Abstract
Immediate and long-term mechanisms interact in the regulation of action. We will examine neurobiology and practical clinical consequences of these interactions. Long-term regulation of immediate behavioural control is based on analogous responses to highly rewarding or stressful stimuli: (i) impulsivity is a failure of the balance between activation and inhibition in the immediate regulation of action. (ii) Sensitization is a persistently exaggerated behavioural or physiological response to highly salient stimuli, such as addictive stimuli or inescapable stress. Sensitization can generalize across classes of stimuli. (iii) Impulsivity, possibly related to poor modulation of catecholaminergic and glutamatergic functions, may facilitate development of long-term sensitized responses to stressful or addictive stimuli. In turn, impulsivity is prominent in sensitized behaviour. (iv) While impulsivity and sensitization are general components of behaviour, their interactions are prominent in the course of bipolar disorder, emphasizing roles of substance-use, recurrent course and stressors. (v) Suicide is a complex and severe behaviour that exemplifies the manner in which impulsivity facilitates behavioural sensitization and is, in turn, increased by it, leading to inherently unpredictable behaviour. (vi) Interactions between impulsivity and sensitization can provide targets for complementary preventive and treatment strategies for severe immediate and long-term behavioural disorders. Progress along these lines will be facilitated by predictors of susceptibility to behavioural sensitization.
This article is part of the theme issue ‘Risk taking and impulsive behaviour: fundamental discoveries, theoretical perspectives and clinical implications’.
Keywords: impulsive behaviour, behavioural sensitization, time factors, bipolar disorder, recurrence, suicide
1. Introduction
Action is regulated by interacting immediate and long-term processes. Both are responses to strong positive or negative stimuli. They appear related to survival mechanisms, and are associated with increased arousal [1,2]. Impulsivity, a breakdown in the balance between behaviour activation and inhibition, results in action that is not governed by deliberate intent, representing failure of immediate behaviour regulation [1]. Behavioural sensitization to addictive or stressful stimuli, where previous exposure to a salient stimulus can result in persistent, exaggerated behavioural and physiological responses to that stimulus class [2], represents failure of long-term behaviour regulation and is also related to arousal [3]. Arousal can predispose to impulsive behaviour that appears unpredictable [1].
Impulsivity, sensitization and their interaction, involving arousal, are basic behavioural mechanisms across species [1–5]. They, and their potentially severe consequences, are prominent in bipolar disorder [6,7]. Susceptibility to sensitization varies widely across individuals. Objective, clinically feasible markers are necessary to identify those at risk for severe behavioural disturbances and develop targets for treatment. Yet, we know of no objective markers that predict susceptibility to sensitization.
We will address (i) impulsivity, its role in the transition to a sensitized state, and relationships between impulsivity and the expression of sensitized behaviour (see §2); (ii) interactions of sensitization and impulsivity in the course of bipolar disorder, which strongly demonstrates interactions between immediate action regulation and behavioural sensitization (see §3); (iii) the role of sensitization–impulsivity interactions in complex and severe behaviours, focusing on suicidality see (§§4a–c); and (iv) potential targets for predictive markers and treatments aimed at interacting short- and long-term mechanisms (see §4d).
2. Immediate and long-term action regulation
(a). Immediate action dysregulation: impulsivity
Action regulation balances the initiation and inhibition of behaviour [4]. Impulsivity, a disruption of this balance, leads to action that does not conform to its context, with potentially severe consequences [5–10]. Impulsivity is increased in affective disorders [7], substance-use disorders (SUD) [8], severe aggression [9] and suicide [6] or other premature death [10].
Impulsivity can be assessed by questionnaires, including the Barratt Impulsiveness Scale (BIS-11), addressing long-term self-reported attitudes and patterns of behaviour associated with the potential for impulsivity [11]. The expression of impulsive behaviour can be measured by behavioural laboratory and neurophysiological methods, which have quantifiable outcomes and animal models [12].
Models of impulsivity include (i) reward-delay or delay-discounting impulsivity (DDI, impulsive choice), an exaggeration of the normal decrease of perceived value of a future (higher) reward over an immediate (smaller) reward [12,13] and (ii) rapid-response impulsivity (RRI, impulsive action), with impaired response inhibition producing responses before adequately processing a stimulus, and inability to adapt behaviour to changing contexts [12]. RRI can be measured as commission (impulsive) errors in continuous performance, go/no-go or stop-signal tasks [7,12–15]. It is associated with disrupted brain responses as early as 50 msec after a stimulus; conscious awareness starts at about 200 msec [8]. RRI precedes conscious awareness of a stimulus or ability to consider its results, while DDI is based on expected results. We will focus on RRI because of its relationship to rapid responses to stimuli and action control. Further, recurrent psychiatric disorders like addictions, psychotic and affective disorders are potentially progressive illnesses, and there is evidence that RRI is related to illness progression [16].
Impulsivity encompasses brain functions including arousal, attention and motivation, with multiple neural substrates [1]. For example, there is a complementary relationship between the anterior cingulate cortex (ACC), involved in action regulation and monitoring, and the locus coeruleus (LC), involved in arousal. Both are related to interactions between immediate and long-term behavioural regulation.
The ACC is involved in monitoring, inhibiting and correcting goal-directed behaviour [17]. Its volume or function correlates with questionnaire-assessed potential impulsivity in cocaine abuse [18], bipolar disorder [19] and controls [20].
The LC releases norepinephrine (NE) throughout the brain and, through effects on arousal, could optimize behaviour via focused processing of relevant information, or interfere with optimal behaviour via intensified processing of information regardless of relevance [21,22]. It can block behavioural regulation by the prefrontal cortex [23]. LC and ACC are mutually inhibitory [22].
NE increases RRI in rats [24] and humans [25,26]. In humans, pharmacologically increased NE increased impulsive errors, accelerated reaction time and increased arousal [26]. Impulsive errors correlated with increased plasma 3-methoxy,4-hydroxyphenylglycol (MHPG), an NE metabolite (β = 0.727 ± 0.243, F2,18 = 4.62, p = 0.02) [25]. Similarly, in bipolar disorder, mania scores correlate with cerebrospinal fluid (CSF) MHPG [27] and with RRI [28].
Consistent with NE effects, impulsivity is related to exaggerated autonomic and neurophysiological arousal elicited by salient stimuli [1–5]. Impulsivity was originally considered a stable moderator of the rate of arousal change; more recent evidence suggests that the relationship between impulsivity and rate of arousal change is unstable and more complex [29].
(b). Long-term action regulation: behavioural sensitization
Sensitization is exaggerated behavioural or physiological responses to repeated or intense salient stimuli. These include inescapable stressors, drugs of abuse, or, possibly, endogenous stimuli potentially including neurotransmitter changes related to illness episodes.
Behavioural sensitization has been demonstrated in species ranging from humans [30,31] and rodents [32,33] to invertebrates [34]. It may represent a class of survival-related behaviours [2]. It is strongly related to arousal [3,35,36]. Most direct data on mechanisms of sensitization–arousal disturbances comes from invertebrates [35,36]. Cross-sensitization between amphetamine and stress-related effects on arousal and dopaminergic function has been demonstrated in humans [37]. The detailed mechanisms may differ across species but results appear similar.
Behavioural sensitization is sequential; table 1 shows examples of sensitizing stimuli and their behavioural expressions. Development involves repeated exposure to a sensitizing stimulus, resulting in exaggerated responses to related stimuli. Susceptibility to sensitization development varies widely across individuals, with important potential effects on the course of psychiatric disorders or consequences of exposure to stressors or addictive stimuli. Expression of sensitization is persistently enhanced responsiveness to repeated stimuli, even after prolonged absence of the stimulus. Expression requires NE and dopamine (DA) release [47–49] and produces increased arousal [3,35–37,47]. Cross-sensitization represents generalization across classes of stimuli, potentially expressed as stress-induced anhedonia [50], arousal [37,51] and impulsivity [52].
Table 1.
Sensitizing stimuli and expressions. HPA, hypothalamic–pituitary–adrenocortical axis activity.
| stimulus type | example | sensitized response |
|---|---|---|
| addictive | stimulants [32,33] | increased locomotor response, self-administration, autonomic and rewarding effects |
| alcohol [38] | ||
| nicotine [39] | ||
| stress-trauma | inescapable stress [40] | increased HPA, autonomic and behaviour responses to stressors; stress-induced arousal and anhedonia |
| episode-related or endogenous | recurrent episodes [41–44] | increased frequency/severity |
| inflammation [45] | increased sensitivity to the same stimulus type | |
| cross-sensitization | addictive × addictive [46] | increased sensitivity to both stimulus classes stress-induced arousal, anhedonia and/or drug sensitivity |
| stressor × addictive [37] | ||
| episode × addictive or stressor [16,43] |
Development and expression involve similar networks, requiring glutamatergic inputs from, respectively, ventral tegmental area and nucleus accumbens to subcortical and prefrontal cortex structures involved in motivation and action [53]. Low doses of the non-competitive N-methyl-d-aspartate receptor (NMDAR) antagonist MK-801, which resembles ketamine, prevents development, expression and cross-sensitization [54].
(c). Interaction between immediate and long-term action regulation
Sensitization and impulsivity are responses to highly salient stimuli that involve increased arousal [1–3,35–37] and require DA [32,55], glutamate [32,54] and NE [48,49,56,57], with inhibition by γ-aminobutyric acid [58] and serotonin [5HT] [59]. Sensitization requires NE–5HT uncoupling [60] with reduced 5HT in the ACC [61]. This increases LC activity directly and through glutamate receptors [32], leading to a potential cycle of increasing LC disinhibition. Sensitization can therefore increase impulsivity and arousal. In addition to NE-related mechanisms, sensitization involves DA-related ‘fluctuating disinhibition’ with increased impulsivity [47,52].
Stress-induced arousal is an important potential consequence of sensitized behaviour [2,3]. Fear-potentiated startle (FPS) is a potential measure of stress-induced activation [62]. Clonidine, which inhibits NE release, reduced FPS, while FPS was increased by compounds that increased NE release [63]. Among depressed inpatients, only those with suicide attempts had increased FPS [64].
(d). Summary
Uncontrollable stress, addictive stimuli or psychiatric illness episodes can increase impulsivity via sensitization and resulting hyperarousal [1,16,43,47]. Conversely, pre-existing impulsivity can facilitate sensitization, via increased stress-related NE and DA release [1,29]. Rats [61] and humans [30] with high pre-existing impulsivity are more readily sensitized. Impulsivity can be readily measured [7,9,11–13]. There are, however, no established markers for sensitization, though potentiated startle is promising [62–64].
3. Course of illness and adaptations to impulsivity in bipolar disorder
(a). Characterizing the course(s) of bipolar disorder
Bipolar disorder may predispose to impulsivity and behavioural sensitization. Like other long-term psychiatric and non-psychiatric illnesses [65], bipolar disorder may have a sequential course with a prodrome (high genetic or environmental risk without overt illness), progressing through early symptoms, syndromal episodes and progressively increasing risk of recurrence, severity and related complications like addictions and severe behavioural disturbances [65–67]. This progression may have at least two basic patterns [66]:
Episodic-stable (or classic) [66]: episodes, while potentially severe, are infrequent and time-limited, without prominent comorbidities; either depressive or manic without combining or rapidly alternating between the two; and with strong short- [68] and long-term [67] response to lithium. Long-term impairment is more strongly related to episode frequency than affective symptom severity [69].
Complicated or unstable-progressive: earlier onset, frequent episodes with partial or transient recovery, and common sensitization-related problems including substance-use and trauma history [70–72].
These descriptions represent patterns along a continuum rather than distinct entities, but their characteristics appear persistent and familial [68]. Trauma or addiction can potentially convert a ‘classic’ to a ‘complicated’ course [43]. Complicated course is associated with impulsive behaviour, including suicidality [6], and sensitizing events, including frequent episodes [41–44], substance-use [29,43] and traumas [43,44].
Mixed affective states combining manic and depressive features, a hallmark of complicated illness course [73–75], are associated with early illness onset, high trait impulsivity and RRI, and sensitization-related characteristics including SUD, early stressors and severe recurrence [73–75]. RRI [73] and CSF MHPG [76] are higher in episodes with mixed characteristics than in more exclusively manic or depressive episodes.
‘Staging’ bipolar disorder describes its progression from a prodromal state, through the development of recurrent episodes, to a potentially more complex illness [65]. This may differ in ‘classic’ versus ‘complicated’ courses [66]. Early stressors, addictions, recurrence and activated depression are consistent with sensitization [70–72].
Episode-sensitization may be a component of illness progression in complicated bipolar disorder [71]. It entails apparent increased sensitivity to stressors and/or addictions, with accelerating episode frequency and treatment resistance [41–44]. This could result from relationships between episodes and substance abuse or stressors [43], or results of episodes themselves like increased NE, glutamate and/or DA [27]. Rather than progressive increases in episode frequency, highly recurrent disorders could have a ‘pre-sensitized’ course with increased episode frequency from the outset or developing early in the course of illness. Re-analysis of data originally suggesting continuous progression in episode frequency showed that, correcting for observational biases, episode frequency increased early in the course of illness and then remained constant, rather than continuously progressing [42]. Recurrence is predicted more strongly by the interaction between early trauma and number of previous episodes than number of episodes alone [72].
(b). Potential and expressed impulsivity
(i). Protection against potential impulsivity
The BIS-11 score represents potential for impulsive behaviour [1]. BIS-11 scores were increased in bipolar disorder (BD) versus healthy controls (Cohen's effect size (ES) = 1.45), even after correction for symptoms (intercept after regression on depression, mania, anxiety and psychosis) and medications [77]. This is also true for those at familial risk for major depressive [78] or psychotic [79] disorders. Regardless of symptoms, treatment or clinical state, BIS-11 impulsivity scores were increased further with early onset, many episodes, SUD, histories of suicidal behaviour (ES = 0.7–0.8) [77] or early trauma [72]. Table 2 summarizes initial adaptation to increased potential impulsivity followed by loss of adaptation with sensitization.
Table 2.
Transition to sensitized illness. ERN, error-related negativity.
| characteristic | example | pre-sensitized | sensitized |
|---|---|---|---|
| ‘trait’ impulsivity [77] | BIS-11 | higher than normal | greater increase |
| structures/circuits [17,18] | ACC volume and activity | normal or increased; negatively correlated with impulsivity | reduced |
| ACC-LC functional connectivity | normal/reduced | increased | |
| response inhibition (RRI) [16] | reaction time | slowed | faster |
| omission errors | increased | decreased to normal | |
| commission errors | normal | increased | |
| behaviour monitoring [80,81] | ERN | normal or greater | decreased |
| symptoms [73] | mixed state index | low | high |
Despite increased potential impulsivity, RRI is only increased in bipolar disorder during mania or with history of potentially sensitizing stimuli [16]. Compared to controls, subjects with uncomplicated bipolar disorder had conservative RRI test performance with more omission errors and slower reaction time, but similar impulsive error rates [16]. This conservative response bias could protect against impulsive behaviour in uncomplicated bipolar disorder. Protection against RRI with increased omission errors and delayed reaction times resembles effects of imbalance between pre- and postsynaptic serotonergic 5HT1a receptor activation [82].
By contrast, either manic subjects with uncomplicated course, or interepisode subjects with history of SUD or many previous episodes, had faster RRI reaction times and increased impulsive errors [16,29]. This suggests loss of apparent adaptation to potential impulsivity (BIS-11), increasing expressed impulsivity (RRI impulsive errors [16]).
(ii). Impulsivity and behaviour regulation
Impulsivity is associated with poor adaptation of behaviour to consequences, implicating the ACC. ACC activity can be measured as error-related negativity (ERN), arising 50–250 ms after a commission error [83]. ERN reflects action-monitoring and behavioural adaptation, reflected by reduction of post-error errors [84]. ERN or ACC activity correlates with stress-sensitized impulsivity in post-traumatic stress disorder (PTSD) [85] and with trait impulsivity across diagnoses [86]. Decreased ERN predicts relapse in cocaine use [87].
ERN amplitude correlates negatively with trait impulsivity [80] and impulsive RRI errors (Kendall's τ > 0.45, p < 0.015). BIS-11 trait impulsivity correlated with post-error errors (τ = 0.43, p = 0.025). Controls, pressured to respond in 300 ms, responded 85 ms faster than without time pressure (F1,15 = 72.49, p < 0.001), with fewer correct responses, more impulsive errors and smaller ERN (F1,15 > 5.30, p < 0.036). Reduced ERN, therefore, can represent stress-related impulsivity [80,81]. Illness severity [88] or stress [18,85] appear related to reduced ACC function or ERN. LC activity correlates with NE metabolites and with increased RRI [26,27], consistent with the increased RRI in complicated versus uncomplicated bipolar disorder [16,73]. Initial compensatory or protective ACC activation in uncomplicated illness may be followed, with recurrence, SUD, or severe stressors, by increased RRI, consistent with ACC inhibition.
(iii). Expression of sensitization in combined bipolar and substance-use disorders
Combined BD and SUD is associated with increased BIS-11 and RRI [16]. Sensitization to recurrent episodes could predispose, via cross-sensitization, to SUD. Subjects (methods in [16]) with bipolar disorder and history of many episodes and SUD had BD onset (15.7 ± 9.3 years) significantly earlier than SUD (20.6 ± 6.3 years) (within-subjects F1,11 = 12.9, p = 0.005). Therefore, SUD could result from cross-sensitization between drug exposure and many episodes, because onset of highly recurrent BD appeared about 5 years earlier than SUD onset. This was consistent with reports that increased episode frequency can emerge early in bipolar disorder [41,42]. Without frequent recurrence, BD onset did not precede SUD. These results are preliminary because they are retrospective and involve a small number of subjects.
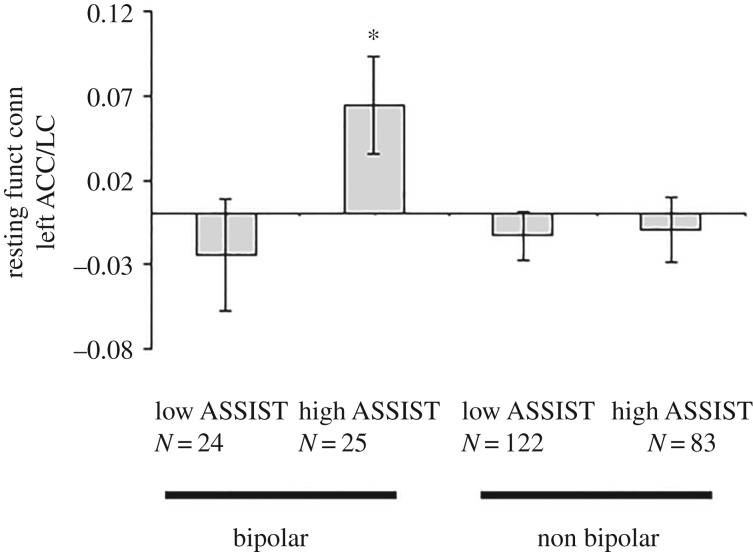
Progression of bipolar disorder, with severe recurrence and/or SUD [45,63], is consistent with a breakdown of reciprocal inhibition balancing LC and ACC activity, with excessive LC-mediated inhibition of ACC. We conducted a pilot study of resting-state functional connectivity between LC and ACC in BD inpatients with past severe versus mild or absent SUD (current negative drug screens; McNair Initiative for Neuroscience Discovery—Menninger/Baylor College of Medicine, MIND-MB (methods in [89])), compared to non-bipolar controls. Non-bipolar subjects' diagnoses included major depressive, anxiety, personality and SUD; BD included bipolar-I, -II or -note otherwise specified (NOS). We used the World Health Organization ASSIST scale to quantify drug use [90], comparing subjects with low (0–30) versus high (greater than 80) ASSIST scores. Analyses used Fisher's z-transformed correlation coefficients between seeds for each subject. Based on the apparent reciprocal relationship between ACC and LC, with severe SUD as a reflection of sensitization, we hypothesized stronger ACC–LC connectivity in bipolar disorder with high, compared with low, ASSIST.
Figure 1 shows that a priori ANOVA comparing connectivity between left ACC and LC for low versus high ASSIST BD subjects (sex and age as covariates) was significant (F48,1 = 4.664, p < 0.036). Multiple analysis of covariance with left and right ACC–LC connectivity as dependent variables; ASSIST (low versus high) and diagnosis (bipolar versus non-bipolar) as independent variables, with sex and age as covariates, revealed a trend toward a significant interaction between ASSIST and diagnosis for left ACC–LC (F1,245 = 3.16, p = 0.078) connectivity. These preliminary results are consistent with SUD-related behavioural sensitization associated with LC-mediated inhibition of the ACC.
Figure 1.
Left ACC–LC resting-state functional connectivity. Subject characterization, procedures and data analysis were as described [89]. ASSIST is an overall measure of substance-use severity, independent of specific substance(s) used [90]. The graph shows resting-state connectivity; error bars are standard error of the mean. *p < 0.05.
(c). Summary
Progression of recurrent affective disorders may involve transition from a compensated state, with slowed, conservative responding protecting against expression of impulsivity, to loss of compensation with faster responses and increased impulsivity. This transition may be associated with multiple illness episodes and/or potential sensitizing stimuli, including drug use or early trauma. Sensitization expression includes impulsivity and stress-induced arousal. Mixed presentations in bipolar disorder appear related to sensitized course with history of stressors, addictions, early onset and severe recurrence, characterized by activated depression, elevated RRI and increased suicidality [75]. Consistent with potential ACC-LC interactions in sensitization, severe SUD is associated with increased resting ACC-LC connectivity in bipolar disorder (figure 1). Bipolar disorder predisposes strongly to activation with negative arousal, and impulsivity with sensitization, but these general behavioural mechanisms extend beyond bipolar disorder.
4. Interaction between impulsivity and sensitization in timing of suicide risk in bipolar disorder
(a). Suicidal behaviour
Depression and impulsivity, combined with susceptibility to behavioural sensitization, can generate severe behavioural risk, including suicidality. Despite advances in characterizing long-term risk and relevant behavioural processes, suicide itself remains difficult to predict. The interaction of complex behavioural systems in impulsivity and expression of sensitization can result in unpredictable behaviour including suicidality [91] and substance-use relapse [47].
Suicide is the leading cause of injury mortality in the USA, exceeding motor vehicle accidents and homicide [92]. Medically severe suicide attempts (MSSA) predict high risk for subsequent suicide and other premature mortality, including homicide and accidents [93]. Post-hospital survival time correlated negatively with impulsivity [10]. In MSSA, 60% of subsequent premature mortality was not suicide, suggesting a more general high-risk process [94]. Impulsivity-linked predisposition to potentially lethal behaviour, with unpredictably variable action dysregulation, extends across diagnoses [95]. Most suicides occur on the first attempt [96]. While MSSA is considered a strong predictor of risk, we must develop predictors that do not automatically exclude half of the suicides. A measure of susceptibility to unpredictably variable action dysregulation may provide such a predictor.
(b). Impulsivity and suicide risk
Impulsivity is woven into suicidal behaviour. Impulsive versus planned attempts is a false dichotomy: trait impulsivity was higher in individuals with both a plan and an attempt than with either an attempt or plan alone [97]. Impulsive individuals are more likely to act on a plan, and state-dependent increases in impulsivity during ongoing depressive episodes potentially increase suicide risk [6]. We studied impulsivity, alcohol use and clinical features in people with self-inflicted gunshot wounds requiring helicopter rescue [98], a CDC/NIAAA-funded case-control study of MSSA at Level I trauma centres [99], and impulsivity in bipolar disorder, personality disorders and controls [6]. MSSA, even planned, were associated with increased RRI [6]; momentary expression of impulsivity could increase the likelihood of initiating a ‘planned’ attempt [6,97,99]. Impulsive attempters had less expectation to die but used more violent methods [99]; they had elevated Beck Hopelessness, but normal depression scores [99]. Impulsivity, with low perseverance, resilience and future sense [12,100], is, operationally, hopelessness. By increasing activation and hopelessness, increased impulsivity, whether intentional (alcohol [98,101]) or unintentional (stress or overstimulation), may be potentiated by sensitization and facilitate suicidal action.
(c). Mechanisms in timing of suicidal behaviour
Predicting fluctuations in behaviour involving immediate or short-term action regulation, such as suicidality, is difficult. Short-term variability of suicidal ideation, in subjects with suicide attempts over the previous year or current hospitalization for suicide risk, showed high and unpredictable within-day variation. Risk factors including hopelessness, burdensomeness and loneliness correlated with suicidal ideation (SI), but did not predict short-term change in SI [95]. Further, attempts typically occur within ten minutes after the onset of SI specifically related to that attempt [102].
Once sensitization has developed, the sensitized characteristic (for example, RRI), appears to be, effectively, a dormant trait, readily and unpredictably activated by environmental or endogenous analogues of sensitizing stimuli. Therefore, an individual with generally unremarkable behaviour can respond to apparently small situational changes with impulsivity and anhedonia. The unpredictability of suicidal behaviour [91,95] may resemble that of fluctuating expression of sensitized behaviour in substance-use [47,103]. Arousal, elicited by sensitization and unpredictably magnified by impulsivity [1,3,29,36], is central to immediate suicide risk. Individuals and contexts with high risk for eventual suicide can be identified, but the behaviour itself is difficult to predict.
Suicide (and similar impulsivity-related behaviours) results from interactions of factors with varying time structures:
-
(i)
Lifetime, including genetic or environmental characteristics.
-
(ii)
Long-term, including behavioural sensitization with early trauma, addiction or chronic recurrent illness.
-
(iii)
Intermediate to short-term, including illness episodes or stressors.
-
(iv)
Immediate, including immediate stressors or reminders of the trauma, overstimulation, addiction-related stimuli and similar circumstances.
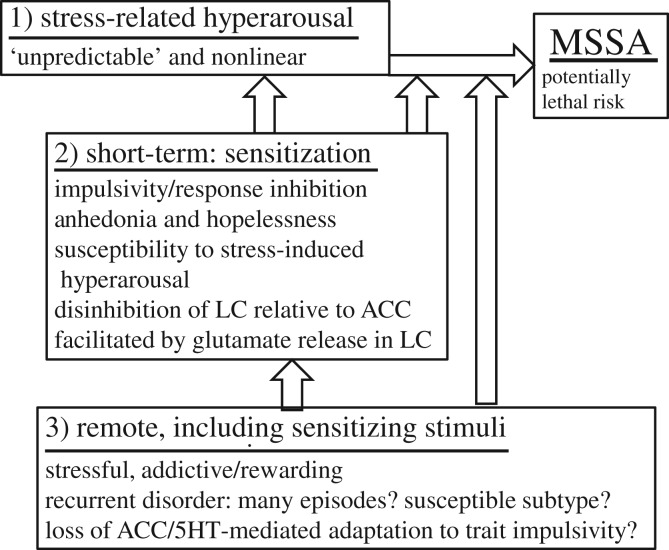
Figure 2 summarizes potential temporal interactions proposed in this paper. Each risk category interacts with the others, generally in a nonlinear manner [91]; immediate risk, the most difficult to predict, is the strongest determinant of exactly when a suicidal act may occur. Real-time monitoring of behaviour, symptoms or arousal may be valuable for measuring the timing of risky internal states [29,47].
Figure 2.
Interactions among mechanisms in illness progression and high-risk behaviour: (1) immediate, including stress-related arousal; (2) short-term, including expression of sensitized behaviour; and (3) long-term, including exposure to potentially sensitizing stimuli. Bipolar disorder is enriched in each risk category. Each risk category can interact with the other categories and can contribute to risk in its own right. MSSA, medically severe suicide attempt.
(d). Treatment considerations
Illness progression and behavioural complications of bipolar disorder resemble those of other progressive/recurrent psychiatric disorders including major depressive disorder, schizophrenia, substance-related disorders and PTSD, all characterized by increased trait impulsivity and course characteristics analogous to those of bipolar disorder, consistent with characteristics of suicidality and lethal behaviour that cross diagnoses [6,93–96]. The complex interaction between long-term and immediate factors calls for multi-tiered management:
-
(i)
Identify risk for recurrent/progressive illness (family and developmental history, biomarkers once developed).
-
(ii)
Identify risk for a progressive, possibly sensitizing illness course. We know of no currently available biomarkers for susceptibility to sensitization, which varies greatly across individuals.
-
(iii)
Prevention by reducing exposure of high-risk individuals to potentially sensitizing stimuli early in life [58], especially with diagnosis or family history of recurrent psychiatric illness; this may involve treatment of the mother and/or the patient with therapies aimed at reframing stressors as escapable and vigorous prevention or treatment of addictive disorders.
-
(iv)
Rapid, crisis-related pharmacological blockade of sensitization expression, for example, by NMDA receptor blockade, shown to prevent expression of sensitization [49], reduce impulsivity [86] and to reduce depression and suicidality [104].
-
(v)
Real-time monitoring of behaviour to identify, and intervene in, high-risk patients [64], or, more practical, to identify individuals susceptible to unpredictably variable behaviour dyscontrol [16,93] for preventive treatment.
-
(vi)
Based on (v), long-term treatment to reduce risk over time. For example, lithium has been shown to reduce suicide mortality across diagnoses [105].
These strategies, combining longer-term effects of psychotherapies, family-based treatments and long-term treatments, with faster-acting but shorter-term treatments including NMDAR antagonists and their congeners, are needed in people at high risk.
(e). Summary
Suicide and related potentially lethal behaviour are related to impulsivity, which can have a decisive role even with an existing plan. Suicidality combines impulsivity and negative affect, both potential expressions of sensitization. The complex interactions by which underlying impulsivity is expressed, combining long- and short-term conditions, can lead to chaotic behaviour requiring nonlinear analysis and fine-grained monitoring. Treatment will require identification of risk, prevention/treatment of long-term risk factors and acute treatment of severe exacerbations.
5. Conclusion
Behavioural sensitization and impulsivity appear to arise from responses to highly salient stimuli, are linked by hyperarousal, and can lead to progression of psychiatric disorders. Illness progression could result from behavioural sensitization by addiction/substance abuse, stressors, recurrent episodes or other sensitizing agents. Expression of sensitization can trigger increased impulsivity, mixed states combining impulsivity and negative affect, and high-lethality behaviour including suicidality. Premorbid impulsivity may predispose to behavioural sensitization, resulting in further increased impulsivity. Sensitization requires uncoupling of the regulatory effects of ACC and serotonergic function over glutamate-related activating effects of LC NE, contributing to increased RRI. This could result from sensitization-associated deterioration of mechanisms otherwise protective against the expression of potential impulsivity. Fluctuations in the expression of sensitization can be rapid, producing unpredictable high-lethality behaviour, including suicide attempts. Effective prevention and treatment require short- and long-term measures, consistent with underlying behavioural mechanisms. Because of the unpredictable timing of sensitized behaviour, identification of susceptibility and preventive treatment are paramount.
Acknowledgements
This work was supported in general by the Program on Mood and Anxiety Disorders, Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, and the Michael E. DeBakey Veterans Affairs Medical Center.
Data accessibility
Neuroimaging data relevant to figure 1 is available from co-author Ramiro Salas (rsalas@bcm.edu).
Competing interests
We declare we have no competing interests.
Funding
Funding for the study in Figure 1 was provided by The Robert and Janice McNair Foundation, Houston, TX, USA. We are also grateful for grant support from National Institutes of Health R61MH10540 (ML, BO, SJM, ACS) and American Foundation for Suicide Prevention STR-1-004-16 (ML, SJM, ACS). ML, SJM, ACS, Department of Defense Congressionally Directed Medical Research Programs, Award no. W81XWH-17-1-0488. Glutamate Receptor and Kynurenine Pathway Functioning in the Pathobiology of Gulf War Illness.
References
- 1.Zhang S, Hu Sien H, Hu J, Wu P-L, Chao HH, Li C-SR. 2015. Barratt impulsivity and neural regulation of physiological arousal. PLoS ONE 10, e0129139 ( 10.1371/journal.pone.0129139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandel ER. 1980. Cellular insights into the multivariant nature of arousal. In Neural mechanisms in behavior (ed. McFadden D.), pp. 290–291. New York, NY: Springer. [Google Scholar]
- 3.Malmo RB. 1959. Activation: a neuropsychological dimension. Psychol. Rev. 66, 367–386. ( 10.1037/h0047858) [DOI] [PubMed] [Google Scholar]
- 4.Fineberg NA, et al. 2010. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35, 591–604. ( 10.1038/npp.2009.185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moeller FG, Barratt ES, Dougherty DM, Schmitz S, Swann AC. 2001. Psychiatric aspects of impulsivity. Am. J. Psychiatry 158, 1783–1793. ( 10.1176/appi.ajp.158.11.1783) [DOI] [PubMed] [Google Scholar]
- 6.Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. 2005. Increased impulsivity associated with severity of suicide attempt history in bipolar disorder. Am. J. Psychiatry 162, 1680–1687. ( 10.1176/appi.ajp.162.9.1680) [DOI] [PubMed] [Google Scholar]
- 7.Swann AC, Lijffijt M, Lane SD, Steinberg JL, Acas MD, Cox B, Moeller FG. 2013. Pre-attentional information processing and impulsivity in bipolar disorder. J. Psychiatr. Res. 47, 1917–1924. ( 10.1016/j.jpsychires.2013.08.018) [DOI] [PubMed] [Google Scholar]
- 8.Moeller FG, Dougherty D, Barratt E, Oderinde V, Mathias C, Andrew HR, Swann A. 2002. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alc. Depend. 68, 105–111. ( 10.1016/S0376-8716(02)00106-0) [DOI] [PubMed] [Google Scholar]
- 9.Mathias CW, Stanford MS, Marsh DM, Frick PJ, Moeller FG, Swann AC, Dougherty DM. 2007. Characterizing aggressive behavior with the impulsive/premeditated aggression scale among adolescents with conduct disorder. Psychiatry Res. 151, 231–242. ( 10.1016/j.psychres.2006.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordstrom P, Gustavsson P, Edman G, Asberg M. 1996. Temperamental vulnerability and suicide risk after attempted suicide. Suicide Life Threat. Behav. 26, 380–394. [PubMed] [Google Scholar]
- 11.Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. 2009. Fifty years of the Barratt impulsiveness scale: an update and review. Pers. Indiv. Diff. 47, 385–395. ( 10.1016/j.paid.2009.04.008) [DOI] [Google Scholar]
- 12.Swann AC, Bjork JM, Moeller FG, Dougherty DM. 2002. Two models of impulsivity: relationship to personality traits and psychopathology. Biol. Psychiatry 51, 988–994. ( 10.1016/S0006-3223(01)01357-9) [DOI] [PubMed] [Google Scholar]
- 13.Grant JE, Chamberlain PR. 2014. Impulsive action and impulsive choice across substance and behavioral addictions: cause or consequence? Addict. Behav. 39, 1632–1639. ( 10.1016/j.addbeh.2014.04.022) [DOI] [PubMed] [Google Scholar]
- 14.Logan GD. 1994. On the ability to inhibit thought and action: a user's guide to to stop signal paradigm. In Inhibitory processes in attention, memory, and language (eds Dagenbach D, Carr TH), pp. 189–239. San Diego, CA: Academic Press. [Google Scholar]
- 15.Winstanley CA, Eagle DM, Robbins TW. 2006. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin. Psychol. Rev. 26, 379–395. ( 10.1016/j.cpr.2006.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. 2009. Severity of bipolar disorder is associated with impaired response inhibition. J. Affect. Disord. 116, 30–36. ( 10.1016/j.jad.2008.10.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiehl KA, Liddle PF, Hopfinger JB. 2000. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology 37, 216–223. ( 10.1111/1469-8986.3720216) [DOI] [PubMed] [Google Scholar]
- 18.Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. 2009. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc. Natl Acad. Sci. USA 106, 9453–9458. ( 10.1073/pnas.0900491106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuo K, et al. 2009. Anterior cingulate volumes associated with trait impulsivity in individuals with bipolar disorder. Bipolar Disord. 11, 628–636. ( 10.1111/j.1399-5618.2009.00732.x) [DOI] [PubMed] [Google Scholar]
- 20.Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. 2009. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum. Brain Mapp. 30, 1188–1195. ( 10.1002/hbm.20588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aston-Jones G, Cohen JD. 2005. Adaptive gain and the role of the locus coeruleus–norepinephrine system in optimal performance. J. Comp. Neurol. 493, 99–110. ( 10.1002/cne.20723) [DOI] [PubMed] [Google Scholar]
- 22.Aston-Jones G, Akaoka H, Charlety P, Chouvet G. 1991. Serotonin selectively attenuates glutamate-evoked activation of noradrenergic locus coeruleus neurons. J. Neurosci. 9, 760–769. ( 10.1523/JNEUROSCI.11-03-00760.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM. 1999. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol. Psychiatry 45, 26–31. ( 10.1016/S0006-3223(98)00296-0) [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Green TA, Theobald DE, Birnbaum SG, Graham DL, Zeeb FD, Nestler EJ, Winstanley CA. 2010. Yohimbine increases impulsivity through activation of cAMP response element binding in the orbitofrontal cortex. Biol. Psychiatry 67, 649–656. ( 10.1016/j.biopsych.2009.11.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swann AC, Lijffijt M, Lane SD, Cox B, Steinberg JL, Moeller FG. 2013. Norepinephrine and impulsivity: effects of acute yohimbine. Psychopharmacology (Berlin) 229, 83–94. ( 10.1007/s00213-013-3088-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swann AC, Birnbaum D, Schneider L, Dougherty DM, Moeller FG. 2005. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol. Psychiatry 57, 1209–1211. ( 10.1016/j.biopsych.2005.02.007) [DOI] [PubMed] [Google Scholar]
- 27.Swann AC, Koslow SH, Katz MM, Maas JW, Javaid J, Secunda SK, Robins E. 1987. Lithium treatment of mania: CSF and urinary monoamine metabolites and treatment outcome. Arch. Gen. Psychiatry 44, 345–354. ( 10.1001/archpsyc.1987.01800160057008) [DOI] [PubMed] [Google Scholar]
- 28.Swann AC, Anderson J, Dougherty DM, Moeller FG. 2001. Measurement of interepisode impulsivity in bipolar disorder. Psychiatry Res. 101, 195–197. ( 10.1016/S0165-1781(00)00249-3) [DOI] [PubMed] [Google Scholar]
- 29.Anderson KJ, Revelle W. 1994. Impulsivity and time of day: is rate of change in arousal a function of impulsivity? J. Pers. Soc. Psychol. 67, 334–344. ( 10.1037/0022-3514.67.2.334) [DOI] [PubMed] [Google Scholar]
- 30.Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. 2006. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch. Gen. Psychiatry 63, 1386–1395. ( 10.1001/archpsyc.63.12.1386) [DOI] [PubMed] [Google Scholar]
- 31.Strakowski SM, Sax KW, Setters MJ, Keck PE Jr. 1996. Enhanced response to repeated d-amphetamine challenge: evidence for behavioral sensitization in humans. Biol. Psychiatry 40, 872–880. ( 10.1016/0006-3223(95)00497-1) [DOI] [PubMed] [Google Scholar]
- 32.Kalivas PW. 1995. Interactions between dopamine and excitatory amino acids in behavioral sensitization to psychostimulants. Drug Alcohol Depend. 37, 95–100. ( 10.1016/0376-8716(94)01063-Q) [DOI] [PubMed] [Google Scholar]
- 33.Robinson TE, Becker JB. 1986. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 396, 157–198. ( 10.1016/0165-0173(86)90002-0) [DOI] [PubMed] [Google Scholar]
- 34.Lee YS, Kim TH, Choi SL, Lee S, Bhak J, Kaang BK. 2009. Transcriptome and protein domain analyses in Aplysia nervous system with evolutionary implications. Commun. Integr. Biol. 2, 21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunelli M, Castellucci V, Kandel ER. 1976. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science 194, 1176–1178. ( 10.1126/science.186870) [DOI] [PubMed] [Google Scholar]
- 36.Valzachi MC, Teodorov E, Marcourakis T, Bailey A, Camarini R. 2013. Enhancement of behavioral sensitization, anxiety-like behavior, and hippocampal and frontal cortical CREB levels following cocaine abstinence in mice exposed to cocaine during adolescence. PLoS ONE 8, e78317 ( 10.1371/journal.pone.0078317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booij L, et al. 2016. Dopamine cross-sensitization between psychostimulant drugs and stress in healthy male volunteers. Transl. Psychiatry 6, 740 ( 10.1038/tp.2016.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broadbent J, Weitemier AZ. 1999. Dizocilpine (MK-801) prevents the development of sensitization to ethanol in DBA/2 J mice. Alcohol Alcohol 34, 283–288. ( 10.1093/alcalc/34.3.283) [DOI] [PubMed] [Google Scholar]
- 39.DiFranza JR, Wellman RJ. 2007. Sensitization to nicotine: how the animal literature might inform future human research. Nicotine Tob. Res. 9, 9–20. ( 10.1080/14622200601078277) [DOI] [PubMed] [Google Scholar]
- 40.Haile CN, Grandpre T, Kosten TA. 2001. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology (Berl.) 154, 213–220. ( 10.1007/s002130000650) [DOI] [PubMed] [Google Scholar]
- 41.Kessing LV, Andersen PK. 2016. Evidence for clinical progression of unipolar and bipolar disorders. Acta Psychiatr. Scand. 135, 51–64. ( 10.1111/acps.12667) [DOI] [PubMed] [Google Scholar]
- 42.Anderson SF, Monroe SM, Rohde P, Lewinsohn PM. 2015. Questioning kindling: an analysis of cycle acceleration in unipolar depression. Clin. Psychol. Sci. 4, 229–238. ( 10.1177/2167702615591951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Post RM. 2016. Epigenetic basis of sensitization to stress, affective episodes, and stimulants: implications for illness progression and prevention. Bipolar Disord. 18, 315–324. ( 10.1111/bdi.12401) [DOI] [PubMed] [Google Scholar]
- 44.Swann AC, Secunda SK, Stokes PE, Croughan J, Davis JM, Koslow SH, Maas JW. 1990. Stress, depression, and mania: relationship between perceived role of stressful events and clinical and biochemical characteristics. Acta Psychiatr. Scand. 81, 389–397. ( 10.1111/j.1600-0447.1990.tb05469.x) [DOI] [PubMed] [Google Scholar]
- 45.Hayley S, Merali Z, Anisman H. 2003. Stress and cytokine-elicited neuroendocrine and neurotransmitter sensitization: implications for depressive illness. Stress 6, 19–32 ( 10.1080/1025389031000091167) [DOI] [PubMed] [Google Scholar]
- 46.Bonate PL, Swann AC, Silverman PB. 1997. Context-dependent cross-sensitization between cocaine and amphetamine. Life Sci. 60, PL1–PL7. ( 10.1016/S0024-3205(97)87488-7) [DOI] [PubMed] [Google Scholar]
- 47.Jones A, Christiansen P, Nederkoorn C, Houben K, Field M. 2013. Fluctuating disinhibition: implications for the understanding and treatment of alcohol and other substance use disorders. Front. Psychiatry 4, 140 ( 10.3389/fpsyt.2013.00140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanderschuren LJ, Beemster P, Schoffelmeer AN. 2003. On the role of noradrenaline in psychostimulant-induced psychomotor activity and sensitization. Psychopharmacology (Berl.) 169, 176–185. ( 10.1007/s00213-003-1509-8) [DOI] [PubMed] [Google Scholar]
- 49.Jiménez-Rivera CA, Feliu-Mojer M, Vázquez-Torres R. 2006. Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Ann. NY Acad. Sci. 1074, 390–402. ( 10.1196/annals.1369.039) [DOI] [PubMed] [Google Scholar]
- 50.Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. 2005. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav. Brain Res. 162, 127–134 ( 10.1016/j.bbr.2005.03.009) [DOI] [PubMed] [Google Scholar]
- 51.Anisman H, Kelly O, Hayley S, Borowski T, Merali Z, McIntyre RC. 2000. Acoustic startle and fear-potentiated startle in rats selectively bred for fast and slow kindling rates: relation to monoamine activity. Eur. J. Neurosci. 12, 4405–4406. ( 10.1046/j.0953-816X.2000.01216.x) [DOI] [PubMed] [Google Scholar]
- 52.Jentsch JD, Taylor JR. 1999. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl.) 146, 373–390. ( 10.1007/PL00005483) [DOI] [PubMed] [Google Scholar]
- 53.Gaytan O, Nason R, Alagugurusamy R, Swann AC, Dafny N. 2000. MK-801 blocks the development of sensitization to the locomotor effects of methylphenidate. Brain Res. Bull. 51, 485–492. ( 10.1016/S0361-9230(99)00268-3) [DOI] [PubMed] [Google Scholar]
- 54.Wanchoo SJ, Swann AC, Dafny N. 2009. Descending glutamatergic pathways from the PFC are involved in acute and chronic actions of methylphenidate. Brain Res. 1301, 68–79. ( 10.1016/j.brainres.2009.08.095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steketee JD. 2003. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res. Brain Res. Rev. 41, 203–228. ( 10.1016/S0165-0173(02)00233-3) [DOI] [PubMed] [Google Scholar]
- 56.Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. 2002. α1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J. Neurosci. 22, 2873–2884. ( 10.1523/JNEUROSCI.22-07-02873.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alttoa A, Eller M, Herm L, Rinken A, Harro J. 2007. Amphetamine-induced locomotion, behavioral sensitization to amphetamine, and striatal D2 receptor function in rats with high or low spontaneous exploratory activity: differences in the role of locus coeruleus. Brain Res. 1131, 138–148. ( 10.1016/j.brainres.2006.10.075) [DOI] [PubMed] [Google Scholar]
- 58.Thayer JF, Friedman BH. 2002. Stop that! Inhibition, sensitization, and their neurovisceral concomitants. Scand. J. Psychol. 43, 123–130. ( 10.1111/1467-9450.00277) [DOI] [PubMed] [Google Scholar]
- 59.Haleem DJ. 2013. Extending therapeutic use of psychostimulants: focus on serotonin-1A receptor. Prog. Neuropsychopharmacoliol. Psychiatry 46, 170–180. ( 10.1016/j.pnpbp.2013.07.015) [DOI] [PubMed] [Google Scholar]
- 60.Salomon L, Lanteri C, Glowinski J, Tassin JP. 2006. Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc. Natl Acad. Sci. USA 103, 7476–7481. ( 10.1073/pnas.0600839103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heidbreder CA, Oertle T, Feldon J. 1999. Dopamine and serotonin imbalances in the left anterior cingulate and pyriform cortices following the repeated intermittent administration of cocaine. Neuroscience 89, 701–715. ( 10.1016/S0306-4522(98)00339-X) [DOI] [PubMed] [Google Scholar]
- 62.Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. 1991. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 28, 588–595. ( 10.1111/j.1469-8986.1991.tb01999.x) [DOI] [PubMed] [Google Scholar]
- 63.Davis M, Walker DL, Miles L, Grillon C. 2010. Phasic vs. sustained fear in rats and humans. Role of the extended amygdala. Neuropyschopharmacology 35, 105–135. ( 10.1038/npp.2009.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ballard ED, et al. 2014. Increased fear-potentiated startle in major depressive disorder patients with lifetime history of suicide attempt. J. Affect. Disord. 162, 34–38. ( 10.1016/j.jad.2014.03.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muneer A. 2016. Staging models in bipolar disorder: a systematic review of the literature. Clin. Psychopharmacol. Neurosci. 14, 117–130. ( 10.9758/cpn.2016.14.2.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duffy A, Vandeleur C, Heffer N, Preisig M. 2017. The clinical trajectory of emerging bipolar disorder among the high-risk offspring of bipolar parents: current understanding and future considerations. Int. J. Bipolar Disord. 5, 37 ( 10.1186/s40345-017-0106-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greil W, Kleindienst N, Erazo N, Müller-Oerlinghausen B. 1998. Differential response to lithium and carbamazepine in the prophylaxis of bipolar disorder. J. Clin. Psychopharmacol. 18, 455–460. ( 10.1097/00004714-199812000-00007) [DOI] [PubMed] [Google Scholar]
- 68.Duffy A, Alda M, Kutcher S, Cavazzoni P, Robertson C, Grof E, Grof P. 2002. A prospective study of offspring of bipolar parents responsive and nonresponsive to lithium treatment. J. Clin. Psychiatry 65, 11 171–11 178. [DOI] [PubMed] [Google Scholar]
- 69.Goldberg JF, Harrow M, Grossman LS. 1995. Recurrence of affective episodes in bipolar and unipolar mood disorders at follow-up. Br. J. Psychiatry 166, 382–385. ( 10.1192/bjp.166.3.382) [DOI] [PubMed] [Google Scholar]
- 70.Post RM, Kalivas P. 2013. Bipolar disorder and substance abuse: pathological and therapeutic implications of their comorbidity and cross-sensitization. Br. J. Psychiatry 202, 172–176. ( 10.1192/bjp.bp.112.116855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dienes KA, Hammen C, Henry RM, Cohen AN, Daley SE. 2006. The stress sensitization hypothesis: understanding the course of bipolar disorder. J. Affect. Disord. 95, 43–49. ( 10.1016/j.jad.2006.04.009) [DOI] [PubMed] [Google Scholar]
- 72.Weiss RB, Stange JP, Boland EM, Black SK, LaBelle DR, Abramson LY, Alloy LB. 2015. Kindling of life stress in bipolar disorder: comparison of sensitization and autonomy models. J. Abnorm. Psychol. 124, 4–16. ( 10.1037/abn0000014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swann AC, Steinberg JL, Lijffijt M, Moeller FG. 2009. Continuum of depressive and manic mixed states in patients with bipolar disorder: quantitative measurement and clinical features. World Psychiatry 8, 166–172. ( 10.1002/j.2051-5545.2009.tb00245.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swann AC, Moeller FG, Steinberg JL, Schneider L, Barratt ES, Dougherty DM. 2007. Manic symptoms and impulsivity during bipolar depressive episodes. Bipolar Disord. 9, 206–212. ( 10.1111/j.1399-5618.2007.00357.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swann AC, Lafer B, Perugi G, Frye MA, Bauer M, Bahk WM, Scott J, Ha K, Suppes T. 2013. Bipolar mixed states: an international society for bipolar disorders task force report of symptom structure, course of illness, and diagnosis. Am. J. Psychiatry 170, 31–42. ( 10.1176/appi.ajp.2012.12030301) [DOI] [PubMed] [Google Scholar]
- 76.Swann AC, Stokes PE, Secunda SK, Maas JW, Bowden CL, Berman N, Koslow SH. 1994. Depressive mania vs agitated depression: biogenic amine and hypothalamic–pituitary–adrenocortical function. Biol. Psychiatry 35, 803–813. ( 10.1016/0006-3223(94)91143-6) [DOI] [PubMed] [Google Scholar]
- 77.Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. 2009. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disord. 11, 280–288. ( 10.1111/j.1399-5618.2009.00678.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanches M, et al. 2014. Impulsivity in children and adolescents with mood disorders and unaffected offspring of bipolar parents. Compr. Psychiatry. 55, 1337–1341. ( 10.1016/j.comppsych.2014.04.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee TY, et al. 2013. Neural correlate of impulsivity in subjects at ultra-high risk for psychosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 45, 165–169. ( 10.1016/j.pnpbp.2013.04.008) [DOI] [PubMed] [Google Scholar]
- 80.Lijffijt M, Swann AC, Moeller FG. 2008. Biological substrates of personality traits associated with aggression. In Personality theories and models (eds Boyle GJ, Matthews G, Saklofske DH), pp. 334–356. Los Angeles, CA: Sage Publishing. [Google Scholar]
- 81.Nieuwenhuis S, Yeung N, van der Wildenberg W, Ridderinkhof KR. 2003. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effect of response conflict and trial type frequency. Cogn. Affect. Behav. Neurosci. 3, 17–26. ( 10.3758/CABN.3.1.17) [DOI] [PubMed] [Google Scholar]
- 82.Carli M, Samanin R. 2000. The 5-HT(1A) receptor agonist 8-OH-DPAT reduces rats' accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT(1A) receptors. Psychopharmacology (Berl.) 49, 259–268. ( 10.1007/s002139900368) [DOI] [PubMed] [Google Scholar]
- 83.Fallgatter AJ, Bartsch AJ, Herrmann MJ. 2002. Electrophysiological measurements of anterior cingulate function. J. Neural Transm. 109, 977–988. ( 10.1007/s007020200080) [DOI] [PubMed] [Google Scholar]
- 84.Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. 2004. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 56, 129–140. ( 10.1016/j.bandc.2004.09.016) [DOI] [PubMed] [Google Scholar]
- 85.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. 1999. Neural correlates of memories of childhood sexual abuse in women with and without post-traumatic stress disorder. Am. J. Psychiatry 156, 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoerst M, Weber-Fahr W, Tunc-Charka N, Ruf M, Bohus M, Schmahl C, Ende G. 2010. Correlations of glutamate levels in the anterior cingulate cortex with self-reported impulsivity in patients with borderline personality disorder and healthy controls. Arch. Gen. Psychiatry 67, 946–954. ( 10.1001/archgenpsychiatry.2010.93) [DOI] [PubMed] [Google Scholar]
- 87.Marhe R, van de Wetering BJM, Franken IHA. 2013. Error-related brain activity predicts cocaine use after treatment at 3-month follow-up. Biol. Psychiatry 73, 782–788. ( 10.1016/j.biopsych.2012.12.016) [DOI] [PubMed] [Google Scholar]
- 88.Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC. 2009. Early adverse events, hypothalamic–pituitary–adrenocortical activity, and rostral anterior cingulate volume in major depressive disorder. PLoS ONE 47, e4887 ( 10.1371/journal.pone.0004887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ambrosi E, Arciniegas DB, Madan A, Curtis KN, Patriquin MA, Jorge RE, Spalletta G, Fowler JC, Frueh BC, Salas R. 2017. Insula and amygdala resting-state functional connectivity differentiate bipolar from unipolar depression. Acta Psychiatr. Scand. 136, 129–139. ( 10.1111/acps.12724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hermeniuk R, et al. 2008. Validation of the alcohol, smoking, and substance involvement screening test (ASSIST). Addiction 103, 1039–1047. ( 10.1111/j.1360-0443.2007.02114.x) [DOI] [PubMed] [Google Scholar]
- 91.Fartacek C, Schiepek G, Kunroth S, Fartacek R, Ploder M. 2016. Real-time monitoring of nonlinear suicidal dynamics: methodology and a demonstrative case report. Front. Psychiatry 7, 1–14. ( 10.3389/fpsyg.2016.00130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rockett IR, et al. 2012. Leading causes of unintentional and intentional injury mortality: United States, 2000–2009. Am. J. Public Health 102, e84-e92. ( 10.2105/AJPH.2012.300960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nordstrom P, Samuelsson M, Asberg M. 1995. Survival analysis of suicide risk after attempted suicide. Acta Psychiatr. Scand. 91, 336–340. ( 10.1111/j.1600-0447.1995.tb09791.x) [DOI] [PubMed] [Google Scholar]
- 94.Beghi M, Rosenbaum JF, Cerri C, Cornaggia CM. 2013. Risk factors for fatal and nonfatal repetition of suicide attempts: a literature review. Neuropsychiatr. Dis. Treat 9, 1725–1736. ( 10.2147/NDT.S40213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kleiman EM, Turner BJ, Fedor S, Beale EE, Huffman JC, Nock MK. 2017. Examination of real-time fluctuations in suicidal ideation and its risk factors: results from two ecological momentary assessment studies. J. Abnorm. Psychol. 126, 726–738. ( 10.1037/abn0000273) [DOI] [PubMed] [Google Scholar]
- 96.Michaelis BH, Goldberg JF, Singer TM, Garno JL, Ernst CL, Davis GP. 2003. Characteristics of first suicide attempts in single versus multiple suicide attempters with bipolar disorder. Compr. Psychiatry 44, 15–20. ( 10.1053/comp.2003.50004) [DOI] [PubMed] [Google Scholar]
- 97.Witte TK, Merrill KA, Stellrecht NE, Bernert RA, Hollar DL, Schatschneider C, Joiner TE Jr. 2008. ‘Impulsive’ youth suicide attempters are not necessarily all that impulsive. J. Affect. Disord. 107, 107–116. ( 10.1016/j.jad.2007.08.010) [DOI] [PubMed] [Google Scholar]
- 98.Peterson LG, Peterson M, O'Shanick G, Swann AC. 1985. Violent suicide attempts: lethality of method vs intent. Am. J. Psychiatry 142, 228–231. ( 10.1176/ajp.142.2.228) [DOI] [PubMed] [Google Scholar]
- 99.Simon TR, Swann AC, Powell KE, Potter LB, Kresnow M-J, O'Carroll PW. 2001. Characteristics of impulsive suicide attempts and attempters. Suicide Life-Thr. Behav. 32, 49–59. ( 10.1521/suli.32.1.5.49.24212) [DOI] [PubMed] [Google Scholar]
- 100.Swann AC, Steinberg JL, Lijffijt M, Moeller FG. 2008. Impulsivity: differential relationship to depression and mania in bipolar disorder. J. Affect. Disord. 106, 241–248. ( 10.1016/j.jad.2007.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Powell KE, Kresnow M-J, Mercy JA, Potter LB, Swann AC, Frankowski RF, Lee RK, Bayer TL. 2001. Alcohol consumption and nearly lethal suicide attempts. Suicide Life-Thr. Behav. 32, 30–41. ( 10.1521/suli.32.1.5.30.24208) [DOI] [PubMed] [Google Scholar]
- 102.Deisenhammer EA, Ing CM, Strauss R, Kemmler G, Hinterhuber H, Weiss EM. 2009. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J. Clin. Psychiatry 70, 19–24. ( 10.4088/JCP.07m03904) [DOI] [PubMed] [Google Scholar]
- 103.Paine TA, Dringenberg HC, Olmstead MC. 2003. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav. Brain Res. 147, 135–147. ( 10.1016/S0166-4328(03)00156-6) [DOI] [PubMed] [Google Scholar]
- 104.Price RB, et al. 2014. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress. Anxiety 31, 335–343. ( 10.1002/da.22253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith KA, Cipriani A. 2017. Lithium and suicide in mood disorders. Update and meta-review of the scientific literature. Bipolar Disord. 19, 575–586. ( 10.1111/bdi.12543) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Neuroimaging data relevant to figure 1 is available from co-author Ramiro Salas (rsalas@bcm.edu).