Abstract
Hypertension is a becoming a major threat to the world. Angiotensin converting enzyme (ACE) is a key part in the renin angiotensin aldosterone system (RAAS) which control blood pressure. Over expression of RAAS is related with vascular hypertension, ACE inhibition has turned into a noteworthy target for controlling hypertension. In the search of lead molecules from plant origin as a substitute for toxic synthetic drugs, 25 Indian medicinal plants and foods were screened for their ACE inhibitory activity. IC50 (50% inhibition of ACE) values of hydroalcoholic crude extracts and fraction were determined by a colorimetric method. Active fractions were further screened to determine the enzyme kinetics, mode, specificity and mechanism of inhibition. Standardization was done by determining total phenolics and flavonoids as gallic acid and quercetin equivalents/mg of extract respectively. Among 25 crude extracts, Cynara scolymus extract showed the best activity, IC50 value 356.62 μg/mL. ACE inhibition resulting from protein precipitation was highest in Coscinium fenestratum. Lineweaver-Burk plots revealed a competitive mode of inhibition for Punica granatum ethyl acetate fraction. Fractions of Cassia occidentalis, Cynara scolymus and Embelia ribes were found to be non-specific inhibitors of ACE. Embelia ribes, Cassia occidentalis and Coscinium fenestratum fractions inhibited the ACE by Zn2+ ion chelation. Research revealed the potential of tested plants fractions as ACE inhibitors along with their inhibition kinetics and mechanism of inhibition. These active plant fractions might find importance in the development of potential antihypertensive agents after further investigations using preclinical and clinical trials.
Keywords: Angiotensin converting enzyme, Lineweaver-burk plots, Enzyme kinetics, Mode of inhibition
Abbreviations: ACE, Angiotensin I Converting Enzyme; BAPNA, a-N-benzoyl-dl-arginine-Pnitroanilide HCl; BP, blood pressure; BSA, bovine serum albumin; BSC, benzene sulphonyl chloride; CH2Cl2, dichloromethane; DMSO, dimethyl sulphoxide; EtOAc, Ethyl acetate; EtOH, ethanol; GAEs, gallic acid equivalents; HA, hippuric acid; HCl, Hydrochloric acid; HHL, hippury-l-histidyl-l-leucine; IC50, half maximal inhibitory concentration; Km, Michaelis-Menten constant; M, Molar; MeOH, methanol; mg, milligram; mL, milli litre; Mm, Milli mole; Mu, Milli units; n-BuOH, n-butanol; ng, nano gram; QEs, quercetin equivalents; RAS, renin-angiotensin system; TCA, Trichloroacetic acid; TFA, trifluoroacetic acid; UV, ultra violet; Vmax, Maximum velocity; Zn2+, Zinc ion; ZnCl2, Zinc chloride
Graphical abstract
1. Introduction
Hypertension is a major risk factor for many cardiovascular diseases such as arteriosclerosis, congestive heart failure, coronary heart disease, end-stage renal diseases, myocardial infarction, and stroke.1 In the year 2000, about 26.4% of the world's population suffered hypertension, and it is increasing at an alarming rate with a prediction of 60% in 2025.2 Blood pressure is regulated by different biochemical pathways, one of the major components of blood pressure regulation physiology is the renin-angiotensin system (RAS). Angiotensin I (an inactive form of decapeptide) is produced by catalytic cleavage by renin from angiotensinogen; Angiotensin I is further cleaved to angiotensin II(octapeptide) by angiotensin I converting enzyme (ACE), angiotensin II is a potent vasoconstrictor and an inhibitor of the bradykinin, which is vasodilator.3 Inhibition of RAS is the mechanism of ACE inhibitors and they are the most commonly used classes of drugs for controlling elevated blood pressure (BP). ACE inhibitor drugs prevent the formation of angiotensin-II which is responsible for constriction of blood vessels and thereby lowers the blood pressure. By controlling hypertension through ACE inhibitors, cardiovascular diseases such as congestive heart failure,4 myocardial infarction,5 and diabetic nephropathy6 has been successfully controlled. Specific inhibitors of ACE have been shown to be useful as antihypertensive drugs. ACE inhibitors (viz., captopril, enalapril, fosinopril and ramipril) currently available in the market for clinical use, exert the antihypertensive effect by competitively binding to the active site of ACE.7 Use of ACE inhibitor is associated with many adverse effects including bronchospasm and cough. ACE inhibitors are contraindicated in pregnancy.8,9 Foods and plants are the perfect sources for exploring novel lead molecules for ACE inhibition. India being rich in biological diversity often been referred to as the “Medicinal Garden of the world’’. India is sitting on gold mine of well recorded and traditionally well-practiced knowledge of herbal medicine.10 These herbs are used for variety of diseases including hypertension and many of them are unexplored for its phytopharmacological uses. Plant extracts and molecules isolated from them have previously shown inhibitory effects on ACE.11,12 In the search for lead molecules for hypertension from plant origin, in this research article we have selected 25 plants for screening ACE inhibitory activity on the basis of their traditional use as cardiotonics, diuretics or modern research exploring cardiovascular potential.11 The article aims toward identifying the potential plants and it fractions as ACE inhibitors, investigating the kinetics of ACE inhibition, thereby revealing the pharmacological mechanism of these fractions.
2. Material and methods
2.1. Enzymes and chemical reagents
ACE from rabbit lung (9015-82-1), chymotrypsin (9004-07-3), trypsin (9002-07-7), bovine serum albumin (BSA)(9048-46-8), hippury-l-histidyl-l-leucine (HHL)(207386-83-2), hippuric acid (HA)(495-69-2), Folin-Ciocalteu's phenol reagent (47641-100 ML-F), Trichloroacetic acid (TCA)(76-03-9), Gallic acid (149-91-7), Quercetin (117-39-5), Aluminium chloride (7446-70-0), trifluoroacetic acid (TFA)(76-05-1), Pyridine (110-86-1), benzene sulphonyl chloride (BSC)(98-09-9), BAPNA (a-N-benzoyl-dl-arginine-Pnitroanilide HCl) (911-77-3), dimethyl sulphoxide (DMSO)(67-68-5), Zinc chloride (ZnCl2)(7646-85-7) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ethyl acetate (EA), n-hexane, n-butanol (n-BuOH), methanol(MeOH), ethanol (EtOH) and dichloromethane (CH2Cl2) were purchased from S.D. Fine Chemical Ltd., Mumbai, India. All other chemicals used were of analytical grade.
2.2. Plant materials
Plant materials were collected from Whitestone Healthcare Pvt. Ltd. Bhopal in central India within the duration of 12 months during 2011–12. All Voucher specimens NIPPHYTO01-NIPPHYTO25 were authenticated by Dr. Brijesh Sahoo of Botanical Survey of India, Govt. of India, Ministry of Environment and Forests, India and Dr. Bhasker Punjani, Professor in P.G., Botany Department, Science college, Talod, Gujarat. All the voucher specimens (NIPPHYTO01-NIPPHYTO25) are deposited at the Herbarium of Institute of Pharmacy Nirma University of Science and Technology Ahmedabad for further reference.
2.3. Extraction and fractionation
Plant materials were dried under shade for a week, segregated, pulverized by a mechanical grinder and passed through a 40 mesh sieve. Extraction was carried out using analytical grade solvents obtained from S.D. Fine Chemical Ltd., Mumbai, India, for which 1 kg powder of each plant material was transferred into a container and hydroalcoholic solvent was added, until the coarse particles of the plant material was completely soaked. The container was gently shaken for 36 h at room temperature with intermittent shaking. The extract obtained was filtered using Whatman filter paper and the residue was again extracted with a fresh solvent for another 12 h. The extract was filtered and both the filtrates were pooled together. The solvent was removed using Buchi type rotary evaporator and the extract was subjected to freeze drying in a lyophilizer till dry powder was obtained for further use. As the plants and their parts were selected based on their traditional uses and modern research, fractionation was done only for those plants whose extract were found to be active, using a different fractionation approach for each plant material to obtain the bioactive enriched fraction. In general, all fractions were obtained by suspending a fixed quantity of crude extract in water forming the slurry which was successively partitioned with solvents of increasing polarity between water and n-hexane or Petroleum ether, followed by chloroform or dichloromethane, ethyl acetate, and n-butanol. The solutions were completely evaporated to give the respective fractions.13
2.4. Quantitative determination of total phenolic and flavonoids contents
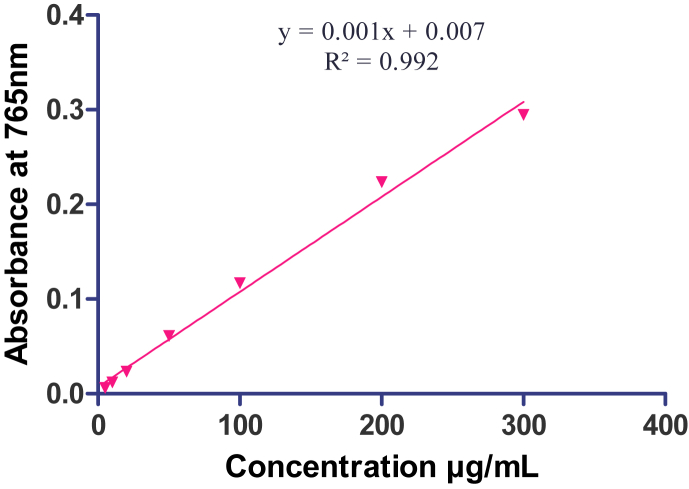
Total phenolic and flavonoids contents were determined for the extracts which were found to be active. Determination of total phenolics compounds was performed on hydroalcoholic extracts by the Folin-Ciocalteu method. Each sample was mixed with 1 mL Folin-Ciocalteu reagent and 0.8 mL of 7.5% Na2CO3. This procedure involves the reduction of Folin-Ciocalteu reagent by phenolic compounds present in the extract, with concomitant formation of a blue complex determined at 765 nm by UV–visible spectrophotometer (UV-1700 Shimadzu, Japan) after 90 min at room temperature. Gallic acid was used for constructing the standard curve (Fig. 1) and the mean of three readings was used to determine the total phenolic content expressed as μg of gallic acid equivalents/mg of extract (GAEs).14
Fig. 1.
Gallic acid standard curve.
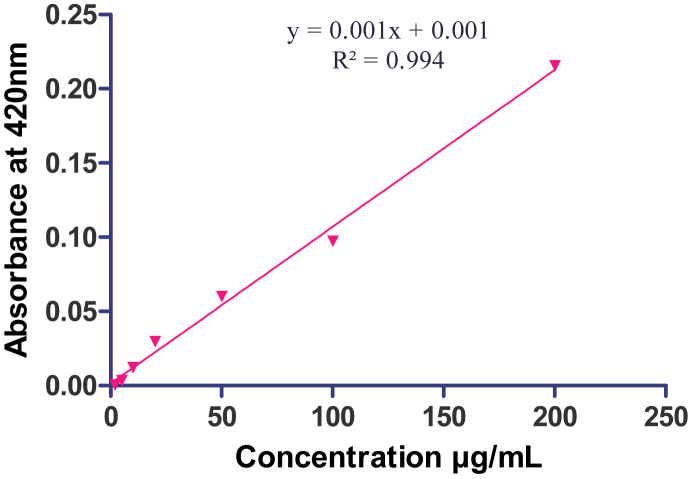
Determination of total flavonoids was done by the aluminum chloride colorimetric method. 0.6 mL of 2% aluminum chloride solution was mixed with 0.6 mL diluted standard quercetin solutions or extracts. The solution was incubated for 60 min at room temperature after proper mixing. The volume of 6% aluminium chloride was substituted by the same volume of distilled water in the blank. Reaction mixtures absorbance was measured against blank at 420 nm wavelength with a UV–visible spectrophotometer (UV-1700 Shimadzu, Japan). Concentration was determined by comparison with the standard calibration curve (2–200 μg/mL) of quercetin. The concentration of total flavonoid content in the test samples was calculated from the calibration plot (Y = 0.0001x + 0.001, R2 = 0.994) (Fig. 2), and expressed as μg quercetin equivalents/mg of extract (QEs). All the quantifications were carried out in triplicate.15
Fig. 2.
Quercetin standard curve.
2.5. Angiotensin I-converting-enzyme (ACE) inhibitory activity
There are several methods to determine the ACE inhibitory activity which can be used to determine the ACE activity. However, the method utilized here is a spectrophotometric method based on the commonly used method introduced by Cushman and Cheung (1971).16 This method is simple, sensitive, and rapid, requiring no solvent extraction and can, therefore, be used for high-throughput screening of ACE inhibitors. ACE inhibitory activity was assayed by measuring the release of HA (Hippuric acid) from the substrate HHL.
Various types of substrates and methods used to analyze ACE inhibition activity are following: Cushman and Cheung Method using a substrate hippuryl-histidyl-leucine (HHL), Holmquist method using a substrate furanacryloyl-tripeptide, Elbl and Wagner method using a substrate benzoil-[l-14C] glicyl-l-histidine-l-leucine, Carmel and Yaron method using a substrate o-aminobenzoylglycyl-p-nitrophenylalanilproline, and Lam method using 3-hydroxybutyrylglycyl-glycyl-glycine as substrate. Methods to measure the results of enzymatic reactions are spectrophotometric, fluorometric, high-performance liquid chromatography, electrophoresis, and radiochemistry. A Radiometric assay using the labeled angiotensin I substrate, wherein the release of radioactive histidine-leucine which serves as an enzymatic activity index.17 Carmel and Yaron (1977–1978) developed a measurement method of the ACE inhibitory activity using an o-aminobenzoylglycine-p-nitrophenylalanylproline as a substrate and then hydrolyzed into o-aminobenzoylglicyl.18 Holmquist et al. in 1979 developed a method using FAPGG as a substrate. This method is based on the absorption spectrum blue shift that occurred on the substrate hydrolysis produces dipeptide and furanacryloyl-blocked amino acid.19 Many of the methods described above require expensive instrumentation and a large volume of organic solvents,20 so utilized a simple, sensitive, and rapid, requiring no solvent extraction method as described by Jimsheena and Gowda, 2009.21
The assay mixture contained 125 μL of a 0.05 M sodium borate buffer (pH 8.2), containing 0.3 M NaCl, 50 μL of 5 mM HHL and 25 μL of ACE (2.5 Milli units (mU)), which was pre-incubated with different sample concentrations of the plant inhibitor. The reaction was stopped after incubation at 37 °C for 30 min by the addition of 0.2 mL of 1 M HCl. Pyridine (0.4 mL) was added followed by 0.2 mL of BSC (the order of addition of reagents is critical) The solution was slowly mixed using a vortex mixer and cooled on ice. The yellow colour developed was measured at 410 nm using UV–visible spectrophotometer (UV-1700 Shimadzu, Japan). One unit of ACE activity is defined as the amount of enzyme, which releases 1 μmol of HA per min at 37 °C (Degree Celsius) and pH 8.2.21
The degree of ACE inhibition (%) was calculated with the following equation
Where.
A1 is absorbance of the ACE solution without an inhibitor (Plant extract and fractions)
A2 is absorbance of the tested sample of extract and fractions
A3 is absorbance of HHL solution (a buffer was added instead of the ACE solution and sample)
The IC50 value is defined as the concentration of inhibitor required to decrease the HA peak area by 50% (indicating 50% inhibition of ACE), and was calculated using a non-linear regression from a plot of activity versus inhibitory concentration of at least five separate concentrations. To obtain the standard deviation during the assay, each assay was performed in triplicate.
2.6. Kinetics of ACE inhibition
Study of enzyme kinetics is an important tool to investigate the chemical mechanism of catalysis. Kinetic studies provide information on substrate and product affinity to the enzyme. Knowledge of the dynamic properties of enzyme catalysis is a prerequisite for the design of inhibitors (drugs) directed against ACE.
Kinetic parameters were evaluated by the Lineweaver–Burk's method. The reaction conditions were the same as ACE inhibitory activity assay as described above. The enzyme activity was measured at various substrate (HHL) concentrations (0.5, 1, 2, 3.5, and 6 mM) in the presence of the inhibitor at three different concentrations (0, 100 μg/mL and 200 μg/mL) which was used to construct the Lineweaver-Burk plot. Classification of inhibition type as competitive, non-competitive or uncompetitive is done on the basis of these plots. In this plot, a linear regression was made using the reciprocals of various HHL concentrations as the independent variable (X-axis) and the reciprocals of HA formation rates as dependent variable (Y-axis). The rate of formation of HA was determined in the presence of different concentrations of the plant inhibitor. The Lineweaver-Burk plot provides information about 1/Vmax (Y axis-intercept of the linear regression) and −1 /Km (X axis-intercept) for the ACE inhibitory kinetics. A competitive inhibitor will have the same Y axis-intercept (1/Vmax) in the presence of inhibitor (at 100 μg/mL and 200 μg/mL) as in the absence of inhibitor (since Vmax is unaffected). A calibration curve for standard HA was constructed for reference. All experiments were conducted in triplicate.22,23
2.7. Assessment of mechanism and specificity of the ACE inhibition
Plants extracts and fractions can conjugate with protein and thus has the ability to non-specifically inhibit enzyme action, taking into consideration ACE inhibition with increasing concentrations of BSA (0, 25, 50, 125, 200 and 250 μg/mL) was accessed. The specificity of the inhibition of ACE was accessed by investigating the effect extract on the activity of chymotrypsin and trypsin as described by Liu et al. (2003).24
Briefly, in the test sample, 6 μL of plant extract in 100% DMSO was mixed with 0.5 mL 20 μg/mL chymotrypsin under 0.1 M phosphate buffer (pH 8.0) and incubated at 37 °C for 5 min. 1000 μl of 2% casein in 0.1 M phosphate buffer (pH 8.0) was then added and incubated for a further 30 min at which time 2 mL of 10% trichloroacetate was added to quench the reaction. After 1 h, Absorbance of the supernatant was measured at 280 nm after the mixture was centrifuged for protein sedimentation. No plant fractions were added to the DMSO in the control group, and in the blank group, no enzyme was added.
Assay for trypsin inhibition was similar to that for chymotrypsin. In the test sample, 6 μL of plant extract or fractions solution was mixed with 0.5 mL (40 μg/mL) trypsin solution and incubated at 37 °C for 5 min 1 mL of BAPNA solution (25 mg BAPNA in 0.5 mL DMSO made up to 50 mL in 0.1 M Tris-HCl buffer) was added and incubated at 37 °C for a further 20 min. To quench the reaction, 0.5 mL of 10% acetic acid was added and the absorbency was measured at 410 nm.24
The Chymotrypsin/trypsin inhibitory activity is expressed as
2.8. The effect of ZnCl2 on the inhibitory activity of ACE
As ACE is a Zn2+ containing enzyme, 1.5 mM ZnCl2 was added to the reaction mixture according to the method of Liu et al. (2003) to assess whether the inhibitory action of the plant fractions resulted from the chelation of the Zn2+ ions. The experimental approaches including the supplementation of the ACE activity test system with or without ZnCl2 were performed.
2.9. Statistical analysis
Each test was conducted in triplicate or otherwise mentioned, all data are presented as the mean (±SD) for the number of determinations shown in the tables and figures.
3. Results
3.1. Total phenolic and flavonoids contents
Phenolic and flavonoids content of tested plants extracts and active fractions are presented in Table 6. Total flavonoids ranged from 92.75 to 4.77(μg QE/mg dry wt). Total phenolic content varied from 94.42 to 3.27 (μg GAE/mg dry wt).
Table 6.
Total Phenolic & flavonoids contents of various plants.
| Plant Hydroalcoholic Extract | Total phenolic content (μg(±)-gallic acid/mg of plant extract or fraction)* | Total flavonoid content (μg (±)-Quercetin/mg of plant extract or fraction)* |
|---|---|---|
| Asparagus racemosus | 73.26 ± 5.65 | 47.26 ± 6.01 |
| Cassia occidentalis | 20.79 ± 1.75 | 58.16 ± 4.31 |
| Coscinium fenestratum | 17.96 ± 0.88 | 11.65 ± 0.66 |
| Crataegus oxyacantha | 19.32 ± 0.85 | 15.13 ± 0.21 |
| Cynara scolymus | 50.96 ± 2.37 | 92.75 ± 5.53 |
| Embelia ribes | 13.16 ± 0.21 | 8.17 ± 0.19 |
| Euphorbia prostrata | 8.4 ± 0.26 | 5.29 ± 0.16 |
| Feronia limonia | 34.76 ± 1.84 | 32.98 ± 2.06 |
| Kalanchoe pinnata | 3.27 ± 0.10 | 5.09 ± 0.24 |
| Morus nigra | 20.19 ± 0.57 | 11.78 ± 0.57 |
| Mucuna pruriens | 38.66 ± 1.77 | 4.77 ± 0.28 |
| Oryza sativa | 06.43 ± 0.16 | 9.69 ± 0.33 |
| Piper betle | 94.42 ± 3.79 | 40.98 ± 1.80 |
| Polyalthia longifolia | 80.76 ± 6.27 | 74.57 ± 3.48 |
| Psoralea corylifolia | 39.41 ± 2.01 | 14.85 ± 0.68 |
| Punica granatum | 27.23 ± 1.58 | 18.46 ± 0.36 |
| Stevia rebaudiana | 77.43 ± 3.26 | 52.36 ± 2.01 |
| Cassia occidentalis | 109.38 ± 3.57 | 26.84 ± 1.14 |
| Ethyl acetate fraction | ||
| Coscinium fenestratum | 14.23 ± 0.64 | 16.09 ± 0.31 |
| Butanol fraction | ||
| Crataegus oxyacantha | 209.16 ± 7.48 | 19.18 ± 0.71 |
| Ethyl acetate fraction | ||
| Cynara scolymus | 61.43 ± 2.06 | 109.68 ± 3.27 |
| Ethyl acetate fraction | ||
| Cynara scolymus | 39.47 ± 1.44 | 72.98 ± 1.07 |
| Butanol fraction | ||
| Embelia ribes | 7.84 ± 0.68 | 5.06 ± 0.23 |
| Butanol fraction | ||
| Morus nigra | 18.42 ± 0.99 | 4.93 ± 0.31 |
| Ethyl acetate fraction | ||
| Mucuna pruriens | 46.56 ± 0.57 | 6.19 ± 0.36 |
| Ethyl acetate fraction | ||
| Piper betle | 51.23 ± 1.77 | 18.63 ± 0.68 |
| Chloroform fraction | ||
| Punica granatum | 17.60 ± 0.48 | 24.12 ± 1.35 |
| Ethyl acetate fraction | ||
| Stevia rebaudiana | 32.6 ± 1.44 | 41.72 ± 1.66 |
| Water fraction |
Each value represents a mean ± SD (n = 3).
3.2. ACE inhibitory activity
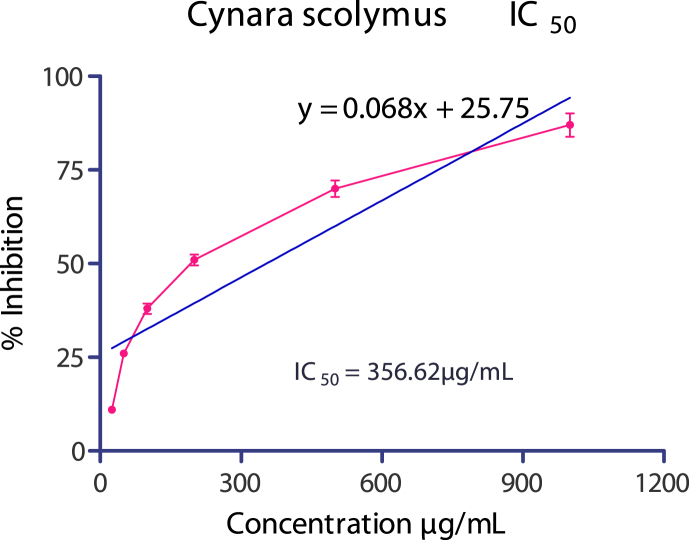
Initially hydroalcoholic extracts of 25 plants were investigated for their IC50 value. Among 25 extracts studied, the best activity was found in Cynara scolymus, IC50 value 356.62 μg/mL(Fig. 3), activity decreases in following order Cynara scolymus > Embelia ribes > Crataegus oxyacantha > Coscinium fenestratum > Stevia rebaudiana > Euphorbia prostrate > Piper betle > Punica granatum > Kalanchoe pinnata > Oryza sativa > Morus nigra > Polyalthia longifolia > Feronia limonia > Mucuna pruriens > Psoralea corylifolia > Cassia occidentalis > Asparagus racemosus > Cyperus rotundus > Carissa carandas > Elaeocarpus ganitrus > Bombax ceiba > Chenopodium album > Nepeta hindostana > Abutilon indicum > Nyctanthes arbortristis. Least activity was found in Nyctanthes arbortristis IC50 value of 4478.01 μg/mL. IC50 values of all the plants are shown in Table 1.
Fig. 3.
IC50 of Cynara scolymus extract.
Table 1.
IC50 Value of Extracts and fractions.
| S.no | Family | Scientific name | Part | V·S | Use | Crude Extract Solvent ratio % Yield w/w (IC50 μg/mL) | Fraction 1% Yield w/w (IC50 μg/mL) | Fraction 2% Yield w/w (IC50 μg/mL) | Fraction 3% Yield w/w (IC50 μg/mL) | Fraction 4% Yield w/w (IC50 μg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Malvaceae | Abutilon indicum L. | Whole plant | NIPPYHTO01 | Diuretic, Anti-hypertensive | Ethanol-Water (70–30) | – | – | – | – |
| 16.43% | ||||||||||
| 3159.46 ± 195.28 | ||||||||||
| 2 | Liliaceae | Asparagus racemosus L. | Roots | NIPPYHTO02 | Diuretic, Cardioprotective | Ethanol-Water (80–20) | Chloroform | Ethyl acetate | Butanol | Water |
| 18.56% | 2.09 | 0.83 | 14.2 | 82.88 | ||||||
| 1065.2 ± 153.46 | NA | 417.26 ± 27.27 | 323.66 ± 17.41 | 6019.23 ± 326.33 | ||||||
| 3 | Bombacaceae | Bombax ceiba L. | Fruits | NIPPYHTO03 | Diuretic | Ethanol-Water (80–20) | – | – | – | – |
| 16.87% | ||||||||||
| 2096.20 ± 167.02 | ||||||||||
| 4 | Caesalpiniaceae | Cassia occidentalis L. | Leaves | NIPPYHTO04 | Diuretic, High Blood Pressure | Ethanol-Water (50-50) | Hexane | Ethyl acetate | Butanol | Water |
| 25.9% | 30.40 | 14.51 | 21.19 | 33.84 | ||||||
| 1035.46 ± 175.61 | NA | 199.48 ± 14.48 | 976.03 ± 28.37 | 1879.25 ± 132.03 | ||||||
| 5 | Chenopodiaceae | Chenopodium album L. | Arial Parts | NIPPYHTO05 | Diuretic, Cardiotonic | Ethanol-Water (60–40) | – | – | – | – |
| 17.49% | ||||||||||
| 2486.54 ± 313.82 | ||||||||||
| 6 | Menispermaceae | Coscinium fenestratum Gaertn. | Stem | NIPPYHTO06 | Diuretic, Hypotensive | Ethanol-Water (80–20) | Hexane | Chloroform | Butanol | Water |
| 15.96% | 12.8 | 23.43 | 16.30 | 47.40 | ||||||
| 837.96 ± 61.30 | NA | 1523.76 ± 104.44 | 150.36 ± 13.8 | 1103.26 ± 41.01 | ||||||
| 7 | Apocynaceae | Carissa carandas L. | Fruits | NIPPYHTO07 | Diuretic | Methanol-Water (90:10) | – | – | – | – |
| 31.13% | ||||||||||
| 1635.23 ± 93.08 | ||||||||||
| 8 | Rosaceae | Crataegus oxyacantha L. | Berries | NIPPYHTO08 | Diuretic, Cardiotonic, Hypotensive | Ethanol-Water (70–30) | Chloroform | Ethyl acetate | Butanol | Water |
| 18.51% | 24.58 | 15.12 | 23.33 | 36.33 | ||||||
| 796.25 ± 70.54 | 3265.23 ± 173.70 | 50.91 ± 3.52 | 369.15 ± 26.78 | 1598.58 ± 104.32 | ||||||
| 9 | Asteraceae | Cynara scolymus L. | Aerial Parts | NIPPYHTO09 | Diuretic, Cardiotonic | Ethanol-Water (70–30) | Chloroform | Ethyl acetate | Butanol | Water |
| 25.67% | 23.67 | 09.23 | 17.37 | 49.7 | ||||||
| 356.62 ± 22.15 | 453.16 ± 27.94 | 63.36 ± 3.62 | 116.99 ± 7.21 | 3952.23 ± 260.56 | ||||||
| 10 | Cyperaceae | Cyperus rotundus L. | Tubers | NIPPYHTO10 | Diuretic | Methanol-Water (70:30) | – | – | – | – |
| 9.17% | ||||||||||
| 1416.39 ± 156.68 | ||||||||||
| 11 | Elaeocarpaceae | Elaeocarpus ganitrus Roxb. | Seeds | NIPPYHTO11 | Antihypertensive | Ethanol-Water (70–30) | – | – | – | – |
| 6.53% | ||||||||||
| 1869.39 ± 144.86 | ||||||||||
| 12 | Myrsinaceae | Embelia ribes Burm. | Fruits | NIPPYHTO12 | Diuretic, Cardioprotective | Ethanol-Water (90–10) | Petroleum ether | Chloroform | Butanol | Water |
| 12.6% | 41.5 | 13.2 | 16.96 | 28.30 | ||||||
| 761.53 ± 36.01 | 39.32 ± 8.94 | NA | 1165.74 ± 63.32 | NA | ||||||
| 13 | Euphorbiaceae | Euphorbia prostrata Ait. | Whole Plant | NIPPYHTO13 | Diuretic | Methanol-Water(80–20) | Dichloromethane | Ethyl acetate | Butanol | Water |
| 6.1% | 2.9 | 10.1 | 30.3 | 56.6 | ||||||
| 863.23 ± 95.54 | NA | 405.46 ± 69.28 | 611.75 ± 58.89 | 963.19 ± 61.94 | ||||||
| 14 | Rutaceae | Feronia limonia L. | Fruits | NIPPYHTO14 | Diuretic, Cardiotonic | Methanol-Water (80:20) | Petroleum ether | Ethyl acetate | Butanol | Water |
| 25.6% | 46.70 | 14.17 | 10.38 | 28.72 | ||||||
| 981.29 ± 148.38 | 498.45 ± 31.14 | 315.17 ± 15.54 | 1633.43 ± 104.18 | 3578.03 ± 214.47 | ||||||
| 15 | Crassulaceae | Kalanchoe pinnata Lam. | Aerial Parts | NIPPYHTO15 | Antihypertensive, Diuretic | Methanol-Water (80:20) | Hexane | Chloroform | Butanol | Water |
| 16.51% | 6.35 | 29.67 | 19.98 | 43.97 | ||||||
| 916.49 ± 92.19 | NA | 365.97 ± 5.70 | 1830.19 ± 160.11 | 230.41 ± 19.40 | ||||||
| 16 | Moraceae | Morus nigra L. | Fruits | NIPPYHTO16 | Diuretic, Hypotensive | Ethanol-Water (80:20) | Hexane | Ethyl acetate | Butanol | Water |
| 28.08% | 6.45 | 11.46 | 21.52 | 60.53 | ||||||
| 961.15 ± 69.90 | NA | 197.15 ± 8.94 | 363.22 ± 5.40 | 3956.95 ± 236.07 | ||||||
| 17 | Fabaceae | Mucuna pruriens L. | Seeds | NIPPYHTO17 | Diuretic, Hypotensive | Ethanol-Water (80:20) | Hexane | Ethyl acetate | Butanol | Water |
| 39.46% | 31.39 | 7.54 | 6.53 | 54.26 | ||||||
| 1003.15 ± 33.19 | NA | 156.45 ± 6.75 | 1101.56 ± 30.36 | 770.56 ± 61.28 | ||||||
| 18 | Lamiaceae | Nepeta hindostana Roth | Aerial Parts | NIPPYHTO18 | Diuretic, Cardiotonic | Ethanol-Water (80:20) | – | – | – | – |
| 28.48% | ||||||||||
| 2561.32 ± 186.86 | ||||||||||
| 19 | Oleaceae | Nyctanthes arbortristis L. | Flowers | NIPPYHTO19 | Diuretic | Ethanol-Water (80:20) | – | – | – | – |
| 17.23% | ||||||||||
| 4478.01 ± 473.53 | ||||||||||
| 20 | Poaceae | Oryza sativa L. | Bran | NIPPYHTO20 | Diuretic, Antihypertensive | Methanol-Water (80:20) | Hexane | Chloroform | Ethyl acetate | Water |
| 18.15% | 28.27 | 1.02 | 2.54 | 67.20 | ||||||
| 956.43 ± 151.78 | 430.98 ± 26.64 | NA | 207.15 ± 11.71 | NA | ||||||
| 21 | Piperaceae | Piper betle L. | Leaves | NIPPYHTO21 | Diuretic, Antihypertensive | Ethanol-Water (80:20) | Hexane | Chloroform | Butanol | Water |
| 09.51% | 32.10 | 12.30 | 8.40 | 44.20 | ||||||
| 889.01 ± 113.39 | NA | 130.35 ± 8.94 | 1135.88 ± 64.48 | 870.56 ± 61.28 | ||||||
| 22 | Annoaceae | Polyalthia longifolia Sonn. | Leaves | NIPPYHTO22 | Diuretic, Hypotensive | Ethanol-Water (70:30) | Hexane | Chloroform | Butanol | Water |
| 14.63% | 40.23 | 5.56 | 23.63 | 29.80 | ||||||
| 965.49 ± 57.4 | 2863.56 ± 57.31 | NA | 401.56 ± 12.52 | 293.01 ± 9.32 | ||||||
| 23 | Fabaceae | Psoralea corylifolia L. | Fruit | NIPPYHTO23 | Diuretic, Vasodilator | Ethanol-Water (90–10) | Hexane | Ethyl acetate | Butanol | Water |
| 22.5% | 13.56 | 30.61 | 18.14 | 20.78 | ||||||
| 1013.98 ± 61.16 | NA | 211.15 ± 8.94 | 363.22 ± 5.40 | 2530.55 ± 137.35 | ||||||
| 24 | Punicaceae | Punica granatum L. | Flowers | NIPPYHTO24 | Diuretic, Cardiotonic | Ethanol-Water (80–20) | Hexane | Ethyl acetate | Butanol | Water |
| 27.3% | 11.2 | 15.7 | 27.8 | 45.3 | ||||||
| 905.94 ± 40.51 | NA | 201.15 ± 5.72 | 189.56 ± 3.76 | 1516.55 ± 12.45 | ||||||
| 25 | Asteraceae | Stevia rebaudiana Bert. | Leaves | NIPPYHTO25 | Diuretic, High Blood Pressure | Ethanol-Water (70–30) | Hexane | DCM | Butanol | Water |
| 10.1% | 6.24 | 8.8 | 70.32 | 10.72 | ||||||
| 854.23 ± 54.09 | NA | 993.16 ± 17.72 | 677.12 ± 9.96 | 137.23 ± 9.40 |
NA: Not active upto 3000 μg concentration.
Positive Control Captopril is 1.33 ± 0.03 ng/mL.
17 out of 25 plants, whose IC50 value was less than 1 mg/mL were selected for fractionation. Based on the extensive literature survey of each plant, fractionation was done very carefully resulting 68 fractions from 17 plants. Out of 68 fractions screened, 11 fractions were found to be very active with the IC50 value less than 200 μg/mL. These active fractions were used for further study of enzyme kinetics (Table 2). Captopril, employed as positive control in the assays, presented IC50 = 1.33 ± 0.03 ng/mL.
Table 2.
Kinetic parameters of the ACE inhibitor activity of most active fractions from various plants.
| S.no | Fraction | Concentration (μg/mL) | Km (mM) | Vmax (μM/min) | Inhibition type |
|---|---|---|---|---|---|
| 1 | Cassia occidentalis | 200 | 3.540 | 0.086 | Non-Competitive |
| Ethyl acetate fraction | |||||
| 2 | Coscinium fenestratum | 200 | 4.065 | 0.088 | Non-Competitive |
| Butanol fraction | |||||
| 3 | Crataegus oxyacantha | 200 | 4.620 | 0.098 | Non-Competitive |
| Ethyl acetate fraction | |||||
| 4 | Cynara scolymus | 200 | 6.323 | 0.063 | Non-Competitive |
| Ethyl acetate fraction | |||||
| 5 | Cynara scolymus | 200 | 3.198 | 0.043 | Non-Competitive |
| Butanol fraction | |||||
| 6 | Embelia ribes | 200 | 6.449 | 0.049 | Non-Competitive |
| Butanol fraction | |||||
| 7 | Morus nigra | 200 | 3.025 | 0.046 | Non-Competitive |
| Ethyl acetate fraction | |||||
| 8 | Mucuna pruriens | 200 | 3.226 | 0.073 | Non-Competitive |
| Ethyl acetate fraction | |||||
| 9 | Piper betle | 200 | 2.236 | 0.053 | Non-Competitive |
| Chloroform fraction | |||||
| 10 | Punica granatum | 200 | 9.950 | 0.015 | Competitive |
| Ethyl acetate fraction | |||||
| 11 | Stevia rebaudiana | 200 | 2.495 | 0.064 | Non-Competitive |
| Water fraction |
Results indicate that out of various solvents selected for fractionation, ethyl acetate fraction was found to be most potent for 7 fractions of plants, in which highest activity was found in ethyl acetate fraction of Crataegus oxyacantha with IC50 value of 50.91 μg/mL. Activity goes on decreasing with the following order of ethyl acetate extract of Crataegus oxyacantha > Cynara scolymus > Mucuna pruriens > Morus nigra > Cassia occidentalis > Punica granatum > Euphorbia prostrata. Butanol fraction of these plants Coscinium fenestratum, Cynara scolymus, Punica granatum was found to be active. Water and Hexane fraction of the plants were found to be least active except for Stevia rebaudiana water fraction(IC50 value of 137.23 μg/mL), Petroleum ether fraction of Embelia ribes was found to be most active (IC50 value of 39.32 μg/mL) among all fractions.
3.3. Kinetics of the ACE inhibition
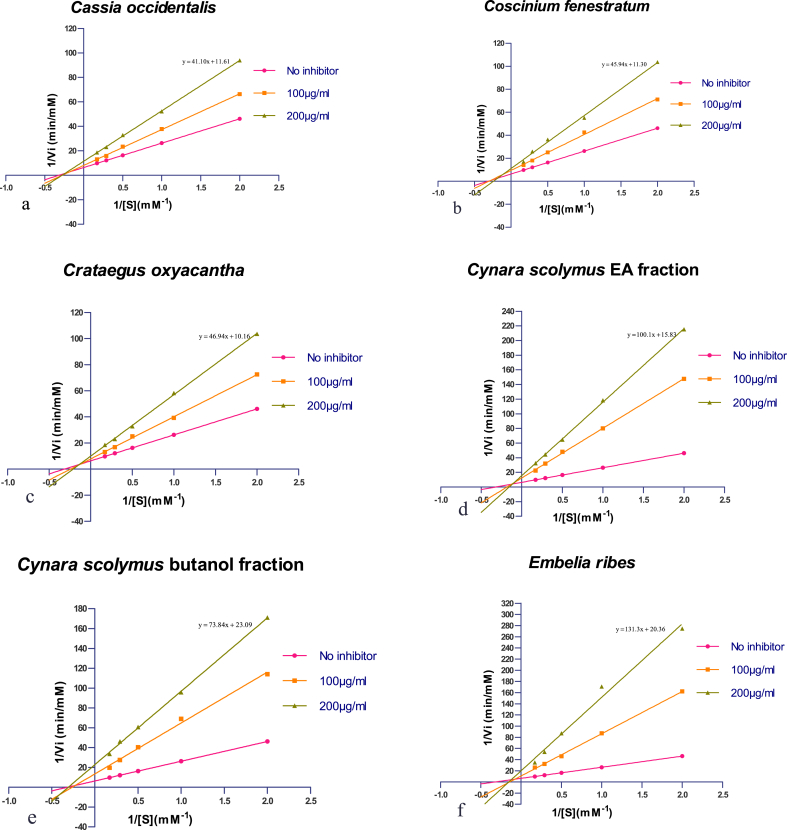
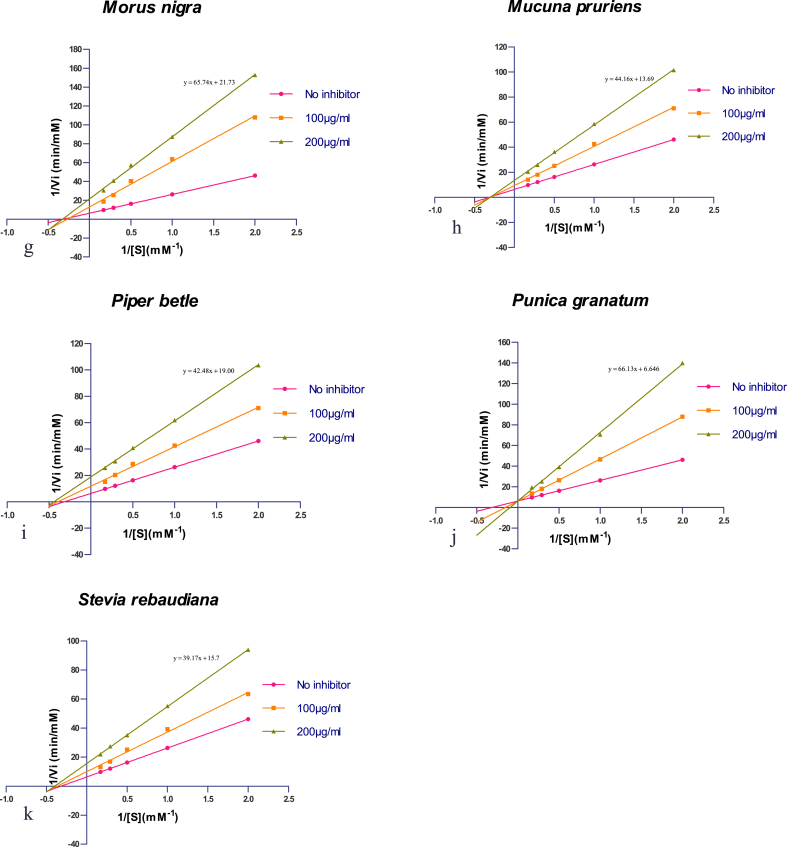
The enzyme kinetics of the ACE in the presence of the inhibitor (eleven fractions) was determined from the Lineweaver-Burk plots. Values of Km (mM) and Vmax (μM/min) were calculated by fitting the slope of linear regression in Michaelis-Menten formula (Fig. 4). All the fractions exhibited a non-competitive mode of inhibition except Punica granatum Ethyl acetate fraction which exhibited a competitive mode of inhibition.
Fig. 4.
Lineweaver–Burk plots derived from the inhibition of ACE by the active extract and fractions. 1/[S] and 1/Vi represent the reciprocal substrate (HHL) concentration and HA formation rate, respectively.
3.4. Mechanism of the ACE inhibition
The ACE inhibitory activity of six fractions was significantly affected by the addition of different concentrations of BSA in the following order Coscinium fenestratum > Mucuna pruriens > Cassia occidentalis > Crataegus oxyacantha > Morus nigra > Punica granatum (Table 3). The ACE inhibition in the four plant fractions, viz Cynara scolymus Ethyl acetate fraction, Embelia ribes Butanol fraction, Piper betle Chloroform fraction and Stevia rebaudiana did not significantly reduce by addition of increasing concentration of BSA (Table 3).
Table 3.
Comparison of inhibitory effects of various plant fractions on angiotensin converting enzyme in the presence or absence of bovine serum albumin (BSA:25 μg/mL).
| Plant Fraction | Concentration (μg/mL) | Inhibition of activity (%) in the absence or presence of BSA |
||
|---|---|---|---|---|
| BSA Absent | BSA Present | % Decrease in activity | ||
| Cassia occidentalis | 200 | 46.59 ± 2.17 | 13.25 ± 0.33 | 71.56 |
| Ethyl acetate fraction | ||||
| Coscinium fenestratum | 200 | 51.30 ± 1.49 | 6.93 ± 0.23 | 86.49 |
| Butanol fraction | ||||
| Crataegus oxyacantha | 50 | 47.63 ± 1.32 | 18.26 ± 0.33 | 61.66 |
| Ethyl acetate fraction | ||||
| Cynara scolymus | 50 | 40.41 ± 3.52 | 39.71 ± 1.58 | 1.73 |
| Ethyl acetate fraction | ||||
| Embelia ribes | 50 | 53.98 ± 3.20 | 50.19 ± 2.48 | 7.02 |
| Butanol fraction | ||||
| Morus nigra | 200 | 43.86 ± 1.94 | 20.01 ± 0.50 | 54.38 |
| Ethyl acetate fraction | ||||
| Mucuna pruriens | 200 | 48.62 ± 1.70 | 10.23 ± 0.57 | 78.96 |
| Ethyl acetate fraction | ||||
| Piper betle | 200 | 54.38 ± 3.07 | 48.76 ± 3.57 | 10.33 |
| Chloroform fraction | ||||
| Punica granatum | 200 | 45.91 ± 2.48 | 23.77 ± 0.99 | 7.02 |
| Ethyl acetate fraction | ||||
| Stevia rebaudiana | 200 | 52.64 ± 3.48 | 49.96 ± 2.96 | 5.09 |
| Water fraction | ||||
Values are given as mean ± SD (n = 3).
3.5. Assessment of specificity of the ACE inhibition
3.5.1. Inhibition of chymotrypsin and trypsin enzymes
Plant fraction of Cassia occidentalis Ethyl acetate fraction, Cynara scolymus Ethyl acetate fraction and Embelia ribes Butanol fraction were able to non-specifically inhibit chymotrypsin and trypsin enzymes, whereas other fractions were not able to significantly inhibit these enzyme (Table 4).
Table 4.
Inhibitory effects of various fractions on the activity of angiotensin converting enzyme, trypsin and chymotrypsin enzyme.
| Plant Fraction | Concentration (μg/mL) | Inhibitory Activity (% inhibition) |
||
|---|---|---|---|---|
| ACE | Trypsin | Chymotrypsin | ||
| Cassia occidentalis | 200 | 46.59 ± 2.17 | 31.60 ± 1.52 | 37.94 ± 1.89 |
| Ethyl acetate fraction | ||||
| Coscinium fenestratum | 200 | 51.30 ± 1.49 | 7.23 ± 0.23 | 4.19 ± 0.14 |
| Butanol fraction | ||||
| Crataegus oxyacantha | 50 | 47.63 ± 1.32 | 11.56 ± 0.57 | 8.69 ± 0.61 |
| Ethyl acetate fraction | ||||
| Cynara scolymus | 50 | 40.41 ± 3.52 | 41.01 ± 2.93 | 24.58 ± 1.94 |
| Ethyl acetate fraction | ||||
| Embelia ribes | 50 | 53.98 ± 3.20 | 37.88 ± 1.65 | 26.13 ± 1.35 |
| Butanol fraction | ||||
| Morus nigra | 200 | 43.86 ± 1.94 | 2.93 ± 0.19 | 5.16 ± 0.35 |
| Ethyl acetate fraction | ||||
| Mucuna pruriens | 200 | 48.62 ± 0.98 | 5.98 ± 0.26 | 7.13 ± 0.52 |
| Ethyl acetate fraction | ||||
| Piper betle Linn | 200 | 54.38 ± 3.07 | 7.56 ± 0.47 | 4.98 ± 0.21 |
| Chloroform fraction | ||||
| Punica granatum | 200 | 45.91 ± 2.48 | 3.46 ± 0.28 | 4.16 ± 0.19 |
| Ethyl acetate fraction | ||||
| Stevia rebaudiana | 200 | 52.64 ± 3.48 | 8.43 ± 0.68 | 11.09 ± 0.92 |
| Water fraction | ||||
Values are given as mean ± SD (n = 3).
3.5.2. Zinc ion chelation
The addition of 1.5 mM ZnCl2 to the assay of the fraction induced ACE inhibitory action showed that Embelia ribes, Cassia occidentalis, Coscinium fenestratum, fractions reduced the inhibition of ACE activity by 58.39% (53.98%–22.46%), 38.91% (46.59%–28.46%) and 35.71% (51.3%–32.98%), respectively. The inhibition of ACE activity of the other fractions was insignificantly changed by the addition of ZnCl2 (Table 5).
Table 5.
Effects of ZnCl2 on the inhibitory activity of tested extracts on angiotensin converting enzyme.
| Plant Fraction | Amount added (μg/mL) | Inhibition of activity (%) in the presence or absence of ZnCl2 |
||
|---|---|---|---|---|
| None | ZnCl2 (1.5 mM) | % Decrease in activity | ||
| Cassia occidentalis | 200 | 46.59 ± 2.17 | 28.46 ± 2.13 | 38.91 |
| Ethyl acetate fraction | ||||
| Coscinium fenestratum | 200 | 51.30 ± 1.49 | 32.98 ± 2.36 | 35.71 |
| Butanol fraction | ||||
| Crataegus oxyacantha | 50 | 47.63 ± 1.32 | 45.37 ± 3.19 | 4.74 |
| Ethyl acetate fraction | ||||
| Cynara scolymus | 50 | 40.41 ± 3.52 | 39.64 ± 1.45 | 1.91 |
| Ethyl acetate fraction | ||||
| Embelia ribes | 50 | 53.98 ± 3.2 | 22.46 ± 1.75 | 58.39 |
| Butanol fraction | ||||
| Morus nigra | 200 | 43.86 ± 1.94 | 42.13 ± 1.85 | 3.94 |
| Ethyl acetate fraction | ||||
| Mucuna pruriens | 200 | 48.62 ± 1.70 | 47.23 ± 3.57 | 2.86 |
| Ethyl acetate fraction | ||||
| Piper betle | 200 | 54.38 ± 3.07 | 49.91 ± 3.26 | 8.22 |
| Chloroform fraction | ||||
| Punica granatum | 200 | 45.91 ± 2.48 | 42.88 ± 1.78 | 6.60 |
| Ethyl acetate fraction | ||||
| Stevia rebaudiana | 200 | 52.64 ± 3.48 | 46.51 ± 3.91 | 11.65 |
| Water fraction | ||||
Values are given as mean ± SD (n = 3).
4. Discussion
Therapeutic potential of ACE inhibitors in the treatment of hypertension and management of cardiovascular diseases is well established.25,26 Antihypertensive activity of some potential Indian medicinal plants and foods were evaluated by the inhibition of the angiotensin converting enzyme (ACE), using a colorimetric assay. In this article we have screened 25 extracts and 68 fractions for their IC50 value, which was a tedious job, so we utilized a simple, sensitive, and rapid method, which requires no solvent extraction and can therefore be utilized for high-throughput screening of ACE inhibitors.21 Pharmacologically ACE removes histidyl-leucine from angiotensin I to form the physiologically active octapeptide, angiotensin II, a potent vasoconstrictor that inactivates the vasodilating nonapeptide, bradykinin. Medicinal plants were selected on the basis of their traditional usage as antihypertensive, cardiotonics and diuretics to obtain the active fractions for ACE inhibition, using hydro-alcoholic extraction. As far as possible, the traditionally used part of the plant was employed for the ACE assay.
Previous report suggest that fractions with a concentration of 330 μg/mL with inhibition rates between 50% and 100% deserve further investigation, aiming at the isolation of the active compound(s).27 From the preliminary calculation of IC50 values, 11 out of the 68 fractions were whose IC50 value was found to be less than 200 μg/mL was used for further study of enzyme kinetics study (Table 2). Captopril, employed as a positive control in the assays, presented IC50 = 1.33 ng/mL. The limit of 200 μg/mL is a hypothetical value which is difficult to justify since crude extracts and fractions are complex mixtures of compounds.
4.1. Enzyme kinetics
Various kinetic parameters obtained from the Lineweaver-Burk plots are shown in Table 2. Km of Angiotensin-converting enzyme varies according to the substrate, as a result, it is known as an unspecific enzyme. Vmax is defined as the rate at which a substrate will be converted to a product once bound to the enzyme. Km reflects how effectively the enzyme binds to the substrate, affinity is reflected by these two parameters. When the enzyme binds strongly to its substrate, Km value will be smaller, which means that lower concentration of substrate is needed to attain Vmax. The lowest Km is usually seen with the ‘natural’ substrates like angiotensin I and/or bradykinin for which the Km value is 16–90 mM and 0.18–1.0 mM respectively.28, 29, 30 Our result in Table 2 indicates that lowest Km value is for Piper betle and highest is for Punica granatum. The maximum rate of substrate hydrolysis (Vmax) and the apparent Michaelis constant (Km) were determined to characterize the kind of inhibition exerted by fractions.31 Table 2 shows the parameter Vmax is significantly different in all the fractions which suggest a mixed inhibition. The Lineweaver-Burk plots Fig. 4 provides information about −1 /Km (x-axis intercept) and 1/Vmax (y-axis intercept of the linear regression) for the ACE inhibitory kinetics. In the presence of competitive inhibitor at different concentration (at 100 μg/mL and 200 μg/mL), the regression lines will have the same y-axis intercept (1/Vmax) and it is same in the absence of inhibitor (since Vmax is unaffected). Fig. 4(j) shows a competitive mode of inhibition for Punica granatum Ethyl acetate fraction. Three lines of the Lineweaver–Burk plot of Punica granatum representing 0, 100 μg/mL and 200 μg/mL of inhibitor concentration with different slopes shared the same y-intercept. This type of inhibition is competitive where inhibitor increases Km and Vmax remains unaffected, this type of inhibition suggest that fraction as well as the substrate interact with key residues in the active site of ACE, and fraction can compete with the substrate for access to the binding site. A competitive inhibitor can firmly bind to the active site and block access to other substrates.32 In other words, competitive inhibitors might be able to enter the ACE protein and compete with HHL to interact with the active sites. Focusing on the Km value, a competitive inhibitor increases the apparent Km for a given substrate meaning that in the presence of a competitive inhibitor more substrate is needed to achieve half Vmax. Fig. 4(a–d) shows Lineweaver-Burk plots for fractions of Cassia occidentalis, Coscinium fenestratum, Crataegus oxyacantha, Cynara scolymus EA, these fractions exhibited mixed inhibition mode, and the plots cross to the left of the 1/V axis but above the 1/S axis which indicates that these fractions showed a competitive-non-competitive inhibition mode with respect to the substrate (HHL). This mixed-type inhibition suggests that the fractions affected the affinity of the enzyme for the HHL substrate but did not bind at the active site.33
Fig. 4(e–i and k) shows Lineweaver-Burk plots for fractions of Cynara scolymus (BtOH), Embelia ribes, Morus nigra, Mucuna pruriens, Piper betle, Stevia rebaudiana. The ACE inhibition pattern of the fractions was analysed by the Lineweaver–Burk plot and was found to be non-competitive. These plots suggest that fractions might be able to enter the catalytic site, but could not bind to the catalytic site of ACE, and that it could not be hydrolyzed by ACE. Increasing angiotensin I to maintain angiotensin II levels would not overcome this mode of inhibition of the ACE. Fraction molecules can combine with an enzyme molecule to produce a dead-end complex by binding to different sites, from the substrate, regardless of whether a substrate molecule is bound or not.
To elucidate the mechanism of ACE inhibition through protein precipitation BSA (bovine serum albumin) was used. When the inhibition of ACE is due to protein precipitation addition of BSA in the test system will result in the decrease of the ACE activity, due to the reason that some of the fraction molecules will also inhibit the BSA, resulting the reduced activity of ACE. ACE inhibition resulting from enzymatic protein precipitation was observed in following fractions with decreasing order Coscinium fenestratum > Mucuna pruriens > Cassia occidentalis > Crataegus oxyacantha > Morus nigra > Punica granatum. Data in the (Table 3) indicates that inhibition of ACE due to above plants in a part may be in part due to precipitation of ACE. To elucidate the specificity of the fraction toward ACE, chymotrypsin and trypsin enzyme were used. Our result in Table 4 indicates that Cassia occidentalis ethyl acetate fraction, Cynara scolymus ethyl acetate fraction and Embelia ribes butanol fraction are non-specific inhibitors of ACE. These fractions were found to have reduced the activity of chymotrypsin and trypsin enzymes, thus, these fractions are non-specific inhibitors of ACE activity. Fractions of plants Crataegus oxyacantha, Stevia rebaudiana, Piper betle Linn, Coscinium fenestratum, Mucuna pruriens, Punica granatum, Morus nigra were not able to significantly inhibit the chymotrypsin and trypsin enzymes suggesting these fractions to be specific inhibitors of ACE.24 ACE is a zinc metallopeptidase, its catalytic active site has zinc ion, which is essential for enzyme activity. This means that non-specific metal chelators may have apparent ACE inhibitor action. The active site of ACE is composed of three parts; a carboxylate binding functionality such as the guanidinium group of Arg, a pocket that accommodates a hydrophobic side chain of C-terminal amino acid residues, and a zinc ion. Supplementation of ZnCl2 in the ACE activity test system is designed to reduce enzyme inhibition resulting from fraction-induced Zn2+ ion chelation. Our result in Table 5 indicates that out of 11 fraction only Embelia ribes, Cassia occidentalis, and Coscinium fenestratum fractions inhibited the ACE by Zn2+ ion chelation, ZnCl2 decreases the inhibitory activity in these fractions suggesting that plant fractions apart from chelating the Zn2+ ion of ACE, they were involved in the chelation of Zn2+ ion released from ZnCl2. Its shows that the chelation of Zn2+ may at least in part to be responsible for the ACE inhibitory activity of the fractions. Presence of flavonoids and other polyphenols is already reported in Embelia ribes,34 Cassia occidentalis35 and Coscinium fenestratum36 and these molecules chelate complexes with the zinc atom within the active centre of zinc-dependent metallopeptidases.37 Results from the previous study also suggest formation of hydrogen bridges between the inhibitor and amino acids near at the active site.38 But the exact molecules responsible for the ACE inhibition by Zn2+ ion chelation from these fractions require further study, which going in our laboratory.
Supplementation of ZnCl2 to the fractions of plant Stevia rebaudiana, Piper betle, Punica granatum, Crataegus oxyacantha, Morus nigra, Mucuna pruriens, Cynara scolymus did not significantly reduce the ACE inhibition suggesting a different mechanism of enzyme inhibition apart from Zn2+ ion chelation.
Our result for the estimation of phenolic and flavonoid content indicates the presence of these compound in the extracts. Previous research shows that plants flavonoids are a wonderful source of ACE inhibitor.39, 40, 41 Similarly, plant phenolics are the good source of ACE inhibitors.42 Presence of high phenolic and flavonoid content may responsible for the bioactivity of these crude hydroalcoholic extracts. Best IC50 value of Cynara scolymus may be directly correlated to the very high content of flavonoid and phenolic compounds.
Out of 68 fractions screened, 11 fractions were found to be very active with the IC50 value less than 200 μg/mL. Most active was petroleum ether fraction of Embelia ribes (IC50 39.32 ± 8.94), chemical profile of n-hexane fraction revealed embelin as the active compound.43 Crataegus oxyacantha ethyl acetate fraction (IC50 50.91 ± 3.52) was also very active with the highest content of the total phenolic compound (209.16 ± 7.48 μg/mg) of the fraction. Moreover several phenolic compounds like hyperoside, isoquercitrin, chlorogenic acid and epicatechin were reported in the extract of Crataegus oxyacantha.44 Ethyl acetate fraction Cassia occidentalis, Mucuna pruriens, Punica granatum and Morus nigra, chloroform fraction of Piper betle, ethyl acetate and butanol fraction of Cynara scolymus, water fraction of Stevia rebaudiana were found to very active with high phenolic and flavonoidal content. Previous reports suggest that ethyl acetate fraction Cassia occidentalis, Mucuna pruriens, Punica granatum and Morus nigra contain anthraquinones,35 l-dopa,45 ellagitannins46 and chlorogenic acid47 respectively. Chloroform fraction of Piper betle reported hydroxychavicol as the bioactive constituent.48 Chemical profile of butanol fraction of Cynara scolymus was reported to contain chlorogenic acid, cynarin, cynaroside, apigenin-7-rutinoside.49 Water extract of Stevia rebaudiana contain phenolic compound pyrogallol.50 Compounds reported in these plants may responsible for the bioactivity of these fractions but the exact molecules responsible for the ACE inhibition requires isolation of compound by various chromatographic techniques along with the animal studies which going in our laboratory.
5. Conclusion
Our study revealed utilization of Indian medicinal plants as a potential source of angiotensin-converting enzyme inhibitors. Among the assayed plants, the fraction of many plants produced significant ACE inhibition. Enzyme kinetics and mechanism of inhibition was determined by various parameters. The ACE inhibitory activity of the tested plants fractions might find importance in the development of potential antihypertensive agents after further investigations of each plant for active compound isolation by various chromatographic techniques and evaluating the activity of extract, fraction and isolated molecule using preclinical animal and human clinical trials.
Conflicts of interest
The authors declare that they have no conflict of interests.
For inhibition of ACE by inhibitors Km is defined as Kmapp since it is affected by the factor (1 + [I])/Ki (Segel, 1975).
Acknowledgements
The authors gratefully acknowledge Whitestone Healthcare Pvt. Ltd. for the gift of the plant materials, and Institute of Pharmacy, Nirma University of Science and Technology, Ahmadabad for providing the facilities to carry out this research. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Vasan R.S., Larson M.G., Leip E.P. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 2.Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005:365217–365223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Weir M.R., Dzau V.J. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens. 1999;12(S9) doi: 10.1016/s0895-7061(99)00103-x. 205S–13S. [DOI] [PubMed] [Google Scholar]
- 4.Carson P.E. Rationale for the use of combination angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker therapy in heart failure. Am Heart J. 2000;140:361–366. doi: 10.1067/mhj.2000.109215. [DOI] [PubMed] [Google Scholar]
- 5.Shlipak M.G., Browner W.S., Noguchi H., Massie B., Frances C.D., McClellan M. Comparison of the effects of angiotensin converting–enzyme inhibitors and beta blockers on survival in elderly patients with reduced left ventricular function after myocardial infarction. Am J Med. 2001;110:425–433. doi: 10.1016/s0002-9343(01)00652-0. [DOI] [PubMed] [Google Scholar]
- 6.Hebert L.A., Falkenhain M.E., Nahman N.S., Jr., Cosio F.G., O'Dorisio T.M. Combination ACE inhibitor and angiotensin II receptor antagonist therapy in diabetic nephropathy. Am J Nephrol. 1999;19:1–6. doi: 10.1159/000013417. [DOI] [PubMed] [Google Scholar]
- 7.Hemels M.E., Bennett H.A., Bonari L., Han D., Traverso M.L., Einarson T.R. HOPE study impact on ACE inhibitors use. Ann Pharmacother. 2003;37:640–645. doi: 10.1345/aph.1C339. [DOI] [PubMed] [Google Scholar]
- 8.Alderman C.P. Adverse effects of the angiotensin-converting enzyme inhibitors. Ann Pharmacother. 1996;30:55–61. doi: 10.1177/106002809603000110. [DOI] [PubMed] [Google Scholar]
- 9.Wood R.I. Bronchospasm and cough as adverse reactions to the ACE inhibitors captopril, enalapril and lisinopril. A controlled retrospective cohort study. Br J Clin Pharmacol. 1995;39:265–270. doi: 10.1111/j.1365-2125.1995.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubey N.K., Kumar R., Tripathi P. Global promotion of herbal medicine: India's opportunity. Curr Sci. 2004;86:37–41. [Google Scholar]
- 11.Somanadhan B., Varughese G., Palpu P. An ethnopharmacological survey for potential angiotensin converting enzyme inhibitors from Indian medicinal plants. J Ethnopharmacol. 1999;65:103–112. doi: 10.1016/s0378-8741(98)00201-3. [DOI] [PubMed] [Google Scholar]
- 12.Wagner H., Elbl G., Lotter H., Guinea M. Evaluation of natural products as inhibitors of angiotension I — converting enzyme (ACE) Pharmacol Lett. 1991;1:15–18. [Google Scholar]
- 13.Machado T.B., Pinto A.V., Pinto M.C. In vitro activity of Brazilian medicinal plants, naturally occurring naphthoquinones and their analogues, against methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2003;21:279–284. doi: 10.1016/s0924-8579(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 14.Dewanto V., Wu X., Liu R.H. Processed sweet corn has higher antioxidant activity. J Agric Food Chem. 2002;50:4959–4964. doi: 10.1021/jf0255937. [DOI] [PubMed] [Google Scholar]
- 15.Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 16.Cushman D.W., Cheung H.S. Spectrophotometric assay and properties of the angiotensin I-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 17.Huggins C.G., Thampi N.S. A simple method for the determination of angiotensin I converting enzyme. Life Sci. 1968;7:633–639. doi: 10.1016/0024-3205(68)90086-6. [DOI] [PubMed] [Google Scholar]
- 18.Carmel A., Yaron A. An intramolecularly quenched fluorescent tripeptide as a fluorogenic substrate of angiotensin-I-converting enzyme and of bacterial dipeptidyl carboxypeptidase. Eur J Biochem. 1978;87:265–273. doi: 10.1111/j.1432-1033.1978.tb12375.x. [DOI] [PubMed] [Google Scholar]
- 19.Holmquist B., Bünning P., Riordan J.F. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal Biochem. 1979;95:540–548. doi: 10.1016/0003-2697(79)90769-3. [DOI] [PubMed] [Google Scholar]
- 20.Wu J., Aluko R.E., Muir A.D. Improved method for direct high-performance liquid chromatography assay of angiotensin-converting enzyme-catalyzed reactions. J Chromatogr A. 2002;950:125–130. doi: 10.1016/s0021-9673(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 21.Jimsheena V.K., Gowda L.R. Colorimetric, high-throughput assay for screening angiotensin I-converting enzyme inhibitors. Anal Chem. 2009;81:9388–9394. doi: 10.1021/ac901775h. [DOI] [PubMed] [Google Scholar]
- 22.Chen J., Liu S., Ye R., Cai G., Ji B., Wu Y. Angiotensin-I converting enzyme (ACE) inhibitory tripeptides from rice protein hydrolysate: purification and characterization. Journal of Functional Foods. 2013;5:1684–1692. [Google Scholar]
- 23.Priyanto A.D., Doerksen R.J., Chang C.I. Screening, discovery, and characterization of angiotensin-I converting enzyme inhibitory peptides derived from proteolytic hydrolysate of bitter melon seed proteins. Journal of proteomics. 2015;128:424–435. doi: 10.1016/j.jprot.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Liu J.C., Hsu F.L., Tsai J.C. Antihypertensive effects of tannins isolated from traditional Chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci. 2003;73:1543–1555. doi: 10.1016/s0024-3205(03)00481-8. [DOI] [PubMed] [Google Scholar]
- 25.Stanton A. Therapeutic potential of renin inhibitors in the management of cardiovascular disorders. Am J Cardiovasc Drugs. 2003;3:389–394. doi: 10.2165/00129784-200303060-00002. [DOI] [PubMed] [Google Scholar]
- 26.Unger T., Gohlke P. Converting enzyme inhibitors in cardiovascular therapy: current status and future potential. Cardiovasc Res. 1994;28:146–158. doi: 10.1093/cvr/28.2.146. [DOI] [PubMed] [Google Scholar]
- 27.Elbl G., Wagner H. A new method for the in vitro screening of inhibitors of angiotensin-converting enzyme (ACE), using the chromophore-and fluorophore-labelled substrate, dansyltriglycine. Planta Med. 1991;57:137–141. doi: 10.1055/s-2006-960050. [DOI] [PubMed] [Google Scholar]
- 28.Dorer F.E., Kahn J.R., Lentz K.E., Levine M., Skeggs L.T. Hydrolysis of bradykinin by angiotensin-converting enzyme. Circ Res. 1974;34:824–827. doi: 10.1161/01.res.34.6.824. [DOI] [PubMed] [Google Scholar]
- 29.Jaspard E., Wei L., Alhenc-Gelas F. Differences in the properties and enzymatic specificities of the two active sites of angiotensin I-converting enzyme (kininase II). Studies with bradykinin and other natural peptides. J Biol Chem. 1993;268:9496–9503. [PubMed] [Google Scholar]
- 30.Campbell D.J. Angiotensin converting enzyme (ACE) inhibitors and kinin metabolism: evidence that ACE inhibitors may inhibit a kininase other than ACE. Clin Exp Pharmacol Physiol. 1995;22:903–911. doi: 10.1111/j.1440-1681.1995.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 31.Segel I.H. Wiley; New York: 1975. Enzyme Kinetics. [Google Scholar]
- 32.Matthews B.W., Weaver L.H., Kester W.R. The conformation of thermolysin. J Biol Chem. 1974;249:8030–8044. [PubMed] [Google Scholar]
- 33.Webb J.L. Academic Press; New York, NY, USA: 1963. Enzyme and Metabolic Inhibitors; pp. 149–188. [Google Scholar]
- 34.Bachmetov L., Gal Tanamy M., Shapira A. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J Viral Hepat. 2012;19:e81–e88. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 35.Yadav J.P., Arya V., Yadav S., Panghal M., Kumar S., Dhankhar S. Cassia occidentalis L.: a review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia. 2010;81:223–230. doi: 10.1016/j.fitote.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Rai R.V., Rajesh P.S., Kim H.M. Medicinal use of Coscinium fenestratum (Gaertn.) Colebr.: an short review. Oriental Pharmacy and Experimental Medicine. 2013;13:1–9. [Google Scholar]
- 37.Chen C.H., Lin J.Y., Lin C.N., Hsu S.Y. Inhibition of angiotensin-I-converting enzyme by tetrahydroxyxanthones isolated from Tripterospermum lanceolatum. J Nat Prod. 1992;55:691–695. doi: 10.1021/np50083a025. [DOI] [PubMed] [Google Scholar]
- 38.Lacaille-Dubois M.A., Franck U., Wagner H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine. 2001;8:47–52. doi: 10.1078/0944-7113-00003. [DOI] [PubMed] [Google Scholar]
- 39.Balasuriya B.N., Rupasinghe H.V. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Functional Foods in Health and Disease. 2011;1:172–188. [Google Scholar]
- 40.Guerrero L., Castillo J., Quiñones M. Inhibition of angiotensin-converting enzyme activity by flavonoids: structure-activity relationship studies. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049493. e49493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Actis-Goretta L., Ottaviani J.I., Fraga C.G. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem. 2006;54:229–234. doi: 10.1021/jf052263o. [DOI] [PubMed] [Google Scholar]
- 42.Al Shukor N., Van Camp J., Gonzales G.B. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: a study of structure activity relationships. J Agric Food Chem. 2013;61:11832–11839. doi: 10.1021/jf404641v. [DOI] [PubMed] [Google Scholar]
- 43.Thippeswamy B.S., Mahendran S., Biradar M.I. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur J Pharmacol. 2011;654:100–105. doi: 10.1016/j.ejphar.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Liu P., Kallio H., Lü D., Zhou C., Yang B. Quantitative analysis of phenolic compounds in Chinese hawthorn (Crataegus spp.) fruits by high performance liquid chromatography–electrospray ionisation mass spectrometry. Food Chem. 2011;127:1370–1377. doi: 10.1016/j.foodchem.2011.01.103. [DOI] [PubMed] [Google Scholar]
- 45.Brain K.R. Accumulation of L-DOPA in cultures from Mucuna pruriens. Plant Sci Lett. 1976;7:157–161. [Google Scholar]
- 46.Machado T.D., Leal I.C., Amaral A.C., Santos K., Silva M.G., Kuster R.M. Antimicrobial ellagitannin of Punica granatum fruits. J Braz Chem Soc. 2002;13:606–610. [Google Scholar]
- 47.Ercisli S., Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007;103:1380–1384. [Google Scholar]
- 48.Ali I., Khan F.G., Suri K.A. In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Ann Clin Microbiol Antimicrob. 2010;9:7. doi: 10.1186/1476-0711-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu X., Zhang H., Lo R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J Agric Food Chem. 2004;52:7272–7278. doi: 10.1021/jf0490192. [DOI] [PubMed] [Google Scholar]
- 50.Kim I.S., Yang M., Lee O.H., Kang S.N. The antioxidant activity and the bioactive compound content of Stevia rebaudiana water extracts. LWT-Food Science and Technology. 2011;44:1328–1332. [Google Scholar]