan BN, Becker JT, Rivadeneira F, Nalls MA, Graff-Radford NR, Campion D, Auerbach S, Rice K, Hofman A, Jonsson PV, Schmidt H, Lathrop M, Mosley TH, Au R, Psaty BM, Uitterlinden AG, Farrer LA, Lumley T, Ruiz A, Williams J, Amouyel P, Younkin SG, Wolf PA, Launer LJ, Lopez OL, van Duijn CM, Breteler MMB; CHARGE Consortium; GERAD1 Consortium; EADI1 Consortium . Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 303: 1832–1840, 2010. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Shams S, Martola J, Granberg T, Li X, Shams M, Fereshtehnejad SM, Cavallin L, Aspelin P, Kristoffersen-Wiberg M, Wahlund LO. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis—the Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol 36: 661–666, 2015. doi: 10.3174/ajnr.A4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Shams S, Wahlund L-O. Cerebral microbleeds as a biomarker in Alzheimer’s disease? A review in the field. Biomarkers Med 10: 9–18, 2016. doi: 10.2217/bmm.15.101. [DOI] [PubMed] [Google Scholar]
- 531.Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, Goate A, Mintun MA. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci 30: 17035–17040, 2010. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu S-C, Quinn JF, Galasko DR, Banks WA, Zhang J. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128: 639–650, 2014. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, Gallardo G, Wang K, Roh J, Robinson G, Finn MB, Jiang H, Sullivan PM, Baufeld C, Wood MW, Sutphen C, McCue L, Xiong C, Del-Aguila JL, Morris JC, Cruchaga C, Fagan AM, Miller BL, Boxer AL, Seeley WW, Butovsky O, Barres BA, Paul SM, Holtzman DM; Alzheimer’s Disease Neuroimaging Initiative . ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549: 523–527, 2017. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X, Xu N, Stetler RA, Zhang F, Liu X, Leak RK, Keep RF, Ji X, Chen J. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun 7: 10523, 2016. doi: 10.1038/ncomms10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106: 1489–1499, 2000. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 6: 393–403, 2010. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, Montresor A, Bolomini-Vittori M, Feigelson SW, Kirchhausen T, Laudanna C, Shakhar G, Alon R. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity 30: 384–396, 2009. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Siegenthaler JA, Sohet F, Daneman R. ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barriergenesis. Curr Opin Neurobiol 23: 1057–1064, 2013. doi: 10.1016/j.conb.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Sigurbjörnsdóttir S, Mathew R, Leptin M. Molecular mechanisms of de novo lumen formation. Nat Rev Mol Cell Biol 15: 665–676, 2014. doi: 10.1038/nrm3871. [DOI] [PubMed] [Google Scholar]
- 540.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27: 1766–1791, 2007. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann Neurol 35: 546–551, 1994. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- 542.Simpson IA, Vannucci SJ, DeJoseph MR, Hawkins RA. Glucose transporter asymmetries in the bovine blood-brain barrier. J Biol Chem 276: 12725–12729, 2001. doi: 10.1074/jbc.M010897200. [DOI] [PubMed] [Google Scholar]
- 543.Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, Kunkle BW, Boland A, Raybould R, Bis JC, Martin ER, Grenier-Boley B, Heilmann-Heimbach S, Chouraki V, Kuzma AB, Sleegers K, Vronskaya M, Ruiz A, Graham RR, Olaso R, Hoffmann P, Grove ML, Vardarajan BN, Hiltunen M, Nöthen MM, White CC, Hamilton-Nelson KL, Epelbaum J, Maier W, Choi S-H, Beecham GW, Dulary C, Herms S, Smith AV, Funk CC, Derbois C, Forstner AJ, Ahmad S, Li H, Bacq D, Harold D, Satizabal CL, Valladares O, Squassina A, Thomas R, Brody JA, Qu L, Sánchez-Juan P, Morgan T, Wolters FJ, Zhao Y, Garcia FS, Denning N, Fornage M, Malamon J, Naranjo MCD, Majounie E, Mosley TH, Dombroski B, Wallon D, Lupton MK, Dupuis J, Whitehead P, Fratiglioni L, Medway C, Jian X, Mukherjee S, Keller L, Brown K, Lin H, Cantwell LB, Panza F, McGuinness B, Moreno-Grau S, Burgess JD, Solfrizzi V, Proitsi P, Adams HH, Allen M, Seripa D, Pastor P, Cupples LA, Price ND, Hannequin D, Frank-García A, Levy D, Chakrabarty P, Caffarra P, Giegling I, Beiser AS, Giedraitis V, Hampel H, Garcia ME, Wang X, Lannfelt L, Mecocci P, Eiriksdottir G, Crane PK, Pasquier F, Boccardi V, Henández I, Barber RC, Scherer M, Tarraga L, Adams PM, Leber M, Chen Y, Albert MS, Riedel-Heller S, Emilsson V, Beekly D, Braae A, Schmidt R, Blacker D, Masullo C, Schmidt H, Doody RS, Spalletta G, Longstreth WT Jr, Fairchild TJ, Bossù P, Lopez OL, Frosch MP, Sacchinelli E, Ghetti B, Yang Q, Huebinger RM, Jessen F, Li S, Kamboh MI, Morris J, Sotolongo-Grau O, Katz MJ, Corcoran C, Dunstan M, Braddel A, Thomas C, Meggy A, Marshall R, Gerrish A, Chapman J, Aguilar M, Taylor S, Hill M, Fairén MD, Hodges A, Vellas B, Soininen H, Kloszewska I, Daniilidou M, Uphill J, Patel Y, Hughes JT, Lord J, Turton J, Hartmann AM, Cecchetti R, Fenoglio C, Serpente M, Arcaro M, Caltagirone C, Orfei MD, Ciaramella A, Pichler S, Mayhaus M, Gu W, Lleó A, Fortea J, Blesa R, Barber IS, Brookes K, Cupidi C, Maletta RG, Carrell D, Sorbi S, Moebus S, Urbano M, Pilotto A, Kornhuber J, Bosco P, Todd S, Craig D, Johnston J, Gill M, Lawlor B, Lynch A, Fox NC, Hardy J, Albin RL, Apostolova LG, Arnold SE, Asthana S, Atwood CS, Baldwin CT, Barnes LL, Barral S, Beach TG, Becker JT, Bigio EH, Bird TD, Boeve BF, Bowen JD, Boxer A, Burke JR, Burns JM, Buxbaum JD, Cairns NJ, Cao C, Carlson CS, Carlsson CM, Carney RM, Carrasquillo MM, Carroll SL, Diaz CC, Chui HC, Clark DG, Cribbs DH, Crocco EA, DeCarli C, Dick M, Duara R, Evans DA, Faber KM, Fallon KB, Fardo DW, Farlow MR, Ferris S, Foroud TM, Galasko DR, Gearing M, Geschwind DH, Gilbert JR, Graff-Radford NR, Green RC, Growdon JH, Hamilton RL, Harrell LE, Honig LS, Huentelman MJ, Hulette CM, Hyman BT, Jarvik GP, Abner E, Jin L-W, Jun G, Karydas A, Kaye JA, Kim R, Kowall NW, Kramer JH, LaFerla FM, Lah JJ, Leverenz JB, Levey AI, Li G, Lieberman AP, Lunetta KL, Lyketsos CG, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Morris JC, Murrell JR, Myers AJ, O’Bryant S, Olichney JM, Pankratz VS, Parisi JE, Paulson HL, Perry W, Peskind E, Pierce A, Poon WW, Potter H, Quinn JF, Raj A, Raskind M, Reisberg B, Reitz C, Ringman JM, Roberson ED, Rogaeva E, Rosen HJ, Rosenberg RN, Sager MA, Saykin AJ, Schneider JA, Schneider LS, Seeley WW, Smith AG, Sonnen JA, Spina S, Stern RA, Swerdlow RH, Tanzi RE, Thornton-Wells TA, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Van Eldik LJ, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Wilhelmsen KC, Williamson J, Wingo TS, Woltjer RL, Wright CB, Yu C-E, Yu L, Garzia F, Golamaully F, Septier G, Engelborghs S, Vandenberghe R, De Deyn PP, Fernadez CM, Benito YA, Thonberg H, Forsell C, Lilius L, Kinhult-Stählbom A, Kilander L, Brundin R, Concari L, Helisalmi S, Koivisto AM, Haapasalo A, Dermecourt V, Fievet N, Hanon O, Dufouil C, Brice A, Ritchie K, Dubois B, Himali JJ, Keene CD, Tschanz J, Fitzpatrick AL, Kukull WA, Norton M, Aspelund T, Larson EB, Munger R, Rotter JI, Lipton RB, Bullido MJ, Hofman A, Montine TJ, Coto E, Boerwinkle E, Petersen RC, Alvarez V, Rivadeneira F, Reiman EM, Gallo M, O’Donnell CJ, Reisch JS, Bruni AC, Royall DR, Dichgans M, Sano M, Galimberti D, St George-Hyslop P, Scarpini E, Tsuang DW, Mancuso M, Bonuccelli U, Winslow AR, Daniele A, Wu C-K, Peters O, Nacmias B, Riemenschneider M, Heun R, Brayne C, Rubinsztein DC, Bras J, Guerreiro R, Al-Chalabi A, Shaw CE, Collinge J, Mann D, Tsolaki M, Clarimón J, Sussams R, Lovestone S, O’Donovan MC, Owen MJ, Behrens TW, Mead S, Goate AM, Uitterlinden AG, Holmes C, Cruchaga C, Ingelsson M, Bennett DA, Powell J, Golde TE, Graff C, De Jager PL, Morgan K, Ertekin-Taner N, Combarros O, Psaty BM, Passmore P, Younkin SG, Berr C, Gudnason V, Rujescu D, Dickson DW, Dartigues J-F, DeStefano AL, Ortega-Cubero S, Hakonarson H, Campion D, Boada M, Kauwe JK, Farrer LA, Van Broeckhoven C, Ikram MA, Jones L, Haines JL, Tzourio C, Launer LJ, Escott-Price V, Mayeux R, Deleuze J-F, Amin N, Holmans PA, Pericak-Vance MA, Amouyel P, van Duijn CM, Ramirez A, Wang L-S, Lambert J-C, Seshadri S, Williams J, Schellenberg GD ARUK Consortium; GERAD/PERADES, CHARGE, ADGC, EADI . Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet 49: 1373–1384, 2017. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Singleton AB, Hall R, Ballard CG, Perry RH, Xuereb JH, Rubinsztein DC, Tysoe C, Matthews P, Cordell B, Kumar-Singh S, De Jonghe C, Cruts M, van Broeckhoven C, Morris CM. Pathology of early-onset Alzheimer’s disease cases bearing the Thr113-114ins presenilin-1 mutation. Brain 123: 2467–2474, 2000. doi: 10.1093/brain/123.12.2467. [DOI] [PubMed] [Google Scholar]
- 545.Skillbäck T, Delsing L, Synnergren J, Mattsson N, Janelidze S, Nägga K, Kilander L, Hicks R, Wimo A, Winblad B, Hansson O, Blennow K, Eriksdotter M, Zetterberg H. CSF/serum albumin ratio in dementias: a cross-sectional study on 1861 patients. Neurobiol Aging 59: 1–9, 2017. doi: 10.1016/j.neurobiolaging.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 546.Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, Blennow K. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer’s disease and vascular dementia. Neurology 50: 966–971, 1998. doi: 10.1212/WNL.50.4.966. [DOI] [PubMed] [Google Scholar]
- 547.Smith AJ, Yao X, Dix JA, Jin B-J, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 6: e27679, 2017. doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Smith DH, Hicks RR, Johnson VE, Bergstrom DA, Cummings DM, Noble LJ, Hovda D, Whalen M, Ahlers ST, LaPlaca M, Tortella FC, Duhaime A-C, Dixon CE. Pre-Clinical Traumatic Brain Injury Common Data Elements: Toward a Common Language Across Laboratories. J Neurotrauma 32: 1725–1735, 2015. doi: 10.1089/neu.2014.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277: 813–817, 1997. doi: 10.1001/jama.1997.03540340047031. [DOI] [PubMed] [Google Scholar]
- 550.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement 11: 710–717, 2015. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Sohet F, Lin C, Munji RN, Lee SY, Ruderisch N, Soung A, Arnold TD, Derugin N, Vexler ZS, Yen FT, Daneman R. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J Cell Biol 208: 703–711, 2015. doi: 10.1083/jcb.201410131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28: 897–916, 1977. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 553.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Soto I, Graham LC, Richter HJ, Simeone SN, Radell JE, Grabowska W, Funkhouser WK, Howell MC, Howell GR. APOE Stabilization by Exercise Prevents Aging Neurovascular Dysfunction and Complement Induction. PLoS Biol 13: e1002279, 2015. doi: 10.1371/journal.pbio.1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp Neurol 273: 57–68, 2015. doi: 10.1016/j.expneurol.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 556.Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer’s disease: a case-control MRI study. Psychiatry Res 171: 232–241, 2009. doi: 10.1016/j.pscychresns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 557.Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J Immunol 185: 4846–4855, 2010. doi: 10.4049/jimmunol.0903732. [DOI] [PubMed] [Google Scholar]
- 558.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322: 1247–1250, 2008. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 559.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev Biol 84: 183–192, 1981. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 560.Stoll J, Wadhwani KC, Smith QR. Identification of the cationic amino acid transporter (System y+) of the rat blood-brain barrier. J Neurochem 60: 1956–1959, 1993. doi: 10.1111/j.1471-4159.1993.tb13428.x. [DOI] [PubMed] [Google Scholar]
- 561.Storck SE, Meister S, Nahrath J, Meißner JN, Schubert N, Di Spiezio A, Baches S, Vandenbroucke RE, Bouter Y, Prikulis I, Korth C, Weggen S, Heimann A, Schwaninger M, Bayer TA, Pietrzik CU. Endothelial LRP1 transports amyloid-β(1-42) across the blood-brain barrier. J Clin Invest 126: 123–136, 2016. doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res 1399: 96–115, 2011. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Sui Y-T, Bullock KM, Erickson MA, Zhang J, Banks WA. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides 62: 197–202, 2014. doi: 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Suidan GL, Dickerson JW, Johnson HL, Chan TW, Pavelko KD, Pirko I, Seroogy KB, Johnson AJ. Preserved vascular integrity and enhanced survival following neuropilin-1 inhibition in a mouse model of CD8 T cell-initiated CNS vascular permeability. J Neuroinflammation 9: 218, 2012. doi: 10.1186/1742-2094-9-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Sun L, Zeng X, Yan C, Sun X, Gong X, Rao Y, Yan N. Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature 490: 361–366, 2012. doi: 10.1038/nature11524. [DOI] [PubMed] [Google Scholar]
- 566.Sun L, Zhou R, Yang G, Shi Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc Natl Acad Sci USA 114: E476–E485, 2017. doi: 10.1073/pnas.1618657114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Sun W, Hu Q, Ji W, Wright G, Gu Z. Leveraging Physiology for Precision Drug Delivery. Physiol Rev 97: 189–225, 2017. doi: 10.1152/physrev.00015.2016. [DOI] [Google Scholar]
- 568.Suri S, Mackay CE, Kelly ME, Germuska M, Tunbridge EM, Frisoni GB, Matthews PM, Ebmeier KP, Bulte DP, Filippini N. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimers Dement 11: 648–57.e1, 2015. doi: 10.1016/j.jalz.2014.05.1755. [DOI] [PubMed] [Google Scholar]
- 569.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14: 133–150, 2018. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19: 771–783, 2016. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Sweeney MD, Sagare AP, Zlokovic BV. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease. J Cereb Blood Flow Metab 35: 1055–1068, 2015. doi: 10.1038/jcbfm.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Szaruga M, Veugelen S, Benurwar M, Lismont S, Sepulveda-Falla D, Lleo A, Ryan NS, Lashley T, Fox NC, Murayama S, Gijsen H, De Strooper B, Chávez-Gutiérrez L. Qualitative changes in human γ-secretase underlie familial Alzheimer’s disease. J Exp Med 212: 2003–2013, 2015. doi: 10.1084/jem.20150892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Szpak GM, Lewandowska E, Wierzba-Bobrowicz T, Bertrand E, Pasennik E, Mendel T, Stepień T, Leszczyńska A, Rafałowska J. Small cerebral vessel disease in familial amyloid and non-amyloid angiopathies: FAD-PS-1 (P117L) mutation and CADASIL. Immunohistochemical and ultrastructural studies. Folia Neuropathol 45: 192–204, 2007. [PubMed] [Google Scholar]
- 574.Taheri S, Rosenberg GA, Ford C. Quantification of blood-to-brain transfer rate in multiple sclerosis. Mult Scler Relat Disord 2: 124–132, 2013. doi: 10.1016/j.msard.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol 1: E52, 2003. doi: 10.1371/journal.pbio.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Tam SJ, Richmond DL, Kaminker JS, Modrusan Z, Martin-McNulty B, Cao TC, Weimer RM, Carano RAD, van Bruggen N, Watts RJ. Death receptors DR6 and TROY regulate brain vascular development. Dev Cell 22: 403–417, 2012. doi: 10.1016/j.devcel.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 577.Tan X, Chen C, Zhu Y, Deng J, Qiu X, Huang S, Shang F, Cheng B, Liu Y. Proteotoxic Stress Desensitizes TGF-beta Signaling Through Receptor Downregulation in Retinal Pigment Epithelial Cells. Curr Mol Med 17: 189–199, 2017. doi: 10.2174/1566524017666170619113435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Tang G, Liu Y, Zhang Z, Lu Y, Wang Y, Huang J, Li Y, Chen X, Gu X, Wang Y, Yang G-Y. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells 32: 3150–3162, 2014. doi: 10.1002/stem.1808. [DOI] [PubMed] [Google Scholar]
- 579.Tanifum EA, Starosolski ZA, Fowler SW, Jankowsky JL, Annapragada AV. Cerebral vascular leak in a mouse model of amyloid neuropathology. J Cereb Blood Flow Metab 34: 1646–1654, 2014. doi: 10.1038/jcbfm.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2: a006296, 2012. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain–implications for Alzheimer diseaser. Nat Rev Neurol 12: 248, 2016. doi: 10.1038/nrneurol.2016.36. [DOI] [PubMed] [Google Scholar]
- 582.Taylor CJ, Nicola PA, Wang S, Barrand MA, Hladky SB. Transporters involved in regulation of intracellular pH in primary cultured rat brain endothelial cells. J Physiol 576: 769–785, 2006. doi: 10.1113/jphysiol.2006.117374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Thakker DR, Weatherspoon MR, Harrison J, Keene TE, Lane DS, Kaemmerer WF, Stewart GR, Shafer LL. Intracerebroventricular amyloid-beta antibodies reduce cerebral amyloid angiopathy and associated micro-hemorrhages in aged Tg2576 mice. Proc Natl Acad Sci USA 106: 4501–4506, 2009. doi: 10.1073/pnas.0813404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 67: 93–98, 2010. doi: 10.1001/archneurol.2009.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Theiler K. The House Mouse. Development and Normal Stages From Fertilization to 4 Weeks of Age. Berlin: Springer-Verlag, 1972, p. 1–168. [Google Scholar]
- 586.Thomas RS, Henson A, Gerrish A, Jones L, Williams J, Kidd EJ. Decreasing the expression of PICALM reduces endocytosis and the activity of β-secretase: implications for Alzheimer’s disease. BMC Neurosci 17: 50, 2016. doi: 10.1186/s12868-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Thompson AG, Gray E, Heman-Ackah SM, Mäger I, Talbot K, Andaloussi SE, Wood MJ, Turner MR. Extracellular vesicles in neurodegenerative disease: pathogenesis to biomarkers. Nat Rev Neurol 12: 346–357, 2016. doi: 10.1038/nrneurol.2016.68. [DOI] [PubMed] [Google Scholar]
- 588.Thompson BJ, Sanchez-Covarrubias L, Slosky LM, Zhang Y, Laracuente ML, Ronaldson PT. Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood-brain barrier: relevance to CNS drug delivery. J Cereb Blood Flow Metab 34: 699–707, 2014. doi: 10.1038/jcbfm.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Tian Y, Chang JC, Fan EY, Flajolet M, Greengard P. Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer’s APP-CTF for terminal degradation via autophagy. Proc Natl Acad Sci USA 110: 17071–17076, 2013. doi: 10.1073/pnas.1315110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Tietz S, Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J Cell Biol 209: 493–506, 2015. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Tikka S, Baumann M, Siitonen M, Pasanen P, Pöyhönen M, Myllykangas L, Viitanen M, Fukutake T, Cognat E, Joutel A, Kalimo H. CADASIL and CARASIL. Brain Pathol 24: 525–544, 2014. doi: 10.1111/bpa.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 164: 1226–1232, 2016. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 593.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136: 2697–2706, 2013. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Toledo JB, Toledo E, Weiner MW, Jack CR Jr, Jagust W, Lee VMY, Shaw LM, Trojanowski JQ; Alzheimer’s Disease Neuroimaging Initiative . Cardiovascular risk factors, cortisol, and amyloid-β deposition in Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 8: 483–489, 2012. doi: 10.1016/j.jalz.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 208: 821–838, 2015. doi: 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Torres-Vázquez J, Gitler AD, Fraser SD, Berk JD, Pham VN, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell 7: 117–123, 2004. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 597.Traub O, Hertlein B, Kasper M, Eckert R, Krisciukaitis A, Hülser D, Willecke K. Characterization of the gap junction protein connexin37 in murine endothelium, respiratory epithelium, and after transfection in human HeLa cells. Eur J Cell Biol 77: 313–322, 1998. doi: 10.1016/S0171-9335(98)80090-3. [DOI] [PubMed] [Google Scholar]
- 598.Treusch S, Hamamichi S, Goodman JL, Matlack KES, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS, Han H, Lindhagen-Persson M, Reiman EM, Evans DA, Bennett DA, Olofsson A, DeJager PL, Tanzi RE, Caldwell KA, Caldwell GA, Lindquist S. Functional links between Aβ toxicity, endocytic trafficking, and Alzheimer’s disease risk factors in yeast. Science 334: 1241–1245, 2011. doi: 10.1126/science.1213210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Troost D, Van den Oord JJ, Vianney de Jong JM. Immunohistochemical characterization of the inflammatory infiltrate in amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol 16: 401–410, 1990. doi: 10.1111/j.1365-2990.1990.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 600.Trougakos IP, Lourda M, Antonelou MH, Kletsas D, Gorgoulis VG, Papassideri IS, Zou Y, Margaritis LH, Boothman DA, Gonos ES. Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex. Clin Cancer Res 15: 48–59, 2009. doi: 10.1158/1078-0432.CCR-08-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou A-P, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci 121: 29–37, 2008. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem 117: 333–345, 2011. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- 603.Ueda H, Baba T, Terada N, Kato Y, Fujii Y, Takayama I, Mei X, Ohno S. Immunolocalization of dystrobrevin in the astrocytic endfeet and endothelial cells in the rat cerebellum. Neurosci Lett 283: 121–124, 2000. doi: 10.1016/S0304-3940(00)00925-3. [DOI] [PubMed] [Google Scholar]
- 604.Uetani H, Hirai T, Hashimoto M, Ikeda M, Kitajima M, Sakamoto F, Utsunomiya D, Oda S, Sugiyama S, Matsubara J, Yamashita Y. Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. AJNR Am J Neuroradiol 34: 984–989, 2013. doi: 10.3174/ajnr.A3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Uhlirova H, Kılıç K, Tian P, Thunemann M, Desjardins M, Saisan PA, Sakadžić S, Ness TV, Mateo C, Cheng Q, Weldy KL, Razoux F, Vandenberghe M, Cremonesi JA, Ferri CG, Nizar K, Sridhar VB, Steed TC, Abashin M, Fainman Y, Masliah E, Djurovic S, Andreassen OA, Silva GA, Boas DA, Kleinfeld D, Buxton RB, Einevoll GT, Dale AM, Devor A. Cell type specificity of neurovascular coupling in cerebral cortex. eLife 5: e14315, 2016. doi: 10.7554/eLife.14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 10: 463–470, 2003. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 607.Urich E, Lazic SE, Molnos J, Wells I, Freskgård P-O. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS One 7: e38149, 2012. doi: 10.1371/journal.pone.0038149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol 36: 157–165, 2014. doi: 10.1016/j.semcdb.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. eLife 4: e06489, 2015. doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554: 475–480, 2018. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 611.Vatine GD, Al-Ahmad A, Barriga BK, Svendsen S, Salim A, Garcia L, Garcia VJ, Ho R, Yucer N, Qian T, Lim RG, Wu J, Thompson LM, Spivia WR, Chen Z, Van Eyk J, Palecek SP, Refetoff S, Shusta EV, Svendsen CN. Modeling Psychomotor Retardation using iPSCs from MCT8-Deficient Patients Indicates a Prominent Role for the Blood-Brain Barrier. Cell Stem Cell 20: 831–843.e5, 2017. doi: 10.1016/j.stem.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol 10: 241–252, 2011. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Verma S, Kumar M, Gurjav U, Lum S, Nerurkar VR. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 397: 130–138, 2010. doi: 10.1016/j.virol.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 348: 1215–1222, 2003. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 615.Verstraete E, Biessels G-J, van Den Heuvel MP, Visser F, Luijten PR, van Den Berg LH. No evidence of microbleeds in ALS patients at 7 Tesla MRI. Amyotroph Lateral Scler 11: 555–557, 2010. doi: 10.3109/17482968.2010.513053. [DOI] [PubMed] [Google Scholar]
- 616.Verstraeten A, Theuns J, Van Broeckhoven C. Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet 31: 140–149, 2015. doi: 10.1016/j.tig.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 617.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol 15: 692–704, 2015. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 618.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol 7: 31–40, 2011. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Villringer K, Sanz Cuesta BE, Ostwaldt A-C, Grittner U, Brunecker P, Khalil AA, Schindler K, Eisenblätter O, Audebert H, Fiebach JB. DCE-MRI blood-brain barrier assessment in acute ischemic stroke. Neurology 88: 433–440, 2017. doi: 10.1212/WNL.0000000000003566. [DOI] [PubMed] [Google Scholar]
- 620.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol 70: 871–880, 2011. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Van Vliet EA, Aronica E, Gorter JA. Role of blood-brain barrier in temporal lobe epilepsy and pharmacoresistance. Neuroscience 277: 455–473, 2014. doi: 10.1016/j.neuroscience.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 622.Van Vliet EA, da Costa Araújo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 130: 521–534, 2007. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 623.Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, Kofler N, Kitajewski J, Weissman I, Red-Horse K. Pericytes are progenitors for coronary artery smooth muscle. eLife 4: e10036, 2015. doi: 10.7554/eLife.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Vorbrodt AW. Ultrastructural cytochemistry of blood-brain barrier endothelia. Prog Histochem Cytochem 18: 1–99, 1988. doi: 10.1016/S0079-6336(88)80001-9. [DOI] [PubMed] [Google Scholar]
- 625.Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist’s view. Brain Res Brain Res Rev 42: 221–242, 2003. doi: 10.1016/S0165-0173(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 626.Vos CMP, Geurts JJG, Montagne L, van Haastert ES, Bö L, van der Valk P, Barkhof F, de Vries HE. Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis 20: 953–960, 2005. doi: 10.1016/j.nbd.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 627.Wada K, Arai H, Takanashi M, Fukae J, Oizumi H, Yasuda T, Mizuno Y, Mochizuki H. Expression levels of vascular endothelial growth factor and its receptors in Parkinson’s disease. Neuroreport 17: 705–709, 2006. doi: 10.1097/01.wnr.0000215769.71657.65. [DOI] [PubMed] [Google Scholar]
- 628.Wälchli T, Wacker A, Frei K, Regli L, Schwab ME, Hoerstrup SP, Gerhardt H, Engelhardt B. Wiring the Vascular Network with Neural Cues: A CNS Perspective. Neuron 87: 271–296, 2015. doi: 10.1016/j.neuron.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 629.Wallin A, Blennow K, Rosengren L. Cerebrospinal fluid markers of pathogenetic processes in vascular dementia, with special reference to the subcortical subtype. Alzheimer Dis Assoc Disord 13, Suppl 3: S102–S105, 1999. [PubMed] [Google Scholar]
- 630.Wang D, Kranz-Eble P, De Vivo DC. Mutational analysis of GLUT1 (SLC2A1) in Glut-1 deficiency syndrome. Hum Mutat 16: 224–231, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 631.Wang H, Golob EJ, Su M-Y. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J Magn Reson Imaging 24: 695–700, 2006. doi: 10.1002/jmri.20669. [DOI] [PubMed] [Google Scholar]
- 632.Wang W, Bodles-Brakhop AM, Barger SW. A Role for P-Glycoprotein in Clearance of Alzheimer Amyloid β-Peptide from the Brain. Curr Alzheimer Res 13: 615–620, 2016. doi: 10.2174/1567205013666160314151012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160: 1061–1071, 2015. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Wang Y, Chang H, Rattner A, Nathans J. Frizzled receptors in development and disease. Curr Top Dev Biol 117: 113–139, 2016. doi: 10.1016/bs.ctdb.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Wang Y, Huang J, Ma Y, Tang G, Liu Y, Chen X, Zhang Z, Zeng L, Wang Y, Ouyang Y-B, Yang G-Y. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J Cereb Blood Flow Metab 35: 1977–1984, 2015. doi: 10.1038/jcbfm.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151: 1332–1344, 2012. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw F-E, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BCM, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1) . Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12: 822–838, 2013. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet 5: 88, 2014. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Wegiel J, Wisniewski HM, Kuchna I, Tarnawski M, Badmajew E, Popovitch E, Kulczycki J, Dowjat WK, Wisniewski T. Cell-type-specific enhancement of amyloid-beta deposition in a novel presenilin-1 mutation (P117L). J Neuropathol Exp Neurol 57: 831–838, 1998. doi: 10.1097/00005072-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 640.Weller RO, Boche D, Nicoll JAR. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer’s disease and their potential impact on therapy. Acta Neuropathol 118: 87–102, 2009. doi: 10.1007/s00401-009-0498-z. [DOI] [PubMed] [Google Scholar]
- 641.Wen PH, De Gasperi R, Sosa MAG, Rocher AB, Friedrich VL Jr, Hof PR, Elder GA. Selective expression of presenilin 1 in neural progenitor cells rescues the cerebral hemorrhages and cortical lamination defects in presenilin 1-null mutant mice. Development 132: 3873–3883, 2005. doi: 10.1242/dev.01946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, Nottebaum AF, Vestweber D. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol 15: 223–230, 2014. doi: 10.1038/ni.2824. [DOI] [PubMed] [Google Scholar]
- 643.Wheeler MJ, Dempsey PC, Grace MS, Ellis KA, Gardiner PA, Green DJ, Dunstan DW. Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimers Dement (N Y) 3: 291–300, 2017. doi: 10.1016/j.trci.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Whetstone WD, Hsu J-YC, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res 74: 227–239, 2003. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Whiteus C, Freitas C, Grutzendler J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature 505: 407–411, 2014. doi: 10.1038/nature12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Wilhelmus MMM, Otte-Höller I, Davis J, Van Nostrand WE, de Waal RMW, Verbeek MM. Apolipoprotein E genotype regulates amyloid-beta cytotoxicity. J Neurosci 25: 3621–3627, 2005. doi: 10.1523/JNEUROSCI.4213-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Wilhelmus MMM, Otte-Höller I, van Triel JJJ, Veerhuis R, Maat-Schieman MLC, Bu G, de Waal RMW, Verbeek MM. Lipoprotein receptor-related protein-1 mediates amyloid-beta-mediated cell death of cerebrovascular cells. Am J Pathol 171: 1989–1999, 2007. doi: 10.2353/ajpath.2007.070050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 14: 1398–1405, 2011. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, Sullivan JS, Zhao Z, Meiselman HJ, Wendy RB, Soto J, Abel ED, Makshanoff J, Zuniga E, De Vivo DC, Zlokovic BV. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 18: 521–530, 2015. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab 32: 1841–1852, 2012. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z, Sullivan JS, Griffin JH, Cleveland DW, Zlokovic BV. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci USA 111: E1035–E1042, 2014. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 125: 111–120, 2013. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.World Health Organization. Epilepsy Fact Sheet [Online]. https://login.libproxy1.usc.edu/login?qurl=http://www.who.int%2fmediacentre%2ffactsheets%2ffs999%2fen%2f.
- 654.Worzfeld T, Schwaninger M. Apicobasal polarity of brain endothelial cells. J Cereb Blood Flow Metab 36: 340–362, 2016. doi: 10.1177/0271678X15608644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Wu H, Deng R, Chen X, Wong WC, Chen H, Gao L, Nie Y, Wu W, Shen J. Caveolin-1 Is Critical for Lymphocyte Trafficking into Central Nervous System during Experimental Autoimmune Encephalomyelitis. J Neurosci 36: 5193–5199, 2016. doi: 10.1523/JNEUROSCI.3734-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med 11: 959–965, 2005. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 657.Wyss L, Schäfer J, Liebner S, Mittelbronn M, Deutsch U, Enzmann G, Adams RH, Aurrand-Lions M, Plate KH, Imhof BA, Engelhardt B. Junctional adhesion molecule (JAM)-C deficient C57BL/6 mice develop a severe hydrocephalus. PLoS One 7: e45619, 2012. doi: 10.1371/journal.pone.0045619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Xiao Q, Gil S-C, Yan P, Wang Y, Han S, Gonzales E, Perez R, Cirrito JR, Lee J-M. Role of phosphatidylinositol clathrin assembly lymphoid-myeloid leukemia (PICALM) in intracellular amyloid precursor protein (APP) processing and amyloid plaque pathogenesis. J Biol Chem 287: 21279–21289, 2012. doi: 10.1074/jbc.M111.338376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science 342: 373–377, 2013. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Xu B, Zhang Y, Du X-F, Li J, Zi H-X, Bu J-W, Yan Y, Han H, Du J-L. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res 27: 882–897, 2017. doi: 10.1038/cr.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci 26: 4985–4994, 2006. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Yamadera M, Fujimura H, Inoue K, Toyooka K, Mori C, Hirano H, Sakoda S. Microvascular disturbance with decreased pericyte coverage is prominent in the ventral horn of patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 16: 393–401, 2015. doi: 10.3109/21678421.2015.1011663. [DOI] [PubMed] [Google Scholar]
- 663.Yamamoto H, Ehling M, Kato K, Kanai K, van Lessen M, Frye M, Zeuschner D, Nakayama M, Vestweber D, Adams RH. Integrin β1 controls VE-cadherin localization and blood vessel stability. Nat Commun 6: 6429, 2015. doi: 10.1038/ncomms7429. [DOI] [PubMed] [Google Scholar]
- 664.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol 172: 521–533, 2008. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Yamamoto T, Ochalski A, Hertzberg EL, Nagy JI. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. J Comp Neurol 302: 853–883, 1990. doi: 10.1002/cne.903020414. [DOI] [PubMed] [Google Scholar]
- 666.Yamamura H, Suzuki Y, Yamamura H, Asai K, Imaizumi Y. Hypoxic stress up-regulates Kir2.1 expression and facilitates cell proliferation in brain capillary endothelial cells. Biochem Biophys Res Commun 476: 386–392, 2016. doi: 10.1016/j.bbrc.2016.05.131. [DOI] [PubMed] [Google Scholar]
- 667.Yao Y, Chen Z-L, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun 5: 3413, 2014. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Yasuda M, Maeda K, Ikejiri Y, Kawamata T, Kuroda S, Tanaka C. A novel missense mutation in the presenilin-1 gene in a familial Alzheimer’s disease pedigree with abundant amyloid angiopathy. Neurosci Lett 232: 29–32, 1997. doi: 10.1016/S0304-3940(97)00569-7. [DOI] [PubMed] [Google Scholar]
- 669.Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, Rowe CC; AIBL Research Group . Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 82: 1266–1273, 2014. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Yemisci M, Caban S, Gursoy-Ozdemir Y, Lule S, Novoa-Carballal R, Riguera R, Fernandez-Megia E, Andrieux K, Couvreur P, Capan Y, Dalkara T. Systemically administered brain-targeted nanoparticles transport peptides across the blood-brain barrier and provide neuroprotection. J Cereb Blood Flow Metab 35: 469–475, 2015. doi: 10.1038/jcbfm.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Yu AS, Hirayama BA, Timbol G, Liu J, Diez-Sampedro A, Kepe V, Satyamurthy N, Huang S-C, Wright EM, Barrio JR. Regional distribution of SGLT activity in rat brain in vivo. Am J Physiol Cell Physiol 304: C240–C247, 2013. doi: 10.1152/ajpcell.00317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Yu YJ, Atwal JK, Zhang Y, Tong RK, Wildsmith KR, Tan C, Bien-Ly N, Hersom M, Maloney JA, Meilandt WJ, Bumbaca D, Gadkar K, Hoyte K, Luk W, Lu Y, Ernst JA, Scearce-Levie K, Couch JA, Dennis MS, Watts RJ. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med 6: 261ra154, 2014. doi: 10.1126/scitranslmed.3009835. [DOI] [PubMed] [Google Scholar]
- 673.Yuen N, Lam TI, Wallace BK, Klug NR, Anderson SE, O’Donnell ME. Ischemic factor-induced increases in cerebral microvascular endothelial cell Na/H exchange activity and abundance: evidence for involvement of ERK1/2 MAP kinase. Am J Physiol Cell Physiol 306: C931–C942, 2014. doi: 10.1152/ajpcell.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Zarranz JJ, Fernandez-Martinez M, Rodriguez O, Mateos B, Iglesias S, Baron J-C. Iowa APP mutation-related hereditary cerebral amyloid angiopathy (CAA): a new family from Spain. J Neurol Sci 363: 55–56, 2016. doi: 10.1016/j.jns.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 675.Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347: 1138–1142, 2015. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 676.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nanì S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, Constantin G. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 21: 880–886, 2015. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 677.Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang C-Y. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol 7: 909–915, 2005. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
- 678.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34: 11929–11947, 2014. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Zhang ZG, Tsang W, Zhang L, Powers C, Chopp M. Up-regulation of neuropilin-1 in neovasculature after focal cerebral ischemia in the adult rat. J Cereb Blood Flow Metab 21: 541–549, 2001. doi: 10.1097/00004647-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 680.Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis 26: 36–46, 2007. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Zhao N, Liu C-C, Qiao W, Bu G. Apolipoprotein E, Receptors, and Modulation of Alzheimer’s Disease. Biol Psychiatry 83: 347–357, 2018. doi: 10.1016/j.biopsych.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163: 1064–1078, 2015. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T, Sugawara T, Ichida JK, Zlokovic BV. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat Neurosci 18: 978–987, 2015. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Zhao Z, Zlokovic BV. Blood-brain barrier: a dual life of MFSD2A? Neuron 82: 728–730, 2014. doi: 10.1016/j.neuron.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Zhao Z, Zlokovic BV. Remote control of BBB: a tale of exosomes and microRNA. Cell Res 27: 849–850, 2017. doi: 10.1038/cr.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Zheng Y, Zhang Y, Barutello G, Chiu K, Arigoni M, Giampietro C, Cavallo F, Holmgren L. Angiomotin like-1 is a novel component of the N-cadherin complex affecting endothelial/pericyte interaction in normal and tumor angiogenesis. Sci Rep 6: 30622, 2016. doi: 10.1038/srep30622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci 11: 420–422, 2008. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S, Zlokovic AB, Liu T, Griffin JH, Chow N, Castellino FJ, Stojanovic K, Cleveland DW, Zlokovic BV. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest 119: 3437–3449, 2009. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell 31: 248–256, 2014. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest 124: 3825–3846, 2014. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging 28: 977–986, 2007. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 692.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57: 178–201, 2008. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 693.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12: 723–738, 2011. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol 70: 440–444, 2013. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin, d-alanine2-d-leucine5-enkephalin and their N-terminal amino acid (tyrosine). Brain Res 336: 125–132, 1985. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- 696.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β-peptide elimination from the brain. J Neurochem 115: 1077–1089, 2010. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci 34: 198–209, 2011. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Zlokovic BV, Hyman S, McComb JG, Lipovac MN, Tang G, Davson H. Kinetics of arginine-vasopressin uptake at the blood-brain barrier. Biochim Biophys Acta 1025: 191–198, 1990. doi: 10.1016/0005-2736(90)90097-8. [DOI] [PubMed] [Google Scholar]
- 699.Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology 141: 1434–1441, 2000. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]
- 700.Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, Frangione B, Ghiso J. Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci USA 93: 4229–4234, 1996. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Zlokovic BV, Segal MB, McComb JG, Hyman S, Weiss MH, Davson H. Kinetics of circulating vasopressin uptake by choroid plexus. Am J Physiol Renal Fluid Electrolyte Physiol 260: F216–F224, 1991. [DOI] [PubMed] [Google Scholar]
- 702.Zonneveld HI, Goos JDC, Wattjes MP, Prins ND, Scheltens P, van der Flier WM, Kuijer JPA, Muller M, Barkhof F. Prevalence of cortical superficial siderosis in a memory clinic population. Neurology 82: 698–704, 2014. doi: 10.1212/WNL.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 703.del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience 158: 972–982, 2009. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF, Iruela-Arispe ML. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell 18: 39–51, 2010. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Zuchero YJY, Chen X, Bien-Ly N, Bumbaca D, Tong RK, Gao X, Zhang S, Hoyte K, Luk W, Huntley MA, Phu L, Tan C, Kallop D, Weimer RM, Lu Y, Kirkpatrick DS, Ernst JA, Chih B, Dennis MS, Watts RJ. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 89: 70–82, 2016. doi: 10.1016/j.neuron.2015.11.024. [DOI] [PubMed] [Google Scholar]