Abstract
In this review, we present a framework to guide the design of surfaces which are resistant to solid fouling, based on the modulus and length scale of the fouling material. Solid fouling is defined as the undesired attachment of solid contaminants including ice, clathrates, waxes, inorganic scale, polymers, proteins, dust and biological materials. We first provide an overview of the surface design approaches typically applied across the scope of solid fouling and explain how these disparate research efforts can be united to an extent under a single framework. We discuss how the elastic modulus and the operating length scale of a foulant determine its ability or inability to elastically deform surfaces. When surface deformation occurs, minimization of the substrate elastic modulus is critical for the facile de-bonding of a solid contaminant. Foulants with low modulus or small deposition sizes cannot deform an elastic bulk material and instead de-bond more readily from surfaces with chemistries that minimize their interfacial free energy or induce a particular repellant interaction with the foulant. Overall, we review reported surface design strategies for the reduction in solid fouling, and provide perspective regarding how our framework, together with the modulus and length scale of a foulant, can guide future antifouling surface designs.
This article is part of the theme issue ‘Bioinspired materials and surfaces for green science and technology’.
Keywords: solid fouling, low adhesion, easy clean surfaces, elastic modulus, surface chemistry, coatings
1. Introduction
The undesired adhesion of solid contaminants to a surface, particularly when the adhering material damages the desired properties of that surface, can be labelled as solid fouling. This broad area includes the surface adhesion of a diverse array of contaminants such as ice, clathrate hydrates of natural gases, wax, inorganic scale deposits, polymers in solution, proteins, dust and dirt, as well as a wide range of biofouling materials. Examples of the industrial problems caused by these materials include the attachment of marine organisms to ship hulls [1,2], icing of power lines and airplanes [3–5], deposition of asphaltenes and wax on systems in petroleum processing [6,7] and biofilm growth on medical devices [8,9]. It is therefore essential to either prevent the attachment and continued deposition of these solids or to facilitate their easy removal from critical surfaces.
Solid fouling materials vary widely in composition and chemical structure, as well as in their physical properties and modes of deposition. Many surfaces have been fabricated to resist the adhesion of these materials; however, research efforts have traditionally focused on only a single type of solid foulant when designing a surface. Therefore, an effort to establish a classification methodology for different solid foulants, particularly one which guides the design of antifouling surfaces, can be quite useful.
(a). Surface design strategies
Surface design approaches which have been shown to lower solid adhesion can be divided simplistically into three categories. The first, we will refer to as chemical functionalization. This strategy involves selecting or creating a surface chemistry which either lowers the substrate surface energy or allows the surface to interact in a particular way with the fouling material. Minimizing the energy of a surface reduces the strength of any potential adhesive interaction between the surface and a fouling material, known as the work of adhesion, Wa. The contribution of the interfacial free energy of a surface to the work of adhesion is commonly represented by the equation , where and are the interfacial free energy for the foulant and the foulant–substrate interface, respectively [10,11]. Surfaces designed with this approach commonly use silicones or fluorinated polymers, which are among the lowest surface energy materials [12]. Beyond interfacial energy considerations, surface chemistries which interact specifically with a fouling material vary more widely, from zwitterionic polymer surfaces designed to repel biofilms and proteins [13–16], to conductive films for the repulsion of dirt and dust [17,18]. Common drawbacks of designing a surface based on chemical functionalization involve the lower attainable limit of surface energy and the complexity of achieving particular desired interactions.
The next design approach which can be used to limit solid fouling is tuning the physical properties of the surface, specifically its elastic modulus. In the case of a rigid material adhered to an elastic surface, the strength of that adhesive bond is proportional to both the work of adhesion and the elastic modulus, E, of the surface material by the relation , where t is the film thickness [19–21]. The rationale for this is that the total energy of a surface consists of both its elastic strain energy (a proportional function of modulus) and its interfacial free energy [19,21]. When the de-bonding of a contaminant from a surface involves the macro-scale deformation of the surface, as in a rigid material de-bonding from an elastic surface, this elastic strain can significantly increase the overall surface energy. Minimizing the elastic strain energy of the surface by reducing its modulus lowers the equilibrium barrier for fracture of the adhesive interface [21]. Therefore, by lowering the modulus of the surface material, the adhesion of solid fouling materials can be reduced dramatically. In fact, while the interfacial free energy difference between smooth surfaces of a high energy metal such as iron (2.4 J m−2) [22] and the lowest energy material C20F42 (0.0067 J m−2) [23] is only a factor of 100, the modulus between a hard and soft surface can vary over as many as 5 orders of magnitude [24,25]. Thus, when bulk deformation occurs, reduction in the surface modulus can have a more significant effect on foulant adhesion than the intrinsic work of adhesion of the surface, which is controlled by chemical functionalization. This approach raises one primary concern: the durability of low-modulus surface materials.
A final approach for surface design is control over surface microtexture. While textural approaches are a critical component of designing for the reduction in liquid fouling [26], in the case of solid fouling, microtexture is typically detrimental as it increases the adhesive/interfacial contact area or creates the potential for an interlocking interface across which de-bonding is significantly hindered [11,27–29]. Some niche applications using textured surfaces will be mentioned where relevant in this review, but overall, the approach is considered to be outside of the design guide proposed.
(b). Influence of foulant material properties
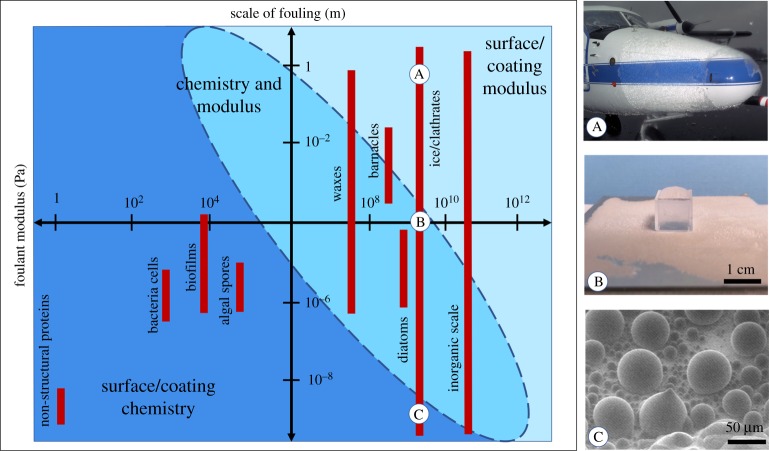
With these key design approaches defined, the question becomes how can the properties of a fouling material be used to determine which is the most effective approach towards antifouling surface design. As was just described, the mechanism for a solid to de-bond from a low-modulus surface involves the deformation of the surface by the fouling material when an external force is applied [19–21]. This deformation increases the elastic strain energy of the surface, which is limited by the modulus of its material [19,21]. However, when considering a broad list of potential foulants, it becomes clear that only foulants with sufficiently high intrinsic modulus will be able to cause the deformation of a plausible range of surface materials and de-bond via this mechanism. In these cases, the minimization of the elastic modulus of a surface will have a significant, and often dominating, effect on foulant adhesion. Therefore, it is apparent that the rigidity of the fouling material, and specifically, its modulus in comparison to potential surface moduli, is critical in directing low adhesion surface design. By categorizing fouling materials according to their elastic modulus, a general guide can be set which allows a researcher to predict the relative impact of chemical versus mechanical factors upon their surface design. This is illustrated in figure 1.
Figure 1.
Fouling materials classified on the basis of modulus. Estimated values of modulus for: soft fouling materials such as bacterial biofilm on a catheter tube [30–32] (obtained from CDC Public Health Image Library under public domain) and marine algae adhered to a ship hull [33], along with hard fouling materials like barnacles [34] (image reproduced with permission from [35], licensed under https://creativecommons.org/licenses/by/4.0/), clathrates in petroleum pipelines [36] (image reproduced from www.usgs.gov under public domain), ice on a wind turbine blade [37] reproduced from [38] (with licence under https://creativecommons.org/licenses/by-nc-sa/3.0/) and inorganic scale deposited in a pipe carrying cooling water (licensed under https://creativecommons.org/licenses/by-sa/3.0/deed.en).
Although the straightforward simplicity of this division between hard and soft foulants is appealing, many surface design approaches do not fit this trend. Examples of this include surfaces designed to repel dry dirt or ice crystal nucleates, which rely on chemical approaches despite the high modulus of the typical components of soil and ice [17,18,39,40]. Clearly, the initial guide in figure 1 is insufficient to capture the scope of solid fouling. In revisiting the criteria for surface modulus-based design, it is evident that not only must the fouling material have adequate intrinsic modulus, but it must be of sufficient size in order to deform the bulk of the surface and bring elastic strain energy into consideration. With this in mind, an extra dimension needs to be added to our guide to account for the length scale of the fouling phenomena. A key example of this scale variation in a fouling material is ice, which can range from centimetres thick sheets with adhesion areas of square metres, to microscopic nucleation sites. Additionally, it is important to note that even for large, high modulus fouling materials, some reduction in adhesion due to the chemical functionalization of the surface can be obtained, as is indicated in the discussion above. What primarily varies across the scope of this design guide is the ability of a fouling material to deform a surface during de-bonding and thus bring elastic strain energy effects into consideration. The larger the degree of deformation of the surface, the more its elastic strain energy will contribute to the equilibrium barrier for fracture of the interface [21]. In these cases, the reduction in the surface modulus will have a dominant effect on minimizing the adhesion strength of the foulant, because the surface modulus can be tuned over many more orders of magnitude than the work of adhesion, which is controlled by chemical functionalization. For foulant materials of moderate modulus or length scale which cause only minor deformation of most elastic surfaces during de-bonding, chemical functionalization and reduction in surface modulus have competing effects on foulant adhesion. The enhanced version of our guide, presented in figure 2, indicates which surface design approach, chemical functionalization or low-modulus substrate, will have a dominant effect in reducing solid adhesion. The delineations between the regions in this guide are estimated based on the predicted ability of foulants at each modulus and length scale to deform a range of elastic bulk materials. This design guide accounts for fouling from materials of moderate modulus or length scale where both strategies can be effective.
Figure 2.
Low solid adhesion surface design guide, which categorizes fouling materials according to their length scale. Fouling length scales are indicated for bacterial cells [41], biofilm thickness [30,42], algal spores [43,44], diatoms [45], barnacles, wax [46] and ice [47–49]. Elastic modulus values for bacterial cells [50], biofilms [30–32], algal spores [33], diatoms [45], barnacles [34], wax [51,52], ice [37,53], clathrates [36,54] and inorganic scale [55,56] are also provided in order to indicate whether the effects of surface chemistry or surface elastic modulus will be most potent in minimizing the fouling of that material. Subset images A, B and C illustrate typical micro-, meso- and macro-scale ice fouling, respectively. Subset image A is an image provided by NASA, and is in the public domain. Subset image B was taken by the authors. Subset image C is adapted with permissions from [57], copyright © 2013 American Chemical Society.
The remainder of this review is dedicated to an overview of a myriad of surfaces which have been designed to limit fouling from a wide range of contaminating solids. Discussion centres around the ways these designer surfaces fit into the framework presented in figure 2, as well as how this framework can guide the future of low adhesion surface design.
2. Ice fouling
One of the most significant examples of solid fouling that affects both commercial and residential activities is the unwanted accumulation of ice on surfaces. From nucleates of frost to sheets of solid glaze [58], ice contamination poses an expensive problem for many major industries. Common applications where ice is a challenge include airplane wings [3,4,59] and power lines [5,60], as well as locks and dams [61] and other mechanical components. Ice fouling also impacts renewable energy technology, impeding the function of solar panels [62] and wind turbines [63]. While a myriad of low ice adhesion (τice) surface designs are proposed in the literature, the primary methods of combatting ice accumulation in industry still involve active deicing. Surface heating, by hot air or electrothermal pulses [4,63], is a common approach, but is extremely energy intensive. Similarly, mechanical shock or vibrations require both energy input and consistent surface monitoring [5]. Particularly for airplanes, organic liquids with low freezing temperatures are routinely sprayed onto surfaces to remove accumulated ice, which requires significant treatment time for a short-term solution [59]. These cases of ice fouling, combined with currently inadequate solutions, demand the development of passive, low ice adhesion surfaces which are durable enough to survive under relatively harsh environmental conditions.
In the context of solid fouling materials addressed in this review, ice has a very high innate material modulus, on the order of 1 GPa [37,53]. However, when placing low adhesion surface designs within the framework of figure 2, it is equally critical to note the exceptionally broad length scale over which ice can act as a foulant. The critical radius for an ice nucleus to become stable and grow is reported by multiple groups to be in the single nanometre range [47–49], while bulk ice can easily accumulate on industrial equipment in sheets, which are metres long and inches thick.
(a). Nucleation prevention
Beginning at the bottom of the length scale, many groups have worked to develop surfaces which delay or prevent the nucleation of ice. The most commonly attempted method in this regard has been the fabrication of superhydrophobic surfaces (SHP). Through a combination of low surface energy and microtexture [64], SHP surfaces support water above entrapped pockets of air (known as the Cassie–Baxter state) [65]. The low surface energy, minimal effective surface area and thermal insulation from entrapped air for an SHP surface in the Cassie–Baxter state is desirable to minimize opportunities for heterogenous ice nucleation. SHPs have displayed the ability to retard ice formation from saturated vapour onto a surface held at −8 to −10°C [66–69]. However, such surfaces were minimally effective at −23°C [66]. One nanotextured SHP designed by Jung et al. [49] was able to generate a 25 h freezing delay at −21°C. However, frost was eventually able to form on all surfaces under freezing conditions. Along with delaying frost formation, the ability of SHPs to bounce or roll water droplets off a surface [70] has led to experimentation and modelling on their capacity to repel freezing rain and condensate before it can nucleate [71]. It was determined by Bahadur et al. [71] that pinning and nucleation would occur before a falling droplet bounced off from an SHP surface at −20°C or below. Multiple groups have, however, reported that the application of sufficient pressure (including Laplace pressure) or exposure to small droplets (i.e. initial condensation) can instigate a transition from the Cassie–Baxter state to the Wenzel state where the contacting liquid displaces all the air present and fully wets the surface [48,70,72–78]. Critical issues with SHPs arise when ice or condensation is able to penetrate the surface texture (figure 3a). Such complete wetting leads to more rapid heterogenous nucleation due to an increase in effective surface area, as well as to the subsequent high adhesion of bulk ice which can interlock mechanically with the texture [27,72,76,81]. Lastly, the highly textured surfaces of SHP surfaces are subject to damage from mechanical abrasion, which increases the presented surface area for nucleation and limits the SHP surfaces' ability to delay ice deposition [72,82]. Ultimately, surface design has shifted away from the SHP approach under the realization that anti-icing designs must optimize the surface wettability while minimizing roughness [49].
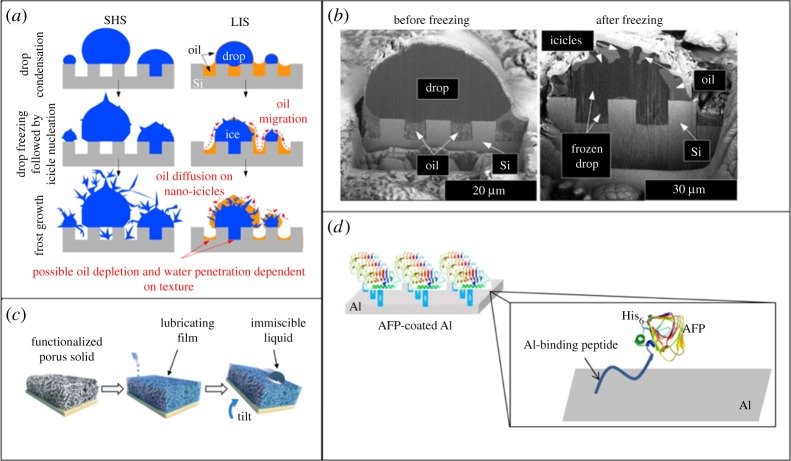
Figure 3.
(a) The mechanism of ice nucleation via drop condensation and freezing on superhydrophobic surfaces and SLIPS-based surfaces. The images demonstrate the texture permeation for superhydrophobic surfaces during frost formation, and lubricant wicking from SLIPS-based surfaces. (b) Cryo-FIB/SEM images of a water droplet before and after freezing on oil impregnated silicon microposts (a,b) reprinted with permission from [57] Copyright © 2013 American Chemical Society. (c) Standard fabrication steps for a SLIPS-based lubricated coating; (c) adapted from [79] with permission. (d) Schematic diagram of AFP functionalized aluminium (adapted from [80] with permission from http://creativecommons.org/licenses/by/4.0/).
Multiple chemical approaches have also been taken to limit ice nucleation onto a surface by engineering a particular interaction between the surface and ice nucleates. The grafting of antifreeze proteins (AFPs), found in organisms with the ability to survive in subzero conditions, has been shown to delay or prevent frost formation on aluminium and glass surfaces (figure 3d) [80,83]. Wong et al. [79] pioneered another novel approach to frost prevention: slippery liquid-infused porous surfaces or SLIPS. This approach is reliant on the inability of ice to nucleate on the molecularly smooth surface presented by an imbibed lubricant (figure 3c). Surfaces designed with this approach include a porous matrix that is functionalized in order to interact strongly with an infused liquid lubricant [79,84]. Within the framework of this review, lubricated surfaces are not considered as ultra-low-modulus solid surfaces. They behave as liquid surfaces which do not possess elastic strain energy when deformed, meaning that solid de-bonding from lubricated surfaces is not governed by the same mechanism as de-bonding from low-modulus elastic solids. SLIPS-based surfaces have succeeded in delaying frost formation [85,86], allowing for the rapid defrosting of surfaces when heated to only 5°C [85], and lowering the nucleation temperature of water as far as −26°C [87]. The drawbacks of this design are the tendency of lubricant, including different perfluorinated oils and silicone oils, to wick out of the porous texture and onto the frost deposits occurring at non-lubricated sites (figure 3a,b), as well as the inability of porous texture asperities/lubricant to withstand mechanical damage [57,88].
In order to combat the issue of depleted lubricant, multiple groups have replaced the infused oil with tethered hygroscopic polymer chains, or simply tethered such polymers to the surface in the absence of porous texture. At low temperature, these chains deliquesce by absorbing water from condensation and swell to form a lubricating surface layer that melts incoming frost nucleates (figure 4a) [82,92–94]. In addition to being ecofriendly with no risk of leaching oils [82], these surfaces have been shown to be abrasion resistant [82], and to function at temperatures as low as −53°C [93]. A final approach to improving on the SLIPS design for antifrost surfaces was proposed by Sun et al. who fabricated a microporous surface impregnated with antifreeze and capped by an SHP layer. This design enabled their surface to repel the majority of condensation and falling droplets, while liquid or frost that penetrated the SHP texture caused the wicking of the antifreeze, which in turn caused their melting [95]. Although this surface is still vulnerable to eventual liquid depletion, the lubricant wicks out selectively, thereby lengthening its usable lifetime [95].
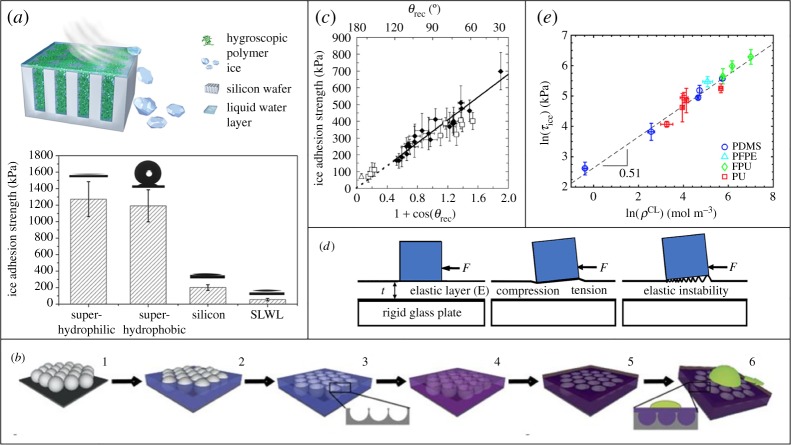
Figure 4.
(a) A schematic illustrating a self-lubricating, water layer (SLWL) surface. The average ice adhesion strength values on four different surface types, superhydrophilic, superhydrophobic, silicon and SLWL are also shown (adapted from [82] Copyright © 2013 American Chemical Society). (b) Construction scheme for colloid templated SLIPS (reproduced with permission from [89]). (c) Average ice adhesion strengths measured at −10°C for bare steel and 21 different coatings plotted versus 1 + cos(θrec)—a variable that scales with the practical work of adhesion, and the receding contact angle θrec for water. (c) Adapted from [90] Copyright © 2010 American Chemical Society. (d) Schematic for a solid de-bonding from an elastic surface layer. (d) Adapted from [19] with permission. (e) Relationship between the elastomer cross-link density ρCL (a variable that is directly proportional to the shear modulus) and the ice adhesion strength (τice) for coatings without interfacial slippage. (e) Adapted from [91] with permission from http://creativecommons.org/licenses/by/4.0/.
(b). Reduction in ice adhesion strength (τice)
Because of the many complexities and lack of success in preventing the adherence of ice nuclei and microscale deposits, especially over long periods of time, many research groups have instead focused on the facile removal of bulk ice, ranging from approximately 1 cm cubes to large sheets. At an intermediate length scale, in accordance with the design framework given in figure 2, the effects of chemical functionalization and bulk modulus compete for dominance in surface design. With regard to chemical functionalization, a variety of methods have been used to obtain low interfacial energy coatings which inhibit hydrogen bonding with the forming ice. Petrenko & Peng [96] used a variety of self-assembled monolayers (SAMs) with a range of surface energies to demonstrate that the shear adhesion of ice scales with the work of adhesion. Meuler et al. [90] arrived at a similar conclusion by studying polymeric coatings filled with varying amounts of fluorinated polyhedral oligomeric silsesquioxane, a low-surface-energy additive (figure 4c). Zou et al. and Sojoudi et al. also confirmed this result using chemical vapour deposition (PECVD) of fluorinated and non-fluorinated components [97,98]. Fluorinated sol gels, and annealed silicone and fluorine containing block copolymers are among other coating types which demonstrate the utility of lowering solid surface energy to lower ice adhesion [99,100]. While these results are encouraging, the theoretical lower limit of τice on a smooth, non-elastic, low-energy surface is only approximately 150 kPa [91]. Therefore, additional strategies are needed to fabricate low τice surfaces.
One design approach is to capitalize on the mobile interface enabled by SLIPS surfaces, which were previously discussed in the context of ice nucleation. Many SLIPS surfaces have demonstrated low τice [85,86,88,101], down to 15 kPa [85]. However, such surfaces are still afflicted by the tendency of the lubricant to leach out of the surface, and of the asperities of the porous matrix to sustain mechanical damage. Multiple modifications to SLIPS surfaces have been adapted in order to improve ice adhesion performance. Once again, surfaces containing hygroscopic polymers have been presented as an alternate method of generating a lubricated surface, and have been successful in reducing τice to as low as 20 kPa (figure 4a) [82,92,93]. Vogel et al. [89] demonstrated ice adhesion values of only τice = 10 kPa by using colloidal templating to generate closed-cell silica microstructures for SLIPS with tunable porosity, improved containment of lubricant and superior mechanical durability (figure 4b). Yin et al. [81] adopted a similar fabrication methodology, with the addition of Fe3O4 nanoparticles for photoresponsive thermogenesis, to enable rapid, low-energy surface heating (up to 50°C after 10 s of exposure to near-infrared light). Lastly, multiple groups have developed self-lubricating organogels with τice values as low as 0.4 kPa [102,103]. The gels consist of a low-modulus, lubricant-impregnated polymer network which contracts at low temperatures to expel a surface oil layer [102,103]. While they are vulnerable to mechanical damage, such surfaces illustrate the potential success of combined chemical and mechanical design strategies in lowering meso-scale ice adhesion.
As the length scale of the fouling ice increases, the success of surface design strategies becomes increasingly dependent on their consideration of the surface elastic modulus. For ice adhered to an elastic surface that it is able to deform, the de-bonding mechanism becomes distinct from that of ice on a rigid surface [104]. As was proposed by Kendall for pull-off of a rigid solid and later extended by Chaudhury to apply to shear detachment, the strength of a solid/elastic interface is governed by the relation where Wa is the work of adhesion, E is the surface elastic modulus, and t is the surface layer thickness (figure 4d) [19–21]. Multiple groups have demonstrated that ice adhesion on such an elastic surface is a function of both surface energy (included in Wa) and surface modulus [105], as well as the thickness of a substrate [106,107]. However, in this region, it is the surface modulus which can be varied over the largest range. To allow macro-scale ice to be shed from a surface, extremely low values of ice adhesion are frequently necessary for a range of applications. For example, in order for a sheet of ice which measures 1 inch thick with 1 m3 of contact area to passively detach from a typical offshore wind turbine, the ice adhesion strength of the surface must be τice < 12 kPa [108].
Beemer et al. [106] have demonstrated that cross-linked PDMS with an extremely low shear modulus of 10.3 kPa can yield an ice adhesion value of only τice = 5 kPa, which it retained after 100 icing/deicing cycles or 1000 abrasion cycles with 400 grit sandpaper. The impact of surface modulus on ice adhesion and durability has been studied most thoroughly by Golovin et al. [91] who tested nearly 60 different compositions of elastomeric coatings to determine the impact of modulus, as well as imbedded miscible oil on surface ice adhesion and durability (figure 4e). They were able to generate multiple coatings with ice adhesion values as low as τice = 0.15 kPa, as well as exceptionally durable coatings with ice adhesion values of τice < 10 kPa that withstood damage from 100 repeat icing–deicing cycles, 5000 Taber abrasion cycles, thermal cycling and acid/base exposure [91]. They grouped elastic surfaces into three categories: no-slip surfaces, surfaces with interfacial slippage and lubricated surfaces. The no-slip condition, which applies to most surfaces, indicates that ice is rigidly adhered to the surface up until the moment of fracture. By filling low-modulus elastomers with a particular amount of oil, they can be rendered sufficiently mobile to allow ice to slip at its interface with the surface, without adding enough oil for the surface to become lubricated, i.e. form a free layer of oil on surfaces which leads to poor durability. In their follow-up work, the group proposed a design framework to predict the icephobic properties of polymeric surfaces, both thermoplastics and thermosets, based on the fraction of oil added, its miscibility and the inherent ice adhesion strength of the polymers [108]. Based on this framework, they fabricated icephobic coatings from a range of polymers such as polystyrene, polyvinyl chloride, natural rubber and polydimethyl siloxane.
In this section, we have summarized the different approaches in the literature that have been applied to the large commercial problem of ice fouling. This topic clearly demonstrates the need for a design framework dependent on the modulus and length scale of the fouling material, as the effective approaches for resisting ice fouling differ as a function of these parameters. The future of low ice adhesion surfaces rests on the development of higher durability surfaces prepared to meet the low-cost, high-scale demands of industrial use.
3. Clathrate fouling
Clathrates, or natural gas hydrates, occur when gas molecules become enclosed in the cage-like structures of water to form a crystalline material under conditions of high pressure and low temperature. The structure is stabilized by intermolecular forces, primarily hydrogen bonding and van der Waals interactions. Typical clathrate gasses include propane, methane, cyclopentane, ethane and carbon dioxide [36,109]. This class of materials poses a significant problem for oil and gas industries where hydrate accumulation inside pipelines can cause blockages, reduce flow and increase pressure build-ups, resulting in loss of production speed, increased energy costs and significant safety hazards [109,110]. Currently employed solutions to address this issue include the addition of methanol or glycol to a flow stream in order to shift its thermodynamic equilibrium, kinetic inhibitors to prevent hydrate nucleation or anti-agglomeration agents [110–113]. All of these additives present continual costs to operators, with annual worldwide methanol costs for this application reaching about $220 million in 2003 [113]. Additionally, flow compositions with high water content, or watercut, limit the effectiveness and exponentially increase the cost of these components [114].
The adhesion of clathrates to common pipe surfaces in a water-free environment is sufficiently low to allow de-bonding by natural flow forces alone [115]. However, Aspenes et al. have shown that in the presence of water, and especially in the case of a hydrated surface, the strength of clathrate–surface adhesion is as much as 10x stronger than clathrate–clathrate adhesion. Surfaces tested by the group included carbon steel, stainless steel, aluminium, brass, glass and epoxy [116]. As water is commonly present in oil and gas pipeline flows, this result demonstrates the clear need for investigations into low clathrate adhesion surfaces.
Within the context of the solid fouling surface design framework presented in figure 2, natural gas hydrates possess an elastic modulus on the order of 1 GPa [36,54] and exist on length scales ranging from nanoscale nucleation sites to macro-scale bulk fouling. Research on hydrate surface adhesion has focused on the meso-scale, using chemical functionalization to minimize surface energy. Smith et al. measured the adhesion of tetrahydrofuran hydrates to surfaces functionalized with a variety of silanes to achieve a range of surface energies. They found that a fourfold reduction in hydrate adhesion could be achieved by the lowest surface energy silane layer, which minimized the work of adhesion [117]. Sojoudi et al. followed up on this work by measuring the adhesion of tetrahydrofuran hydrates and cyclopentane hydrates to CVD fabricated surfaces of polydivinylbenzene capped by poly(perfluorodecyl acrylate). Again, it was found that hydrate adhesion scaled with the work of adhesion of the surface, reaching as low as τclathrate = 20 kPa [97,118].
Despite the high modulus of natural gas hydrates in the context of solid fouling materials, to the best of our knowledge, no work has been done to investigate the effects of surface modulus on meso- and macro-scale adhesion of these materials. Owing to the extreme similarity in modulus and chemical composition between ice and clathrates [36], it is reasonable to assume that many surface design strategies may be shared effectively between the two fouling scenarios. In fact, the CVD surfaces fabricated by Sojoudi et al. [97] were also applied to mitigate ice fouling; however, these surfaces were able to reduce ice adhesion strength to only τice = 185.3 kPa. In comparison, as discussed previously, durable, low-modulus surfaces achieved values of τice < 10 kPa [108]. Therefore, by considering the high modulus of this foulant and incorporating surface elasticity into low hydrate adhesion surface design, significant additional reduction in adhesion strength may be achieved.
4. Inorganic scaling
The deposition of inorganic scale [119] is a major challenge in a range of industries including oil and gas, water desalination and power generation [120–122]. The uncontrolled build-up of scale can cause flow restriction and increased friction in pipes (figure 5a), under-scale surface corrosion of metal parts and loss of heat transfer efficiency through fouled surfaces [120,122,126]. In water desalination applications, equipment design must allow as much as 40% additional heat transfer area to account for fouling by inorganic scale [127]. The costs incurred by industry as a result of scale fouling can be enormous, stemming from both operational losses and increased energy demands, as well as the continued maintenance of fouled systems [120,126]. Current approaches to combat inorganic scaling require mechanical removal and the use of chemical inhibitors to delay or prevent nucleation [120–122,126]. Mechanical approaches can be costly and are difficult to apply to many equipment configurations, especially without incurring significant equipment down time [120,121]. Chemical inhibitors and cleaning agents also present high costs, while introducing potentially damaging contamination to both products and the environment [120–122,126]. Consequently, the need for passive, antiscale adhesion surfaces is clear to limit costs and improve efficiency in a myriad of critical industries that use heat exchangers.
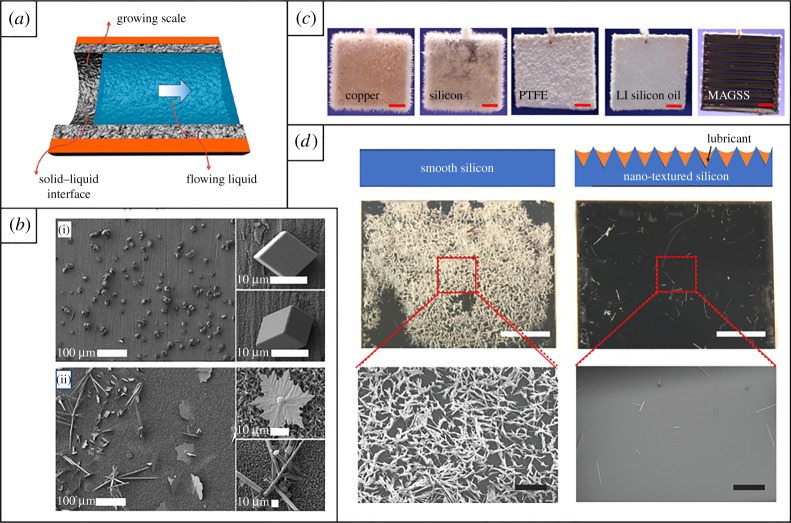
Figure 5.
(a) A schematic illustrating scale deposition on the interior of a pipe. (b) SEM images of CaCO3 crystals after 1 h of crystallization on samples of (i) pure Cu foil and (ii) superhydrophobic CuO nanowire film. (c) Forty-eight hours of calcium sulfate scale formation on copper, silicon, PTFE, liquid-infused silicon oil surfaces and MAGSS surface. The scale bar is 5 mm. (a, c) Adapted from [123] Copyright © 2017 American Chemical Society. (b) Adapted from [124] Copyright © 2015 American Chemical Society. (d) Calcium sulfate scale formation after approximately 80 h residence time on a smooth uncoated silicon substrate, and a lubricant-impregnated nanotextured silicon substrate. White scale bar 5 mm. Black scale bar 1 mm. (d) Adapted from [125] with permission.
In order to place inorganic scale deposits within the fouling material framework presented in figure 2, it is critical to recognize that inorganic scaling exists over a broad size range, from microscopic nucleation sites to deposits which are inches thick with square metres of surface area. The modulus of inorganic scale is typically estimated from the modulus of CaCO3, one of the most common scaling materials observed in industry [124]. With a value on the order of 10 GPa [55,56], CaCO3 is the highest modulus fouling material addressed in this review. Therefore, the length scale of fouling will be critical in determining whether chemical- or modulus-based surface designs will have the greatest impact on reducing CaCO3 adhesion strength.
Multiple surfaces have been proposed which use chemical functionalization to inhibit deposition during nucleation and early-stage adhesion. Research efforts have widely confirmed that reduction in the interfacial free energy of a surface results in: an increase in the final heat transfer coefficient for that surface in scaling environments [121,128], a reduction in the adhesion strength of scale deposits [120–122,124,126,129–131], a reduction in the nucleation rate of scale on the surface [120,124,130,132,133] and increased porosity of the scale deposits which do form (figure 5b) [126]. Specifically, multiple groups have identified the minimization of the polar component of the surface energy for a surface as the most impactful factor in limiting fouling [120,129]. Microscale roughness was found to increase both the rate of heterogeneous surface nucleation and the adhesion strength of deposited scale due to the increased surface area and mechanical locking effect of the impregnated texture [122,127,129,130,132–137]. Roughness also led to higher density deposits due to enhanced nucleation density and the orientation of the crystals [135,136].
While some previous works reported low-energy surface designs that use SAMs [120,124] or fluorinated polymers [122,129,130,132], the harsh environmental conditions of heat transfer applications require that surface coatings be thermally conductive, heat-resistant, and resistant to mechanical abrasion [121,131]. Diamond-like carbon coatings have been used by multiple groups for this application because they are chemically inert, thermally conductive, durable and have low roughness values [128,130–132]. Zhao & Wang [131] demonstrated that fluorine-doped diamond-like carbon (DLC) can significantly reduce the adhesion of scale deposits. The electric double-layer theory states that the implantation of low metal character elements such as F, Si, H and C into a metal layer will reduce the ionic nature of its surface and consequently lower the surface energy [121]. Multiple groups have capitalized on this effect and demonstrated the correlation between low scale adhesion and increased elemental implantation into metal heat-exchanger parts [121,133,137]. Other ultra-durable, thermally conductive coatings in the literature include fluorine-doped tantalum carbide [128] and NI-P-PTFE composites [126,133].
Despite the high modulus of inorganic scale as a fouling material, and its frequent occurrence at relatively high length scales, to the best of our knowledge, no experimental literature exists on the effect of surface modulus on scale adhesion. This can be attributed to the unique requirements of conductivity, heat-resistance and mechanical durability for heat-exchanger coatings which eliminate virtually all elastic bulk materials from consideration. Müller-Steinhagen and Zhao reported that due to the thermal resistivity of polymeric materials, effective heat transfer would require a coating thickness less than 5 µm [121], which would eliminate the impact of its elastic properties. As an alternative strategy, multiple groups have investigated lubricated surfaces, which possess a molecularly smooth surface to limit potential nucleation sites and a liquid surface interface to minimize scale adhesion. Subramanyam et al. [125] fabricated etched silicon samples impregnated with silicone oil and successfully demonstrated an over 48 h delay in scale nucleation, as well as a 10x reduction in adhesion strength when compared with bare silicon (figure 5d). Masoudi et al. demonstrated significant improvement in delaying scale nucleation, even over lubrication infused silicon oil substrates, by fabricating gel magnetic slippery surface (MAGSS). MAGSS are composed of styrene–ethylene–butylene–styrene triblock copolymer infused with a ferrofluid (figure 5c). These surfaces demonstrated scaling nucleation prevention for over 120 h under flow [123].
In this section, we have summarized multiple approaches in the literature which have been designed to address the issue of inorganic scaling. Ultimately, the future of low scale adhesion surfaces rests on the innovative development of high durability, high conductivity, heat resistant, low surface energy surfaces that can meet the low-cost, large-scale demands of industrial heat-exchanger applications.
5. Dust fouling
The adhesion of dust and particulate matter to surfaces represents another key type of solid fouling. Dust accumulation has the potential to dramatically reduce the output of solar panels by scattering and absorbing solar rays [138–143]. This impediment is particularly damaging in arid climates where infrequent rainfall and water shortages limit cleaning, high temperatures and dust storms exacerbate fouling, and low humidity increases static build-up [138,141,143,144]. Additionally, the heat transfer efficiency of heat exchangers in multiple industries is impacted by surface dust fouling, as well as the resultant air-side pressure drop within the exchanger [145,146]. Dust fouling has also been posed as a significant impediment to space science; expeditions to both the Moon and to Mars have experienced complications as a result of dust contamination [147–149]. While many self-cleaning surfaces have been developed which allow water to undercut adhered dust and rinse it from a surface [150–152], this review will focus only on the adhesion of dry dust as a solid foulant.
The elastic modulus of dust is virtually impossible to assign as the term encompasses particulates of a broad range of compositions. Dust primarily includes organic minerals, but also other materials such as pollen, hair or even human and animal cells [143]. What unites these components is their scale; dust particles are less than 500 µm in diameter [143]. This small length scale indicates that the chemical functionalization of a potential low adhesion surface is critical to its design. In 1980, Cuddihy [153] identified five surface properties which could be used to limit dust adhesion: hardness, roughness, hydrophobicity, surface energy and chemical cleanliness (particularly from water soluble salts). Of these, surface hydrophobicity/surface energy, as well as roughness, have been researched most thoroughly. Researchers have identified two primary factors influencing dust adhesion: van der Waals and electrostatic forces [147,153–156]. van der Waals interactions can be reduced by lowering a surface material's polarity and interfacial free energy, while electrostatic interactions are increased when surface charge induces coulombic interactions with particulates [147,153]. Additionally, Said et al. [141] demonstrated that the effect of surface texture depends strongly on its scale. Features larger than contaminating particulates will trap dust, while features on a smaller length scale limit the surface area which is presented for adhesion.
Jang et al. [139] used nanoscale texture, in addition to low-energy fluorinated surface functionalization to fabricate a superhydrophobic coating which reduced dust adhesion by 400% when compared with an untreated surface. Quan & Zhang [157] demonstrated that the texture required to achieve superhydrophobicity is not necessary to achieve dust repellency by fabricating and testing smooth tetraethoxysilane sol–gel coatings which minimize van der Waals effects and eliminate the risk of particle trapping. Multiple groups have confirmed that dust adhesion scales with the interfacial free energy of the surface, primarily by testing low surface energy fluorinated surfaces [18,147,158].
Surface conductivity also plays a critical role in the reduction of dust fouling, as it prevents charge build-up which will increase electrostatic forces [143]. Dove et al. [147] demonstrated that conductivity can have a much larger impact on the attraction of dust particles than surface energy alone. Sueto et al. [156] created another conductive coating from a combination of WO3 and partially hydrolysed tetraethyl orthosilicate and demonstrated low dust adhesion. Joseph et al. [155] demonstrated Indium Tin Oxide as a dust-repellant, conductive material with exceptional durability against abrasion, humidity, salt spray and thermal cycling. Fenero et al. [18] used laponite clay nanoparticles, partially functionalized with perfluoroalkylsilane, to fabricate an antidust surface which is both low energy and conductive. Continuing to investigate surfaces which are mechanically durable, low surface energy and conductive will enable researchers to improve the technology available to address the wide occurrences of dust fouling in both commercial and residential settings.
6. Paraffins and wax deposition
Wax and paraffin deposition can cause plugging of crude oil transportation pipelines and lead to production losses, cleaning downtimes and increased labour costs for oil and gas industries [159,160]. Much of the attention in petroleum fouling mitigation is given to deposit-inhibiting additives such as polymers [161], n-alkanes [162], wax crystal modifiers [163], dispersants [164,165] and even microbes [166,167]. However, such chemical additions to crude oil can be costly and require additional separation steps amidst an already complex and expensive purification process. Reports from field testing provide an indication of what pipeline coating strategies are commonly adopted, and offer clues for future antifouling strategies [168,169]. It is important to note that coatings designed to prevent wax deposition must withstand the harsh conditions inside of a petroleum pipeline, including severe abrasion, high temperature, high fluid shear stresses, and avoid chemical reactions with a myriad of organic compounds such as crude oil, resins, asphaltenes, waxes, scales, fines and water. Laboratory experimental designs in this case typically do not consistently mimic the technical complexities associated with on-site field application [170].
It is difficult to obtain a concrete modulus and length-scale range for paraffin waxes due to the diversity in their chemical composition, as a result of variability in processing conditions. However, reported values of modulus range between 61 and 250 MPa [51,52]. Although our design guide (figure 2) predicts that surface modulus and chemical functionality would have comparable effects on lowering the adhesion of foulants in this range of modulus, most efforts in the literature have been directed towards controlling surface free energy. The earliest antiwax coatings date back to the 1960s and use Bakelite lacquer, epoxy resins and enamel as a form of wax control [169,171]. Jorda investigated paraffin deposition on mill scale steel, as well as steel coated with smooth epoxy-phenolic and polyurethane coatings. They observed that after passing a wax-oil solution over these surfaces, less weight of paraffin was deposited on both of the smooth polymeric coatings, indicating an effect of both surface chemistry and texture on paraffin deposition [168].
Many early studies demonstrate surface energy and roughness as co-dependent factors in paraffin adhesion [168,172,173]. Quintella et al. [174] showed the dependence of wax deposition on the contact angle of a surface with paraffin oil. They observed high wax deposition rates for vinyl acetate copolymers with 28% oxygen content (EVA28) when compared with the more hydrophobic polymers, polypropylene (PP) and high-density polyethylene. Zhang et al. [175] tested paraffin deposition in crude oil for different surfaces. Low-energy surfaces such as polyvinylidene fluoride, poly(vinylidene fluoride)-chlorotrifluoroethylene and a methyl acrylate-styrene copolymer were tested and compared with higher surface energy polyurethane and epoxy surfaces. They demonstrated a decrease in paraffin deposition (of up to 75% compared to uncoated aluminium) with decreasing surface energy of the coating. They claimed that a combination of surface composition, chemical functionality and surface density of the functional groups affects wax wettability as measured by contact angle hysteresis with crude oil. This in turn controls the work of adhesion of paraffin on the surface. Rashidi et al. [176] recently showed approximately 86% reduction in grams of paraffin wax deposition from Malaysian (Tapis) crude oil on ethylene-tetrafluoroethylene versus steel and PVC. Finally, substrates coated with DLC (surface energy of approx. 30 mN m−1) also allow for low paraffin deposition, with the additional advantages of being able to withstand the harsh abrasive and thermal environments within an oil pipeline [177].
The impact of surface energy has been typically investigated within the context of mass deposition rates of wax and paraffin on different industrial surfaces, as opposed to direct surface-wax bond/adhesion measurements. Our design framework suggests that alteration of surface modulus may offer an avenue for continued improvement. Further research into the correlation between coating stiffness and wax deposition rates, as well as bond adhesion, may demonstrate the benefits of lowering modulus and perhaps offer avenues to use the synergistic advantages of low surface energy, highly elastic materials. The high durability demands for surfaces applied to oil transportation pipelines will likely present the greatest hindrance in this development.
7. Protein fouling
The contamination of surfaces by biological matter and organisms, or biofouling, is problematic for a multitude of fields and applications [119]. Within the medical realm, biocontamination can be dangerous for implants [178–181], surgical equipment [182–184], catheters [185–188] and hospital beds [189]. Biofouling is also problematic for food packaging and storage [190–193], filtration [194–198], as well as marine vessels [1,199,200] and infrastructure [201,202]. It is therefore imperative to develop antifouling surfaces which resist the attachment of biological organisms.
Unlike other forms of undesired solid adhesion, biological fouling is multiphasic, with each phase building on, or aiding the next. Biological organisms also possess the ability to divide, and to adjust based on the properties of the underlying surface [203]. The fouling process typically begins with protein adsorption on a surface, which serves as a conditioning layer for larger order organisms [203–205]. This process can occur within seconds, or minutes [203]. Besides acting as a conditioning layer for attachment of larger organisms, the adsorbed proteins can hamper the performance of immunological assays [206,207], lead to thrombosis for medical implants [208,209] and aid in the formation of platelets within certain microfluidic devices [208]. It has been demonstrated that even 10 ng cm−2 of the protein fibrinogen adsorbed on a surface can lead to blood platelet formation [208]. Surface design should thus be focused not only on the reduction in large bio-organisms, but also on protein fouling independently.
Proteins are complex biomaterials which consist of one or more ordered polypeptide chains [210]. These polypeptides can have a wide array of functionalities, causing either hydrophilic or hydrophobic character. Within aqueous solutions, proteins take on a metastable globular form where the majority of the hydrophobic functionality is ordered within the interior, while the hydrophilic side groups are oriented to the exterior [210]. The metastability of proteins is a result of a decrease in conformational entropy for the ordered interior non-polar groups which is somewhat overcome by their hydrophobic interactions, as well as by the enthalpic stabilization of the outer hydrophilic groups by surrounding water molecules [210]. This makes proteins susceptible to chemical, thermal or mechanical perturbations, which can lead to unfolding, and adsorption. Low surface energy hydrophobic surfaces, although able to repel high surface tension fluids, will cause protein adsorption by disrupting their metastable state. Additionally, even though proteins vary broadly in modulus [211,212], their small length scales do not allow for the low surface modulus approaches previously discussed to be a sufficient solution to combat adhesion. Regardless of the inherent challenges, chemistry-based approaches can be useful in rendering certain surfaces protein-resistant.
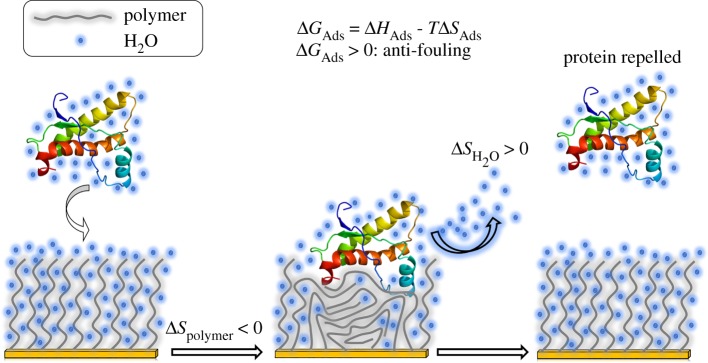
Poly(ethylene glycol) (PEG)-based grafted polymers are among the most commonly studied surface treatments used for the prevention of protein adsorption [213–221]. Jeon et al. [213] were one of the first groups to study the antifouling characteristics of grafted PEG. With a simple model, the group found that as a protein approaches a substrate, the PEG chains are compressed, leading to a decrease in conformational entropy, translating to compressive elastic forces [213]. Water molecules attracted to the hydrophilic PEG are displaced as a result of this compression, leading to a thermodynamically unfavourable osmotic barrier [207]. The coupling of both forces acts as a net repulsive force to the incoming proteins [213,219]. Figure 6 illustrates this process. The extent to which proteins will not adsorb to the surface monotonically increases with an increase in PEG grafting density and chain length [213]. Jeon et al.'s theoretical findings were later confirmed with a more refined model [219], validating the importance of grafting density, chain length, and polymer chain hydrophilicity.
Figure 6.
Protein repellency mechanism for a hydrophilic, brush-like grafted polymer [210,213,219]. Protein adsorption onto grafted PEG results in the release of the hydration barrier from both the polymer and the protein. This process increases the entropy for water, but is outweighed by a decrease in conformational entropy for the polymer. The net result is entropically unfavourable for protein adsorption. (ΔHAds) for the protein–polymer interaction can either be favourable or unfavourable depending on the paired species [210,222]. More strongly bound water results in less favourable protein adsorption.
Using SAMs, Prime and Whitesides were able to attach high grafting density monolayers presenting oligo(ethylene oxide) terminal groups onto gold substrates [207,214,223]. An array of proteins was introduced to the fabricated surfaces to prove the surface's protein resistance. These proteins included: fibrinogen, pyruvate kinase, lysozyme, and ribonuclease [223]. All tested proteins were repelled by the SAMs [223]. Contrary to previous studies claiming the importance of long grafted chain lengths, high-density SAMs presenting only two monomer groups of ethylene oxide displayed notable protein resistance [223]. This discovery does not contradict the theory proposed by Jeon et al. [213,219], as it demonstrates the importance of high grafting density which was previously fulfilled by substantially longer PEG chains [207]. Additionally, this finding reinforced the importance of polymer hydrophilicity in order to create a hydrated barrier for impinging proteins.
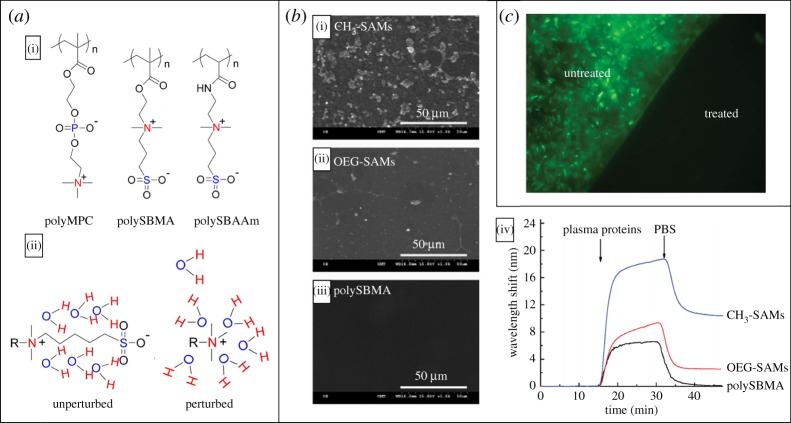
To take this understanding further, many groups have studied zwitterionic grafted polymers to impart protein resistance [13,15,16,224–226]. Zwitterionic polymers tightly bind to water due to the strong dipole created by anionic moieties [228]. It has also been suggested that the structure of zwitterionic groups forces water into a highly ordered array [229]. As a consequence, relatively high amounts of energy are required to disrupt the organized water molecule network supported by the zwitterionic moieties (figure 7a) [229]. Early studies were conducted by Holmlin et al. [15] and Tegoulia et al. [224] on the protein fouling resistance of phosphorylcholine-based zwitterionic SAMs. As expected, both groups observed low comparative levels of protein adsorption on the zwitterionic SAMs due to the strongly bound hydration layer. Chang and co-workers [225] studied blood plasma and platelet adsorption to zwitterionic grafted surfaces, as well as methyl and oligo(ethylene glycol) terminated SAMs [226]. The group observed ultra-low levels of both platelet and blood plasma proteins adsorbed to the zwitterionic surface when compared with the controls. Scanning electron microscopy and surface plasmon resonance (SPR) were used to confirm these findings (figure 7b). Beyond protein antifouling, grafted zwitterionic polymers have displayed notable long-term bacterial resistance as shown by multiple groups [14,230–232]. Li et al. [232] developed a sophisticated, easily applied, bacteria- and protein-resistant zwitterionic polymer termed pSBMA300-catechol. This polymer, when grafted to NH2 functionalized glass, resisted the adsorption of a wide range of proteins, in addition to bacteria over a 70 h period (figure 7c) [232]. Zwitterionic polymers have also proven to be more robust than PEG and are not limited to being implemented as a weakly bound SAM [233–238]. Moreover, PEG auto oxidizes within air and aqueous solutions into aldehyde-terminated chains, severely diminishing its protein resistance [238]. Zwitterionic grafted polymers thus have proven to be an overall advantageous route to achieving durable, long-term, protein- and bacteria-resistant surfaces.
Figure 7.
(a)(i) Three examples of zwitterionic polymers: poly-(methacryloyloxylethyl phosphorylcholine), polyMPC; poly-(sulfobetaine methacrylate), polySBMA; and poly-(sulfobetaineacrylamide), polySBAAm. (ii) Graphic representation of two ordering mechanisms for water by a charged group, and zwitterionic group. The zwitterionic polymer allows water's highly ordered structure to remain ‘unperturbed’, while a single charge disrupts the hydrogen bonds of water into a more disordered state. Images adapted from [229] and reprinted with permission Copyright © 2014 American Chemical Society. (b)(i–iii) Minimization of platelet adhesion by using zwitterionic polySBMA SAMs when compared with SAMs presenting terminal methyl and oligo(ethylene glycol) groups. (iv) An SPR plot comparing adsorption of blood plasma proteins at 37°C. A 1 nm wavelength shift is equivalent to a deposition of 15 ng cm−2. Images adapted from [227] with permission. Copyright © 2008 American Chemical Society. (c) Fluorescence microscopy image displaying Pseudomonas aeruginosa attached to a partially coated NH2-glass substrate after 70 h of bacterial exposure. A section of the surface was treated with a zwitterionic polymer pSBMA300-catechol (treated) while a portion of the surface remained unmodified (untreated). Adapted from [16] with permission. Copyright © 2009 John Wiley and Sons.
8. Polymer fouling
Unlike protein fouling, organic polymer fouling has been infrequently studied. Polymer fouling can lead to: contamination during polymerization reaction processes [239], down time during reactor cleaning cycles [239], ineffective filtration [240] and inefficient heat exchangers [241]. In biological settings, algae and diatoms secrete extracellular polymeric substance (EPS) when adhering to a given surface [205,242]. EPS strengthens their attachment, but can be problematic for a variety of marine applications [205,242]. Polymers in solution, although unlike the metastable proteins, exhibit a similar surface repellency mechanism shown in figure 6. Organic fouling within an organic solvent was studied extensively by Wang et al. [243]. The group investigated the comparative polymer repellency of a single fluorine atom terminated alkyne monolayer, with alkyne monolayers containing 17, 3 and 0 fluorine atoms [243]. The single fluorine terminated monolayer proved to be highly repellent to a broad range of polymers with varying functionalities [243]. This finding closely matches the models proposed for protein adsorption as the strong dipole created by the single fluorine terminated monolayer strongly attracted the dimethylformamide (DMF) solvent, creating a barrier to adsorption of polymer chains. The single fluorine atom monolayer did however foul when encountering P2VP/DMF [243]. As confirmed by simulations, the interaction between protonated pyridine moieties, and polarized C–F groups was strong enough to break through the solvent layer and overcome the decrease in conformational entropy of the grafted monolayer [243]. The study of polymer antifouling in solution is still in its infancy, allowing for new opportunities to develop techniques to mitigate this phenomenon.
9. Marine fouling
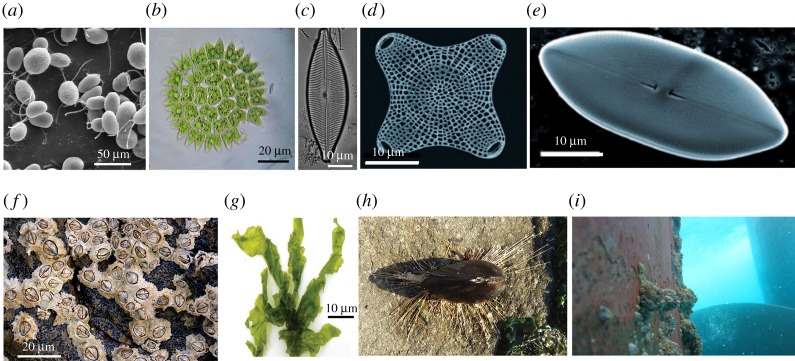
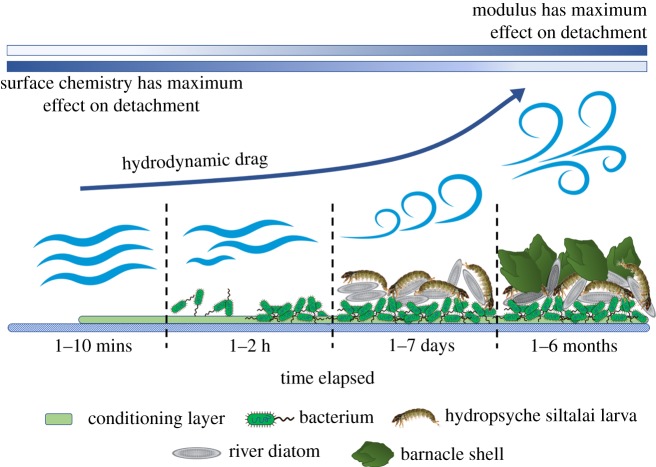
Marine fouling materials are a special class of foulants in that they represent a diverse community of organisms with varying sizes, preferences to surface settlement, attachment as well as release mechanisms (figure 8a–i). This causes marine fouling to be time-dependent, and possess a unique evolutionary attachment sequence. Marine organisms are an important part of the biosphere; however, they can be highly damaging to underwater sensors and increase drag experienced by marine vessels several fold [247,248]. This in turn raises their fuel consumption [1,200], and environmental pollution. Furthermore, ship maintenance for removal of these organisms extends down time and cleaning expenditures [1]. When a surface is submerged underwater in a marine environment, it provides a fresh surface on which an ecosystem can develop. First, an organic layer composed of humic acids, uronic acids, carbohydrates, amino acids and proteins is formed within the first few minutes of fouling [242,249,250]. This film provides an ‘environment signal’ that activates production of the necessary genes, proteins or even structural changes [250] (metamorphosis) that encourages planktonic bacteria attachment on top of the film. This attachment in turn provides a nutrition layer for unicellular organisms such as protozoa and diatoms which bind via mucus or EPS secretion [205,242]. This surface environment becomes a breeding ground for multicellular species of larvae, algae and algae spores which colonize within several days, followed by increasingly complex organisms such as tunicates, mussels and barnacles over the course of several weeks [205,251]. It is this dependence on foulant size, geographical location and time that makes marine fouling (figure 9) an intractable issue to solve.
Figure 8.
Images highlighting the diversity of marine foulants, including (a) SEM image of green algae, adapted from (http://remf.dartmouth.edu/imagesindex.html under public domain notice), (b) non-motile coenobium (group of cells) of Pediastrum duplex, a species of freshwater green algae, licensed under (https://creativecommons.org/licenses/by-sa/3.0/deed.en). (c) Diatom frustule of Cymbella inaequalis (adapted with permission from [244]). (d,e) SEM images of marine diatom species (adapted with permission from [245]). (f) Chthamalus stellatus barnacle photographed near the upper shoreline, Lundy Island, UK, reproduced with permission from (https://creativecommons.org/licenses/by-sa/3.0/deed.en). (g) Ulva lactuca a.k.a. sea lettuce (adapted from [246]. Copyright © 2014 licensed under https://creativecommons.org/licenses/by/3.0/). (h) A mussel (genus Mytilus), attached to a rock by its secreted filaments called byssus, licensed under https://creativecommons.org/licenses/by-sa/3.0/deed.en. (i) Biofouling on a ship hull adapted from [35] licensed under https://creativecommons.org/licenses/by/4.0/.
Figure 9.
The marine fouling sequence observed on a surface underwater. Within the first few minutes, a layer of organic macro-molecules, known as the conditioning layer, is formed and fouls the surface [242,249,250]. After 1-2 h of submersion, planktonic bacteria colonizes the conditioning layer [250]. Within several days, the substrata become a habitat for larger unicellular organisms such as diatoms [205,242] followed by multicellular species of larvae, algae and algae spores (image licensed under https://creativecommons.org/licenses/by/3.0/deed.en). After several weeks, the surface experiences fouling from increasingly complex organisms such as tunicates, mussels and barnacles [205,251]. A consequence of fouling on a ship hull is the increased drag experienced by the vessel in motion which increases costs and extends downtime during hull maintenance [200].
(a). Microbial fouling
One solution to marine fouling that has been explored previously is blocking the initial food supply for the fouling species, namely proteins and bacteria [207,252]. Given the extremely low modulus of biofilms, between 10 Pa and 600 kPa [30–32,253,254], these foulants are incapable of deforming any reasonably robust solid substrate. Therefore, consistent with our guiding framework, the role of surface functionalization has been extensively studied to mitigate initial bacterial or protein attachment onto a surface [252,255,256]. The relationship between bacterial retention on a surface and the surface energy of the substrata is well represented by the Baier curve [257]. For many attached biofouling organisms present in numerous natural environments (bacterial suspensions, fresh, brackish and sea water), minimum fouling is achieved between a substratum surface energy of γsv ∼ 20–30 mN m−1. This surface energy is approximately equal to the dispersive force contribution of water (approx. 22 mN m−1 and 50 mN m−1 for the dispersive and polar force contributions, respectively). Hence, the thermodynamic cost for water to wet a solid (from a hydration layer) with surface energy of approximately 22 mN m−1 is minimum [257]. If much higher or lower surface energy materials are selected, fouling would be more likely.
Several groups continue to investigate the utility of low surface energy of materials such as polydimethylsiloxane (PDMS) and fluorinated polymers, as foul-release (FR) coatings [258,259]. Hydrophobic antifouling coatings, such as Intersleek 1100SR, work by exploiting a fluorinated, low surface energy material that minimizes the surface interaction with slime and subsequent foulants. However, such a design is only effective in dynamic operation (i.e. when a marine vessel is moving) and not for extended static conditions, when the ship may be docked. During docking, such surfaces can be fouled by the adhesion of complex and hard organisms, significantly increasing the ship drag [247,258]. The argument for hydrophilic surfaces is that the presence of a strongly bound hydration layer will prevent the attachment of foulants. A good example is the Hempasil X3 FR paint which forms a water-retaining PDMS hydrogel network and shows less attachment to marine foulants compared to PDMS and a PDMS coating with fluorinated oils [260]. As discussed previously, zwitterionic polymers work by a similar principle in that they develop a hydration layer that inhibits bacterial attachment by diminishing electrostatic interactions [261–263]. Additionally, the effect of surface chemistry seems to be species-dependent, i.e. one type of foulant may adhere better than another for a particular surface [264–266]. Nonetheless, appreciable removal of organic molecules and microorganisms depends more strongly on fine tuning of surface chemistry than on the surface physical properties such as stiffness. Bakker et al. [267,268] showed minor but observable decreases in the attachment of marine bacteria while lowering the modulus of stiff substrates (from 2.2 to 1.5 GPa). Unless the surface modulus is lowered sufficiently to render marine foulants capable of deforming the fouling surface (E < 50 kPa), the stiffness of the substrate will not affect adhesion to an observable extent [269,270].
(b). Diatomaceous fouling
There is considerable literature agreement with the strategy to arrest the initial settlement of larger single-celled microorganisms such as diatoms and protozoa [250,258,271]. Species of diatoms such as Navicula pelliculosa were found by nanoindentation to have a hard shell (called a frustule) with modulus varying from 7 to hundreds of GPa, similar to silicas [272,273]. Losic et al. [274] reported an elastic modulus of approximately 3.4 GPa for Coscinodiscus sp. diatom frustule's perforations. The diatom frustule, or outer shell, is hard with softer mucilage layers underneath (figure 10). Diatoms can bind on different surfaces by secreting mucilage consisting of EPS [275]. Higgins et al. [276] have reported the elastic modulus of the softer mucilage (secreted fluids enabling attachment) layers in Pinnularia viridis and Craspedostauros australis diatoms to be between 0.54–0.76 and 0.25–0.45 MPa, respectively. Marine and freshwater diatoms span minute sizes from 102 to 109 µm3 and 10 to 106 µm3, respectively [277]. At this length scale, based on figure 2, limiting the adhesion of these foulants will again require the tuning of surface chemistry.
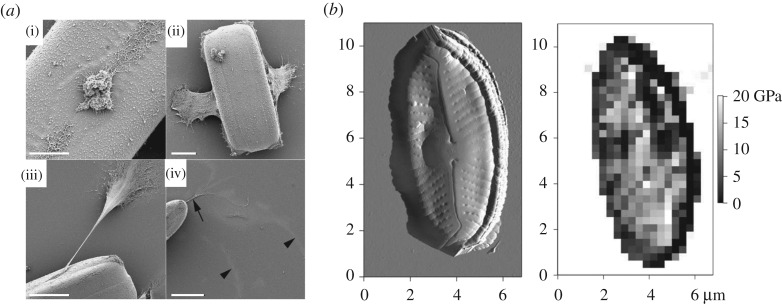
Figure 10.
(a) SEM of chemically fixed Pinnularia viridis cells after settling for 30 min on the substratum (glass coverslips). (i) A large mass of mucilage (sticky substance) that is tenuously attached along the diatom slits (called raphe) is observed at the centre (central nodule). (ii) Two sheets of mucilage that extend down from the diatom to form a connection with the substratum. (iii) A long thin mucilaginous tether extending from the diatom surface and forming a connection with the substratum. (iv) The tethers are observed on the substratum (arrow), in addition to fine diffuse trails that form curved patterns (arrowheads). Scale bars: 5 mm (a(i)), 10 mm (a(ii)), 15 mm (a(iii) and a(iv)) (adapted with permission from [273]). (b) Atomic force microscopy mapping of a diatom shell (frustule) showing modulus values at different locations. A region of stronger material spans along the centre of the body (reproduced with permission from [45]).
Amphiphilic coatings, which possess both hydrophobic and hydrophilic domains, disturb the settlement of hydrophobic and hydrophilic foulants such as Navicula diatoms and Ulva linza zoospores, respectively. Krishnan et al. [266] used block copolymers with ethoxylated fluoroalkyl side chains and outperformed PDMS surfaces in lowering adhesion for these two species. Several other reports also demonstrate the utility of using amphiphilic polymer coatings to reduce the attachment of different marine species [278–282].
Since most marine foulants are organic, free radical or reactive oxygen species generators may be useful in causing their degradation and thereby reduce fouling. Free radical generators such as titanium dioxide, zinc oxide and tungsten oxide have been previously used to reduce fouling. Although they are usually equipped with photosensitizer dyes to induce more efficient absorption of light, their performance is limited by that incident light's intensity [207]. Another mechanism to cause degradation is biocide deployment. Zargiel et al. published an extensive study on the multi-species diatom fouling of a myriad of coatings including commercial foul-releasers, copper-based surfaces and non-copper biocidal coatings. They found that certain diatoms like Amphora adhere more strongly to FR coatings than on biocide-based coatings demonstrating the inefficacies of modern FR coatings [271]. Commercial coatings, such as Hempaguard X7, use copper pyrithione as a biocide and provide ship protection from fouling marine organisms for up to 120 idle days of operation [283]. Dow's Sea-Nine™ coating is functionalized with biocidal 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one, and targets the adhesion of diatoms, freshwater and marine bacteria, algae, and barnacles. This material displays rapid biodegradability when compared with previously used toxic biocides such as tributyltin (TBT) [284]. However, the biocide is still toxic to numerous non-target species such as tunicates [285]. Several attempts have been made in developing other less toxic biocidal antifouling paints, however, such biocides display varied toxicity among the mixed diatom community [271]. Researchers have also investigated the integration of biocidal and foulant-release coatings. However, getting the biocide to be miscible within the FR silicone polymer networks has proven to be extremely challenging and has hindered this approach [258].
(c). Macro-fouling
For the prevention of macro-scale biofouling, surface chemistry has been observed to have a limited effect on fouling, while the modulus of the substrate begins to have a more important role (as shown in figure 2). Barnacle and mussel adhesion is a complex process, involving the attachment of proteins to any organic substrata via high strength covalent bonding [286]. Lowering the modulus of an elastic foulant-release coating has been shown by several researchers as an effective deterrent against algal plants [33] and barnacles (or pseudo-barnacles) [20,34]. A significant decrease in detachment force was observed when the coating modulus was reduced 10–1000 times lower than the foulant modulus [20,33,34,287–289]. Chaudhury et al. observed that the attachment of Ulva sporelings (young plants) was reduced by approximately 80% when modulus of a PDMS coating was reduced 12-fold. The attachment of Ulva spores (size approx. 5–7 µm), however, required a 47-fold reduction in modulus to observe the same per cent detachment [33]. The reason for this is that the sporelings have a well-developed cell wall that increases their overall rigidity. Shultz et al. showed increased removal of Enteromorpha biofilms under shear as they grew in size over a 6-day growth period over a surface coated with Veridian (a commercial FR coating from International Paint). They observed approximately 90% removal for 6-day-old biofilm compared to only approximately 15% removal for 1-day-old biofilm [290]. This shows that FR becomes more prominent for larger biofilms due to increased hydrodynamic shear, as the film grows larger.
The problem of marine fouling draws participation from species of varying sizes and modulus. Minute foulants including soft organic layers and bacteria are incapable of appreciably deforming the coating surface. Therefore, the modulus of the underlying substrata has a much lower effect on settlement and adhesion of such foulants when compared with tuning substrata surface chemistry, which has proven to be the most effective pathway in fouling mitigation. Owing to the vastness in fouling species present in the aquatic environment, with preferential adhesion and breadth in size and rigidity, a single antifouling solution currently does not exist. As for much larger macro-foulants, such as algal plants, barnacles and mussels, and smaller but harder micro-foulants, such as diatoms, substrata stiffness starts to contribute to adhesion as well. An antifouling coating would thus need to have a modulus that is significantly lower than these foulants. However, the coating stiffness must be sufficiently high to maintain durability requirements during its operation lifetime. Hence, extremely soft materials would not suffice as a feasible coating option.
10. Conclusion
In this review, we have broadly discussed solid fouling, and the various surface design solutions required for effective prevention. Fouling, defined as the undesired adhesion or adsorption of solid contaminants to a given surface, included but was not limited to: ice, scale deposits, wax and asphaltenes, polymers, dust and a complex range of biological matter and organisms. Each form of solid fouling is problematic or dangerous for multiple applications and industrial processes. Some examples covered in this text include ice on airplane wings, clathrates in industrial oil and gas piping, barnacles and mussels on ship hulls, scale on heat exchangers, dust formation on solar cells and protein adsorption to medical implants.
No single engineered surface has been developed which can broadly solve the problem of solid fouling. Many groups have designed surfaces to combat fouling, but typically only a single fouling material is studied at a time. However, certain similarities between each study can help draw a relation between the foulant size and modulus, and the most effective direction for antifouling surface engineering. At small length scales, ice and calcite nucleates, bacteria, algal spores and proteins may be repelled or greatly inhibited by surfaces with carefully designed chemistries. Lowering the surface free energy is often sufficient to reduce fouling at this scale; however, biological matter such as proteins are most effectively repelled by high surface energy molecular brushes. Regardless, the antifouling effects of chemistry-based design approaches dominate for relatively small-scale foulants. Larger fouling scenarios such as ice sheets, barnacles and calcite deposits benefit from low-modulus surfaces for facile de-bonding. These foulants when large enough can elastically deform a rigid surface when under an applied force, and therefore benefit from low-modulus surfaces which reduce the surface elastic energy during the de-bonding process. Some materials with intermediate size or modulus, including diatoms, barnacles and small ice nucleates, may require surface designs which consider both chemistry and elastic modulus for non-fouling surface properties. For example, some diatom species have lengths on the order of micrometres, but possess a high modulus shell that could reach hundreds of GPa. Diatoms and other foulants of this nature therefore require surfaces with low modulus and fine-tuned chemistry for antifouling properties. Thus, there remains an interplay between the foulant modulus and size which dictates the final surface engineering considerations.
To conclude, we have broadly reviewed many modes of solid fouling, as well as the surface design strategies in the literature which have demonstrated effective prevention and/or release. Design considerations primarily include the intrinsic modulus and the length scale of the foulant, as well as the chemical functionality and elastic modulus of the surface. For many situations, when a foulant is low in modulus and relatively small in dimension, chemistry-based approaches to prevent fouling are dominant. Conversely for larger, more rigid forms of fouling, a low-modulus surface will tend to be most effective for facile removal. Many real-word applications constrain this framework further with the need for a surface to resist high temperatures, chemical damage and mechanical deformation. Therefore, surface durability offers a critical dimension to be implemented within our framework. Many groups have made great strides in the development of durable antifouling surfaces for a range of different applications, and with this continuous progress, we look forward to new developments which will significantly impact this important area.
Data accessibility
This article has no additional data.
Authors' contributions
All authors drafted, read, edited and approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We thank Dr Ki-Han Kim and the Office of Naval Research (ONR) for financial support under grant no. N00014-12-1-0874. We also thank Dr Kenneth Caster and the Air Force Office of Scientific Research (AFOSR) for financial support under grant no. FA9550-10-1-0523. We also thank the National Science Foundation and the Nanomanufacturing program for supporting this work through grant no. 1351412.
References
- 1.Schultz M, Bendick J, Holm E, Hertel W. 2011. Economic impact of biofouling on a naval surface ship. Biofouling 27, 87–98. ( 10.1080/08927014.2010.542809) [DOI] [PubMed] [Google Scholar]
- 2.Schultz M, Walker J, Steppe C, Flack K. 2015. Impact of diatomaceous biofilms on the frictional drag of fouling-release coatings. Biofouling 31, 759–773. ( 10.1080/08927014.2015.1108407) [DOI] [PubMed] [Google Scholar]
- 3.Agency EP. 2012. Effluent limitation guidelines and new source performance standards for the airport deicing category, p. 37. Federal Register.
- 4.Gent R, Dart N, Cansdale J. 2000. Aircraft icing. Phil. Trans. R. Soc. Lond. A 358, 2873–2911. ( 10.1098/rsta.2000.0689) [DOI] [Google Scholar]
- 5.Laforte J, Allaire M, Laflamme J. 1998. State-of-the-art on power line de-icing. Atmos. Res. 46, 143–158. ( 10.1016/S0169-8095(97)00057-4) [DOI] [Google Scholar]
- 6.Aiyejina A, Chakrabarti DP, Pilgrim A, Sastry M. 2011. Wax formation in oil pipelines: a critical review. Int. J. Multiphase Flow 37, 671–694. ( 10.1016/j.ijmultiphaseflow.2011.02.007) [DOI] [Google Scholar]
- 7.Leontaritis KJ, Mansoori GA. 1988. Asphaltene deposition: a survey of field experiences and research approaches. J. Petrol. Sci. Eng. 1, 229–239. ( 10.1016/0920-4105(88)90013-7) [DOI] [Google Scholar]
- 8.Donlan RM. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7, 277 ( 10.3201/eid0702.010226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Percival SL, Suleman L, Vuotto C, Donelli G. 2015. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J. Med. Microbiol. 64, 323–334. ( 10.1099/jmm.0.000032) [DOI] [PubMed] [Google Scholar]
- 10.Chaudhury MK. 1996. Interfacial interaction between low-energy surfaces. Mater. Sci. Eng.: R: Rep. 16, 97–159. ( 10.1016/0927-796X(95)00185-9) [DOI] [Google Scholar]
- 11.Packham DE. 2003. Surface energy, surface topography and adhesion. Int. J. Adhes. Adhes. 23, 437–448. ( 10.1016/S0143-7496(03)00068-X) [DOI] [Google Scholar]
- 12.Callow ME, Fletcher RL. 1994. The influence of low surface energy materials on bioadhesion—a review. Int. J. Biodeterior. Biodegred. 34, 333–348. ( 10.1016/0964-8305(94)90092-2) [DOI] [Google Scholar]
- 13.Chen S, Zheng J, Li L, Jiang S. 2005. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: insights into nonfouling properties of zwitterionic materials. J. Am. Chem. Soc. 127, 14 473–14 478. ( 10.1021/ja054169u) [DOI] [PubMed] [Google Scholar]
- 14.Cheng G, Zhang Z, Chen S, Bryers JD, Jiang SJB. 2007. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. 28, 4192–4199. ( 10.1016/j.biomaterials.2007.05.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmlin RE, Chen X, Chapman RG, Takayama S, Whitesides GM. 2001. Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir 17, 2841–2850. ( 10.1021/la0015258) [DOI] [PubMed] [Google Scholar]
- 16.Jiang S, Cao Z. 2010. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 22, 920–932. ( 10.1002/adma.200901407) [DOI] [PubMed] [Google Scholar]
- 17.Baytekin HT, Baytekin B, Hermans TM, Kowalczyk B, Grzybowski BA. 2013. Control of surface charges by radicals as a principle of antistatic polymers protecting electronic circuitry. Science 341, 1368–1371. ( 10.1126/science.1241326) [DOI] [PubMed] [Google Scholar]
- 18.Fenero M, Palenzuela J, Azpitarte I, Knez M, Rodríguez J, Tena-Zaera R. 2017. Laponite-based surfaces with holistic self-cleaning functionality by combining antistatics and omniphobicity. ACS Appl. Mater. Interfaces 9, 39 078–39 085. ( 10.1021/acsami.7b13535) [DOI] [PubMed] [Google Scholar]
- 19.Chaudhury M, Kim K. 2007. Shear-induced adhesive failure of a rigid slab in contact with a thin confined film. Eur. Phys. J. E 23, 175–183. ( 10.1140/epje/i2007-10171-x) [DOI] [PubMed] [Google Scholar]
- 20.Chung JY, Chaudhury MK. 2005. Soft and hard adhesion. J. Adhesion 81, 1119–1145. ( 10.1080/00218460500310887) [DOI] [Google Scholar]
- 21.Kendall K. 1971. The adhesion and surface energy of elastic solids. J. Phys. D: Appl. Phys. 4, 1186 ( 10.1088/0022-3727/4/8/320) [DOI] [Google Scholar]
- 22.Popov VL. 2010. Contact mechanics and friction. Berlin, Germany: Springer. [Google Scholar]
- 23.Chen Z, Nosonovsky M. 2017. Revisiting lowest possible surface energy of a solid. Surf. Topogr. 5, 045001 ( 10.1088/2051-672X/aa84c9) [DOI] [Google Scholar]
- 24.Callister D, Rethwisch DG. 2007. Materials science and engineering: an introduction, 7th edn New York, NY: John Wiley and Sons, Inc. [Google Scholar]
- 25.Johnston I, McCluskey D, Tan C, Tracey M. 2014. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 24, 035017 ( 10.1088/0960-1317/24/3/035017) [DOI] [Google Scholar]
- 26.Tuteja A, Choi W, Mabry JM, McKinley GH, Cohen RE. 2008. Robust omniphobic surfaces. Proc. Natl Acad. Sci. USA 105, 18 200–18 205. ( 10.1073/pnas.0804872105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, et al. 2012. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 101, 111603 ( 10.1063/1.4752436) [DOI] [Google Scholar]
- 28.Gent A, Lai S. 1995. Adhesion and autohesion of rubber compounds: effect of surface roughness. Rubber Chem. Technol. 68, 13–25. ( 10.5254/1.3538725) [DOI] [Google Scholar]
- 29.Kreder MJ, Alvarenga J, Kim P, Aizenberg J. 2016. Design of anti-icing surfaces: smooth, textured or slippery? Nat. Rev. Mater. 1, 15003 ( 10.1038/natrevmats.2015.3) [DOI] [Google Scholar]
- 30.Abe Y, Polyakov P, Skali-Lami S, Francius G. 2011. Elasticity and physico-chemical properties during drinking water biofilm formation. Biofouling 27, 739–750. ( 10.1080/08927014.2011.601300) [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal S, Hozalski RM. 2010. Determination of biofilm mechanical properties from tensile tests performed using a micro-cantilever method. Biofouling 26, 479–486. ( 10.1080/08927011003793080) [DOI] [PubMed] [Google Scholar]
- 32.Stoodley P, Cargo R, Rupp CJ, Wilson S, Klapper I. 2002. Biofilm material properties as related to shear-induced deformation and detachment phenomena. J. Ind. Microbiol. Biotechnol. 29, 361–367. ( 10.1038/sj.jim.7000282) [DOI] [PubMed] [Google Scholar]
- 33.Chaudhury MK, Finlay JA, Chung JY, Callow ME, Callow JA. 2005. The influence of elastic modulus and thickness on the release of the soft-fouling green alga Ulva linza (syn. Enteromorpha linza) from poly(dimethylsiloxane) (PDMS) model networks. Biofouling 21, 41–48. ( 10.1080/08927010500044377) [DOI] [PubMed] [Google Scholar]
- 34.Berglin M, Lönn N, Gatenholm P. 2003. Coating modulus and barnacle bioadhesion. Biofouling 19, 63–69. ( 10.1080/0892701021000048774) [DOI] [PubMed] [Google Scholar]
- 35.Oliveira D, Granhag L. 2016. Matching forces applied in underwater hull cleaning with adhesion strength of marine organisms. J. Mar. Sci. Eng. 4, 66 ( 10.3390/jmse4040066) [DOI] [Google Scholar]
- 36.Sloan ED Jr, Koh C. 2007. Clathrate hydrates of natural gases. Boca Raton, FL: CRC Press. [Google Scholar]
- 37.Petrenko VF, Whitworth RW. 1999. Physics of ice. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.Yirtici O, Tuncer IH, Ozgen S. 2016. Ice accretion prediction on wind turbines and consequent power losses. J. Phys: Conf. Ser. 753, 022022 ( 10.1088/1742-6596/753/2/022022) [DOI] [Google Scholar]
- 39.Boreyko JB, Collier CP. 2013. Delayed frost growth on jumping-drop superhydrophobic surfaces. ACS Nano 7, 1618–1627. ( 10.1021/nn3055048) [DOI] [PubMed] [Google Scholar]
- 40.He M, Wang J, Li H, Jin X, Wang J, Liu B, Song Y. 2010. Super-hydrophobic film retards frost formation. Soft Matter 6, 2396–2399. ( 10.1039/c0sm00024h) [DOI] [Google Scholar]
- 41.Katz A, Alimova A, Xu M, Rudolph E, Shah MK, Savage HE, Rosen RB, McCormick SA, Alfano RR. 2003. Bacteria size determination by elastic light scattering. IEEE J. Sel. Top. Quantum Electron. 9, 277–287. ( 10.1109/JSTQE.2003.811284) [DOI] [Google Scholar]
- 42.Mueller LN, De Brouwer JF, Almeida JS, Stal LJ, Xavier JB. 2006. Analysis of a marine phototrophic biofilm by confocal laser scanning microscopy using the new image quantification software PHLIP. BMC Ecol. 6, 1 ( 10.1186/1472-6785-6-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barsanti L, Gualtieri P. 2014. Algae: anatomy, biochemistry, and biotechnology. Boca Raton, FL: CRC Press. [Google Scholar]
- 44.Ngan Y, Price IR. 1979. Systematic significance of spore size in the Florideophyceae (Rhodophyta). Br. Phycol. J. 14, 285–303. ( 10.1080/00071617900650311) [DOI] [Google Scholar]
- 45.Almqvist N, Delamo Y, Smith B, Thomson N, Bartholdson Å, Lal R, Brzezinski M, Hansma P. 2001. Micromechanical and structural properties of a pennate diatom investigated by atomic force microscopy. J. Microsc. 202, 518–532. ( 10.1046/j.1365-2818.2001.00887.x) [DOI] [PubMed] [Google Scholar]
- 46.Towler BF, Rebbapragada S. 2004. Mitigation of paraffin wax deposition in cretaceous crude oils of Wyoming. J. Petrol. Sci. Eng. 45, 11–19. ( 10.1016/j.petrol.2004.05.006) [DOI] [Google Scholar]
- 47.Eberle P, Tiwari MK, Maitra T, Poulikakos D. 2014. Rational nanostructuring of surfaces for extraordinary icephobicity. Nanoscale 6, 4874–4881. ( 10.1039/C3NR06644D) [DOI] [PubMed] [Google Scholar]
- 48.Heydari G, Thormann E, Jarn M, Tyrode E, Claesson PM. 2013. Hydrophobic surfaces: topography effects on wetting by supercooled water and freezing delay. J. Phys. Chem. C 117, 21 752–21 762. ( 10.1021/jp404396m) [DOI] [Google Scholar]
- 49.Jung S, Dorrestijn M, Raps D, Das A, Megaridis CM, Poulikakos D. 2011. Are superhydrophobic surfaces best for icephobicity? Langmuir 27, 3059–3066. ( 10.1021/la104762g) [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Norde W, van der Mei HC, Busscher HJ. 2012. Bacterial cell surface deformation under external loading. MBio 3, e00378-12 ( 10.1128/mBio.00378-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Severtson SJ, Stein A. 2006. Significant and concurrent enhancement of stiffness, strength, and toughness for paraffin wax through organoclay addition. Adv. Mater. 18, 1585–1588. ( 10.1002/adma.200502615) [DOI] [Google Scholar]
- 52.DeSain J, Brady B, Metzler K, Curtiss T, Albright T. 2009. Tensile tests of paraffin wax for hybrid rocket fuel grains In 45th AIAA/ASME/SAE/ASEE Joint Propulsion Conf. & Exhibit, p. 5115 August 2009, Denver, Colorado: Aerospace Research Central. [Google Scholar]
- 53.Tatinclaux J-C, Hirayama K-I. 1982. Determination of the flexural strength and elastic modulus of ice from in situ cantilever-beam tests. Cold Reg. Sci. Technol. 6, 37–47. ( 10.1016/0165-232X(82)90043-X) [DOI] [Google Scholar]
- 54.Ecker C, Dvorkin J, Nur AM. 2000. Estimating the amount of gas hydrate and free gas from marine seismic data. Geophysics 65, 565–573. ( 10.1190/1.1444752) [DOI] [Google Scholar]
- 55.Belkofsi R, Adjaoud O, Bellabbas I. 2018. Pressure induced phase transitions and elastic properties of CaCO3 polymorphs: a density functional theory study. Model. Simul. Mater. Sci. Eng. 26, 065004 ( 10.1088/1361-651x/aacbed) [DOI] [Google Scholar]
- 56.Zhang J, Reeder RJ. 1999. Comparative compressibilities of calcite-structure carbonates: deviations from empirical relations. Am. Mineral. 84, 861–870. ( 10.2138/am-1999-5-620) [DOI] [Google Scholar]
- 57.Rykaczewski K, Anand S, Subramanyam SB, Varanasi KK. 2013. Mechanism of frost formation on lubricant-impregnated surfaces. Langmuir 29, 5230–5238. ( 10.1021/la400801s) [DOI] [PubMed] [Google Scholar]
- 58.Sojoudi H, Wang M, Boscher N, McKinley G, Gleason K. 2016. Durable and scalable icephobic surfaces: similarities and distinctions from superhydrophobic surfaces. Soft Matter 12, 1938–1963. ( 10.1039/C5SM02295A) [DOI] [PubMed] [Google Scholar]
- 59.Thomas SK, Cassoni RP, MacArthur CD. 1996. Aircraft anti-icing and de-icing techniques and modeling. J. Aircraft 33, 841–854. ( 10.2514/3.47027) [DOI] [Google Scholar]
- 60.Farzaneh M, Volat C, Leblond A. 2008. Anti-icing and de-icing techniques for overhead lines. In Atmospheric icing of power networks, pp. 229–268. Berlin, Germany: Springer. [Google Scholar]
- 61.Frankenstein S, Tuthill AM. 2002. Ice adhesion to locks and dams: past work; future directions? J. Cold Regions Eng. 16, 83–96. ( 10.1061/(ASCE)0887-381X(2002)16:2(83) [DOI] [Google Scholar]
- 62.Fillion R, Riahi A, Edrisy A. 2014. A review of icing prevention in photovoltaic devices by surface engineering. Renew. Sustain. Energy Rev. 32, 797–809. ( 10.1016/j.rser.2014.01.015) [DOI] [Google Scholar]
- 63.Parent O, Ilinca A. 2011. Anti-icing and de-icing techniques for wind turbines: critical review. Cold Reg. Sci. Technol. 65, 88–96. ( 10.1016/j.coldregions.2010.01.005) [DOI] [Google Scholar]
- 64.Tuteja A, Choi W, McKinley GH, Cohen RE, Rubner MF. 2008. Design parameters for superhydrophobicity and superoleophobicity. MRS Bull. 33, 752–758. ( 10.1557/mrs2008.161) [DOI] [Google Scholar]
- 65.Cassie A, Baxter S. 1944. Wettability of porous surfaces. Trans. Faraday Soc. 40, 546–551. ( 10.1039/tf9444000546) [DOI] [Google Scholar]
- 66.Boinovich L, Emelyanenko AM, Korolev VV, Pashinin AS. 2014. Effect of wettability on sessile drop freezing: when superhydrophobicity stimulates an extreme freezing delay. Langmuir 30, 1659–1668. ( 10.1021/la403796g) [DOI] [PubMed] [Google Scholar]
- 67.Guo P, Zheng Y, Wen M, Song C, Lin Y, Jiang L. 2012. Icephobic/anti-icing properties of micro/nanostructured surfaces. Adv. Mater. 24, 2642–2648. ( 10.1002/adma.201104412) [DOI] [PubMed] [Google Scholar]
- 68.He M, Wang J, Li H, Song Y. 2011. Super-hydrophobic surfaces to condensed micro-droplets at temperatures below the freezing point retard ice/frost formation. Soft Matter 7, 3993–4000. ( 10.1039/c0sm01504k) [DOI] [Google Scholar]
- 69.Tourkine P, Le Merrer M, Quéré D. 2009. Delayed freezing on water repellent materials. Langmuir 25, 7214–7216. ( 10.1021/la900929u) [DOI] [PubMed] [Google Scholar]
- 70.Bartolo D, Bouamrirene F, Verneuil E, Buguin A, Silberzan P, Moulinet S. 2006. Bouncing or sticky droplets: impalement transitions on superhydrophobic micropatterned surfaces. EPL 74, 299 ( 10.1209/epl/i2005-10522-3) [DOI] [Google Scholar]
- 71.Bahadur V, Mishchenko L, Hatton B, Taylor JA, Aizenberg J, Krupenkin T. 2011. Predictive model for ice formation on superhydrophobic surfaces. Langmuir 27, 14 143–14 150. ( 10.1021/la200816f) [DOI] [PubMed] [Google Scholar]
- 72.Kulinich S, Farhadi S, Nose K, Du X. 2010. Superhydrophobic surfaces: are they really ice-repellent? Langmuir 27, 25–29. ( 10.1021/la104277q) [DOI] [PubMed] [Google Scholar]
- 73.Liu B, Lange FF. 2006. Pressure induced transition between superhydrophobic states: configuration diagrams and effect of surface feature size. J. Colloid Interface Sci. 298, 899–909. ( 10.1016/j.jcis.2006.01.025) [DOI] [PubMed] [Google Scholar]
- 74.Narhe R, Beysens D. 2007. Growth dynamics of water drops on a square-pattern rough hydrophobic surface. Langmuir 23, 6486–6489. ( 10.1021/la062021y) [DOI] [PubMed] [Google Scholar]
- 75.Reyssat M, Yeomans J, Quéré D. 2007. Impalement of fakir drops. EPL 81, 26006 ( 10.1209/0295-5075/81/26006) [DOI] [Google Scholar]
- 76.Varanasi KK, Deng T, Smith JD, Hsu M, Bhate N. 2010. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 97, 234102 ( 10.1063/1.3524513) [DOI] [Google Scholar]
- 77.Wier KA, McCarthy TJ. 2006. Condensation on ultrahydrophobic surfaces and its effect on droplet mobility: ultrahydrophobic surfaces are not always water repellant. Langmuir 22, 2433–2436. ( 10.1021/la0525877) [DOI] [PubMed] [Google Scholar]
- 78.Yin L, Xia Q, Xue J, Yang S, Wang Q, Chen Q. 2010. In situ investigation of ice formation on surfaces with representative wettability. Appl. Surf. Sci. 256, 6764–6769. ( 10.1016/j.apsusc.2010.04.086) [DOI] [Google Scholar]
- 79.Wong T-S, Kang SH, Tang SK, Smythe EJ, Hatton BD, Grinthal A, Aizenberg J. 2011. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 477, 443 ( 10.1038/nature10447) [DOI] [PubMed] [Google Scholar]
- 80.Gwak Y, Park J-I, Kim M, Kim HS, Kwon MJ, Oh SJ, Kim Y-P, Jin E. 2015. Creating anti-icing surfaces via the direct immobilization of antifreeze proteins on aluminum. Sci. Rep. 5, 12019 ( 10.1038/srep12019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin X, Zhang Y, Wang D, Liu Z, Liu Y, Pei X, Yu B, Zhou F. 2015. Integration of self-lubrication and near-infrared photothermogenesis for excellent anti-icing/deicing performance. Adv. Funct. Mater. 25, 4237–4245. ( 10.1002/adfm.201501101) [DOI] [Google Scholar]
- 82.Chen J, et al. 2013. Robust prototypical anti-icing coatings with a self-lubricating liquid water layer between ice and substrate. ACS Appl. Mater. Interfaces 5, 4026–4030. ( 10.1021/am401004t) [DOI] [PubMed] [Google Scholar]
- 83.Esser-Kahn AP, Trang V, Francis MB. 2010. Incorporation of antifreeze proteins into polymer coatings using site-selective bioconjugation. J. Am. Chem. Soc. 132, 13 264–13 269. ( 10.1021/ja103038p) [DOI] [PubMed] [Google Scholar]
- 84.Stone HA. 2012. Ice-phobic surfaces that are wet. ACS Nano 6, 6536–6540. ( 10.1021/nn303372q) [DOI] [PubMed] [Google Scholar]
- 85.Kim P, Wong T-S, Alvarenga J, Kreder MJ, Adorno-Martinez WE, Aizenberg J. 2012. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 6, 6569–6577. ( 10.1021/nn302310q) [DOI] [PubMed] [Google Scholar]
- 86.Liu Q, Yang Y, Huang M, Zhou Y, Liu Y, Liang X. 2015. Durability of a lubricant-infused electrospray silicon rubber surface as an anti-icing coating. Appl. Surf. Sci. 346, 68–76. ( 10.1016/j.apsusc.2015.02.051) [DOI] [Google Scholar]
- 87.Wilson PW, Lu W, Xu H, Kim P, Kreder MJ, Alvarenga J, Aizenberg J. 2013. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS). Phys. Chem. Chem. Phys. 15, 581–585. ( 10.1039/C2CP43586A) [DOI] [PubMed] [Google Scholar]
- 88.Subramanyam SB, Rykaczewski K, Varanasi KK. 2013. Ice adhesion on lubricant-impregnated textured surfaces. Langmuir 29, 13 414–13 418. ( 10.1021/la402456c) [DOI] [PubMed] [Google Scholar]
- 89.Vogel N, Belisle RA, Hatton B, Wong T-S, Aizenberg J. 2013. Transparency and damage tolerance of patternable omniphobic lubricated surfaces based on inverse colloidal monolayers. Nat. Commun. 4, 2176 ( 10.1038/ncomms3176) [DOI] [PubMed] [Google Scholar]
- 90.Meuler AJ, Smith JD, Varanasi KK, Mabry JM, McKinley GH, Cohen RE. 2010. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2, 3100–3110. ( 10.1021/am1006035) [DOI] [PubMed] [Google Scholar]
- 91.Golovin K, Kobaku SP, Lee DH, DiLoreto ET, Mabry JM, Tuteja A. 2016. Designing durable icephobic surfaces. Sci. Adv. 2, e1501496 ( 10.1126/sciadv.1501496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J, Luo Z, Fan Q, Lv J, Wang J. 2014. Anti-ice coating inspired by ice skating. Small 10, 4693–4699. ( 10.1002/smll.201401557) [DOI] [PubMed] [Google Scholar]
- 93.Dou R, Chen J, Zhang Y, Wang X, Cui D, Song Y, Jiang L, Wang J. 2014. Anti-icing coating with an aqueous lubricating layer. ACS Appl. Mater. Interfaces 6, 6998–7003. ( 10.1021/am501252u) [DOI] [PubMed] [Google Scholar]
- 94.Lee H, Alcaraz ML, Rubner MF, Cohen RE. 2013. Zwitter-wettability and antifogging coatings with frost-resisting capabilities. ACS Nano 7, 2172–2185. ( 10.1021/nn3057966) [DOI] [PubMed] [Google Scholar]
- 95.Sun X, Damle VG, Liu S, Rykaczewski K. 2015. Bioinspired stimuli-responsive and antifreeze-secreting anti-icing coatings. Adv. Mater. Interfaces 2, 1400479 ( 10.1002/admi.201400479) [DOI] [Google Scholar]
- 96.Petrenko V, Peng S. 2003. Reduction of ice adhesion to metal by using self-assembling monolayers (SAMs). Can. J. Phys. 81, 387–393. ( 10.1139/p03-014) [DOI] [Google Scholar]
- 97.Sojoudi H, McKinley GH, Gleason KK. 2015. Linker-free grafting of fluorinated polymeric cross-linked network bilayers for durable reduction of ice adhesion. Mater. Horiz. 2, 91–99. ( 10.1039/C4MH00162A) [DOI] [Google Scholar]
- 98.Zou M, Beckford S, Wei R, Ellis C, Hatton G, Miller M. 2011. Effects of surface roughness and energy on ice adhesion strength. Appl. Surf. Sci. 257, 3786–3792. ( 10.1016/j.apsusc.2010.11.149) [DOI] [Google Scholar]
- 99.Li X, Zhao Y, Li H, Yuan X. 2014. Preparation and icephobic properties of polymethyltrifluoropropylsiloxane–polyacrylate block copolymers. Appl. Surf. Sci. 316, 222–231. ( 10.1016/j.apsusc.2014.07.097) [DOI] [Google Scholar]
- 100.Susoff M, Siegmann K, Pfaffenroth C, Hirayama M. 2013. Evaluation of icephobic coatings—screening of different coatings and influence of roughness. Appl. Surf. Sci. 282, 870–879. ( 10.1016/j.apsusc.2013.06.073) [DOI] [Google Scholar]
- 101.Zhu L, Xue J, Wang Y, Chen Q, Ding J, Wang Q. 2013. Ice-phobic coatings based on silicon-oil-infused polydimethylsiloxane. ACS Appl. Mater. Interfaces 5, 4053–4062. ( 10.1021/am400704z) [DOI] [PubMed] [Google Scholar]
- 102.Urata C, Dunderdale GJ, England MW, Hozumi A. 2015. Self-lubricating organogels (SLUGs) with exceptional syneresis-induced anti-sticking properties against viscous emulsions and ices. J. Mater. Chem. A 3, 12 626–12 630. ( 10.1039/C5TA02690C) [DOI] [Google Scholar]
- 103.Wang Y, Yao X, Chen J, He Z, Liu J, Li Q, Wang J, Jiang L. 2015. Organogel as durable anti-icing coatings. Sci. China Mater. 58, 559–565. ( 10.1007/s40843-015-0069-7) [DOI] [Google Scholar]
- 104.Makkonen L. 2012. Ice adhesion—theory, measurements and countermeasures. J. Adhes. Sci. Technol. 26, 413–445. [Google Scholar]
- 105.Jellinek H, Kachi H, Kittaka S, Lee M, Yokota R. 1978. Ice releasing block-copolymer coatings. Colloid Polym. Sci. 256, 544–551. ( 10.1007/BF01639199) [DOI] [Google Scholar]
- 106.Beemer DL, Wang W, Kota AK. 2016. Durable gels with ultra-low adhesion to ice. J. Mater. Chem. A 4, 18 253–18 258. ( 10.1039/C6TA07262C) [DOI] [Google Scholar]
- 107.Wang C, Fuller T, Zhang W, Wynne KJ. 2014. Thickness dependence of ice removal stress for a polydimethylsiloxane nanocomposite: Sylgard 184. Langmuir 30, 12 819–12 826. ( 10.1021/la5030444) [DOI] [PubMed] [Google Scholar]
- 108.Golovin K, Tuteja A. 2017. A predictive framework for the design and fabrication of icephobic polymers. Sci. Adv. 3, e1701617 ( 10.1126/sciadv.1701617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gbaruko B, Igwe J, Gbaruko P, Nwokeoma R. 2007. Gas hydrates and clathrates: flow assurance, environmental and economic perspectives and the Nigerian liquified natural gas project. J. Petrol. Sci. Eng. 56, 192–198. ( 10.1016/j.petrol.2005.12.011) [DOI] [Google Scholar]
- 110.Sum AK, Koh CA, Sloan EDJI, Research EC. 2009. Clathrate hydrates: from laboratory science to engineering practice. Ind. Eng. Chem. Res. 48, 7457–7465. ( 10.1021/ie900679m) [DOI] [Google Scholar]
- 111.Devarakonda S, Groysman A, Myerson AS. 1999. THF–water hydrate crystallization: an experimental investigation. J. Cryst. Growth 204, 525–538. ( 10.1016/S0022-0248(99)00220-1) [DOI] [Google Scholar]
- 112.Koh C, Westacott R, Zhang W, Hirachand K, Creek J, Soper A. 2002. Mechanisms of gas hydrate formation and inhibition. Fluid Phase Equilib. 194, 143–151. ( 10.1016/S0378-3812(01)00660-4) [DOI] [Google Scholar]
- 113.Sloan ED., Jr 2003. Fundamental principles and applications of natural gas hydrates. Nature 426, 353 ( 10.1038/nature02135) [DOI] [PubMed] [Google Scholar]
- 114.Gao S. 2009. Hydrate risk management at high watercuts with anti-agglomerant hydrate inhibitors. Energy Fuels 23, 2118–2121. ( 10.1021/ef8009876) [DOI] [Google Scholar]
- 115.Nicholas JW, Dieker LE, Sloan ED, Koh CA. 2009. Assessing the feasibility of hydrate deposition on pipeline walls—adhesion force measurements of clathrate hydrate particles on carbon steel. J. Colloid Interface Sci. 331, 322–328. ( 10.1016/j.jcis.2008.11.070) [DOI] [PubMed] [Google Scholar]
- 116.Aspenes G, Dieker L, Aman Z, Høiland S, Sum A, Koh C, Sloan E. 2010. Adhesion force between cyclopentane hydrates and solid surface materials. J. Colloid Interface Sci. 343, 529–536. ( 10.1016/j.jcis.2009.11.071) [DOI] [PubMed] [Google Scholar]
- 117.Smith JD, Meuler AJ, Bralower HL, Venkatesan R, Subramanian S, Cohen RE, McKinley GH, Varanasi KK. 2012. Hydrate-phobic surfaces: fundamental studies in clathrate hydrate adhesion reduction. Phys. Chem. Chem. Phys. 14, 6013–6020. ( 10.1039/c2cp40581d) [DOI] [PubMed] [Google Scholar]
- 118.Sojoudi H, Walsh MR, Gleason KK, McKinley GH. 2015. Designing durable vapor-deposited surfaces for reduced hydrate adhesion. Adv. Mater. Interfaces 2, 1500003 ( 10.1002/admi.201500003) [DOI] [Google Scholar]
- 119.Bhushan B. 2016. Biomimetics: bioinspired hierarchical-structured surfaces for green science and technology. Berlin, Germany: Springer. [Google Scholar]
- 120.Azimi G, Cui Y, Sabanska A, Varanasi KK. 2014. Scale-resistant surfaces: fundamental studies of the effect of surface energy on reducing scale formation. Appl. Surf. Sci. 313, 591–599. ( 10.1016/j.apsusc.2014.06.028) [DOI] [Google Scholar]
- 121.Müller-Steinhagen H, Zhao Q. 1997. Investigation of low fouling surface alloys made by ion implantation technology. Chem. Eng. Sci. 52, 3321–3332. ( 10.1016/S0009-2509(97)00162-0) [DOI] [Google Scholar]
- 122.Rankin B, Adamson W. 1973. Scale formation as related to evaporator surface conditions. Desalination 13, 63–87. ( 10.1016/S0011-9164(00)80092-2) [DOI] [Google Scholar]
- 123.Masoudi A, Irajizad P, Farokhnia N, Kashyap V, Ghasemi H. 2017. Antiscaling magnetic slippery surfaces. ACS Appl. Mater. Interfaces 9, 21 025–21 033. ( 10.1021/acsami.7b05564) [DOI] [PubMed] [Google Scholar]
- 124.Jiang W, He J, Xiao F, Yuan S, Lu H, Liang B. 2015. Preparation and antiscaling application of superhydrophobic anodized CuO nanowire surfaces. Ind. Eng. Chem. Res. 54, 6874–6883. ( 10.1021/acs.iecr.5b00444) [DOI] [Google Scholar]
- 125.Subramanyam SB, Azimi G, Varanasi KK. 2014. Designing lubricant-impregnated textured surfaces to resist scale formation. Adv. Mater. Interfaces 1, 1300068 ( 10.1002/admi.201300068) [DOI] [Google Scholar]
- 126.Zhao Q, Liu Y, Wang S. 2005. Surface modification of water treatment equipment for reducing CaSO4 scale formation. Desalination 180, 133–138. ( 10.1016/j.desal.2005.01.002) [DOI] [Google Scholar]
- 127.Alahmad M. 2008. Factors affecting scale formation in sea water environments—an experimental approach. Chem. Eng. Technol.: Ind. Chem. - Plant Equip. - Process Eng. – Biotechnol. 31, 149–156. [Google Scholar]
- 128.Bornhorst A, Muller-Steinhagen H, Zhao Q. 1999. Reduction of scale formation under pool boiling conditions by ion implantation and magnetron sputtering on heat transfer surfaces. Heat Transfer Eng. 20, 6–14. ( 10.1080/014576399271529) [DOI] [Google Scholar]
- 129.Bargir S, Dunn S, Jefferson B, Macadam J, Parsons S. 2009. The use of contact angle measurements to estimate the adhesion propensity of calcium carbonate to solid substrates in water. Appl. Surf. Sci. 255, 4873–4879. ( 10.1016/j.apsusc.2008.12.017) [DOI] [Google Scholar]
- 130.Cheong W, Gaskell P, Neville A. 2013. Substrate effect on surface adhesion/crystallisation of calcium carbonate. J. Cryst. Growth 363, 7–21. ( 10.1016/j.jcrysgro.2012.09.025) [DOI] [Google Scholar]
- 131.Zhao Q, Wang X. 2005. Heat transfer surfaces coated with fluorinated diamond-like carbon films to minimize scale formation. Surf. Coat. Technol. 192, 77–80. ( 10.1016/j.surfcoat.2004.02.030) [DOI] [Google Scholar]
- 132.Vazirian MM, Charpentier TV, de Oliveira Penna M, Neville A. 2016. Surface inorganic scale formation in oil and gas industry: as adhesion and deposition processes. J. Petrol. Sci. Eng. 137, 22–32. ( 10.1016/j.petrol.2015.11.005) [DOI] [Google Scholar]
- 133.Zettler H, Wei M, Zhao Q, Müller-Steinhagen H. 2005. Influence of surface properties and characteristics on fouling in plate heat exchangers. Heat Transfer Eng. 26, 3–17. ( 10.1080/01457630590897024) [DOI] [Google Scholar]
- 134.Förster M, Bohnet M. 2000. Modification of molecular interactions at the interface crystal/heat transfer surface to minimize heat exchanger fouling. Int. J. Therm. Sci. 39, 697–708. ( 10.1016/S1290-0729(00)00229-5) [DOI] [Google Scholar]
- 135.Herz A, Malayeri M, Müller-Steinhagen H. 2008. Fouling of roughened stainless steel surfaces during convective heat transfer to aqueous solutions. Energy Convers. Manage. 49, 3381–3386. ( 10.1016/j.enconman.2007.09.034) [DOI] [Google Scholar]
- 136.Keysar S, Semiat R, Hasson D, Yahalom J. 1994. Effect of surface roughness on the morphology of calcite crystallizing on mild steel. J. Colloid Interface Sci. 162, 311–319. ( 10.1006/jcis.1994.1044) [DOI] [Google Scholar]
- 137.Müller-Steinhagen H, Zhao Q, Helali-Zadeh A, Ren XG. 2000. The effect of surface properties on CaSO4 scale formation during convective heat transfer and subcooled flow boiling. Can. J. Chem. Eng. 78, 12–20. ( 10.1002/cjce.5450780105) [DOI] [Google Scholar]
- 138.Elminir HK, Ghitas AE, Hamid R, El-Hussainy F, Beheary M, Abdel-Moneim KM. 2006. Effect of dust on the transparent cover of solar collectors. Energy Convers. Manage. 47, 3192–3203. ( 10.1016/j.enconman.2006.02.014) [DOI] [Google Scholar]
- 139.Jang GG, Smith DB, List FA, Lee DF, Ievlev AV, Collins L, Park J, Polizos G. 2018. The anti-soiling performance of highly reflective superhydrophobic nanoparticle-textured mirrors. Nanoscale 10, 14 600–14 612. ( 10.1039/C8NR03024C) [DOI] [PubMed] [Google Scholar]
- 140.Ju F, Fu X. 2011. Research on impact of dust on solar photovoltaic (PV) performance In 2011 Int. Conf. on Electrical and Control Engineering (ICECE), pp. 3601–3606, IEEE. [Google Scholar]
- 141.Said SA, Al-Aqeeli N, Walwil HM. 2015. The potential of using textured and anti-reflective coated glasses in minimizing dust fouling. Sol. Energy 113, 295–302. ( 10.1016/j.solener.2015.01.007) [DOI] [Google Scholar]
- 142.Said SA, Walwil HM. 2014. Fundamental studies on dust fouling effects on PV module performance. Sol. Energy 107, 328–337. ( 10.1016/j.solener.2014.05.048) [DOI] [Google Scholar]
- 143.Sarver T, Al-Qaraghuli A, Kazmerski LL. 2013. A comprehensive review of the impact of dust on the use of solar energy: History, investigations, results, literature, and mitigation approaches. Renew. Sustain. Energy Rev. 22, 698–733. ( 10.1016/j.rser.2012.12.065) [DOI] [Google Scholar]
- 144.Corn M. 1961. The adhesion of solid particles to solid surfaces. I. A review. J. Air Pollut. Contr. Assoc. 11, 523–528. ( 10.1080/00022470.1961.10468032) [DOI] [PubMed] [Google Scholar]
- 145.Bell IH, Groll EA. 2011. Air-side particulate fouling of microchannel heat exchangers: experimental comparison of air-side pressure drop and heat transfer with plate-fin heat exchanger. Appl. Therm. Eng. 31, 742–749. ( 10.1016/j.applthermaleng.2010.10.019) [DOI] [Google Scholar]
- 146.Bott T, Bemrose C. 1983. Particulate fouling on the gas-side of finned tube heat exchangers. J. Heat Transfer 105, 178–183. ( 10.1115/1.3245538) [DOI] [Google Scholar]
- 147.Dove A, Devaud G, Wang X, Crowder M, Lawitzke A, Haley C. 2011. Mitigation of lunar dust adhesion by surface modification. Planet. Space Sci. 59, 1784–1790. ( 10.1016/j.pss.2010.12.001) [DOI] [Google Scholar]
- 148.Sabri F, Werhner T, Hoskins J, Schuerger A, Hobbs A, Barreto J, Britt D, Duran R. 2008. Thin film surface treatments for lowering dust adhesion on Mars Rover calibration targets. Adv. Space Res. 41, 118–128. ( 10.1016/j.asr.2007.06.074) [DOI] [Google Scholar]
- 149.Stubbs TJ, Vondrak RR, Farrell WM. 2007. Impact of dust on lunar exploration. See http://hefd.jsc.nasa.gov/files/StubbsImpactOnExploration.
- 150.He G, Zhou C, Li Z. 2011. Review of self-cleaning method for solar cell array. Procedia Eng. 16, 640–645. ( 10.1016/j.proeng.2011.08.1135) [DOI] [Google Scholar]
- 151.Liu K, Jiang L. 2012. Bio-inspired self-cleaning surfaces. Annu. Rev. Mater. Res. 42, 231–263. ( 10.1146/annurev-matsci-070511-155046) [DOI] [Google Scholar]
- 152.Ragesh P, Ganesh VA, Nair SV, Nair AS. 2014. A review on ‘self-cleaning and multifunctional materials'. J. Mater. Chem. A 2, 14 773–14 797. ( 10.1039/C4TA02542C) [DOI] [Google Scholar]
- 153.Cuddihy EF. 1980. Theoretical considerations of soil retention. Solar Energy Mater. 3, 21–33. ( 10.1016/0165-1633(80)90047-7) [DOI] [Google Scholar]
- 154.Bowling RA. 1985. An analysis of particle adhesion on semiconductor surfaces. J. Electrochem. Soc. 132, 2208–2214. ( 10.1149/1.2114320) [DOI] [Google Scholar]
- 155.Joseph S, Ytzhaki T, Kasis E, Sheinman A, Yadin I. 2016. Transparent dust repellent coating for sapphire. In Optical interference coatings, p. TB. 10 Optical Society of America. [Google Scholar]
- 156.Sueto T, Ota Y, Nishioka K. 2013. Suppression of dust adhesion on a concentrator photovoltaic module using an anti-soiling photocatalytic coating. Sol. Energy 97, 414–417. ( 10.1016/j.solener.2013.09.006) [DOI] [Google Scholar]
- 157.Quan Y-Y, Zhang L-Z. 2017. Experimental investigation of the anti-dust effect of transparent hydrophobic coatings applied for solar cell covering glass. Sol. Energy Mater. Sol. Cells 160, 382–389. ( 10.1016/j.solmat.2016.10.043) [DOI] [Google Scholar]
- 158.Crowder MS, Haley C. 2008. Mitigating molecular and particulate contamination via surface energy. In Optical system contamination: effects, measurements, and control, San Diego, California, USA, 2 September 2008. p. 706909 Washington, USA: International Society for Optics and Photonics. [Google Scholar]
- 159.Sanjay M, Simanta B, Kulwant S. 1995. Paraffin problems in crude oil production and transportation: a review. SPE Prod. Facil. 10, 50–54. ( 10.2118/28181-PA) [DOI] [Google Scholar]
- 160.Kelechukwu EM, Al-Salim HS, Saadi A. 2013. Prediction of wax deposition problems of hydrocarbon production system. J. Petrol. Sci. Eng. 108, 128–136. ( 10.1016/j.petrol.2012.11.008) [DOI] [Google Scholar]
- 161.Al-Yaari M. 2011. Paraffin wax deposition: mitigation and removal techniques In SPE Saudi Arabia Section Young Professionals Technical Symposium Society of Petroleum Engineers. [Google Scholar]
- 162.Wang J, Buckley JS, Creek JL. 2004. Asphaltene deposition on metallic surfaces. J. Dispersion Sci. Technol. 25, 287–298. ( 10.1081/DIS-120037697) [DOI] [Google Scholar]
- 163.Patil AO, Zushma S, Berluche E, Varma-Nair M. 2002. Wax crystal modifiers (LAW657). (Google Patents).
- 164.Pedersen KS, Rønningsen HP. 2003. Influence of wax inhibitors on wax appearance temperature, pour point, and viscosity of waxy crude oils. Energy Fuels 17, 321–328. ( 10.1021/ef020142%2B) [DOI] [Google Scholar]
- 165.Kraiwattanawong K, Fogler HS, Gharfeh SG, Singh P, Thomason WH, Chavadej S. 2009. Effect of asphaltene dispersants on aggregate size distribution and growth. Energy Fuels 23, 1575–1582. ( 10.1021/ef800706c) [DOI] [Google Scholar]
- 166.Etoumi A. 2007. Microbial treatment of waxy crude oils for mitigation of wax precipitation. J. Petrol. Sci. Eng. 55, 111–121. ( 10.1016/j.petrol.2006.04.015) [DOI] [Google Scholar]
- 167.Brown F. 1992. Microbes: The practical and environmental safe solution to production problems, enhanced production, and enhanced oil recovery In Permian Basin Oil and Gas Recovery Conf Society of Petroleum Engineers. [Google Scholar]
- 168.Jorda R. 1966. Paraffin deposition and prevention in oil wells. J. Petrol. Technol. 18, 605–601, 612 ( 10.2118/1598-PA) [DOI] [Google Scholar]
- 169.Zholondz IA, Finkelstein MI. 1962. Lakokrasochnye Materialy i Ikh Primenenie pp. 40–44.
- 170.Paso K, Kompalla T, Aske N, Rønningsen HP, Øye G, Sjöblom J. 2009. Novel surfaces with applicability for preventing wax deposition: a review. J. Dispersion Sci. Technol. 30, 757–781. ( 10.1080/01932690802643220) [DOI] [Google Scholar]
- 171.Zaitseva TA, Gonik AA, Maksheyev Y, Permyakov NG. 1968. Trudy, Ufimskii Neftyanoi Nauchno-Issledovatel’skii Institut, pp. 141–144. [Google Scholar]
- 172.Patton CC, Casad BM. 1970. Paraffin deposition from refined wax-solvent systems. Soc. Petrol. Eng. J. 10, 17–24. ( 10.2118/2503-PA) [DOI] [Google Scholar]
- 173.Jun T, Qunji X. 1998. Effect on the wettability between doating and liquid paraffin by the dispersion component and polar component of coating surface energy. Chem. Eng. Oil Gas 2, 018. [Google Scholar]
- 174.Quintella CM, Musse APS, Castro MT, Scaiano J, Mikelsons L, Watanabe YN. 2006. Polymeric surfaces for heavy oil pipelines to inhibit wax deposition: PP, EVA28, and HDPE. Energy Fuels 20, 620–624. ( 10.1021/ef050267p) [DOI] [Google Scholar]
- 175.Zhang X, Tian J, Wang L, Zhou Z. 2002. Wettability effect of coatings on drag reduction and paraffin deposition prevention in oil. J. Petrol. Sci. Eng. 36, 87–95. ( 10.1016/S0920-4105(02)00267-X) [DOI] [Google Scholar]
- 176.Rashidi M, Mombekov B, Marhamati M. 2016. A study of a novel inter pipe coating material for paraffin wax deposition control and comparison of the results with current mitigation technique in oil and gas industry In Offshore Technology Conf. Asia (Offshore Technology Conf.). [Google Scholar]
- 177.Dotto M, Martins R, Ferreira M, Camargo S Jr. 2006. Influence of hydrogenated amorphous carbon coatings on the formation of paraffin deposits. Surf. Coat. Technol. 200, 6479–6483. ( 10.1016/j.surfcoat.2005.11.061) [DOI] [Google Scholar]
- 178.Bixler GD, Bhushan B. 2012. Biofouling: lessons from nature. Phil. Trans. R. Soc. A 370, 2381–2417. ( 10.1098/rsta.2011.0502) [DOI] [PubMed] [Google Scholar]
- 179.Engelsman AF, Saldarriaga-Fernandez IC, Nejadnik MR, van Dam GM, Francis KP, Ploeg RJ, Busscher HJ, van der Mei HC. 2010. The risk of biomaterial-associated infection after revision surgery due to an experimental primary implant infection. Biofouling 26, 761–767. ( 10.1080/08927014.2010.515027) [DOI] [PubMed] [Google Scholar]
- 180.Ward WK. 2008. A review of the foreign-body response to subcutaneously-implanted devices: the role of macrophages and cytokines in biofouling and fibrosis. Beverley Hills, CA: SAGE Publications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wisniewski N, Reichert M. 2000. Methods for reducing biosensor membrane biofouling. Colloids Surf. B: Biointerfaces 18, 197–219. ( 10.1016/S0927-7765(99)00148-4) [DOI] [PubMed] [Google Scholar]
- 182.Edmiston CE, Seabrook GR, Goheen MP, Krepel CJ, Johnson CP, Lewis BD, Brown KR, Towne JB. 2006. Bacterial adherence to surgical sutures: can antibacterial-coated sutures reduce the risk of microbial contamination? J. Am. Coll. Surg. 203, 481–489. ( 10.1016/j.jamcollsurg.2006.06.026) [DOI] [PubMed] [Google Scholar]
- 183.Kluge RM, Calia FM, McLaughlin JS, Hornick RB. 1974. Sources of contamination in open heart surgery. J.Am. Med. Assoc. 230, 1415–1418. ( 10.1001/jama.1974.03240100033023) [DOI] [PubMed] [Google Scholar]
- 184.Schmalzried TP, Amstutz HC, Au M-K, Dorey FJ. 1992. Etiology of deep sepsis in total hip arthroplasty. The significance of hematogenous and recurrent infections. Clin. Orthopaed. Relat. Res. 280, 200–207. ( 10.1097/00003086-199207000-00026) [DOI] [PubMed] [Google Scholar]
- 185.Chan J, Wong S. 2010. Biofouling: types, impact and anti-fouling. New York, NY: Nova Science Publishers, Incorporated. [Google Scholar]
- 186.Chopra V, O'horo JC, Rogers MA, Maki DG, Safdar N. 2013. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 34, 908–918. ( 10.1086/671737) [DOI] [PubMed] [Google Scholar]
- 187.Maki DG, Tambyah PA. 2001. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 7, 342 ( 10.3201/eid0702.010240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Peters G, Locci R, Pulverer G. 1982. Adherence and growth of coagulase-negative Staphylococci on surfaces of intravenous catheters. Emerg. Infect. Dis. 146, 479–482. ( 10.1093/infdis/146.4.479) [DOI] [PubMed] [Google Scholar]
- 189.Attaway HH III, Fairey S, Steed LL, Salgado CD, Michels HT, Schmidt MG. 2012. Intrinsic bacterial burden associated with intensive care unit hospital beds: effects of disinfection on population recovery and mitigation of potential infection risk. Am. J. Infect. Control 40, 907–912. ( 10.1016/j.ajic.2011.11.019) [DOI] [PubMed] [Google Scholar]
- 190.Appendini P, Hotchkiss JH. 2002. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 3, 113–126. ( 10.1016/S1466-8564(02)00012-7) [DOI] [Google Scholar]
- 191.Han JH. 2003. Antimicrobial food packaging. In Novel food packaging techniques, vol. 8 (ed. R Ahvenainen), pp. 50–70. Boca Raton, FL: CRC Press. [Google Scholar]
- 192.Petersen K, Nielsen PV, Bertelsen G, Lawther M, Olsen MB, Nilsson NH, Mortensen G. 1999. Potential of biobased materials for food packaging. Trends Food Sci. Technol. 10, 52–68. ( 10.1016/S0924-2244(99)00019-9) [DOI] [Google Scholar]
- 193.Quintavalla S, Vicini L. 2002. Antimicrobial food packaging in meat industry. Meat Sci. 62, 373–380. ( 10.1016/S0309-1740(02)00121-3) [DOI] [PubMed] [Google Scholar]
- 194.Flemming H-C. 1997. Reverse osmosis membrane biofouling. Exp. Therm. Fluid Sci. 14, 382–391. ( 10.1016/S0894-1777(96)00140-9) [DOI] [Google Scholar]
- 195.Hyun J, Jang H, Kim K, Na K, Tak T. 2006. Restriction of biofouling in membrane filtration using a brush-like polymer containing oligoethylene glycol side chains. J. Membr. Sci. 282, 52–59. ( 10.1016/j.memsci.2006.05.008) [DOI] [Google Scholar]
- 196.Lee W, Ahn CH, Hong S, Kim S, Lee S, Baek Y, Yoon J. 2010. Evaluation of surface properties of reverse osmosis membranes on the initial biofouling stages under no filtration condition. J. Membr. Sci. 351, 112–122. ( 10.1016/j.memsci.2010.01.035) [DOI] [Google Scholar]
- 197.Mansouri J, Harrisson S, Chen V. 2010. Strategies for controlling biofouling in membrane filtration systems: challenges and opportunities. J. Mater. Chem. 20, 4567–4586. ( 10.1039/b926440j) [DOI] [Google Scholar]
- 198.Potts D, Ahlert RC, Wang SS. 1981. A critical review of fouling of reverse osmosis membranes. Desalination 36, 235–264. ( 10.1016/S0011-9164(00)88642-7) [DOI] [Google Scholar]
- 199.Hellio C, Yebra D. 2009. Advances in marine antifouling coatings and technologies. Cambridge, UK: Woodhead Publishing. [Google Scholar]
- 200.Townsin R. 2003. The ship hull fouling penalty. Biofouling 19, 9–15. ( 10.1080/0892701031000088535) [DOI] [PubMed] [Google Scholar]
- 201.Edyvean R, Terry L, Picken G. 1985. Marine fouling and its effects on offshore structures in the North Sea: a review. Int. Biodeterior. 21, 277–284. [Google Scholar]
- 202.Houghton D. 1978. Marine fouling and offshore structures. Ocean Manage. 4, 347–352. ( 10.1016/0302-184X(78)90033-1) [DOI] [Google Scholar]
- 203.Magin CM, Cooper SP, Brennan AB. 2010. Non-toxic antifouling strategies. Mater. Today 13, 36–44. ( 10.1016/S1369-7021(10)70058-4) [DOI] [Google Scholar]
- 204.Chambers LD, Stokes KR, Walsh FC, Wood RJ. 2006. Modern approaches to marine antifouling coatings. Surface Coat. Technol. 201, 3642–3652. ( 10.1016/j.surfcoat.2006.08.129) [DOI] [Google Scholar]
- 205.Wahl M. 1989. Marine epibiosis. I. Fouling and antifouling: some basic aspects. Mar. Ecol. Prog. Ser. 58, 175–189. ( 10.3354/meps058175) [DOI] [Google Scholar]
- 206.Hucknall A, Rangarajan S, Chilkoti A. 2009. In pursuit of zero: polymer brushes that resist the adsorption of proteins. Adv. Mater. 21, 2441–2446. ( 10.1002/adma.200900383) [DOI] [Google Scholar]
- 207.Banerjee I, Pangule RC, Kane RS. 2011. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 23, 690–718. ( 10.1002/adma.201001215) [DOI] [PubMed] [Google Scholar]
- 208.Zhang H, Chiao M. 2015. Anti-fouling coatings of poly (dimethylsiloxane) devices for biological and biomedical applications. J. Med. Biol. Eng. 35, 143–155. ( 10.1007/s40846-015-0029-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Amiji M, Park K. 1992. Prevention of protein adsorption and platelet adhesion on surfaces by PEO/PPO/PEO triblock copolymers. Biomaterials 13, 682–692. ( 10.1016/0142-9612(92)90128-B) [DOI] [PubMed] [Google Scholar]
- 210.Gong P, Grainger DW. 2007. Nonfouling surfaces. In Microarrays pp. 59–92. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 211.Ji B, Gao H. 2004. Mechanical properties of nanostructure of biological materials. J. Mech. Phys. Solids 52, 1963–1990. ( 10.1016/j.jmps.2004.03.006) [DOI] [Google Scholar]
- 212.Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. 2002. Elastic proteins: biological roles and mechanical properties. Phil. Trans. R. Soc. Lond. B 357, 121–132. ( 10.1098/rstb.2001.1022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jeon S, Lee J, Andrade J, De Gennes P, Science I. 1991. Protein–surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 142, 149–158. ( 10.1016/0021-9797(91)90043-8) [DOI] [Google Scholar]
- 214.Prime KL, Whitesides GM. 1991. Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces. Science 252, 1164–1167. ( 10.1126/science.252.5009.1164) [DOI] [PubMed] [Google Scholar]
- 215.Bearinger J, Terrettaz S, Michel R, Tirelli N, Vogel H, Textor M, Hubbell J. 2003. Chemisorbed poly (propylene sulphide)-based copolymers resist biomolecular interactions. Nat. Mater. 2, 259 ( 10.1038/nmat851) [DOI] [PubMed] [Google Scholar]
- 216.Mrksich M, Whitesides GM. 1996. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annu. Rev. Biophys. Biomol. Struct. 25, 55–78. ( 10.1146/annurev.bb.25.060196.000415) [DOI] [PubMed] [Google Scholar]
- 217.Li L, Chen S, Zheng J, Ratner BD, Jiang S. 2005. Protein adsorption on oligo (ethylene glycol)-terminated alkanethiolate self-assembled monolayers: the molecular basis for nonfouling behavior. J. Phys. Chem. B 109, 2934–2941. ( 10.1021/jp0473321) [DOI] [PubMed] [Google Scholar]
- 218.Andrade J, Hlady V, Wei A, Ho C, Lea A, Jeon S, Lin Y, Stroup E. 1992. Proteins at interfaces: principles, multivariate aspects, protein resistant surfaces, and direct imaging and manipulation of adsorbed proteins. In Biologically modified polymeric biomaterial surfaces, pp. 67–84. Berlin, Germany: Springer. [Google Scholar]
- 219.Jeon S, Andrade J. 1991. Protein—surface interactions in the presence of polyethylene oxide: II. Effect of protein size. J. Colloid Interface Sci. 142, 159–166. ( 10.1016/0021-9797(91)90044-9) [DOI] [Google Scholar]
- 220.Jeon S, Andrade J. 1991. Protein—surface interactions in the presence of polyethylene oxide: II. Effect of protein size. J. Colloid Interface Sci. 142, 149–158. [Google Scholar]
- 221.Sofia SJ, Premnath V, Merrill EW. 1998. Poly (ethylene oxide) grafted to silicon surfaces: grafting density and protein adsorption. Macromolecules 31, 5059–5070. ( 10.1021/ma971016l) [DOI] [PubMed] [Google Scholar]
- 222.Norde W. 2003. Driving forces for protein adsorption at solid surfaces. In Biopolymers at interfaces, vol. 110 (ed. M Malmsten) New York, NY: Marcel Dekker. [Google Scholar]
- 223.Prime KL, Whitesides GM. 1993. Adsorption of proteins onto surfaces containing end-attached oligo (ethylene oxide): a model system using self-assembled monolayers. J. Am. Chem. Soc. 115, 10 714–10 721. ( 10.1021/ja00076a032) [DOI] [Google Scholar]
- 224.Tegoulia VA, Rao W, Kalambur AT, Rabolt JF, Cooper SL. 2001. Surface properties, fibrinogen adsorption, and cellular interactions of a novel phosphorylcholine-containing self-assembled monolayer on gold. Langmuir 17, 4396–4404. ( 10.1021/la001790t) [DOI] [Google Scholar]
- 225.He Y, Hower J, Chen S, Bernards MT, Chang Y, Jiang S. 2008. Molecular simulation studies of protein interactions with zwitterionic phosphorylcholine self-assembled monolayers in the presence of water. Langmuir 24, 10 358–10 364. ( 10.1021/la8013046) [DOI] [PubMed] [Google Scholar]
- 226.Keefe AJ, Jiang S. 2012. Poly (zwitterionic) protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat. Chem. 4, 59 ( 10.1038/nchem.1213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chang Y, Liao S-C, Higuchi A, Ruaan R-C, Chu C-W, Chen W-Y. 2008. A highly stable nonbiofouling surface with well-packed grafted zwitterionic polysulfobetaine for plasma protein repulsion. Langmuir 24, 5453–5458. ( 10.1021/la800228c) [DOI] [PubMed] [Google Scholar]
- 228.Laughlin RG. 1991. Fundamentals of the zwitterionic hydrophilic group. Langmuir 7, 842–847. ( 10.1021/la00053a006) [DOI] [Google Scholar]
- 229.Schlenoff JB. 2014. Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 30, 9625–9636. ( 10.1021/la500057j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hsiao S-W, Venault A, Yang H-S, Chang Y. 2014. Bacterial resistance of self-assembled surfaces using PPOm-b-PSBMAn zwitterionic copolymer–concomitant effects of surface topography and surface chemistry on attachment of live bacteria. Colloids Surf. B: Biointerfaces 118, 254–260. ( 10.1016/j.colsurfb.2014.03.051) [DOI] [PubMed] [Google Scholar]
- 231.Mi L, Jiang S. 2014. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. 53, 1746–1754. ( 10.1002/anie.201304060) [DOI] [PubMed] [Google Scholar]
- 232.Li G, Cheng G, Xue H, Chen S, Zhang F, Jiang S. 2008. Ultra low fouling zwitterionic polymers with a biomimetic adhesive group. Biomaterials 29, 4592–4597. ( 10.1016/j.biomaterials.2008.08.021) [DOI] [PubMed] [Google Scholar]
- 233.Zhao W, Ye Q, Hu H, Wang X, Zhou F. 2014. Grafting zwitterionic polymer brushes via electrochemical surface-initiated atomic-transfer radical polymerization for anti-fouling applications. J. Biomed. Mater. Res. B Appl. Biomater. 2, 5352–5357. [DOI] [PubMed] [Google Scholar]
- 234.Chen S, et al. 2016. Durable antibacterial and nonfouling cotton textiles with enhanced comfort via zwitterionic sulfopropylbetaine coating. Small 12, 3516–3521. ( 10.1002/smll.201600587) [DOI] [PubMed] [Google Scholar]
- 235.Nguyen AT, Baggerman J, Paulusse JM, van Rijn CJ, Zuilhof H. 2011. Stable protein-repellent zwitterionic polymer brushes grafted from silicon nitride. Langmuir 223, 2587–2594. ( 10.1021/la104657c) [DOI] [PubMed] [Google Scholar]
- 236.Yeh S-B, Chen C-S, Chen W-Y, Huang C-J. 2014. Modification of silicone elastomer with zwitterionic silane for durable antifouling properties. Langmuir 30, 11 386–11 393. ( 10.1021/la502486e) [DOI] [PubMed] [Google Scholar]
- 237.Jiang H, Wang X, Li C, Li J, Xu F, Mao C, Yang W, Shen J. 2011. Improvement of hemocompatibility of polycaprolactone film surfaces with zwitterionic polymer brushes. Langmuir 27, 11 575–11 581. ( 10.1021/la202101q) [DOI] [PubMed] [Google Scholar]
- 238.Qin G, Cai C. 2009. Oxidative degradation of oligo (ethylene glycol)-terminated monolayers. Chem. Commun. 34, 5112–5114. ( 10.1039/b911155g) [DOI] [PubMed] [Google Scholar]
- 239.Saikhwan P, Chew JY, Paterson WR, Wilson DI. 2007. Swelling and its suppression in the cleaning of polymer fouling layers. Ind. Eng. Chem. Res. 228, 4846–4855. ( 10.1021/ie0615943) [DOI] [Google Scholar]
- 240.Metzger U, Le-Clech P, Stuetz RM, Frimmel FH, Chen V. 2007. Characterisation of polymeric fouling in membrane bioreactors and the effect of different filtration modes. J. Membr. Sci. 301, 180–189. ( 10.1016/j.memsci.2007.06.016) [DOI] [Google Scholar]
- 241.Watkinson AP. 1992. Chemical reaction fouling of organic fluids. Chem. Eng. Technol.: Ind. Chem. -Plant Equip. -Process Eng. -Biotechnol. 15, 82–90. [Google Scholar]
- 242.Callow ME, Callow JA. 2002. Marine biofouling: a sticky problem. Biologist 49, 1–5. [PubMed] [Google Scholar]
- 243.Wang Z, Pujari SP, van Lagen B, Smulders MM, Zuilhof H. 2016. Highly polymer-repellent yet atomically flat surfaces based on organic monolayers with a single fluorine atom. Adv. Mater. Interfaces 3, 1500514 ( 10.1002/admi.201500514) [DOI] [Google Scholar]
- 244.Smol JP,, Stoermer EF. 2010. The diatoms: applications for the environmental and earth sciences. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 245.Losic D, Mitchell JG, Voelcker NH. 2009. Diatomaceous lessons in nanotechnology and advanced materials. Adv. Mater. 21, 2947–2958. ( 10.1002/adma.200803778) [DOI] [Google Scholar]
- 246.Ibrahim WM, Ali RM, Hemida KA, Sayed MA. 2014. Role of Ulva lactuca extract in alleviation of salinity stress on wheat seedlings. Sci. World J. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Schultz MP. 2007. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 23, 331–341. ( 10.1080/08927010701461974) [DOI] [PubMed] [Google Scholar]
- 248.Schultz MP, Kavanagh CJ, Swain GW. 1999. Hydrodynamic forces on barnacles: implications on detachment from fouling-release surfaces. Biofouling 13, 323–335. ( 10.1080/08927019909378388) [DOI] [Google Scholar]
- 249.Jain A, Bhosle NB. 2009. Biochemical composition of the marine conditioning film: implications for bacterial adhesion. Biofouling 25, 13–19. ( 10.1080/08927010802411969) [DOI] [PubMed] [Google Scholar]
- 250.Cooksey K, Wigglesworth-Cooksey B. 1995. Adhesion of bacteria and diatoms to surfaces in the sea: a review. Aquat. Microb. Ecol. 9, 87–96. ( 10.3354/ame009087) [DOI] [Google Scholar]
- 251.Fletcher RL, Callow ME. 1992. The settlement, attachment and establishment of marine algal spores. Br. Phycol. J. 27, 303–329. ( 10.1080/00071619200650281) [DOI] [Google Scholar]
- 252.Hellio C, De La Broise D, Dufosse L, Le Gal Y, Bourgougnon N. 2001. Inhibition of marine bacteria by extracts of macroalgae: potential use for environmentally friendly antifouling paints. Mar. Environ. Res. 52, 231–247. ( 10.1016/S0141-1136(01)00092-7) [DOI] [PubMed] [Google Scholar]
- 253.Picioreanu C, Van Loosdrecht MC, Heijnen JJ. 2001. Two-dimensional model of biofilm detachment caused by internal stress from liquid flow. Biotechnol. Bioeng. 72, 205–218. ( 10.1002/1097-0290(20000120)72:2%3C205::AID-BIT9%3E3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 254.Pavlovsky L, Younger JG, Solomon MJ. 2013. In situ rheology of Staphylococcus epidermidis bacterial biofilms. Soft Matter 9, 122–131. ( 10.1039/C2SM27005F) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Baier RE. 1970. Surface properties influencing biological adhesion. In Adhesion in biological systems, pp. 15–48. New York, NY: Academic Press. [Google Scholar]
- 256.Ista LK, Callow ME, Finlay JA, Coleman SE, Nolasco AC, Simons RH, Callow JA, Lopez GP. 2004. Effect of substratum surface chemistry and surface energy on attachment of marine bacteria and algal spores. Appl. Environ. Microbiol. 70, 4151–4157. ( 10.1128/AEM.70.7.4151-4157.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Baier RE. 2006. Surface behaviour of biomaterials: the theta surface for biocompatibility. J. Mater. Sci.: Mater. Med. 17, 1057 ( 10.1007/s10856-006-0444-8) [DOI] [PubMed] [Google Scholar]
- 258.Ciriminna R, Bright FV, Pagliaro M. 2015. Ecofriendly antifouling marine coatings. ACS Sustain. Chem. Eng. 3, 559–565. ( 10.1021/sc500845n) [DOI] [Google Scholar]
- 259.Bressy C, Lejars M. 2014. Marine fouling: an overview. J. Ocean Technol. 9, 19–28. [Google Scholar]
- 260.Thorlaksen P, Yebra DM, Catala P. 2010. Hydrogel-based third generation fouling release coatings.
- 261.Higaki Y, Nishida J, Takenaka A, Yoshimatsu R, Kobayashi M, Takahara A. 2015. Versatile inhibition of marine organism settlement by zwitterionic polymer brushes. Polym. J. 47, 811 ( 10.1038/pj.2015.77) [DOI] [Google Scholar]
- 262.Zhang Z, Finlay JA, Wang L, Gao Y, Callow JA, Callow ME, Jiang S. 2009. Polysulfobetaine-grafted surfaces as environmentally benign ultralow fouling marine coatings. Langmuir 25, 13 516–13 521. ( 10.1021/la901957k) [DOI] [PubMed] [Google Scholar]
- 263.Callow JA, Callow ME. 2011. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2, 244 ( 10.1038/ncomms1251) [DOI] [PubMed] [Google Scholar]
- 264.Peter Thorlaksen DMY, Catala P. 2013. Hydrogel based third generation fouling release coatings. World Patent WO 2013000479.
- 265.Ozkan A, Berberoglu H. 2013. Adhesion of algal cells to surfaces. Biofouling 29, 469–482. ( 10.1080/08927014.2013.782397) [DOI] [PubMed] [Google Scholar]
- 266.Krishnan S, et al. 2006. Anti-biofouling properties of comblike block copolymers with amphiphilic side chains. Langmuir 22, 5075–5086. ( 10.1021/la052978l) [DOI] [PubMed] [Google Scholar]
- 267.Bakker DP, Huijs FM, de Vries J, Klijnstra JW, Busscher HJ, van der Mei HC. 2003. Bacterial deposition to fluoridated and non-fluoridated polyurethane coatings with different elastic modulus and surface tension in a parallel plate and a stagnation point flow chamber. Colloids Surf., B 32, 179–190. ( 10.1016/S0927-7765(03)00159-0) [DOI] [Google Scholar]
- 268.Bakker DP, Busscher HJ, van Zanten J, de Vries J, Klijnstra JW, van der Mei HC. 2004. Multiple linear regression analysis of bacterial deposition to polyurethane coatings after conditioning film formation in the marine environment. Microbiology 150, 1779–1784. ( 10.1099/mic.0.26983-0) [DOI] [PubMed] [Google Scholar]
- 269.Guégan C, Garderes J, Le Pennec G, Gaillard F, Fay F, Linossier I, Herry J-M, Fontaine M-NB, Réhel KV. 2014. Alteration of bacterial adhesion induced by the substrate stiffness. Colloids Surf. B 114, 193–200. ( 10.1016/j.colsurfb.2013.10.010) [DOI] [PubMed] [Google Scholar]
- 270.Kolewe KW, Zhu J, Mako NR, Nonnenmann SS, Schiffman JD. 2018. Bacterial adhesion is affected by the thickness and stiffness of poly (ethylene glycol) hydrogels. ACS Appl. Mater. Interfaces 10, 2275–2281. ( 10.1021/acsami.7b12145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zargiel KA, Coogan JS, Swain GW. 2011. Diatom community structure on commercially available ship hull coatings. Biofouling 27, 955–965. ( 10.1080/08927014.2011.618268) [DOI] [PubMed] [Google Scholar]
- 272.Francius G, Tesson B, Dague E, Martin-Jézéquel V, Dufrêne YF. 2008. Nanostructure and nanomechanics of live Phaeodactylum tricornutum morphotypes. Environ. Microbiol. 10, 1344–1356. ( 10.1111/j.1462-2920.2007.01551.x) [DOI] [PubMed] [Google Scholar]
- 273.Higgins MJ, Molino P, Mulvaney P, Wetherbee R. 2003. The structure and nanomechanical properties of the adhesive mucilage that mediates diatom-substratum adhesion and motility 1. J. Phycol. 39, 1181–1193. ( 10.1111/j.0022-3646.2003.03-027.x) [DOI] [Google Scholar]
- 274.Losic D, Short K, Mitchell JG, Lal R, Voelcker NH. 2007. AFM nanoindentations of diatom biosilica surfaces. Langmuir 23, 5014–5021. ( 10.1021/la062666y) [DOI] [PubMed] [Google Scholar]
- 275.Anderson C, Atlar M, Callow M, Candries M, Milne A, Townsin R. 2003. The development of foul-release coatings for seagoing vessels. Proc. Inst. Mar. Eng., Sci. Technol. Part B, J. Mar. Des. Oper. 2003, 11–23. [Google Scholar]
- 276.Higgins MJ, Sader JE, Mulvaney P, Wetherbee R. 2003. Probing the surface of living diatoms with atomic force microscopy: the nanostructure and nanomechanical properties of the mucilage layer 1. J. Phycol. 39, 722–734. ( 10.1046/j.1529-8817.2003.02163.x) [DOI] [Google Scholar]
- 277.Litchman E, Klausmeier C, Yoshiyama K. 2009. Contrasting size evolution in marine and freshwater diatoms. Proc. Natl Acad. Sci. USA 106, 2665–2670 ( 10.1073/pnas.0810891106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Martinelli E, et al. 2008. Nanostructured films of amphiphilic fluorinated block copolymers for fouling release application. Langmuir 24, 13 138–13 147. ( 10.1021/la801991k) [DOI] [PubMed] [Google Scholar]
- 279.Weinman CJ, et al. 2009. ABC triblock surface active block copolymer with grafted ethoxylated fluoroalkyl amphiphilic side chains for marine antifouling/fouling-release applications. Langmuir 25, 12 266–12 274. ( 10.1021/la901654q) [DOI] [PubMed] [Google Scholar]
- 280.van Zoelen W, et al. 2014. Sequence of hydrophobic and hydrophilic residues in amphiphilic polymer coatings affects surface structure and marine antifouling/fouling release properties. ACS Macro Lett. 3, 364–368. ( 10.1021/mz500090n) [DOI] [PubMed] [Google Scholar]
- 281.Gudipati CS, Finlay JA, Callow JA, Callow ME, Wooley KL. 2005. The antifouling and fouling-release performance of hyperbranched fluoropolymer (HBFP)− poly (ethylene glycol)(PEG) composite coatings evaluated by adsorption of biomacromolecules and the green fouling alga Ulva. Langmuir 21, 3044–3053. ( 10.1021/la048015o) [DOI] [PubMed] [Google Scholar]
- 282.Feng S, Wang Q, Gao Y, Huang Y, Qing FL. 2009. Synthesis and characterization of a novel amphiphilic copolymer capable as anti-biofouling coating material. J. Appl. Polym. Sci. 114, 2071–2078. ( 10.1002/app.30779) [DOI] [Google Scholar]
- 283. Hempel. See http://www.hempel.com/en/products/hempaguard-x7-89900 .
- 284.Jacobson AH, Willingham GL. 2000. Sea-nine antifoulant: an environmentally acceptable alternative to organotin antifoulants. Sci. Total Environ. 258, 103–110. ( 10.1016/S0048-9697(00)00511-8) [DOI] [PubMed] [Google Scholar]
- 285.Cima F, Bragadin M, Ballarin L. 2008. Toxic effects of new antifouling compounds on tunicate haemocytes: I. Sea-Nine 211™ and chlorothalonil. Aquat. Toxicol. 86, 299–312. ( 10.1016/j.aquatox.2007.11.010) [DOI] [PubMed] [Google Scholar]
- 286.Lee H, Scherer NF, Messersmith PB. 2006. Single-molecule mechanics of mussel adhesion. Proc. Natl Acad. Sci. USA 103, 12 999–13 003. ( 10.1073/pnas.0605552103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sun Y, Guo S, Walker GC, Kavanagh CJ, Swain GW. 2004. Surface elastic modulus of barnacle adhesive and release characteristics from silicone surfaces. Biofouling 20, 279–289. ( 10.1080/08927010400026383) [DOI] [PubMed] [Google Scholar]
- 288.Wynne K, Swain G, Fox R, Bullock S, Uilk J. 2000. Two silicone nontoxic fouling release coatings: hydrosilation cured PDMS and CaCO3 filled, ethoxysiloxane cured RTV11. Biofouling 16, 277–288. ( 10.1080/08927010009378451) [DOI] [Google Scholar]
- 289.Kaffashi A, Jannesari A, Ranjbar Z. 2012. Silicone fouling-release coatings: effects of the molecular weight of poly(dimethylsiloxane) and tetraethyl orthosilicate on the magnitude of pseudobarnacle adhesion strength. Biofouling 28, 729–741. ( 10.1080/08927014.2012.702342) [DOI] [PubMed] [Google Scholar]
- 290.Schultz M, Finlay J, Callow M, Callow J. 2003. Three models to relate detachment of low form fouling at laboratory and ship scale. Biofouling 19, 17–26. ( 10.1080/0892701031000089516) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.