Abstract
Background
Previous studies with a limited number of patients have reported divergent findings on whether screening can detect small cell lung cancer (SCLC) at an earlier stage and whether there might be a survival benefit.
Methods
This study examined the characteristics of SCLC detected by using low-dose CT (LDCT) screening in the National Lung Screening Trial, a randomized study of individuals at high risk for developing lung cancer comparing LDCT imaging vs chest radiography. SCLC was denoted as screen detected if diagnosed ≤ 1 year of a positive screen or after a longer period but with no time gap between diagnostic procedures of > 1 year; interval detected if diagnosed ≤ 1 year of a negative screen; and nonscreen detected if the subject did not receive any screens or otherwise as postscreening.
Results
A total of 143 cases of SCLC were diagnosed, including 49 (34.2%) screen detected, 15 (10.5%) interval detected, and 79 (55.2%) nonscreened/postscreening. Of the screening phase-diagnosed cases (ie, screen or interval detected), a higher proportion of SCLC cases compared with NSCLC cases were interval detected (23% vs 5%; P < .0001). A higher proportion of all SCLC cases compared with NSCLC cases were advanced stage (III/IV: 86% vs 36%; P < .0001). The unfavorable SCLC stage distribution extended across screen-detected (80% stage III/IV), interval-detected (86%), and nonscreened/postscreening (90%) cancers. Among screen-detected SCLC, only 63.3% had ≥ 1 noncalcified nodule in the cancer lobe compared with 85.4% of NSCLC cases (P < .0001). Even with very small LDCT screen-detected nodules, a high proportion of SCLC cases were late stage. There was no significant difference in survival between screen- and interval-detected or postscreening SCLC.
Conclusions
“Early detection” with the use of LDCT imaging had no impact on SCLC outcomes. A successful screening modality should ideally detect SCLC earlier than when it can be detected on LDCT scans.
Key Words: low-dose CT scan, lung cancer screening, non-small cell lung cancer, small cell lung cancer
Abbreviations: CXR, chest radiography; I-ELCAP, International Early Lung Cancer Action Program; LDCT, low-dose CT; NCN, noncalcified nodule; NLST, National Lung Screening Trial; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer
FOR EDITORIAL COMMENT, SEE PAGE 1265
Small cell lung cancer (SCLC) is an aggressive cancer with a poor prognosis. Annually, approximately 34,000 new patients are diagnosed with SCLC in the United States. Patients are routinely staged as having either limited or extensive-stage disease.1 Chemotherapy is the mainstay of therapy for patients with both extensive and limited-stage disease due to the disseminated nature of the cancer at presentation. Although highly responsive to chemotherapy, SCLC relapses quickly and becomes refractory to treatment within a few months. Fewer than 5% of patients survive 2 years, and < 2% of patients are alive 5 years following diagnosis. Given its widely metastatic nature at diagnosis and the lack of effective therapies, early detection could theoretically have a beneficial influence on SCLC patient survival. Previous studies with limited number of patients have reported divergent findings on whether screening can detect SCLC at an earlier stage and whether there might be a survival benefit.2, 3
The National Lung Screening Trial (NLST) showed that screening with low-dose CT (LDCT) imaging compared with chest radiography (CXR) reduced lung cancer mortality, but the benefit was limited to non-small cell lung cancer (NSCLC).4, 5 The goal of the present study was to determine whether LDCT imaging could detect SCLC and whether such screen detection offered a stage and/or survival benefit. To address this question, we examined the characteristics of LDCT imaging-detected SCLC in the NLST. To our knowledge, this study is the largest analysis to date of SCLC detected in a screening study.
Materials and Methods
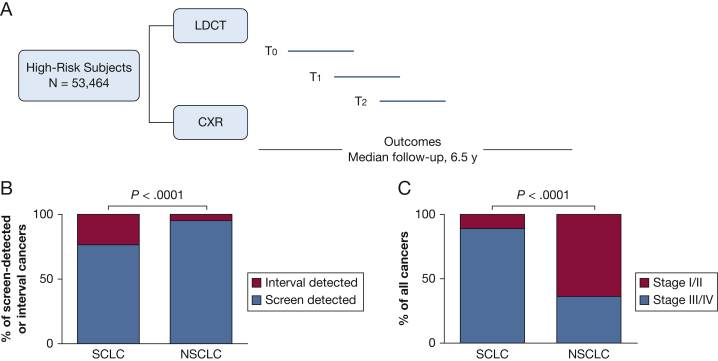
The NLST was a randomized screening trial comparing LDCT vs CXR (Fig 1A).4, 5 Subjects were enrolled from August 2002 through April 2004 and were randomly assigned to undergo three annual screenings with either LDCT scanning or CXR. Screening occurred from August 2002 through September 2007. Eligible participants were 55 to 74 years of age, had a history of cigarette smoking of at least 30 pack-years, and, if former smokers, had quit within the previous 15 years. Individuals who had previously received a diagnosis of lung cancer, had undergone chest CT scanning within 18 months prior to enrollment, had hemoptysis, or had an unexplained weight loss > 6.8 kg in the preceding year were excluded. After randomization, participants completed a questionnaire that included demographic characteristics and smoking behavior.5
Figure 1.
A, The National Lung Screening Trial design. Subjects were enrolled and randomly assigned to undergo three annual screenings with either LDCT imaging or CXR. B, Mode of detection of screen-detected or interval-detected lung cancers. Compared with NSCLC cases, a significantly higher proportion of SCLC cases were interval detected than screen detected. C, Stage distribution of all cancers. Compared with NSCLC cases, a significantly higher proportion of SCLC cases were detected in stages III and IV than stages I and II. CXR = chest radiograph; LDCT = low-dose CT; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer.
Each CT study was interpreted by a single NLST radiologist. Any noncalcified nodule (NCN) ≥ 4 mm in the axial plane or, less frequently, other abnormalities such as adenopathy or pleural effusion defined a positive screen. Size, lobe location, and attenuation of each NCN ≥ 4 mm were recorded. Subjects were followed up with annual surveys to ascertain incident cancers. All reported cancers were verified with medical records, with stage and histologic features recorded. “Best” stage was defined as pathologic stage if available; otherwise, clinical stage was used. Deaths were tracked with the annual surveys and supplemented by National Death Index searches. Participants were followed up for events occurring through December 31, 2009.
A cancer was denoted as screen detected if diagnosed within 1 year of a positive screen or if it was diagnosed after a longer period but with no time gap between diagnostic procedures of > 1 year. It is noteworthy that not all positive screens were cancers. Screen-detected cancers were assigned a nodule size if one (and only one) NCN was reported in the lobe of the cancer on the last positive screen. An interval-detected cancer was defined as a cancer diagnosed within 1 year of a negative screen. Nonscreen-detected or interval-detected cancers were denoted as nonscreened if the subject did not receive any NLST screens or otherwise as postscreening.
Lung cancer-specific and overall survival rates were estimated by using the Kaplan-Meier method. The statistical significance of differences in proportions was computed by using the χ2 test.
Results
There were 26,722 subjects randomized to the LDCT imaging arm, and the median follow-up was 6.5 years (Fig 1A). A total of 59% were men, and the median age at enrollment was 62 years. A total of 143 SCLC cases and 926 NSCLC cases were detected in the LDCT arm.
Table 1 presents LDCT arm SCLC cases according to stage and mode of detection. Of 143 SCLC cases, 49 (34.2%) were screen-detected, 15 (10.5%) were interval-detected, and 79 were nonscreened or postscreening (55.2%) cancers. Of 64 interval- or screen-detected cases, 49 (76.5%) were screen detected, which can be taken as an estimate of test sensitivity of LDCT imaging for SCLC. In contrast, for NSCLC, test sensitivity was 95.3% based on 591 screen-detected and 29 interval-detected cases (P < .0001 SCLC vs NSCLC) (Fig 1B).
Table 1.
Small Cell Lung Cancers in the NLST LDCT Imaging Arm According to Best Stage
| Stage | Screen Detected |
Interval Detecteda |
Postscreening/Never Screened |
All |
|---|---|---|---|---|
| No. (Column %) | No. (Column %) | No. (Column %) | No. (Column %) | |
| All | 49 (100) | 15 (100) | 79 (100) | 143 (100) |
| Stage I | 2 (4) | 2 (13) | 4 (5) | 8 (6) |
| Stage IIA | 4 (6) | 0 | 1 (1) | 5 (3) |
| Stage IIB | 1 (2) | 0 | 1 (1) | 2 (1) |
| Stage III | 18 (37) | 5 (33) | 22 (28) | 45 (31) |
| Stage IV | 21 (43) | 8 (53) | 49 (62) | 78 (55) |
| Occult | 0 | 0 | 1 (1) | 1 (1) |
| Unknown | 3 (6) | 0 | 1 (1) | 4 (3) |
LDCT = low-dose CT; NLST = National Lung Screening Trial.
Interval-detected cancers are within 1 year of a negative screen; only one case from the LDCT imaging arm was never screened.
Of 143 SCLC cases, 123 (86%) were diagnosed at late stages (stage III/IV) (Table 1). The unfavorable stage distribution was noted among screen-detected (80% stage III/IV), interval-detected (86%), and nonscreened/postscreening (90%) cases. Only 15 (10.5%) SCLC cases were diagnosed in early stages (stage I/II). The findings were similar when pathologic stage was examined (data not shown). There were no differences in sex distribution, age, smoking status (current vs former), or pack-years of smoking between those with early-stage SCLC compared with late-stage SCLC. In contrast to the data for SCLC, the stage distribution for NSCLC was more favorable (64% were stage I/II; P < .0001 SCLC vs NSCLC) (Fig 1C).
Examining findings on the LDCT imaging of screen-detected SCLC (Table 2), an associated nodule could be determined for 24 cases (49%); in contrast, for NSCLC, an associated nodule could be determined for 421 cases (71%). Most SCLC cases, including those detected when the size of the associated nodule was very small, had stage III/IV disease. For example, only 14% (1 of 7) of screen-detected SCLC cases were stage I when the average nodule size was 3 to < 7 mm. In contrast, 73% of screen-detected NSCLC cases were stage I when the average nodule size was 3 to < 7 mm, and 34% were stage I when the nodule size was ≥ 30 mm; the proportion of stage I cancers remained largely the same through an average nodule size < 30 mm. The median interval (days) from the last screen to diagnosis (for cases with associated nodules) was 54 for the SCLC and 70 for the NSCLC screen-detected cases.
Table 2.
Stage Distribution of LDCT Screen-Detected Cancers According to Nodule Size on Screen
| Average Nodule Sizea | NSCLC |
SCLC |
||||
|---|---|---|---|---|---|---|
| No. | % Stage I | % Stage III/IV | No. | % Stage I | % Stage III/IV | |
| 3 to < 7 mm | 40 | 73 | 15 | 7 | 14 | 86 |
| 7 to < 10 mm | 83 | 81 | 14 | 1 | 0 | 100 |
| 10 to < 15 mm | 122 | 79 | 16 | 4 | 0 | 75 |
| 15 to < 20 mm | 79 | 75 | 14 | 5 | 0 | 80 |
| 20 to < 30 mm | 55 | 69 | 24 | 3 | 0 | 67 |
| ≥ 30 mm | 32 | 34 | 59 | 3 | 0 | 100 |
| Allb | 587 | 67 | 25 | 46 | 4 | 85 |
Three unknown-stage small cell lung cancer (SCLC) cases were excluded, including one case with one noncalcified nodule (NCN) in the cancer lobe (size, 10-14 mm). Nine unknown-stage non-small cell lung cancer (NSCLC) cases were excluded, including seven cases with one NCN in the cancer lobe (two 7-9 mm, two 10-14 mm, two 15-19 mm, and one 20-29 mm). Also excluded were three NSCLC cases with one NCN in the cancer lobe but unknown size of the NCN. See Table 1 legend for expansion of other abbreviation.
Average of longest diameter and longest perpendicular. Analysis limited to subjects with one (and only one) NCN in the lobe of the cancer at screen detection.
Includes subjects with 0 or ≥ 2 NCNs in the cancer lobe, and with unknown lobe of the cancer.
Of 49 screen-detected SCLC, only 31 (63.3%) had at least one NCN in the lobe of the cancer (Table 3). The remaining screen-detected SCLC cases either had no NCNs in the cancer lobe (20.4%; eg, nodules in lobes other than the cancer lobe), had non-lobe cancer locations (8.1%; hilum, main stem bronchus, mediastinum, and carina), or the cancer location was unknown (8.1%). In comparison, 85.4% of screen-detected NSCLC cases had at least one NCN in the cancer lobe at screen (P < .0001). When an NCN was not detected in the cancer lobe at screening, it took longer for an SCLC diagnosis to be established: a median of 54 days (from the LDCT screen) when at least one NCN was identified in the lobe where SCLC was eventually diagnosed vs a median of 166 days when no NCN was identified there (P = .054). There were no differences in the stage distribution of cancer and survival depending on the presence or absence of NCN in the cancer lobe.
Table 3.
Screen-Detected SCLC and NSCLC Cases (LDCT Arm)
| NCN Characteristic | SCLC |
NSCLC |
||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Median Time (d) to Dx | Stage I/II No. (%) | 3-y Survival | No. (%) | Median Time (d) to Dx | Stage I/II No. (%) | 3-y Survival | |
| ≥1 NCN in cancer lobe | 31 (63.3) | 54 | 4 (13.3) | 18.6% | 505 (85.4) | 73 | 394 (78.3) | 80.7% |
| <1 NCN in cancer lobea | 18 (36.7) | 166 | 3 (18.8) | 8.3% | 86 (14.6) | 116 | 42 (50.0) | 56.9% |
| All | 49 | 591 | ||||||
Survival NSCLC ≥ 1 NCN vs other, P < .0001. Stage I/II NSCLC ≥ 1 NCN vs other, P < .0001. Time to Dx, P = .09. Survival SCLC ≥1 NCN vs other, P = .7. Stage I/II SCLC ≥ 1 NCN vs other, P = .63. Time to Dx, P = .054. Percentage with ≥ 1 NCN NSCLC vs SCLC, P < .0001. Nonlobe locations: L,R hilar, L,R main stem bronchus, mediastinum, carina. Dx = diagnosis. See Table 1 and 2 legends for expansion of other abbreviations.
Includes the following: (1) no NCNs in cancer lobe (SCLC, 10 [20.4]; NSCLC, 67 [11.3]); (2) non-lobe cancer location (SCLC, 4 [8.1]; NSCLC, 9 [1.5]); and (3) unknown cancer location (SCLC, 4 [8.1]; NSCLC, 10 [1.7]).
Of the 49 screen-detected SCLC cases, 24 (49%) had only one NCN in the lobe of the cancer (e-Table 1). In cases in which a single NCN in the lobe of the cancer was small (< 1 cm; n = 8), most were eventually diagnosed with advanced-stage disease (seven stage IIIB/IV and one stage IA); had a discrepantly large tumor size based on pathologic analysis, operative report, or radiology report (range, 7-100 mm); and experienced a prolonged time to diagnosis from the positive screen (median, 173 days; range, 55-789 days).
Treatments are summarized in e-Table 2. Eleven of 16 patients (69%) with early-stage SCLC underwent resection compared with two of 123 patients (2%) with late-stage SCLC. Radiation was used in nine patients (56%) with early-stage disease compared with 64 patients (52%) with late-stage disease. Rates of use of chemotherapy were 69% and 89%, respectively, for early and late stages. Among the 16 patients detected with early-stage disease, five patients (31%) underwent surgery only; the remaining patients were treated with a combination of chemotherapy and radiation (n = 5; 31%); surgery, radiation, and chemotherapy (n = 4; 25%); or surgery and chemotherapy (n = 2; 13%).
Three-year lung cancer-specific survival was 83% for patients with early-stage disease and 8% for those with late-stage disease. Three-year cancer-specific survival rates were 15.3%, 20.0%, and 13.8% among screen-detected, interval-detected, and nonscreened/postscreening cases, respectively (Table 4). These differences were not statistically significant (P = .35 for screen detected vs nonscreen detected). There were too few early-stage cases to determine whether screen-detected, early-stage cases had survival different from other early-stage cases.
Table 4.
Survival of SCLCs Detected in the NLST LDCT Arm
| Mode of Detection | No. | 3-y Cancer-Specific Survival | 3-y All-Cause Survival |
|---|---|---|---|
| Screen detected | 49 | 15.3% | 14.9% |
| Interval detected | 15 | 20.0% | 20.0% |
| Postscreening/never screened | 79 | 13.8% | 13.8% |
Discussion
Data from the NLST were analyzed to assess the characteristics and outcomes of SCLC with a focus on LDCT-detected cases. Novel observations from this analysis are as follows: (1) compared with NSCLC, a significantly higher proportion of SCLC cases were interval cancers, diagnosed within 1 year following a negative screening, and only one third of cases were screen detected; (2) as expected, the majority of SCLC cases were late-stage cancers but, surprisingly, the unfavorable stage distribution was present regardless of whether the cancer was screen, interval, or postscreen detected; (3) there was no significant difference in survival between screen-detected cases and interval-detected or postscreening cases; and (4) even with very small LDCT screen-detected tumor-associated nodules, a high proportion of cases were late stage.
This analysis represents the largest report to date of the characteristics of SCLC diagnosed according to screening. Our findings underscore and provide further granularity to the premise that SCLC is a very aggressive neoplasm and, contrary to smaller previous studies, indicate that LDCT imaging is an ineffective tool to screen for SCLC. Our results also highlight the early and widespread dissemination of SCLC by the time it is detected on LDCT imaging. If early detection of SCLC were to be realistic, it would need to be detected before the nodules are visible on LDCT scans.
The lack of survival advantage for SCLC cases in the LDCT vs the CXR arm is known.4 In the present analysis, only 15 patients were diagnosed with early-stage SCLC. Three-year lung cancer-specific survival for these patients was 83%. In comparison, survival of patients with early-stage SCLC from population-based studies appears lower. Weksler et al6 reported a median survival of 34 months for patients from the Surveillance, Epidemiology, and End Results database with stage I or II SCLC who underwent lung resection. The differences in the populations studied and the varying frequencies of surgical resections between the studies (50% in this study vs 25% in the Surveillance, Epidemiology, and End Results database) might explain the observed differences in outcome. One- and 5-year survival rates for stages IA-IIB SCLC ranged between 79% and 90% and 35% and 53% in another report.7 There were too few patients with stage I/II disease in the screen-detected group (n = 7) in this study to determine whether they have better long-term survival.
Lung cancers not detected by screening but diagnosed during the screening interval, known as interval-detected cancers, might have been missed at screening or might have developed between screening and detection. Intuitively, these cancers (especially if they developed between screening and detection) are likely more aggressive. For example, rapidly growing and aggressive breast tumors account for a substantial proportion of screening mammographic failures, especially among younger women, who have a high proportion of aggressive cancers.8 The overall survival in our study was the same for screen- vs interval- vs postscreen-detected cancers, suggesting similarly aggressive biology between the cancers detected in these different ways. These low survival rates were driven largely by the late stages of most SCLC cases in our study. These results further underscore the aggressive nature of SCLC.
The literature offers limited descriptions of SCLC detected by screening.2, 3, 9, 10, 11 Our results differ from previous screening studies involving smaller number of participants in terms of the number of cases detected, stage distribution at diagnosis, and correlations between nodule size and stage of screen-detected SCLC. In the Toronto and Mayo lung cancer-screening studies,2 15 cases of SCLC were diagnosed among 6,392 individuals screened. Of 10 patients with available clinical information, eight were screen detected and two interval detected; four had extensive-stage disease. In the International Early Lung Cancer Action Program (I-ELCAP),3 48 cases of SCLC were diagnosed among 48,037 individuals screened. Most cases (92% [44 of 48]) were screen detected, and 8% were interval detected. The respective proportions for NLST (LDCT arm) SCLC cases were 77% (screen detected) vs 23% (interval detected). In contrast to our findings, the screen-detected SCLC cases had a much more favorable stage distribution in I-ELCAP: clinical stages at presentations were I, II, III, and IV in 16 (33%), 5 (11%), 20 (42%), and 7 (15%) patients, respectively. Furthermore, the I-ELCAP study found a correlation between smaller screen-detected nodules and earlier stage disease, an association that was not found in our study. In two reports that detected approximately 10 SCLC cases each, screen detection did not affect survival.9, 11
The differences between our study and previous smaller studies may in part be a result of the different populations screened. Both the Toronto and Mayo lung cancer- screening studies included individuals aged ≥ 50 years.2 The Toronto study participants had a 10 pack-year smoking history, whereas the Mayo Clinic study enrolled those with a 20 pack-year smoking history. I-ELCAP screened asymptomatic individuals aged > 40 years who had variable smoking histories.3 In contrast, NLST screened individuals with clearly defined risk factors that included age 55 to 74 years with at least a 30 pack-year smoking history and, if former smokers, had quit within the previous 15 years.5
An interesting observation from this analysis was that in approximately 20% of screen-detected SCLC cases, no NCNs were present on a positive screen in the lobe where cancer was eventually diagnosed. Even when a single NCN was found in the cancer lobe, it did not necessarily represent the cancer; rather, another abnormality detected in the evaluation following the positive screen was likely diagnosed as cancer, as indicated by the prolonged time to diagnosis from a positive screen and the discordance between NCN size and tumor size. As one would expect, it took a longer time from the positive screen to diagnose these cancers. Possibly due to the small number of cases, we observed no differences in stage distribution or survival depending on whether the screen-detected case had an NCN in the cancer lobe. In contrast, most NSCLC cases had at least one NCN in the cancer lobe at screen. Compared with NSCLC cases with no NCNs in the cancer lobe at a positive screen, a significantly higher proportion of these cases were early stage and had an improved survival.
Conclusions
Analysis of SCLC diagnosed during LDCT screening in the NLST shows that yearly LDCT screens detected a significant number of SCLC cases. Compared with NSCLC cases, a higher proportion of SCLC cases were interval detected than screen detected. However, no stage shift or survival benefit for screen-detected SCLC cases compared with interval- or postscreen-detected cases was observed. Even for screen-detected SCLC for which the lesion observed on the LDCT screen was small, the proportion of late-stage SCLC was very high. To our knowledge, this study is the largest analysis to date of SCLC diagnosed during a screening study. Our results suggest that for a screening modality to be successful in reducing SCLC mortality, SCLC should be detectable earlier than it is with LDCT imaging.
Acknowledgments
Author contributions: A. T., E. S., and P. Pinsky conceived and designed the analysis, collected the data, performed the analysis and wrote the paper. P. Pattanayak collected the data, performed the analysis and contributed to manuscript writing. All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The funding agencies had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Dr Pattanayak is currently at Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD.
FUNDING/SUPPORT: This work was supported in part by the Center for Cancer Research, National Cancer Institute at the National Institutes of Health [Grant ZIA BC 011793].
Supplementary Data
References
- 1.van Meerbeeck J.P., Fennell D.A., De Ruysscher D.K. Small-cell lung cancer. Lancet. 2011;378(9804):1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 2.Cuffe S., Moua T., Summerfield R., Roberts H., Jett J., Shepherd F.A. Characteristics and outcomes of small cell lung cancer patients diagnosed during two lung cancer computed tomographic screening programs in heavy smokers. J Thorac Oncol. 2011;6(4):818–822. doi: 10.1097/JTO.0b013e31820c2f2e. [DOI] [PubMed] [Google Scholar]
- 3.Austin J.H., Yip R., D'Souza B.M., Yankelevitz D.F., Henschke C.I. International Early Lung Cancer Action Program Investigators. Small-cell carcinoma of the lung detected by CT screening: stage distribution and curability. Lung Cancer. 2012;76(3):339–343. doi: 10.1016/j.lungcan.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Pinsky P.F., Church T.R., Izmirlian G., Kramer B.S. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119(22):3976–3983. doi: 10.1002/cncr.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aberle D.R., Adams A.M., Berg C.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. New Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weksler B., Nason K.S., Shende M., Landreneau R.J., Pennathur A. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg. 2012;94(3):889–894. doi: 10.1016/j.athoracsur.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd F.A., Crowley J., Van Houtte P. The International Association for the Study of Lung Cancer-Lung Cancer Staging Project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2(12):1067–1077. doi: 10.1097/JTO.0b013e31815bdc0d. [DOI] [PubMed] [Google Scholar]
- 8.Gilliland F.D., Joste N., Stauber P.M. Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer I. 2000;92(9):743–749. doi: 10.1093/jnci/92.9.743. [DOI] [PubMed] [Google Scholar]
- 9.Silva M., Galeone C., Sverzellati N. Screening with low-dose computed tomography does not improve survival of small cell lung cancer. J Thorac Oncol. 2016;11(2):187–193. doi: 10.1016/j.jtho.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Kondo R., Yoshida K., Kawakami S. Different efficacy of CT screening for lung cancer according to histological type: analysis of Japanese-smoker cases detected using a low-dose CT screen. Lung Cancer. 2011;74(3):433–440. doi: 10.1016/j.lungcan.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima T., Tateishi K., Yamamoto H., Hanaoka M., Kubo K., Koizumi T. Clinical characteristics and outcomes of patients with small cell lung cancer detected by CT screening. Med Oncol. 2013;30(3) doi: 10.1007/s12032-013-0623-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.