Abstract
Foraging and operant models suggest that animals will tolerate uncertainty or risk to obtain food quickly. In modern food environments, sustained access to quick energy-dense foods can promote weight gain. Here, we used a discrete-choice procedure to examine peoples' decisions about when next to eat high-value, palatable food rewards, probabilistically delivered immediately or following longer delays. In Experiment 1, moderately hungry young females showed consistent preferences for a variable delay option that delivered food rewards immediately or following long delays over a fixed delay option that delivered the same rewards following intermediate delays. These preferences were stronger in females with higher BMIs compared with lower BMIs, suggesting that quick food can enhance the value of uncertain or ‘risky’ food-seeking strategies in individuals vulnerable to future weight gain. In Experiment 2, prior exposure to a subtle and not easily identifiable food aroma increased selections of the variable delay option following delayed food rewards in a mixed sample of male and female adults, providing preliminary evidence that food cues can sustain uncertain food-seeking strategies. These data highlight a working hypothesis that the rapid delivery and consumption of food rewards, and food cues, can increase risk-tolerance in the food-seeking behaviours of individuals who are vulnerable to weight gain.
This article is part of the theme issue ‘Risk taking and impulsive behaviour: fundamental discoveries, theoretical perspectives and clinical implications’.
Keywords: food, foraging, risk, food-seeking, obesity, variable delays
1. Introduction
Evolutionary perspectives posit that the current population prevalence of obesity (and its broader health consequences) reflects the persistence of inherited food-seeking strategies that favour the over-consumption of energy-dense foods in food-enriched environments [1–3]. Specifically, activation of these strategies in environments in which energy-dense foods are readily available (at vastly reduced travel and energy costs) promotes positive energy budgets and facilitates weight gain [1]. Possibly, this food-seeking/food environment mismatch reflects the continuance of ‘thrifty’ genes [4], selectively neutral genetic ‘drift’ (which accounts for the varying incidence of obesity across individuals) [5,6] or the moderation of genetic influences upon food-seeking behaviours by climate change [7]. Despite the interest that these ideas have attracted [3] and, arguably, their face validity against evidence that some eating behaviours can contribute to obesity [8,9]—there has been relatively little experimental investigation of peoples' food-seeking strategies and their relationships with risk factors for longer-term weight gain.
One way to investigate such a connection is to examine the decisions that people make about when they will next eat; hereafter, called ‘food-scheduling behaviours’. Animals tend to make risk-averse selections for small and certain food rewards (on the one hand) over larger uncertain food rewards (on the other hand). However, animals also tend to show risk-seeking selections for food rewards that might be available very quickly or following longer delays [10–12]. Notwithstanding uncertainty about whether these risk-seeking biases reflect fluctuating (and negative) energy budgets (as indicated by Risk Sensitivity Theory) [13–15] or the greater salience of shorter delays compared with prolonged delays in memory (as in Scalar Expectancy Theory) [16], animals' food-seeking behaviours typically place a distinct premium upon obtaining food quickly, which sometimes wins out against the risks of sometimes sustaining longer delays to food and its energy pay-offs.
Within operant settings too, animals consistently exhibit strongly biased responding towards variable (VI) over fixed interval (FI) reinforcement schedules, reflecting the heightened expectancy of quick rewards [17–22]. In addition, we have demonstrated, using a discrete-choice method in rats, that preferences for variable over fixed delays to opportunities to earn food rewards are mediated by activity within corticolimbic circuitry [23] and its monoamine neuromodulation [24]. Humans too can show preferences for variable delays to non-food rewards in ways that reflect the relative probability (and distributions) of shorter over longer delays [21,22,25] and, possibly, sensitivity to (analogue) energy budgets [26]. To date though, there have been no tests of preferences for variable over fixed delays for edible food rewards in humans.
In a clinical context, investigations of choices involving delays to food rewards have focused on delay discounting and observations that, for humans and animals alike, the value of rewards tends to diminish (or be discounted) with the delay to receipt or consumption [27,28]. These delay discounting rates can be faster in groups at risk of weight gain, or in clinical groups with obesity, metabolic or eating disorders [29–37], possibly influencing the evaluation of food portions over inter-meal intervals [38]. However, while tests of delay discounting highlight links between impulsiveness and obesity [32], they do not help us to understand peoples’ tolerance of risk for variable over fixed delays to high-value edibles, or how the experience of high-value foods delivered and consumed immediately might influence subsequent food-seeking behaviours in individuals at elevated risk of weight gain.
Here, we explored a novel discrete-choice computerized ‘food-scheduling’ procedure in order to assess individuals’ decisions about when next to eat; and their risk-tolerance as preferences for variable delay options (that might deliver food rewards quickly or following longer delays) over fixed (intermediate) delays to high-value (i.e. energy-dense and palatable) food rewards. We tested preferences for ‘risky’ variable delays against a simple risk factor for further weight gain: body mass index (BMI) (Experiment 1) and their modulation by prior exposure to external food cues, here operationalized as a food (chocolate) aroma (Experiment 2).
Obesity and weight gain may be associated with specific difficulties in learning about food rewards [39]. Therefore, we were particularly interested in testing whether food rewards delivered and consumed immediately enhance preferences for behavioural options that offer variable delays, as a way to model how the availability of quick food might strengthen uncertain or risky food-seeking behaviours. Our results lay the foundations for investigations in clinical populations and investigations of the neural and neuroscientific basis of these behaviours in human and animal models [24] (see also Humby et al. [40]).
2. Experiment 1
To begin with, we sought to test the hypothesis that healthy adult volunteers would tolerate risk as preferences for variable delay options (that might deliver food rewards immediately or following longer delays) over fixed (intermediate) delays to high-value food rewards (as either confectionary or savoury snacks). To maximize sensitivity to detect such risk-tolerance, we sought to remove some likely confounding variables. First, because there are significant gender differences in attitudes to food and calorie estimation that might be relevant to our food rewards [41,42] and in attitudes to risk/uncertainty per se [43–45], we restricted our sample to females.
Second, we also excluded individuals with severe obesity (as indicated by a BMI of 40 or more) or who reported at least potential significant eating disorder symptoms. Finally, because low mood can alter eating behaviours [46], we excluded individuals with recent depressive symptoms of at least moderate severity. In this way, Experiment 1 was intended to provide (boundary-condition) information about individuals' preferences for variable over fixed delays for high-value rewards in the absence of some obvious confounding clinical factors.
(a). Method
Experiment 1 was approved by Bangor University (School of Psychology) Ethics Committee. All participants provided written informed consent.
(i). Participants
Sixty healthy adult female volunteers (mean age: 25 ± 1.4 years (standard error)) took part. Fifty participants were recruited from the Bangor University School of Psychology student panel or through word-of-mouth and were compensated with course credits. Ten local community participants received £15 for their time.
Exclusion criteria included (i) severe obesity as a BMI of 40 or more; (ii) moderate depressive symptoms as indicated by scores of 19 or more on the Beck Depression Inventory-II [47]; (iii) ‘caseness’ for DSM-IV eating disorders indicated by scores of 4 or more on any subscale of the Eating Disorders Examination-Questionnaire [48].
(ii). Psychometric questionnaires and self-report scales
Participants also completed the Barratt Impulsiveness Scale (BIS-11) [49] and the 18-item version of the Three-Factor Eating Questionnaire-Revised/TFEQ-R [50] to assess eating attitudes and behaviours. In Experiments 1 and 2, we found only modest associations between preferences for variable over fixed delays and BIS-11 scores. We also found inconsistent associations involving the restrained and uncontrolled eating subscales of the TFEQ-R [50], possibly reflecting differences in sample selection criteria and sample sizes. Therefore, we have chosen not to report these findings here, pending further investigation in carefully selected samples.
Finally, participants completed the Raven's Progressive Matrices-Short Form as a quick measure of cognitive ability [51]. There were no marked associations between preferences for variable over fixed delays and cognitive ability.
(iii). Food-scheduling assessment
In a discrete-choice procedure, participants completed 39 selections involving preferred food rewards or ‘treats’. On each selection, participants were presented with one green and one blue box (both 40 × 40 mm) on a standard touch-sensitive display (figure 1). The boxes were positioned 40 mm apart, subtending a viewing angle of approximately 7.26° at a viewing distance of approximately 630 mm.
Figure 1.
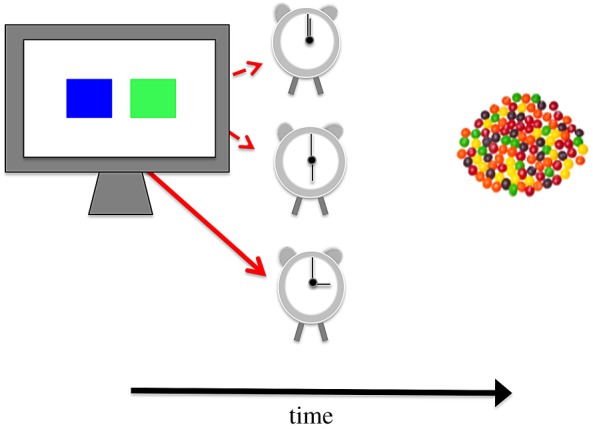
Schematic representation of the selection options and sequence of events in the discrete-choice food-scheduling procedure. On each selection, participants were presented with one green and one blue box, side by side on a touch-sensitive computer display. Touch-responses on one box (e.g. green) delivered food rewards either immediately (0 s) or following long delays (30 s). Touching the other box (e.g. blue) delivered food rewards following fixed intermediate delays (15 s). Participants made 39 such selections.
Touching one of the boxes (e.g. the green box), with the index finger of the preferred hand, delivered a single food reward following variable delays of 0 or 30 s (each scheduled with probabilities of 0.5), while touching the other box delivered a single reward following a fixed delay of 15 s. Food rewards were delivered by a bespoke motorized dispenser into a plastic ‘hopper’ positioned within easy reach on participants' right-hand side. A randomly jittered interval of 20 to 30 s allowed participants sufficient time to consume each reward before the next selection. Participant instructions are included in the electronic supplementary material.
The variable delay (e.g. green) and the fixed delay (e.g. blue) boxes appeared randomly on the left-hand or the right-hand side of the display over successive selections. The assignment of the colour of box (green or blue) to the variable or fixed delay options was counterbalanced across the participant sample.
(iv). Procedure
Participants were asked to fast for at least 2 h following breakfast or lunch prior to testing sessions scheduled for 11.00 or 16.00. On arrival, participants provided informed consent and completed the questionnaires. Their height and weight (to the nearest 0.1 cm kg−1) were measured in light clothing without shoes to calculate BMI as weight (kg)/(height (cm))2. Participants then provided ratings of hunger using a simple seven-point Likert scale with anchor points of ‘Not at all hungry’ to ‘Extremely hungry’.
Next, participants were shown small paper dishes of five sweet (Maltesers, Minstrels, Jelly Beans, Skittles and Revels) and five savoury (Hula Hoops Original, Cheese Puffs, Cheese Savouries, Pretzels and Twiglets) food rewards, and asked to rank them in order of preference from 1 to 5 for each food type. Participants chose between their highest-ranking sweet and savoury food rewards to determine their preferred treat for the experiment, and 39 of these ‘treats’ were loaded into the food dispenser.
Participants were left alone to complete the food-scheduling assessment in their own time. On its completion, participants were asked to rate again how hungry they felt using the seven-point Likert scale and complete a brief questionnaire about their awareness of the variable and fixed delay contingencies in the food-scheduling assessment, before being paid (if recruited from the community) and discharged.
(v). Data analysis
Statistical analysis (for Experiment 1 and Experiment 2) was completed with R-Studio (v. 1.0.1.136). Experiment 1 yielded two dependent measures: (i) the proportion of (risky) variable delay over fixed delay selections and (ii) the latencies for selections between the two delay options. Participants' proportions of variable delay selections were analysed with a sequence of mixed-effects binomial logistic models with both participant and selection (1 through 39) included as random effects in the intercepts. These models yield β-coefficients and standard errors; dividing the former by the latter yields Z-scores, allowing convenient significance tests (p < 0.05). As Experiment 1 (and Experiment 2) were exploratory, there was no correction for multiple comparisons. Full details of the model sequences are provided in the electronic supplementary materials.
Participants’ latencies as selection times (s) were analysed with normal-distribution models that included the same predictors, entered in the same sequence, as the logistic models. These models yielded β-coefficients and standard errors; this time, tested with t-statistics against estimated degrees of freedom. Preferences for the variable delay over fixed delay options were tested against individuals' questionnaire estimates of the contingencies of the food-scheduling assessment in simple binomial models.
(b). Results
(i). Demographic, morphometric and psychometric sample characteristics
Participants’ demographic, recent mood and eating characteristics are shown in table 1. Forty participants showed BMI scores within the healthy weight range (18.5 to 24.9); 18 showed BMIs in the overweight range (25.0 to 29.9) and two showed BMIs in the obese range (30 to 39.9). Participants were screened to ensure only modest depressive symptoms scored with the BDI-II [47] and eating disorder symptoms scored with EDE-Q [48]. Participants reported slightly fewer concerns about eating, shape, weight or restrained eating compared with unselected norms: 0.62 ± 0.06 (eating); 2.15 ± 0.10 (shape); 1.59 ± 0.06 (weight); and 1.25 ± 0.09 (restraint) [54].
Table 1.
Demographic, anthropometric and psychometric characteristics for Experiment 1 (n = 60) and Experiment 2 (n = 35 × 2 groups). BMI: body mass index; BDI-II: Beck's Depression Inventory-II (Beck et al. [47]); EDE-Q: Eating Disorder Examination-Questionnaire (Fairburn et al. [48]); TFEQ-R: Three-factor Eating Questionnaire-Revised (de Lauzon et al. [50]); BIS-11: Barratt's Impulsiveness Scale (Patton et al. [49]; Raven's Progressive Matrices-Short Form (Arthur et al. [51]); PANAS: Positive and Negative Affect Scale (Watson et al. [52]); PAD: Pleasure Arousal Dominance scale (Mehrabian [53]).
| Experiment 1 | Experiment 2 |
||
|---|---|---|---|
| scent-primed | scent-absent/control | ||
| gender (M : F) | 0 : 60 | 10 : 25 | 15 : 20 |
| age | 24.78 ± 1.44 | 20.69 ± 0.73 | 20.80 ± 0.71 |
| BMI | 23.38 ± 0.40 | 23.09 ± 0.44 | 23.09 ± 0.57 |
| BDI-II | 6.59 ± 0.67 | 7.86 ± 1.06 | 8.69 ± 1.18 |
| EDE-Q restraint subscale | 1.12 (0.14) | 0.73 ± 0.20 | 0.66 ± 0.16 |
| EDE-Q eating concern subscale | 0.57 (0.09) | 0.72 ± 0.15 | 0.54 ± 0.11 |
| EDE-Q shape concern subscale | 1.70 (0.14) | 1.85 ± 0.27 | 1.57 ± 0.21 |
| EDE-Q weight concern subscale | 1.24 (0.13) | 1.34 ± 0.23 | 1.23 ± 0.18 |
| TFEQ-R | 29.79 (1.83) | 24.22 ± 2.40 | 26.30 ± 3.12 |
| TFEQ-R | 28.84 (1.61) | 31.43 ± 3.08 | 28.09 ± 3.07 |
| TFEQ-R | 32.92 (2.92) | 28.84 ± 2.03 | 30.56 ± 2.51 |
| BIS-11 total score | 61.39 ± 1.14 | 63.20 ± 1.60 | 64.93 ± 2.00 |
| Raven's Matrices-Short Form | 12.16 ± 0.47 | 11.91 ± 0.39 | 11.44 ± 0.46 |
| PANAS state positive affect | — | 27.43 ± 2.03 | 28.24 ± 1.16 |
| PANAS state negative affect | — | 12.29 ± 0.62 | 13.47 ± 0.66 |
| PAD arousal | — | 17.68 ± 0.52 | 18.51 ± 0.63 |
(ii). Proportionate selections of the (risky) variable delay option
Preferences for the variable over the fixed delay option were not moderated by the colour of box assigned to either option, side of the screen on which the box assigned to the variable delay option was presented across selections, time of day of the testing session, or type of food reward chosen by participants (sweet confectionary or savoury snacks) (−0.14 ± 0.39 < β < 0.19 ± 0.37; electronic supplementary materials, table S1).
Overall, participants showed marginal preferences for the variable compared with fixed delay option (0.55 ± 0.03) (electronic supplementary material, table S1/Model 1; β = −0.72 ± 0.61). Those who reported being more hungry before the food-scheduling assessment did not select the variable delay option significantly more frequently than participants who reported being less hungry (electronic supplementary material, table S1/Model 1; β = 0.19 ± 0.11). However, compared with having chosen the fixed delay option and waiting for 15 s for the delivery of a food reward, participants were significantly more likely to select the variable delay option if, having done so on previous selection, they received (and consumed) a food reward immediately (0.60±0.03 versus 0.55±0.03) (electronic supplementary material, table S1/Model 2; β = 0.23 ± 0.11, Z = 2.09, p < 0.05). By contrast, participants were less likely to repeat their selections of the variable delay option if, on the previous selection, they had received a food reward only after the longer delay of 30 s (0.49 ± 0.03 versus 0.55 ± 0.03) (β = −0.27 ± 0.12, Z = −2.25, p < 0.05).
Participants with higher BMIs were slightly, and non-significantly, less likely to choose the fixed delay option twice in succession than participants with lower BMIs (figure 2) (electronic supplementary material, table S1/Model 4; β = −0.07 ± 0.05). By comparison, they were more likely to opt again for the variable delay option following immediate food rewards (figure 2) (electronic supplementary material, table S1/Model 4; β = 0.12 ± 0.03, Z = 4.00, p < 0.01) and at least as likely following rewards delivered after delays of 30 s (β = 0.10 ± 0.04, Z = 2.50, p < 0.05).
Figure 2.
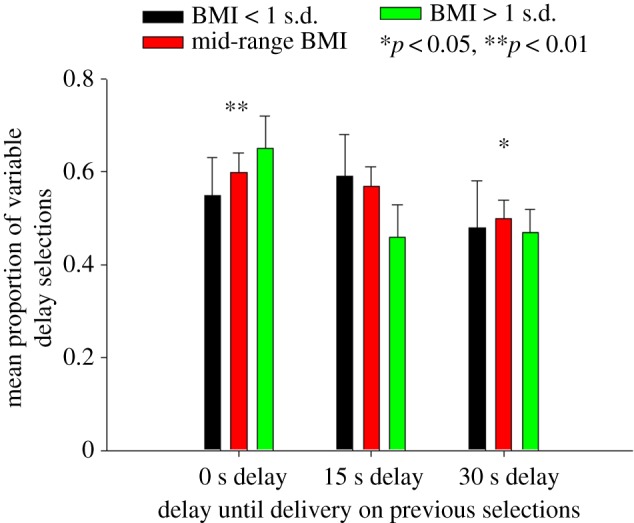
Mean proportion (and standard errors) of variable delay selections for low BMI participants (less than 20.2; less than 1 s.d. less than the mean; n = 10), mid-range (n = 39) and high BMI participants (greater than 26.5; less than 1 s.d. greater than the mean; n = 11) following delays of 0 s (variable delay), 15 s (fixed) or 30 s (variable delay) on previous selections. *p < 0.05 and **p < 0.01 selections of the variable delay option following delays of 0 or 30 s to food rewards as compared with selections of the variable delay option following fixed delays of 15 s. (BMIs categorized by ± 1 s.d. for illustration only.)
(iii). Selection times between (risky) variable and fixed delay options
Participants were faster to select between the two delay options following selections of the variable delay option that delivered immediate food rewards compared with selections of the fixed delay option (2.09 ± 0.09 s versus 2.38 ± 0.12 s, respectively) (electronic supplementary material, table S2/Model 2; β = −0.44 ± 0.16, t = −2.75, p < 0.01). Selections times did not differ much following selections of the variable delay option that delivered (delayed) food rewards after 30 s compared with delays of 15 s (2.30 ± 0.11 s versus 2.38 ± 0.12 s) (β = −0.09 ± 0.18). Finally, participants with higher BMIs were not markedly faster or slower than participants with lower BMIs to select between the delay options following selections of the variable delay option that delivered immediate food rewards (electronic supplementary material, table S2/Model 4; β = 0.04 ± 0.05) or following the longer delays of 30 s (β = 0.02 ± 0.06).
(iv). Participants’ self-reported estimates of food-scheduling contingencies
Forty (/60) participants identified the variable delay option as their favourite of the two; unsurprisingly, they made more selections of this option (β = 1.17 ± 0.23, Z = 5.09, p < 0.01). At a group level, participants' estimates of their proportionate choices of the variable over the fixed delay option were quite accurate at 0.55 ± 0.03 (median = 0.60). Estimates of their own proportion of variable delay choices were strongly associated with higher numbers of such selections (β = 3.51 ± 0.40, Z = 8.77, p < 0.01).
Participants markedly underestimated the average delay of the variable delay option (i.e. 0+30 s/2) at 9.05 ± 1.09 s (median = 6 s) compared with its actual value of 15 s. By contrast, participants’ estimates of the duration of the fixed option's delay were more accurate at 14.53 ± 1.60 s (median = 10 s). Participants who provided shorter estimates of the average variable delays tended to select that option more frequently than those who reported longer estimates (β = −0.04 ± 0.02, Z = −2.00, p < 0.05). There was little sign that these participants selected the variable delay option more frequently following the delivery of immediate food rewards (β = 0.03 ± 0.02. Overall, participants dramatically underestimated the number of food rewards consumed: a mean of 24.75 ± 1.46 (median = 20) compared with the actual value at 39 treats.
(c). Discussion
Evolutionary perspectives on weight gain and obesity posit a mismatch between persisting food selection strategies that favour over-consumption of energy-dense food and an obesogenic environment in which such foods are easily accessed and consumed [1,2]. Foraging [10–16] and operant models [17–21,23,24] highlight animals' tolerance of risk as a preference for variable delays over fixed intervals to food rewards. To the best of our knowledge, Experiment 1 is the first to provide evidence (i) that moderately hungry humans show preferences for variable over fixed delays for high-value food rewards (consumed on-the-spot); (ii) that these preferences are strengthened by the quick delivery and consumption of food rewards; and (iii) that these risk-prone biases are, at least across the healthy/overweight range, enhanced in individuals at risk of weight gain by dint of higher rather than lower BMIs.
Obesity is associated with increased preferences for small immediate rewards (including, for example, money) at the expense of large delayed rewards, indicating a potential role for impulsivity in over-eating and weight gain [29–38]. From this perspective, preferences for variable over fixed delay options may reflect the higher combined (and non-discounted) value of immediate food rewards (delivered at 0 s) and the more heavily discounted food rewards (at 30 s) compared with intermediately discounted food rewards (at 15 s). Our observation that the immediate delivery of high-value food rewards can sustain selections of variable delays (to a greater extent in individuals with high BMIs rather than lower BMIs) supports a preliminary, working hypothesis that the consumption of quick food produces transient increases in their relative reward value in individuals vulnerable to longer-term weight gain.
Experiment 1 has several strengths. Our participants were free of significant recent depressive symptoms (which can interfere with eating behaviours) [46] and clinically significant symptoms for eating disorders. Thus, our demonstration that individuals’ preference for variable delays is strengthened by the delivery of immediate food rewards on prior selections (i.e. as quick foods) is unlikely to reflect co-occurring overt mood or eating-related psychopathology. Our participants completed the food-scheduling assessment with palatable food rewards (treats) picked out of a menu of five confectionary and five savoury snacks, ensuring that participants were responding for preferred high-value palatable foods. Finally, there was no indication that preferences for variable delays, selection times and the observed relationships with BMI were specific to particular food types or time-of-day.
Finally, we note that, consistent with scalar models of interval timing [16], our participants tended to underestimate the average value of the variable delays (9.05 ± 1.09 s compared with the actual value of 15 s). Moreover, underestimation of these delays was associated with increased preference for the variable delay option, suggesting that risk-seeking choices, as operationalized here, reflect (at least partially) the biased estimates of the available delays to food rewards [16].
In Experiment 2, we sought to extend the above findings by testing whether individuals' food-scheduling behaviours, operationalized here as preferences for variable over fixed delays, are sensitive to environmental cues that signal the availability of a particular high-value food reward: chocolate.
3. Experiment 2
Modern food environments contain a plethora of food cues or stimuli that signal the easy availability of food [1,55,56]. However, these cues are more salient to some individuals than others [57,58] and more salient in certain situations or motivational states (such as deprivation; [59]). Food aromas can trigger food-seeking behaviours [60,61]. Experiment 1 demonstrated that moderately hungry healthy young females show small but consistent preferences for variable delays to food rewards but that these preferences can be enhanced following immediate food delivery and consumption. In Experiment 2, we conducted a preliminary investigation of whether preferences for variable delays to food rewards can be modulated by prior exposure to food cues.
Seventy adult participants were randomized to one of two groups. One group (scent-primed) was exposed to a subtle, not easily identifiable, chocolate aroma in a waiting room prior to completion of the food-scheduling assessment, altered to deliver small chocolate pieces as rewards. The other group (scent-absent/‘control’) were not exposed to any aroma in the waiting room prior to the food-scheduling assessment for the same chocolate rewards. We exposed participants to the chocolate aroma in the waiting room prior to the food-scheduling task in line with previous ‘priming’ protocols in food research [61]. We used a chocolate aroma as the olfactory cue and Cadbury's chocolate pieces™ as the reward because our pilot testing had identified a reliable protocol in which the chocolate aroma reached a discreet, discernible intensity that could be identified only once participants were aware of its presence.
Experiment 2 included several other design amendments. First, Experiment 1 had implemented relatively stringent inclusion/exclusion criteria to remove or mitigate some confounding factors. In particular, because males and females can differ in their attitudes to food and calorie estimation [41,42] and attitudes to risk [43–45], this meant using only female participants. In Experiment 2, we relaxed our gender, mood and eating-disorder symptom exclusions. This allowed us to examine whether preferences for variable over fixed delays to palatable food rewards are evident in a mixed gender and (relatively unrestricted) sample.
Second, Experiment 1 included participants who were moderately hungry. However, food cues can sometimes promote eating behaviour even when people are sated [62]. Therefore, in Experiment 2, we allowed hunger and the time of day of the testing session to vary freely. Third, in addition to measuring the time needed to select between the variable and fixed delay options during the food-scheduling assessment, we also measured how long it took participants to collect food rewards from the hopper. This allowed us to examine whether prior exposure to an olfactory cue had similar impacts on both consummatory behaviours and variable versus fixed delay selections.
Finally, olfactory cues can be highly arousing [63]. Therefore, we included the Pleasure Arousal Dominance (PAD) scale [64] to measure any differences in arousal between the scent-primed and scent-absent/control participants. The PAD scale has been used in retail, to measure changes in consumers' behaviour in response to environmental factors that constitute ‘store atmospherics’ [65,66]. We also included the state version of the Positive and Negative Affect Scale (PANAS) [52] and a measure of chocolate attitudes and liking [67] to capture individual differences in the valuation of chocolate.
(a). Method
Ethical approval was granted by Bangor University School of Psychology Research Ethics committee. All participants provided informed, written consent.
(i). Participants
Twenty-five healthy male and 45 female adults (mean age 20.74 ± 0.50 years) were recruited from Bangor University psychology student participant panel and were compensated with course credits. Their mean BMI was 23.09 ± 0.36 (19 to 33.5). Exclusion criteria were relaxed compared with Experiment 1 and consisted of any self-reported food allergies and/or a BMI above 40 indicating severe obesity.
(ii). Psychometric questionnaires and self-report scales
Participants completed the same measures as in Experiment 1 (table 1) and the PAD scale [53], PANAS [52] and chocolate scale [67].
(iii). Food aroma primes
Thirty-five participants were exposed to a subtle non-identifiable chocolate aroma or scent. This prime was delivered in a small waiting room next door to the room in which the food-scheduling task was to be completed. To deliver the prime, we used a chocolate scented cartridge (www.scentair.co.uk/) and a small desk fan. Pilot testing (n = 20) allowed us to identify an optimal exposure that involved leaving the fan to disperse the scent actively for 65 s, followed by free dispersal for 3 min before the participants entered the room. Under these conditions, participants were able to identify that an aroma was present but were not able to identify reliably that aroma as chocolate in free-recall. However, when given the forced-choice of chocolate, Haribo sweets, toffee or cinnamon, participants tended to identify chocolate reliably; see the ‘Manipulation check’ section below. Participants remained in the scented room for 6 min to allow enough time to complete the PAD (to measure arousal) [53], the PANAS (to measure state affect) [52] and the BIS-11 questionnaires [49].
(iv). Food-scheduling assessment
The food-scheduling assessment was the same as reported in Experiment 1. However, all participants completed the assessment for half-squares of Cadbury's Dairy Milk chocolate (to be congruent with the scent prime). We also collected latencies for the time taken to reach for and retrieve the chocolate pieces by means of a light-sensitive (infrared) diode positioned just inside the mouth of the food hopper.
(v). Procedure
On arrival, participants completed the protocol questionnaires and the Raven's Progressive Matrices-Short Form [51], before providing anthropometric measurements and a single rating of their current hunger using the same seven-point Likert scale as in Experiment 1. Next, participants were taken to the waiting room (which had been scented with a chocolate aroma for participants in the scent-primed group to be exposed to the prime for 6 min) while completing the PANAS [52], the PAD [64] and the BIS-11 [49] questionnaires. Participants in the scent-absent/control group followed exactly the same procedure. However, the same waiting room where they completed the extra questionnaires was not scented with a chocolate aroma.
Following this, participants were moved to the testing room (which was free of chocolate aroma for both groups) and completed the food-scheduling assessment. Participants started the food-scheduling assessment as soon as they were ready and the experimenter exited the room. On completion of the food-scheduling assessment, participants provided a second hunger rating and answered a debriefing questionnaire about the contingencies of the variable and fixed delay options. Finally, as a manipulation check, all participants answered questions about their awareness of the chocolate aroma (see below) before being thanked and discharged.
(vi). Manipulation check
First, we asked both participant groups if they could smell anything (coded as a binary variable, with ‘yes’ and ‘no’ responses) in the waiting room. Second, participants were presented with a forced-choice from chocolate, Haribo sweets, toffee or cinnamon as to which they thought best described the aroma that had been circulated in the room.
(vii). Data analysis
Group-matching for demographic, anthropometric characteristics and manipulation checks were assessed with χ2 statistics and standard linear models. All participants were included in the data analyses. Proportions of variable delay over fixed delay selections were assessed with a sequence of mixed-effects binomial logistic models. Variable over fixed delay selections were tested against gender and hunger in two preliminary models; see electronic supplementary materials for more details. Selection and food collection latencies were tested using normal-distribution models with equivalent structures; see the electronic supplementary material for more details.
Experiment 2 produced somewhat noisier data than Experiment 1. We found the same associations between variable delay selections following immediate food rewards (on the one hand) and BMI (on the other hand) in the scent-absent/control participants as observed in Experiment 1 (β = 0.39 ± 0.15, Z = 2.6, p < 0.01). However, selections as a function of BMI were markedly disrupted in the scent-primed participants and the models that tested the higher-order interactive effects of group (scent-primed versus scent-absent/control), delay to reward delivery on previous selections and BMI were not robust as assessed by fit statistics. Therefore, in light of the relatively low statistical power offered by Experiment 2 (which was principally intended to test the effects of prior exposure to food cues), the models involving BMI are not described here. However, they are available from the corresponding author.
(b). Results
(i). Group-matching: demographic, morphometric and psychometric features
Demographic, anthropometric and psychometric data for the scent-primed and scent-absent participants are displayed in table 1. Within the scent-absent/control group, 25 participants showed BMI scores within the healthy weight range, nine showed BMIs in the overweight range and one showed a BMI score in the obese range. Within the scent-primed group, 26 participants showed BMI scores within the healthy weight range, nine showed BMIs in the overweight range and two showed BMI scores in the obese range.
As expected, participants' mean scores on the BDI-II [47] and EDE-Q [48] indicated low or mild eating or mood concerns overall. At baseline, the two participant groups were closely matched in their hunger ratings prior to the food-scheduling assessment (4.29 ± 0.23 versus 3.89 ± 0.26, respectively) (β = 0.03 ± 0.07). The scent-primed and the scent-absent/control participants showed no significant differences in their (PAD) state arousal (17.68 ± 0.52 versus 18.51 ± 0.63) (β = 0.84 ± 0.8). State positive affect was unchanged but the scent-primed participants showed a small reduction in their negative affect (12.29 ± 0.62 versus 13.47 ± 0.66) (β = 0.−1.19 ± 0.15, t(7.28)= −2.05, p < 0.05).
(ii). Manipulation checks
Twenty-two out of the 35 (63%) of the scent-present participants reported that they detected an aroma in the waiting room prior to the food-scheduling assessment, compared with five out of 35 participants (15%) of the scent-absent/control participants (as probed by the question ‘Could you smell anything?’, , p < 0.001). Participants reported smelling chocolate more frequently than the other aromas in both the scent-primed (χ2(3) = 40.31, p < 0.01) and scent-absent groups (χ2(3) = 8.31, p = 0.04) (see electronic supplementary material, table S3). While the number of scent-primed participants who correctly identified chocolate as a forced-choice was elevated in comparison with the scent-absent participants (25 versus 16 out of 35), this was not significant (, p = 0.18).
(iii). Proportionate selections of the (risky) variable delay option
Gender and hunger: Overall, preference for variable delays to chocolate rewards was only very marginally influenced by gender and hunger. Preferences for the variable over the fixed delay option did not vary much between males and females (see electronic supplementary material, table S4 for details), either overall (0.52 ± 0.04 versus 0.53 ± 0.03) (β = 0.04 ± 0.07), following chocolate rewards delivered immediately (0.61 ± 0.06 versus 0.59 ± 0.04) (β = 0.02 ± 0.21), following delays of 30 s (0.46 ± 0.04 versus 0.48 ± 0.04) (β = 0.09 ± 0.22) or following exposure to the chocolate aroma (β = −0.19 ± 0.41). Neither did selections of the variable delay option differ much between males and females in the scent-primed groups compared with the scent-absent groups following delays of 0 or 30 s (β = 0.71 ± 0.43 and β = 0.55 ± 0.46).
In contrast to Experiment 1, preference for the variable delay option was slightly increased with hunger but only following 30 s delays (see electronic supplementary material, table S5) (β = 0.31 ± 0.08, Z = 3.88). There was no significant change in variable delay selections over fixed delay selections in relation to state hunger following exposure to the chocolate aroma (see electronic supplementary material, table S5 for the data) (β = 0.07 ± 0.14) or in the scent-present compared with scent-absent groups following chocolate rewards delivered after 0 or 30 s (electronic supplementary material, table S5) (β = 0.23 ± 0.15 and β = 0.17 ± 0.15).
As expected, preferences for the variable over fixed delays were not modulated much by the colour of box assigned to either option or time of day (−0.08 ± 0.25 < all βs < 0.80 ± 0.85). But, participants did choose the variable delay option more frequently when presented on the right-hand side compared with the left-hand side of the display (0.55 ± 0.01 versus 0.51 ± 0.01), (β = 0.21 ± 0.08, Z = 2.43, p < 0.05). Therefore, this predictor was retained in all subsequent models (see electronic supplementary material, table S6).
Effects of food aroma: As we found in Experiment 1, participants were more likely to choose the variable delay option when, having selected that option on the previous opportunity, they had received chocolate immediately (0.60 ± 0.03 versus 0.53 ± 0.03) (electronic supplementary material, table S6/Model 2; β = 0.47 ± 0.10, Z = 4.70, p < 0.01). Exposure to the chocolate aroma was not associated with clear shifts in overall preference for the variable delays over the fixed delay (0.52 ± 0.03 versus 0.53 ± 0.03) (electronic supplementary material, table S6/Model 3; β = −0.03 ± 0.19). However, participants in the scent-primed group were significantly more likely than participants in the scent-absent (control group) to select the variable delay option again if, having done so on previous selections, they had received chocolate rewards following delays of 30 s (figure 3) (0.52 ± 0.04 versus 0.43 ± 0.04) (electronic supplementary material, table S6/Model 4; β = 0.62 ± 0.22, Z = 2.87, p < 0.05). By contrast, there were no marked changes in the frequency of variable delay selections following immediate delivery and consumption of chocolate rewards in the scent-primed compared with the scent-absent/control participants (0.59 ± 0.05 versus 0.61 ± 0.04) (electronic supplementary material, table S6/Model 4; β = 0.17 ± 0.21).
Figure 3.
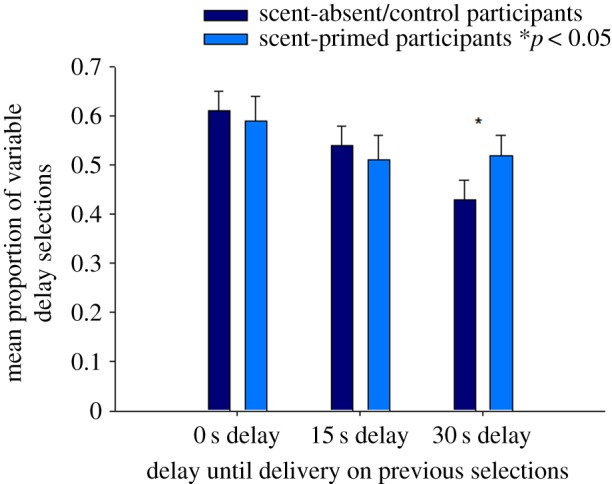
Mean proportion (and standard errors) of variable delay schedule selections over fixed delay schedule selections for chocolate food rewards in the scent-primed participants (exposed previously to a chocolate aroma; n = 35) and scent-absent/control participants (n = 35) following delays to reward delivery of 0, 15 or 30 s on previous selections. *p < 0.05, selections of the variable delay option following delays to food rewards of 30 s compared with the selections of variable delay option following the fixed delay of 15 s in the scent-primed compared with scent-absent participants.
(iv). Selection times for variable (risky) and fixed delay options
Participants made faster selections between the variable and fixed delay options when they had received chocolate rewards following delays of 0 s compared with fixed delays of 15 s on preceding selections (2.30 ± 0.11 versus 2.94 ± 0.12) (electronic supplementary material, table S7/Model 2; β = −0.54 ± 0.16, t2562.10 = −3.38, p < 0.01) and, in contrast to Experiment 1, following delays of 30 s (2.42 ± 0.08 versus 2.94 ± 0.12) (β = −0.39 ± 0.17; t2560.40 = −2.32, p < 0.05). These patterns were not changed in the scent-primed compared with the scent-absent/control participants (electronic supplementary material, table S7/Model 4; −0.55 ± 0.34 < all βs < 0.47 ± 0.32).
(v). Collection times for variable and fixed delay options
Females were slower to retrieve their food rewards than males (electronic supplementary material, table S8/Model 1; β = 0.48 ± 0.19, t4580.00 = 2.58, p < 0.05). (This predictor was retained in all models.) Overall, participants were quicker to collect chocolate rewards on selections that followed delays of 0 s compared with delays of 15 s (2.43 ± 0.08 versus 2.65 ± 0.09) (electronic supplementary material, table S8/Model 2; β = −0.21 ± 0.05, t1775.10 = −4.71, p < 0.001). Collection latencies were not much affected by exposure to the chocolate aroma for the scent-primed compared with scent-absent participants (2.34 ± 0.05 versus 2.39 ± 0.05) (electronic supplementary material, table S8/Model 3; β = −0.17 ± 0.17). There were no substantial changes in food collection times for the scent-primed compared with the scent-absent/control participants following selections that delivered chocolate rewards immediately or after delays of 30 s (see electronic supplementary material, table S8/Model 3; −0.16 ± 0.17 < βs < −0.04 ± 0.09).
(vi). Self-reported choice between variable and fixed delay options
Finally, associations between participants' preferences for the variable delay option over the fixed delay option (on the one hand) and their estimates of the food-scheduling contingencies (on the other hand) were comparable to those of Experiment 1. This included the observation that participants who provided shorter estimates of the combined (i.e. average) variable delays selected that option more frequently than those who estimated longer delays following immediate rewards (β = −0.01 ± 0.00, Z = −2.57, p < 0.05) and following rewards delivered after 30 s (β = −0.02 ± 0.01, Z = −2.00, p < 0.05). Other details can be found in the electronic supplementary material.
(c). Discussion
Experiment 2 provides an exploratory investigation of the effects of environmental food cues—operationalized as a subtle chocolate aroma—on food-scheduling behaviours for high-value chocolate rewards. We hypothesized that prior exposure to a chocolate aroma would increase preferences for the variable delay option delivering chocolate rewards compared with non-exposure. We found a modest increase in the proportion of variable delay selections over fixed delay selections in the scent-primed participants compared with the scent-absent participants but only following extended delays of 30 s. Selection times were also speeded following choice of the variable delay. However, pre-exposure to the chocolate aroma did not alter selection times or collection times. Although clearly preliminary, this is the first report of links between preferences for variable over fixed delays to palatable food rewards and prior exposure to food primes in human experimental subjects.
Broadly speaking, these results replicate those of Experiment 1. Participants chose the variable delay option more frequently following the delivery of immediate food rewards on previous selections. Participants were also faster to make their next selections and collect subsequent food rewards, following the immediate delivery and consumption of food rewards. Although the scent-primed participants showed a small reduction in state negative affect compared with the scent-absent participants following exposure to the aroma, the groups reported equivalent arousal (as measured by the PAD questionnaire [53,63,68]). Therefore, the modestly altered preferences for the variable compared with fixed delay options in the former participants cannot be attributed to differences in arousal following exposure to the chocolate aroma. Similarly, there were no marked differences between the scent-primed and scent-absent/control participants in terms of demographic and anthropometric characteristics, impulsiveness (as measured by the BIS-11), recent depressive symptomology (as measured by the BDI), cognitive ability (as measured by the short form of the Raven's Matrices) or concerns involving eating, body shape or weight (as indicated by the EDE-Q).
Experiment 2 extends the findings of Experiment 1 in several respects. First, pilot testing allowed us to achieve an intensity of chocolate aroma in response to which more scent-primed than scent-absent participants reported being able to ‘smell something’ (22 versus 5 out of 35), but showed only a slight increase in the ability to identify chocolate in a forced-choice test with three sweet aroma distractors (25 versus 16). This demonstrates that, while the chocolate aroma was identifiable to the level of awareness, it was not sufficiently strong to influence the food-scheduling behaviour through the conscious expectations of chocolate as a powerful, high-value reward.
Second, Experiment 2 demonstrated preferences for variable over fixed delays to food rewards in a mixed sample of men and women. Although we found little evidence that these preferences were stronger or weaker in one gender compared with the other, a larger experiment will be needed to test this possibility properly. Third, in contrast to Experiment 1, participants' hunger was left uncontrolled to vary over testing sessions that might have occurred at any time of the working day. Other evidence suggests that exposure to food cues can stimulate consumption in people who are already sated [62]. Experiment 2 shows that food cues may also modulate preferences between variable and fixed delays in participants with varying levels of state hunger.
Our environment contains a plethora of food cues or stimuli that signal easy access to food [57–59]. Some of these, such as food aromas, can trigger food-seeking behaviours [60,61]. Our finding that prior exposure to a subtle chocolate aroma did not increase selections of the variable over the fixed delay option following the delivery of immediate food rewards on previous selections but did so following delivery of those same rewards after 30 s, suggests a more generalized enhancement of preference rather than one driven by solely the value of immediate or quick food. Possibly, the magnitude of this enhancement could be further increased by stronger aromas, by visual and olfactory cues or by manipulations of motivational state such as hunger.
Animal models of delay discounting indicate that the presence of conditioned cues (CS+) during prolonged delays to rewards can reduce discounting rates in comparison with when the cue (CS+) is not presented during delays [69–71]. Possibly, prior exposure to the chocolate aroma that signalled the availability of a high-value reward (chocolate pieces) acted as a cue, or prime, to sustain tolerance of the longer delays of 30 s, sustaining subsequent selections of the variable delay option.
Finally, Experiment 2 included an additional measure of the latencies to collect food rewards from the food hopper where the chocolate rewards were delivered. Collection times were faster when participants received and consumed their food rewards immediately on the previous selections. This suggests that the impact of quick food extends beyond the selection of variable over fixed delay options to facilitate consummatory behaviours as participants reach for and eat high-value food rewards.
4. General discussion
Evolutionary perspectives on obesity (and its broader health consequences) posit a mismatch between persisting food-seeking strategies that favour over-consumption of energy-dense foods and environments that afford these foods at massively reduced travel and energy costs, facilitating positive energy budgets and weight gain [1–3]. While the theoretical background for these proposals has been discussed widely [3–7,9], there has been relatively little experimental work around peoples’ food-seeking strategies and their relationships with relevant risk factors for weight and metabolic problems. In two experiments with (non-clinical) human adults, we explored a prominent food-seeking bias observed in foraging and operant contexts across species, i.e. preferences for opportunities that afford the possibility of immediate access to high-value food rewards at the risk of relatively prolonged delays [10–25] and the modulation of these preferences by BMI and food cues.
Operationalized in a ‘food-scheduling’ assessment that involved decisions about when next to eat, the preliminary results demonstrate (i) that males and females (without severe obesity) show modest but consistent preferences for variable delays that offer rewards delivered immediately or following prolonged delays over fixed intermediate delays; (ii) that these preferences, the speed of selections between these options, and the collection of high-value food rewards are all enhanced following the immediate delivery and consumption of these food rewards on previous selections; (iii) that the enhanced preferences for variable delays following immediate food rewards show some further enhancement in individuals with higher rather than lower BMI; and (iv) that preferences for variable delays can be enhanced following prior exposure to olfactory food cues. These data demonstrate that humans, like animals, will tolerate degrees of risk (as uncertainty) when making decisions about when next to eat.
Preferences for variable delays over fixed delays may be mediated by several mechanisms. Possibly, the variable delay option sustained a higher combined value of immediate food rewards (delivered at 0 s) and heavily discounted food rewards (delivered at 30 s) compared with the fixed delay option intermediately discounted food rewards (delivered at 15 s). Our observation that the delivery of quick foods sustained subsequent selections of the variable delay option, speeded subsequent selections and speeded the collection (and consumption) of food rewards suggests transient increases in the value of the variable delay option. Individuals who are vulnerable to obesity, weight gain and associated metabolic disorders or certain eating disorders tend to discount rewards (including food rewards) rapidly [29–38] and also show changes in how they learn about food rewards [39]. Experiment 1's finding that preferences for variable delays over fixed delays were further enhanced in individuals with higher BMIs relative to lower BMIs following the quick delivery of food rewards supports the tentative hypothesis that vulnerability to weight gain is associated with changes in the evaluation of uncertain food-seeking strategies.
Food-seeking and consumption can also be driven by environmental cues including food aromas [60–62]. Experiment 2's finding that prior exposure to a chocolate aroma increased the selection of the variable delay option following chocolate rewards delivered after delays of 30 s suggests a generalized enhancement of preference rather than one driven by the value of quick food. Conditioned cues that predict the eventual delivery of rewards can support preferences over prolonged delays [69,70]. In a complementary way, our data suggest that pre-exposure to cues that signal the availability of high-value foods can sustain food-seeking strategies that turn on the relative balance of immediate/uncertain rewards versus delayed/certain rewards.
Foraging models suggest that animals' biases towards variable delay over fixed delay reinforcement opportunities can reflect energy budgets that once depleted—for example, following food deprivation—promote risk-tolerance (as described in Risk Sensitivity Theory) [13–15]. None of our experiments manipulated energy budgets directly and there was only weak evidence that preferences for variable delays reflected participants’ ratings of state hunger (as a crude indicator of negative energy budgets). This is broadly in line with the operant evidence in other species [17–19].
In addition, foraging perspectives attribute risk-seeking behaviour (over delays to food) to the more variable representations of longer time-intervals in memory compared with shorter time-intervals so that the latter delays are over-weighted in selections between food-seeking options (as in Scalar Expectancy Theory) [16]. Consistent with this, we note that participants in Experiment 1 tended to underestimate the combined value of the variable delays (9.05 ± 1.09 s compared with the actual value of 15 s). Further, this underestimation was linked to increased preferences for the variable delays, suggesting that our food-scheduling behaviour reflects (in part) participants' explicit (or otherwise) estimates of delays to food rewards.
Finally, operant perspectives might posit that variability of individuals’ preferences for variable delays reflect a ‘matching’ operation with the experienced rate per unit time of (discounted) rewards delivered [17]. Our current work is testing between these possibilities but, in particular, focusing upon what individuals learn in our food-scheduling assessment and how this varies with risk factors for weight gain.
Notwithstanding the above possibilities, our results lay the foundations for investigations both in clinical populations and of the neural and neuroscientific basis of these behaviours in human and animal models. Recently, using a comparable discrete-choice task, we demonstrated that administration of the D2 receptor antagonist (but not the D1 receptor agonist, SCH23390) and the 5-HT1A receptor agonist, 8-OH-DPAT, dose-dependently attenuates rats' preferences for risky options that might minimize delays to earn food rewards but at the risk of longer and increasing delays [24]. Future work, using analogues of the food-scheduling assessment introduced here can help us to understand the neurochemistry of food-seeking strategies and identify therapeutic targets in relation to obesity and weight gain [40].
Supplementary Material
Data accessibility
Primary data available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.81hn422 [72].
Authors' contributions
L.-J.G.S. contributed to the experimental design, data collection and analysis, and the manuscript preparation. A.D. contributed to the data collection and analysis. P.L. and C.W. contributed to the conceptual development of the research and the manuscript preparation. R.D.R. contributed to the conceptual development of the research along with the experimental design, the data analysis and manuscript preparation.
Competing interests
The authors have no relevant conflicts of interest.
Funding
L.-J.G.S. was supported by an ESRC PhD studentship.
References
- 1.Lieberman L. 2006. Evolutionary and anthropological perspectives on optimal foraging in obesogenic environments. Appetitie 47, 3–9. ( 10.1016/j.appet.2006.02.011) [DOI] [PubMed] [Google Scholar]
- 2.Pinel JP, Assanand S, Lehman DR. 2000. Hunger, eating, and ill health. Am. Psychol. 55, 1105–1116. ( 10.1037/0003-066X.55.10.1105) [DOI] [PubMed] [Google Scholar]
- 3.Albuquerque D, Stice E, Rodriguez-Lopez R, Manco L, Nobrega C. 2015. Current review of genetics of human obesity: from molecular mechanisms to an evolutionary perspective. Mol. Genet. Genomics 290, 1191–1221. ( 10.1007/s00438-015-1015-9) [DOI] [PubMed] [Google Scholar]
- 4.Neel JV. 1962. Diabetes mellitus: a ‘thrifty’ genotype rendered detrimental by ‘progress’? Am. J. Hum. Genet. 14, 353. [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen R. 2005. Molecular signatures of natural selection. Annu. Rev. Genet. 39, 197–218. ( 10.1146/annurev.genet.39.073003.112420) [DOI] [PubMed] [Google Scholar]
- 6.Speakman JR. 2013. Evolutionary perspectives on the obesity epidemic: adaptive, maladaptive, and neutral viewpoints. Annu. Rev. Nutr. 33, 289–317. ( 10.1146/annurev-nutr-071811-150711) [DOI] [PubMed] [Google Scholar]
- 7.Sellayah D, Cagampang FR, Cox RD. 2014. On the evolutionary origins of obesity: a new hypothesis. Endocrinology 155, 1573–1588. ( 10.1210/en.2013-2103) [DOI] [PubMed] [Google Scholar]
- 8.Mesas AE, Muñoz-Pareja M, López-García E, Rodríguez-Artalejo F. 2012. Selected eating behaviours and excess body weight: a systematic review. Obes. Rev. 13, 106–135. ( 10.1111/j.1467-789X.2011.00936.x) [DOI] [PubMed] [Google Scholar]
- 9.Brunstrom JM, Drake AC, Forde CG, Rogers PJ. 2018. Undervalued and ignored: are humans poorly adapted to energy-dense foods? Appetite 120, 589–595. ( 10.1016/j.appet.2017.10.015) [DOI] [PubMed] [Google Scholar]
- 10.Marsh B, Kacelnik A. 2002. Framing effects and risky decisions in starlings. Proc. Natl Acad. Sci. USA 99, 3352–3355. ( 10.1073/pnas.042491999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateson M, Kacelnik A. 1997. Starlings' preferences for predictable and unpredictable delays to food. Anim. Behav. 53, 1129–1142. ( 10.1006/anbe.1996.0388) [DOI] [PubMed] [Google Scholar]
- 12.Bateson M, Kacelnik A. 1995. Preferences for fixed and variable food sources: variability in amount and delay. J. Exp. Anal. Behav. 63, 313–329. ( 10.1901/jeab.1995.63-313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caraco T, Blanckenhorn WU, Gregory GM, Newman JA, Recer GM, Zwicker SM. 1990. Risk-sensitivity: ambient temperature affects foraging choice. Anim. Behav. 39, 338–345. ( 10.1016/S0003-3472(05)80879-6) [DOI] [Google Scholar]
- 14.Stephens DW. 1981. The logic of risk-sensitive foraging preferences. Anim. Behav. 29, 628–629. ( 10.1016/S0003-3472(81)80128-5) [DOI] [Google Scholar]
- 15.Shafir S. 2000. Risk-sensitive foraging: the effect of relative variability. Oikos 88, 663–669. ( 10.1034/j.1600-0706.2000.880323.x) [DOI] [Google Scholar]
- 16.Kacelnik A, Bateson M. 1996. Risky theories – the effects of variance on foraging decisions. Am. Zool. 36, 402–434. ( 10.1093/icb/36.4.402) [DOI] [Google Scholar]
- 17.Herrnstein RJ. 1964. Aperiodicity as a factor in choice. J. Exp. Anal. Behav. 7, 179–182. ( 10.1901/jeab.1964.7-179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Case D, Nichols P, Fantino E. 1995. Pigeons’ preference for variable-interval water reinforcement under widely varied water budgets. J. Exp. Anal. Behav. 64, 299–311. ( 10.1901/jeab.1995.64-299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killeen P. 1968. On the measurement of reinforcement frequency in the study of preference. J. Exp. Anal. Behav. 11, 263–269. ( 10.1901/jeab.1968.11-263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bateson M, Kacelnik A. 1996. Rate currencies and the foraging starling: the fallacy of the averages revisited. Behav. Ecol. 7, 341–352. ( 10.1093/beheco/7.3.341) [DOI] [Google Scholar]
- 21.Lagorio CH, Hackenberg TD. 2010. Risky choice in pigeons and humans: a cross-species comparison. J. Exp. Anal. Behav. 93, 27–44. ( 10.1901/jeab.2010.93-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohn A, Kohn WK, Staddon J. 1992. Preferences for constant duration delays and constant sized rewards in human subjects. Behav. Process. 26, 125–142. ( 10.1016/0376-6357(92)90008-2) [DOI] [PubMed] [Google Scholar]
- 23.Tremblay M, Cocker PJ, Hosking JG, Zeeb FD, Rogers RD, Winstanley CA. 2014. Dissociable effects of basolateral amygdala lesions on decision making biases in rats when loss or gain is emphasized. Cogn. Affect. Behav. Neurosci. 14, 1184–1195. ( 10.3758/s13415-014-0271-1) [DOI] [PubMed] [Google Scholar]
- 24.Rogers RD, Wong A, McKinnon C, Winstanley CA. 2013. Systemic administration of 8-OH-DPAT and eticlopride, but not SCH23390, alters loss-chasing behaviour in the rat. Neuropsychopharmacology 38, 1094 ( 10.1038/npp.2013.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locey ML, Pietras CJ, Hackenberg TD. 2009. Human risky choice: delay sensitivity depends on reinforcer type. J. Exp. Psychol. Anim. Behav. Process. 35, 15–22. ( 10.1037/a0012378) [DOI] [PubMed] [Google Scholar]
- 26.Pietras CJ, Locey ML, Hackenberg TD. 2003. Human risky choice under temporal constraints: tests of an energy-budget model. J. Exp. Anal. Behav. 80, 59–75. ( 10.1901/jeab.2003.80-59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. 2012. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol. Ther. 134, 287–297. ( 10.1016/j.pharmthera.2012.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazur JE. 1987. An adjusting procedure for studying delayed reinforcement. In Quantitative analysis of behaviour. The effect of delay and of intervening events on reinforcement value (eds Commons M, Mazur JE, Nevin JA, Rachlin H), pp. 55–73. Sussex, UK: Psychology Press. [Google Scholar]
- 29.Rasmussen E, Lawyer S, Reilly W. 2010. Percent body fat is related to delay and probability discounting for food in humans. Behav. Process. 89, 23–30. ( 10.1016/j.beproc.2009.09.001) [DOI] [PubMed] [Google Scholar]
- 30.Appelhans BM, Waring ME, Schneider KL, Pagoto SL, DeBiasse MA, Whited MC, Lynch EB. 2012. Delay discounting and intake of ready-to-eat and away-from-home foods in overweight and obese women. Appetite 59, 576–584. ( 10.1016/j.appet.2012.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manwaring J, Green L, Myerson J, Strube M, Wilfley D. 2011. Discounting of various types of rewards by women with and without binge eating disorder: evidence for general rather than specific differences. Psychol. Record 21, 561–582. ( 10.1007/BF03395777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stojek MM, MacKillop J. 2017. Relative reinforcing value of food and delayed reward discounting in obesity and disordered eating: a systematic review. Clin. Psychol. Rev. 55, 1–11. ( 10.1016/j.cpr.2017.04.007) [DOI] [PubMed] [Google Scholar]
- 33.Barlow P, Reeves A, McKee M, Galea G, Stuckler D. 2016. Unhealthy diets, obesity and time discounting: a systematic literature review and network analysis. Obes. Rev. 17, 810–819. ( 10.1111/obr.12431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elfhag K, Morey LC. 2008. Personality traits and eating behaviour in the obese: poor self-control in emotional and external eating but personality assets in restrained eating. Eat. Behav. 9, 285–293. ( 10.1016/j.eatbeh.2007.10.003) [DOI] [PubMed] [Google Scholar]
- 35.Jansen A, Nederkoorn C, van Baak L, Keirse C, Guerrieri R, Havermans R. 2009. High-restrained eaters only overeat when they are also impulsive. Behav. Res. Ther. 47, 105–110. ( 10.1016/j.brat.2008.10.016) [DOI] [PubMed] [Google Scholar]
- 36.Rollins BY, Dearing KK, Epstein LH. 2010. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite 55, 420–425. ( 10.1016/j.appet.2010.07.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weller RE, Cook EW III, Avsar KB, Cox JE. 2008. Obese women show greater delay discounting than healthy-weight women. Appetite 51, 563–569. ( 10.1016/j.appet.2008.04.010) [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman AR, Mason A, Rogers PJ, Brunstrom JM. 2017. Obese and overweight individuals are less sensitive to information about meal times in portion size judgements. Int. J. Obes. 42, 905–910. ( 10.1038/ijo.2017.275) [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Manson KF, Schiller D, Levy I. 2014. Impaired associative learning with food rewards in obese women. Curr. Biol. 24, 1731–1736. ( 10.1016/j.cub.2014.05.075) [DOI] [PubMed] [Google Scholar]
- 40.Humby T, Patel Y, Carter J, Stokes L-JG, Rogers RD, Wilkinson LS. 2019. Feeding behaviour, risk-sensitivity and response control: effects of 5-HT2C receptor manipulations. Phil. Trans. R. Soc. B 374, 20180144 ( 10.1098/rstb.2018.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carels RA, Konrad K, Harper J. 2007. Individual differences in food perceptions and calorie estimation: an examination of dieting status, weight, and gender. Appetite 49, 450–458. ( 10.1016/j.appet.2007.02.009) [DOI] [PubMed] [Google Scholar]
- 42.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. 2010. Sex-based differences in the behavioral and neuronal responses to food. Physiol. Behav. 99, 538–543. ( 10.1016/j.physbeh.2010.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warshawsky-Livne L, Novack L, Rosen A, Downs SM, Shkolnik-Inbar J, Pliskin JS. 2014. Gender differences in risk attitudes. In Preference measurement in health (Advances in Health Economics and Health Services Research, vol. 24) (eds Blomquist GC, Bolin K), pp. 123–140. Bingley, UK: Emerald Group Publishing. [PubMed] [Google Scholar]
- 44.Charness G, Gneezy U. 2012. Strong evidence for gender differences in risk taking. J. Econ. Behav. Organ. 83, 50–58. ( 10.1016/j.jebo.2011.06.007) [DOI] [Google Scholar]
- 45.Anbarci N, Arin KP, Okten C, Zenker C. 2016. Is Roger Federer more loss averse than Serena Williams? J. Appl. Econ. 49, 3546–3559. ( 10.1080/00036846.2016.1262527) [DOI] [Google Scholar]
- 46.Blaine B. 2008. Does depression cause obesity? A meta-analysis of longitudinal studies of depression and weight control. J. Health Psychol. 13, 1190–1197. ( 10.1177/1359105308095977) [DOI] [PubMed] [Google Scholar]
- 47.Beck AT, Steer RA, Brown GK. 1996. Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 48.Fairburn C, Beglin S. 1994. Assessment of eating disorders: interview or self-report questionnaire? Int. J. Eat. Disord. 16, 363–370. [PubMed] [Google Scholar]
- 49.Patton J, Stanford M, Barratt E. 1995. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 51, 768–774. ( 10.1002/1097-4679(199511)51:6%3C768::AID-JCLP2270510607%3E3.0.CO;2-1) [DOI] [PubMed] [Google Scholar]
- 50.de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J, Ducimetiere P, Charles MA. 2004. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J. Nutr. 134, 2372–2380. ( 10.1093/jn/134.9.2372) [DOI] [PubMed] [Google Scholar]
- 51.Arthur W, Day D. 1994. Development of a short form for the Raven Progressive Matrices test. Educ. Psychol. Measur. 54, 394–403. ( 10.1177/0013164494054002013) [DOI] [Google Scholar]
- 52.Watson D, Clark LA, Tellegen A. 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. ( 10.1037/0022-3514.54.6.1063) [DOI] [PubMed] [Google Scholar]
- 53.Mehrabian A. 1996. Pleasure-arousal-dominance: a general framework for describing and measuring individual differences. Curr. Psychol. 14, 261–292. ( 10.1007/BF02686918) [DOI] [Google Scholar]
- 54.Fairburn CG, Cooper Z, O'Connor M. 2008. Eating disorder examination. In Cognitive behavior therapy and eating disorders (ed. CG Fairburn) New York, NY: Guilford Press. [Google Scholar]
- 55.Malik VS, Willett WC, Hu FB. 2013. Global obesity: trends, risk factors and policy implications. Nat. Rev. Endocrinol. 9, 13–27. ( 10.1038/nrendo.2012.199) [DOI] [PubMed] [Google Scholar]
- 56.Burton P, Smit HJ, Lightowler HJ. 2007. The influence of restrained and external eating patterns on overeating. Appetite 49, 191–197. ( 10.1016/j.appet.2007.01.007) [DOI] [PubMed] [Google Scholar]
- 57.Schachter S. 1971. Some extraordinary facts about obese humans and rats. Am. Psychol. 26, 129–144. ( 10.1037/h0030817) [DOI] [PubMed] [Google Scholar]
- 58.Polivy J, Herman CP, Coelho JS. 2008. Caloric restriction in the presence of attractive food cues: external cues, eating, and weight. Physiol. Behav. 94, 729–733. ( 10.1016/j.physbeh.2008.04.010) [DOI] [PubMed] [Google Scholar]
- 59.Polivy J, Coleman J, Herman CP. 2005. The effect of deprivation on food cravings and eating behavior in restrained and unrestrained eaters. Int. J. Eat. Disord. 38, 301–309. ( 10.1002/eat.20195) [DOI] [PubMed] [Google Scholar]
- 60.Rouby C. 2002. Olfaction, taste, and cognition. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 61.Fedoroff I, Polivy J, Herman CP. 1997. The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite 28, 33–47. ( 10.1006/appe.1996.0057) [DOI] [PubMed] [Google Scholar]
- 62.Cornell CE, Roddin J, Weingarten H. 1989. Stimulus-induced eating when satiated. Physiol. Behav. 45, 695–704. ( 10.1016/0031-9384(89)90281-3) [DOI] [PubMed] [Google Scholar]
- 63.Mattila AS, Wirtz J. 2001. Congruency of scent and music as a driver of in-store evaluations. J. Retail. 77, 273–289. ( 10.1016/S0022-4359(01)00042-2) [DOI] [Google Scholar]
- 64.Mehrabian A, Russell JA. 1974. An approach to environmental psychology. Cambridge, MA: MIT Press. [Google Scholar]
- 65.Donovan RJ, Rossiter JR, Marcoolyn G, Nesdale A. 1994. Store atmosphere and purchasing behaviour. J. Retail. 70, 283–294. ( 10.1016/0022-4359(94)90037-X) [DOI] [Google Scholar]
- 66.Spence C, Puccinelli NM, Grewal D, Roggeveen AL. 2014. Store atmospherics: a multisensory perspective. Psychol. Mark. 31, 472–488. ( 10.1002/mar.20709) [DOI] [Google Scholar]
- 67.Gibson EL, Desmond E. 1999. Chocolate craving and hunger state: implications for the acquisition and expression of appetite and food choice. Appetite 32, 219–240. ( 10.1006/appe.1998.0207) [DOI] [PubMed] [Google Scholar]
- 68.Donovan RJ, Rossiter JR. 1982. Store atmosphere: an environmental psychology approach. J. Retail. 58, 34–57. [Google Scholar]
- 69.Cardinal RN, Robbins TW, Everitt BJ. 2000. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl.) 152, 362–375. ( 10.1007/s002130000536) [DOI] [PubMed] [Google Scholar]
- 70.Winstanley CA, Dalley JW, Theobald DE, Robbins TW. 2003. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl.) 170, 320–331. ( 10.1007/s00213-003-1546-3) [DOI] [PubMed] [Google Scholar]
- 71.Zeeb FD, Floresco SB, Winstanley CA. 2010. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl.) 211, 87–98. ( 10.1007/s00213-010-1871-2) [DOI] [PubMed] [Google Scholar]
- 72.Stokes L-JG, Davies A, Lattimore P, Winstanley C, Rogers RD. 2019. Data from: Exploring preferences for variable delays over fixed delays to high-value food rewards as a model of food-seeking behaviours in humans Dryad Digital Repository. ( 10.5061/dryad.81hn422) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Stokes L-JG, Davies A, Lattimore P, Winstanley C, Rogers RD. 2019. Data from: Exploring preferences for variable delays over fixed delays to high-value food rewards as a model of food-seeking behaviours in humans Dryad Digital Repository. ( 10.5061/dryad.81hn422) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Primary data available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.81hn422 [72].


