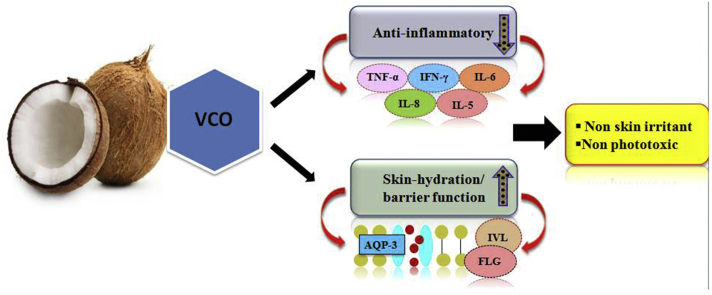
Abstract
Virgin coconut oil (VCO) has been traditionally used as moisturizer since centuries by people in the tropical region. Clinical studies have revealed that VCO improves the symptoms of skin disorders by moisturizing and soothing the skin. However, the mechanistic action of VCO and its benefits on skin has not been elucidated in vitro. The cytotoxicity (CTC50) of VCO was 706.53 ± 2.1 and 787.15 ± 1.1 μg/mL in THP-1 (Human monocytes) and HaCaT (Human keratinocytes) cells respectively. VCO inhibited TNF-α (62.34 ± 3.2 %), IFN-γ (42.66 ± 2.9 %), IL-6 (52.07 ± 2.0 %), IL-8 (53.98 ± 1.8 %) and IL-5 (51.57 ± 2.6 %) respectively in THP-1 cells. Involucrin (INV) and filaggrin (FLG) content increased by 47.53 ± 2.1 % and 40.45 ± 1.2 % respectively in HaCaT cells. VCO increased the expression of Aquaporin-3 (AQP3), involucrin (INV) and filaggrin (FLG) and showed moderate UV protection in HaCaT cells. In vitro skin irritation studies in Reconstructed human epidermis (RHE) and NIH3T3 cells showed that VCO is a non skin irritant (IC50 > 1000 μg/mL) and non phototoxic (PIF < 2). Our study demonstrated the anti inflammatory activity of VCO by suppressing inflammatory markers and protecting the skin by enhancing skin barrier function. This is the first report on anti-inflammatory and skin protective benefits of VCO in vitro. Overall, the results warrant the use of VCO in skin care formulations.
Keywords: Virgin coconut oil, Non-irritant, Anti-inflammatory, Skin barrier function, Skin hydration, Skin protection
Graphical abstract
1. Introduction
Skin is the most extensive and diverse organ of the human body.1 General skin condition is one of the important indicators of health. Its ill appearance resulting from dermatitis affects the mental condition of the patient, and above factors play a vital role in the progression and treatment of chronic skin diseases.1 Atopic dermatitis is classified as a chronic inflammatory skin disease, distinguished by invasive leukocytes, restructuring in skin barrier function, high percentage of water loss, decreased water retention capacity, a low percentage of lipids and ceramides.2 Such events results in distressful symptoms associated with the chronic disorders like inflammation and itching that leads to skin irritation.2, 3 Xerosis, a common skin condition displays several symptoms like external aggression, senescence to drugs, infection, and atopic dermatitis.4 The stratum corneum of patients suffering from atopic dermatitis shows low hydration, altered barrier function, increase in trans-epidermal water loss, and increase in thickness of stratum corneum.4, 5
Inflammation occurs in response to stimuli such as, infections and tissue injury.6 However, uncontrolled or sustained inflammation triggers several pathophysiological conditions like bacterial sepsis, rheumatoid arthritis, and skin inflammation.6, 7 The skin as a prime boundary between the body and external abiotic conditions, acts as the first line of defence against trauma-related injuries and invasion by microbial pathogens. In addition to physical barrier function, the skin possess several active defence mechanisms8 and regulation of these mechanisms is crucial, as inappropriate or misdirected immune activity is involved in the pathogenesis of several inflammatory skin disorders. Some of these conditions are medicated with ease, whereas treatments for chronic inflammatory diseases like psoriasis and atopic dermatitis are not 100% successful.9 Levels of cytokines and reactive oxygen species proffer in contributing to the mechanistic actions related with various inflammatory skin disorders.10
Cytokines, prostaglandins, leukotrienes and the pro-inflammatory molecules trigger the release of inflammatory mediators to the site of infection that is widely recognized by cutaneous inflammation.11, 12
Recently skin therapies highlight on combinational treatment like use of moisturizer, antibiotics, anti-histamine and corticosteroids for the treatment of skin inflammation in order to repair altered skin barrier function and reducing itch.13 However the use of steroids for immunosuppression and long term topical application lowers the collagen percentage causing atrophy of the skin.13 Because of these risks, new therapeutic approaches are being intensively investigated. Various plant species contain several bioactive components displaying health beneficial roles like anti-oxidative, anti-inflammatory, antimicrobial effects, thus increasing their use for therapeutic purposes. For development of anti inflammatory therapeutics, several plant-derived natural products have been tested in animal models.14, 15 Thus, naturally-derived plant products are emerging as a novel alternative to present certain diseases produced by free-radicals either in humans, food and cosmetics.16 Research revealed that essential oils are natural volatile compounds that exhibit strong odours and are produced as secondary metabolites by aromatic plants.17 Historically, they have been used for various medicinal purposes, ranging from skin problems to cancer treatment and are known for their antimicrobial, anti-inflammatory, sedative and analgesic properties.18
Virgin coconut oil (VCO) is processed natural oil obtained from fresh, mature coconut kernel.19 It displays several biological activities like anticancer, antimicrobial, analgesic, antipyretic, and anti-inflammatory properties in vivo.20, 21, 22 Traditionally, coconut oil is used to moisturize and treat skin infections. The emollient effect of coconut oil has been successfully demonstrated in atopic dermatitis patients, thereby showing that coconut oil is a potent natural emollient to be used in treatment of xerosis.23 The effectiveness and safe use of VCO for its application as a therapeutic moisturizer has been reported earlier for mild to moderate xerosis.24 However, there is no report available on anti-inflammatory and skin barrier function of VCO in vitro.
The aim of this study was to investigate the effect of VCO on selected inflammatory cytokines and to study its effect on skin hydration and barrier function in vitro. The in vitro skin irritation, UV protection and phototoxicity potential of VCO was also evaluated.
2. Material & methods
2.1. Cell lines and its maintenance
HaCaT (Human keratinocytes), THP-1 (Human monocytes) and NIH3T3 (mouse embryonic fibroblasts) were obtained from National Centre for Cell Science, Pune, India. HaCaT, NIH3T3 and THP-1 cells were grown in Ham's F12 DMEM (high glucose) and RPMI 1640, respectively. Reconstructed human epidermis was obtained from Skin Ethic, France. All media were supplemented with 10% heat inactivated fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 μg/mL) and cultured under a humidified atmosphere (95% air and 5% CO2) at 37 °C and the monolayer cultures were routinely subcultured by using trypsin–EDTA.
2.2. Chemicals
Ham's F12, DMEM (high glucose) and RPMI 1640 medium, 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), 2′,7′-dichlorofluorescin diacetate (DCFDA), Neutral red dye, dimethyl sulfoxide (DMSO) were obtained from Sigma Chemicals, Bangalore, India. Fetal Bovine Serum (FBS) was purchased from Invitrogen, USA. Glacial acetic acid, absolute ethanol, ethylene diamine tetra acetic acid (EDTA) was procured from Merck, India. Trypan blue was purchased from HiMedia Labs, India. ELISA kits for human TNF-α, IFN-γ, IL-6, IL-8 and IL-5 were purchased from Krishgen Biosystems, Mumbai, India. ELISA kits for human Involucrin and Filaggrin were purchased from Cloud-Clone Corp., USA. All the molecular biology reagents for PCR were obtained from Bio Rad, USA. Virgin Coconut Oil was obtained from Central Plantation Crops Research Institute (CPCRI), Indian Council of Agricultural Research, Government of India, Kasaragod, India.
2.3. Gas chromatography-FID analysis
The solvent extracts were analyzed by Gas Chromatography (GC) using a GC system 2014 Shimadzu, Japan equipped with AOC 5000 auto injector system, fitted out with a 30 m L × 0.25 mm i.d., 0.25 μm film thickness, BPX-70 analytical column, connected to a flame ionization detector (FID). Split ratio was 1: 20, with nitrogen as carrier gas at a flow rate of 1 mL/min, while the damping gas flow was 0.3 mL/min. The initial oven temperature was set to 40 °C for 1 min. The temperature for GC oven was as follows: 120 °C hold for 4 min and ramping at 5 °C/min to final temperature 230 °C and hold for 5 min at 230 °C. The injector temperature was 250 °C and the detector temperature was 275 °C. The detectable peaks were recorded, and the retention time was confirmed.25, 26
For identification of the compounds present in VCO, a solution of standard Fatty Acid Methyl Ester (FAME) grain mix (Sigma) was injected into the GC using the same instrumental conditions. The data was evaluated by comparing the retention times of FAMEs of the sample with those of standard FAME grain mix. The areas were computed for all the peaks, and percent composition was calculated by taking area percentage of total chromatogram.
2.4. Cell viability
MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay was used to determine cell viability, that reflects initial cell death. THP-1 and HaCaT cells were cultured in 96-well plates (1 × 104 cells/mL) and treated with various concentrations (15.625–1000 μg/mL) of VCO. After 24 h incubation, cytotoxicity was tested by MTT (10 μL/well containing 100 μL of cell suspension; 5 mg/mL of stock in PBS) solution and the absorbance were read at 540 nm using Synergy HT multi-detection microplate reader (Bio-Tek, Winooski, VT). The non-toxic concentrations (200 and 100 μg/mL) of the test sample were taken for further experiments.
Trypan blue assay was also used to determine cell viability. HaCaT cells were cultured in 6-well plate (1 × 105 cells/mL) and treated with various concentrations (100–1000 μg/mL) of VCO. After 24 h incubation, cells were trypsinized and stained with Trypan blue dye and the live cells were counted using Cell counter (Countess, Life Technologies).
2.5. ELISA for cytokine measurement
For the cytokine measurements of TNF-α, IFN-γ, IL-8, IL-6, & IL-5, THP-1 cells were seeded in 40 mm well plates with 1 × 105 cells and tested in duplicates. Further, cells were subjected to LPS (1 μg/mL) for the cytokine secretion, followed by treatment with two non-toxic concentrations of VCO for cytokine suppression. The conditioned media was removed from petri plates after 24 h incubation and stored at −80 °C for further use. The conditioned media was taken for all the assays, and the amount of cytokine for each sample was determined in duplicates and the assays were conducted as per the manufacturer's instructions. The concentration of cytokines was determined for the unknown using a standard curve.
2.6. ELISA for involucrin and filaggrin
HaCaT cells were used for estimation of involucrin and filaggrin activity. The cells were seeded into 40 mm petri plates, treated with non-toxic concentrations of VCO and then incubated for 96 h at 37 °C respectively. Kinetin (100 μmol/mL) was used as positive control for involucrin and filaggrin ELISA. After the completion of incubation period, cell lysates for involucrin and filaggrin were collected for determination of their content respectively. All the assays were performed in duplicates as per the manufacturer's protocol. The concentration of involucrin and filaggrin were determined for the unknown using a standard curve.
2.7. Semi-quantitative RT-PCR
THP-1 cells and HaCaT cells were treated for 24 h with two different non-toxic concentrations (200 μg, 100 μg/mL) of VCO. Total cellular RNA was extracted from THP-1 and HaCaT cells with RNA isolation kits according to the manufacturer's protocol for studying the expression of TNF-α, IL-6, IL-8, involucrin (INV), filaggrin (FLG) and aquaporin (AQP3). The samples were DNase treated, and first strand cDNA was synthesized using Superscript III and an oligo dT primer. Semi quantitative PCR was performed by rapid cycling using the master mix as a ready-to-use reaction mix according to the manufacturer's instructions. The RNA expression levels were normalized to the level of GAPDH expression. The primers used are listed in Table 1. The amplified PCR products were run in 1.5% agarose gel electrophoresis, stained with ethidium bromide and gel picture captured under UV light. Band intensities were quantified using IMAGE J software (Rasband, USA) and densitometric analysis was carried out.
Table 1.
List of Primers used in this study.
| Gene | Primer sequence |
|---|---|
| TNF-α(Human) | Fwd 5′ - ATGAGCACTGAAAGCATGATC - 3′ |
| Rev 5′ - TCACAGGGCAATGATCCCAAAGTAGACCTGCCC - 3′ | |
| IL-6 (Human) | Fwd 5′ - GTACCCCCAGGAGAAGATTC - 3′ |
| Rev 5′ - CAAACTGCATAGCCACTTTC - 3′ | |
| IL-8 (Human) | Fwd 5′- GCTTTCTGATGGAAGAGAGC - 3′ |
| Rev 5′ - GGCACAGTGGAACAAGGACT - 3′ | |
| AQP3 (Human) | Fwd 5′- GGTTGATGGTGAGGAAACCA - 3′ |
| Rev 5′ - GGGACCCTCATCCTGGTG - 3′ | |
| FLG (Human) | Fwd 5′ - TTTCGTGTTTGTCTGCTTGC - 3′ |
| Rev 5′ - CTGGACACTCAGGTTCCCAT - 3′ | |
| INV (Human) | Fwd 5′ - ACTGAGGGCAGGGGAGAG - 3′ |
| Rev 5′ - TCTGCCTCAGCCTTACTGTG - 3′ |
2.8. Skin irritation and phototoxicity assay
Skin irritation and phototoxicity assay was performed using NIH3T3 cells as per OECD guideline 432. Neutral red uptake assay was carried out in NIH3T3 cells to assess the cytotoxicity as described by us previously.27, 28 Phototoxicity assay was carried out with NIH3T3 cells cultured in 96 well plates. The culture medium was decanted and replenished with 100 μl of VCO with 7 different concentrations ranging from 15.625 μg/mL to 1000 μg/ml. Cell control and SDS (reference standard) control was also maintained. The culture plates were incubated for one hour at 37 °C. Of the treated plates, one was exposed to UV radiation at room temperature with the highest dose of radiation which is non-cytotoxic (i.e., 5 J/cm2). Other plate was kept in dark box for 50 min (UV exposure time) to determine cytotoxicity (-Irr) (i.e., the control plate). Test solution was decanted and washed twice with 150 μl of buffer solution and incubated with culture medium for 18–22 h. After incubation, NRU assay was followed to determine the cytotoxicity. Further, PIF value (photo irritation factor) was determined by using formula,
In vitro skin irritation study was also performed on the Reconstructed Human Epidermis (RHE) model as per the OECD guideline 439. The experiment was conducted as per the INVITTOX SKINETHIC™ SKIN IRRITATION TEST-42bis protocol. Briefly, VCO were weighed approximately 16 μl and transferred on the top of epidermis tissue, and incubated for 42 min at 37 °C with 5% CO2. After incubation, the treatment was washed with PBS and traces of PBS was drained with filter paper and further incubated in growth medium for 42 h at 37 °C with 5% CO2. After incubation, the treated tissues were transferred in the prefilled MTT solution and incubated for 3 h at 37 °C. The formazan was extracted by isopropanol and the absorbance was measured at 570 nm. The percentage viability was calculated from absorbance values at 540 nm of treated and control groups.
2.9. UV inhibitory study by reactive oxygen species assay
Cells were seeded in 96-well plate with a density of 3 × 104 cells per well using HAM'S F-12 media containing 10% Fetal bovine serum and incubated overnight in CO2 incubator. Drug treatment was done at various concentrations (100–500 μg/mL) using HAM'S F-12 media containing 2% Fetal Bovine Serum and incubated for 1 h in CO2 incubator. After drug incubation, cells were washed with sterile PBS (100 μl) and irradiated the cells in serum free HAM'S media with a radiation dose of 200 mJ/cm2 of UVB irradiation. The cells were incubated with DCFDA (10 μM) containing 2% fetal bovine serum in phenol free HAM’S media and incubated in CO2 incubator for 30 min. After incubation, the media was removed and washed with PBS (100 μl), after which 50 μl of PBS was added and fluorescence was taken at an excitation wavelength of 488 nm and emission wavelength of 530 nm.
3. Results
3.1. Chemical composition of VCO by GC-FID analysis
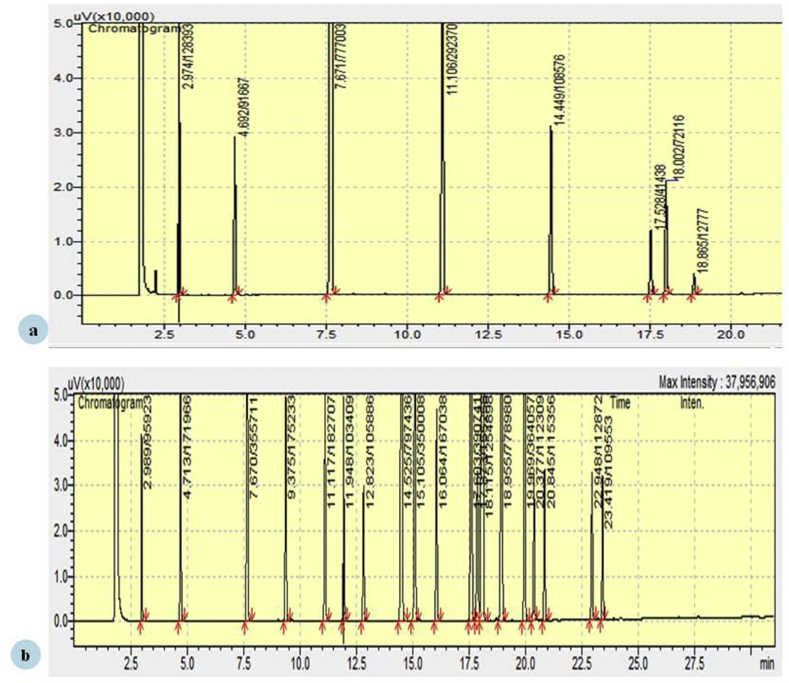
The composition of Virgin coconut oil (VCO) was determined by GC-FID analysis. A total of 8 fatty acids were qualitatively identified in VCO against the standard FAME mix. The constituents of VCO with their retention time and percentage composition are given in Table 2 and Fig. 1.
Table 2.
Chemical composition of Virgin coconut oil.
| Fatty acid | Retention time (min) | % |
|---|---|---|
| Caprylic acid C8:0 | 2.97 | 8.42 |
| Capric acid C10:0 | 4.69 | 6.01 |
| Lauric acid C12:0 | 7.67 | 50.97 |
| Myristic acid C14:0 | 11.10 | 19.18 |
| Palmitic acid C16:0 | 14.44 | 7.12 |
| Stearic acid C18:0 | 17.52 | 2.71 |
| Oleic acid C18:1 | 18.00 | 4.73 |
| Linoleic acid C18:2 | 18.86 | 0.83 |
Fig. 1.
(a) GC-FID chromatogram showing compounds of Virgin Coconut Oil. (b) GC-FID chromatogram showing compounds of FAME grain mix.
3.2. Cell viability assay by MTT and Trypan blue
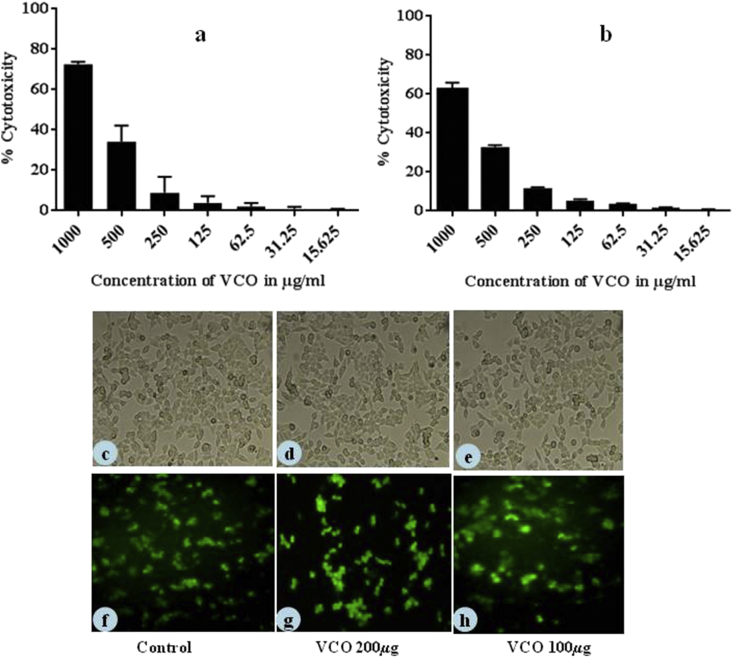
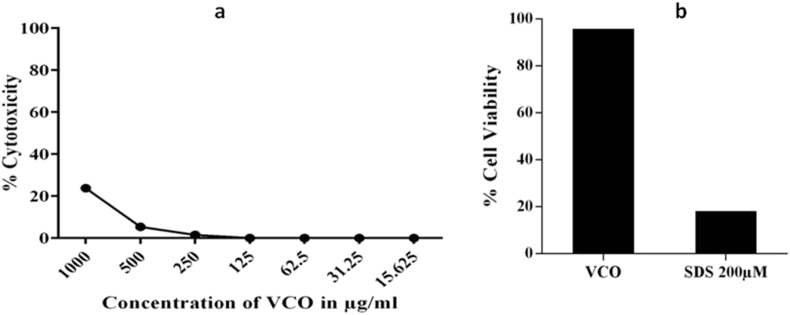
To determine the cytotoxic effect of VCO on THP-1 and HaCaT cells, MTT assay was carried out. The results showed that VCO at 706.53 and 787.15 μg/mL caused 50% cytotoxicity to THP-1 and HaCaT cells respectively (Fig. 2).
Fig. 2.
Cytotoxicity effect of VCO on (a) THP-1, (b) HaCaT cells. Both the cells were treated with different concentrations of VCO and the cell viability was assessed by using MTT assay. Data are expressed as percentage of control (n = 3). Morphology of cells with/without VCO treatment under phase contrast microscope (c) Control cells (d, e) Cells treated with VCO at 200 μg/mL and 100 μg/mL respectively. Cell viability on treatment of VCO assessed by Live/Dead cell assay. The green fluorescence cells are viable cells. (f) Cell control, Cells treated with VCO (g) 200 μg/mL (h) 100 μg/mL.
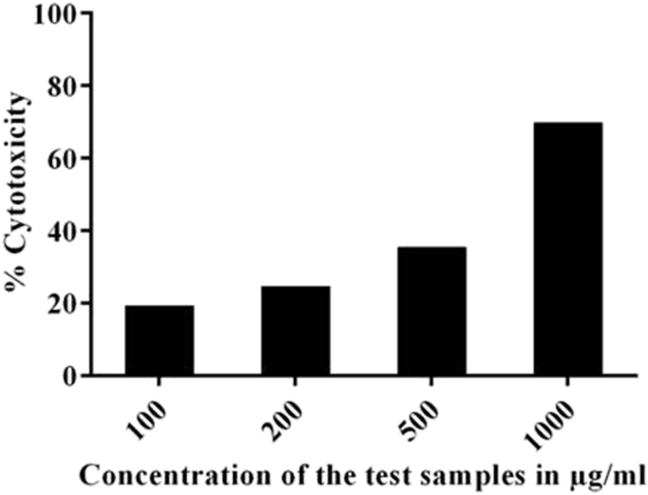
The cell viability of VCO on HaCaT cells were also determined using Trypan blue assay (Fig. 3). The results showed that IC50 of VCO was found to be 712.62 μg/mL. The nontoxic concentrations of VCO was further taken for the evaluation of its anti-inflammatory activity and skin barrier function.
Fig. 3.
Cytotoxic effect of VCO on HaCaT cells using Trypan blue staining method. Cells were treated with different concentrations of VCO (100–1000 μg/mL) and the cell viability was assessed by using Trypan blue staining. The stained cells were counted using a cell counter. Data is expressed as percentage of control (n = 2).
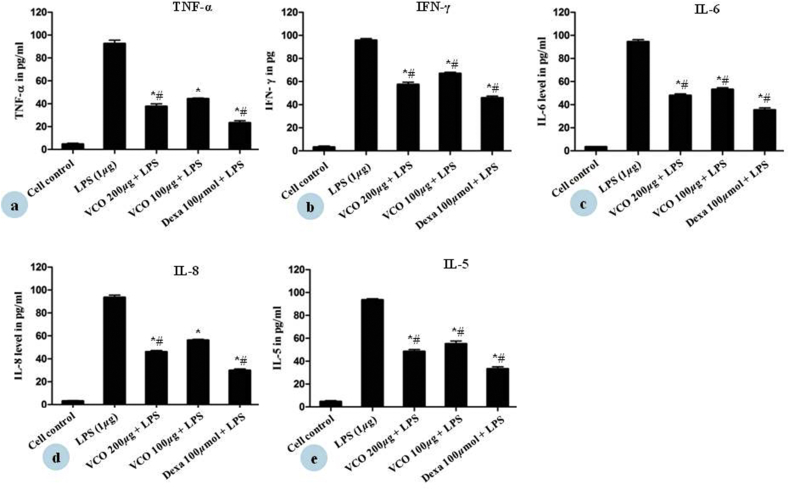
3.3. Effect of VCO on inflammatory cytokines measurements
We examined whether VCO could regulate pro inflammatory cytokines such as TNF-α, IFN-γ, IL-6, IL-5, and IL-8 in THP-1 cells. In order to activate the various cytokine secretions, THP-1 cells were exposed to 1 μg/mL of LPS for 24 h. LPS increased the cytokine secretion in THP-1 cells, whereas the cytokines level was reduced in dose dependent manner by VCO in LPS stimulated THP-1 cells, the results are shown in Table 3. VCO showed 62.34 ± 3.2%, 42.66 ± 2.9%, 52.07 ± 2.0%, 53.98 ± 1.8%, and 51.57 ± 2.6% inhibition of TNF-α, IFN-γ, IL-5, IL-6 and IL-8 at higher test concentration respectively (Fig. 4). Dexamethasone (100 μM) was used as positive control which showed 76.78 ± 2.8%, 64.16 ± 2.2%, 64.62 ± 2.5%, 70.26 ± 1.9% and 66.80 ± 2.8% inhibition of TNF-α, IFN-γ, IL-6, IL-8 and IL-5 respectively.
Table 3.
Inhibitory role of VCO on cytokines production.
| Cytokines | VCO concentration (μg/mL) | % inhibition |
|---|---|---|
| TNF-α | 200 | 62.34 ± 3.2 |
| 100 | 55.79 ± 1.0 | |
| IFN-γ | 200 | 42.66 ± 2.9 |
| 100 | 33.08 ± 1.6 | |
| IL-6 | 200 | 52.07 ± 2.0 |
| 100 | 46.87 ± 2.1 | |
| IL-8 | 200 | 53.98 ± 1.8 |
| 100 | 43.81 ± 1.1 | |
| IL-5 | 200 | 51.57 ± 2.6 |
| 100 | 44.94 ± 3.5 |
Fig. 4.
Effect of VCO on inhibition of inflammatory cytokines (a) TNF-α, (b) IFN-γ (c) IL-6 (d) IL-8 (e) IL-5 in THP-1 cells was done by ELISA. THP-1 cells were incubated with LPS alone, LPS with test samples in media containing 2% FBS for 24 h at 37 °C. Each values represented Mean ± SE of two determinations. *p < 0.05 statistical comparison with cell control was performed by student t-test. #p < 0.05 statistical comparison with LPS was performed by student t-test (n = 2).
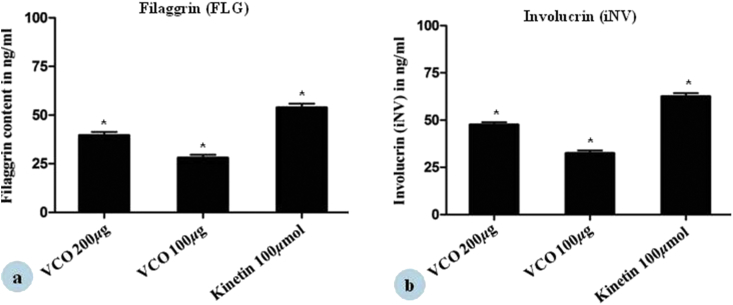
3.4. Effect of VCO on various epidermal markers
ELISA measurements revealed a steady increase in involucrin and filaggrin content in HaCaT cells after 4 days. Treatment of HaCaT cells with VCO increased involucrin content by 47.53 ± 2.1% and 32.36 ± 2.3% at 200 μg and 100 μg/mL respectively (Fig. 5 (a)). Kinetin was used as positive control and increased the involucrin content by 62.44 ± 2.6% at 100 μmol concentration. VCO increased filaggrin level by 40.45 ± 1.2% and 27.95 ± 2.3% at 100 and 50 μg/mL respectively (Fig. 5 (b)). Kinetin increased the filaggrin content by 53.72 ± 3.1% at 100 μmol concentration.
Fig. 5.
Effect of VCO on stimulation of epidermal differentiation marker (a) Filaggrin, (b) Involucrin in HaCaT cells was done by ELISA. HaCaT cells were incubated with VCO in media containing 2% FBS for 96 h at 37 °C. Kinetin (100 μmol)/mL was maintained as control. Each values represented Mean ± SE of two determinations. *p < 0.05 statistical comparison with cell control was performed by student t-test (n = 2).
3.5. Semi-quantitative RT-PCR
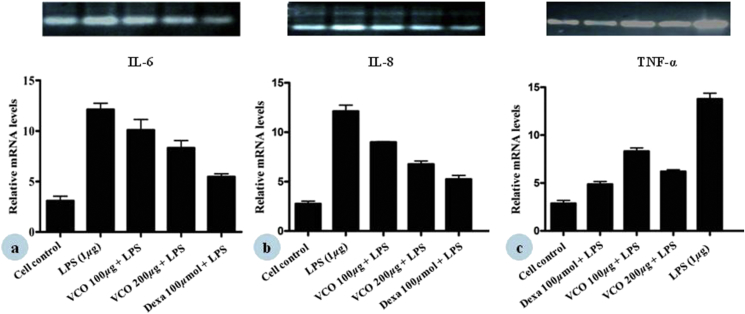
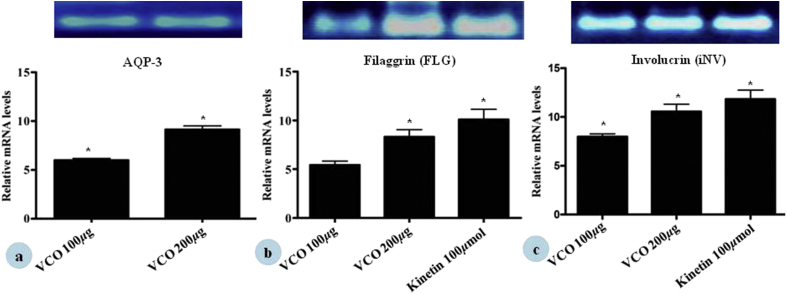
The isolated cDNA from THP-1 and HaCaT cells upon the treatment with VCO were amplified with specific primers for TNF-α, IL-6, IL-8 and involucrin, filaggrin and aquaporin-3. The results of semi-quantitative PCR showed the expression of TNF-α, IL-6, and IL-8 that became more pronounced on addition of 1 μg/mL LPS. Treatment with VCO at 200 and 100 μg/mL clearly reduced all cytokine mRNA expression as compared with LPS control cells. Similarly, dexamethasone showed reduced mRNA expression of TNF-α, IL-6, and IL-8 (Fig. 6). To further establish the skin barrier role of VCO, the mRNA levels for skin-related proteins/markers were checked. VCO at 200 and 100 μg/mL increased the gene expression of AQP-3, FLG, and INV in HaCaT cells (Fig. 7). The amplified products were visualized by ethidium bromide staining, that confirmed the results of semi-quantitative PCR. GAPDH gene has a constant rate of expression in the cell and hence, was used as an internal control to correct variations in different samples and also for establishing the degree of expression of given cytokines and skin related proteins by mRNA expression to the level of GAPDH mRNA expression and the densitometry analysis was carried out using Image J software.
Fig. 6.
Effect of VCO on (a) IL-6, (b) IL-8, (c) TNF-α, gene expression in THP-1 cells. Densitometric analysis of gene transcripts shown here. For each study, cells were treated with LPS alone, LPS + test samples (at indicated concentrations), Dexamethasone (100 μg/mL) + LPS for 24 h. RNA was then isolated from the cells and RT-PCR carried out using random hexamer primers and gene specific primers as described in the Materials and methods. The image shown is a representative from among three replicates. The relative level of gene expression is normalized to GAPDH (n = 3).
Fig. 7.
Effect of VCO on (a) Aquaporin, (b) Filaggrin, (c) Involucrin, gene in HaCaT cells. Densitometric analysis of gene transcripts shown here. For each study, cells were treated with different concentration of VCO and Kinetin (100 μmol/mL). RNA was then isolated from the cells and RT-PCR carried out using random hexamer primers and gene specific primers as described in the Materials and methods. The image shown is a representative from among three replicates. The relative level of gene expression is normalized to GAPDH (n = 3).
3.6. Skin irritation and phototoxicity assay
VCO was non toxic to NIH3T3 cells, the IC50 value was found more than 1000 μg/mL as evident from NRU cytotoxicity assay (Fig. 8a). Cytotoxicity conducted in RHE also confirmed that VCO is a non skin irritant (>50% non-irritant) (Fig. 8b). In photo toxicity studies, IC50 value was found to be more than 1000 μg/mL in both irradiated and non irradiated plates. PIF value was found to be 1.00 which corresponds to non-phototoxic category (PIF < 2.00 for non-phototoxic compounds) according to OECD guideline 432. These studies clearly established that VCO is a non skin irritant and non phototoxic.
Fig. 8.
In vitro skin irritation assay for VCO. a) Cytotoxic effect of VCO on NIH3T3 cells. The cells were treated with different concentrations of VCO and the cell viability was assessed by using NRU assay. b) Cell viability in RHE model. RHE was treated with VCO and the cell viability was assessed by using MTT assay.
3.7. Protection against UVB irradiation induced intracellular ROS
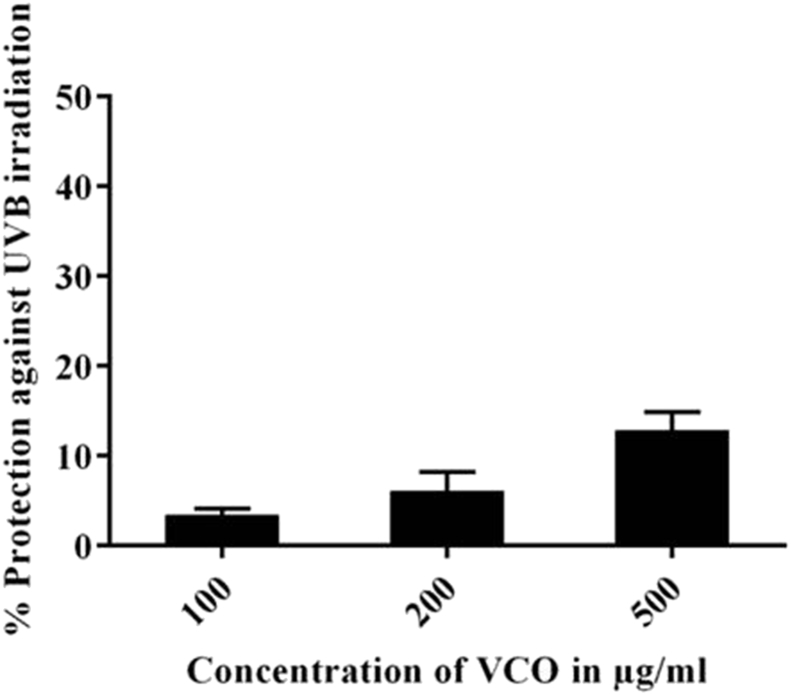
UVB induced ROS generation plays an important role in skin ageing, inflammation and carcinogenesis. To investigate the protective effect of VCO in UVB irradiated HaCaT cells, the intracellular ROS generation was estimated using a fluorescent probe DCFDA. The UVB dose of 200 mJ/cm2 showed increased concentration of ROS generation compared to normal cells. As shown in Fig. 9, VCO showed concentrated dependent ROS inhibition with highest protection at 500 μg/mL.
Fig. 9.
Effect of VCO on UVB induced reactive oxygen species (ROS) in HaCaT cells. The cells were treated with different concentrations of VCO and the percentage protection against UVB induced ROS was measured using DCFDA fluorescent probe. Data are expressed as percentage of control (n = 3).
4. Discussion
The present study investigated the anti-inflammatory activity and skin protective effects of VCO. Our study showed that VCO can alter the expression of several genes concerned with inflammatory response and skin beneficiary effects. Monocytes/Macrophages play a vital role in delivering effective immune function as part of innate immunity.29, 30 These cells play an important role in immunomodulation by secreting cytokines which show acute inflammatory response and inhibitory effects.31 The cytokines TNF-α, IL-1, IL-6, IL-8 and IL-10 are secreted by monocytes and have been found to be responsible for normal immune response and immunopathogenesis.32 Our research work described the effect of VCO on skin inflammation that VCO was capable of suppressing LPS induced pro inflammatory cytokine stimulation in Human monocytic leukaemia (THP-1) cells. We found that VCO suppressed the pro inflammatory cytokines in both protein and gene expression level.
Tumor necrosis factors (TNF-α) and Interleukins (IL) are pro-inflammatory cytokines responsible for inflammation, fever, tissue damage and cell death.33 TNF-α is widely studied pro inflammatory cytokine which play a key role in the pathogenesis of numerous inflammatory diseases.34 TNF is an important initiator cytokine of inflammatory responses. TNF-α has been reported to be a crucial therapeutic target in various inflammatory diseases.35 The release of TNF-α after stimulation of THP-1 cells with LPS is best availed model system to test compounds for potential anti-inflammatory effects. This study demonstrated that VCO significantly decreases the LPS-induced TNF-α production in THP-1 cells and VCO as such had very high impact on TNF-α reduction in the cell culture medium and showed significant inhibition of both TNF- α secretion and mRNA expression.
Monocytes secretes IFN-γ, IL-10, IL-12 cytokines which actively participate in cross regulatory role in the pathogenesis of infection and various inflammatory diseases.36 The most vital biological activity of interferon (IFN) is immune cell activation. IFN has been found to stimulate the activation of pro-inflammatory transcription factor Nuclear factor B (NF-kB), under particular conditions. Epidermal keratinocytes, react to proinflammatory cytokines like tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) by involving in the expression of many inflammatory mediators during the chronic inflammatory skin disorders like psoriasis and atopic dermatitis. The chemokines and the growth factors produced by these keratinocytes act as a driving force behind the accumulation and proliferation of inflammatory cells to the skin, in turn maintaining the chronic skin inflammation.37 IFN-γ plays an essential role in the generation and regulation of the immune response and it is the earliest detectable cytokine at the site of immunization with protein antigens. Apart from these effects, IFN-γ priming and activation of macrophages lead to enhanced production of proinflammatory cytokines in response to several stimuli.38 In this study, we focused on IFN-γ inhibitory activity of VCO on LPS stimulation. VCO was potent in reducing the LPS induced IFN-γ secretion and our results have demonstrated the anti-inflammatory effects of VCO on IFN-γ inhibition in THP-1 cells.
Interleukin – 6 (IL-6), a pro-inflammatory cytokine which plays a key role in the host defense mechanism, is released by macrophages, T and B cells, endothelial cells, monocytes, fibroblasts and keratinocytes upon stimulation.39 It is also involved in growth and differentiation of dermal, epidermal and cytotoxic cells.40 IL-6 is found to be involved in epidermal hyperplasia in psoriatic epithelium, influences the functioning of dermal inflammatory cells and induces the differentiation of human Th17 cells.41 IL-6 which is induced by LPS similar to TNF and IL-1 is also involved in the programmed activation of proinflammatory cytokine.42 Our study demonstrates that VCO mediate its anti inflammatory activity by reducing the secretion of IL-6 level in LPS stimulated THP-1 cells.
IL-8, a member of chemotactic cytokine family is central to the chemotactic migration and activation of neutrophils and other cell types like monocytes, lymphocytes, basophils and eosinophils at the site of inflammation.43, 44 It is one of the widely studied chemokine and hence used as a prototype for demonstrating the biological properties of the rapidly growing family of inflammatory mediators.45 In our study, we found that lipopolysaccharide (LPS) has significantly up regulated IL-8 expression in THP-1 cells. Here VCO significantly reduced the LPS induced IL-8 gene expression in a dose dependent manner.
The secretion of IL-5 is vital for eosinophilic inflammation as demonstrated in animal and human models respectively. The activated mast cells produce IL-5 leading to hypersensitive allergic reaction and activation of eosinophil.46 IL-5 in humans is not present in significant level, it is found to be in higher levels in the circulation, tissue and bone marrow under various disease conditions which include respiratory tract, hematopoietic system, gut, skin and also in food and drug allergies, atopic dermatitis, aspirin sensitivity and allergic or non allergic respiratory diseases.47 In our study LPS significantly increased IL-5 level in THP-1 supernatant medium indicating that inflammatory mechanisms were involved. This study demonstrated that VCO significantly decreases the LPS-induced IL-5 in THP-1 cells, which further confirm its anti-inflammatory effect.
On the contrary, VCO augments the protein coding genes present in the topmost layer of the epidermis which include involucrin (IVL), filaggrin (FLG), aquaporin (AQP-3) which play a major role in keratinocyte differentiation and skin barrier function. Cytokines secreted by keratinocytes and other skin inhabited cells are involved in cellular communication thus ending up with an altered barrier function of the skin. For example, cytokines manipulate the keratinocyte proliferation and differentiation, by amending the gene expression within these cells. The regulated expression of cytokines results in the complex cascade of signaling moieties which influence the keratinocyte physiology and barrier function of the skin. Uncontrolled cytokine expression can lead to dysfunction of epidermal barrier as seen in diseases like atopic dermatitis (AD) and psoriasis. The study reported that the influence of different epidermal marker which is responsible for the skin barrier/beneficial function in human keratinocytes.48
Aquaporins (AQPs) are molecular water channel which are expressed in epithelia and endothelia in fluid transporting organs and other cell types, where they are involved in numerous physiological functions.49 The glycerol transporting function of aquaglyceroporins is involved in fat metabolism, skin hydration, wound healing, and cellular energy metabolism.50, 51 Aquaporins (AQPs) are trans-membrane channels that facilitate the transport of water and small moieties across the epidermal cell membrane thus maintaining the water-ion balance within the cell. Out of thirteen AQPs found in human, AQP3 found in epidermal basal cells is the most widely studied AQP.52, 53 AQP3 which is a hydrating agent also plays a vital role in maintaining osmotic gradient across viable layers of the skin.54 AQP3 has been reported to be a novel therapeutic agent in amending the immune response in numerous infectious and inflammatory conditions.55 The clinical trial results showed that VCO reduced cutaneous inflammation and increased the epidermal barrier function and hydration property by decreasing transepidermal water loss in Atopic dermatitis condition.56 Here, we found that the expression of AQP3 on HaCaT cells was up regulated upon VCO induction facilitating its functions that permits better distribution and maintenance of water, glycerol which helps in hydrating the skin.
Filaggrin is an essential protein that is required for the development of epidermal corneocytes and its intracellular metabolic generation leads to hydration of stratum corneum and balance in physiological pH. The insufficient amount of filaggrin content in patients is an important cause for pathogenic skin condition of atopic dermatitis.57 The keratins and filaggrin related proteins are important for skin barrier function. In this present study, we observed that VCO increased the filaggrin content level and our results are agreement with significant increase in filaggrin potential thereby moisturising the skin and improving the barrier function of skin.
Involucrin, a cell envelope protein is secreted in the early stages of keratinocyte terminal differentiation.58 The cross-linking of proteins with lipids to develop into cornified cell envelopes occurs in the upper layers of stratified epithelia during late stages of differentiation.58 Involucrin in human is an important precursor for envelope formation and cellular cohesion acting as a major substrate for enzymes.59 The down regulation of INV gene leads to structural anomalies of skin and hair.58, 60 Higher levels of involucrin expression in epidermis accounts for enhanced skin barrier function. Our study revealed that VCO increased the involucrin content level and also up regulated the mRNA expression level in HaCaT cells and thereby it can promote the cell envelop formation and cohesion.
Cell cytotoxicity and phototoxicity assays are commonly used in vitro bioassay methods to predict the toxicity of substances in various tissues and the ability of a chemical to elicit a corrosive response is easily predicted using appropriate end points because they demonstrate the degree of damage caused by the chemical.61 In the present study, we used RHE model and NIH3T3 cells to determine the cytotoxic effect of VCO by MTT and NRU assays respectively. MTT and NRU assays on RHE and NIH3T3 cells respectively is an OECD approved technique to determine the cytotoxicity of test materials to determine skin irritation.27 Photo irritation factor (PIF) helps determine the photoxicity potential of topical formulations. In the present study, VCO was found to be non-cytotoxic with IC50 value above 1000 μg/mL and non phototoxic with PIF value lesser than two. These studies showed that VCO is non skin irritant and non phototoxic and safe to be used for topical applications.
Intracellular reactive oxygen species (ROS) produced due to UVB irradiation plays a key role in inflammation, aging and cancer. The photoprotective effects of plants and plant extracts are well reported.62, 63 Also earlier reports shows that herbal oils has the ability to protect against UVB irradiation.64, 65 In the present study, VCO showed concentration dependent protection against intracellular ROS produced by UVB irradiation. The result shows that VCO protects against UVB irradiation and has anti-inflammatory action which makes it an important ingredient in formulations and warrants further clinical studies.
In summary, the present study demonstrates that topical application of VCO bring anti inflammatory activity by inhibiting the various cytokine levels including TNF-α, IFNγ, IL-6, IL-5 and IL-8 and improves skin barrier function by up regulating AQP-3, filaggrin and involucrin mRNA expression and also by protecting against UVB irradiation. Therefore, VCO could be useful in treating skin disorders with permeability barrier dysfunction, especially those accompanied by reduced epidermal protein expression, such as atopic dermatitis, eczema.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
We are grateful to Dr. Abheepsa Mishra, Department of Cell Biology, The Himalaya Drug Company, for helping us in drafting the article.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Weiss R., Fintelmann V. Herb Med. 2000:10–12. [Google Scholar]
- 2.Boguniewicz Mark, Leung Donald, Dermatitis Atopic. A disease of altered skin barrier and imune dysregulation. Imunnol Rev. 2011;242(1):233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinhoff M., Bienenstock J., Schmelz M., Maurer M., Wei E., Bíró T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006;126(8):1705–1718. doi: 10.1038/sj.jid.5700231. [DOI] [PubMed] [Google Scholar]
- 4.Tagami H., Kobayashi H., O’goshi K., Kikuchi K. Atopic xerosis: employment of noninvasive biophysical instrumentation for the functional analyses of the mildly abnormal stratum corneum and for the efficacy assessment of skin care products. J Cosmet Dermatol. 2006;5(2):140–149. doi: 10.1111/j.1473-2165.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 5.Lebwohl M., Herrmann L.G. Impaired skin barrier function in dermatologic disease and repair with moisturization. Cutis. 2005;76(6 Suppl.):7–12. [PubMed] [Google Scholar]
- 6.Dinarello C.A. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 1997;112(6 Suppl.):133–165. doi: 10.1378/chest.112.6_supplement.321s. [DOI] [PubMed] [Google Scholar]
- 7.Palladino M.A., Bahjat F.R., Theodorakis E.A., Moldawer L.L. Anti-TNF-alpha therapies: the next generation. 2003;2:736–746. doi: 10.1038/nrd1175. [DOI] [PubMed] [Google Scholar]
- 8.Kupper T., Fuhlbrigge R. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4(3):211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi Y.S., Lim H., Park H., Kim H.P. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: in vivo regulation of inflammation-associated gene expression. Biochem Pharmacol. 2003;66(7):1271–1278. doi: 10.1016/s0006-2952(03)00463-5. [DOI] [PubMed] [Google Scholar]
- 10.Trouba K.J., Hamadeh H.K., Amin R.P., Germolec D.R. Oxidative stress and its role in skin disease. Redox Signal. 2002;4(4) doi: 10.1089/15230860260220175. [DOI] [PubMed] [Google Scholar]
- 11.Briganti S., Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol. 2003;17(6):663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.L., Mukhtar H., Bickers D.R., Kopelovich L., Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003;192(3):294–306. doi: 10.1016/s0041-008x(03)00301-6. doi. [DOI] [PubMed] [Google Scholar]
- 13.Oikarinen A., Haapasaari K.M., Sutinen M., Tasanen K. The molecular basis of glucocorticoid-induced skin atrophy: topical glucocorticoid apparently decreases both collagen synthesis and the corresponding collagen mrna level in human skin in vivo. Br J Dermatol. 1998;139(6):1106–1110. doi: 10.1046/j.1365-2133.1998.02646.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin C.T., Chen C.J., Lin T.Y., Tung J.C., Wang S.Y. Anti-inflammation activity of fruit essential oil from Cinnamomum insularimontanum Hayata. Bioresour Technol. 2008;99(18):8783–8787. doi: 10.1016/j.biortech.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Lee D.Y., Choi G., Yoon T., Cheon M.S., Choo B.K., Kim H.K. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009;123(1):149–154. doi: 10.1016/j.jep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Lim Y.Y., Lim T., Tee J. Antioxidant properties of several tropical fruits: a comparative study. Food Chem. 2007;103:1003–1008. [Google Scholar]
- 17.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils–a review. Food Chem Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 18.de Sousa Damiao Pergentino. “Medicinal Essential Oils: Chemical, Pharmacological and Therapeutic Aspects.,” Nova Science Publishers.
- 19.Villariano B.J., Dy L., Lizada C.C. Descriptive sensory evaluation of virgin coconut oil and refined, blached, and deodorized coconut oil. LWT - Food Sci Technol. 2007;40:193–199. [Google Scholar]
- 20.Winarsi H., Purwanto A. Virgin coconut oil (VCO) enriched with Zn as immunostimulator for vaginal candidiasis patient. HAYATI J Biosci. 2008;15(4):135–139. [Google Scholar]
- 21.Intahphuak S., Khonsung P., Panthong A. Anti-inflammatory, analgesic, and antipyretic activities of virgin coconut oil. Pharm Biol. April 2008;2010(48):151–157. doi: 10.3109/13880200903062614. [DOI] [PubMed] [Google Scholar]
- 22.Nevin K.G., Rajamohan T. Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal wound healing in young rats. Skin Pharmacol Physiol. 2010;23(6):290–297. doi: 10.1159/000313516. [DOI] [PubMed] [Google Scholar]
- 23.Verallo-Rowell V.M., Dillague K.M., Syah-Tjundawan B.S. Novel antibacterial and emollient effects of coconut and virgin olive oils in adult atopic dermatitis. Dermatitis. 2008;19(6):308–315. [PubMed] [Google Scholar]
- 24.Agero A.L., Verallo-Rowell V.M. A randomized double-blind controlled trial comparing extra virgin coconut oil with mineral oil as a moisturizer for mild to moderate xerosis. Dermatitis. 2004;15(3):109–116. doi: 10.2310/6620.2004.04006. [DOI] [PubMed] [Google Scholar]
- 25.Wirasnita R., Hadibarata T., Novelina Y.M., Yusoff A.R.M., Yusop Z. A modified methylation method to determine fatty acid content by gas chromatography. Bull Korean Chem Soc. 2013;34(11):3239–3242. [Google Scholar]
- 26.Misra B.B., Dey S. Evaluation of in vivo anti-hyperglycemic and antioxidant potentials of α-santalol and sandalwood oil. Phytomedicine. 2013;20(5):409–416. doi: 10.1016/j.phymed.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Varma R.S., Godavarthi A., Vidyashankar S., Nandakum K.S., Patki P.S. Evaluation of in vitro toxicity of Rumalaya liniment using mouse embryonic fibroblasts and human keratinocytes Full Text Introduction. Int J Green Pharm. 2011 March:95–97. [Google Scholar]
- 28.Varma R.S., Shamsia S., Thiyagarajan O.S., Vidyashankar S., Patki P.S. Yashada bhasma (Zinc calx) and Tankana (Borax) inhibit Propionibacterium acne and suppresses acne induced inflammation in vitro. Int J Cosmet Sci. 2014;36(4):361–368. doi: 10.1111/ics.12134. [DOI] [PubMed] [Google Scholar]
- 29.Louie A., Baltch A.L., Smith R.P. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun. 1994;62(7):2761–2772. doi: 10.1128/iai.62.7.2761-2772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith J.G., Magee D.M., Williams D.M., Graybill J.R. Tumor necrosis factor-alpha plays a role in host defense against Histoplasma capsulatum. J Infect Dis. 1990;162(6):1349–1353. doi: 10.1093/infdis/162.6.1349. [DOI] [PubMed] [Google Scholar]
- 31.Sadeghi H.M., Schnelle J.F., Thomas J.K., Nishanian P., Fahey J.L. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Exp Gerontol. 1999;34(8):959–970. doi: 10.1016/s0531-5565(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 32.Dooley D.P., Cox R.A., Hestilow K.L., Dolan M.J., Magee D.M. Cytokine induction in human coccidioidomycosis. Infect Immun. 1994;62(9):3980–3983. doi: 10.1128/iai.62.9.3980-3983.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinarello C.A. Proinflammatory cytokines. Chest. 2000;118(2):503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 34.Eigler A., Sinha B., Hartmann G., Endres S., Taming T.N.F. Strategies to restrain this proinflammatory cytokine. Immunol Today. 1997;18(10):487–492. doi: 10.1016/s0167-5699(97)01118-3. [DOI] [PubMed] [Google Scholar]
- 35.Neurath M.F., Fuss I., Pasparakis M. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol. 1997;27(7):1743–1750. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- 36.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-??) Curr Opin Immunol. 1997;9(1):17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 37.Pastore S., Mascia F., Mariotti F., Dattilo C., Mariani V., Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005;174(8):5047–5056. doi: 10.4049/jimmunol.174.8.5047. [DOI] [PubMed] [Google Scholar]
- 38.Ponzoni M., Casalaro A., Lanciotti M., Montaldo P.G., Cornaglia Ferraris P. The combination of gamma-interferon and tumor necrosis factor causes a rapid and extensive differentiation of human neuroblastoma cells. Cancer-Res. 1992;52(4):931–5472. [PubMed] [Google Scholar]
- 39.Bartold P.M., Haynes D.R. Interleukin-6 production by human gingival fibroblasts. J Periodontal Res. 1991;26(4):339–345. doi: 10.1111/j.1600-0765.1991.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 40.Hirano T. Interleukin 6 and its receptor: ten years later. 1998;16 doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 41.Ishigame H., Kakuta S., Nagai T. Differential roles of interleukin-17A and-17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30(1):108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992;62(1 Part 1) doi: 10.1016/0090-1229(92)90042-m. [DOI] [PubMed] [Google Scholar]
- 43.Miller M.D., Krangel M.S. Biology and biochemistry of the chemokines - a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12(1–2):17–46. [PubMed] [Google Scholar]
- 44.Stoeckle M.Y., Barker K.A. Two burgeoning families of platelet factor 4-related proteins: mediators of the inflammatory response. New Biol. 1990;2(4):313–323. [PubMed] [Google Scholar]
- 45.Van Damme J. 1994. Interleukin-8 and Related Chemotactic Cytokines. [Google Scholar]
- 46.Cohn L., Elias J.A., Chupp G.L. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22(1):789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 47.Khan M. Role of cytokines. Immunopharmacology. 2008:33–60. [Google Scholar]
- 48.Proksch E., Brandner J.M., Jensen J.M. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 49.Verkman A. Mammalian aquaporins: diverse physiological roles and potential clinical significance. Expert Rev Mol Med. 2008;10(13):1–18. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeda N., Hibuse T., Funahashi T. Role of aquaporin-7 and aquaporin-9 in glycerol metabolism; Involvement in obesity. Handb Exp Pharmacol. 2009;190:233–249. doi: 10.1007/978-3-540-79885-9_12. [DOI] [PubMed] [Google Scholar]
- 51.Maeda N., Funahashi T., Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metab. 2008;4(11):627–634. doi: 10.1038/ncpendmet0980. [DOI] [PubMed] [Google Scholar]
- 52.Hara M., Verkman A.S. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc Natl Acad Sci U S A. 2003;100(12):7360–7365. doi: 10.1073/pnas.1230416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takata K., Matsuzaki T., Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem. 2004;39(1):1–83. doi: 10.1016/j.proghi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Fluhr J., Darienski R., Surber C. Glycerol and skin: holisic approach to its origin and function. Br J Dermatol. 2008;159(1):23–24. doi: 10.1111/j.1365-2133.2008.08643.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhu N., Feng X., He C. Defective macrophage function in aquaporin-3 deficiency. Faseb J. 2011;25(12):4233–4239. doi: 10.1096/fj.11-182808. [DOI] [PubMed] [Google Scholar]
- 56.Evangelista M., Casintahan F., Villafuerte L. The effect of topical virgin coconut oil on scorad, transepidermal water loss and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomized, double-blind clinical trial. Int J Dermatol. 2014;53(4):100–108. doi: 10.1111/ijd.12339. [DOI] [PubMed] [Google Scholar]
- 57.Thyssen J.P., Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134(4):792–799. doi: 10.1016/j.jaci.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Eckert R.L., Welter J.F. Transcription factor regulation of epidermal keratinocyte gene expression. Mol Biol Rep. 1996;23(1):59–70. doi: 10.1007/BF00357073. [DOI] [PubMed] [Google Scholar]
- 59.Steinert P.M., Marekov L.N. Direct evidence that involucrin is a major early isopeptide cross- linked component of the keratinocyte cornified cell envelope. J Biol Chem. 1997;272(3):2021–2030. doi: 10.1074/jbc.272.3.2021. [DOI] [PubMed] [Google Scholar]
- 60.Eckert R.L., Yaffe M.B., Crish J.F., Murthy S., Rorke E.A., Welter J.F. Involucrin–structure and role in envelope assembly. J Invest Dermatol. 1993;100:613–617. doi: 10.1111/1523-1747.ep12472288. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez L., Mitjans M., Infante M.R., Vinardell M.P. Assessment of the potential skin irritation of lysine-derivative anionic surfactants using mouse fibroblasts and human keratinocytes as an alternative to animal testing. Pharm Res. 2004;21(9):1637–1641. doi: 10.1023/b:pham.0000041459.63362.6f. [DOI] [PubMed] [Google Scholar]
- 62.Gui M., Du J., Guo J., Xiao B., Yang W., Li M. Aqueous extract of Chrysanthemum morifolium (Ju Hua) enhances the antimelanogenic and antioxidative activities of the mixture of soypeptide and collagen peptide. J Tradit Complement Med. 2014;4(3):171–176. doi: 10.4103/2225-4110.128897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thongrakard V., Ruangrungsi N., Ekkapongpisit M., Isidoro C., Tencomnao T. Protection from UVB toxicity in human keratinocytes by Thailand native herbs extracts. Photochem Photobiol. 2014;90:214–224. doi: 10.1111/php.12153. [DOI] [PubMed] [Google Scholar]
- 64.Gause S., Chauhan A. UV-blocking potential of oils and juices. Int J Cosmet Sci. 2016;38(4):354–363. doi: 10.1111/ics.12296. [DOI] [PubMed] [Google Scholar]
- 65.Saraf S., Kaur C. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn Res. 2010;2(1):22. doi: 10.4103/0974-8490.60586. [DOI] [PMC free article] [PubMed] [Google Scholar]