Summary
It is thought that corneal epithelial injuries resolve by leading-edge cells “sliding” or “rolling” into the wound bed. Here, we challenge this notion and show by real-time imaging that corneal wounds initially heal by “basal cell migration.” The K14CreERT2-Confetti multi-colored reporter mouse was employed to spatially and temporally fate-map cellular behavior during corneal wound healing. Keratin-14+ basal epithelia are forced into the wound bed by increased population pressure gradient from the limbus to the wound edge. As the defect resolves, centripetally migrating epithelia decelerate and replication in the periphery is reduced. With time, keratin-14+-derived clones diminish in number concomitant with their expansion, indicative that clonal evolution aligns with neutral drifting. These findings have important implications for the involvement of stem cells in acute tissue regeneration, in key sensory tissues such as the cornea.
Keywords: cornea wound healing, limbal epithelial stem cells, K14CreERT2-Confetti transgenic mice, basal cell migration, organ-culture system, spatiotemporal image correlation spectroscopy, computational modeling
Highlights
-
•
Basal limbal epithelial cell proliferation is increased following a corneal injury
-
•
Corneal epithelial wounds initially heal by K14+ basal cell migration
-
•
STICS accurately measures clonal dynamics during wound closure
-
•
Computational modeling confirms the pivotal role of LESCs in wound repair
In this article, Di Girolamo and colleagues visualized the contribution of K14+ limbal epithelial precursors in resolving corneal epithelial debridement wounds. They noted that population pressure from the limbal perimeter is the main driver of K14+ basal cell displacement during the initial phase of injury repair.
Introduction
The cornea is the most anterior tissue of the eye, and its transparency is critical for vision. The outermost layer consists of a stratified squamous epithelium that protects against fluid loss, microbial invasion, ultraviolet radiation, and physical and chemical trauma (Di Girolamo, 2015, Richardson et al., 2016). Stem cells of the cornea reside in an annular transition zone known as the limbus and are otherwise known as limbal epithelial stem cells (LESCs). LESCs give rise to transit amplifying cells (TACs) that have high but limited proliferative potential, and subsequently terminally differentiated cells, which are exfoliated from the ocular surface. LESCs have important functional traits including an ability to self-renew, cycle slowly, and divide symmetrically and asymmetrically to produce daughter SCs or TACs (Beebe and Masters, 1996, Castro-Muñozledo and Gómez-Flores, 2011, Lamprecht, 1990, Lobo et al., 2016). During homeostasis, the corneal epithelium is continuously replenished in accord with the XYZ hypothesis, in which desquamating superficial cells (Z) must be replenished by proliferating basal cells (X) that migrate in a centripetal direction (Y) (Thoft and Friend, 1983). Under these conditions, the centripetal velocity of murine corneal epithelia ranges from 10 to 26 μm/day (Buck, 1985, Di Girolamo et al., 2015, Nagasaki and Zhao, 2003). Upon injury, cell displacement is accelerated to fast-track healing, and is likely influenced by proliferating LESCs and their early progeny (Lobo et al., 2016, Mort et al., 2009).
Following a superficial epithelial wound, three basic events transpire. In the initial phase, no active cell proliferation or migration occurs; instead, proteins are synthesized (Gipson and Kiorpes, 1982, Zieske and Gipson, 1986) and a temporary extracellular matrix is deposited (Fujikawa et al., 1981). Second, suprabasal wing cells “slide” into the wound bed to cover the defect (Crosson et al., 1986, Gipson and Kiorpes, 1982, Kuwabara et al., 1976). Finally, epithelial layers are restored by cells proliferating, differentiating, and stratifying (Lehrer et al., 1998, Stepp et al., 2014, Zhao et al., 2003).
After corneal epithelial debridement, cell migration is accelerated 40-fold compared with steady state (Kuwabara et al., 1976, Mort et al., 2009, Ramaesh et al., 2006), and slow-cycling LESCs can be stimulated to proliferate (Chung et al., 1999, Cotsarelis et al., 1989), thereby supporting the proposition that these cells provide the impetus to restore epithelial integrity. Although these studies offer fundamental mechanistic insights into cellular behavior, they are fraught with obvious limitations. A major challenge has been determining whether LESCs truly partake in corneal wound healing, and if so, what experimental paradigms could be employed to visualize their contribution in real-time. Despite attempts to resolve this shortcoming through the use of adenoviral vectors carrying a GFP reporter (Danjo and Gipson, 2002) and non-specific transgenic reporters (Mort et al., 2009, Ramaesh et al., 2006), the origins of marked cells has been difficult to reconcile.
Herein, we employed the K14CreERT2-Confetti (Confetti) mouse, which delivers stable, long-term labeling of keratin-14+ (K14+) limbal epithelia, along with their ensuing progeny. We provide the real-time spatial-temporal recordings of LESCs during the initial phase of wound repair, and visualized elevated clonal activity emanating from the limbus, along with streaming basal limbal epithelia into the wound bed. After using bromodeoxyuridine (BrdU) labeling and applying a computational model, we confirmed that LESC proliferation generates population pressure in the periphery that drives basal cell displacement to seal the wound in a timely fashion. Finally, through the application of spatiotemporal image correlation spectroscopy (STICS) (Toplak et al., 2012), and after correcting for corneal curvature, we determined the velocity and direction of migrating clones during wound closure with greater precision.
Results
Resolution of a Phenotypically Normal Corneal Epithelium after Mechanical Debridement
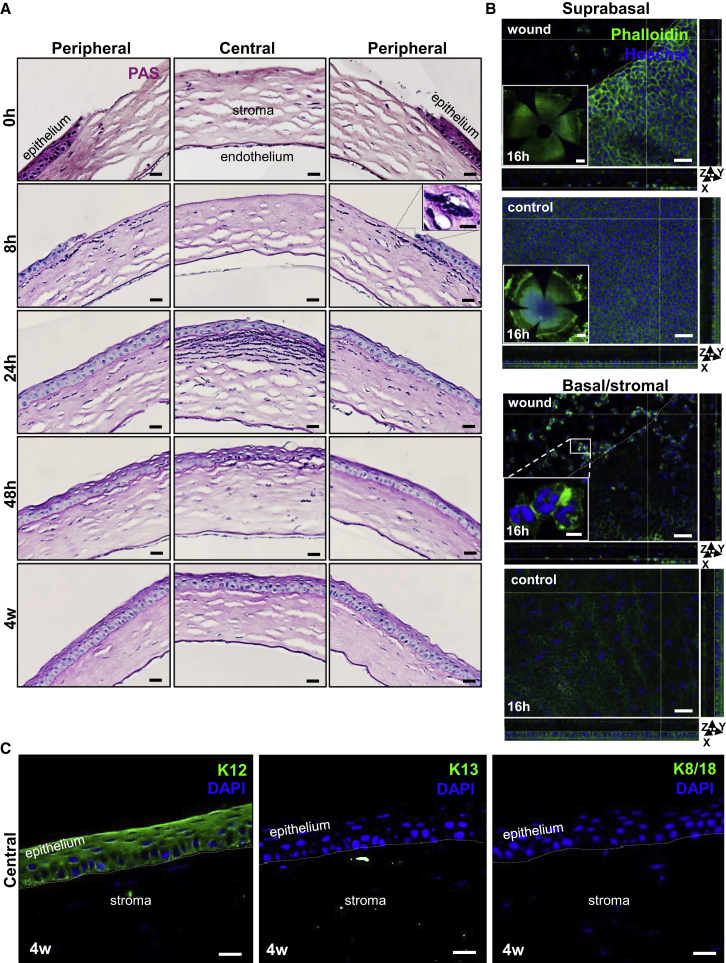
To assess the extent of epithelial debridement, we euthanized mice at regular intervals and examined their corneas after staining sections with periodic acid-Schiff (PAS) (Figure 1A). Corneas inflicted with a 2-mm wound displayed no central epithelium; however, the peripheral and limbal epithelium remained intact (Figure 1A, first row). At 8 hr post wounding, immune cells with multi-lobed nuclei infiltrated the stroma from the periphery (Figure 1A, second row, inset). Confocal microscopy on phalloidin-stained flat-mounted corneas at 16 hr post wounding suggested that these cells were mostly neutrophils (Li et al., 2006, Petrescu et al., 2007) confined to the superficial stroma (Figure 1B, second row, inset). By 24 hr post injury, the central cornea was covered by at least one layer of epithelia, stromal inflammation adjacent the regenerating epithelium was heightened, and the basement membrane was more pronounced (Figure 1A, third row, middle panel). Notably, this coincided with the disappearance of neutrophils from the periphery (Figure 1A, third row, left and right panels). At 48 hr post wounding, the peripheral epithelium returned to near steady state, with reduced inflammation (Figure 1A, fourth row). Epithelial stratification was restored prior to 4 weeks post injury (Figure 1A, fifth row), with no signs of “conjunctivalization” (Huang and Tseng, 1991, Lin et al., 2013, Pajoohesh-Ganji et al., 2012). At this point in time, the regenerated central epithelium displayed corneal-specific K12 immunoreactivity but lacked conjunctival K13 and K8/18 expression (Figure 1C), indicating that the limbal barrier had not been breached and corneal epithelial regeneration likely ensued via proliferating LESCs and/or TACs.
Figure 1.
Restoration of a Phenotypically Normal Corneal Epithelium after Mechanical Debridement
(A) Seven-week-old WT mice (n = 3/time point) had their right corneal epithelium mechanically debrided. Histological features of the central and peripheral cornea are displayed after staining 4-μm sections with PAS at 0 hr, 8 hr, 24 hr, 48 hr and 4 weeks post injury. Scale bars represent 20 μm. Inset presents a magnified view of the region encompassed by the hatched box and confirms a stromal neutrophilic infiltrate (scale bar, 10 μm).
(B) Phalloidin (green)-stained flat-mounted whole corneas at 16 hr post wounding and control (n = 5/group). White hatched lines demarcate the wound margin. y-z and x-z planes display epithelial thickness. Scale bars, 40 μm. Insets of suprabasal layers (first and second panels) provide a global view of flat-mounted corneas (scale bars, 500 μm). Inset (third panel) shows cells with multi-lobed nuclei within the corneal stroma directly beneath the wound bed, indicative of neutrophils (scale bar, 10 μm). Nuclei were counterstained with Hoechst (blue). See also Table S1.
(C) Immunofluorescence for K12 (green), K13 (green), and K8/18 (green) in the central cornea 4 weeks post wounding (n = 3/group). Hatched white lines demarcate the epithelial basement membrane. Nuclei are counterstained with DAPI (blue). Scale bars, 20 μm.
After staining specimens with phalloidin, we assessed cell morphology in and around the injury site just prior to wound closure (Figure 1B). At 16 hr post injury, cell size adjacent to the wound margin was significantly increased (Figure 1B [x-y plane] and Table S1); this was congruent with decreased cell density and epithelial thickness (Figure 1B, orthogonal views; x-z plane and y-z planes; and Table S1), implying the defect was covered by a monolayer or dual layer of epithelial cells that changed shape.
Accelerated Re-epithelialization of Corneal Wounds by K14+ Limbal Progenitor-Derived Clones
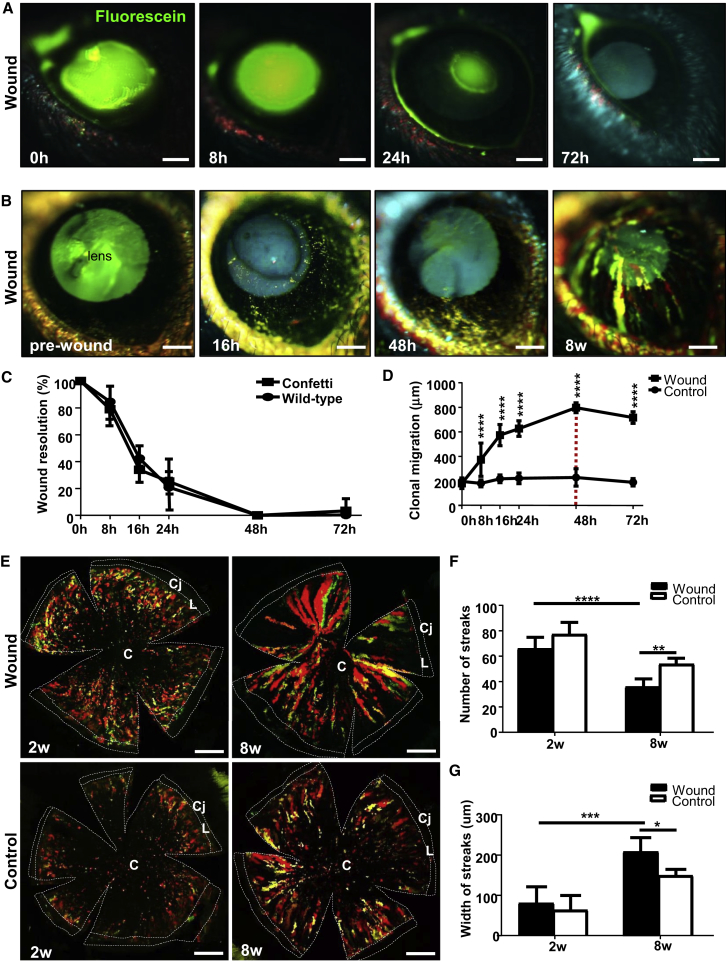
One advantage of utilizing an inducible transgenic model is the ability to visualize clonal evolution and expansion during wound healing in real time with intra-vital microscopy. However, to confirm that the genetic alterations in Confetti mice did not alter the wound-healing response, we compared wound-closure rates with those of wild-type (WT) mice. Fluorescein staining demonstrated that epithelial defects in Confetti (Figure 2A) and WT (not shown) mice healed at near identical rates (Figure 2C; p = 0.964) and were generally closed by 24–48 hr. The percentage wound closure at 8, 16, and 24 hr in Confetti mice was 79.0% ± 6.9%, 33.9% ± 9.9%, and 25.2% ± 4.8%, respectively, which was comparable with 84.6% ± 15.9%, 42.3% ± 9.8%, and 36.6% ± 18.1% in WT mice (Figure 2C).
Figure 2.
Accelerated Re-epithelialization by K14+ Limbal Progenitor-Derived Clones
(A) In vivo staining with sodium fluorescein (green) post injury in Confetti corneas (n = 6/group). Scale bars, 400 μm.
(B) Long-term intra-vital monitoring Confetti clones after injury (n = 6/group). The intraocular lens autofluoresces (blue-green hue). Scale bars, 400 μm.
(C) Percentage wound resolution (i.e., re-epithelialization). by measuring the size of the defect at t = 0 hr compared with other time points. Line graphs represent mean ± SD (n = 3/group/time point). No statistically significant difference was noted between Confetti and WT corneas at any time point (unpaired two-tailed Welch's t test and Sidak's multiple comparisons test).
(D) Clonal displacement in wounded versus unwounded Confetti corneas (mean ± SD, n = 3/group/time point; ∗∗∗∗p < 0.0001, Sidak's multiple comparisons test). The red hatched line indicates wound closure.
(E) Confocal images of flat-mounted Confetti corneas at 2 and 8 weeks post 2-mm central injury. Scale bars, 500 μm. C, cornea; L, limbus; Cj, conjunctiva.
(F and G) Streak number (F) and width (G) at 2 and 8 weeks post injury (mean ± SD, n = 3/group/time point). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001, one-way ANOVA with Tukey's multiple comparisons test.
Following epithelial debridement, fluorescent patches derived from K14+ transgenic cells emerged from the limbus in wounded eyes (Figure 2B), and within 1 week developed into multi-colored clonal streaks that migrated at 19.8 ± 3.7 μm/hr (Figure 2D) and persisted beyond 8 weeks post injury (Figure 2B). Fluorescent clones were displaced 180.5 ± 42.0 μm, 374.1 ± 135.4 μm, 574.1 ± 86.3 μm, 627.0 ± 63.4 μm, and 797.6 ± 40.6 μm after 0, 8, 16, 24, and 48 hr, respectively (Figure 2D). In contrast, they were relatively stationary over the same time course in the contralateral control eye, traveling at a rate of 0.53 ± 0.52 μm/hr (p = 0.015), meaning cells in the injured eye moved 37.7-fold faster than under steady state (Figure 2D). Confocal microscopy on flat-mounted corneas provided a higher-resolution perspective of clonal dynamics in wounded and uninjured Confetti corneas (Figure 2E). There was no statistically significant difference in the number of multi-colored clonal streaks at 2 weeks post wounding compared with steady state (67.6 ± 6.2 versus 76.8 ± 4.6; p = 0.14). However, after 8 weeks there were significantly less in the injured compared with unwounded corneas (36.5 ± 6.2 versus 53.8 ± 4.5; p = 0.0003) (Figures 2E and 2F). Furthermore, streak number was reduced at 8 weeks compared with 2 weeks post wounding (p < 0.0001) (Figure 2F) and broadened from 149.9 ± 43.5 μm to 210.0 ± 75.4 μm (p = 0.044) after 8 weeks (Figure 2G). Notably, clonal dynamics at day 0 was excluded from the analysis due to our inability to accurately discriminate fluorescent streaks from undeveloped multi-colored patches. There were few TUNEL+ cells detected during wound healing (not shown), and there was no difference compared with steady state (Richardson et al., 2017), suggesting that streak loss was not due to elevated apoptosis. Previous studies showed that loss of limbal clones, concomitant with their widening and/or merging, is suggestive of either increased symmetric division or an accelerated rate of symmetric/asymmetric division after trauma (Klein and Simons, 2011, Richardson et al., 2017).
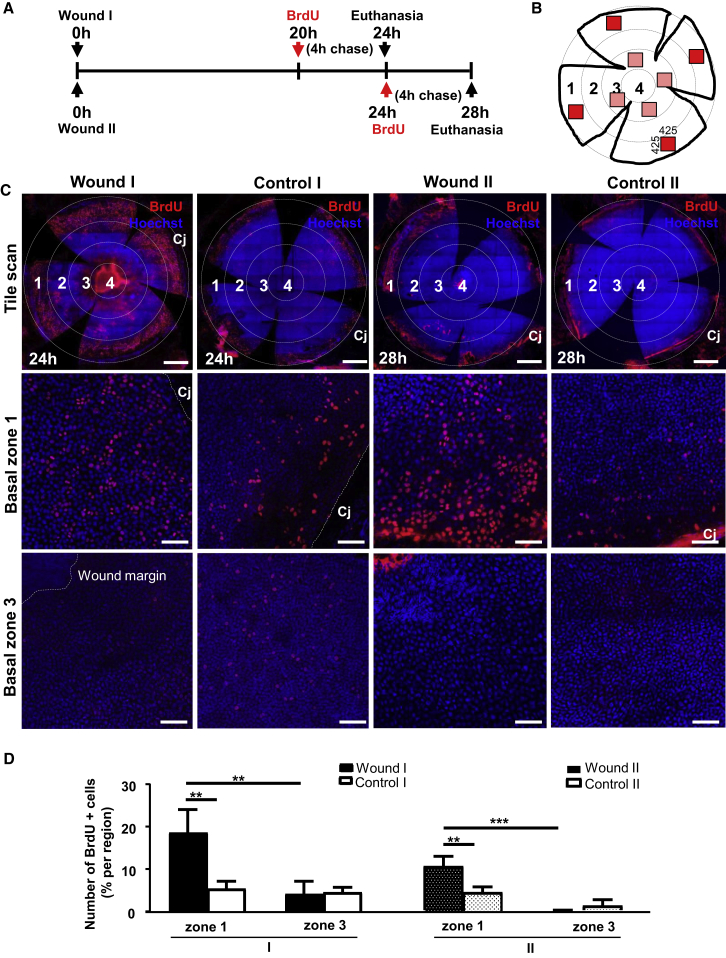
Proliferation in the Periphery Drives Centripetal Migration to Expedite Wound Closure
To determine how LESCs partake in corneal wound healing, we assessed basal cell proliferation before (wound I) and just after (wound II) wound closure (Figure 3A) in four randomly selected regions within numerically specified concentric zones (Figure 3B). Irrespective of the time post wounding, BrdU+ basal epithelia increased within the peripheral (zone 1) compared with the para-central (zone 3) region (Figure 3C, first and third columns). This contrasted with the uninjured contralateral eye in which there was no significant difference (Figure 3C, second and fourth columns). Overall, wounding significantly increased basal limbal epithelial cell proliferation by 3.6-fold at 24 hr (19.2% ± 4.4% versus 5.4% ± 1.8%, p = 0.0016) and 2.4-fold at 28 hr (10.8% ± 2.4% versus 4.5% ± 1.41%, p = 0.0099) post wounding compared with steady state (Figure 3D). Moreover, proliferation between 24 and 28 hr was reduced by 1.8-fold post wounding (p = 0.0439) (Figure 3D), indicating that peripheral replication attenuated once the defect sealed.
Figure 3.
Proliferation of Basal Limbal Epithelia after Inflicting a Central Wound
(A) Schematic of BrdU administration in relation to corneal wounding.
(B) Schematic of a flat-mounted cornea displaying randomly distributed regions (red boxes) that represent areas imaged and analyzed in selected concentric zones that were numbered 1–4.
(C) Confocal images of wounded and control corneas stained for BrdU (red) and counterstained with Hoechst (blue). Scale bars, 500 μm (upper panels) and 100 μm (middle and lower panels). Cj, conjunctiva.
(D) Percentage BrdU+ basal epithelial cells between zone 1 (limbal) and zone 3 (para-central), comparing wounded with control corneas (mean ± SD, n = 3/group/time point; ∗∗p < 0.01 and ∗∗∗p < 0.001, two-way ANOVA with a Turkey's multiple comparisons test).
Corneal Epithelial Wound Closure by Basal Cell Migration
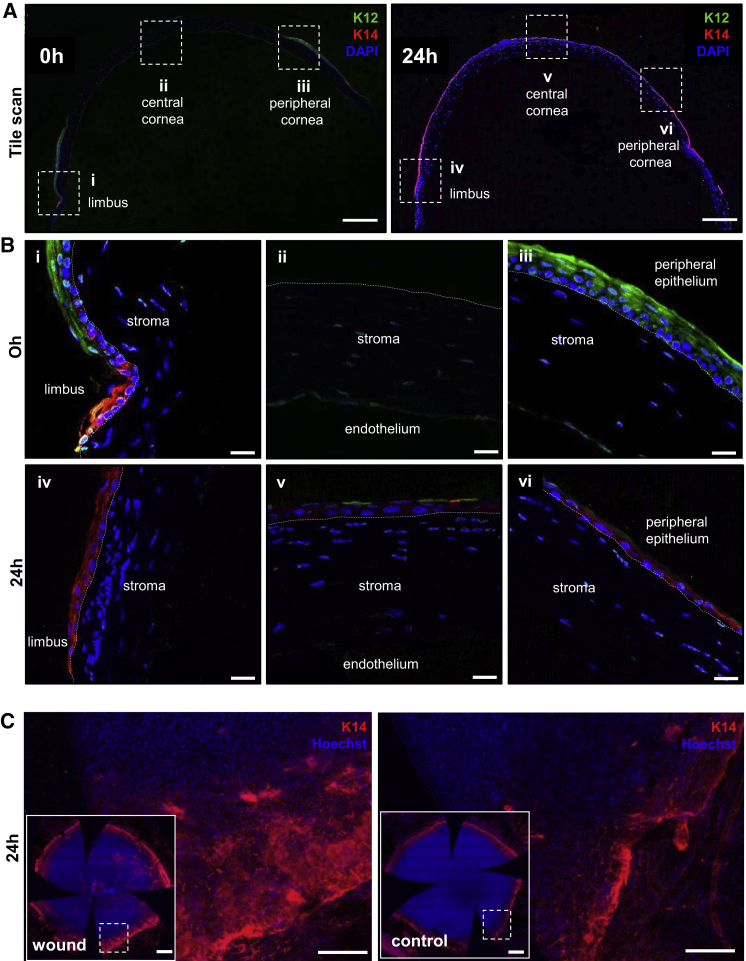
To determine which cells contribute to corneal epithelial wound closure, we examined their phenotype at 0 hr and 24 hr post wounding (Figure 4). At 0 hr post injury, corneal-specific K12 expression was displayed throughout the intact peripheral corneal epithelium, and K14 was restricted to the limbus (Figure 4A, first row, left panel and second row; i and iii). However, K14 was highly expressed within the layer of epithelial cells that covered the defect at 24 hr post injury (Figure 4A, first row, right panel and third row; iv, v, and vi), while K12 was occasionally expressed in superficial cells within the central and peripheral cornea (Figure 4A, first row, right panel and third row; v and vi). This suggests that the regenerated epithelium was derived from K14+ basal limbal cells, an observation confirmed in flat-mounted whole corneas, which showed a broader region of K14-stained basal cells at 24 hr post wounding compared with controls (Figure 4B).
Figure 4.
K14 Expression in the Regenerated Corneal Epithelium after Wound Closure
(A) Immunofluorescence was conducted on paraffin-embedded tissue sections (n = 3/group/time point) procured at 0 hr and 24 hr post wounding. Representative corneas were double immunostained for K12 (green) and K14 (red), and counterstained with DAPI (blue). Images of transverse sections provide a panoramic view of the staining across the entire cornea. Scale bars, 200 μm.
(B) Images are magnified views of the region encompassed by the hatched boxes in (A), which focus on the limbal transition zone (i and iv), as well as the central (ii and v) and peripheral (iii and vi) cornea. Hatched white lines demarcate the epithelial basement membrane. Scale bars, 20 μm.
(C) Representative images of whole-mounted corneas displaying K14 expression in limbal/peripheral zone. Insets in each panel provide a global appreciation of the entire cornea (n = 3/group). Specimens were counterstained with Hoechst (blue) to highlight cell nuclei. Scale bars, 100 μm and 500 μm (insets).
Measuring Clonal Displacement with Spatial-Temporal Vector Flow Maps
To accurately map the spatial-temporal dynamics of K14+ cells within clones during wound closure, we developed a method to maintain and image corneas in short-term organ culture, after which quantitative STICS analysis was applied. Wounded corneas were imaged by light-sheet microscopy, and cells within K14+-Confetti clones migrated in a manner comparable with those in live animals (Video S1). Furthermore, to visualize basal cell migration at a single cell level, we imaged wounded corneas in an ex vivo organ culture by confocal microscopy under higher magnification (Video S2). Epithelial cells located in and around the wound edge became elongated and moved into the defective area to form a monolayer; these observations certainly align with our in vivo results (Figures 1 and 2; Table S1), and suggests that moving cells were likely basal epithelia as indicated by our phenotypic analyses (Figure 4).
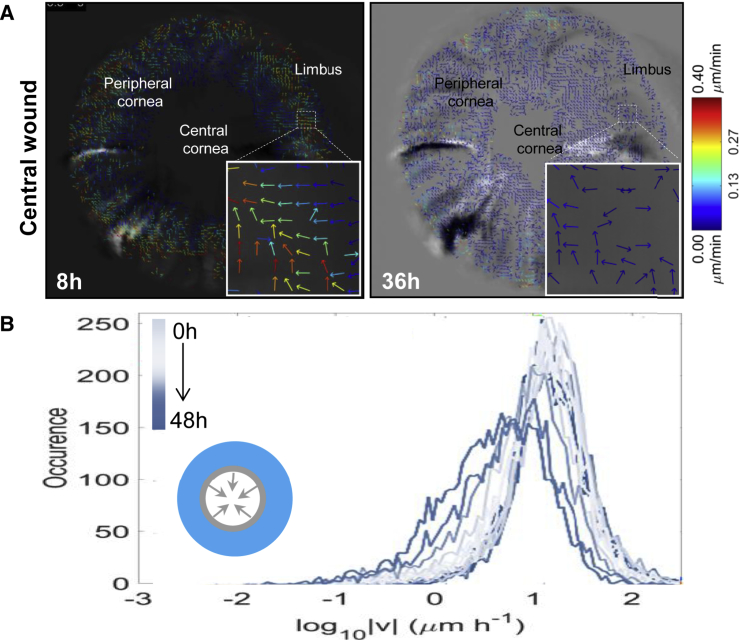
At 8 and 36 hr post injury, the direction and velocity of clonal migration was calculated and displayed as vector flow maps (Figure 5A). This analysis indicated that although groups of cells within clones move in a multi-directional manner (Figure 5A, insets), the overall motion was centripetal (Video S3). Upon closer inspection, clones traveled faster from the limbal margin after 8 hr compared with 36 hr post wounding (Figure 5A). As wounds resolved, clones gradually decelerated from 49.0 ± 23.4 μm/hr at approximately 14 hr to 15.2 ± 4.9 μm/hr from the periphery at 36 hr (Figure 5B) while little or no migration was observed in control eyes. The data are displayed as a spectrum of histograms ranging from light blue (earliest time point of 0 hr) to dark blue (latest time point of 48 hr); the height of each represents the speed of movement.
Figure 5.
Velocity of Migrating Clones during Ex Vivo Wound Healing Measured by Quantitative STICS Analysis
(A) Eyes from Confetti mice (n = 3/group) were enucleated immediately post wounding, placed in organ culture, and imaged by light-sheet microscopy every 2 hr for 48 hr. Vector flow maps represent cell migration and direction of clone movement from images taken at 8 hr and 36 hr. Insets show the multi-directional movement of groups of cells within a clone, depicted as colored arrows; associated with these images is a heatmap that acts as a speed gauge.
(B) STICS was applied on a time series of 2D maximum-intensity projections of 3D image stacks. The speed of peripherally located clones every 2 hr for a representative cornea is displayed by colored histogram (from light to dark blue). The inset is a schematic representation of the wound, with arrows indicating the direction of closure.
See also Video S3.
Dynamics of Corneal Epithelial Wound Healing through Computational Modeling
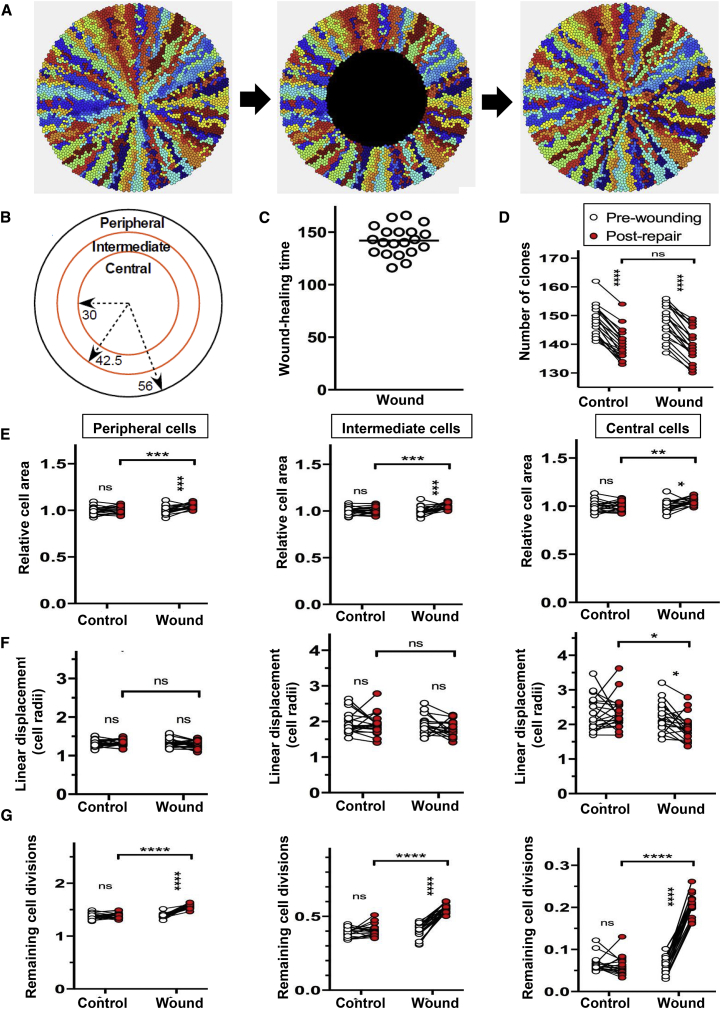
Using animal and computational modeling, we previously showed that population density-driven pressure is sufficient to promote centripetal clonal migration during homeostasis (Lobo et al., 2016, Richardson et al., 2017). To determine whether a similar mechanism plays a role in wound healing, we adapted our mathematical model to incorporate an epithelial injury (Figure 6). Wounds were simulated by replacing cells in the intermediate and central zones, respectively, with “blank” cells (Figures 6A and 6B), which offer minimal resistance to the movement of adjacent epithelia (see Experimental Procedures). Simulated corneas completely healed the wound within 200 time steps (Figure 6C and Video S4), and there was no difference in the number of clones between wounded and control, or pre-wounding and post repair (Figure 6D). At the end of the healing process, cell size significantly increased in the peripheral, intermediate, and central zones (Figure 6E and Table S2); results that agreed with our in vivo observations (Figure 1 and Table S1).
Figure 6.
Computational Modeling to Investigate the Effect of Wounding on the Composition and Structure of the Repaired Corneal Epithelium
(A) Examples of simulated corneas before and during wounding, and immediately after complete wound repair (left to right).
(B) Wounds equivalent in area were made by removing epithelial cells from the central zone within 30 cell radii of the center of the cornea. Cell properties were measured in these regions and in the peripheral zone.
(C) The wound was completely repaired in less than 200 time steps.
(D) The number of clones was counted before wounding and after repair.
(E) Cell size was measured for all cells in each zone before and after wounding, and immediately after healing was complete or, in the case of unwounded corneas, at t = 2,300 (“pre-wounding”) and t = 2,500 (“post wounding”). Cell size was adjusted for differences in pre-wounding size, which varied within the three zones. The mean cell area was plotted for each cornea in the peripheral (left), intermediate (middle), and central (right) zone.
(F) The mean linear displacement, a measure of clonal dispersion, was determined for all cells within the peripheral (left), intermediate (middle), and central (right) zone in corneas subjected to a wound, as indicated below the x axis.
(G) The number of remaining cell divisions of the TACs in corneas was determined for all cells in the peripheral (left), intermediate (middle), and central (right) zone in corneas subjected to a wound, as indicated below the x axis.
Data represent mean ± SD, n = 20/group; ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, ns = p > 0.05 by repeated-measures (2,300-time unit) one-way ANOVA with Sidak’s multiple comparisons correction. See also Video S4.
The computational model was also used to examine the effects of wounding on the organization of the corneal epithelium. We quantified the mean linear displacement (Lobo et al., 2016) a measure of clonal dispersion, and found that clones were slightly more cohesive in the peripheral and intermediate zones, consistent with the steeper population density pressure gradient drawing cells faster through these zones toward the central cornea (Figure 6F). In addition, cell division was high in peripheral cells and gradually decreased in intermediate and central cells (Figure 6G). Thus, while not ruling out a role for signaling molecules released by inflammatory other cells to enhance the epithelial wound-healing response, population pressure seems sufficient to mediate many changes in epithelial behavior that accompany the process of wound healing.
Discussion
Herein we describe the contribution of K14+-Confetti precursor cells and their progeny to corneal epithelial repair using real-time visual monitoring in live mice. After wounding, centripetal migration of limbal-derived clones was accelerated, and this coincided with elevated mitosis at the periphery. Our intra-vital analysis was supported by data collated from ex vivo organ-cultured corneas, an advantageous system because clonal dynamics could be visualized and rendered with high-resolution microscopy and robust image analysis tools. Using this model system, we also provide direct evidence that basal limbal epithelia are the predominant cells involved in the initial phase of injury repair. These cells seem to stream along the basement membrane in a centripetal manner, most likely propelled by population pressure that arises from heightened proliferation in and around the LESC niche upon injury, an observation supported by computational modeling.
It is generally accepted that LESCs undergo asymmetric division as a means of maintaining corneal tissue mass. In this scenario, two daughters are produced, including one that remains within the SC pool and the other (a TAC) that leaves the niche, ascending through the epithelial layers before being sloughed from the ocular surface (Di Girolamo, 2015, Richardson et al., 2016, Richardson et al., 2017). However, it is likely that a proportion of divisions are symmetric, whereby both daughters either remain or are evicted from the repository (Lamprecht, 1987, Lamprecht, 1990, Beebe and Masters, 1996, Castro-Muñozledo and Gómez-Flores, 2011). We recently affirmed this notion through mathematical modeling (Lobo et al., 2016) and via aging experiments in which K14+-Confetti clones either disappeared from the trace and/or became broader with time (Richardson et al., 2017). Herein we observed an accentuated clonal pattern (Figure 2E), concordant with spokes widening, especially so at 8 weeks post injury (Figures 2E–2G), a result that aligns with the concept of neutral evolutionary drift (Doupé et al., 2012). A limitation of our study was that gender was not factored into the experimental paradigm and may have influenced some of the readouts. Certainly, there have been several reports of sex differences in relation to corneal wound healing that could be hormonally regulated (Krishnan et al., 2012, Wang et al., 2012). Notably, most aspects of our in silico model (Figure 6) recapitulated observations made in vivo, with the exception that there was no statistically significant difference in clone number between wounded and control corneas. This suggests that the model may not be capturing clone loss or that the time and space restrictions placed upon the model preclude the manifestation of this phenomenon.
During the initial 24 hr post wounding, epithelial movement (without proliferation) proceeded adjacent to the wound edge (Figures 2 and 3; Videos S1, S2, S3, and S4) while in the periphery, cell replication predominated (Figure 3C). These data support our proposition that increased limbal population pressure is the principal driving force that propels K14+ basal cells toward the wound bed to seal the defect (Figure 4 and Video S2). The speed at which basal cells move into the injury site suggests that adhesive connection between these cells and the immature basement membrane are weak and/or diminished; alternatively, secretion of matrix metalloproteinases by these cells would render them more “fluid” (Aragona et al., 2017). In future studies we would like to define the molecular signature of stationary limbal epithelia compared to migratory cells at the wound margin. Certainly, K14 was found to be one of nine upregulated genes in moving rat epithelia after cornea wounding (Yu et al., 1995). In agreement with this study and our K14+ migratory cells, Chung et al. (1995) identified the steady-state expression of α-enolase in basal limbal epithelia. After creating a debridement injury in rabbit corneas (like the one our mice endured), the first cells to migrate into the wound were flat, elongated, α-enolase+ epithelia thought to be displaced from the intact limbal zone. Furthermore, by 4 weeks post wounding α-enolase expression regressed from the central epithelia, and this was concomitant with basal cells becoming cuboidal to coincide with maturation and restratification. Once the defect is covered by a monolayer of epithelia (Figures 1 and 4), suprabasal cells located around the wound edge can crawl over newly migrated basal cells. Alternatively, basal cells themselves could differentiate into K12+ wing cells (Figures 4 and 7A–7D) to restore epithelial hierarchy. Notably, our results are incompatible with long-standing models of epithelial wound healing in either the skin or cornea, which include the “cell-sliding” model, in which epithelial cells move into the wound as a sheet (Figure 7E), and the “cell-rolling” model (also known as the “leap-frog” model), in which suprabasal cells roll over leading-edge cells to become new basal cells (Figure 7F) (Crosson et al., 1986, Danjo and Gipson, 2002, Kuwabara et al., 1976). Our observations are more in line with recent studies conducted in skin, which demonstrated K14+ basal keratinocytes moving into central cutaneous wounds, behind which are concentric regions of proliferation (Park et al., 2017, Safferling et al., 2013). Furthermore, if superficial K14−/K12+ corneal epithelia are the first cells arriving in the wound bed, they would likely require ample replicative activity to contribute to vertical epithelial restoration; this could occur if they had the ability to dedifferentiate (Nasser et al., 2018).
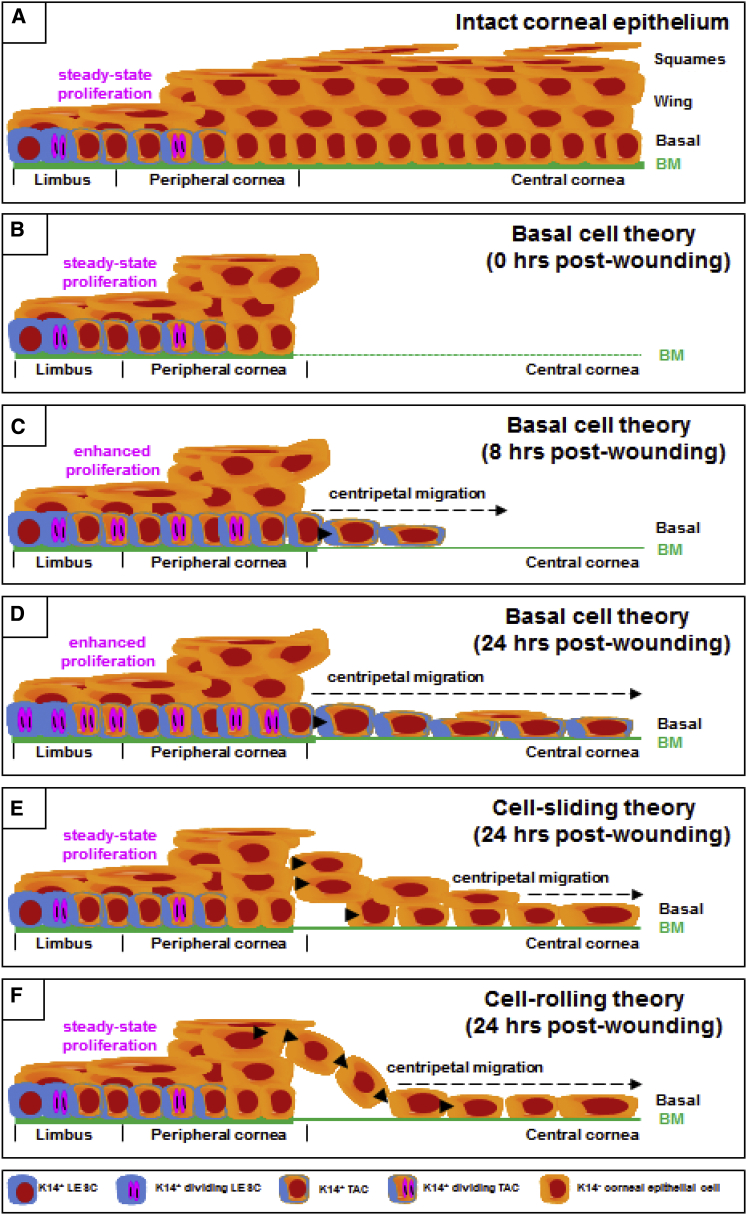
Figure 7.
Basal Cell Migration: A Hypothesis for the Early Phase of Re-epithelialization
(A) Under homeostasis the multi-layered squamous epithelium of the mammalian cornea is maintained by slow-cycling, self-sustaining, K14+ LESCs located in the limbus.
(B) A mechanical debridement injury removes the epithelium and basement membrane (BM) within a confined region (t = 0 hr).
(C and D) The basal cell migration hypothesis. Peripherally located K14+ LESCs are activated to proliferate, forcing generations of newly propagated K14+ basal TACs into the wound bed, during which they synthesize an immature BM (solid green line) over the first 8 hr (C) and 24 hr (D). See also Figures 1, 2, 3, and 4; Video S2.
(E) Our basal cell migration hypothesis challenges the “sliding-cell” theory in which epithelia surrounding the wound progressively move into the debrided zone as a block or sheet of cells.
(F) Our basal cell migration hypothesis also challenges the “cell-rolling” proposition whereby suprabasal epithelia role over leading-edge basal cells to form new leader cells.
Finally, we employed STICS to quantify the dynamics of corneal epithelial cells during wound healing in an ex vivo setting. The advantage of STICS over manually measuring cell migration is that it is readily automated, and vector speeds can be extracted from an image for every spatial and temporal region of data acquired. The inherent computational simplicity of this approach makes it a versatile tool for measuring the dynamics of molecular complexes, for example organelles (Ashdown et al., 2017, Meddens et al., 2016, Toplak et al., 2012) and cells in the context of migration (Tanner et al., 2009). Since this method does not require segmentation and detection of single cells but relies on measuring pixel intensity fluctuations, it is more adaptable to scenarios whereby migrating objects change size and shape over time. It is therefore a preferable choice in complex processes such as during wound healing (Figure 5; Videos S1 and S3). However, STICS is currently limited to 2D mapping, and applying this system on in vivo data imposes technical and ethical barriers that must be overcome, such as the frequency of sampling and the inherent challenges of intra-vital imaging (i.e., limited ketamine dosing and animal movement under anesthesia). In the future, we will extend these investigations to include a full 3D plus time vector mapping of cell migration, allowing us to visualize cell movement in all epithelial layers during each phase of wound healing.
In this study, we visualized the deployment of K14+-derived limbal epithelial cells into the wound bed and propose the “basal cell migration” theory, which was substantiated in an organ-culture system and with computational modeling. Our results indicate that there is cellular activity beyond what occurs in the immediate vicinity of the wound, and to this end we provide evidence that heightened proliferative population pressure from the limbal perimeter is the main driver of centripetal epithelial cell movement upon injury.
Experimental Procedures
Mice
K14CreERT2-Confetti (n = 28 eyes) and age-matched WT C57BL/6 (n = 63 eyes) male and female mice were housed under pathogen-free conditions and fed standard chow (Gordon's Specialty Feeds, Yanderra, Australia). All studies were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. All procedures were approved by the UNSW Animal Care and Ethics Committee (Approval No. 17/81A).
Six-week-old male and female transgenic mice (heterozygous for K14CreERT2 and homozygous for Confetti) were injected intraperitoneally with 50 μg/g body weight of tamoxifen (Sigma-Aldrich, St Louis, MO) dissolved in 10% ethanol and 90% olive oil (Sigma-Aldrich) over 3 consecutive days. Mice were used at 1 or 24 weeks post tamoxifen injection, rendering them 7–30 weeks of age for experimentation. This facilitated the development of a multi-colored fluorescent streaking pattern arising from marginally located K14+ LESCs (Di Girolamo et al., 2015, Lobo et al., 2016, Richardson et al., 2017).
Inducing, Monitoring, and Assessing Corneal Epithelial Debridement Wounds
Mice were anesthetized by an intraperitoneal injection of 100 μg/g ketamine (Provet, Eastern Creek, NSW, Australia) and 10 μg/g xylazine (Sigma-Aldrich), after which they were placed under an OPMI pico (Carl Zeiss, Jena, Germany) surgical microscope. Epithelial debridement was performed with an Algerbrush II (Katena Products, Denville, NJ) as previously described (Chan and Werb, 2015). In brief, a central corneal debridement injury was created by marking a limbal-sparing central circle using a 2-mm trephine (KAI Medical, Solingen, Germany), then removing the epithelium using an Algerbrush II attached to a 1-mm burr. After wounding, the right eye was rinsed with saline to remove cell debris; the left eye was uninjured and acted as the internal control. Saline was instilled in both eyes to maintain hydration while mice recovered. Eyes were monitored by fluorescence microscopy (3i VIVO; Intelligent Imaging Innovations, Denver, CO). Flat-mounted corneas were imaged by confocal fluorescence microscopy (Zeiss LSM 780; Carl Zeiss). Histological and phenotypical assessments were performed with PAS and immunofluorescence staining (see details in Supplemental Information: Histological Assessment, Immunofluorescence, Measuring Clonal Migration and Wound Closure by Intra-vital Microscopy and Confocal Microscopy).
Monitoring Corneal Wound Resolution in Organ Culture
Confetti mice were wounded as described above. The animals were euthanized and their eyes immediately enucleated. Paired (wounded and intact) globes were embedded in sterile 1.5% agarose and placed within the microscope's imaging/incubation chamber, set to 37°C and 5% CO2 prior to immersion in defined keratinocyte serum-free medium (Gibco, Fremont, CA) containing 50 nM recombinant human epidermal growth factor (Peprotech, Rocky Hill, NJ), 500 nM cholera toxin (List Biological Labs, Campbell, CA) and 100 U/mL penicillin-streptomycin (Gibco). Eyes were imaged every 2 hr over 48 hr by light-sheet microscopy (Zeiss Lightsheet Z.1) using a 5×/0.16 detection lens and 5×/0.1 illumination lens as described above.
Cell Proliferation by BrdU Incorporation
To determine the level of proliferation, we wounded WT mice (as described above) and injected them intraperitoneally with BrdU (100 μg/g body weight) (Sigma-Aldrich) at 20 or 24 hr post wounding. After 4 hr, mice were euthanized, and their eyes enucleated and fixed in 4% paraformaldehyde overnight at 4°C. Corneas were dissected and stained for BrdU as previously described (Pajoohesh-Ganji et al., 2006, Richardson et al., 2017). In brief, corneas were treated with 2 N HCl for 20 min at room temperature, washed four times (5 min each) in PBS at room temperature, blocked in 20% goat serum diluted in PBS containing 2% BSA and 0.1% Triton X-100 (PBS-BT) overnight at 4°C, then reacted with a rat anti-BrdU antibody (5 μg/mL; ab6326, Abcam) in PBS-BT for 48 hr at 4°C. Corneas were washed thrice (20 min each) in PBS-BT at room temperature, incubated with a goat anti-rat Alexa-Fluor633-conjugated secondary antibody (5 μg/mL; Life Technologies) at 4°C, counterstained with Hoechst 33342 (1 μg/mL; Life Technologies), and flat-mounted as described above in ProLong Gold anti-fade reagent. To analyze the number of BrdU+ basal cells, we randomly selected four regions (425 × 425 μm), each within the limbal margin and central cornea, from confocal scans, then counted BrdU+ cells over unlabeled cells and generated a labeling index. A grid with four equally distant concentric rings was drawn over each cornea, and zones numbered accordingly: 1 (limbus), 2 (peripheral cornea), 3 (para-central), and 4 (central). BrdU+ basal epithelia within zones 1 and 3 were counted in each of four randomly selected regions that measured 425 × 425 μm in area.
Spatiotemporal Image Correlation Spectroscopy of Clonal Migration in Ex Vivo Wounded Corneas
As an alternative and more accurate means of measuring cell movement, spatiotemporal image correlation spectroscopy (STICS) (Ashdown et al., 2017, Meddens et al., 2016, Toplak et al., 2012) was applied on a series of 2D maximum-intensity projections of 3D image stacks that were acquired via light-sheet microscopy at different time points post injury in ex vivo organ-cultured murine corneas. The raw data consisted of a temporal sequence (i.e., time point per 2 hr) of z stacks from corneas imaged through a 5×/0.16 detection lens and 5×/0.1 illumination lens as described above (pixel size 2.28 μm) (see details in Supplemental Information: Spatiotemporal Image Correlation Spectroscopy of Clonal Migration in Ex Vivo Wounded Corneas).
Computational Model of Corneal Epithelial Wound Healing
A mathematical model was used to investigate the role that population pressure-driven motility plays in corneal wounding and repair and to compare spatial distributions of clonally related cells in corneas, following cases of no wounding and wounding of the central cornea. It was based on a previous model (Grimm et al., 2010, Lobo et al., 2016), and modified to incorporate regions in which epithelial cells were removed by wounding. This was achieved by substituting a new type of agent, “blank cells,” for the epithelial cells of the wound area. Blank cells exerted less pressure on neighboring cells than epithelial cells. Blank cells were further classified as internal blank cells, which were only adjacent to other blank cells, and edge blank cells, which were adjacent to at least one epithelial cell.
The model space was a circular region representing the corneal epithelium and adjacent limbus, which collectively is in one of three states: a “healthy state,” an “early wounded state,” and a “late wounded state.” The healthy state represents a cornea whose dynamics did not incorporate any wound or any active response to previous wounding. The early wounded state represents a cornea that was undergoing repair, where the key driver of wound repair was cell movement into the vacant wounded area before the wound approaches closure. The late wounded state represents a cornea that had almost transitioned to the healthy state, where the key component of wound repair was cells coming together spatially, which results in final closure of the wound. LESCs had a 2.5-fold increase in proliferation as a response to wounding, which was sufficient to maintain the epithelium outside of the wound and supply cells for the wounded area. In the early wounded state, epithelial cells more effectively pushed into blank cells than into other cells in the cornea, due to the reduced pressure exerted by blank cells. This produced the preference for cells to move into unoccupied wounded areas of the cornea over competing for space with other cells. Blank cells remained in the cornea and could occupy less of the basal layer, but they were not removed in the early wounded state. The cornea moved from an early wounded state to a late wounded state when there were more edge blank cells than internal blank cells. In the late wounded state, blank cells were removed automatically when the distance between two internal blank cells was less than 0.15 idealized cell radii. At this time they were replaced by a single blank cell whose position takes the value of the average position of the two internal cells being replaced. During the late wounded state, blank cells were also removed stochastically with p = 0.2 per blank cell per time step and were not replaced (see details in Supplemental Information: Overview, Design Concepts, and Details of the Mathematical Model).
Statistical Analysis
Data are presented as mean ± SD (n denotes sample size). Unpaired two-tailed Welch's t test with unequal variance and ANOVA with Sidak's or Tukey's multiple comparisons was used to compare wound closure between WT and Confetti mice, and the velocity of K14+ clonal migration, clone number, and streak width between wounded and contralateral control eyes. A p value of <0.05 was considered statistically significant.
Author Contributions
Conceptualization, M.P. and N.D.; Methodology, M.P., A.R., E.P., E.P.L., and S.L.W.; Investigation, M.P., A.R., E.P., and E.P.L.; Writing – Original Draft, M.P. and N.D.; Writing – Review and Editing, M.P., A.R., E.P., E.P.L., R.W., S.L.W., J.G.L., D.W., and N.D.; Funding Acquisition, N.D., D.W., and S.L.W.; Resources, R.W., D.W., and N.D.; Supervision, N.D., D.W., J.G.L., R.W., and S.L.W.
Acknowledgments
This work was supported by the Australian National Health and Medical Research Council (APP1101078) to N.D., S.L.W., and D.W. The authors thank Richard Francis, Sandra Fok, Iveta Slapetova, and Fei Shang (Biomedical Imaging Facility, University of NSW, Sydney, Australia) for their assistance with intra-vital, light-sheet, and confocal imaging and analysis, and assistance with histological sectioning.
Published: December 13, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, one figure, two tables, and four videos and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.11.014.
Supplemental Information
References
- Aragona M., Dekoninck S., Rulands S., Lenglez S., Mascré G., Simons B.D., Blanpain C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017;8:14684. doi: 10.1038/ncomms14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashdown G.W., Burn G.L., Williamson D.J., Pandžić E., Peters R., Holden M., Ewers H., Shao L., Wiseman P.W., Owen D.M. Live-cell super-resolution reveals F-actin and plasma membrane dynamics at the T cell synapse. Biophys. J. 2017;112:1703–1713. doi: 10.1016/j.bpj.2017.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D.C., Masters B.R. Cell lineage and the differentiation of corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 1996;37:1815–1825. [PubMed] [Google Scholar]
- Buck R.C. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest. Ophthalmol. Vis. Sci. 1985;26:1296–1299. [PubMed] [Google Scholar]
- Castro-Muñozledo F., Gómez-Flores E. Challenges to the study of asymmetric cell division in corneal and limbal epithelia. Exp. Eye Res. 2011;92:4–9. doi: 10.1016/j.exer.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Chan M.F., Werb Z. Animal models of corneal injury. Bio Protoc. 2015;5:e1516. doi: 10.21769/bioprotoc.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E.-H., DeGregorio P.G., Wasson M., Zieske J.D. Epithelial regeneration after limbus-to-limbus debridement. Expression of alpha-enolase in stem and transient amplifying cells. Invest. Ophthalmol. Vis. Sci. 1995;36:1336–1343. [PubMed] [Google Scholar]
- Chung E.H., Hutcheon A.E., Joyce N.C., Zieske J.D. Synchronization of the G1/S transition in response to corneal debridement. Invest. Ophthalmol. Vis. Sci. 1999;40:1952–1958. [PubMed] [Google Scholar]
- Cotsarelis G., Cheng S.-Z., Dong G., Sun T.-T., Lavker R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Crosson C., Klyce S., Beuerman R. Epithelial wound closure in the rabbit cornea. A biphasic process. Invest. Ophthalmol. Vis. Sci. 1986;27:464–473. [PubMed] [Google Scholar]
- Danjo Y., Gipson I.K. Specific transduction of the leading edge cells of migrating epithelia demonstrates that they are replaced during healing. Exp. Eye Res. 2002;74:199–204. doi: 10.1006/exer.2001.1115. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N. Moving epithelia: tracking the fate of mammalian limbal epithelial stem cells. Prog. Retin. Eye Res. 2015;48:203–225. doi: 10.1016/j.preteyeres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N., Bobba S., Raviraj V., Delic N., Slapetova I., Nicovich P., Halliday G., Wakefield D., Whan R., Lyons J. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells. 2015;33:157–169. doi: 10.1002/stem.1769. [DOI] [PubMed] [Google Scholar]
- Doupé D.P., Alcolea M.P., Roshan A., Zhang G., Klein A.M., Simons B.D., Jones P.H. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337:1091–1093. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa L., Foster C., Harrist T., Lanigan J., Colvin R. Fibronectin in healing rabbit corneal wounds. Lab. Invest. 1981;45:120–129. [PubMed] [Google Scholar]
- Gipson I.K., Kiorpes T.C. Epithelial sheet movement: protein and glycoprotein synthesis. Dev. Biol. 1982;92:259–262. doi: 10.1016/0012-1606(82)90170-1. [DOI] [PubMed] [Google Scholar]
- Grimm V., Berger U., DeAngelis D.L., Polhill J.G., Giske J., Railsback S.F. The ODD protocol: a review and first update. Ecol. Model. 2010;221:2760–2768. [Google Scholar]
- Huang A., Tseng S. Corneal epithelial wound healing in the absence of limbal epithelium. Invest. Ophthalmol. Vis. Sci. 1991;32:96–105. [PubMed] [Google Scholar]
- Klein A.M., Simons B.D. Universal patterns of stem cell fate in cycling adult tissues. Development. 2011;138:3103–3111. doi: 10.1242/dev.060103. [DOI] [PubMed] [Google Scholar]
- Krishnan T., Prajna N.V., Gronert K., Oldenburg C.E., Ray K.J., Keenan J.D., Lietman T.M., Acharya N.R. Gender differences in re-epithelialisation time in fungal corneal ulcers. Br. J. Ophthalmol. 2012;96:137–138. doi: 10.1136/bjophthalmol-2011-300441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T., Perkins D.G., Cogan D.G. Sliding of the epithelium in experimental corneal wounds. Invest. Ophthalmol. Vis. Sci. 1976;15:4–14. [PubMed] [Google Scholar]
- Lamprecht J. Mitosis in the corneal epithelium. A preliminary communication on the coexistence of differential and equivalent cell divisions. Cell Biol. Int. Rep. 1987;11:449–455. doi: 10.1016/0309-1651(87)90078-6. [DOI] [PubMed] [Google Scholar]
- Lamprecht J. Symmetric and asymmetric cell division in rat corneal epithelium. Cell Prolif. 1990;23:203–216. doi: 10.1111/j.1365-2184.1990.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Lehrer M.S., Sun T.-T., Lavker R.M. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci. 1998;111:2867–2875. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- Li Z., Burns A.R., Smith C.W. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Invest. Ophthalmol. Vis. Sci. 2006;47:1947–1955. doi: 10.1167/iovs.05-1193. [DOI] [PubMed] [Google Scholar]
- Lin Z., He H., Zhou T., Liu X., Wang Y., He H., Wu H., Liu Z. A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chlorideA model of limbal stem cell deficiency. Invest. Ophthalmol. Vis. Sci. 2013;54:6314–6325. doi: 10.1167/iovs.12-10725. [DOI] [PubMed] [Google Scholar]
- Lobo E.P., Delic N.C., Richardson A., Raviraj V., Halliday G.M., Di Girolamo N., Myerscough M.R., Lyons J.G. Self-organized centripetal movement of corneal epithelium in the absence of external cues. Nat. Commun. 2016;7:12388. doi: 10.1038/ncomms12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddens M.B., Pandzic E., Slotman J.A., Guillet D., Joosten B., Mennens S., Paardekooper L.M., Houtsmuller A.B., Van Den Dries K., Wiseman P.W. Actomyosin-dependent dynamic spatial patterns of cytoskeletal components drive mesoscale podosome organization. Nat. Commun. 2016;7:13127. doi: 10.1038/ncomms13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort R.L., Ramaesh T., Kleinjan D.A., Morley S.D., West J.D. Mosaic analysis of stem cell function and wound healing in the mouse corneal epithelium. BMC Dev. Biol. 2009;9:4. doi: 10.1186/1471-213X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki T., Zhao J. Centripetal movement of corneal epithelial cells in the normal adult mouse. Invest. Ophthalmol. Vis. Sci. 2003;44:558–566. doi: 10.1167/iovs.02-0705. [DOI] [PubMed] [Google Scholar]
- Nasser W., Amitai-Lange A., Soteriou D., Hanna R., Tiosano B., Fuchs Y., Shalom-Feuerstein R. Corneal-committed cells restore the stem cell pool and tissue boundary following injury. Cell Rep. 2018;22:323–331. doi: 10.1016/j.celrep.2017.12.040. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A., Pal-Ghosh S., Simmens S.J., Stepp M.A. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells. 2006;24:1075–1086. doi: 10.1634/stemcells.2005-0382. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A., Pal-Ghosh S., Tadvalkar G., Stepp M.A. Corneal goblet cells and their niche: implications for corneal stem cell deficiency. Stem Cells. 2012;30:2032–2043. doi: 10.1002/stem.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Gonzalez D.G., Guirao B., Boucher J.D., Cockburn K., Marsh E.D., Mesa K.R., Brown S., Rompolas P., Haberman A.M. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat. Cell Biol. 2017;19:155–163. doi: 10.1038/ncb3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrescu M.S., Larry C.L., Bowden R.A., Williams G.W., Gagen D., Li Z., Smith C.W., Burns A.R. Neutrophil interactions with keratocytes during corneal epithelial wound healing: a role for CD18 integrins. Invest. Ophthalmol. Vis. Sci. 2007;48:5023–5029. doi: 10.1167/iovs.07-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaesh T., Ramaesh K., Leask R., Springbett A., Riley S.C., Dhillon B., West J.D. Increased apoptosis and abnormal wound-healing responses in the heterozygous Pax6+/− mouse cornea. Invest. Ophthalmol. Vis. Sci. 2006;47:1911–1917. doi: 10.1167/iovs.05-1028. [DOI] [PubMed] [Google Scholar]
- Richardson A., Lobo E.P., Delic N.C., Myerscough M.R., Guy Lyons J., Wakefield D., Di Girolamo N. Keratin-14-positive precursor cells spawn a population of migratory corneal epithelia that maintain tissue mass throughout life. Stem Cell Reports. 2017;9:1081–1096. doi: 10.1016/j.stemcr.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A., Wakefield D., Di Girolamo N. Fate mapping mammalian corneal epithelia. Ocul. Surf. 2016;14:82–99. doi: 10.1016/j.jtos.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Safferling K., Sütterlin T., Westphal K., Ernst C., Breuhahn K., James M., Jäger D., Halama N., Grabe N. Wound healing revised: a novel reepithelialization mechanism revealed by in vitro and in silico models. J. Cell Biol. 2013;203:691–709. doi: 10.1083/jcb.201212020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp M.A., Zieske J.D., Trinkaus-Randall V., Kyne B.M., Pal-Ghosh S., Tadvalkar G., Pajoohesh-Ganji A. Wounding the cornea to learn how it heals. Exp. Eye Res. 2014;121:178–193. doi: 10.1016/j.exer.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner K., Ferris D.R., Lanzano L., Mandefro B., Mantulin W.W., Gardiner D.M., Rugg E.L., Gratton E. Coherent movement of cell layers during wound healing by image correlation spectroscopy. Biophys. J. 2009;97:2098–2106. doi: 10.1016/j.bpj.2009.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoft R.A., Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest. Ophthalmol. Vis. Sci. 1983;24:1442–1443. [PubMed] [Google Scholar]
- Toplak T., Pandzic E., Chen L., Vicente-Manzanares M., Horwitz A.R., Wiseman P.W. STICCS reveals matrix-dependent adhesion slipping and gripping in migrating cells. Biophys. J. 2012;103:1672–1682. doi: 10.1016/j.bpj.2012.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.B., Hu K.M., Seamon K.J., Mani V., Chen Y., Gronert K. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 2012;26:1506–1516. doi: 10.1096/fj.11-198036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.X., Gipson I.K., Guo Y. Differential gene expression in healing rat corneal epithelium. Invest. Ophthalmol. Vis. Sci. 1995;36:1997–2007. [PubMed] [Google Scholar]
- Zhao M., Song B., Pu J., Forrester J.V., McCAIG C.D. Direct visualization of a stratified epithelium reveals that wounds heal by unified sliding of cell sheets. FASEB J. 2003;17:397–406. doi: 10.1096/fj.02-0610com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske J., Gipson I. Protein synthesis during corneal epithelial wound healing. Invest. Ophthalmol. Vis. Sci. 1986;27:1–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.