Abstract
Purpose
To evaluate retinal dysfunction in diabetic patients who have mild or no non-proliferative diabetic retinopathy (NPDR) using the high-frequency flicker electroretinogram (ERG).
Methods
Light-adapted flicker ERGs were recorded from 15 diabetics who have no clinically-apparent retinopathy, 15 diabetics who have mild NPDR, and 15 non-diabetic, age-equivalent controls. ERGs were elicited by full-field flicker at two temporal frequencies, 31.25 Hz and 62.5 Hz, and were recorded using conventional techniques. Amplitude and timing of the flicker responses were compared among the groups and correlated with clinical characteristics including age, acuity, disease duration, and HbA1c.
Results
The 31.25 Hz flicker amplitude was slightly, but non-significantly, smaller for no DR and mild NPDR subjects, compared to the control group (both t < 1.38, p > 0.31); small, non-significant response delays for both patient groups were also observed (both t < 1.57, p > 0.12). In contrast, there were significant amplitude reductions for the 62.5 Hz flicker stimulus: mean amplitude was reduced by 32% for subjects with no DR and by 41% for subjects with mild NPDR (both t > 2.92, p < 0.01). Response timing at 62.5 Hz did not differ significantly from control for either group (both t < 1.2, p > 0.39). ERG amplitude and timing were not correlated significantly with clinical characteristics.
Conclusions
The 62.5 Hz flicker ERG is useful for evaluating retinal dysfunction in diabetics who have mild or no DR, as this response can be significantly reduced. Attenuation of the high frequency flicker ERG, which is primarily generated by bipolar cells, suggests a relatively early retinal site of neural dysfunction.
Keywords: diabetes, diabetic retinopathy, electroretinogram, retinal function
INTRODUCTION
Diabetic retinopathy (DR) is the most serious ocular complication of diabetes mellitus (DM) and is the leading cause of new cases of legal blindness among working-age adults worldwide.1 Although current international standards classify DR stage based on the severity of clinically-apparent vascular abnormalities,2, 3 there is mounting evidence supporting neural dysfunction prior to clinically observable vascular changes in these individuals. For example, the pattern electroretinogram (ERG) has been shown to be reduced in amplitude in diabetics who have no clinically apparent retinopathy.4 However, the amplitude reductions are relatively small and have not been observed in all studies.5 Additionally, multifocal ERG abnormalities have been reported prior to the onset of clinically-apparent retinopathy6–8 and locations of new lesions can be predicted based on localized changes in implicit time in eyes with DR.9 The full-field ERG obtained under standard clinical conditions, which is more commonly recorded than the pattern ERG and multifocal ERG, is generally normal until moderate to severe DR is apparent.10 However, some, but not all, studies have reported changes in the oscillatory potentials, which are high-frequency components of the single-flash response, in individuals who have no clinically-apparent retinopathy (reviewed by Tzekov11).
There has been renewed interest recently in recording the flicker ERG as a means to screen for DR.12–14 In these studies, the flicker ERG was elicited by full-field periodic flashes of light at a flicker rate of approximately 30 Hz. These studies are largely in agreement that the implicit time of the flicker ERG can be delayed in advanced DR and that the flicker ERG may be a useful screening tool for sight threatening DR. By contrast, these studies have shown that the 30 Hz flicker response is minimally affected in early stage DR. The selection of a flicker rate near 30 Hz used in these studies was based on international standards,15 but there may be value in recording the response elicited by higher flicker rates. For example, in some inherited retinal diseases, the high frequency flicker ERG can show greater abnormalities than the 30 Hz flicker response.16, 17 High frequency flicker abnormalities are typically attributed to abnormal bipolar cell function, as bipolar cells are the dominant generator of the flicker ERG at moderate to high flicker frequencies.18
The purpose of the present study was to evaluate retinal dysfunction in diabetics who have no nonproliferative DR (NPDR) or mild NPDR using the flicker ERG. The flicker ERG was recorded at the standard 31.25 Hz flicker rate and compared to that measured at twice the standard: 62.5 Hz. Conventional analyses were performed on the steady-state flicker responses to derive amplitude and timing measures. In addition, the response to the first stimulus cycle at the two stimulus frequencies was evaluated, as this response may be used to approximate that obtained with a single, brief flash. Finally, ERG responses were compared to clinical characteristics such as HbA1C percentage, disease duration, visual acuity, and age.
METHODS
Subjects
Thirty subjects diagnosed with type-2 DM participated in the study. The diabetic subjects were recruited from the Retina and General Eye Clinics of the University of Illinois at Chicago Department of Ophthalmology and Visual Sciences. The majority of the subjects were African American (67%), with 13% Asian, 13% Caucasian, and 7% Hispanic subjects constituting the remainder of the sample. For all subjects, a comprehensive history was obtained from the medical record and an examination of each eye was performed by a retina specialist (authors NB, FC, JL, or YL), with particular attention to the optic nerve, retina, and its vasculature. No subject had systemic disease (other than DM) or ocular disease known to affect the retina, such as retinal vascular occlusions, sickle cell disease, age-related macular degeneration, glaucoma, or high myopia. The stage of NPDR was graded and the subjects were clinically classified as diabetic with no DR (N = 15) or diabetic with mild NPDR (N = 15) according to the early treatment of diabetic retinopathy study (ETDRS) scale.2 Subjects classified as mild NPDR had one or more of the following vascular abnormalities: microaneurysms, hard exudates, cotton-wool spots and/or mild retinal hemorrhage (equivalent to ETDRS level 35 or less2). Subject characteristics including age, sex, visual acuity, estimated diabetes duration, and HbA1c percentage are provided in Table 1. No subject had a history of ocular treatment for diabetic eye disease.
Table 1.
Subject characteristics
| Control (N = 15) | No DR (N = 15) | Mild NPDR (N = 15) | |
|---|---|---|---|
| Age (yr) | 50.1 ± 13.6 | 52.3 ± 7.7 | 52.9 ± 8.9 |
| Sex | 7M 8F | 6M 9F | 7M 8F |
| Log MAR acuity (Snellen) | −0.07 ± 0.07 (20/17) | −0.01 ± 0.05 (20/20) | −0.02 ± 0.07 (20/19) |
| Disease duration (yr) | 9.7 ± 8.9 | 14.1± 9.1 | |
| HbA1c (%) | 7.4 ± 1.2 | 8.4± 1.7 |
yr is years; M is male and F is female; MAR is minimum angle of resolution; HbA1c is glycated hemoglobin
Fifteen visually-normal, non-diabetic, control subjects also participated. The mean age of the control subjects did not differ significantly from that of the diabetic subjects (F = 0.30, p = 0.74). All control subjects had best-corrected visual acuity of 0.06 log MAR (equivalent to approximately 20/23 Snellen acuity) or better, as assessed with the Lighthouse distance visual acuity chart, and normal letter contrast sensitivity as measured with a Pelli-Robson chart. The research followed the tenets of the Declaration of Helsinki and was approved by an institutional review board of the University of Illinois at Chicago. All subjects provided written informed consent.
Apparatus, stimuli, and procedure
An LED-driven ganzfeld system that we have used previously and described elsewhere19 was used for stimulus generation and display (Diagnosys LLC, Lowell, MA). The subject was adapted to a uniform field for approximately 2 minutes that was composed of equal luminances (100 cd/m2) of middle-wavelength (516-nm peak) and long-wavelength (632-nm peak) light. During the ERG recording, the adapting field was modulated sinusoidally at 31.25 Hz for 32 cycles (1024 ms) or at 62.50 Hz for 64 cycles (1024 ms). The Michelson contrast of the sinusoidal modulation was 100%.
Measurements from both the control and diabetic groups were performed monocularly, with the fellow eye patched. Prior to the ERG recordings, the pupil of the tested eye was dilated with 2.5% phenylephrine hydrochloride and 1% tropicamide drops. Data were obtained from the right eye of each control subject. For the diabetic subjects, measurements were performed on the eye with the lower NPDR stage; in cases were the two eyes had the same stage, the right eye was tested.
ERGs were recorded with DTL electrodes, and gold-cup electrodes were used as reference (ear) and ground (forehead). Five responses at each flicker frequency were acquired from each subject with an Espion E3 electroretinography console and were averaged for analysis. Amplifier bandpass settings were 0.30 to 500 Hz and the sampling frequency was 2 kHz. The fundamental amplitude and phase of the mean ERG was derived by Fast Fourier Transform (FFT). For waveforms analyzed by FFT, the “steady-state” response was analyzed by omitting the initial and final few cycles of the waveforms, as these cycles can contain onset and offset transients.
RESULTS
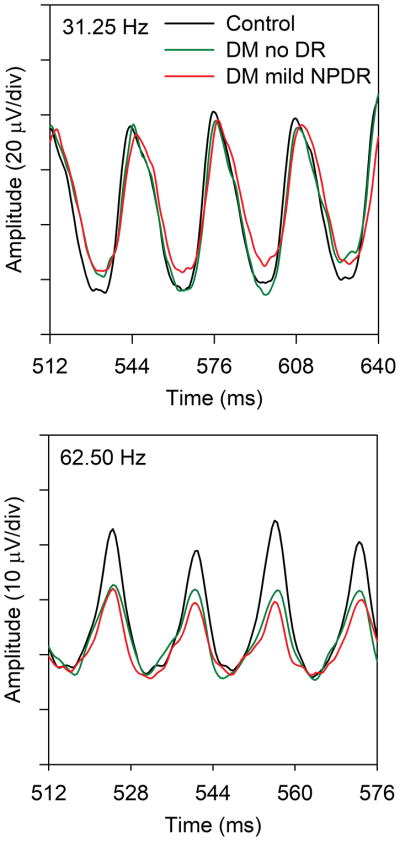
Fig. 1 shows the mean ERG traces recorded at 31.25 Hz (top) and 62.5 Hz (bottom) from the three subject groups. For clarity, only four stimulus cycles recorded near the middle of the flicker train are shown. The shape of the 31.25 Hz response for the two diabetic groups was similar to that of the control group, but the amplitude was somewhat smaller for the diabetic groups compared to the controls (9% reduction for the no DR group and 15% reduction for the mild NPDR group). Additionally, the waveform for the mild NPDR group was shifted slightly rightward (delayed) relative to the controls. In comparison, there was a clear amplitude reduction for the 62.5 Hz response (bottom) for both diabetic groups compared to the control group (average reduction of 35% and 40% for the no DR and mild NPDR groups, respectively). There was no apparent difference in timing among the waveforms recorded for the three groups at 62.5 Hz. The ERG traces shown in Fig. 1 are intended to provide examples of the responses at the two temporal frequencies for the three subject groups; amplitude and timing for the individual subjects are discussed below.
Figure 1.
Mean ERG waveforms recorded at 31.25 Hz (top) and 62.5 Hz (bottom) from the control subjects (black), diabetics who have no DR (green), and diabetics who have mild NPDR (red). For clarity, only four response cycles that were recorded near the middle of the flicker train are shown.
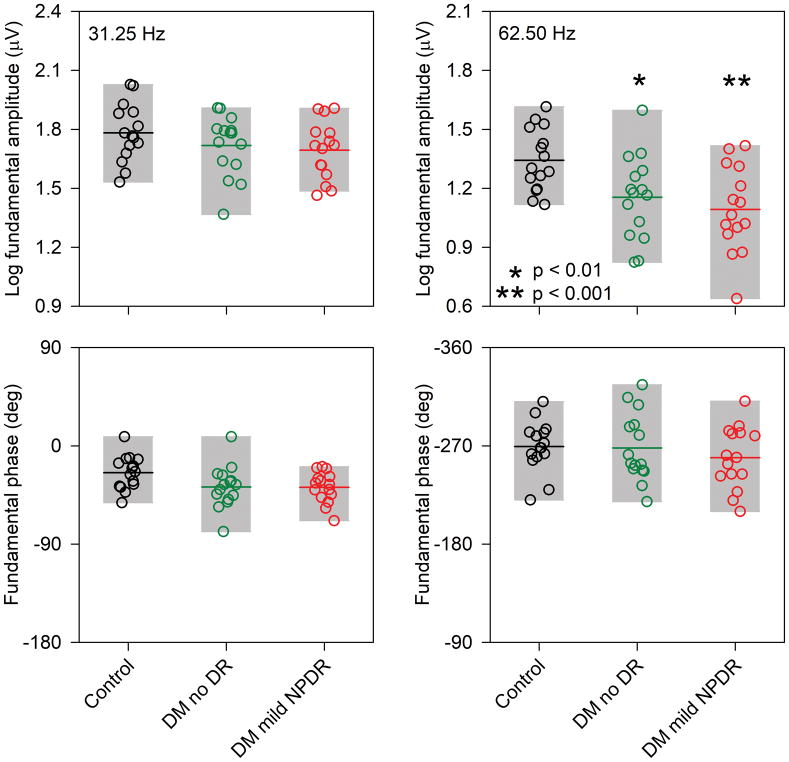
Fig. 2 shows the log fundamental amplitude (top) and phase (bottom) measured at 31.25 Hz (first column) and 62.5 Hz (second column) obtained by FFT for the steady-state waveform. The amplitude recorded at 31.25 Hz for the diabetic subjects was generally within the range of normal, with the exception of one no DR subject and three mild NPDR subjects who had slightly attenuated responses. Similarly, there was a slight decrease in the mean fundamental phase (delay) for the diabetic groups, but with few exceptions the individual diabetic subjects were within the range of normal. In contrast to the results obtained at 31.25 Hz, many of the diabetic subjects had fundamental amplitude reductions for the 62.5 Hz flicker stimulus: 33% of the no DR group and 53% of the mild NPDR group had amplitudes that fell below the lower limit of the control range. The 62.5 Hz phase, however, was generally normal for the diabetic subjects, with the exception of a few subjects who had slight phase delays or advances.
Figure 2.
Log fundamental amplitude (top row) and phase (bottom row) measured at 31.25 Hz (first column) and 62.5 Hz (second column) are shown. Each circle represents a different subject, with control subjects shown in black (leftmost data sets), diabetics with no DR shown in green (middle data sets), and diabetics with mild NPDR shown in red (rightmost data sets). The gray regions indicate the range of data for each group (5th to 95th percentile) and the horizontal lines show the mean for each group. Asterisks mark statistically significant differences from the control group. Other conventions are as in Fig. 2.
A repeated measures two-way analysis of variance (ANOVA), with main effects of group (control, DM no DR, DM mild NPDR) and stimulus frequency (31.25 Hz vs 62.5 Hz), was performed to compare the amplitudes among the groups. There were significant effects of group (F = 4.32, p = 0.02) and stimulus frequency (F = 812.39, p < 0.001), as well as a significant interaction between these main effects (F = 6.78, p = 0.003). Tukey pairwise comparisons indicated that the 31.25 Hz response amplitude did not differ significantly between the diabetic and control groups (both t < 1.38, p > 0.32). There was also no significant difference in amplitude between the no DR and mild NPDR groups (t = 0.37, p = 0.71). However, for the 62.5 Hz response, there was a significant amplitude reduction for the no DR group (t = 2.93, p = 0.005) and mild NPDR group (t = 3.91, p < 0.001) compared to the controls. There was no significant difference in amplitude between the no DR and mild NPDR groups (t = 0.98, p = 0.33). A repeated measures two-way ANOVA, with main effects of group (control, DM no DR, DM mild NPDR) and stimulus frequency (31.25 Hz vs 62.5 Hz) was also performed to compare the phases among the groups. There was no significant effect of group (F = 0.50, p = 0.61), but there was a significant effect of stimulus frequency (F = 2531.94, p < 0.001); the interaction between these effects was not significant (F = 2.21 p = 0.12). There were no significant phase differences between the control and diabetic groups.
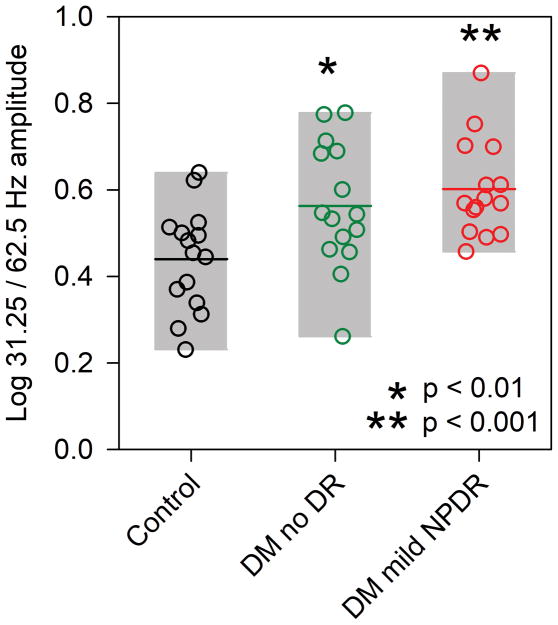
As an additional approach to examine amplitude differences among the three groups, the log ratio of the 31.25 Hz and 62.5 Hz amplitude was computed, which is equivalent to normalizing each subject’s 62.5 Hz response by his/her 31.25 Hz response. This minimizes the potential effects of overall ERG amplitude differences among the subjects and highlights the difference in amplitude measured at the two temporal frequencies. The results of this analysis are shown in Fig. 3 for each subject. In this figure, larger ratios represent a larger difference between the 31.25 Hz and 62.5 Hz responses, indicating a high frequency ERG attenuation. On average, the amplitude at 31.25 Hz was 2.75x larger than the amplitude at 62.5 Hz for the control subjects. In comparison, the amplitude was 3.67x and 4.05x larger for the no DR and mild NPDR subjects at 31.25 Hz compared to 62.5 Hz. A one-way ANOVA was performed to examine the differences in the amplitude ratios between the control and diabetic groups. ANOVA indicated that the ratio was significantly different among the groups (F = 6.78, p = 0.003) and that the no DR and mild NPDR groups had significantly larger ratios than the controls (both t > 2.69, p < 0.01). This indicates that increasing the flicker frequency decreased the response amplitude for the diabetic groups more than for the control group.
Figure 3.
Log ratio of the 31.25 Hz amplitude to 62.5 Hz amplitude. Other conventions are as in Fig. 2.
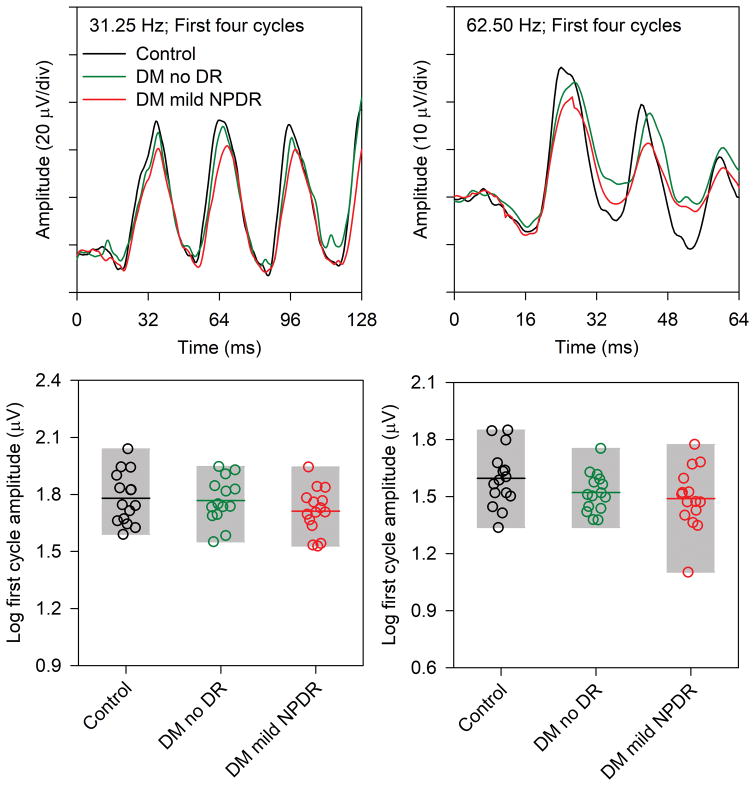
The analyses presented above were based on the steady-state flicker response, which does not permit an analysis of responses elicited at different times throughout the flicker train. For example, it might be expected that the response amplitude for the initial stimulus cycle recorded at 62.5 Hz would be normal for the diabetic groups, assuming the first stimulus cycle approximates a single-flash response. The waveforms for the first four stimulus cycles (three response cycles) and the log trough-to-peak response amplitude for the first stimulus cycle are shown in Fig. 4. The first four response cycles of the 31.25 Hz waveform (top left) generally had the same shape for the three groups, but the amplitude was slightly reduced for the diabetic groups compared to the controls. The trough-to-peak amplitude (measured from the trough appearing at approximately 16 ms to the peak occurring at approximately 40 ms) was calculated for each subject and is presented in Fig. 4 (lower left). With few exceptions, the amplitude of the first cycle was within the range of the controls for both diabetic groups.
Figure 4.
The response to the first four stimulus cycles recorded at 31.25 Hz (left) and 62.5 Hz (right). The waveforms represent the mean of the control subjects (black), diabetics who have no DR (green), and diabetics who have mild NPDR (red). The log trough-to-peak amplitude of the response to the first stimulus cycle is shown in the bottom row for the 31.25 Hz stimulus (left) and 62.5 Hz (right). Other conventions are as in Fig. 2.
For the 62.5 Hz response, there were also relatively small differences among the three groups in the response to the first stimulus cycle of the waveform. The initial trough of the waveform (appearing at approximately 16 ms) was similar for all subjects, but there were small differences in peak amplitude (appearing at approximately 28 ms) among the three groups. However, there was a clear amplitude reduction for both diabetic groups compared to the control for the second stimulus cycle and for the subsequent cycles. The trough-to-peak amplitude for the first cycle recorded at 62.5 Hz was calculated for each subject and is presented in Fig. 4 (lower right). The trough-to-peak amplitude of the first cycle was generally within the range of normal for the diabetic subjects, with the exception of one diabetic with mild NPDR who had a substantial amplitude reduction for the first cycle. A repeated measures ANOVA, with main effects of group (control, DM no DR, DM mild NPDR) and stimulus frequency (31.25 Hz vs 62.5 Hz) was performed to compare the first cycle amplitudes among the groups. There was no significant effect of group (F = 2.19, p = 0.12), but there was a significant effect of frequency (F = 119.73, p < 0.001); the interaction between these main effects was not significant (F = 0.828, p = 0.45).
The relationship between the flicker response amplitude at both temporal frequencies and subject characteristics including age, acuity, disease duration, and HbA1c were examined by computing Pearson correlation coefficients. The relationships among these parameters were relatively weak (the r values were between −0.39 and 0.00) and were not statistically significant after correcting for multiple correlations. There were also no statistically significant relationships between the log 31.25 Hz/62.5 Hz amplitude ratio and age, acuity, disease duration, or HbA1c.
DISCUSSION
The purpose of the present study was to evaluate retinal dysfunction in diabetic subjects who have mild or no NPDR using the flicker ERG. Our focus was on comparing the high frequency flicker ERG recorded at 62.5 Hz to that recorded at the standard 31.25 Hz flicker rate. The results show significant amplitude reductions in the 62.5 Hz flicker response that were not apparent at 31.25 Hz or in the response elicited by the first stimulus cycle at these two frequencies. Additionally, there were no statistical differences in mean response timing (phase) at either temporal frequency for the diabetic groups, compared to the controls. Taken together, the results indicate that the flicker ERG abnormalities observed in this study are restricted to the steady-state high frequency flicker (62.5 Hz) amplitude.
Analysis of the amplitude ratio between the 31.25 Hz and 62.5 Hz responses also showed an abnormality for the diabetic groups. That is, increasing the flicker rate from 31.25 Hz to 62.5 Hz decreased the response amplitude for all subjects, but the decrease was significantly larger for the diabetic groups compared to the control group. Although analyzing the ratio has the advantage of minimizing the effects of potential confounding variables, such as electrode placement and axial length that can affect the overall ERG amplitude, the conclusions based on the ratios are similar to those based on the amplitude values. The elevated amplitude ratios further indicate that the 62.5 Hz response attenuation cannot be attributed to an overall reduction in ERG amplitude for the diabetic subjects compared to the controls.
There are several potential explanations for the greater reduction in the 62.5 Hz amplitude compared to the 31.25 Hz amplitude in our sample of diabetic subjects. For example, the 62.5 Hz response is primarily driven by OFF (hyperpolarizing) bipolar cells, whereas the 31.25 Hz response is produced by a combination of ON (depolarizing) and OFF pathway activity.18 As such, a selective OFF pathway defect may be expected to attenuate the 62.5 Hz flicker ERG more than the 31.25 Hz flicker ERG. A recent study that examined ON and OFF pathway function in DM subjects using the “long duration” mfERG found evidence for altered interactions between the ON and OFF pathways, potentially due to reduced antagonism of the ON pathway by the OFF pathway.20 Alterations in retinal oxygenation and blood flow provide a second possible explanation for the 62.5 Hz amplitude loss. It has been shown that vascular function (assessed by blood flow at the optic nerve head) and neural function (assessed by the flicker ERG) are strongly associated.21 Diabetic individuals, who can have altered retinal hemodynamics, may be unable to maintain adequate oxygenation in response to rapid flicker, possibly decreasing response amplitude at high flicker rates. Although there is evidence of abnormal flicker-induced vascular and hemoglobin oxygen saturation responses in diabetics22 the flicker rates used in studies of retinal hemodynamics are typically much lower than those used in the present study.22, 23 Finally, reduced photoreceptor sensitivity may underlie the high frequency amplitude loss in the diabetic subjects. Previous work in a small sample of diabetics showed a loss of photoreceptor sensitivity, as derived from ERG measures, possibly due to transduction abnormalities.24 Photoreceptor sensitivity loss has been shown to have a greater effect on the high frequency flicker ERG compared to ERG responses measured at low to moderate temporal frequencies.17 Thus, a photoreceptor sensitivity loss would be expected to have a larger impact on the 62.5 Hz ERG compared to the 31.25 Hz ERG. At present, the explanation for the 62.5 Hz amplitude loss in the diabetic subjects is unclear and additional work is needed to evaluate these speculative explanations and to determine the frequency range over which the flicker response is abnormal.
Although the mean 62.5 Hz steady-state flicker amplitude was significantly reduced in the diabetic groups, the response to the initial stimulus cycle recorded at 31.25 Hz and 62.5 Hz was not significantly reduced. Likewise, the light-adapted single flash response is also generally unaffected in diabetics who have mild or no DR.10, 25 This finding may be understood by considering the response to the first stimulus cycle as an approximation of the single flash response, with the following caveats: 1) the energy contained in the first stimulus cycle differs from that used in the standard clinical single flash stimulus; 2) the adaptation level used in the present study (200 cd/m2) is substantially higher than that recommended for standard clinical ERGs (30 cd/m2); 3) the stimulus waveforms differ (sinusoidal modulation versus a luminance pulse). Nevertheless, if the response to the initial stimulus cycle approximates a single flash response, then it would be expected to be unaltered in diabetics who have mild or no NPDR, as was observed in the present study. To examine this further, light-adapted single flash responses were obtained from a subset of the subjects in the present study (10 control, 13 no DR, 13 mild NPDR subjects). As expected, the single flash responses were generally normal for these subjects, with only two diabetic subjects having a b-wave amplitude that was below the lower limit of normal. Of note, these two subjects also had an amplitude reduction for the first cycle of the flicker response.
We also show that the 62.5 Hz flicker amplitude abnormalities cannot be predicted based on typical clinical characteristics including age, acuity, disease duration, or HbA1c. The poor correlation between disease duration and the 62.5 Hz ERG flicker abnormality may, at least in part, be attributed to difficulty in accurately determining diabetes duration, as individuals can be diabetic for several years prior to a clinical diagnosis. The non-significant correlation between HbA1c and the 62.5 Hz flicker ERG abnormality is not necessarily surprising; previous ERG studies have shown non-significant26, 27 or moderate28, 29 correlations with HbA1c. Nevertheless, the generally weak correlations with standard clinical parameters suggest that the ERG may provide insight into neural aspects of the disease beyond those provided by typical clinical measures.
One limitation of the present study is that all DM subjects were staged based on clinical examination. Fluorescein angiography was not performed in DM subjects who were classified as no DR. As such, it is possible that subtle vascular abnormalities not apparent on clinical examination or standard fundus photography may have been overlooked. The use of additional imaging modalities, such as ultra-wide-field fluorescein angiography30 and red-free fundus photography,31 may be useful to better detect subtle vascular abnormalities for sub-grouping diabetic subjects in future studies. The use of advanced approaches to subgroup subjects may enhance the ERG differences between the no DR and mild NPDR groups.
In summary, the 62.5 Hz flicker ERG is useful for evaluating retinal dysfunction in diabetics who have mild or no NPDR, as the response amplitude at this high frequency can be significantly abnormal. Although the source of the abnormality requires further study, the results indicate a relatively early retinal site of neural dysfunction in diabetes, as the high frequency flicker ERG is believed to be generated primarily by retinal bipolar cells. Regardless of the source, the high frequency flicker ERG may be of use as a non-invasive, objective outcome measure for future clinical trials.
SUMMARY.
Retinal dysfunction was evaluated in diabetic patients who have mild or no non-proliferative diabetic retinopathy (NPDR) using the high-frequency flicker electroretinogram. The results show that the 62.5 Hz flicker electroretinogram is a useful, clinically-relevant measure of dysfunction and the pattern of response abnormalities suggests an early retinal site of disease.
Acknowledgments
This work was supported by: National Institutes of Health research grants R01EY026004 (JM), P30EY001792 (core grant), unrestricted departmental grant and a Dolly Green Scholar award (JM) from Research to Prevent Blindness. These funding organizations had no role in the design or conduct of this research.
Footnotes
No author has a proprietary interest in the data presented in this manuscript.
References
- 1.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 2.Davis MD, et al. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci. 1998;39:233–252. [PubMed] [Google Scholar]
- 3.Wilkinson CP, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 4.Falsini B, et al. Steady-state pattern electroretinogram in insulin-dependent diabetics with no or minimal retinopathy. Doc Ophthalmol. 1989;73:193–200. doi: 10.1007/BF00155037. [DOI] [PubMed] [Google Scholar]
- 5.Coupland SG. A comparison of oscillatory potential and pattern electroretinogram measures in diabetic retinopathy. Doc Ophthalmol. 1987;66:207–218. doi: 10.1007/BF00145234. [DOI] [PubMed] [Google Scholar]
- 6.Han Y, et al. Multifocal electroretinogram and short-wavelength automated perimetry measures in diabetic eyes with little or no retinopathy. Arch Ophthalmol. 2004;122:1809–1815. doi: 10.1001/archopht.122.12.1809. [DOI] [PubMed] [Google Scholar]
- 7.Chan HH, et al. Detection of early functional changes in diabetic retina using slow double-stimulation mfERG paradigm. Br J Ophthalmol. 2011;95:1560–1563. doi: 10.1136/bjo.2010.192476. [DOI] [PubMed] [Google Scholar]
- 8.Lung JC, Swann PG, Chan HH. Early local functional changes in the human diabetic retina: a global flash multifocal electroretinogram study. Graefes Arch Clin Exp Ophthalmol. 2012;250:1745–1754. doi: 10.1007/s00417-012-2010-z. [DOI] [PubMed] [Google Scholar]
- 9.Ng JS, et al. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;49:1622–1628. doi: 10.1167/iovs.07-1157. [DOI] [PubMed] [Google Scholar]
- 10.Bresnick GH. Diabetic Retinopathy. St Louis: Mosby Year Book; 1991. [Google Scholar]
- 11.Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44:53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- 12.Al-Otaibi H, et al. Validity, Usefulness and Cost of RETeval System for Diabetic Retinopathy Screening. Transl Vis Sci Technol. 2017;6:3. doi: 10.1167/tvst.6.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuo M, et al. Screening for diabetic retinopathy using new mydriasis-free, full-field flicker ERG recording device. Sci Rep. 2016;6:36591. doi: 10.1038/srep36591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maa AY, et al. A novel device for accurate and efficient testing for vision-threatening diabetic retinopathy. J Diabetes Complications. 2016;30:524–532. doi: 10.1016/j.jdiacomp.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCulloch DL, et al. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 16.Alexander KR, Barnes CS, Fishman GA. High-frequency attenuation of the cone ERG and ON-response deficits in X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2001;42:2094–2101. [PubMed] [Google Scholar]
- 17.Alexander KR, et al. Activation phase of cone phototransduction and the flicker electroretinogram in retinitis pigmentosa. Vision Res. 2006;46:2773–2785. doi: 10.1016/j.visres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Kondo M, Sieving PA. Primate photopic sine-wave flicker ERG: vector modeling analysis of component origins using glutamate analogs. Invest Ophthalmol Vis Sci. 2001;42:305–312. [PubMed] [Google Scholar]
- 19.Park JC, et al. Rod and cone contributions to the dark-adapted 15-Hz flicker electroretinogram. Doc Ophthalmol. 2015;130:111–119. doi: 10.1007/s10633-015-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lung JC, Swann PG, Chan HH. The Multifocal On- and Off-Responses in the Human Diabetic Retina. PLoS One. 2016;11:e0155071. doi: 10.1371/journal.pone.0155071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falsini B, Riva CE, Logean E. Flicker-evoked changes in human optic nerve blood flow: relationship with retinal neural activity. Invest Ophthalmol Vis Sci. 2002;43:2309–2316. [PubMed] [Google Scholar]
- 22.Felder AE, et al. The Effects of Diabetic Retinopathy Stage and Light Flicker on Inner Retinal Oxygen Extraction Fraction. Invest Ophthalmol Vis Sci. 2016;57:5586–5592. doi: 10.1167/iovs.16-20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garhofer G, et al. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004;88:887–891. doi: 10.1136/bjo.2003.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holopigian K, et al. Evidence for photoreceptor changes in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997;38:2355–2365. [PubMed] [Google Scholar]
- 25.Jansson RW, Raeder MB, Krohn J. Photopic full-field electroretinography and optical coherence tomography in type 1 diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253:989–997. doi: 10.1007/s00417-015-3034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyrberg M, Ponjavic V, Lovestam-Adrian M. Multifocal electroretinography (mfERG) in insulin dependent diabetics with and without clinically apparent retinopathy. Doc Ophthalmol. 2005;110:137–143. doi: 10.1007/s10633-005-4187-5. [DOI] [PubMed] [Google Scholar]
- 27.Baget-Bernaldiz M, et al. Multifocal electroretinography changes at the 1-year follow-up in a cohort of diabetic macular edema patients treated with ranibizumab. Doc Ophthalmol. 2017;135:85–96. doi: 10.1007/s10633-017-9601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakhani E, et al. Multifocal ERG defects associated with insufficient long-term glycemic control in adolescents with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:5297–5303. doi: 10.1167/iovs.10-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klemp K, et al. The multifocal ERG in diabetic patients without retinopathy during euglycemic clamping. Invest Ophthalmol Vis Sci. 2005;46:2620–2626. doi: 10.1167/iovs.04-1254. [DOI] [PubMed] [Google Scholar]
- 30.Wessel MM, et al. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32:785–791. doi: 10.1097/IAE.0b013e3182278b64. [DOI] [PubMed] [Google Scholar]
- 31.Venkatesh P, et al. Detection of retinal lesions in diabetic retinopathy: comparative evaluation of 7-field digital color photography versus red-free photography. Int Ophthalmol. 2015;35:635–640. doi: 10.1007/s10792-012-9620-7. [DOI] [PubMed] [Google Scholar]