Abstract
Epidemiological data suggest that risk taking in the real world increases from childhood into adolescence and declines into adulthood. However, developmental patterns of behaviour in laboratory assays of risk taking and impulsive choice are inconsistent. In this article, we review a growing literature using behavioural economic approaches to understand developmental changes in risk taking and impulsivity. We present findings that have begun to elucidate both the cognitive and neural processes that contribute to risky and impulsive choice, as well as how age-related changes in these neurocognitive processes give rise to shifts in choice behaviour. We highlight how variability in task parameters can be used to identify specific aspects of decision contexts that may differentially influence risky and impulsive choice behaviour across development.
This article is part of the theme issue ‘Risk taking and impulsive behaviour: fundamental discoveries, theoretical perspectives and clinical implications’.
Keywords: risk taking, impulsivity, development, behavioural economics
1. Introduction
Although even young children are proficient at making many simple value-based choices, children's and adolescents' decision-making differs in important qualitative ways from that of adults [1]. Such developmental differences in decision making are particularly consequential during adolescence, when individuals transition from childhood to adulthood. Although adolescence is both a biological and cultural construct that cannot be strictly defined on the basis of age, this period is thought to begin with the onset of puberty (as early as age 10) and to extend into the third decade of life, when individuals reach cultural milestones such as economic independence or marriage [2]. During adolescence, increased autonomy confers many opportunities to make independent choices. Learning to make self-directed choices is critical for successfully transitioning toward independence from one's parents. The pronounced neurobiological restructuring of the reward system during pubertal maturation [3,4] may drive increased exploratory behaviour as well as facilitate adolescents' ability to learn about positive outcomes in the environment [5,6]. However, adolescents often exhibit a greater propensity toward risky actions that carry potential negative consequences [7,8] and seemingly shortsighted choices that prioritize immediate rewards over longer-term beneficial outcomes [9].
Such risky and impulsive decision making represents one of the greatest perils of adolescence. Criminal behaviour is higher in adolescents than in any other age group [10]. Over half of new sexually transmitted infections diagnosed are in individuals aged 15–24 [11,12]. Mortality rates increase markedly from childhood to adolescence, with about three quarters of deaths attributable to preventable risky or impulsive actions (e.g. reckless driving, suicide; [11]). The propensity to take risks varies substantially across individual adolescents [13]. The peak age for engaging in specific risky or impulsive behaviours also varies [14], in part owing to differential opportunities for risk taking [15]. However, theoretical accounts suggest that an overall increase in shortsighted and risky choices during adolescence, whether advantageous or not, may stem from underlying neural and cognitive changes [15,16]. Although a growing body of literature examines the development of decision making, motivated by a broad relevance to public policy, adolescent health and juvenile justice, extant studies have yet to converge on a consistent account of the mechanisms underlying developmental changes in risky and impulsive choice [17].
Inconsistencies in empirical findings may stem in part from inconsistent definitions of risk and impulsivity. Colloquial ideas about risky and impulsive choice often diverge from formal definitions of these constructs stemming from the field of behavioural economics. The public tends to discuss risks in terms of actions that might lead to negative outcomes. Economists, on the other hand, consider risk taking as the choice of an option with higher variability in possible outcomes [18]. Lay definitions of impulsivity typically refer to difficulty controlling one's impulses (e.g. in purchasing an item), or acting without adequately considering future consequences. In contrast, the field of behavioural economics posits that such behaviours arise from idiosyncratic time preferences for reward receipt, without invoking notions such a self-control failure [19]. While some studies test hypotheses stemming from lay definitions of risk and impulsivity, others employ definitions based in behavioural economics. Varied definitions lead to differences in experimental design and data analysis, which in turn can dictate the patterns of results [20]. In order to arrive at a convergent account of risky and impulsive choice, it is important to try to understand how features of disparate experimental paradigms (e.g. the probabilities and relative values associated with taking a risk) influence the patterns of behaviour that are reported. Such careful examination of extant studies in the field may clarify our understanding of the mechanisms and contextual drivers of risky and impulsive choice.
Many developmental studies of risky decision making have employed tasks that capture features of naturalistic risk taking (e.g. the Stoplight driving task, the Balloon Analogue Risk Task; [21,22]) or situations in which impulse control proves challenging (e.g. antisaccade, stop signal and go/no-go tasks; [23–25]). While behaviour in such tasks often reproduces developmental patterns of real-world risk taking and impulsive choice [22,26–31], a shortcoming of these tasks is that it is often unclear how to quantify the key features of the choice context and the underlying cognitive process that influence naturalistic decision making [20].
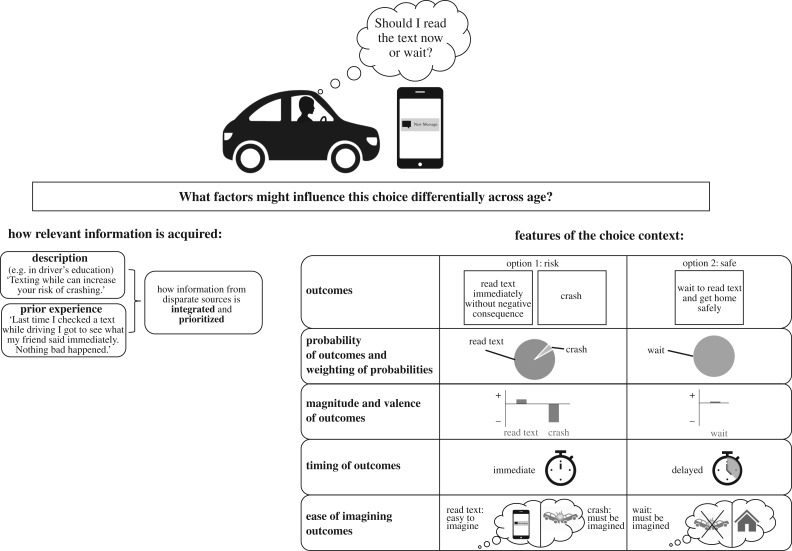
To illustrate the myriad factors that might inform real-world risky choice, consider the example of mobile phone use while driving (figure 1), a behaviour to which we will return periodically below. This behaviour is more common in adolescents than adults, and is problematic because auto accidents (often resulting from distractions) are the leading cause of death in adolescents [32,33]. A teen deciding whether or not to check a text while driving might have acquired information relevant to this choice context in a variety of ways. This teen might have learned statistics in drivers' education class about the dangers of distracted driving, or might have directly experienced the reward of successfully reading a message from a friend without crashing. Information obtained from these explicitly described and experientially learned sources may be differentially weighted. A variety of contextual factors might also influence the teen's choice relative to an adult's, including the magnitude of an immediately available reward (e.g. high reward if the text is from a crush), the relative difficulty of envisioning more distal outcomes (calling to mind the option of returning home safely, not crashing, and then reading the text may be more difficult than imagining immediately reading the text), the relative probability of potential outcomes (the text is likely to be rewarding, a crash is unlikely) and timing of the outcomes (the need to see the rewarding text now, versus getting home safely but delaying receipt of the message). Understanding how these component processes of decision making change over development is essential in learning how best to promote adaptive choice behaviour in adolescence.
Figure 1.
A schematic representing a common naturalistic choice and highlighting contextual features that may differentially influence choice across development.
In recent years, behavioural and neuroscientific studies of risky and impulsive choice have adopted theoretical models, experimental paradigms and analytical frameworks from the fields of behavioural economics that allow risky and impulsive behaviours to be decomposed into relevant cognitive processes [20]. In behavioural economics, risk taking and impulsive action are conceptualized as value-based decisions [34,35]. For instance, a paradigm assessing risk preferences might ask participants to choose between an 80% chance of $10, otherwise $0, versus $8 for sure; while one assessing time preferences might present a choice between $5 immediately versus $15 in three months (we will also return to these examples below). Properties of the decision problems (e.g. the magnitude, valence, probability or delay of the decision outcome) can be varied systematically to isolate the influence of specific aspects of the choice context on decision making.
While the vast majority of behavioural economics research has focused on decision making in adulthood, a burgeoning literature has begun to apply these approaches to the study of developmental changes in risk taking and impulsive behaviour from childhood to adulthood [7,8,14,36,37]. In this article, we review this developmental literature, highlighting patterns of age-related change in risk taking and impulsive choice. We specifically focus on aspects of the decision context in each study that might influence an individual's likelihood of making a risky or impulsive choice, and how this effect might vary across development. To be consistent in our interpretation of studies inspired by varied definitions of risk and impulsivity, we discuss these constructs in terms of behavioural economic definitions, with risk taking defined as choosing the option with highest outcome variability [18] and an impulsive choice defined as choosing the option that will result in a smaller but sooner reward [19]. Importantly, although we acknowledge that social context is a critical factor influencing risky and impulsive choice across development [38], we do not address this topic within the scope of the current review. Further, although we briefly discuss some of the key neural mechanisms that may underpin risk taking and impulsivity across development, we direct readers to other sources for a more comprehensive discussion [17,39,40]. Here, we present findings that have begun to elucidate both the cognitive and neural processes that contribute to risky and impulsive choice, as well as how age-related changes in these neurocognitive processes give rise to shifts in choice behaviour.
2. Decisions under risk
Individuals sometimes make choices based (at least partially) on described information about possible options and the outcomes and probabilities associated with those options (e.g. statistical information about outcomes of distracted driving). ‘Decisions under risk’, a commonly used risk-taking paradigm, are a laboratory model for choices based on described information [41]. Typically, participants in decisions under risk experiments are asked to make a choice between two monetary gambles (e.g. the aforementioned choice between an 80% chance of $10, and 20% chance of $0, versus $8 for sure). Many developmental studies of risk taking employ such paradigms [8,14,37,39]. Developmental versions of these tasks typically present decision problems as ‘wheels of fortune’, where probabilities of each decision outcome are both written and visually depicted to aid younger participants in understanding the task [42]. Developmental patterns in decisions under risk are quite variable [7,8,14]. In studies that are limited to adolescent and adult age groups, over half do not report higher risk taking in adolescents [8]. Studies that also include children sometimes show linear decreases in risk taking from childhood, across adolescence and into adulthood [43–45], while others have demonstrated that risk taking does not vary across this age range [46–50]. Very few studies using decisions under risk paradigms have found results that mirror epidemiological risk patterns, with increases in risk taking from childhood to adolescence and decreasing into adulthood [51,52]. In one of these studies, this inverted-U shaped pattern was limited to choices for gains, not losses [52].
Although the developmental findings in decisions under risk are highly variable, age patterns in risky choice can be better understood when examined in terms of contextual factors that vary across choice problems and paradigms. For example, choices are likely influenced by expected values, or the relative amount one can expect to win or lose from each option (calculated by adding the values of each potential outcome, weighted by their probabilities of occurring). Because computational and probabilistic reasoning abilities increase from childhood into adulthood [53,54], the ability to compute which option has a higher expected value may also increase, which in turn may affect choice patterns across age [14]. Indeed, several studies have shown that older participants are more likely to choose the option with higher expected value, regardless of the riskiness of that option [51,55–59]. However, several studies have not demonstrated age differences in choosing the higher expected-value option [60–62]. Yet another study found that adolescents are more likely than adults to choose the option with higher expected value. In this study, adolescents’ (versus adults’) neural activity in the ventral striatum, an area strongly implicated in valuation [63], also tracked more closely with expected value when rendering a decision [64]. Notably, all of the studies that did not show age-related increases in choosing the option with higher expected value tested only adolescents and adults. Based on the available evidence, it seems plausible that one's ability to identify and choose the option with the higher expected value increases from childhood through adolescence, but does not change considerably from adolescence into adulthood.
While the likelihood of choosing the option with higher expected value typically increases from childhood into adulthood, adults often do not make decisions under risk based on which option has a higher expected value [65]. Rather, adults' choices vary based on a variety of additional contextual factors [66,67]. One such factor is valence, or whether the problem involves gains or losses (or both). Specifically, losses are thought to ‘loom larger’, or sway behaviour more, than gains of the same magnitude [67]. This behaviourally manifests as an increased tendency to take risks to avoid losses than to increase wins, or in greatly reduced risk taking if a risky option involves a possible loss when there is a positive (but potentially smaller) safe outcome available.
How might loss aversion contribute to developmental patterns of risk taking in the real world? To return to the distracted driving example, it is possible that an adolescent decides to check the text message in part resulting from lower loss-aversion relative to adults. In other words, while loss-averse adults might be much less likely to read a text for fear that the distraction would result in a crash, adolescents would be less influenced by this potential loss. Consistent with this idea, results from several studies suggest that children and adolescents do not exhibit loss aversion [57,58,61]. However, other studies have not found evidence of age differences in loss aversion [52,60,68,69]. Yet others suggest that in some cases, younger individuals demonstrate ‘reverse framing’, or increased risk taking in gain compared to loss problems [70,71]. Notably, such reverse framing only occurs when gain or loss magnitude is large, demonstrating that the specific features of the choice environment are likely to substantially influence the patterns observed with respect to loss aversion across age groups.
Another key factor that affects adults' decision making under risk is the probability structure of the risky option. Specifically, if a problem contains infrequent outcomes (i.e. less than 30% likely) adults tend to behave as if they overweight those outcomes when rendering a decision [67]. To return to the monetary gamble example presented above, when choosing between an 80% chance of winning $10 and 20% chance of gaining nothing, versus a sure $8 gain, the majority of adults will choose the sure gain. This pattern results from a general tendency to overrepresent the infrequent outcome (20% chance of $0) in the decision process [67]. This is analogous to the driving example as well—adults may be less likely to check a message on their phone because they tend to overweight the possibility of an unfavourable crash outcome. Critically, whether such overweighting of rare outcomes results in a bias towards choosing the risky or safe option depends on the favourability of the rare outcome. In the case of a 10% chance to win $90 (otherwise $0) versus a sure $9 win, adults more often choose the risky option because the infrequent (10% chance to win $90) option that is overweighted is more favourable than the frequent (90% chance of $0) option. That is, probability weighting biases in decisions under risk result in more risk taking when the infrequent option is favourable, and less risk taking when the infrequent option is unfavourable. Risk taking is context-dependent [72] and rare-outcome weighting patterns are helpful in explaining such context-dependencies in adults' naturalistic risk taking [67,73]. For instance, adults are risk averse in many domains (e.g. buying insurance to avoid a small probability loss) but are risk seeking in the context of unlikely large gains (e.g. buying lottery tickets); both patterns can be explained by overweighting rare outcomes.
Very few developmental studies have explicitly examined probability weighting in developmental samples. However, findings tend to show that children and adolescents underweight (rather than overweight) infrequent outcomes in decisions under risk ([56,74,75]; but see [76]). Consistent with this pattern, an adolescent who checks a text when driving may be underweighting the unlikely crash outcome in rendering a decision. Possibly as a result of such rare outcome underweighting, a recent study found that adolescents perceived a variety of risky behaviours to be ‘less risky’ than did children or adults [77]. Importantly, a simple summary statistic of overall risk taking would fail to capture differential weighting biases across age. In contrast, leveraging computational models of valuation and choice enables the estimation of such decision biases [37]. Future studies testing developmental patterns in decisions under risk that are designed with computational modelling analyses in mind will be helpful in understanding how probability weighting biases develop from childhood into adulthood.
3. Decisions under uncertainty
(a). Decisions under ambiguity
Decisions under uncertainty involve circumstances in which the underlying probabilities and/or values of an option are unknown. In ‘decisions under ambiguity’, participants are told the values that may result from a given choice, but part or all of the probability distribution is unknown. Statistically, individuals should be indifferent between a 50/50 lottery and a fully ambiguous lottery (i.e. a lottery with an unknown probability distribution). However, adults strongly prefer a labelled to an unlabelled lottery, demonstrating aversion to ambiguity [78,79].
Ambiguity likely plays a role in the distracted driving scenario framed above. The teen who checks a text while driving may have an idea of the range of positive and negative outcomes that can result from the behaviour, but the exact probabilities of each outcome are unknown. If the adolescent is more tolerant to ambiguity (i.e. has a more optimistic evaluation of the unknown outcome probabilities) than adults, this may increase the likelihood of checking the text. Until recently, attitudes toward ambiguity had not been studied developmentally. Several studies have now identified patterns of developmental change in ambiguity tolerance, but such patterns are not consistently observed. Tymula and colleagues found that adolescents were less ambiguity-averse than adults in the gain domain [75], but not in the loss domain [69]. Conversely, van den Bos & Hertwig [52] found uniform ambiguity aversion in the gain domain from childhood to adulthood, but in the loss domain adolescents were ambiguity neutral, while children and adults were ambiguity averse. In yet another study Blankenstein and colleagues [46] found linear increases in ambiguity aversion from childhood through adulthood, while a follow-up study using a similar paradigm in participants in a similar age range found no age differences in ambiguity attitude [47]. Other studies have shown that younger children (ages 5 and 8) are ambiguity-neutral [80,81]. Given the lack of consistency in these results, future research will be required to better understand what factors might underlie these disparate patterns of age-related choice behaviour.
(b). Decisions from experience
‘Decisions from experience’, another type of decision from uncertainty, require individuals to make repeated draws from unmarked lotteries to learn about their underlying probability distributions. A participant may, for instance, draw from one lottery several times and observe the following sequence of outcomes: $10, $10, $0, $10, $10. Another lottery would yield $8 on every draw. Cumulatively, feedback from multiple draws provide similar information to that provided in the risk example above, with the major difference between risk and experienced uncertainty being the way in which information was acquired (descriptions or direct experience). Research on the ‘description–experience gap’ directly contrasts choice patterns often observed in decisions under described risk with those seen in decisions under experienced uncertainty [82]. While adults tend to overweight infrequent outcomes in description-based decisions under risk, as described above, they often show the opposite choice pattern in experience-based decisions under uncertainty: they make choices consistent with an underweighting of rare outcomes [18,41,83].
The contrast between described risk and experienced uncertainty may also be important in disentangling when adolescents do and do not take more risks than children and adults [8]. After all, it is likely that outcomes of prior experience in similar situations directly influence subsequent choices. The adolescent who chooses to read a text message while driving is likely to have read a text while driving in the past. Therefore, decisions under uncertainty paradigms that require learning from experience may be a better model for risk taking in the real world, and behaviour observed in these paradigms may better approximate many real-world behaviours. Further, the problematic risks that adolescents often take in the real world involve rare unfavourable outcomes (e.g. the rare likelihood of a car accident), so a pronounced pattern of underweighting rare unfavourable outcomes might be a contributing factor underlying adolescent risk behaviour. Consistent with this idea, and in contrast to the highly variable results observed in developmental studies of decisions under risk, almost all studies of adolescent risk taking that use experience-based uncertainty paradigms evince heightened risk taking in adolescents relative to adults [8]. Further, the first developmental study to systematically test decision making under both risk and uncertainty found that when rare outcomes are unfavourable, adolescents' choices are consistent with increased underweighting of rare outcomes in uncertainty relative to risk problems, a pattern that was not observed in children or adults [52]. Therefore, in understanding real-world risk in adolescence, it may be useful to further examine behaviour within these experience-based assays of decisions under uncertainty.
The majority of studies that explore developmental trends in experiential risk taking employ variants of the Iowa Gambling Task (IGT) [84]. In the IGT, participants make repeated choices between four decks of cards. Two of the decks are ‘good’, always providing small gains paired with either frequent (50%) or infrequent (10%) small losses, but importantly, resulting in net gains when chosen repeatedly. The other two decks are ‘bad’, always resulting in a slightly larger gain (relative to the good decks), but also coinciding with frequent (50%) or infrequent (10%) larger losses, resulting in a net loss when chosen repeatedly. IGT studies consistently demonstrate age-related increases in choosing good relative to bad decks from childhood through adulthood [55,85–93]. The ability to learn which decks are good is thought to rely on the ventromedial prefrontal cortex (PFC) ([84]; but see [94]), an area that is often implicated in reward valuation [64], and that is increasingly recruited from adolescence to adulthood as IGT performance improves [87].
Given evidence of age-related differences in experience-based paradigms, an important question is how the integration and evaluation of experienced positive and negative outcomes might change across development. The computational field of reinforcement learning has defined algorithms formalizing how the value of an action can be learned experientially through trial-and-error [95]. Central to this learning process is a prediction-error signal, reflecting the degree to which an outcome is better or worse than one's initial expectation. The activity of dopaminergic neurons, projecting from the midbrain to the ventral striatum, is thought to approximate such a prediction-error signal [96]. Some studies have suggested that prediction-error related ventral striatal responses are heightened in adolescents [97,98], however this finding has not been consistently replicated. Other studies have observed age differences in ventral striatal connectivity during reinforcement learning [99,100], suggesting that age differences in ventral striatal signals likely reflect differences in reward learning computations across a more extensive network of brain areas [101].
After receiving feedback about an action, reward prediction error signals can be used to revise, upward or downward, one's estimated value of the action. In reinforcement-learning models, the extent to which this value estimate is altered depends on one's learning rate. Higher learning rates give an increased weighting to a recent outcome, leading to large changes in value, whereas lower learning rates result in a small adjustment. Several recent studies have found that models containing separate learning rates for positive and negative prediction errors provide a better characterization of choice behaviour than models with a single learning rate [87,100,102]. Critically, the introduction of two learning rates enables the model to capture asymmetries in the influence of previous positive and negative outcomes on one's choices. Studies examining developmental differences in positive and negative learning rates have observed valence-dependent asymmetries that differ by age [87,100,103,104]. While the age-related patterns of asymmetry observed vary across studies, any interpretation of these findings must also take into consideration how specific weightings of positive and negative feedback might produce better performance given the reinforcement statistics of a given task. The application of reinforcement-learning models in future studies that explore developmental change in responses to the structure of the choice environment will be useful to better characterize how age-related changes in feedback-based learning influence experiential decisions from uncertainty.
Interestingly, theoretical accounts of adolescent risk taking implicate the same ventral striatal circuitry thought to be involved in reinforcement learning. Specifically, models of adolescent risky choice suggest that heightened ventral striatum activation may drive adolescent reward seeking, resulting in increased risk taking [15,105]. Empirical tests of age differences in neural responses during risky choice have inconsistently yielded such ventral striatum hyperresponsivity [17]. However, in understanding variability across studies, it is important to consider the tasks used to index these responses, and the stage of the task (e.g. anticipation, feedback) at which responses are assessed [64]. Studies in which participants experience repeated rewards or rewarding feedback (e.g. reinforcement-learning or experiential-choice tasks) typically demonstrate some degree of adolescent ventral striatal hyperresponsivity [45,97,106–108]. While some studies have demonstrated lower ventral striatal responses in adolescents relative to adults [26,109,110], these studies typically measure responsivity during predictable reward anticipation, which does not involve learning. Further, despite the absence of a learning component in these tasks, adolescent ventral striatal responses were similar to [109,110] or increased [26] relative to adults' after successfully receiving a reward. These findings suggest that adolescent ventral striatal hyperactivation may be most pronounced when receiving reward feedback. Future neuroimaging studies using paradigms that are amenable to computational modelling are needed to elucidate the nature of these developmental differences in ventral striatal reward processing. Moreover, these studies should examine how the adolescent ventral striatum interacts with a broader corticostriatal and subcortical (e.g. hippocampal–striatal) circuitry to influence decision making from experience.
In real-world decision making, individuals often acquire information about a choice through both descriptions and experience. For instance, the adolescent deciding whether to read a text while driving may have learned about the dangers about this behaviour in a course, and may have also engaged in the behaviour in the past. How information from these disparate sources is integrated and prioritized can bias the decision. In paradigms that present both described and experienced information, adults readily integrate both sources of information ([111–114]; but see [115]). Children's and adolescents' learning, on the other hand, is more strongly informed by prior experience [93,116], which may contribute to heightened risk taking when rewarding outcomes are frequent and negative outcomes are rarely experienced ([49] but see [117]). For instance, if the adolescent deciding whether to check a text has read messages from friends while driving in the past, the rewarding result may bias the teen toward repeating the behaviour in the future, without consideration of descriptive warnings about the possible dangers.
4. Decisions over time
The term ‘impulsivity’ is commonly used to refer to actions that are premature in their execution, or reflect a lack of foresight [118]. This definition encompasses a range of behaviours including a tendency to prioritize near-term rewards over higher-valued delayed outcomes, failure to wait for delayed rewards, acting without consideration of the consequences of one's actions, and failure to inhibit inappropriate responses. While often grouped under the same heading, experimental studies of impulsivity suggest that these measures do not reflect a single underlying construct [119,120]. Convergent evidence in adult humans and animal models has dissociated, both behaviourally and neurally, measures of motor-response inhibition (e.g. antisaccade, stop signal and go/no-go tasks) from measures derived from choice behaviour, suggesting that these measures reflect different forms of impulsivity [118,121]. Studies examining developmental changes in impulsivity across each of these behavioural domains have observed pronounced patterns of age-related change [9,122]. However, while some developmental studies have corroborated this dissociation between measures of choice impulsivity and motor response inhibition [123], others have observed correlation between these measures [124,125]. One possible interpretation of this inconsistency is that these forms of impulsivity become more differentiated with age, however this remains an open question best answered through future longitudinal studies. In this review, we focus on the development of impulsivity as it relates to value-based choice behaviour, leveraging insights that can be derived through behavioural-economic studies of intertemporal choice. We present findings that reveal developmental changes in this assay of impulsive-choice behaviour in humans and discuss the cognitive and neural mechanisms that might underlie these age-related changes.
In intertemporal choice paradigms, participants make choices between smaller rewards that are available sooner and larger rewards that are only available at a future point in time (e.g. $5 now or $15 in three months). Economic models propose that such intertemporal choices are made by comparing the subjective value of an immediate reward with that of a delayed reward, which is diminished, or ‘discounted’, by the length of time we must wait for it. Choice behaviour in these tasks can be fit to mathematical models that represent hypothesized cognitive processes that lead to discounting behaviour. These models can be used to derive precise estimates of an individual's temporal discounting rate. Such experimental measures of temporal discounting appear to have construct validity for real-world impulsive behaviours, predicting a range of measures that reflect prioritization of delayed rewards, including academic performance [126], drug abuse [127], creditworthiness [128], diet and exercise habits [129] and marital fidelity [130].
Convergent findings across developmental studies of intertemporal choice suggest that discount rates decline linearly with age from childhood into adolescence, reflecting increasing patience, reaching an asymptote in early adulthood [123,131–134]. Changes in functional dynamics and white matter connectivity, particularly within and between the striatum and PFC, appear to play a central underlying role in this gradual increase in patient choice [123,125,131,133]. Age-related increases in patient choice across childhood have been associated with greater connectivity between the ventromedial and dorsolateral PFC regions [125]. From early adolescence into young adulthood, increased strength of tracts connecting the dorsolateral PFC and the striatum predicts decreases in discounting [123]. In adults, ventromedial PFC activity has been found to track subjective value, both during intertemporal choice [19,135], as well as in other value-based learning and decision making tasks [64], while dorsolateral regions are broadly implicated in the deployment of cognitive control processes that support goal-directed decision making [136]. The increased integration between these prefrontal regions and the striatum—a region centrally implicated in reward processing and action selection [137]—may reflect the deployment of these cognitive-control processes in a manner that increases the subjective value of delayed rewards.
In younger children, impulsive choice has been assayed using a delay-of-gratification task [138] that involves actually waiting for a delayed reward, as opposed to choosing between rewards that are available at different timepoints. In this task, one faces a reward that is immediately available (e.g. one marshmallow), with the prospect of obtaining a larger reward (e.g. two marshmallows) if one is willing to forego the immediate reward and wait for a longer duration of time. In this task, children exhibit an increasing tendency to wait for a larger reward at older ages [139,140]. As in discounting tasks, choice behaviour in the delay of gratification task appears to have construct validity for real-world choices. Preschoolers' choices in the task have been found to predict academic performance in adolescence [138,141], and relate to differences in frontostriatal recruitment in adulthood [142]. While such findings suggest the presence of early-developing trait-like impulsivity phenotypes, studies in children have also shown that willingness to wait in the task is sensitive to contextual features such as the reliability of the individual controlling reward delivery [143]. This is consistent with a theoretical proposal in the animal behavioural-ecology literature that ‘collection risk’, or the level of uncertainty that a delayed reward will actually be delivered, may promote impulsive intertemporal choices [144]. This notion also suggests a potential developmental mechanism underlying the association between steeper temporal discounting and poverty [145], which may impose greater collection risks for delayed rewards stemming from increased environmental instability.
Further evidence of the adaptability of discount rates comes from research highlighting how cognitive factors and aspects of the choice context can alter intertemporal choices [146]. One such factor is the degree to which individuals vividly simulate the future when making intertemporal choices [147]. Recent work in adults suggests that when individuals are cued to situate intertemporal choices in the context of personal events scheduled to occur in the future (e.g. ‘Would you prefer $5 now, or $40 in 6 months during your vacation in Florida?’), their discount rates are reduced [148,149]. The act of calling to mind this future timepoint, and the potential utility of a larger reward in that context, appears to increase the subjective value of the delayed reward. Moreover, adults' self-reported simulation vividness, as well as the strength of functional coupling between the PFC and the hippocampus—a region implicated in episodic memory and prospective simulation [150,151]—predict the degree to which episodic simulation alters discount rates [148,149]. Collectively, this work suggests that individuals who spontaneously engage in such future-directed mentalizing behaviour might exhibit more patient choices (but see [152]) and that age-related changes in episodic simulation might contribute to developmental differences in intertemporal choice.
A large literature suggests that future-directed thinking and episodic simulation of the future exhibit a protracted developmental trajectory [153,154]. Self-reported future-oriented cognition increases linearly with age from childhood to adulthood [154] and adolescents report more event details for simulated future events than children, suggesting an increase in the vividness of episodic future simulation with age [155]. Consistent with a role for future simulation in patient intertemporal choices, adolescents who report a greater tendency to imagine future events vividly [156] and to engage in future-oriented thinking [123,154] also exhibit less temporal discounting. Importantly, while children and adolescents may be less likely than adults to engage in spontaneous cognition about future time points and may experience less vivid simulations, when cued to make intertemporal choices in the context of planned personal future events, they also exhibit decreased discount rates for future rewards situated at those timepoints [157,158]. These findings suggest that children and adolescents can richly simulate the future when cued to do so. However, a decreased tendency to engage in spontaneous future simulation, potentially reflecting the protracted development of PFC–hippocampal functional connectivity [159,160], may contribute to increased impulsive choice at younger ages. It is possible that adolescents' decreased ventral striatal activity during reward anticipation [26,109,110], as discussed previously, might also reflect a decreased tendency to spontaneously simulate future events.
While intertemporal-choice paradigms are widely used assays of impulsive behaviour, the task fails to capture an important aspect of typical real-world intertemporal choices. Whereas the task explicitly specifies the availability of a choice between a smaller sooner and larger later option, in real-world situations one often is faced with an immediately rewarding option, but must spontaneously call to mind an alternative course of action that might have a higher future value. Consider the example of an adolescent deciding whether to read a text message while driving. The prospect of reading the message presents a tangible potential immediate reward. However, the potential ‘larger later’ reward of reading the text after arriving safely at one's destination, coupled with the large relative value of a decreased crash likelihood, must be spontaneously mentally generated to be evaluated in comparison with this near-term rewarding outcome. Thus, cognitive processes including the spontaneous retrieval of future events, causal learning of the relationship between actions and delayed rewards or negative outcomes [161], counterfactual reasoning about the potential future consequences of unchosen actions [162,163], and deliberative evaluation processes [164,165] are likely to be important components of real-world intertemporal choices.
Developmental improvements in these cognitive processes may contribute to age-related decreases in impulsive choice. Prominent models of decision making distinguish two types of evaluative processes that can inform one's choices [164,165]. A ‘model-based’ evaluation process relies on an individual's cognitive model or ‘map’ of the causal relationships between actions and outcomes that apply in a given context. This mental model can be consulted to evaluate candidate actions and their potential consequences, and select and an action most likely to yield a desired outcome. In contrast, ‘model-free’ learning uses the positive and negative outcomes of past actions as reinforcing feedback that can respectively strengthen or weaken one's propensity to reflexively perform that action again in the future (i.e. the previous rewarding experiences of checking a text message while driving will increase the estimated ‘value’ of text message checking). When considering a choice between two actions, model-based evaluation is proposed to depend on a prospective-simulation process [166], through which potential future actions and outcomes are ‘sampled’ and evaluated [167,168]. While it is unclear to what extent this sort of prospective simulation involves vivid mental imagery of future events, greater reliance on model-based evaluation might decrease impulsive decision making though spontaneous engagement of a simulation process that augments the value of future outcomes [169,170]. Across development, in tasks designed to dissociate reliance on model-based and model-free evaluation strategies, the recruitment of model-based evaluation has been found to increase linearly from childhood into adulthood [116,171], whereas model-free evaluation exhibits developmental invariance. Thus, gradual increases in the reliance on deliberative action-selection strategies may contribute to age-related reductions in impulsive choice by promoting the engagement of future simulation.
Collectively, a convergent literature suggests that with age, individuals exhibit a greater prioritization of delayed higher-value outcomes over near-term rewards and these changes in intertemporal choice preferences appear to have construct validity for real-world impulsive choices. Intertemporal choice preferences in adulthood are highly variable across individuals [172], reflecting both genetic variability [173], as well as sensitivity to many aspects of the choice context [146]. An important question for future longitudinal studies will be to understand how such factors interact over the course of development to shape individual differences in intertemporal choice preferences, as well as whether the malleability of such preferences changes over development, suggesting the presence of ‘sensitive periods’ for altering impulsive-choice behaviour.
5. Conclusion
Here, we reviewed a nascent developmental literature using experimental paradigms and analytical approaches from behavioural economics to understand risky and impulsive decision making. Importantly, these findings suggest that specific aspects of decision contexts exert dissociable effects on choice behaviour. Specifically, decision parameters such as the probability, magnitude and valence of outcomes, and whether information about these outcomes is derived through experience or explicitly conveyed, can influence risky choices in a way that may vary as a function of age. Similarly, aspects of intertemporal choice contexts, such as whether intertemporal choices need to be spontaneously represented or are explicitly presented, or whether delayed rewards are situated in the context of future personal events can shift intertemporal-choice preferences, and may differentially affect impulsive choice across age.
To refine our understanding of the developmental differences in risky and impulsive choice evident in real-world epidemiological statistics, future laboratory studies must aim to better identify and isolate the specific task parameters that elicit these behaviours. To date, very few studies have examined developmental changes in decision making using the formal approaches discussed here. Given the lack of consistency in the extant findings, it will be important going forward for researchers to explicitly report the task parameters used in their studies in order to systematically understand their influence on behaviour. Additionally, future work can concurrently use physiological and neuroimaging techniques, as well as experimental manipulations, to gain a better understanding of how factors such as emotional states or cognitive abilities influence choice behaviour differentially across development. Sharing raw data will also facilitate meta-analysis of study results to clearly isolate contextual influences on risky and impulsive choice.
To understand the complexity of decision making in naturalistic choice contexts, it will also be critical for future studies to consider how the myriad factors that influence risky and impulsive-choice behaviour interact. The teen deciding whether to check a text while driving likely has acquired both described information and limited direct experience concerning the potential costs and benefits associated with this action. As in many contexts in which adolescents take risks, prior encounters with this choice context are likely to have involved frequent rewards, but infrequent negative consequences, thus requiring foresight and simulation to bring to mind potential negative consequences. Consideration of the contextual factors that are likely to play a role in specific real-world choice contexts may also help to distinguish between environments in which adolescents are likely to make adult-like choices (e.g. contexts in which future outcomes are easy to call to mind, or potential rewarding outcomes are infrequent) and those in which they may be vulnerable to increased risky and impulsive choice [174].
Ultimately, this improved understanding of both the specific contextual factors and underlying mechanisms that modulate risk taking and impulsive choice across different stages of development can be leveraged to help teens in the real world. For instance, it may be possible to steer adolescents toward taking positive, prosocial risks (e.g. auditioning for a play or running for student government; [5]) with a similar probability and reward structure, rather than risks that are illegal or that can lead to dire consequences. Moreover, providing opportunities to engage in future simulation (e.g. opportunities for reflection on long-term goals) may improve adolescents' ability to make less impulsive choices. Such interventions based in behavioural economics may be effective in preventing problematic manifestations of risky and impulsive choices in adolescence (e.g. problem gambling [175]; substance abuse [176]).
Data accessibility
This article has no additional data.
Authors' contributions
G.M.R. and C.A.H. drafted and revised the manuscript.
Competing interests
We have no competing interests.
Funding
C.A.H. is supported by a Klingenstein-Simons Fellowship Award, National Science Foundation CAREER grant no. 1654393, a Brain and Behavior Research Foundation Young Investigator Grant, and a Jacobs Foundation Early Career Fellowship. G.M.R. is supported by an F32 DA047047-01 from the National Institute on Drug Abuse.
References
- 1.Jacobs JE, Klaczynski PA. 2002. The development of judgment and decision making during childhood and adolescence. Curr. Dir. Psychol. Sci. 11, 145–149. ( 10.1111/1467-8721.00188) [DOI] [Google Scholar]
- 2.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. 2018. The age of adolescence. Lancet Child Adolesc. Health 2, 223–228. ( 10.1016/S2352-4642(18)30022-1) [DOI] [PubMed] [Google Scholar]
- 3.Spear LP. 2000. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463. ( 10.1016/S0149-7634(00)00014-2) [DOI] [PubMed] [Google Scholar]
- 4.Urošević S, Collins P, Muetzel R, Lim K, Luciana M.. 2012. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev. Psychol. 48, 1488 ( 10.1037/a0027502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duell N, Steinberg L. 2018. Positive risk taking in adolescence. Child Dev. Perspect. 66, 93 ( 10.1111/cdep.12310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romer D, Reyna VF, Satterthwaite TD. 2017. Beyond stereotypes of adolescent risk taking: placing the adolescent brain in developmental context. Dev. Cogn. Neurosci. 27, 19–34. ( 10.1016/j.dcn.2017.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Defoe IN, Dubas JS, Figner B, Van Aken MAG. 2015. A meta-analysis on age differences in risky decision making: adolescents versus children and adults. Psychol. Bull. 141, 29 ( 10.1037/a0038088) [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum GM, Venkatraman V, Steinberg L, Chein JM. 2017. The influences of described and experienced information on adolescent risky decision-making. Dev. Rev. 47, 23–43. ( 10.1016/j.dr.2017.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green L, Myerson J, Ostaszewski P. 1999. Discounting of delayed rewards across the life span: age differences in individual discounting functions. Behav. Processes 46, 89–96. ( 10.1016/S0376-6357(99)00021-2) [DOI] [PubMed] [Google Scholar]
- 10.Steinberg L. 2013. The influence of neuroscience on US Supreme Court decisions about adolescents’ criminal culpability. Nat. Rev. Neurosci. 14, 513–518. ( 10.1038/nrn3509) [DOI] [PubMed] [Google Scholar]
- 11.Kann L, et al. 2018. Youth risk behavior surveillance—United States, 2017. MMWR Surveill. Summ. 67, 1–114. ( 10.15585/mmwr.mm6701a1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satterwhite CL, et al. 2013. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex. Transm. Dis. 40, 187–193. ( 10.1097/OLQ.0b013e318286bb53) [DOI] [PubMed] [Google Scholar]
- 13.Bjork JM, Pardini DA. 2014. Who are those ‘risk-taking adolescents’? Individual differences in developmental neuroimaging research. Dev. Cogn. Neurosci. 11, 56–64. ( 10.1016/j.dcn.2014.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li R. 2017. Flexing dual-systems models: how variable cognitive control in children informs our understanding of risk-taking across development. Dev. Cogn. Neurosci. 27, 91–98. ( 10.1016/j.dcn.2017.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L. 2016. The dual systems model: review, reappraisal, and reaffirmation. Dev. Cogn. Neurosci. 17, 103–117. ( 10.1016/j.dcn.2015.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey BJ. 2015. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev. Psychol. 66, 295–319. ( 10.1146/annurev-psych-010814-015156) [DOI] [PubMed] [Google Scholar]
- 17.Sherman L, Steinberg L, Chein J. 2017. Connecting brain responsivity and real-world risk taking: strengths and limitations of current methodological approaches. Dev. Cogn. Neurosci. 33, 27–41. ( 10.1016/j.dcn.2017.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber EU, Shafir S, Blais A.-R. 2004. Predicting risk sensitivity in humans and lower animals: risk as variance or coefficient of variation. Psychol. Rev. 111, 430–445. ( 10.1037/0033-295X.111.2.430) [DOI] [PubMed] [Google Scholar]
- 19.Kable J, Glimcher P. 2007. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 10, 1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schonberg T, Fox CR, Poldrack RA. 2011. Mind the gap: bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn. Sci. 15, 11–19. ( 10.1016/j.tics.2010.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lejuez CW, Aklin W, Daughters S, Zvolensky M, Kahler C, Gwadz M. 2007. Reliability and validity of the youth version of the Balloon Analogue Risk Task (BART-Y) in the assessment of risk-taking behavior among inner-city adolescents. J. Clin. Child Adolesc. Psychol. 36, 106–111. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. 2008. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev. Psychol. 44, 1764–1778. ( 10.1037/a0012955) [DOI] [PubMed] [Google Scholar]
- 23.Casey BJ, et al. 1997. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J. Cogn. Neurosci. 9, 835–847. ( 10.1162/jocn.1997.9.6.835) [DOI] [PubMed] [Google Scholar]
- 24.Logan GD, Schachar RJ, Tannock R. 1997. Impulsivity and inhibitory control. Psychol. Sci. 8, 60–64. ( 10.1111/j.1467-9280.1997.tb00545.x) [DOI] [Google Scholar]
- 25.Luna B, et al. 2001. Maturation of widely distributed brain function subserves cognitive development. Neuroimage 13, 786–793. ( 10.1006/nimg.2000.0743) [DOI] [PubMed] [Google Scholar]
- 26.Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. 2010. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex 20, 1613–1629. ( 10.1093/cercor/bhp225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim-Spoon J, Kahn R, Deater-Deckard K, Chiu PH, Steinberg L, King-Casas B. 2015. Risky decision making in a laboratory driving task is associated with health risk behaviors during late adolescence but not adulthood. Int. J. Behav. Dev. 10, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lydon-Staley DM, Geier CF. 2017. Age-varying associations between cigarette smoking, sensation seeking, and impulse control through adolescence and young adulthood. J. Res. Adolesc. 28, 354–367. ( 10.1111/jora.12335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacPherson L, Magidson JF, Reynolds EK, Kahler CW, Lejuez CW. 2010. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcohol. Clin. Exp. Res. 34, 1400–1408. ( 10.1111/j.1530-0277.2010.01223.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somerville LH, Hare T, Casey BJ. 2012. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 23, 2123–2134. ( 10.1162/jocn.2010.21572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinbeis N, Crone EA. 2016. The link between cognitive control and decision-making across child and adolescent development. Curr. Opin. Behav. Sci. 10, 28–32. ( 10.1016/j.cobeha.2016.04.009) [DOI] [Google Scholar]
- 32.National Center for Statistics and Analysis 2018 2017 fatal motor vehicle crashes: overview. (Traffic safety facts research note. Report No. DOT HS 812 603) Washington, DC: National Highway Traffic Safety Administration; (https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/812603) [Google Scholar]
- 33.Stavrinos D, Pope CN, Shen J, Schwebel DC. 2017. Distracted walking, bicycling, and driving: systematic review and meta-analysis of mobile technology and youth crash risk. Child Dev. 89, 118–128. ( 10.1111/cdev.12827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glimcher PW. 2004. Decisions, uncertainty, and the brain: the science of neuroeconomics. Cambridge, MA: MIT Press. [Google Scholar]
- 35.Rangel A, Camerer C, Montague PR. 2008. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 9, 545–556. ( 10.1038/nrn2357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartley CA, Somerville LH. 2015. The neuroscience of adolescent decision-making. Curr. Opin. Behav. Sci. 5, 108–115. ( 10.1016/j.cobeha.2015.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Bos W, Bruckner R, Nassar MR, Mata R, Eppinger B. 2017. Computational neuroscience across the lifespan: promises and pitfalls. Dev. Cogn. Neurosci. 33, 42–53. ( 10.1016/j.dcn.2017.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blakemore S.-J. 2018. Avoiding social risk in adolescence. Curr. Dir. Psychol. Sci. 27, 116–122. ( 10.1177/0963721417738144) [DOI] [Google Scholar]
- 39.Crone EA, Van Duijvenvoorde ACK, Peper JS. 2016. Annual research review: neural contributions to risk-taking in adolescence—developmental changes and individual differences. J. Child Psychol. Psychiatry 57, 353–368. ( 10.1111/jcpp.12502) [DOI] [PubMed] [Google Scholar]
- 40.van Duijvenvoorde ACK, Peters S, Braams BR, Crone EA. 2016. What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neurosci. Biobehav. Rev. 70, 135–147. ( 10.1016/j.neubiorev.2016.06.037) [DOI] [PubMed] [Google Scholar]
- 41.Hertwig R, Barron G, Weber EU, Erev I. 2004. Decisions from experience and the effect of rare events in risky choice. Psychol. Sci. 15, 534–539. ( 10.1111/j.0956-7976.2004.00715.x) [DOI] [PubMed] [Google Scholar]
- 42.Ernst M, et al. 2004. Choice selection and reward anticipation: an fMRI study. Neuropsychologia 42, 1585–1597. ( 10.1016/j.neuropsychologia.2004.05.011) [DOI] [PubMed] [Google Scholar]
- 43.Paulsen DJ, Carter RM, Platt ML, Huettel SA, Brannon EM. 2012. Neurocognitive development of risk aversion from early childhood to adulthood. Front. Hum. Neurosci. 5, 1–17. ( 10.3389/fnhum.2011.00178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulsen DJ, Platt ML, Huettel SA, Brannon EM. 2011. Decision-making under risk in children, adolescents, and young adults. Front. Psychol. 2, 72 ( 10.3389/fpsyg.2011.00072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Leijenhorst L, Gunther Moor B, de Macks ZA Op, Rombouts SARB, Westenberg PM, Crone EA. 2010. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage 51, 345–355. ( 10.1016/j.neuroimage.2010.02.038) [DOI] [PubMed] [Google Scholar]
- 46.Blankenstein NE, Crone EA, van den Bos W, van Duijvenvoorde ACK. 2016. Dealing with uncertainty: testing risk- and ambiguity-attitude across adolescence. Dev. Neuropsychol. 41, 77–92. ( 10.1080/87565641.2016.1158265) [DOI] [PubMed] [Google Scholar]
- 47.Blankenstein NE, Schreuders E, Peper JS, Crone EA, van Duijvenvoorde AC. K. 2018. Individual differences in risk-taking tendencies modulate the neural processing of risky and ambiguous decision-making in adolescence. Neuroimage 172, 663–673. ( 10.1016/j.neuroimage.2018.01.085) [DOI] [PubMed] [Google Scholar]
- 48.Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. 2007. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia 45, 1270–1279. ( 10.1016/j.neuropsychologia.2006.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Figner B, Mackinlay RJ, Wilkening F, Weber EU. 2009. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. J. Exp. Psychol. Learn. Mem. Cogn. 35, 709–730. ( 10.1037/a0014983) [DOI] [PubMed] [Google Scholar]
- 50.Van Leijenhorst L, Westenberg PM, Crone EA. 2008. A developmental study of risky decisions on the cake gambling task: age and gender analyses of probability estimation and reward evaluation. Dev. Neuropsychol. 33, 179–196. ( 10.1080/87565640701884287) [DOI] [PubMed] [Google Scholar]
- 51.Burnett S, Bault N, Coricelli G, Blakemore SJ. 2010. Adolescents’ heightened risk-seeking in a probabilistic gambling task. Cogn. Dev. 25, 183–196. ( 10.1016/j.cogdev.2009.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Bos W, Hertwig R. 2017. Adolescents display distinctive tolerance to ambiguity and to uncertainty during risky decision making. Sci. Rep. 7, 40962 ( 10.1038/srep40962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donati MA, Panno A, Chiesi F, Primi C. 2014. A mediation model to explain decision making under conditions of risk among adolescents: the role of fluid intelligence and probabilistic reasoning. J. Clin. Exp. Neuropsychol. 36, 588–595. ( 10.1080/13803395.2014.918091) [DOI] [PubMed] [Google Scholar]
- 54.Schlottmann A, Anderson NH. 1994. Children's judgments of expected value. Dev. Psychol. 30, 56 ( 10.1037/0012-1649.30.1.56) [DOI] [Google Scholar]
- 55.Crone EA, van der Molen MW. 2004. Developmental changes in real life decision making: performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Dev. Neuropsychol. 25, 251–279. ( 10.1207/s15326942dn2503_2) [DOI] [PubMed] [Google Scholar]
- 56.Harbaugh WT, Krause K, Vesterlund L. 2002. Risk attitudes of children and adults: choices over small and large probability gains and losses. Exp. Econ. 5, 53–84. ( 10.1023/A:1016316725855) [DOI] [Google Scholar]
- 57.Levin IP, Bossard EA, Gaeth GJ, Yan H. 2014. The combined role of task, child's age and individual differences in understanding decision processes. Judgm. Decis. Mak. 9, 274–286. [Google Scholar]
- 58.Levin I, Weller J, Pederson A, Harshman L. 2007. Age-related differences in adaptive decision making: sensitivity to expected value in risky choice. Retrieved from http://scholarsbank.uoregon.edu/xmlui/handle/1794/22039.
- 59.Weller JA, Levin IP, Denburg NL. 2011. Trajectory of risky decision making for potential gains and losses from ages 5 to 85. J. Behav. Decis. Mak. 24, 331–344. ( 10.1002/bdm.690) [DOI] [Google Scholar]
- 60.Barkley-Levenson EE, Van Leijenhorst L, Galván A. 2013. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Dev. Cogn. Neurosci. 3, 72–83. ( 10.1016/j.dcn.2012.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galván A, McGlennen KM. 2012. Daily stress increases risky decision-making in adolescents: a preliminary study. Dev. Psychobiol. 54, 433–440. ( 10.1002/dev.20602) [DOI] [PubMed] [Google Scholar]
- 62.Keulers EHH, Stiers P, Jolles J. 2011. Developmental changes between ages 13 and 21 years in the extent and magnitude of the BOLD response during decision making. Neuroimage 54, 1442–1454. ( 10.1016/j.neuroimage.2010.08.059) [DOI] [PubMed] [Google Scholar]
- 63.Bartra O, McGuire JT, Kable JW. 2013. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76, 412–427. ( 10.1016/j.neuroimage.2013.02.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barkley-Levenson EE, Galván A. 2014. Neural representation of expected value in the adolescent brain. Proc. Natl Acad. Sci. USA 111, 1646–1651. ( 10.1073/pnas.1319762111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon HA. 1955. A behavioral model of rational choice. Q. J. Econ. 69, 99–118. ( 10.2307/1884852) [DOI] [Google Scholar]
- 66.Kahneman D, Tversky A. 1979. Prospect theory: an analysis of decision under risk. Econometrica 47, 263–292. ( 10.2307/1914185) [DOI] [Google Scholar]
- 67.Tversky A, Kahneman D. 1992. Advances in prospect theory: cumulative representation of uncertainty. J. Risk Uncertain. 5, 297–323. ( 10.1007/BF00122574) [DOI] [Google Scholar]
- 68.Levin IP, Hart SS. 2003. Risk preferences in young children: early evidence of individual differences in reaction to potential gains and losses. J. Behav. Decis. Mak. 16, 397–413. ( 10.1002/bdm.453) [DOI] [Google Scholar]
- 69.Tymula A, Rosenberg Belmaker LA, Ruderman L, Glimcher PW, Levy I. 2013. Like cognitive function, decision making across the life span shows profound age-related changes. Proc. Natl Acad. Sci. USA 110, 17 143–17 148. ( 10.1073/pnas.1309909110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reyna VF, Ellis SC. 1994. Fuzzy-trace theory and framing effects in children's risky decision making. Psychol. Sci. 5, 275–279. ( 10.1111/j.1467-9280.1994.tb00625.x) [DOI] [Google Scholar]
- 71.Reyna VF, Estrada SM, Demarinis JA, Myers RM, Stanisz JM, Mills BA. 2011. Neurobiological and memory models of risky decision making in adolescents versus young adults. J. Exp. Psychol. Learn. Mem. Cogn. 37, 1125–1142. ( 10.1037/a0023943) [DOI] [PubMed] [Google Scholar]
- 72.Weber E, Blais A, Betz N. 2002. A domain-specific risk-attitude scale: measuring risk perceptions and risk behaviors. J. Behav. Decis. Mak. 15, 263–290. ( 10.1002/bdm.414) [DOI] [Google Scholar]
- 73.Lieder F, Griffiths TL, Hsu M. 2018. Overrepresentation of extreme events in decision making reflects rational use of cognitive resources. Psychol. Rev. 125, 1–32. ( 10.1037/rev0000074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Engelmann JB, Moore S, Capra CM, Berns GS. 2012. Differential neurobiological effects of expert advice on risky choice in adolescents and adults. Soc. Cogn. Affect. Neurosci. 7, 557–567. ( 10.1093/scan/nss050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tymula A, Rosenberg BLA, Roy AK, Ruderman L, Manson K, Glimcher PW, Levy I. 2012. Adolescents’ risk-taking behavior is driven by tolerance to ambiguity. Proc. Natl Acad. Sci. USA 109, 17 135–17 140. ( 10.1073/pnas.1207144109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steelandt S, Broihanne M-H, Romain A, Thierry B, Dufour V. 2013. Decision-making under risk of loss in children. PLoS ONE 8, e52316 ( 10.1371/journal.pone.0052316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knoll LJ, Magis-Weinberg L, Speekenbrink M, Blakemore S.-J. 2015. Social influence on risk perception during adolescence. Psychol. Sci. 26, 583–592. ( 10.1177/0956797615569578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camerer C, Weber M.. 1992. Recent developments in modeling preferences: uncertainty and ambiguity. J. Risk Uncertainty 5, 325–370. ( 10.1007/BF00122575) [DOI] [Google Scholar]
- 79.Ellsberg D. 1961. Risk, ambiguity, and the Savage axioms. Q. J. Econ. 75, 643–669. ( 10.2307/1884324) [DOI] [Google Scholar]
- 80.Li R, Brannon EM, Huettel SA. 2014. Children do not exhibit ambiguity aversion despite intact familiarity bias. Front. Psychol. 5, 1519 ( 10.3389/fpsyg.2014.01519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li R, Roberts RC, Huettel SA, Brannon EM. 2017. Five-year-olds do not show ambiguity aversion in a risk and ambiguity task with physical objects. J. Exp. Child Psychol. 159, 319–326. ( 10.1016/j.jecp.2017.02.013) [DOI] [PubMed] [Google Scholar]
- 82.Wulff DU, Canseco MM, Hertwig R. 2017. A meta-analytic review of two modes of learning and the description-experience gap. Psychol. Bull. 144, 140–176. ( 10.1037/bul0000115) [DOI] [PubMed] [Google Scholar]
- 83.Barron G, Erev I. 2003. Small feedback-based decisions and their limited correspondence to description-based decisions. J. Behav. Decis. Mak. 16, 215–233. ( 10.1002/bdm.443) [DOI] [Google Scholar]
- 84.Bechara A, Damasio AR, Damasio H, Anderson SW. 1994. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50, 7–15. ( 10.1016/0010-0277(94)90018-3) [DOI] [PubMed] [Google Scholar]
- 85.Almy B, Kuskowski M, Malone SM, Myers E, Luciana M. 2018. A longitudinal analysis of adolescent decision-making with the Iowa Gambling Task. Dev. Psychol. 54, 689–702. ( 10.1037/dev0000460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, Woolard J. 2010. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Dev. Psychol. 46, 193–207. ( 10.1037/a0016128) [DOI] [PubMed] [Google Scholar]
- 87.Christakou A, Gershman SJ, Niv Y, Simmons A, Brammer M, Rubia K. 2013. Neural and psychological maturation of decision-making in adolescence and young adulthood. J. Cogn. Neurosci. 26, 194–198. ( 10.1162/jocn_a_00447) [DOI] [PubMed] [Google Scholar]
- 88.Crone EA, van der Molen MW. 2007. Development of decision making in school-aged children and adolescents: evidence from heart rate and skin conductance analysis. Child Dev. 78, 1288–1301. ( 10.1111/j.1467-8624.2007.01066.x) [DOI] [PubMed] [Google Scholar]
- 89.Huizenga HM, Crone EA, Jansen BJ. 2007. Decision-making in healthy children, adolescents and adults explained by the use of increasingly complex proportional reasoning rules. Dev. Sci. 10, 814–825. ( 10.1111/j.1467-7687.2007.00621.x) [DOI] [PubMed] [Google Scholar]
- 90.Icenogle G, et al. 2016. Puberty predicts approach but not avoidance on the Iowa Gambling Task in a multinational sample. Child Dev. 88, 1598–1614. ( 10.1111/cdev.12655) [DOI] [PubMed] [Google Scholar]
- 91.Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. 2004. Performance on the IOWA card task by adolescents and adults. Neuropsychologia 42, 1838–1851. ( 10.1016/j.neuropsychologia.2004.03.014) [DOI] [PubMed] [Google Scholar]
- 92.Prencipe A, Kesek A, Cohen J, Lamm C, Lewis MD, Zelazo PD. 2011. Development of hot and cool executive function during the transition to adolescence. J. Exp. Child Psychol. 108, 621–637. ( 10.1016/j.jecp.2010.09.008) [DOI] [PubMed] [Google Scholar]
- 93.van Duijvenvoorde ACK, Jansen BRJ, Bredman JC, Huizenga HM. 2012. Age-related changes in decision making: comparing informed and noninformed situations. Dev. Psychol. 48, 192–203. ( 10.1037/a0025601) [DOI] [PubMed] [Google Scholar]
- 94.Dunn BD, Dalgleish T, Lawrence AD. 2006. The somatic marker hypothesis: a critical evaluation. Neurosci. Biobehav. Rev. 30, 239–271. ( 10.1016/j.neubiorev.2005.07.001) [DOI] [PubMed] [Google Scholar]
- 95.Sutton RS, Barto AG. 1998. Reinforcement learning: an introduction, vol. 1 Cambridge, MA: MIT press. [Google Scholar]
- 96.Schultz W, Dayan P, Montague PR. 1997. A neural substrate of prediction and reward. Science 275, 1593–1599. ( 10.1126/science.275.5306.1593) [DOI] [PubMed] [Google Scholar]
- 97.Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. 2010. A unique adolescent response to reward prediction errors. Nat. Neurosci. 13, 669–671. ( 10.1038/nn.2558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peters S, Crone EA. 2017. Increased striatal activity in adolescence benefits learning. Nat. Commun. 8, 1983 ( 10.1038/s41467-017-02174-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davidow JY, Foerde K, Galvan A, Shohamy D. 2016. An upside to reward sensitivity: the hippocampus supports enhanced reinforcement learning in adolescence. Neuron 92, 93–99. ( 10.1016/j.neuron.2016.08.031) [DOI] [PubMed] [Google Scholar]
- 100.van den Bos W, Cohen MX, Kahnt T, Crone EA. 2012. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cereb. Cortex 22, 1247–1255. ( 10.1093/cercor/bhr198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DePasque S, Galván A. 2017. Frontostriatal development and probabilistic reinforcement learning during adolescence. Neurobiol. Learn. Mem. 143, 1–7. ( 10.1016/j.nlm.2017.04.009) [DOI] [PubMed] [Google Scholar]
- 102.Niv Y, Edlund JA, Dayan P, O'Doherty JP. 2012. Neural prediction errors reveal a risk-sensitive reinforcement-learning process in the human brain. J. Neurosci. 32, 551–562. ( 10.1523/JNEUROSCI.5498-10.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hauser TU, Iannaccone R, Walitza S, Brandeis D, Brem S. 2015. Cognitive flexibility in adolescence: neural and behavioral mechanisms of reward prediction error processing in adaptive decision making during development. Neuroimage 104, 347–354. ( 10.1016/j.neuroimage.2014.09.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van der Schaaf ME, Warmerdam E, Crone EA, Cools R. 2011. Distinct linear and non-linear trajectories of reward and punishment reversal learning during development: relevance for dopamine's role in adolescent decision making. Dev. Cogn. Neurosci. 1, 578–590. ( 10.1016/j.dcn.2011.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Casey BJ, Galvan A, Somerville LH. 2016. Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Dev. Cogn. Neurosci. 17, 128–130. ( 10.1016/j.dcn.2015.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Braams BR, van Duijvenvoorde ACK, Peper JS, Crone EA. 2015. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J. Neurosci. 35, 7226–7238. ( 10.1523/JNEUROSCI.4764-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. 2006. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 26, 6885–6892. ( 10.1523/JNEUROSCI.1062-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schreuders E, Braams BR, Blankenstein NE, Peper JS, Güroğlu B, Crone EA. 2018. Contributions of reward sensitivity to ventral striatum activity across adolescence and early adulthood. Child Dev. 89, 797–810. ( 10.1111/cdev.13056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. 2004. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 24, 1793–1802. ( 10.1523/JNEUROSCI.4862-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bjork JM, Smith AR, Chen G, Hommer DW. 2010. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS ONE 5, e11440 ( 10.1371/journal.pone.0011440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barron G, Leider S, Stack J. 2008. The effect of safe experience on a warnings’ impact: sex, drugs, and rock-n-roll. Organ. Behav. Hum. Decis. Process. 106, 125–142. ( 10.1016/j.obhdp.2007.11.002) [DOI] [Google Scholar]
- 112.Doll BB, Hutchison KE, Frank MJ. 2011. Dopaminergic genes predict individual differences in susceptibility to confirmation bias. J. Neurosci. 31, 6188–6198. ( 10.1523/JNEUROSCI.6486-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Doll BB, Jacobs WJ, Sanfey AG, Frank MJ. 2009. Instructional control of reinforcement learning: a behavioral and neurocomputational investigation. Brain Res. 1299, 74–94. ( 10.1016/j.brainres.2009.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jessup RK, Bishara AJ, Busemeyer JR. 2008. Feedback produces divergence from prospect theory in descriptive choice. Psychol. Sci. 19, 1015–1022. ( 10.1111/j.1467-9280.2008.02193.x) [DOI] [PubMed] [Google Scholar]
- 115.Lejarraga T, Gonzalez C. 2011. Effects of feedback and complexity on repeated decisions from description. Organ. Behav. Hum. Decis. Process. 116, 286–295. ( 10.1016/j.obhdp.2011.05.001) [DOI] [Google Scholar]
- 116.Decker JH, Otto AR, Daw ND, Hartley CA. 2016. From creatures of habit to goal-directed learners. Psychol. Sci. 27, 848–858. ( 10.1177/0956797616639301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Duijvenvoorde ACK, et al. 2015. Neural correlates of expected risks and returns in risky choice across development. J. Neurosci. 35, 1549–1560. ( 10.1523/JNEUROSCI.1924-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dalley JW, Everitt BJ, Robbins TW. 2011. Impulsivity, compulsivity, and top–down cognitive control. Neuron 69, 680–694. ( 10.1016/j.neuron.2011.01.020) [DOI] [PubMed] [Google Scholar]
- 119.Bari A, Robbins TW. 2013. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 108, 44–79. ( 10.1016/j.pneurobio.2013.06.005) [DOI] [PubMed] [Google Scholar]
- 120.Friedman NP, Miyake A. 2004. The relations among inhibition and interference control functions: a latent-variable analysis. J. Exp. Psychol. Gen. 133, 101–135. ( 10.1037/0096-3445.133.1.101) [DOI] [PubMed] [Google Scholar]
- 121.Reynolds B, Ortengren A, Richards JB, de Wit H. 2006. Dimensions of impulsive behavior: personality and behavioral measures. Pers. Individ. Dif. 40, 305–315. ( 10.1016/j.paid.2005.03.024) [DOI] [Google Scholar]
- 122.Tamm L, Menon V, Reiss AL. 2002. Maturation of brain function associated with response inhibition. J. Am. Acad. Child Adolesc. Psychiatry 41, 1231–1238. ( 10.1097/00004583-200210000-00013) [DOI] [PubMed] [Google Scholar]
- 123.van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. 2015. Adolescent impatience decreases with increased frontostriatal connectivity. Proc. Natl Acad. Sci. USA 115, E3765–E3774. ( 10.1073/pnas.1423095112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olson EA, Hooper CJ, Collins P, Luciana M. 2007. Adolescents’ performance on delay and probability discounting tasks: contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Pers. Individ. Dif. 43, 1886–1897. ( 10.1016/j.paid.2007.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Steinbeis N, Haushofer J, Fehr E, Singer T. 2014. Development of behavioral control and associated vmPFC–DLPFC connectivity explains children's increased resistance to temptation in intertemporal choice. Cereb. Cortex 26, 32–42. ( 10.1093/cercor/bhu167) [DOI] [PubMed] [Google Scholar]
- 126.Kirby KN, Winston GC, Santiesteban M. 2005. Impatience and grades: delay-discount rates correlate negatively with college GPA. Learn. Individ. Differ. 15, 213–222. ( 10.1016/j.lindif.2005.01.003) [DOI] [Google Scholar]
- 127.Bickel WK, Marsch LA. 2001. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96, 73–86. ( 10.1046/j.1360-0443.2001.961736.x) [DOI] [PubMed] [Google Scholar]
- 128.Meier S, Sprenger CD. 2012. Time discounting predicts creditworthiness. Psychol. Sci. 23, 56–58. ( 10.1177/0956797611425931) [DOI] [PubMed] [Google Scholar]
- 129.Chabris CF, Laibson D, Morris CL, Schuldt JP, Taubinsky D. 2008. Individual laboratory-measured discount rates predict field behavior. J. Risk Uncertain. 37, 237–269. ( 10.1007/s11166-008-9053-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reimers S, Maylor EA, Stewart N, Chater N. 2009. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Pers. Individ. Dif. 47, 973–978. ( 10.1016/j.paid.2009.07.026) [DOI] [Google Scholar]
- 131.Christakou A, Brammer M, Rubia K. 2011. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage 54, 1344–1354. ( 10.1016/j.neuroimage.2010.08.067) [DOI] [PubMed] [Google Scholar]
- 132.de Water E, Cillessen AHN, Scheres A. 2014. Distinct age-related differences in temporal discounting and risk taking in adolescents and young adults. Child Dev. 85, 1881–1897. ( 10.1111/cdev.12245) [DOI] [PubMed] [Google Scholar]
- 133.Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. 2009. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J. Cogn. Neurosci. 21, 1406–1421. ( 10.1162/jocn.2009.21107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. 2006. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia 44, 2092–2103. ( 10.1016/j.neuropsychologia.2005.10.012) [DOI] [PubMed] [Google Scholar]
- 135.Pine A, Seymour B, Roiser JP, Bossaerts P, Friston KJ, Curran HV, Dolan RJ. 2009. Encoding of marginal utility across time in the human brain. J. Neurosci. 29, 9575–9581. ( 10.1523/JNEUROSCI.1126-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miller E, Cohen J. 2001. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. ( 10.1146/annurev.neuro.24.1.167) [DOI] [PubMed] [Google Scholar]
- 137.Haber SN, Knutson B. 2009. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 1–23. ( 10.1038/npp.2009.146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mischel W, Shoda Y, Rodriguez MI. 1989. Delay of gratification in children. Science 244, 933–938. ( 10.1126/science.2658056) [DOI] [PubMed] [Google Scholar]
- 139.Miller DT, Weinstein SM, Karniol R. 1978. Effects of age and self-verbalization on children's ability to delay gratification. Dev. Psychol. 14, 569 ( 10.1037/0012-1649.14.5.569) [DOI] [Google Scholar]
- 140.Yates GCR, Lippett RMK, Yates SM. 1981. The effects of age, positive affect induction, and instructions on children's delay of gratification. J. Exp. Child Psychol. 32, 169–180. ( 10.1016/0022-0965(81)90101-6) [DOI] [Google Scholar]
- 141.Shoda Y, Mischel W, Peake PK. 1990. Predicting adolescent cognitive and self-regulatory competencies from preschool delay of gratification: identifying diagnostic conditions. Dev. Psychol. 26, 978 ( 10.1037/0012-1649.26.6.978) [DOI] [Google Scholar]
- 142.Casey BJ, et al. 2011. Behavioral and neural correlates of delay of gratification 40 years later. Proc. Natl Acad. Sci. USA 108, 14 998–15 003. ( 10.1073/pnas.1108561108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kidd C, Palmeri H, Aslin RN. 2013. Rational snacking: young children's decision-making on the marshmallow task is moderated by beliefs about environmental reliability. Cognition 126, 109–114. ( 10.1016/j.cognition.2012.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stevens JR, Stephens DW. 2010. The adaptive nature of impulsivity. Retrieved from https://digitalcommons.unl.edu/psychfacpub/519/.
- 145.Green L, Myerson J, Lichtman D, Rosen S, Fry A. 1996. Temporal discounting in choice between delayed rewards: the role of age and income. Psychol. Aging 11, 79–84. ( 10.1037/0882-7974.11.1.79) [DOI] [PubMed] [Google Scholar]
- 146.Lempert KM, Phelps EA. 2016. The malleability of intertemporal choice. Trends Cogn. Sci. 20, 64–74. ( 10.1016/j.tics.2015.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bulley A, Henry J, Suddendorf T. 2016. Prospection and the present moment: the role of episodic foresight in intertemporal choices between immediate and delayed rewards. Rev. Gen. Psychol. 20, 29 ( 10.1037/gpr0000061) [DOI] [Google Scholar]
- 148.Benoit RG, Gilbert SJ, Burgess PW. 2011. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J. Neurosci. 31, 6771–6779. ( 10.1523/JNEUROSCI.6559-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peters J, Büchel C. 2010. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal–mediotemporal interactions. Neuron 66, 138–148. ( 10.1016/j.neuron.2010.03.026) [DOI] [PubMed] [Google Scholar]
- 150.Buckner RL. 2007. Prospection and the brain. Behav. Brain Sci. 30, 318–319. ( 10.1017/S0140525X07002038) [DOI] [Google Scholar]
- 151.Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. 2012. The future of memory: remembering, imagining, and the brain. Neuron 76, 677–694. ( 10.1016/j.neuron.2012.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parthasarathi T, McConnell MH, Luery J, Kable JW. 2017. The vivid present: visualization abilities are associated with steep discounting of future rewards. Front. Psychol. 8, 289 ( 10.3389/fpsyg.2017.00289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nurmi J-E. 1991. How do adolescents see their future? A review of the development of future orientation and planning. Dev. Rev. 11, 1–59. ( 10.1016/0273-2297(91)90002-6) [DOI] [Google Scholar]
- 154.Steinberg L, Graham S, O'Brien L, Woolard J, Cauffman E, Banich M. 2009. Age differences in future orientation and delay discounting. Child Dev. 80, 28–44. ( 10.1111/j.1467-8624.2008.01244.x) [DOI] [PubMed] [Google Scholar]
- 155.Gott C, Lah S. 2014. Episodic future thinking in children compared to adolescents. Child Neuropsychol. 20, 625–640. ( 10.1080/09297049.2013.840362) [DOI] [PubMed] [Google Scholar]
- 156.Bromberg U, Wiehler A, Peters J. 2015. Episodic future thinking is related to impulsive decision making in healthy adolescents. Child Dev. 86, 1458–1468. ( 10.1111/cdev.12390) [DOI] [PubMed] [Google Scholar]
- 157.Bromberg U, Lobatcheva M, Peters J. 2017. Episodic future thinking reduces temporal discounting in healthy adolescents. PLoS ONE 12, e0188079 ( 10.1371/journal.pone.0188079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Daniel TO, Said M, Stanton CM, Epstein LH. 2015. Episodic future thinking reduces delay discounting and energy intake in children. Eat. Behav. 18, 20–24. ( 10.1016/j.eatbeh.2015.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Menon V, Boyett-Anderson JM, Reiss AL. 2005. Maturation of medial temporal lobe response and connectivity during memory encoding. Cogn. Brain Res. 25, 379–385. ( 10.1016/j.cogbrainres.2005.07.007) [DOI] [PubMed] [Google Scholar]
- 160.Ofen N, Chai XJ, Schuil KDI, Whitfield-Gabrieli S, Gabrieli JD. E. 2012. The development of brain systems associated with successful memory retrieval of scenes. J. Neurosci. 32, 10 012–10 020. ( 10.1523/JNEUROSCI.1082-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Walsh MM, Anderson JR. 2011. Learning from delayed feedback: neural responses in temporal credit assignment. Cogn. Affect. Behav. Neurosci. 11, 131–143. ( 10.3758/s13415-011-0027-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gleicher F, Boninger DS, Strathman A, Armor D, Hetts J, Ahn M. 1995. With an eye toward the future: the impact of counterfactual thinking on affect, attitudes, and behavior. In What might have been: the social psychology of counterfactual thinking (eds Roese NJ, Olson JM), pp. 283–304. Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- 163.Schacter DL, Benoit RG, De Brigard F, Szpunar KK. 2015. Episodic future thinking and episodic counterfactual thinking: intersections between memory and decisions. Neurobiol. Learn. Mem. 117, 14–21. ( 10.1016/j.nlm.2013.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Daw N, Niv Y, Dayan P. 2005. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat. Neurosci. 2005, 1704 ( 10.1038/nn1560) [DOI] [PubMed] [Google Scholar]
- 165.Dolan RJ, Dayan P. 2013. Goals and habits in the brain. Neuron 80, 312–325. ( 10.1016/j.neuron.2013.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Doll BB, Duncan KD, Simon DA, Shohamy D, Daw ND. 2015. Model-based choices involve prospective neural activity. Nat. Neurosci. 18, 767–772. ( 10.1038/nn.3981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van der Meer M, Kurth-Nelson Z, Redish AD. 2012. Information processing in decision-making systems. Neuroscientist 18, 342–359. ( 10.1177/1073858411435128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Verschure PFMJ, Pennartz CMA, Pezzulo G. 2014. The why, what, where, when and how of goal-directed choice: neuronal and computational principles. Phil. Trans. R. Soc. B 369, 20130483 ( 10.1098/rstb.2013.0483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kurth-Nelson Z, Bickel W, Redish AD. 2012. A theoretical account of cognitive effects in delay discounting. Eur. J. Neurosci. 35, 1052–1064. ( 10.1111/j.1460-9568.2012.08058.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Story GW, Vlaev I, Seymour B, Darzi A, Dolan RJ. 2014. Does temporal discounting explain unhealthy behavior? A systematic review and reinforcement learning perspective. Front. Behav. Neurosci. 8, 76 ( 10.3389/fnbeh.2014.00076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Potter TCS, Bryce NV, Hartley CA. 2017. Cognitive components underpinning the development of model-based learning. Dev. Cogn. Neurosci. 25, 272–280. ( 10.1016/j.dcn.2016.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Peters J, Büchel C. 2011. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn. Sci. 15, 227–239. ( 10.1016/j.tics.2011.03.002) [DOI] [PubMed] [Google Scholar]
- 173.Anokhin AP, Grant JD, Mulligan RC, Heath AC. 2015. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol. Psychiatry 77, 887–894. ( 10.1016/j.biopsych.2014.10.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Steinberg L, Cauffman E, Woolard J, Graham S, Banich M. 2009. Are adolescents less mature than adults?: minors’ access to abortion, the juvenile death penalty, and the alleged APA ‘flip-flop’. Am. Psychol. 64, 583–594. ( 10.1037/a0014763) [DOI] [PubMed] [Google Scholar]
- 175.Chambers RA, Potenza MN. 2003. Neurodevelopment, impulsivity, and adolescent gambling. J. Gambl. Stud. 19, 53–84. ( 10.1023/A:1021275130071) [DOI] [PubMed] [Google Scholar]
- 176.Stanger C, Budney AJ, Bickel WK. 2013. A developmental perspective on neuroeconomic mechanisms of contingency management. Psychol. Addict. Behav. 27, 403–415. ( 10.1037/a0028748) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.