Abstract
Croton roxburghii and Croton sublyratus have been used as skin treatments in traditional medicine. The objective of the present study was to investigate the antimelanogenic effect of ethanol extracts of Croton roxburghii (CRE) and Croton sublyratus (CSE) leaves on cellular melanin content and cellular tyrosinase activity as mediated by the action of microthalmia transcription factor (MITF) and melanogenic enzymes. Croton roxburghii and Croton sublyratus leaves were extracted by petroleum ether, dichloromethane and absolute ethanol, sequentially. The ethanolic crude extracts were examined for antimelanogenic activity by their ability to decrease melanin content and cellular tyrosinase activity in alpha-melanocyte-stimulating hormone-stimulated B16F10 melanoma cells. In addition, the extracts were evaluated to determine a plausible mechanism of melanogenesis suppression through determining the activation of MITF transcription factor and melanogenic proteins (tyrosinase, tyrosinase-related protein 1 or TRP-1 and tyrosinase-related protein 2 or TRP-2) at the transcriptional and translation levels in α-MSH-induced B16F10 cells. Upon treatment with CRE and CSE, the cells showed significant decreases in melanin content and cellular tyrosinase activity. CRE and CSE also suppressed MITF, tyrosinase, TRP-1 and TRP-2 at the transcription and translation levels in α-MSH-stimulated melanin biosynthesis in B16F10 cells. Our finding shows that CRE and CSE inhibit melanin content and cellular tyrosinase activity through suppressing MITF and melanogenic enzymes. CRE and CSE may be useful to combine with skin whitening agents for cosmetic uses.
Keywords: Croton roxburghii, Croton sublyratus, Melanin, Tyrosinase, B16F10 mouse melanoma cells
Graphical abstract
1. Introduction
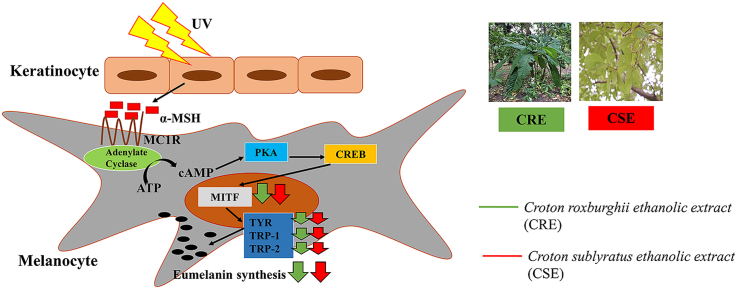
Melanin is the major component of skin color and is synthesized by melanocytes in melanosomes.1 The overproduction and accumulation of melanin content in skin can lead to pigmentation disorders as well as aesthetic problems. Melanin synthesis is regulated by various environmental, hormonal and genetic factors such as ultraviolet radiation (UVR), alpha-melanocyte stimulating hormone (α-MSH), cAMP-elevating agents, and estrogen.2 Overproduction of melanin is caused directly by the response of melanocyte to ultraviolet radiation (UVR), but it is affected indirectly by paracrine and autocrine factors including hormones, cytokines, and growth factors, whose synthesis in epidermal cells is influenced by UVR.3 UV stimulates the secretion of α-MSH from keratinocytes which then induces melanogenesis in melanocytes.4 α-MSH or adrenocorticotropic hormone (ACTH) binds to the melanocortin 1 receptor (MC1R) in melanocytes, and then activates intracellular adenylyl cyclase via G proteins, followed by increased intracellular cyclic AMP (cAMP) levels from adenosine triphosphate (ATP).5 Cyclic AMP contributes its function through protein kinase A (PKA). PKA phosphorylates and activates the cAMP-response element binding protein (CREB) which binds to the cAMP response element present in the M promoter of the microphthalmia-associated transcription factor (MITF) gene. The transient increase of MITF leads to the up-regulation of tyrosinase, TRP-1 and TRP-2.6
Three major enzymes are involved in eumelanin synthesis, including tyrosinase, tyrosinase-related protein 1 (TRP-1) and tyrosinase-related protein 2 (TRP-2).3 In the processes of melanogenesis, tyrosinase is the key enzyme in the rate-limiting step in which l-tyrosine is hydroxylated to l-DOPA, which is further oxidized into DOPAquinone. In addition, tyrosinase-related protein (TRP)-1 and TRP-2 are major targets induced by MITF. TRP-2 catalyzes the rearrangement of dopachrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA), whereas TRP-1 oxidizes DHICA to a carboxylated indole-quinone, which is eventually converted into eumelanin.7 Down regulation of tyrosinase activity has been proposed to be responsible for decreased melanin production.8
Treatment of hyperpigmentation disorders depends on whether the pigmentation is epidermal or dermal. However, only epidermal pigmentation responds well to treatment. Hydroquinone is one of the most popular depigmenting agents which act by reducing melanin content through suppressing tyrosinase activity. However, adverse effects of hydroquinone application may occur such as erythema, stinging, colloid milium, irritation and allergic contact dermatitis, nail discoloration, transient hypochromia, and paradoxical postinflammatory hypermelanosis.9 Moreover, the prolonged usage of hydroquinone may lead to ochronosis.10 Therefore, natural sources have recently become of interest for the development of safe depigment agents for the management of hyperpigmentation disorders.
Croton roxburghii N.P.Balakr and Croton sublyratus Kurz. belong to the Euphorbiceae family. Aqueous and alcoholic extracts of Croton roxburghiii bark and leaf have antibacterial properties against Staphylococcus aureus and Escherichia coli.11 Moreover, the stem, bark and leaves of C. roxburghiii are used as herbal treatments for ringworm, wounds, scabies, skin diseases, liver diseases, diarrhea, fever, and headache.12 Croton roxburghii leaves contain many phytochemical compounds such as tannins, phenolics, flavonoids, carbohydrates, proteins, and amino acids in polar extracts (ethanol, methanol, and aqueous).13 Plaunotol from Croton sublyratus leaves has antibacterial activity against Staphylococcus aureus isolated from patients' skin with atopic dermatitis, and has anti-angiogenic effect on human umbilical vein endothelial cells (HUVEC).14,15 To the best of our knowledge, there are no reports about any antimelanogenic activity of extracts from Croton roxburghii and Croton sublyratus.
In this study, we investigated the effects of CRE and CSE in α-MSH stimulated B16F10 mouse melanoma cells. We examined toxicity of CRE and CSE and further investigated the molecular mechanism by which CRE and CSE suppress melanogenesis through determining the activation of MITF transcription factor and melanogenic proteins (tyrosinase, TRP-1 and TRP-2) in α-MSH induced B16F10 melanoma cells.
2. Materials and methods
2.1. Chemicals and reagents
α-MSH, dimethyl sulfoxide (DMSO), 3.4-dihydroxyphenylalanine (l-DOPA), bovine serum albumin, ethanol, kojic acid, Triton X-100, polyvinylidene fluoride (PVDF), protease inhibitor and melanin were purchased from Sigma (St. Louis, MO). Sodium hydroxide (NaOH), methanol, RIPA buffer and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Merck (Darmstadt, Germany). Dulbecco's Modified Eagle Medium/High glucose (DMEM/HG), penicillin/streptomycin, phosphate buffer saline (PBS) and trypsin/EDTA were purchase from Thermo Scientific Hyclone (Logan, Utah). Sodium dodecyl sulfate and Bradford reagent were obtained from Bio-rad (Hercules, CA). Anti-tyrosinase (ab180753), TRP-1 (ab3312) and TRP-2 (ab74073) antibodies were purchased from Abcam (Cambridge, UK). Anti-GAPDH (14C10), MITF (D5G7V), anti-rabbit IgG, HRP-linked antibody (7074S) and anti-mouse IgG, HRP-linked antibody (7076S) were obtained from Cell Signaling Technology (Beverly, MA).
2.2. Preparation of Croton roxburghii and Croton sublyratus
Leaves of Croton roxburghii and Croton sublyratus were collected from the HRH Princess Sirindhorn Herb Garden, Rayong province, Thailand. These leaves were authenticated and voucher numbers were deposited at the Herbarium, Department of Botany, Faculty of Science, Chulalongkorn University, Thailand.
Fresh leaves were washed and dried in a hot air oven at 45 °C. Dried leaves were blended into a fine powder. Dried powder of the leaves (10 g) was extracted in the organic solvents (1:40, w/v) petroleum ether, dichloromethane and absolute ethanol, sequentially. The extracts from petroleum ether and dichloromethane fractions were discarded. The ethanol fractions were concentrated using a MiVac Quattro concentrator. The ethanol extracts were finally dissolved in dimethyl sulfoxide (DMSO) at a concentration of 100 mg/ml and kept with protection from light at −20 °C.
2.3. Cell culture
The mouse melanoma cell line B16F10 was obtained from American Type Culture Collection (ATCC). The cells were maintained in Dulbecco's Modified Eagle Medium/High glucose (DMEM/HG), supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 U/mL penicillin and 100 μg/ml streptomycin) at 37 °C in a humidified atmosphere of 5% CO2.
2.4. Cell viability assay
Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) as previously described.16 Briefly, cells at 5000 cells/well were grown in 96-well flat-bottom-plates in 200 μl of DMEM with 10% FBS and allowed to attach overnight. Then the cells were exposed to 1 μM of α-MSH and various concentration of ethanol extracts from Croton roxburghii (3.125–100 μg/ml) and Croton sublyratus (3.125–200 μg/ml) leaves or kojic acid (62.5–500 μg/ml) for 48 h. After treatment, the cells were incubated with MTT solution (0.5 mg/ml) for 4 h at 37 °C. The medium was removed and 200 μl of DMSO was added. The absorbance was measured at 550 nm using an ELISA plate reader (Biotek, USA). Results are expressed as a percentage of the control cells.
2.5. Measurement of melanin content
Melanin content was measured using a previously described method.17 Briefly, B16F10 cells were seeded at a density of 100,000 cells/well in 6-well plates for overnight. After attachment, the cells were treated 1 μM of α-MSH and ethanol extracts or kojic acid for 48 h. At the end of the treatment, the cells were washed twice with phosphate buffer saline. Then, the cells were detached with 0.25% (%v/v) trypsin/EDTA solution and subsequently centrifuged at 13,000 g for 10 min. The cell pellets were solubilized in 1N NaOH at 80 °C. Each sample was determined by comparison of the sample OD475 to a standard curve of synthetic melanin. Melanin content of each sample was calculated by comparing with control cells as 100%.
2.6. Intracellular tyrosinase activity
Tyrosinase activity was examined by measuring the rate of l-DOPA oxidation as described by others.18 B16F10 cells at a density of 100,000 cells/well were cultured in 6-well plates and allowed to attach overnight. Then the cells were exposed to 1 μM of α-MSH in the presence of CRE and CSE or kojic acid for 48 h. After treatment, the cells were washed twice with phosphate buffer and lysed with 1% Triton X-100/PBS. The cell lysates were centrifuged at 13,000 g for 10 min. After quantifying protein levels and adjusting protein concentrations with 1% Triton X-100/PBS, the supernatants of each cell lysate (40 μg) were dissolved in 100 μl of 0.1 mM sodium phosphate buffer pH 6.8 and mixed with 100 μl of 5 mM l-DOPA in a 96-well plate. The mixture was incubated at 37 °C for 1 h. The absorbance was measured at 475 nm. Cellular tyrosinase activity of each sample was calculated by comparing with the control cells (100%).
2.7. RNA extraction and quantitative-reverse-transcription polymerase chain reaction analysis
The effect of CRE and CSE extracts was determined by using real-time PCR. B16F10 cells were stimulated with 1 μM of α-MSH in the presence of each ethanol extract for 48 h. Total RNA was isolated using Ribozol™ reagent (AMRESCO) following the manufacturer's instructions. Reverse transcription and cDNA amplification was carried out with 1 μg of isolated RNA using AccuPower® RT premix. Real-time PCR was performed using an Exicycler™ 96 Quantitative Real-Time PCR System (Bioneer, Korea). The reaction was cycled 40 times for GAPDH, MITF, tyrosinase, TRP-1 and TRP-2 for 10 min at 95 °C, 15 s at 95 °C and 30 s at 56–58 °C. The primers used for quantitative real-time PCR were as follows: for GAPDH (133 bp) 5′ CTTTGTCAAGCTCATTTCCTGG 3’ (forward) and 5′ TCTTGCTCA GTGTCCTTGC 3’ (reverse); for MITF (116 bp) 5′AGGACCTTGAAAACCGACAG 3’ (forward) and 5′ GGTGGATGGGATAAGGGAAAG 3’ (reverse); for tyrosinase (150 bp) 5′ CTAACTTACTCAGCCCAGCATC 3’ (forward) and 5′ GGGTTTTGGCTTTGTCATGG 3’ (reverse); for TRP-1 (134 bp) 5′AGCCCCAACTCTGTCTTTTC 3’ (forward) and 5′ GGTCTCCCTACATTTCCAGC 3’ (reverse); for TRP-2 (135 bp) 5′ TCCAGAAGTTTGACAGCCC 3’ (forward) and 5′ GGAAGGAGTGAGCCAAGTTATG 3’ (reverse). The fold change in mRNA levels was calculated using the ΔΔct method (2−ΔΔct) for relative quantification between samples and control.
2.8. Western blot analysis
B16F10 cells were treated with 1 μM of α-MSH and ethanol extracts for 48 h. After treatment, the cells were washed twice with PBS and lysed with RIPA buffer with protease inhibitor. Protein concentrations were determined using the Bradford assay (Bio-Rad, USA). Samples containing equal amounts of protein (40 μg/sample) were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% skim milk and incubated with a 1:1000 dilution of a rabbit polyclonal anti-tyrosinase antibody (Abcam, UK), a 1:2000 dilution of a mouse monoclonal anti-TRP-1 antibody (Abcam, UK), a 1:2000 dilution of a mouse monoclonal anti-TRP-2 antibody (Abcam, UK), a 1:10000 dilution of a rabbit monoclonal anti-GAPDH antibody (Cell Signaling Technology, Berverly MA), or a 1:1000 dilution of a rabbit monoclonal anti-MITF antibody (Cell Signaling Technology, Berverly MA). All bands were visualized using an HRP-linked anti-rabbit IgG antibody or an HRP-linked anti-mouse IgG, antibody (Cell Signaling Technology, Beverly, MA). Bound antibodies were detected using an enhanced chemiluminescence kit (Thermo Scientific, USA). Equal loading was assessed using an anti-GAPDH antibody to normalize the amount of total protein.
2.9. Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM) derived from three independent experiments. Statistical significance was determined by one-way analysis of variance (ANOVA) with Dunnett's post-hoc test using SPSS program version 20.0. A P value < .05 was considered to indicate statistical significance.
3. Results
3.1. Effects of CRE and CSE on cell viability
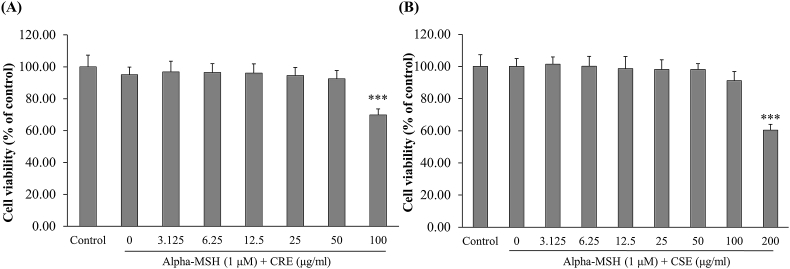
We first determined the cytotoxic effects of CRE and CSE on α-MSH-stimulated B16F10 cells. The cells were treated with various concentrations (3.125–100 μg/ml) of CRE and CSE and 1 μM of α-MSH for 48 h. As shown in Fig. 1A, CRE did not show cytotoxic effects at concentrations of 3.125–50 μg/m, while, CSE was not toxic to the cells in a concentration range of 3.125–100 μg/ml (Fig. 1B). The four highest non-cytotoxic concentrations of each extract were selected for the next experiments.
Fig. 1.
Cytotoxicity of CRE and CSE in B16F10 cells. B16F10 cells were incubated with (A) CRE at concentrations of 3.125–100 μg/ml in the presence of α-MSH (1 μM) for 48 h and (B) with CSE at 3.125–200 μg/ml and α-MSH (1 μM) for 48 h. Each percentage values for treated cells are reported relative to that of the control group. Data are the mean ± SEM from three independent experiments. ***P < .001: statistically significant vs α -MSH group.
3.2. Effects of CRE and CSE on melanin content in α-MSH induced B16F10 cells
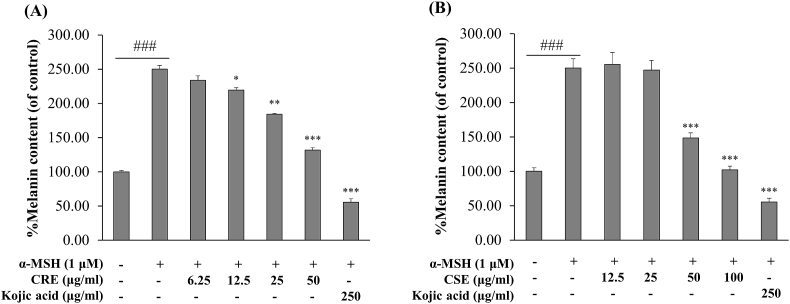
To determine the effects of CRE and CSE on intracellular melanin content in α-MSH induced B16F10 cells, the cells were treated with 1 μM of α-MSH and non-cytotoxic concentrations of each ethanol extracts for 48 h, and absorbance at 475 nm was determined. As shown in Fig. 2, the amount of melanin content in α-MSH-treated cells significantly increased to 2.5-fold over control cells (no treatment). Kojic acid, a positive control at 250 μg/ml significantly reduced melanin content when compared with α-MSH group. As shown in Fig. 2A, CRE at concentrations between 12.5, 25 and 50 μg/ml significantly inhibited melanin production by α-MSH stimulation in a dose dependent manner by 30.56%, 65.88% and 118.28%, respectively (compared with α-MSH-treated control cells). As shown in Fig. 2B, intracellular melanin content was also decreased about 117.35% and 168.45% at the tested concentrations (50 and 100 μg/ml). These results suggest that CRE and CSE can suppress cellular melanin synthesis in α-MSH-stimulated B16F10 cells.
Fig. 2.
Effects of CRE and CSE on cellular melanin content in B16F10 cells. B16F10 cells were treated with (A) CRE (6.25–50 μg/ml) in the presence of 1 μM of α-MSH for 48 h and (B) CSE (12.5–100 μg/ml) and exposed to 1 μM of α-MSH for 48 h before analysis of melanin content. Percentage values for treated cells are reported relative to that of the control group. Data are the mean ± SEM from three independent experiments. ###P < .001: statistically significant vs Control group. *P < .05, **P < .01, ***P < .001: statistically significant vs α -MSH group.
3.3. Effects of CRE and CSE on tyrosinase activity in α-MSH induced B16F10 cells
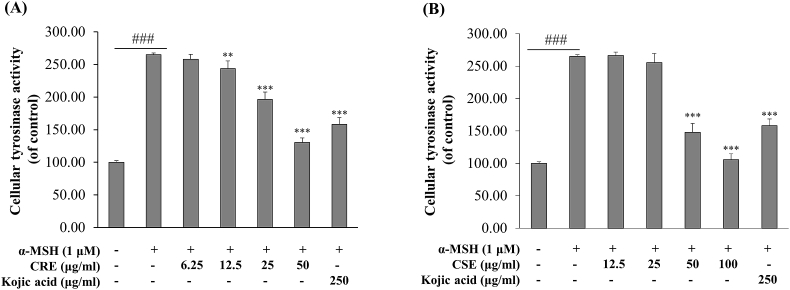
Because tyrosinase is a key enzyme for melanin synthesis, we investigated the effect of CRE and CSE on tyrosinase activity in α-MSH-stimulated B16F10 cells. Tyrosinase activity in α-MSH-stimulated B16F10 cells was significantly increased by 2.6-fold as compared with control cells. Kojic acid significantly reduced cellular tyrosinase activity as compared with α-MSH treated cells. As shown in Fig. 3A, the ethanol extract of Croton roxburghii leaves significantly inhibited cellular tyrosinase activity in a concentration-dependent manner. Similarly, cellular tyrosinase was significantly decreased with 117.16% inhibition at 50 μg/ml and 159.33% inhibition at 100 μg/ml (Fig. 3B). These results suggest that CRE and CSE can suppress cellular tyrosinase activity, and that this inhibitory effect leads to reduced melanin synthesis in α-MSH-stimulated B16F10 cells.
Fig. 3.
Effects of CRE and CSE on cellular tyrosinase activity. B16F10 cells were treated with (A) CRE (at 6.25–50 μg/ml) in the presence of 1 μM of α-MSH for 48 h and (B)CSE (at 12.5–100 μg/ml) and α-MSH for 48 h before analysis of cellular tyrosinase activity. Percentage values for treated cells are reported relative to that of the control group. Data are the mean ± SEM from three independent experiments. ###P < .001: statistically significant vs control group. **P < .01, ***P < .001: statistically significant vs α -MSH group.
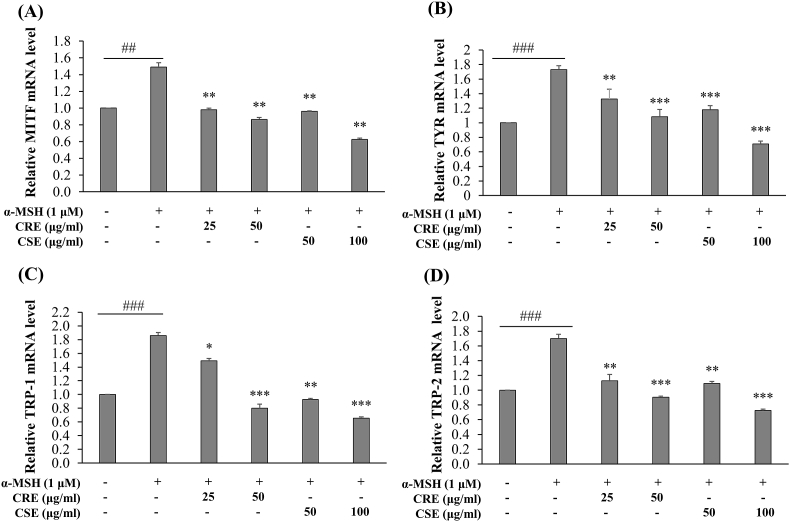
3.4. Effects of CRE and CSE on mRNA expression involved melanogenesis
To elucidate the inhibitory mechanism of CRE and CSE on melanogenesis, we examined mRNA levels of MITF, tyrosinase, TRP-1 and TRP-2. B16F10 cells were stimulated by α-MSH for 48 h with or without ethanol extracts, and mRNA levels of MITF, tyrosinase, TRP-1 and TRP-2 were examined. As shown in Fig. 4A, 4B, mRNA levels of MITF and tyrosinase were significantly decreased by treatment with CRE (at concentrations of 25 and 50 μg/ml) and CSE (at concentrations of 50 and 100 μg/ml) in a concentration-dependent manner. Moreover, CSE and CRE also significantly reduced mRNA levels of TRP-1 and TRP-2 in α-MSH stimulated cells (Fig. 4C, 4D).
Fig. 4.
Effects of CRE and CSE on mRNA expression of genes involved in melanogenesis. Total RNA from B16F10 cells treated with CRE and CSE extracts were collected at 48 h, and levels of (A) MITF (B) TYR (C) TRP-1 (D) TRP-2 mRNA were determined by qPCR, using GAPDH as an internal control. ##P < .01, ###P < .001: statistically significant vs control group and *P < .05, *P < .01, ***P < .001 vs α -MSH group.
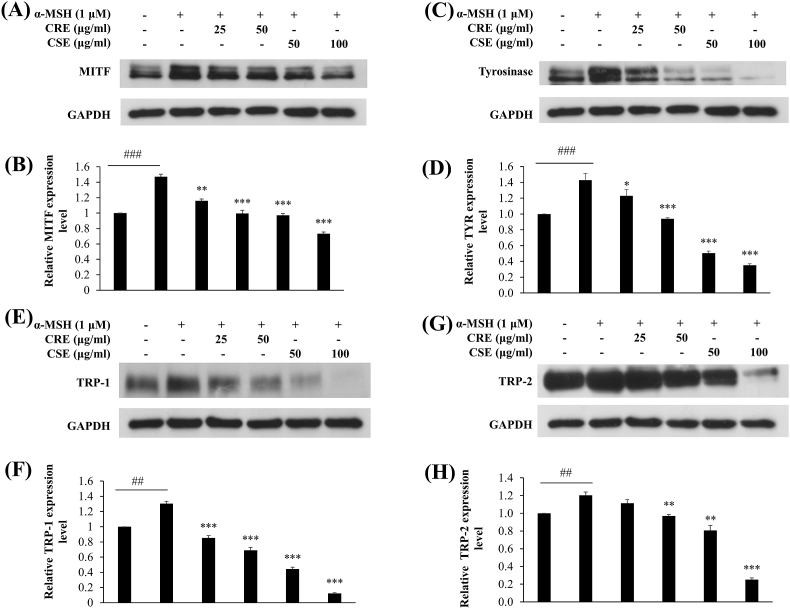
3.5. Effects of CRE and CSE on the expression of melanogenic proteins
The effects of CRE and CSE on the expression of the melanogenic proteins MITF, tyrosinase, TRP-1 and TRP-2, were determined. B16F10 cells were treated with the ethanol extracts and 1 μM of α-MSH for 48 h. Protein levels of MITF were clearly decreased after 48 h of treatment with CRE at concentrations of 25 and 50 μg/ml, and by CSE at concentrations of 50 and 100 μg/ml in a dose-dependent manner (Fig. 5A, 5B). Furthermore, tyrosinase, TRP-1, and TRP-2 proteins in α-MSH stimulated B16F10 cells were also suppressed by CRE and CSE treatment (Fig. 5C-5H). These results suggest that CRE and CSE suppressed MITF, tyrosinase, TRP-1 and TRP-2 at both the transcriptional and translational levels, which led to the reduction of cellular tyrosinase activity and melanin content (Fig. 6).
Fig. 5.
Effects of CRE and CSE on expression of melanogenic proteins. B16F10 cells were treated with CRE and CSE in the presence of 1 μM α-MSH for 48 h. After treatment, total protein was extracted with RIPA buffer and levels of (A) MITF (C) tyrosinase (E) TRP-1 and (G) TRP-2 proteins were determined by western blot analysis, using GAPDH as a loading control. Results (B, D, F, H) from three independent experiments are expressed as mean ± SEM. ##P < .01, ###P < .001 vs control cells and **P < .01, ***P < .001 vs α-MSH group.
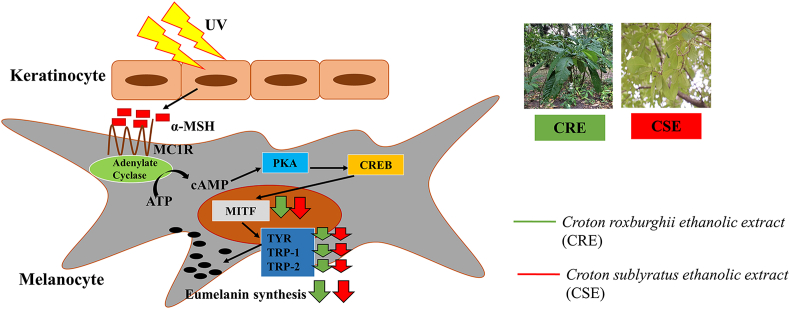
Fig. 6.
Possible inhibitory mechanisms of CRE and CSE on melanogenesis.
4. Discussion
Careful consideration should be given to safety in the formulation of plant extracts or phytochemical compounds as functional foods or cosmetic agents. Hydroquinone, which is used as a skin whitening agent for treating hyperpigmentation disorders in the cosmetics industry, has a lot of side effects including skin irritation, contact dermatitis and exogenous ochronosis in dark-skinned people. Because of these side effects, sale of hydroquinone was prohibited in the US market by the Food and Drug Administration in 2006.19 Kojic acid, which inhibits tyrosinase activity and is also used as a depigmenting agent can induce dermatitis.20 Thus, many researchers seek new, safer agents for the treatment of hyperpigmentation disorders with less adverse effects. Natural products from herbal extracts and active compounds include potential agents such as phenolics, flavonoids, gallic acid, epigallocatechin, aloesin, hydroxystillbene and ellagic acid.21
Tyrosinase is a key enzyme in mammalian melanin synthesis.22 So, tyrosinase is a target for skin whitening agents. The inhibition of mushroom tyrosinase (Agaricus bisporus) is often used as an enzymatic in vitro model for screening skin whitening agents.23 To screen for new compounds, popular depigmenting agents, such as hydroquinone, kojic acid, or arbutin, serve as positive controls.24 However, the inhibition of mushroom tyrosinase was not related with the inhibition of cellular tyrosinase activity in a melanoma cell line.25 Aoki et al. have found that oolong tea decreased intracellular tyrosinase at the mRNA level in B16 mouse melanoma cells, but this extract did not show any inhibitiory effect on mushroom tyrosinase activity in a cell free system.26 In order to identify new depigmenting agents, the inhibitory effects of CRE and CSE on melanin biosynthesis were determined in B16F10 mouse melanoma cells. A mouse melanoma cell line was used in this study because it produces melanin and contains tyrosinase activity which is the rate limiting enzyme for melanin synthesis. These cells respond to α-MSH, and are easy to culture in vitro.27
Ultraviolet radiation (UV) directly affects the function of epidermal cells through secretion of α-MSH.28 On binding to MC1R, α-MSH activates adenylate cyclase, which increases cAMP levels, leading to PKA and CREB activation and CRE binding to the MITF gene promoter, and the induction of MITF transcription. The increase of MITF leads to de-novo transcription of tyrosinase, TRP-1 and TRP-2.29 Eumelanin within melanocytes is synthesized by an enzymatic cascade which is controlled by tyrosinase, TRP-1 and TRP-2.30 We hypothesized that CRE and CRE-induced inhibition of melanogenic enzymes involved the MITF signaling cascade. As expected, CRE (at 25 and 50 μg/ml) and CSE significantly decreased MITF at the mRNA and protein levels in α-MSH-stimulated B16F10 cells (Fig. 5A. 5.B.). Moreover, CRE and CSE also suppressed melanogenic enzymes, including tyrosinase, TRP-1 and TRP-2 at the transcriptional and translation levels in a concentration-dependent manner (Fig. 5C-5H). These results strongly suggested that the decrease in melanin content by the treatment with CRE and CSE is a result of inhibition of MITF and melanogenic enzymes.
Terpenoid is a secondary metabolite which is found in the genus Croton, especially diterpenoids. Triterpenoids, either pentacyclic or steroidal, have usually been found in Croton species.31 In phytochemical analysis, methanol extract of Croton roxburghii (whole plant) contain alkaloid, flavonoid, carbohydrate, glycoside, tannin and flavonoid. There are many phytochemical compounds such as tannins, phenolics, flavonoids, carbohydrates, proteins, and amino acids in polar extracts (ethanol, methanol, and aqueous).13 C. roxburghii is traditionally used for would healing and antidote of snake venom.32 While, plaunotol, a diterpenoid is a major compound in ethanol extracts of Croton sublyratus leaves.33 It had antibacterial activity against Staphylococcus aureus isolated from patients' skin with atopic dermatitis.15 Moreover, plaunotol from ethanol extract of C. sublyratus had the highly effective antigastric ulcer properties in rats.34 So, there is no study on the anti-melanogenic effect of C. roxburghii and C. sublyratus leaves.
In a previous study, we tested total phenolic content, total flavonoid content, DPPH and ABTS scavenging activity of an ethanol extract of Croton roxburghii and Croton sublyratus leaves. CRE and CSE had a phenolic content of about 19.41 and 16.28 mg GAE/g dry material, respectively. Flavonoid content was found in CRE (7.54 mg QE/g dry material) and CSE (14.85 mg QE/g dry material). Scavenging activity as assessed by ABTS was observed in CRE (73.86% scavenging activity) and CSE (60.36% scavenging activity).35 These antioxidant compounds are useful for the skin by attenuating reactive oxygen species (ROS). UVB results in indirect damage to macromolecules via generation of reactive oxygen species.36 Thus, CRE and CSE contain phytochemical compounds which are capable of reducing ROS generated from exposure to sunlight. Significantly, CRE and CSE seem to be potential sources for novel depigmenting agents. In future studies we will separate the active compounds from these crude extracts by High Performance Liquid Chromatography (HPLC) which will then be tested for anti-melanogenic activity. This study is limited to use in human skin, but the two plant extracts will be tested in an animal model for determining toxicity, efficiency and absorption. After that, CRE and CSE in cream or lotion will be tested on human skin for skin irritation, absorption test and anti-melanogenic activity.
5. Conclusions
Based on the results, we report for the first time that CRE and CSE inhibit cellular melanin content and tyrosinase activity in α-MSH-stimulated B16F10 cells by suppressing MITF, tyrosinase, TRP-1 and TRP-2. These extracts can be further developed into a combination of a skin whitening agent or antimelanogenic agent for cosmetic uses. Further studies are necessary to investigate the active components and safety of CRE and CSE.
Conflicts of interest
None to declare.
Acknowledgements
This study was supported financially by Doctoral Degree Chulalongkorn University 100th Year Birthday Anniversary, Overseas Research Experience Scholarship for Graduate Student and the Research fund project 58002, Faculty of Allied Health Sciences, Chulalongkorn University, Thailand. This research was also supported by the National Research University Project, Office of Higher Education Commission (NRU59-007-HR). We appreciated the assistance of the HRH Princess Sirindhorn Herb Garden to give the plants for this project. Finally, we would like to express gratitude to Prof. Dr. Duncan R. Smith (Institute of Molecular Biosciences, Mahidol University) for his critical reading of this manuscript.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Slominski A., Tobin D.J., Shibahara S., Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 2.Hirobe T. Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes. Pigm Cell Res. 2005;18(1):2–12. doi: 10.1111/j.1600-0749.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 3.Choi W., Miyamura Y., Wolber R. Regulation of human skin pigmentation in situ by repetitive UV exposure: molecular characterization of responses to UVA and/or UVB. J Invest Dermatol. 2010;130(6):1685–1696. doi: 10.1038/jid.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt G., Todd C., Cresswell J.E., Thody A.J. Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci. 1994;107(Pt 1):205–211. doi: 10.1242/jcs.107.1.205. [DOI] [PubMed] [Google Scholar]
- 5.Im S., Moro O., Peng F. Activation of the cyclic AMP pathway by alpha-melanotropin mediates the response of human melanocytes to ultraviolet B radiation. Canc Res. 1998;58(1):47–54. [PubMed] [Google Scholar]
- 6.Tachibana M. MITF: a stream flowing for pigment cells. Pigm Cell Res. 2000;13(4):230–240. doi: 10.1034/j.1600-0749.2000.130404.x. [DOI] [PubMed] [Google Scholar]
- 7.Solano F. Melanins: skin pigments and much more—types, structural models, biological functions, and formation routes. New Journal of Science. 2014;2014:28. [Google Scholar]
- 8.Sarangarajan R., Apte S.P. The polymerization of melanin: a poorly understood phenomenon with egregious biological implications. Melanoma Res. 2006;16(1):3–10. doi: 10.1097/01.cmr.0000195699.35143.df. [DOI] [PubMed] [Google Scholar]
- 9.Picardo M., Carrera M. New and experimental treatments of cloasma and other hypermelanoses. Dermatol Clin. 2007;25(3):353–362. doi: 10.1016/j.det.2007.04.012. (ix) [DOI] [PubMed] [Google Scholar]
- 10.Levin C.Y., Maibach H. Exogenous ochronosis. An update on clinical features, causative agents and treatment options. Am J Clin Dermatol. 2001;2(4):213–217. doi: 10.2165/00128071-200102040-00002. [DOI] [PubMed] [Google Scholar]
- 11.Panda S.K., Dutta S.K., Bastia A.K. Antibacterial activity of Croton roxburghii Balak. against the enteric pathogens. “J Adv Pharm Technol Research”“(JAPTR)”. 2010;1(4):419–422. doi: 10.4103/0110-5558.76442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel E., Padiya R., Acharya R.N., Shukla V. Preliminary Phytochemical evaluation of stem bark, root, leaf of Croton roxburghii Balak. Pharmaceutical and Biological Evaluations. 2014;1:29–34. [Google Scholar]
- 13.Panda S.K., Bastia A.K., Dutta S.K. Anticandidal activity of Croton roxburghii Balak. Int J Curr Pharmaceut Res. 2010;2(4):55–59. doi: 10.4103/0110-5558.76442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai K., Tsuno N.H., Kitayama J. Anti-angiogenic properties of plaunotol. Anti Canc Drugs. 2005;16(4):401–407. doi: 10.1097/00001813-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto Y., Hamashima H., Masuda K., Shiojima K., Sasatsu M., Arai T. The antibacterial activity of plaunotol against Staphylococcus aureus isolated from the skin of patients with atopic dermatitis. Microbios. 1998;96(385):149–155. [PubMed] [Google Scholar]
- 16.van Meerloo J., Kaspers G.J., Cloos J. Cell sensitivity assays: the MTT assay. Meth Mol Biol. 2011;731:237–245. doi: 10.1007/978-1-61779-080-5_20. (Clifton, N.J.) [DOI] [PubMed] [Google Scholar]
- 17.Su T.R., Lin J.J., Tsai C.C. Inhibition of melanogenesis by gallic acid: possible involvement of the PI3K/Akt, MEK/ERK and Wnt/beta-catenin signaling pathways in B16F10 cells. Int J Mol Sci. 2013;14(10):20443–20458. doi: 10.3390/ijms141020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyu Han S, Hee Choi W, Soo Ann H, Me Ahn R, Yoon Yi S. Effects of EGb 761 and Korean Red Ginseng on Melanogenesis in B16F10 Melanoma Cells and Protection against UVB Irradiation in Murine Skin;vol. 42008.
- 19.Tse T.W. Hydroquinone for skin lightening: safety profile, duration of use and when should we stop? J Dermatol Treat. 2010;21(5):272–275. doi: 10.3109/09546630903341945. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa M., Kawai K., Kawai K. Contact allergy to kojic acid in skin care products. Contact Dermatitis. 1995;32(1):9–13. doi: 10.1111/j.1600-0536.1995.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 21.Ebanks J.P., Wickett R.R., Boissy R.E. Mechanisms regulating skin pigmentation: the rise and fall of complexion coloration. Int J Mol Sci. 2009;10(9):4066–4087. doi: 10.3390/ijms10094066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tief K., Hahne M., Schmidt A., Beermann F. Tyrosinase, the key enzyme in melanin synthesis, is expressed in murine brain. Eur J Biochem. 1996;241(1):12–16. doi: 10.1111/j.1432-1033.1996.0012t.x. [DOI] [PubMed] [Google Scholar]
- 23.Nokinsee D., Shank L., Lee V.S., Nimmanpipug P. Estimation of inhibitory effect against tyrosinase activity through homology modeling and molecular docking. Enzym Res. 2015;2015:262364. doi: 10.1155/2015/262364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillaiyar T., Manickam M., Namasivayam V. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J Enzym Inhib Med Chem. 2017;32(1):403–425. doi: 10.1080/14756366.2016.1256882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song T-Y, Chen C-H, Yang N-C, Fu C-S, Chang Y-T, Chen C-L. The Correlation of in Vitro Mushroom Tyrosinase Activity with Cellular Tyrosinase Activity and Melanin Formation in Melanoma Cells A2058;vol. 172009.
- 26.Aoki Y., Tanigawa T., Abe H., Fujiwara Y. Melanogenesis inhibition by an oolong tea extract in B16 mouse melanoma cells and UV-induced skin pigmentation in brownish Guinea pigs. Biosci Biotechnol Biochem. 2007;71(8):1879–1885. doi: 10.1271/bbb.70099. [DOI] [PubMed] [Google Scholar]
- 27.Chan Y.Y., Kim K.H., Cheah S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J Ethnopharmacol. 2011;137(3):1183–1188. doi: 10.1016/j.jep.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 28.Schiller M., Brzoska T., Bohm M. Solar-simulated ultraviolet radiation-induced upregulation of the melanocortin-1 receptor, proopiomelanocortin, and alpha-melanocyte-stimulating hormone in human epidermis in vivo. J Invest Dermatol. 2004;122(2):468–476. doi: 10.1046/j.0022-202X.2004.22239.x. [DOI] [PubMed] [Google Scholar]
- 29.Videira I.F., Moura D.F., Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88(1):76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kippenberger S., Loitsch S., Solano F., Bernd A., Kaufmann R. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR–regulation by steroid hormones. J Invest Dermatol. 1998;110(4):364–367. doi: 10.1038/jid.1998.1. [DOI] [PubMed] [Google Scholar]
- 31.Salatino A., Salatino M.L.F., Negri G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae) J Braz Chem Soc. 2007;18(1):11–33. [Google Scholar]
- 32.Mandal L., Bose S. Pharmacognostic standardization and quantitative estimation of some isolated phytoconstituents from Croton oblongifolius roxb. J PharmaSciTech. 2011;1(1):10–15. [Google Scholar]
- 33.Wungsintaweekul J., De-Eknamkul W. Biosynthesis of plaunotol in Croton stellatopilosus proceeds via the deoxyxylulose phosphate pathway. Tetrahedron Lett. 2005;46(12):2125–2128. [Google Scholar]
- 34.Chaotham C., Chivapat S., Chaikitwattana A., De-Eknamkul W. Acute and chronic oral toxicity of a partially purified plaunotol extract from Croton stellatopilosus ohba. BioMed Research International. 2013;2013:12. doi: 10.1155/2013/303162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatatikun M., Chiabchalard A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Compl Alternative Med. 2017;17:487. doi: 10.1186/s12906-017-1994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pattison D.I., Davies M.J. Actions of ultraviolet light on cellular structures. Exs. 2006;(96):131–157. doi: 10.1007/3-7643-7378-4_6. [DOI] [PubMed] [Google Scholar]