Abstract
Background
There is an association between overweight status and life span in kenneled dogs, but a similar association has not been reported for pet dogs.
Objectives
To examine the effects of being overweight in middle age on the life span of neutered client‐owned dogs.
Animals
Fifty‐thousand seven‐hundred eighty seven middle‐aged neutered client‐owned dogs attending a network of approximately 900 veterinary hospitals across North America.
Methods
Retrospective case‐control study. For each of 12 breeds, groups of dogs aged between 6.5 and 8.5 years were identified as being in “overweight” or “normal” body condition. Within each breed and sex, differences in life span between dogs in normal body condition and overweight body condition in the 2 groups were then analyzed by Cox proportional hazards models.
Results
For all breeds, instantaneous risk of death for dogs in overweight body condition was greater than those in normal body condition throughout the age range studied, with hazard ratios ranging from 1.35 (99.79% confidence interval [CI] 1.05‐1.73) for German Shepherd dog to 2.86 (99.79% CI 2.14‐3.83) for Yorkshire Terrier. In all breeds, median life span was shorter in overweight compared with normal weight dogs, with the difference being greatest in Yorkshire Terriers (overweight: 13.7 years, 99.79% CI 13.3‐14.2; normal: 16.2 years, 99.79% CI 15.7‐16.5) and least in German Shepherd dogs (overweight: 12.1 years, 99.79% CI 11.8‐12.4; normal: 12.5 years, 99.79% CI 12.2‐12.9).
Conclusions and Clinical Importance
Veterinary professionals should consider promoting healthy body condition for dogs, particularly from midlife onward.
Keywords: canine, longevity, nutrition, obesity, survival
Abbreviations
- BCS
body condition score
- CEM
coarsened exact matching
- CI
confidence interval
- HR
hazard ratio
- PH
proportional hazards
- WAT
white adipose tissue
1. INTRODUCTION
Obesity is characterized by an expansion of white adipose tissue (WAT)1 and is now a major health concern in pet dogs,1 with recent evidence suggesting a rapidly increasing prevalence.2 Dogs that are overweight or have obesity are at increased risk of developing a range of chronic diseases including orthopedic diseases, diabetes mellitus, and certain types of neoplasia.1, 3 Metabolic derangements,4, 5 functional impairment (most notably respiratory, cardiovascular, and renal function),6, 7, 8 and adverse effects on quality of life also occur.9 Parallels exist between canine and human obesity because both are outbred species that share a similar environment,1 and similar disease associations are seen including diabetes mellitus, cardiovascular disease, and hypertension.10 As such, both the international medical and veterinary communities have advised formally classifying obesity as a disease.11 The expansion of WAT causes secondary disease in 2 ways: through the “mechanical” impact of increased tissue mass or volume on function and through the effects of perturbed endocrine function.1 Both pro‐inflammatory cytokines and acute phase proteins are produced by WAT, and both their tissue expression and circulating concentration are altered by obesity in humans and dogs.12, 13 The chronic low‐grade systemic inflammation that results is thought to provide the link among obesity, insulin resistance, and the metabolic syndrome.12
In addition to increasing disease risk and causing functional impairment, having an overweight body condition increases mortality risk in humans worldwide.14 All‐cause mortality is least in adults with a body mass index of 20.0‐25.0 and increases significantly and incrementally throughout the overweight range.14 In veterinary studies, there are negative associations between life span and both underweight and obese body condition in cats from a single veterinary practice in Sydney, Australia,15 and evidence for overweight condition having an adverse effect on life span effects in a lifelong feeding study involving a colony of Labrador Retriever dogs.16, 17, 18, 19 In this latter study, dogs were paired, with 1 dog in each pair being fed ad libitum, whereas the other dog was fed 25% less food than its pair‐mate from 8 weeks of age until death. Ad‐libitum‐fed dogs had a greater body condition and a shorter median life span than dogs of the restricted‐feeding group.17 Although this suggests a possible association between overweight condition and shortened life span in dogs maintained in a controlled colony environment, to date, similar effects have not been studied in client‐owned domesticated dogs. Therefore, the aim of the current study was to explore possible associations between body condition and life span using a large database of veterinary health records from pet dogs in North America.
2. METHODS
2.1. Study design
This was a retrospective case‐control study to investigate longevity in dogs, utilizing demographic, geographic, and clinical data from dogs registered with a North American veterinary hospital network (BANFIELD Pet Hospitals).
2.2. Data extraction and study population
The network comprised 900 veterinary hospitals located predominantly in the United States (BANFIELD Pet Hospitals), which have electronic records dating back to 1994. All medical notes from these records were anonymized by removing client‐identifying details and then stored in an object‐related database management system (Oracle 11g release 2, Oracle Corporation, Redwood Shores, California), hereafter referred to as the “records database.” Available records included those from April 1994 to September 2015. Data were extracted for purebred individuals from 12 of the most popular breeds representing the 5 size classes defined by similarities in patterns of growth.20 The breeds studied were American Cocker Spaniel, Beagle, Boxer, Chihuahua, Dachshund, German Shepherd dog, Golden Retriever, Labrador Retriever, Pit Bull Terrier type, Pomeranian, Shih Tzu, and Yorkshire Terrier. The data extracted included demographic (breed, sex, neuter status, and date of birth) and geographic (latitude and longitude of the owner's zip code) variables, plus data collected during in‐clinic visits (date of visit, bodyweight, and if available body condition), and date of death. Pedigree status and date of birth are both owner‐reported parameters and were not verified by the veterinary staff.
2.3. Eligibility criteria
Dogs were eligible for inclusion when they had at least 1 “in‐clinic” visit (defined as an appointment when both the owner and dog were in attendance), were between 6.5 and 8.5 years, and whose veterinary care had not ceased (eg, because of death or moving to a different veterinary practice) before 9.5 years. In this respect, there had to be some contact with the owner after this time, for example, an in‐clinic visit or a phone consultation about the dog. This was necessary so as to minimize any possible influence of life‐threatening disease on the body condition assessment, as is customary in similar human studies comparing the association between overweight status and mortality.14 In addition, dogs had to be neutered and from 1 of the 12 most common breeds in the database (see above). Because most dogs were neutered before 2 years, only data from visits after this age were used in modeling studies to ensure that neuter status would not change during follow‐up. Furthermore, for the group‐matching process (see below), a single in‐clinic visit was chosen for each dog as the representative visit, and this was the 1 that was closest to 7.5 year. This ensured that each dog was only included once during matching.
2.4. Age, date of birth, and date of death
The age of the dog at each visit was calculated from the visit date and the date of birth of the dog. Date of birth is a field within the computer electronic records and must be recorded for all dogs; the field is completed at the time the dog is registered with the practice, based on information provided by the owner. Date of death is also a field within the computer electronic records and was completed when dogs died or were euthanized, along with the reason for death. If the euthanasia is conducted by a veterinarian at the practice, the field is manually completed. Neither the date of birth nor date of death fields are verified for accuracy. For dogs with a recorded death date, life span was calculated from date of birth to date of death; where no date of death was not recorded, survival data were censored at the date of the last contact (clinic visit or phone consultation).
2.5. Body condition assessment
Before 2010, body condition was assessed by a subjective 3‐category classification (“thin,” “normal,” or “heavy”). After 2010, a 5‐category body condition score (BCS) was used, based on visual characteristics and palpation as previously described.3 To ensure consistency between the pre‐2010 body condition categories and post‐2010 BCS data, the 5 category BCS was mapped to the 3‐category classification with the same approach as that used in a previous study.20 Briefly, the “very thin” and ‘thin’ categories were mapped to the pre‐2010 “thin” category, whereas the “overweight” and “markedly obese” categories were mapped to the pre‐2010 “heavy” category. In addition, the records database contains weight‐related diagnoses, which veterinarians can use when classifying the nature of any consultation. The use of these diagnoses was also examined, and if it did not agree with the body condition assessment (eg, dog assigned a normal body condition, but given a diagnosis of “thin”), the body condition assessment was altered to bring it in line with the stated diagnosis.
One further issue for pre‐2013 data was that the body condition field defaulted to “normal” if a body condition category was not entered. An extrapolation process was used to correct errors arising from this issue, where a non‐default body condition assessment either preceded or followed a default body condition assessment, the default assessment was changed to the value of the non‐default assessment provided that the bodyweight of the dog had remained within ±5% between visits. The ±5% rule was used because changes in bodyweight of >5% are typically required for changes in BCS to be seen.21
Body condition data were used to produce 2 groups of dogs, “normal body condition” and “overweight body condition,” that were matched on sex, visit age, visit year, latitude, and longitude (see below). The “overweight body condition” group comprised dogs with a body condition category of “heavy” at every visit between 5.5 and 9.5 years. The “normal body condition” group comprised dogs whose body condition was never classified as “thin” or “heavy,” and whose body condition was classified as “normal” between 5.5 and 9.5 years.
2.6. Data handling and statistical analysis
Six substantial data cleaning steps were used to ensure both eligibility criteria were met and data were accurate and reliable (Figure 1). First, dogs younger than 5.5 years or older than 9.5 years at their visit were excluded to ensure the selection of middle‐aged dogs and to maintain a balance between numbers of dogs and knowing with certainty that dogs were still alive at a specific age. Second, outlying visits with extreme ages (which might have been keyed in by mistake) were removed by a method based on box and whisker statistics for skewed data.22 Third, groups of dogs in overweight and normal body condition were selected (as described above) to create a single dataset for matching purposes. Fourth, dogs whose veterinary care ceased before 9.5 years, either because of death, euthanasia, or moving to a different veterinary practice were excluded, and the in‐clinic visit which was closest to 7.5 years in age was chosen as the single representative visit for the subsequent matching process, ensuring that dogs with multiple visits between 6.5 and 8.5 years were only included once. Fifth, dogs listed as sexually intact were removed given their relative paucity within the dataset. Finally, to mitigate any possible lack of balance between groups, a “statistical matching” technique was applied to all 12 data subsets, whereby normal and overweight groups were matched on sex, visit year, or visit age by the coarsened exact matching (CEM) method,23 using a bespoke package (Package “cem,” version 1.1.17, Iacus SM) within an open‐source statistical software environment (R, version 3.2.0, R Foundation for Statistical Computing, Vienna, Austria). This method temporarily coarsens the data and then finds exact matches for dogs in the 2 groups. Each observed variable is coarsened into meaningful groups (eg, using age groups instead of exact birth dates). Then, visits with the same values for all the coarsened variables are placed in a single stratum. Strata without at least 1 “normal” and 1 “overweight” visit are dropped. Only the original un‐coarsened values of the matched data are retained. It should be emphasized that the exact matching procedure is applied to the coarsened data to find the matches and discard unmatched units before the un‐coarsened values of the matched data are returned. Therefore, similar to other matching techniques, CEM does not produce a 1:1 ratio of normal to overweight dogs; to do so might lead to a large number of observations being discarded, leading to reduced statistical power. Supporting Information Table S1 outlines the improvement in balance, using the L1 norm for each of the breeds.24 After matching, the median number of dogs with a death date was 911 across the 12 breeds.
Figure 1.
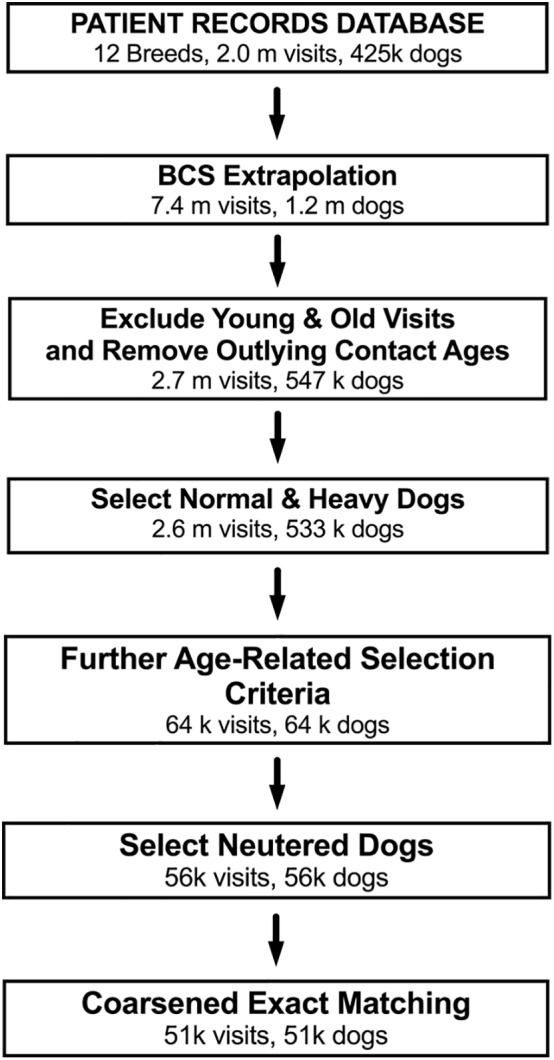
Summary of the data cleaning process. Flow diagram illustrating the data cleaning process to create the final study population. The number of dogs eligible at each stage is depicted, where m denotes million(s) and k denotes thousand(s)
Given that the records database did not include death dates for all dogs, the data were right censored. As a result, comparisons of survival between normal and overweight dogs were made by generating Cox proportional hazards (PH) models given their ability to deal with left and right censored data. A bespoke package (Package “survival,” version 2.38‐3, Therneu TM) of a statistical software environment (R, version 3.2.0) was used to produce models for male and female dogs, and survival predictions from the Cox PH models were used to compare the median life span of normal and overweight dogs. The PH assumption holds if the hazard functions for 2 individuals remain proportional over time, that is, constant relative hazard. This assumption was tested for all explanatory variables (sex, BCS, visit year, and visit age) using weighted residuals.25 When body condition violated the PH assumption, it was managed by adding a time‐dependent body condition variable. This indicator variable depended on the visit age at which the PH assumption failed, specifically 10.5 (when violation of PH could not be mitigated), 12.5, or 14.5 years. Stratification was used for instances for which the PH assumption did not hold for sex, visit year, or visit age. Hazard ratios (HR) and 99.79% confidence intervals (CI) were calculated for the body condition term in each Cox PH model. For each HR, the “hazard” was the instantaneous event rate in an overweight dog compared with a dog in normal body condition, with the event in question being death. Binomial tests were used to assess propensity in the direction of any association between body condition and risk change in all 12 breeds. A Bonferroni adjustment was used to correct for the effects of multiple testing, with the adjusted significance level being P = .002 (eg, 0.05/24) for 2‐sided analyses.
Median life span was calculated for each breed using predictions from the Cox PH models, and these were stratified both by sex (male versus female) and for both normal and overweight groups stratified by sex. We tested the hypothesis that there would be a difference in median life span between dogs with normal body condition and overweight body condition. The percentage difference in median life span for the overweight group relative to the normal group was compared for all 12 breeds and both male and female. The effect of body condition on life span was tested by the Cox PH model for each of the 12 breeds by the same open‐source statistical software environment (R, version 3.2.0). A Bonferroni adjustment was again used to correct for the effects of multiple testing, with the adjusted significance level being P = .002 (eg, 0.05/24) for 2‐sided analyses. Binomial tests were again used to assess trends in the direction of any association between body condition and survival across all 12 breeds. For example, where no underlying trend existed, the overweight group would be expected to have a 50% chance of having a longer median life span than the normal group and a 50% chance of having a shorter median life span.
3. RESULTS
3.1. Final study population
Before applying eligibility criteria and performing matching, the records database contained 5.4 × 106 visits available from 1.2 × 106 dogs (Figure 1). After filtering the dataset and matching, the total number of dogs available was 50 787, and date of death was recorded in 14 316 of these (28.2%). The number of dogs available for each breed is shown in Table 1. After data cleaning, the median number of dogs available per breed was 3865 (range 1273‐11 867), whereas the median number of dogs with a known death date was 911 (range 328‐4520).
Table 1.
Details of the final study population, stratified according to breed, sex, and body condition status
| All dogs | Male dogs | Female dogs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breed | Size class | Total | Normal1 | Overweight2 | Total | Normal1 | Overweight2 | Total | Normal1 | Overweight2 |
| Chihuahua | I | 6306 (1098) | 2460 (210) | 3846 (888) | 2977 (524) | 1227 (109) | 1750 (415) | 3329 (574) | 1233 (101) | 2096 (473) |
| Pomeranian | I | 2297 (515) | 1123 (140) | 1174 (375) | 1280 (281) | 623 (76) | 657 (205) | 1017 (234) | 500 (64) | 517 (170) |
| Yorkshire Terrier | I | 4065 (574) | 2540 (212) | 1525 (362) | 2287 (313) | 1447 (112) | 840 (201) | 1778 (261) | 1093 (100) | 685 (161) |
| Shih Tzu | II | 5488 (873) | 3329 (355) | 2159 (518) | 2915 (469) | 1733 (179) | 1182 (290) | 2573 (404) | 1596 (176) | 977 (228) |
| American Cocker Spaniel | III | 2997 (949) | 927 (179) | 2070 (770) | 1398 (402) | 452 (85) | 946 (317) | 1599 (547) | 475 (94) | 1124 (453) |
| Beagle | III | 3665 (1095) | 703 (106) | 2962 (989) | 1756 (518) | 342 (55) | 1414 (463) | 1909 (577) | 361 (51) | 1548 (526) |
| Dachshund | III | 4799 (984) | 1538 (152) | 3261 (832) | 2396 (491) | 765 (76) | 1631 (415) | 2403 (493) | 773 (76) | 1630 (417) |
| Boxer | IV | 1659 (718) | 807 (264) | 852 (454) | 740 (315) | 348 (109) | 392 (206) | 919 (403) | 459 (155) | 460 (248) |
| Pit Bull | IV | 1273 (328) | 530 (99) | 743 (229) | 484 (119) | 215 (46) | 269 (73) | 789 (209) | 315 (53) | 474 (156) |
| German Shepherd dog | V | 1811 (729) | 833 (262) | 978 (467) | 777 (309) | 383 (122) | 394 (187) | 1034 (420) | 450 (140) | 584 (280) |
| Golden Retriever | V | 4560 (1933) | 876 (242) | 3684 (1691) | 2166 (940) | 407 (117) | 1759 (823) | 2394 (993) | 469 (125) | 1925 (868) |
| Labrador Retriever | V | 11 867 (4520) | 2672 (598) | 9195 (3922) | 5511 (2194) | 1238 (299) | 4273 (1895) | 6356 (2326) | 1434 (299) | 4922 (2027) |
| Total | … | 50 787 (14316) | 18 338 (2819) | 32 449 (11497) | 24 687 (6875) | 32 449 (1385) | 15 507 (5490) | 26 100 (7441) | 9158 (1434) | 16 942 (6007) |
Data reported are total numbers of dogs and dogs with a recorded death age in brackets.1 Dogs were classified as “normal” when their body condition was recorded as “normal” between 5.5 and 9.5 years and if they were never recorded as “thin” or “heavy” at any age2; dogs were classified as “overweight” when their body condition was recorded as “heavy” at every visit between 5.5 and 9.5 years.
3.2. Survival predictions and hazards ratios
Body condition score violated the PH assumption and required the introduction of a time‐dependent body condition variable for 8 of 12 breeds (American Cocker Spaniel, Beagle, Chihuahua, Dachshund, Labrador Retriever, Pomeranian, Shih Tzu, and Yorkshire Terrier). For Labrador Retriever and Shih Tzu, the PH violation occurred at 12.5 and 10.5 years, respectively. The PH violation could not be reduced for Shih Tzu. For other breeds, violation occurred at 14.5 years. Survival probability predictions for male and female dogs of all 12 breeds were then produced from the Cox PH models; examples for 5 breeds (Yorkshire Terrier, Shih Tzu, Dachshund, Boxer, and German Shepherd dog) are depicted in Figures 2, 3, 4, 5, 6, whereas survival curves for all other breeds are included in Supporting Information (Figures S1‐S7). For all 12 breeds, the survival probability for dogs in overweight body condition was less than for dogs in ideal body condition throughout the age range studied.
Figure 2.
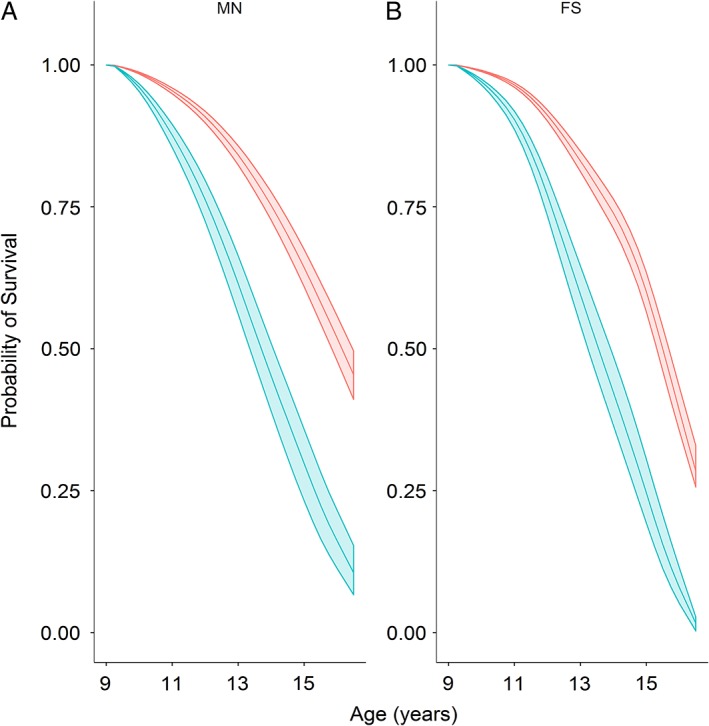
Survival probability models for male neutered (A) and female spayed (B) Yorkshire Terriers. Middle lines depict the probability of survival for a dog at 7.5 years age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in red, whereas that of the overweight group is show in blue
Figure 3.
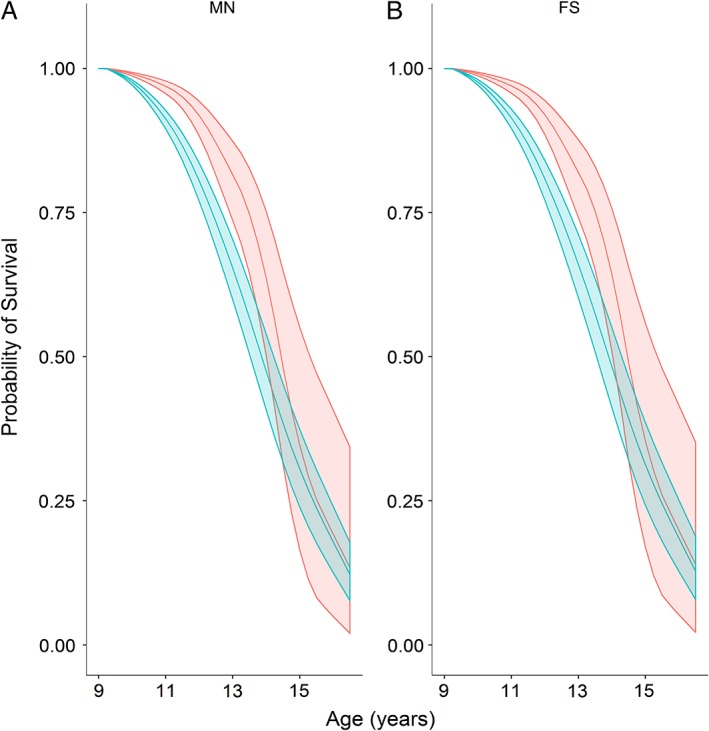
Survival probability models for male neutered (A) and female spayed (B) Shih Tzus. Middle lines depict the probability of survival for a dog at 7.5 years age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in red, whereas that of the overweight group is show in blue
Figure 4.
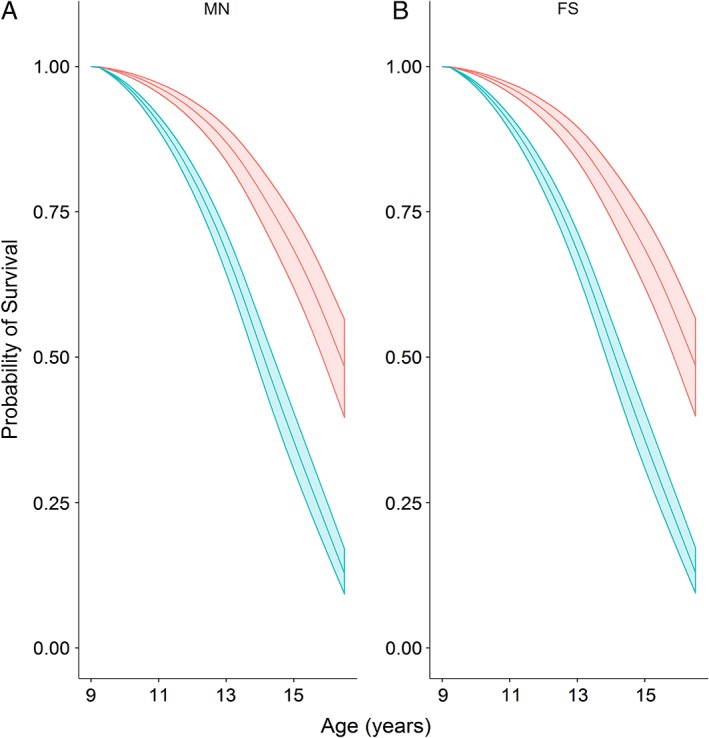
Survival probability models for male neutered (A) and female spayed (B) Dachshunds. Middle lines depict the probability of survival for a dog at 7.5 years age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in red, whereas that of the overweight group is show in blue
Figure 5.
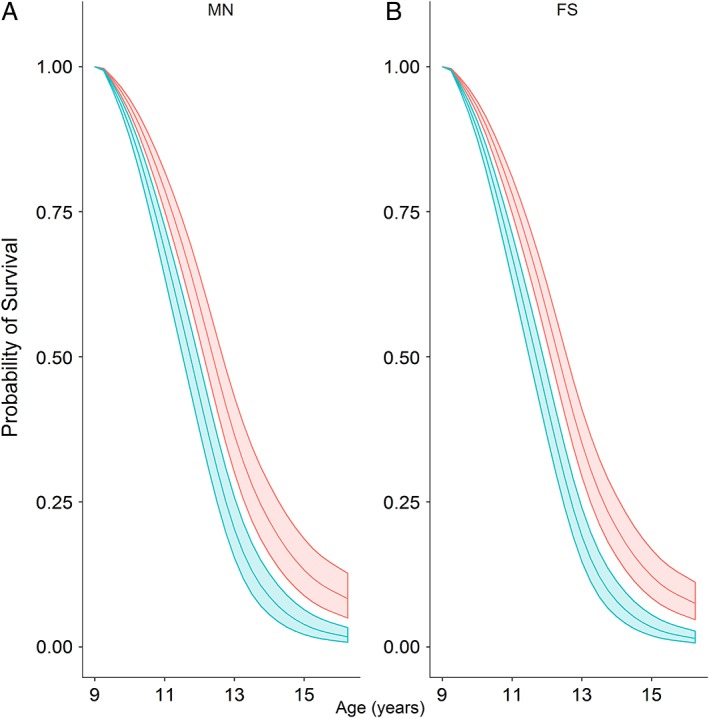
Survival probability models for male neutered (A) and female spayed (B) Boxers. Middle lines depict the probability of survival for a dog at 7.5 years age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in red, whereas that of the overweight group is show in blue
Figure 6.
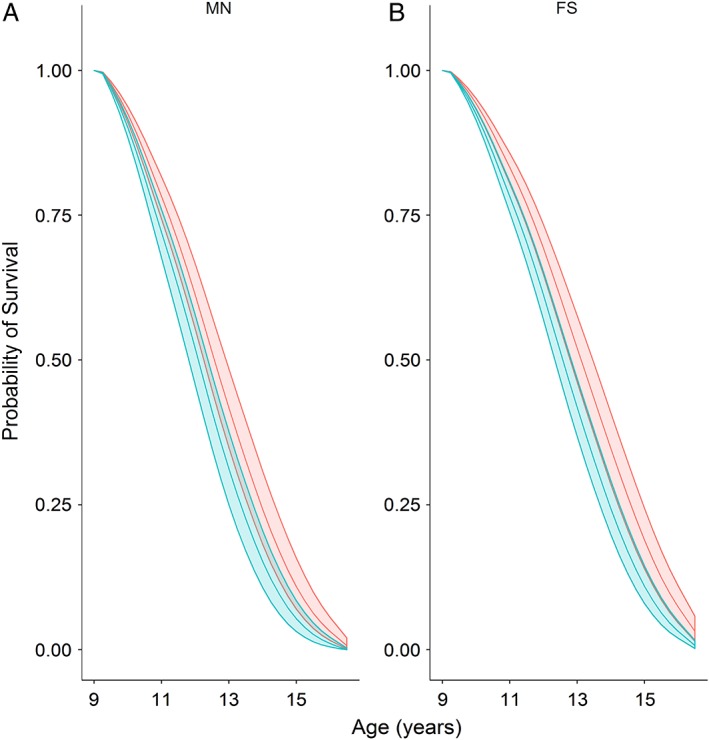
Survival probability models for male neutered (A) and female spayed (B) German Shepherd dogs. Middle lines depict the probability of survival for a dog at 7.5 years age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in red, whereas that of the overweight group is show in blue
Hazard ratios and 99.79% CIs for the body condition term (overweight condition relative to normal condition) from the Cox PH models produced for all 12 breeds are shown in Table 2. The body condition HRs ranged from 1.35 (99.79% CI 1.05‐1.73) for German Shepherd dog to 2.86 (99.79% CI 2.14‐3.83) for Yorkshire Terrier. Binomial testing confirmed an increase in relative risk of dying for overweight dogs compared to normal body condition dogs across all 12 breeds (P < .001 for all) at the Bonferroni‐corrected 2‐sided test level.
Table 2.
Hazard ratios for the effect of overweight body condition on risk of death in pet dogs
| Breed | Size class | Hazard ratio | 99.79% CI | P value |
|---|---|---|---|---|
| Chihuahua | I | 2.42 | 1.87‐3.13 | <.001 |
| Pomeranian | I | 2.25 | 1.62‐3.12 | <.001 |
| Yorkshire Terrier | I | 2.86 | 2.14‐3.83 | <.001 |
| Shih Tzu | II | 2.19 | 1.39‐3.45 | <.001 |
| American Cocker Spaniel | III | 2.21 | 1.66‐2.93 | <.001 |
| Beagle | III | 2.40 | 1.69‐3.43 | <.001 |
| Dachshund | III | 2.77 | 2.03‐3.79 | <.001 |
| Boxer | IV | 1.62 | 1.27‐2.07 | <.001 |
| Pit Bull | IV | 1.57 | 1.08‐2.29 | <.001 |
| German Shepherd | V | 1.35 | 1.05‐1.73 | <.001 |
| Golden Retriever | V | 1.56 | 1.26‐1.94 | <.001 |
| Labrador Retriever | V | 1.83 | 1.54‐2.17 | <.001 |
Data reported are hazard ratios, 99.79% confidence interval (CI), and associated P values for risk of death for dogs in overweight body condition relative to dogs in normal body condition.
3.3. Life span of dogs in ideal and overweight body condition
Median life spans (with multiple testing adjusted CIs) for dogs in overweight and normal body condition from the 12 breeds are shown in Table 3, whereas Figure 7 illustrates the reduction in median life span for all 12 breeds and both male and female dogs. Binomial testing confirmed a shorter survival for overweight dogs of all 12 breeds would not be expected by chance, indicating the presence of a nonrandom directional trend (P < .001). The estimated reduction in median life span for the overweight group relative to the normal group ranged from 5 months, for male German Shepherd dogs, to 2 years 6 months for male Yorkshire Terriers (Table 3).
Table 3.
Differences in life span between dogs in normal and overweight body condition
| Male dogs | Female dogs | ||||
|---|---|---|---|---|---|
| Breed | Size class | Normal1 | Overweight2 | Normal1 | Overweight2 |
| Chihuahua | I | 16.0 (15.6, 16.4) | 13.9 (13.5, 14.2) | 16.1 (15.7, 16.7) | 14.0 (13.6, 14.3) |
| Pomeranian | I | 15.5 (15.2, 16.3) | 13.7 (13.3, 14.1) | 15.5 (15.0, 15.9) | 13.6 (13.2, 14.0) |
| Yorkshire Terrier | I | 16.2 (15.7, 16.5) | 13.7 (13.3, 14.2) | 15.5 (15.3, 15.7) | 13.5 (13.2, 14.0) |
| Shih Tzu | II | 14.5 (14.5, 15.3) | 13.8 (13.6, 14.3) | 14.5 (14.5, 15.4) | 13.9 (13.6, 14.3) |
| American Cocker Spaniel | III | 14.9 (14.4, 15.6) | 13.4 (13.2, 13.6) | 14.8 (14.3, 15.4) | 13.3 (13.0, 13.4) |
| Beagle | III | 15.2 (14.5, 16.1) | 13.2 (13.0, 13.5) | 15.3 (14.6, 16.2) | 13.3 (13.1, 13.6) |
| Dachshund | III | 16.4 (15.8, 16.8) | 14.1 (13.8, 14.4) | 16.4 (15.9, 16.8) | 14.1 (13.8, 14.4) |
| Boxer | IV | 12.4 (12.2, 12.6) | 11.8 (11.5, 12.0) | 12.3 (12.1, 12.6) | 11.7 (11.4, 11.9) |
| Pit Bull | IV | 13.8 (13.3, 14.5) | 13.0 (12.5, 13.5) | 13.8 (13.3, 14.3) | 12.9 (12.6, 13.4) |
| German Shepherd dog | V | 12.5 (12.2, 12.9) | 12.1 (11.8, 12.4) | 13.1 (12.7, 13.5) | 12.5 (12.3, 12.8) |
| Golden Retriever | V | 13.3 (13.0, 13.6) | 12.5 (12.4, 12.7) | 13.5 (13.1, 13.8) | 12.7 (12.6, 12.9) |
| Labrador Retriever | V | 13.3 (12.8, 13.6) | 12.7 (12.6, 12.8) | 13.6 (13.2, 14.0) | 13.0 (12.9, 13.2) |
Data reported are median (99.79% confidence interval) life span for male and female dogs of the 12 breeds in the study.1 Dogs were classified as ‘normal’ when their body condition was recorded as “normal” between 5.5 and 9.5 years and if they were never recorded as “thin” or “heavy” at any age2; dogs were classified as “overweight” when their body condition was recorded as “heavy” at every visit between 5.5 and 9.5 years.
Figure 7.
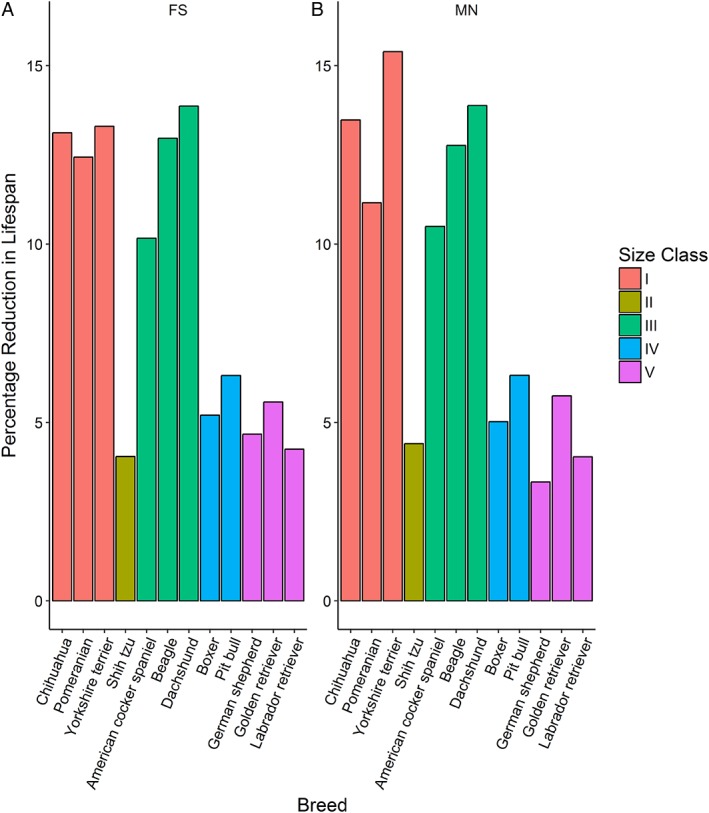
Estimated effect of overweight body condition on life span in male (A) and female (B) dogs. The 12 breeds studied have been ordered by size class,20 with columns representing the median, for overweight dogs compared with dogs in normal body condition. The columns indicate medians of 12 breeds and both sexes (MN, male neutered; FS, female spayed). Breeds have been ordered by size class, starting with small breeds
4. DISCUSSION
Our study demonstrates an adverse effect of overweight body condition on life span in client‐owned dogs of a range of breeds, thereby extending the findings from an earlier colony study in Labrador Retrievers.16, 17, 18, 19 These results emphasize the need for veterinarians to implement steps to prevent the development of obesity in dogs under their care. Specifically, veterinarians could use the study findings in discussions with owners of new puppies, to highlight the risk that overweight status poses to health and the need for prevention. The findings could also be used in discussions with owners of already‐obese dogs to convince them of the need to implement a controlled weight loss program.
Overweight body condition was associated with a shorter life span in all 12 breeds studied, but the magnitude of the effect varied being least for large‐breed dogs (eg, 5 months) and greatest for dogs of the smallest breed (eg, greater than 2 years in dogs from size class I). Hazards ratios for estimated risk of death in overweight dogs relative to those in normal body condition mirrored these findings. The reason for these results is unclear, but 1 possibility would be a difference in natural prevalence of the various obesity‐associated diseases among the breeds studied. For example, orthopedic diseases such as osteoarthritis are more common in larger breeds.26 Alternatively, individual breed characteristics might influence the impact of any functional impairments that arise from obesity, for example, metabolic dysfunction or impaired quality of life.5, 6, 7, 8, 9, 18 Whatever the reasons, the importance of this life span effect should not be ignored. Indeed, even in the breeds for which the effect was least pronounced, such shortening is likely to be important because most owners would wish to ensure that their dog lives a long and healthy life.
In the authors' opinion, although only 12 breeds were studied, the fact that the negative association between overweight status and life span was apparent in all breeds implies that this same effect is likely to be present in any breed. Nonetheless, such extrapolations should be made cautiously until studies including a wider breed range are undertaken. In a similar manner, the inclusion of neutered dogs only and of dogs between 5.5 and 9.5 years means that extrapolation to sexually intact or younger dogs should also be made cautiously.
Given that the study was retrospective and observational, it was not possible to determine the reasons for the association between overweight body condition and life span, and causality cannot necessarily be assumed. One possibility is that overweight status is only indirectly associated with life span, for example, by predisposing to diseases that are themselves fatal (eg, neoplasia).3, 27, 28 Alternatively, overweight body condition might exacerbate other diseases that have a negative impact on health (eg, osteoarthritis), thereby prompting a decision for euthanasia. Indeed, in the previous lifelong feeding study of dogs, chronic diseases including various types of neoplasia and osteoarthritis were diagnosed at an earlier age in the ad libitum‐fed group that were overweight, compared with the calorie‐restricted group that remained in ideal body condition.17 Furthermore, obese dogs have a poorer quality of life than dogs in ideal condition.9 A 2nd possibility for the life span difference between overweight and ideal weight dogs is that weight status is a proxy measure for caloric intake. In this respect, calorie restriction without malnutrition can increase longevity in a wide variety of species including spiders, fish, invertebrates, and rodents.29, 30 Furthermore, biomarkers associated with longevity (eg, fasting insulin concentration and body temperature) were decreased by prolonged calorie restriction in human beings.31 Given that, in our study, information on concurrent disease, diet, and energy intake were not assessed, further work would be required to determine the reason for the difference in life span between overweight dogs and those in ideal condition.
In analyzing study data, we chose an approach involving matching rather than, for example, using PH models with weight status as the variable of interest, and all other covariates considered as potential confounders. The main disadvantage of using matching methods is that they can be less “powerful” and, therefore, might increase the chances of type II statistical error.32 However, this was arguably not a concern in the current study because significant effects were demonstrated even after applying a Bonferroni correction. The main advantage of using a matching method is that it can better deal with observational data that are unbalanced in one or more covariates that themselves might be associated with the dependent variable of interest. For example, in a situation where 1 of the covariates is a causal variable in its own right but is also correlated with the independent variable of interest, a model without matching can struggle to separate the effect of the independent variable of interest from that of the covariate. Furthermore, even when a significant effect of the independent variable of interest is found, it might simply be related to its association with the covariate. Matching methods can separate these effects thereby avoiding a “second‐hand” association of the independent variable of interest with a noise variable.33 Therefore, although other methods could have been considered, the advantages of the method chosen outweighed the disadvantages.
A strength of our study is its size in that, by using a large veterinary hospital network, data from over 50 000 dogs were available for assessment even after data cleaning. This enabled differences in life span to be identified in male and female neutered dogs in all 12 breeds. A further advantage of the approach taken was the fact that client‐owned dogs living in a home environment could be studied and, as such, results are likely to be generalizable to the general pet dog population. However, a disadvantage of the approach is the fact that data were collected by many veterinary professionals in many locations, meaning potential discrepancies in assessment of body condition. Furthermore, data were not specifically collected for this scientific study, rather data were gathered by many veterinarians for clinical reasons, and there might have been errors or omissions in terms of data inputting.
Several other study limitations should also be acknowledged. First, the study was retrospective with data collected over a period of 20 years. As well as both the prevalence and awareness of body condition during this time frame, it is likely that there were numerous changes in practice protocols, expertise, technology, and data recording. Such factors are likely to impact on the reliability of the study data. Moreover, some information that owners provided, such as breed and date of birth, were not verified for accuracy at the time it was recorded, for instance by examining pedigree records. Furthermore, owners might not have known the exact date of birth for their dog, for example, if it had been re‐homed. There might also have been inaccuracies with date of death when this was owner‐reported (if the dog died at home), because some owners might understandably have delayed informing the practice until a time that they could cope with such a difficult conversation. It is also possible that veterinary practice staff might have made errors when entering data into the records database. To mitigate such limitations, extensive data cleaning was undertaken, with exclusion of any data thought to be unreliable. One consequence of this cautious approach is the reduction of available data (Figure 1), meaning that the final dataset might be less representative of the original study population, and the number of breeds where sufficient data were available for analysis is reduced. That said, the original datasets were large enough to accommodate this.
A 2nd limitation is that we cannot be certain whether or not the association between body condition and survival is truly related to a shortened natural life span. Survival studies are difficult to conduct in companion animals because, unlike in humans, pet dogs can be euthanized rather than allowed to die from natural causes. The reasons for euthanasia and the timing of the decision are variable with many factors involved. Decisions might relate to animal factors such as the development of disease (not least if the disease is a terminal one), presence of multiple concurrent diseases, perceived poor quality of life, aggression, or other behavioral disorders. A final possible reason for euthanasia is financial, whereby the costs of pet ownership and, sometimes, costs of treatment might sway the decision for euthanasia. In a recent review, the financial impact of a dog having obesity and obesity‐related disease was estimated to be approximately $2000 per year.34 Given that the current study was retrospective, the reasons for death or euthanasia were not always recorded.
A 3rd study limitation was the fact that a different approach was used to assess body condition at the start of the study compared with the end. Initially, a 3‐category system was used, which was replaced by a more conventional 5‐category system in 2010. To maximize the available study data, 5‐point scores used after 2010 were mapped onto the 3‐category scores used before 2010. This approach has been used in a previous study,20 and because it was straightforward (involving merging categories), it is unlikely that errors arose at this stage. That said, it is unclear whether the categories were truly equivalent. A 2nd concern regarding body condition was that it was not a mandatory field within the computer records and, if not completed, the system automatically recorded the body condition as “normal.” It could be argued that failure to complete this field is more likely to occur when the dog already has a normal body condition than when the body condition is abnormal (overweight or underweight). It cannot be guaranteed that this would always be the case given the relative infrequency with which veterinarians spontaneously record body condition35 or use the terms overweight and obese36 in electronic records. Therefore, to mitigate errors arising at this stage, an extrapolation process was used whereby all default values were compared with assessments that immediately preceded or followed them. Furthermore, body condition assessments were cross‐checked for consistency against diagnosis categories that the veterinarian had recorded (eg, when the veterinarian recorded the diagnosis as underweight, overweight, or obesity) and corrected if they deviated. Although it is likely that these stringent data cleaning methods improved the accuracy of the final dataset, some uncertainty over accuracy remains. That said, because most errors would involve dogs with abnormal body condition being erroneously classified as normal rather than the other way around, the effect would be to decrease differences between groups rather than increase them. Further studies should be considered, involving similarly large datasets to confirm the findings of the current study.
A 4th study limitation was the fact that the decision about whether dogs were selected for the overweight and normal groups was made when dogs were middle aged (between 5.5 and 9.5 years), rather than earlier in life. As a result, the kinetics of weight change throughout life could not be assessed, as they had been in a previous cohort study.16, 17, 18, 19 This was necessary because selecting dogs earlier would have meant fewer dogs with usable data for the study. First, younger dogs are generally healthier than middle‐aged dogs (because chronic diseases typically manifest later in life), hence they visit the veterinarian less frequently. Second, it is common to have notable attrition from owners moving practices, meaning far fewer selected dogs would have had a recorded death dates available for analysis. However, the disadvantage of this approach is that the impact of timing, speed, and duration of weight gain could not be examined; for example, whether weight gain early in life has more impact than weight gain later in life. Likewise, we did not examine the effect of any methods used to achieve weight loss or prevent weight gain on life span. Moreover, as only 2 groups were compared (normal condition and overweight condition), we did not consider the impact of magnitude of excess weight. Therefore, additional studies would be required to examine these aspects in more detail.
5. CONCLUSIONS
There is a negative association between overweight body condition and life span in client‐owned dogs from 12 common breeds. These findings emphasize the need for veterinary professionals to promote a healthy body condition for dogs, particularly in midlife onward.
CONFLICT OF INTEREST DECLARATION
Carina Salt, Penelope J. Morris, and Derek Wilson are employees of WALTHAM, whereas Elizabeth M. Lund was an employee of BANFIELD Pet Hospitals at the time the study was conducted. Both companies both are owned by Mars Inc. Alexander J. German is an employee of the University of Liverpool, but his post is financially supported by Royal Canin, which is also owned by Mars Petcare. Alexander J. German has also received financial remuneration for providing educational material, speaking at conferences, and consultancy work for Mars Petcare; all such remuneration has been for projects unrelated to the work reported in this article. The funder, Mars, did not have any role in the study design, data collection, data analysis, results, or preparation of the manuscript.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study protocol was approved by the WALTHAM ethical review committee, and owners of all participating dogs gave consent for data to be used.
HUMAN ETHICS APPROVAL DECLARATION
The study protocol was approved by the WALTHAM ethical review committee, and all owners gave consent for data to be used.
Supporting information
Supplementary Table S1. Changes in imbalance from Coarsened Exact Matching.
Supplementary Figure S1. Survival probability models for male neutered (a) and female spayed (b) Boxer dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S2. Survival probability models for male neutered (a) and female spayed (b) Golden retriever dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S3. Survival probability models for male neutered (a) and female spayed (b) Pomeranian dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S4. Survival probability models for male neutered (a) and female spayed (b) Beagle dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S5. Survival probability models for male neutered (a) and female spayed (b) American cocker spaniel dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S6. Survival probability models for male neutered (a) and female spayed (b) Dachshund dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S7. Survival probability models for male neutered (a) and female spayed (b) Labrador retriever dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S8. Survival probability models for male neutered (a) and female spayed (b) Pit bull dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S9. Survival probability models for male neutered (a) and female spayed (b) Shih tzu dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S10. Survival probability models for male neutered (a) and female spayed (b) Yorkshire terrier dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Emi Saito (BANFIELD Pet Hospitals) for help with veterinary interpretation and provision of information regarding clinical practices at BANFIELD Pet Hospitals. The data used in the study were gathered from the BANFIELD Pet Hospitals, and data analysis was conducted at the WALTHAM Centre for Pet Nutrition, Melton Mowbray, United Kingdom. Preliminary findings for part of the dataset used in this study were presented at the WALTHAM International Nutritional Sciences Symposium 2013, October 1‐4, Portland.
Salt C, Morris PJ, Wilson D, Lund EM, German AJ. Association between life span and body condition in neutered client‐owned dogs. J Vet Intern Med. 2019;33:89–99. 10.1111/jvim.15367
REFERENCES
- 1. German AJ, Ryan VH, German AC, Wood IS, Trayhurn P. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J. 2010;185:4‐9. [DOI] [PubMed] [Google Scholar]
- 2. BANFIELD® Pet Hospitals . In: Obesity in dogs and cats – state of pet health report [1 screen; cited November 24, 2017]. Available from: https://www.banfield.com/state-of-pet-health/obesity.
- 3. Lund EM, Armstrong PJ, Kirk CA, Klausner JS. Prevalence and risk factors for obesity in adult dogs from private US veterinary practices. Int J Appl Res Vet Med. 2006;4:177‐186. [Google Scholar]
- 4. German AJ, Hervera M, Hunter L, et al. Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest Anim Endocrinol. 2009;37:214‐226. [DOI] [PubMed] [Google Scholar]
- 5. Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Obesity‐related metabolic dysfunction in dogs: a comparison with human metabolic syndrome. BMC Vet Res. 2012;8:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Effect of weight loss in obese dogs on indicators of renal function or disease. J Vet Intern Med. 2013;27:31‐38. [DOI] [PubMed] [Google Scholar]
- 7. Mosing M, German AJ, Holden SL, et al. Oxygenation and ventilation characteristics in obese sedated dogs before and after weight loss: a clinical trial. Vet J. 2013;198:367‐371. [DOI] [PubMed] [Google Scholar]
- 8. Tropf M, Nelson OL, Lee PM, Weng HY. Cardiac and metabolic variables in obese dogs. J Vet Intern Med. 2017;31:1000‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. German AJ, Holden SL, Wiseman‐Orr ML, et al. Quality of life is reduced in obese dogs but improves after successful weight loss. Vet J. 2012;192:428‐434. [DOI] [PubMed] [Google Scholar]
- 10. Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635‐643. [DOI] [PubMed] [Google Scholar]
- 11. Day MJ. One health approach to preventing obesity in people and their pets. J Comp Pathol. 2017;156:293‐295. [DOI] [PubMed] [Google Scholar]
- 12. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347‐355. [DOI] [PubMed] [Google Scholar]
- 13. Manco M, Fernandez‐Real JM, Equitani F, et al. Effect of massive weight loss on inflammatory adipocytokines and the innate immune system in morbidly obese women. J Clin Endocrinol Metabol. 2007;92:483‐490. [DOI] [PubMed] [Google Scholar]
- 14. The Global BMI Mortality Collaboration . Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388:776‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teng KT, McGreevy PD, Toribio JL, et al. Strong associations of 9‐point body condition scoring with survival and lifespan in cats. J Feline Med Surg 2018. (In press). doi: 10.1177/1098612X17752198, 1098612X1775219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kealy RD, Lawler DF, Ballam JM, et al. Evaluation of the effect of limited food consumption on radiographic evidence of osteoarthritis in dogs. J Am Vet Med Assoc. 2000;217:1678‐1680. [DOI] [PubMed] [Google Scholar]
- 17. Kealy RD, Lawler DF, Ballam JM, et al. Effects of diet restriction on life span and age‐related changes in dogs. J Am Vet Med Assoc. 2002;220:1315‐1320. [DOI] [PubMed] [Google Scholar]
- 18. Larson BT, Lawler DF, Spitznagel EL, Kealy RD. Improved glucose tolerance with lifetime diet restriction favorably affects disease and survival in dogs. J Nutr. 2003;133:2887‐2892. [DOI] [PubMed] [Google Scholar]
- 19. Lawler DF, Evans RH, et al. Influence of lifetime food restriction on causes, time, and predictors of death in dogs. J Am Vet Med Assoc. 2005;226:225‐231. [DOI] [PubMed] [Google Scholar]
- 20. Salt C, Morris PJ, German AJ, et al. Growth standard charts for monitoring bodyweight in dogs of different sizes. PLoS One. 2017;12:e0182064 10.1371/journal.pone.0182064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. German AJ, Holden SL, Bissot T, Morris PJ, Biourge V. Use of starting condition score to estimate changes in body weight and composition during weight loss in obese dogs. Res Vet Sci. 2009;87:249‐254. [DOI] [PubMed] [Google Scholar]
- 22. Hubert M, Vandervieren E. An adjusted boxplot for skewed distributions. Comput Stat Data Anal. 2008;52:5186‐5201. [Google Scholar]
- 23. Iacus SM, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal. 2012;20:1‐24. [Google Scholar]
- 24. Iacus SM, King G, Porro G. Multivariate matching methods that are monotonic imbalance bounding. J Am Stat Assoc. 2011;106:345‐361. [Google Scholar]
- 25. Grambsch PM, Therenau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515‐526. [Google Scholar]
- 26. Bland SD. Canine osteoarthritis and treatments: a review. Vet Sci Dev. 2005;5:5931. [Google Scholar]
- 27. Sonnenschein EG, Glickman LT, Goldschmidt MH, McKee LJ. Body conformation, diet, and risk of breast cancer in pet dogs: a case‐control study. Am J Epidemiol. 1991;133:694‐703. [DOI] [PubMed] [Google Scholar]
- 28. Glickman LT, Schofer FS, McKee LJ, et al. Epidemiologic study of insecticide exposures, obesity, and risk of bladder cancer in household dogs. J Toxicol Environ Health. 1989;28:407‐414. [DOI] [PubMed] [Google Scholar]
- 29. McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size: one figure. J Nutr. 1935;10:63‐79. [PubMed] [Google Scholar]
- 30. Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol. 1996;24:742‐745. [DOI] [PubMed] [Google Scholar]
- 31. Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6‐month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brazauskas R, Logan BR. Observational studies: matching or regression? Biol Blood Marrow Transplant. 2016;22:557‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. King G, Nielsen R. Why propensity scores should not be used for matching [cited July 28, 2018]. In: King G, ed. Working Paper; 2018. Available from: https://gking.harvard.edu/publications/why‐propensity‐scores‐should‐not‐be‐used‐formatching [2 screens].
- 34. Bomberg E, Birch L, Endenburg E, et al. The financial costs, behaviour and psychology of obesity: a one health analysis. J Comp Pathol. 2017;156:310‐325. [DOI] [PubMed] [Google Scholar]
- 35. German AJ, Morgan LE. How often do veterinarians assess the bodyweight and body condition of dogs? Vet Rec. 2008;163:503‐505. [DOI] [PubMed] [Google Scholar]
- 36. Rolph NC, Noble PJM, German AJ. How often do primary care veterinarians record the overweight status of dogs? J Nutr Sci. 2014;3:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Changes in imbalance from Coarsened Exact Matching.
Supplementary Figure S1. Survival probability models for male neutered (a) and female spayed (b) Boxer dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S2. Survival probability models for male neutered (a) and female spayed (b) Golden retriever dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S3. Survival probability models for male neutered (a) and female spayed (b) Pomeranian dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S4. Survival probability models for male neutered (a) and female spayed (b) Beagle dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S5. Survival probability models for male neutered (a) and female spayed (b) American cocker spaniel dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S6. Survival probability models for male neutered (a) and female spayed (b) Dachshund dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S7. Survival probability models for male neutered (a) and female spayed (b) Labrador retriever dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S8. Survival probability models for male neutered (a) and female spayed (b) Pit bull dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S9. Survival probability models for male neutered (a) and female spayed (b) Shih tzu dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.
Supplementary Figure S10. Survival probability models for male neutered (a) and female spayed (b) Yorkshire terrier dogs. Middle lines depict the probability of survival for a dog at 7.5y age in 2003 (assuming survival to at least 9.5 years), with the upper and lower lines depicting 99.79% confidence intervals. The survival of dogs in the normal body condition group is shown in blue, whilst that of the overweight group is show in red.


