Abstract
Polyamines (PAs) are low molecular weight aliphatic nitrogenous bases containing two or more amino groups. They are produced by organisms during metabolism and are present in almost all cells. Because they play important roles in diverse plant growth and developmental processes and in environmental stress responses, they are considered as a new kind of plant biostimulant. With the development of molecular biotechnology techniques, there is increasing evidence that PAs, whether applied exogenously or produced endogenously via genetic engineering, can positively affect plant growth, productivity, and stress tolerance. However, it is still not fully understood how PAs regulate plant growth and stress responses. In this review, we attempt to cover these information gaps and provide a comprehensive and critical assessment of the published literature on the relationships between PAs and plant flowering, embryo development, senescence, and responses to several (mainly abiotic) stresses. The aim of this review is to summarize how PAs improve plants' productivity, and to provide a basis for future research on the mechanism of action of PAs in plant growth and development. Future perspectives for PA research are also suggested.
Keywords: polyamines, flowering, embryonic development, senescence, abiotic stress
Introduction
Polyamines (PAs) are low molecular weight aliphatic nitrogenous bases containing two or more amino groups, and they have potent biological activity (Xu et al., 2009; Vuosku et al., 2018). They are widely distributed in eukaryotic and prokaryotic cells (Liu et al., 2017; Mustafavi et al., 2018). In living organisms, PAs mainly exist in free (F-PAs), covalently conjugated (CC-PAs) or non-covalently conjugated (NCC-PAs) forms (Gholami et al., 2013). The CC-PAs can be divided into perchloric acid-soluble covalently conjugated polyamines (PSCC-PAs) and perchloric acid-insoluble covalently conjugated polyamines (PISCC-PAs).
In higher plants, PAs are mainly present in their free form. Putrescine (Put), spermidine (Spd), and spermine (Spm) are the main PAs in plants, and they are involved in the regulation of diverse physiological processes (Xu et al., 2014b; Mustafavi et al., 2018), such as flower development, embryogenesis, organogenesis (Xu, 2015), senescence, and fruit maturation and development. They are also involved in responses to biotic and abiotic stresses (Vuosku et al., 2012; de Oliveira et al., 2016; Reis et al., 2016; Mustafavi et al., 2018).
Free polyamines covalently combine with a small molecular substance, such as a phenolic compound and a derivative thereof in the amide bond to form a binding PA, which is also known as a PSCC-PA. The phenolic compound may be hydroxy cinnamic acid, coumaric acid, caffeic acid, or ferulic acid (Luo et al., 2009; Martin-Tanguy, 2010). This kind of PA forms the largest pools of PAs in plants (Kusano et al., 2008; Bassard et al., 2010). Many studies have confirmed that PSCC-PAs act as secondary metabolites, and participate not only in the local allergic reaction of plants against external infestation (Kumar et al., 1997), but also in plant morphogenesis (de Oliveira et al., 2016; De Oliveira et al., 2018; Mustafavi et al., 2018).
Free polyamines covalently bind to biomacromolecules, such as proteins, nucleic acids, uronic acids, or lignin by ionic and hydrogen bonds to form bound PAs, also known as PISCC-PAs. In the physiological pH range, F-PAs are fully protonated and positively charged, and can electrostatically combine with negatively charged biomacromolecules, such as acidic proteins, membrane phospholipids, and nucleic acids in the organism to become NCC-PAs (Igarashi and Kashiwagi, 2015). The NCC-PAs are associated with the regulation of enzyme activity, DNA replication, gene transcription, cell division and membrane stability, and have a wide range of biological functions in plant growth and development. Generally, the more the amino groups, the stronger the physiological activity.
Recent studies using exogenous PAs, PA synthesis inhibitors, and transgenic methods have intensively investigated the role of PAs in plant development and their mechanism of action. Such studies have shown that PAs are closely associated with plant growth, the stability of nucleic acids and membrane structure, stress resistance, and even plant survival (Agudelo-Romero et al., 2013; Pál et al., 2015; Sequeramutiozabal et al., 2016).
In this review, we provide a comprehensive and critical assessment of the published literature on the relationship between plant PAs and plant growth and development. We summarize recent research on the effects of PAs on the development of plants from flowering to embryonic development to senescence, and explore their roles in the responses to several stresses. The aim of this paper is to reveal the roles that PAs play in plant growth and development and provide a basis for future research on the mechanism of action of PAs in plant growth and development. We also discuss the ways in which exogenous PAs can be used regulate and promote plant growth and development in production.
Distribution and Metabolism of PAs in Plants
PAs Distribution
Polyamines are ubiquitous in eukaryotic and prokaryotic cells (Liu et al., 2016, 2017), and are found even in plant RNA viruses and plant tumors. They have potent biological activities. There are numerous forms of PAs. In higher plants, PAs are predominantly present in their free form. The most common PAs in higher plants are Put, Spd, Spm, thermospermine (Tspm) (Kim et al., 2014; Sobieszczuk-Nowicka, 2017; Takahashi et al., 2017b), and cadaverine (Cad) (Regla-Márquez et al., 2015; Nahar et al., 2016) (Table 1). Other PAs are found only in certain plants or under special conditions.
Table 1.
Polyamine structure and distribution.
| Name | Structure | Molecular formula | Source |
|---|---|---|---|
| Agm | 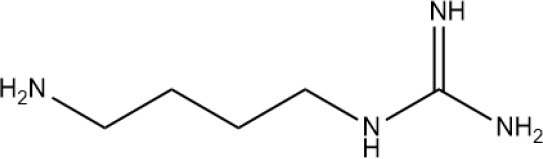 |
C5 H14N4 | ubiquitous |
| Put |  |
C4 H12N2 | Ubiquitous |
| Spd | 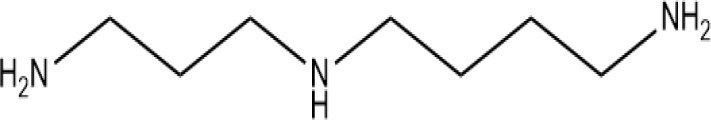 |
C7H19N3 | Ubiquitous |
| Spm | 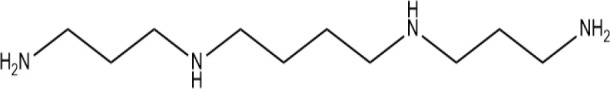 |
C10H26N4 | Ubiquitous |
| Cad | 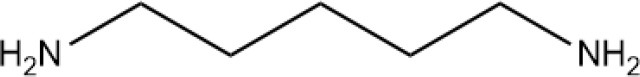 |
C5H14N2 | Legume plants |
| Tspm | 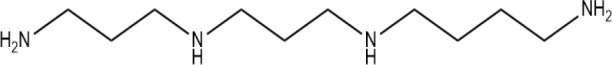 |
C10H26N4 | – |
Polyamines show tissue- and organ-specific distribution patterns in plants. For example, the most abundant PA in leaves was found to be Put, and its levels were three times higher than those of Spd and Spm, whereas Spd was found to be the most abundant PA in other organs (Takahashi et al., 2017b). Different types of PAs also show different localization patterns within cells. In carrot cells, Put was found to accumulate in the cytoplasm, and Spm in the cell wall (Cai et al., 2006). The distribution patterns of PAs may be related to their unique functions. In general, more vigorous plant growth and metabolism is associated with greater PA biosynthesis and higher PA contents (Zhao et al., 2004; Cai et al., 2006).
Polyamine Biosynthesis
Putrescine is the central product of the common PA biosynthetic pathway. It contains two amino groups and is a synthetic precursor of Spd and Spm (Xu et al., 2009). There are three different routes of Put biosynthesis in plants (Figure 1). In the first route, the No. 8 carbon atom is removed from arginine (Arg) by arginine decarboxylase (ADC) to form agmatine (Agm) and CO2; the No. 2 nitrogen atom is removed from Agm to form N-carbamoyl Put (NCPA) and NH3; and then NCPA is hydrolyzed by N-carbamoylputreseine amidohydrolase (NCPAH) and its carbamoyl group is removed to form Put, CO2, and NH3. This is the main Put synthesis pathway in plants (Docimo et al., 2012; Pegg, 2016). In the second route, ornithine (Orn) is produced from Arg by arginase; and then ornithine decarboxylase (ODC) removes the carboxyl group of the no.1 carbon atom of Orn to form Put and CO2 (Docimo et al., 2012; Pegg, 2016). The ODC gene has been lost from Arabidopsis thaliana and many members of the Brassicaceae (Hanfrey et al., 2010), indicating that the ornithine pathway is not essential for normal growth. In the third route, Arg is first converted into citrulline (Cit), which is then decarboxylated by citrulline decarboxylase (CDC) to form Put (Han, 2016; Ouyang et al., 2017; De Oliveira et al., 2018). To date, the Cit pathway has only been found in sesame, and so the first two pathways are more common in plants. The activities of ADC and ODC can be inhibited by the irreversible competitive inhibitors difluoromethylarginine (DFMA) and difluoromethylornithine (DFMO), respectively (Grossi et al., 2016; Yamamoto et al., 2016). Spermidine and Spm are produced from Put and aminopropyl residues, which are gradually provided by methionine (Vuosku et al., 2018) (Figure 1).
Figure 1.
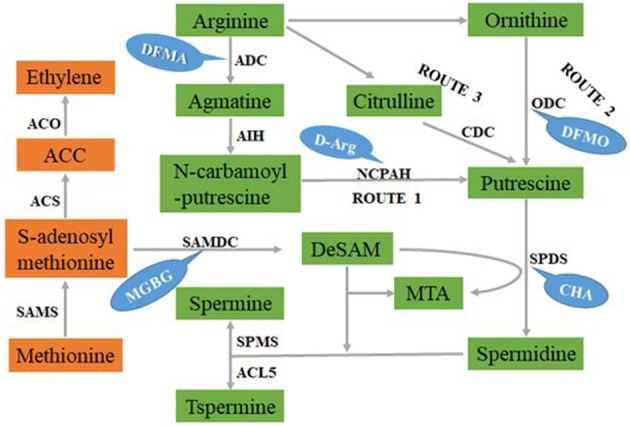
The pathway of PAs biosynthesis in plant. The orange part is the ethylene synthesis pathway, and the green part is the polyamine synthesis pathway (There are three routes of putrescine synthesis, route 1, route 2, and route 3), and the blue part is the corresponding enzyme inhibitor.
Polyamine Catabolism
The catabolism of PAs in plants is mainly dependent on the action of amine oxidases. The known amine oxidases include diamine oxidase (DAO) and PA oxidase (PAO) (Figure 2). Diamine oxidase, which relies on Cu2+ and pyridoxal phosphate as its cofactors, catalyzes the formation of H2O2, ammonia, and 4-aminobutanal from Put. Then, 4-aminobutanal undergoes cyclization to form pyrroline (PYRR), which is converted into γ-aminobutyric acid (GABA) by the action of pyrroline dehydrogenase (PYRR-DH). Then, GABA is further converted into succinate, which enters the Krebs cycle. Dicots contain high contents of DAO, but its encoding gene has been found in only a few species (Cona et al., 2006). Unlike DAO, PAO is linked to flavin adenine dinucleotide (FAD) by non-covalent bonds and is found at high levels in monocots (Takahashi et al., 2017a; Hao et al., 2018). Its substrates are advanced PAs, such as Spd, Spm, and Tspm. There are multiple PAO families in many plants (Liu et al., 2014; Takahashi et al., 2017a). Some PAOs catalyze the production of metabolic end-products of PAs; for example, the wheat PAO oxidizes Spd and Spm to form 4-aminobutanal,3-aminopropyl-4-aminobutanal,1,3-diaminopropane (Dap) and H2O2 (Cona et al., 2006; Liu et al., 2014). Some PAOs catalyze the reverse reaction of PA synthesis in the PA back-conversion pathway (PBCP) (Liu et al., 2014; Takahashi et al., 2017a). Del Duca and Tassoni et al. found that exogenous Spd applied to Helianthus tuberosis and A. thaliana was transformed into Put (Tassoni et al., 2000). In Arabidopsis, PAO1 and PAO4 were able to convert Spm to Spd; and PAO2 and PAO3 catalyzed the production of Spd from Spm and then produced Put (Moschou et al., 2008). The PAO2 of Brachypodium distachyon catalyzed the conversion of Spm or Tspm to Spd, and Spd to Put, with Spd as the preferred substrate. In contrast, BdPAO3 preferentially utilized Spm as the substrate and catalyzed the conversion of tetraamines to Spd (Takahashi et al., 2017a) (Figure 2).
Figure 2.
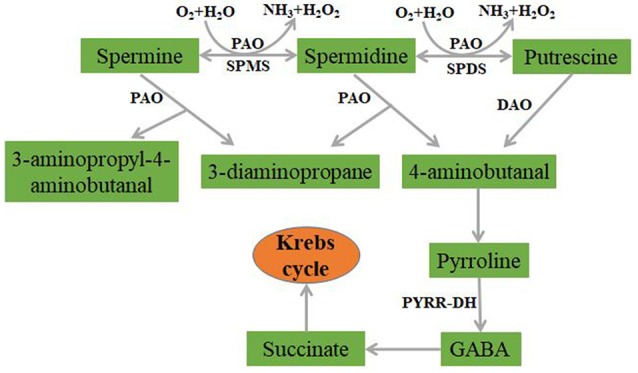
The pathway of polyamine catabolism in plant.
The metabolism of PAs in plants is closely connected to many other metabolic pathways. The H2O2 produced by PA oxidation functions in the signal transduction process of plants during biotic and abiotic stress responses (Freitas et al., 2017; Mellidou et al., 2017), and affects stomatal closure induced by abscisic acid (ABA) (Cona et al., 2006; Tun et al., 2006; An et al., 2008). S-adenosylmethionine (SAM) in the PA biosynthetic pathway is also a precursor for ethylene synthesis (Figure 1), and studies have demonstrated that PAs synthesis competes with ethylene synthesis (Lasanajak et al., 2014). In addition, the metabolism of PAs is related to the production of NO (Pál et al., 2015), which is an essential signaling component for plant growth (Agurla et al., 2017). Therefore, the roles of PAs in plant growth and development and the mechanisms underlying their function can be explored by studying the relationship between PA metabolism and plant hormones, and the effects of PA metabolism on plant signaling substances.
Polyamines and Plant Development
Polyamines and Flowering
After a period of vegetative growth, higher plants enter a period of reproductive growth; that is, leaf bud tissue changes its physiological state to become flower bud tissue, and then develops into a floral organ. This process is called flower bud differentiation (Guo et al., 2015). Flower bud differentiation is a complex process of morphogenesis. It is triggered by various factors, such as photoperiod, vernalization, nutrition, and water status, and is accomplished by the interaction and coordination of hormones and PAs (Xu, 2015).
Polyamines are considered to be a class of growth regulators in plants (Xu et al., 2014b). Many studies have shown that exogenous PAs and PA synthesis inhibitors can affect flower bud differentiation. Exogenous PAs were shown to accelerate the process of flower bud differentiation, and high PA contents in apical buds were beneficial for the initiation and maintenance of flower bud differentiation in Chrysanthemum (Xu, 2015) (Table 3). In Arabidopsis, PAs were found to be more abundant in flowers than in any other organ, and the addition of exogenous PAs to poorly flowering plants significantly promoted their flowering response (Applewhite et al., 2010) (Table 3). The application of Spm (10 ppm) improved flower quality and extended vase life by 3 days in cut rose flowers (Tatte et al., 2015). Lower contents of PAs (mainly Put and Spd) in rapeseed were found to be conducive to the initiation of flower bud differentiation, and an increased PAs content was beneficial for flower bud development. Earlier peaking of PA contents in tissues led to earlier bolting time (Ai et al., 2011). Similar results were observed in Dendrobium nobile, where plants with higher levels of Put and Spd in the leaves had more flower buds, more flowers, and a larger mean floral diameter (Li et al., 2014). The recombinant proteins of GtSPDS and GtSPMS from Gentiana triflora (homologs of two Arabidopsis PA biosynthetic enzymes) had SPDS and SPMS activity, respectively. The expression levels of GtSPDS and GTSPMS transiently increased from the vegetative to the reproductive growth phase, and overexpression of these genes hastened flowering (Applewhite et al., 2010; Imamura et al., 2015) (Table 2).
Table 3.
Effects of polyamines on plant growth and development.
| Plant species | Polyamine treatment | Effect | Outcome | Citation |
|---|---|---|---|---|
| Arabidopsis thaliana | Spd (0.3 or 3 mM) CHA + DFMO (4 mM) | Inhibitors preventing bolting and flowering, exogenous PAs to poorly flowering plants can significantly add to their flowering response | PAs promote flowering | Applewhite et al., 2010 |
| Dendranthema morifolium | Spd (0.1 mM/L) | Significantly affect endogenous polyamines (Spd, Spm) and endogenous hormones (IAA, ZR, IPA, GA) | Accelerate the process of flower bud differentiation | Xu, 2015 |
| Wheat | Spd or Spm (1 mM) | In wheat grains, endogenous Spd, Spm, ABA, and IAA contents ↑, ETH content↓ | Increased the grain filling rate and the grain weight | Liu et al., 2013 |
| Sugarcane | Put (500 μM) | Somatic embryos in embryogenic callus↑ | Induces somatic embryo development | Reis et al., 2016 |
| Seedless grapevine | PAs (0.3–3 mM) | Embryo germination rate↑ | Efficiency of embryo rescue in vitro↑ | Jiao et al., 2017 |
| Indica rice | Put (30 mg/l) | Spm and Spd contents↑, affect the expression levels of ADC1 gene and SAMDC gene | Improve the growing state and the callus embryogenic traits | Tan et al., 2017 |
↓: Indicates a decrease in substance content or enzyme activity; ↑: Indicates an increase in substance content or enzyme activity.
Table 2.
Genes related to polyamines on regulating plant growth.
| Plant species | Gene | Effect | Outcome | Citation |
|---|---|---|---|---|
| Citrus sinensis | CsPAO3 | Overexpression of CsPAO3 in tobacco, Spd and Spm↓, Put↑ | CsPAO3 plays a potential role in PAs back conversion | Wang and Liu, 2015 |
| Gossypium hirsutum L | GhPAO3 | In transgenic Arabidopsis(GhPAO3), Spm↓, Put↑ | GhPAO3 plays a potential role in the conversion of Spd and Spm | Cheng et al., 2017 |
| Transgenic rice | OsSAMDC2 | Transcript levels of OsSAMDC1, OsSAMDC2, and OsSAMDC4 were all reduced in transgenic rice, Spd, Spm, and PAs oxidase activity↓ | Spd and Spm are essential for maintenance of normal plant growth, pollen viability, seed setting rate, grain yield and stress tolerance in rice | Chen et al., 2014 |
| Transgenic tomato | Mouse ODC | Put, Spd and Spm↑, ethylene, respiration rate and physiological loss of water↓ | Enhances fruit quality in tomato | Pandey et al., 2015 |
| Gentiana triflora | GtSPDS or GtSPMS | The expression levels of GtSPDS and GtSPMS increased transiently during vegetative to reproductive growth phase | Hasten flowering | Imamura et al., 2015 |
| Pyrus betulaefolia | PbrMYB21 | Modulate the PAs synthesis by regulating the ADC expression | Plays a positive role in drought tolerance | Li et al., 2017 |
| Medicago falcata | MfERF1 | Up-regulates the genes associated with PAs synthesis and catabolism, promotes PAs turnover, antioxidant protection | Confers cold tolerance | Zhuo et al., 2018 |
↓: Indicates a decrease in substance content or enzyme activity; ↑: Indicates an increase in substance content or enzyme activity.
Applying polyamine synthase inhibitors to the growth medium reduced the Spd content in Arabidopsis, and almost completely inhibited bolting and flowering. When the plants were transferred to medium without inhibitors, bolting and flowering were restored (Applewhite et al., 2010; Xu et al., 2014b; Xu, 2015) (Table 3). However, feeding Spd via the roots under permissive flowering conditions resulted in delayed flowering in Arabidopsis (Applewhite et al., 2010; Ahmed et al., 2017). Overexpression of ADC resulted in Put accumulation in the leaves, and plants showed a dwarf and delayed-flowering phenotype (Ahmed et al., 2017). Endogenous Put was found to be closely related to IAA and gibberellin (GA) contents, and high levels of Put and Spd were not conducive to the accumulation of IAA and GA (Xu, 2015). The effects of exogenous PAs and PA synthesis inhibitors on GA were mainly observed at the inflorescence differentiation and floret differentiation stages (Xu, 2015). Both the dwarf and delayed-flowering phenotypes were alleviated by spraying leaves with GA. Under short-day conditions, exogenous Spd significantly promoted PAO activity and lignin synthesis during flower bud differentiation. D-arginine inhibits flower bud differentiation, and reduces PAO activity and lignin synthesis (Xu et al., 2014a). Lignin is a secondary metabolite in plant growth and development, and it is of great significance in the growth, differentiation, and resistance of plant cells (Smita and Upendranath, 2008).
Polyamines and Embryo Development
Polyamines have typical polycation characteristics. They bind to negatively charged nucleic acids, proteins, and phospholipids by ionic and hydrogen bonds through their amino and imino groups, and participate in zygote polarity establishment, apical axis formation, cell layer differentiation, and establishment of the meristem (Cangahuala-Inocente et al., 2014; Tiburcio et al., 2014). Polyamines are generally regarded as regulators in the process of embryogenesis in both angiosperms and gymnosperms (de Oliveira et al., 2016), and an increase in PAs content is required for embryogenesis. Studies have shown that the normal development of plant embryos requires a well-maintained dynamic balance of PAs in vivo. The types and abundance of PAs vary among different stages of embryonic development, from the multi-cell proembryo, globular, heart-shaped, and torpedo stages to the cotyledon stage (Krasuska et al., 2013). It is possible to regulate nucleic acid synthesis and protein translation in both directions by applying exogenous PAs and PA synthesis inhibitors. This can affect the development of organelles, such as endoplasmic reticulum, plastids, and mitochondria, and the structures of microtubules (Vondráková et al., 2015).
Generally, efficient somatic embryogenesis and the growth of embryos into complete plantlets are closely related to the levels of endogenous hormones, such as IAA, cytokinins (Cyt), ethylene, ABA, and PAs. Many studies have shown that PAs play a vital role in inducing cell division and promoting regeneration in plant tissues and cell cultures (Minocha and Minocha, 1995; Yadav and Rajam, 1997; Vondráková et al., 2015). In general, PAs are more abundant in embryogenic callus and somatic and zygotic immature embryos than in mature and germinating embryos. Putrescine stimulates somatic embryogenesis, and reduced concentrations of Put and Spd result in fewer somatic embryos. In cultured Panax ginseng somatic embryos, the addition of PAs at different concentrations (10–1,000 μm) to induction or regeneration media affected the formation of embryogenic structures. A 5- and 4-fold increase in the number of embryogenic structures was obtained by adding Spd (1,000 μm) to induction and regeneration medium, respectively (Kevers et al., 2000). In the embryogenic suspensor mass (ESM) of Norway spruce, the Put and Spd contents were approximately equal at the early stage of proliferation, but after 4 weeks, the Spd level was significantly higher than the Put level (Vondráková et al., 2015). In a range of hybrid combinations of seedless grapevine, the addition of 3 mM Put, 0.5 mM Spd, or 0.3 mM Spm to the culture medium significantly promoted plantlet development or the embryo germination rate. This indicated that addition of appropriate amounts of PAs to the culture medium could significantly increase the efficiency of in vitro embryo rescue for seedless grapevine (Jiao et al., 2017) (Table 3). A study on embryo development in litchi showed that the contents of Put, Spd, and Spm were higher in normal ovules than in abortive ovules during embryonic development (Chen and Lv, 2000).
Other studies have used PA synthesis inhibitors to explore the roles of PAs in plant embryogenesis. The addition of PA biosynthesis inhibitors (DFMO and DFMA) to induction and regeneration media at all tested concentrations (10–1,000 μm) significantly reduced the number of P. ginseng somatic embryos (Kevers et al., 2000). The concentrations of Spm and Spd were 11 times and 3 times higher, respectively, in embryogenic callus than in non-embryogenic callus of Coffea canephora, but the Put content did not differ significantly between embryogenic callus and non-embryogenic callus. Exogenous PAs resulted in a 58% explant response for embryogenesis, compared with a 42% response in the control. The PA biosynthesis inhibitors DFMO and DFMA caused an 83% decrease in the embryogenic response (Kumar et al., 2008). These results were consistent with those of other studies (Bais and Sudha Gravishankar, 2001).
With the development of molecular biology techniques, genes encoding key enzymes in PA biosynthesis have been successfully isolated and cloned from plants, such as rice, tobacco, and Arabidopsis, and the corresponding mutants have been obtained by T-DNA insertion mutation (Su et al., 2012; Miller-Fleming et al., 2015). Analyses of these genes and signal transduction regulators in wild-type and mutant Arabidopsis revealed that one mutant had a blocked PA signal transduction pathway, which in turn affected cell division and differentiation (Gallois et al., 2013; Molesini et al., 2015).
Polyamines and Plant Senescence
The activities of PA metabolic enzymes and PAs contents change throughout the stages of plant growth. In whole plants, endogenous PAs and PA synthetase activity were found to be highest in the meristem and growing cells, and lowest in senescent tissues. As leaves senescence, the chlorophyll content gradually decreases, and the activities of ADC and ODC decrease, while the activities of PAO and hydrolases, such as ribonuclease and protease increase rapidly. All of these changes can be inhibited by the application of exogenous PAs (Duan, 2000; Cai, 2009) A reduction in PA levels seems to be a significant prelude to senescence signals, or it may be that a decrease in PAs content is the senescence signal (Duan et al., 2006).
Exogenous Spd and Spm treatments can increase the PAs content in cut flowers, and delay their senescence and improve quality (Yang and He, 2001; Cao, 2010). In Anthurium andraeanum, the application of GA3 + Spm by spraying delayed the senescence of cut flowers stored at 20°C, and improved the quality of the inflorescences (Simões et al., 2018). Delayed leaf senescence was found to be associated with a higher Spm level, reduced reactive oxygen species (ROS) production, and increased NO levels (Sobieszczuk-Nowicka, 2017). Polyamines appeared to delay senescence by inhibiting ethylene biosynthesis (Woo et al., 2013; Anwar et al., 2015).
Gerbera flowers sprayed with 0.1 mM Spd or treated with 10 mM Spd in vase water showed delayed senescence, while those sprayed with 1 mM Spd, 10 mM Spd, 0.1 mM Spm, 1 mM Spm, or mixed solution of 0.1 mM each of Put, Spd, Spm showed accelerated senescence, with brown spots and yellowing of the petal rims starting from day 2 of treatment (Bagni and Tassoni, 2006). Legocka and Serafni-Fracassini et al. found that chlorophyll rapidly degraded and Put accumulated during senescence, while the exogenous addition of Spd or Spm inhibited protein degradation and reduced chlorophyll losses (Serafini-Fracassini et al., 2010; Cai et al., 2015). In peony, a PA synthesis inhibitor (0.1 Mm) extended the lifespan and delayed the senescence of cut flowers, while PAs shortened the lifespan and accelerated flower senescence (Han, 2016).
Polyamines and Abiotic Stress Responses
Polyamines and Temperature Stress
There are two major categories of temperature stress; low and high temperature stress. Low temperature stress can be further divided into cold stress and freezing stress. To date, few studies have focused on the physiological functions of PAs in plants under high temperature stress. High temperature stress significantly affected PA synthesis in the leaves of Chinese kale; after 6 days of high temperature treatment, the total PAs and Put contents had increased, but the increases were not sustained over longer treatment times (Yang and Yang, 2002). Under high temperature stress, PAs can promote photosynthesis, and increase the antioxidant capacity and osmotic adjustment ability of plants (Tian, 2012). Antioxidant enzymes can scavenge ROS to prevent membrane lipid peroxidation and stabilize membrane structure (Ouyang et al., 2017). Shao et al. reported that the heat tolerance of alfalfa was related to higher Spd contents and lower Put and Spm contents (Shao et al., 2015). The PAs have many different functions in plants, and the main physiological mechanisms of high temperature tolerance differ among plant species. This explains why the various PAs show different patterns of change in different plant species under high temperature stress (Shao et al., 2015).
Polyamines can bind to the phospholipid site of the cell membrane to prevent cytolysis and improve cold resistance (Li and He, 2012) (Table 2). However, there are several different viewpoints on the relationship between Put and plant chilling stress (Wu and Yuan, 2008). When sweet pepper and zucchini fruits were stored at chilling temperature, the Put content increased exponentially, accompanied by chilling damage. Storage under CO2 modified atmosphere reduced the extent of cold damage and inhibited the accumulation of Put, suggesting that Put accumulated as a result of chilling stress (Serrano et al., 1997, 1998). In contrast, Roy et al. proposed that Put accumulation caused chilling damage, and increased Spm may be a defense response to cold damage. They found that the Put, Spm, and Spd contents gradually increased in loquat fruit stored at low temperatures. The application of exogenous Spm maintained high levels of endogenous Spm and Spd, inhibiting Put accumulation and reducing chilling damage (Zhen et al., 2000; Roy and Wu, 2001). Another opinion was that Put may accumulate as a defense response of plants to chilling damage, because Put accumulation was found to be positively correlated with the cold resistance of plants (Wang et al., 2003b).
Sun et al. studied the effect of Put and D-Arg at different concentrations (0.5, 1.0, 1.5, and 2.0 mmol/L) on the physiological and biochemical indexes of Anthurium andraeanum under chilling stress at 6°C in winter. They found that Put application resulted in increased antioxidant enzyme activities, root activity, nitrogen metabolism, chlorophyll content, and proline content, and a decrease in malondialdehyde content. Treatment with 1.0 mmol/L Put had the strongest effect, and chilling damage was reduced by treatment with D-Arg (Sun et al., 2018b). Similar results were obtained for stevia plants, where PA supplementation increased their tolerance to cold conditions (Peynevandi et al., 2018). When an SPDS cDNA from Cucurbita ficifolia was introduced into Arabidopsis (Kasukabe et al., 2004), the transgenic plants exhibited a significant increase in SPDS activity and Spd content in leaves together with enhanced tolerance to various stresses including chilling and freezing (Groppa and Benavides, 2008). Recent studies have suggested that abiotic stress tolerance is mainly affected by the role of PAs in signal transduction rather than their accumulation (Pál et al., 2015).
PAs and Water Stress
Most studies on the relationship between PAs and water stress have focused on drought resistance (Ebeed et al., 2017), and few have focused on waterlogging resistance. Polyamines (Spm, Spd, and Put) can regulate the size of the potassium channel and the size of pores in the plasma membrane of guard cells, thereby strongly regulating pore opening and closing. In this way, PAs can control water loss in plants (Liu et al., 2000). Many studies have shown that foliar application of Put at an appropriate level can trigger physiological processes and induce the biosynthesis of osmotic adjustment substances, such as free amino acids, soluble sugars, and proline. This may compensate for the negative impacts of drought stress on plant biomass and increase the quality and quantity of certain bioactive substances (Sánchezrodríguez et al., 2016; Mohammadi et al., 2018). In alfalfa, a Put treatment was shown to improve seed germination and increase all growth indexes (hypocotyl length, root and shoot fresh and dry mass) under drought stress caused by different concentrations of polyethylene glycol (PEG 4000), both in vitro and in a pot experiment (Zeid and Shedeed, 2006) (Table 4).
Table 4.
Effects of polyamines on plant abiotic stress.
| Plant species | Stress | Polyamine treatment | Effect | Outcome | Citation |
|---|---|---|---|---|---|
| Alfalfa | PEG (4,000) | Put (0.01 mM) | Germination, polysaccharide, protein, photosynthetic pigment contents and all growth criteria↑ | Reduces the sensitivity of alfalfa to drought stress | Zeid and Shedeed, 2006 |
| Wheat | Drought stress | PAs | Spd and Spm relieve the inhibition caused by drought stress, and Put has the opposite effect | Grain filling and drought resistance↑ | Yang et al., 2016 |
| Agrostis stolonifera | Drought stress | Spm (1 mM) Spd (5 mM) | Turf quality, relative water content, photochemical efficiency and membrane health↑, GA1, GA4, and ABA↑ | Enhance the drought stress tolerance and growth of plant | Krishnan and Merewitz, 2017 |
| Thymus vulgaris L. | Water stress | Put (20 mg/L) | Leaf water content, dry matter and antioxidant enzyme activities↑, cell injury indices↓ | The negative impacts of drought stress on plants ↓ | Mohammadi et al., 2018 |
| Panax ginseng | NaCl (150 mM) | Spd (0.01, 0.1, 1 mM) | Chlorophyll degradation↓, Spd, Spm and the activities of enzyme scavenging system↑ | Enhance salt tolerance | Parvin et al., 2014 |
| Zoysia japonica Steud | Salt stress (200 mM) | Spd (0.3 mM) | Polyamine biosynthetic enzyme levels↑, H2O2 and MDA levels↓ | Improved tolerance to salinity stress | Li et al., 2016 |
| Bakraii citrus seedlings | NaCl (75 mM) | PAs (0.5–1, 0.5 mM Spd best) | The negative effects of salinity stress↓, growth parameters↑ | Improve plant salinity tolerance | Khoshbakht et al., 2017 |
| Cucumis sativus | NaCl (75 mM) | Spd (0.1 mM) | PAs, H2O2, SOD, POD and CAT↑, antioxidant defense↑, oxidative damage↓ | Improve salt tolerance in cucumber seedlings | Wu et al., 2018 |
| Cerasus humilis seedlings | Oxidative stress | Spd or Spm (0.2 mM) | The activities of ADC, ODC, SAMDC and antioxidant systems↑, endogenous free Put, Spd and Spm↑ | Prevent oxidative damage induced by drought | Yin et al., 2014 |
| Muskmelon | Ca(NO3)2 (80 mM) | GABA (50 mM) | The activities of ADC, ODC, SAMDC, PAO and DAO↑, Spd and Spm ↑, Put↓ | Improve muskmelon seedling tolerance to Ca(NO3)2 stress | Hu et al., 2015 |
| Wheat and Sunflower | CdCl2 or CuCl2 (1 mM) | PAs (0.1 mM) | Pevent the deleterious effect caused by Cd and Cu during plant development | Improve tolerance to heavy metal | Benavides et al., 2018 |
↓: Indicates a decrease in substance content or enzyme activity; ↑: Indicates an increase in substance content or enzyme activity.
The Arabidopsis mutant acl5/Spms, which cannot produce Spm, is hypersensitive to high salt and drought. This phenotype was cured by a Spm pretreatment but not by pretreatments with Put and Spd, suggesting that the drought-hypersensitivity of the mutant is due to Spm deficiency (Yamaguchi et al., 2007). A high Spm content and a high ratio of (Spd + Spm)/Put were associated with the drought resistance of mycorrhizal masson pine (Xu et al., 2009). Among the three main endogenous PAs, Spm was most strongly related to drought resistance apple (Liu et al., 2010). Similar results were obtained for cherry tomato (Montesinos-Pereira et al., 2015). However, Yang et al. found that Spd and Spm relieved the inhibitory effects of drought stress and promoted grain filling and drought resistance in wheat, while Put had the opposite effect (Yang et al., 2016) (Table 4).
The above results indicate that the function of PAs can differ among different plants and even different parts of the same plant, whether under osmotic stress or water stress (Sen et al., 2018). Therefore, the response of plants to exogenous PAs under osmotic stress and water stress will depend on the plant species.
PAs and Salt Stress
Salt and drought stress are the two major abiotic stresses in agriculture, and both of them lead to reduced water potential in plants. Salinity is a complex environmental constraint. A high salt concentration reduces membrane integrity, decreases the activity of various enzymes, and impairs the function of the photosynthetic apparatus. Plants adapt to such unfavorable environmental conditions by accumulating low molecular-weight osmolytes, such as proline and PAs. The application of different types and concentrations of exogenous PAs has been shown to alleviate the effects of NaCl stress on various plants, and reduce damage (Verma and Mishra, 2005; Li et al., 2008) (Table 4). Plants rich in PAs usually show strong salt tolerance.
It has been suggested that the level of Spm in plants is an important indicator of salt tolerance (Li and He, 2012). The free, acid-soluble bound, and total Spm contents in leaf tissues of sunflower plants increased under 50, 100, or 150 mM NaCl treatments (Mutlu and Bozcuk, 2005). Exogenous PAs, especially Spm and Spd, resulted in increased reactive oxygen metabolism and photosynthesis, which improved plant growth and reduced the inhibitory effects of salt stress (Meng et al., 2015; Baniasadi et al., 2018). Similar results were obtained in a study on soybean seedlings (Wang and Bo, 2014). Li et al. produced a cucumber line with up-regulated SAMDC expression and down-regulated ADC and ODC expression, resulting in increased accumulation of Spd and Spm and decreased accumulation of Put under salt stress. As a result, the inhibition of plant growth under salt stress was alleviated in the transgenic seedlings (Li et al., 2011; Takahashi et al., 2017b). Several metabolic pathways are affected by Spm and Spd (Paul and Roychoudhury, 2017). Sun et al. showed that PAs and ABA together alleviated salt stress in grape seedlings (Sun et al., 2018a).
Recent studies have explored the relationship between PAs and plant drought resistance by using genetic engineering techniques. Malabika et al. transformed the oat ADC gene into rice, and found that the ADC activity, biological yield, and Put contents were higher in the transgenic rice and its progeny than in non-transgenic rice under NaCl stress (Roy and Wu, 2001). Later, they introduced the SAMDC gene of the durum wheat × barley hybrid Tritordeum into rice. A Southern blot analysis showed that the SAMDC gene was stably integrated. Under NaCl stress, the growth potential of transgenic rice seedlings was better than that of non-transgenic rice, and the contents of Spd and Spm were 3–4 times higher in transgenic lines than in non-transgenic lines (Roy and Wu, 2002). Similarly, micropropagated transgenic Lotus tenuis plants expressing ADC were healthier than wild-type plants under salinity stress and showed better osmotic adjustment (5.8-fold) (Espasandin et al., 2018). An ADC2 deletion mutant of Arabidopsis showed extreme sensitivity to salt stress, which was alleviated by applying exogenous Put (Naka et al., 2010).
PAs and Oxidative Stress
Polyamines play a complex role in plant oxidative stress (Minocha et al., 2014). On one hand, polyamines can increase the activity of various antioxidant enzymes in plants, so that it can effectively regulate oxidative stress in plants caused by various environmental factors. Maize leaves pretreated with Spm and Put showed increased tolerance to oxidative stress induced by paraquat (Durmu and Kadioglu, 2005). Exogenous Spd significantly increased the contents of Spd and Spm and reduced the content of Put in the roots of cucumber seedlings under hypoxia stress. These changes were related to increased antioxidant enzyme activity, enhanced ROS scavenging ability, and less membrane lipid peroxidation, which ultimately led to enhanced hypoxia stress tolerance (Jia et al., 2008; Wu et al., 2018). Under cadmium- and copper-induced oxidative stress, lipid peroxidation in sunflower leaf discs increased, while the activities of glutathione reductase (GR) and superoxide dismutase (SOD) decreased (Groppa et al., 2001). When plants were treated with exogenous PAs (1 mM), Spm treatment reduced the effects of Cd2+ and Cu2+ on lipid peroxidation almost to control values (Tajti et al., 2018). In addition, GR activity was completely restored by Spm or Spd treatments, and SOD activity under Cu2+ treatment was restored by Spm treatment (Table 3).
On the other hand, PAs are a source of reactive oxygen species. Because their catabolism produces the strong oxidizers H2O2 and acrolein, PAs can potentially be the cause of cellular harm under stress conditions (Minocha et al., 2014). However, H2O2 is also a signaling molecule that can enter the stress signal transduction chain and activate an antioxidant defense response (Groppa and Benavides, 2008). Thus, it seems that PAs are regulators of redox homeostasis that play a dual role in plant oxidative stress (Saha et al., 2015).
Other
Plants can also be affected by other stresses, such as acid stress, radiation stress, wounding, heavy metals (Tajti et al., 2018), and diseases and pests (Khajuria and Ohri, 2018). Few studies have focused on these topics, but current data indicate that PAs are important in the responses to these stresses. Exogenous Put was shown to regulate the balance of active oxygen metabolism under acid stress and stabilize membrane system structure, thereby protecting plants from acid stress and improving their acid resistance (Li et al., 1995). Arabidopsis plants subjected to mechanical injury showed increased expression of ADC2 (Perezamador et al., 2002). Similarly, mechanical wounding of the first leaves of oilseed rape led to a significant increase in free Put content in the wounded first leaf and the unwounded second leaf (Cowley and Walters, 2010). Treatment with the heavy metals Hg2+ and Cr6+ led to reduced contents of Spd and Spm and decreased activities of SOD, catalase, and peroxidase in amaranth leaves, leading to excessive accumulation of membrane lipid peroxides (malondialdehyde) and a significant decrease in chlorophyll and soluble protein contents. Exogenous Spd ameliorated these negative effects of Hg2+ and Cr6+ (Wang et al., 2003a; Wang and Shi, 2004).
As well as being involved in abiotic stress responses, PAs are also closely related to biotic stress responses in plants. Plant tissues infected with pathogens accumulate large amounts of PAs, which inhibit the growth of bacteria and viruses and inactivate viruses. When pathogens invade plant cells, they induce PA accumulation and PA oxidase activity; this leads to increased H2O2 content, which prevents pathogens from infecting cells (Yordanova et al., 2003). Overexpression of the ADC gene from trifoliate orange significantly increased resistance to ulcer disease in citrus (Wang, 2009). Similarly, a higher Put content was found to be associated with greater insect resistance in Chinese cabbage (Wang, 2007).
Conclusions and Future Prospects
This paper represents a comprehensive review of the published literature on the relationship between PAs and plant growth, development and stress tolerance. We explored the role of PAs in plant developmental processes ranging from flowering to senescence, and discussed the effects of PAs on plant growth and development. This information provides a reference for the future research on the regulation mechanism of PAs and the use of exogenous PAs to regulate plant growth in production. In recent years, many studies have focused on the relationship between PAs and plant growth and development, but most of them have been relatively simple and similar. Almost all of them have focused the effects of exogenous PAs on the growth and development of fruit or vegetable crops or model plants. However, it is becoming increasingly popular to increase endogenous PA production via genetic manipulation to regulate plant growth. There are still many questions to answer regarding the roles of PAs in regulating plant growth and development. It is still largely unknown how the biosynthetic and catabolic pathways are regulated at the transcriptional, translational, and post-transcriptional levels. Further research is required to uncover the exact mechanism of PA accumulation to improve plant stress resistance. Similarly, there is still much to learn about the metabolic relationship between PAs and other hormones during the growth and development of higher plants, especially the relationship between PAs and ethylene. With the advancement of molecular biology techniques, research is now focusing on events at the molecular level. The contents of intracellular PAs have been modulated by altering the expression of ADC, ODC, and SAMDC. The use of transgenic methods to manipulate PA metabolism has become an effective tool to study the physiological functions of PAs in higher plants. Illuminating the regulation mechanism of PAs at the molecular level should be a major research direction in the future.
Author Contributions
DC read a lot of literatures and wrote the paper. QS provided the writing direction and revised the paper. LY provided some suggestions for the paper. AY and BZ helped in polish the language of this article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81673531), Major Science and Technology Projects of Breeding New Varieties of Agriculture in Zhejiang Province (2016C02058). We thank Jennifer Smith, Ph.D., from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for polishing the English text of this manuscript, and we thank the reviewers for carefully reviewing our manuscript and making many valuable suggestions.
Glossary
Abbreviations
- PAs
polyamines
- F-PAs
free polyamines
- CC-PAs
covalently conjugated polyamines
- NCC-PAs
non-covalently conjugated polyamines
- PSCC-PAs
perchloric acid-soluble covalently conjugated polyamines
- PISCC-PAs
perchloric acid-insoluble covalently conjugated polyamines
- Put
putrescine
- Spd
spermidine
- Spm
spermine
- Tspm
thermospermine
- Cad
cadaverine
- Arg
arginine
- ADC
arginine decarboxylase
- Agm
agmatine
- NCPA
N-carbamoylputrescine
- NCPAH
N-carbamoylputreseine amidohydrolase
- Orn
ornithine
- ODC
ornithine decarboxylase
- Cit
citrulline
- CDC
citrulline decarboxylase
- DFMA
difluoromethylarginine
- DFMO
difluoromethylornithine
- AIH
agmatine iminohydrolase
- SPDS
spermidine synthase
- CHA
clohexylamine, the inhibitor of SPDS
- SPMS
spermine synthase
- ACL5
thermospermine synthase
- SAM
s-adenosylmethionine
- SAMDC
S-adenosylmethionine decarboxylase
- SAMS
S-adenosylmethionine synthase
- ACC
1-amino-1-carboxycyclopropane
- ACS
1-aminocyclopropane-1-carboxylate synthase
- ACO
1-aminocyclopropane-1-carboxylate oxidase
- MGBG
methylglyoxal bis (guanylhy-drazone), the inhibitor of SAMDC
- DeSAM
decarboxylated SAM
- MTA
5′-methyl-thioadenosine
- DAO
diamine oxidase
- PAO
polyamine oxidase
- PYRR
pyrroline
- GABA
γ-aminobutyric acid
- PYRR-DH
pyrroline dehydrogenase
- FAD
flavin adenine dinucleotide
- Dap, 1
3-diaminopropane
- PBCP
polyamines back-conversion pathway
- ABA
abscisic acid
- IAA
auxin
- ETH
ethylene
- GA
gibberellic acid
- Cyt
cytokinins
- ESM
embryogenic suspensor mass
- ROS
reactive oxygen species
- PEG
polyethylene glycol
- CAT
catalase
- SOD
superoxide dismutase
- GR
glutathione reductase.
References
- Agudelo-Romero P., Bortolloti C., Pais M. S., Al E. (2013). Study of polyamines during grape ripening indicate an important role of polyamine catabolism. Plant Physiol. Biochem. 67, 105–119. 10.1016/j.plaphy.2013.02.024 [DOI] [PubMed] [Google Scholar]
- Agurla S., Gayatri G., Raghavendra A. S. (2017). Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 255, 153–162. 10.1007/s00709-017-1139-3 [DOI] [PubMed] [Google Scholar]
- Ahmed S., Ariyaratne M., Patel J., Al E. (2017). Altered expression of polyamine transporters reveals a role for spermidine in the timing of flowering and other developmental response pathways. Plant Sci. 258, 146–155. 10.1016/j.plantsci.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Ai Y., Chen G., Zhou Y. (2011). The study on polyamine metabolism in leaves during flower formation in the early-maturing mutant of Brassica Napus L. Chin. Agric. Sci. Bull. 27, 101–105. [Google Scholar]
- An Z., Jing W., Liu Y., Zhang W. (2008). Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 59, 815–825. 10.1093/jxb/erm370 [DOI] [PubMed] [Google Scholar]
- Anwar R., Mattoo A. K., Handa A. K. (2015). Polyamine interactions with plant hormones: crosstalk at several levels. Int. J. Hydrogen Energy 38, 1039–1051. 10.1007/978-4-431-55212-3_22 [DOI] [Google Scholar]
- Applewhite P. B., Kaur-Sawhney R., Galston A. W. (2010). A role for spermidine in the bolting and flowering of Arabidopsis. Physiol. Plant 108, 314–320. 10.1034/j.1399-3054.2000.108003314.x [DOI] [Google Scholar]
- Bagni N., Tassoni A. (2006). The role of polyamines in relation to flower senescence. Floricult. Ornament. Plant Biotechnol. 1536, 855–856. [Google Scholar]
- Bais H. P., Sudha Gravishankar G. A. (2001). Influence of putrescine, silver nitrate and polyamine inhibitors on the morphogenetic response in untransformed and transformed tissues of Cichorium intybus and their regenerants. Plant Cell Rep. 20, 547–555. 10.1007/s002990100367 [DOI] [Google Scholar]
- Baniasadi F., Saffari V. R., Moud A. A. M. (2018). Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci. Horticult. 234, 312–317. 10.1016/j.scienta.2018.02.069 [DOI] [Google Scholar]
- Bassard J. E., Ullmann P., Bernier F., Al E. (2010). Phenolamides: bridging polyamines to the phenolic metabolism. Phytochemistry 71, 1808–1824. 10.1016/j.phytochem.2010.08.003 [DOI] [PubMed] [Google Scholar]
- Benavides M. P., Groppa M. D., Recalde L., Verstraeten S. V. (2018). Effects of polyamines on cadmium- and copper-mediated alterations in wheat (Triticum aestivum L) and sunflower (Helianthus annuus L) seedling membrane fluidity. Arch. Biochem. Biophys. 654, 27–39. 10.1016/j.abb.2018.07.008 [DOI] [PubMed] [Google Scholar]
- Cai G., Sobieszczuknowicka E., Aloisi I., Al E. (2015). Polyamines are common players in different facets of plant programmed cell death. Amino Acids 47, 27–44. 10.1007/s00726-014-1865-1 [DOI] [PubMed] [Google Scholar]
- Cai Q. (2009). Progress in physiology of plant polyamines. Fujian Sci. Technol. Rice Wheat 27, 37–40. 10.3969/j.issn.1008-9799.2009.01.020 [DOI] [Google Scholar]
- Cai Q., Zhang J., Guo C., Al E. (2006). Reviews of the physiological roles and molecular biology of polyamines in higher plants. J. Fujian Educ. Coll. 7, 118–124. 10.3969/j.issn.1673-9884.2006.10.039 [DOI] [Google Scholar]
- Cangahuala-Inocente G. C., Silveira V., Caprestano C. A., Al E. (2014). Dynamics of physiological and biochemical changes during somatic embryogenesis of Acca sellowiana. Vitro Cell. Deve. Biol. 50, 166–175. 10.1007/s11627-013-9563-3 [DOI] [Google Scholar]
- Cao D. (2010). Effects of Polyamines on Seed Quality and Germination of Super Sweet Corn Seeds during Development. Hangzhou: Zhejiang University. [Google Scholar]
- Chen M., Chen J., Fang J., Al E. (2014). Down-regulation of S -adenosylmethionine decarboxylase genes results in reduced plant length, pollen viability, and abiotic stress tolerance. Plant Cell Tissue Organ Cult. 116, 311–322. 10.1007/s11240-013-0405-0 [DOI] [Google Scholar]
- Chen W., Lv L. (2000). Changes in polyamine content in relation to embryo development in Litchi Ovules. J. Trop. Subtrop. Bot. 8, 229–234. 10.3969/j.issn.1005-3395.2000.03.008 [DOI] [Google Scholar]
- Cheng X. Q., Zhu X. F., Tian W. G., Al E. (2017). Genome-wide identification and expression analysis of polyamine oxidase genes in upland cotton (Gossypium hirsutum L.). Plant Cell Tissue Organ Cult. 129, 237–249. 10.1007/s11240-017-1172-0 [DOI] [Google Scholar]
- Cona A., Rea G., Angelini R., Al E. (2006). Functions of amine oxidases in plant development and defence. Trends Plant Sci. 11, 80–88. 10.1016/j.tplants.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Cowley T., Walters D. R. (2010). Local and systemic changes in arginine decarboxylase activity, putrescine levels and putrescine catabolism in wounded oilseed rape. N. Phytol. 165, 807–811. 10.1111/j.1469-8137.2004.01280.x [DOI] [PubMed] [Google Scholar]
- de Oliveira L. F., Elbl P., Navarro B. V., Al E. (2016). Elucidation of the polyamine biosynthesis pathway during Brazilian pine (Araucaria angustifolia) seed development. Tree Physiol. 37, 116–130. 10.1093/treephys/tpw107 [DOI] [PubMed] [Google Scholar]
- De Oliveira L. F., Navarro B. V., Cerruti G., Al E. (2018). Polyamines and amino acid related metabolism: the roles of arginine and ornithine are associated with the embryogenic potential. Plant Cell Physiol. 59, 1084–1098. 10.1093/pcp/pcy049 [DOI] [PubMed] [Google Scholar]
- Docimo T., Reichelt M., Schneider B., Al E. (2012). The first step in the biosynthesis of cocaine in Erythroxylum coca: the characterization of arginine and ornithine decarboxylases. Plant Mol. Biol. 78, 599–615. 10.1007/s11103-012-9886-1 [DOI] [PubMed] [Google Scholar]
- Duan G. (2000). Effect of speridine on protein contents and protease during senescence of fxicsed wheat leaves. J. Sichuan Teach. Coll. 21, 44–47. 10.3969/j.issn.1673-5072.2000.01.009 [DOI] [Google Scholar]
- Duan G., Huang Z., Lin H. (2006). The role of polyamines in the ontogeny of higher plants. Acta Agric. Boreali Occidentalis Sinica 15, 190–194. 10.7606/j.issn.1004-1389.2006.02.050 [DOI] [Google Scholar]
- Durmu N., Kadioglu A. (2005). Spermine and putrescine enhance oxidative stress tolerance in maize leaves. Acta Physiol. Plant. 27, 515–522. 10.1007/s11738-005-0057-8 [DOI] [Google Scholar]
- Ebeed H. T., Hassan N. M., Aljarani A. M. (2017). Exogenous applications of Polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 118, 438–448. 10.1016/j.plaphy.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Espasandin F. D., Calzadilla P. I., Maiale S. J., Al E. (2018). Overexpression of the arginine decarboxylase gene improves tolerance to salt stress in Lotus tenuis plants. J. Plant Growth Regul. 37, 156–165. 10.1007/s00344-017-9713-7 [DOI] [Google Scholar]
- Freitas V. S., Miranda R. D. S., Costa J. H., Al E. (2017). Ethylene triggers salt tolerance in maize genotypes by modulating polyamine catabolism enzymes associated with H2O2 production. Environ. Exp. Bot. 145, 75–86. 10.1016/j.envexpbot.2017.10.022 [DOI] [Google Scholar]
- Gallois J. L., Drouaud J., Lécureuil A., Al E. (2013). Functional characterization of the plant ubiquitin regulatory X (UBX) domain-containing protein AtPUX7 in Arabidopsis thaliana. Gene 526, 299–308. 10.1016/j.gene.2013.05.056 [DOI] [PubMed] [Google Scholar]
- Gholami M., Fakhari A. R., Ghanati F. (2013). Selective regulation of nicotine and polyamines biosynthesis in tobacco cells by enantiomers of ornithine. Chirality 25, 22–27. 10.1002/chir.22107 [DOI] [PubMed] [Google Scholar]
- Groppa M. D., Benavides M. P. (2008). Polyamines and abiotic stress: recent advances. Amino Acids 34, 35–45. 10.1007/s00726-007-0501-8 [DOI] [PubMed] [Google Scholar]
- Groppa M. D., Tomaro M. L., Benavides M. P. (2001). Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci. 161, 481–488. 10.1016/S0168-9452(01)00432-0 [DOI] [Google Scholar]
- Grossi M., Phanstiel O., Rippe C., Al E. (2016). Inhibition of polyamine uptake potentiates the anti-proliferative effect of polyamine synthesis inhibition and preserves the contractile phenotype of vascular smooth muscle cells. J. Cell. Physiol. 231, 1334–1342. 10.1002/jcp.25236 [DOI] [PubMed] [Google Scholar]
- Guo J., Tian L., Sun X. Z., Al E. (2015). Relationship between endogenous polyamines and floral bud differentiation in Chrysanthemum morifolium under short-day conditions. Wonye kwahak kisulchi 33, 31–38. 10.7235/hort.2015.14043 [DOI] [Google Scholar]
- Han L. (2016). Studies on Mechanism of Low Temperature Storage and Polyamine Impact in Cut Flowers of Herbaceous Peony Postharvest Senescence. Shandong: Shandong Agricultural University. [Google Scholar]
- Hanfrey C., Sommer S., Mayer M. J., Al E. (2010). Arabidopsis polyamine biosynthesis: absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant J. 27, 551–560. 10.1046/j.1365-313X.2001.01100.x [DOI] [PubMed] [Google Scholar]
- Hao Y., Huang B., Jia D., Al E. (2018). Identification of seven polyamine oxidase genes in tomato (Solanum lycopersicum L.) and their expression profiles under physiological and various stress conditions. J. Plant Physiol. 228, 1–11. 10.1016/j.jplph.2018.05.004 [DOI] [PubMed] [Google Scholar]
- Hu X., Xu Z., Xu W., Al E. (2015). Application of γ-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca(NO3)2 stress. Plant Physiol. Biochem. 92, 1–10. 10.1016/j.plaphy.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K. (2015). Modulation of protein synthesis by polyamines. Iubmb Life 67, 160–169. 10.1002/iub.1363 [DOI] [PubMed] [Google Scholar]
- Imamura T., Fujita K., Tasaki K., Al E. (2015). Characterization of spermidine synthase and spermine synthase–The polyamine-synthetic enzymes that induce early flowering in Gentiana triflora. Biochem. Biophys. Res. Commun. 463, 781–786. 10.1016/j.bbrc.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Jia Y., Guo S., Li J. (2008). Effects of exogenous putrescine on polyamines and antioxidant system in cucumber seedlings under root-zone hypoxia Stress. Acta Bot. Boreali Occidentalia Sinica 28, 1654–1662. [Google Scholar]
- Jiao Y., Li Z., Xu K., Al E. (2017). Study on improving plantlet development and embryo germination rates in in vitro embryo rescue of seedless grapevine. N. Z. J. Crop Horticult. Sci. 46, 39–53. 10.1080/01140671.2017.1338301 [DOI] [Google Scholar]
- Kasukabe Y., He L., Nada K., Al E. (2004). Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 45, 712–722. 10.1093/pcp/pch083 [DOI] [PubMed] [Google Scholar]
- Kevers C., Gal N. L., Monteiro M., Al E. (2000). Somatic embryogenesis of Panax ginseng in liquid cultures: a role for polyamines and their metabolic pathways. Plant Growth Regul. 31, 209–214. 10.1023/A:1006344316683 [DOI] [Google Scholar]
- Khajuria A., Ohri P. (2018). Exogenously applied putrescine improves the physiological responses of tomato plant during nematode pathogenesis. Sci. Hortic. 230, 35–42. 10.1016/j.scienta.2017.11.021 [DOI] [Google Scholar]
- Khoshbakht D., Asghari M. R., Haghighi M. (2017). Influence of foliar application of polyamines on growth, gas exchange characteristics, and chlorophyll fluorescence in Bakraii citrus under saline conditions. Photosynthetica 56, 731–742. 10.1007/s11099-017-0723-2 [DOI] [Google Scholar]
- Kim D. W., Watanabe K., Murayama C., Al E. (2014). Polyamine oxidase5 regulates Arabidopsis growth through thermospermine oxidase activity. Plant Physiol. 165, 1575–1590. 10.1104/pp.114.242610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasuska U., Ciacka K., Bogatek R., Al E. (2013). Polyamines and nitric oxide link in regulation of dormancy removal and germination of Apple (Malus domestica Borkh.) embryos. J. Plant Growth Regul. 33, 590–601. 10.1007/s00344-013-9408-7 [DOI] [Google Scholar]
- Krishnan S., Merewitz E. B. (2017). Polyamine Application effects on gibberellic acid content in creeping bentgrass during drought stress. J. Am. Soc. Horticult. Sci. 142, 135–142. 10.21273/JASHS03991-16 [DOI] [Google Scholar]
- Kumar A., Taylor M., Altabella T., Al E. (1997). Recent advances in polyamine research. Trends Plant Sci. 2, 124–130. 10.1016/S1360-1385(97)01013-3 [DOI] [Google Scholar]
- Kumar V., Giridhar P., Chandrashekar A., Al E. (2008). Polyamines influence morphogenesis and caffeine biosynthesis in vitro cultures of Coffea canephora P. Acta Physiol. Plant. 30, 217–223. 10.1007/s11738-007-0110-x [DOI] [Google Scholar]
- Kusano T., Berberich T., Tateda C., Al E. (2008). Polyamines: essential factors for growth and survival. Planta 228, 367–381. 10.1007/s00425-008-0772-7 [DOI] [PubMed] [Google Scholar]
- Lasanajak Y., Minocha R., Minocha S. C., Al E. (2014). Enhanced flux of substrates into polyamine biosynthesis but not ethylene in tomato fruit engineered with yeast S-adenosylmethionine decarboxylase gene. Amino Acids 46, 729–742. 10.1007/s00726-013-1624-8 [DOI] [PubMed] [Google Scholar]
- Li B., Guo S., Sun J., Al E. (2011). Effects of exogenous spermidine on free polyamine content and polyamine biosynthesis gene expression in cucumber seedlings under salt Stress. Plant Sci. J. 29, 480–485. [Google Scholar]
- Li C., Pei Z., Gan L. (2014). Effects of photoperiod on flowering and polyamine contents of nobile-type Dendrobium. Plant Physiol. J. 1167–1170. 10.13592/j.cnki.ppj.2012.0435 [DOI] [Google Scholar]
- Li K., Xing C., Yao Z., Al E. (2017). PbrMYB21, a novel MYB protein of Pyrus betulaefolia, functions in drought tolerance and modulates polyamine levels by regulating arginine decarboxylase gene. Plant Biotechnol. J. 15, 1186–1203. 10.1111/pbi.12708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Ge H., Hu S. (2008). Effects of exogenous putrescine and calciumon ion uptake of strawberry seedling under NaCI stress. J. Plant Nutr. Fertil. 14, 540–545. 10.3724/SP.J.1005.2008.01083 [DOI] [Google Scholar]
- Li R., Shen H., Li M. (1995). Effects of acid stress on the contents of proline and putrescine in several forest trees. J. Nanjing Forestry Univ. 9, 88–93. [Google Scholar]
- Li S., Jin H., Zhang Q. (2016). The effect of exogenous spermidine concentration on polyamine metabolism and salt tolerance inzoysiagrass (Zoysia japonica Steud) subjected to short-term salinity stress. Front. Plant Sci. 7:1221. 10.3389/fpls.2016.01221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., He J. (2012). Advance in metabolism and response to stress of polyamines in plant. Acta Agric. Boreali Sinica 27, 240–245. 10.3969/j.issn.1000-7091.2012.z1.048 [DOI] [Google Scholar]
- Liu K., Fu H., Bei Q., Al E. (2000). Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 124, 1315–1325. 10.1104/pp.124.3.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Nishibori N., Imai I., Al E. (2016). Response of polyamine pools in marine phytoplankton to nutrient limitation and variation in temperature and salinity. Mar. Ecol. Prog. 544, 93–105. 10.3354/meps11583 [DOI] [Google Scholar]
- Liu T., Kim D. W., Niitsu M., Al E. (2014). Polyamine oxidase 7 is a terminal catabolism-type enzyme in Oryza sativa and is specifically expressed in anthers. Plant Cell Physiol. 55, 1110–1122. 10.1093/pcp/pcu047 [DOI] [PubMed] [Google Scholar]
- Liu W., Tan M., Zhang C., Al E. (2017). Functional characterization of murB-potABCD operon for polyamine uptake and peptidoglycan synthesis in Streptococcus suis. Microbiol. Res. 207, 177–187. 10.1016/j.micres.2017.11.008 [DOI] [PubMed] [Google Scholar]
- Liu Y., Gu D., Wu W., Al E. (2013). The relationship between polyamines and hormones in the regulation of wheat grain. Filling 8:e78196. 10.1371/journal.pone.0078196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zuo Z., Hu J. (2010). Effects of exogenous polyamines on growth and drought resistance of Apple seedlings. J. Northwest Forestry Univ. 25, 000039–000042. [Google Scholar]
- Luo J., Fuell C., Parr A., Al E. (2009). A novel polyamine acyltransferase responsible for the accumulation of spermidine conjugates in Arabidopsis seed. Plant Cell 21, 318–333. 10.1105/tpc.108.063511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Tanguy J. (2010). Conjugated polyamines and reproductive development: biochemical, molecular and physiological approaches. Physiol. Plant 100, 675–688. 10.1111/j.1399-3054.1997.tb03074.x [DOI] [Google Scholar]
- Mellidou I., Karamanoli K., Beris D., Al E. (2017). Underexpression of apoplastic polyamine oxidase improves thermotolerance in Nicotiana tabacum. J. Plant Physiol. 218, 171–174. 10.1016/j.jplph.2017.08.006 [DOI] [PubMed] [Google Scholar]
- Meng D., Hou L., Yang S. (2015). Exogenous polyamines alleviating salt stress on peanuts (Arachis hypogaea) grown in pots. Chin. J. Plant Ecol. 39, 1209–1215. 10.17521/cjpe.2015.0117 [DOI] [Google Scholar]
- Miller-Fleming L., Olin-Sandoval V., Campbell K., Al E. (2015). Remaining mysteries of molecular biology: the role of polyamines in the cell. J. Mol. Biol. 427, 3389–3406. 10.1016/j.jmb.2015.06.020 [DOI] [PubMed] [Google Scholar]
- Minocha R., Majumdar R., Minocha S. C. (2014). Polyamines and abiotic stress in plants: a complex relationship. Front. Plant Sci. 5:175. 10.3389/fpls.2014.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha S. C., Minocha R. (1995). Role of Polyamines in Somatic Embryogenesis. Berlin; Heidelberg: Springer; 10.1007/978-3-662-03091-2_5 [DOI] [Google Scholar]
- Mohammadi H., Ghorbanpour M., Brestic M. (2018). Exogenous putrescine changes redox regulations and essential oil constituents in field-grown Thymus vulgaris L. under well-watered and drought stress conditions. Ind. Crops Prod. 122, 119–132. 10.1016/j.indcrop.2018.05.064 [DOI] [Google Scholar]
- Molesini B., Mennella G., Martini F., Al E. (2015). Involvement of the putative N-acetylornithine deacetylase from Arabidopsis thaliana in flowering and fruit development. Plant Cell Physiol. 56, 1084–1096. 10.1093/pcp/pcv030 [DOI] [PubMed] [Google Scholar]
- Montesinos-Pereira D., Barrameda-Medina Y., Romero L., Ruiz J., M Sánchez-Rodríguez, E. (2015). Genotype differences in the metabolism of proline and polyamines under moderate drought in tomato plants. Plant Biol. 16, 1050–1057. 10.1111/plb.12178 [DOI] [PubMed] [Google Scholar]
- Moschou P. N., Paschalidis K. A., Roubelakis-Angelakis K. A. (2008). Plant polyamine catabolism. Plant Signal. Behav. 3, 1061–1066. 10.4161/psb.3.12.7172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafavi S. H., Badi H. N., Sekara A., Al E. (2018). Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol. Plant. 40, 102 10.1007/s11738-018-2671-2 [DOI] [Google Scholar]
- Mutlu F., Bozcuk S. (2005). Effects of salinity on the contents of polyamines and some other compounds in sunflower plants differing in salt tolerance. Russian J. Plant Physiol. 52, 29–34. 10.1007/s11183-005-0005-x [DOI] [Google Scholar]
- Nahar K., Hasanuzzaman M., Rahman A., Al E. (2016). Polyamines confer salt tolerance in Mung Bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front. Plant Sci. 7:1104. 10.3389/fpls.2016.01104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka Y., Watanabe K., Sagor G. H. M., Al E. (2010). Quantitative analysis of plant polyamines including thermospermine during growth and salinity stress. Plant Physiol. Biochem. 48, 527–533. 10.1016/j.plaphy.2010.01.013 [DOI] [PubMed] [Google Scholar]
- Ouyang J., Song C., Chen D. (2017). Research progress on heat-tolerance mechanism and transports of polyamfines in plant. Mol. Plant Breed. 15, 3286–3294. 10.13271/j.mpb.015.003286 [DOI] [Google Scholar]
- Pál M., Szalai G., Janda T. (2015). Speculation: polyamines are important in abiotic stress signaling. Plant Sci. 237, 16–23. 10.1016/j.plantsci.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Pandey R., Gupta A., Chowdhary A., Al E. (2015). Over-expression of mouse ornithine decarboxylase gene under the control of fruit-specific promoter enhances fruit quality in tomato. Plant Mol. Biol. 87, 249–260. 10.1007/s11103-014-0273-y [DOI] [PubMed] [Google Scholar]
- Parvin S., Lee O. R., Sathiyaraj G., Al E. (2014). Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Gene 537, 70–78. 10.1016/j.gene.2013.12.021 [DOI] [PubMed] [Google Scholar]
- Paul S., Roychoudhury A. (2017). Seed priming with spermine and spermidine regulates the expression of diverse groups of abiotic stress-responsive genes during salinity stress in the seedlings of indica rice varieties. Plant Gene 11, 124–132. 10.1016/j.plgene.2017.04.004 [DOI] [Google Scholar]
- Pegg A. E. (2016). Functions of polyamines in mammals. J. Biol. Chem. 291, 14904–14912. 10.1074/jbc.R116.731661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perezamador M. A., Leon J., Green P. J., Al E. (2002). Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol. 130, 1454–1463. 10.1104/pp.009951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peynevandi K. M., Razavi S. M., Zahri S. (2018). The ameliorating effects of polyamine supplement on physiological and biochemical parameters of Stevia rebaudiana Bertoni under cold stress. Plant Prod. Sci. 21, 123–131. 10.1080/1343943X.2018.1437756 [DOI] [Google Scholar]
- Regla-Márquez C. F., Canto-Flick A., Avilés-Viñas S. A., Al E. (2015). Cadaverine: a common polyamine in zygotic embryos and somatic embryos of the species Capsicum chinense Jacq. PCTOC 124, 253–264. 10.1007/s11240-015-0889-x [DOI] [Google Scholar]
- Reis R. S., Vale E. M., Heringer A. S., Al E. (2016). Putrescine induces somatic embryo development and proteomic changes in embryogenic callus of sugarcane. J. Proteomics 130, 170–179. 10.1016/j.jprot.2015.09.029 [DOI] [PubMed] [Google Scholar]
- Roy M., Wu R. (2001). Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci. 160, 869–875. 10.1016/S0168-9452(01)00337-5 [DOI] [PubMed] [Google Scholar]
- Roy M., Wu R. (2002). Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci. 163, 987–992. 10.1016/S0168-9452(02)00272-8 [DOI] [Google Scholar]
- Saha J., Brauer E. K., Sengupta A., Al E. (2015). Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 3:21 10.3389/fenvs.2015.00021 [DOI] [Google Scholar]
- Sánchezrodríguez E., Romero L., Ruiz J. M. (2016). Accumulation of free polyamines enhances the antioxidant response in fruits of grafted tomato plants under water stress. J. Plant Physiol. 190, 72–78. 10.1016/j.jplph.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Sen S., Ghosh D., Mohapatra S. (2018). Modulation of polyamine biosynthesis in Arabidopsis thaliana by a drought mitigating Pseudomonas putida strain. Plant Physiol. Biochem. 129, 180–188. 10.1016/j.plaphy.2018.05.034 [DOI] [PubMed] [Google Scholar]
- Sequeramutiozabal M. I., Erban A., Kopka J., Al E. (2016). Global metabolic profiling of Arabidopsis polyamine oxidase 4 (AtPAO4) loss-of-function mutants exhibiting delayed dark-induced senescence. Front. Plant Sci. 7:173 10.3389/fpls.2016.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini D., Sandro A. D., Duca S. D. (2010). Spermine delays leaf senescence in Lactuca sativa and prevents the decay of chloroplast photosystems. Plant Physiol. Biochem. 48, 602–611. 10.1016/j.plaphy.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Serrano M., Mc M.-M., Mt P. (1997). Modified atmosphere packaging minimizes increases in putrescine and abscisic acid levels caused by chilling injury in pepper fruit. J. Agric. Food Chem. 45, 1668–1672. 10.1021/jf960866h [DOI] [Google Scholar]
- Serrano M., Pretel M. T., Martinezmadrid M. C. (1998). CO2 treatment of zucchini squash reduces chilling-induced physiological changes. J. Agric. Food Chem. 46, 2465–2468. 10.1021/jf970864c [DOI] [Google Scholar]
- Shao C. G., Wang H., Yu-Fen B. I. (2015). Relationship between endogenous polyamines and tolerance in Medicago sativa L.under heat stress. Acta Agrestia Sinica. 23, 1214–1219. 10.11733/j.issn.1007-0435 [DOI] [Google Scholar]
- Simões A. D. N., Diniz N. B., Vieira M. R. D. S., Al E. (2018). Impact of GA3 and spermine on postharvest quality of anthurium cut flowers (Anthurium andraeanum) cv. Arizona. Sci. Horticult. 241, 178–186. 10.1016/j.scienta.2018.06.095 [DOI] [Google Scholar]
- Smita R., Upendranath D. (2008). Manipulation of lignin in plants with special reference to O-methyltransferase. Plant Sci. 174, 264–277. 10.1016/j.plantsci.2007.11.014 [DOI] [Google Scholar]
- Sobieszczuk-Nowicka E. (2017). Polyamine catabolism adds fuel to leaf senescence. Amino Acids 49, 49–56. 10.1007/s00726-016-2377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. J., Wi S. J., Choi Y. J., Al E. (2012). Increased polyamine biosynthesis enhances stress tolerance by preventing the accumulation of reactive oxygen species: T-DNA mutational analysis of oryza sativa lysine decarboxylase-like Protein 1. Molecules Cells 34, 251–262. 10.1007/s10059-012-0067-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Yu S., Zhao F. (2018a). Effects of salt stress on polyamines and hormone metabolism in Grape seedlings. Xinjiang Agric. Sci. 55, 66–73. 10.6048/j.issn.1001-4330 [DOI] [Google Scholar]
- Sun X., Wang Y., Tan J., Al E. (2018b). Effects of exogenous putrescine and D-Arg on physiological and biochemical indices of anthurium under chilling stress. Jiangsu J. Agric. Sci. 34, 152–157 10.3969/j.issn.1000-4440.2018.01.022 [DOI] [Google Scholar]
- Tajti J., Janda T., Majláth I., Szalai G., Pál M. (2018). Comparative study on the effects of putrescine and spermidine pre-treatment on cadmium stress in wheat. Ecotoxicol. Environ. Safety 148, 546–554. 10.1016/j.ecoenv.2017.10.068 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Ono K., Akamine Y., Asano T., Ezaki M., Mouri I. (2017a). Highly-expressed polyamine oxidases catalyze polyamine back conversion in Brachypodium distachyon. J. Plant Res. 131, 341–348. 10.1007/s10265-017-0989-2 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Tahara M., Yamada Y., Al E. (2017b). Characterization of the polyamine biosynthetic pathways and salt stress response in Brachypodium distachyon. J. Plant Growth Regul. 37, 625–634. 10.1007/s00344-017-9761-z [DOI] [Google Scholar]
- Tan Y., Hu W., Xu X., Al E. (2017). Polyamine plays a role in subculture growth of in vitro callus of indica rice. Acta Biol. Cracoviensia S Botanica 59, 105–112. 10.1515/abcsb-2017-0001 [DOI] [Google Scholar]
- Tassoni A., Buuren M. V., Franceschetti M., Al E. (2000). Polyamine content and metabolism in Arabidopsis thaliana and effect of spermidine on plant development. Plant Physiol. Biochem. 38, 383–393. 10.1016/S0981-9428(00)00757-9 [DOI] [Google Scholar]
- Tatte S., Alka, S.ingh, Ahlawat T. R. (2015). Effect of PAs on postharvest quality and vaselife of rose var. Samurai The Bioscan 10, 675–678. [Google Scholar]
- Tian J. (2012). Physiological regulation function and proteomics research of exogenous spermidine on alleviating high temperature stressthe of cucumber seedlings. Nanjing Agric. Unive. 7–21. [Google Scholar]
- Tiburcio A. F., Altabella T., Bitrián M., Alcázar R. (2014). The roles of polyamines during the lifespan of plants: from development to stress. Planta 240, 1–18. 10.1007/s00425-014-2055-9 [DOI] [PubMed] [Google Scholar]
- Tun N. N., Santa-Catarina C., Begum T., Silveira V., Handro W., Floh E. I. (2006). Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 47, 346–354. 10.1093/pcp/pci252 [DOI] [PubMed] [Google Scholar]
- Verma S., Mishra S. N. (2005). Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 162, 669–677. 10.1016/j.jplph.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Vondráková Z., Eliášová K., Vágner M., Al E. (2015). Exogenous putrescine affects endogenous polyamine levels and the development of Picea abies somatic embryos. Plant Growth Regul. 75, 405–414. 10.1007/s10725-014-0001-2 [DOI] [Google Scholar]
- Vuosku J., Karppinen K., Muilu-Mäkelä R., Kusano T., Sagor G. H. M., Avia K. (2018). Scots pine aminopropyltransferases shed new light on evolution of the polyamine biosynthesis pathway in seed plants. Ann. Bot. 121, 1243–1256. 10.1093/aob/mcy012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuosku J., Suorsa M., Ruottinen M., Sutela S., Muilu-Mäkelä R., Julkunen-Tiitto R. (2012). Polyamine metabolism during exponential growth transition in Scots pine embryogenic cell culture. Tree Physiol. 32, 1274–1287. 10.1093/treephys/tps088 [DOI] [PubMed] [Google Scholar]
- Wang J. (2009). Changes in polyamine contents in Citrus and its closely related species under abitic stresses and isolation, characterization of two polyamine biosynthetic genes. Huazhong Agric. Univ. 1–8 10.7666/d.y1995975 [DOI] [Google Scholar]
- Wang Q., Bo Y. (2014). Alleviative effects of different kinds of exogenous polyamines on salt inj ury of Soybean seedlings. J. Henan Agric. Sci. 43, 48–50. 10.3969/j.issn.1004-3268.2014.04.011 [DOI] [Google Scholar]
- Wang W., Liu J. H. (2015). Genome-wide identification and expression analysis of the polyamine oxidase gene family in sweet orange (Citrus sinensis). Gene 555, 421–429. 10.1016/j.gene.2014.11.042 [DOI] [PubMed] [Google Scholar]
- Wang X. (2007). Studies on the evaluation methods and the mechanism of resistance of Chinese Cabbage (Brassica campestris L.) to Diamondback Moth (Plutella xylostella). Chin. Acad. Agric. Sci. 24–31 10.7666/d.Y1057065 [DOI] [Google Scholar]
- Wang X., Shi G. (2004). Effect of exogenous spermidine on anti-Hg2+ stress ability of Amaranth. J. Plant Physiol. Mol. Biol. 30, 69–74. 10.3321/j.issn:1671-3877.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Wang X., Shi G., Xu Q. (2003a). Toxic effects of Cr6+ on nymphoides peltatum mitigated by exogenous polyamine. Acta Sci. Circumst. 23, 689–693. 10.3321/j.issn:0253-2468.2003.05.024 [DOI] [Google Scholar]
- Wang Y., Lu W., Zhang Z. (2003b). ABA and putrescine treatment alleviate the chilling damage of banana fruit. J. Plant Physiol. Mol. Biol. 29, 549–554. 10.3321/j.issn:1671-3877.2003.06.011 [DOI] [Google Scholar]
- Woo H.R, Kim H.J, Nam H.G, Lim P. O. (2013). Plant leaf senescence and death–regulation by multiple layers of control and implications for aging in general. J. Cell Sci. 126, 4823–4833. 10.1242/jcs.109116 [DOI] [PubMed] [Google Scholar]
- Wu J., Shu S., Li C., Sun J., Guo S. (2018). Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol. Biochem. 128, 152–162. 10.1016/j.plaphy.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Wu M., Yuan L. (2008). Research progress in the relationship between polyamine and plant resistance. J. Anhui Agric. Sci. 36, 3516–3518. 10.3969/j.issn.0517-6611.2008.09.020 [DOI] [Google Scholar]
- Xu C., Wu X., Zhang H. (2009). Impact of D-Arg on drought resistance and endogenous polyamines in mycorrhizal Pinus massoniana. J. Nanjing Forestry Univ. 33, 019–023. 10.3969/j.issn.1000-2006.2009.04.004 [DOI] [Google Scholar]
- Xu D., Guo J., Xu L. (2014a). The relationship between polyamine oxidase activity and lignin deposition and chrysanthemum flower bud differentiation. Acta Agric. Boreali Sinica 29, 164-169. 10.7668/hbnxb.2014.03.030 [DOI] [Google Scholar]
- Xu L. (2015). The effect of polyamineon flower bud differentiation and bud germination of chrysanthemum. Shandong Agric. Univ. 31–36. [Google Scholar]
- Xu L., Xing S. T, Sun X. (2014b). Effects of polyamines on hormones contents and the relationship with the flower bud differentiation in chrysanthemum. Plant Physiol. J. 50, 1195–1202. 10.13592/j.cnki.ppj.2014.0212 [DOI] [Google Scholar]
- Yadav J. S., Rajam M. V. (1997). Spatial distribution of free and conjugated polyamines in leaves of Solanum melongena L. associated with differential morphogenetic capacity: efficient somatic embryogenesis with putrescine. J. Exp. Bot. 48, 1537–1545. 10.1093/jxb/48.8.1537 [DOI] [Google Scholar]
- Yamaguchi K., Takahashi Y., Berberich T., Al E. (2007). A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem. Biophys. Res. Commun. 352, 486–490. 10.1016/j.bbrc.2006.11.041 [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Shim I. S., Fujihara S. (2016). Inhibition of putrescine biosynthesis enhanced salt stress sensitivity and decreased spermidine content in rice seedlings. Biol. Plant. 61, 385–388. 10.1007/s10535-016-0676-5 [DOI] [Google Scholar]
- Yang C., He S. (2001). The relationship between polyamine and membrane lipid peroxidase during the senescence of cut rose flowers. Acta Botanica Boreali Occidentalia Sinica 21, 1157–1161. 10.1109/8.244640 [DOI] [Google Scholar]
- Yang L., Liang H., Lv X., Liu D., Wen X., Liao Y. (2016). Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol. Biochem. 100, 113–129. 10.1016/j.plaphy.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Yang Y., Yang X. (2002). Effect of temperature on endogenous polyamine content in leaves of Chinese Kale Seedlings. J. South China Agric. Univ. 23, 9–12. 10.3969/j.issn.1001-411X.2002.03.003 [DOI] [Google Scholar]
- Yin Z. P., Li S., Ren J., Al E. (2014). Role of spermidine and spermine in alleviation of drought-induced oxidative stress and photosynthetic inhibition in Chinese dwarf cherry (Cerasus humilis) seedlings. Plant Growth Regul. 74, 209–218. 10.1007/s10725-014-9912-1 [DOI] [Google Scholar]
- Yordanova R. Y., Alexieva V. S., Popova L. P. (2003). Influence of root oxygen deficiency on photosynthesis and antioxidant status in barley plants1. Russian J. Plant Physiol. 50, 163–167. 10.1023/A:1022908811211 [DOI] [Google Scholar]
- Zeid I. M., Shedeed Z. A. (2006). Response of alfalfa to putrescine treatment under drought stress. Biol. Plant. 50, 635–640. 10.1007/s10535-006-0099-9 [DOI] [PubMed] [Google Scholar]
- Zhao W., Sun G., Li S. (2004). Polyamines and plant stress resistance. J. Southern Agric. 35, 443–447. 10.3969/j.issn.2095-1191.2004.06.003 [DOI] [Google Scholar]
- Zhen Y., Li S., Su X. (2000). Polvamine changes and chilling injury in cold-stored Loquat fruits. J. Integr. Plant Biol. 42, 824–827. 10.3321/j.issn:1672-9072.2000.08.007 [DOI] [Google Scholar]
- Zhuo C., Liang L., Zhao Y., Guo Z, Lu S. (2018). A cold responsive ethylene responsive factor from Medicago falcata confers cold tolerance by up-regulation of polyamine turnover, antioxidant protection, and proline accumulation. Plant Cell Environ. 41, 2021–2032. 10.1111/pce.13114 [DOI] [PubMed] [Google Scholar]


