Abstract
Background
Little is known about normal heart rate variability (HRV) in horses during exercise. It can be difficult to separate premature beats from normal beat‐to‐beat variation at higher heart rates.
Objectives
The aim was to quantify HRV in healthy horses during a high‐speed treadmill‐standardized exercise test (HSET) and to compare with the HRV in horses observed to have arrhythmias during exercise.
Animals
Thirteen healthy horses (Group H), 30 horses with arrhythmias (Group A), and 11 horses with poor performance but no observed arrhythmias (Group O).
Methods
Prospective, observational study. All horses performed a HSET with simultaneous electrocardiograph (ECG) recorded. The ECGs were corrected for artifacts, and arrhythmias noted. Percent instantaneous beat‐to‐beat cycle length variation (% R‐R variation) was calculated, and HRV analyses were performed on trot, canter, and recovery segments.
Results
Group H showed between −4.4 and +3.8% R‐R variation during trot and between −6.1 and +5.4% R‐R variation during the canter phase of the HSET. Group A had significantly larger maximum and 1st percentile R‐R shortening and lengthening compared with Group H and Group O during the recovery phase where most arrhythmias were observed. During recovery, a cutoff of 6% maximum % R‐R shortening predicted the presence of arrhythmia with 88% sensitivity and 97% specificity and likelihood ratio of 26.
Conclusions and Clinical Importance
Healthy horses have little instantaneous R‐R variation during exercise. If a cardiac cycle shortens more than 6% from the previous cycle during the recovery phase, this R‐R interval is likely to represent an arrhythmic event.
Keywords: beat‐to‐beat variation, dysrhythmia, HRV, performance
Abbreviations
- % R‐R variation
percent instantaneous beat‐to‐beat cycle length variation
- [lactate]b
whole blood lactate concentration
- AF
atrial fibrillation
- ANOVA
analysis of variance
- ANS
autonomic nervous system
- APC
atrial premature complex
- AUC
area under the curve
- AVB
atrio‐ventricular block
- CI
confidence intervals
- HR
heart rate
- HR2
heart rate at a whole blood lactate concentration of 2 mmol/L
- HR4
heart rate at a whole blood lactate of 4 mmol/L
- HRV
heart rate variability
- HSET
high‐speed treadmill‐standardized exercise test
- PD
punctuated deceleration
- RMSSD
square root of mean‐squared differences in successive R‐R intervals
- ROC
receiver operating characteristic
- SD
standard deviation
- SD1
SD quantifying the dispersal of data points in a Poincaré plot perpendicular to the line of identity
- SD2
SD quantifying the dispersal of data points in a Poincaré plot along the line of identity
- SDRR
SD of RR intervals
- Sn
sensitivity
- Sp
specificity
- TI
triangular index
- TIRR
triangular interpolation of the R‐R interval histogram
- VPC
ventricular premature complex
1. INTRODUCTION
Resting heart rate variability (HRV) has been well described in horses.1, 2, 3 However, during exercise, publications documenting the normal R‐R variation are limited.4 It is challenging during analysis of an exercising electrocardiograph (ECG) to separate truly premature beats from normal beat‐to‐beat variation, when the degree of normal variation is unclear, P‐QRS‐T morphology is difficult to differentiate and motion artifacts impair ECG quality.
Heart rate (HR) is closely regulated by the autonomic nervous system (ANS).5 In the horse at rest, tonic vagal inhibition decreases the HR and increases short‐term HRV. At the onset of exercise, withdrawal of vagal tone increases HR to approximately 100‐120 beats/min. Sympathetic tone and neurohumoral regulation further elevate the HR above 120 beats/min.4, 5, 6, 7 As HR rises, differences between R‐R intervals become less apparent. Once a horse enters the recovery phase after exercise, sympathetic tone withdraws and parasympathetic tone again predominates. This recovery phase is frequently associated with vagally mediated arrhythmias such as 1st and 2nd degree atrio‐ventricular block (AVB) or marked sinus arrhythmias which have been referred to as “punctuated decelerations (PDs)” by some authors.8 In addition, premature complexes are commonly reported in horses during the recovery phase, which may also increase HRV.8, 9, 10, 11
Studies assessing HRV indices for normally performing horses during exercise have predominantly focused on the normal‐normal (N‐N) interval time series processed using time, frequency, or nonlinear domain analyses. Many of these studies were performed using a HR monitor rather than an ECG, and arrhythmic beats were either not mentioned or excluded from the analysis using automated or manual filtering. None of these older studies have provided clear information on percent instantaneous beat‐to‐beat cycle length variation (% R‐R variation), which would be helpful to differentiate individual premature beats from normal variability.6, 7, 12, 13 A recently published study suggests that normal % R‐R variation of falls between −4 and +4% of the preceding R‐R interval at HRs above 100/min. This is based on data from 11 healthy Arabian endurance horses during ridden exercise, obtained from computer modeling of the width of a cluster of “normal” beats within an R‐R deviation plot, but it is unknown if this variation is consistent across breeds and types of exercise.4
The aims of our current study were to (1) establish the normal % R‐R variation and HRV in horses (both healthy and poorly performing) during a high‐speed treadmill‐standardized exercise test (HSET), (2) compare HRV of horses without arrhythmias to those observed to have arrhythmias, and (3) establish cutoffs in % R‐R variation for R‐R‐based detection of arrhythmias.
The a priori hypothesis was that horses without arrhythmias during exercise would have the narrowest % R‐R deviation and HRV, followed by horses with poor performance (but no arrhythmias), whereas horses with arrhythmias would have the widest % R‐R deviation and HRV.
2. MATERIAL AND METHODS
2.1. Study population
Cases were selected retrospectively from medical records at the Equine Department of the University of Zurich, Switzerland, between 2010 and 2016. Inclusion criteria were the availability of stress ECG recordings (warm up, exercise test, and recovery phase), the presence of underlying sinus rhythm throughout the test (horses with atrial fibrillation [AF] were excluded), and having at least 5 completed HSET steps. A HSET was performed in healthy horses with no arrhythmias (Group H, n = 13), horses with arrhythmias (Group A, n = 30), and horses with poor performance but no arrhythmias (Group O, n = 11). Horses were considered healthy and free of any arrhythmia based on medical and performance history and physical examination findings, including cardiothoracic auscultation. Echocardiography was performed in 11 of 13 healthy horses and was normal.14, 15, 16 Echocardiography was also performed in 24 of 30 horses with arrhythmias and 8 of 11 horses with poor performance. Details of the echocardiographic diagnoses can be found in Supporting Information Table S1. Horses were included in the arrhythmia group if at least 1 arrhythmia was detected during any of the exercise test phases. Group O included horses with a variety of diagnoses related to poor performance but had no arrhythmias detected during the exercise test. Further information on these horses and their final diagnoses is found in Supporting Information Table S1.
On presentation, horses were assessed by clinical examination (including lameness evaluation) to ensure they could perform the HSET. All horses were acclimated to the treadmill over a 2‐day period with at least 3 training sessions. For the exercise test, a jugular vein catheter was placed for blood sample collection. Seventeen horses were additionally equipped with an intracardiac catheter (2 of 13 horses in Group H, 14 of 30 horses in Group A, and 1 of 11 horses in Group O) because of participation in another study.17
All examinations were performed according to institutional ethical standards. Healthy horses were enrolled with owner consent in studies at the University of Zurich's Equine Department that had been approved by the District Veterinary Office of the Canton of Zurich (Animal Use Licenses 208/2008 and 116/2014). Owner of clinical patients consented to the treadmill examinations as part of the diagnostic evaluation of the presenting problem.
2.2. Electrocardiographic recordings
Continuous ECG recordings were obtained using a telemetric ECG recording system (Televet 100, Engel Engineering Service GmbH, Germany) recorded at a sampling frequency of 500 Hz. Self‐adhesive electrodes (Kruuse ECG electrodes, Kruuse A/S, Denmark) were placed in a modified base‐apex lead configuration.18 ECG quality was subjectively assessed (for appropriate QRS detection during the exercise period), and any nondiagnostic ECGs or ECG periods (eg, loss of electrode contact) were excluded.
2.3. Exercise testing
Horses underwent a submaximal fatigue HSET as described previously.17 The test consisted of 2 steps at trot and depending on the horse's performance 3 or 4 steps of canter. The velocity increased by approximately 1 m/s after every step (1st step 120 seconds, following steps 90 seconds duration). The treadmill slope was 6%. Criteria for terminating the test were a HR >200/min or a whole blood lactate concentration ([lactate]b) of >4 mmol/L. After termination, the treadmill was stationary for 5 minutes of passive recovery before horses walked for 30 minutes of active recovery. In addition to the ECG recording, [lactate]b were collected (before the test start, after each test step, and at 30, 60, 120, 180, and 300 seconds after exercise). The following performance indices were calculated: velocity at HR 150/min and 200/min, velocity at [lactate]b of 2 mmol/L and 4 mmol/L, and HR at [lactate]b of 2 mmol/L (HR2) and 4 mmol/L (HR4).19
2.4. ECG analysis
ECG data were analyzed using a standard analysis software (Televet 100, Engel Engineering Service GmbH, software version 6.0.0) set to perform negative S‐wave detection (referred to as “R” waves to simplify terminology). Raw ECG files were shortened to include the duration of the exercise test plus 5 minutes of immediate recovery, excluding the initial increase of HR at the beginning of the exercise test. Manual page‐by‐page verification and correction of the ECG R‐wave detection were performed by a single operator (L. Frick). An assessment of ECG quality (good, moderate, and poor) was made based on the frequency of artifacts that did not allow optimal manual R‐wave identification (ie, rounded, blunt, or fragmented R waves). A good quality ECG had all QRS's appropriately detected. A moderate quality ECG had occasional (approximately 1/min) suboptimal QRS identification, whereas a poor quality ECG had frequent (>1/min) suboptimal QRS identification. These nonoptimally identified R waves were included in the downstream analysis. With this version of software, all QRS complexes could be detected and no data were excluded from analysis. Based on the sampling frequency of 500 Hz, the temporal resolution for correction was 2 milliseconds. For the maximal reported HR of 345/min (R‐R interval of 174 milliseconds), this created a potential inherent variation of 1.8%, whereas at HRs of 225, 150, 100, and 30/min (R‐R intervals of 267, 400, 600, and 2000 milliseconds), this is a potential inherent variation of 0.8, 0.5, 0.3, and 0.1%, respectively.
In the general approach of HRV analyses, abnormal depolarizations such as atrial premature complexes (APCs) or ventricular premature complexes (VPCs) are excluded, as traditionally the focus of HRV analysis is the influence of the ANS on sinus node activity. In our study, all beats, including premature beats, were included in the analyses to establish the effect (if any) of exercising arrhythmias on traditional HRV parameters. Arrhythmias were counted and categorized as physiological (eg, marked sinus arrhythmia and sinus pause/block [grouped together as “PDs”], 1st and 2nd degree AVB, typical during the recovery phase) and pathological (eg, APC, VPC, or ventricular tachycardia). For our study, all premature complexes were grouped together regardless of their apparent origin, as the study was not designed to try and differentiate APCs from VPCs. No interpolated premature complexes (an APC or VPC occurring between 2 sinus beats with no interruption to the sinus rhythm and no pause) or fusion beats (premature fused with a sinus beat) were identified in our study. Specific changes to P‐QRS‐T morphology and polarity associated with premature complexes were difficult to assess given the motion artifacts associated with exercising ECGs. Some premature complexes were not identified by the automated R‐R interval analysis but only from visual inspection of the ECG tachogram (obtained from within the ECG software or following export of the R‐R time series into the downstream software). For analysis, horses with any arrhythmia (physiological or pathological) during any phase of the exercise test were placed together in Group A.
The corrected R‐R time series was exported from the ECG analysis software as a csv file. This file was imported into a spreadsheet. The R‐R time series was then cut into 2 distinct test phase HR plateaus (trot and canter) where the sharp increase of HR at the start of the phase was excluded. The recovery phase was cut to include the first 5 minutes of recovery only. The R‐R data for each plateau phase was imported into statistical software for further analysis (Graphpad Prism version 7.0 for Windows, Graphpad Software, La Jolla, California). Here, % R‐R variation was calculated as the percentage difference between R‐Rn and R‐Rn + 1 (the R‐R time series offset by 1 cycle) across the time series from each phase, using the formula:
Any R‐Rn + 1 cycle shorter than the preceding R‐Rn would be designated negative, whereas R‐Rn + 1 cycles longer than the preceding R‐Rn would be designated positive. The minimum and 1st percentile of the % R‐R variation (ie, the maximum and 1st percentile of % R‐R shortening), the median % R‐R variation, and the 99th percentile and maximum % R‐R variation (ie, the 1st percentile and maximum of % R‐R lengthening) were calculated during each phase.
Using HRV analysis software, the whole test R‐R time series was processed (Kubios version 2.2 for Windows, MATLAB, The MathWorks Inc, Kuopio, Finland). Trot, canter plateau, and recovery segments were identified and analyzed separately in the software. No artifact or smoothing filters were applied. Time domain parameters (mean HR, standard deviation [SD] of R‐R intervals [SDRR], triangular index [TI, calculated as the integral of the R‐R interval histogram divided by the height of the histogram], triangular interpolation of R‐R interval histogram [TIRR], square root of mean‐squared differences between successive R‐R intervals [RMSSD]), and nonlinear domain parameters (SD quantifying the dispersion of data points in a Poincaré plot perpendicular to the line of identity [SD1] and along the line of identity [SD2]) were calculated for each phase. The SDRR, TI, and TIRR are considered parameters of overall HRV. The parameters RMSSD and SD1 are considered markers of short‐term HRV (ie, variability in instantaneous HR) and are mathematically identical20; therefore, only RMSSD is reported in the manuscript. The parameter SD2 is considered an indicator of long‐term HRV. These data were exported as text files for further data handling and statistical analyses.
2.5. Statistics
Distribution and variance of the raw data were assessed by inspection of dot plots, histograms, and normal probability plots. Parametric data were reported as mean ± SD; nonparametric data were described as median [minimum‐maximum].
Population characteristics and performance indices were compared among the healthy (H), arrhythmia (A), and other (O) groups using a one‐way analysis of variance (anova) for parametric or Kruskal‐Wallis test for nonparametric data. Two‐way mixed‐model repeated measures (RM) anova was performed for comparison of groups (H, A, and O) and phases (trot, canter, and recovery) for the beat‐to‐beat parameters (number of R‐R values, median % R‐R variation, maximum % R‐R shortening, 1st percentile of % R‐R shortening, maximum % R‐R lengthening, and 1st percentile of % R‐R lengthening) and traditional HRV parameters (mean HR, RMSSD, SD2, SDRR, TI, and TIRR). Natural log transformation of nonparametric data was applied before these statistical analyses. The Holm‐Sidak method was used for post hoc multiple comparison analysis. A one‐way anova was used to compare ECG quality (good, moderate, and poor) and maximum % R‐R shortening and RMSSD during the canter phase.
Receiver operating characteristic (ROC) curve analysis for the maximum % R‐R shortening and RMSSD was performed by combining Groups H and O together and comparing with Group A data in which only the horses with arrhythmias during each individual phase were included for that phase's ROC analysis. Area under the curve (AUC), cutoff values, and likelihood ratios for maximum % R‐R shortening and RMSSD were reported for the highest sensitivity (Sn), the highest specificity (Sp), and the highest combined Sp and Sn (Youden Index), respectively. The data are presented with the 95% confidence intervals (95% CIs) for each cutoff.
The level of significance was set at P < .05.
Statistical analysis was performed using GraphPad Prism (version 7.0) and Sigmaplot (version 12.3 for Windows, Systat Software GmbH, Erkrath, Germany).
3. RESULTS
3.1. Population characteristics
The study population comprised 54 horses (37 Geldings, 16 Mares, 1 Stallion; 45 Warmbloods, 4 Thoroughbreds, 3 Arabs, 1 Quarter Horse, and 1 Pony) aged 11 ± 5 years. Two horses were excluded because of extremely poor ECG quality that precluded clear identification of R waves and any subsequent rhythm analysis.
3.2. Performance indices
All horses exercised to the set end point of the HSET (HR > 200/min, [lactate]b > 4 mmol/L). As the study population was heterogeneous in the fitness level, most horses were not fit enough to reach step 7 of the exercise test (range 5‐7 steps/test). Performance data were collected in 51 of 54 horses and did not differ significantly among groups. (These data can be seen in Table 1.) Blood lactate concentrations (and therefore performance indices) were not measured during the HSET in 3 horses.
Table 1.
Population characteristics and performance values of the high‐speed treadmill‐standardized exercise test (HSET); a one‐way anova test was performed for parametric values
| Group H (n = 13) | Group A (n = 30) | Group O (n = 11) | P‐value | |
|---|---|---|---|---|
| Age (y) | 10 ± 4 | 11 ± 5 | 9 ± 5 | 0.56 |
| Sex | 8 mc | 22 mc | 7 mc | nt |
| 5 f | 8 f | 3 f | ||
| 0 m | 0 m | 1 m | ||
| Breed | 12 Warmbloods | 26 Warmbloods | 7 Warmbloods | nt |
| 1 Thoroughbred | 1 Thoroughbred | 2 Thoroughbreds | ||
| 0 Other | 3 Other | 2 Other | ||
| Completed test steps | 6 (5, 7) | 5 (5, 7) | 6 (5, 7) | 0.38 |
| Performance values (n = 51) | (n = 13) | (n = 27) | (n = 11) | |
| V150 (m/s) | 5.2 ± 0.7 | 5.2 ± 0.9 | 5.1 ± 0.9 | 0.93 |
| V200 (m/s) | 8.6 (6.6, 9.8) | 8.5 (6.6, 11.4) | 8.4 (7.3, 10.9) | 0.68 |
| V2 (m/s) | 6.3 ± 1.1 | 6.0 ± 1.1 | 5.9 ± 0.9 | 0.63 |
| V4 (m/s) | 7.3 (6.2, 10.0) | 7.4 (4.9, 9.3) | 6.7 (6.1, 9.6) | 0.66 |
| HR2 (/min) | 168 (130, 182) | 165 (135, 190) | 165 (152, 176) | 0.50 |
| HR4 (/min) | 183 ± 15 | 181 ± 13 | 180 ± 9 | 0.79 |
| Peak lactate (mmol/L) | 7.2 ± 2.3 | 8.0 ± 2.1 | 8.7 ± 2.1 | 0.28 |
| Peak velocity (m/s) | 8 (7.3, 9.7) | 8 (5, 10) | 8 (7.5, 11) | 0.83 |
| Max instantaneous HR (/min) | 201 (185, 222) | 208 (181, 345) | 203 (189, 217) | 0.43 |
Abbreviations: ANOVA, analysis of variance; f, female; Group A, arrhythmia; Group H, healthy; Group O, other; HR, heart rate; HR2, HR at [lactate]b of 2 mmol/L; HR4, HR at [lactate]b of 4 mmol/L; m, male; Max instantaneous HR, the highest measured instantaneous heart rate; mc, male‐castrated; nt, not tested; V2, velocity at [lactate]b of 2 mmol/L; V4, velocity at [lactate]b of 4 mmol/L; V150, velocity at HR 150/min; V200, velocity at HR 200/min.
Nonparametric values were analyzed using a Kruskal‐Wallis test. Level of significance was set at P < .05. Values are reported as mean ± standard deviation or as median (minimum, maximum).
3.3. Arrhythmias
Thirty of 54 horses (56%) showed arrhythmias at least once during exercise or immediately after the exercise test. During trot, 6 of 54 horses (11%) showed premature complexes (median [min‐max],1 [1‐5] premature complexes). Similar numbers were seen during canter, with 8 of 54 horses (15%) showing one or more premature complexes (3 [1‐7] premature complexes). Three horses had premature complexes during both trot and canter phases. None of the horses had physiological arrhythmias during exercise.
Arrhythmias were most frequently detected during recovery (24 of 54 horses [44%]). Fifteen horses showed only premature complexes (2 [1‐29] premature complexes). Six horses showed only physiological arrhythmias (PDs or AVBs) during the recovery phase, whereas 3 horses had a combination of both premature beats and physiological arrhythmias during recovery. Figure 1 shows an example of the graphical HRV analysis performed on a horse with arrhythmias occurring during the HSET.
Figure 1.
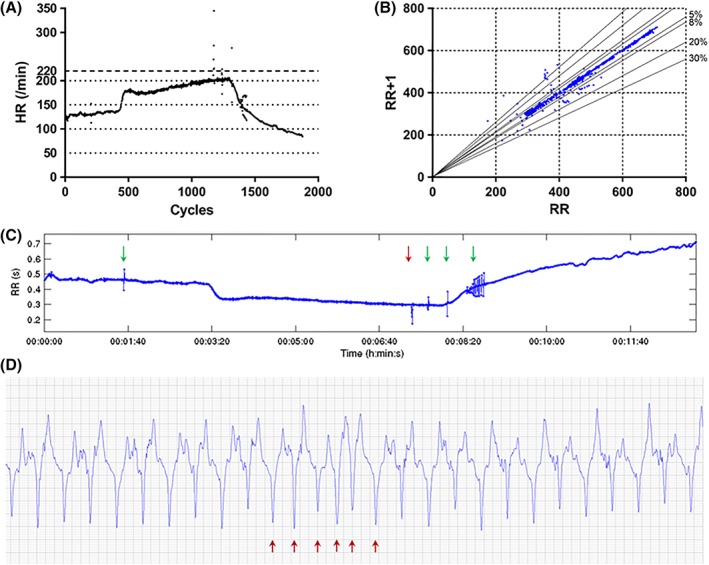
(A) HR tachogram of a horse from Group A, where premature beats are observed as outliers during the exercise test. (B) Poincaré plot of the same study horse. Outliers represent premature beats and related pauses. Solid lines represent 5, 8, 20, and 30% R‐R deviation; (C) R‐R time series of the exercise test as exported from the HRV analysis software (Kubios HRV analysis software, Finland), showing premature beats and subsequent pauses as peaks. The red arrow indicates the run of NST during canter (shown in D), the green arrows indicate VPCs seen during the trot, canter and recovery phases; (D) ECG recording of the short run of NST (indicated by the red arrows), including “near R‐on‐T,” during canter. Abbreviations: ECG, electrocardiograph; HR, heart rate; HRV, heart rate variability; NST, non‐sinus tachycardia; VPCs, ventricular premature complexes
3.4. Percent R‐R variation
The parameters characterizing % R‐R variation are displayed in Figure 2 and Table 2. There was little variation throughout the exercise period, with a median % R‐R variation of 0% for all groups during each phase. Within Group H, increased variation was seen during the canter phase when compared with the recovery phase (max % R‐R shortening, C versus R, P = .01, 1st percentile % R‐R shortening, C versus R, P = .002). Within Group O, similar findings were observed within increased variation within the canter phase compared with recovery (1st percentile % R‐R shortening, C versus R, P = .03).
Figure 2.
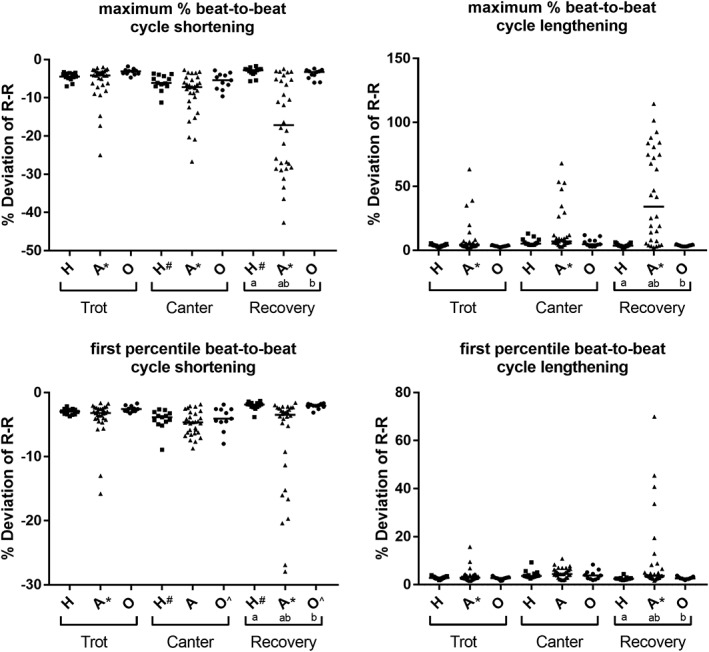
Dot plots of the maximum % R‐R shortening and lengthening and of the 1st percentile % R‐R shortening and lengthening, respectively. The solid line represents the median. Groups (a,b) or phases (#,*,^) that are significantly different (P < .05) from one another are indicated with the same superscript. Abbreviations: H, Group “healthy”; A, Group “arrhythmia”; O, Group “other”
Table 2.
(A) Instantaneous beat‐to‐beat cycle length variation parameters during test phases and among groups. Parameters are reported as mean ± standard deviation (parametric data) or as median (minimum, maximum) (nonparametric data); (B) F‐test and Holm‐Sidak post hoc P‐values of the group and phase comparison in a 2‐way repeated measures analysis of variance (nonparametric data was natural log transformed before analysis). The group‐phase interactions when significant are indicated. Not significant (P > .05) comparisons are not reported
| (A) | Trot | Canter | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group H (n = 13) | Group A (n = 30) | Group O (n = 11) | Group H (n = 13) | Group A (n = 30) | Group O (n = 11) | Group H (n = 13) | Group A (n = 30) | Group O (n = 11) | |
| Number of R‐R intervals | 418 ± 50 | 417 ± 48 | 402 ± 41 | 962 ± 159 | 884 ± 179 | 894 ± 139 | 517 ± 40 | 519 ± 60 | 540 ± 33 |
| Max R‐R shortening (%) | −4.4 (−7.0, −3.3) | −4.2 (−25.0, −2.0) | −3.1 (−4.7, −1.8) | −6.1 (−11, −3.6) | −7.2 (−27, −2.7) | −5.4 (−9.6, −2.8) | −2.8 (−5.6, −1.7) | −17 (−43, −2.4) | −3.2 (−6, −2.4) |
| 1st percentile R‐R shortening (%) | −2.9 (−3.7, −2.1) | −3.2 (−16, −1.6) | −2.6 (−3.2, −1.7) | −3.8 (−8.9, −2.6) | −4.6 (−8.7, −1.8) | −4.1 (−8, −1.9) | −1.9 (−3.8, −1.3) | −3.5 (−28.0, −1.6) | −2 (−3.1, −1.7) |
| Median (%) | 0 (0, 0) | 0 (−0.4, 0.2) | 0 (0, 0) | 0 (0, 0) | 0 (−0.5, 0) | 0 (0, 0) | 0 (0, 0.3) | 0 (0, 0.4) | 0 (0, 0.3) |
| 1st percentile R‐R lengthening (%) | 2.8 (1.9, 4.0) | 2.9 (1.6, 16) | 2.8 (1.6, 3.7) | 3.6 (2.6, 9.3) | 4.4 (1.9, 11) | 3.8 (1.9, 8.4) | 2.5 (1.9, 4.4) | 3.7 (1.6, 70.0) | 2.6 (1.9, 3.8) |
| Max R‐R lengthening (%) | 3.8 (2.5, 5.6) | 4.4 (1.6, 64) | 3.3 (2.3, 4.2) | 5.4 (4.2, 13) | 7.1 (2.9, 68) | 4.8 (2.9, 12) | 3.7 (2.3, 6.3) | 34 (2.3, 115) | 3.8 (3.1, 4.8) |
| (B) | Group comparison | Phase comparison | Group × phase interaction | ||
|---|---|---|---|---|---|
| F‐test P‐value | F‐test P‐value | Holm‐Sidak post hoc P‐value | F‐test P‐value | Holm‐Sidak post hoc P‐value | |
| Number of R‐R values | .41 | <.001 | T versus C, <.001; T versus R, <.001; C versus R, <.001 | .43 | |
| Median (%) | 1.0 | 1.0 | 1.0 | ||
| Max. R‐R shortening (%) | <.001 | .003 | <.001 | Group A: T versus C, .002; T versus R, .001; C versus R, .002 Group H: C versus R, .013 Phase R: Group A versus Group H, <.001; Group A versus Group O, <.001 |
|
| Max. R‐R lengthening (%) | <.001 | .002 | <.001 | Group A: T versus C, .007; T versus R, <.0001; C versus R, <.001 Phase R: Group A versus Group H, <.001; Group A versus Group O, <.001 |
|
| 1% R‐R shortening (%) | <.001 | .001 | .003 | Group A: T versus C, .025 Group H: C versus R, .002 Group O: C versus R, .032 Phase R: Group A versus Group H, <.001; Group A versus Group O, <.001 |
|
| 1% R‐R lengthening (%) | <.001 | .032 | .048 | Group A: T versus R, .002 Phase R: Group A versus Group H, <.001; Group A versus Group O, <.001 |
|
Abbreviations: C, canter; Group A, arrhythmia; Group H, healthy; Group O, other; R, recovery; T, trot.
For all % R‐R parameters, horses with arrhythmias were different to the other 2 groups during the recovery phase (all comparisons among groups within recovery phase, P < .001). For Group A horses, the maximum % R‐R shortening and lengthening were also different among trot, canter, and recovery phases (max % R‐R shortening, T versus C, P = .002, T versus R, P < .001, C versus R, P = .002; max % R‐R lengthening, T versus C, P = .007, T versus R, P < .001, C versus R, P < .001). For the Group A horses, the 1st percentile shortening and lengthening were only different between trot and recovery (1st percentile R‐R shortening, T versus R, P = .02, 1st percentile R‐R lengthening, T versus R, P = .002).
3.5. HRV parameters
The data describing the HRV parameters are displayed in Figure 3A,B and Table 3. The parameter of short‐term variability (RMSSD) was increased in Group A during the recovery phase compared with the other groups (Group A versus Group H, Group O, P < .001). Indices of long‐term variability (SD2) and overall variability (SDRR, TI, and TIRR) were not different among groups but increased significantly among trot, canter, and recovery phase.
Figure 3.
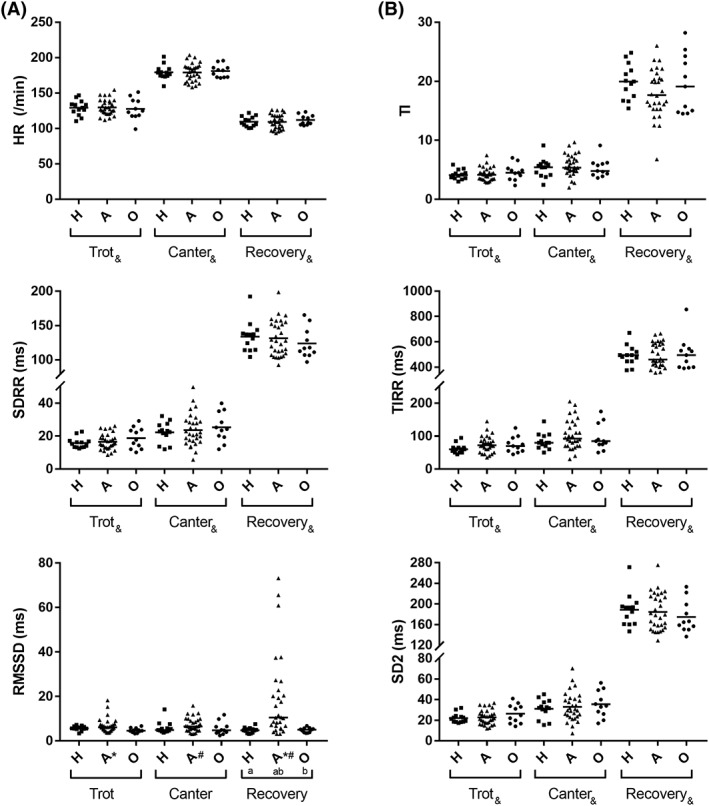
(A) Dot plots of HR, SDRR, and RMSSD. (B) Dot plots of TI, TIRR, SD2. The solid line represents the mean for HR, SDRR, TI, TIRR, and SD2 and the median for RMSSD. Groups (a,b) or phases (#,*,&) that are significantly different (P < .05) from one another are indicated with the same superscript. Abbreviations: HR, heart rate; RMSSD, root mean square of successive R‐R interval differences; SD2, standard deviation quantifying the dispersion of data points in a Poincaré plot along the line of identity; SDRR, standard deviation of R‐R intervals; TI, triangular index; TIRR, triangular interpolation of R‐R‐intervals; H, Group “healthy”; A, Group “arrhythmia”; O, Group “other”
Table 3.
(A) HRV parameters. Parameters are reported as mean ± standard deviation (parametric data) or as median (minimum, maximum) (nonparametric data); (B) F‐test and Holm‐Sidak post hoc P‐values of the group and phase comparison in a 2‐way repeated measures analysis of variance (nonparametric data was natural log transformed before analysis). The group‐phase interactions when significant are indicated. Not significant (P > .05) comparisons are not reported
| (A) | Trot phase | Canter phase | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group H (n = 13) | Group A (n = 30) | Group O (n = 11) | Group H (n = 13) | Group A (n = 30) | Group O (n = 11) | Group H (n = 13) | Group A (n = 30) | Group O (n = 11) | |
| Mean HR (/min) | 129 ± 11 | 130 ± 11 | 128 ± 15 | 179 ± 10 | 179 ± 13 | 175 ± 9 | 110 ± 7 | 109 ± 10 | 112 ± 7 |
| RMSSD (ms) | 5.6 (3.3, 7.2) | 6.1 (3.7, 18) | 4.6 (3.3, 6.7) | 4.9 (3.7, 14) | 6.5 (3.1, 16) | 4.8 (2.5, 12) | 4.8 (3.1, 7.5) | 11 (3.1, 73) | 5.1 (3.5, 6.8) |
| SD2 (ms) | 22 ± 4.7 | 23 ± 7.1 | 26 ± 9.2 | 31.0 ± 9.3 | 33 ± 14 | 35 ± 13 | 189 ± 32 | 184 ± 35 | 175 ± 31 |
| TI | 4.1 (3, 5.9) | 4.2 (2.8, 7.5) | 4.5 (2.4, 7.0) | 5.4 (2.5, 9.1) | 5.4 (2, 9.7) | 4.8 (3.6, 9.1) | 20 (15, 25) | 18 (6.8, 26) | 19 (15, 28) |
| TIRR (ms) | 60 (45, 95) | 73 (35, 145) | 70 (45, 125) | 80 (50, 145) | 93 (30, 205) | 85 (50, 175) | 495 (375, 670) | 460 (355, 665) | 495 (390, 855) |
| SDRR (ms) | 16 ± 3.2 | 17 ± 4.8 | 19 ± 6.5 | 22 ± 6.3 | 24 ± 9.7 | 25 ± 8.9 | 134 ± 22 | 131 ± 26 | 124 ± 22 |
| (B) | Group comparison | Phase comparison | Group × phase interaction | ||
|---|---|---|---|---|---|
| F‐test P‐value | F‐test P‐value | Holm‐Sidak post hoc P‐value | F‐test P‐value | Holm‐Sidak post hoc P‐value | |
| Mean HR (/min) | .97 | <.001 | T versus C, <.001; T versus R, <.001; C versus R, <.001 | .62 | |
| RMSSD (ms) | <.001 | .26 | .002 | Group A: T versus R, <.001, C versus R, <.001 Phase R: Group A versus Group H, <.001, Group A versus Group O, <.001 |
|
| SD2 (ms) | .95 | <.001 | T versus C, .022; T versus R, <.001; C versus R, <.001 | .42 | |
| TI | .87 | <.001 | T versus C, <.001; T versus R, <.001; C versus R, <.001 | .47 | |
| TIRR (ms) | .31 | <.001 | T versus C, <.001; T versus R, <.001; C versus R, <.001 | .76 | |
| SDRR (ms) | .93 | <.001 | T versus C, .024; T versus R, <.001; C versus R, <.001 | .44 | |
Abbreviations: C, canter; Group A, arrhythmia; Group H, healthy; Group O, other; HR, heart rate; HRV, heart rate variability; R, recovery; RMSSD, root mean square of successive R‐R interval differences; SD2, standard deviation 2; SDRR, standard deviation of R‐R intervals; T, trot; TI, triangular index; TIRR, triangular interpolation of R‐R‐intervals.
3.6. ECG quality
Subjectively, ECG quality was considered poor in 7 horses, moderate in 20 horses, and good in 27 horses. An example of a poor and good quality ECG tracing with R‐R identification is seen in Supporting Information Figure S1. Quality was equally distributed among the groups: Group H, 6 good, 6 moderate, 1 poor quality; Group A, 15 good, 11 moderate, 4 poor quality; and Group O, 6 good, 3 moderate, 2 poor quality ECGs (chi‐squared test, P = .88). During canter, variability increased with decreasing ECG quality, with both maximum % R‐R shortening (good versus moderate P = .007, good versus poor P = .002) and RMSSD higher in poor quality ECGs (good versus poor P < .001, moderate versus poor P = .002). For RMSSD, moderate and poor quality ECGs were not different to those containing arrhythmias. This is shown in Figure 4.
Figure 4.
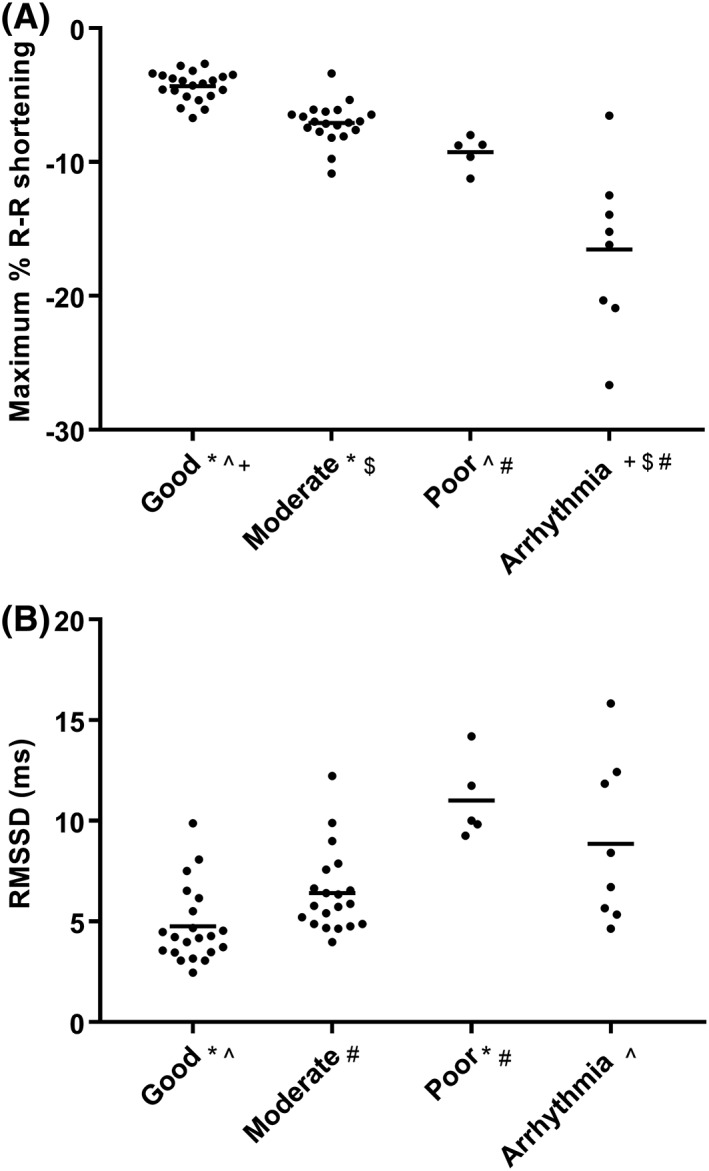
Dot plots of maximum % R‐R shortening (A) and RMSSD (B) with data from all horses during the canter phase, grouped by subjective assessment of electrocardiograph quality or if an arrhythmia was detected during canter. The solid lines represent the mean. Groups (#,*,^,+,$) that are significantly different (P < .05) from one another are indicated with the same superscript. Abbreviation: RMSSD, root mean square of successive R‐R interval differences
3.7. Receiver operating characteristic curve analysis
The results of the ROC curve analysis for maximum % R‐R shortening and RMSSD during each phase are shown in Tables 4 and 5. A range of cutoffs are reported for each parameter, to allow individual selection of a cutoff based on the need for higher Sn or Sp when detecting arrhythmias. The recovery phase analysis showed the highest AUC for both parameters.
Table 4.
ROC curve statistics for maximum % R‐R shortening to predict the presence of arrhythmia (including only horses with arrhythmias occurring in each separate phase)
| Phase AUC (95% CI) P‐value | Max % R‐R shortening cutoff | Sn (%) (95% CI) | Sp (%) (95% CI) | LR | |
|---|---|---|---|---|---|
| Trot 0.81 (0.52‐1.1) P = .01 | High Sn | 1.9 | 100 (54%‐100%) | 2.1 (0.05%‐11%) | 1 |
| Youden index | 5.7 | 83 (36%‐100%) | 85 (72%‐94%) | 5.7 | |
| High Sp | 12 | 50 (12%‐88%) | 100 (93%‐100%) | na | |
| Canter 0.95 (0.85‐1) P < .0001 | High Sn | 6.5 | 100 (63%‐100%) | 59 (43%‐73%) | 2.4 |
| Youden index | 11 | 88 (47%‐100%) | 98 (88%‐100%) | 40 | |
| High Sp | 12 | 88 (47%‐100%) | 100 (92%‐100%) | na | |
| Recovery 0.98 (0.95‐1) P < .0001 | High Sn | 4.1 | 100 (86%‐100%) | 80 (61%‐92%) | 5 |
| Youden index | 6 | 88 (68%‐97%) | 97 (83%‐100%) | 26 | |
| High Sp | 6.3 | 88 (68%‐97%) | 100 (88%‐100%) | na |
Abbreviations: AUC, area under curve; 95% CI, 95% confidence intervals; LR, likelihood ratio; na, not available; R‐R, R‐R interval; ROC curve, receiver operating characteristic curve; Sn, sensitivity; Sp, specificity.
Table 5.
ROC curve statistics for RMSSD to predict the presence of arrhythmia (including only horses with arrhythmias occurring in each separate phase)
| Phase AUC (95% CI) P‐value | RMSSD cutoff | Sn (%) (95% CI) | Sp (%) (95% CI) | LR | |
|---|---|---|---|---|---|
| Trot 0.82 (0.55‐1.1) P = .01 | High Sn | 3.7 | 100 (54%‐100%) | 10 (3.5%‐23%) | 1.1 |
| Youden index | 6.7 | 83 (36%‐100%) | 83 (70%‐93%) | 5 | |
| High Sp | 8.8 | 67 (22%‐96%) | 98 (89%‐100%) | 32 | |
| Canter 0.72 (0.54‐0.91) P = .04 | High Sn | 4.6 | 100 (63%‐100%) | 33 (20%‐48%) | 1.5 |
| Youden index | 6.7 | 63 (24%‐91%) | 72 (57%‐84%) | 2.2 | |
| High Sp | 12 | 25 (3.2%‐65%) | 98 (88%‐100%) | 12 | |
| Recovery 0.98 (0.95‐1) P < .0001 | High Sn | 5.2 | 100 (86%‐100%) | 67 (47%‐83%) | 3 |
| Youden index | 6.4 | 96 (79%‐100%) | 93 (78%‐99%) | 14 | |
| High Sp | 7.3 | 83 (63%‐95%) | 97 (83%‐100%) | 25 |
Abbreviations: AUC, area under curve; LR, likelihood ratio; RMSSD, square root of mean‐squared differences in successive R‐R intervals; ROC curve, receiver operating characteristic curve; Sn, sensitivity; Sp, Specificity.
4. DISCUSSION
Our study identifies that there is little % R‐R variation in healthy horses during a HSET. Interestingly, in the horses without arrhythmias, the variability during the canter phase was greater than that during the recovery phase, likely in part because of reduced ECG quality and difficulty in precise R‐R detection. Horses with arrhythmias showed more variation, particularly during the recovery phase when compared with the other 2 groups, corresponding to when the majority of arrhythmias were observed.
4.1. Exercise capacity and the influence of arrhythmias
All horses performed similarly during the HSET, despite many of the horses presenting for evaluation of poor performance and 11 horses having pathological arrhythmias detected during the high‐intensity exercise period. This could be because most horses came from a similar training background as privately owned horses with predominant leisure use. Rider perception of poor performance is subjective, and frequently a dichotomy exists between the rider expectations and horse ability. In addition, this HSET protocol is designed as a submaximal exercise test. It could be speculated that even historically “poor performing” horses or horses with arrhythmias during exercise did not exceed the reserve capacity of their CV system, resulting in similar performance indices among the groups. The highest arrhythmia frequency in a single horse was 7 premature complexes during the canter phase, which may not have been frequent enough to have hemodynamic consequences resulting in an appreciable decrease in performance. As a result, comparisons in HRV can be made across the groups, as all horses exercised to a similar intensity and peak HR.
In the conventional approach of HRV analysis, arrhythmias are excluded.21 Although a recent equine study highlighted the somewhat unconventional use of HRV analyses to aid in identification of AF. In their study, HRV analyses (particularly RMSSD) were used as a way to differentiate NSR from AF in horses at rest and during lunging exercise.22
In our current study, arrhythmias were intentionally included to assess their effect on HRV. We suggest that arrhythmias should be included in HRV analysis when applying this technique in the clinical setting with the goal to detect and quantify arrhythmia and characterize the temporal distribution of arrhythmia. In particular, visual assessment of the HR tachogram, R‐R interval time series, or Poincaré graphs can be very useful in helping to identify abnormal beats and determining their timing and frequency (ie, differentiating between peak exercise and early recovery). Figure 1 provides an example of this. It should be noted that most horses in our study only had occasional single arrhythmias during exercise (maximum 7 premature complexes/phase, <1% of the total beats/phase).
It should also be pointed out that a large number of horses in our study had intracardiac catheters in place during the HSET, because of participation in another project. Although these horses were considered to be performing normally, they were included in the arrhythmia group as a result of the arrhythmias observed. This higher‐than‐expected number of arrhythmias in otherwise “healthy” horses is likely related to the intracardiac catheters irritating the myocardium during exercise.17 Care should be taken not to overinterpret the frequency of arrhythmias in this population of horses. Investigations of any difference in effect of these “induced” arrhythmias compared with naturally occurring arrhythmias on HRV parameters were not part of our study.
4.2. Percent R‐R variation and HRV parameters
The study by Flethøj et al showed healthy Arab horses have very narrow % R‐R variation of approximately 4% at a HR of greater than 100/min during ridden exercise.4 Data from our study provide similar results using a different analysis technique. Maximum % R‐R shortening and lengthening, respectively, describe the percent deviation of the shortest and the longest R‐R interval in a time series compared to the preceding R‐R interval. The percent deviation at the 1st percentile % R‐R shortening and lengthening was calculated to exclude outliers, so that 98% of all calculated % R‐R variations in the time series fall between these 2 values. Given that the average number of cycles (ie, heart beats) during the trot and canter phases was between 400 and 1000 beats, this corresponds to 4‐10 beats falling outside of the 1st percentile % R‐R shortening and lengthening range. With the low frequency of arrhythmias observed, this explains why the maximum and 1st percentile % R‐R shortening or lengthening values were fairly similar.
Our study suggests maximum % R‐R variation of between −6.1 and +5.4% in healthy horse during treadmill exercise. This information on normal % R‐R variation is useful for helping decide when to consider an individual complex truly premature or delayed by an inappropriately long pause. A commonly used ECG software package (Televet, Engel Engineering Service GmbH) for stress ECG analysis in horses utilizes R‐R analysis where a predetermined percent variation in consecutive R‐R intervals can be automatically detected and displayed.
The ROC curve analysis identified a potential number of cutoffs that could be used during ECG analysis. Depending on the desired outcome (detecting arrhythmias or confirming regular R‐R intervals), the threshold for abnormal R‐R detection can be manipulated. Setting a lower threshold (ie, <3% R‐R variation) will result in most arrhythmias being correctly detected but will also result in a large number of normal beats being identified (high false positive rate). Setting a higher threshold (ie, <12% R‐R variation) will reduce the false positive rate; however, many arrhythmias will not be detected. An optimal threshold for normal R‐R detection does not exist, but rather should be determined based on the individual situation. This should include an assessment of risk related to missing an arrhythmia or over interpreting normal R‐R variation. The Youden index provides the cutoff value with the highest combined Sn and Sp, and the respective cutoff value may provide a good starting point for clinicians.
Automated R‐R detection can improve the efficiency of ECG analysis but should not replace manual verification of the ECG. Including evaluation of graphical HR representations into manual ECG analysis can be extremely helpful, where premature complexes and inappropriate pauses (and their timing) can be more easily visualized when represented graphically.
It is also important to realize that the maximum % R‐R shortening represents the shortest R‐R interval in a time series but does not reflect the frequency of arrhythmic events during that series. A HRV parameter like RMSSD incorporates data from the entire time series in the calculation and is a more robust measure of short‐term variability throughout the data series. The ROC curve analysis for RMSSD performed similarly to the maximum % R‐R variation analysis during the recovery phase but was less discriminating for arrhythmia particularly during the canter phase. This is likely in part because of the effects of ECG quality on R‐R detection during the canter phase.
Another consideration when extrapolating the results of this research into clinical practice is that for this proof of principle study, all arrhythmias (physiological and pathological premature beats and pauses) were grouped together in the analysis. Using the maximum % R‐R shortening or RMSSD for detection of a R‐R interval, QRS complex or data series that falls outside of the “normal” range still requires manual visualization of the ECG to interpret the findings correctly.
Variation in HR (outside that caused by premature beats or artificially created by motion artifacts) is driven by changes in the sinus node depolarization rate or in AV node conduction. Many competing factors are involved in the control of HR during exercise. Horses are primarily under the influence of the parasympathetic nervous system at rest. During low intensity exercise, there is withdrawal of the vagal tone resulting in increasing HR up to approximately 100/min. The change of HR regulation from parasympathetic to sympathetic (and other neurohumoral mechanisms) control occurs around the transition from trot to canter (with the exception of trotting‐bred horses).5, 6, 7
As exercise intensity increases, there are interactions between respiratory frequency and stride length, which in healthy horses are usually coupled at the canter and gallop. This entrainment does not often occur at the trot (except in trotting‐bred horses).12, 23 As fatigue occurs, stride and respiratory frequency may become uncoupled (stride frequency decreases while respiratory frequency increases) resulting in increased variability. This may in part explain the increased beat‐to‐beat variation observed during the canter phase. In addition, if the tidal volume of respiration increases, negative thoracic pressures can result in greater right heart filling, causing stretching of the right atrial myocardium resulting in spontaneous depolarization within the sinus node tissue. This “mechano‐electric” feedback can result in coupling of the respiratory rate with HR during periods of hyperpnea, leading to increased variability like that seen during early recovery.7
Although it was hypothesized that Group O horses would have increased HRV compared with the healthy horses without arrhythmia, as a result of the effects of pain, lameness, or respiratory disease creating more variability, no differences were seen between Group H and Group O at any time point for any parameter. Further research is required to fully investigate these influences on exercising HRV.
The PDs previously reported to occur in the early recovery phase8 as a result of autonomic instability (returning vagal tone and high sympathetic activity) can result in high R‐R variability during the early recovery phase. During peak exertion, catecholamines (eg, adrenaline and noradrenaline) are released, which can suppress HRV completely.24, 25 Although designed to be a submaximal exercise test, it is possible that fatigue and increased respiratory effort masked the effects of any catecholamine‐induced suppression of HRV, especially in the horses with reduced performance associated with respiratory disease. The influence of changing AV node conduction remains an additional factor to consider. Given the difficulty in detecting clear P waves on an exercising surface ECG, it is hard to assess whether the variability seen in horses is driven primarily by the sinus node or if variable AV conduction played a role.
When comparing traditional time domain and nonlinear HRV parameters, we were particularly interested in the short‐term variability parameter RMSSD. During trot and canter, RMSSD was low in horses without arrhythmias and similar to those previously reported during ridden exercise or in trotters.12, 13 This value increased during recovery as parasympathetic tone increased, although there was a wide inter‐horse variation seen during this recovery phase. The variation in this phase is in part because of a combination of vagally induced physiological arrhythmias (eg, 1st and 2nd degree AVB, sinus pauses) and premature complexes (and subsequent pauses) seen in 24 horses during the early recovery period. Measures of overall and long‐term variability were different among all phases, with less variation in the trot and more variation seen during the canter phase. This may be influenced by the number of steps in each phase (2 steps in the trot, 3‐4 steps in the canter phase) resulting in significantly more R‐R intervals over a range of HRs being evaluated during the canter phase.
4.3. Limitations
The study population was selected retrospectively and therefore assessment and classification of disease could not be influenced. With a prospective study concept, a standardized protocol could be followed and a uniform classification of disease achieved, although a very high caseload would be necessary to acquire sufficient cases. In our study, all ECGs were reanalyzed in a standardized way by a single‐trained operator (L. Frick) specifically for our study to reduce the ECG interpretation bias.
When evaluating ECG recordings, ECG quality is crucial to provide meaningful analysis. If ECG quality is poor, imprecise detection of S (“R”) waves occurs, which are difficult to manually correct. This could lead to artificially increased R‐R variability or potential exclusion of horses because of poor quality data sets. This could be demonstrated in Figure 4, where during the canter, maximum % R‐R shortening and RMSSD were significantly different between the good and poor quality ECGs. Based on these data, it is likely that part of the increased variability seen during the canter phase is associated with imprecise R‐R detection resulting from motion artifacts.
An additional limitation of our study is the potential misidentification or misclassification of arrhythmias. Visual representations of data were frequently checked for the presence of outliers, and the ECGs reassessed to determine if outlier R‐R intervals were the result of arrhythmias or artifacts. Fusion complexes not resulting in a visually obvious deviation from the R‐R interval baseline may have been missed. Interpolated premature complexes would have resulted in shorter R‐R intervals than expected (but not necessarily pauses and therefore would have been detected during analysis). Our study was not designed to use HRV analysis to differentiate between APCs and VPCs; therefore, they were classified together in the arrhythmia group. Further research is necessary to determine if HRV analysis can detect, for example, the subtle differences in timing between complete and incomplete pauses.
5. CONCLUSION
Assessment of R‐R cycle length variation and HRV analysis have appeared as an interesting tool for researchers and cardiologists. The knowledge that % R‐R deviation varies between −6.1 and +5.4% in healthy horses during treadmill exercise is particularly helpful when trying to assess an ECG for premature complexes and other pathological events. If a cardiac cycle shortens more than 6% from the previous cycle during the recovery phase, this R‐R interval is likely to represent an arrhythmic event. Differentiation between normal beat‐to‐beat variation and prematurity during exercise remains poorly defined, and further research is required in this area.
CONFLICT OF INTEREST DECLARATION
Colin C. Schwarzwald serves as Associate Editor for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The authors confirm that ethical approval from the District Veterinary Office of the Canton of Zurich was obtained as appropriate (Animal use licenses 208/2008 and 116/2014). Owners of clinical patients consented to the treadmill examinations as part of the diagnostic evaluation of the presenting problem.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supporting Information Tables
Supporting information Figure S1 Modified base‐apex ECG lead II and III recordings taken during the canter phase of the exercise test. Examples of a poor quality ECG (top), with frequent motion artifacts, small, rounded and at times fragmented R‐waves that resulted in inaccurate R‐wave detection (indicated by the green arrows), and a good quality ECG (bottom), where all R‐waves were correctly identified. The calculated instantaneous HR is displayed in red. Abbreviations: HR, Heart rate.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of the veterinarians and technicians in the Equine Department and Equine Sports Medicine Section of the Equine Department and Equine Sports Medicine Section of the University of Zurich's Equine Hospital in performing the treadmill examinations and obtaining ECG recordings. The study was performed at the University of Zurich's Equine Department, Zurich, Switzerland. Data from this project was presented as a poster at the 2017 ACVIM Forum, National Harbor, Maryland.
Frick L, Schwarzwald CC, Mitchell KJ. The use of heart rate variability analysis to detect arrhythmias in horses undergoing a standard treadmill exercise test. J Vet Intern Med. 2019;33:212–224. 10.1111/jvim.15358
REFERENCES
- 1. Eggensperger BH, Schwarzwald CC. Influence of 2nd‐degree AV blocks, ECG recording length, and recording time on heart rate variability analyses in horses. J Vet Cardiol. 2017;19:160‐174. [DOI] [PubMed] [Google Scholar]
- 2. Ille N, Erber R, Aurich C, et al. Comparison of heart rate and heart rate variability obtained by heart rate monitors and simultaneously recorded electrocardiogram signals in nonexercising horses. J Vet Behav. 2014;9:341‐346. [Google Scholar]
- 3. van Vollenhoven E, Grant CC, Fletcher L, et al. Repeatability and reliability of heart rate variability in healthy, adult pony mares. J Equine Vet Sci. 2016;46:73‐81. [Google Scholar]
- 4. Flethoj M, Kanters JK, Pedersen PJ, et al. Appropriate threshold levels of cardiac beat‐to‐beat variation in semi‐automatic analysis of equine ECG recordings. BMC Vet Res. 2016;12:266 10.1186/s12917-016-0894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamlin RL, Klepinger WL, Gilpin KW, et al. Autonomic control of heart rate in the horse. Am J Phys. 1972;222:976‐978. [DOI] [PubMed] [Google Scholar]
- 6. Physick‐Sheard PW, Marlin DJ, Thornhill R, et al. Frequency domain analysis of heart rate variability in horses at rest and during exercise. Equine Vet J. 2000;32:253‐262. [DOI] [PubMed] [Google Scholar]
- 7. Cottin F, Medigue C, Lopes P, et al. Effect of exercise intensity and repetition on heart rate variability during training in elite trotting horse. Int J Sports Med. 2005;26:859‐867. [DOI] [PubMed] [Google Scholar]
- 8. Physick‐Sheard PW, McGurrin MK. Ventricular arrhythmias during race recovery in Standardbred racehorses and associations with autonomic activity. J Vet Intern Med. 2010;24:1158‐1166. [DOI] [PubMed] [Google Scholar]
- 9. Jose‐Cunilleras E, Young LE, Newton JR, et al. Cardiac arrhythmias during and after treadmill exercise in poorly performing thoroughbred racehorses. Equine Vet J. 2006;(Suppl 36):163‐170. [DOI] [PubMed] [Google Scholar]
- 10. Martin BB Jr, Reef VB, Parente EJ, et al. Causes of poor performance of horses during training, racing, or showing: 348 cases (1992‐1996). J Am Vet Med Assoc. 2000;216:554‐558. [DOI] [PubMed] [Google Scholar]
- 11. Ryan N, Marr CM, McGladdery AJ. Survey of cardiac arrhythmias during submaximal and maximal exercise in Thoroughbred racehorses. Equine Vet J. 2005;37:265‐268. [DOI] [PubMed] [Google Scholar]
- 12. Cottin F, Barrey E, Lopes P, et al. Effect of repeated exercise and recovery on heart rate variability in elite trotting horses during high intensity interval training. Equine Vet J. 2006;38:204‐209. [DOI] [PubMed] [Google Scholar]
- 13. Younes M, Robert C, Barrey E, et al. Effects of age, exercise duration, and test conditions on heart rate variability in young endurance horses. Front Physiol. 2016;7:155 10.3389/fphys.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwarzwald CC, Schober KE, Bonagura JD. Methods and reliability of tissue doppler imaging for assessment of left ventricular radial wall motion in horses. J Vet Intern Med. 2009;23:643‐652. [DOI] [PubMed] [Google Scholar]
- 15. Schwarzwald CC, Schober KE, Bonagura JD. Methods and reliability of echocardiographic assessment of left atrial size and mechanical function in horses. Am J Vet Res. 2007;68:735‐747. [DOI] [PubMed] [Google Scholar]
- 16. Schefer KD, Bitschnau C, Weishaupt MA, et al. Quantitative analysis of stress echocardiograms in healthy horses with 2‐dimensional (2D) echocardiography, anatomical M‐mode, tissue doppler imaging, and 2D speckle tracking. J Vet Intern Med. 2010;24:918‐931. [DOI] [PubMed] [Google Scholar]
- 17. Trachsel DS, Schwarzwald CC, Bitschnau C, et al. Atrial natriuretic peptide and cardiac troponin I concentrations in healthy Warmblood horses and in Warmblood horses with mitral regurgitation at rest and after exercise. J Vet Cardiol. 2013;15:105‐121. [DOI] [PubMed] [Google Scholar]
- 18. Young LE, van Loon G. Diseases of the heart and vessels In: Hinchcliff KW, Kaneps AJ, Geor RJ, eds Equine Sports Medicine and Surgery, 2nd Ed. St Louis: Saunders Elsevier; 2014:695–743. [Google Scholar]
- 19. Bitschnau C, Wiestner T, Trachsel DS, et al. Performance parameters and post exercise heart rate recovery in Warmblood sports horses of different performance levels. Equine Vet J. 2010;42:17‐22. [DOI] [PubMed] [Google Scholar]
- 20. Ciccone AB, Siedlik JA, Wecht JM, et al. Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve. 2017;56:674‐678. [DOI] [PubMed] [Google Scholar]
- 21. Bowen IM. Ambulatory electrocardiography and heart rate variability In: Marr CM, Bowen M, eds. Cardiology of the Horse. 2nd ed. Edinburgh: Saunders Elsevier; 2010:127‐137. [Google Scholar]
- 22. Broux B, De Clercq D, Decloedt A, et al. Heart rate variability parameters in horses distinguish atrial fibrillation from sinus rhythm before and after successful electrical cardioversion. Equine Vet J. 2017;49:723‐728. [DOI] [PubMed] [Google Scholar]
- 23. Padilla DJ, McDonough P, Kindig CA, et al. Ventilatory dynamics and control of blood gases after maximal exercise in the Thoroughbred horse. J Appl Physiol. 2004;96:2187‐2193. [DOI] [PubMed] [Google Scholar]
- 24. Snow DH, Harris RC, MacDonald IA, et al. Effects of high‐intensity exercise on plasma catecholamines in the thoroughbred horse. Equine Vet J. 1992;24:462‐467. [DOI] [PubMed] [Google Scholar]
- 25. Breuer HW, Skyschally A, Schulz R, et al. Heart rate variability and circulating catecholamine concentrations during steady state exercise in healthy volunteers. Br Heart J. 1993;70:144‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Tables
Supporting information Figure S1 Modified base‐apex ECG lead II and III recordings taken during the canter phase of the exercise test. Examples of a poor quality ECG (top), with frequent motion artifacts, small, rounded and at times fragmented R‐waves that resulted in inaccurate R‐wave detection (indicated by the green arrows), and a good quality ECG (bottom), where all R‐waves were correctly identified. The calculated instantaneous HR is displayed in red. Abbreviations: HR, Heart rate.


