Abstract
Objective:
Using primary lymphatic muscle cells (LMCs) in vitro we sought to characterize the impact of LMC remodeling on their functional and molecular response to mechanical loading and culture conditions.
Methods:
Primary “wounded leg” LMCs were derived from the hindlimb of three sheep who underwent lymphatic injury six weeks prior, while “control leg” LMCs were derived from the contralateral, unwounded, limb. Function of the LMCs were characterized in response to media of variable levels of serum (10% vs 0.2%) and glucose (4.5g/L vs 1g/L). Functional and proteomic data was evaluated in LMCs exposed to cyclic stretch (0.1Hz, 7.5% elongation) for 1 week.
Results:
LMCs were sensitive to changes in serum levels, significantly reducing overall activity and collagen synthesis under low serum conditions. LMCs from the remodeled vessel had higher baseline levels of metabolic activity but not collagen synthesis. Cyclic loading induced cellular alignment perpendicular to the axis of stretch and alterations in signaling pathways associated with metabolism. Remodeled LMCs had consistently higher levels of metabolic activity and were more resistant to strain induced apoptosis.
Conclusions:
LMCs exist on a functional spectrum, becoming more active in response to stretching and maintaining phenotypic remodeling in response to local lymphatic/tissue damage.
Keywords: Lymphatics, Vascular Remodeling, Lymphatic Muscle Cells, Mechanical Loading
Introduction
The lymphatic system is responsible for maintaining fluid homeostasis in all the soft tissue of the body. Through its role of fluid transport, it also plays a critical role in transport of cells, macromolecules, and information through the form of exosomes and tissue soluble antigens1–8. Through this transport the lymphatics not only return fluid to blood circulation but actively participate in the priming of immune response and transport of macromolecules.
The lymphatic vasculature transports approximately 8L of fluid back to the blood vasculature every day through direct flow into the subclavian veins and through fluid exchange at the lymph nodes9,10. There is no sustained pressure gradient that would allow for passive drainage of fluid from the interstitial tissue space back into blood circulation. Experimental measurements of pressures in-vivo show that lymphatic transport regularly overcomes adverse passive pressure gradients of up to 30 cmH2O11,12. To accomplish this, the lymphatic system relies on both extrinsic and intrinsic factors to maintain fluid homeostasis1,13,14. Extrinsic factors, such as the pulsation of blood vasculature and the contractions of surrounding muscle tissue constricts lymphatic vessel walls propelling lymph downstream. One-way valves ensure the unidirectional flow of lymph, physically preventing backflow. However, the primary drive of lymphatic flow is the intrinsic pumping of the lymphatic units separated by valves and termed lymphangions. The specialized smooth muscle cells, commonly referred to as lymphatic muscle cells (LMCs), that comprise the outer layer of lymphatic collecting vessels express a unique combination of contractile proteins that allow them contract regularly and rapidly, a property that distinguishes them from vascular smooth muscle cells15,16.
Despite their unique phenotype and importance in generating fluid transport, the role of lymphatic muscle in mediating or propagating pathologies such as lymphedema has been largely understudied. Functionally, it has been demonstrated that lymphatic collecting vessels become more permeable in inflammatory environments and significantly remodel17–19. Vessels removed from lymphedema patients undergoing lymphaticovenous anastomosis displayed a thicker media and intima when the stage of lymphedema was advanced, decreased ratio of myosin-heavy-chain-2 to alpha-smooth muscle actin and increased collagen20,21. In the lymphedemic environment, the phenotype of the lymphatic collecting vessel is sensitive to various macrophages and inflammatory signaling that regulates tissue remodeling through fibrosis21,22. Understanding the remodeling response of lymphatic muscle to injury and pathology, and the role that the mechanical tissue microenvironment plays in this process, may provide useful insight into the chronic nature of lymphedema.
LMC’s response to local injury and disease is likely to be similar in many ways to vascular smooth muscle cells (VSMCs). The behavior of VSMCs have been characterized in much greater detail both in-vivo and in-vitro. Depending on the combination of chemical and mechanical cues, VSMC phenotype is typically described as “contractile” or “synthetic”23–35. In the contractile phenotype, VSMCs tend to be quiescent; with little to no migration or proliferation. A contractile VSMC’s primary function is to modulate the diameter of a blood vessel through constricting or relaxing in response to biomechanical cues such as endocrine signaling or pressure changes. Commonly in response to pathological stimuli, such as disease or vessel damage, VSMCs convert to a synthetic phenotype. In this phenotype, VSMCs are highly proliferative and migratory. In addition, they begin to excrete a variety of extracellular matrix (ECM) proteins, such as collagen. In combination, proliferation and ECM synthesis from high levels of synthetic VSMCs thicken and alter the walls of blood vasculature in disease states. Studies have demonstrated that the contractile and synthetic phenotypes do not exist exclusively, and in-vitro assays using a variety of different chemical and mechanical cues can induce a VSMC phenotype that exhibit features of both phenotypes36. While much of the LMC pathological response could be characterized as existing on this contractile to synthetic spectrum as well, it is unclear how the distinct functions of LMCs may lead to differences in their pathological responses.
At this time, LMC phenotype and function has mostly been explored while part of an intact vessel37–41, limiting the opportunity for systematic, high throughput, analysis of multiple factors and stimuli. Therefore, with this study we aim to assess the impact of common biochemical and mechanical culture conditions on the phenotype of LMCs in-vitro. Primarily we explore the chronic phenotypic changes that occur from lymphatic smooth muscle derived from a limb where surrounding lymphatic vessels were surgically removed and compare those responses to LMCs isolated from the contralateral control. We determine the sensitivity of LMC phenotype to common changes in culture media thought to regulate phenotype. Specifically, we examine how changes in medium serum and glucose levels, in addition to cyclic loading, alter LMC morphology, metabolic activity, and synthesis of collagen. Furthermore, we examined how cyclic stretching of LMCs influence orientation and the expression of various molecular pathways. We further demonstrate that LMCs derived from an inflammatory in-vivo environment, following local tissue damage, chronically alter the state of lymphatic muscle and their response to bio-mechanical cues.
Materials and Methods
Cell Culture
Primary cells lines were established form a sheep lymphatic damage model. In brief, sheep were anesthetized with midazolam 0.25 mg/kg and ketamine 5 mg/kg, intubated and maintained under anesthesia at 1–3% sevoflurane in oxygen. The hind limbs of the sheep were shaved with electric sheers, and sterilized with three alternating scrubs of 2% chlorhexidine and 70% isopropyl alcohol. A 4-cm incision was made on the lateral aspect of one of the hind limbs proximal to the tarsus, exposing a collecting lymphatic vessel running in parallel to the saphenous vein. A 2-cm portion of the vessel along with the vein was ligated with sutures and resected. Animals were euthanized after 6 weeks and primary lymphatic muscle cell (LMC) lines were formed from collecting lymphatic vessels isolated from wounded and intact hind limbs. Once isolated, the collecting vessels were cleaned from all adipocytes and rinsed in a physiological saline before being transferred to a tissue culture dish where it was attached by gently pressing the ends to the surface with forceps. Vessels were cultured in Dulbecco’s Modified Eagle’s Medium (ThermoFisher Scientific, Waltham, MA), 4,500mg/L of glucose, with 10% Fetal Bovine Serum (FBS), along with Antibiotic-Antmycotic (ThermoFisher Scientific). Media was exchanged every 2–3 days. Muscle cells began to migrate and proliferate from the vessel around day 3. The vessel was gently discarded, leaving only the newly migrated cells. Cells were split once confluent using Trypsin, 0.25% EDTA, and frozen in liquid nitrogen at passage 3. Experiments were conducted on LMCs between passage 5–6. All following experiments were done with LMCs derived from 3 separate sheep, all who underwent the same lymphatic injury surgery, creating of 6 cells lines. For statistical analysis experimental conditions were grouped into cells isolated from the wounded leg (3 sheep) and cells isolated from the control leg (3 sheep).
Lymphatic endothelial cells were isolated by first inverting the collecting lymphatic vessel using fine forceps so that the previously luminal endothelial cells would be on the exterior. One end of the collecting vessel was tied to the end of a long glass micropipette and inverted using suction and removed from the micropipette. The inverted lymphatic vessel was then gently pressed to a petri dish and cultured in Endothelial Basal Medium (Lonza, Basel, CH), supplemented with 1% penicillin-streptomycin-amphotericin B, 1% Glutamax, 0.1% DBcAMP, and 0.1% hydrocortisone acetate, until the LECs began to migrate away from the vessel, usually around day 2–3. The vessel was then discarded and the cells were cultured until confluent. Cells were split using Trypsin, 0.25% EDTA, and frozen in liquid nitrogen at passage 3. Immunohistochemistry was conducted on LECs at passage 5.
Marker Assessment
Purity of the cell culture lines was established through expression of key protein markers including endothelial nitric oxide synthase (Abcam, Cambridge, UK), alpha-Smooth Muscle Actin (Sigma, St Louis, MO), Collagen Type 1 (Sigma), and Tropomyosin (BD Biosciences, San Jose, CA). Samples were permeabilized with methanol (−20C) for 2 minutes then rinsed three times with phosphate buffered saline (PBS). Blocking of samples were done in a PBS solution containing 2% bovine serum albumin, BSA and 10% goat serum for 1 hour at room temperature. Following this, a primary antibody, suspended in the 2% BSA solution (1:100), was applied to the samples for one hour on a plate rocker. The samples were rinsed in PBS before application of a fluorescent secondary antibody solution in 2% BSA (1:200) for 1 hour at room temperature on a shaker. Samples were rinsed two times in PBS before imaging.
Media Assay
To explore the role of serum levels and glucose levels in the phenotype of LMCs, cells were seeded into a cell culture treated 48-well plate at a density of 14800 cells/cm2 in our standard DMEM formulation. This DMEM contained 4,500mg/L glucose and was supplemented with 10% FBS. Cells were allowed to settle and attach overnight before being serum starved for 24 hours in standard DMEM supplemented with 0.2% serum to arrest cell cycle progression. After serum starvation, cells were grown in one of three types of media for two days; the standard DMEM formulation (4,500 mg/L of glucose and 10% FBS), a low serum DMEM formulation (LS-DMEM) with only 0.2% FBS, or a low glucose DMEM formulation (LG-DMEM) containing only 1000mg/L of glucose.
Cellular Metabolic Activity
A reduction-oxidation indicator known as alamarBlue® (ThermoFisher Scientific) both fluoresces and undergoes a colorimetric change in response to cellular metabolic activity. Specifically, resazurin, a blue cell permeable compound with low fluorescence is reduced to resorufin, a red shifted and highly fluorescent compound. This reduction of resazurin to resorufin is a byproduct of normally occurring reduction reactions in metabolically active cells. Each well from the stretch experiment was incubated in media containing 10% alamarBlue® reagent for 4 hours. After the 4 hours, 100μL of the media from each well was transferred to a 96-well plate. The fluorescent intensity of each sample-including well was measured using a plate reader (Synergy H4) after excitation with a 562nm wavelength laser.
Collagen Expression Assay
A Sirius red/fast green assay (Chondrex, Redmond, WA) was used to determine the amount of collagen produced by cultured LMCs. In short LMCs were cultured on a tissue culture treated 48-well plate. Cells were cultured at a density of 15,000 cells/cm2. Cells attached overnight and then serum starved for 24 hours. Following the serum starvation, media of a specific formulation was applied and the cells were cultured for two days before being fixed in 4% PFA. 75μL of the Sirius red/fast green dye was then incubated with each sample for 30 minutes. Samples were rinsed with 0.5mL of PBS twice, and then 0.3mL of the dye extraction buffer was applied and pipetted briefly to mix. 100μL of the extraction dye was added to a 96-well plate and optical density was analyzed with the Synergy H4 plate reader at 540nm and 605nm.
To calculate the amount of collagen, the OD 540 value was corrected by subtracting the contribution of Fast Green at 540 nm, which is 29.1% of the OD 605 value. The color equivalence (OD values/μg protein) is 0.0378 for collagen and 0.00204 for non-collagenous proteins at OD 540 and 605, respectively.
Stretch Device
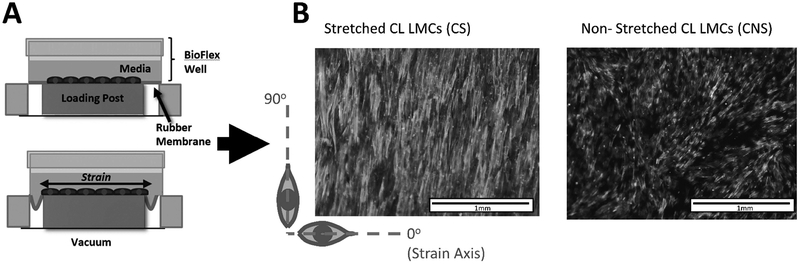
Hardware from a commercially available Flexcell® system (Flexcell® International Corporation, Burlington, NC) was interfaced with a vacuum pump from Parker Hannifin Corporation and a custom control code using National Instruments LabVIEW program to cyclically strain a monolayer of cells. In brief, 70,000 LMCs (~7400 cells/cm2) were seeded into each well of the Uniflex® Culture Plate onto a rubber membrane treated with type I collagen. The plate was loaded above loading posts which allow the cells to be deformed in a uniaxial direction through the manipulation of a vacuum below the wells, see Figure 1.
Figure 1:
(A) Stretch Device Schematic. (B) Alignment of F-actin fibers for stretch and non-stretched CL LMCs to axis of strain
For all stretch experiments, the membranes which made the basement substrate of the LMCs were stretched to 7.5% elongation at 0.1Hz for 1 week. The deformation profile was roughly a square wave with up to 2% oscillation around the set vacuum pressure. This translates to about a 0.3% variation in the percent elongation. Ultimately there were four stretch conditions; Control Leg LMCs - Non Stretched (CNS) Control Leg LMCs - Stretched (CS), Wounded Leg LMCs - Non Stretched (WNS), Wounded Leg LMCs - Stretched (WS). Cells isolated from all three sheep are present in each of the 4 experimental groups.
Blebbistatin (Abcam), an inhibitor to myosin II, was added to the cell culture media (5μM) to determine the sensitivity of the cyclic stretch induced behavior changes to the cell-matrix adhesion.
Actin Fiber Alignment
Images were taken of the wells post a stretching treatment for 1 week. The wells were immediately fixed in 4% paraformaldehyde once the experiment finished and stained with a F-actin stain (Molecular Probes, Eugene OR). Each image was analyzed with a Matlab code that utilizes built-in functions to determine the magnitude and direction of a vector indicating the path of maximum intensity change. In practice, this gradient vector is oriented perpendicular to a fiber at any given point along the fiber. The direction of the vector is given as an angle from −180° to 180°, referred to as the intensity angle. We then calculated an alignment score for each pixel based on how well the intensity gradient vector aligned to an arbitrary angle from 0° to 90° using the following formula:
Values were normalized to the minimum score in any direction for the specific image to correct for slight variations in brightness from image to image.
Statistical Analysis of Functional Cell Assays
All data was tested using GraphPad prism software. Data was initially tested for normality using D’Agostino & Pearson normality test. The normality test revealed that the alamarBlue results in the control leg LMCs cultured in the standard DMEM were not normally distributed. In addition, the alamarBlue and the collagen synthesis results for the wounded leg LMCs cultured in the low serum DMEM were not normal. Therefore, multiple one-way, nonparametric, Kruskal-Wallis tests were used to assess statistical differences for a single LMC type across the three media conditions. Dunn’s test was used to correct for multiple comparisons. Differences between CL and WL LMCs in a shared media conditions were tested with the Mann-Whitney test.
Proteomic Analysis
One well of LMCs from each stretch condition (CNS, WNS, CS, or WS) was collected at the end of 5 individual stretch experiments to be used as a sample for proteomic analysis, with the cells that made up these samples being derived from 3 different sheep. Each of the 5 samples was run through the protocols listed below in duplicate. To prepare samples for proteomic analysis, monolayers of LMCs were rinsed once with PBS and then trypsinized. The cell solution was centrifuged for 5mins at 4°C and 800RCF. The resulting cell pellet was re-suspended in PBS and then spun down again under the same conditions. After aspirating the PBS, the cell pellet was then suspended in 0.5mL of RIPA lysis buffer (Thermo Scientific) and incubated on ice for 30 minutes. After 30 minutes, the samples were centrifuged at 13,000–14,000 RPM for 10 minutes at 4°C. The protein containing supernatant was collected and the pellet of cell debris was discarded. Protein content of the cell lysate was measured using a microplate BCA protein assay kit (Thermo Scientific) and aliquots containing 20μg of protein were stored in a −20C freezer.
Proteins in the supernatant are reduced, alkylated and digested with trypsin according to the FASP protocol42. The peptides were analyzed by high-performance liquid chromatography-coupled tandem mass spectrometry (HPLC-MS/MS), separated on a 2 micron, 15 cm × 75 μm C18 column, and analyzed with a Q Exactive Plus mass spectrometer.
Protein Identification
The protein IDs were assigned by searching the raw files from each technical and biological replicate against the sheep (Ovis Aries) Swiss-Prot database (December 2017; 788 entries) after being filtered and “de novo” sequenced in Peaks 8.0 software (Bioinformatics Solutions, Waterloo, Canada). The following search parameters were applied: trypsin restriction for enzyme and one allowed missed cleavages. The parent mass tolerance was set to 15 ppm using monoisotopic mass, and fragment ion mass tolerance was set to 0.05 Da, for the samples run on a Q Exactive Plus mass spectrometer. Carbamidomethyl cysteine (+57.0215 on C) was specified in PEAKS 8.0 as a fixed modification. Methionine, lysine, proline, arginine, cysteine and asparagine oxidations (+15.99 on CKMNPR), deamidation of asparagine and glutamine (NQ-0.98) and pyro-Glu from glutamine (Q-18.01 N-term) were set as variable modifications. The results were validated using the FDR method built in PEAKS 8.0 and protein identifications were accepted if they could be characterized with a confidence score (−10lgP)>15 for peptides and (−10lgP)>15 for proteins. The FDR for proteins was further adjusted to less than 1% (p<0.05) after removing contaminants like serum albumin, hemoglobin, keratins and immunoglobulin and further allowing a minimum of 1 peptide per protein. An independent validation of the MS/MS-based peptides and protein identification was performed with the Scaffold (version Scaffold_4.7.3, Proteome Software Inc., Portland, OR) using the compatible “. mzid” files exported from PEAKS 8.0. for each “control” and “wounded” stretched or non-stretched lymphatic muscles samples. The Scaffold built in option “MuDPIT” was used to combine multiple files from technical replicates for each sample defining the “control stretched or non-stretched” and “wounded stretched or non-stretched” samples (see Supplementary Table 1). Peptide identifications were accepted if they could be established at greater than 95.0% probability by the Peptide Prophet algorithm with Scaffold delta-mass correction. Protein identifications were accepted if they could be established at greater than 90.0% probability and contained at least 1 identified peptide. Protein probabilities were assigned by the Protein Prophet algorithm. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony as described originally elsewhere43,44.
Label-free relative peptide quantification (LFQ) and analysis of differential protein expression profiles across the stretch and non-stretched control and wounded sample
Label-free quantitative (LFQ) methods used the raw spectral data from parallel MS runs to determine relative protein abundances. We used both “MS/MS (MS2) spectral counting” and “precursor MS1 area” methods for LFQ analysis and further contrasting the differentially expressed proteomic profiles across the control and wounded lymph (stretched or non-stretch) muscle samples. The label-free quantification based on the precursor intensity (area) was performed using the quantification algorithm supported by the PEAKS Q module (Bioinformatics Solution Inc., version 8.0). The data were filtered, smoothed, and aligned in retention time, followed by feature detection based on peak volume and isotopic clustering using the algorithm of PEAKS 8.0. An additional LFQ analysis using the spectral counts intensity (MS/MS or MS2) was employed for the same set of samples using the normalized weighted spectrum count option provided in “perSPECtives”, (version 2.0.6, Proteome Software Inc., Portland, OR). The corresponding “.mzIdentML” files generated in Scaffold (version 4.7.3) for each “control” and “wounded” in both “stretched and non-stretched” sample categories were imported in “perSPECtives” where the threshold of 1 unique peptide/sample and the significance level of P<0.05 were set up with the standard Benjamini-Hochberg procedure.
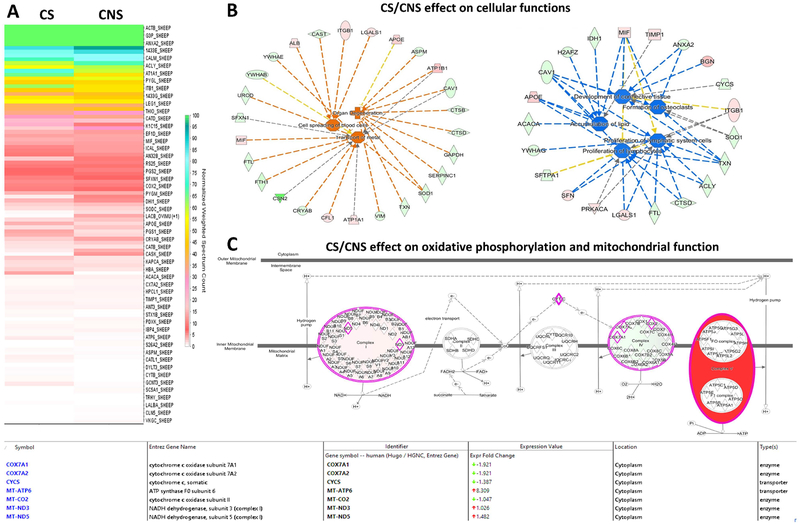
The relative protein abundances were displayed as heat maps including representative proteins of each protein group after normalization of the corresponding weighted spectral counts using the built-in algorithm from “perSPECtives” (see figures 1A and 6A). Only proteins, which passed a selected significance statistical threshold (ANOVA, p<0.05 and FDR <1% for protein and peptide expression) and are shown in the representative heat maps.
Figure 6: Comparative protein expression profiling of “control stretched” vs “control non-stretched” in the lymphatic muscle cells.
(A) Heat map highlights the change in the protein expression profiles in the “control-stretched (CS))” relative to the “control non-stretched (CNS)” lymph muscle cells. (B) Oxidative phosphorylation and mitochondrial function is displayed as one of the major pathways up-regulated (red-color) in the CS vs CNS sample. The “expression value” associated with each protein found in the experimental data set presented in supplementary table 1 highlights the “ATP synthase F0 subunit” as being top up-regulated protein in CS vs CNS (characterized by more than eight-fold increase in the expression profile). (C) The IPA analysis of cellular networks identified the increased in cellular spreading, transport of metals and organ degeneration up-regulated in the CS vs CNS (z>1.5 and orange/red color). By contrast, the down-regulated cellular pathways in the CS vs CNS (z<−1.5 and blue/green color) identified the development of connective tissue together with the cellular proliferation. The proteins in red and green had a significantly up- or down-regulated expression (p < 0.05), respectively. The shape of symbols denotes the molecular class of the proteins. A solid line indicates a direct molecular interaction, whereas a dashed line indicates an indirect molecular interaction.
Gene ontology (GO), molecular and cellular pathways enrichment analysis
The biochemical and cellular pathways together with the GO assignments were generated by the ingenuity pathway analysis (IPA; Ingenuity Systems, Redwood City, CA, USA) on the list of differentially abundant proteins extracted from LFQ analyses (weighted and normalized MS2 spectral counts generated in Perspectives software) and presented categorized in the Supplementary Table 2. Specifically, the experimentally determined protein ratios, quantified using ≥ 1 peptides/sample, were used to calculate the experimental fold changes by rescaling their values using a log2 transformation, such that positive values reflected fold increases while the negative values reflected fold decreases. For network generation, datasets containing gene identifiers (gene symbols) for the “control” and “wounded”, for each “stretched” and “non-stretched” sample category samples were uploaded into the IPA application together with their rescaled log2 transformation MS2 normalized spectral counts ratios. The networks with qualified molecules were then algorithmically generated based on their connectivity index using the built-in the IPA algorithm. The probability of having a relationship between each IPA indexed biological function and the experimentally determined genes was calculated by a right-tailed Fisher’s exact test. The level of significance was set to a P-value of <0.05. Accordingly, the IPA analysis identified the molecular and cellular pathways from the IPA library of canonical pathways that were most significant to the dataset (-log (p value)>2.0). For the quantitative analysis of protein expression profiles, IPA assigned the “z-score” function to all eligible canonical and cellular pathways (where a “z<−1.5 represent significant down-regulation while a z>1.5 represent a significant up-regulation of the selected pathway) (see Supplementary Figures 1 and 2 and Supplementary Table 2).
Results
Establishment of Primary Lymphatic Muscle Cell Line
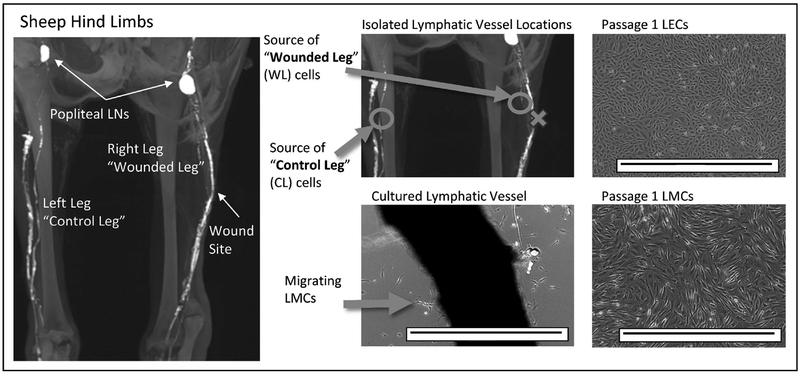
Lymphatic cells were isolated from the hindlimb of an adult sheep as outlined in the methods. As shown in Figure 2, the leg in which a section of a parallel lymphatic vessel had previously been resected is referred to as the “wounded leg” (WL). The contralateral limb was left uninjured and referred to as the “control leg” (CL). Cells usually began to migrate away from the vessel after 2–3 days in culture. Cells were initially identified through morphology; LMCs which migrated off the vessel plated in the non-inverted configuration had a spindle like morphology while lymphatic endothelial cells (LECs) which migrated off the vessel plated in the inverted configuration had a cobblestone-like morphology.
Figure 2: Isolation and morphological identification of lymphatic muscle cells (LMC) and lymphatic endothelial cells (LEC) :
MRI image of the lymphatics in the hindlimb of an adult sheep. There are two primary collecting lymphatic vessels that drain to the popliteal lymph nodes (LNs). The intact lymphatic vessel in the wounded leg (WL) of the adult sheep and the corresponding intact vessel in the control leg (CL) were isolated and plated in our standard DMEM with 4,500 mg/L of glucose and 10% FBS to produce lymphatic cell lines. Primary LMCs began to migrate from the non-inverted vessel by day 2–3 and had a spindle-like morphology. Primary LECs had a cobblestone-like morphology and were identified using similar methodology to LMCs but the vessel was inverted before plating. Both lines formed a monolayer once passaged. Scale bar is 1mm.
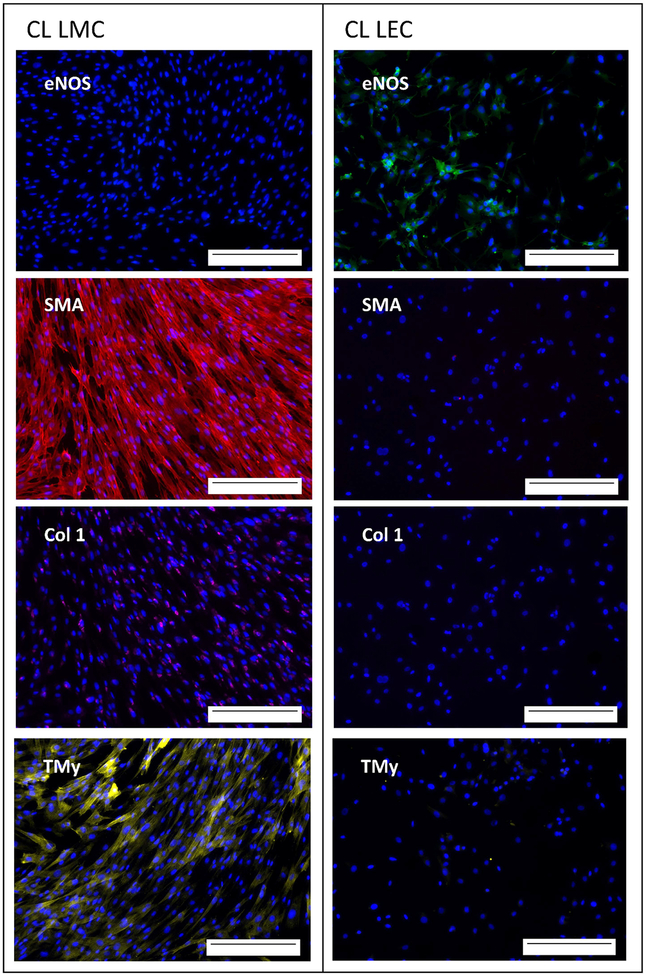
The purity of the primary lymphatic muscle line was determined using expression of four relevant proteins to differentiate endothelial cells from smooth muscle, cells as seen in Figure 3. Wells of lymphatic muscle cells were positive for smooth muscle actin (SMA), collagen type 1 (Col 1), and tropomyosin (TMy), while being negative for endothelial nitic oxide synthase (eNOS). LECs were positive for eNOS while being negative for the other 3 markers (Figure 3). The differing protein expressions indicate successful separation of LECs and LMCs through the culturing and expansion technique used.
Figure 3: Purity of LMC and LEC lines derived from the control leg of adult sheep :
CL LMC (left) had positive expression of smooth muscle actin (SMA) labeled red, collagen type 1 (Col 1) labeled magenta, and tropomyosin (TMy) labeled yellow after one week of culture. CL LEC (right) had positive expression of endothelial nitric oxide synthase (eNOS) labeled green after one week of culture. Nucleus stained in blue. Scale bar is 0.25mm.
Optimizing Growth Conditions
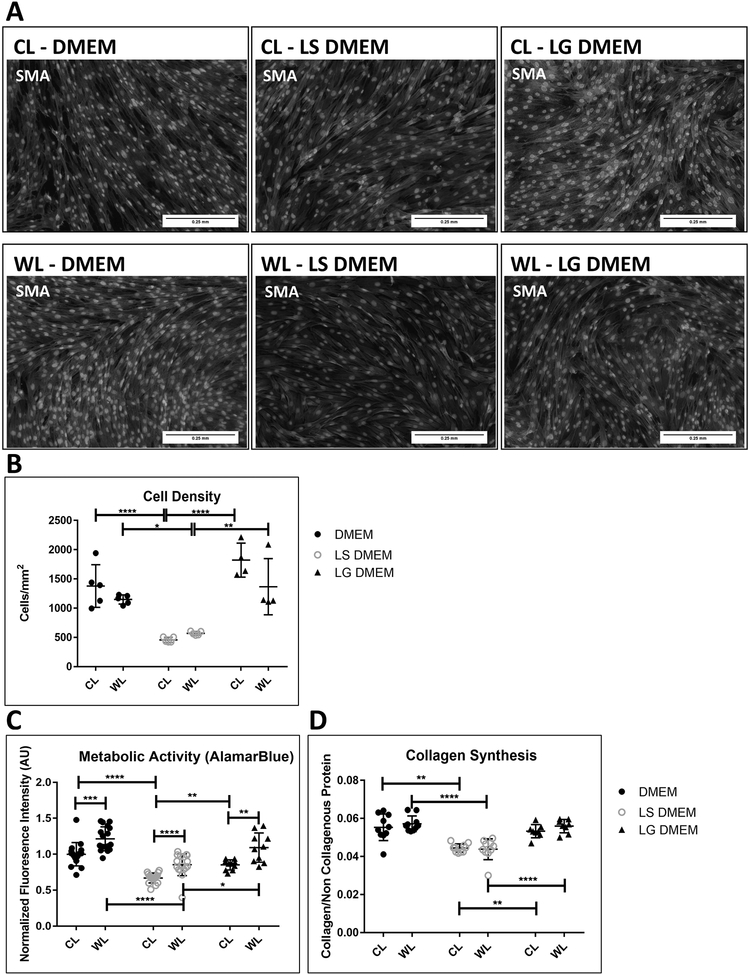
The impact of changing levels of glucose and serum (FBS) within the DMEM on the phenotype of in-vitro cultured LMCs was systematically explored. Culture in low serum DMEM (LS DMEM), drastically impacted the phenotype of LMCs after two days. The cells took on a shorter spindle morphology and aligned less densely as seen by the SMA staining (Figure 4A & B). Low glucose DMEM (LG DMEM), had a negligible impact on morphology cell density. Furthermore, metabolic activity and production of collagen was significantly reduced upon reducing serum concentrations but were unchanged when changing glucose levels alone (Figure 4C & D).
Figure 4: Impact of culture media on non-stretched CL and WL LMC phenotype:
(A) CL LMC and WL LMC SMA expression (red) depict shorter spindle morphology and (B) lower cell density after two day culture in low serum DMEM (LS-DMEM) with 0.2% FBS compared to standard DMEM (10% FBS, 4500g glucose/L) or low glucose DMEM (LG-DMEM) with 1000g glucose/L. (C) alamarBlue assay show reduction in fluorescence intensity and by extension, metabolic activity and (D) Sirius red/fast green assay show reduction in collagen production in CL and WL LMCs after two-day culture in LS-DMEM compared to standard DMEM and LG-DMEM. Error bars reflect standard deviation. *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001
Despite similar cell densities (Figure 4B), wounded leg LMCs consistently had higher metabolic activity levels (Figure 4C) than CL LMCs, but their response to changes in the culture media followed a similar trend to the response of the control leg LMCs. The cellular source of the LMCs (WL vs. CL) had no measurable impact on expression of collagen after two-day cultures (Figure 4D).
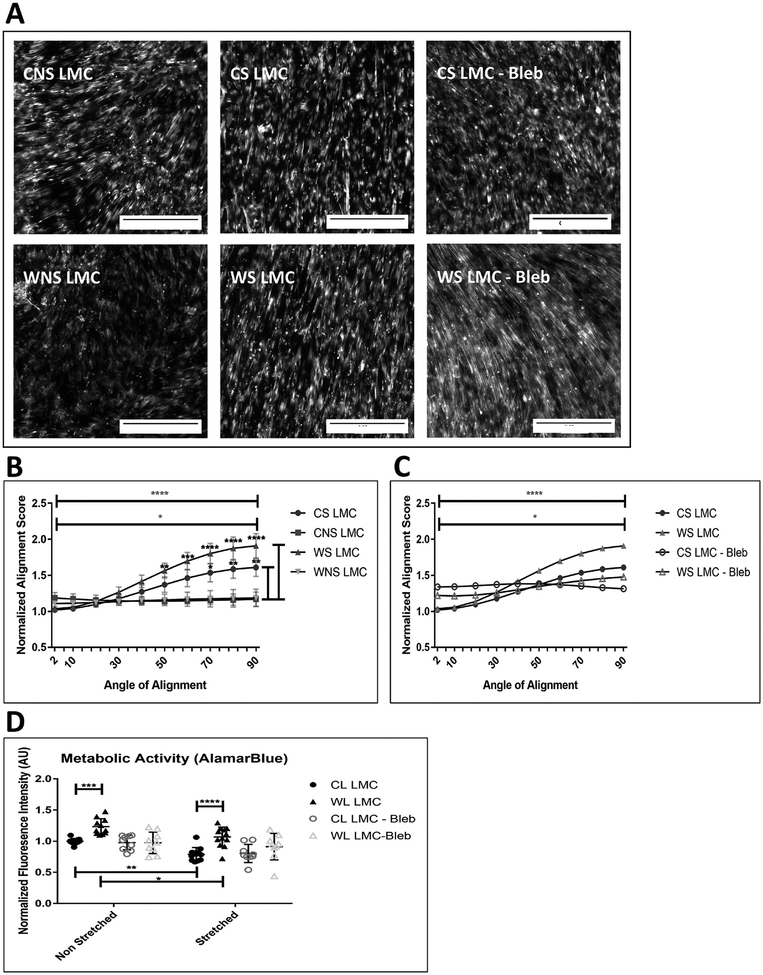
Cyclic Stretching Impacts Alignment and Viability of LMCs
Cells were cultured in standard DMEM on the stretch device as shown in Figure 1A. After exposure to cyclic stretch for one-week, LMCs from control and wounded legs (CS and WS LMCs, respectively) were fixed and stained for F-actin (Figure 5A). Control leg or wounded leg LMCs that were not stretched (CNS and WNS LMCs respectively), did not align in any particular direction. When LMCs were stretched in media containing blebbistatin (abbreviated as “Bleb”), an inhibitor for myosin, there was not a dominant axis to which the cells regularly oriented. Images were processed and the extent to which all the fibers fell along a specific orientation was assigned a score as outlined in the methods section. Stretched LMCs cultured in standard DMEM were much more likely to be oriented in the 90o direction compared to non-stretched cells and this orientation was lost with blebbistatin (Figure 5B&C).
Figure 5: Impact of cyclic stretching on the alignment and metabolic activity in CL and WL LMCs.
(A) Representative images of F-actin fiber orientation of stretched (CS) and non-stretched (CNS) CL LMCs, and stretched (WS) and non-stretched (WNS) WL LMCs taken after 1 week. Scale bar is 0.25mm. (B) Quantification of F-actin fiber alignment for stretched and non-stretched LMCs. Preferential orientation perpendicular to strain (along 90o axis) is shown by comparing alignment score at 2o to alignment score at 90o for WS LMCs (top bar) and CS LMCs (2nd bar). Comparisons along the side are between the CS vs CNS LMCs and the WNS vs WS LMCs respectively. Error bars reflect standard error. There was no significant difference in the response between control and wounded LMCs (C) Blebbistatin (5μM) inhibited preferential alignment along 90o axis after cyclic strain. Error bars are removed for clarity. (D) Impact of cyclic stretching on metabolic activity of LMCs cultured in Standard DMEM, and DMEM supplemented with Blebbistatin (5μM). *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001
Results of alamarBlue assay showed significantly reduced fluorescent intensity, metabolic activity by extension, in both CS and WS LMCs compared to CNS and WNS, respectively. Culture in media containing blebbistatin inhibited any difference between the cell groups and their response to stretching (Figure 5D).
Phenotypic Impact of Cyclic Stretching
Proteomic analysis reveals much about the changing phenotype of the LMCs in response to cyclic stretch. Oxidative phosphorylation and mitochondrial function is displayed as one of the major pathways up-regulated for CL LMCs in response to stretch (Figure 6). “ATP synthase F0 subunit” is one of the highest up-regulated proteins in CS vs CNS (characterized by more than eight-fold increase in the expression profile). The F0 subunit of ATP synthase is a transmembrane, proton powered, rotating motor, which provides the energy to convert ADP to ATP in the F1 subunit. The IPA analysis of cellular networks identified the increased in cellular spreading, transport of metals and organ degeneration up-regulated in the CS vs CNS (z>1.5 and orange/red color). By contrast, the down-regulated cellular pathways in the CS vs CNS (z<−1.5 and blue/green color) identified the development of connective tissue together with cellular proliferation. Elevation of proteins such as cathepsin B (CTSB) and cathepsin D (CTSB) contribute to pathways associated with tissue degeneration. Reductions in the expression of tissue inhibitor of metalloprotease 1(TIMP1) allow for additional local matrix remodeling.
As outlined previously, both CL LMCs and WL LMCs generally become less active and more aligned in response to cyclic stretch, but notably the WL LMCs were found to maintain higher levels of oxidative phosphorylation and mitochondrial dysfunction after one week of stretch (Figure 7). Comparing stretched WL and stretched CL LMCs, the stretched WL LMCs are subjected to some biochemical and cellular changes characterized by significant changes in functions related as cell growth, survival, movement, and free radical scavenging (Figure 8A). Figure 8 B&C indicate proteins contributing to a down-regulation in cellular movement, ions transport and homeostasis and significant up-regulation in some metabolic pathways, related to the generation of energy from lipids and nucleotides as well as activation of reactive oxygen species (ROS) and regulation of ROS (Figure 8).
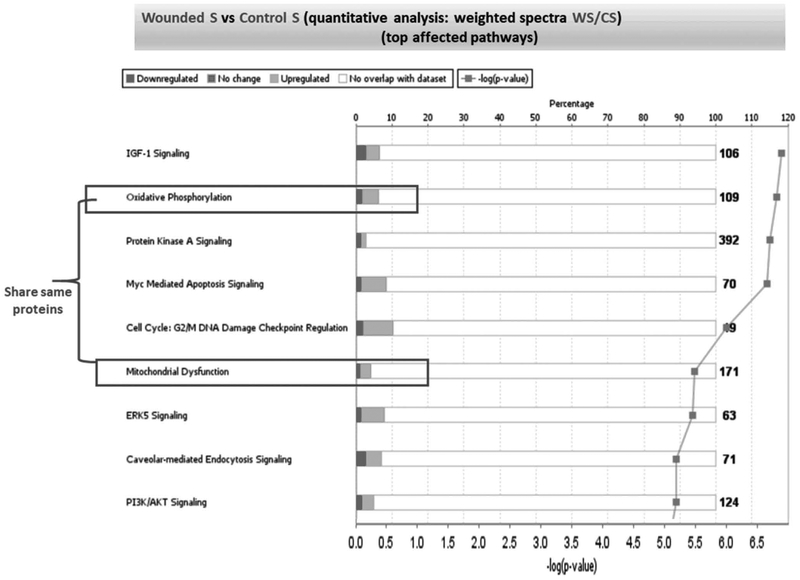
Figure 7: Quantitative analysis of protein expression profiles in the WS vs CS samples.
The main canonical pathways affected by the top 105 protein genes presented in the supplementary table 2 for the WS vs CS samples were subjected to the analysis by IPA algorithm. The % of total molecules up- (light gray) or downregulated (dark grey) in each pathway are displayed. White is the total number of proteins in each pathway; which does not appear in our proteome. Each of the square dots on the histograms correspond to the -log(p-value), which act as a scoring function associated with each pathway and reflect the confidence of having the selected number of identified proteins IDs fitted to the IPA Knowledge-based canonical pathways.
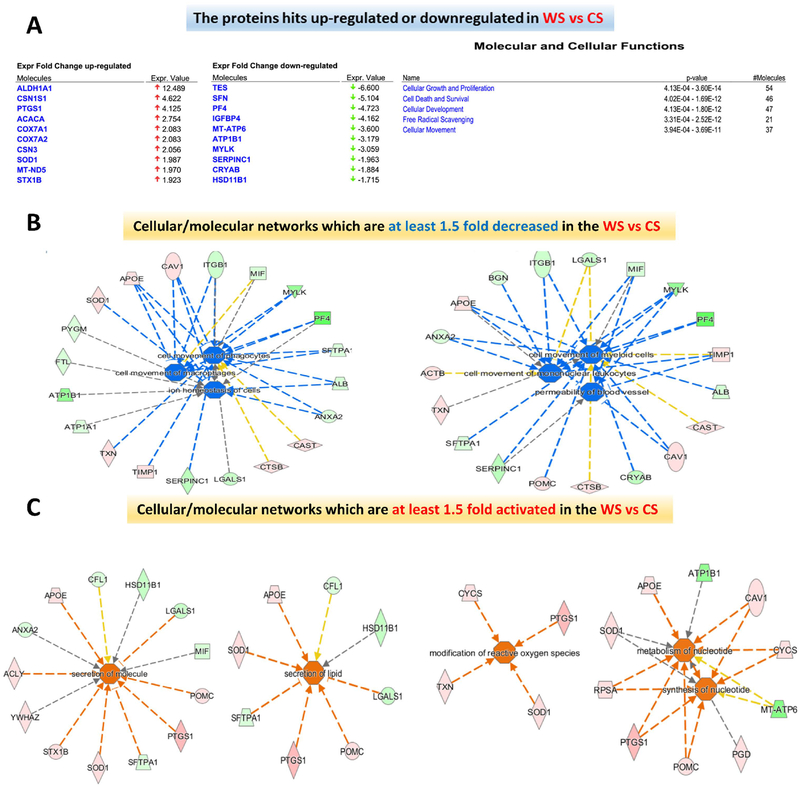
Figure 8: Protein Expression and protein networks of the WS vs CS samples.
(A) Top protein hits characterized by at least 1.5-fold up- or down-regulation in the WS vs CS samples are displayed together with the molecular and cellular functions predicted by IPA to be affected by changes in these protein expression profiles. (B) Significantly down-regulated protein networks (p< 0.05) in the “WS” relative to the “CS” lymph muscle cells are displayed as IPA predicted diseases and cellular functions using the z score (down-regulated corresponding to z<−1.5 and blue color). (C) Significantly up-regulated protein networks (p< 0.05) in the “WS” relative to the “CS” lymph muscle cells are displayed as IPA predicted diseases and cellular functions using the z score (up-regulated corresponding to z>1.5 and orange color).
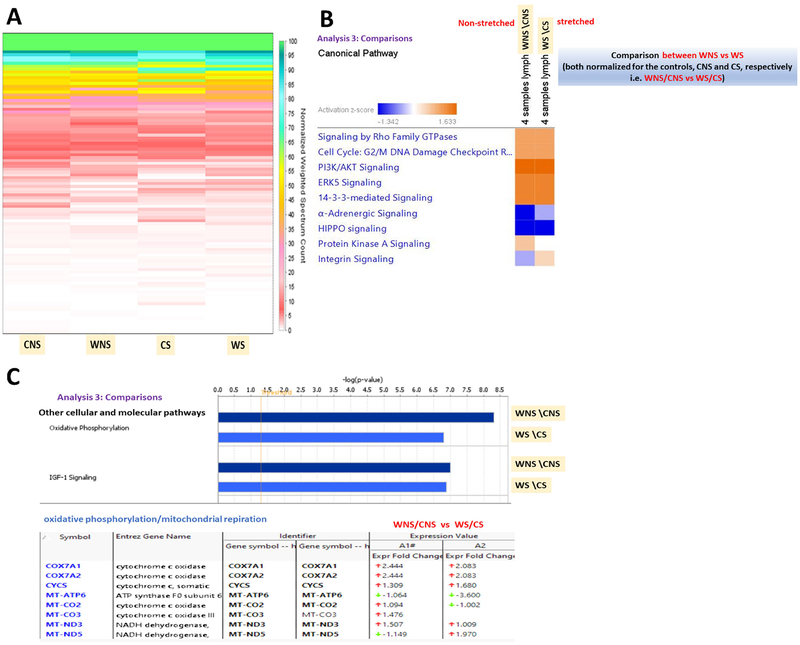
By normalizing the signaling expression of the WL LMCs by the CL LMCs expression we can further demonstrate the distinct response of WL LMC to stretch when compared to control cells (Figure 9). Overall, proteomic analysis indicates that wounded stretched sample are predicted to be subjected to the loss of many intracellular signaling pathways responsible for preserving the ability of cells to move, proliferate or perform the normal metabolic reactions. Figure 9 outlines variably expressed proteins and canonical signaling pathways. Remarkably, the wounded and stretched state (WS) was characterized by a significant fivefold down-regulation of stratifin (SFN) (Supplementary Figure 3 A&C), an adapter protein implicated in the regulation of a large spectrum of both general and specialized signaling pathways, including Phosphoinositide 3-kinase (PI3K) and Protein Kinase A (PKA) signalling24. In addition, at least a threefold down-regulation of myosin light chain kinase was induced in the WS as compared with the WNS lymph muscle cells (see expression profiles in Supplementary Figure 3 F). Another important change induced in the WS samples was the threefold down-regulation of the ATP synthase F0 subunit (Figure 9C) that suggests impairment in the mitochondrial ability to couple ATP synthesis with the respiration.
Figure 9: Comparative protein expression profiling of “wounded/control stretched” vs “wounded/control non-stretched” in the lymphatic muscle cells.
(A) Un-biased, non-clustered heat map generated in “perSPECtives” using the normalized weighted MS/MS spectral counts highlighting the change in the protein expression profiles in the stretched (CS) and non-stretched (CNS) controls vs stretched (WS) and non-stretched (WNS) wounded lymph muscle cells. (B) IPA generated comparison of the canonical biochemical pathways characterizing the WNS relative to the WS samples, each normalized to the corresponding controls (CNS and CS, respectively). Significantly regulated protein networks (p< 0.05) in the “non-stretched WNS” relative to the “stretched WS” lymph muscle cells are displayed as a heat map using the z score (down-regulated corresponding to z<−1.5 and blue color; up-regulated, corresponding to z>+1.5 and orange color). (C) Comparisons of other cellular and molecular pathways predicted by IPA display the oxidative phosphorylation/mitochondrial function and IGF-signaling as the major possible mechanisms mediating the wounded-stretched (WS) induced changes in the lymph muscle cells.
Discussion
Studies have begun to show that collecting lymphatic vessels remodel during the progression of disease states such as lymphedema. Collecting vessels isolated from patients with advanced lymphedema have been shown to become stenotic in lymphedemic limbs, with severity of lumen stenosis related directly to the stage of lymphedema45. This wall thickening seems to be due to remodeling of the lymphatic muscle cells, increasing in both cell proliferation and collagen synthesis within the walls as well as changing the protein composition of myosin46. This study presents a platform to both study the functional and molecular implications of this remodeling and to induce some of the features of the remodeling phenotype for further exploration in-vitro. We were able to successfully isolate LMCs from a lymphatic dysfunction model and established that the LMC lines used for this study were free of eNOS expressing cells such as lymphatic endothelial cells. We demonstrate that LMCs’ behavior seems to change from a more quiescent phenotype to a more proliferative and active phenotype after vessel injury and that these differences persist in culture. A more quiescent phenotype seems to be restored partly in culture under low serum, reducing the LMC’s metabolic activity, as indicated through the alamarBlue assay, and reducing the production of collagen. The LMC line derived from the wounded leg of the adult sheep seem chronically differentiated, expressing high cell metabolic activity. Functional assay results, in combination with elevated signaling pathways highlighted in the proteomic analysis, indicate the WL LMCs may be in a more dedifferentiated, proliferative phenotype. While collagen expression was not quantifiably higher, other types of ECM protein synthesis and proteins integral to ECM remodeling should be explored in future studies.
In alignment with other work in smooth muscle cells, LMCs were particularly sensitive to serum levels. Lymph is rich in a variety of growth factors, and lymph spillage in addition to bleeding following lymphatic vessel or node resection may in part contribute to dedifferentiating the LMCs surrounding lymphatic vasculature47,48. While very little has been published on the effect of growth factors on LMC, vascular smooth muscle cell (VSMC) are very responsive to components of serum such as platelet-derived growth factor and insulin-like growth factor (IGF), which have been shown to induce proliferation, and migration in VSMCs. VSMC proliferation is accompanied by a loss in contractile force generation and increase in ECM synthesis26,49,50. Our work demonstrates that reducing serum levels restores aspects of a quiescent phenotype, similar to what’s been documented with VSMCs in-vitro23. While growth factors have a beneficial effect on expansion of lymphatic endothelial cells, it is possible that their sustained release could impair LMC function and contractility, although such statements are currently speculative due to the lack of research currently published on LMC signaling. Targeted and strategically timed interventions to conserve or restore the contractile phenotype of LMCs may contribute to treating or preventing future lymphatic complications.
Recent work has thoroughly explored the phenotype of LMCs after being cultured in hyperglycemic and hyperinsulinemic conditions51. As the LMCs developed a resistance to insulin they also developed significant impairment to glucose uptake, mitochondrial function, ATP production, and other functional changes. The results from our study are largely in agreement with these findings as the response to changing glucose levels that were previously observed were only apparent in the presence of insulin. Otherwise, the LMCs behaved quite similarly, regardless of glucose concentration in the media. Initially, introduction of high glucose media (25mM) lead to a short-term increase in glucose uptake, but glucose uptake lowered to initial rates within one-hour. This is slightly divergent to early work that explored in-vitro VSMC response to high glucose concentrations, which demonstrated that glucose levels alone lead to cell cycle progression and growth52,53.
Exposure to cyclic stretch has been demonstrated to have a complex impact on VSMC phenotype36 and this seems to hold true for LMCs as well. Cellular response and sensitivity to stretch depends on many factors including the magnitude and frequency of elongation, loading profile, coating of the wells, direction of the strain, duration of stretch, and the phenotypic state of the cell prior to loading36. A notable study showed that by using a stretching waveform that replicates the heart pressure waveform proliferation was actually inhibited by cyclic strain54, despite sinusoidal strain typically enhancing proliferation in the majority of other 2D cyclic stretch studies55–57. With this in mind, our study attempted to design a waveform that was reflective of lymphatic physiology through choosing a frequency of 0.1 Hz and a percent elongation of 7.5%. These values reflect commonly reported of frequencies of lymphatic pumping in-vivo58 and the passive diameter changes from pressurizing an isolated collecting vessel from low pressures (~2cmH2O) to high pressures (≥6cmH2O)59. Furthermore, the initial phenotype of a cell strongly influences their response to stretch. We demonstrate a notable differential response to stretch of the CL LMCs compared to WL LMCs, presumably due to different phenotypes of the LMCs prior to stretch, a phenomena noted in VSMCs as well60.
Proteomic analysis of the CL LMCs and their response to cyclic stretching outlined in figures 7–9, under normal conditions LMC are more quiescent and contractile than the LMC derived from vessels that have undergone active compensation due to a lymphatic injury. Higher expression of signaling pathways associated with oxidative phosphorylation and insulin-like growth factor-1 in WL LMCs, regardless of stretch, indicate that the differentiation of the WL LMCs in response to a local injury persist 6 weeks after the initial insult and are maintained even after several passages in vitro (Figure 9C). Both of these pathways have been found to be critical to the growth, proliferation, and survival of VSMCs49,50,61,62. This, in part may explain the increased sensitivity of CL LMCs to tissue degeneration and apoptosis in response to stretch (Figure 8). Furthermore, the WL LMCs consistently expressed lower levels of α-adgrenergic signaling proteins (Figure 9B), a pathway critical for regulation of vascular tone and constriction of vascular smooth muscle63. In response to stretch, WL LMCs down regulate MLCK, which may be correlated with a weaker contractile state and increased migration or growth41. The changes in PKA signaling further support this notion as PKA signaling has been found to inhibit proliferation in response to vascular injury in VSMC64. Down regulation of PKA signaling in the WL LMCs (Figure 9B) further indicate a commitment to growth, a common reaction to vessel damage, in response to cyclic loading.
Given the unique phenotype and function of LMCs, it is still unclear exactly how LMC remodeling impacts the unique lymphatic role of fluid drainage and immune cell regulation. Characterization of this sheep wound mode in-vivo and the functional impact on intact lymphatic vessels will be the focus of future studies from our lab. Future experiments can build on this stretch platform to see how LECs influence LMC response to stretch and to look for specific signaling pathways that can be interrupted to promote a specific response. This platform and the established findings provide a framework to begin to explore how interventions to maintain or restore a given LMC phenotype in the presence of pathological cues. The effect of interventions adopted from vascular research, such as rapamycin or calcium channel agonist, on LMCs can be effectively and systematically explored in future work.
Perspectives
Increases in ATP synthase by the CL LMCs after stretching along with the measured long-term remodeling of WL LMCs, indicate that LMCs become more active and mobile after an injury or mechanical perturbation. The described stretching platform allows for future studies in which we can determine the implications of this injury response, including how it may contribute to the chronic nature of lymphatic diseases, including the hyperproliferation of LMCs that has been observed in a small number of clinical studies. Furthermore, we now have the ability, and baseline knowledge, to systematically explore what combination of loading and molecular therapies can be used to manipulate LMC responses and prevent maladaptive remodeling.
Supplementary Material
Acknowledgments
We would like to acknowledge the Systems Mass Spectrometry Core at Georgia Institute of Technology for the with initial proteomic analysis of samples.
Grants
NIH R01 HL 113061
Georgia Partners in Regenerative Medicine Seed Grant
List of Abbreviations
- LMCs
Lymphatic Muscle Cells
- LECs
Lymphatic Endothelial Cells
- VSMCs
Vascular Smooth Muscle Cells
- CL
Control Leg
- WL
Wounded Leg
- CS
CL LMCs – Stretched
- WS
WL LMCs – Stretched
- CNS
CL LMCs - Non stretched
- WNS
WL LMCs - Non stretched
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
Fetal Bovine Serum
Footnotes
Competing Interests
There are no competing interests to disclose.
References
- 1.Swartz MA The physiology of the lymphatic system. Adv. Drug Deliv. Rev 50, 3–20 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Randolph GJ & Miller NE Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Invest 124, 929–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasan S, Vannberg FO & Dixon JB Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep 6, 24436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clement CC et al. Protein expression profiles of human lymph and plasma mapped by 2D-DIGE and 1D SDS-PAGE coupled with nanoLC-ESI-MS/MS bottom-up proteomics. J. Proteomics 78, 172–187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clement CC et al. The dendritic cell Major Histocompatibility Complex II (MHC II) peptidome derives from a variety of processing pathways and includes peptides with a broad spectrum of HLA-DM sensitivity. J. Biol. Chem 291, 5576–5595 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement CC et al. An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma. PLoS One 5, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clement CC, Rotzschke O & Santambrogio L The lymph as a pool of self-antigens. Trends Immunol. 32, 6–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement CC & Santambrogio L The lymph self-antigen repertoire. Front. Immunol 4, 1–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jafarnejad M, Woodruff MC, Zawieja DC, Carroll MC & Moore JE Modeling Lymph Flow and Fluid Exchange with Blood Vessels in Lymph Nodes. Lymphat. Res. Biol 13, 234–47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levick JR & Michel CC Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res 87, 198–210 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Von Der Weid P & Zawieja DC Lymphatic smooth muscle : the motor unit of lymph drainage. 36, 1147–1153 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Davis MJ et al. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am. J. Physiol. Heart Circ. Physiol 303, H795–808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swartz MA et al. Mechanics of interstitial-lymphatic fluid transport: theoretical foundation and experimental validation. J. Biomech 32, 1297–1307 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Gashev AA Physiological aspects of lymphatic contractile function. Ann. N. Y. Acad. Sci (2002). [DOI] [PubMed] [Google Scholar]
- 15.Muthuchamy M, Gashev A & Boswell N Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. (2003). [DOI] [PubMed] [Google Scholar]
- 16.Muthuchamy M & Zawieja D Molecular regulation of lymphatic contractility. Ann. N. Y. Acad. Sci 1131, 89–99 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Scallan JP, Davis MJ & Huxley VH Permeability and contractile responses of collecting lymphatic vessels elicited by atrial and brain natriuretic peptides. J. Physiol 591, 5071–81 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuan EL et al. Collecting Lymphatic Vessel Permeability Facilitates Adipose Tissue Inflammation and Distribution of Antigen to Lymph Node-Homing Adipose Tissue Dendritic Cells. J. Immunol 6389, 0–3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov S et al. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J. Clin. Invest 126, 1581–1591 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogata F et al. Phenotypic modulation of smooth muscle cells in lymphedema. 5411, (2014). [DOI] [PubMed] [Google Scholar]
- 21.Avraham T et al. Fibrosis is a key inhibitor of lymphatic regeneration. Plast. Reconstr. Surg 124, 438–450 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Mihara M et al. Pathological steps of cancer-related lymphedema: histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 7, e41126 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Sims S, Jiao Y, Chow LH & Pickering JG Evidence From a Novel Human Cell Clone That Adult Between Noncontractile and Contractile Phenotypes. Circ. Res 85, 338–348 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Martin KA et al. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol 286, C507–C517 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Rensen SSM, Doevendans PAFM & van Eys GJJM Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J 15, 100–8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kottke-marchant K & Ph D Molecular Regulation of Contractile Smooth Muscle Cell Phenotype : Implications for Vascular. 16, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J et al. Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. J. R. Soc. Interface 11, 20130852 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abouhamed M, Reichenberg S, Robenek H & Plenz G Tropomyosin 4 expression is enhanced in dedifferentiating smooth muscle cells in vitro and during atherogenesis. Eur. J. Cell Biol 82, 473–482 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Hultgårdh-Nilsson A, Lövdahl C, Blomgren K, Kallin B & Thyberg J Expression of phenotype- and proliferation-related genes in rat aortic smooth muscle cells in primary culture. Cardiovasc. Res 34, 418–30 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Gallagher PJ, Jin Y, Killough G, Blue EK & Lindner V Alterations in expression of myosin and myosin light chain kinases in response to vascular injury. Am J Physiol Cell Physiol 279, 1–17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin KA et al. Rapamycin Promotes Vascular Smooth Muscle Cell Feedback Signaling *. 282, 36112–36120 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Fisher SA Vascular smooth muscle phenotypic diversity and function. Physiol. Genomics 42A, 169–187 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang DG et al. Culture of smooth muscle cells from guinea pig mesenteric lymphatic vessels. Lymphology 40, 14–18 (2007). [PubMed] [Google Scholar]
- 34.Moiseeva EP Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc. Res 52, 372–386 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Eberini I et al. A proteomic portrait of atherosclerosis. J. Proteomics 82, 92–112 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Mantella L, Quan A & Verma S Variability in vascular smooth muscle cell stretch-induced responses in 2D culture. Vasc. Cell 1–9 (2015). doi: 10.1186/s13221-015-0032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogata F et al. Phenotypic modulation of smooth muscle cells in lymphedema. (2013). [DOI] [PubMed] [Google Scholar]
- 38.Munn LL Mechanobiology of lymphatic contractions. Semin. Cell Dev. Biol 38, 67–74 (2015). [DOI] [PubMed] [Google Scholar]
- 39.von der Weid P-Y, Lee S, Imtiaz MS, Zawieja DC & Davis MJ Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphat. Res. Biol 12, 66–75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gashev AA, Zhang RZ, Muthuchamy M, Zawieja DC & Davis MJ Regional heterogeneity of length-tension relationships in rat lymph vessels. Lymphat Res Biol 10, 14–19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muthuchamy M Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. (2003). doi: 10.1096/fj.02-0626fje [DOI] [PubMed] [Google Scholar]
- 42.Wiśniewski JR, Zougman A, Nagaraj N & Mann M Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Nesvizhskii AI, Keller A, Kolker E & Aebersold R A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem 75, 4646–4658 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Keller A, Nesvizhskii AI, Kolker E & Aebersold R Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem 74, 5383–5392 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Mihara M et al. Pathological steps of cancer-related lymphedema: Histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 7, 1–10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogata F, Fujiu K, Koshima I, Nagai R & Manabe I Phenotypic modulation of smooth muscle cells in lymphoedema. Br. J. Dermatol 172, 1286–1293 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Lv S et al. A review of the postoperative lymphatic leakage. Oncotarget 8, 69062–69075 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen KC, D’Alessandro A, Clement CC & Santambrogio L Lymph formation, composition and circulation: A proteomics perspective. Int. Immunol 27, 219–227 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Bornfeldt KE et al. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J. Clin. Invest 93, 1266–74 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rzucidlo EM, Martin K. a. & Powell RJ Regulation of vascular smooth muscle cell differentiation. J. Vasc. Surg 45, 25–32 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Lee Y, Fluckey JD, Chakraborty S & Muthuchamy M Hyperglycemia- and hyperinsulinemia-induced insulin resistance causes alterations in cellular bioenergetics and activation of inflammatory signaling in lymphatic muscle. FASEB J. 31, 2744–2759 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiong M et al. Influence of glucose metabolism on vascular smooth muscle cell proliferation. Vasa (2013). doi: 10.1024/0301-1526/a000243 [DOI] [PubMed] [Google Scholar]
- 53.Natarajan R, Gonzales N, Xu L & Nadler JL Vascular smooth muscle cells exhibit increased growth in response to elevated glucose. Biochem. Biophys. Res. Commun 187, 552–560 (1992). [DOI] [PubMed] [Google Scholar]
- 54.Morrow D et al. Cyclic Strain Inhibits Notch Receptor Signaling in Vascular Smooth Muscle Cells In Vitro. (2005). doi: 10.1161/01.RES.0000159182.98874.43 [DOI] [PubMed] [Google Scholar]
- 55.Iwasaki H, Eguchi S, Ueno H, Marumo F & Hirata Y Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. 521–529 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Mata-greenwood E et al. Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-  1 and reactive oxygen species : a requirement for NAD (P) H oxidase. 59802, 288–298 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Song L et al. Downregulation of miR-223 and miR-153 mediates mechanical stretch-stimulated proliferation of venous smooth muscle cells via activation of the insulin-like growth factor-1 receptor. Arch. Biochem. Biophys 528, 204–211 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Weiler M, Kassis T & Dixon JB Sensitivity analysis of near-infrared functional lymphatic imaging. J. Biomed. Opt 17, 066019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caulk AW, Dixon JB & Gleason RL A lumped parameter model of mechanically mediated acute and long-term adaptations of contractility and geometry in lymphatics for characterization of lymphedema. Biomech. Model. Mechanobiol 15, 1601–1618 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su BY, Shontz KM, Flavahan NA & Nowicki PT The effect of phenotype on mechanical stretch-induced vascular smooth muscle cell apoptosis. J. Vasc. Res 43, 229–237 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Chiong M et al. Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Front. Cell Dev. Biol 2, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bennett MR, Evan G & Schwartz SM Apoptosis of Human Vascular Smooth Muscle Cells Derived from Normal Vessels and Coronary Atherosclerotic Plaques. (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brummelen P, Jie K & Zwieten P Alpha-adrenergic receptors in human blood vessels. Br. J. Clin. Pharmacol 21, 33S–39S (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Indolfi C et al. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med 3, 775–779 (1997). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.