Abstract
Objective:
To characterize the excess risk for death, grade 3–4 intraventricular hemorrhage (IVH), bronchopulmonary dysplasia (BPD), and stage 3–5 retinopathy of prematurity independently associated with birth small-for-gestational-age (SGA) among very preterm infants, stratified by completed weeks of gestation.
Methods:
Retrospective cohort study using the Optum Neonatal Database. Study infants were born < 32 weeks gestation without severe congenital anomalies. SGA was defined as a birth weight <10th percentile. The excess outcome risk independently associated with SGA birth among SGA babies was assessed using adjusted risk differences (aRD).
Results:
Of 6,708 infants sampled from 717 US hospitals, 743 (11.1%) were SGA. SGA compared to non-SGA infants experienced higher unadjusted rates of each study outcome except grade 3–4 IVH among survivors. The excess risk independently associated with SGA birth varied by outcome and gestational age. The highest aRD for death (0.27; 95% CI 0.13, 0.40) occurred among infants born at 24 weeks gestation and declined as gestational age increased. In contrast, the peak aRDs for BPD among survivors (0.32; 95% CI 0.20, 0.44) and the composites of death or BPD (0.35; 95% CI 0.24, 0.46) and death or major morbidity (0.35; 95% CI 0.24, 0.45) occurred at 27 weeks gestation. The risk-adjusted probability of dying or developing one or more of the evaluated morbidities among SGA infants was similar to that of non-SGA infants born approximately 2–3 weeks less mature.
Conclusion:
The excess risk for neonatal morbidity and mortality associated with being born SGA varies by adverse outcome and gestational age.
INTRODUCTION
Severe fetal growth restriction and in-utero stress can result in infants being born small for gestational age (SGA). Multiple epidemiological studies have investigated the association between SGA birth and the risk for adverse neonatal outcomes among very preterm infants.(1–10) While most reports indicate that SGA compared to non-SGA very preterm infants are at increased risk for mortality, the strength and direction of the associations between SGA birth and prognostically important neonatal morbidities are not fully established.(1–10) Limitations of the current literature examining the effects of SGA birth among very preterm infants include infrequent assessment of morbidity-mortality composite outcomes, few data on infants born at or near the current limits of viability, and scant comparisons of SGA to non-SGA infants stratified by gestational age. Moreover, most population based studies that compared SGA to non-SGA very preterm infants within gestational age week strata evaluated infants delivered over 15 years ago.(1, 7) Contemporary data on the potential adverse effects associated with SGA birth may help inform current perinatal counseling, obstetrical decision making, and prediction of long-term outcomes.
We conducted the present study to characterize the independent association between SGA birth and neonatal morbidity and mortality in a recent, multicenter cohort of very preterm infants. We examined variability in the risks for the following 3 well-established predictors of long-term neurodevelopment, stratified by completed weeks of gestation: severe intraventricular hemorrhage, bronchopulmonary dysplasia, and severe retinopathy of prematurity.(13, 14) We limited our analyses to these outcomes, and their composites with mortality, as they are likely to be among the most important prognostic factors considered during the perinatal period.
METHODS
Data Source and Population
We performed a retrospective cohort study using data collected prospectively for the Optum Neonatal Database (Eden Prairie, MN). The Optum Corporation provides neonatal care management services for multiple private, government, and self-insured employer health plans throughout the United States. The database is comprised of predefined clinical information that is abstracted from the primary medical record 3–4 times per week by trained neonatal nurses. Infants whose insurer contracts with the Optum Corporation to provide care management services and are cared for in a level II or higher neonatal intensive care unit (NICU) are captured in the database. Clinical data are collected throughout each hospitalization, regardless of whether an infant is cared for in a single institution or transferred between hospitals. For the present analyses, we evaluated infants with gestational ages < 32 weeks born between January 1, 2010 and October 31, 2016 who survived in the delivery room and were admitted to a NICU. Infants with severe congenital anomalies were excluded. The Thomas Jefferson University Institutional Review Board certified the use of this de-identified dataset as non-human subjects research.
Exposure and Outcome Definitions
The exposure of interest was birth SGA, defined as a birth weight < 10th percentile based on gestational age (week and day) and sex according to the Olsen growth curves.(15) SGA infants were compared to non-SGA infants, defined as those with birth weights ≥ 10th percentile. Gestational age and all maternal data were abstracted from the neonatal record. The method used to define each infant’s gestational age and details of the mother’s menstrual history and ultrasonographic findings are not recorded in the Optum Neonatal Database.
The study outcomes were death; bronchopulmonary dysplasia (BPD), defined as supplemental oxygen use at 36 weeks postmenstrual age; severe (stage 3–5) retinopathy of prematurity (ROP); and severe (grade 3–4) intraventricular hemorrhage (IVH) among surviving infants. We also evaluated the composites of each morbidity with mortality and a combined outcome of death or major morbidity, defined as the presence of 1 or more of the evaluated morbidities.
Statistical Analysis
Infant and maternal characteristics and the study outcome rates were compared between SGA and non-SGA infants using standard descriptive statistics. For our primary analyses, the risks for the study outcomes were compared between the two groups using gestational age defined as: (1) the number of completed weeks of gestation considered as a categorical variable and, (2) the gestational age day and week considered as a continuous variable. Owing to smaller samples sizes at the lowest gestational ages, we performed a post-hoc analysis categorizing gestational age according the following 3 strata: 23 0/7 – 25 6/7 weeks, 26 0/7 – 28 6/7 weeks, and 29 6/7 – 31 6/7 weeks. For each comparison, the association between birth SGA and the study outcomes was assessed using logistic regression. Each regression model included an interaction term between the SGA and gestational age variables and was adjusted for the following prespecified maternal and infant characteristics previously shown to be associated with one or more of study outcomes: infant sex, race, insurance status, cesarean birth, multiple gestation pregnancy, maternal initiation of prenatal care during the first trimester, preeclampsia or eclampsia, gestational diabetes, gestational hypertension, treatment with antenatal corticosteroids, and rupture of amniotic membranes ≥ 18 hours prior to delivery. Covariates that may contribute to fetal growth restriction were included in the regression models to enable use of the study results regardless of whether the etiology for SGA is known or unknown. A robust variance estimator for cluster-correlated data was used in all models to account for potential within hospital outcome correlation.(16) C-statistics were calculated to assess multivariable model fit.
For gestational age week considered as a categorical variable, we calculated adjusted risk differences (aRD) using the post-regression Stata command adjrr.(17, 18) The adjrr function first determined risk-adjusted outcome probabilities at each gestational age week stratum for SGA and non-SGA infants using marginal standardization, holding all independent covariates at their original values.(18, 19) aRD values were then calculated by subtracting the adjusted outcome probability for SGA infants from that of non-SGA infants.(18) The 95% confidence intervals (CI) were calculated using delta-method standard errors. In our primary analyses, we determined risk differences, instead of a relative measure of effect such as an odds ratio, to quantify the excess outcome risk among SGA babies that is independently associated with SGA birth. Here, an aRD value above zero indicates greater risk for the study outcome associated with SGA birth while a value below zero indicates lower risk. For completeness, we also report adjusted relative risks for the study outcomes stratified by gestational age week.
Finally, for gestational age considered as a continuous variable, we used post-estimation linear prediction from the logistic regression models to calculate the adjusted probability of developing the study outcomes among SGA and non-SGA babies. These results, with 95% CI, were summarized graphically using kernel-weighted local polynomial regression. A p-value < 0.05 was used to define statistical significance. No adjustment for multiple comparisons was performed in this exploratory analysis. All statistical testing was conducted using Stata 13.1 (StataCorp, College Station, Texas).
RESULTS
Of 6,708 infants without severe congenital anomalies sampled from 717 US hospitals, 743 (11.1%) were SGA. Demographic, baseline clinical, and unadjusted outcome data are compared between SGA and non-SGA infants in Table 1. Mothers of SGA infants were more commonly diagnosed with gestational hypertension and preeclampsia or eclampsia, and more frequently gave birth via cesarean section. Multiple gestation pregnancies and prolonged rupture of amniotic membranes were more common among the mothers of non-SGA babies. The frequencies of all study outcomes, except for severe IVH, were significantly higher among SGA than non-SGA infants (Table 1).
Table 1.
Characteristics and study outcomes of SGA and non-SGA infants
| SGA N=743 |
Non-SGA N=5,965 |
P | |
|---|---|---|---|
| Infant Characteristics | |||
| Gestational age, week mean (SD) | 28.5 (2.4) | 28.7 (2.4) | 0.01 |
| Birth weight, g mean (SD) | 767 (235) | 1241 (379) | <0.001 |
| Male, n (%) | 412 (55.5) | 3123 (52.4) | 0.11 |
| Race/ethnicity, n (%) | 0.32 | ||
| Caucasian | 241 (32.4) | 2009 (33.7) | |
| African American | 154 (20.7) | 1069 (17.9) | |
| Hispanic | 132 (17.8) | 1078 (18.1) | |
| Asian/Biracial/Other | 216 (29.1) | 1809 (30.3) | |
| Insurance, n (%) | 0.96 | ||
| Commercial/Private | 302 (40.7) | 2455 (41.2) | |
| HMO | 125 (16.8) | 992 (16.6) | |
| Medicaid/Medicaid HMO | 316 (42.5) | 2518 (42.2) | |
| Maternal Characteristics | |||
| Cesarean section, n (%) | 652 (87.8) | 3826 (64.1) | <0.001 |
| Multiple gestation pregnancy, n (%) | 150 (20.2) | 1737 (29.1) | <0.001 |
| Full prenatal care, n (%) | 630 (84.8) | 4707 (78.9) | <0.001 |
| Pre/eclampsia, n (%) | 217 (29.2) | 619 (10.4) | <0.001 |
| Gestational diabetes, n (%) | 26 (3.5) | 253 (4.2) | 0.34 |
| Gestational hypertension, n (%) | 166 (22.3) | 532 (8.9) | <0.001 |
| Antenatal corticosteroids, n (%) | 362 (48.7) | 3026 (50.7) | 0.30 |
| Rupture of amniotic membranes ≥ 18hr, n (%) | 46 (6.2) | 1008 (16.9) | <0.001 |
| Infant Outcomes | |||
| Death, n (%) | 112 (15.1) | 332 (5.6) | <0.001 |
| Grade 3–4 IVH, n (%)a | 25 (4.0) | 251 (4.5) | 0.57 |
| BPD, n (%)a | 288 (45.6) | 1494 (26.5) | <0.001 |
| Stage 3–5 ROP, n (%)a | 44 (7.0) | 210 (3.7) | <0.001 |
| Death or grade 3–4 IVH, n (%) | 137 (18.4) | 583 (9.8) | <0.001 |
| Death or BPD, n (%) | 400 (53.8) | 1826 (30.6) | <0.001 |
| Death or stage 3–5 ROP, n (%) | 156 (21.0) | 542 (9.1) | <0.001 |
BPD, bronchopulmonary dysplasia; HMO, health maintenance organization; IVH, intraventricular hemorrhage; ROP, retinopathy of prematurity; SGA, small for gestational age
Determined for 631 surviving SGA and 5,633 surviving non-SGA infants
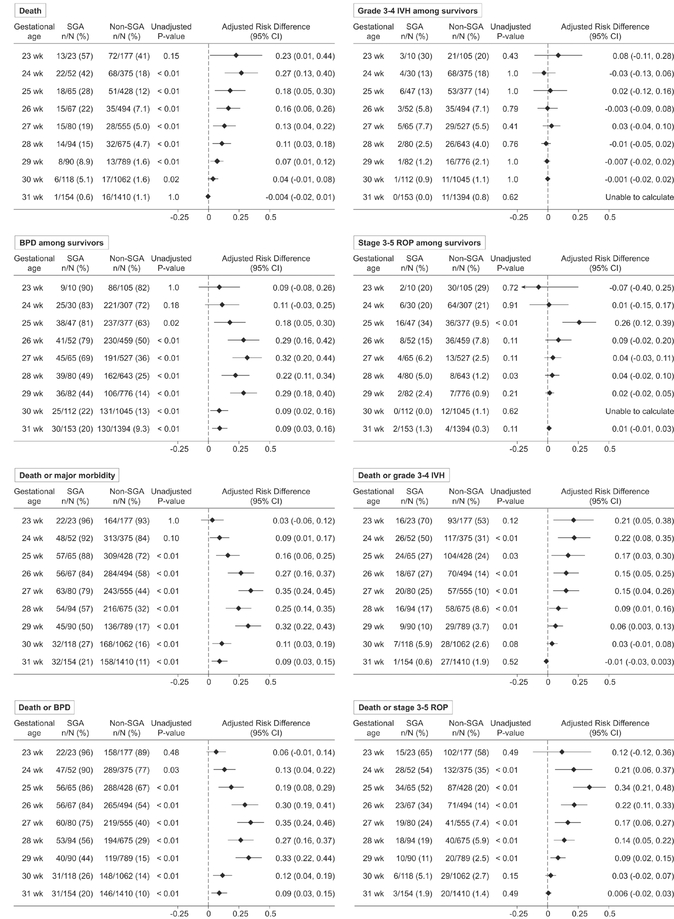
Unadjusted outcome rates and adjusted risk differences for each study outcome, stratified by completed weeks of gestation, are shown in Figure 1 (adjusted outcome rates and relative risks are shown in Supplemental Tables 1 and 2). Model fit for each outcome was excellent (c-statistics >0.8; see Supplemental Tables 1 and 2 for individual values). The risks for the study outcomes associated with SGA varied by outcome and week of gestation. For death, the adjusted risk difference was highest among infants born at 24 weeks gestation (0.27; 95% CI 0.13, 0.40) and decreased with each subsequent week of gestation until 29 weeks (Figure 1). SGA birth was not associated with increased mortality risk among babies born at 30 and 31 completed weeks. In contrast, the adjusted risk differences peaked at 27 weeks gestation for BPD among survivors (32; 95% CI 0.20, 0.44) and the composites of death or BPD (0.35; 95% CI 0.24, 0.46) and death or major morbidity (0.35; 95% CI 0.24, 0.45)(Figure 1). SGA was not associated with higher or lower risk of severe IVH among survivors at any gestational age (Figure 1).
Figure 1. Unadjusted rates and adjusted risk differences for death and the individual morbidities among survivors and morbidity-mortality composite outcomes stratified by gestational age week.
The adjusted risk difference values above zero quantify the excess proportion of risk for the study outcome independently associated with SGA birth among SGA babies. Risk difference values are adjusted for infant sex, race/ethnicity, insurance type, cesarean birth, multiple gestation pregnancy, maternal initiation of prenatal care during the first trimester, preeclampsia or eclampsia, gestational diabetes, gestational hypertension, treatment with antenatal corticosteroids, and rupture of amniotic membranes ≥ 18 hours prior to delivery. A robust variance estimator for cluster-correlated data was used in all models to account for potential within hospital outcome correlation.
With gestational age stratified into 3 subgroups, SGA birth was associated with the greatest excess risk for mortality and stage 3–5 ROP among the least mature infants (gestational ages: 23 0/7 – 25 6/7 weeks)(Table 2). The adjusted risk differences for BPD among survivors, death or BPD, and death or major morbidity were highest among infants in the middle subgroup (gestational ages: 26 0/7 – 28 6/7 weeks).
Table 2.
Unadjusted outcome rates and adjusted risk differences utilizing 3 gestational age strata
| Outcome (by gestational age subgroup) | SGA n/N (%) |
Non-SGA n/N (%) |
p-value | Adjusted RD (95% CI) |
|---|---|---|---|---|
| Individual Outcomes | ||||
| Death | ||||
| 23 0/7 – 25 6/7 weeks | 53/140 (37.9) | 191/980 (19.5) | <0.001 | 0.23 (0.14, 0.31) |
| 26 0/7 – 28 6/7 weeks | 44/241 (18.3) | 95/1724 (5.5) | <0.001 | 0.14 (0.08, 0.19) |
| 29 0/7 – 31 6/7 weeks | 15/362 (4.1) | 46/3261 (1.4) | <0.001 | 0.03 (0.01, 0.05) |
| Grade 3–4 IVH among survivors | ||||
| 23 0/7 – 25 6/7 weeks | 13/87 (14.9) | 123/789 (15.6) | 0.87 | 0.009 (−0.07, 0.09) |
| 26 0/7 – 28 6/7 weeks | 10/197 (5.1) | 90/1629 (5.5) | 0.79 | 0.002 (−0.03, 0.03) |
| 29 0/7 – 31 6/7 weeks | 2/347 (0.6) | 38/3215 (1.2) | 0.31 | −0.006 (−0.02, 0.004) |
| BPD among survivors | ||||
| 23 0/7 – 25 6/7 weeks | 72/87 (82.8) | 544/789 (69.0) | 0.007 | 0.14 (0.05, 0.23) |
| 26 0/7 – 28 6/7 weeks | 125/197 (63.5) | 583/1629 (35.8) | <0.001 | 0.27 (0.19, 0.35) |
| 29 0/7 – 31 6/7 weeks | 91/347 (26.2) | 367/3215 (11.4) | <0.001 | 0.14 (0.09, 0.18) |
| Stage 3–5 ROP among survivors | ||||
| 23 0/7 – 25 6/7 weeks | 24/87 (27.6) | 130/789 (16.5) | 0.01 | 0.13 (0.03, 0.23) |
| 26 0/7 – 28 6/7 weeks | 16/197 (8.1) | 57/1629 (3.5) | 0.002 | 0.05 (0.002, 0.10) |
| 29 0/7 – 31 6/7 weeks | 4/347 (1.2) | 23/3215 (0.7) | 0.37 | 0.005 (−0.007, 0.02) |
| Composite Outcomes | ||||
| Death or grade 3–4 IVH | ||||
| 23 0/7 – 25 6/7 weeks | 66/140 (47.1) | 314/980 (32.0) | <0.001 | 0.20 (0.11, 0.28) |
| 26 0/7 – 28 6/7 weeks | 54/241 (22.4) | 185/1724 (10.7) | <0.001 | 0.13 (0.07, 0.19) |
| 29 0/7 – 31 6/7 weeks | 17/362 (4.7) | 84/3261 (2.6) | 0.02 | 0.02 (0.001, 0.05) |
| Death or BPD | ||||
| 23 0/7 – 25 6/7 weeks | 125/140 (89.3) | 735/980 (75.0) | <0.001 | 0.14 (0.08, 0.21) |
| 26 0/7 – 28 6/7 weeks | 169/241 (70.1) | 678/1724 (39.3) | <0.001 | 0.30 (0.23, 0.37) |
| 29 0/7 – 31 6/7 weeks | 106/362 (29.3) | 413/3261 (12.7) | <0.001 | 0.16 (0.141 0.21) |
| Death or stage 3–5 ROP | ||||
| 23 0/7 – 25 6/7 weeks | 77/140 (55.0) | 321/980 (32.8) | <0.001 | 0.26 (0.16, 0.36) |
| 26 0/7 – 28 6/7 weeks | 60/241 (24.9) | 152/1724 (8.8) | <0.001 | 0.17 (0.11, 0.23) |
| 29 0/7 – 31 6/7 weeks | 19/362 (5.3) | 69/3261 (2.1) | <0.001 | 0.03 (0.01, 0.06) |
| Death or major morbidity | ||||
| 23 0/7 – 25 6/7 weeks | 127/140 (90.7) | 786/980 (80.2) | 0.003 | 0.11 (0.05, 0.17) |
| 26 0/7 – 28 6/7 weeks | 173/241 (71.8) | 743/1724 (43.1) | <0.001 | 0.29 (0.22, 0.35) |
| 29 0/7 – 31 6/7 weeks | 109/362 (30.1) | 462/3261 (14.2) | <0.001 | 0.16 (0.11, 0.20) |
BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; RD, risk difference; ROP, retinopathy of prematurity; SGA, small for gestational age
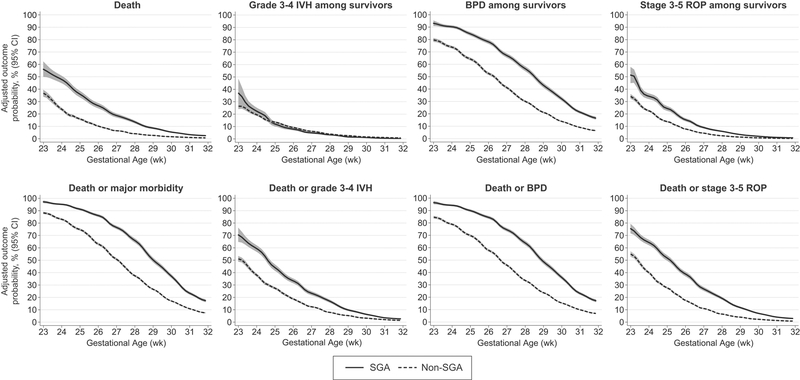
Lastly, Figure 2 shows the adjusted probabilities for developing the study outcomes according to gestational age, defined as a continuous variable. These data indicate that the risk-adjusted probability of mortality, BPD, and death or major morbidity among SGA infants was similar to that of non-SGA infants born approximately 2–3 weeks less mature.
Figure 2. Risk adjusted probability and 95% confidence interval for the study outcomes among small for gestational age (SGA) and non-SGA infants.
Outcome probabilities adjusted for infant sex, race/ethnicity, insurance type, cesarean birth, multiple gestation pregnancy, maternal initiation of prenatal care during the first trimester, preeclampsia or eclampsia, gestational diabetes, gestational hypertension, treatment with antenatal corticosteroids, and rupture of amniotic membranes ≥ 18 hours prior to delivery. A robust variance estimator for cluster-correlated data was used in all models to account for potential within hospital outcome correlation. Model c-statistics: death 0.827, grade 3–4 IVH among survivors 0.809, BPD among survivors 0.800, stage 3–5 ROP among survivors 0.857, death or grade 3–4 IVH 0.820; death or BPD 0.814; death or stage 3–5 ROP 0.842; death or major morbidity 0.819.
DISCUSSION
In this contemporary, multicenter cohort of very preterm infants who survived to NICU admission, SGA birth was independently associated with increased risk for death, BPD, and the composite of death or major morbidity at most gestational ages between 23 and 31 completed weeks. Severe IVH among survivors was the only study outcome for which SGA babies were not at increased risk, both when considering data from the cohort as a whole and within individual gestational age week subgroups. Prior studies postulated that the higher morbidity and mortality rates among SGA compared to non-SGA preterm infants result from an additive “double insult” owing to diminished nutrient delivery to the growing fetus and postnatal sequelae attributed to prematurity.(1, 20–22) Our data support this hypothesis and further suggest that the relative importance of these two factors varies depending on the outcome and the gestational age at birth.
For mortality, the adjusted risk differences associated with SGA birth were greatest among the infants born at the lowest gestational ages. Between 24 and 29 weeks gestation, the excess mortality risk associated with SGA birth decreased among SGA babies with each subsequent week of maturation. This suggests that the mechanisms underlying fetal growth restriction such as impaired mitochondrial function, oxidative stress, decreased substrate availability, and in utero hypoxia and acidosis, when combined with extreme prematurity, may be most injurious among SGA infants born at the limits of viability.(22–24)
In contrast to death, the excess risk for BPD associated with SGA birth was greatest among SGA infants born between 26 0/7 and 29 6/7 weeks. Data from animal models suggest a potential mechanism for this finding. Growth restriction in fetal sheep impairs surfactant production and maturation and hinders postnatal growth of the terminal, gas exchanging airways.(25–27) At 23 and 24 weeks, the human lung is in the canalicular stage of development, prior to when most surfactant production and terminal air sac expansion begins.(28) At this stage, exposure to an unfavorable intrauterine environment may have less impact on the risk for BPD than if exposure continues into the early saccular phase (25–28 weeks).(28) However, if pregnancy progresses further, up to or beyond the mid point in the saccular phase (30–32 weeks), sufficient lung development may have occurred such that the excess risk for BPD associated with birth SGA begins to decrease.(28) Of note, the similar trend in adjusted risk differences observed for the composite outcome of death or BPD suggests this finding is not solely the result of censoring by deaths prior to 36 weeks PMA.
Similar to several previous studies, we found no difference in the risk for severe IVH among SGA compared to non-SGA infants.(1, 2, 7, 12, 29) Although the etiology for this observation is unknown, the higher rates of maternal hypertension and pre-eclampsia among SGA babies may protect against IVH and offset the adverse effects of other factors contributing to fetal growth restriction.(30, 31) There may also be fetal compensatory mechanisms that spare brain growth and nutrient delivery at a cost to other organ systems.(32–34)
In the analysis stratified by gestational week, the adjusted risk difference for stage 3–5 ROP among survivors was significantly increased only among SGA babies born at 25 weeks. It is unknown whether this represents a true biological phenomenon or a spurious finding due to chance. The higher mortality but lower ROP rates among SGA infants born at 23 and 24 weeks raises concern for possible censoring of this outcome by earlier death in these infants.
For each study outcome, the peak adjusted risk difference and peak adjusted relative risk values occurred at different gestational ages. This finding reflects the inherent differences in the two measures. The relative risk is useful to characterize the strength of the association between SGA birth and the study outcomes, but it can be misleading when the absolute difference in outcome rates between SGA and non-SGA infants is low. The adjusted risk difference, assuming a cause and effect relationship, quantifies the excess outcome risk among SGA babies that may be independently “attributable” to SGA birth and fetal growth restriction. The higher adjusted risk differences for mortality among infants born at 23 and 24 weeks gestation suggest that prevention of poor in-utero growth during the first or second trimester would proportionally result in the largest reduction in mortality among infants born at these gestations. However, as with all observational studies, we cannot determine whether SGA birth was causative of the observed associations or whether there is residual, unmeasured confounding.
We acknowledge several other study limitations. Only infants whose insurer contracts with the Optum Corporation are included in the dataset. While the cohort was sampled across a broad range of hospitals and geographic locations, the study results may be less generalizable to infants who are uninsured and those receiving care through a health maintenance organization. We utilized SGA as a marker of fetal growth restriction as obstetrical ultrasonographic findings were not captured in the database. However, SGA has been shown to be a reliable proxy for intrauterine growth restriction among infants born at early preterm gestations.(35) Our dataset does not include certain anthropometric measures, such as head circumference, preventing assessment of whether the SGA infants were symmetrically or asymmetrically small. Lastly, all infant and maternal data were abstracted from the infant medical record and some values, including gestational ages estimated after the first trimester, may be imprecise.(36)
In summary, the findings from this large, contemporary cohort of very preterm infants suggest that the excess risk for prognostically important complications of prematurity independently associated with SGA birth varies between outcomes and by gestational age. The risk-adjusted probability of developing one or more of the evaluated study outcomes among SGA infants was generally equivalent to non-SGA infants born approximately 2–3 weeks less mature. These new data may help inform perinatal care decision making, neonatal outcome prediction, and family counseling in the setting of second trimester fetal growth restriction, particularly among threatened preterm deliveries occurring near the current limits of viability
Supplementary Material
What is already known on this topic?
Very preterm small for gestational age (SGA) infants are at increased risk for death and neonatal morbidity.
What this study adds?
The excess risk for neonatal morbidity and mortality associated with being born SGA varies by outcome and gestational age among very preterm infants admitted to the neonatal intensive care unit.
The excess risk for death independently associated with SGA birth was highest at 24 weeks. For bronchopulmonary dysplasia, the excess risk peaked at 27 weeks.
The risks for death and prognostically important morbidity among SGA infants were generally equivalent to non-SGA babies born 2–3 weeks less mature.
Acknowledgments
Funding Source: EAJ was supported by a grant from the National Health, Lung, and Blood Institute (K23HL136843). EEF was supported by a grant from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (K23HD084727).
Footnotes
Financial Disclosure: The authors have no relevant financial relationships to disclose.
Conflict of Interest: The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Regev RH, Lusky A, Dolfin T, et al. Excess mortality and morbidity among small-for-gestational-age premature infants: a population-based study. J Pediatr. 2003;143:186–91. [DOI] [PubMed] [Google Scholar]
- 2.Tsai L, Chen Y, Tsou K, et al. The impact of small-for-gestational-age on neonatal outcome among very-low-birth-weight infants. Pediatr Neonatol. 2015;56:101–7. [DOI] [PubMed] [Google Scholar]
- 3.Zaw W, Gagnon R, da Silva O. The risks of adverse neonatal outcome among preterm small for gestational age infants according to neonatal versus fetal growth standards. Pediatrics. 2003;111:1273–7. [DOI] [PubMed] [Google Scholar]
- 4.Qiu X, Lodha A, Shah P, et al. Neonatal outcomes of small for gestational age preterm infants in Canada. Am J Perinatol. 2012;29:87–94. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein I, Horbar J, Badger G, et al. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000;182:198–206. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, McKay K, Rosenkrantz T, et al. Comparisons of mortality and pre-discharge respiratory outcomes in small-for-gestational-age and appropriate-for-gestational-age premature infants. BMC Pediatr. 2004;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartels D, Kreienbrock L, Dammann O, et al. Population based study on the outcome of small for gestational age newborns. Arch Dis Child Fetal Neonatal Ed. 2005;90:F53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korhonen P, Tammela O, Koivisto A, et al. Frequency and risk factors in bronchopulmonary dysplasia in a cohort of very low birth weight infants. Early Hum Dev. 1999;54:245–58. [DOI] [PubMed] [Google Scholar]
- 9.Baud O, Zupan V, Lacaze-Masmonteil T, et al. The relationships between antenatal management, the cause of delivery and neonatal outcome in a large cohort of very preterm singleton infants. BJOG. 2000;107:877–84. [DOI] [PubMed] [Google Scholar]
- 10.Piper J, Xenakis E, McFarland M, et al. Do growth-retarded premature infants have different rates of perinatal morbidity and mortality than appropriately grown premature infants? Obstet Gynecol. 1996;87:169–74. [DOI] [PubMed] [Google Scholar]
- 11.Clausson B, Cnattingius S, Axelsson O. Preterm and term births of small for gestational age infants: a population-based study of risk factors among nulliparous women. Br J Obstet Gynaecol. 1998;105:1011–7. [DOI] [PubMed] [Google Scholar]
- 12.Giapros V, Drougia A, Krallis N, et al. Morbidity and mortality patterns in small-for-gestational age infants born preterm. J Matern Fetal Neonatal Med. 2012;25:153–7. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt B, Roberts RS, Davis PG, et al. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr. 2015;167:982–6.e2. [DOI] [PubMed] [Google Scholar]
- 14.Synnes A, Luu T, Moddemann D, et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed. 2017;102:F235. [DOI] [PubMed] [Google Scholar]
- 15.Olsen IE, Groveman SA, Lawson ML, et al. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24. [DOI] [PubMed] [Google Scholar]
- 16.Williams R A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6. [DOI] [PubMed] [Google Scholar]
- 17.Kleinman L, Norton E. What’s the Risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44:288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norton E, Miller M, Kleinman L. Computing adjusted risk ratios and risk differences in Stata. Stata J. 2013;13:492–509. [Google Scholar]
- 19.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristensen S, Salihu H, Keith L, et al. SGA subtypes and mortality risk among singleton births. Early Hum Dev. 2007;83:99–105. [DOI] [PubMed] [Google Scholar]
- 21.Teberg A, Walther F, Pena I. Mortality, morbidity, and outcome of the small-for-gestational age infant. Semin Perinatol. 1988;12:84–94. [PubMed] [Google Scholar]
- 22.Robinson J, Moore V, Owens J, et al. Origins of fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. 2000;92:13–9. [DOI] [PubMed] [Google Scholar]
- 23.Ream M, Ray A, Chandra R, et al. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol. 2008;295:R583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishimoto H, Natori M, Tanaka M, et al. Role of oxygen-derived free radicals in free growth retardation induced by ischemia-reperfusion in rats. Am J Physiol. 1997;272:H701–5. [DOI] [PubMed] [Google Scholar]
- 25.Orgeig S, Crittenden T, Marchant C, et al. Intrauterine growth restriction delays surfactant protein maturation in the sheep fetus. Am J Physiol Lung Cell Mol Physiol. 2010;298:L575–83. [DOI] [PubMed] [Google Scholar]
- 26.Wignarajah D, Cock M, Pinkerton K, et al. Influence of intrauterine growth restriction on airway development in fetal and postnatal sheep. Pediatr Res. 2002;51:681–8. [DOI] [PubMed] [Google Scholar]
- 27.Gagnon R, Langridge J, Inchley K, et al. Changes in surfactant-associated protein mRNA profile in growth-restricted fetal sheep. Am J Physiol. 1999;276:L459–65. [DOI] [PubMed] [Google Scholar]
- 28.Wert S. Normal and Abnormal Structural Development of the Lung In: Polin R, Fox W, Abman S, eds. Fetal and Neonatal Physiology. Philadelphia, PA: Elsevier; 2011:864–77. [Google Scholar]
- 29.Simchen M, Beiner M, Strauss-Liviathan N, et al. Neonatal outcome in growth-restricted versus appropriately grown preterm infants. Am J Perinatol. 2000;17:187–92. [DOI] [PubMed] [Google Scholar]
- 30.Doshi H, Moradiya Y, Roth P, et al. Variables associated with the decreased risk of intraventricular haemorrhage in a large sample of neonates with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 2016;101:F223–9. [DOI] [PubMed] [Google Scholar]
- 31.Ancel P, Marret S, Larroque B, et al. Are maternal hypertension and small-for-gestational age risk factors for severe intraventricular hemorrhage and cystic periventricular leukomalacia? Results of the EPIPAGE cohort study. Am J Obstet Gynecol. 2005;193:178–84. [DOI] [PubMed] [Google Scholar]
- 32.Simmons R, Gounis A, Bangalore S, et al. Intrauterine growth retardation: fetal glucose transport is diminished in lung but spared in brain. Pediatr Res. 1992;31:59–63. [DOI] [PubMed] [Google Scholar]
- 33.Barker D Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–83. [DOI] [PubMed] [Google Scholar]
- 34.Gluckman P, Cutfield W, Hofman P, et al. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev. 2005;81:51–9. [DOI] [PubMed] [Google Scholar]
- 35.Ananth C, Vintzileos A. Distinguishing pathological from constitutional small for gestational age births in population-based studies. Early Hum Dev. 2009;85:653–8. [DOI] [PubMed] [Google Scholar]
- 36.Butt K, Lim K, Society of Obstetricians and Gynaecologists of Canada. Determination of gestational age by ultrasound. J Obstet Gynaecol Can. 2014;36:171–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.