Abstract
Heparin-binding epidermal growth factor (EGF)-like growth factor (HBEGF) is expressed in the embryo and uterus at the implantation site, stimulating trophoblast invasive activity essential for placentation. The effect of extraembryonic HBEGF deficiency on placental development was investigated by breeding mice heterozygous for the Hbegf null mutation. On gestation day 13.5, the average placental weights of the wild-type (Hbegf+/+) and heterozygous (Hbegf+/−) mice were approximately 76 and 77 mg, respectively, as opposed to reduced average placental weights of approximately 61 mg in homozygous null (Hbgef−/−) females. In contrast, fetal weights were not significantly affected by genotype. HBEGF immunostaining in placental sections was Hbegf gene dosage-dependent, while expression of other EGF family members was comparable in Hbegf+/+ and Hbegf−/− placentas. Histological analysis revealed no apparent differences in trophoblast giant cells, but the spongiotrophoblast region was reduced compared to labyrinth (P < 0.05) in Hbegf null placentas. While no differences in cell apoptosis were noted, proliferation as assessed by nuclear Ki67 staining was elevated in the labyrinth and decreased in the spongiotrophoblast region of Hbegf−/− placentas. Labyrinth morphology appeared disrupted in Hbegf −/– placentas stained with laminin, a marker for capillary basement membrane, and the capillary density was reduced. Immunohistochemical staining revealed reduced vascular endothelial growth factor (VEGF) levels in both spongiotrophoblast and labyrinth (P < 0.01) regions of Hbegf−/− placentas. In vitro, HBEGF supplementation increases the expression of VEGF in a human trophoblast cell line. These findings suggest that trophoblast HBEGF promotes placental capillary formation by inducing VEGF in the developing placenta of mice.
Keywords: angiogenesis, growth factors, placenta, transgenic/knockout model, trophoblast
Studies using a transgenic mouse model reveal HBEGF deficiency in extraembryonic tissues reduces placental size.
Introduction
Heparin-binding EGF-like growth factor (HBEGF) is a member of the epidermal growth factor (EGF) family of peptide growth and differentiation factors [1, 2]. The EGF family includes EGF, HBEGF, transforming growth factor-alpha (TGFA), amphiregulin (AREG), epiregulin, betacellulin, neuregulins, and the NRG-2 peptides [1]. Like other members of this family, HBEGF is synthesized as a transmembrane precursor (proHBEGF) that is processed by a metalloproteinase to release the soluble ectodomain (sHBEGF) from the cell surface [2]. Both proHBEGF and sHBEGF can activate its cognate receptors, EGFR/ERBB1 and ERBB4, with requisite binding to cell surface heparin sulfate proteoglycans as cofactors [1–3]. The family of ERBB receptor tyrosine kinases includes four transmembrane proteins, EGFR/ERBB1, ERBB2, ERBB3, and ERBB4, which bind EGF family ligands. The cytoplasmic domains become autophosphorylated through receptor homo- or heterodimerization upon ligation, thus transmitting downstream signaling to regulate cell growth and differentiation.
HBEGF is involved in a number of physiological and pathological processes that include wound healing [4], heart development [5, 6], oncogenic transformation [7], and blastocyst implantation [8–10]. HBEGF is expressed in both the blastocyst and the luminal epithelium during implantation in several species, including humans and rodents [9]. It is also expressed in the decidua and placental trophoblast cells [8]. HBEGF is a critical signaling molecule for pregnancy success that directs trophoblast survival and invasion during implantation and placentation [10–12]. The in vivo function of HBEGF during development was examined in Hbegf mutant mice (Hbegf−/−), revealing that it is essential for embryonic heart development, but embryo implantation goes forward [5]. The Hbegf−/− embryos appear to implant normally and Hbegf+/− embryos develop into healthy adults. However, HBEGF expression in the uterus and decidua appears to be critical during implantation. Using Hbegf−/− mice it was found that loss of maternal HBEGF deferred on-time implantation and compromised pregnancy outcome [10]. In humans, HBEGF is expressed in the placenta throughout pregnancy [13–16]. Notably, HBEGF expression is deficient in trophoblast cells of placentas of women with preeclampsia [13, 16], a hypertensive disorder associated with poor trophoblast invasion [17] and elevated apoptosis [18, 19]. These findings suggest that in addition to its role during blastocyst implantation, HBEGF could provide essential functions during placental development. Although there is reason to suspect that HBEGF deficiency directly impedes normal heart development [5], developmental defects of the fetus, including heart, can arise secondarily from gene deletions that disrupt the structural organization and function of the placenta [20–22].
In humans, HBEGF appears to regulate trophoblast invasion and survival. In vitro studies using an immortalized first trimester human trophoblast cell line demonstrated that EGF family members EGF, TGFA, and HBEGF could induce its differentiation to an extravillous trophoblast phenotype [11]. HBEGF and other members of the EGF family have cytoprotective activity in trophoblast cells exposed to various forms of oxidative stress [23, 24]. Survival of trophoblast cells at low O2 concentration (2%) is dependent specifically on HBEGF signaling [12, 25]. These in vitro studies are consistent with HBEGF having a critical role in human placentation, and are supported by the observation of its disruption in vivo associated with preeclampsia [13, 16]. Unfortunately, a direct role of HBEGF dysfunction in pathologies stemming from poor placentation is difficult to establish in humans. However, the Hbegf−/− mouse model offers an opportunity to investigate the in vivo function of HBEGF in trophoblast cells during placenta development.
In rodents, the fully developed placenta is composed of three major layers. In mice, they are the maternal decidua, the placenta junctional zone, and the innermost labyrinth [26]. The outer maternal layer includes decidual cells of the uterus, and maternal vasculature [26]. The decidua is devoid of trophoblast cells until mid-gestation when trophoblast cells invade into the decidua and spiral arteries, replacing the endothelium and thereby promoting the transition from endothelial cell- to trophoblast cell-lined maternal blood spaces [27]. The middle junctional region attaches the fetal placenta to the uterus and contains fetoplacental (trophoblast) cells that invade the uterine wall and maternal vessels [26]. The junctional zone consists of spongiotrophoblast cells (spongiotrophoblast region) and a layer of trophoblast giant cells that delimit the implantation site [27]. The inner labyrinth layer is composed of highly branched maternal blood sinuses intertwined with fetal capillaries for efficient nutrient exchange [26, 28]. The sinuses contain maternal blood and are composed of outer epithelial layers that are derived from the trophoblast cell lineage (mononuclear layer of trophoblast cell, layer I) and a bilayer of syncytiotrophoblast cells (layers II and III), and fetal blood vessels [26]. The nutrients, gases, and waste must diffuse or be transported across the four layers to get from one blood compartment to the next [26].
We hypothesize that placental development is compromised in the absence of intercellular signaling by HBEGF, which in turn compromises trophoblast differentiation and survival. Therefore, we compared placentation in Hbegf+/+ and Hbegf−/− mouse conceptuses developing in heterozygous dams, focusing on trophoblast differentiation and signaling in placentas on day 13.5 of gestation.
Materials and methods
Animal
All mice used in this investigation were housed in the Vanderbilt Animal Care Facility according to National Institutes of Health and institutional guidelines for laboratory animals. HBEGF heterozygous (Hbegf+/−) mice on a C57BL/6J genetic background were generated as previously described [5]. Mouse Hbegf cDNA containing the polyadenylation sequence flanked by loxP sequences was fused with the first exon of the mouse Hbegf gene. Cre-mediated recombination led to deletion of Hbegf cDNA concomitant with expression of the lacZ inserted downstream. Mice with systemic deletion of Hbegf were generated by breeding Hbegfflox/lox mice with CAG-Cre mice [10]. Hbegf+/− females were mated with Hbegf+/− males to induce pregnancy (day 0.5 = vaginal plug).
Conceptuses were removed on gestation day 13.5 from Hbegf+/− dams. Each fetus was separated, weighed, and genotyped using genomic DNA by PCR [5]. Placentas were weighed, fixed, and embedded in paraffin for sectioning. Sections were stained with hematoxylin and eosin (H&E).
Immunohistochemistry
Immunohistochemistry was performed using a DAKO (Carpinteria, CA) Autostainer Universal Staining System, as previously described [11, 13, 15]. Rehydrated sections of placenta were labeled for 1 h at 25°C with either 10 μg/ml nonimmune goat IgG (Jackson Immunoresearch Laboratories, West Grove, PA) or 5 μg/ml goat polyclonal antibody against human HBEGF (AF259), EGF (AF2028), TGFA (AF239), AREG (AF989), laminin (EMD Millipore AB2034), or vascular endothelial growth factor (VEGF) (AF293) (R&D systems unless otherwise indicated). Tissues were then incubated for 1 h at 25°C with 0.1 μg/ml rabbit anti-goat IgG (Jackson Immunoresearch). To visualize and quantify antigen, an Envision System peroxidase anti-mouse/rabbit kit (DAKO) was used in conjunction with image analysis, according to our published procedure [13]. Images were processed using Simple PCI (C-Imaging Corp., Cranberry Township, PA), and the average gray level was obtained for semiquantitative analysis, as previously described [13, 29]. Values obtained with IgG substituted for primary antibody were subtracted from each sample.
In brief, microscopic images were obtained at ×10 magnification with a DM IRB inverted microscope (Leica, Wetzlar, Germany) at the same light settings and processed for printing with Photoshop version 5.0 using identical conditions of enlargement, color balance, contrast, and brightness. Images were semiquantitatively analyzed using Simple PCI. The 8-bit monochrome images were obtained from the antibody labeled slides at ×40 magnification with blue and green filtered light. The white level was obtained from the region without any tissue, while the average gray level of staining was established from the region with placenta tissue. The stain intensity was calculated by subtraction of the gray level from 255. The staining intensity of each antibody was averaged from triplicate readings of a target area after subtracting the background from nine slides incubated with IgG. In our preliminary experiments, the concentration of the primary antibody was selected that produced a stain intensity within the linear response range in stained trophoblast cells cultured on the slides.
Competition experiments were done by incubating sections of placenta with anti-HBEGF (mab2591), anti-EGF (AF2028), anti-TGFA (AF239), and anti-AREG (AF989), all from R&D Biosystems, adsorbed with a 10-fold molar excess of mouse recombinant HBEGF (Novus Biosciences), EGF (Novus Biosciences), rat TGFA, and mouse AREG, performed as described above.
Morphometric analysis
Images of central cross sections through the placenta were used. The spongiotrophoblast region and labyrinth layers of the entire placental area were circumscribed, and the areas were measured by image analysis, using Simple PCI in 10 Hbegf+/+ placentas, 18 Hbegf+/– placentas, and 10 Hbegf–/– placentas, and reported as a fraction of the total placenta area, including the labyrinth. The size of spongiotrophoblast region or labyrinth layer in proportion to the total area from these two layers was also reported. In addition, laminin-highlighted capillaries were counted in images taken from laminin-stained sections and recorded as the average number from three fields for each of 8 Hbegf+/+ placentas and 8 Hbegf −/− placentas (one placenta was randomly selected from each mouse).
Proliferation and cell death assays
Proliferation was quantified by immunohistochemical staining of nuclear Ki67, as previously described [15, 30]. Sections labeled by immunohistochemistry with a mouse monoclonal antibody against Ki-67 (DAKO) were counterstained with hematoxylin and similarly assessed for the percentage of Ki-67/hematoxylin-labeled nuclei as an index of cell proliferation. Cell death was detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL), using an alkaline phosphatase-based kit from Roche Applied Science (Indianapolis, IN) and counterstaining with hematoxylin as previous described [15, 30]. Slides were viewed at ×200 magnification and digital images were analyzed to determine the percentage of TUNEL/hematoxylin-labeled nuclei (TUNEL index) from triplicate fields. Positive and counterstained cells were counted to obtain the Ki67 and TUNEL indices (%+).
Cell culture and treatments
The HTR-8/SVneo cytotrophoblast cell line [31] was maintained at passages 29–45 in a 1:1 mixture of DMEM and Ham's F12 media (DMEM/F12; Sigma Chemical Co., St. Louis, MO) containing 10% donor calf serum and cultured at 37°C in a humidified, 5% CO2/95% air incubator. HTR-8/SVneo cells were cultured for 24 h in serum-free medium containing 5 mg/ml BSA (Sigma) in six-well microtiter plates (Becton Dickinson Labware, Bedford, MA). The cells were then treated with 0, 0.1 nM, 1 nM, or 10 nM HBEGF (R&D system) for 24 h.
VEGF ELISA
HTR-8/SVneo cells treated for 24 h with recombinant human HBEGF were extracted with 600 μl/plate of PBS containing 0.5% Tween 20 (Sigma) and protease inhibitors (1 μg/ml each of leupeptin, chymostatin, and pepstatin, 25 KIU/ml aprotinin, 2 μg/ml antipain, and 10 μg/ml benzamidine; all from Sigma). Extracts were centrifuged at 5000 g and the supernatants were stored frozen at −70°C. Total cellular protein concentrations were determined using a Pierce BCA protein assay kit (ThermoFisher Scientific, Waltham, MA) for all cell lysates. ELISA was performed using the VEGF Quantikine ELISA kit (R&D Systems). The optical density of the final reaction product was determined at 450 nm using a programmable multiplate spectrophotometer (Power Wave Workstation; Bio-Tek Instruments) with automatic wavelength correction. VEGF concentrations were calculated from the corresponding standard curve.
Statistics
The data were analyzed for any differences between the means by two-tailed t-test for two independent groups and ANOVA for three or more independent groups by SPSS 21. A Tukey HSD post hoc test was performed if significant difference was identified in ANOVA. A P value of 0.05 and less was deemed to be statistically significant.
Results
Hbegf-null mice have smaller placentas with normal size fetuses
In mice, the definitive placental structure is established by gestation day 12.5. In our current study, 9 Hbegf heterozygous (Hbegf+/–) females were mated with Hbegf+/– males and 68 conceptuses were collected on gestation day 13.5 and genotyped. The ratio of Hbegf+/+: Hbegf+/−: Hbegf−/- conceptuses was 14:36:18. The average weights of Hbegf+/+, Hbegf+/−, and Hbegf−/– fetuses were 0.151 ± 0.042 g, 0.143 ± 0.041 g, and 0.139 ± 0.027 g, respectively (Figure 1). No significant difference (P > 0.05) was found among the three groups. The average placental weights of Hbegf+/+, Hbegf+/−, and Hbegf−/− conceptuses were 0.076 ± 0.017 (n = 14) g, 0.077 ± 0.014 (n = 36) g, and 0.060 ± 0.015 (n = 18) g (Figure 1). The Hbegf−/− placentas were significantly lighter than Hbegf+/+ or heterozygous placentas (P < 0.01). No significant difference was found between Hbegf+/+ and Hbegf+/− placentas (P > 0.05). These results show a 21% decrease in placental weight was not sufficient to significantly reduce fetal weight.
Figure 1.
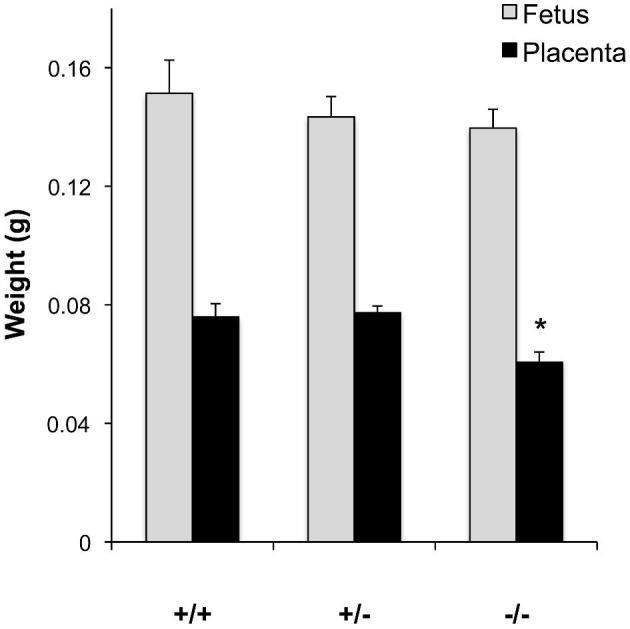
Fetal weight and placental weights of Hbegf+/+, Hbegf+/−, and Hbegf−/− conceptuses. * P < 0.01.
EGF family expression in Hbegf−/− placentas
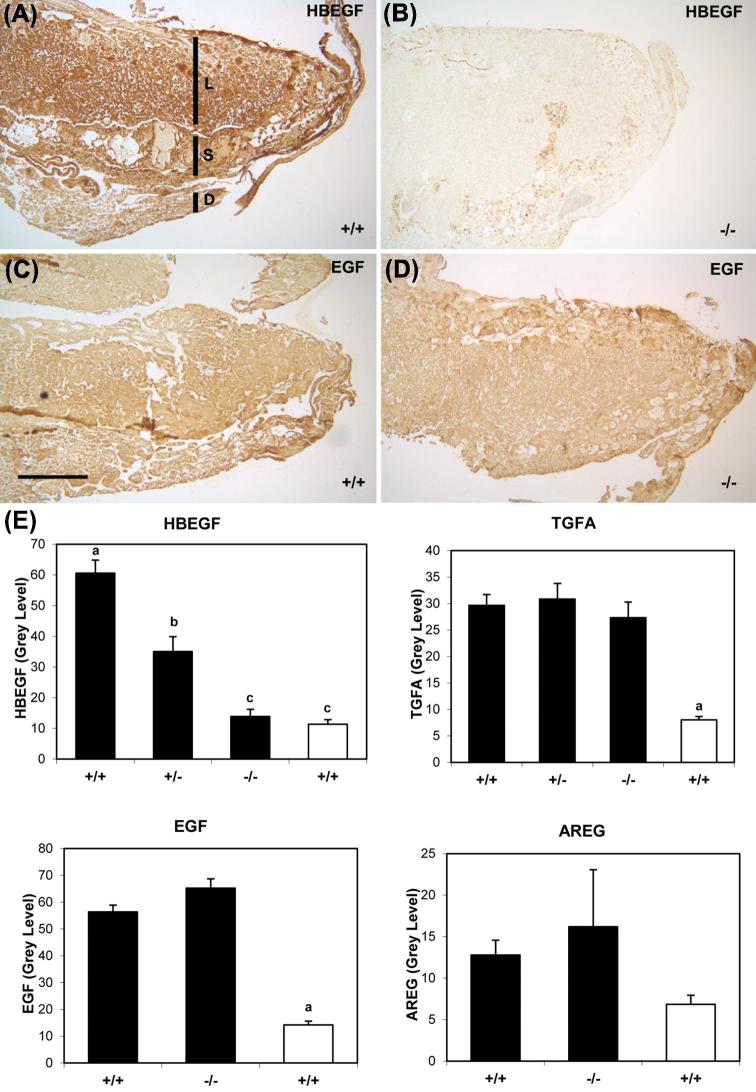
Members of the EGF family, including EGF, HBEGF, TGFA, AREG, BTC, and EREG, are expressed in the human placenta, and regulate implantation and placentation [11, 16]. Immunohistochemistry was performed to evaluate their expression in mouse placentas of the three genotypes, as it provides important information on the location, as well as the amount, of expressed protein. In Hbegf+/+, the expression of EGF family members (HBEGF, TGFA, EGF, and AREG) was stronger (P < 0.05) than background (nonimmune IgG) controls (Supplemental Figures S1 and S2) or labeling with antibody preadsorbed with the corresponding recombinant growth factor (Figure 2E), according to semiquantitative analysis. HBEGF was present throughout Hbegf+/+ placentas (n = 24; Figure 2A and E), moderately expressed in Hbegf+/− placentas (n = 17, P < 0.05; Figure 2E), but was not detected in Hbegf−/− placentas (n = 21, P < 0.05; Figure 2B and E). However, the placental expression of TGFA (Hbegf+/+ n = 24, Hbegf+/− n = 9, Hbegf−/− n = 21; Figure 2E), EGF (Hbegf+/+ and Hbegf−/− n = 12; Figure 2C–E), and AREG (Hbegf+/+ n = 13, Hbegf−/− n = 11) did not differ (P > 0.05) among the three genotypes.
Figure 2.
Expression of EGF family proteins in placentas with different Hbegf genotypes. Immunohistochemistry of HBEGF (A, B) and EGF (C, D) in Hbegf+/+ (A, C) and Hbegf−/−(B, D) placentas. Size bar in C, 500 μm. S, spongiotrophoblast region; L, labyrinth; D, decidua. Semiquantification of expression of HBEGF and TGFA in placentas of the three genotypes (E, top panels), and the growth factors EGF and AREG in Hbegf+/+ and Hbegf–/− genotypes (E, bottom panels) are shown. The open bars indicate values for placentas in which the antibody had been incubated with recombinant antigen, as described in competition experiment of Materials and Methods section (n = 3). Values are compared to IgG control shown in Supplemental Figure 1. Nonmatching letters over bars indicate P < 0.05.
Spongiotrophoblast region is reduced in Hbegf−/− placentas
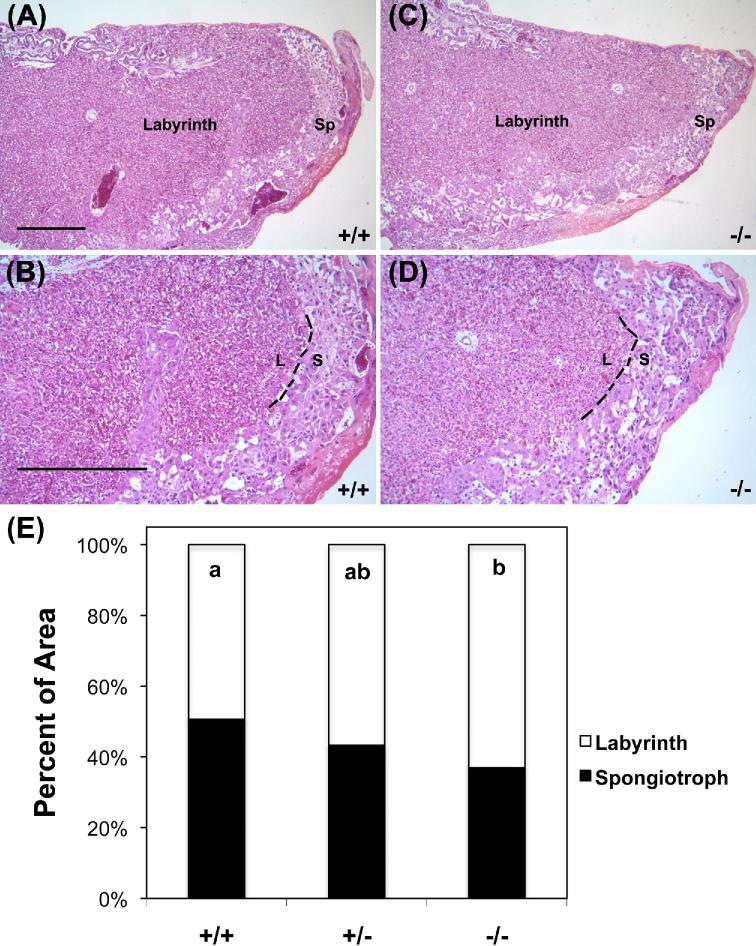
To explore morphological changes in Hbegf−/− conceptuses, placentas of the three genotypes were sectioned and stained with H&E. The size of the spongiotrophoblast region in proportion to the whole placental area including spongiotrophoblast region and labyrinth layer was examined (Figure 3A–D). Morphometric analysis demonstrated the relative size of the spongiotrophoblast region to be 50.6%, 43.2%, and 36.9%, as a fraction of the total placental measured area in Hbegf+/+, Hbegf +/−, and Hbegf−/− placentas (n = 8 for all groups), respectively, including the labyrinth (Figure 3E). The relative size of the spongiotrophoblast region in Hbegf−/− placentas was significantly reduced compared with Hbegf+/+ placentas (P < 0.05). The relative size of the spongiotrophoblast region in Hbegf+/− placenta was intermediate and did not differ from the other genotypes (P > 0.05). The absolute size of labyrinth layer did not significantly differ among the three phenotypes, but the absolute size of spongiotrophoblast region demonstrated the same pattern of change as its relative size (data not shown).
Figure 3.
Morphology of placentas with different Hbegf genotypes. (A–D) H&E staining of Hbegf+/+ and Hbegf−/− placentas at low (×20) and high (×200) magnifications. Size bars, 500 μm. Sp or S, spongiotrophoblast region; L, labyrinth. E. Relative cross-sectional size of the labyrinth and spongiotrophoblast region. Nonmatching letters indicate P < 0.05, while bars that share a letter are not statistically different due to genotype.
Proliferation is altered in Hbegf−/− placentas
To examine the cause of reduction of the spongiotrophoblast region in Hbegf−/− placentas, apoptosis and proliferation were evaluated by TUNEL and Ki67 immunostaining, respectively. There were no differences in apoptosis in either the spongiotrophoblast regions or labyrinth layers between Hbegf+/+ and Hbegf−/– placentas (not shown). However, proliferative capacity was significantly reduced in the spongiotrophoblast region and increased in labyrinth layer of Hbegf−/– placentas (n = 10) compared with Hbegf+/+ placentas (n = 11; Figure 4).
Figure 4.
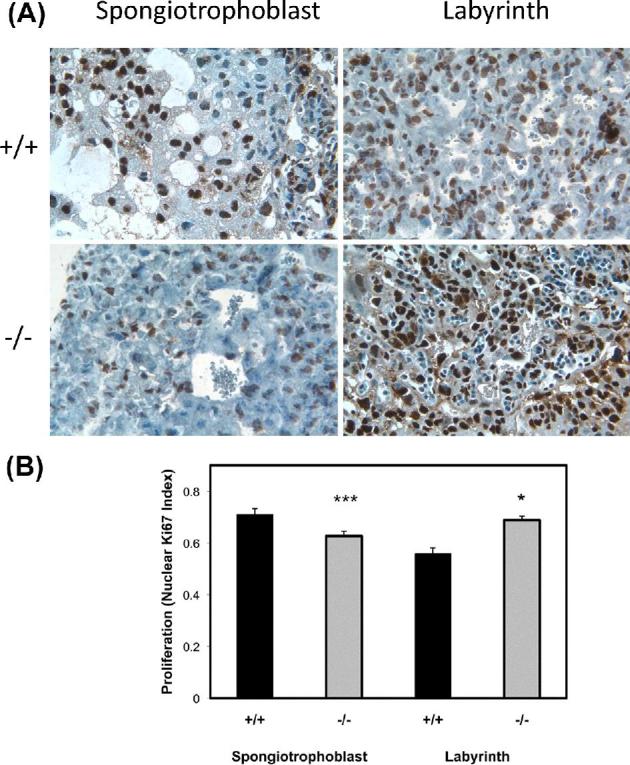
Cell proliferation in labyrinth and spongiotrophoblast regions of placentas with different Hbegf genotypes. Proliferation was measured by immunohistochemistry for Ki67. Images of sections stained for Ki67 (A) were semiquantified (B), as described in the Materials and Methods section. WT: wild type; KO: knockout. *, P < 0.05; ***, P < 0.001
Labyrinth morphology is disrupted in Hbegf−/− placentas
The labyrinth layer contains trophoblasts and blood vessels. To highlight labyrinth structure, capillaries were labeled with anti-laminin by immunohistochemistry (Figure 5A). Examples of two fields from Hbegf+/+ placentas appear in the left panels and examples from Hbegf–/– placentas appear in the right panels. In Hbegf+/+ placentas, fetal capillaries (fc) were clearly circumscribed by a basement membrane containing laminin. Capillaries in the Hbegf−/− placentas were poorly defined and contained disorganized laminin structures (arrows in Figure 5A). Capillaries were counted to quantify the density of capillaries in the labyrinth (Figure 5B). Analysis demonstrated a reduced density of laminin-lined capillaries (n = 8, P < 0.02) in Hbegf−/− placentas (57.7 ± 11.5/field), compared to the Hbegf+/+ placentas (n = 8, 71.3 ± 8.9/field).
Figure 5.
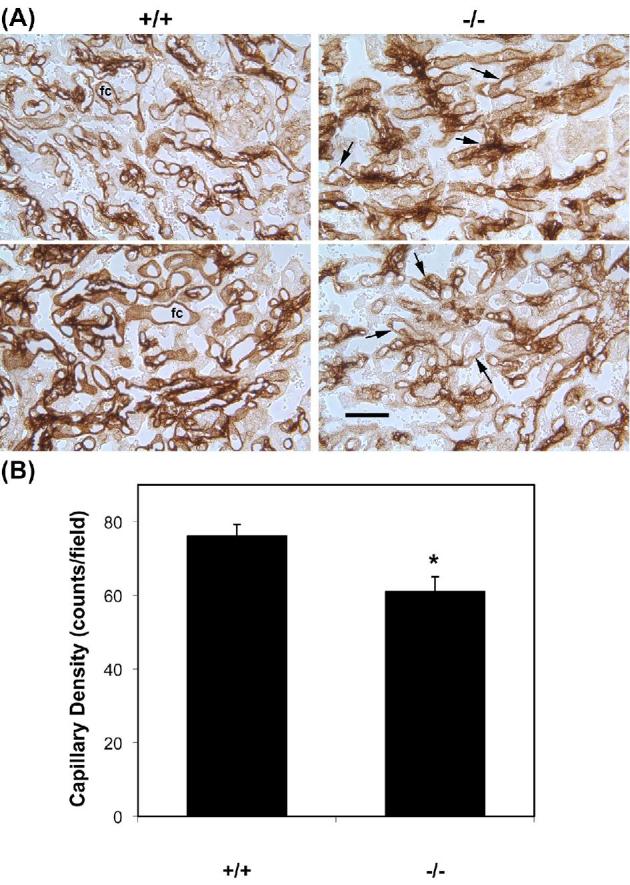
Density of capillary vessels in placentas with different Hbegf genotypes. (A) Capillary vessels were labeled with an antibody against laminin and vessels in the labyrinth are shown. Size bars, 500 μm. (B) The density of laminin-labeled capillary beds in the labyrinth determined as described in the Materials and Methods section. * P < 0.05.
Expression of VEGF is reduced in Hbegf−/− placentas
VEGF is a key factor regulating blood vessel genesis. Immunostaining was used to detect VEGF expression in trophoblast cells. VEGF expression was significantly reduced in the labyrinth layer (P < 0.01) of Hbegf−/− placentas (n = 9) compared with Hbegf+/+ placentas (n = 7) (Figure 6), and exhibited a trend toward reduction in spongiotrophoblast (P = 0.06).
Figure 6.
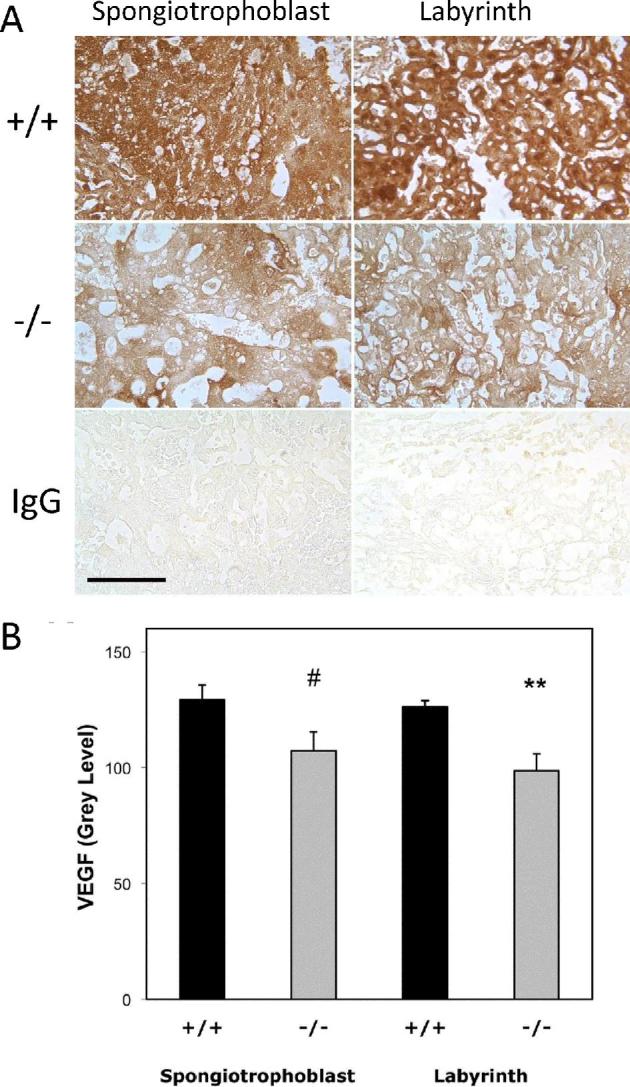
The effect of Hbegf genotype on expression of VEGF in the mouse placenta. Immunohistochemical labeling with anti-VEGF antibody or nonimmune IgG (A) was subjected to semiquantitative analysis (B), as described in the Materials and Methods section. VEGF expression was measured in sections of mouse placentas with the indicated genotype. P values of comparisons between Hbegf+/+ and Hbegf−/− in the labyrinth and spongiotrophoblast region (#, P = 0.06; **P < 0.01).
HBEGF upregulates VEGF expression in a human trophoblast cell line
To explore the role of HBEGF in regulating the expression of VEGF in trophoblast cells, an in vitro study with placental trophoblast was performed, using the human trophoblast cell line, HTR-8/SVneo. HBEGF supplementation (≥0.1 nM) increased the expression of VEGF after 24 h of treatment (P < 0.01) as measured by ELISA (Figure 7). Of note, 1 nM HBEGF is the concentration achieved within 4 h during hypoxic treatment of HTR-8/SVneo cells [12].
Figure 7.
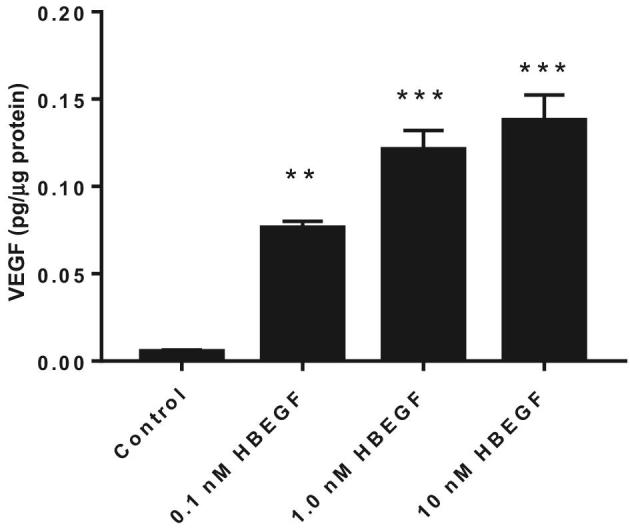
The effect of HBEGF on expression of VEGF in HTR-8/SVneo cells as measured by ELISA. Cells were treated with the indicated concentrations of HBEGF and ELISA was performed for VEGF, as described in Materials and Methods section. **, P < 0.01 and ***, P < 0.001, compared to the nontreated control.
Discussion
In this study, we explored the role of extraembryonic HBEGF during placentation in mice. During pregnancy, the placenta is exposed to both maternal and fetal (extraembryonic and embryonic) HBEGF. Therefore, it is difficult to isolate the effects of extraembryonic HBEGF from maternal and embryonic HBEGF. The ideal approach to study the effect of HBEGF on placentation would be to transfer Hbegf+/+ or null embryos to Hbegf−/− dams. Unfortunately, Hbegf−/– females rarely survive to reproductive age (our unpublished data). Thus, we mated Hbegf+/– males and females and compared the status of placentas among the three resulting fetal genotypes. As expected, the expression of extraembryonic HBEGF decreased in proportion to Hbegf gene dosage in placentas. Other members of the EGF family, including EGF, TGFA, and AREG, failed to compensate for the loss of HBEGF in the placenta, since their expression was not altered with Hbegf gene dosage in the placentas, and Hbegf−/– placentas weighed less than Hbegf+/– and Hbegf+/+ placentas. Our data show that extraembronic HBEGF has important roles in mouse placentation, even under the influence of maternal HBEGF.
The mouse placenta is comprised of three major layers: maternal decidua, junctional zone consisting of parietal giant trophoblast cell layer and spongiotrophoblast region, and labyrinth layer. The labyrinth layer is exposed to both maternal and fetal HBEGF. However, the spongiotrophoblast is not directly exposed to maternal and embryonic HBEGF. Therefore, the effects of HBEGF on the development of spongiotrophoblast mainly reflect extraembryonic contributions. We found that the spongiotrophoblast region was smaller in Hbegf−/− placentas, suggesting that extraembryonic HBEGF deficiency restricts the full development of the spongiotrophoblast region. This is also consistent with our observation that Ki67 staining is reduced in the spongiotrophoblast, compared with labyrinth, region.
HBEGF receptors, EGFR/ERBB1 and ERBB4 [1–3], are both expressed in murine placentas. There was no difference in the expression level and pattern for these receptors among HBEGF wild type, heterozygous, and null placenta (unpublished data). EGFR is expressed primarily in the decidua, trophoblast giant cells, and spongiotrophoblast cells, with low levels detected in the labyrinth [32]. Homozygosity for a null allele of EGFR in mice leads to periimplantation or postnatal lethality that is strongly dependent on genetic background [33–35]. EGFR null fetuses, which die at mid-gestation or postnatal stage, have reduced spongiotrophoblast region and small placentas [33, 34]. Our data demonstrating smaller placentas and reduced spongiotrophoblast region in Hbegf−/− mice are consistent with the above findings. However, the effects of loss of HBEGF on the spongiotrophoblast region are milder than in the EGFR knockout, probably due to some compensatory effects of other EGFR agonists such as EGF and/or TGFA in Hbegf−/− mice. ERBB4 is expressed in the murine placenta, specifically in maternal decidua and trophoblast giant cells [32]. Mice lacking ERBB4 die during mid-embryogenesis from the aborted development of myocardial trabeculae in the heart ventricle [36]. The placental changes in Errb4−/− mice have not been reported. Since ERBB4 is only expressed in trophoblast giant cells, its role in murine placentation, especially in the spongiotrophoblast region, might be limited. Therefore, our data are consistent with the idea that HBEGF regulates murine placentation predominantly through EGFR.
In Egfr−/− mice, the reduction in the spongiotrophoblast region is caused by reduced proliferation; there is no difference in apoptosis between Egfr−/− and Egfr+/+ mice [32]. Our data suggest that HBEGF regulates murine placentation mainly through EGFR, which is consistent with the observation that Ki67 staining, a marker of proliferation, was reduced in the spongiotrophoblast region in Hbegf−/− mice. Note that in Egfr−/– mice, the proliferative capacity of the total placenta was compared [32], while in our study, we used immunohistochemistry to compare proliferation of each region. We found that the proliferative capacity was significantly reduced in the spongiotrophoblast region, and increased in the labyrinth region of Hbegf−/− placentas. The decreased proliferation in the spongiotrophoblast region is consistent with its reduced size; however, the significance and cause of increased proliferation in the labyrinth layer in Hbegf−/− placentas is not clear. The lack of differences in apoptosis between Hbegf+/+ and Hbegf−/− placentas in our study is consistent with a previous report in Egfr−/− mice [32].
The labyrinth is a highly vascularized component of the mouse placenta that mediates efficient transfer of gases, nutrients, wastes, and other molecules between the maternal and embryonic circulations. Egfr−/− placentas had a disorganized labyrinthine trophoblast layer, with reduced cell numbers [34]. Our results support the idea that loss of HBEGF, an agonist of EGFR, might disorganize the labyrinth. In this study, we examined the vascular complexity in the labyrinth by laminin staining. We found that Hbegf−/− placentas have reduced vascular density compared with Hbegf+/+ placentas. In the labyrinth, laminin is expressed only in basement membrane of fetal-derived blood vessels [37]. Since fetal labyrinth blood vessels are separated from maternal blood by three layers of trophoblast cells, maternal HBEGF is unlikely to readily impact fetal blood vessels. Therefore, it was most likely that fetal (extraembryonic and embryonic) HBEGF deficiency disrupted the development of those vessels.
VEGF and its receptors are important in these processes during placentation [38]. We observed reduced VEGF expression in both spongiotrophoblast and labyrinth of Hbegf−/− placentas although HBEGF has not been reported to exhibit appreciable biological effects on vasculogenesis and angiogenesis. The low expression of VEGF is probably related to the low density of vessels in the labyrinth. Although cross talk between VEGF and HBEGF pathways has not been reported in the literature, cross talk between VEGF and EGFR pathways is well established [39]. To test the effect of HBEGF on VEGF, we used a human trophoblast cell line, HTR-8/SVneo [31]. We found that HBEGF supplementation increased expression of VEGF. This supports the existence of cross talk between HBEGF and VEGF pathways. It suggests that placentation could rely on HBEGF induction of VEGF signaling in the vascularization and branching of the chorionic villi.
The relationship between placenta weight and fetal body weight has been explored widely because the placenta is critical in delivery maternal nutrients, oxygen, and hormones to the fetus. In the human, there is a significant association between placenta volume in the second trimester and birth weight percentile [40] and significant correlation between placenta weight and fetal birth weight at delivery [41]. In mouse there was a significant correlation between fetal and placental weights during mid-gestation, but there was no correlation during late gestation [42]. Demographic, environmental, and medical factors influence the placental weight to birthweight ratio in the human [41]. The effect of individual genes on placentation has been mainly explored in mice [43, 44]. The function of the junctional zone is poorly understood. However, it could act as structural support for the developing villous structures of the labyrinth [45]. Of note, the overgrowth of spongiotrophoblast in lpl-null placenta does not confer a fetal growth advantage in mice mid-gestation [44]. In our study, no significant change in fetal body weight was observed even though the placenta was smaller due to reduced spongiotrophoblast region in Hbegf−/– mice in midgestation. These data are consistent with a supportive role of spongiotrophoblast region in placenta. However, the labryinth layer is very important for nutrient, gas, and waste exchange. Its disorganization or malfunction will have a dramatic effect in fetal development. The reduced capillary density of the labyrinth in Hbegf−/– mice did not compromise fetal growth, since the fetal weights in all three genotypes were similar, which suggests that the observed changes in capillary density were not severe enough to compromise fetal growth and development at gestational day 13.5. However, the effect of HBEGF in fetal birth weight needs further evaluation.
In summary, this is the first study to explore the role of HBEGF in murine placentation. Our findings demonstrate that extraembryonic HBEGF regulates the growth of the spongiotrophoblast region and angiogenesis in the labyrinth during placentation at gestation day 13.5. Moreover, our results support the idea that extraembryonic HBEGF most likely exerts these biological activities via EGFR, possibly through cross talk with VEGF. These results, in turn, are consistent with our previous report of a role for HBEGF deficiency in human placental insufficiency [13, 16].
Supplementary data
Supplemental Figure S1. Nonimmune IgG staining in the Hbegf+/+ placenta.
Supplemental Figure S2. Semiquantitative expression of EGF, HBEGF, TGFA, and AREG compared with IgG alone in the Hbegf+/+ placenta, determined as described in Materials and Methods section. *, value for the IgG control was significantly different than all other values.
Acknowledgments
The authors would like to thank Po Jen Chiang, Anelia Petkova and Huirong Xie for their expert technical assistance.
Footnotes
Grant support: Supported by NIH grants HD12304 and HD071408, and a grant from the March of Dimes.
References
- 1. Riese DJ 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays 1998; 20(1):41–48. [DOI] [PubMed] [Google Scholar]
- 2. Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta 1997; 1333:F179–F199. [DOI] [PubMed] [Google Scholar]
- 3. Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol 2004; 44(1):195–217. [DOI] [PubMed] [Google Scholar]
- 4. Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E, Higashiyama S, Hashimoto K. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci 2005; 118(11):2363–2370. [DOI] [PubMed] [Google Scholar]
- 5. Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, Miyado K, Adachi S, Kitakaze M, Hashimoto K, Raab G, Nanba D, Higashiyama S et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci USA 2003; 100(6):3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iwamoto R, Mekada E. ErbB and HB-EGF signaling in heart development and function. Cell Struct Funct 2006; 31(1):1–14. [DOI] [PubMed] [Google Scholar]
- 7. Miyamoto S, Yagi H, Yotsumoto F, Kawarabayashi T, Mekada E. Heparin-binding epidermal growth factor-like growth factor as a novel targeting molecule for cancer therapy. Cancer Sci 2006; 97(5):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jessmon P, Leach RE, Armant DR. Diverse functions of HBEGF during pregnancy. Mol Reprod Dev 2009; 76(12):1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim HJ, Dey SK. HB-EGF: a unique mediator of embryo-uterine interactions during implantation. Exp Cell Res 2009; 315(4):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA 2007; 104(46):18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol 2004; 266(2):223–237. [DOI] [PubMed] [Google Scholar]
- 12. Armant DR, Kilburn BA, Petkova A, Edwin SS, Duniec-Dmuchowski ZM, Edwards HJ, Romero R, Leach RE. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development 2006; 133(4):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn BA, Das SK, Dey SK, Johnson A, Qureshi F, Jacques S, Armant DR. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet North Am Ed 2002; 360(9341):1215–1219. [DOI] [PubMed] [Google Scholar]
- 14. Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab 1999; 84:3355–3363. [DOI] [PubMed] [Google Scholar]
- 15. Imudia AN, Kilburn BA, Petkova A, Edwin SS, Romero R, Armant DR. Expression of heparin-binding EGF-like growth factor in term chorionic villous explants and its role in trophoblast survival. Placenta 2008; 29(9):784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armant DR, Fritz R, Kilburn BA, Kim YM, Nien JK, Maihle NJ, Romero R, Leach RE. Reduced expression of the epidermal growth factor signaling system in preeclampsia. Placenta 2015; 36(3):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972; 1:177–191. [PubMed] [Google Scholar]
- 18. Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstetrics & Gynecology 2000; 96:271–276. [DOI] [PubMed] [Google Scholar]
- 19. DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol 1999; 155(1):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu L, de Bruin A, Saavedra HI, Starovic M, Trimboli A, Yang Y, Opavska J, Wilson P, Thompson JC, Ostrowski MC, Rosol TJ, Woollett LA et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 2003; 421(6926):942–947. [DOI] [PubMed] [Google Scholar]
- 21. Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell 2000; 6(1):109–116. [PubMed] [Google Scholar]
- 22. Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 1999; 4(4):585–595. [DOI] [PubMed] [Google Scholar]
- 23. Leach RE, Kilburn BA, Petkova A, Romero R, Armant DR. Diminished survival of human cytotrophoblast cells exposed to hypoxia/reoxygenation injury and associated reduction of heparin-binding epidermal growth factor-like growth factor. Am J Obstet Gynecol 2008; 198(4):471.e1–471.e8e471-477; discussion 471 e477-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolff GS, Chiang PJ, Smith SM, Romero R, Armant DR. Epidermal growth factor-like growth factors prevent apoptosis of alcohol-exposed human placental cytotrophoblast cells. Biol Reprod 2007; 77(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jain CV, Jessmon P, Barrak CT, Bolnick AD, Kilburn BA, Hertz M, Armant DR. Trophoblast survival signaling during human placentation requires HSP70 activation of MMP2-mediated HBEGF shedding. Cell Death Differ 2017; 24(10):1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005; 20:180–193. [DOI] [PubMed] [Google Scholar]
- 27. Hu D, Cross JC. Development and function of trophoblast giant cells in the rodent placenta. Int J Dev Biol 2010; 54(2–3):341–354. [DOI] [PubMed] [Google Scholar]
- 28. Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet 2001; 2(7):538–548. [DOI] [PubMed] [Google Scholar]
- 29. Leach RE, Jessmon P, Coutifaris C, Kruger M, Myers ER, Ali-Fehmi R, Carson SA, Legro RS, Schlaff WD, Carr BR, Steinkampf MP, Silva S et al. High throughput, cell type-specific analysis of key proteins in human endometrial biopsies of women from fertile and infertile couples. Hum Reprod 2012; 27(3):814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel S, Kilburn B, Imudia A, Armant DR, Skafar DF. Estradiol elicits proapoptotic and antiproliferative effects in human trophoblast cells. Biol Reprod 2015; 93(3):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993; 206(2):204–211. [DOI] [PubMed] [Google Scholar]
- 32. Dackor J, Strunk KE, Wehmeyer MM, Threadgill DW. Altered trophoblast proliferation is insufficient to account for placental dysfunction in Egfr null embryos. Placenta 2007; 28(11-12):1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 1995; 269(5221):234–238. [DOI] [PubMed] [Google Scholar]
- 34. Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 1995; 269(5221):230–234. [DOI] [PubMed] [Google Scholar]
- 35. Strunk KE, Amann V, Threadgill DW. Phenotypic variation resulting from a deficiency of epidermal growth factor receptor in mice is caused by extensive genetic heterogeneity that can be genetically and molecularly partitioned. Genetics 2004; 167(4):1821–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 1995; 378(6555):390–394. [DOI] [PubMed] [Google Scholar]
- 37. Kim ST, Adair-Kirk TL, Senior RM, Miner JH. Functional consequences of cell type-restricted expression of laminin alpha5 in mouse placental labyrinth and kidney glomerular capillaries. PLoS One 2012; 7(7):e41348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Demir R, Seval Y, Huppertz B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem 2007; 109(4):257–265. [DOI] [PubMed] [Google Scholar]
- 39. Larsen AK, Ouaret D, El Ouadrani K, Petitprez A. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther 2011; 131(1):80–90. [DOI] [PubMed] [Google Scholar]
- 40. Derwig IE, Akolekar R, Zelaya FO, Gowland PA, Barker GJ, Nicolaides KH. Association of placental volume measured by MRI and birth weight percentile. J Magn Reson Imaging 2011; 34(5):1125–1130. [DOI] [PubMed] [Google Scholar]
- 41. Williams LA, Evans SF, Newnham JP. Prospective cohort study of factors influencing the relative weights of the placenta and the newborn infant. BMJ 1997; 314(7098):1864–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ishikawa H, Seki R, Yokonishi S, Yamauchi T, Yokoyama K. Relationship between fetal weight, placental growth and litter size in mice from mid- to late-gestation. Reprod Toxicol 2006; 21(3):267–270. [DOI] [PubMed] [Google Scholar]
- 43. Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice–a review. Placenta 2005; 26(Suppl A):S3–S9. [DOI] [PubMed] [Google Scholar]
- 44. Frank D, Fortino W, Clark L, Musalo R, Wang W, Saxena A, Li CM, Reik W, Ludwig T, Tycko B. Placental overgrowth in mice lacking the imprinted gene Ipl. Proc Natl Acad Sci USA 2002; 99:7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol 2005; 284:12–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.