Abstract
Cisplatin, a widely used anticancer drug, damages hair cells in cochlear organotypic cultures at low doses, but paradoxically causes little damage at high doses resulting in a U-shaped dose-response function. To determine if the cisplatin dose-response function for vestibular hair cells follows a similar pattern, we treated vestibular organotypic cultures with doses of cisplatin ranging from 10 to 1000 μM. Vestibular hair cell lesions progressively increased as the dose of cisplatin increased with maximum damage occurring around 50–100 μM, but the lesions progressively decreased at higher doses resulting in little hair cell loss at 1000 μM. The U-shaped dose-response function for cisplatin-treated vestibular hair cells in culture appears to be regulated by copper transporters, Ctr1, ATP7A and ATP7B, that dose-dependently regulate the uptake, sequestration and extrusion of cisplatin.
Keywords: Cisplatin, Ototoxicity, Copper transporters, Vestibular organotypic cultures
1. Introduction
Cisplatin, one of the most widely used anti-cancer drugs, is activated after entering the cell's cytoplasm when the chloride atoms on cisplatin are displaced by water molecules. Hydrated cisplatin becomes a potent electrophile that reacts with nucleic acids in DNA, resulting in intrastrand and interstrand cross linking of DNA leading to cell cycle arrest which blocks tumor proliferation (Cepero et al., 2007). Cisplatin is not only nephrotoxic, neurotoxic, but also damages the sensory hair cells and neurons in the cochlea and vestibular system. Cisplatin initially damages the cochlear outer hair cells (OHC) followed by inner hair cells (IHC) and damage spreads from the high-frequency base of the cochlea towards the apex with increasing dose or duration of treatment (Ding et al., 2012a; Fleischman et al., 1975; Saito et al., 1995). In cochlear cultures, cisplatin damages hair cells throughout the cochlea as well as other structures (Laurell and Bagger-Sjoback, 1991; Meech et al., 1998) including spiral ganglion neurons (SGN) (Alam et al., 2000; Ding et al., 2012a; Gabaizadeh et al., 1997).
For toxicity to occur cisplatin must first be transported into the cytoplasm. The pathways that regulate the uptake and translocation of cisplatin into sensory hair cells, neurons, and supporting cells in the inner ear are poorly understood. However, recent evidence suggests that cisplatin uptake is mediated by copper transporters that regulate the import, export and sequestration of platinum (Ding et al., 2011b, 2012a, 2013b; Holzer et al., 2004b; Katano et al., 2004; Komatsu et al., 2000; Kuo et al., 2007; Safaei, 2006; Safaei et al., 2008; Samimi and Howell, 2006; Yoshizawa et al., 2007).
Copper is an essential metal involved in important biological processes such as oxidative phosphorylation (cytochrome oxidase), catecholamine synthesis, antioxidant defenses (Cu/Zn superoxide dismutase) and iron homeostasis (Puig and Thiele, 2002). High intracellular concentrations of copper are toxic (Olivari et al., 2008), therefore cells tightly regulate intracellular copper homeostasis mainly through Ctr1, ATP7A and ATP7B (Holzer et al., 2004a; Katano et al., 2002; Komatsu et al., 2000; Samimi et al., 2004; Wang and Lippard, 2005). Copper uptake through the plasma membrane is regulated by Ctr1 (Lee et al., 2002) (Lee et al., 2002; Safaei, 2006), which also imports platinum-based compounds (Holzer et al., 2004a; Kuo et al., 2007). ATP7A sequesters excess copper or platinum, and ATP7B expels these compounds from the cytoplasm (He et al., 2011; Kalayda et al., 2008; Kuo et al., 2007; Safaei and Howell, 2005; Samimi et al., 2004). These results suggest that changes in Ctr1, ATP7A and ATP7B could modulated the toxic effects of cisplatin on vestibular hair cell. To test this hypothesis, we treated postnatal vestibular explants with various doses of cisplatin to determine if high doses of cisplatin were less toxic than low doses and to examine cisplatin uptake.
2. Materials and methods
2.1. Vestibular organ cultures
Postnatal day 3 Sprague-Dawley rat pups (Charles River, Wilmington, MA) were used to prepare vestibular organ cultures according to procedures outlined in detail in our previous publications (Ding et al., 2012b, 2013a; Dong et al., 2014). Briefly, rat pups were decapitated and the macula of the utricle, superior and lateral crista ampullae were micro-dissected out along with the macula of saccule and posterior crista ampulla. The otolithic membranes on the macula of utricle and saccule were removed and then the vestibular explants were placed on the collagen gel on the bottom of a 30 mm culture dish and sufficient serum-free medium added to promote the attachment of the explant to the gel. The culture dish was then placed in an incubator at 37 °C in 5% CO2 for 1 h. Afterwards, 0.7 ml of serum-free medium was added to cover the explants. The cultures were placed in an incubator and maintained at 37 °C in 5% CO2 overnight. On the second day, the vestibular explants were treated with 0, 10, 50, 100, 400 or1000 μM cisplatin in culture medium for 48 h.
2.2. Hair cell labeling
Vestibular explants were fixed with 10% formalin for 3 h, rinsed in PBS, and stained with Alexa Fluor 488 conjugated phalloidin (Sigma P1951, 1:200). Specimens were rinsed in PBS and mounted on glass slides in glycerin. Stained specimens were examined with a confocal microscope (Zeiss LSM-510) using appropriate filters to detect Alexa-488 labeled vestibular hair cells. The numbers of vestibular hair cells with an intact cilia bundle and cuticular plate were counted in a 141 × 141 μm square (∼0.02 mm2). Hair cell counts were obtained from four regions in each explants and a mean computed for each specimen.
2.3. Vestibular ganglion quantification
Specimens were fixed for 3 h with 10% formalin, stained with mouse anti-β-tubulin III antibody to label the vestibular neurons and processes. ToPro-3 was used to label the nuclei. Specimens were immersed in a mouse anti-β-tubulin III antibody (Covance, TUJ1, MMS-435P, 1:100) solution containing 1% Triton X-100 and 5% goat serum in PBS for 24 h at 4 °C. Specimens were rinsed with PBS, then incubated for 2 h in goat anti-mouse secondary antibody conjugated with Alexa Fluor 555 (Abcam, ab150114, 1:200 dilution). Specimens were rinsed, stained with 1 μM ToPro-3 (Invitrogen T3605) for 1 h, mounted on glass slide, cover slipped, and examined with a confocal microscope (Zeiss LSM-510) with appropriate filters.
Images were evaluated with Zeiss LSM image Examiner and post-processed with Adobe Photoshop software and used to identify the nuclei, soma and processes of vestibular ganglion neurons. We measured the size of the ganglion neurons and counted the numbers of neurons with condensed or fragmented nuclei (Fu et al., 2013; Wu et al., 2011). Multiple layers of images (630X) of vestibular neurons were collected and merged. The numbers of layers were adjusted so that: (1) the largest cross sectional area of each ganglion neuron was included in the analysis and (2) the overlap among different ganglion neurons was minimized. A polygon was drawn around the perimeter of the soma of ganglion neurons and Zeiss LSM Image Examiner software was used to calculate the enclosed area.
2.4. Uptake of labeled cisplatin
Alexa Fluor 488 hydrazide (Thermo Fischer, A10436) was conjugated to a carbohydrate-linked platinum (II) complex cis-dichloro [(2-b-D-glucopyranosidyl) propane-1,3-diamine]platinum following the methods described previously (Chen et al., 1999). Vestibular explants were treated with Alexa Fluor 488-cisplatin (50, or 1000 μM) for 48 h, fixed with 10% formalin in PBS for 4 h, and stained with Alexa Fluor 555-labeled phalloidin. Specimens were examined under a confocal microscope (Zeiss LSM-510) with appropriate filters. Image layers were collected and organized with Zeiss LSM image Examiner and post-processed with Adobe Photoshop software (version 5.0).
2.5. AM1-43 uptake
In the first series of experiments, vestibular explants were treated with 0, 10, 50, or 1000 μM cisplatin for 48 h. Afterwards, the explants were incubated for 30 min in freshly made AM1-43 (Biotium, #70024; 30 μg/ml in serum-free medium) to assess the functional status of the hair cells and uptake of cisplatin into vestibular hair cells (Ding et al., 2011b; Gale et al., 2001; Meyers et al., 2003). Afterwards, the explants were rinsed with PBS, fixed with 10% formalin for 2 h, stained with Alexa Fluor 488-labeled phalloidin for 30 min and mounted on glass slides. In the second series of experiments aimed at determining if cisplatin permanently blocked the uptake of AM1-43 into hair cells, explants were pre-treated with 50 μM, or 1000 μM cisplatin for 18 h. Then, the culture medium containing cisplatin was removed and replaced by normal serum free medium for another 18 h. In both series of experiments, the specimens were subsequently fixed and labeled with Alexa Fluor 488 conjugated phalloidin and examined under a confocal microscope to detect the green fluorescence of the phalloidin-labeled hairs cells and the red fluorescence of the labeled AM1-43 (excitation 510 nm, emission 625 nm) taken up by vestibular hair cells.
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of University at Buffalo, and conform to the guidelines issued by the National Institutes of Health.
3. Results
3.1. Cisplatin dose-response
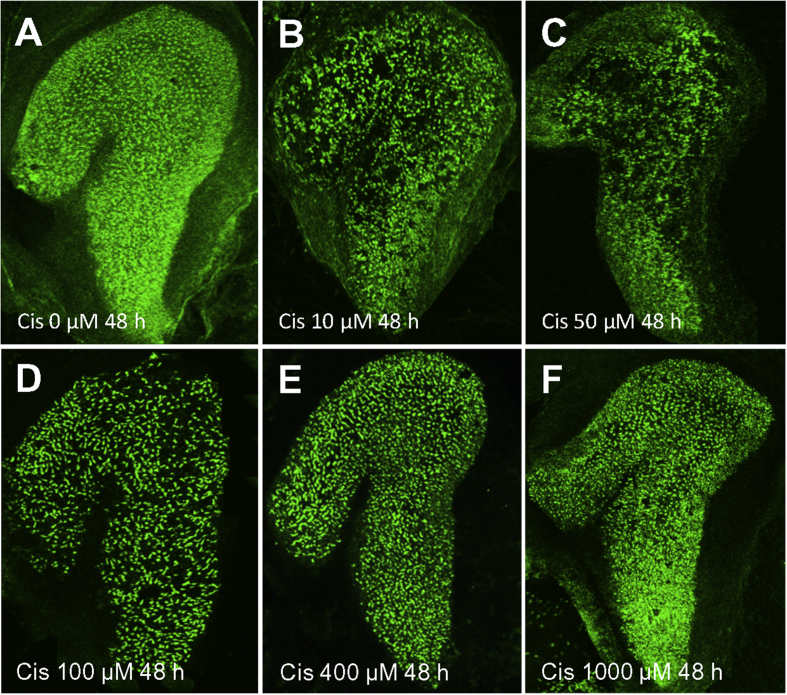
Fig. 1 shows six representative surface preparation (100X) of the whole macula of the saccule stained with Alexa 488-phalloidin after 48 h treatment with 0, 10, 50, 100, 400 and 1000 μM of cisplatin. There is dense, uniform labeling of hair cells in the untreated control culture (Fig. 1A); however, hair cell labeling initially increased as the cisplatin dose increased from 10 to 50 μM resulting in regions largely devoid of hair cells (Fig. 1B–C), but hair cell survival progressively increased as the dose of cisplatin increased from 100 to 1000 μM (Fig. 1D–F).
Fig. 1.
Photomicrographs showing the whole macula of saccule after 48 h treatment (A) without (control) and with cisplatin (Cis) at concentrations of 10–1000 μM (B–F). Specimens stained with Alexa 488-conjugated phalloidin which preferentially labels the stereocilia bundles and cuticular plate of vestibular hair cells. (A) Note high density of hair cell stereocilia in specimen cultured without cisplatin for 48 h. (B–D) Many hair cells were missing after treatment with 10, 50, or 100 μM cisplatin for 48 h. (E–F) Hair cell density in cultures treated with 400 or 1000 μM cisplatin were greater than with 10 or 50 μM cisplatin.
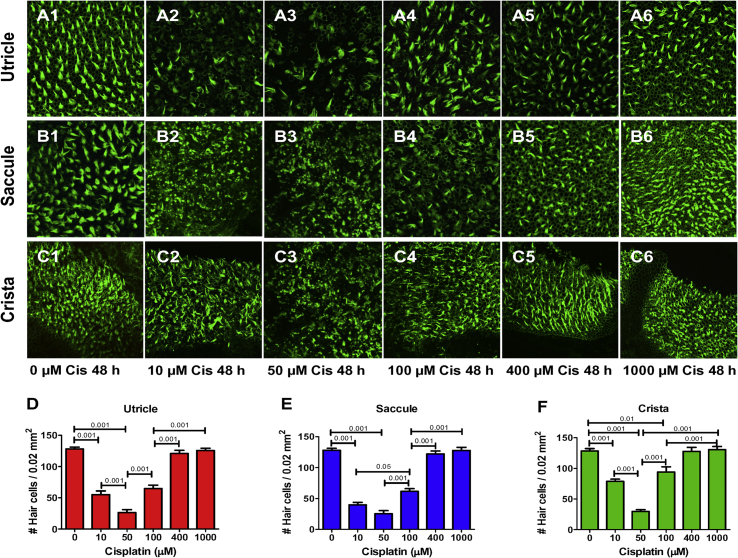
Fig. 2 shows high magnification (650X) images of vestibular hair cells in the macula of the utricle and saccule and crista ampullaris after 48 h treatment with 0, 10, 50, 100, 400 and 1000 μM of cisplatin. The densities of phalloidin-labeled stereocilia bundles in the utricle (Fig. 2, A2-4), saccule (Fig. 2, B2-4) and crista ampullaris (Fig. 2, Fig. 3, Fig. 4) decreased as the cisplatin dose increased from 10 to 100 μM, but then hair cell density increased at higher doses (Fig. 2, Fig. 5, Fig. 6, Fig. 1, Fig. 2, Fig. 5, Fig. 6C and Fig. 2, Fig. 5, Fig. 6). We quantified these effects, by counting the numbers of hair cells with an intact stereocilia bundle; counts were obtained from four separate regions of 0.02 mm2 in each explant. Mean (+SEM, n = 6) hair cell counts were plotted as a function of cisplatin dose in the utricle, saccule and crista ampullaris (Fig. 2D–F). The mean numbers of hair cells initially decreased as the cisplatin dose increased from 0 to 50 μM, but then gradually increased from 100 to 1000 μM. There was a significant effect of cisplatin dose for the utricle (F5,30 df = 83.6), saccule (F5,30 df = 109.8) and crista (F5,50 df = 5.2.5); a Bonferroni post-hoc analysis identified treatments that were significantly different from one another (See Fig. 2D–F and figure legend for details).
Fig. 2.
Representative photomicrographs of vestibular explants from utricle, saccule and crista cultured for 48 h without cisplatin (Cis) or with 10, 50, 100, 400 or 1000 μM cisplatin. Specimens stained with Alexa 488-conjugated phalloidin which labels the hair cell stereocilia bundles. Macula of utricle (A1), macula of saccule (B1) and ampulla of crista (C1) cultured with 0 μM cisplatin for 48 h; note high density of stereocilia bundles in control cultures. Density of hair cell stereocilia reduced at cisplatin concentrations of 10–50 μM in utricle (A2-3), saccule (B2-3) and crista (C2-3), but hair cell density gradually increased as the concentration increased from 100 to 1000 μM (A4,5,6; B4,5,6; C4,5,6). D–F: Histograms showing mean (n = 6, +/−) number of hair cells per 0.22 μm2 in utricle, saccule and ampula. Horizontal lines with p values above indicate significant differences between conditions (p < 0.05, 0.01 or 0.001, one-way ANOVA, Bonferroni post-hoc comparisons).
Fig. 3.
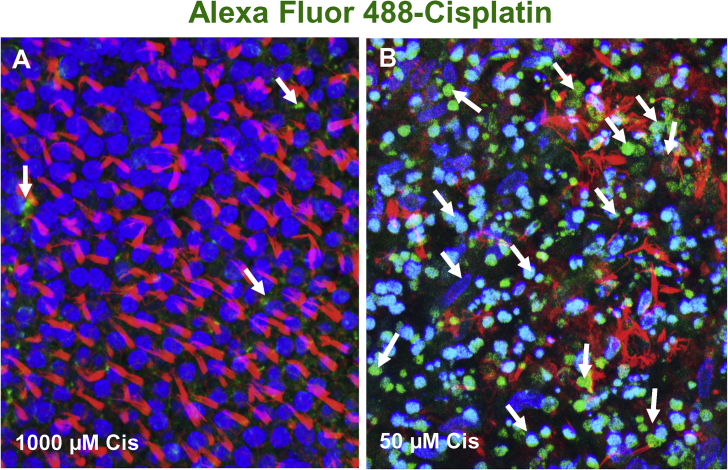
Photomicrographs showing accumulation of Alexa Fluor 488-conjugated cisplatin (green near arrows) in hair cells in the macula of utricle. Nuclei labeled with ToPro-3 (blue); hair cell stereocilia labeled with Alex Fluor 555-conjugated phalloidin (red). (A) In cultures treated with 1000 μM cisplatin, stereocilia bundles (red) were arranged in tufts and ToPro-3 nuclei were large and round, morphological features of healthy hair cells. Only a few puncta of Alex 488-cisplatin (arrow) present in hair cells. (B) In cultures treated with 50 μM cisplatin, extensive Alexa 488-cisplatin (green/turquoise) labeling was evident in vestibular hair cells. The stereocilia bundles (red) were either missing or in disarray and many hair cell nuclei were condensed (blue/turquoise) indicative of cells undergoing apoptosis.
Fig. 4.
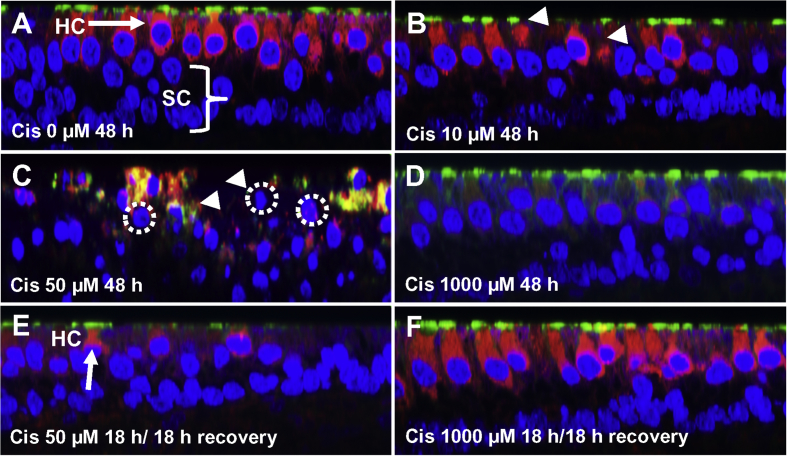
Representative Z-plane photomicrographs showing uptake of AM1-43 (red) into vestibular hair cells (HC) in macula of utricle. Nuclei of cells labeled with ToPro-3 (blue) to label nuclei and Alex Fluor 488-conjugated phalloidin (green) to identify hair cell stereocilia and cuticular plate. (A) In controls (0 μM cisplatin), robust AM1-43 labeling was present in hair cell cytoplasm, but was absent from support cells (SC) beneath the hair cells. (B) After 48 h treatment with 10 μM cisplatin, AM1-43 was reduced or absent from hair cells with damaged or missing stereocilia (arrowhead). After 48 h treatment with 50 μM cisplatin, many hair cells were damaged (dotted circles around shrunken nuclei) and only small, scattered puncta of AM1-43 were present in damaged areas of the epithelium. (D) After 48 h treatment with 1000 μM cisplatin, most hair cells were intact (large nuclei, phalloidin label on apical surface of hair cells); however, AM1-43 was absent from hair cells. (E) Cultures treated for 18 h with 50 μM cisplatin; then culture medium was replaced with standard culture medium and cultured an additional 18 h after which AM1-43 was added to medium. Note damage to stereocilia and shrunken nuclei (dotted circle) due to low-dose cisplatin. (F) Same conditions as E except cisplatin dose was 1000 μM cisplatin. Note robust AM1-43 labeling in cytoplasm and phallodin labeling of cuticular plate of hair cells, which appear normal.
Fig. 5.
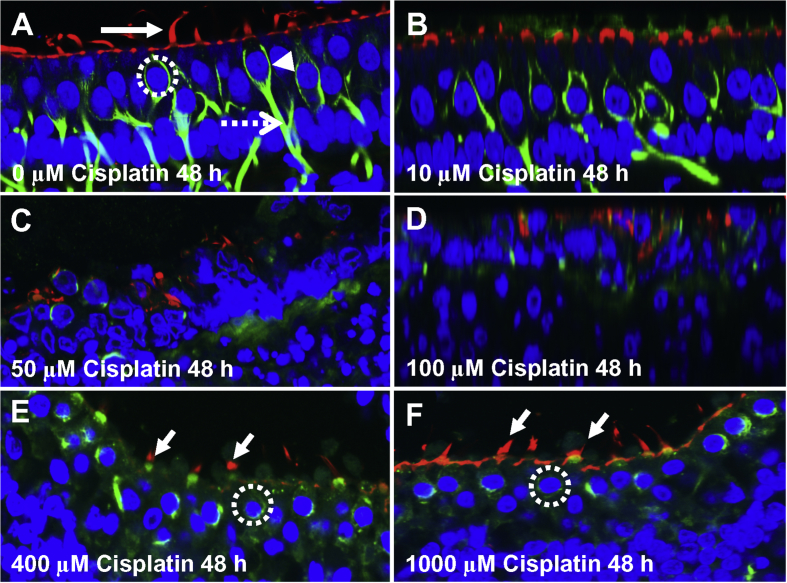
Photomicrographs of vestibular hair cells and nerve endings. (A) Control vestibular explant cultured for 48 h. Stereocilia and cuticular plate labeled with Alexa Fluor 555-phalloidin (red, arrow), nuclei labeled with ToPro-3 (blue) and nerve fibers labeled with beta-tubulin (green). Note chalice shaped afferent ending (arrowhead) around hair cells, afferent nerve fiber (dashed arrow) and large round nucleus (dotted circle) of hair cells. (B): After 48 h treatment with 10 μM cisplatin, no major changes observed in ToPro-3 labeled nuclei, phalloidin labeled hair cells or tubulin-labeled nerve fibers. (C–D) After 48 h treatment with 50 and 100 μM cisplatin treatment, most vestibular hair and afferent nerve fibers missing. (E) In vestibular explant treated with 400 μM cisplatin for 48 h, most hair cell nuclei present, but nuclei generally smaller than controls. Nerve fibers missing and chalice afferent terminals largely absent. (F) Note phalloidin labeling of apical pole of hair cell and stereocilia and round nuclei of sensory hair cell (dotted circle) after 48 treatment with 1000 μM cisplatin. Nerve fibers missing and chalice shaped afferent terminal shrunken or largely absent.
Fig. 6.
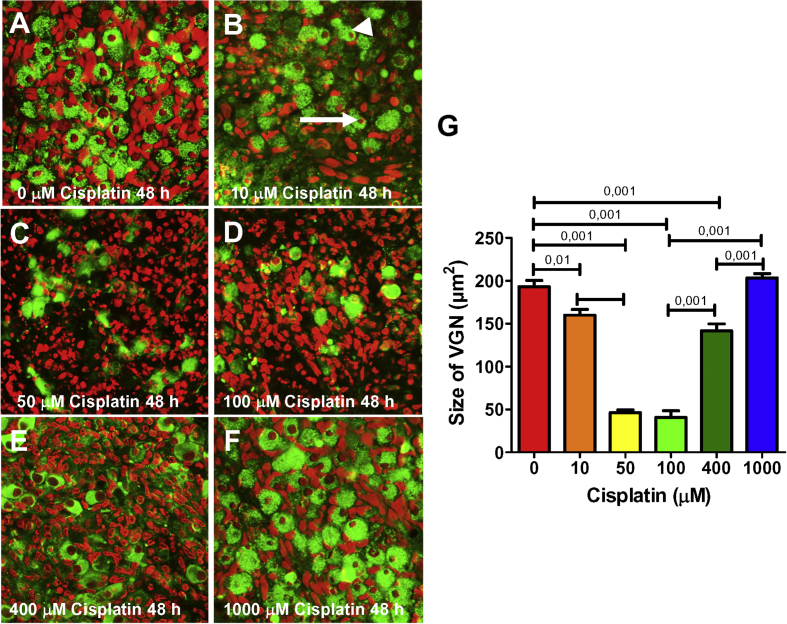
Vestibular ganglion neurons (VGN) cultured for 48 h without (A) (0 μm) or with (B–F) 10, 50, 100, 400 and 1000 μM cisplatin respectively. Samples labeled with β−tubulin III (green) to label neurons and ToPro-3 (red) to label nuclei. (A) Note large round soma and round nucleolus in the absence of cisplatin. (B) Treatment with 10 μM cisplatin resulted shrinkage of nucleolus (arrow) and soma (arrowhead) of some neurons. (C–D) Treatment with 50 and 100 μM cisplatin resulted in soma loss, and shrinkage, condensation and fragmentation of the nucleolus. (E–F) Note increases in the number of neurons and size of the soma and nucleolus as the cisplatin dose increased from 400 to 1000 μM. (G) Mean size of vestibular ganglion soma initially decreased as the cisplatin dose increased from zero to 100 μM cisplatin, but soma size incresed as the cisplatin dose increased from 400 to 1000 μM. Horizontal bars show conditions that were significantly different from one another (Bonferroni p values shown above bar).
3.2. Hair cell uptake of cisplatin
We compared the uptake of Alexa Fluor 488 conjugated-cisplatin into vestibular hair cells treated either with a high, 1000 μM dose of cisplatin and low 50 μM dose of labeled cisplatin. When 1000 μM of labeled-cisplatin was applied to the cultures, very little cisplatin was taken up into vestibular hair cells (Fig. 3A); the stereocilia bundles remained intact and the nuclei of the hair cells were large and round, indicative of healthy cells. When the dose of Alexa Fluor 488 conjugated-cisplatin was lowered to 50 μM, there was extensive uptake of labeled cisplatin into hair cell (Fig. 3B, arrows). This low dose of cisplatin was associated with stereocilia damage and condensation of hair cell nuclei, morphological features of apoptosis. These results indicate that hair cell death is associated with robust uptake of labeled-cisplatin at low doses whereas hair cell survival is associated with little cisplatin uptake at high drug does.
3.3. Cisplatin-induced endocytosis
AM1-43, rapidly enters hair cells through the divalent, mechanically-gated channel, but dye entry is blocked when the channel is disrupted (Meyers et al., 2003). AM1-43 was taken up by normal vestibular hair cells (Fig. 4A, red), but dye uptake was reduced when vestibular cultures were treated for 48 h with 10 μM cisplatin, particularly in hair cells with damaged or missing stereocilia (Fig. 4B). When cultures were treated with 50 μM cisplatin, AM1-43 uptake was almost completely eliminated, but with this dose, many of the hair cells were damaged (missing or damaged stereocilia or cuticular plate, shrunken hair cell hair nuclei) (Fig. 4C). When cultures were treated with 1000 μM cisplatin for 48 h, AM1-43 uptake was largely abolished; in this case the stereocilia, cuticular plate, and cell nuclei had a normal appearance (Fig. 4D). However, when vestibular cultures were pretreated for 18 h with 50 μM cisplatin, returned to normal culture medium for 18 h, and then challenged with AM1-43, dye uptake was mainly confined to a few hair cells with remnants of stereocilia and/or cuticular plate (Fig. 4E, arrow). In contrast. when cultures were pretreated with 1000 μM cisplatin for 18 h, returned to normal culture medium for 18 h and then challenged with AM1-43, dye uptake was restored (Fig. 4F) in these normal appearing hair cells (Fig. 4F, arrow).
3.4. Cisplatin damage to vestibular nerve fibers and synapses
To determine if cisplatin damaged vestibular nerve fibers and nerve terminals in a dose-dependent, vestibular explants were treated with 0, 10, 50, 100, 400, or 1000 μM cisplatin for 48 h and then stained with ToPro-3 (nuclei, blue), Alex 555-phaloidin (ciliary bundle, cuticular plate, red) and β-tubulin III (nerve fibers, afferent synapse, green). β-tubulin protein was heavily expressed in the fibers and nerve endings in control vestibular explants (Fig. 5A). The vestibular nerve fibers and terminals in cultures treated for 48 h with 10 μM cisplatin were similar to control cultures (Fig. 5B). In contrast, 48 h treatment with 50, 100, 400, or 1000 μM cisplatin resulted in substantial loss of vestibular nerve fibers and nerve terminals (Fig. 5C and F). In contrast, the vestibular hair cells treated with 50 or 100 μM cisplatin for 48 h were severely damaged (Fig. 5C and D) whereas the vestibular hair cells treated with 1000 μM (Fig. 5F) were essentially normal. These results indicate that damage to vestibular nerve fibers and synapses continues to increase as the dose of cisplatin increases, unlike the U-shaped dose-response function for vestibular hair cells (Fig. 2E–F).
3.5. Cisplatin damage to vestibular ganglion neurons
We treated vestibular explants with 0, 10, 50, 100, 400, or 1000 μM cisplatin for 48 h and used a β-tubulin III antibody to label the soma of vestibular ganglion neurons and ToPro-3 to stain the nuclei. In untreated (0 μM) control explants (A), the soma and nucleolus of the vestibular neurons were large and round, but when the explants were treated with 10 μM cisplatin, the soma and nucleolus of some neurons were shrunken and distorted (Fig. 6B). As the dose of cisplatin increased from 50 to 100 μM, many neurons disappeared (Fig. 6C-D) and ToPro-3 labeling of residual nuclear DNA became condensed and fragmented, morphological features of apoptosis. However, as the dose of cisplatin increased from 400 to 1000 μM (Fig. 6E–F), the numbers of neurons increased and the size of the soma and nucleolus of these neurons also increased. To quantify the results, mean soma size was assessed after 48 h treatment with cisplatin doses from 0 to 1000 μM. As shown in Fig. 6G, mean soma size (+SEM, n = 17) initially decreased from approximately 195 μm2 in the absence of cisplatin to roughly 45 μm2 at 100 μM and then rapidly increased to approximately 200 μm2 as the cisplatin dose increased to 1000 μM. The effect of cisplatin dose on vestibular ganglion soma size was statistically significant (one-way ANOVA, F5,96 df = 117.9, p < 0.0001, Bonferroni post-hoc comparison for between group differences shown in Fig. 6). Interestingly, soma size for the 1000 μM dose of cisplatin was similar to that of the untreated controls. The U-shaped cisplatin dose-response function for VGN is similar to that seen for cochlear spiral ganglion neurons (Ding et al., 2011a, 2012a).
4. Discussion
4.1. U-shaped dose-response
When we treated organotypic cultures of the utricle, saccule and crista ampullaris with increasing concentrations of cisplatin for 48 h, we observed an unusual U-shaped dose-response function. Damage to vestibular hair cells and neurons was greatest at intermediate doses of cisplatin whereas damage was least at very low and very high doses. Maximum hair cell loss in the utricle, saccule and crista ampullaris occurred when the dose of cisplatin was approximately 50 μM (Fig. 2D–F) and the dose-response functions were similar for all three vestibular organs. In contrast, vestibular ganglion damage was greatest at 100 μM cisplatin (Fig. 6G). What is most remarkable is that there was little damage to vestibular hair cells or neurons following treatment with 1000 μM cisplatin, the highest dose tested. The nonlinear cisplatin dose-response function for vestibular hair cells and vestibular neurons was similar to that seen for hair cells and neurons in postnatal cochlear organotypic cultures (Ding et al., 2011b, 2012a). Unlike hair cells or ganglion neurons which showed little evidence of damage after treatment with 1000 μM cisplatin, most of vestibular nerve fibers and peripheral nerve terminals were still missing after this high dose (Fig. 5F). These results suggest that the peripheral nerve fibers and terminals are more sensitive to cisplatin damage than the hair cells or the soma of neurons.
4.2. Cisplatin and AM1-43 uptake
The U-shaped dose-response function was linked to the nonlinear uptake of fluorescently labeled cisplatin. When cultures were treated with 1000 μM cisplatin, there was minimal uptake of cisplatin and little hair cell damage (Fig. 3A). Conversely, when cultures were treated with 50 μM cisplatin, there was significant hair cell loss and considerable uptake of fluorescently labeled cisplatin into hair cells (Fig. 3B). These results suggest that the uptake of cisplatin into hair cells is a critical step in hair cell death. AM1-43 rapidly enters hair cells in the absence of cisplatin (Fig. 4A), but as the concentration of cisplatin in the culture medium increased less AM1-43 entered the hair cells with complete block occurring at 1000 μM (Fig. 4B–D). These results show that vestibular hair cells prevent the uptake of divalent cations like AM1-43 in the presence of high concentrations of cisplatin. When cisplatin was removed from the medium for 18 h, AM1-43 uptake increased significantly when the stereocilia were intact (Fig. 4F), but less uptake occurred when the stereocilia and hair cells were damaged (Fig. 4E). These results suggest that high dose cisplatin disrupts drug uptake through the apical pole of the hair cell. We did not explore potential routes of cisplatin uptake into vestibular neurons; however, because the cisplatin dose-response function for neurons was similar to hair cells, it seems likely that uptake occurs through the same pathway.
Summary: The U-shaped cisplatin dose-response function observed in our vestibular organ cultures is similar to that seen in the cochlea. We previously reported that three copper transport proteins Ctr1, ATP7A and ATP7B were highly expressed in cochlear hair cells and neurons (Ding et al., 2011b, 2012a) and that altering these transporters suppressed cisplatin damage to cochlear hair cells (Ding et al., 2011b, 2012a). When copper sulfate was added to cochlear cultures medium, it competitively inhibited the uptake of cisplatin through Ctr1 and attenuated cisplatin damage in the cochlea (Ding et al., 2011b; More et al., 2010). Cimetidine, a competitive inhibitor of the organic cation transporter 2, also reduced cisplatin damage in the cochlea; however, our results showed that cimetidine also reduced Ctr1 suggesting that cimetidine suppressed cisplatin damage by inhibiting the uptake of cisplatin (He et al., 2011). Cisplatin also increased the expression of ATP7B, a major copper export pump (He et al., 2011). Because cochlear and vestibular hair cells are quite similar, we believe that the same three copper transporter are likely responsible for the U-shaped cisplatin dose-response function in our vestibular organ cultures.
Acknowledgements
Research supported in part by a grant from NIOSH (R01OH010235), in part by NIH grant 5R01DC011808, in part by grant NIH R01DC014437, and in part by foundation of Science and Technology Commission of Shanghai Municipality (NO 15140900900).
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Alam S.A., Ikeda K., Oshima T., Suzuki M., Kawase T., Kikuchi T., Takasaka T. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear. Res. 2000;141:28–38. doi: 10.1016/s0378-5955(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Cepero V., Garcia-Serrelde B., Moneo V., Blanco F., Gonzalez-Vadillo A.M., Alvarez-Valdes A., Navarro-Ranninger C., Carnero A. Trans-platinum(II) complexes with cyclohexylamine as expectator ligand induce necrosis in tumour cells by inhibiting DNA synthesis and RNA transcription. Clin. Transl. Oncol. 2007;9:521–530. doi: 10.1007/s12094-007-0096-2. [DOI] [PubMed] [Google Scholar]
- Chen Y., Heeg M.J., Braunschweiger P.G., Xie W., Wang P.G. A carbohydratee-linked cisplatin analogue having antitumor activity. Angew. Chem. Int. Ed. 1999;38:1768–1769. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1768::AID-ANIE1768>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ding D., Allman B.L., Salvi R. Review: ototoxic characteristics of platinum antitumor drugs. Anat. Rec. 2012;295:1851–1867. doi: 10.1002/ar.22577. [DOI] [PubMed] [Google Scholar]
- Ding D., Allman B.L., Yin S., Sun H., Salvi R. Cisplatin ototoxicity. In: Dupont J., editor. Hearing Loss: Classification, Causes and Treatment. Nova Science Publishers, Inc.; Hauggauge, NY: 2011. pp. 39–63. [Google Scholar]
- Ding D., Jiang H., Fu Y., Li Y., Salvi R. Ototoxic model of oxaliplatin and protection from nocitinamide adenine dinucleotide. Journal of Otology. 2013;8:22–30. doi: 10.1016/s1672-2930(13)50009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Jiang H., Fu Y., Salvi R., Someya S., Tanokura M. Ototoxic effects of carboplatin in cochlear organotypic cultures in chinchillas and rats. Journal of Otology. 2012;7:92–101. doi: 10.1016/S1672-2930(12)50023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., He J., Yu D., Jiang H., Li Y., Salvi R.J. New insights on cisplatin ototoxicity. Can. Hear. Rep. 2013;8:29–31. [Google Scholar]
- Ding D., He J., Allman B.L., Yu D., Jiang H., Seigel G.M., Salvi R.J. Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear. Res. 2011;282:196–203. doi: 10.1016/j.heares.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Ding D., Jiang H., Shi J.R., Salvi R., Roth J.A. Ototoxicity of paclitaxel in rat cochlear organotypic cultures. Toxicol. Appl. Pharmacol. 2014;280:526–533. doi: 10.1016/j.taap.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Fleischman R.W., Stadnicki S.W., Ethier M.F., Schaeppi U. Ototoxicity of cis-dichlorodiammine platinum (II) in the Guinea pig. Toxicol. Appl. Pharmacol. 1975;33:320–332. doi: 10.1016/0041-008x(75)90098-8. [DOI] [PubMed] [Google Scholar]
- Fu Y., Ding D., Wei L., Jiang H., Salvi R. Ouabain-induced apoptosis in cochlear hair cells and spiral ganglion neurons in vitro. BioMed Res. Int. 2013;2013:628064. doi: 10.1155/2013/628064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaizadeh R., Staecker H., Liu W., Kopke R., Malgrange B., Lefebvre P.P., Van de Water T.R. Protection of both auditory hair cells and auditory neurons from cisplatin induced damage. Acta Otolaryngol. 1997;117:232–238. doi: 10.3109/00016489709117778. [DOI] [PubMed] [Google Scholar]
- Gale J.E., Marcotti W., Kennedy H.J., Kros C.J., Richardson G.P. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Ding D., Yu D., Jiang H., Yin S., Salvi R. Modulation of copper transporters in protection against cisplatin-induced cochlear hair cell damage. Journal of Otology. 2011;6:53–61. [Google Scholar]
- Holzer A.K., Katano K., Klomp L.W., Howell S.B. Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clin. Canc. Res. 2004;10:6744–6749. doi: 10.1158/1078-0432.CCR-04-0748. [DOI] [PubMed] [Google Scholar]
- Holzer A.K., Samimi G., Katano K., Naerdemann W., Lin X., Safaei R., Howell S.B. The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol. Pharmacol. 2004;66:817–823. doi: 10.1124/mol.104.001198. [DOI] [PubMed] [Google Scholar]
- Kalayda G.V., Wagner C.H., Buss I., Reedijk J., Jaehde U. Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Canc. 2008;8:175. doi: 10.1186/1471-2407-8-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano K., Safaei R., Samimi G., Holzer A., Tomioka M., Goodman M., Howell S.B. Confocal microscopic analysis of the interaction between cisplatin and the copper transporter ATP7B in human ovarian carcinoma cells. Clin. Canc. Res. 2004;10:4578–4588. doi: 10.1158/1078-0432.CCR-03-0689. [DOI] [PubMed] [Google Scholar]
- Katano K., Kondo A., Safaei R., Holzer A., Samimi G., Mishima M., Kuo Y.M., Rochdi M., Howell S.B. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Canc. Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- Komatsu M., Sumizawa T., Mutoh M., Chen Z.S., Terada K., Furukawa T., Yang X.L., Gao H., Miura N., Sugiyama T., Akiyama S. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Canc. Res. 2000;60:1312–1316. [PubMed] [Google Scholar]
- Kuo M.T., Chen H.H., Song I.S., Savaraj N., Ishikawa T. The roles of copper transporters in cisplatin resistance. Canc. Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- Laurell G., Bagger-Sjoback D. Dose-dependent inner ear changes after i.v. administration of cisplatin. J. Otolaryngol. 1991;20:158–167. [PubMed] [Google Scholar]
- Lee J., Pena M.M., Nose Y., Thiele D.J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002;277:4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- Meech R.P., Campbell K.C., Hughes L.P., Rybak L.P. A semiquantitative analysis of the effects of cisplatin on the rat stria vascularis. Hear. Res. 1998;124:44–59. doi: 10.1016/s0378-5955(98)00116-6. S0378-5955(98)00116-6 [pii] [DOI] [PubMed] [Google Scholar]
- Meyers J.R., MacDonald R.B., Duggan A., Lenzi D., Standaert D.G., Corwin J.T., Corey D.P. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J. Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More S.S., Akil O., Ianculescu A.G., Geier E.G., Lustig L.R., Giacomini K.M. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 2010;30:9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivari F.A., Hernandez P.P., Allende M.L. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res. 2008;1244:1–12. doi: 10.1016/j.brainres.2008.09.050. [DOI] [PubMed] [Google Scholar]
- Puig S., Thiele D.J. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 2002;6:171–180. doi: 10.1016/s1367-5931(02)00298-3. [DOI] [PubMed] [Google Scholar]
- Safaei R. Role of copper transporters in the uptake and efflux of platinum containing drugs. Canc. Lett. 2006;234:34–39. doi: 10.1016/j.canlet.2005.07.046. [DOI] [PubMed] [Google Scholar]
- Safaei R., Howell S.B. Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit. Rev. Oncol. Hematol. 2005;53:13–23. doi: 10.1016/j.critrevonc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Safaei R., Otani S., Larson B.J., Rasmussen M.L., Howell S.B. Transport of cisplatin by the copper efflux transporter ATP7B. Mol. Pharmacol. 2008;73:461–468. doi: 10.1124/mol.107.040980. [DOI] [PubMed] [Google Scholar]
- Saito T., Manabe Y., Honda N., Yamada T., Yamamoto T., Saito H. Semiquantitative analysis by scanning electron microscopy of cochlear hair cell damage by ototoxic drugs. Scanning Microsc. 1995;9:271–280. discussion 280-1. [PubMed] [Google Scholar]
- Samimi G., Howell S.B. Modulation of the cellular pharmacology of JM118, the major metabolite of satraplatin, by copper influx and efflux transporters. Canc. Chemother. Pharmacol. 2006;57:781–788. doi: 10.1007/s00280-005-0121-5. [DOI] [PubMed] [Google Scholar]
- Samimi G., Katano K., Holzer A.K., Safaei R., Howell S.B. Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol. Pharmacol. 2004;66:25–32. doi: 10.1124/mol.66.1.25. [DOI] [PubMed] [Google Scholar]
- Wang D., Lippard S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- Wu X., Ding D., Sun H., Liu H., Jiang H., Salvi R. Lead neurotoxicity in rat cochlear organotypic cultures. J. Otology. 2011;6:45–52. [Google Scholar]
- Yoshizawa K., Nozaki S., Kitahara H., Ohara T., Kato K., Kawashiri S., Yamamoto E. Copper efflux transporter (ATP7B) contributes to the acquisition of cisplatin-resistance in human oral squamous cell lines. Oncol. Rep. 2007;18:987–991. [PubMed] [Google Scholar]