Abstract
Background
The understanding of ovarian cancer pathogenesis has recently shifted to recognize distinct changes in how ovarian cancer histotypes are defined. Using the 2014 World Health Organization (WHO) diagnostic guidelines, we classified ovarian cancer histotypes in Surveillance, Epidemiology, and End Results (SEER) cancer registry data and examined survival patterns by histotype and disease stage.
Methods
We extracted data on 28 118 incident epithelial ovarian cancer cases diagnosed in 2004–2014 from SEER and defined histotype using the 2014 WHO guidelines (high-grade serous, low-grade serous, endometrioid, clear cell, mucinous, carcinosarcoma, and malignant Brenner tumors). By histotype and disease stage, we estimated Kaplan-Meier survival curves and calculated age-adjusted overall and cause-specific survival estimates. Cox proportional hazards regression models were used to estimate histotype-specific hazard ratios (HRs) and 95% confidence intervals (CIs) by disease stage while adjusting for age at diagnosis, region, race/ethnicity, and receipt of surgery.
Results
Within two years after diagnosis, localized/regional-stage carcinosarcoma and distant-stage mucinous, clear cell, and carcinosarcoma had a higher risk of mortality compared with high-grade serous, with the most pronounced association for localized/regional carcinosarcoma (>1–2-year time period: HR = 3.81, 95% CI = 2.74 to 5.30) and distant-stage mucinous (0–1-year time period: HR = 3.87, 95% CI = 3.45 to 4.34). In the time period more than four to 10 years after diagnosis, hazard ratios for all histotypes relative to high-grade serous, irrespective of disease stage, were less than 1.00. Cumulatively, both localized/regional and distant-stage low-grade serous and endometrioid carcinomas had the most favorable outcomes.
Conclusions
Our large study, which is representative of the United States population and incorporates the most current knowledge of ovarian cancer pathogenesis, highlights the need to recognize ovarian cancer as a set of distinct diseases and not a single entity. Only then will we be able to effectively target the unique features of each histotype to reduce ovarian cancer mortality.
Ovarian cancer is the most lethal cancer of the female reproductive system, with an estimated 14 070 deaths to occur in the United States in 2018 (1). Although advances in treatment over the past few decades have substantially improved median survival, cure rates remain relatively unchanged (2,3). Approximately half of women diagnosed with ovarian cancer survive five years after diagnosis (47%), and among women diagnosed with distant-stage disease, five-year survival is only 29% (1). Several factors are associated with survival, including stage (1,3), size of residual tumor after cytoreductive surgery (4,5), histotype (6–9), and race/ethnicity (10–13).
Epithelial ovarian cancer (EOC) is a heterogeneous disease, comprising several histotypes with distinct epidemiologic, molecular, and clinical features (14). While serous carcinomas were previously believed to progress along a disease continuum, recent evidence suggests that high-grade and low-grade serous carcinomas are separate disease processes that develop via independent pathways and have different behavior and prognosis (15). Additionally, many tumors historically designated as high-grade endometrioid show immunohistochemical similarities to high-grade serous and would be more accurately classified as such (16). Pathologists are now able to reproducibly assign these categories with judicious use of ancillary immunohistochemistry (17). The World Health Organization (WHO) classification guidelines for female reproductive tumors (18) were modified in 2014 to incorporate these findings, and they delineate seven EOC histotypes: high-grade serous, low-grade serous, endometrioid, mucinous, clear cell, carcinosarcoma (analogous to malignant mixed Mullerian/mesodermal tumors), and malignant Brenner tumors. Given that these refined definitions more accurately reflect EOC pathogenesis, in the present study, we applied the 2014 WHO guidelines (18) to nationally representative, population-based cancer registry data to evaluate survival patterns by histotype. Due to the markedly different outcomes by extent of disease (1), we present survival patterns separately by disease stage.
Methods
Study Population
Ovarian cancer cases were identified through the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, which collects data on every cancer case reported from 20 geographic areas of the United States (approximately 28% of the population) that represent the underlying demographics for the entire US population (19). Women meeting the following criteria were extracted from the SEER 18 registries in the November 2016 data submission (20): microscopically confirmed invasive ovarian cancer (International Classification of Diseases for Oncology, 3rd ed. [ICD-O-3] [21], primary site: C56.9 ovary), diagnosed between 2004 and 2014, age 20 to 84 years, and known race/ethnicity, surgical status, and stage (Supplementary Figure 1, available online). Stage is defined by the derived SEER Summary Stage 2000 variable (22), which uses the Collaborative Staging algorithm to combine clinical and pathologic information on the extent of disease to assign stage for diagnoses in 2004 and later. The SEER staging system corresponds to the commonly used International Federation of Gynecology and Obstetrics (FIGO) staging system in the following way: localized (FIGO I-A, I-B, I-not otherwise specified [NOS]), regional (FIGO I-C, II-A, II-B, II-C, II-NOS), distant (FIGO III-A, III-B, III-C, III-NOS, IV) (22). Cases identified only through an autopsy or death certificate were excluded due to the lack of follow-up time for survival analyses.
Histotype Classification
An expert gynecological pathologist (MK) reviewed the ICD-O-3 morphology codes for cases meeting the inclusion criteria and grouped them into the 2014 WHO EOC histotypes: serous, endometrioid, clear cell, mucinous, carcinosarcoma, and malignant Brenner tumors (Table 1). Nonepithelial (eg, germ cell, sex cord-stromal) and other miscellaneous epithelial tumors were excluded. As recommended by the 2014 WHO guidelines, we used a combination of grade and histology to further classify serous carcinomas into high-grade (grades 2–4) and low-grade (grade 1) serous, and we reclassified high-grade endometrioid tumors (grades 3–4, n = 1239) as high-grade serous carcinomas (18,23). All serous or endometrioid cases missing grade were excluded (n = 4854 and n = 500, respectively).
Table 1.
Histotype classification scheme based on the ICD-O-3 morphology/behavior codes, histology, and grade*
| Histology | ICD-O morphology/behavior codes | No. |
|---|---|---|
Serous
|
8020/3: Carcinoma, undifferentiated, NOS | 149 |
| 8021/3: Carcinoma, anaplastic, NOS | 33 | |
| 8022/3: Pleomorphic carcinoma | — | |
| 8050/3: Papillary carcinoma, NOS | 69 | |
| 8120/3: Transitional cell carcinoma, NOS | 121 | |
| 8130/3: Papillary transitional cell carcinoma | — | |
| 8260/3: Papillary adenocarcinoma, NOS | 164 | |
| 8441/3: Serous cystadenocarcinoma, NOS | 8160 | |
| 8442/3: Proliferating serous carcinoma, malignant | — | |
| 8450/3: Papillary cystadenocarcinoma, NOS | 49 | |
| 8460/3: Papillary serous cystadenocarcinoma | 7304 | |
| 8461/3: Serous surface papillary carcinoma | 1236 | |
| 8462/3: Papillary serous cystadenocarcinoma | — | |
| 8463/3: Serous surface papillary carcinoma | — | |
| 9014/3: Serous adenocarcinofibroma | 14 | |
Endometrioid
|
8380/3: Endometrioid carcinoma | 3924 |
| 8381/3: Endometrioid adenofibroma, malignant | 17 | |
| 8382/3: Endometrioid adenocarcinoma, secretory variant | 9 | |
| 8383/3: Endometrioid adenocarcinoma, ciliated cell variant | 10 | |
| 8482/3: Mucinous adenocarcinoma, endocervical type (24) | 13 | |
| 8570/3: Adenocarcinoma with squamous metaplasia | 48 | |
| Mucinous | 8470/3: Mucinous cystadenocarcinoma, NOS | 737 |
| 8471/3: Papillary mucinous cystadenocarcinoma | 69 | |
| 8472/3: Mucinous cystadenocarcinoma | — | |
| 8480/3: Mucinous adenocarcinoma | 1729 | |
| 8481/3: Mucin-producing adenocarcinoma | 92 | |
| 9015/3: Mucinous adenocarcinofibroma | 13 | |
| Clear cell | 8290/3: Oxyphilic adenocarcinoma | 5 |
| 8310/3: Clear cell adenocarcinoma, NOS | 2667 | |
| 8313/3: Clear cell adenocarcinofibroma | 20 | |
| 8443/3: Clear cell cystadenocarcinoma | — | |
| 8444/3: Clear cell cystic tumor, malignant | — | |
| Carcinosarcoma | 8575/3: Metaplastic carcinoma, NOS | — |
| 8950/3: Mullerian mixed tumor | 634 | |
| 8951/3: Mesodermal mixed tumor | 107 | |
| 8980/3: Carcinosarcoma, NOS | 635 | |
| 8981/3: Carcinosarcoma, embryonal | — | |
| Malignant Brenner | 9000/3: Brenner tumor, malignant | 74 |
| Carcinoma, NOS | 8010/3: Carcinoma, NOS | 1481 |
| 8046/3: Non-small cell carcinoma | 70 | |
| 8140/3: Adenocarcinoma, NOS | 4702 | |
| 8230/3: Solid carcinoma, NOS | 5 | |
| 8440/3: Cystadenocarcinoma, NOS | 113 | |
| Mixed | 8255/3: Adenocarcinoma with mixed subtypes | 198 |
| 8323/3: Mixed adenocarcinoma | 2008 |
— = number of cases not shown due to fewer than five cases during the time period; NOS = not otherwise specified.
Carcinoma, NOS, and mixed histotypes were historically used but are no longer represented in the 2014 WHO guidelines. As many epidemiologic studies of EOC were conducted prior to 2010 (25) with data on these historic histotypes, we include these histotypes for reference but not as a focus of the present study.
Statistical Analysis
Survival was defined as time from diagnosis until death or until time last followed. One-year, five-year, and 10-year age-standardized overall survival estimates were calculated by histotype and stage (localized, regional, and distant) using the actuarial method in SEER*Stat (26), and 95% confidence intervals (CIs) were calculated using the log(-log()) transformation. Age standardization was completed using the International Cancer Survival Standard weights for cancer sites with increasing incidence by age (27). Where age-adjusted survival estimates could not be calculated due to small numbers, crude survival estimates are provided.
We also calculated cause-specific survival, which represents the probability of surviving EOC in the absence of other causes of death. A cancer registry–derived algorithm processes death certificate data to assign a single cause of death, which is then used to define cause-specific death as any cancer death due to the primary site of origin, the general organ system of the primary site, other malignant tumors, or a death from AIDS with cancer (28). While all cases were included to calculate overall survival estimates, other causes of death were considered censored observations, and cases with a missing or unknown cause of death (n = 204) were excluded to calculate cause-specific survival estimates.
Using Stata, version 14.0 (Stata Corporation, College Station, TX), we estimated unadjusted Kaplan-Meier survival curves by histotype and stage and used Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% confidence intervals for the association between histotype and survival (with high-grade serous as the reference group), separately by stage. For these analyses, localized and regional were combined due to small numbers. Cox models were adjusted for age at diagnosis, region, race/ethnicity, and surgery. Localized/regional models were additionally adjusted for stage (localized, regional). Likelihood ratio tests were used to assess heterogeneity by histotype. We evaluated the proportional hazards assumption for these models overall and for each covariate using Schoenfeld residuals. Irrespective of stage, a violation of proportional hazards was observed for histotype (P < .05), primarily due to time-varying survival patterns for mucinous, clear cell, and carcinosarcoma. Therefore, we separated the Cox models into four time periods: zero to one, more than one to two, more than two to four, and more than four to 10 years after diagnosis. However, a violation of proportional hazards was still present for the zero to one– and more than one to two–year time periods for distant disease. Surgical status also violated proportional hazards for the zero to one–year time period and was included as a strata variable in the Cox models to allow for different baseline hazard functions by surgical status.
Results
The present analysis included 28 118 EOC cases (Table 2). The most common histotype was high-grade serous (63.4%), followed by endometrioid (9.9%), clear cell (9.6%), mucinous (9.4%), carcinosarcoma (4.9%), low-grade serous (2.5%), and malignant Brenner tumors, which are quite rare (0.3%). In comparison with other histotypes, low-grade serous, endometrioid, clear cell, and mucinous were diagnosed at younger ages, and a higher percentage of Asian/Pacific Islanders were diagnosed with clear cell. About half of low-grade serous were diagnosed at distant stage, while the majority of high-grade serous and carcinosarcoma were diagnosed at distant stage, and all other histotypes were more commonly diagnosed at localized/regional stage. A higher percentage of women diagnosed with carcinosarcoma and mucinous did not have surgery compared with other histotypes (10.9% and 7.8% vs 0.0%–3.0%, respectively). The majority of women who did not have surgery were diagnosed with distant-stage disease (91.5%; data not shown).
Table 2.
Characteristics of patients diagnosed with invasive epithelial ovarian cancer in 2004–2014 by histotype, SEER 18 registries
| Patient characteristics | High-grade serous | Low-grade serous | Endometrioid | Clear cell | Mucinous | Carcinosarcoma | Malignant Brenner |
|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Age at diagnosis, y | |||||||
| Mean ± SD | 61.2 ± 11.6 | 54.7 ± 14.5 | 54.2 ± 12.3 | 55.7 ± 10.9 | 53.5 ± 14.9 | 64.7 ± 11.5 | 61.3 ± 11.7 |
| 20–39 | 535 (3.0) | 106 (15.0) | 299 (10.7) | 167 (6.2) | 468 (17.7) | 28 (2.0) | — |
| 40–44 | 835 (4.7) | 70 (9.9) | 290 (10.4) | 231 (8.6) | 239 (9.1) | 38 (2.8) | — |
| 45–49 | 1612 (9.0) | 84 (11.9) | 434 (15.6) | 364 (13.5) | 283 (10.7) | 72 (5.2) | 6 (8.1) |
| 50–54 | 2332 (13.1) | 85 (12.0) | 476 (17.1) | 489 (18.1) | 393 (14.9) | 133 (9.6) | 13 (17.6) |
| 55–59 | 2613 (14.7) | 88 (12.4) | 411 (14.8) | 502 (18.6) | 373 (14.1) | 164 (11.9) | 6 (8.1) |
| 60–64 | 2802 (15.7) | 82 (11.6) | 279 (10.0) | 409 (15.2) | 274 (10.4) | 205 (14.8) | 11 (14.9) |
| 65–69 | 2466 (13.8) | 77 (10.9) | 243 (8.7) | 230 (8.5) | 195 (7.4) | 216 (15.6) | 14 (18.9) |
| 70–74 | 2066 (11.6) | 45 (6.4) | 161 (5.8) | 145 (5.4) | 158 (6.0) | 206 (14.9) | 8 (10.8) |
| 75–79 | 1593 (8.9) | 46 (6.5) | 116 (4.2) | 100 (3.7) | 138 (5.2) | 183 (13.3) | 8 (10.8) |
| 80–84 | 983 (5.5) | 25 (3.5) | 73 (2.6) | 58 (2.2) | 120 (4.5) | 136 (9.8) | — |
| Region of residence* | |||||||
| Northeast | 4493 (25.2) | 191 (27.0) | 708 (25.4) | 731 (27.1) | 684 (25.9) | 411 (29.8) | 14 (18.9) |
| Northwest | 1178 (6.6) | 11 (1.6) | 139 (5.0) | 202 (7.5) | 110 (4.2) | 69 (5.0) | — |
| Southeast | 3455 (19.4) | 181 (25.6) | 505 (18.2) | 356 (13.2) | 558 (21.1) | 252 (18.2) | 15 (20.3) |
| Southwest | 8711 (49.8) | 325 (45.9) | 1430 (51.4) | 1406 (52.2) | 1289 (48.8) | 649 (47.0) | 41 (55.4) |
| Race/ethnicity | |||||||
| Non-Hispanic white | 13 286 (74.5) | 535 (75.6) | 1950 (70.1) | 1795 (66.6) | 1733 (65.6) | 1013 (73.4) | 48 (64.9) |
| Non-Hispanic black | 1160 (6.5) | 49 (6.9) | 130 (4.7) | 110 (4.1) | 223 (8.4) | 117 (8.5) | 8 (10.8) |
| Non-Hispanic Asian/ Pacific Islander | 1254 (7.0) | 24 (3.4) | 308 (11.1) | 488 (18.1) | 283 (10.7) | 85 (6.2) | 6 (8.1) |
| Non-Hispanic American Indian/Alaskan Native | 123 (0.7) | 5 (0.7) | 20 (0.7) | 13 (0.5) | 19 (0.7) | 13 (0.9) | 0 (0.0) |
| Hispanic—all races | 2014 (11.3) | 95 (13.4) | 374 (13.4) | 289 (10.7) | 383 (14.5) | 153 (11.1) | 12 (16.2) |
| Disease stage | |||||||
| Localized | 882 (5.0) | 144 (20.3) | 1275 (45.8) | 929 (34.5) | 1274 (48.2) | 75 (5.4) | 42 (56.8) |
| Regional | 3057 (17.1) | 186 (26.3) | 1177 (42.3) | 1021 (37.9) | 661 (25.0) | 267 (19.3) | 19 (25.7) |
| Distant | 13 898 (78.9) | 378 (53.4) | 330 (11.9) | 745 (27.6) | 706 (26.7) | 1039 (75.2) | 13 (17.6) |
| Surgery of primary site | |||||||
| No | 535 (3.0) | 16 (2.3) | 7 (0.3) | 71 (2.6) | 205 (7.8) | 150 (10.9) | 0 (0.0) |
| Yes | 17 302 (97.0) | 692 (97.7) | 2775 (99.7) | 2624 (97.4) | 2436 (92.2) | 1231 (89.1) | 74 (100.0) |
| Total | 17 837 (63.4) | 708 (2.5) | 2782 (9.9) | 2695 (9.6) | 2641 (9.4) | 1381 (4.9) | 74 (0.3) |
Northeast includes Connecticut, Iowa, Michigan, New Jersey. Northwest includes Alaska and Washington. Southeast includes Georgia, Kentucky, and Louisiana. Southwest includes California, Hawaii, New Mexico, and Utah. — = statistic not shown due to fewer than five cases during the time period; NOS = not otherwise specified; SEER = Surveillance, Epidemiology, and End Results.
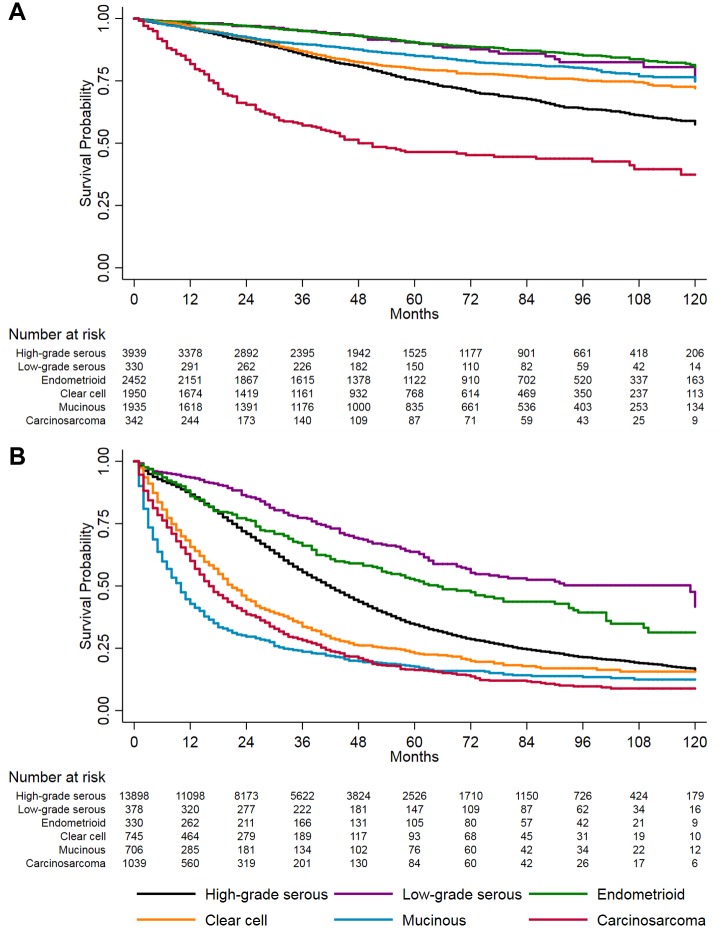
Irrespective of stage, the best survival outcomes were observed for endometrioid and low-grade serous (Figure 1). Among women with localized/regional disease, carcinosarcoma cases experienced the worst survival, especially within two years after diagnosis. For distant disease, survival for clear cell, mucinous, and carcinosarcoma was poor and fairly similar, with a markedly high rate of mortality within the first two years after diagnosis.
Figure 1.
Kaplan-Meier survival curves of invasive epithelial ovarian cancer survival by stage and histotype, 2004–2014, SEER 18 registries. A) Localized and regional-stage disease. B) Distant-stage disease. Malignant Brenner tumors were excluded due to small sample size.
The one-year, five-year, and 10-year age-standardized overall survival estimates by histotype and stage are provided in Table 3. For localized disease, one-year and five-year survival estimates were greater than 80% for all histotypes, except carcinosarcoma, which had worse outcomes at five years after diagnosis (70.7%, 95% CI = 56.3% to 81.1%). At 10 years, malignant Brenner tumors had the worst survival (59.1%, 95% CI = 31.9% to 78.5%), and survival for mucinous, carcinosarcoma, and high-grade serous declined to less than 70%. For regional disease, one-year survival estimates were approximately 80% or greater for all histotypes. At five years after diagnosis, all histotypes except low-grade serous and endometrioid had survival estimates lower than 80%, with the worst outcomes for carcinosarcoma (38.6%, 95% CI = 32.0% to 45.1%). Carcinosarcoma still had the worst survival at 10 years (29.0%, 95% CI = 22.4% to 35.9%). Across each time interval and irrespective of disease stage, low-grade serous had the best outcomes. For distant disease, survival estimates were less than 40% within the first year after diagnosis for mucinous (37.9%, 95% CI = 33.9% to 41.8%) and about 60% for clear cell and carcinosarcoma (63.3%, 95% CI = 58.5% to 67.6%, and 60.0%, 95% CI = 56.8% to 63.0%, respectively). All other histotypes had survival estimates greater than 80% during this time period. By five years after diagnosis, most histotypes had a survival of less than 35%, with clear cell, carcinosarcoma, and mucinous at or less than 22%. The 10-year survival estimates for clear cell, mucinous, and carcinosarcoma (which are not age-adjusted due to small numbers) were generally similar to the five-year age-adjusted survival estimates. For all other histotypes, survival continued to decline five to 10 years after diagnosis. Low-grade serous had the best survival of all distant-stage histotypes, but still, only 37.3% (95% CI = 29.0% to 45.7%) survived 10 years after diagnosis.
Table 3.
One-year, five-year, and 10-year age-standardized overall survival estimates for invasive epithelial ovarian cancer by histotype and stage, 2004–2014, SEER 18 registries
| Overall survival (95% CI) |
|||||
|---|---|---|---|---|---|
| Stage | Histotype | No. | 1-y | 5-y | 10-y |
| Localized | High-grade serous | 882 | 96.8 (94.7 to 98.0) | 84.0 (80.4 to 87.0) | 67.5 (61.4 to 72.8) |
| Low-grade serous | 144 | 98.0 (92.0 to 99.5)* | 93.2 (86.5 to 97.3) | 90.4 (80.2 to 95.5) | |
| Endometrioid | 1275 | 96.9 (94.9 to 98.1) | 87.1 (83.6 to 90.1) | 72.5 (65.9 to 78.0) | |
| Clear cell | 929 | 96.6 (93.1 to 98.3) | 81.7 (76.1 to 86.0) | 71.3 (62.9 to 78.1) | |
| Mucinous | 1274 | 95.1 (92.5 to 96.8) | 82.9 (78.6 to 86.3) | 69.2 (61.6 to 75.6) | |
| Carcinosarcoma | 75 | 92.3 (82.4 to 96.7)† | 70.7 (56.3 to 81.1)† | 67.6 (52.5 to 78.9)†,‡ | |
| Malignant Brenner | 42 | 95.9 (76.8 to 99.3)* | 87.9 (70.3 to 95.4) | 59.1 (31.9 to 78.5)‡ | |
| Regional | High-grade serous | 3057 | 93.7 (92.5 to 94.7) | 67.7 (65.4 to 69.8) | 48.8 (45.5 to 52.0) |
| Low-grade serous | 186 | 98.6 (96.1 to 99.5) | 82.7 (72.4 to 89.4) | 71.2 (57.6 to 81.2) | |
| Endometrioid | 1177 | 97.5 (95.1 to 98.7) | 83.9 (79.3 to 87.5) | 68.4 (60.3 to 75.2) | |
| Clear cell | 1021 | 92.6 (89.4 to 94.9) | 69.0 (63.1 to 74.1) | 55.7 (46.9 to 63.7) | |
| Mucinous | 661 | 89.7 (85.7 to 92.7) | 69.5 (63.5 to 74.7) | 66.2 (60.3 to 71.5) | |
| Carcinosarcoma | 267 | 78.2 (72.2 to 83.0) | 38.6 (32.0 to 45.1) | 29.0 (22.4 to 35.9) | |
| Malignant Brenner | 19 | 100.0 (N/A) | 55.8 (30.4 to 75.2) | 49.3 (18.4 to 74.4) | |
| Distant | High-grade serous | 13 898 | 84.3 (83.6 to 85.0) | 32.1 (31.1 to 33.0) | 15.0 (13.9 to 16.1) |
| Low-grade serous | 378 | 90.3 (85.7 to 93.5) | 54.2 (47.3 to 60.6) | 37.3 (29.0 to 45.7) | |
| Endometrioid | 330 | 82.9 (76.8 to 87.5) | 44.7 (37.3 to 51.9) | 25.9 (18.1 to 34.4) | |
| Clear cell | 745 | 63.3 (58.5 to 67.6) | 22.3 (18.2 to 26.8) | 15.5 (12.0 to 19.4)‡ | |
| Mucinous | 706 | 37.9 (33.9 to 41.8) | 13.9 (11.2 to 16.8) | 12.4 (9.5 to 15.6)‡ | |
| Carcinosarcoma | 1039 | 60.0 (56.8 to 63.0) | 15.9 (13.2 to 18.7) | 8.7 (6.3 to 11.6)‡ | |
| Malignant Brenner | 13 | 100.0 (N/A)‡ | 28.9 (5.8 to 58.2)‡ | § | |
The width of the confidence interval is more than 25% larger than if the normal approximation was applied. Survival estimates were calculated using the actuarial method, and 95% confidence intervals were calculated using the log(-log()) transformation. Age-standardized to the International Cancer Survival Standard 1—age ≥15 years. CI = confidence interval; SEER = Surveillance, Epidemiology, and End Results.
Even though the one-year and five-year age-standardized overall survival estimates for localized stage carcinosarcoma could be calculated, we present the crude one-year and five-year overall survival estimates in the table for consistency with the 10-year overall crude survival estimates. The one-year age-standardized overall survival estimate is 92.3 (95% CI = 81.3 to 97.0), and the five-year age-standardized overall survival estimate is 65.6 (95% CI = 51.1 to 76.8).
Numbers were too small to calculate age-standardized rates, so crude overall survival estimates are presented.
The statistic could not be calculated.
Cause-specific survival estimates were similar to overall survival estimates, although slightly greater in magnitude (Supplementary Table 1, available online). The difference between these two estimates was more pronounced for localized and regional disease, as the duration of survival time increased. The largest differences between the cause-specific and overall survival estimates were observed for localized and regional mucinous and endometrioid.
Similar survival patterns by histotype and stage persisted after adjustment for demographic and clinical characteristics (Table 4). For localized/regional disease, endometrioid and low-grade serous had a lower risk of mortality compared with high-grade serous across the entire survival period. In comparison with high-grade serous, women with early-stage carcinosarcoma had the highest risk of mortality in the first four years after diagnosis, with the most pronounced hazard ratio in the more than one to two–year time period (HR = 3.81, 95% CI = 2.74 to 5.30). In the more than four to 10–year time interval, hazard ratios for all histotypes relative to high-grade serous were less than 1.00; however, the cumulative mortality for carcinosarcoma remained notably lower than for high-grade serous during these years (Figure 1A).
Table 4.
Estimated hazard ratios and 95% confidence intervals for invasive epithelial ovarian cancer by histotype* and stage, 2004–2014, SEER 18 registries
| Stage and histotype | Survival time intervals† |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0–1 y‡ |
>1–2 y |
>2–4 y |
>4–10 y |
|||||
| Cases (deaths) | HR (95% CI) | Cases (deaths) | HR (95% CI) | Cases (deaths) | HR (95% CI) | Cases (deaths) | HR (95% CI) | |
| Localized/regional | ||||||||
| High-grade serous | 3939 (161) | 1.00 (ref) | 3330 (159) | 1.00 (ref) | 2857 (284) | 1.00 (ref) | 1914 (333) | 1.00 (ref) |
| Low-grade serous | 330 (6) | 0.55 (0.24 to 1.25) | 289 (—) | 0.25 (0.08 to 0.78) | 261 (9) | 0.42 (0.22 to 0.81) | 181 (16) | 0.64 (0.38 to 1.06) |
| Endometrioid | 2452 (39) | 0.60 (0.42 to 0.86) | 2121 (28) | 0.38 (0.25 to 0.58) | 1847 (68) | 0.52 (0.40 to 0.68) | 1357 (97) | 0.53 (0.42 to 0.67) |
| Mucinous | 1935 (73) | 1.54 (1.15 to 2.06) | 1604 (57) | 1.11 (0.81 to 1.53) | 1376 (64) | 0.76 (0.57 to 1.01) | 989 (81) | 0.64 (0.50 to 0.83) |
| Clear cell | 1950 (60) | 1.13 (0.84 to 1.54) | 1652 (77) | 1.38 (1.04 to 1.83) | 1395 (128) | 1.36 (1.10 to 1.69) | 922 (69) | 0.58 (0.44 to 0.75) |
| Carcinosarcoma | 342 (58) | 3.38 (2.49 to 4.58) | 239 (46) | 3.81 (2.74 to 5.30) | 171 (37) | 2.24 (1.59 to 3.15) | 105 (15) | 0.73 (0.44 to 1.23) |
| Distant | ||||||||
| High-grade serous | 13 898 (1782) | 1.00 (ref) | 10 877 (1888) | 1.00 (ref) | 7972 (2733) | 1.00 (ref) | 3702 (1560) | 1.00 (ref) |
| Low-grade serous | 378 (24) | 0.49 (0.33 to 0.74) | 318 (25) | 0.45 (0.30 to 0.67) | 271 (49) | 0.51 (0.38 to 0.67) | 179 (42) | 0.47 (0.35 to 0.64) |
| Endometrioid | 330 (44) | 1.16 (0.86 to 1.56) | 254 (27) | 0.66 (0.45 to 0.96) | 208 (44) | 0.59 (0.44 to 0.80) | 129 (40) | 0.62 (0.45 to 0.84) |
| Mucinous | 706 (390) | 3.87 (3.45 to 4.34) | 272 (82) | 1.80 (1.47 to 2.29) | 177 (55) | 0.90 (0.69 to 1.17) | 99 (27) | 0.53 (0.36 to 0.78) |
| Clear cell | 745 (245) | 2.82 (2.47 to 3.23) | 442 (136) | 2.03 (1.71 to 2.42) | 264 (99) | 1.17 (0.96 to 1.43) | 114 (34) | 0.59 (0.42 to 0.84) |
| Carcinosarcoma | 1039 (393) | 2.62 (2.35 to 2.93) | 531 (181) | 2.00 (1.72 to 2.33) | 307 (126) | 1.16 (0.97 to 1.38) | 121 (54) | 0.96 (0.73 to 1.26) |
Malignant Brenner tumors were excluded due to small sample size. All models adjusted for age at diagnosis (five-year age categories), region of residence (Northeast: Connecticut, Iowa, Michigan, New Jersey; Northwest: Alaska and Washington; Southeast: Georgia, Kentucky, and Louisiana; Southwest: California, Hawaii, New Mexico, and Utah), race/ethnicity (non-Hispanic white, non-Hispanic black, non-Hispanic Asian/Pacific Islander, non-Hispanic American Indian/Alaskan Native, Hispanic—all races), and surgery of primary site (yes, no). Models for localized/regional stage disease additionally adjusted for stage. — = number of cases not shown due to fewer than five cases during the time period; CI = confidence interval; NOS = not otherwise specified; HR = hazard ratio; SEER: Surveillance, Epidemiology, and End Results.
For each analysis by stage and time period, all P values for heterogeneity by histotype were <.0001.
To meet the proportional hazards assumption, baseline hazards were allowed to vary by surgical status.
Among women with distant disease, a higher risk of mortality was observed within the first two years after diagnosis for mucinous, clear cell, and carcinosarcoma compared with high-grade serous, with the most striking hazard ratio observed in the first year after diagnosis for mucinous (HR = 3.87, 95% CI = 3.45 to 4.34) (Table 4). Within certain time intervals after diagnosis, hazard ratios for some histotypes indicated a reduced risk of mortality relative to high-grade serous (eg, >4–10 years for mucinous and clear cell); nevertheless, cumulative survival remained lower among those histotypes than for high-grade serous (Figure 1B). Throughout the entire survival period, low-grade serous had better survival than high-grade serous.
Supplementary Figure 2 and Supplementary Tables 2 and 3 (available online) provide the Kaplan-Meier survival curves, survival estimates, and hazard ratios for carcinoma, NOS, and mixed histotypes by stage, respectively. Survival patterns for carcinoma and NOS were poor irrespective of stage and closely resemble carcinosarcoma. For mixed histotypes, survival patterns were similar to endometrioid for localized/regional stage but high-grade serous for distant stage.
Sensitivity Analyses
Due to the likely common pathogenesis of ovarian, fallopian tube, and primary peritoneal cancers (29) and the recent understanding that most high-grade serous likely originate in the fallopian tube (30), we estimated overall and cause-specific survival including these additional primary sites (ICD-O-3 primary site: C57.0 fallopian tube, C48.1 peritoneum) (Supplementary Table 4, available online). Generally, survival estimates were similar, with the greatest magnitude of change for high-grade serous, where 2136 cases were added to the analysis, and survival estimates were slightly lower than those for primary ovary only.
To evaluate whether excluding serous and endometrioid carcinomas with unknown grade impacted our results, we repeated the analyses assuming serous cases missing grade (n = 4854) were high-grade serous, while endometrioid cases missing grade were high-grade serous if distant stage (n = 123) or endometrioid if localized/regional stage (n = 377). The survival patterns were practically identical to our main analyses, with only a slightly poorer survival observed for high-grade serous (Supplementary Figure 3, available online).
Discussion
The present analysis of SEER data comprehensively assessed EOC histotype-specific survival using the current WHO classification and provided data on the largest sample of carcinosarcoma and malignant Brenner tumors to date. We observed the most favorable outcomes for low-grade serous and endometrioid irrespective of stage and strikingly high mortality rates for carcinosarcoma and distant-stage mucinous and clear cell, especially within two years after diagnosis. However, in multivariable analyses, both localized/regional and distant-stage high-grade serous had higher mortality four or more years after diagnosis in comparison with the other histotypes.
Previously published data on histotype-specific survival patterns have been somewhat inconsistent and suffer from several limitations. The majority of studies (6–9,12,31–36) were published prior to the 2014 WHO guidelines and do not reflect the current knowledge of EOC pathogenesis. Even a study published in 2017 (13) examining racial and histologic differences in EOC survival did not delineate between high- and low-grade serous. Furthermore, histotype-specific survival estimates were not always presented by disease stage, and as a result, it was reported that women with serous carcinoma had worse outcomes than mucinous and clear cell (6,7). Our study, along with several others (9,31–34,36–38), observed markedly higher mortality among distant-stage mucinous and clear cell; however, the prior studies focused on specific subgroups of women with EOC (eg, stage IV disease, clear cell only) and do not offer a comprehensive assessment of histotype by stage. Another limitation of previous reports is the use of relative survival estimates (7,8,13), which compare the observed survival of cancer patients with the expected survival of a group of cancer-free individuals, typically the general population. Utilizing the general population is biased because the general population includes women who have had an oophorectomy and are no longer at risk for ovarian cancer.
Histotype-specific survival patterns are likely a result of differences in tumor biology and treatment effectiveness by histotype. The recommended primary treatment for EOC is currently cytoreductive surgery and a combination of platinum and taxane-based chemotherapy (39). Late-stage mucinous and clear cell often progress with platinum-based chemotherapy (40–42). This is reflected in the survival curves for these histotypes, where the mortality rate is particularly high in the two years following diagnosis but eventually levels off and remains constant. The dismal survival pattern for carcinosarcoma is likely due to the aggressive clinical behavior of these neoplasms and the lack of effective therapy (43). In comparison, an initial responsiveness to firstline chemotherapy for high-grade serous (41) and the more indolent nature of low-grade serous and endometrioid is reflected in the gradual decline in survival a few years after diagnosis.
Although the majority of women diagnosed with EOC die of their disease, varying proportions survive more than 10 years after diagnosis, depending on histotype and stage. Recent studies (44–47) have reported that several factors are suggestively associated with long-term survival, including a younger age at diagnosis, nonserous histotypes, early-stage disease, no gross residual disease after cytoreductive surgery, absence of ascites, and lower CA-125 levels. While our results show that women with some of the nonserous histotypes are more likely to survive long-term than those with the high-grade serous subtype, distant-stage mucinous, clear cell, and carcinosarcoma have similar or worse 10-year survival estimates than distant-stage high-grade serous. Thus, future investigations of characteristics associated with long-term survival should be evaluated separately by histotype and stage.
We observed a higher prevalence of early-stage low-grade serous compared with other reports (15,48), which may be due to a portion of these that would now be considered serous borderline tumors. Furthermore, this histotype was only recently introduced into the WHO guidelines, and we inferred it based on reported grade. Thus, in our study, some low-grade serous were likely missed as historically they may have been assigned to grade 2. Nevertheless, the survival estimates are within the expected range. Likewise, the proportion of mucinous carcinoma is relatively high, and influx of misclassified metastatic gastrointestinal carcinomas is likely. However, Zaino, et al. (49) showed no difference in survival between distant primary or metastatic mucinous carcinoma.
We recognize that our study has some limitations. The proportional hazards assumption was not met for distant disease during the zero to one– and more than one to two–year time periods, which may result in biased estimates. However, the observed survival patterns were consistent across all analytic approaches. Treatment data in SEER during the time period studied are limited, with information only available for receipt of surgery, and no data were available for chemotherapy or the extent of residual disease after cytoreductive surgery. Moreover, SEER does not have data on additional prognostic factors (eg, smoking status, obesity), precluding our ability to evaluate confounding. We were unable to assess the impact of histotype on progression-free survival, which is important for EOC considering that tumors will recur in approximately 70% of women with advanced disease (50). The accuracy of cause of death on death certificates may be questionable (51); however, a notably high accuracy for EOC has been reported (52,53), suggesting that the degree of bias in the present study is likely minimal. Also, histotype classification may not completely align with current practice. Gilks et al. (54) identified that upon re-review, some high-grade endometrioid were reclassified as high-grade serous. We reduced the impact of this known problem by classifying high-grade endometrioid as high-grade serous. We acknowledge that a small group of true high-grade endometrioid do exist, but future studies with modern classification are required to assess their behavior. The large numbers, particularly for high-grade serous, also allow for robust results unlikely to be confounded by misclassification. With the adoption of the 2014 WHO guidelines in clinical practice and improved diagnostic reproducibility of histotype diagnosis among pathologists (16,55), our data provide reference survival estimates to monitor treatment success by histotype and stage.
In summary, our study was able to overcome considerable limitations of previous work to provide survival patterns by histotype that reflect our current knowledge of EOC pathogenesis. We observed substantial histotype-specific survival differences, with a markedly high mortality rate among carcinosarcoma and distant mucinous and clear cell a few years after diagnosis. These findings underscore the need to develop therapeutics targeting the unique molecular features of each histotype. Recognizing that EOC represents several distinct disease processes remains an important goal to reducing the mortality of this deadly disease.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health (K99 CA218681 to LCP, R01 CA168758 to MAR and JAD, R01 CA200854 to JAD and JMS, R01 CA207260 to JMS, K22 CA193860 to HRH), Calgary Laboratory Services internal research support (RS11-508 to MK; P30 CA042014), and the Huntsman Cancer Foundation (JAD).
Notes
Affiliations of authors: Department of Public Health Sciences, University of Virginia, Charlottesville, VA (LCP, JMS); Program in Epidemiology, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA (KLCH, HRH, MAR, JAD); Department of Pathology and Laboratory Medicine, University of Calgary, Calgary Laboratory Services, Calgary, Alberta, Canada (MK); Department of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC (AB); Huntsman Cancer Institute and Department of Population Health Sciences, University of Utah, Salt Lake City, UT (JAD).
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin. 2018;681:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Coleman RL, Monk BJ, Sood AK, Herzog TJ.. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;104:211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engel J, Eckel R, Schubert-Fritschle G, et al. Moderate progress for ovarian cancer in the last 20 years: Prolongation of survival, but no improvement in the cure rate. Eur J Cancer. 2002;3818:2435–2445. [DOI] [PubMed] [Google Scholar]
- 4. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ.. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J Clin Oncol. 2002;205:1248–1259. [DOI] [PubMed] [Google Scholar]
- 5. Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;1141:26–31. [DOI] [PubMed] [Google Scholar]
- 6. Brun JL, Feyler A, Chêne G, Saurel J, Brun G, Hocké C.. Long-term results and prognostic factors in patients with epithelial ovarian cancer. Gynecol Oncol. 2000;78:21–27. [DOI] [PubMed] [Google Scholar]
- 7. Barnholtz-Sloan JS, Schwartz AG, Qureshi F, Jacques S, Malone J, Munkarah AR.. Ovarian cancer: Changes in patterns at diagnosis and relative survival over the last three decades. Am J Obstet Gynecol. 2003;1894:1120–1127. [DOI] [PubMed] [Google Scholar]
- 8. Kosary CL. Figo stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: An analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;101:31–46. [DOI] [PubMed] [Google Scholar]
- 9. Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS.. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–376. [DOI] [PubMed] [Google Scholar]
- 10. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;1099:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuh KC, Shin JY, Kapp DS, et al. Survival differences of Asian and Caucasian epithelial ovarian cancer patients in the United States. Gynecol Oncol. 2015;1363:491–497. [DOI] [PubMed] [Google Scholar]
- 12. McGuire V, Jesser C, Whittemore AS.. Survival among US women with invasive epithelial ovarian cancer. Gynecol Oncol. 2002;843:399–403. [DOI] [PubMed] [Google Scholar]
- 13. Park HK, Ruterbusch JJ, Cote ML.. Recent trends in ovarian cancer incidence and relative survival in the United States by race/ethnicity and histologic subtypes. Cancer Epidemiol Biomarkers Prev. 2017;2610:1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soslow RA. Histologic subtypes of ovarian carcinoma. Int J Gynecol Pathol. 2008;27:161–174. [DOI] [PubMed] [Google Scholar]
- 15. Vang R, Shih I-M, Kurman RJ.. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;165:267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobel M, Kalloger S, Baker P, et al. Diagnosis of ovarian carcinoma cell type is highly reproducible: A transcanadian study. Am J Surg Pathol. 2010;347:984–993. [DOI] [PubMed] [Google Scholar]
- 17. Köbel M, Rahimi K, Rambau PF, et al. An immunohistochemical algorithm for ovarian carcinoma typing. Int J Gynecol Pathol. 2016;355:430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurman RJ, Carcangiu ML, Herrington CS, Young RH.. WHO Classification of Tumours of Female Reproductive Organs 4th ed Lyon: IARC; 2014. [Google Scholar]
- 19. National Cancer Institute. Overview of the SEER program. https://seer.cancer.gov/about/overview.html. Accessed August 17, 2017.
- 20.Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Research Data, Nov 2016 Sub (2000-2014) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total US, 1969-2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program. Released April 2017, based on November 2016 submission.
- 21. Fritz A, Percy C, Jack A, et al. , eds. International Classification of Diseases for Oncology. 3rd ed Geneva: World Health Organization; 2000. [Google Scholar]
- 22. Young JLJ, Roffers SD, Ries LAG, Fritz AG, Hurlburt AA, eds. SEER Summary Staging Manual - 2000: Codes and Coding Instructions NIH Pub. No. 01-4969. Bethesda, MD: National Institute of Health; 2001. https://seer.cancer.gov/tools/ssm/breast_femgen.pdf. Accessed August 17, 2017. [Google Scholar]
- 23. Clarke BA, Gilks B.. Ovarian carcinoma : Recent developments in classification of tumour histological subtype. Can J Pathol. 2011:33–42.21432852 [Google Scholar]
- 24. Rambau PF, McIntyre JB, Taylor J, et al. Morphologic reproducibility, genotyping, and immunohistochemical profiling do not support a category of seromucinous carcinoma of the ovary. Am J Surg Pathol. 2017;415:685–695. [DOI] [PubMed] [Google Scholar]
- 25. Cannioto RA, Trabert B, Poole EM, Schildkraut JM.. Ovarian cancer epidemiology in the era of collaborative team science. Cancer Causes Control. 2017;285:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surveillance Research Program, National Cancer Institute SEER*Stat software, version 8.3.4. seer.cancer.gov/seerstat.
- 27. Corazziari I, Quinn M, Capocaccia R.. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;4015:2307–2316. [DOI] [PubMed] [Google Scholar]
- 28. Howlader N, Ries LAG, Mariotto AB, Reichman ME, Ruhl J, Cronin KA.. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;10220:1584–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goodman MT, Shvetsov YB.. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiol Biomarkers Prev. 2009;181:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 2013;24(suppl 10):x16–x21. [DOI] [PubMed] [Google Scholar]
- 31. Makar AP, Baekelandt M, Tropé CG, Kristensen GB.. The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol. 1995;562:175–180. [DOI] [PubMed] [Google Scholar]
- 32. Winter WE, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2007;2524:3621–3627. [DOI] [PubMed] [Google Scholar]
- 33. MacKay HJ, Brady MF, Oza AM, et al. Prognostic relevance of uncommon ovarian histology in women with stage III/IV epithelial ovarian cancer. Int J Gynecol Cancer. 2010;206:945–952. [DOI] [PubMed] [Google Scholar]
- 34. Akahira J, Yoshikawa H, Shimizu Y, et al. Prognostic factors of stage IV epithelial ovarian cancer: A multicenter retrospective study. Gynecol Oncol. 2001;81:398–403. [DOI] [PubMed] [Google Scholar]
- 35. Chan J, Fuh K, Shin J, et al. The treatment and outcomes of early-stage epithelial ovarian cancer: Have we made any progress? Br J Cancer. 2008;987:1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schiavone MB, Herzog TJ, Lewin SN, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol. 2011;2055:480.e1–480.e8. [DOI] [PubMed] [Google Scholar]
- 37. Goff BA, Sainz de la Cuesta R, Muntz HG, et al. Clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol Oncol. 1996;60:412–417. [DOI] [PubMed] [Google Scholar]
- 38. Mizuno M, Kikkawa F, Shibata K, et al. Long-term follow-up and prognostic factor analysis in clear cell adenocarcinoma of the ovary. J Surg Oncol. 2006;94:138–143. [DOI] [PubMed] [Google Scholar]
- 39. Motzer RJ, Jonasch E, Agarwal N, et al. Ovarian cancer, version 2. 2014 featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2014;122:175–181. [Google Scholar]
- 40. Hess V, A’Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: A separate entity requiring specific treatment. J Clin Oncol. 2004;226:1040–1044. [DOI] [PubMed] [Google Scholar]
- 41. Jelovac D, Armstrong DK.. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;613:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pectasides D, Pectasides E, Psyrri A, Economopoulos T.. Treatment issues in clear cell carcinoma of the ovary: A different entity? Oncologist. 2006;11:1089–1094. [DOI] [PubMed] [Google Scholar]
- 43. Del Carmen MG, Birrer M, Schorge JO.. Carcinosarcoma of the ovary: A review of the literature. Gynecol Oncol. 2012;1251:271–277. [DOI] [PubMed] [Google Scholar]
- 44. Hamilton CA, Miller A, Casablanca Y, et al. Clinicopathologic characteristics associated with long-term survival in advanced epithelial ovarian cancer: An NRG Oncology/Gynecologic Oncology Group ancillary data study. Gynecol Oncol. 2018;1482:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoppenot C, Eckert MA, Tienda SM, Lengyel E.. Who are the long-term survivors of high grade serous ovarian cancer? Gynecol Oncol. 2018;1481:204–212. [DOI] [PubMed] [Google Scholar]
- 46. Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS.. Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol. 2015;1263:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dao F, Schlappe BA, Tseng J, et al. Characteristics of 10-year survivors of high-grade serous ovarian carcinoma. Gynecol Oncol. 2016;1412:260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McCluggage WG. Morphological subtypes of ovarian carcinoma: A review with emphasis on new developments and pathogenesis. Pathology. 2011;435:420–432. [DOI] [PubMed] [Google Scholar]
- 49. Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA.. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: A Gynecologic Oncology Group study. Cancer. 2011;1173:554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herzog TJ, Pothuri B.. Ovarian cancer: A focus on management of recurrent disease. Nat Clin Pr Oncol. 2006;311:604–611. [DOI] [PubMed] [Google Scholar]
- 51. Smith Sehdev AE, Hutchins GM.. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med. 2001;1612:277–284. [DOI] [PubMed] [Google Scholar]
- 52. Percy C, Stanek E, Gloeckler L.. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;713:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. German RR, Fink AK, Heron M, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;352:126–131. [DOI] [PubMed] [Google Scholar]
- 54. Gilks CB, Ionescu DN, Kalloger SE, et al. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008;398:1239–1251. [DOI] [PubMed] [Google Scholar]
- 55. Kommoss S, Gilks CB, du Bois A, Kommoss F.. Ovarian carcinoma diagnosis: The clinical impact of 15 years of change. Br J Cancer. 2016;1158:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.