Abstract
Background
A novel equine parvovirus (EqPV‐H) was recently discovered in the equine liver with Theiler's disease.
Objectives
To determine the prevalence of EqPV‐H infection in naturally occurring Theiler's disease cases and in‐contact horses in the absence of historical equine biologic product administration.
Animals
Ten cases of Theiler's disease from 6 separate properties were included in the study, based on the criteria of acute onset of clinical signs of liver failure with laboratory or histopathologic findings characteristic of Theiler's disease and no history of receiving an equine biologic product within the preceding 4 months. In addition, 37 in‐contact horses from 4 of the 6 properties were screened for EqPV‐H infection and hepatitis.
Methods
In prospective case series, cases were diagnosed with Theiler's disease by the attending veterinarian and were tested for EqPV‐H by PCR of liver or serum. In‐contact horses were assessed via serum chemistry and PCR at the attending veterinarian's discretion. Hepatitis was defined as serum gamma‐glutamyltransferase activity above reference interval. The association of EqPV‐H with hepatitis was determined by Fisher's exact test.
Results
Nine of 10 (90%) Theiler's disease cases and 54% of tested in‐contact horses were EqPV‐H positive. Hepatitis was significantly associated with EqPV‐H infection (P = .036).
Conclusions and Clinical Importance
Although further study is required to identify EqPV‐H as the causative agent of Theiler's disease, EqPV‐H appears strongly associated with cases of fatal Theiler's disease and subclinical hepatitis in horses in contact with those cases. The prevalence of EqPV‐H infection on affected properties can be high.
Keywords: acute hepatitis, hepatic failure, hepatic insufficiency, horses, liver, serum hepatitis
Abbreviations
- EqPV‐H
equine parvovirus‐hepatitis
- EPgV
equine pegivirus
- GGT
gamma‐glutamyltransferase
- NPHV
non‐primate hepacivirus
- RI
reference interval
- TDAV
Theiler's disease–associated virus.
1. INTRODUCTION
Theiler's disease, also known as equine serum hepatitis or idiopathic acute hepatic necrosis, is a serious and often life‐threatening fulminant hepatitis of horses. The disorder was 1st described in 1918, in horses that developed fatal hepatic failure after receiving hyperimmune equine serum to prevent African Horse Sickness.1 Since then, Theiler's disease has been reported after administration of a variety of equine biologic products. In North America, it has been most commonly associated with tetanus antitoxin.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The incidence of fulminant hepatitis in horses receiving the same lot of contaminated equine biologic product is estimated to range between 1.4% and 18%.1, 8 Subclinical liver disease has also been reported in horses receiving biologics of equine origin.4, 5 Theiler's disease frequently occurs 4‐10 weeks after administration of an equine biologic product.1, 8, 12 However, spontaneous cases without a history of recent administration of equine origin biologics occur, typically in the late summer and fall.16, 17 Spontaneous disease can also occur in horses that are in contact with horses affected with equine biologic‐associated Theiler's disease.1, 8, 12 These findings suggest that Theiler's disease could be both infectious and contagious.
A novel equine parvovirus, equine parvovirus‐hepatitis (EqPV‐H), was recently discovered in the equine liver with Theiler's disease and was demonstrated to induce hepatitis in 2 experimentally infected horses.18 In the same report, a retrospective reanalysis of samples from an outbreak of Theiler's disease that was previously attributed to the pegivirus, Theiler's disease–associated virus (TDAV),5 demonstrated coinfection with EqPV‐H.18 The primary objective of our study was to determine the prevalence of EqPV‐H infection in horses with Theiler's disease that had no history of treatment with an equine biologic product. Secondary objectives were (1) to determine the prevalence of EqPV‐H among horses on the same premises as horses with Theiler's disease and (2) to determine if there was an association between EqPV‐H infection and hepatitis.
2. MATERIAL AND METHODS
2.1. Prospective clinical case study
A prospective study of clinical cases was performed to assess the possible role of 4 newly identified viruses in the etiology of acute Theiler's disease. A letter was sent to American College of Veterinary Internal Medicine Large Animal Diplomates at teaching and large referral hospitals requesting cases. Case inclusion criteria consisted of (1) acute onset of clinical signs of fulminant hepatic failure with clinicopathologic or liver histopathology findings characteristic of Theiler's disease (serum hepatitis)4, 7, 10, 11, 12, 15 and (2) no history of receiving an equine biologic product within the preceding 4 months. In each case, a diagnosis of Theiler's disease or serum hepatitis was made at the referral practice before submitting samples to Cornell University for viral testing. Cases were enrolled between January 2014 and February 2018.
2.2. Sample and data collection
Serum samples collected from the 10 Theiler's disease cases were frozen or kept on ice before being shipped from the clinic of origin to Cornell University. Liver samples were shipped fresh on ice, frozen, or in formalin‐fixed blocks. All samples were tested for EqPV‐H by quantitative‐PCR (qPCR). Samples were also tested for the flaviviruses non‐primate hepacivirus (NPHV, classified as hepacivirus A), equine pegivirus (EPgV, classified as pegivirus E), and TDAV (classified as pegivirus D).19 Case history, serum chemistry data, and necropsy reports were collected as available. The attending veterinarian and owners of in‐contact horses on 4 of 6 properties from which the 10 cases originated elected to test additional horses by serum PCR for viral infection and chemistry for detection of liver disease. Only the data from in‐contact horses that had PCR and serum gamma‐glutamyltransferase (GGT) performed within 2 weeks of each other were included in the analysis. Horses that had biochemical evidence of hepatitis were not counted as Theiler's disease cases unless there was evidence of liver failure. In all cases, prospective sampling and analysis was approved in full by the Cornell University Institutional Animal Care and Use Committee IACUC #2012‐0154.
2.3. Polymerase chain reaction
Viral nucleic acids were extracted from serum or liver with Qiagen Viral RNA Mini Kit (catalog no. 52906) according to the manufacturer's instructions. No DNase treatment was applied. All PCR mixtures used the Path ID multiplex RT‐qPCR kit (catalog no. 4442137; Thermo Fisher Scientific, Waltham, MA, USA) and 4 μL of extracted nucleic acids in a 25‐μL reaction volume. Utilized primers are listed in Table 1. Two primer pairs were used for EPgV; a positive result in either pair was considered positive. Primers were used at 0.4 μM concentration and probes at 0.12 μM. All PCR reactions were run on the ABI Step‐One‐Plus Real‐Time system and analyzed with StepOne software (Thermo Fisher Scientific, Waltham, MA, USA). Real‐time PCR conditions included an initial incubation at 48°C for 10 minutes, then 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and 60°C for 60 seconds.
Table 1.
Quantitative reverse transcription PCR primer‐probe pairs used to detect hepatitis‐associated viruses in equine liver or serum samples
| Name | Specificity | Sequence | Source |
|---|---|---|---|
| TDAV‐UTR171F | TDAV forward | AGGGTTCTTCGGGTAAATCC | Chandriani et al5 |
| TDAV‐UTR336Rd | TDAV reverse | CCCTCGGACTGAATTrTAGGC | Modified from Chandriani et al5 |
| TDAV‐UTR274 | TDAV probe | ACCTCCCTCACGAAAGGTCGCCAC | Current study |
| QANTI‐5UF1 | NPHV forward | GAGGGAGCTGRAATTCGTGAA | Burbelo et al32 |
| QANTI‐5UR1 | NPHV reverse | GCAAGCATCCTATCAGACCGT | Burbelo et al32 |
| NPHV‐UTR288 | NPHV probe | CCACGAAGGAAGGCGGGGGC | Burbelo et al32 |
| EPgV‐80F | EPgV forward1 | ACCGAGCCGCCCTGTAG | Current study |
| EPgV‐163R | EPgV reverse1 | CCTGCCACCGCGATCA | Current study |
| EPgV‐UTR122 | EPgV probe1 | TCCTGGCACTGGCCCGAAGC | Current study |
| EPgV‐127F | EPgV forward2 | GCACTGGCCCGAAGCAT | Current study |
| EPgV‐210R | EPgV reverse2 | CTGCCCTAACACAATCACAACAC | Current study |
| EPgV‐UTR165 | EPgV probe2 | TTCTTCGGGTAAATCCCGGCCG | Current study |
| EqPV‐3218F | EqPV‐H forward | ATGCAGATGCTTTCCGACC | Current study |
| EqPV‐3386R | EqPV‐H reverse | GCCCCAGAAACATATGGAAA | Current study |
| EqPV‐3310 | EqPV‐H probe | ACCGTAGCGGATTCGGGATCTGC | Current study |
Abbreviations: EPgV, equine pegivirus; EqPV‐H, equine parvovirus‐hepatitis; NPHV, non‐primate hepacivirus; TDAV, Theiler's disease–associated virus.
2.4. Definition of hepatitis
Hepatitis was defined as serum GGT activity above the laboratory‐specific reference interval (RI). The majority of serum chemistry data that was provided did not include the hepatocellular leakage enzyme, succinate dehydrogenase (SDH). GGT was included in all available serum chemistry panels. Although increase in GGT does not indicate a specific etiology, it is highly specific for liver damage. In addition, although GGT comes from the biliary tract, it is frequently increased even when the primary damage is hepatocellular. This is consistently observed in cases of Theiler's disease.4, 7, 10, 11, 12, 15
2.5. Statistical analysis
See Figure 1 for an outline of case numbers and analyses applied. Fisher's exact test (GraphPad Prism) was used to determine whether EqPV‐H viremia was associated with clinical Theiler's disease by comparing EqPV‐H PCR status of Theiler's disease cases (n = 10) to in‐contact horses with PCR and serum chemistry testing (n = 37), regardless of biochemical evidence of hepatitis. Fisher's exact test was also used to determine whether EqPV‐H viremia was associated with any degree of hepatitis by comparing EqPV‐H PCR status of all hepatitis cases (n = 20) to all horses without hepatitis (n = 27).
Figure 1.
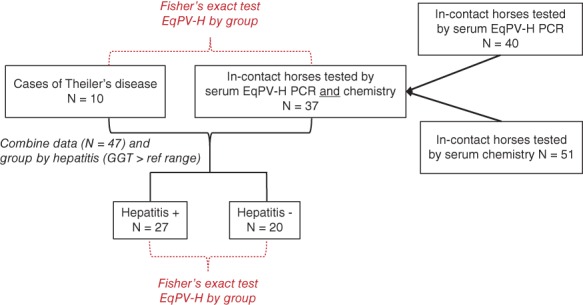
Case analysis outline. The numbers of Theiler's disease and in‐contact horses and the groupings are shown in the boxes. The analyses applied are indicated in red and italics
3. RESULTS
3.1. Theiler's disease cases demographic data
Between January 2014 and February 2018, 28 cases of acute fulminant hepatic failure consistent with and reported as Theiler's disease by the attending practice were submitted. Of those 28 cases, 18 were excluded from this report because of administration history of an equine biologic product within the preceding 4 months; these cases are described in the companion article.20 Ten horses were diagnosed with Theiler's disease without any history of receiving a biologic product of equine blood origin. These 10 horses were from 6 different farms, and case details are provided in Supporting Information Supplemental Table 1. The farms are identified by a letter (A‐F), and each case on a farm was identified with the farm letter and a number, for example, e1, e2, and so forth. Theiler's disease cases included 2 Arabians, 2 Quarter Horses, 2 Welsh Ponies, and 4 Standardbreds. There were 5 mares and 5 geldings. All 5 affected mares were pregnant and between 5 and 10 months gestation. The ages ranged from 5 to 20 years old (median, 8 years). All 10 cases died or were euthanized.
3.2. Theiler's disease cases clinical data
Although not a primary aim of our study, information on clinical signs, biochemical findings, and ancillary testing was available for many of the 10 Theiler's disease cases and are reported here for clinical interest. The primary clinical sign that necessitated the initial veterinary examination in affected horses was acute onset of neurologic signs. Four of 10 horses were described as ataxic, 3 were cortically blind, 3 were obtunded, 1 was head pressing, and 1 was recumbent and moribund. Other early clinical findings provided in case records included icterus (n = 5) and bleeding diathesis (n = 2). Fever was not reported in any cases. One horse (case c1) was being treated for guttural pouch empyema and had a normal physical examination 5 days before onset of liver failure. This was the only horse in the study receiving medical treatments at the time of onset of liver failure. Duration of clinical signs before death or euthanasia was available in all 10 horses, with a median duration of disease of 1 day (range <24 hours to 4 days).
Serum biochemistry abnormalities at admission to referral institutions are summarized in Table 2, and individual case details are reported in Supporting Information Supplemental Table 1. Blood glucose concentration was moderately to severely reduced in all horses (Table 2).
Table 2.
Serum biochemistry of 10 Theiler's disease cases
| Number of cases | Median (range) | Reference interval | |
|---|---|---|---|
| AST (U/L) | 8 | 2925 (1239‐6177) | 222‐489 |
| GGT (U/L) | 9 | 117 (97‐185) | 8‐33 |
| Total bilirubin (mg/dL) | 8 | 20.1 (8.7‐21.7) | 0.5‐2.1 |
| Direct bilirubin (mg/dL) | 3 | 2.3 (1.7‐7.9) | 0.1‐0.3 |
| Ammonia (mmol/L) | 5 | 479 (70‐959) | Not determined |
| Bile acids (μmol/L) | 3 | 90.4 (75.6‐176) | 2‐10 |
| Glucose (mg/dL) | 6 | 39 (10‐74) | 71‐113 |
| Hematocrit (%) | 7 | 55 (49‐68) | 34‐46 |
Data are from the 1st serum biochemistry performed on each horse. Reference range provided is a general range from the New York State Animal Health Diagnostic Center. Tests were run at multiple laboratories and laboratory‐specific reference ranges varied.
Abbreviations: AST, aspartate aminotransferase; GGT, gamma‐glutamyltransferase.
Liver histopathology reports were available for 9 of the Theiler's disease cases. Microscopic findings in 8 horses consistently described centrilobular to massive necrosis, collapse of the lobular architecture, and replacement with cellular debris and sometimes hemorrhage. Periportal hepatocytes were consistently described as degenerate, swollen, and containing cytoplasmic vacuoles or lipidosis. Mild to moderate lymphocytic scattered or periportal infiltration was noted in all but 1 of these cases. Biliary hyperplasia was noted in 4 cases. One case, e4, showed prominent proliferation of hepatic progenitor cells in the canals of Hering, compatible with a ductular response. Microscopic findings in the 9th case (case e1) were obscured by severe autolysis but were compatible with severe necrosis and lipidosis with moderate portal fibrosis. Alzheimer type 2 cells in the brain were present in 1 of 3 reports that included microscopic examination of the brain. All necropsy reports except for cases e1 (diagnosis: autolyzed) and e3 (diagnosis: necrotizing and vacuolar hepatopathy with bile duct hyperplasia) summarized the histologic features as being most suggestive of, presumptive of, or at least compatible with Theiler's disease.
Five cases had additional testing of feed or liver samples to exclude other causes of acute hepatitis (Supporting Information Supplemental Table 2). Liver tissues from cases e2 and e3 were tested for heavy metal content. Although there were mild deviations from RIs for some metals, none were expected to be the cause of the fulminant liver disease. Although bacterial culture of the liver from case f1 grew 2 types of bacteria, neither bacteria nor neutrophilic infiltrates were reported on histopathologic evaluation. There was no history or suspicion of pyrrolizidine alkaloid ingestion in any case, and megalocytosis was not observed in any case.
3.3. Virology of Theiler's disease cases
Nine of the 10 Theiler's disease cases were EqPV‐H PCR positive (Table 3). PCR was performed on a serum sample from 1 horse, liver samples from 7 horses, and paired liver and serum samples from 2 horses. The case that was EqPV‐H negative (case f1) only had frozen liver tissue tested. All samples were negative for NPHV and TDAV. Two horses were EPgV positive, with 1 horse EPgV positive on serum but not liver, and the other horse positive on liver (serum not tested). Details of PCR testing for each horse can be found in Supporting Information Supplemental Table 1.
Table 3.
Summary of EqPV‐H qRT‐PCR and serum GGT results among the 10 cases of clinical Theiler's disease and 37 in‐contact horses
| EqPV‐H+/Theiler's disease cases | EqPV‐H+/in‐contact horses | Hepatitis+/in‐contact horses | |
|---|---|---|---|
| Property | |||
| A | 1/1 | NA | NA |
| B | 1/1 | 3/5 | 0/5 |
| C | 1/1 | 3/3 | 2/3 |
| D | 2/2 | 1/9 | 4/9a |
| E | 4/4 | 13/20 | 4/20 |
| F | 0/1 | NA | NA |
| Total | 9/10 (90%) | 20/37 (54%) | 10/37 (27%) |
Hepatitis was defined as GGT above reference interval. Not all in‐contact horses on each property were tested by PCR and GGT. On property B, only the other horses (n = 5) belonging to the owner of the Theiler's disease case were tested. On property C, only horses with clinical suspicion of illness were tested (n = 3). On property D, all in‐contact horses were tested (n = 9). On property E, a random selection of approximately half the Broodmare herd was tested (n = 20). No statistical association was observed between EqPV‐H and Theiler's disease (P = .06).
Abbreviations: EqPV‐H, equine parvovirus‐hepatitis; GGT, gamma‐glutamyltransferase; NA, not applicable; qRT‐PCR, quantitative reverse transcription PCR.
All horses in this group were tested at a laboratory with an upper limit of reference interval for GGT of 19 U/L, whereas the upper limit at other laboratories ranged from 30 to 35 U/L.
3.4. Demographic data and virologic testing of in‐contact horses
Testing of in‐contact horses was neither random nor encompassed all in‐contact horses. Details regarding the tested in‐contact horses are included in the Supplemental Information. Fifty‐one horses were screened for hepatitis with serum chemistry, and 40 horses were screened by serum PCR. This resulted in 37 horses that were tested for both EqPV‐H infection and hepatitis at the same time (Figure 1). In‐contact horses were tested within 24 days (3‐24 days, median 9 days) of recognition of the last Theiler's disease case on the property. These horses were located on 4 of the 6 properties (Table 3). More than half (29/37, 78%) of the in‐contact horses that were tested came from the 2 properties that had multiple cases of Theiler's disease (properties D and E).
A high prevalence of EqPV‐H infection was observed in the tested in‐contact horses (20/37, 54%). A high prevalence of hepatitis was also observed (10/37, 27%; Table 3). Seven of the 10 in‐contact horses with hepatitis tested positive for EqPV‐H (Table 4). Four of 9 horses on property D were classified as having hepatitis. These horses were tested at a laboratory with an upper limit of RI for GGT of 19 U/L. This limit was notably lower than the RIs at other laboratories (upper limits 30‐35 U/L). All 4 horses had GGT <35 U/L. Three of these horses were EqPV‐H negative, and only 1 horse was positive.
Table 4.
Serum GGT activities of horses in contact with clinical cases of Theiler's disease
| GGT (hepatitis) | ||||||
|---|---|---|---|---|---|---|
| Increased | n = 10 | Within reference interval | n = 22 | All | ||
| EqPV‐H | Detected n = 20 | 48 U/L (20‐98) | n = 7 | 13 U/L (10‐16) | n = 13 | 16 U/L (10‐98) |
| Not detected n = 12 | 29 U/L (26‐32)a | n = 3 | 15 U/L (9‐21) | n = 9 | 16 U/L (9‐32) | |
| All | 39.5 U/L (20‐98) | 14 U/L (9‐21) | ||||
Hepatitis was defined as serum GGT above reference interval within 2 weeks of serum PCR testing for EqPV‐H. An additional 5 horses were reported to have normal serum GGT at the time of PCR testing, and 3 of 5 horses were EqPV‐H PCR+, but specific GGT values were not available. GGT is expressed as median (range) in U/L.
Abbreviations: EqPV‐H, equine parvovirus‐hepatitis; GGT, gamma‐glutamyltransferase.
All horses in this group were tested at a laboratory with an upper limit of reference interval for GGT of 19 U/L, whereas the upper limit at other laboratories ranged from 30 to 35 U/L.
3.5. Statistical analysis
No statistically significant association was documented between EqPV‐H infection and clinical Theiler's disease when compared to in‐contact horses (Figure 1, Table 3, P = .065). When the EqPV‐H status of all 20 cases of clinical and subclinical hepatitis was compared with that of in‐contact horses with normal serum GGT values, EqPV‐H infection was found to be significantly associated with hepatitis (Table 5, P = .036).
Table 5.
Association of serum EqPV‐H PCR status with clinical or biochemical evidence of hepatitis
| Hepatitis | ||||
|---|---|---|---|---|
| Detected | Not detected | Total | ||
| EqPV‐H | Detected | 16 | 13 | 29 |
| Not detected | 4 | 14 | 18 | |
| Total | 20 | 27 | 47 | |
Hepatitis was defined as clinical diagnosis of Theiler's disease or serum GGT above reference interval within 2 weeks of serum PCR testing for EqPV‐H. Primary cases of Theiler's disease (n = 10) and in‐contact horses with paired serum PCR and serum biochemistry (obtained within 2 weeks of each other, n = 37) are included. EqPV‐H infection was significantly associated with hepatitis by Fisher's exact test (P = .03).
Abbreviations: EqPV‐H, equine parvovirus‐hepatitis; GGT, gamma‐glutamyltransferase.
3.6. Timeline for hepatitis
All cases of hepatitis, including clinical Theiler's disease and in‐contact horses with hepatitis, occurred between May and December (Figure 2). To construct a hypothetical timeline of viral exposure, we used the assumption that non‐biologic product–associated cases of Theiler's disease have a similar time frame between exposure to the virus and onset of disease as is observed for biologic product–associated cases. Cases of Theiler's disease that are associated with the administration of equine biologic products typically occur 4‐10 weeks after treatment.1, 8, 12 The predicted earliest exposure period for each property was calculated as 10 weeks before the onset of the 1st case of hepatitis. The predicted latest exposure was calculated as 4 weeks before the onset of the last case. This encompasses the range of likely exposure if biologic and non‐biologic cases have similar incubation periods. By this calculation, exposure was predicted to have occurred between March and the end of October (Figure 2).
Figure 2.
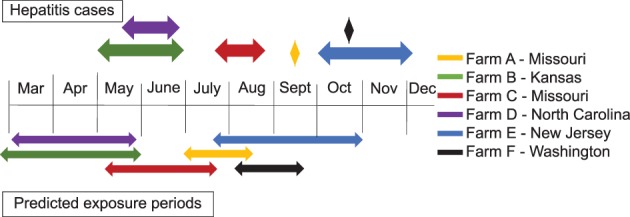
Timing of non‐biologic product–associated cases of Theiler's disease and predicted exposure periods. Ten non‐biologic‐associated cases of Theiler's disease were diagnosed on 6 properties (properties A‐F) between January 2014 and February 2018. Each property is indicated by a different color. On 4 properties, there was follow‐up monitoring of in‐contact horses by examination, serum chemistry, and equine parvovirus‐hepatitis quantitative‐PCR. The time span during which new cases of hepatitis were identified on each farm is shown above the timeline (diamonds indicate that only 1 case occurred on that farm). In equine biologic product–associated cases, hepatitis typically occurs 4‐10 weeks after administration of an equine biologic product. Assuming a similar time frame between exposure and disease for non‐biologic‐associated cases, a predicted timeline of exposure for each farm was constructed, calculated as 10 weeks before the 1st case to 4 weeks before the last case
4. DISCUSSION
It has long been suspected that Theiler's disease could be caused by a blood‐borne virus, similar to serum hepatitis (hepatitis B) in people, because it can occur in association with or in the absence of a history of equine biologic product administration and because it appears to spread within some herds.1, 12 In this prospective case series, 9 of 10 horses diagnosed with Theiler's disease were EqPV‐H positive. All 10 cases had clinical findings consistent with prior descriptions of both biologic product–associated and idiopathic Theiler's disease in horses.1, 4, 6, 8, 10, 11, 12, 13, 14, 15 There are other known toxic causes of acute hepatic failure in horses that produce centrilobular necrosis, which could explain the single case that was EqPV‐H negative. Examples include ingestion of pyrrolizidine alkaloids (acute form), aflatoxins, fumonisins, and phenol.17, 21, 22, 23 Unfortunately, feed samples were not available for analysis.
Three additional viruses that have been implicated in equine hepatitis were absent or rarely present. NPHV has been demonstrated to cause subclinical liver disease in experimental equine models24, 25, 26, 27 but has not been documented to cause fulminant hepatic failure. NPHV was not detected in any case in our study and was only rarely detected in 18 biologic‐associated Theiler's disease cases reported in the companion article.20 The pegivirus, TDAV, was identified during an outbreak of serum hepatitis in horses in 2011. Although it was initially suspected to be a causative agent of Theiler's disease, TDAV was not found in subsequent cases of Theiler's disease.5, 18, 20 TDAV was also not identified in any case in the current study. The related pegivirus, EPgV, has not been associated with disease in clinical cases28 or after experimental inoculation of horses (personal communication). Likewise, EPgV was not commonly identified in the Theiler's disease cases of this present study.
Equine parvovirus‐hepatitis infection was positively associated with the occurrence of hepatitis among the 10 Theiler's disease cases and 37 in‐contact horses. The prevalence of EqPV‐H infection in horses with biochemical evidence of hepatitis was high (16/20, 80%). In contrast, only 48% (13/27) of in‐contact horses without hepatitis were EqPV‐H positive, and only 13% of a convenience sample of 100 serum samples from presumably healthy horses in another study were EqPV‐H positive.18 These findings are highly suggestive that EqPV‐H is a cause of both subclinical and clinical hepatitis in horses.
A major limitation of our study is that it was not a case‐control study. We utilized available data from in‐contact horses that was generated at the attending veterinarians' discretion as a control group. Because these horses were not prospectively sampled as part of the study, there was bias regarding which horses were sampled. The bias erred toward properties with multiple cases of Theiler's disease and horses showing any clinical signs of illness. It is most likely that this bias would result in a higher detected prevalence of hepatitis, EqPV‐H, or both than the true rate if the entire population were sampled. In addition, the biochemical tests performed were inconsistent, and GGT was the only marker of liver damage that was consistently available among the in‐contact horses. Although a specific hepatocellular enzyme such as SDH could have provided more accurate information about ongoing hepatocellular damage, GGT is a reasonable marker of hepatitis for these purposes. GGT has a longer half‐life than SDH, potentially allowing detection of acute cases and those that might have recently recovered. GGT is also highly liver specific. Despite coming from the biliary tract, it is also typically increased in hepatocellular disease. Although there are some causes of high GGT other than hepatitis, such as noninflammatory liver conditions like hepatic lipidosis or extrahepatic compression of the bile duct from large colon displacement, there are typically other clinical signs associated with such conditions and none of these were reported for any in‐contact horse.
Cases of non‐biologic‐associated Theiler's disease in our study presented in the summer through fall season16, 17 and occurred across the United States, with cases originating in Missouri, Kansas, North Carolina, New Jersey, and Washington. Assuming the typical 4‐ to 10‐week incubation period that is reported for biologic‐associated cases, this would imply a transmission period between March and the end of October (Figure 2). Because EqPV‐H can be transmitted via blood products,18 vector‐borne mechanical transmission is hypothesized to be a possible mode of natural horizontal transmission. For example, horse flies (Tabanids) serve as mechanical vectors for over 35 pathogens of livestock.29 Biting insects are active between March and October and tend to be absent in the winter,30 which would be consistent with this theory and could explain the lack of cases in the winter season. Insect transmission studies should be performed to investigate this possibility. An alternative mechanism could be iatrogenic transfer by the use of medical equipment (needles, dental equipment, etc.), which is an important mode of transmission of equine infectious anemia, another blood‐borne equine virus.31
5. CONCLUSIONS
Our study documents EqPV‐H infection in horses with Theiler's disease in the absence of preceding administration of an equine origin biologic product. Further study is needed to unequivocally demonstrate that EqPV‐H is a cause of acute hepatitis and Theiler's disease in horses and to determine the mode(s) of transmission of the virus.
CONFLICT OF INTEREST DECLARATION
Melissa Laverack, Randall Renshaw, and Edward Dubovi are employees of the New York Animal Health Diagnostic Center where equine hepatitis panel PCR testing is offered as fee‐for‐service. These authors were instrumental in viral testing development, validation, and performance but did not contribute to the specific analysis of results. Joy E. Tomlinson received speaker honoraria for presenting parts of this data at the 2018 ACVIM Forum, Seattle, Washington.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All work was approved by the Cornell University IACUC #2012‐0154.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplemental Table 1 Clinical data and virology results for each Theiler's disease case. Chemistry values in bold were outside the reference interval
Supplemental Table 2 Additional diagnostic testing on Theiler's disease cases. HPLC, high performance liquid chromatography; RI, reference interval
Supplemental Figure 1 Timeline of herd testing after cases of Theiler's disease on Property B in Kansas (A) and Property E in New Jersey (B). Cases of Theiler's disease are indicated in gray or black boxes above the timeline. The time of diagnosis of additional cases of clinical or subclinical hepatitis is indicated in red above the timeline. Predicted exposure periods for each respective set of cases are shown below the timeline. Serum PCR data for in‐contact horses is displayed below the timeline. (A) After the index case on Property B, b1, was euthanized, 5 in‐contact horses were screened for hepatitis (serum chemistry) and EqPV‐H (serum PCR) in May. No horses had hepatitis at that time, although 3 (b3, b4 and b5) were PCR+. Three in‐contact horses (b2, b3 and b4) developed non‐specific signs of illness in late June and two (b2 and b3) were confirmed to have biochemical evidence of hepatitis at that time. The 5 horses were re‐tested for EqPV‐H in July. A sixth in‐contact horse was tested by PCR in June but did not have serum chemistry performed. (B) After 2 index cases of Theiler's disease (e1 and e2) were euthanized at the end of September, the farm instituted serial serum biochemistry screening of 35 mares. Cases of mild clinical or subclinical hepatitis were diagnosed between the beginning of October and early December (red arrows above the timeline), although monitoring continued into January. Two additional cases of Theiler's disease (e3 and e4) developed among these 35 monitored mares. EqPV‐H PCR was performed on 20 mares at a single timepoint in early December.
Supplemental Figure 2: Nine horses in contact with cases d1 and d2 were monitored by serial serum GGT, SDH, total bilirubin, and bile acids for 3 months following the death of case d2. GGT and SDH are shown. Total bilirubin and bile acids remained within reference interval in all horses at all timepoints. At day zero, all 9 horses were screened for EqPV‐H by serum PCR and only one horse, denoted in red, was PCR positive. The upper limit of the reference interval is denoted by the horizontal dotted line.
Supplemental Table 3: Clinical data for the horses from Property B. QH, Quarter Horse; G, gelding; F, female; ND, not detected; Ct, cycle threshold (ie, number of cycles required for the fluorescent signal to exceed background level). On May 12, horses b2‐b6 all had normal serum chemistry.
Supplemental Table 4: Clinical data for the horses from Property C. QH, Quarter Horse; G, gelding; F, female; ND, not detected; Ct, cycle threshold (ie, number of cycles required for the fluorescent signal to exceed background level).
Supplemental Table 5: Hepatitis status of 20 horses screened for EqPV‐H by serum PCR after 4 herd‐mates died of Theiler's disease. Hepatitis was classified as “Active” if GGT was >35 U/L within 2 weeks of the PCR; “Resolved” if GGT had been >35 U/L within the prior 2 months but was normal at the time of PCR; and “Not detected” if GGT was <35 U/L at all times tested within the previous 2 months.
Supplemental Table 5: EqPV‐H status of 20 horses that developed biochemical evidence of liver disease between September and December of 2015, including 4 cases that died of Theiler's disease. Hepatitis was classified as “Active” if GGT was >35 U/L within 2 weeks of the PCR or “Resolved” if GGT had been >35 U/L within the preceding 2 months but was normal at the time of PCR
ACKNOWLEDGMENTS
We acknowledge Dr Kirsty Husby, Department of Clinical Sciences, Oregon State University for her care of a case included in this study. Samples were submitted to the New York State Animal Health Diagnostic Center for qPCR testing. The analysis and manuscript preparation were performed at Cornell University College of Veterinary Medicine. Aspects of this work presented at the 2018 ACVIM Forum, Seattle WA. While the viral testing in this case series was performed on a prospective basis, the individual case management was not determined by the study parameters and was performed by the attending clinicians.
Tomlinson JE, Tennant BC, Struzyna A, et al. Viral testing of 10 cases of Theiler's disease and 37 in‐contact horses in the absence of equine biologic product administration: A prospective study (2014‐2018). J Vet Intern Med. 2019;33:258–265. 10.1111/jvim.15362
Present addressCamilla Jamieson, Equine Veterinary Medical Center, Doha, Qatar.
REFERENCES
- 1. Theiler A. Acute liver‐atrophy and parenchymatous hepatitis in horses Union of South Africa. Dept. of Agriculture. 5th and 6th Repts. of the Director of Veterinary Research; 1918. [Google Scholar]
- 2. Meyer K. Equine encephalomyelitis. North Am Vet. 1933;14:30‐48. [Google Scholar]
- 3. Madsen D. Equine encephalomyelitis. Utah Acad Sci, Arts, Lett. 1934;11:95‐99. [Google Scholar]
- 4. Guglick MA, MacAllister CG, Ely RW, Edwards WC. Hepatic disease associated with administration of tetanus antitoxin in eight horses. J Am Vet Med Assoc. 1995. Jun 1;206(11):1737‐1740. [PubMed] [Google Scholar]
- 5. Chandriani S, Skewes‐Cox P, Zhong W, et al. Identification of a previously undescribed divergent virus from the Flaviviridae family in an outbreak of equine serum hepatitis. Proc Natl Acad Sci USA. 2013;110(15):E1407‐E1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aleman M, Nieto JE, Carr EA, Carlson GP. Serum hepatitis associated with commercial plasma transfusion in horses. J Vet Intern Med. 2005;19:120‐122. [DOI] [PubMed] [Google Scholar]
- 7. Panciera RJ. Serum hepatitis in the horse. J Am Vet Med Assoc. 1969;155(2):408‐410. [PubMed] [Google Scholar]
- 8. Marsh H. Losses of undetermined cause following an outbreak of equine encephalomyelitis. J Am Vet Med Assoc. 1937;91:88‐93. [Google Scholar]
- 9. Marsh H. Supplementary note to article on equine encephalomyelitis. J Am Vet Med Assoc. 1937;81:330‐331. [Google Scholar]
- 10. Hjerpe C. Serum hepatitis in the horse. J Am Vet Med Assoc. 1964;144:734‐740. [PubMed] [Google Scholar]
- 11. Rose J, Immenschuh R, Rose E. Serum hepatitis in the horse. In: Proceeding Annual Conference of the American Association of Equine Practice; 1974:175‐185.
- 12. Thomsett LR. Acute hepatic failure in the horse. Equine Vet J. 1971;3(15):15‐19. [DOI] [PubMed] [Google Scholar]
- 13. Step D, Blue J, Dill S. Penicillin‐induced hemolytic anemia and acute hepatic failure following treatment of tetanus in a horse. Cornell Vet. 1991;81(1):13‐18. [PubMed] [Google Scholar]
- 14. Messer NT, Johnson PJ. Serum hepatitis in two brood mares. J Am Vet Med Assoc. 1994;204(11):1790‐1792. [PubMed] [Google Scholar]
- 15. Messer NT, Johnson PJ. Idiopathic acute hepatic disease in horses: 12 cases (1982‐1992). J Am Vet Med Assoc. 1994. Jun 15;204(12):1934‐1937. [PubMed] [Google Scholar]
- 16. Davis JL. Infectious, toxic, and parasitic liver disease acute hepatitis in horses In: Smith BP, ed. Large Animal Internal Medicine. 5th ed. London: Mosby Elsevier; 2014:848‐849. [Google Scholar]
- 17. Tennant BC. Acute hepatitis in horses: problems differentiating toxic and infectious causes in adults. In: Proceedings of the Annual Convention of the American Association of Equine Practitioners; 1978:465‐471.
- 18. Divers TJ, Tennant BC, Kumar A, et al. A new parvovirus associated with serum hepatitis in horses following inoculation of a common equine biological. Emerg Infect Dis. 2018;24(2):303‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith DB, Becher P, Bukh J, et al. Proposed update to the taxonomy of the genera Hepacivirus and Pegivirus within the Flaviviridae family. J Gen Virol. 2016;97(11):2894‐2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomlinson J, Kapoor A, Kumar A, et al. Viral testing of 18 consecutive cases of equine serum hepatitis – a prospective study (2014‐2018). J Vet Intern Med. 2018; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caloni F, Cortinovis C. Toxicological effects of aflatoxins in horses. Vet J. 2011;188(3):270‐273. [DOI] [PubMed] [Google Scholar]
- 22. Voss KA, Smith GW, Haschek WM. Fumonisins: toxicokinetics, mechanism of action and toxicity. Anim Feed Sci Technol. 2007;137(3–4):299‐325. [Google Scholar]
- 23. Smith BP, ed. Large Animal Internal Medicine. 5th ed. St. Louis, MO: Mosby Elsevier; 2014:854‐857. [Google Scholar]
- 24. Ramsay JD, Evanoff R, Wilkinson TE, Divers TJ, Knowles DP, Mealey RH. Experimental transmission of equine hepacivirus in horses as a model for hepatitis C virus. Hepatology. 2015;61(5):1533‐1546. [DOI] [PubMed] [Google Scholar]
- 25. Pfaender S, Cavalleri JMV, Walter S, et al. Clinical course of infection and viral tissue tropism of hepatitis C virus‐like nonprimate hepaciviruses in horses. Hepatology. 2015;61(2):447‐459. [DOI] [PubMed] [Google Scholar]
- 26. Pfaender S, Walter S, Grabski E, et al. Immune protection against reinfection with nonprimate hepacivirus. Proc Natl Acad Sci USA. 2017;114(12):E2430‐E2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scheel TKH, Kapoor A, Nishiuchi E, et al. Characterization of nonprimate hepacivirus and construction of a functional molecular clone. Proc Natl Acad Sci USA. 2015;112(7):2192‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kapoor A, Simmonds P, Cullen JM, et al. Identification of a pegivirus (GB virus‐like virus) that infects horses. J Virol. 2013;87(12):7185‐7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foil LD, Hogsette JA. Biology and control of tabanids, stable flies, and horn flies. Rev Sci Tech. 1994;13(4):1125‐1158. [DOI] [PubMed] [Google Scholar]
- 30. Mullens BA. In: Mullen FR, Durden LA, eds. Medical and Veterinary Entomology [E‐book]. 2nd ed. Amsterdam: Elsevier Ltd; 2009:258. [Google Scholar]
- 31. Sponseller B. Equine infectious anemia In: Smith BP, ed. Large Animal Internal Medicine [E‐book]. 5th ed. London: Mosby; 2014:1060‐1061. [Google Scholar]
- 32. Burbelo PD, Dubovi EJ, Simmonds P, et al. Serology‐enabled discovery of genetically diverse hepaciviruses in a new host. J Virol. 2012;86(11):6171‐6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Clinical data and virology results for each Theiler's disease case. Chemistry values in bold were outside the reference interval
Supplemental Table 2 Additional diagnostic testing on Theiler's disease cases. HPLC, high performance liquid chromatography; RI, reference interval
Supplemental Figure 1 Timeline of herd testing after cases of Theiler's disease on Property B in Kansas (A) and Property E in New Jersey (B). Cases of Theiler's disease are indicated in gray or black boxes above the timeline. The time of diagnosis of additional cases of clinical or subclinical hepatitis is indicated in red above the timeline. Predicted exposure periods for each respective set of cases are shown below the timeline. Serum PCR data for in‐contact horses is displayed below the timeline. (A) After the index case on Property B, b1, was euthanized, 5 in‐contact horses were screened for hepatitis (serum chemistry) and EqPV‐H (serum PCR) in May. No horses had hepatitis at that time, although 3 (b3, b4 and b5) were PCR+. Three in‐contact horses (b2, b3 and b4) developed non‐specific signs of illness in late June and two (b2 and b3) were confirmed to have biochemical evidence of hepatitis at that time. The 5 horses were re‐tested for EqPV‐H in July. A sixth in‐contact horse was tested by PCR in June but did not have serum chemistry performed. (B) After 2 index cases of Theiler's disease (e1 and e2) were euthanized at the end of September, the farm instituted serial serum biochemistry screening of 35 mares. Cases of mild clinical or subclinical hepatitis were diagnosed between the beginning of October and early December (red arrows above the timeline), although monitoring continued into January. Two additional cases of Theiler's disease (e3 and e4) developed among these 35 monitored mares. EqPV‐H PCR was performed on 20 mares at a single timepoint in early December.
Supplemental Figure 2: Nine horses in contact with cases d1 and d2 were monitored by serial serum GGT, SDH, total bilirubin, and bile acids for 3 months following the death of case d2. GGT and SDH are shown. Total bilirubin and bile acids remained within reference interval in all horses at all timepoints. At day zero, all 9 horses were screened for EqPV‐H by serum PCR and only one horse, denoted in red, was PCR positive. The upper limit of the reference interval is denoted by the horizontal dotted line.
Supplemental Table 3: Clinical data for the horses from Property B. QH, Quarter Horse; G, gelding; F, female; ND, not detected; Ct, cycle threshold (ie, number of cycles required for the fluorescent signal to exceed background level). On May 12, horses b2‐b6 all had normal serum chemistry.
Supplemental Table 4: Clinical data for the horses from Property C. QH, Quarter Horse; G, gelding; F, female; ND, not detected; Ct, cycle threshold (ie, number of cycles required for the fluorescent signal to exceed background level).
Supplemental Table 5: Hepatitis status of 20 horses screened for EqPV‐H by serum PCR after 4 herd‐mates died of Theiler's disease. Hepatitis was classified as “Active” if GGT was >35 U/L within 2 weeks of the PCR; “Resolved” if GGT had been >35 U/L within the prior 2 months but was normal at the time of PCR; and “Not detected” if GGT was <35 U/L at all times tested within the previous 2 months.
Supplemental Table 5: EqPV‐H status of 20 horses that developed biochemical evidence of liver disease between September and December of 2015, including 4 cases that died of Theiler's disease. Hepatitis was classified as “Active” if GGT was >35 U/L within 2 weeks of the PCR or “Resolved” if GGT had been >35 U/L within the preceding 2 months but was normal at the time of PCR


