Abstract
Background
Chronic hepatitis (CH) in dogs is common and has the tendency to progress to liver cirrhosis (LC). Circulating microRNAs might have the potential as markers for disease progression.
Objectives
To investigate whether concentration of specific microRNAs in serum correlate with the stage and grade of CH in Labrador Retrievers.
Animals
Twenty‐two Labrador Retrievers with histological CH (n = 8), LC (n = 7), and normal liver (NL, n = 7).
Methods
In this retrospective study, serum concentrations of miR‐122, miR‐29a, miR‐133a, miR‐181b, and miR‐17‐5p were measured by quantitative real‐time PCR and evaluated using univariate linear regression in dogs. A multivariate model was fit including the grade of hepatitis and the stage of fibrosis.
Results
Of the 5 microRNAs, only circulating miR‐122 and miR‐29a were significantly associated with the grade of hepatitis and the stage of fibrosis. A positive correlation was identified between the grade of hepatitis with miR‐122 (rs = 0.79, P < .001) and miR‐29a (rs = 0.78, P < .001). Both miR‐122 (rs = 0.81, P < .001) and miR‐29a (rs = 0.67, P < .001) showed a significant positive correlation with the stage of fibrosis. MiR‐122 concentrations were significantly higher in the CH (P < .01) and LC groups (P < .001) compared to the NL group. MiR‐29a concentrations were significantly higher in the CH (P < .001) and LC (P < .001) groups compared to the NL group.
Conclusions and Clinical Importance
Circulating miR‐122 and miR‐29a concentrations might be useful for monitoring the response to treatment and progression of canine CH.
Keywords: canine, cirrhosis, dog, fibrosis, liver injury
Abbreviations
- ALT
alanine aminotransferase
- BA
bile acids
- CH
chronic hepatitis
- ECM
extracellular matrix
- HSCs
hepatic stellate cells
- LC
liver cirrhosis
- NL
normal liver
- TGF‐β1
transforming growth factor β1.
1. INTRODUCTION
Chronic hepatitis (CH) is a common liver disease in dogs and is histologically characterized by hepatocellular apoptosis or necrosis, a variable mononuclear or mixed inflammatory cell infiltrate, and the presence of fibrosis.1, 2 Liver biopsy is the gold standard for diagnosing chronic liver diseases, and necro‐inflammatory activity (grade) and amount of fibrosis (stage) can be scored histopathologically.3 Liver fibrosis is defined as increased deposits of extracellular matrix (ECM) proteins.4 Deposited ECM proteins include fibrillary collagens, nonfibrillary collagens, glycosaminoglycans, and proteoglycans.5 Liver cirrhosis (LC) is the result of an accumulation of ECM accompanied by disruption of the hepatic architecture and the presence of hyperplastic nodules.1 The presence of LC is a negative prognostic factor of CH.6, 7, 8
Labrador Retrievers have increased risk for developing CH.2, 8, 9, 10, 11 This breed is predisposed to copper‐associated hepatitis, and genetic and dietary factors play a role in its pathogenesis.12 Copper toxicosis in Labrador Retrievers was identified as a complex hereditary disease affected by mutations in the genes coding for the copper transporters ATP7B and ATP7A.13 In addition, dietary uptake of copper is associated with progression of CH,14, 15 and D‐penicillamine treatment is effective in dogs with copper‐associated hepatitis in this breed.10 Furthermore, idiopathic CH has higher incidence in Labrador Retrievers.6, 9, 11
Alanine aminotransferase (ALT) is commonly considered to be the most liver‐specific enzyme.16 However, it has a low sensitivity for detecting hepatitis in Labrador Retrievers.17 In addition, ALT activity in advanced fibrosis might be within the reference range as a result of a decrease in the number of hepatocytes.16 Histopathologic evaluation is required not only to make a diagnosis but also to monitor progression during treatment. However, liver biopsies are an inherently invasive method for dogs and carry some risk.18 Thus, the evaluation of the applicability of minimal invasive biomarkers of disease severity is an important effort in dogs with CH.
MicroRNAs are a family of noncoding RNAs that play an important role in a variety of cell processes and are remarkably stable in blood.19, 20 Circulating microRNAs have been investigated in humans as noninvasive biomarkers of liver inflammation, fibrosis, and cancer.21, 22, 23 MiR‐122 is the most abundant miRNA in the liver, and the serum concentrations increase in humans with various liver injuries.22, 23 Recently, it is reported that circulating miR‐122 has the potential utility as a sensitive biomarker in early detection of liver injury in Labrador Retrievers.24 In addition, a panel of circulating microRNAs might be used to distinguish among parenchymal, biliary, and neoplastic hepatobiliary diseases in dogs.25 In human, several microRNAs have been reviewed as potential biomarkers for the diagnosis of liver fibrosis and disease progression.26 To our knowledge, there is no report about the relationship of specific circulating microRNAs and progression of fibrosis in canine CH.
The aim of our study was to investigate whether specific microRNAs in serum correlate with the stage and grade of CH in Labrador Retrievers. We hypothesized that circulating microRNAs would be different between healthy dogs and those with CH or LC.
2. MATERIALS AND METHODS
2.1. Animals
Our study included 15 client‐owned Labrador Retrievers with histologically CH and LC, referred to the Department of Clinical Sciences of Companion Animals, Utrecht University, between 2010 and 2016 either because of liver‐related clinical signs or increased liver enzyme activities. In addition, clinically healthy dogs related to affected Labrador Retrievers were recruited to participate in the ongoing research program into copper‐associated hepatitis of the Faculty of Veterinary Medicine, Utrecht University.14 To confirm that these dogs were clinical healthy or subclinically affected, liver biopsies and blood samples were collected according to the Act on Veterinary Practice, as required under Dutch legislation. As a result, 7 client‐owned Labrador Retrievers without histological abnormalities in liver were included in our study as normal liver (NL) group. Data concerning signalment and laboratory and histopathology findings were retrospectively identified from medical records. Samples were taken with informed consent of the owners, and all procedures were known and approved by the Animal Welfare Body of the University of Utrecht.
2.2. Blood samples
Blood samples were obtained from all dogs before the liver biopsy. ALT and fasting bile acids (BA) concentrations were measured in heparinized plasma at the time of diagnostic workup at the Veterinary Diagnostic Laboratory of Utrecht University (UVDL). Reference range for ALT and fasting BA were established by UVDL and are <70 U/L and <10 μmol/L, respectively. Serum samples were stored at −20°C or −70°C until microRNA analysis.
2.3. Histopathology
Liver biopsies were taken with a 14‐G needle using a Tru‐Cut device under ultrasound guidance and processed as described previously.10 Samples were taken from the liver lobes that could be safely reached under ultrasound guidance (in most cases, this was the left lateral liver lobe). From 2 dogs, at 2 occasions, biopsies were available of which the 2nd biopsy was obtained in a later stage of their disease. Based on histological evaluation according to the World Small Animal Veterinary Association standards,1 dogs were classified into 3 groups, NL, CH, and LC, by a board‐certified veterinary pathologist. Histopathological assessments were based on a published method.27, 28 Grading of hepatitis was defined on a scale of 0‐5 for hepatic necro‐inflammatory activity grade: absent (0), slight (1), mild (2), moderate (3), marked (4), and very marked (5), and staging of fibrosis using the reticulin stain was defined on a scale of 0‐4 for the degree of fibrosis: absent (0), mild (1), moderate (2), marked (3), and very marked (4).3 A semi‐quantitative scoring of hepatic copper–stained rubeanic acid was defined on a grading scale of 0‐5.12, 29
2.4. RNA isolation
Total RNA was isolated from 50 μL of serum according to the manufacturer's protocol using a miRNeasy Serum/Plasma kit (Qiagen, Hiden, Germany). After addition of QIAzol lysis reagent (250 μL) to sera and incubation at room temperature for 10 minutes, 5.6 × 108 copies of synthetic Caenorhabditis elegans miR‐39 (Qiagen) was added as a spike‐in control. Of note, 50 μL of chloroform was added and incubated at room temperature for 2 minutes. After centrifugation at 12 000g for 15 minutes at 4°C, the aqueous phase was transferred to a fresh tube and 1.5 volumes of ethanol was added. RNA was purified on an RNeasy minElute spin column (Qiagen), eluted in 14 μL RNase‐free water, and stored at −20°C.
2.5. Quantitative real‐time PCR
Mature microRNA was reverse transcribed using miScript II RT kit (Qiagen) according to the manufacturer's instructions by polyadenylation in combination with oligo‐dT primers and 5× miScript HiSpec Buffer that facilitates the selective conversion of mature microRNAs into cDNA. The obtained cDNA was diluted to a total volume of 200 μL. Based on the current human literature, the serum concentrations of miR‐122, miR‐29a, miR‐133a, miR‐181b, and miR‐17‐5p were chosen to be measured in dogs.26, 30, 31, 32, 33, 34 Quantitative real‐time PCR was performed using the miScript SYBR Green PCR kit (Qiagen). All PCRs were carried out in duplicate on a CFX‐384 Real‐Time PCR Detection System (BioRad, Veenendaal, The Netherlands). Each reaction consisted of 5 μL 2× QuantiTect SYBR Green PCR Master Mix, 1 μL 10× miScript Universal Primer, 1 μL 10× miR‐specific primer (Qiagen), and 1 μL of the previously diluted cDNA. The total reaction volume of each PCR was adjusted to 10 μL by adding 2 μL RNase‐free water. The concentrations of all microRNAs were quantified using absolute quantification via a standard curve, with quantities normalized to the synthetic C. elegans miR‐39 as spike‐in control.35
2.6. Statistical analysis
All data of dog characteristics were reported as median (range). The overall difference among groups was determined using the nonparametric Kruskal‐Wallis test, followed by Dunn's multiple comparison. Linear regression was performed to investigate the relationship between the grade of hepatitis and the stage of fibrosis with microRNA expression levels. A univariate analysis was performed with the microRNA expression levels as dependent variable and the grade or stage as independent variables. In addition, a multivariate analysis was performed, including grade, stage, and copper scoring. Copper scoring was added as a confounder in our study. The natural logarithm of the different microRNAs was taken to ensure the validity of all models, which was checked by studying the residuals on normality and constant variance. Spearman rank‐correlation coefficients were calculated between microRNA expression levels and grade of hepatitis, stage of fibrosis, and copper score. A Wilcoxon rank sum test was performed to test the differences between microRNA expression levels and histological subgroups. P <.05 was considered statistically significant. Data were analyzed in GraphPad PRISM for Mac OS X version 5.0b software (GraphPad Software Inc., La Jolla, California) and R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Characteristics of Labrador Retrievers
Serum samples of 22 Labrador Retrievers were analyzed. Characteristics of the NL group (n = 7), CH group (n = 8), and LC group (n = 7) are shown in Table 1.
Table 1.
Dog characteristics
| NL (n = 7) | CH (n = 8) | LC (n = 7) | P‐value | |
|---|---|---|---|---|
| Sex | 1M, 3MC, 3FS | 1F, 7FS | 6MC, 1FS | |
| Age (y) | 4.8 (1.6‐8.1) | 8.7 (5.2‐11.4)* | 9.2 (6.1‐12.1)* | .01 |
| ALT (U/L) | 44 (27‐56) | 416 (68‐779)** | 508 (78‐712)** | <.001 |
| BA (μmol/L) | 1 (1‐2) | 12 (1‐95) | 72 (40‐161)*** | <.001 |
| Grade of hepatitis | 0 (0‐0) | 2 (0‐3)* | 2 (1‐3)** | .001 |
| Stage of fibrosis | 0 (0‐0) | 2 (1‐3) | 4 (3‐4)*** | <.001 |
| Copper scoring | 0 (0‐1) | 2 (1‐2.5)* | 2 (0‐4)* | <.001 |
Data are expressed as median (range).
Abbreviations: ALT, alanine aminotransferase; BA, bile acids; CH, chronic hepatitis; F, female; FS, female spayed; LC, liver cirrhosis; NL, normal liver; M, male; MC, male castrated.
P < .05 compared with NL.
P < .01 compared with NL.
P < .001 compared with NL.
3.2. Serum microRNAs are associated with liver fibrosis in Labrador Retrievers
The analysis of circulating microRNAs associated with the grade of hepatitis and the stage of fibrosis is provided in Table 2. Upon univariate analysis, both miR‐122 and miR‐29a were significantly associated with the grade of hepatitis and the stage of fibrosis (P < .001 for all analyses). These microRNAs were also statistically significant upon multivariate analysis. Neither of the microRNAs was significantly associated to copper scoring upon multivariate analysis.
Table 2.
Univariate and multivariate analysis of microRNA variables associated with the grade of hepatitis and the stage of fibrosis in Labrador Retrievers
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Estimate | P‐value | Estimate | P‐value | ||
| Grade of hepatitis | miR‐122 | 1.460 | <.001 | 0.870 | .023 |
| miR‐29a | 2.030 | <.001 | 1.086 | .006 | |
| miR‐181b | 0.190 | .366 | 0.140 | .665 | |
| miR‐133a | 0.240 | .491 | 0.620 | .250 | |
| miR‐17‐5p | 0.200 | .255 | 0.330 | .195 | |
| Stage of fibrosis | miR‐122 | 1.110 | <.001 | 0.600 | .046 |
| miR‐29a | 1.580 | <.001 | 0.800 | .009 | |
| miR‐181b | 0.180 | .256 | 0.210 | .374 | |
| miR‐133a | −0.080 | .775 | −0.385 | .342 | |
| miR‐17‐5p | −0.004 | .977 | −0.240 | .222 | |
A positive correlation was identified between the grade of hepatitis with miR‐122 (rs = 0.79, P < .001; Figure 1A) and miR‐29a (rs = 0.78, P < .001; Figure 1B). Both miR‐122 (rs = 0.81, P < .001; Figure 2A) and miR‐29a (rs = 0.67, P < .001; Figure 2B) showed a significant positive correlation with the stage of fibrosis. On histological stage, circulating miR‐122 concentrations were significantly higher in the LC group than in the NL (P < .001) and CH (P < .05) groups (Figure 3A) and were significantly higher in the CH group than in the NL group (P < .01). Circulating miR‐29a concentrations were not detected in the NL group and were highly expressed in the CH and LC groups (Figure 3B).
Figure 1.
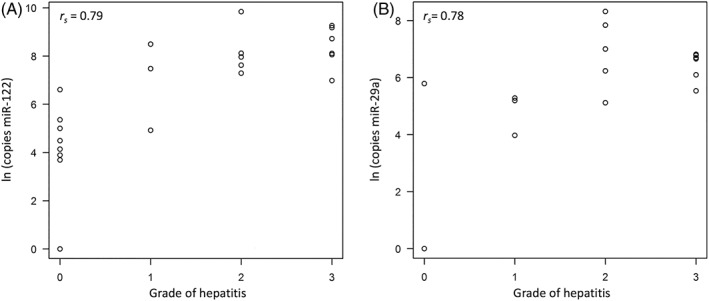
Scatter plot with Spearman correlation coefficient (rs) of circulating miR‐122 (A) and miR‐29a (B) concentrations with the grade of hepatitis in Labrador Retrievers
Figure 2.
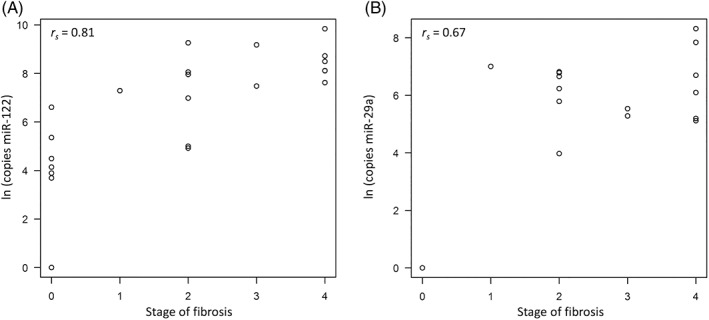
Scatter plot with Spearman correlation coefficient (rs) of circulating miR‐122 (A) and miR‐29a (B) concentrations with the stage of fibrosis in Labrador Retrievers
Figure 3.
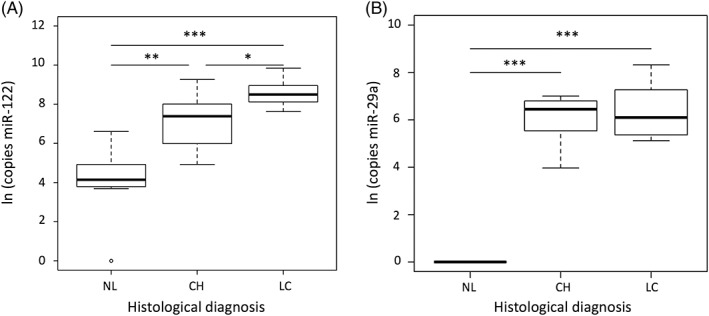
Circulating miR‐122 (A) and miR‐29a (B) concentrations in Labrador Retrievers with normal liver (NL, n = 7), chronic hepatitis (CH, n = 8), and liver cirrhosis (LC, n = 7). *P < .05, **P < .01, ***P < .001
Circulating miR‐122 and miR‐29a concentrations in 2 Labrador Retrievers with different disease stage are shown in Table 3. Circulating miR‐122 concentrations were increased in dog 1 upon progression form NL to CH and were decreased in dog 2 (progression CH to LC) in parallel with plasma ALT concentrations. Circulating miR‐29a concentrations were increased in both dogs with a worsened stage of fibrosis.
Table 3.
Circulating miR‐122 and miR‐29a concentrations in 2 Labrador Retrievers with different disease stage
| Dog 1 | Dog 2 | |||
|---|---|---|---|---|
| NL | CH | CH | LC | |
| Grade of hepatitis | 0 | 1 | 2 | 2 |
| Stage of fibrosis | 0 | 3 | 2 | 3 |
| Copper scoring | 1 | 1 | 2 | 2 |
| ALT (U/L) | 107 | 439 | 779 | 172 |
| BA (μmol/L) | 1 | 57 | 95 | 53 |
| miR‐122 (copies) | 235 | 1776 | 2873 | 1187 |
| miR‐29a (copies) | 1 | 197 | 510 | 644 |
Abbreviations: ALT, alanine aminotransferase; BA, bile acids; CH, chronic hepatitis; LC, liver cirrhosis; NL, normal liver.
4. DISCUSSION
Liver cirrhosis, the end stage of CH, carries a very poor prognosis in dogs.5, 7, 8 Therefore, a liver biopsy is often recommended for early diagnosis and treatment although this invasive technique carries some risk.6, 18 Therefore, efforts have been made to investigate the possibilities for using noninvasive biomarkers to monitor disease progression. Mature microRNAs are emerging as biomarkers for hepatobiliary diseases in humans because they can be detected and quantified in the blood.21, 22, 23 MicroRNAs can be used as diagnostic markers for hepatocellular injury and hepatobiliary diseases in Labrador Retrievers and other canine breeds.24, 25 Because various microRNAs also play essential roles in hepatic fibrogenesis such as hepatic stellate cell (HSC) activation, HSC proliferation, and ECM deposition, microRNAs may serve as markers of disease progression.26, 36 Thus, we assessed in our study whether circulation microRNAs can be used as biomarkers to monitor the grade and stage of CH in Labrador Retrievers.
Circulating microRNAs have been reported as biomarkers of liver injury in various forms of human liver diseases such as chronic viral hepatitis, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, cholestasis, and hepatocellular carcinoma, and their concentrations have been correlated with the severity of the disease.21, 22, 23, 26 Because most microRNAs show a high level of similarity in dogs and humans,37 there is a possibility that circulating microRNAs in dogs can also be used as biomarkers for disease progression in CH. Thus, we selected 5 candidates of circulating microRNAs as biomarkers for fibrosis progression including miR‐122, miR‐29a, miR‐133a, miR‐181b, and miR‐17‐5p based on the current available human literature.30, 31, 32, 33, 34 As a result, we observed that miR‐122 and miR‐29a correlated with the grade of hepatitis and the stage of fibrosis. The serum concentrations of miR‐133a, miR‐181b, and miR‐17‐5p were increased in human patients with LC.31, 33, 34 However, these circulating microRNAs were not significantly associated with the grade and stage in our dogs. In a study on circulating microRNAs as biomarkers of chronic heart diseases in Dachshunds, Hulanicka et al concluded that the microRNA findings from dogs were not directly reflected in human medicine.38 Thus, circulating microRNA concentrations in Labrador Retrievers with CH might also differ between species and disease type.
Mature miR‐122 has been characterized as a hepatocyte‐derived microRNA, accounting for about 70% of all hepatic microRNAs.23 Serum miR‐122 concentrations were increased in acute liver injury and was an earlier marker of hepatocellular damage than serum ALT activity in humans.22, 23 Circulating miR‐122 values were increased in Beagle dogs with acute hepatocellular necrosis induced by hepatotoxic compounds in the absence of increases in ALT.37, 39 Our previous reports demonstrated that circulating miR‐122 is a highly sensitive marker for the detection of hepatocellular injury in Labrador Retrievers,24 and it is increased in dogs with acute and CH.25 In our study, circulating miR‐122 showed a positive correlation with the stage of fibrosis and grade of hepatitis in Labrador Retrievers. This is in agreement with findings in humans where increased serum concentrations of miR‐122 correlated with fibrosis stage and inflammation activity in patients with CH‐C and nonalcoholic fatty liver disease.40 However, circulating miR‐122 concentrations correlated negatively with increasing stages of fibrosis in patients with CH‐C.41 This suggests that reduced concentrations of circulating miR‐122 might be caused by their downregulation caused by liver damage and the loss of hepatocytes.41 Circulating miR‐122 concentrations have been reported to be increased and to correlate with histological liver stage and inflammation grade in human patients with various chronic liver diseases.40, 42 Serum miR‐122 concentrations in dogs were higher in the LC group than the CH group on histological stage. However, in humans with LC, lower miR‐122 concentrations in serum might be an indicator of hepatic functional capacity and a useful prognostic parameter.32 Circulating miR‐122 showed a positive correlation with ALT activity in liver injury and hepatic copper accumulation in Labrador Retrievers and tended to be higher in CH compared to AH in dogs.24, 25 Plasma ALT activity in our study was high in dogs with the LC group as well as the CH group. This suggests that the inflammatory activity was maintained in dogs with LC and indicates that the release of miR‐122 from hepatocytes into the systemic circulation continues in our dogs, even with severe stages of fibrosis.
Mature miR‐29 family is 1 of the most extensively studied microRNAs in the pathophysiology of human liver fibrosis.26 It not only plays an essential role in HSC activation and regulators of hepatic fibrosis but is also associated with the development of renal, pulmonary, and cardiac fibrosis in humans.30, 36 Transforming growth factor β1 (TGF‐β1) is a key mediator in inducing HSC activation and transformation to fibroblastic cells in dogs.4, 5 Although there are microRNAs that control the expression of TGF‐β1, their role in liver fibrosis is not well understood. Serum miR‐29a concentrations were down‐regulated in humans with liver fibrosis/cirrhosis compared to a healthy control group.30 The molecular process that leads to lower serum concentrations of miR‐29a in humans with LC is not clear.30 Interestingly, circulating miR‐29a concentrations in our dogs were significantly higher in the CH and LC groups compared to the NL group. In addition, circulating miR‐29a concentrations were positively correlated with the grade of hepatitis and the stage of fibrosis. Mature miR‐29a molecules are produced in similar amounts by hepatocytes and HSCs in the liver.26 Thus, miR‐29a might be released form hepatocytes in the same manner as miR‐122 concentrations are released in both CH and LC groups. Because circulating miR‐29a concentrations are virtually lacking in histologically NLs, we believe that it is a more unequivocal marker for the presence of inflammation and fibrosis.
Although there was a correlation between circulating miR‐122 and miR‐29a concentrations with the grade of hepatitis and the stage of fibrosis in our study, their levels overlapped in each grade and stage, respectively. In addition, we measured these microRNAs longitudinally in 2 dogs with different disease phase. Circulating miR‐122 concentrations in these 2 dogs fluctuated with ALT activity, reflecting liver injury, as was shown in previous studies.24, 25 In addition, circulating miR‐29a concentrations were increased with a worsened stage of fibrosis. MiR‐122 and miR‐29a might be useful to serially monitor for disease progression or response to treatment in Labrador Retrievers with CH.
Our study has some limitations. This study was a retrospective study only conducted in Labrador Retrievers, and a small number of dogs were included. Therefore, the possibility of type II error cannot be excluded. Because there were age differences between the NL group and the CH and LC groups, this might be a confounding factor. Furthermore, longitudinal follow‐up data were only available from 2 Labrador Retrievers. Because the stage of fibrosis only changed from 2 to 3 in 1 dog, it might be caused by variation in liver tissue samples. In addition, only 1 pathologist involved in our study, and the size of liver specimens collected by 14‐G needle, and the fact that not all liver lobes were sampled might influence the assessment of the grade of hepatitis and the stage of fibrosis because of a low representative value of the biopsy.
In conclusion, we report that circulating miR‐122 and miR‐29a concentrations were related to the grade and stage in Labrador Retrievers with CH. Based on these results, we believe that these circulating microRNAs might be useful for monitoring the response to treatment and progression of disease in dogs with CH. However, further work, including a larger number of dogs, different breeds, and follow‐up data over time, is needed to determine if specific microRNAs can be used as a biomarker for the diagnosis of liver fibrosis and disease progression in dogs with CH.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
This work was completed in the Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands.
Sakai M, Spee B, Grinwis GCM, et al. Association of circulating microRNA‐122 and microRNA‐29a with stage of fibrosis and progression of chronic hepatitis in Labrador Retrievers. J Vet Intern Med. 2019;33:151–157. 10.1111/jvim.15366
REFERENCES
- 1. Van den Ingh TS, Van Winkle TJ, Cullen JM, et al. Morphological classification of parenchymal disorders of the canine and feline liver WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. 1st ed. Philadelphia: Saunders Elsevier; 2006:85‐101. [Google Scholar]
- 2. Poldervaart JH, Favier RP, Penning LC, van den Ingh TSGAM, Rothuizen J. Primary hepatitis in dogs: a retrospective review (2002–2006). J Vet Intern Med. 2009;23:72‐80. [DOI] [PubMed] [Google Scholar]
- 3. Lidbury JA, Rodrigues Hoffmann A, Ivanek R, et al. Interobserve agreement using histological scoring of the canine liver. J Vet Intern Med. 2017;31:778‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Favier RP. Idiopathic hepatitis and cirrhosis in dogs. Vet Clin North Am Small Anim Pract. 2009;39:481‐488. [DOI] [PubMed] [Google Scholar]
- 5. Eulenberg VM, Lidbury JA. Hepatic fibrosis in dogs. J Vet Intern Med. 2018;32:26‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Favier RP, Poldervaart JH, van den Ingh TS, et al. A retrospective study of oral prednisolone treatment in canine chronic hepatitis. Vet Q. 2013;33:113‐120. [DOI] [PubMed] [Google Scholar]
- 7. Raffan E, McCallum A, Scase TJ, Watson PJ. Ascites is a negative prognostic indicator in chronic hepatitis in dogs. J Vet Intern Med. 2009;23:63‐66. [DOI] [PubMed] [Google Scholar]
- 8. Shih JL, Keating JH, Freeman LM, Webster CR. Chronic hepatitis in Labrador Retrievers: clinical presentation and prognostic factors. J Vet Intern Med. 2007;21:33‐39. [DOI] [PubMed] [Google Scholar]
- 9. Bexfield NH, Buxton RJ, Vicek TJ, et al. Breed, age and gender distribution of dogs with chronic hepatitis in the United Kingdom. Vet J. 2012;193:124‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fieten H, Dirksen K, van den Ingh TS, et al. D‐penicillamine treatment of copper‐associated hepatitis in Labrador Retrievers. Vet J. 2013;196:522‐527. [DOI] [PubMed] [Google Scholar]
- 11. Hirose N, Uchida K, Kanemoto H, et al. A retrospective histopathological survey on canine and feline liver diseases at the University of Tokyo between 2006 and 2012. J Vet Med Sci. 2014;76:1015‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dirksen K, Fieten H. Canine copper‐associated hepatitis. Vet Clin North Am Small Anim Pract. 2017;47:631‐644. [DOI] [PubMed] [Google Scholar]
- 13. Fieten H, Gill Y, Martin AJ, et al. The Menkes and Wilson disease genes counteract in copper toxicosis in Labrador Retrievers: a new canine model for copper‐metabolism disorders. Dis Model Mech. 2016;9:25‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fieten H, Hooijer‐Nouwens BD, Biourge VC, et al. Association of dietary copper and zinc levels with hepatic copper and zinc concentration in Labrador Retrievers. J Vet Intern Med. 2012;26:1274‐1280. [DOI] [PubMed] [Google Scholar]
- 15. Johnston AN, Center SA, McDonough SP, et al. Hepatic copper concentrations in Labrador Retrievers with and without chronic hepatitis: 72 cases (1980‐2010). J Am Vet Med Assoc. 2013;242:372‐380. [DOI] [PubMed] [Google Scholar]
- 16. Dirksen K, Burgener IA, Rothuizen J, et al. Sensitivity and specificity of plasma ALT, ALP, and bile acids for hepatitis in Labrador Retrievers. J Vet Intern Med. 2017;31:1017‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawrence YA, Steiner JM. Laboratory evaluation of the liver. Vet Clin North Am Small Anim Pract. 2017;47:539‐553. [DOI] [PubMed] [Google Scholar]
- 18. Lidbury JA, Suchodolski JS. New advances in the diagnosis of canine and feline liver and pancreatic disease. Vet J. 2016;215:87‐95. [DOI] [PubMed] [Google Scholar]
- 19. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597‐610. [DOI] [PubMed] [Google Scholar]
- 21. Arrese M, Eguchi A, Feldstein AE. Circulating microRNAs: emerging biomarkers of liver disease. Semin Liver Dis. 2015;35:43‐54. [DOI] [PubMed] [Google Scholar]
- 22. Enache LS, Enache EL, Ramière C, et al. Circulating RNA molecules as biomarkers in liver disease. Int J Mol Sci. 2014;15:17644‐11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roderburg C, Luedde T. Circulating microRNAs as markers of liver inflammation, fibrosis, and cancer. J Hepatol. 2014;61:1434‐1437. [DOI] [PubMed] [Google Scholar]
- 24. Dirksen K, Verzijl T, van den Ingh TS, et al. Hepatocyte‐derived microRNAs as sensitive serum biomarkers of hepatocellular injury in Labrador Retrievers. Vet J. 2016;211:75‐81. [DOI] [PubMed] [Google Scholar]
- 25. Dirksen K, Verzijl T, Grinwis GC, et al. Use of serum microRNAs as biomarker for hepatobiliary diseases in dogs. J Vet Intern Med. 2016;30:1816‐1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kitano M, Bloomston PM. Hepatic stellate cells and microRNAs in pathogenesis of liver fibrosis. J Clin Med. 2016;16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishak KG, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696‐699. [DOI] [PubMed] [Google Scholar]
- 28. Ishak KG. Pathologic feautres of chronic hepatitis. A review and update. Am J Clin Pathol. 2002;113:40‐55. [DOI] [PubMed] [Google Scholar]
- 29. Hoffmann G, Jones PG, Biourge V, et al. Dietary management of hepatic copper accumulation in Labrador Retrievers. J Vet Intern Med. 2009;23:957‐963. [DOI] [PubMed] [Google Scholar]
- 30. Roderburg C, Urban GW, Bettermann K, et al. Micro‐RNA profiling reveals a role for miR‐29 in human and murine liver fibrosis. Hepatology. 2011;53:209‐218. [DOI] [PubMed] [Google Scholar]
- 31. Roderburg C, Luedde M, Vargas Cardenas D, et al. MiR‐133a mediates TGF‐β‐dependent derepression of collagen synthesis in hepatic stellate cells during liver fibrosis. J Hepatol. 2013;58:736‐742. [DOI] [PubMed] [Google Scholar]
- 32. Waidmann O, Köberle V, Brunner F, Zeuzem S, Piiper A, Kronenberger B. Serum microRNA‐122 predicts survival in patients with liver cirrhosis. PLoS One. 2012;7:e45652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang B, Li W, Guo K, Xiao Y, Wang Y, Fan J. MiR181‐b promotes hepatic stellate cells proliferation by targeting p27 and is elevated in the serum of cirrhosis patients. Biochem Biophys Res Commun. 2012;421:4‐8. [DOI] [PubMed] [Google Scholar]
- 34. Yu F, Guo Y, Chen B, Dong P, Zheng J. Micro‐RNA‐17‐5p activates hepatic stellate cells through targeting of Smad7. Lab Invest. 2015;95:781‐789. [DOI] [PubMed] [Google Scholar]
- 35. Krol EM, Parkin RK, Michell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription‐PCR (qRT‐PCR). Methods. 2010;50:298‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. MicroRNAs and the regulation of fibrosis. FEBS J. 2010;277:2015‐2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koenig EM, Fisher C, Bernard H, et al. The beagle dog microRNA tissue atlas: identifying translatable biomarkers of organ toxicity. BMC Genomics. 2016;17:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hulanicka M, Garncarz M, Parzeniecka‐Jaworska M, Jank M. Plasma miRNAs as potential biomarkers of chronic degenerative valvular disease in Dachshunds. BMC Vet Res. 2014;10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harrill AH, Eaddy JS, Rose K, et al. Liver biomarker and in vitro assessment confirm the hepatic origin of aminotransferase elevations lacking histopathological correlate in beagle dogs treated with GABAA receptor antagonist NP260. Toxicol Appl Pharmacol. 2014;277:131‐137. [DOI] [PubMed] [Google Scholar]
- 40. Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non‐alcoholic fatty liver disease. PLoS One. 2011;6:e23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trebicka J, Anadol E, Elfimova N, et al. Hepatic and serum levels of miR‐122 after chronic HCV‐induced fibrosis. J Hepatol. 2013;58:234‐239. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y, Jia Y, Zheng R, et al. Plasma microRNA‐122 as a biomarker for viral‐, alcohol‐, and chemical‐related hepatic diseases. Clin Chem. 2010;56:1830‐1838. [DOI] [PubMed] [Google Scholar]


