Abstract
Background
Acute pancreatitis in dogs is an under‐diagnosed disease. Current diagnostic methods are insufficient at identifying sequelae and lack prognostic capability. Computed tomographic angiography (CTA) is accurate for diagnosis and prognostication of pancreatitis in humans.
Objectives
In comparison to ultrasound (US), CTA will (1) better diagnose more severe pancreatitis and sequelae and (2) provide assessment of patient outcome by identification of pancreatic contrast enhancement patterns.
Animals
Twenty‐six client‐owned dogs suspected to have acute pancreatitis.
Methods
US and CTA examinations performed at time of admission were compared to determine the detection of pancreatic changes and sequelae. CTA findings also were compared to outcome indicators for prognosis of dogs with acute pancreatitis. Specific canine pancreatic lipase (cPL) samples were obtained and compared with CTA findings.
Results
Ten of 26 dogs had heterogeneous contrast enhancement of the pancreas. Compared to US, CTA better identified portal vein thrombosis (P = .003). Patients with heterogeneous contrast enhancement had longer hospitalization (P = .01), including hospital stays for >5 days (P = .02), had more relapses, and were more likely to have portal vein thrombosis (P = .002). Patients with heterogeneous contrast enhancement had increased spec cPL (P = .006).
Conclusions and Clinical Importance
In comparison to US, CTA better identified dogs with more severe acute pancreatitis and those with portal vein thrombosis, factors that may predict longer hospitalization and increased risk of relapse. The presence of heterogeneous contrast enhancement and portal vein thrombosis may change therapy for patients with acute pancreatitis.
Keywords: portal vein thrombosis, ultrasound
Abbreviations
- CTA
computed tomographic angiography
- US
ultrasound
- cPL
canine pancreatic lipase
- FNA
fine‐needle aspiration
1. INTRODUCTION
Acute pancreatitis in dogs is an under‐diagnosed and potentially fatal disease. The true prevalence of pancreatitis in the canine population is unknown, but a previous study involving 200 dogs presented to 1st opinion clinics found histological evidence of chronic or acute disease in 37% of pancreata at necropsy.1 In a recent review of the current literature, the reported mortality rate of dogs with acute pancreatitis ranged from 27% to 58%, indicating that early and accurate diagnosis of acute pancreatitis is essential.2 Current methods of diagnosing acute pancreatitis in dogs include a combination of abdominal ultrasound (US) examination, clinical history, physical examination, and serum biochemistry, including the Spec cPL assay for canine pancreatic lipase (cPL). The reported sensitivities and specificities of these tests are variable and relatively low3, 4, 5; thus, these tests can be insufficient for accurate diagnosis of acute pancreatitis and cannot adequately diagnose concurrent local or remote sequelae, including portal vein thromboses.
Three‐phase computed tomographic angiography (CTA) has been shown to be a rapid and accurate method for the diagnosis of acute pancreatitis in people.6 In a previous study, people with CT evidence of pancreatic necrosis (ie, heterogeneous contrast enhancement) had a 23% mortality rate and 82% morbidity rate compared to 0% mortality rate and 6% morbidity in the absence of necrosis (ie, homogeneous pancreatic contrast enhancement).7, 8 In addition, CTA has shown overall accuracy of 87% and sensitivity of 100% in diagnosing surgically confirmed pancreatic necrosis in humans.8, 9, 10, 11 The CTA findings are utilized to determine the course of treatment in human patients diagnosed with acute pancreatitis.6, 7, 8, 9
A previous pilot study at our institution using CTA to evaluate 10 dogs with acute pancreatitis identified multiple changes in the CT appearance of the pancreas, peripancreatic tissues, and adjacent vessels that may be important in the prognostication of affected patients.12 Specifically, those patients with more heterogeneous contrast enhancement appeared to have worse outcome than those with homogeneous contrast enhancement.12 Computed tomographic angiography also diagnosed 3 of the 10 dogs from the aforementioned study as having portal vein thrombosis, a sequela of acute pancreatitis, which was not seen during ultrasonographic examination.12 A larger study is necessary to further elucidate how these changes in contrast enhancement correspond with prognosis and outcome and to further evaluate and identify sequelae of pancreatitis (eg, venous and arterial thromboses) using CTA.
Our goals were 2‐fold: first, to compare CTA to US as a diagnostic tool in a larger population of dogs with suspected acute pancreatitis and, second, to evaluate CTA as a prognostic tool in this same population of dogs. We hypothesized that, in comparison to US, CTA in sedated dogs will allow for better visualization of the entire pancreas, better identify sequelae, including portal vein thrombosis, and better identify changes indicative of necrotizing pancreatitis, specifically a heterogeneous contrast enhancement pattern. We also hypothesized that CTA can be used to predict patient outcome and that specific findings identified on CTA (ie, heterogeneous contrast enhancement) will be associated with worse prognosis.
2. MATERIALS AND METHODS
2.1. Study overview
This cross‐sectional, prospective study consisted of 26 dogs, 10 of which had been included in a previous pilot study.12 The 2nd portion of the study was conducted from March 2016 to April 2017. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC), and all dog owners gave informed consent. Dogs were considered for inclusion if 2 of 3 of the following criteria were fulfilled: (1) strong suspicion of acute pancreatitis based on history, clinical signs, and physical examination findings, (2) positive SNAP cPL results and laboratory findings indicative of acute pancreatitis, and (3) US findings suggestive of acute pancreatitis. Historical and clinical findings included anorexia, acute vomiting, diarrhea, pain on abdominal palpation, or some combination of these. Laboratory findings included an inflammatory leukogram, hypocalcemia, increases in cholestatic liver enzyme activities, increased spec cPL, or some combination of these. Ultrasonographic findings included a hypoechoic, enlarged pancreas with surrounding hyperechoic mesentery with or without adjacent free fluid. Dogs were excluded from the study if sonographic evidence of gastrointestinal, hepatic, or splenic neoplasia was identified because these conditions can have clinical signs similar to those of pancreatitis.
2.2. Abdominal ultrasonography
An abdominal ultrasonographic examination was performed for inclusion or repeated within 24 hours of inclusion using a B‐mode US machine (Siemens Accuson Antares; Siemens Medical Solutions, Mountain View, California or Toshiba Aplio 500; Canon Medical Systems, Tustin, California) by a 2nd‐ or 3rd‐year radiology resident or a board‐certified radiologist. All US examinations were performed using a standard scanning protocol developed at our institution, and examinations performed by a radiology resident were later reviewed by a board‐certified radiologist. All examinations were performed with the patient in dorsal or lateral recumbency.
2.3. Computed tomographic angiography
A 3‐phase CTA was performed under sedation using a 16‐slice PET‐CT scanner (Philips Gemini TF Big Bore System; Philips Medical System, Cleveland, Ohio) within 3 days of the ultrasonographic examination, with 21 of 26 examinations occurring within 12‐24 hours of the initial US examination. The attending clinician determined sedation protocol. Twenty‐three of 26 dogs received a constant rate infusion (CRI) of propofol (Zoetis; Kalamazoo, Michigan) with an initial administration of 2‐4 mg/kg followed by a CRI of 0.2 mg/kg/min. The remaining 3 dogs were sedated with either butorphanol and midazolam or fentanyl and midazolam. The CTA examinations were performed with the patient in either sternal or dorsal recumbency and included pre‐contrast and post‐contrast arterial, venous, and delayed phases of the abdomen from the diaphragm to the caudal aspect of the left kidney. The locator for the contrast medium bolus tracer was placed over the aorta at approximately the middle aspect of the liver for determination of timed delivery of contrast in the arterial and venous phases. Two milliliters per kilogram body weight of nonionic positive contrast (350 mg I/mL Omnipaque; GE Healthcare Inc., Princeton, New Jersey) medium was administered by a CT power injector via the cephalic vein at a flow rate of 3‐4 mL/s. Arterial phase images were acquired in a cranial to caudal direction followed by venous phase images in a caudal to cranial direction. Delayed phase images were acquired approximately 2‐3 minutes post‐contrast injection. All images (pre‐contrast, arterial, venous, and delayed) were acquired using 2‐mm thick slices in a standard algorithm.
2.4. Additional tests
All dogs had a CBC, serum biochemistry, and spec cPL performed at the time of enrollment, if not previously performed. US‐guided aspirates of the pancreas using 22‐gauge needles were performed after CTA in an attempt to identify pancreatic necrosis, fibrosis, inflammation, or abscess and to eliminate the possibility of pancreatic neoplasia. When different contrast enhancement patterns were identified in regions of the pancreas, an attempt was made to sample these regions. The clinical pathologists on duty reviewed the cytologic samples.
2.5. Data collection
A radiologist (A.J.M.) and a 3rd‐year radiology resident (J.M.F.) reviewed all CTA and US examinations with careful attention to the pancreas and peripancreatic tissues. The 2 imaging procedures were compared to determine the potential added diagnostic value and clinical relevance of CTA compared to US. The CTA and US findings were analyzed in terms of severity of disease, attenuation and echogenicity of the pancreas, contrast enhancement of the pancreas, size of the pancreas, changes to the peripancreatic tissues, and the presence of specific sequelae. Ultrasonographic and CTA pancreatic enlargement were determined using previously reported reference ranges.13, 14 All CTA height and width measurements of the pancreas were made on a transverse plane image at the thickest portion of each region of the pancreas. The lobes of the pancreas were identified as the portions of the pancreas at least 5 mm caudal to the incisura pancreatitis and the body as the portion cranial to the incisura pancreatitis. Because not all regions of the pancreas were visualized by US in all dogs, the thickest portion of the pancreas identified on US images was measured.
Specific changes evaluated on both CT and US examinations included the presence of portal vein (or other venous) thrombosis, biliary tract dilatation, biliary mineralization, gastrointestinal dilatation, gastric or intestinal wall thickening, abnormal mesentery or peripancreatic fat (ie, hyperechoic mesentery on US and fat stranding on CTA), and free peritoneal fluid. The presence of lymphadenopathy was evaluated solely using CTA and according to previously reported reference ranges for size.15 The dogs recruited in the 2nd portion of the study were followed for a minimum of 9 months, and all owners were contacted at that time. Medical records also were evaluated in all dogs to determine outcome variables, including duration of hospitalization, number of relapses, time until death, and long‐term complications that may be associated with pancreatitis (eg, exocrine pancreatic insufficiency). Clinical outcome was determined by review of the medical records and client communications.
2.6. Statistical analysis
The data from CTA and US were dependent data and categorical; hence, most of the analyses were performed using a McNemar's test for categorical dependent data. Fisher's exact test was used whenever the former was not applicable to find associations. If the continuous data were normally distributed, a t‐test was used; for non‐normally distributed continuous data, a Wilcoxon test was used to compare the groups. The computer program SAS v9.4 (SAS Institute Inc., Cary, North Carolina) was used for all statistical analysis. A P‐value of .05 was defined as statistically significant.
3. RESULTS
Twenty‐six animals were evaluated: 12 female spayed, 12 male castrated, and 1 each of intact male and female dogs. The age range was 5‐12 years old (mean, 8.8 years old: median, 9.0). Breeds included 7 mixed breed, 5 miniature schnauzers, and 1 each of a Collie, Welsh Terrier, Airedale Terrier, Labrador Retriever, Australian Shepherd, Petit Basset Griffon Vendeen, Eskimo, Pug, Corgi, Keeshond, Miniature Pinscher, Italian Greyhound, Golden Retriever, and Cocker Spaniel. The most common presenting complaints were acute vomiting, anorexia, lethargy, and abdominal pain with diarrhea, hematochezia, with hematemesis being less commonly reported. No complications occurred with sedation or fine‐needle aspiration (FNA) of the pancreas.
Eight of the 26 dogs had a spec cPL result within the reported reference range (0‐200 μg/L), 4 of the 26 dogs had a spec cPL result within the equivocal range (200‐400 μg/L), and 14 of the 26 dogs had increased spec cPL (>400 μg/L). The average spec cPL was approximately 775 μg/L, with 10 of the 26 dogs having a spec cPL >1000 μg/L. Cytology showed mixed results with 9 of the 26 dogs having definitive evidence of suppurative, pyogranulomatous, or mixed inflammation and 11 of the 26 dogs having inconclusive results including nondiagnostic samples, no clinically relevant abnormalities, or mostly blood and fat. Two of the 26 dogs had possible suppurative inflammation on cytology and, in 4 of the 26 dogs, cytology was not attempted because of concern for the safety of the procedure and patient care.
3.1. Ultrasound imaging findings
The entirety of the pancreas was sonographically visualized in 19 of the 26 (73%) dogs. One dog had the left lobe and body of the pancreas previously removed because of concern for insulinoma, which was not confirmed by histopathology. The majority of dogs, 16 of the 26 dogs, had hypoechoic pancreata, 7 of the 26 dogs had heterogeneously echogenic pancreata, and 3 of the 26 dogs had normal echogenicity of the pancreas. No dog had a hyperechoic pancreas. Thirteen of the 26 dogs had enlargement of the pancreas, with a mean thickness of approximately 21.3 mm (reference range, 3.5‐16 mm).13 The most commonly affected portion of the pancreas as determined by US was the right lobe (16/26 dogs). Portal vein thrombosis was identified in 1 of the 26 dogs. Bile duct dilatation was identified in 7 of the 26 dogs. Biliary mineralization was identified in 1 of the 26 dogs. Evidence of gastric or intestinal wall thickening in the region of the inflamed pancreas was seen in 8 of the 26 dogs. Twenty‐three of the 26 dogs had hyperechoic mesentery surrounding the pancreas. Seventeen of the 26 dogs had free peritoneal fluid.
3.2. Computed tomographic angiography imaging findings
The entirety of the pancreas was visualized in all dogs using CTA. The right lobes, left lobes, and bodies were evaluated separately. The most commonly thickened region of the pancreas was the body. Fourteen of the 26 dogs had a thickened pancreatic body, with a mean height of approximately 25.8 mm. The width of the pancreatic body was not measured because of the shape of the pancreas in this region. Nine of the 26 dogs had a thickened left lobe with a mean height of approximately 22.1 mm and mean width of 24.6 mm. Eight of the 26 dogs had a thickened right lobe with a mean height of 18.9 mm and mean width of 20.0 mm.
Eleven of the 26 dogs had hypoattenuation of the left lobe, and the remaining dogs had normal attenuation pre‐IV contrast administration. Eight of the 26 dogs had heterogeneous contrast enhancement of the left lobe, and the remaining dogs were homogeneously contrast enhancing. Thirteen of the 26 dogs had hypoattenuation of the pancreatic body, and the remaining dogs were normally attenuating pre‐contrast. Nine of the 26 dogs had heterogeneous contrast enhancement of the pancreatic body, and the remaining dogs were homogeneously contrast enhancing. Seven of the 26 dogs had hypoattenuation of the right lobe, and the remaining dogs were normally attenuating pre‐contrast. Three of the 26 dogs had heterogeneous contrast enhancement of the right lobe, and the remaining dogs were homogeneously contrast enhancing. In total, 10 of the 26 dogs had heterogeneous contrast enhancement of some portion of the pancreas. Comparative images of normal pancreas, homogeneous contrast enhancement of the pancreas, and heterogeneous contrast enhancement are shown in Figure 1.
Figure 1.
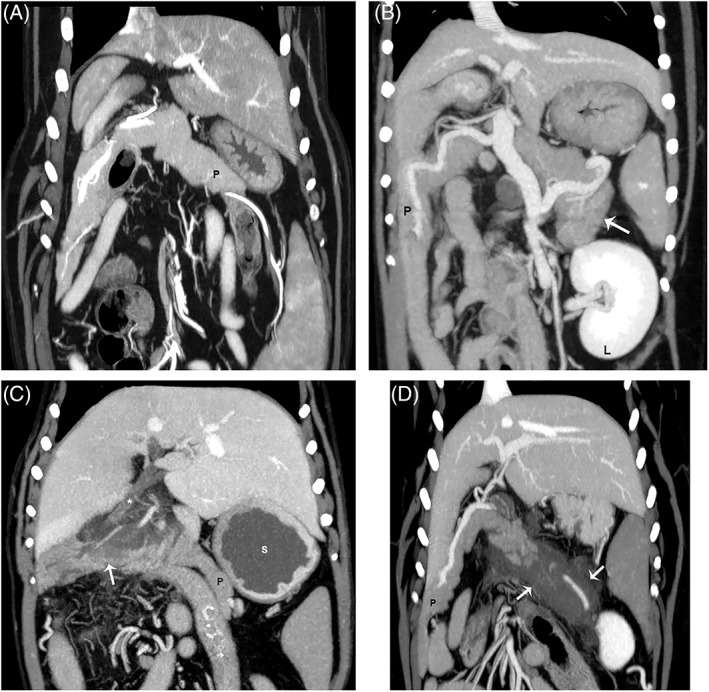
Dorsal plane reconstructions of the pancreas from different dogs in venous phase for all images. (A) Comparison of a normal pancreas (P); (B) a pancreas with homogeneous contrast enhancement and thickening of the left lobe (white arrow). The left kidney is visible (L); (C) a pancreas with heterogeneous contrast enhancement (arrow) of the mid body, and (D) a pancreas with a severe form of heterogeneous contrast enhancement (arrows). Notice the dilatation of the common bile duct (*) and fluid filled stomach (s) likely indicating gastric ileus in C.
Portal vein thrombosis was identified in 10 of the 26 dogs (Figure 2). Biliary duct dilatation was identified in 6 of the 26 dogs. Biliary mineralization was identified in 11 of the 26 dogs (Figure 3). Twelve of the 26 dogs had gastric or intestinal wall thickening or dilatation in the region of the inflamed pancreas. Fat stranding or other evidence of inflammation within the mesentery surrounding the pancreas was identified in 20 of the 26 dogs. Peritoneal effusion was present on CT in 15 of the 26 dogs. Lymphadenomegaly was identified in 11 of the 26 dogs.
Figure 2.
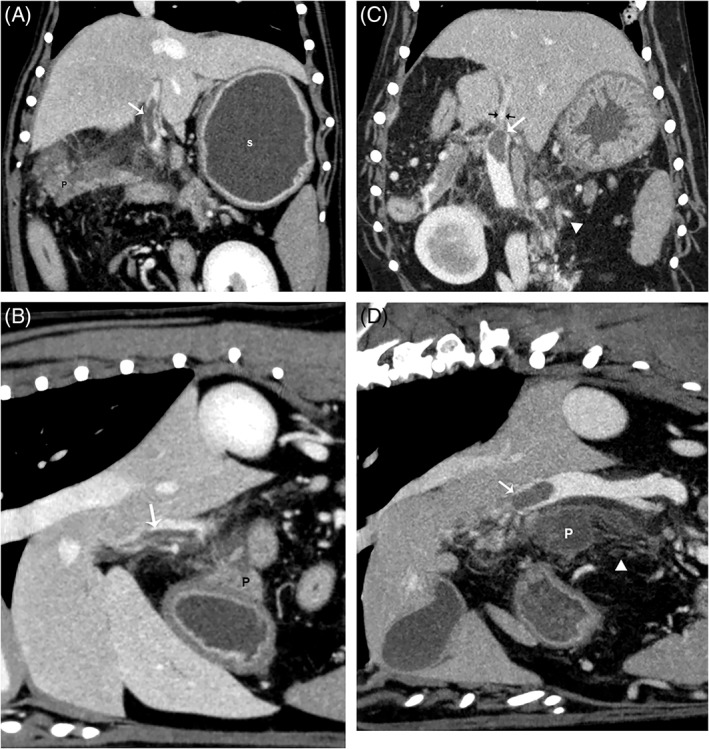
(A, C) Dorsal and (B, D) sagittal plane reconstructions from different dogs in a venous phase for all images. There are large filling defects (arrows) within the main portal vein immediately adjacent to the body of the pancreas (P). This was the most common location of thrombus formation identified. Notice in C (black arrows), the marked tapering of the portal vein immediately cranial to the filling defect likely indicating occlusion of the vessel. Also notice the heterogeneous contrast enhancement of the pancreas in A and the fluid filled stomach (s) likely indicating gastric ileus. In C, D, there is marked fat stranding indicating peripancreatic inflammation (white arrowheads in C and D).
Figure 3.
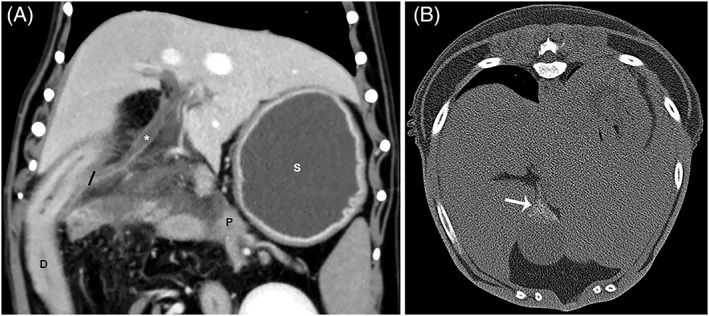
(A), Dorsal plane reconstruction in venous phase of biliary duct dilatation (*). The black line indicates the level of the insertion of the common bile duct on the duodenum. (B) Transverse non‐contrast image in a bone window with the dog in dorsal recumbency highlighting mineralization within the gall bladder (arrow). In image A, the pancreas (P) is seen immediately adjacent to the insertion of the common bile duct on the duodenum (D). Also notice the fluid filled stomach (S) indicating gastric ileus. Biliary mineralization was significantly more likely to be identified by CTA than ultrasound.
3.3. Comparison of Computed tomographic angiography and US imaging findings
Overall, CTA better visualized the entirety of the pancreas than did US. The entirety of the pancreas was visualized in 100% of the dogs by CTA, whereas the entirety of the pancreas was only visualized by US in 73% of the dogs. Computed tomographic angiography did not significantly identify changes indicative of pancreatitis, including pancreatic enlargement, mesenteric changes (P = .12), or changes in attenuation and echogenicity better than US (P = .14).
Comparison between detection of sequelae by CTA versus US is shown in Table 1. Portal vein thrombosis was significantly more likely to be detected by CTA when compared to US (P = .004). Computed tomographic angiography identified portal vein thrombi in 10 dogs. Among those 10 dogs, US examination was only able to detect a portal vein thrombus in 1 dog. Computed tomographic angiography was significantly more likely to detect biliary mineralization than was US (P = .002). Computed tomographic angiography detected biliary mineralization in 10 dogs, and of those 10 dogs, only 1 dog had biliary mineralization detected by US examination. No significant difference was found in the detection of the remaining dogs of peripancreatic changes or sequelae evaluated between CTA and US (ie, peritoneal effusion, gastric or intestinal wall thickening, biliary dilatation, or mesenteric changes).
Table 1.
Comparison of sequelae detection by ultrasound (US) and CT angiography (CTA)
| US | CTA | |
|---|---|---|
| Entire pancreas visualized | 19/26 (73%) | 26/26 (100%) |
| Pancreatic enlargement | 13/26 (50%) | 18/26 (69%) |
| Portal vein thrombosis | 1/26 (4%) | 10/26 (38%) |
| Extra‐hepatic biliary obstruction | 6/26 (23%) | 7/26 (27%) |
| Cholelithiasis | 1/26 (4%) | 11/26 (42%) |
| Gastric/intestinal wall thickening | 8/26 (31%) | 12/26 (46%) |
| Free fluid | 17/26 (65%) | 15/26 (58%) |
| Mesenteric changes | 23/26 (88%) | 20/26 (77%) |
3.4. Outcome and CTA as a prognostic tool
Twelve of the 26 dogs had confirmed outcomes with a known cause of death or were still alive at the time of review with 14 of 26 dogs lost to follow‐up with the last communications ranging between immediately after hospitalization to 1184 days after hospitalization. Causes of death were variable with 4 dogs dying from acute pancreatitis or complications of acute pancreatitis. One dog died 371 days after hospitalization from acute necrotizing pancreatitis. The remaining dogs with known outcomes were still alive at the time of review or died from diseases unrelated to pancreatitis. The average time until death was 269 days. The average time until death for the dogs with heterogeneous contrast enhancement was 287 days and for dogs with homogeneous contrast enhancement was 254 days. The time until death was not significantly different between the 2 groups (P = .78). Other outcome variables assessed included number of relapses and duration of hospitalization. Eight dogs experienced a relapse in pancreatitis. The mean duration of hospitalization for all dogs was 3.6 days.
When evaluating CTA as a prognostic tool, heterogeneous contrast enhancement and the presence of specific sequelae were analyzed individually to determine a correlation between these variables and outcome variables, including duration of hospitalization, number of relapses, and time until death. This analysis is shown in Table 2. Heterogeneous contrast enhancement was significantly associated with longer hospitalization (P = .01). Dogs with homogeneous contrast enhancement of the pancreas had a mean hospitalization of 2.8 days, whereas those dogs with heterogeneous contrast enhancement of any portion of the pancreas had a mean duration of hospitalization of 4.8 days. Dogs with heterogeneous contrast enhancement also were significantly more likely to be hospitalized for ≥5 days (P = .03).
Table 2.
CT angiography prognostication based on various imaging findings
| <4 d hospitalization | ≥5 d hospitalization | 0 relapses | ≥1 relapse | Mean time until death (d) | |
|---|---|---|---|---|---|
| Heterogeneous contrast enhancement | 4/25 (16%) | 6/25a (24%) | 3/23 (13%) | 5/23 (22%) | 287 |
| Homogeneous contrast enhancement | 13/25 (52%) | 2/25 (8%) | 12/23 (52%) | 3/23 (13%) | 254 |
| Portal vein thrombosis+ | 4/26 (15%) | 6/26b (23%) | 4/24 (17%) | 4/24 (17%) | 288 |
| Portal vein thrombosis− | 14/26 (54%) | 2/26 (8%) | 12/24 (50%) | 4/24 (17%) | 257 |
| Lymphadenomegaly+ | 4/26 (15%) | 7/26c (27%) | 5/24 (20%) | 5/24 (20%) | 409d |
| Lymphadenomegaly− | 14/26 (54%) | 1/26 (4%) | 11/24 (46%) | 3/24 (13%) | 167 |
| Biliary dilatation+ | 4/26 (15%) | 2/26 (8%) | 2/24 (8%) | 3/24 (13%) | 345 |
| Biliary dilatation− | 14/26 (54%) | 6/26 (23%) | 14/24 (58%) | 5/24 (20%) | 247 |
| Cholelithiasis+ | 8/26 (31%) | 3/26 (12%) | 8/24 (33%) | 3/24 (13%) | 399 |
| Cholelithiasis− | 10/26 (38%) | 5/26 (19%) | 8/24 (33%) | 5/24 (20%) | 175 |
| Gastric/intestinal wall thickening+ | 6/26 (23%) | 6/26 (23%) | 6/24 (25%) | 4/24 (17%) | 249 |
| Gastric/intestinal wall thickening− | 12/26 (46%) | 2/26 (8%) | 10/24 (42%) | 4/24 (17%) | 287 |
| Mesenteric changes+ | 13/26 (50%) | 7/26 (27%) | 11/24 (46%) | 7/24 (29%) | 298 |
| Mesenteric changes− | 5/26 (19%) | 1/26 (4%) | 5/24 (20%) | 1/24 (4%) | 173 |
| Free fluid+ | 9/26 (35%) | 6/26 (23%) | 7/24 (29%) | 6/24 (25%) | 359 |
| Free fluid− | 9/26 (35%) | 2/26 (8%) | 9/24 (38%) | 2/24 (8%) | 148 |
P‐value = .03.
P‐value = .03.
P‐value = .003.
P‐value = .03.
Dogs with portal vein thrombosis had a significantly longer duration of hospitalization. Mean hospitalization was 3 days in dogs without portal vein thrombosis and 4.5 days in dogs with portal vein thrombosis (P = .05), and dogs with portal vein thrombosis were significantly more likely to be hospitalized for ≥5 days (P = .03). Finally, dogs with lymphadenomegaly identified by CT also were significantly more likely to be hospitalized for ≥5 days (P = .003), and median time until death was significantly longer in dogs with lymphadenomegaly (P = .03).
The remaining variables evaluated were not significantly associated. However, when evaluating pancreatic contrast enhancement and number of relapses, patients with heterogeneous contrast enhancement were somewhat more likely to have ≥1 relapse than were patients with homogeneous contrast enhancement but nonsignificantly (P = .07). The relative risk of relapse among dogs with heterogeneous contrast enhancement was 3.13 times that of dogs with homogeneous contrast enhancement. A similar result was found in patients with portal vein thrombosis. Patients with portal vein thrombosis were somewhat more likely to have ≥1 relapse but nonsignificantly (P = .09).
3.5. Pancreatic contrast enhancement, portal vein thrombosis, and spec CPL
When comparing the presence of CT contrast enhancement to the presence of portal vein thrombosis, a significantly higher proportion of subjects with portal vein thrombosis was found in the heterogeneous contrast enhancement group (P = .002). Eighty percent of dogs with heterogeneous contrast enhancement had portal vein thrombosis, whereas only 13% of dogs with homogeneous contrast enhancement had portal vein thrombosis.
Finally, when comparing pancreatic contrast enhancement to spec cPL results, a significantly higher median cPL was observed in dogs with heterogeneous contrast enhancement (P = .007). Dogs with homogeneous contrast enhancement had a median spec cPL of 381 μg/L (range, 30‐1000 μg/L), whereas those with heterogeneous contrast enhancement had a median spec cPL of 1631.5 μg/L (range, 122‐2000 μg/L).
4. DISCUSSION
Similar to human patients with pancreatitis, 3‐phase CTA is a useful tool in the diagnosis and prognostication of acute pancreatitis in dogs.8, 16, 17 Evidence of heterogeneous pancreatic contrast enhancement on CTA is associated with pancreatic necrosis and worse prognosis in dogs as in people.7, 8, 18, 19, 20 In our study, dogs with heterogeneous contrast enhancement on CTA also had a significantly longer duration of hospitalization including the likelihood to be hospitalized for ≥5 days, had an increased number of relapses, and were significantly more likely to have portal vein thrombosis. Our findings also confirm that CTA is significantly more likely to detect the presence of portal vein thrombosis than is US. Also, patients with heterogeneous contrast enhancement had significantly increased spec cPL when compared to those with homogeneous pancreatic contrast enhancement. To the best of our knowledge, ours is the 1st reported correlation between a serum assay for the detection of pancreatitis and CTA findings in dogs.
The 1st goal of our study was to determine if CTA is more accurate than US in the detection of pancreatitis, pancreatic changes that may indicate necrosis (ie, heterogeneous contrast enhancement), and sequelae of pancreatitis. Although CTA allowed better visualization of the entire pancreas than did US, it was not better at identifying those animals suffering from acute pancreatitis. Because US examination is operator dependent, this finding may indicate that experienced sonographers were performing the US studies at our institution. Less experienced sonographers may have more difficulty in identifying the entire pancreas, and CTA may be a better diagnostic imaging tool in certain settings. The increased utility of CTA when compared to US is a result of its ability to detect changes that may indicate more severe forms of acute pancreatitis and its ability to detect sequelae, most importantly portal vein thrombosis. In our study, heterogeneous pancreatic contrast enhancement and portal vein thrombosis were strongly correlated, and portal vein thrombosis was significantly more likely to be detected by CTA than US.
Portal vein thrombosis may be the result of a coagulopathy associated with pancreatitis or other comorbidities or a consequence of local endothelial injury secondary to regional inflammation.21, 22 In the cranial abdomen, the portal vein lies in close proximity to the pancreas just before entering the liver. The majority of the patients in our study developed thrombi immediately adjacent to the body of the pancreas within the main portal vein. Given the anatomy of this region and the surrounding structures, it is not surprising that portal vein thrombi were not commonly detected by US because this area is deep in the abdomen. In addition, most patients undergoing US for acute pancreatitis are severely painful in the cranial abdomen and have other pathologic changes associated with this disease that make evaluation of the cranial abdomen difficult, such as gastric ileus, peritoneal effusion, regional steatitis, and peritonitis, all of which may attenuate the US beam.23 Portal vein thrombosis was detected by US in only 1 of the 26 (4%) dogs in our study.
In a previous study evaluating risk factors in dogs with fatal acute pancreatitis, 96% of dogs had evidence of pancreatic necrosis at necropsy.24 Thrombus formation also was found more commonly in dogs with fatal acute pancreatitis than in control dogs.24 This finding is consistent with the human medical literature that has not only shown higher mortality and morbidity in patients with evidence of pancreatic necrosis on CTA but also has shown a strong correlation between these variables and extent of pancreatic necrosis. A previous report showed that human patients with <30% necrosis had 0% mortality and 48% morbidity, whereas those patients with >30% necrosis had morbidity of 94% and mortality of 29%.7, 8 In our study, heterogeneous contrast enhancement and portal vein thrombosis were both significantly associated with longer hospitalization, with the majority of these patients being hospitalized for >5 days. These findings may be useful to determine treatment recommendations for dogs with more severe forms of pancreatitis and to advise owners of duration of hospitalization and possible complications.
Interestingly, 2 of the 4 dogs that died or were euthanized during the acute stage of disease also were dogs with heterogeneous contrast enhancement of all portions of the pancreas (ie, right lobe, left lobe, and body). One of those dogs had severe focal infarction of the proximal duodenum resulting in duodenal necrosis and subsequent perforation (Figure 4). Necropsy and histopathology confirmed that this dog was suffering from acute, extensive, necrosuppurative pancreatitis with hemorrhage and fat necrosis. In addition, gastritis, hepatitis, and severe cholestasis with possible necrosis of the bile duct were identified at necropsy. This type of duodenal infarction also was reported in a previous clinicopathologic survey of dogs and cats with pancreatitis. In that study, a single dog had complete infarction of the cranial mesenteric artery with gangrenous necrosis of the proximal duodenum.25 Identifying diffuse heterogeneous pancreatic enhancement on CTA may indicate a grave prognosis in dogs with acute pancreatitis.
Figure 4.
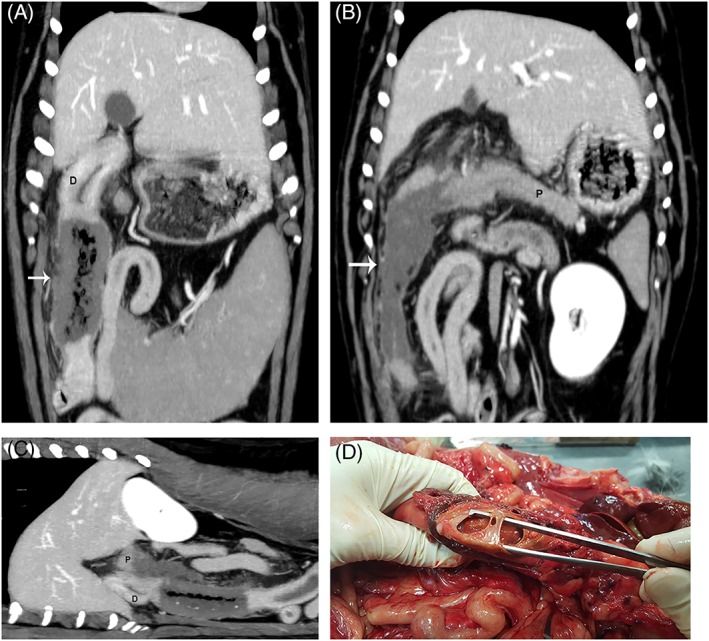
(A, B) Dorsal plane reconstructions in venous phase of the same dog. (A) Markedly thickened and non‐contrast enhancing portion (arrow) of the proximal duodenum (D) consistent with infarction. (B) Image of the non‐contrast enhancing portion (arrow) of the pancreas (P) adjacent to the infarcted duodenum. (C), Sagittal oblique plane reconstruction of the pancreas (P) and duodenum (D) to highlight the pancreatic and duodenal changes. (D) Gross image of the infarcted duodenum with a large perforation within the duodenal wall (forceps). Necropsy and histopathology confirmed that this dog was suffering from acute, extensive, necrosuppurative pancreatitis with hemorrhage, and fat necrosis.
Finally, those dogs with heterogeneous contrast enhancement had significantly increased median spec cPL compared to dogs with homogeneous contrast enhancement. The median result in dogs with heterogeneous contrast enhancement was 1631.5 μg/L compared to 381 μg/L in dogs with homogeneous contrast enhancement. This finding is interesting because it is the 1st time that a correlation between CT findings and a pancreatic serum assay has been noted. Outside histopathology, the spec cPL test has been shown in multiple studies to be the most sensitive and specific indicator of pancreatic acinar cell damage, with previously reported sensitivity and specificity of 71.7%‐77.8% and 80.5%‐88.0%, respectively.4, 26, 27 This information may be utilized by clinicians to indicate the severity of pancreatitis in dogs with acute pancreatitis and allow treatment and prognostic recommendations that are more individualized for each patient, if imaging is not possible or in conjunction with imaging. However, the imaging of dogs with pancreatitis remains critical for diagnosis because the extent of disease and the presence and type of sequelae can only be determined by imaging. This information adds additional prognostic value and may alter treatment options.
Additional findings in our study that were unexpected were the increased detection of biliary mineralization by CTA and the significantly longer hospitalization and longer time until death seen with lymphadenomegaly. Biliary mineralization was most commonly in the form of sediment or “sand‐like” cholelithiasis, with no dogs in our study having larger choleliths. Cholelithiasis previously has been associated with biliary stasis, altered dietary composition, and cholecystitis.28, 29, 30 Whether this finding is a result of decreased gall bladder emptying or inflammation associated with pancreatitis or is associated with some comorbidity that may contribute to both diseases is unknown, but previous studies have shown pancreatitis to be the most common cause of extra‐luminal biliary tract obstruction.30 Because US is considered a good diagnostic tool for detecting cholelithiasis, this finding was surprising. Perhaps, the better soft tissue resolution of CT and the non‐dependence on patient comfort and compliance as well as the small volume of mineralization in most dogs played a role in the better detection of biliary mineralization by CT imaging.
Based on CT imaging, 11 of the 26 dogs in our study had lymph node enlargement. The most commonly affected nodes were the hepatic, pancreaticoduodenal, and mesenteric. None of the affected nodes were sampled, but all lymph nodes were mildly enlarged with normal smooth contour and normal homogeneous parenchyma, which have been shown to be more consistent with reactive lymphadenopathy.15, 31, 32 The correlation between lymphadenomegaly and longer hospitalization time likely indicates a stronger immune and inflammatory response in those animals with more severe pancreatitis. The longer time until death in these patients was, however, unexpected. We suspect this finding is likely the result of small sample size and the fact that a large number of patients survived beyond the acute stage of the disease, were lost to follow‐up, or both.
A shorter time until death was expected but not found in patients with pancreatic necrosis, portal vein thrombosis, or both. This result was also likely a consequence of the small sample size and the large number of animals that were lost to follow‐up. Additional notable sequelae that were identified in a number of animals by both CT and US included gastric and intestinal wall thickening, likely secondary to regional inflammation, biliary dilatation, and peritoneal effusion.
One of the major limitations of our study was the lack of histopathology of the pancreas and definitive confirmation of thrombosis in the majority of patients. The only patients in which histopathology results were available were the relatively few animals that died or were euthanized as a consequence of their disease, but CTA findings were confirmed in these dogs. Histopathology is the gold standard for diagnosis of pancreatic necrosis, but studies have shown the negative complications associated with surgical biopsy of the pancreas, and it is commonly accepted that pancreatic biopsy during acute pancreatitis may further injure the pancreas.33, 34 Alternatively, FNA of the pancreas is a safe and easy procedure. In a previous study of healthy Beagles undergoing both fine needle and clamshell biopsy of the pancreas, no increase in serum trypsin‐like immunoreactivity was observed after FNA of the pancreas, whereas an increase occurred after surgical biopsy.35 An additional study of 73 cats with clinical or sonographic evidence of pancreatic disease undergoing FNA of the pancreas showed no increased risk of complications when compared to cats undergoing FNA of other organs or when compared to control cats.36
Another limitation of our study was the variability of clinician experience in performing US examinations. Ultrasonography is a highly operator‐dependent modality. This factor may have influenced the overall ability of US to evaluate all regions of the pancreas. It is a standard practice in our institution that all US scans performed by a radiology resident are carefully reviewed by a staff radiologist and complicated examinations or examinations in which not all organs are visualized usually are repeated by a staff radiologist. In addition, it was our intention to limit the degree of intervention in the clinical course of these patients, because we wanted to mimic the normal clinical examination as closely as possible.
A final limitation was the lack of blinding of the imaging studies. Both reviewers knew these dogs were suspected to have pancreatitis based on enrollment in the study, which introduced an inherent bias. However, all studies were reviewed months after enrollment to minimize recall of the initial imaging findings.
In conclusion, CTA identified dogs with a heterogeneously contrast‐enhancing pancreas, a more severe form of acute pancreatitis. Heterogeneous pancreatic enhancement along with portal vein thrombosis may predict longer hospitalization and increased risk of relapse, which may affect the clinical management of patients with acute pancreatitis. Our findings support the use of CTA as an additional and superior diagnostic modality in the evaluation of acute pancreatitis in dogs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
This work was performed at Colorado State University. A portion of this work was presented as an abstract at the American College of Veterinary Radiology Annual Meeting, October 2017, Phoenix, Arizona. A portion of the research was presented at the ECVIM‐CA 28th Annual Congress, September 2018, Rotterdam, The Netherlands.
French JM, Twedt DC, Rao S, Marolf AJ. Computed tomographic angiography and ultrasonography in the diagnosis and evaluation of acute pancreatitis in dogs. J Vet Intern Med. 2019;33:79–88. 10.1111/jvim.15364
REFERENCES
- 1. Watson PJ, Roulois AJA, Scase T, Johnston PEJ, Thompson H, Herrtage ME. Prevalence and breed distribution of chronic pancreatitis at post‐mortem examination in first‐opinion dogs. J Small Anim Pract. 2007;48(11):609‐618. [DOI] [PubMed] [Google Scholar]
- 2. Mansfield C. Acute pancreatitis in dogs: advances in understanding, diagnostics, and treatment. Top Companion Anim Med. 2012;27(3):123‐132. [DOI] [PubMed] [Google Scholar]
- 3. Hess RS, Saunders HM, Van Winkle TJ, Shofer FS, Washabau RJ. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986‐1995). J Am Vet Med Assoc. 1998;213(5):665‐670. [PubMed] [Google Scholar]
- 4. Mccord K, Morley PS, Armstrong J, et al. A multi‐institutional study evaluating the diagnostic utility of the spec cPL. J Vet Intern Med. 2012;26(4):888‐896. [DOI] [PubMed] [Google Scholar]
- 5. Steiner JM. Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract. 2003;33(5):1181‐1195. [DOI] [PubMed] [Google Scholar]
- 6. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102‐111. [DOI] [PubMed] [Google Scholar]
- 7. Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174(2):331‐336. [DOI] [PubMed] [Google Scholar]
- 8. Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology. 2002;223(3):603‐613. [DOI] [PubMed] [Google Scholar]
- 9. Maier W. Early objective diagnosis and staging of acute pancreatitis by contrast‐enhanced computed tomography Acute Pancreatitis. Berlin, Heidelberg: Springer Berlin Heidelberg; 1987:132‐140. [Google Scholar]
- 10. Bradley EL, Murphy F, Ferguson C. Prediction of pancreatic necrosis by dynamic pancreatography. Ann Surg. 1989;210(4):495‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beger HG, Maier W, Block S, Büchler M. How do imaging methods influence the surgical strategy in acute pancreatitis? Diagnostic Procedures in Pancreatic Disease. Berlin, Heidelberg: Springer Berlin Heidelberg; 1986:54‐60. [Google Scholar]
- 12. Adrian AM, Twedt DC, Kraft SL, Marolf AJ. Computed tomographic angiography under sedation in the diagnosis of suspected canine pancreatitis: a pilot study. J Vet Intern Med. 2015;29(1):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Penninck DG, Zeyen U, Taeymans ON, Webster CR. Ultrasonographic measurement of the pancreas and pancreatic duct in clinically normal dogs. Am J Vet Res. 2013;74(3):433‐437. [DOI] [PubMed] [Google Scholar]
- 14. Caceres AV, Zwingenberger AL, Hardam E, et al. Helical computed tomographic angiography of the normal canine pancreas. Vet Radiol Ultrasound. 2006;47(3):270‐278. [DOI] [PubMed] [Google Scholar]
- 15. Beukers M, Grosso FV, Voorhout G. Computed tomographic characteristics of presumed normal canine abdominal lymph nodes. Vet Radiol Ultrasound. 2013;54(6):610‐617. [DOI] [PubMed] [Google Scholar]
- 16. London N, Neoptolemos J, Lavelle J, Bailey I, James D. Contrast‐enhanced abdominal computed tomography scanning and prediction of severity of acute pancreatitis: a prospective study. Br J Surg. 1989;76:268‐272. [DOI] [PubMed] [Google Scholar]
- 17. Bollen TL, Singh VK, Maurer R, et al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107(4):612‐619. [DOI] [PubMed] [Google Scholar]
- 18. Paulson E, Vitellas K, Keogan M, Low V, Nelson R. Acute pancreatitis complicated by gland necrosis: spectrum of findings on contrast‐enhanced CT. Am J Roentgenol. 1999;172:609‐613. [DOI] [PubMed] [Google Scholar]
- 19. Johnson C, Stephens D, Sarr M. CT of acute pancreatitis: correlation between lack of contrast and pancreatic necrosis. Am J Roentgenol. 1991;156:93‐95. [DOI] [PubMed] [Google Scholar]
- 20. Balthazar E, Ranson J, Naidich D, Megibow A, Caccavale R, Cooper M. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156(3):767‐772. [DOI] [PubMed] [Google Scholar]
- 21. Van Winkle TJ, Bruce E. Thrombosis of the portal vein in 11 dogs. Vet Pathol. 1993;30:28‐35. [DOI] [PubMed] [Google Scholar]
- 22. Respess M, O'Toole TE, Taeymans O, Rogers CL, Johnston A, Webster CRL. Portal vein thrombosis in 33 dogs: 1998‐2011. J Vet Intern Med. 2012;26(2):230‐237. [DOI] [PubMed] [Google Scholar]
- 23. Penninck D. Atlas of Small Animal Ultrasonography. 2nd ed. Ames, IA: Wiley‐Blackwell; 2015:459‐465. [Google Scholar]
- 24. Hess RS, Kass PH, Shofer FS, Van Winkle TJ, Washabau RJ. Evaluation of risk factors for fatal acute pancreatitis in dogs. J Am Vet Med Assoc. 1999;214(1):46‐51. [PubMed] [Google Scholar]
- 25. Schaer M. A clinicopathologic survey of acute pancreatitis in 30 dogs and 5 cats. J Am Anim Hosp Assoc. 1979;15:681‐687. [Google Scholar]
- 26. Trivedi S, Marks SL, Kass PH, et al. Sensitivity and specificity of canine pancreas‐specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med. 2011;25(6):1241‐1247. [DOI] [PubMed] [Google Scholar]
- 27. Steiner JM, Newman S, Xenoulis P, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther. 2008;9:263‐273. [PubMed] [Google Scholar]
- 28. Center SA. Diseases of the gallbladder and biliary tree. Vet Clin North Am Small Anim Pract. 2009;39(3):543‐598. [DOI] [PubMed] [Google Scholar]
- 29. Mattoon JSNT. Small Animal Diagnostic Ultrasound. 3rd ed. St. Loius, MO: Elsevier Inc.; 2015:438‐443. [Google Scholar]
- 30. Fahie M, Martin R. Extrahepatic biliary tract obstruction: a retrospective study of 45 cases (1983‐1993). J Am Anim Hosp Assoc. 1995;31:478‐482. [DOI] [PubMed] [Google Scholar]
- 31. Kinns J, Mai W. Association between malignancy and sonographic heterogeneity in canine and feline abdominal lymph nodes. Vet Radiol Ultrasound. 2007;48(6):565‐569. [DOI] [PubMed] [Google Scholar]
- 32. Ballegeer EA, Adams WM, Dubielzig RR, Paoloni MC, Klauer JM, Keuler NS. Computed tomography characteristics of canine tracheobronchial lymph node metastasis. Vet Radiol Ultrasound. 2010;51(4):397‐403. [DOI] [PubMed] [Google Scholar]
- 33. Lidbury JA, Suchodolski JS. New advances in the diagnosis of canine and feline liver and pancreatic disease. Vet J. 2016;215:87‐95. [DOI] [PubMed] [Google Scholar]
- 34. Krogvold L, Edwin B, Buanes T, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. 2014;57(4):841‐843. [DOI] [PubMed] [Google Scholar]
- 35. Cordner AP, Armstrong PJ, Newman SJ. Effect of pancreatic tissue sampling on serum pancreatic enzyme levels in clinically healthy dogs. J Vet Diagn Invest. 2016;707:1‐6. [DOI] [PubMed] [Google Scholar]
- 36. Crain SK, Sharkey LC, Cordner AP, Knudson C, Armstrong PJ. Safety of ultrasound‐guided fine‐needle aspiration of the feline pancreas: a case‐control study. J Feline Med Surg. 2015;17(10):858‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]


