Summary
Human pluripotent stem cell-derived vascular smooth muscle cells (hPSC-VSMCs) are of great value for disease modeling, drug screening, cell therapies, and tissue engineering. However, producing a high quantity of hPSC-VSMCs with current cell culture technologies remains very challenging. Here, we report a scalable method for manufacturing hPSC-VSMCs in alginate hydrogel microtubes (i.e., AlgTubes), which protect cells from hydrodynamic stresses and limit cell mass to <400 μm to ensure efficient mass transport. The tubes provide cells a friendly microenvironment, leading to extremely high culture efficiency. We have shown that hPSC-VSMCs can be generated in 10 days with high viability, high purity, and high yield (∼5.0 × 108 cells/mL). Phenotype and gene expression showed that VSMCs made in AlgTubes and VSMCs made in 2D cultures were similar overall. However, AlgTube-VSMCs had higher expression of genes related to vasculature development and angiogenesis, and 2D-VSMCs had higher expression of genes related to cell death and biosynthetic processes.
Keywords: human pluripotent stem cells, alginate hydrogel tube, 3D culture, vascular smooth muscle cells
Graphical Abstract
Highlights
-
•
hPSCs are expanded and differentiated into VSMCs in alginate hydrogel microtubes
-
•
The hydrogel tubes create a cell-friendly microenvironment for efficient cell culture
-
•
hPSC-VSMCs made in alginate hydrogel tubes are closer to a contractile phenotype
Human pluripotent stem cell-derived vascular smooth muscle cells (hPSC-VSMCs) are of great value for regenerative medicine. In this article, Lei and colleagues describe a method to manufacture hPSC-VSMCs in alginate hydrogel microtubes, which provide cells a cell-friendly microenvironment, leading to extremely high culture efficiency. The method is scalable and has potential to make hPSC-VSMCs broadly accessible.
Introduction
Vascular smooth muscle cells (VSMCs) are critical in the development, function, and maintenance of blood vessels (Carmeliet, 2000, Owens et al., 2004). VSMC dysfunction can lead to a variety of diseases (Melanie et al., 2014, Porter and Riches, 2013). VSMCs are important, and required in large numbers for drug discovery and disease modeling (Biel et al., 2015, Ge et al., 2012, Granata et al., 2017, Ji et al., 2017, Kinnear et al., 2013a, Liu et al., 2011, Zhang et al., 2014, Zhang et al., 2011), tissue engineering (Ferreira et al., 2007, Hibino et al., 2012, Karamariti et al., 2013, Levenberg, 2005, Park et al., 2010, Patsch et al., 2015, Wang et al., 2016, Wang et al., 2014, Yoo et al., 2013), and cell therapies (Cheung et al., 2012, Emilio et al., 2006, Hattan et al., 2004, Hong et al., 2017, Li et al., 1999, Matsubayashi et al., 2003, Staudacher et al., 2006, Wang et al., 2016, Wanjare et al., 2013, Ye et al., 2014). It is, however, a great challenge to retrieve enough primary VSMCs from fetal or adult human tissues (Cheung et al., 2014, Owens et al., 2004, Poh et al., 2005). This difficulty could potentially be resolved by using human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) (Thomson et al., 1998) and induced pluripotent stem cells (iPSCs) (Levenberg et al., 2007, Takahashi et al., 2007, Yu et al., 2007). Large numbers of hPSCs can be grown due to their ability to continually proliferate in vitro. They are also capable of differentiating into all somatic cell types (Chen et al., 2011, Lei and Schaffer, 2013). Also, patient-specific derived iPSCs retain the patient's genetic information, which is important in modeling many human diseases (Okita et al., 2011, Takahashi et al., 2007). This also allows these cells to produce little or no immune response in the patient (Lalit et al., 2014). Protocols for effectively differentiating hPSCs into VSMCs have been developed in the past decade, through either embryoid bodies (Biel et al., 2015, Ge et al., 2012, Kinnear et al., 2013b, Lee et al., 2010, Lin et al., 2012, Wang et al., 2014, Yoo et al., 2013, Zhang et al., 2014) or monolayer culture (Bajpai et al., 2012, Bao et al., 2015, Cheung et al., 2012, Karamariti et al., 2013, Lian et al., 2014, Liu et al., 2011, Melanie et al., 2014, Park et al., 2010, Patsch et al., 2015, Taura et al., 2009, Wanjare et al., 2015, Wanjare et al., 2013, Yang et al., 2016, Zhang et al., 2011). For instance, researchers are able to generate developmental origin-specific VSMC subtypes from hPSCs, including neuroectodermal VSMCs, lateral plate mesodermal VSMCs, and paraxial mesodermal VSMCs (Bernardo et al., 2011, Cheung et al., 2012, Granata et al., 2017, Iyer et al., 2016, Raphel et al., 2012, Sinha et al., 2004), or synthetic (Granata et al., 2017, Wanjare et al., 2013, Wanjare et al., 2015) and contractile VSMCs from hPSCs (Bajpai et al., 2012, Eoh et al., 2017, Gerecht et al., 2010, Patsch et al., 2015, Pei et al., 2010, Vo et al., 2010, Wanjare et al., 2015, Yang et al., 2016). Synthetic VSMCs are at a proliferative state, while contractile VSMCs are at a quiescent yet functional state (Ayoubi et al., 2017). Contractile VSMCs are critical for maintaining proper blood flow and normal vessel tone (Alexander and Owens, 2012). The synthetic phenotype has an unfavorable role in human blood vessels, and is associated with conditions such as atherosclerosis and hypertension (Lacolley et al., 2012, Louis and Zahradka, 2010). Under certain conditions, VSMCs can actually switch between the two phenotypes (Mark and Christine, 2012). Patsch and colleagues have recently demonstrated a protocol that can differentiate hPSCs into mesoderm and then VSMCs, using a two-dimensional (2D) culture system, in just 6 days (Patsch et al., 2015). Their protocol is efficient and simple, making it incredibly useful for making VSMCs for a range of biomedical procedures, especially for clinical applications.
Although it is possible to readily make hPSC-VSMCs on a small scale in basic research laboratories, it is still very difficult to produce hPSC-VSMCs at the larger quantities needed by many biomedical applications. The current 2D methods for culturing cells (e.g., growing cells on 2D surfaces like plates or flasks) generally produce such a low cell yield that they are typically utilized when small numbers of cells are needed (e.g., <1010) (Jenkins and Farid, 2015, Kropp et al., 2017). Three-dimensional suspension culturing methods (e.g., culturing cells in agitated medium in stirred-tank bioreactors) are being studied to scale up the cell production, and this technique has been shown to have encouraging results. However, 3D culture systems do have significant limitations (Jenkins and Farid, 2015, Kropp et al., 2017, Lei et al., 2014, Lei and Schaffer, 2013, Serra et al., 2012, Steiner et al., 2010, Wurm, 2004). hPSCs have both strong cell-to-cell and strong cell-to-matrix adhesions. They frequently form large cellular agglomerates by aggregating in 3D suspension (Kropp et al., 2017). Due to the fact that the diffusion limit in human tissue is in the range of 300–400 μm, the mass transport in cellular agglomerates larger than 400 μm (in diameter) becomes limited. This restriction in mass transport can increase apoptosis, cause uncontrolled differentiation, and slow cell growth (Kropp et al., 2017). Mass transport and medium mixing can be enhanced, and cell agglomeration can be reduced by simply agitating the culture. While agitation has its benefits, it can also generate significant, negative, and uncontrollable hydrodynamic stresses (e.g., shear force), which can greatly increase cell death (Fridley et al., 2012, Kinney et al., 2011, Kropp et al., 2017). This can result in decreased cell viability leading to slower growth and lower volumetric yield, all of which are common in 3D suspension culturing (Lei and Schaffer, 2013).
To address the challenge, we previously developed a scalable and high-yield cell culture method for expanding hPSCs and other human cells (Li et al., 2018a, Li et al., 2018b, Lin et al., 2018a, Lin et al., 2018b). With this method, cells are processed into microscale alginate hydrogel tubes (AlgTubes) that are suspended in the cell culture medium. These tubes significantly improved the hPSC culture efficiency and consistency. Under optimized culture conditions, hPSCs could be cultured in these hydrogel tubes long term (e.g., >10 passages, >50 days) with high cell viability, high purity (>95% pluripotency markers, e.g., OCT4, NANOG, ALP, and SSEA4), and high yield (∼5.0 × 108 cells/mL of microspace). However, whether hPSCs can be efficiently differentiated into VSMCs in the alginate hydrogel tubes and if alginate hydrogel tubes could affect the phenotypes of produced VSMCs have not been studied. In this paper, we systematically address these questions. Consequently, we have developed a highly efficient and scalable method for producing hPSC-VSMCs with high volumetric yield, high viability, and high purity.
Results
Processing Cells into Alginate Hydrogel Tubes
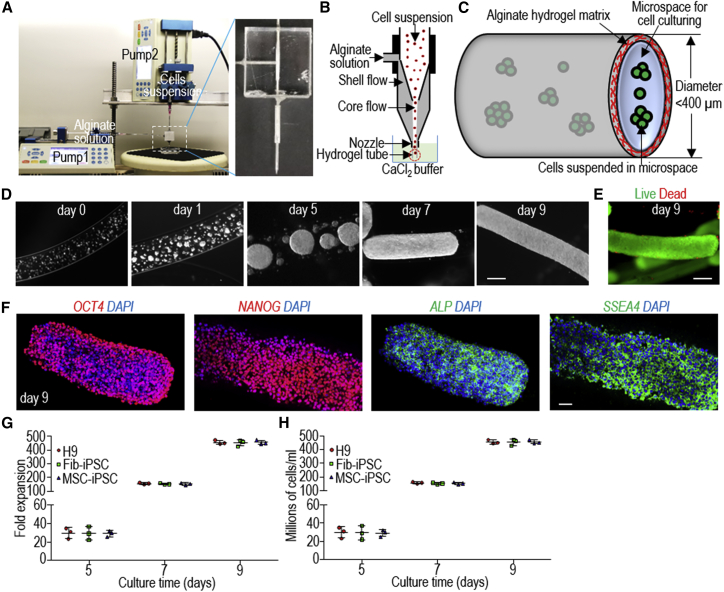
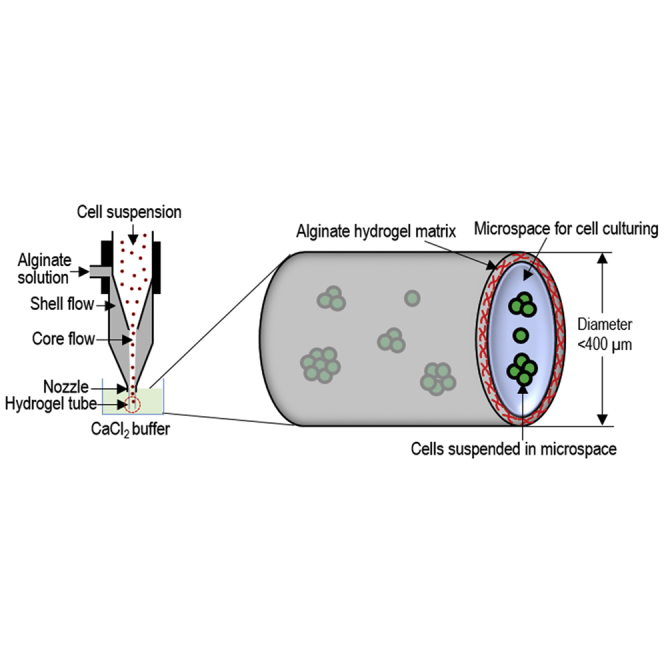
A microextruder was used to process hPSCs into AlgTubes according to our previous study (Li et al., 2018a) (Figures 1A–1C). In the hydrogel tubes, single hPSCs first associated to form small clusters that subsequently grew into spheroids that eventually filled the tube (Figure 1D). Very few dead cells were detected during the culture (Figure 1E). Immunostaining and flow cytometry analyses revealed >95% cells positive for the pluripotency markers OCT4, NANOG, ALP, and SSEA4 (Figure 1F). When seeded at 1.0 × 106 cells/mL, H9s, Fib-iPSCs (iPSCs reprogrammed from dermal fibroblasts), and MSC-iPSCs (iPSCs reprogrammed from bone marrow mesenchymal stem cells) expanded ∼30-, ∼150-, and ∼480-fold to yield ∼30, 150, and 480 × 106 cells/mL of microspace on days 5, 7, and 9, respectively (Figures 1G and 1H). For comparison, typically 2–3 million cells can be generated in one well of a six-well plate. To generate massive numbers of hPSCs, the day 9 cell masses can be released by dissolving the hydrogel tubes with 0.5 mM EDTA solution (5 min at room temperature), and dissociated into single cells with Accutase and processed into new hydrogel tubes for a second round of expansion. Once the targeted cell number is reached, hPSCs can be differentiated into VSMCs within 5 days.
Figure 1.
Culturing hPSCs in Alginate Hydrogel Tubes (AlgTubes)
(A–C) Overview of alginate hydrogel culture system. (A) A microextruder is built for processing cells into microscale alginate hydrogel tubes. (B) A cell suspension and an alginate solution are pumped into the central channel and side channel of the microextruder, respectively, to form coaxial core-shell flows that are extruded through the nozzle into a CaCl2 buffer. (C) The hydrogel tubes protect cells from hydrodynamic stresses and confine the cell mass to <400 μm (in radial diameter) to ensure efficient mass transport. The tubes provide uniform and cell-friendly microspaces that allow cells to interact with one another and expand.
(D) Phase images of H9 hESCs in hydrogel tubes on days 0, 1, 5, 7, and 9. Scale bar, 200 μm.
(E) Live/dead cell staining of day 9 cells in hydrogel tubes. Scale bar, 200 μm.
(F) Immunostaining of day 9 H9 hESCs for pluripotency markers OCT4, NANOG, SSEA4, and ALP. Scale bar, 200 μm.
(G and H) Seeded at 1.0 × 106 cells/mL, H9s, Fib-iPSCs, and MSC-iPSCs expanded (G) ∼30-, 150-, and 480-fold to yield (H) ∼30, 150, and 480 × 106 cells/mL of microspace on days 5, 7, and 9. Data are represented as mean ± SD (n = 3).
Making hPSC-VSMCs in Alginate Hydrogel Tubes
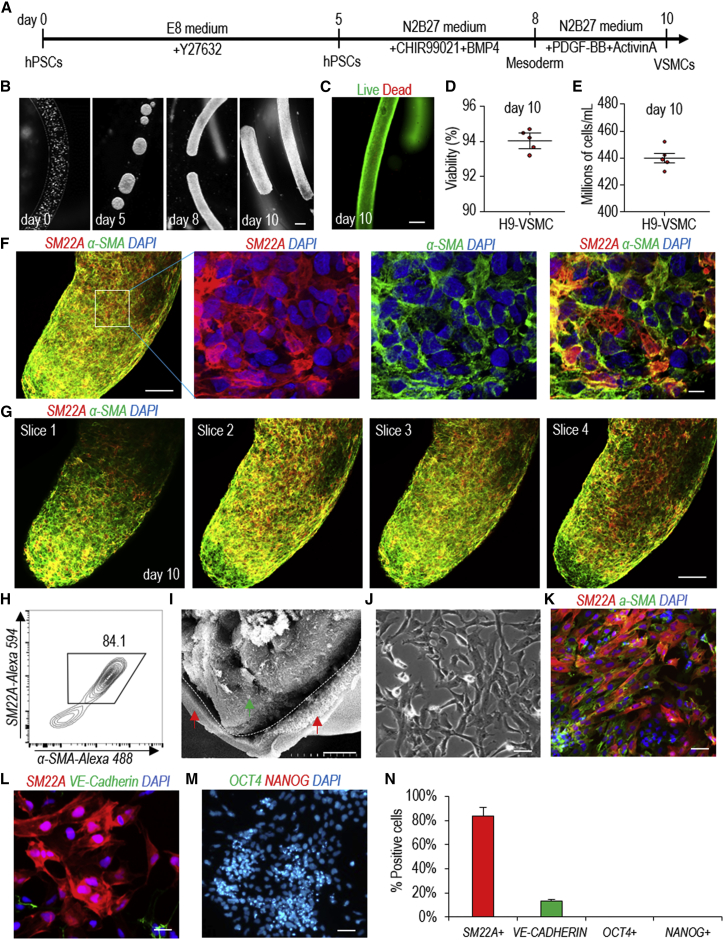
We first checked and confirmed that our starting cells, including H9 hESCs, Fib-iPSCs, and MSC-iPSCs (Park et al., 2008), were high-quality pluripotent stem cells (Figure S1). Patsch et al. reported an efficient protocol that could differentiate hPSCs into VSMCs in 6 days in 2D culturing (Patsch et al., 2015). This protocol is simple and quick, and thus is very appealing for making VSMCs for various biomedical applications, especially for clinical applications. We successfully repeated this protocol (Figure S2). We termed VSMCs made in 2D culturing as 2D-VSMCs.
We then studied whether the protocol could be used to generate VSMCs in alginate hydrogel tubes (Figure 2A). Single hPSCs were processed into the hydrogel tubes and expanded for 5 days to generate small spheroids. On day 5, the differentiation medium was applied. Cells were harvested for analysis on day 10 (Figure 2B). Live/dead staining and cell viability analysis showed very high cell viability on day 10 (Figures 2C and 2D). About 5.0 × 108 cells/mL of microspace were produced on day 10 (Figure 2E). Immunostaining and confocal imaging showed that the majority of the produced cells were positive for VSMC markers SM22A and α-SMA (Figures 2F and 2G). VSMCs were uniformly distributed, and no cysts were found in the cell mass, indicating no or little cell death during the differentiation. Flow cytometry analysis found 84.1% of the cells were SM22A+ and α-SMA+ (Figure 2H). Scanning electron microscopy (SEM) showed there was no adhesion between AlgTube-VSMCs and alginate hydrogel tubes (Figure 2I). When the day 10 cells were released from the tubes and dissociated into single cells and plated on Matrigel-coated plates at high density, they formed a monolayer with tight cell-cell interactions (Figure 2J). The majority of the cells were SM22A+ and α-SMA+ VSMCs (Figure 2K), ∼10% of cells were VE-CADHERIN+ endothelial cells (Figures 2L and 2N), and no OCT4+ and NANOG+ undifferentiated hPSCs were detected (Figures 2M and 2N). H9s, Fib-iPSCs, and MSC-iPSCs had similar outcomes (Figures S3 and S4). We found that the differentiation efficiencies in 2D culturing and in hydrogel tubes were very close. We termed VSMCs made in hydrogel tubes as AlgTube-VSMCs.
Figure 2.
Differentiating H9 hESCs into VSMCs in Alginate Hydrogel Tubes
(A) The VSMC differentiation protocol.
(B) Phase images of day 0, 5, 8, and 10 cells. Scale bar, 200 μm.
(C and D) (C) Live/dead staining and (D) cell viability of day 10 cells. Data are represented as mean ± SD (n = 5). Scale bar, 200 μm.
(E) Volumetric yield on day 10. Data are represented as mean ± SD (n = 5).
(F–H) (F) Immunostaining and (H) flow cytometry analysis of day 10 cells for VSMC markers SM22A and α-SMA. (G) Confocal microscope images of four slices of one fibrous cell mass stained with VSMC markers α-SMA and SM22A. Scale bars, 100 and 10 μm, respectively.
(I) SEM image of hPSC-VSMCs in hydrogel tubes on day 10 (green arrow, cells; red arrows, hydrogel tubes). Scale bar, 10 μm.
(J–N) The day 10 cell masses were dissociated into single cells and plated on 2D surface overnight. (J) Phase image and (K) immunostaining showed the majority of the cells were SM22A+ and α-SMA+ VSMCs, and (L and N) ∼10% were VE-CADHERIN+ endothelial cells, but (M and N) there were no OCT4+/NANOG+ undifferentiated hPSCs. Data are represented as mean ± SD (n = 3). Scale bar, 50 μm.
To study whether the diameter of the hydrogel tubes affected the differentiation efficiency, we differentiated hPSCs into VSMCs in hydrogel tubes with a diameter of 120, 250, or 330 μm. The diameter of the hydrogel tubes can be precisely controlled through adjusting the nozzle diameter of the microextruder (Li et al., 2018a). hPSCs were successfully differentiated into VSMCs in all three tubes with similar cell viability, differentiation efficiency, and yield (Figures S5A and S5B). Quantitative RT-PCR analysis revealed that cells in 250 μm hydrogel tubes had significantly higher expression for some important VSMC genes, including α-SMA, SM22A, CALPONIN, VEGFA, FN, COL4A5, and COL4A6 (Figures S5C–S5E). We thus decided to use 250 μm hydrogel tubes for the rest of the studies.
Properties of hPSC-VSMCs Made in Alginate Hydrogel Tubes and 2D Culture
Our culture system provides cells a 3D microenvironment. Recent research on organoids demonstrates that 3D microenvironments promote the formation of structured tissues during the differentiation of pluripotent stem cells (Hattori, 2014, Jo et al., 2016, Takahashi et al., 2018). Therefore, we asked if AlgTube-VSMCs and 2D-VSMCs were similar in phenotypes, functions, and gene expression. VSMCs were replated in six-well plates for phenotype assays after 5 days differentiation. The fibronectin deposition assay showed that AlgTube-VSMCs and 2D-VSMCs had similar fibronectin production in response to transforming growth factor β (TGF-β) stimulation (Figures 3A–3C). When co-cultured with human umbilical vein endothelial cells (HUVECs), both types of VSMCs attached to the tubular network formed by the HUVECs (Figure 3D). AlgTube-VSMCs had more contraction in response to carbachol treatment than 2D-VSMCs in vitro (Figures 3E–3G), although the carbachol-induced intracellular calcium levels were similar (Figure 3H). When subcutaneously injected with HUVECs into immunodeficient mice for 2 weeks, both AlgTube-VSMCs and 2D-VSMCs contributed to the newly formed blood vessels (Figure 3I). The numbers of VSMCs attached to the vessels were similar (Figure 3J). Similar results were found for Fib-iPSC-derived VSMCs (Figures S6A–S6G). These results show that AlgTube-VSMCs and 2D-VSMCs are similar and both have the typical VSMC phenotypes.
Figure 3.
Properties of hPSC-VSMCs Made in 2D Culture (2D-VSMCs) and Alginate Hydrogel Tubes (AlgTube-VSMCs)
(A–C) Immunostaining of fibronectin production of (A) 2D-VSMCs and (B) AlgTube-VSMCs after 24 hr of 2.5 ng/mL TGF-β treatment. (C) Quantification of produced fibronectin. Data are represented as mean ± SD (n = 5). Scale bar, 50 μm.
(D) Co-culture of VSMCs and HUVECs. Scale bar, 50 μm.
(E–G) (E) Phase images, (F) surface area, and (G) percentage change in cell surface area of 2D-VSMCs and AlgTube-VSMCs in response to carbachol treatment. Data are represented as mean ± SD. ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bar, 50 μm.
(H) The relative fluorescence units (ΔRFU) of Fluo-4-loaded VSMCs over 10 min after adding carbachol. Data are represented as mean ± SD (n = 3).
(I and J) When VSMCs and HUVECs were co-transplanted subcutaneously, both 2D-VSMCs and AlgTube-VSMCs (I) formed nice vascular structures with (J) similar numbers of VSMCs attached to the vessels. Scale bar, 50 μm.
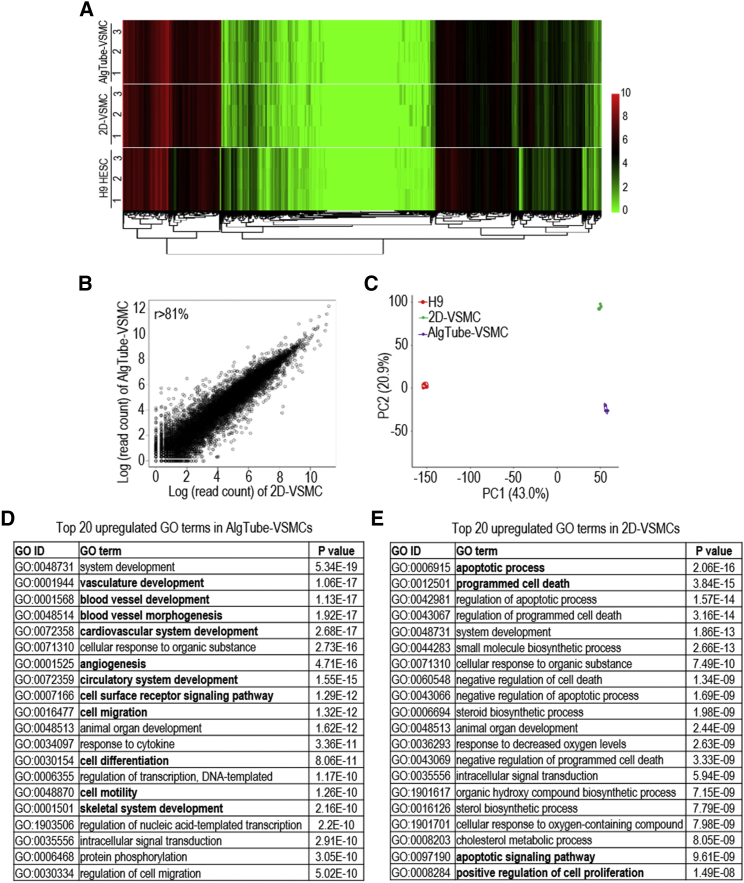
Transcriptome Analysis of AlgTube-VSMCs and 2D-VSMCs
We sequenced the mRNAs of AlgTube-VSMCs and 2D-VSMCs derived from H9s and undifferentiated H9s (three biological replicates for each) to analyze their global gene expression. Hierarchical clustering analysis showed that AlgTube-VSMCs and 2D-VSMCs clustered closely (Figure 4A). The genome-wide gene expression profile correlation coefficients between the AlgTube-VSMCs and the 2D-VSMCs were >0.81, indicating that they had similar global gene expressions (Figure 4B). However, the separation of 2D-VSMCs and AlgTube-VSMCs in PC2 of the principal component analysis (PCA) indicated these cells had some differences in gene expression (Figure 4C), which drove us to perform differential gene expression analysis.
Figure 4.
Whole Transcriptome Analysis of AlgTube-VSMCs and 2D-VSMCs Derived from H9 hESCs
(A) Global heatmap of expressed genes.
(B) The scatterplot in log scale of global gene expression between AlgTube-VSMCs and 2D-VSMCs.
(C) Principal component analysis of AlgTube-VSMCs, 2D-VSMCs, and H9s. Three biological replicates were used for each sample.
(D and E) Top 20 GO terms of upregulated genes in (D) AlgTube-VSMCs and (E) 2D-VSMCs.
Differential gene expression analysis identified 807 genes significantly upregulated in AlgTube-VSMCs, and 719 genes significantly upregulated in 2D-VSMCs (Table S3). Gene Ontology (GO) terms upregulated in AlgTube-VSMCs were mainly related to vasculature development and angiogenesis (Figure 4D). GO terms upregulated in 2D-VSMCs were related to apoptotic process and biosynthesis (Figure 4E). Detailed gene expression analysis using the RNA sequencing (RNA-seq) data showed that AlgTube-VSMCs and 2D-VSMCs had significant differences in the expression of genes related to extracellular matrices, such as those for COLLAGENS, LAMININS, INTEGRINS, and PROTEASES (Figures S7A–S7E); VSMC secreted factors (Figure S7F); EPHRIN signaling (Figure S7G); angiogenesis (Figure S7H); vasculature development (Figure S7I); NOTCH signaling (Figure S7J); cell differentiation (Figure S7K); and cell cycle and proliferation (Figure S7L). These results show that the 3D microenvironment of AlgTubes promoted vasculature development during the differentiation.
We also briefly compared the global gene expression profiles of AlgTube-VSMCs and 2D-VSMCs with those of primary human aortic smooth muscle cells (HASMCs). The RNA-seq data of primary HASMCs were obtained from the literature (Pan et al., 2018). It should be noted that these HASMCs had been cultured in 2D plates in serum-containing medium before RNA sequencing and the 2D culturing may have altered their global gene expression profiles. The genome-wide gene expression profile correlation coefficients were 0.74 between AlgTube-VSMCs and HASMCs, and were 0.63 between 2D-VSMCs and HASMCs (Figures S7M and S7N). These results indicate that AlgTube-VSMCs are closer to HASMCs than 2D-VSMCs. However, both AlgTube-VSMCs and 2D-VSMCs have differences from HASMCs in terms of global gene expression. It is likely due to the fact that VSMCs derived from hPSCs are not as mature as primary VSMCs. Further studies to identify the differences in gene expression, phenotypes, and functions between hPSC-VSMCs and primary VSMCs (especially those that have not been cultured in vitro) should be done in the future.
Using quantitative RT-PCR analysis, we found that both AlgTube-VSMCs and 2D-VSMCs had very low expression levels of the pluripotency markers OCT4 and NANOG (Figure 5A). 2D-VSMCs had higher expression of the synthetic VSMC genes such as α-SMA, SM22A, and CALPONIN (Figure 5B), and AlgTube-VSMCs had higher expression of the mature and contractile VSMC markers such as SMOOTHELIN (SMTN), MYOSIN HEAVY CHAIN 11 (MYH11), and ELASTIN (Figure 5C). These results indicate that 2D-VSMCs are closer to a synthetic phenotype and AlgTube-VSMCs are closer to a contractile phenotype. AlgTube-VSMCs had enhanced expression of extracellular matrix (ECM) genes (FN, COL4A6, and MMP9) (Figure 5D), growth factors (VEGFA and VEGFC) (Figure 5E), and other genes related to angiogenesis (PECAM1, ANGPT2, NOTCH4, and DLL4) compared with 2D-VSMCs (Figure 5F). Using Ki-67 immunostaining and flow cytometry analysis, we found more cells were proliferating in 2D-VSMCs (Figures 5G and 5H). These analyses well supported the RNA-seq data.
Figure 5.
Gene Expression and Cell Proliferation Comparison of 2D-VSMCs and AlgTube-VSMCs
(A–F) qRT-PCR analyses of 2D-VSMCs, AlgTube-VSMCs, and H9 hESCs for (A) pluripotency markers OCT4 and NANOG, (B) synthetic VSMC markers α-SMA, SM22A, and CALPONIN, (C) contractile VSMC markers SMTN, MYH11, and ELASTIN, (D) ECM genes FN, COL4A, and MMP9, (E) growth factors VEGFA and VEGFC, and (F) other genes related to angiogenesis, PECAM1, ANGPT2, NOTCH4, and DLL4. Data are represented as mean ± SD (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(G and H) Immunostaining and flow cytometry of Ki67 showed that (G) 2D-VSMCs had more proliferating cells than (H) AlgTube-VSMCs. Scale bar, 50 μm.
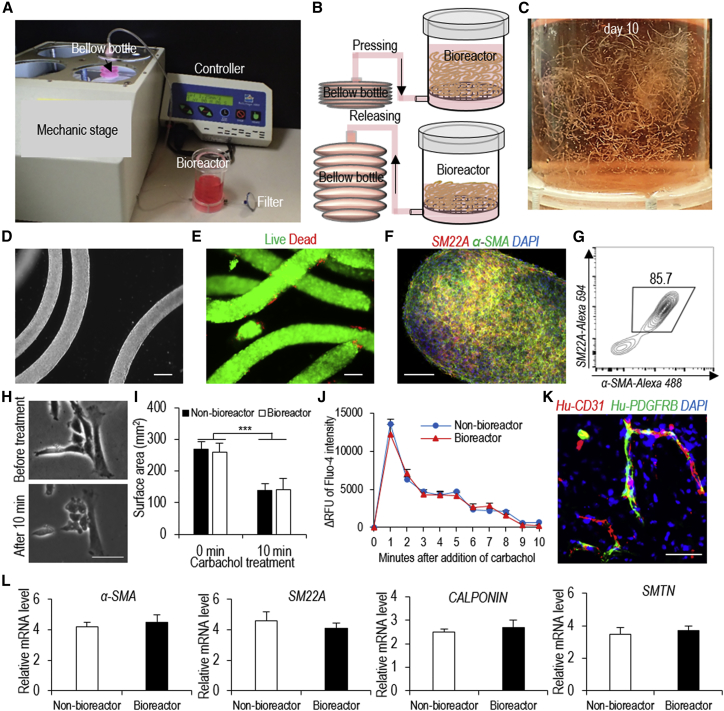
Scalable Manufacturing of hPSC-VSMCs in a Bioreactor
The above studies were done using a small volume (e.g., <100 μL) of AlgTubes suspended in a six-well plate. We asked if AlgTubes could be suspended in a scalable bioreactor (Li et al., 2018a) for making hPSC-VSMCs at various scales (Figures 6A and 6B). Cells were expanded for 5 days and differentiated for 5 days in the bioreactor (Figure 6C). On day 10, hydrogel tubes were dissolved with EDTA and cells were harvested. Phase image and live/dead cell staining showed that the majority of cells were live (Figures 6D and 6E). Flow cytometer analysis and immunostaining showed that 85.7% of the produced cells were VSMCs (Figures 6F and 6G). VSMCs produced in the bioreactor (bioreactor-VSMCs) contracted in response to carbachol treatment in vitro (Figures 6H and 6I). Calcium imaging revealed that carbachol induced similar levels of intracellular calcium between bioreactor-VSMCs and non-bioreactor-VSMCs (i.e., AlgTube-VSMCs made using tissue culture plates) (Figure 6J). A transplantation study showed that bioreactor-VSMCs contributed to blood vessel formation in vivo (Figure 6K). Quantitative RT-PCR analysis showed that bioreactor-VSMCs had similar expression levels of VSMC markers compared with non-bioreactor-VSMCs (Figure 6L). We also showed that bioreactor-VSMCs could be cultured for multiple passages without losing their markers using the conventional 2D cultures (Figures S5F and S5G). These results show that AlgTubes are appropriate for preparing hPSC-VSMCs in a bioreactor. This prototype bioreactor can be scaled up for large-scale production in the future.
Figure 6.
Making hPSC-VSMCs in a Bioreactor
(A) The bioreactor.
(B) Illustration of medium exchange.
(C) Day 10 cells in the bioreactor.
(D–G) (D) Phase, (E) live/dead cell staining, (F) immunostaining, and (G) flow cytometry analysis of day 10 cells from the bioreactor. Scale bars, 200, 50, and 20 μm, respectively.
(H and I) (H) Phase and (I) surface area of AlgTube-VSMCs made using the bioreactor in response to carbachol treatment. Scale bar, 50 μm. Data are represented as mean ± SD (n = 3). ∗∗∗p < 0.001.
(J) The relative fluorescence units (ΔRFU) of Fluo-4-loaded VSMCs made with the bioreactor over 10 min after adding carbachol. Data are represented as mean ± SD (n = 3).
(K) When VSMCs made with the bioreactor and HUVECs were co-transplanted subcutaneously, they formed nice vascular structures. Scale bar, 50 μm.
(L) qRT-PCR analysis showed that AlgTube-VSMCs made using the bioreactor had similar expression levels of VSMC markers compared with AlgTube-VSMCs made using tissue culture plates (non-bioreactor). Data are represented as mean ± SD (n = 3).
Discussion
Generating enough hPSCs and their derivatives continues to be challenging. At present, hPSC-VSMCs are primarily made in 2D cultures (Patsch et al., 2015), which utilize growth conditions that are considerably different from the in vivo 3D microenvironment where cells reside (Chen et al., 2014b, Chen et al., 2014a, Kraehenbuehl et al., 2011, Thomson et al., 1998, Wong et al., 2010). Also, 2D culturing is not conducive to preparing large-scale cultures because it is labor, time, space, and reagent consuming (Kropp et al., 2017). Three-dimensional suspension culturing has successfully been shown to be an attractive method to scale up the production of hPSCs and their derivatives (Fridley et al., 2012, Kinney et al., 2011, Kropp et al., 2017). However, suspension growth of hPSCs continues to suffer from severe cellular agglomeration (Fridley et al., 2012, Kinney et al., 2011, Kropp et al., 2017). Applying agitation (stirring or shaking) to the culture can reduce agglomeration and recover mass transport (Fridley et al., 2012, Kinney et al., 2011, Kropp et al., 2017). However, agitation generates critical shear stress near the bioreactor wall and impeller tip that induces a large amount of cell death, leading to low cell expansion and yield (Fridley et al., 2012, Ismadi et al., 2014, Jenkins and Farid, 2015, Kinney et al., 2011, Kropp et al., 2017, Lei et al., 2014, Lei and Schaffer, 2013, Serra et al., 2012, Steiner et al., 2010). Agitation also generates complicated hydrodynamic conditions (e.g., the medium flow direction, velocity, shear force, and chemical environment) that vary spatially and temporally, and can be affected by a number of variables, including the design of the bioreactor (e.g., impeller geometry, size and position, vessel geometry and size, and positions of probes for pH, temperature, oxygen), the viscosity of the medium, and also the rate of agitation (Ismadi et al., 2014, Kropp et al., 2017). The hydrodynamic conditions are not very well understood yet, which makes them difficult to control (Fridley et al., 2012, Ismadi et al., 2014, Kinney et al., 2011, Kropp et al., 2017). This lack of understanding and technological issues causes considerable variability between culture batches, leading to further difficulty in scaling up culture size. For example, in recent 3D culture studies to generate cardiomyocytes from hPSCs, the cellular yield varied dramatically (from 40 million to 125 million cells) and the cellular purity varied from 28% to 88% in six different batches (100 mL culture volume) (Chen et al., 2015, Jara-avaca et al., 2014). When adjusting the culture volume from ∼100 mL to ∼1000 mL, both the efficiency of differentiation and the final product yield were altered significantly, which led to the need for reoptimization (Chen et al., 2015, Jara-avaca et al., 2014). This demonstrates the difficulty in scaling up further (e.g., hundreds of liters), due to the cost of optimizing multiple factors in a large volume. To date, <10 L of hPSC culture is the highest volume successfully produced (Kempf et al., 2016, Kropp et al., 2017). As a result, hydrodynamic conditions, and the associated critical stresses in combination with the uncontrolled cell aggregation, make it very challenging to utilize a 3D suspension culture system to generate hPSCs and their derivatives at large scales.
The alginate hydrogel tubes could eliminate both the cell agglomeration and the hydrodynamic stresses and their related adverse effects (Figures 1A–1C). Consequently, the culture efficiency (e.g., cell viability and yield) and consistency are significantly improved (Figures 1G and 1H). The volumetric yield reached 5.0 × 108 cells/mL of microspace, which is 250 times the current 3D suspension culturing. This high yield has enormous impact on large-scale cell production (Li et al., 2018a). Our calculations show that the cells would need to undergo passaging 10 times, ∼32,753 L total bioreactor volume, and 45 days to produce ∼1.1 × 1013 hPSC-VSMCs using 1.0 × 108 hPSCs as starting material. This production is technically and economically not feasible. Using AlgTubes, the same cells can be made using merely 320 L of hydrogel tubes, one passage, and 21 days. The reduction in culture volume, time, and passaging makes the production technically and economically feasible.
Our research showed that the differentiation efficiencies in 2D and AlgTubes were similar (Figures 2 and S2), indicating that the differentiation protocol developed by Patsch (Patsch et al., 2015) using 2D cultures can be used for 3D culture. Phenotype assays and genome-wide gene expression analysis showed that AlgTube-VSMCs and 2D-VSMCs were similar overall. Both of them had typical VSMC phenotypes (Figure 3). Transplantation studies showed that AlgTube-VSMCs and 2D-VSMCs contributed to blood vessel formation similarly in vivo (Figures 3I and 3J). However, they do have some differences in global gene expression. AlgTube-VSMCs had higher expression of genes related to vasculature development, angiogenesis, and cell migration (Figure 4D). This agrees with recent research on organoids, which shows that 3D microenvironments promote tissue formation (Hattori, 2014, Jo et al., 2016, Takahashi et al., 2018). 2D-VSMCs are closer to the synthetic phenotype. For instance, they had higher proliferation (Figure 5G) and higher expression of the synthetic VSMC genes (Figure 5B). Two-dimensional culturing on a stiff substrate induces cell proliferation, which is a frequently observed phenomenon when culturing primary human cells (Chamley et al., 1977, Timraz et al., 2015, Wazen et al., 2013). Both AlgTube-VSMCs and 2D-VSMCs had some differences in global gene expression from the primary HASMCs. If and how the differences in global gene expression between the three types of VSMCs affect their phenotypes and functions should be made clear in the future using more sensitive assays and disease models.
Conclusion
In summary, we have developed a scalable method for manufacturing VSMCs from hPSCs with high viability, high purity (>80%), and high yield (∼5.0 × 108 cells/mL of microspace). The method will make VSMCs broadly available and affordable for various applications.
Experimental Procedures
RNA Sequencing and Data Analysis
Total RNA of day 6 VSMCs from 2D culture and day 10 VSMCs from AlgTubes (note: both VSMCs were differentiated for 5 days) was prepared with an RNeasy mini kit (Cat. No. 74,104; QIAGEN) according to the manufacturer's instructions. Prior to RNA-seq, magnetic beads coated with anti-CD144 antibodies were added to remove CD144+ hPSC-ECs with a magnetic cell separator. VSMCs reached 95% purity after purification. Libraries were prepared with a TruSeq Stranded mRNA Library Prep Kit and sequenced with Illumina NextSeq 500. Twenty million 75 bp paired-end reads were generated for each sample. The thresholds for differential expression were set at fold-change > 2 and adjusted p < 0.001 for the null hypothesis. Methods for the data processing, generation of heatmaps, PCA, and differential gene expression analysis have been described in our previous publication (Li et al., 2018a).
Statistical Analysis
The data are presented as the mean ± SD from three independent experiments. We used an unpaired t test to compare two groups and one-way ANOVA to compare more than two groups. A sample size of 3 was selected so that at a significance level of 0.05 there was at least a 95% chance of detecting a 2 SD difference in outcome between the groups. All data were processed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA).
Author Contributions
Y.L. and H.L. conceived the idea, designed the experiments, and wrote the manuscript. L.A. made the device. H.L., X.Q., Q.L., and O.W. performed the experiments. H.L., X.Q., Q.D., K.L., and C.Z. analyzed the data. D.A. contributed to flow cytometry analysis. Z.W. and L.G. revised the manuscript.
Acknowledgments
This work was partially funded by the UNL-UNMC Science Engineering and Medicine (SEM) initiative grant. Confocal microscope imaging was done in the Morrison Microscopy Core Research Facility at the University of Nebraska, Lincoln. Dr. You Zhou and Dr. Christian Elowsky assisted in the confocal imaging. Flow cytometry was done in the Morrison Center, the Flow Cytometry Service Center, University of Nebraska, Lincoln, with the assistance of Dirk Anderson. RNA sequencing was done at the UNMC deep sequencing core.
Published: December 6, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and three tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.11.009.
Accession Numbers
The final processed data and raw fastq files were submitted to Gene Expression Omnibus (GEO) with the accession numbers GEO: GSE99776 and GSE109683. All other data supporting the findings of this study are available within the paper and its Supplemental Information.
Supplemental Information
and S2
References
- Alexander M.R., Owens G.K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- Ayoubi S., Sheikh S.P., Eskildsen T.V. Human induced pluripotent stem cell-derived vascular smooth muscle cells: differentiation and therapeutic potential. Cardiovasc. Res. 2017;113:1282–1293. doi: 10.1093/cvr/cvx125. [DOI] [PubMed] [Google Scholar]
- Bajpai V.K., Mistriotis P., Loh Y.-H., Daley G.Q., Andreadis S.T. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc. Res. 2012;96:391–400. doi: 10.1093/cvr/cvs253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X., Lian X., Dunn K.K., Shi M., Han T., Qian T., Bhute V.J., Canfield S.G., Palecek S.P. Chemically-defined albumin-free differentiation of human pluripotent stem cells to endothelial progenitor cells. Stem Cell Res. 2015;15:122–129. doi: 10.1016/j.scr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo A.S., Faial T., Gardner L., Niakan K.K., Ortmann D., Senner C.E., Callery E.M., Trotter M.W., Hemberger M., Smith J.C. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell. 2011;9:144–155. doi: 10.1016/j.stem.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel N.M., Santostefano K.E., DiVita B.B., El Rouby N., Carrasquilla S.D., Simmons C., Nakanishi M., Cooper-DeHoff R.M., Johnson J.A., Terada N. Vascular smooth muscle cells from hypertensive patient-derived induced pluripotent stem cells to advance hypertension pharmacogenomics. Stem Cells Transl. Med. 2015;4:1380–1390. doi: 10.5966/sctm.2015-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Chamley J.H., Campbell G.R., McConnell J.D., Gröschel-Stewart U. Comparison of vascular smooth muscle cells from adult human, monkey and rabbit in primary culture and in subculture. Cell Tissue Res. 1977;177:503–522. doi: 10.1007/BF00220611. [DOI] [PubMed] [Google Scholar]
- Chen G., Gulbranson D.R., Hou Z., Bolin J.M., Ruotti V., Probasco M.D., Smuga-Otto K., Howden S.E., Diol N.R., Propson N.E. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.G., Mallon B.S., Johnson K.R., Hamilton R.S., Mckay R.D.G., Robey P.G. Developmental insights from early mammalian embryos and core signaling pathways that influence human pluripotent cell growth and differentiation. Stem Cell Res. 2014;12:610–621. doi: 10.1016/j.scr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.G., Mallon B.S., McKay R.D.G., Robey P.G. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell. 2014;14:13–26. doi: 10.1016/j.stem.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen V.C., Ye J., Shukla P., Hua G., Chen D., Lin Z., Liu J., Chai J., Gold J., Wu J. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 2015;15:365–375. doi: 10.1016/j.scr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C., Bernardo A.S., Trotter M.W.B., Pedersen R.A., Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin–dependent disease susceptibility. Nat. Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C., Bernardo A.S., Pedersen R.A., Sinha S. Directed differentiation of embryonic origin-specific vascular smooth muscle subtypes from human pluripotent stem cells. Nat. Protoc. 2014;9:929–938. doi: 10.1038/nprot.2014.059. [DOI] [PubMed] [Google Scholar]
- Emilio R., Antonio G.-M., Eugenia P., Santiago R., Enrique R., Fernando R., Ana M.B., Cornelis van B., Elena O., Teresa T. Human vascular smooth muscle cells from diabetic patients are resistant to induced apoptosis due to high Bcl-2 expression. Diabetes. 2006;55:1243–1251. doi: 10.2337/db05-0949. [DOI] [PubMed] [Google Scholar]
- Eoh J.H., Shen N., Burke J.A., Hinderer S., Xia Z., Schenke-Layland K., Gerecht S. Enhanced elastin synthesis and maturation in human vascular smooth muscle tissue derived from induced-pluripotent stem cells. Acta Biomater. 2017;52:49–59. doi: 10.1016/j.actbio.2017.01.083. [DOI] [PubMed] [Google Scholar]
- Ferreira L.S., Gerecht S., Shieh H.F., Watson N., Rupnick M.A., Dallabrida S.M., Vunjak-novakovic G., Langer R. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ. Res. 2007;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- Fridley K.M., Kinney M.A., Mcdevitt T.C. Hydrodynamic modulation of pluripotent stem cells. Stem Cell Res. Ther. 2012;3:45. doi: 10.1186/scrt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X., Ren Y., Bartulos O., Lee M.Y., Yue Z., Kim K.Y., Li W., Amos P.J., Bozkulak E.C., Iyer A. Modeling supravalvular aortic stenosis syndrome with human induced pluripotent stem cells. Circulation. 2012;126:1695–1704. doi: 10.1161/CIRCULATIONAHA.112.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecht S., Ferreira L.S., Langer R. Vascular differentiation of human embryonic stem cells in bioactive hydrogel-based scaffolds. Methods Mol. Biol. 2010;584:333–354. doi: 10.1007/978-1-60761-369-5_18. [DOI] [PubMed] [Google Scholar]
- Granata A., Serrano F., Bernard W.G., McNamara M., Low L., Sastry P., Sinha S. An iPSC-derived vascular model of Marfan syndrome identifies key mediators of smooth muscle cell death. Nat. Genet. 2017;49:97–109. doi: 10.1038/ng.3723. [DOI] [PubMed] [Google Scholar]
- Hattan N., Warltier D., Gu W., Kolz C., Chilian W.M., Weihrauch D. Autologous vascular smooth muscle cell-based myocardial gene therapy to induce coronary collateral growth. Am. J. Physiol. Circ. Physiol. 2004;287:H488–H493. doi: 10.1152/ajpheart.00145.2004. [DOI] [PubMed] [Google Scholar]
- Hattori N. Cerebral organoids model human brain development and microcephaly. Mov. Disord. 2014;29:185. doi: 10.1002/mds.25740. [DOI] [PubMed] [Google Scholar]
- Hibino N., Duncan D.R., Nalbandian A., Yi T., Qyang Y., Shinoka T., Breuer C.K. Evaluation of the use of an induced pluripotent stem cell sheet for the construction of tissue-engineered vascular grafts. J. Thorac. Cardiovasc. Surg. 2012;143:696–703. doi: 10.1016/j.jtcvs.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X., Margariti A., Le Bras A., Jacquet L., Kong W., Hu Y., Xu Q. Transdifferentiated human vascular smooth muscle cells are a new potential cell source for endothelial regeneration. Sci. Rep. 2017;7:5590. doi: 10.1038/s41598-017-05665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismadi M., Gupta P., Fouras A., Verma P., Jadhav S. Flow characterization of a spinner flask for induced pluripotent stem cell culture application. PLoS One. 2014;9:e106493. doi: 10.1371/journal.pone.0106493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer D., Gambardella L., Bernard W.G., Serrano F., Mascetti V.L., Pedersen R.A., Sinha S., Talasila A. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Development. 2016;143:904. doi: 10.1242/dev.136143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara-avaca M., Kempf H., Olmer R., Kropp C., Ru M., Robles-diaz D., Franke A., Elliott D.A., Wojciechowski D., Fischer M. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Reports. 2014;3:1132–1146. doi: 10.1016/j.stemcr.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.J., Farid S.S. Human pluripotent stem cell-derived products: advances towards robust, scalable and cost-effective manufacturing strategies. Biotechnol. J. 2015;10:83–95. doi: 10.1002/biot.201400348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Kim H.S., Kim H.-W., Leong K.W. Application of induced pluripotent stem cells to model smooth muscle cell function in vascular diseases. Curr. Opin. Biomed. Eng. 2017;1:38–44. doi: 10.1016/j.cobme.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J., Xiao Y., Sun A.X., Cukuroglu E., Tran H.D., Göke J., Tan Z.Y., Saw T.Y., Tan C.P., Lokman H. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell. 2016;19:248–257. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamariti E., Margariti A., Winkler B., Wang X., Hong X., Baban D., Ragoussis J., Huang Y., Han J.D.J., Wong M.M. Smooth muscle cells differentiated from reprogrammed embryonic lung fibroblasts through dkk3 signaling are potent for tissue engineering of vascular grafts. Circ. Res. 2013;112:1433–1443. doi: 10.1161/CIRCRESAHA.111.300415. [DOI] [PubMed] [Google Scholar]
- Kempf H., Andree B., Zweigerdt R. Large-scale production of human pluripotent stem cell derived cardiomyocytes. Adv. Drug Deliv. Rev. 2016;96:18–30. doi: 10.1016/j.addr.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Kinnear C., Chang W.Y., Khattak S., Hinek A., Thompson T., Rodrigues D., de C., Kennedy K., Mahmut N., Pasceri P. Modeling human protein aggregation cardiomyopathy using murine induced pluripotent stem cells. Stem Cell Transl. Med. 2013;2:161–166. doi: 10.5966/sctm.2012-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear C., Chang W., Khattak S., Hinek A., Thompson T., de Carvalho Rodrigues D., Kennedy K., Mahmut N., Pasceri P., Stanford W. Modeling and rescue of the vascular phenotype of Williams-Beuren syndrome in patient induced pluripotent stem cells. Stem Cells Transl. Med. 2013;2:2–15. doi: 10.5966/sctm.2012-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney M.A., Sargent C.Y., Mcdevitt T.C. The multiparametric effects of hydrodynamic environments on stem cell culture. Tissue Eng. Part B Rev. 2011;17:249–262. doi: 10.1089/ten.teb.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuehl T.P., Langer R., Ferreira L.S. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat. Methods. 2011;8:731–736. doi: 10.1038/nmeth.1671. [DOI] [PubMed] [Google Scholar]
- Kropp C., Massai D., Zweigerdt R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process. Biochem. 2017;59:244–254. [Google Scholar]
- Lacolley P., Regnault V., Nicoletti A., Li Z., Michel J.B. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc. Res. 2012;95:194–204. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- Lalit P.A., Hei D.J., Raval A.N., Kamp T.J. Induced pluripotent stem cells for post -myocardial infarction repair. Circ. Res. 2014;114:1328–1345. doi: 10.1161/CIRCRESAHA.114.300556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.-H., Song S.-H., Kim K.L., Yi J.-Y., Shin G.-H., Kim J.Y., Kim J., Han Y.-M., Lee S.H., Lee S.-H. Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ. Res. 2010;106:120–128. doi: 10.1161/CIRCRESAHA.109.207902. [DOI] [PubMed] [Google Scholar]
- Lei Y., Schaffer D.V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl. Acad. Sci. U S A. 2013;110:E5039–E5048. doi: 10.1073/pnas.1309408110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Jeong D., Xiao J., Schaffer D.V. Developing defined and scalable 3D culture systems for culturing human pluripotent stem cells at high densities. Cell. Mol. Bioeng. 2014;7:172–183. doi: 10.1007/s12195-014-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S. Engineering blood vessels from stem cells: recent advances and applications. Curr. Opin. Biotechnol. 2005;16:516–523. doi: 10.1016/j.copbio.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Levenberg S., Zoldan J., Basevitch Y., Langer R. Endothelial potential of human embryonic stem cells. Blood. 2007;110:806–814. doi: 10.1182/blood-2006-08-019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Lin H., Du Q., Liu K., Wang O., Evans C., Christian H., Zhang C., Lei Y. Scalable and physiologically relevant microenvironments for human pluripotent stem cell expansion and differentiation. Biofabrication. 2018;10:025006. doi: 10.1088/1758-5090/aaa6b5. [DOI] [PubMed] [Google Scholar]
- Li Q., Lin H., Rauch J., Deleyrolle L.P., Reynolds B.A., Viljoen H.J., Zhang C., Zhang C., Gu L., Van Wyk E. Scalable culturing of primary human glioblastoma tumor-initiating cells with a cell-friendly culture system. Sci. Rep. 2018;8:3531. doi: 10.1038/s41598-018-21927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.-K., Jia Z.-Q., Weisel R.D., Merante F., Mickle D.A.G. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J. Mol. Cell. Cardiol. 1999;31:513–522. doi: 10.1006/jmcc.1998.0882. [DOI] [PubMed] [Google Scholar]
- Lian X., Bao X., Al-Ahmad A., Liu J., Wu Y., Dong W., Dunn K.K., Shusta E.V., Palecek S.P. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports. 2014;3:804–816. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B., Kim J., Li Y., Pan H., Carvajal-Vergara X., Salama G., Cheng T., Li Y., Lo C.W., Yang L. High-purity enrichment of functional cardiovascular cells from human iPS cells. Cardiovasc. Res. 2012;95:327–335. doi: 10.1093/cvr/cvs185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Du Q., Li Q., Wang O., Wang Z., Liu K., Elowsky C., Zhang C., Lei Y. A hydrogel-based bioprocess for scalable manufacturing of human pluripotent stem cells-derived neural stem cells. ACS Appl. Mater. Interfaces. 2018;10:29238–29250. doi: 10.1021/acsami.8b05780. [DOI] [PubMed] [Google Scholar]
- Lin H., Li Q., Wang O., Rauch J., Harm B., Viljoen H.J., Zhang C., Van Wyk E., Zhang C., Lei Y. Automated expansion of primary human T cells in scalable and cell-friendly hydrogel microtubes for adoptive immunotherapy. Adv. Healthc. Mater. 2018;7:e1701297. doi: 10.1002/adhm.201701297. [DOI] [PubMed] [Google Scholar]
- Liu G.H., Barkho B.Z., Ruiz S., Diep D., Qu J., Yang S.L., Panopoulos A.D., Suzuki K., Kurian L., Walsh C. Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature. 2011;472:221–227. doi: 10.1038/nature09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis S.F., Zahradka P. Vascular smooth muscle cell motility: from migration to invasion. Exp. Clin. Cardiol. 2010;15:e75–e85. [PMC free article] [PubMed] [Google Scholar]
- Mark W.M., Christine L.M. Smooth muscle diversity from human pluripotent cells. Nat. Biotechnol. 2012;30:152–154. doi: 10.1038/nbt.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi K., Fedak P., Mickle D., Weisel R., Ozawa T., Li R. Improved left ventricular aneurysm repair with bioengineered vascular smooth muscle grafts. Circulation. 2003;108:II219–II225. doi: 10.1161/01.cir.0000087450.34497.9a. [DOI] [PubMed] [Google Scholar]
- Melanie M., Erica K.A., Smruti M.P., Michael T.L., John P.C., Bertha C. Concurrent generation of functional smooth muscle and endothelial cells via a vascular progenitor. Stem Cells Transl. Med. 2014;3:91–97. doi: 10.5966/sctm.2013-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., Hong H., Nakagawa M., Tanabe K., Tezuka K. A more efficient method to generate integration-free human iPS cells. Nat. Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Owens G.K., Kumar M.S., Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pan X., Wang B., Yuan T., Zhang M., Kent K.C., Guo L.W. Analysis of combined transcriptomes identifies gene modules that differentially respond to pathogenic stimulation of vascular smooth muscle and endothelial cells. Sci. Rep. 2018;8:395. doi: 10.1038/s41598-017-18675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I.H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., Lerou P.H., Lensch M.W., Daley G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Park S., Koh Y.J., Jeon J., Cho Y., Jang M., Kang Y., Kim M., Choi C., Cho Y.S., Chung H. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2010;116:5762–5772. doi: 10.1182/blood-2010-04-280719. [DOI] [PubMed] [Google Scholar]
- Patsch C., Challet-Meylan L., Thoma E.C., Urich E., Heckel T., O’Sullivan J.F., Grainger S.J., Kapp F.G., Sun L., Christensen K. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015;17:994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei D., Xu J., Zhuang Q., Tse H.-F., Esteban M.A. Induced pluripotent stem cell technology in regenerative medicine and biology. Adv. Biochem. Eng. Biotechnol. 2010;123:127–141. doi: 10.1007/10_2010_72. [DOI] [PubMed] [Google Scholar]
- Poh M., Boyer M., Solan A., Dahl S.L., Pedrotty D., Banik S.S., McKee J.A., Klinger R.Y., Counter C.M., Niklason L.E. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- Porter K.E., Riches K. The vascular smooth muscle cell: a therapeutic target in Type 2 diabetes? Clin. Sci. (Lond.) 2013;125:167–182. doi: 10.1042/CS20120413. [DOI] [PubMed] [Google Scholar]
- Raphel L., Talasila A., Cheung C., Sinha S. Myocardin overexpression is sufficient for promoting the development of a mature smooth muscle cell-like phenotype from human embryonic stem cells. PLoS One. 2012;7:e44052. doi: 10.1371/journal.pone.0044052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M., Brito C., Correia C., Alves P.M. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012;30:350–358. doi: 10.1016/j.tibtech.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Sinha S., Hoofnagle M.H., Kingston P.A., Mccanna M.E., Owens G.K. Transforming growth factor-beta 1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am. J. Physiol. Cell Physiol. 2004;287:C1560–C1568. doi: 10.1152/ajpcell.00221.2004. [DOI] [PubMed] [Google Scholar]
- Staudacher D.L., Preis M., Lewis B.S., Grossman P.M., Flugelman M.Y. Cellular and molecular therapeutic modalities for arterial obstructive syndromes. Pharmacol. Ther. 2006;109:263–273. doi: 10.1016/j.pharmthera.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Steiner D., Khaner H., Cohen M., Even-Ram S., Gil Y., Itsykson P., Turetsky T., Idelson M., Aizenman E., Ram R. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat. Biotechnol. 2010;28:361–364. doi: 10.1038/nbt.1616. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Sato S., Kurashima Y., Yamamoto T., Kurokawa S., Yuki Y., Takemura N., Uematsu S., Lai C., Otsu M. A refined culture system for human induced pluripotent stem cell-derived intestinal epithelial organoids. Stem Cell Reports. 2018;10:314–328. doi: 10.1016/j.stemcr.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura D., Sone M., Homma K., Oyamada N., Takahashi K., Tamura N., Yamanaka S., Nakao K. Induction and isolation of vascular cells from human induced pluripotent stem cells—brief report. Arterioscler. Thromb. Vasc. Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Timraz S.B.H., Rezgui R., Boularaoui S.M., Teo J.C.M. Stiffness of extracellular matrix components modulates the phenotype of human smooth muscle cells in vitro and allows for the control of properties of engineered tissues. Procedia Eng. 2015;110:29–36. [Google Scholar]
- Vo E., Hanjaya-Putra D., Zha Y., Kusuma S., Gerecht S. Smooth-muscle-like cells derived from human embryonic stem cells support and augment cord-like structures in vitro. Stem Cell Rev. Rep. 2010;6:237–247. doi: 10.1007/s12015-010-9144-3. [DOI] [PubMed] [Google Scholar]
- Wang Y., Hu J., Jiao J., Liu Z., Zhou Z., Zhao C., Chang L.J., Chen Y.E., Ma P.X., Yang B. Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials. 2014;35:8960–8969. doi: 10.1016/j.biomaterials.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wen Y., Li Y.H., Wei Y., Green M., Wani P., Zhang P., Pera R.R., Chen B. Smooth muscle precursor cells derived from human pluripotent stem cells for treatment of stress urinary incontinence. Stem Cells Dev. 2016;25:453–461. doi: 10.1089/scd.2015.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjare M., Kuo F., Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc. Res. 2013;97:321–330. doi: 10.1093/cvr/cvs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjare M., Agarwal N., Gerecht S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2015;309:C271–C281. doi: 10.1152/ajpcell.00366.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazen R.M., Kuroda S., Nishio C., Sellin K., Brunski J.B., Nanci A. The effect of substrate modulus on the growth and function of matrix-embedded endothelial cells. Biomaterials. 2013;34:677–684. doi: 10.1016/j.biomaterials.2012.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.C., Loewke K.E., Bossert N.L., Behr B., De Jonge C.J., Baer T.M., Pera R.A.R. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat. Biotechnol. 2010;28:1115–1121. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- Wurm F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- Yang L., Geng Z., Nickel T., Johnson C., Gao L., Dutton J., Hou C., Zhang J. Differentiation of human induced-pluripotent stem cells into smooth-muscle cells: two novel protocols. PLoS One. 2016;11:e0147155. doi: 10.1371/journal.pone.0147155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Chang Y.H., Xiong Q., Zhang P., Zhang L., Somasundaram P., Lepley M., Swingen C., Su L., Wendel J.S. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo C.H., Na H.J., Lee D.S., Heo S.C., An Y., Cha J., Choi C., Kim J.H., Park J.C., Cho Y.S. Endothelial progenitor cells from human dental pulp-derived iPS cells as a therapeutic target for ischemic vascular diseases. Biomaterials. 2013;34:8149–8160. doi: 10.1016/j.biomaterials.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-otto K., Antosiewicz-bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang H., Xiong Z.-M., Cao K. Mechanisms controlling the smooth muscle cell death in progeria via down-regulation of poly(ADP-ribose) polymerase 1. Proc. Natl. Acad. Sci. U S A. 2014;111:E2261–E2270. doi: 10.1073/pnas.1320843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Lian Q., Zhu G., Zhou F., Sui L., Tan C., Mutalif R.A., Navasankari R., Zhang Y., Tse H.F. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8:31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
and S2