Abstract
Background
Proteinuria is a marker of chronic kidney disease in dogs and a risk factor for increased morbidity and death. Predictive models using the results of readily available screening tests could foster early recognition.
Objective
To determine whether urine specific gravity (USG) and semiquantitative category of dipstick protein can be used to predict urinary protein‐to‐creatinine ratio (UP : C) and to examine the effect of urine culture results on UP : C in dogs.
Animals
Three hundred ninety‐four dogs (482 visits) presented to a university Community Practice Clinic or Veterinary Teaching Hospital between January 2011 and November 2015.
Methods
Retrospective study. Medical records were searched to identify dogs for which urinalysis, UP : C measurement, and urine culture testing were performed during a single hospital visit. Urine specific gravity, UP : C, dipstick protein concentration, and findings of urine sediment analysis and urine culture were recorded. Regression or Spearman correlation analysis was used to test for relationships between UP : C and USG within dipstick categories and between UP : C and bacterial colony‐forming units per milliliter, respectively. Cohen's kappa test was used to evaluate agreement between urine culture and UP : C testing.
Results
There were significant (P < .05) weak negative correlations (R2 range, 0.14‐0.37) between UP : C and USG for all nonnegative urine protein dipstick categories. The presence of a positive urine culture did not agree with the presence of abnormal UP : C (κ = −0.06).
Conclusions and Clinical Importance
Within dipstick protein categories, UP : C cannot be accurately predicted from USG. Repeating UP : C measurement after resolution of urinary tract infection is advisable.
Keywords: dog, proteinuria, urinalysis, urinary tract infection
Abbreviations
- CFU/mL
number of bacterial colony‐forming units per milliliter of urine
- CPC
Community Practice Clinic
- hpf
high‐power field
- IRIS
International Renal Interest Society
- RBC
red blood cell
- UP : C
urinary protein‐to‐creatinine ratio
- USG
urine specific gravity
- VTH
Veterinary Teaching Hospital
- WBC
white blood cell
1. INTRODUCTION
Proteinuria is a marker of chronic kidney disease in dogs and a recognized risk factor for morbidity and death.1, 2 Colorimetric or turbidimetric evaluation of urine via dipstick or sulfosalicylic acid methods, respectively, are the most commonly used baseline screening tools for proteinuria.3 Often, the next diagnostic step for a dog that has evidence of proteinuria is measurement of the urinary protein‐to‐creatinine ratio (UP : C).4, 5 This test more precisely quantifies proteinuria but involves additional expense to the client and potentially a second sample collection, which might require an additional clinic visit.
Urinary protein loss, as quantified by dipstick analysis, must be interpreted in light of urine specific gravity (USG); that is, at low USG values, the presence and degree of proteinuria could be concealed, whereas at higher values, these might be overestimated. In one study, the positive predictive value of urine dipstick analysis for the detection of proteinuria (ie, UP : C ≥ 0.2) was 86.1% and 52.9% in samples for which USG was <1.030 and ≥1.030, respectively. In the same study, negative predictive value was 77.0% and 97.6% when USG was <1.030 and ≥1.030, respectively.6 If a reliable relationship between urine dipstick protein and UP : C could be identified while taking into account USG, the clinician could use this relationship to determine whether submission of a UP : C is likely to be of additional diagnostic value. Similarly, it could enable the clinician to make more informed therapeutic decisions when UP : C cannot be obtained.
Identification of persistent renal proteinuria requires exclusion of pre‐renal or post‐renal causes.5 However, post‐renal causes such as urinary tract infection might be superimposed on renal disease, and it could be challenging to discern the contribution of these factors to total proteinuria.1, 5 Thus, it would be valuable to clarify the relationship between naturally occurring bacteriuria and UP : C in particular, and to determine whether the number of colony‐forming units of bacteria per milliliter (CFU/mL) in bacteriuric urine is predictive of UP : C increase. This could allow clinicians to better interpret UP : C measurements from urine obtained at the time of bacteriuria.
The purpose of the study reported here was to determine whether USG might be used to predict UP : C when dipstick protein category is known. We also sought to examine the effect of the presence and severity of bacteriuria, as approximated by CFU/mL, on UP : C. We hypothesized that within a given protein dipstick category, USG and UP : C would be significantly negatively correlated, with a strength of relationship that would allow for the generation of useful predictive models. We further hypothesized that UP : C would be positively correlated with the severity of bacteriuria as indicated by CFUs/mL, and that the presence of confirmed bacteriuria would be associated with abnormal UP : C.
2. MATERIALS AND METHODS
2.1. Sample population
Our samples are from the electronic medical records of a university‐affiliated (University of Georgia) Community Practice Clinic (CPC) and Veterinary Teaching Hospital (VTH). The samples were retrospectively searched for all dogs for which urinalysis, UP : C measurement, and urine culture testing were requested during a single hospital visit between May 25, 2011 and November 12, 2015 (CPC), and between January 4, 2011 and November 12, 2015 (VTH). Each medical record was reviewed to identify those dogs for which tests were performed on the same day, regardless of presenting complaint. Cases were excluded if UP : C results were not available. If either urinalysis or urine culture was not performed on the same day as the UP : C, then only the test performed concurrently was used for analysis. Urine samples could be collected for any reason deemed necessary by the supervising veterinarian. Samples from more than 1 visit for a single dog could be used as long as inclusion criteria were satisfied at each visit.
The following data were recorded for each canine visit fulfilling the study criteria: signalment, method of urine sampling for urinalysis, date of test submission, and results of urinalysis, UP : C, and urine culture testing. Recorded urinalysis results included USG, urine protein concentration category as assessed by dipstick analysis, and findings of sediment analysis. For the purposes of this study, an inactive urine sediment was defined as a sample in which <5 white blood cells (WBCs)/high‐power field (hpf) and <10 red blood cells (RBCs)/hpf were observed. Samples for which ≥5 WBC/hpf or ≥10 RBC/hpf were observed were therefore considered to have an active urine sediment. The presence or absence of bacteriuria was recorded separately. Urine sample collection method is recorded for each urinalysis submission at our institution. As sample collection method is not routinely documented on the electronic submission requests and final reports for urine culture and UP : C, this information could not be confirmed in all cases. For the purposes of analysis, method of sample collection for urine culture and UP : C testing was taken to be the same as for urinalysis. Urinary protein‐to‐creatinine ratio data were recorded both as categorical (ie, normal [≤0.5] or abnormal [>0.5]) and as continuous variables. Designation of UP : C as “normal” or “abnormal” was based on the proposed substaging system of the International Renal Interest Society (IRIS).7 For the purposes of this study, given the IRIS recommendation for further investigation in dogs with UP : C > 0.5, samples classified as nonproteinuric (UP : C < 0.2) or borderline proteinuric (0.2 ≤ UP : C ≤ 0.5) were considered “normal,” and those classified as proteinuric based on the IRIS scheme (UP : C > 0.5) were considered “abnormal.” Recorded urine culture data included the presence or absence of bacterial growth (“positive” when CFU/mL > 0; “negative” when CFU/mL = 0), number of bacterial CFU/mL, and specific bacterial species isolated. If more than 1 organism was cultured, colony counts were summed for the purposes of statistical analysis. For visits in which a positive urine culture was documented, the presence or absence of clinical signs (ie, owner‐disclosed pollakiuria, stranguria, hematuria, malodorous urine, or all) was also recorded. The term “bacteriuria” is used for the description of all positive urine cultures in this article. The term “urinary tract infection” is increasingly interpreted to indicate the presence of clinical signs, which were not documented in all cases of positive culture, and is only used here to describe those instances in which lower urinary tract clinical signs were documented.
2.2. Urine assays
All urinalyses and UP : C evaluations were performed by the same laboratory (University of Georgia Clinical Pathology Laboratory, Athens, GA), whose standard procedures are as follows. Samples are routinely stored at 2°C‐8°C and processed within 1‐3 hours of collection. Depending on the volume of urine submitted, 1‐3 mL of urine is centrifuged at 2597 rpm for 10 minutes; the supernatant is then used for dipstick analysis and UP : C determination, and to measure USG by refractometer (Digital clinical refractometer; Reichert Technologies, Depew, New York). A commercially available colorimetric dipstick test (Multistix reagent strips for urinalysis; Siemens Healthcare Diagnostics, Tarrytown, New York) is used to categorize urine protein concentration according to the manufacturer's instructions and using the following scale: negative (0 mg/dL), trace, 1+ (30 mg/dL), 2+ (100 mg/dL), 3+ (300 mg/dL), and 4+ (≥2000 mg/dL). Urinary protein‐to‐creatinine ratio is determined using an automated chemistry analyzer. Urine samples with protein concentration less than the lower limit of detection of the analyzer (6 mg/dL) are assigned a value of 1 mg/dL for calculation of UP : C. Two different analyzers were used by this laboratory during the time frame evaluated by this study (Roche P‐Modular Analyzer [1/11‐3/15], Roche Cobas c501 [3/15‐11/15]; Roche Diagnostics, Indianapolis, Indiana). The new analyzer was validated, and dog samples and quality control material were run as correlation to determine if any bias existed to alter the reference intervals. No adjustment was necessary for the canine reference intervals.
All urine cultures were performed by the same laboratory (University of Georgia Veterinary Diagnostic Laboratory, Athens, GA). Routinely, urine samples are inoculated onto bacterial growth media (Tryptic Soy Agar with 5% Sheep Blood; Remel, Lenexa, Kansas) using a 1 μL calibrated loop, on the same day that they are received by the laboratory. Plates are then incubated at 35°C ± 2°C and examined approximately 24 and 48 hours later for colony formation. For calculation of CFU/mL, number of colonies per plate are counted and multiplied by 1000.
2.3. Statistical analysis
Statistical analyses were performed by 1 of 2 commercially available software packages (SAS V 9.4, Cary, North Carolina; SAS JMP 12 Pro, Cary, North Carolina). Data were tested for normality of distribution using a Shapiro‐Wilk test. Urine samples were grouped according to dipstick protein category. Within each dipstick category, univariable regression analysis by linear, Michaelis‐Menten, and logistic 3P modeling was used to test for a relationship between UP : C and USG. Logarithmic transformation of data, followed by regression analyses as described above, was also performed in an effort to improve goodness‐of‐fit. Spearman's rank‐order correlation was used to test for association between UP : C and nonzero CFUs/mL. Fisher's exact test was used to test for association between urinalysis sample collection method and urine culture positivity. Chi‐square and Fisher's exact tests were used to compare age and sex distributions and the proportion of bacteriuric samples between proteinuric and nonproteinuric dogs. The median UP : C for dogs with a positive urine culture displaying versus not displaying urinary tract signs was compared using the Mann‐Whitney U test.
Agreement between urine culture and UP : C results was determined by the use of Cohen's kappa test. Strength of agreement was further classified as less than chance for kappa values <0, poor for kappa values from 0.01 to 0.20, fair for values from 0.2 to 0.4, moderate for values from 0.41 to 0.60, substantial for values from 0.61 to 0.80, almost perfect for values from 0.81 to 0.99, and perfect for a value of 1.0.8
Using UP : C as the reference for the presence (UP : C > 0.5) or absence (UP : C ≤ 0.5) of proteinuria, the diagnostic performance of urine dipstick testing was evaluated by calculation of sensitivity, specificity, and negative and positive predictive values. For these calculations, a negative dipstick result was considered a “negative” test, and any dipstick result ≥ trace was considered a “positive” test. The diagnostic performance of UP : C for the detection of a positive or negative urine culture result was similarly evaluated; for these calculations, UP : C ≤ 0.5 was considered a “negative” test and UP : C > 0.5 was considered a “positive” test.
Where appropriate, data are reported as mean ± SD or median (minimum – maximum) for normally and non‐normally distributed data, respectively. For all tests, a P‐value of <.05 was considered to indicate statistical significance.
3. RESULTS
A total of 524 dog visits were identified by medical records search. Of these, 30 were excluded because UP : C determination was not performed on the same day as urinalysis and urine culture; 5 were excluded because UP : C was requested but not performed; 3 were excluded because requests for urine testing were canceled; 3 were excluded because there was no record of the dog having visited the hospital on the day of sample submission; and 1 was excluded because 2 different urinalyses were submitted on the same day (precluding the ability to compare UP : C to a single urine sediment), leaving a total of 482 visits, from a total of 394 individual dogs, for analysis. Three hundred seventeen visits were to the VTH and 165 visits were to the CPC. Two hundred seventy‐two of the 317 total visits (85.8%) to the VTH that met our inclusion criteria yielded a finding of proteinuria based on UP : C. Twenty‐six of 165 visits (15.8%) to our institution's CPC that met our inclusion criteria yielded a finding of proteinuria based on UP : C. Of the 482 included total visits, 28 did not have culture results from the same day as urinalysis and were therefore used only for USG and UP : C association testing; likewise, 15 did not have urinalysis results from the same day as urine culture and UP : C analysis and were therefore used only for culture and UP : C association testing.
The mean ± SD age of dogs from which urine samples were obtained was 9.8 ± 2.9 years. Two hundred seven dogs (52.5%) were spayed females, 157 dogs (39.8%) were castrated males, 11 dogs (2.8%) were intact females, and 19 dogs (4.8%) were intact males. A total of 89 breeds were represented, with dogs of mixed (n = 51), Labrador Retriever (n = 45), Golden Retriever (n = 24), Beagle (n = 22), and Yorkshire Terrier (n = 22) breeds comprising those most commonly represented. No difference in age or sex distribution between proteinuric and nonproteinuric dogs was found. For the 467 visits in which a urinalysis was completed contemporaneously with a UP : C, culture, or both, urine sample collection method for urinalysis was reported as cystocentesis, free‐catch, or direct catheterization for 421, 38, and 8 samples, respectively.
Urine specific gravity and UP : C for samples included in each protein dipstick category are presented in Table 1. Univariable regression analysis was performed for each of the 6 categories of dipstick protein (negative, trace, 1+, 2+, 3+, 4+). For each nonnegative dipstick category and each regression analysis performed, UP : C and USG were negatively correlated in a statistically significant but relatively weak manner. Relative strength of correlation for each type of univariable regression analysis varied by dipstick category; however, no analysis generated an R2 value exceeding 0.56 (ie, the value achieved by Michaelis‐Menten modeling in 1+ dipstick category; data not shown). The results of linear regression analysis within each dipstick protein category are presented in Table 1 and Figure 1. Logarithmic transformation of the data before regression analysis resulted in only modest improvement of goodness of fit (ie, increased R2 values by 0.03‐0.17; data not shown), with no analysis resulting in an R2 value exceeding 0.59. Intercept and slope values for each dipstick category to complete the predictive equation, UP : C = intercept + slope × USG, are included in Table 1.
Table 1.
Urinary protein‐to‐creatinine ratio (UP : C) and urine specific gravity (USG) of 467 canine urine samples stratified according to urinary protein dipstick category, including results of univariable linear regression analysis
| Dipstick category | No. samples | USG | UP : C | P‐value | R2 | Intercept | Slope |
|---|---|---|---|---|---|---|---|
| Negative | 67 | 1.017 (1.004‐1.053) | 0.10 (0.02‐5.63) | 0.28 | 0.02 | −8.8 | 8.8 |
| Trace | 72 | 1.025 (1.004‐1.048) | 0.18 (0.06‐1.28) | <0.0001* | 0.37 | 13.3 | −12.7 |
| 1+ | 67 | 1.022 (1.005‐1.054) | 0.38 (0.06‐3.96) | <0.0001* | 0.36 | 35.8 | −34.2 |
| 2+ | 97 | 1.016 (1.003‐1.059) | 1.68 (0.07‐9.93 | <0.0001* | 0.24 | 101.9 | −97.3 |
| 3+ | 119 | 1.021 (1.007‐1.050) | 4.56 (0.07‐32.95) | <0.0001* | 0.18 | 256.5 | −245.1 |
| 4+ | 45 | 1.029 ± 0.01 | 5.72 (0.8‐21.34) | 0.01* | 0.14 | 198.9 | −186.0 |
Data are presented as mean ± SD or median (range) where appropriate. * denotes statistically significant correlation between UP : C and USG.
Figure 1.
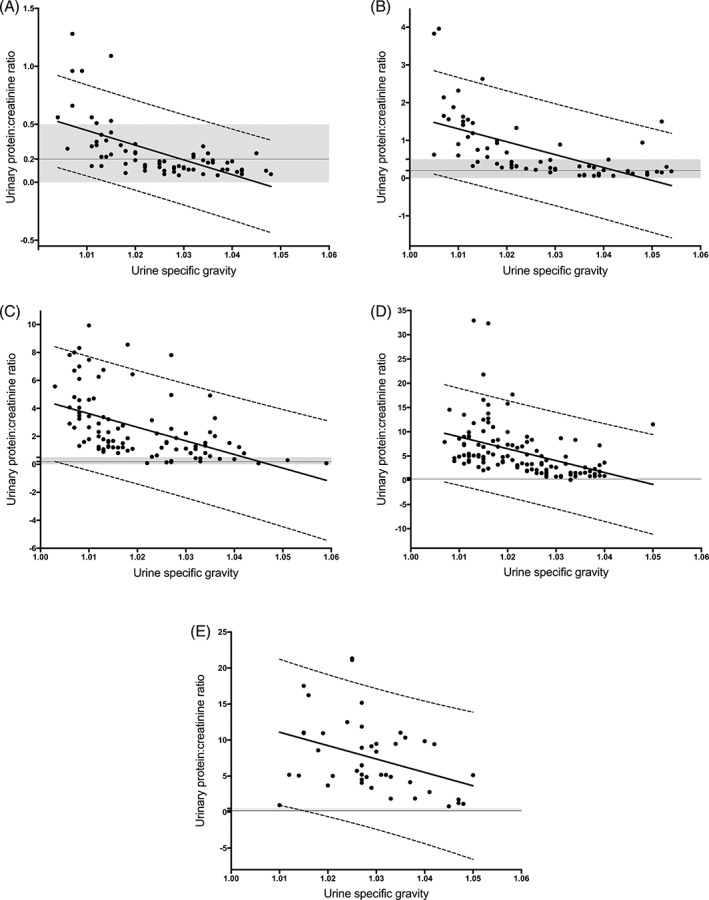
Scatterplots of urinary protein‐to‐creatinine ratio (UP : C) as a function of urine specific gravity (USG) for urine dipstick categories of trace (A), 1+ (B), 2+ (C), 3+ (D), and 4+ (E). For each, the solid line represents the line of best fit based on univariable linear regression analysis. Dashed lines represent the 95% prediction limits (ie, limits within which 95% of future measurements would be expected to lie). The shaded area represents the nonproteinuric range (ie, 0 < UP : C < 0.5), and the solid horizontal line at UP : C = 0.2 represents the cutoff value commonly applied to distinguish borderline proteinuria, as advocated by the International Renal Interest Society
Thirty‐nine (21.5%) of the 181 nonproteinuric samples and 62 (21.6%) of the 286 proteinuric samples from dogs for which urinalysis was performed concurrently with either urine culture or UP : C had an active urine sediment. The presence or absence of white blood cells was not recorded on sediment analysis for 1 dog in each proteinuria category. For the remainder of samples, sediments were considered inactive. Bacteriuria was noted in 23 (12.7%) of the 181 nonproteinuric samples and 23 (8.0%) of the 286 proteinuric samples. The presence or absence of bacteria was not recorded for 1 proteinuric dog. No bacteria were present in the remainder of dogs. The proportion of samples for which bacteria were present, absent, or not recorded was not statistically significantly different between proteinuric and nonproteinuric samples (P = .15). Twelve (26%) of the 46 bacteriuric samples had an inactive sediment.
Forty‐six (10%) of 454 samples in which urine culture testing was performed were positive; in the majority (37/46) of these, a single bacterial species was isolated, with the most commonly represented pathogens being Escherichia coli (n = 17), Streptococcus (n = 4), Staphylococcus intermedius (n = 3), Klebsiella pneumoniae ss. pneumoniae (n = 3), and Enterococcus (n = 3). The remaining 9 samples contained 2 or more bacterial isolates. Reported CFU/mL for all positive culture tests ranged from 3000 to >300 000 and were below 100 000 in 7 submissions originating from 4 individual dogs. At the time of sample collection, dogs from which 15 (32.6%) of the 46 culture‐positive samples were obtained were reportedly displaying clinical signs suggestive of a urinary tract infection, with pollakiuria disclosed most frequently. Frequency of specific clinical signs, CFU/mL, and UP : C in dogs with or without reported clinical signs of urinary tract infection are shown in Table 2. Only 5 (16.7%) of the 30 samples obtained from apparently asymptomatic dogs had low colony counts (ie, <100 000 CFU/mL), whereas the remainder (83.3%) had high colony counts (ie, ≥100 000 CFU/mL). For 1 asymptomatic case only “heavy growth,” but not CFU/mL, was reported. Similarly, only 2 (13.3%) of the 15 samples taken from symptomatic dogs had low colony counts, whereas 13 (86.6%) had high colony counts. Urinary protein‐to‐creatinine ratio values for samples with positive urine culture results are depicted in Figure 2. The median UP : C values for dogs with positive urine culture displaying versus not displaying urinary tract signs were not significantly different (P = .7). Although there was a statistically significant, but weak, positive correlation between severity of bacteriuria, as approximated by CFU/mL, and UP : C (P = .04; ρ = 0.31), there was no overall diagnostic agreement between urine culture results (positive/negative) and UP : C results (abnormal/normal) (P = .01; κ coefficient = −0.06). The diagnostic performances of urine dipstick testing for the detection of abnormal UP : C and of abnormal UP : C to detect a positive urine culture result are summarized in Table 3.
Table 2.
Bacterial colony counts, frequency of reported clinical signs, and UP : C in dogs from which 46 culture‐positive urine samples were obtained
| Number of cases | CFU/mL† | Clinical signs | UP : C | |||||
|---|---|---|---|---|---|---|---|---|
| 46 | <100 000 | ≥100 000 | Stranguria | Pollakiuria | Hematuria | Malodorous urine | ||
| Asymptomatic positive urine culture | 31 | 5 | 25 | 0 | 0 | 0 | 0 | 0.31 (0.07‐9.14) |
| Symptomatic positive urine culture | 15 | 2 | 13 | 1 | 12 | 2 | 2 | 0.43 (0.08‐32.34) |
Cases are stratified by the presence or absence of clinical signs at the time of sample collection. Data are presented as number or median (range) where appropriate.
Abbreviations: CFU/mL, number of bacterial colony‐forming units per milliliter of urine; UP : C, urinary protein‐to‐creatinine ratio.
Excludes 1 asymptomatic case for which “heavy growth,” but not CFU/mL, was reported.
Figure 2.
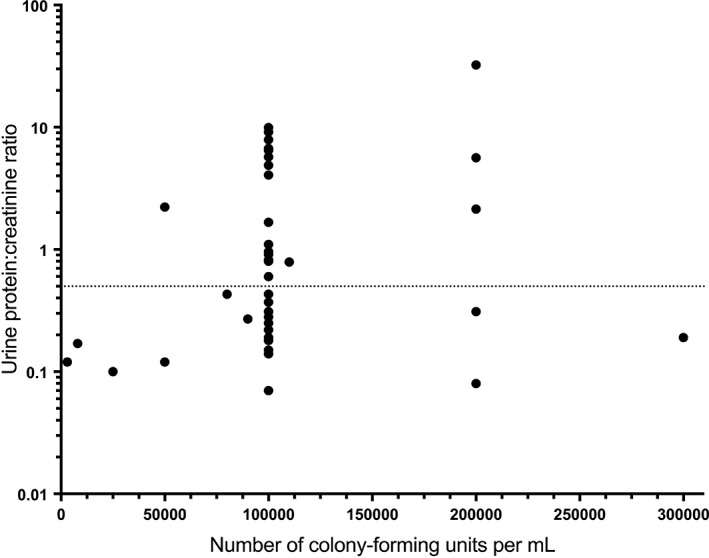
Urinary protein‐to‐creatinine ratio (UP : C) values for 46 canine urine samples in which a positive urine culture result was obtained. The dotted line at UP : C = 0.5 represents the cut‐off used to define proteinuria. CFUs/mL, number of bacterial colony‐forming units per milliliter
Table 3.
Diagnostic performance of urine dipstick testing to correctly classify UP : C results and of UP : C testing to correctly classify urine culture results, as assessed in canine urine samples taken on 467 (dipstick) and 455 (UP : C) occasions
| Diagnostic test | Outcome predicted | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Dipstick protein | UP : C | 99.3 | 48.4 | 81.6 | 96.7 |
| UP : C | Urine culture | 43.5 | 37.2 | 7.2 | 85.4 |
For dipstick testing, UP : C > 0.5 and ≤0.5 were taken to indicate the presence and absence of proteinuria, respectively; dipstick results were taken to be negative when = 0, and positive when ≥ trace. For UP : C testing, the reference criterion was the presence of positive urine culture results; UP : C results were taken to be negative when ≤0.5 and positive when >0.5.
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; UP : C, urinary protein‐to‐creatinine ratio.
Nineteen (3.9%) of 482 cases, 16 from the VTH population and 3 from the CPC population, had positive urine culture results characterized by heavy growth (ie, ≥100 000 CFU/mL), with normal UP : C findings; of these, 9 (47%) were classified as nonproteinuric (ie, UP : C < 0.2) and 10 (53%) were classified as borderline proteinuric (ie, 0.2 ≤ UP : C < 0.5), according to the IRIS substaging system. Thirteen (68.4%) of these 19 cases were not presented with clinical signs suggestive of a urinary tract infection despite having a positive urine culture.
Sample collection method is recorded for urinalyses but is not recorded for UP : C measurements and urine cultures at our institution; thus, it cannot be determined whether sample collection method was the same for UP : C and culture samples from the same dog in the present study. Collection via voiding was reported for urinalysis samples in 9 (19.5%) of 46 cases with positive urine culture results. The reported CFU/mL were below 100 000 for 3 of these 9 cases.
4. DISCUSSION
One purpose of the study reported here was to determine whether USG might be used to predict UP : C when dipstick protein category is known, so as to provide a simple means by which the practitioner could use the urinalysis results to determine the value of submission of urine for UP : C analysis. The results of the present study indicate that the values of USG within dipstick protein categories cannot be used to reliably predict UP : C in dogs. The use of univariable linear regression analysis to test for relationships between USG and UP : C within each dipstick category poorly described the data, as evidenced by the nonrandom distribution of points above and below the lines of best fit (Figure 1). For this reason, nonlinear analyses and log‐transformation of UP : C data were evaluated as potential methods to improve prediction. Although it was possible to variably improve goodness‐of‐fit with these approaches, these univariable analyses are still not recommended for at least 2 reasons. First, the greatest R2 value achieved by any analysis was only 0.59, indicating relatively weak correlation at best. Second, relative goodness‐of‐fit for each of the applied regression analyses varied widely by dipstick category (eg, R2 was greatest for samples in the 1+ dipstick category when the Michaelis‐Menten analysis was applied to log‐transformed UP : C data, whereas it was greatest for samples in the 2+ dipstick category when a logistic 3P analysis was applied to log‐transformed UP : C data). This variability in the “best” univariable analysis among dipstick categories precludes the clinical utility of univariable analysis as a way to predict specific UP : C. Although predictive equations associating UP : C and USG within categories of dipstick proteinuria were able to be generated by certain univariable analyses, these equations do not appear to be clinically useful because they do not reliably predict whether submission of urine for UP : C determination would likely result in an abnormal finding (ie, UP : C > 0.5) when USG and dipstick category are known.
Documentation of contemporaneous negative dipstick protein and abnormal UP : C results was uncommon in the present study, as reflected by the high observed negative predictive value (96.7%) of dipstick testing; however, it did occur on 2 occasions. These cases had UP : C values of 5.63 and 0.79, respectively. In the former, >100 000 CFU/mL of each of 2 different E. coli morphologies were isolated on urine culture. Urine specific gravity was 1.030 and urine pH was 6.0. The latter case had a USG of 1.010, a urine pH of 7.5, and a negative urine culture. The dog in that case was diagnosed with both chronic renal disease and heartworm disease. These examples illustrate the importance of direct measurement of UP : C and caution against assuming that UP : C can be reliably predicted based on USG and dipstick protein testing.
Our data suggest poor agreement between the presence or absence of an abnormal UP : C and the presence or absence of bacteriuria. These results are to be expected, as UP : C can be increased for a multitude of reasons, only 1 of which is the presence of bacteriuria.5 In addition, for culture‐positive samples, there was a significant but weak positive correlation (P = .04; ρ = 0.31) between our chosen measure of infection severity (CFU/mL) and UP : C. The low correlation coefficient may be due, at least in part, to the fact that there were very few dogs with extremely high CFU/mL values, making it difficult to determine whether the single dog with a UP : C of 32.34 and 200 000 CFU/mL represents an outlier. It is also possible that CFU/mL might not represent the best measure of infection severity, as some bacterial species could incite a more intense inflammatory reaction than others.9 Nevertheless, if CFU/mL is used to estimate the severity of an infection, the results of our study suggest that the presence or absence of an active urinary sediment is a poor predictor of the severity of a urinary tract infection.
The presence of a normal UP : C in the setting of a documented urinary tract infection would be unexpected. It is therefore noteworthy that 19 cases included in the present study were characterized by positive urine culture with heavy growth (≥100 000 CFU/mL) and UP : C within the reference interval (n = 9 with UP : C < 0.2; n = 10 with UP : C of 0.2‐0.5). In clinical practice, documentation of high UP : C in conjunction with a positive urine culture might often be attributed solely to the presence of a bacterial infection; however, these cases suggest that positive urine culture results might not fully explain concurrently increased UP : C values. Therefore, the opportunity to diagnose alternate causes of proteinuria could be missed if dogs with urinary tract infections were to fail to undergo further investigation after resolution of the infection. The practice of routinely performing a urine culture in an asymptomatic, proteinuric dog has been questioned in human medicine.10 This is a relevant question for dogs; the present study demonstrates that for a substantial number (68.4%) of the 19 culture‐positive cases, owner‐reported clinical signs suggestive of urinary tract infection were absent. There was no apparent difference in the proportion of cases with high colony counts between dogs with and without clinical signs of a urinary tract infection in this study. It was beyond the scope of this retrospective study to ascertain the effects of treatment of a urinary tract infection on the presence or severity of proteinuria. Future work should address this question by ensuring that dogs for which abnormal UP : C and positive urine culture results are found undergo repeat UP : C measurement after effective elimination of the urinary tract infection, to determine whether the proteinuria was caused by infection alone or instead persists after resolution of the infection because of an alternate cause. Finally, whether these dogs are more likely to be asymptomatic deserves additional investigation.
Various studies have evaluated the effect of active urine sediment findings on UP : C. Increases in UP : C have been demonstrated after gross11 and microscopic12 blood contamination. Another study demonstrated the potential for macroscopic hematuria to lead to increased urinary albumin concentrations.13 The present data suggest that the presence of an active sediment does not have a predictable effect on the UP : C measurement, with approximately one‐fifth (21.5%) of samples with a normal UP : C value displaying an active sediment.
There are several limitations to this study. First, because of its retrospective nature, the attending clinician's reasoning for pursuing urine testing cannot be known. However, because the goal of the study was to assess relationships among dipstick protein, bacteriuria, USG, and UP : C, dogs that had a urinalysis, urine culture, and UP : C performed concurrently would have been included regardless of the reason for performing them. At the authors' institution, a senior “wellness panel”—comprising complete blood count, serum biochemistry panel, urinalysis, UP : C, urine culture, blood pressure, and fecal and heartworm antigen testing—exists as a diagnostic option for geriatric dogs presented to the CPC. This panel is intended for screening of geriatric dogs and is commonly performed on ostensibly well animals. Consequently, a substantial proportion, but not all, of the visits included in the present study involved apparently asymptomatic dogs. Conversely, it is likely that additional dogs with proteinuria seen at the VTH during this time were not included in the study because their urine was not sampled and therefore their proteinuria went undetected, or because clinicians detected a urinary tract infection and elected not to pursue UP : C testing, but it is unlikely that enrollment of additional dogs from either category would have enabled the generation of a strongly predictive univariable equation for UP : C within dipstick protein categories. Additionally, severity of urinary tract infection was only assessed by 1 variable (CFU/mL). Total number of CFU, regardless of the number of infecting species or specific infecting species, was used. It is possible that certain infecting bacterial species induce a larger inflammatory response, and therefore a higher UP : C, than other species. In the future, development of a grading system for severity of urinary tract infection may be valuable. Because of the retrospective study design, information about the presence or absence of clinical signs associated with a urinary tract infection (stranguria, pollakiuria, hematuria, and malodorous urine) could only be extracted from review of the history recorded in the medical record rather than being queried of every owner in an identical manner.
Because of the retrospective nature of this study and reporting practices within our institution, we are unable to confirm that all urine samples submitted for culture and UP : C were taken by the same method as reported for urinalysis. Because it is our practice to perform culture on urine samples obtained by cystocentesis when possible, the most likely discrepancy among the collection methods would arise when UP : C values were obtained from voided samples in culture‐positive dogs (whose samples for culture might have been collected by cystocentesis). Nine of 39 positive urine cultures came from dogs with voided collection of their urinalyses. Assuming that urine cultures were also collected by voiding in these 9 dogs, culture positivity may indicate sample contamination during voided urine collection or may indicate that voiding was the only way to obtain urine for UP : C analysis from a pollakiuric dog with a painful urinary tract infection. However, based on the authors' experience at this institution, it is more likely that a screening urinalysis was initially performed on a voided sample, and a new urine sample was then collected via cystocentesis so that a urine culture could be performed in these cases once urinary sediment analysis supportive of infection became available to the clinician. When culture‐positive samples from dogs whose urinalysis samples were collected by voiding were compared to a random selection of an equal number of culture‐positive samples from dogs whose urinalysis samples were collected by cystocentesis, UP : C was abnormal in 3/8 (37.5%) and 5/8 (62.5%), respectively. Colony counts were similar between groups, and a diversity of bacterial isolates was observed in each. Because previous studies have demonstrated that there is good agreement in measured UP : C values between paired samples collected by cystocentesis or voided methods,14 we did not believe that exclusion of the 9 cases known to have had urinalysis samples collected by voiding was warranted in the present study. Importantly, the goal of the study reported here was to determine whether it is practical for clinicians to use USG to predict UP : C when dipstick protein category is known or to use urine culture positivity to explain abnormal UP : C. Even assuming that it was feasible for clinicians to collect urine for all assays by a single method from all dogs, the poor performance of either dipstick protein or culture positivity as a predictor of UP : C would not be rescued by assessing it for only a single method of urine collection.
In a population of 165 canine visits to our institution's CPC in which urinalysis, UP : C, and urine culture were performed concurrently, 15.8% of the visits led to a diagnosis of proteinuria. Although determination of prevalence was not a primary aim of the study reported here and cannot be performed using this study design, the reported proportion of proteinuric samples (15.8%) was consistent with data presented in 2 recent studies, which report overt proteinuria in 11%15 and 13.4%16 of apparently healthy geriatric dogs. Based on the results of this study and consistent with recent findings of others, general practitioners should expect to encounter proteinuria with moderate frequency among that population of dogs for which an indication exists to perform diagnostic screening comparable to our institution's Senior Wellness Panel and also for those dogs for whom diagnostic testing is warranted owing to clinical signs, known comorbidities, or both. If proteinuria at any level is encountered on the urine dipstick, there is no meaningful way to estimate its quantity from the USG. Urinary protein‐to‐creatinine ratio testing must be performed if proteinuria is to be quantified to assess its significance.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
The authors gratefully acknowledge Deborah Keys, PhD, Manuel da Costa, DVM, PhD, and Steeve Giguère, DVM, PhD for assistance with statistical analysis.
Meindl AG, Lourenço BN, Coleman AE, Creevy KE. Relationships among urinary protein‐to‐creatinine ratio, urine specific gravity, and bacteriuria in canine urine samples. J Vet Intern Med. 2019;33:192–199. 10.1111/jvim.15377
Present address Kate E. Creevy, Department of Small Animal Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX 77843.
REFERENCES
- 1. Grauer GF. Measurement, interpretation, and implications of proteinuria and albuminuria. Vet Clin North Am Small Anim Pract. 2007;37:283‐295. vi‐vii. [DOI] [PubMed] [Google Scholar]
- 2. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc. 2005;226:393‐400. [DOI] [PubMed] [Google Scholar]
- 3. Lyon SD, Sanderson MW, Vaden SL, Lappin MR, Jensen WA, Grauer GF. Comparison of urine dipstick, sulfosalicylic acid, urine protein‐to‐creatinine ratio, and species‐specific ELISA methods for detection of albumin in urine samples of cats and dogs. J Am Vet Med Assoc. 2010;236:874‐879. [DOI] [PubMed] [Google Scholar]
- 4. Grauer GF. Proteinuria: measurement and interpretation. Top Companion Anim Med. 2011;26:121‐127. [DOI] [PubMed] [Google Scholar]
- 5. Lees GE, Brown SA, Elliott J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (small animal). J Vet Intern Med. 2005;19(3):377‐385. [DOI] [PubMed] [Google Scholar]
- 6. Zatelli A, Paltrinieri S, Nizi F, Roura X, Zini E. Evaluation of a urine dipstick test for confirmation or exclusion of proteinuria in dogs. Am J Vet Res. 2010;71:235‐240. [DOI] [PubMed] [Google Scholar]
- 7. IRIS staging of CKD. 2015. Accessed February 28, 2017.
- 8. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐174. [PubMed] [Google Scholar]
- 9. de Man P, Jodal U, Lincoln K, Eden CS. Bacterial attachment and inflammation in the urinary tract. J Infect Dis. 1988;158:29‐35. [DOI] [PubMed] [Google Scholar]
- 10. Carter JL, Tomson CR, Stevens PE, et al. Does urinary tract infection cause proteinuria or microalbuminuria? A systematic review. Nephrol Dial Transplant. 2006;21:3031‐3037. [DOI] [PubMed] [Google Scholar]
- 11. Bagley RS, Center SA, Lewis RM, et al. The effect of experimental cystitis and iatrogenic blood contamination on the urine protein/creatine ratio in the dog. J Vet Intern Med. 1991;5:66‐70. [DOI] [PubMed] [Google Scholar]
- 12. Vientos‐Plotts AI, Behrend EN, Welles EG, et al. Effect of blood contamination on results of dipstick evaluation and urine protein‐to‐urine creatinine ratio for urine samples from dogs and cats. Am J Vet Res. 2018;79:525‐531. [DOI] [PubMed] [Google Scholar]
- 13. Vaden SL, Pressler BM, Lappin MR, Jensen WA. Effects of urinary tract inflammation and sample blood contamination on urine albumin and total protein concentrations in canine urine samples. Vet Clin Pathol. 2004;33:14‐19. [DOI] [PubMed] [Google Scholar]
- 14. Beatrice L, Nizi F, Callegari D, et al. Comparison of urine protein‐to‐creatinine ratio in urine samples collected by cystocentesis versus free catch in dogs. J Am Vet Med Assoc. 2010;236:1221‐1224. [DOI] [PubMed] [Google Scholar]
- 15. Marynissen SJ, Willems AL, Paepe D, et al. Proteinuria in apparently healthy elderly dogs: persistency and comparison between free catch and cystocentesis urine. J Vet Intern Med. 2017;31:93‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willems A, Paepe D, Marynissen S, et al. Results of screening of apparently healthy senior and geriatric dogs. J Vet Intern Med. 2017;31:81‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]


