Abstract
Ion channels are pore-forming protein complexes in membranes that play essential roles in a diverse array of biological activities. Ion channel activity is strictly regulated at multiple levels and by numerous cellular events to selectively activate downstream effectors involved in specific biological activities. For example, ions, binding proteins, nucleotides, phosphorylation, the redox state, channel subunit composition have all been shown to regulate channel activity and subsequently allow channels to participate in distinct cellular events. While these forms of modulation are well documented and have been extensively reviewed, in this article, we will first review and summarize channel proteolysis as a novel and quite widespread mechanism for altering channel activity. We will then highlight the recent findings demonstrating that proteolysis profoundly alters Inositol 1,4,5 trisphosphate receptor activity, and then discuss its potential functional ramifications in various developmental and pathological conditions.
Keywords: proteolysis; ion channels; Inositol 1,4,5 trisphosphate receptor
Introduction
Cells and intracellular organelles are bounded by biological membranes that function as electrical insulators. Ion channels are pore-forming protein complexes embedded in biological membranes that allow the rapid flux of ions in a direction dependent on their electrochemical gradient [1, 2]. By virtue of selectivity and exquisite control over activity, ion channels establish the resting cellular membrane potential and subsequently their behavior in response to cellular stimuli controls a multitude of physiological and pathological events [3–5].
In order to selectively control a diverse array of biological processes with fidelity and selectivity, ion channel activity is strictly regulated [1, 3]. Fundamentally, channel gating can be initiated following the binding of ligands, in response to changes in membrane potential or pH, together with sensing of mechanical stretch [1–3, 6] (Figure 1A). Channel activity is also frequently further fine-tuned by a myriad of regulatory molecules and inputs. A common theme is that the activity of specific channels is selectively regulated by the binding of ions, nucleotides and accessory proteins, together with phosphorylation and redox status and additional post-translational modifications [3, 7, 8]. A further consideration is that many ion channels are protein complexes assembled from multiple individual channel subunits [9, 10]. Thus, the particular subunit composition and their specific regulation can be a major determinant of the overall biophysical properties of an individual channel [3, 9].
Figure 1.
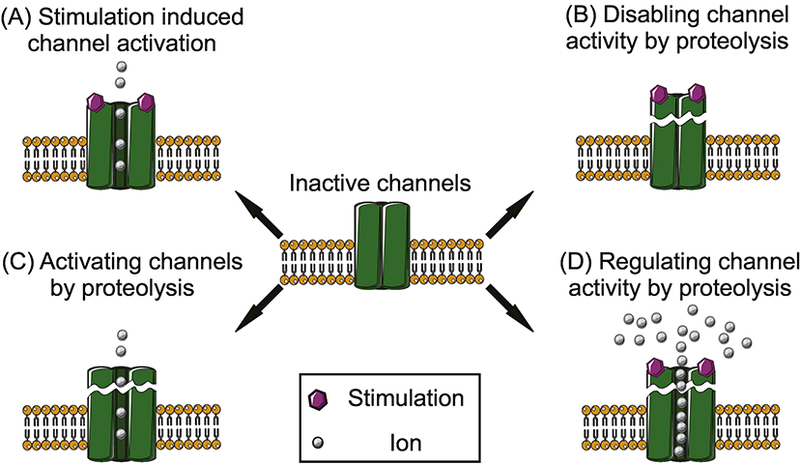
Schematic diagram summarizing different potential forms of proteolytic regulation of ion channels. In normal condition, ion channels are activated by the corresponding stimulus (A). Proteolytic fragmentation functions as a regulatory event, which can disable (B), activate (C), or regulate (C) channel activity in a channel specific manner.
While these “conventional” forms of regulation are well documented, recently a less orthodox, novel form of regulation has emerged as an event, widely employed to alter ion channel activity. Intracellular proteases, including caspase and calpain introduce peptide cleavage at specific sites and result in channel and isoform specific regulation of channel activity. Although, intuitively it might be expected that proteolysis might simply always function as a binary switch to deactivate ion channels, proteolysis can also alter the biophysical properties of channels to enhance or attenuate activity or influence the pharmacological properties of specific ion channels [11–15]. With major reference to proteins involved in Ca2+ signaling, or where proteolysis is regulated by Ca2+, we first highlight the diversity of ion channels subject to proteolysis as regulatory events and discuss the corresponding functional consequences of receptor cleavage. We then focus on recent findings which suggest that proteolytic cleavage of the ubiquitous intracellular Ca2+ release channel, the inositol 1,4,5 trisphosphate receptor (IP3R), profoundly alters activity in a subtype-specific fashion.
Disabling channel activities by proteolysis
Somewhat instinctively, a major consequence of ion channel proteolysis is the abrogation of activity as the proteins are disabled following proteolytic cleavage (Figure 1B). In this scenario, the abundance of functional ion channels and thus the macroscopic current density decrease with no influence per se on their individual biophysical properties. Examples are found for various types of ion channels including voltage-gated potassium channels (hERG) [16–18], acid-sensing ion channels (ASIC-1) [19], NMDA receptors (NR2A subunits) [20], the Store-Operated Calcium Entry (SOCE) machinery (STIM1) [21, 22], and chloride channels (CFTR) [23]. (See Table 1). Exemplars illustrative of the disabling of channel function from the calcium signaling field are described below.
Table 1:
Summary of the proteolytic regulation of ion channels
| Protein | Protease(s) | Cleavage site(s) | Functional outcome of proteolysis |
|---|---|---|---|
| hERG [16] | calpain, proteinase K, proteinase XIV and XXIV |
In the S5-pore linerGly- 603 for calpain |
Disable channel activity |
| NMDA receptor: NR2A subunit [20] |
calpain | After the amino acid 1051 in the C-terminal region |
Disable channel activity |
| STIM1 [21, 22, 26] |
calpain, γ-secretase and casepase-3 |
Disable channel activity | |
| CFTR [23] | calpain | Between the first nucleotide-binding site and the regulatory domain |
Disable channel activity |
| Voltage gated sodium channel: b2 subunit [89] |
BACE1 and γ secretase |
Disable channel activity | |
| ENaC [90] | furin | Arginine 205 and arginine 231 on the a subunit; arginine 143 on the γ subunit for furin |
Activate the channel |
| TRPC5 [31] | calpain | Threonine 857 | Activate the channel |
| Nav. 1.6 [11] | calpain | Decrease the activation threshold of INaP and increase its amplitude |
|
| ASIC-1a [12, 91] | trypsin, chymotrypsin and proteinase K |
Arginine 145 for trypsin | Shift both the pH dependence of channel activation and the steady- state channel inactivation to lower pH values; be resistant to the inhibition of venom of P. cambridgei and display faster recovery from inactivation than wild type channels |
| EAG2 [14] | calpain | Decrease current density; a positive shift in voltage dependence of channel activation; a lack of EAG2 signature fast activation |
|
| Cav 1.2 [32, 34] | calpain, proteasome | Alter channel voltage-current relationship and voltage-dependent channel inactivation |
|
| TRPM7 [13] | caspase | Aspartic acid 1510 | Potentiate TRPM7 channel activity |
| NMDA receptors: NR1 and NR2B subunits [39, 40] |
tissue plasminogen activator |
Arginine 260 in NR1 Arginine 67 in NR2B |
Enhance NMDA-mediated Ca2+ influx for NR1; reduce ifenprodil inhibition and increase glycine EC50 for NR2B |
| CNG channel [36] | metalloproteinase 2 and 9 |
Increase the apparent affinity for cGMP and the efficacy of cAMP to regulate CNG channel activity |
|
| R1 [15] | caspase and calpain | Aspartic acid 1891 for caspase; Glutamic acid 1917 for calpain |
Increase the frequency of Ca2+ oscillations and augment single channel open probability; abolish the PKA regulation of R1 |
| R2 and R3 [65] | Digestive enzymes | Third and fourth solvent exposed regions |
Decrease the frequency of Ca2+ oscillations; decrease the single channel open probability. |
NMDA receptors (NR2A subunits).
Prolonged stimulation of NMDA receptors results in calpain activation and the NR2A subunit of the NMDA receptor is a substrate for this protease [20]. Calpain activity ultimately leads to a reduction in NR2A protein levels. Inhibition of calpain by calpastatin expression prevents NMDA receptor degradation, promotes Ca2+ uptake, and subsequently potentiates agonist-induced cell death. These observations strongly suggest that NR2A proteolysis by calpain is protective and that this event prevents Ca2+ overload and thus abrogates excitotoxicity [20].
Store operated Ca2+ entry machinery: STIM1.
STIM1 is an ER transmembrane protein that closely monitors the Ca2+ concentration in the endoplasmic reticulum (ER) [24]. Upon ER Ca2+ depletion, STIM1 aggregates to form oligomers which interact with Orai1, the pore-forming subunit on the plasma membrane to result in Ca2+ influx. This process is termed store operated Ca2+ entry (SOCE) [25]. Calpain, γ-secretase and casepase-3 can cleave STIM1 thus regulating SOCE [21, 22, 26]. The Michalak group reported that calpain dynamically regulated STIM1 turnover and consequently SOCE through proteolysis of STIM1 proteins in both basal and apoptotic conditions. Silencing the regulatory subunit of calpain, CAPN4, inhibited calpain activity and blocked STIM1 fragmentation. This was associated with a substantial increase in the SOCE [22]. The transmembrane region of STIM1 has a similar sequence motif to that of amyloid precursor protein (GGVXIX) which is a substrate of γ-secretase. Indeed, this protease can cleave STIM1 to markedly reduce STIM1 oligomerization, and the degree of SOCE. Altered proteolysis of STIM1 is associated with detrimental effects. For example, mutations in PS1, a component of γ-secretase, were discovered in Familial Alzheimer and these patients exhibited a higher γ-secretase activity, enhanced STIM1 cleavage and lower SOCE [21]. Further, cultured hippocampal neurons expressing mutant γ-secretase had destabilized dendritic spines that were rescued by inhibition of γ-secretase activity or overexpression of STIM1 [21]. In addition, it has been shown that in submandibular gland acinar cells, exposure to ionizing radiation led to caspase-3 activation and consequently STIM1 cleavage through a TRPM2-dependent pathway. Proteolysis of STIM1 further resulted in a marked loss of SOCE, and consequently decrease in fluid secretion [26]. In summary, proteolytic fragmentation of STIM1 by calpain, γ-secretase and caspase-3 regulates the amount of functional STIM1 on the ER membrane and hence the extent of Ca2+ influx via the SOCE mechanism.
Cystic fibrosis transmembrane conductance regulator (CFTR).
CFTR is a chloride channel that plays a crucial role in fluid transport across epithelial cells [27, 28]. Calpain has been reported to initiate the turnover of CFTR by cleaving the full-length channel [23]. Although the fragmentation site was not identified, based on the size of the proteolytic products, the cleavage was predicted to occur between the first nucleotide-binding site and the regulatory domain. Intriguingly, calpain proteolysis does not lead to the immediate disassembly of the protein fragments but appears to only “mark” the protein for internalization. This concept was supported by the observation that fragmented CFTR peptides remained associated and were mainly evident in cytosolic fractions [23]. Subsequently, internalized CFTR was completely degraded via the lysosomal pathway. The turnover of CFTR is a dynamic process and is regulated by [Ca2+]i and the chaperone HSP90. A role for [Ca2+]i in calpain mediated CFTR turnover is not unexpected because Ca2+ is a major regulator of calpain activity. This also implies a correlation between CFTR function and intracellular Ca2+ homeostasis. In addition, in the presence of the chaperone, HSP90, degradation of fragmented CFTR was almost completely inhibited while the removal of full length CFTR was markedly decreased. These regulatory events define the ratio of full-length channel to the fragmented channel. In the future, it will be important to investigate whether the fragmented channel preserved by HSP90 has altered biophysical properties. Further, given that dysregulation of CFTR localization, expression and function are implicated in several serious diseases [27], it is conceivable that calpain mediated CFTR turnover may have fundamental biological significance.
Activating channels by proteolysis
The structures of the majority of ion channels, can be generally divided into two domains: 1) a channel domain, containing the ion conducting pore and 2) a regulatory domain that receives modulatory input and in so doing adjusts channel activity. In many cases, the regulatory domain inhibits channel activity by maintaining the channel in a closed state in the absence of the stimulatory input. Interestingly, there are examples showing that region specific proteolysis cleaves in the regulatory domain to eliminate any inhibitory effect, and thereby facilitates channel function (Figure 1C). Examples where cleavage results in an increase in activity include the electroneutral (epithelial) sodium channel (ENaC) and the Ca2+ conducting channel TRPC5 (see Table 1).
Transient receptor potential canonical 5 (TRPC5).
TRPC5 is a non-selective cation channel that is involved in regulating the number of neuronal processes and growth cones [29, 30]. Calpain 1 and 2 cleave TRPC5 at the C-terminus to produce a fragmented receptor approximately 15 kD smaller than the full-length channel [31]. Threonine 857 appears to be an essential sites for calpain 2 as mutation of this residue to alanine greatly reduced proteolysis [31]. Calpain mediated receptor fragmentation has been convincingly demonstrated to increase TRPC5 channel activity in multiple experimental settings. First, whole cell patch clamp recording showed that co-expression of constitutive active calpain with TRPC5 significantly increased the basal channel activity. This result was then confirmed in excised patches where purified calpains activated TRPC5 in a manner dependent on the catalytic activity of calpain. Furthermore, truncated TRPC5 channel was constitutively active, and the activity was further potentiated by an increase in [Ca2+] following stimulation of muscarinic receptors. Proteolytic fragmentation of TRPC5 has also been suggested to participate in semaphoring 3A-induced neuronal growth cone collapse as either inhibition of calpain activity or genetic knockout of TRPC5 attenuated this process [31].
Regulation of ion channels activities by proteolysis
In contrast to the previously described roles of proteolytic cleavage, emerging evidence suggests that region specific fragmentation can regulate channel activity by altering the biophysical properties or susceptibility of channels to pharmacological reagents (Figure 1D). It is likely that particular regulatory events require peptide continuity for the appropriate communication between the regulatory domains and the ion conducting pores. In this scenario, disruption of peptide continuity may eliminate, or alter, propagation of regulatory signals to the channel domain and thereby impact channel activity. To date, Na+ channels (Nav 1.6, ASIC-1) [11, 12], K+ channels (EAG2) [14], Ca2+ channels (Cav 1.2, TRPM7) [13, 32–34], and non-selective cation channels (CNG channels, NMDA receptors) [35, 36] have been reported as substrates whose biophysical properties are regulated by proteolysis (see Table 1). We describe several examples of Ca2+ conducting channels where proteolysis alters their properties and finally highlight how this form of regulation alters IP3R activity in a subtype dependent manner.
Voltage gated Ca2+ channel: Cav1.2.
Cav1.2 is a voltage-gated Ca2+ channel that contributes to Ca2+ influx in excitable cells [37]. Multiple sites in the Cav1.2 pore-forming subunit are subject to calpain and proteasome mediated proteolytic cleavage in an activity dependent manner [32–34]. Despite diverse fragmentation sites, all cleaved Cav1.2 channels share the common feature that they remain functional and membrane associated but nevertheless display a remarkable reduction in macroscopic current density [32]. Further, by expression of channels assembled from various complementary peptides representing cleavage products, it was shown that proteolysis at particular sites also dictates the current-voltage relationship and the extent of voltage-dependent channel inactivation [32]. Moreover, by simultaneously visualizing the N- and C- termini of these channels using confocal microscopy, it was shown that cleaved Cav1.2 could either remain associated or become separated into its peptide constituents. Remarkably, the dissociated channel fragments could also associate with intact channels and alter their biophysical properties [32]. Altogether, both the fragmentation site and the mobility of the fragmented channel contribute to regulation of Cav1.2 activity by proteolysis.
Transient receptor potential melastatin 7 (TRPM7).
Transient receptor potential melastatin 7 (TRPM7) is a unique Ca2+ channel which harbors serine/threonine kinase activity at its C-terminus. Knockout of TRPM7 inhibited activation and Fas-induced T cell apoptosis, thus indicating a prominent role of TRPM7 in this process [13]. Fas stimulation resulted in caspase mediated channel fragmentation at aspartic acid 1510, with the N-terminal fragment containing the complete sequence of the channel domain and the C-terminal fragment comprising the kinase domain. Cleaved TRPM7 channels displayed a significant higher current density but showed no alteration in their current-voltage relationship [13]. This suggests that caspase cleaves and potentiates TRPM7 channel activity. Further, while TRPM7 knockout T cells ectopically expressing a caspase cleavage resistant TRPM7 were impervious to Fas application, expression of cleaved TRPM7 effectively induced apoptosis even in the absence of Fas stimulation [13]. These data suggest that in response to Fas stimulation, caspase mediated channel fragmentation promotes TRPM7 channel activity and this event subsequently contributes to T cell apoptosis.
NMDA receptors (NR1 and NR2B subunit).
Tissue plasminogen activator (tPA) forms a complex with the NR1 subunit of the NMDA receptor and regulates the receptor activity through proteolytic cleavage [38, 39]. The Buisson group reported that tPA activity was dynamically increased in cortical neurons [38]. In response to depolarization, biologically active tPA was released into the extracellular space and fragmented NR1 subunit at Arginine 260 in the N-terminus (39). tPA pretreatment enhanced NMDA-mediated Ca2+ influx, which was blocked by mutation of Arg 260, which renders the channel resistant to tPA proteolysis (39). More importantly, tPA potentiated the NMDA mediated excitotoxicity in a dose-dependent manner presumably as a result of NMDA receptor fragmentation.
The NR2B subunit of the NMDA receptor is also subject to proteolytic regulation by tissue plasminogen activator (tPA) [40]. A truncated NR2B protein, designed based on the predicted site of tPA cleavage was unaffected in terms of glutamate activation but exhibited reduced propensity to be inhibited by the antagonist ifenprodil and displayed an increased affinity for glycine. In total, this information demonstrates that tPA can cleave both NR1 and NR2B, but exerts distinct regulation of individual subunits; fragmentation of NR1 markedly enhances channel activity while cleavage of NR2B dramatically alters this subunits pharmacological properties.
Cyclic nucleotide-gated channel (CNG channel).
Cyclic nucleotide gated ion channels (CNG channels) couple the light induced alteration in cGMP concentration with electrical signals and underlie mammalian phototransduction [41, 42]. It has been reported that metalloproteinase 2 and 9 (MMP2 and MMP9) cleave, and thereby alter, both the biophysical and the pharmacological properties of CNG channels [35, 36]. Specifically, extracellular exposure to MMP2 or MMP9, significantly increased the apparent affinity for cGMP and the efficacy of cAMP to regulate CNG channel activity [36]. This is consistent with the observation that exposure of CNG channels to MMP9 substantially augmented the single channel open probability following cGMP exposure [29]. Interestingly, the degree of MMP-dependent CNG channels fragmentation is dictated by the extent of channel glycosylation, as this post-translational modification prevents CNG channel cleavage [35]. Of note, in mouse retina, aged mice exhibited an elevated level of MMP9 activity with a concomitant higher level of fragmented CNGA1 channels [36] and thus, the impact of cleaved CNG channels in the retina during aging remains an exciting field for further investigation.
Proteolytic regulation of IP3R
IP3R are ubiquitous, intracellular Ca2+ release channels predominantly expressed on the endoplasmic reticulum membrane [3, 7, 43] IP3R are capable of regulating a diverse array of cellular activities including, gene expression, cell proliferation, differentiation, migration, muscle contraction, digestive enzyme secretion, and cell death [3, 7, 44–46]. To regulate the diverse activities mentioned above with high specificity and fidelity, IP3R activate downstream signaling cascades by generating Ca2+ signals with distinct spatial and temporal characteristics. The versatility of Ca2+ signals mediated by IP3R is conferred by a series of complex regulatory events. For example, in addition to IΡ3 [47], Ca2+ acts as a co-agonist and regulates IP3R activity in a biphasic manner [48]. In particular, low concentrations of Ca2+ activate, whereas high concentrations of Ca2+, inhibit IP3R activity. This underlies the ability of IP3R to contribute to the generation of oscillatory Ca2+ signals even when the IP3 concentration is constant [49]. Further, nucleotides can bind to and modulate IP3R channel activity in the presence of elevated IP3 and Ca2+ [8, 48]. For example, ATP regulates IP3R in an isoform-specific manner. Specifically, IP3R type 1 (R1) and IP3R type 3 (R3) are regulated by ATP at all concentrations of IP3, while the activity of IP3R type 2 (R2) is only regulated at sub-maximal concentration of IP3 [8, 48]. Numerous proteins function as binding partners and modulate IP3R properties including the IP3 binding affinity, Ca2+ dependency, cellular localization, receptor turnover, and channel activity [7, 44, 50]. In addition, post-translational modification such as phosphorylation, ubiquitination, and redox state also have profound effects to alter and fine-tune IP3R activity [8, 51, 52].
In the last decade, our lab and others have investigated how proteolytic cleavage of IP3R influences activity [15, 53–56]. All three isoforms of IP3R are substrates of proteases. Early in vitro studies investigating IP3R domain structure demonstrated that exposure of R1 to low concentrations of trypsin resulted in five predictable and reproducible receptor fragments (Figure 2A/B) [57–59]. These data were interpreted to indicate that R1 comprises five compact globular domains, interconnected by four solvent exposed linker regions. Notably, the first four tryptic fragments comprise the IΡ3 binding core and much of the cytosolic domain, while the fifth fragment constitutes the transmembrane domain consisting of six helices encompassing the Ca2+ permeation pore and the cytosolic C-terminal tail [57]. Notably, IP3R can also be cleaved in vivo and in vitro by intracellular proteases. Both caspase and calpain have been shown to cleave R1 at the fourth solvent exposed linker region and produce at least two fragments (Figure 2C/D) [56, 60, 61].
Figure 2.
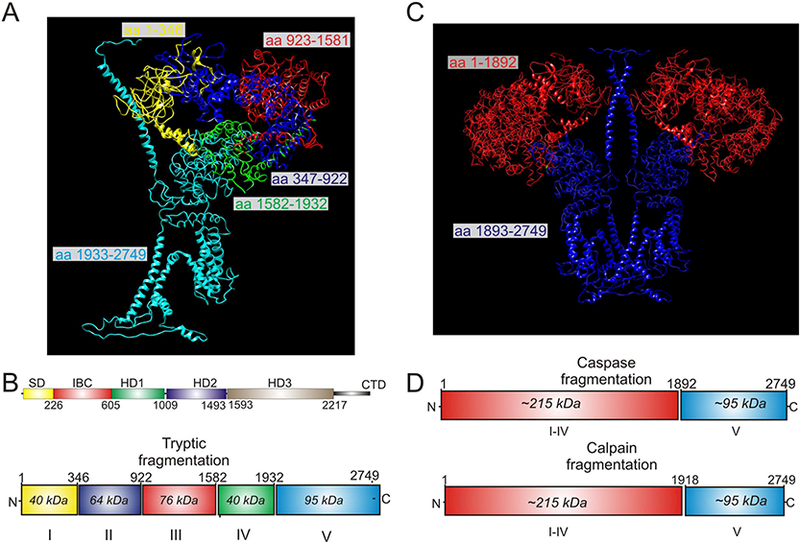
Proteolytic fragments mapped to the Cryo-EM structure of R1. A and C are based on pdb 3JAV. A depicts a single subunit of R1 in which the five tryptic fragments generated by limited trypsin digestion are color coded. B shows these fragments in the linear structure. C Depicts a dimer of R1 in which the soluble (red) and membrane fragments (blue) formed by calpain and caspase activity in vivo are shown. D shows the calpain and caspase fragments in the linear structure of R1.
Given the high sequence homology among all three isoforms, R2 and R3 are predicted to have the same overall structure as R1 and be subject to protease cleavage [57, 62, 63]. For example, although the proteases were not definitively identified, in an early study it was shown that R2 and R3 were proteolytically processed into lower mass products in pancreatic acinar cells in models of the inflammatory disease, acute pancreatitis [64]. Remarkably, inhibition of proteasome activity failed to eliminate receptor fragmentation, indicating that other proteases, likely inappropriately and prematurely activated digestive enzymes, account for the proteolytic cleavage in acinar cells. Consistent with this idea, using an in vitro acute pancreatitis model where isolated pancreatic acinar cells were incubated with bile salts, R2/3 fragmentation was also readily evident [65]. Notably, in this case, fragmentation was ameliorated by pre-incubation with cell permeable trypsin inhibitors. These data indicate that IP3R fragmentation may be a common event in acute pancreatitis and that trypsin activity is either directly or indirectly involved in the process of R2 and R3 fragmentation. Furthermore, these observations provide evidence that proteases can cleave R2 and R3 at specific sites and produce receptor fragments in “primary” cells. R2 and R3 have also been reported to be substrates of caspase and calpain [66]. However, whether there are reproducible fragmentation patterns resulting from the activity of these enzymes requires further investigation.
The biochemical characteristics of fragmented IP3R were first thoroughly investigated in cells expressing R1 in isolation. Staurosporine, a general kinase inhibitor, which promotes caspase and calpain activation and apoptosis resulted in IP3R fragmentation in DT40–3KO cells (null for IP3R) stably re-expressing R1. Notably, native gel analysis demonstrated that all fragmented R1 migrated at an identical molecular weight to the intact R1 tetramer, indicating that receptors retain tetrameric architecture even after proteolysis [56]. These data are consistent with a previous report showing that R1 fragments generated following exposure to trypsin remained associated, presumably through non-covalent interactions [58]. Moreover, receptor fragments were not present in the cytosol, but were retained in ER membranes [56]. Taken together, these data demonstrated that R1 are retained in the ER as a tetramer after proteolytic fragmentation. The native structure of the proteolytically cleaved R2 and R3 was also investigated. Given the same overall topology is shared among all three isoforms of IP3R, it was not unexpected that R2 and R3, like R1 were shown to retain tetrameric architecture and remain membrane associated after proteolysis [65].
Studying the function of fragmented IP3R is a major challenge for a number of reasons. Principally, following protease activation, many proteins and molecules associated with IP3R regulation may also be protease substrates and thus any functional readout is likely influenced by cleavage of a multitude of factors which potentially influence IP3R activity. In addition, induction of protease activation using conventional methods, such as by staurosporine treatment and in acute pancreatitis models [56, 64], only cleaves an undefined portion of the IP3R present. Thus, the functional readout is the net output of the activities of full length and fragmented IP3R. These issues can be largely negated by expression of complementary receptor fragments from individual cDNAs, designed based on predicted protease sites and studying their function following stable expression in IP3R null cells. By exploiting this strategy, we aimed to answer a number of major unanswered questions. For example, are tetrameric IP3R assembled from complementary peptide chains and are fragmented IP3R functionally gated by IP3? Further, do fragmented IP3R have distinct biophysical properties compared to full length IP3R? To address the first issue, co-expression of pairs of complementary peptide chains was demonstrated to result in the assembly of the tetrameric IP3R as determined by the native gel analysis [56]. This finding also suggested that the function of the fragmented IP3R could be investigated using this general approach. Indeed, activation of cell surface Gq protein-coupled receptors were shown to evoke robust Ca2+ signals in cells expressing any pair of complementary IP3R fragments representing various proteolytic cleavage events [15, 56, 67]. More strikingly, it was demonstrated that functional IP3R can be assembled from 20 individual polypeptides, encoded by individual cDNA’s designed based on the five fragments that result from in vitro trypsin activity [15]. An important general implication of these data is that peptide continuity per se is obviously not necessary for IP3R gating.
While R1 gating does not appear to require peptide continuity, it is possible that particular forms of allosteric regulation require communication of a conformational change throughout the peptide chain. It is well established that when IP3R isoforms are expressed in isolation, that individual subtypes support characteristic temporal patterns of agonist-evoked Ca2+ release. For example, stimulation of cells solely expressing R1 always results in a few, irregular Ca2+ transients, (Figure 3A) whereas stimulation of R2 and R3 initiates robust Ca2+oscillations [68–70]. We interpret this observation as reflecting the net integrated output of the specific regulation of the activity of each isoform. One intriguing question is whether disruption of peptide continuity, while not markedly impacting IP3-induced gating of the channel, influences communication between regulatory inputs and the channel domain, and consequently regulates overall IP3R activity. To address this question, we expressed complementary peptide R1 fragments designed based on proteolytic products observed under conditions where R1 is fragmented in vivo. Intriguingly, cells expressing complementary fragments representing caspase, calpain or tryptic (I-III+IV-V) (Figure 2) fragmented R1, supported, in stark contrast to the intact R1, robust oscillatory Ca2+ signals following stimulation (Figure 3C and D) [15, 71]. This effect appears to be site specific, as receptor fragments mimicking fragmentation introduced at the second solvent exposed linker region showed no alteration in the temporal profile of Ca2+ signals (Figure 2B) [67]. In addition, caspase mediated receptor fragmentation significantly increased single channel open probability [15]. In total, these data suggest strongly that proteolytic fragmentation increases R1 channel activity and subsequent alterations in the effect of regulatory input results in an increase in oscillatory activity.
Figure 3.
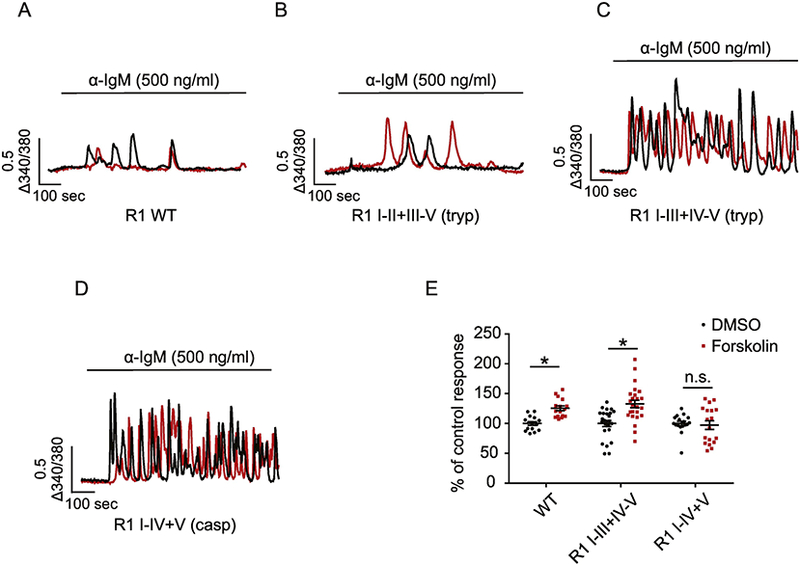
Region-specific proteolysis regulates R1 activity. Single cell Ca2+ imaging was performed using cells expressing DT$) 3KO cells stably expressing R1 WT (A), R1 I-II+III-V (tryptic fragments) (B), R1 I-III+IV-V (tryptic fragments) (C) and R1 I-IV+V (caspase fragments) (D). Cells were loaded with fura-2AM followed by anti-IgM stimulation, which cross-linked the cell surface B cell receptors and induced continuous production of intracellular IP3. In response to anti-IgM stimulation, cells expressing R1 WT (A) and R1 I-II+III-V (tryptic fragments) (B) evoked few Ca2+ transients while cells expressing R1 I-III+IV-V (tryptic fragments) (C) and R1 I-V+V (caspase fragments) (D) exhibited robust Ca2+ oscillations. Two representative single cell calcium traces (black and red) were shown for each condition. In (E), pre-incubation of cells with 20 μM forskolin for 2 min activated PKA and phosphorylated R1. This resulted in significant increases in the amplitude of Ca2+ signals mediated by the full length R1 and fragmented R1 I-III+IV-V, but not R1 I-IV+V. Each point represents one experiment. * indicates p< 0.05. Adapted from [15] with permission.
Based on these observations, we asked whether specific forms of regulation were altered by fragmentation. Phosphorylation by protein kinase A (PKA) is an established and important regulator of R1 activity that potentiates R1 mediated Ca2+ signals [15, 72]. PKA-phosphorylation in tryptic fragment IV at S1755 and S1589 is responsible for this effect. Interestingly, receptor fragmentation introduced to separate the phosphorylation sites and the channel domain into discrete fragments (e.g. R1 I-IV+V) abolished PKA regulation. Notably, when fragmentation was introduced more toward to the N-terminus (e.g. R1 I-III+IV-V), which left the phosphorylation sites and the channel domain in the same peptide fragment, PKA regulation was maintained [15]. One explanation for this region-specific receptor regulation is that some forms of regulation rely on direct peptide continuity for appropriate communication between the regulatory sites and the channel domain. In this scenario, proteolysis disrupts this communication and subsequently either abolishes or alters the regulatory outcome.
Notably proteolytic fragmentation also regulates R2 and R3 activity in a region-specific manner [66]. Using a similar experimental strategy, it was shown that while intact R2 and R3 support robust Ca2+ oscillations in response to sustained production of IP3 (Figure 4A and D), receptor fragmentation introduced at the third or fourth, but not the second, solvent exposed regions suppress the Ca2+ oscillatory activity (Figure 4B, C, E, F). Thus, remarkably IP3R fragmentation results in isoform specific effects. Based on the cryo-EM structure of R1 [73], these data suggest that proteolytic fragmentation must occur more toward to the C-terminal domain, between the ARM (armadillo solenoid folds) domains and the channel domain, to alter the temporal profile of Ca2+ signals mediated by IP3R. The ARM domains are proposed to generate interfaces which bind regulatory molecules. Similar to the effect on PKA regulation of R1, proteolytic fragmentation in the solvent exposed region three and four may disrupt the appropriate transmission of the regulatory inputs from the ARM domains to the channel domain, which impact the signature temporal profile of Ca2+ signals of all three isoforms of IP3R, but in an isoform specific manner.
Figure 4.
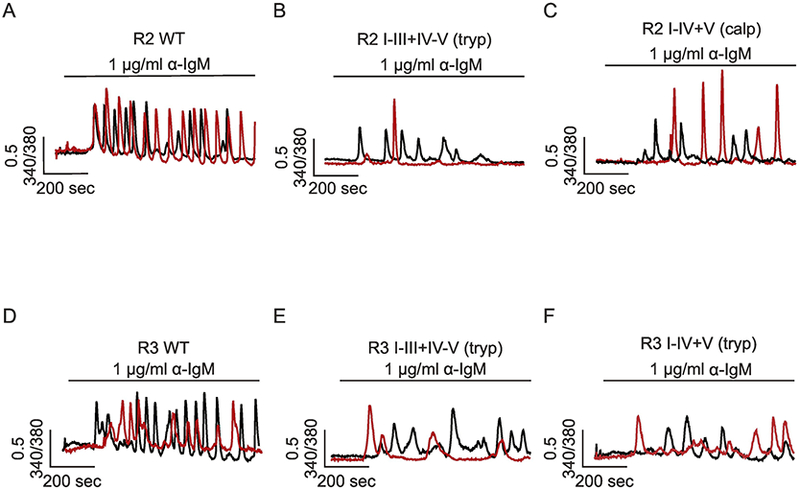
Region-specific proteolysis regulates R2 and R3 activity. Single cell Ca2+ imaging was performed using cells DT40–3KO cells stably expressing R2 WT (A), R2 I-III+IV-V (tryptic fragments) (B), R2 I-V+V (calpain fragments) (C), R3 WT (D), R3 I-III+IV-V (tryptic fragments) (E), R3 I-IV+V (tryptic fragments) (F). In response to anti-IgM stimulation, cells expressing R2 and R3 WT (A and D) evoked robust Ca2+ oscillations, while cells expressing R2 I-III+IV-V (tryptic fragments) (B), R2 I-V+V (calpain fragments) (C), R3 I-III+IV-V (tryptic fragments) (E), or R3 I-IV+V (tryptic fragments) (F) exhibited only a few irregular transients. Two representative single cell calcium traces (black and red) were shown for each condition. Adapted from [15] with permission.
Why does receptor fragmentation differentially regulate different isoforms of IP3R? A current mathematical model suggests that the frequency of oscillatory Ca2+ signals mediated by IP3R can be dictated by the rate at which Ca2+ activates and inactivates the receptor [71]. Full-length IP3R may be regulated by Ca2+ in a subtype-specific manner and therefore show distinct temporal characteristics of Ca2+ signals [69, 74]. We speculate that proteolysis differentially affects the Ca2+ regulation of each isoform and exerts subtype-specific regulation. Support for this hypothesis is suggested by the alteration in the subtype-specific effect of single channel open probability (Po) in response to receptor fragmentation [15]. Specifically, upon receptor fragmentation, an increase in the Po for R1 or a decrease for R2 may result from the alteration of the rate of IP3R to be activated or inactivated by Ca2+.
Physiological and pathophysiological roles for fragmented IP3R.
The differential regulation of each IP3R by proteolysis may have both physiological and pathological ramifications. For example, emerging evidence shows that both caspase and calpain are activated and play essential roles in developmental processes such as neural differentiation and skeletal muscle myoblast differentiation [75–77]. Of note, in these tissues, the activity of R1 is also pivotal. For example, mice with global knockout of R1 largely died in utero, and the surviving animals developed severe ataxia and epileptic seizures, and eventually died soon after weaning [78]. This strongly suggests that R1 is necessary for brain development and function. Consistent with this idea, Ca2+ release through R1 is critical for neuronal sprouting and arborization [79, 80]. It is tempting to speculate that low level caspase and/or calpain leads to R1 fragmentation, altered Ca2+ signals and initiation of a developmental gene program. Similarly, there also appears to be a role for R1 in skeletal muscle myoblast differentiation which occurs on a background of increased cellular protease activity. Interestingly, both the mRNA and protein levels of R1 are greatly increased during myoblast differentiation and knockdown of R1 results in a marked reduction in the expression of muscle-specific transcription factors [81]. These experimental systems provide potential platforms to investigate crosstalk between R1 and protease activity and future work is required to investigate the potential functional role of fragmented R1 during these developmental processes.
Fragmentation of both R2 and R3 were first described in pancreatic acinar cells in a model of acute pancreatitis [64], however, the functional significance has remained elusive. Ca2+signals, specifically those that result in the prolonged cytosolic Ca2+ overload, have been suggested to play important roles in the etiology of acute pancreatitis [82–84]. In support of this contention, various strategies aimed at inhibiting the Ca2+ overload, including inhibition of IP3R, show protective effects [85–88]. Of note, region-specific receptor fragmentation occurs at an early stage during acute pancreatitis and substantially decreases the total Ca2+ release mediated by IP3R, which consequently reduces the possibility of ER Ca2+ depletion and the subsequent Ca2+ influx [65]. These data suggest that proteolytic fragmentation of R2 and R3 may have a protective effect by preventing pathological Ca2+ signals and consequently delaying the progress of acute pancreatitis.
Summary
In conclusion, we summarize evidence showing that proteolysis acts as a regulatory event for a diverse array of ion channels. Proteolytic fragmentation can induce, inhibit, or mediate channel activity both in physiological and pathological conditions. This modulation is accomplished by cleaving either the pore-forming subunits or auxiliary proteins. Further, proteolytic regulation has a high specificity and selectivity; many proteases only cleave channels harboring unique motifs and thus leave others intact. This ensures precise regulation of downstream signaling cascades without uncontrolled cellular damage. We also summarize the evidence showing that all three isoforms of IP3R are substrates of proteases and fragmented IP3R are still properly gated by IP3 binding. Further, region-specific receptor fragmentation differentially regulates all three isoforms of IP3R. While proteolytic fragmentation significantly increases the frequency of Ca2+oscillations mediated through R1 activation, disruption of peptide continuity can decrease the oscillatory activity mediated through R2 and R3. In total, burgeoning evidence indicates that proteolysis may be a common regulatory event for a large number of ion channels. This irreversible form of modification adds a unique regulatory mechanism by expanding the repertoire of modes of channel regulation that a particular channel is subject to.
Highlights.
Ion channels are exquisitely regulated to fine tune function.
A novel form of channel regulation is by proteolysis
Proteolysis alters channel function to disable, activate of change channel activity
Calcium regulated and permeable channels are subject to this form of regulation
As examples, Inositol 1,4,5 trisphosphate receptors are regulated in a subtype specific manner following proteolysis by calpain, caspase and trypsin.
Acknowledgements
This work was supported by NIH grants: RO1 DE14756 and DE019245.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hille B, Ion channels of excitable membranes, Sinaur Associates, (2001) 1–22. [Google Scholar]
- [2].Hille B, Ion channels, scholarpedia, 3 (2008) 6051. [Google Scholar]
- [3].Chandrasekhar R, Yule DI, Wang L, Inositol 1,4,5-trisphosphate receptors (InsP3R), PANCREAPEDIA (Exocrine Pancreas Knowledge Base), 2017. [Google Scholar]
- [4].Hisatsune C, Mikoshiba K, IP3 receptor mutations and brain diseases in human and rodents, Journal of neurochemistry, 141 (2017) 790–807. [DOI] [PubMed] [Google Scholar]
- [5].Berridge MJ, Lipp P, Bootman MD, The versatility and universality of calcium signalling, Nat Rev Mol Cell Biol, 1 (2000) 11–21. [DOI] [PubMed] [Google Scholar]
- [6].Ranade SS, Syeda R, Patapoutian A, Mechanically Activated Ion Channels, Neuron, 87 (2015) 1162–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Foskett JK, White C, Cheung KH, Mak DO, Inositol trisphosphate receptor Ca2+ release channels, Physiol Rev, 87 (2007) 593–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yule DI, Betzenhauser MJ, Joseph SK, Linking structure to function: Recent lessons from inositol 1,4,5-trisphosphate receptor mutagenesis, Cell calcium, 47 (2010) 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chandrasekhar R, Alzayady KJ, Wagner LE 2nd, Yule DI, Unique Regulatory Properties of Heterotetrameric Inositol 1,4,5-Trisphosphate Receptors Revealed by Studying Concatenated Receptor Constructs, The Journal of biological chemistry, 291 (2016) 4846–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, Hendrickson WA, Frank J, Marks AR, Structure of a mammalian ryanodine receptor, Nature, 517 (2015)44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brocard C, Plantier V, Boulenguez P, Liabeuf S, Bouhadfane M, Viallat-Lieutaud A, Vinay L, Brocard F, Cleavage of Na(+) channels by calpain increases persistent Na(+) current and promotes spasticity after spinal cord injury, Nat Med, 22 (2016) 404–411. [DOI] [PubMed] [Google Scholar]
- [12].Vukicevic M, Weder G, Boillat A, Boesch A, Kellenberger S, Trypsin cleaves acid-sensing ion channel 1a in a domain that is critical for channel gating, The Journal of biological chemistry, 281 (2006) 714–722. [DOI] [PubMed] [Google Scholar]
- [13].Desai BN, Krapivinsky G, Navarro B, Krapivinsky L, Carter BC, Febvay S, Delling M, Penumaka A, Ramsey IS, Manasian Y, Clapham DE, Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis, Dev Cell, 22 (2012) 1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shimizu N, Sato N, Kikuchi T, Ishizaki T, Kobayashi K, Kita K, Takimoto K, A sustained increase in the intracellular Ca(2)(+) concentration induces proteolytic cleavage of EAG2 channel, Int J Biochem Cell Biol, 59 (2015) 126–134. [DOI] [PubMed] [Google Scholar]
- [15].Wang L, Wagner LE 2nd, Alzayady KJ, Yule DI, Region-specific proteolysis differentially regulates type 1 inositol 1,4,5-trisphosphate receptor activity, The Journal of biological chemistry, 292 (2017) 11714–11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lamothe SM, Guo J, Li W, Yang T, Zhang S, The Human Ether-a-go-go-related Gene (hERG) Potassium Channel Represents an Unusual Target for Protease-mediated Damage, The Journal of biological chemistry, 291 (2016) 20387–20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang N, Kang HS, Ahmmed G, Khan SA, Makarenko VV, Prabhakar NR, Nanduri J, Calpain activation by ROS mediates human ether-a-go-go-related gene protein degradation by intermittent hypoxia, Am J Physiol Cell Physiol, 310 (2016) C329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rajamani S, Anderson CL, Valdivia CR, Eckhardt LL, Foell JD, Robertson GA, Kamp TJ, Makielski JC, Anson BD, January CT, Specific serine proteases selectively damage KCNH2 (hERG1) potassium channels and I(Kr), Am J Physiol Heart Circ Physiol, 290 (2006) H1278–1288. [DOI] [PubMed] [Google Scholar]
- [19].Clark EB, Jovov B, Rooj AK, Fuller CM, Benos DJ, Proteolytic cleavage of human acid-sensing ion channel 1 by the serine protease matriptase, The Journal of biological chemistry, 285 (2010) 27130–27143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guttmann RP, Sokol S, Baker DL, Simpkins KL, Dong Y, Lynch DR, Proteolysis of the N-methyl-d-aspartate receptor by calpain in situ, J Pharmacol Exp Ther, 302 (2002) 1023–1030. [DOI] [PubMed] [Google Scholar]
- [21].Tong BC, Lee CS, Cheng WH, Lai KO, Foskett JK, Cheung KH, Familial Alzheimer’s disease-associated presenilin 1 mutants promote gamma-secretase cleavage of STIM1 to impair store-operated Ca2+ entry, Sci Signal, 9 (2016) ra89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Prins D, Michalak M, STIM1 is cleaved by calpain, FEBS Lett, 589 (2015) 3294–3301. [DOI] [PubMed] [Google Scholar]
- [23].Averna M, Stifanese R, Grosso R, Pedrazzi M, De Tullio R, Salamino F, Pontremoli S, Melloni E, Role of calpain in the regulation of CFTR (cystic fibrosis transmembrane conductance regulator) turnover, The Biochemical journal, 430 (2010) 255–263. [DOI] [PubMed] [Google Scholar]
- [24].Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD, STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane, Nature, 437 (2005) 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hogan PG, Rao A, Store-operated calcium entry: Mechanisms and modulation, Biochem Biophys Res Commun, 460 (2015) 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu X, Gong B, de Souza LB, Ong HL, Subedi KP, Cheng KT, Swaim W, Zheng C, Mori Y, Ambudkar IS, Radiation inhibits salivary gland function by promoting STIM1 cleavage by caspase-3 and loss of SOCE through a TRPM2-dependent pathway, Sci Signal, 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Saint-Criq V, Gray MA, Role of CFTR in epithelial physiology, Cell Mol Life Sci, 74 (2017) 93–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Frizzell RA, Hanrahan JW, Physiology of epithelial chloride and fluid secretion, Cold Spring Harb Perspect Med, 2 (2012) a009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Greka A, Navarro B, Oancea E, Duggan A, Clapham DE, TRPC5 is a regulator of hippocampal neurite length and growth cone morphology, Nat Neurosci, 6 (2003) 837–845. [DOI] [PubMed] [Google Scholar]
- [30].Davare MA, Fortin DA, Saneyoshi T, Nygaard S, Kaech S, Banker G, Soderling TR, Wayman GA, Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons, J Neurosci, 29 (2009) 9794–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kaczmarek JS, Riccio A, Clapham DE, Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse, Proceedings of the National Academy of Sciences of the United States of America, 109 (2012) 7888–7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Michailidis IE, Abele-Henckels K, Zhang WK, Lin B, Yu Y, Geyman LS, Ehlers MD, Pnevmatikakis EA, Yang J, Age-related homeostatic midchannel proteolysis of neuronal L-type voltage-gated Ca(2)(+) channels, Neuron, 82 (2014) 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hell JW, Westenbroek RE, Breeze LJ, Wang KK, Chavkin C, Catterall WA, N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons, Proceedings of the National Academy of Sciences of the United States of America, 93 (1996) 3362–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Groth RD, Tirko NN, Tsien RW, CaV1.2 calcium channels: just cut out to be regulated?, Neuron, 82 (2014) 939–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Meighan SE, Meighan PC, Rich ED, Brown RL, Varnum MD, Cyclic nucleotide-gated channel subunit glycosylation regulates matrix metalloproteinase-dependent changes in channel gating, Biochemistry, 52 (2013) 8352–8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meighan PC, Meighan SE, Rich ED, Brown RL, Varnum MD, Matrix metalloproteinase-9 and −2 enhance the ligand sensitivity of photoreceptor cyclic nucleotide-gated channels, Channels (Austin), 6 (2012) 181–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J, International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels, Pharmacol Rev, 57 (2005) 411–425. [DOI] [PubMed] [Google Scholar]
- [38].Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A, The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling, Nat Med, 7 (2001) 59–64. [DOI] [PubMed] [Google Scholar]
- [39].Fernandez-Monreal M, Lopez-Atalaya JP, Benchenane K, Cacquevel M, Dulin F, Le Caer JP, Rossier J, Jarrige AC, Mackenzie ET, Colloc’h N, Ali C, Vivien D, Arginine 260 of the amino-terminal domain of NR1 subunit is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-D-aspartate receptor signaling, The Journal of biological chemistry, 279 (2004) 50850–50856. [DOI] [PubMed] [Google Scholar]
- [40].Ng KS, Leung HW, Wong PT, Low CM, Cleavage of the NR2B subunit amino terminus of N-methyl-D-aspartate (NMDA) receptor by tissue plasminogen activator: identification of the cleavage site and characterization of ifenprodil and glycine affinities on truncated NMDA receptor, The Journal of biological chemistry, 287 (2012) 25520–25529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaupp UB, Seifert R, Cyclic nucleotide-gated ion channels, Physiol Rev, 82 (2002) 769–824. [DOI] [PubMed] [Google Scholar]
- [42].Matulef K, Zagotta WN, Cyclic nucleotide-gated ion channels, Annu Rev Cell Dev Biol, 19 (2003)23–44. [DOI] [PubMed] [Google Scholar]
- [43].Alzayady KJ, Sebe-Pedros A, Chandrasekhar R, Wang L, Ruiz-Trillo I, Yule DI, Tracing the Evolutionary History of Inositol, 1, 4, 5-Trisphosphate Receptor: Insights from Analyses of Capsaspora owczarzaki Ca2+ Release Channel Orthologs, Mol Biol Evol, 32 (2015) 2236–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Patterson RL, Boehning D, Snyder SH, Inositol 1,4,5-trisphosphate receptors as signal integrators, Annu Rev Biochem, 73 (2004) 437–465. [DOI] [PubMed] [Google Scholar]
- [45].Mound A, Vautrin-Glabik A, Foulon A, Botia B, Hague F, Parys JB, Ouadid-Ahidouch H, Rodat-Despoix L, Downregulation of type 3 inositol (1,4,5)-trisphosphate receptor decreases breast cancer cell migration through an oscillatory Ca(2+) signal, Oncotarget, 8 (2017) 72324–72341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Luyten T, Welkenhuyzen K, Roest G, Kania E, Wang L, Bittremieux M, Yule DI, Parys JB, Bultynck G, Resveratrol-induced autophagy is dependent on IP3Rs and on cytosolic Ca(2), Biochimica et biophysica acta, 1864 (2017) 947–956. [DOI] [PubMed] [Google Scholar]
- [47].Alzayady KJ, Wang L, Chandrasekhar R, Wagner LE 2nd, Van Petegem F, Yule DI, Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release, Sci Signal, 9 (2016) ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wagner LE 2nd, Yule DI, Differential regulation of the InsP(3) receptor type-1 and −2 single channel properties by InsP(3), Ca(2)(+) and ATP, J Physiol, 590 (2012) 3245–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dupont G, Combettes L, Bird GS, Putney JW, Calcium oscillations, Cold Spring Harb Perspect Biol, 3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schulman JJ, Wright FA, Kaufmann T, Wojcikiewicz RJ, The Bcl-2 protein family member Bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage, The Journal of biological chemistry, 288 (2013) 25340–25349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wright FA, Wojcikiewicz RJ, Chapter 4 - Inositol 1,4,5-Trisphosphate Receptor Ubiquitination, Prog Mol Biol Transl Sci, 141 (2016) 141–159. [DOI] [PubMed] [Google Scholar]
- [52].Bansaghi S, Golenar T, Madesh M, Csordas G, RamachandraRao S, Sharma K, Yule DI, Joseph SK, Hajnoczky G, Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IΡ3) receptors by reactive oxygen species, The Journal of biological chemistry, 289 (2014) 8170–8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Assefa Z, Bultynck G, Szlufcik K, Nadif Kasri N, Vermassen E, Goris J, Missiaen L, Callewaert G, Parys JB, De Smedt H, Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis, The Journal of biological chemistry, 279 (2004) 43227–43236. [DOI] [PubMed] [Google Scholar]
- [54].Elkoreh G, Blais V, Beliveau E, Guillemette G, Denault JB, Type 1 inositol-1,4,5-trisphosphate receptor is a late substrate of caspases during apoptosis, Journal of cellular biochemistry, 113 (2012) 2775–2784. [DOI] [PubMed] [Google Scholar]
- [55].Kopil CM, Siebert AP, Foskett JK, Neumar RW, Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor impairs ER Ca(2+) buffering and causes neurodegeneration in primary cortical neurons, Journal of neurochemistry, 123 (2012) 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Alzayady KJ, Chandrasekhar R, Yule DI, Fragmented inositol 1,4,5-trisphosphate receptors retain tetrameric architecture and form functional Ca2+ release channels, The Journal of biological chemistry, 288 (2013) 11122–11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bezprozvanny I, The inositol 1,4,5-trisphosphate receptors, Cell calcium, 38 (2005) 261–272. [DOI] [PubMed] [Google Scholar]
- [58].Yoshikawa F, Iwasaki H, Michikawa T, Furuichi T, Mikoshiba K, Trypsinized cerebellar inositol 1,4,5-trisphosphate receptor. Structural and functional coupling of cleaved ligand binding and channel domains, The Journal of biological chemistry, 274 (1999) 316–327. [DOI] [PubMed] [Google Scholar]
- [59].Joseph SK, Pierson S, Samanta S, Trypsin digestion of the inositol trisphosphate receptor: implications for the conformation and domain organization of the protein, The Biochemical journal, 307 ( Pt 3) (1995) 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hirota J, Furuichi T, Mikoshiba K, Inositol 1,4,5-trisphosphate receptor type 1 is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner, The Journal of biological chemistry, 274 (1999) 34433–34437. [DOI] [PubMed] [Google Scholar]
- [61].Kopil CM, Vais H, Cheung KH, Siebert AP, Mak DO, Foskett JK, Neumar RW, Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor (InsP(3)R1) has InsP(3)-independent gating and disrupts intracellular Ca(2+) homeostasis, The Journal of biological chemistry, 286 (2011)35998–36010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Maes K, Missiaen L, Parys JB, De Smet P, Sienaert I, Waelkens E, Callewaert G, De Smedt H, Mapping of the ATP-binding sites on inositol 1,4,5-trisphosphate receptor type 1 and type 3 homotetramers by controlled proteolysis and photoaffinity labeling, The Journal of biological chemistry, 276 (2001) 3492–3497. [DOI] [PubMed] [Google Scholar]
- [63].Soulsby MD, Wojcikiewicz RJ, The type III inositol 1,4,5-trisphosphate receptor is phosphorylated by cAMP-dependent protein kinase at three sites, The Biochemical journal, 392 (2005) 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wojcikiewicz RJ, Ernst SA, Yule DI, Secretagogues cause ubiquitination and down-regulation of inositol 1, 4,5-trisphosphate receptors in rat pancreatic acinar cells, Gastroenterology, 116 (1999) 1194–1201. [DOI] [PubMed] [Google Scholar]
- [65].Wang L, Wagner LE 2nd, Alzayady KJ, Yule DI, Region-specific proteolysis differentially modulates type 2 and type 3 inositol 1,4,5-trisphosphate receptor activity in models of acute pancreatitis. J Biol Chem. 2018. doi: 10.1074/jbc.RA118.003421. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Diaz F, Bourguignon LY, Selective down-regulation of IP(3)receptor subtypes by caspases and calpain during TNF alpha -induced apoptosis of human T-lymphoma cells, Cell calcium, 27 (2000)315–328. [DOI] [PubMed] [Google Scholar]
- [67].Wang L, Alzayady KJ, Yule DI , Proteolytic fragmentation of inositol 1,4,5-trisphosphate receptors: a novel mechanism regulating channel activity?, J Physiol, 594 (2016) 2867–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Betzenhauser MJ, Wagner LE 2nd, Iwai M, Michikawa T, Mikoshiba K, Yule DI, ATP modulation of Ca2+ release by type-2 and type-3 inositol (1, 4, 5)-triphosphate receptors. Differing ATP sensitivities and molecular determinants of action, The Journal of biological chemistry, 283 (2008) 21579–21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, lino M, Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes, The EMBO journal, 18 (1999) 1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wagner, LE 2nd Li WH, Joseph SK, Yule DI, Functional consequences of phosphomimetic mutations at key cAMP-dependent protein kinase phosphorylation sites in the type 1 inositol 1,4,5-trisphosphate receptor, The Journal of biological chemistry, 279 (2004) 46242–46252. [DOI] [PubMed] [Google Scholar]
- [71].Sneyd J, Han JM, Wang L, Chen J, Yang X, Tanimura A, Sanderson MJ, Kirk V, Yule DI, On the dynamical structure of calcium oscillations, Proceedings of the National Academy of Sciences of the United States of America, 114 (2017) 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wagner, LE 2nd, Li WH, Yule DI, Phosphorylation of type-1 inositol 1,4,5-trisphosphate receptors by cyclic nucleotide-dependent protein kinases: a mutational analysis of the functionally important sites in the S2+ and S2− splice variants, The Journal of biological chemistry, 278 (2003) 45811–45817. [DOI] [PubMed] [Google Scholar]
- [73].Fan G, Baker ML, Wang Z, Baker MR, Sinyagovskiy PA, Chiu W, Ludtke SJ, Serysheva II, Gating machinery of InsP3R channels revealed by electron cryomicroscopy, Nature, 527 (2015)336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chandrasekhar R, Alzayady KJ, Yule DI, Using concatenated subunits to investigate the functional consequences of heterotetrameric inositol 1,4,5-trisphosphate receptors, Biochem Soc Trans, 43 (2015)364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fernando P, Brunette S, Megeney LA, Neural stem cell differentiation is dependent upon endogenous caspase 3 activity, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 19 (2005) 1671–1673. [DOI] [PubMed] [Google Scholar]
- [76].Balcerzak D, Poussard S, Brustis JJ, Elamrani N, Soriano M, Cottin P, Ducastaing A, An antisense oligodeoxyribonucleotide to m-calpain mRNA inhibits myoblast fusion, Journal of cell science, 108 ( Pt 5) (1995) 2077–2082. [DOI] [PubMed] [Google Scholar]
- [77].Larsen BD, Rampalli S, Burns LE, Brunette S, Dilworth FJ, Megeney LA, Caspase 3/caspase-activated DNase promote cell differentiation by inducing DNA strand breaks, Proceedings of the National Academy of Sciences of the United States of America, 107 (2010) 4230–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, Takano H, Minowa O, Kuno J, Sakakibara S, Yamada M, Yoneshima H, Miyawaki A, Fukuuchi Y, Furuichi T, Okano H, Mikoshiba K, Noda T, Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor, Nature, 379 (1996) 168–171. [DOI] [PubMed] [Google Scholar]
- [79].Takei K, Shin RM, Inoue T, Kato K, Mikoshiba K, Regulation of nerve growth mediated by inositol 1,4,5-trisphosphate receptors in growth cones, Science, 282 (1998) 1705–1708. [DOI] [PubMed] [Google Scholar]
- [80].Fiedler MJ, Nathanson MH, The type I inositol 1,4,5-trisphosphate receptor interacts with protein 4.1N to mediate neurite formation through intracellular Ca waves, Neuro-Signals, 19 (2011) 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Antigny F, Konig S, Bernheim L, Frieden M, Inositol 1,4,5 trisphosphate receptor 1 is a key player of human myoblast differentiation, Cell calcium, 56 (2014) 513–521. [DOI] [PubMed] [Google Scholar]
- [82].Yule DI, Pancreatic acinar cells: molecular insight from studies of signal-transduction using transgenic animals, Int J Biochem Cell Biol, 42 (2010) 1757–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sah RP, Saluja A, Molecular mechanisms of pancreatic injury, Curr Opin Gastroenterol, 27 (2011)444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Saluja AK, Lerch MM, Phillips PA, Dudeja V, Why does pancreatic overstimulation cause pancreatitis?, Annu Rev Physiol, 69 (2007) 249–269. [DOI] [PubMed] [Google Scholar]
- [85].Wen L, Voronina S, Javed MA, Awais M, Szatmary P, Latawiec D, Chvanov M, Collier D, Huang W, Barrett J, Begg M, Stauderman K, Roos J, Grigoryev S, Ramos S, Rogers E, Whitten J, Velicelebi G, Dunn M, Tepikin AV, Criddle DN, Sutton R, Inhibitors of ORAI1 Prevent Cytosolic Calcium-Associated Injury of Human Pancreatic Acinar Cells and Acute Pancreatitis in 3 Mouse Models, Gastroenterology, 149 (2015) 481–492 e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Huang W, Cane MC, Mukherjee R, Szatmary P, Zhang X, Elliott V, Ouyang Y, Chvanov M, Latawiec D, Wen L, Booth DM, Haynes AC, Petersen OH, Tepikin AV, Criddle DN, Sutton R, Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release, Gut, 66 (2017) 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kruger B, Albrecht E, Lerch MM, The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis, Am J Pathol, 157 (2000) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Criddle DN, Murphy J, Fistetto G, Barrow S, Tepikin AV, Neoptolemos JP, Sutton R, Petersen OH, Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis, Gastroenterology, 130 (2006) 781–793. [DOI] [PubMed] [Google Scholar]
- [89].Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM, Woolf CJ, Kovacs DM , BACE1 regulates voltage-gated sodium channels and neuronal activity, Nature cell biology, 9 (2007) 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR, Epithelial sodium channels are activated by furin-dependent proteolysis, The Journal of biological chemistry, 279 (2004) 18111–18114. [DOI] [PubMed] [Google Scholar]
- [91].Poirot O, Vukicevic M, Boesch A, Kellenberger S, Selective regulation of acid-sensing ion channel 1 by serine proteases, The Journal of biological chemistry, 279 (2004) 38448–38457. [DOI] [PubMed] [Google Scholar]


