Abstract
Background
Supraphysiological insulin and incretin responses to a cereal‐based diet have been described in horses and ponies with insulin dysregulation (ID). However, the hormonal responses to grazing have not yet been described.
Objectives
To determine if there is a difference in the insulin and incretin responses to grazing pasture between insulin‐dysregulated and healthy ponies.
Animals
A cohort of 16 ponies comprising 5 with normal insulin regulation (NIR), 6 with moderate ID (MID), and 5 with severe ID (SID).
Methods
In this case‐control study, an oral glucose test (OGT) was used to determine the insulin responsiveness of each pony to PO carbohydrate before grazing pasture (4 hours) for 3 consecutive days. Serial blood samples collected during grazing were analyzed for glucose, insulin, glucose‐dependent insulinotropic peptide (GIP) and active glucagon‐like peptide‐1 (aGLP‐1), and compared among pony groups and day of pasture access.
Results
The area under the insulin curve when grazing increased with ID severity (P < .03). The median (range) maximal insulin concentration was greater in the MID (72.5 [129] μIU/mL) and SID (255 [338.5] μIU/mL) groups, compared to the NIR (11.7 [24.9] μIU/mL) group (P < .03) and occurred within 2‐4 hours of grazing. Postprandial OGT insulin concentration was positively correlated with 2 hours post‐grazing insulin across all 3 grazing days (P ≤ .03). The aGLP‐1 and GIP concentrations increased in response to grazing but did not differ among groups.
Conclusions and Clinical Importance
Grazing pasture provoked an increased insulin and incretin response in insulin‐dysregulated ponies within 4 hours of grazing. The pasture and OGT insulin concentrations were correlated.
Keywords: equine metabolic syndrome, glucagon‐like peptide‐1, glucose‐dependent insulinotropic polypeptide, hyperinsulinemia, laminitis, pasture
Abbreviations
- aGLP‐1
active glucagon‐like peptide‐1
- BCS
body condition score
- BW
body weight
- CNS
cresty neck score
- DM
dry matter
- GIP
glucose‐dependent insulinotropic polypeptide
- ID
insulin dysregulation
- MID
moderately insulin‐dysregulated
- NIR
normally insulin‐regulated
- NSC
nonstructural carbohydrate
- OGT
oral glucose test
- PPID
pituitary pars intermedia dysfunction
- SID
severely insulin‐dysregulated
1. INTRODUCTION
A dysregulated insulin response to the ingestion of nonstructural carbohydrate (NSC) results in hyperinsulinemia.1, 2 Hyperinsulinemia is a component of insulin dysregulation (ID), which is a central feature of equine metabolic syndrome (EMS).1, 2 An increased risk of endocrinopathic laminitis is associated with EMS.1 Amplified insulin secretion in response to PO NSC is partly because of enhanced glucose absorption and partly because of intestinally derived incretin hormones, which encompasses the enteroinsular axis.2, 3, 4, 5 The main insulinogenic incretin hormones, glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic peptide (GIP), boost postprandial insulin release and have been demonstrated to partially augment insulin secretion after carbohydrate consumption in ponies.2 The oral glucose test (OGT) assesses this enteroinsular axis6 and is used to identify hyperinsulinemia in response to PO carbohydrate.5, 6 Furthermore, the insulin response to an OGT has been shown to predict the development of laminitis in ponies after excessive NSC consumption.7 However, the accuracy of this test for predicting the insulin response of grazing animals to a pasture diet has not been determined.
Endocrinopathic laminitis has been reported anecdotally to occur within the 1st few days of unrestricted grazing by insulin‐dysregulated ponies or horses. Rapid induction of laminitis occurs within 48‐72 hours in horses and ponies maintained at supraphysiological insulin concentrations.8, 9 Ponies fed a high NSC diet also developed laminitis within 5‐18 days, when postprandial insulin concentrations were approximately 396 μIU/mL.7 The initial insulin and incretin responses to grazing have not been described and may be a key factor in determining the onset of laminitis.
Our aims were to determine whether insulin and incretin secretion in response to grazing differs with the degree of ID in ponies and to determine whether these responses change over 3 consecutive days of pasture access. A 2nd aim was to identify whether the insulin and incretin responses to the OGT correlated with those measured during voluntary pasture grazing.
2. MATERIALS AND METHODS
2.1. Animals
The animal work was approved by the Animal Ethics Committee of the University of Queensland (QUT/SVS/316/16) and Queensland University of Technology (1600000825). Sixteen mixed‐breed ponies (6 Shetland/Shetland crosses, 3 Welsh/Welsh crosses, and 7 other breeds) owned by Queensland University of Technology were subjected to veterinary examination, including CBC and blood biochemistry before the study.
The ponies were tested for insulin responsiveness using an OGT, and ponies were designated as insulin‐dysregulated if their serum insulin concentration exceeded 80 μIU/mL 2 hours after glucose consumption.5, 10 Briefly, after an overnight fast, an 8 am meal was provided comprising 0.3% body weight (BW) lucerne chaff and 200 g bran. The bran was used as a carrier for 0.75 g/kg BW dextrose dissolved in 500 mL water. Blood samples were collected by venipuncture before and 2 hours after the test meal. Tests for measuring tissue resistance to insulin were not performed. A thorough clinical examination was performed, and basal plasma adrencocorticotropic hormone (ACTH) concentration measured to evaluate each animal for pituitary pars intermedia dysfunction (PPID).
Body condition score (BCS) was assessed on the Henneke scale11 of 1 (very poor) to 9 (extremely fat), and cresty neck score (CNS) was determined as previously described12 on a 0‐5 scale, by an experienced assessor. The ponies were acclimated to a daily diet of 2% BW lucerne hay, plus a low NSC vitamin and mineral supplement (Kentucky Equine Research, Mulgrave, Victoria, Australia), before the start of the study. During the study, the ponies had a daily clinical examination performed, which included an assessment of digital pulses, as well as gait and hoof morphology to detect any potential abnormalities, including laminitis.
2.2. Study design
The study was performed in spring as 2 successive replicates (within 30 days), with 8 ponies randomly allocated to each phase. The ponies were maintained individually in pasture‐free yards when not accessing pasture. Each phase consisted of an initial 10 days when the ponies were fed the hay diet outlined above, followed by 3 consecutive days of grazing native Australian pasture in individual strips (20 m × 5 m) for 4 hours between 8 am and 12 pm. At midday on each day, the ponies were returned to their stable and received an evening meal of lucerne hay (0.7% BW) and a vitamin and mineral premix at 5 pm. New strips were used within the same grazing area for phase 2, so that no pasture was re‐grazed.
2.3. Samples
The pasture was sampled at 10 am on the day before pasture access in each phase. Briefly, the diagonal of the whole grazing area was walked, and a 0.25 m2 set square was thrown randomly 6 times. The entire pasture content of each square was rapidly harvested to a grazing height of 1 cm, pooled and dried immediately using a microwave to arrest any carbohydrate metabolism. The dry matter (DM) content of the pooled sample was determined. The lucerne hay was sampled using a bale corer on 12 bales, and the samples pooled. All forage samples were analyzed at a commercial laboratory accredited by the National Association of Testing Authorities (DPI, New South Wales, Australia).
Blood samples (6 mL) were collected at 0, 1, 2, 4, and 8 hours on each day of grazing, from an indwelling jugular catheter (Mila Int., Kentucky) placed aseptically under local anesthetic the evening before the 1st day of pasture access. Blood glucose concentration was measured immediately using a hand‐held glucometer (Accu‐Check, Roche, New South Wales, Australia) previously validated for use in ponies by the investigators. The remaining blood was separated into clot activator (serum) and dipeptidyl peptidase 4 (DPP4) inhibitor‐containing (plasma) vacutainer tubes (Becton Dickinson, New Jersey). Clot activator tubes were left to clot at room temperature for 30 minutes, centrifuged at 1500g for 10 minutes, and the serum collected and stored at −20°C. The DPP4 inhibitor tubes were immediately placed on ice for 10 minutes, centrifuged at 1500g for 10 minutes, and the plasma collected and stored at −20°C. Samples were transferred to −80°C within 48 hours of collection, before analysis. Serum insulin and plasma ACTH concentrations were measured at a commercial laboratory (VetPath, Western Australia, Australia) using chemiluminescent assays (Immulite; Siemens Healthcare, Brisbane, Queensland, Australia) previously validated for horses.13, 14 Plasma incretin concentrations were measured in duplicate using ELISAs validated for use in horses2 and included active GLP‐1 (aGLP‐1) (intra‐assay CV 3.4% and inter‐assay CV 13.8%) and GIP (intra‐assay CV 4.4% and inter‐assay CV 8.5%).
Pasture intake during the 4‐hour grazing period was estimated by measuring the total fecal DM output per pony over the 24‐hour period after pasture access and accounting for the evening hay meal. The digestible organic matter of the dry matter of each feed component was accounted for as previously described15 where:
The total fecal matter for each pony was weighed, subsampled, dried for 20 hours in a drying cabinet at 58°C, and reweighed to calculate fecal DM. An attempt to corroborate the estimation of pasture intake also was undertaken by determining live weight before and after pasture access.16 However, this approach was confounded by excessive water loss associated with sweating on some days because of high ambient temperatures and therefore is not reported.
2.4. Data analyses
The diet phase estimates of pasture and NSC intake (total g and g/kg BW) were tested for normality (Shapiro‐Wilk test) and when normally distributed were analyzed parametrically using a t‐test or if not normally distributed were analyzed nonparametrically using a Mann‐Whitney rank sum test. The area under the curve (AUC) for hormone concentration versus time was calculated using the trapezoidal method, with a baseline of zero. The AUC, maximum concentration (Cmax), and time to maximum concentration (Tmax) for glucose, insulin, GIP‐1, and GIP were tested for normality (Shapiro‐Wilk test), and when normally distributed were analyzed parametrically using 1‐way anova or 2‐way repeated‐measures anova, as appropriate. Nonparametric data were log10 transformed, and if normally distributed, the data analysis proceeded using parametric methods, or if not, they were analyzed using a nonparametric test, the Kruskal‐Wallis analysis on ranks. Thus, the data are presented either as mean ± SD or median (interquartile range) in tables and mean ± standard error of the mean in figures. Correlations were tested using Pearson's correlation test. Multiple linear regression was used to assess the relative contribution of glucose and the incretin hormones (AUC) to insulin AUC. Significance was set at P < .05. The data were analyzed with SigmaPlot v.13 (Systat, San Jose, California).
3. RESULTS
3.1. Animals
The 16 ponies (8 females and 8 males; 13 ± 6 years) were classified into 3 metabolically distinct groups (P < .03) based on their serum insulin response to the OGT (Table 1): normally insulin‐regulated (NIR; n = 5; 2 hours [insulin] < 60 μIU/mL10, 17); moderately insulin‐dysregulated (MID; n = 6; 2 hours [insulin] 60‐279 μIU/mL); and severely insulin‐dysregulated (SID; n = 5; 2 hours [insulin] ≥ 280 μIU/mL). The cutoff value of 280 μIU/mL for the SID ponies was selected based on a previous study in which this value represented the upper 95% confidence interval (CI) of postprandial insulin in a cohort of ponies and was used in the current study to account for the wide range of ID severity in the cohort.7 No difference in BCS or CNS was found among the groups, but the NIR ponies were heavier than both the MID and SID groups (Table 1). The NIR group consisted of 1 Shetland type and 4 “other” breeds; the MID group consisted of 4 Shetland types, 1 Welsh type, and 1 “other” breeds; and the SID group consisted of 2 Shetland types, 2 Welsh types, and 1 “other” breed. None of the ponies exhibited clinical signs of PPID.18 Clinical signs of acute laminitis were not evident before or during the study, based on digital pulses, gait, and hoof morphology. Four ponies (2 NIR and 2 SID) were excluded from sampling on day 3 of pasture access because of technical problems with their IV catheters.
Table 1.
The mean (±SD) postprandial serum insulin concentration during an oral glucose test, plasma ACTH concentration, and morphometric parameters of 16 ponies separated into 3 groups based on their insulin response to the oral glucose test
| Group | n | Insulin 0 h (μIU/mL) | Insulin 2 h (μIU/mL) | ACTH (pg/mL) | BW | BCS | CNS |
|---|---|---|---|---|---|---|---|
| NIR | 5 | 2 ± 0a | 27.2 ± 10.8c | 15.6 ± 4.8 | 348 ± 96.3f | 6.6 ± 1.8 | 1 [3] |
| MID | 6 | 2 ± 0a | 147 ± 93.4d | 23.2 ± 16.7 | 150 ± 58.2g | 6.7 ± 1.4 | 2 [1] |
| SID | 5 | 7.73 ± 2.46b | 438 ± 97.9e | 11.4 ± 3.3 | 184 ± 39.1g | 6.2 ± 0.8 | 3 [0] |
Abbreviations: BCS, body condition score; BW, body weight; CNS, cresty neck score; MID, moderate insulin dysregulation; NIR, normal insulin regulation; SID, severe insulin dysregulation.
The CNS is reported as the median [IQR]. Letters denote statistical differences between groups within variable: a versus b, P < .03; c versus d versus e, P < .03; f versus g, P < .004. 2 μIU/mL is the limit of detection of the insulin assay.
3.2. Diet
Chemical analyses of the lucerne hay and pasture are reported on a dry matter basis and are shown in Table 2. Over the 4‐hour grazing period, estimated pasture intake for the cohort ranged from 2.07 to 25.6 g/kg BW (8.83 [4.94] g/kg BW). The estimated median (IQR) pasture intake increased from day 1 to day 2 (P = .04; Figure 1A). Intake during 4 hours of grazing was not different (P = .09) from intake when on the hay diet (Figure 1B). Furthermore, it was estimated that the MID group consumed a similar (P = .06) amount of grass to the SID group, during the same grazing period.
Table 2.
Analysis of the 2 forage components of a diet fed to 16 ponies
| LOR | Lucerne hay | Pasture (phase 1) | Pasture (phase 2) | |
|---|---|---|---|---|
| Dry matter (%) | 0.5 | 91.2 | 92.2 | 93.8 |
| Neutral detergent fiber (%) | 10 | 44 | 66 | 67 |
| Acid detergent fiber (%) | 4 | 30 | 36 | 38 |
| Crude protein (%) | 2.0 | 19.3 | 13.5 | 11.6 |
| Inorganic ash (%) | 3 | 9 | 9 | 9 |
| Digestible organic matter in the dry matter (%) | 38 | 61 | 56 | 58 |
| Metabolizable energy (MJ/kg DM) | 4.3 | 9.3 | 8.4 | 8.8 |
| Nonstructural carbohydrate (%) | 7.3 | 6 | 10.9 |
Abbreviations: LOR, limit of reporting; MJ, megajoule; ppm, parts per million.
Figure 1.
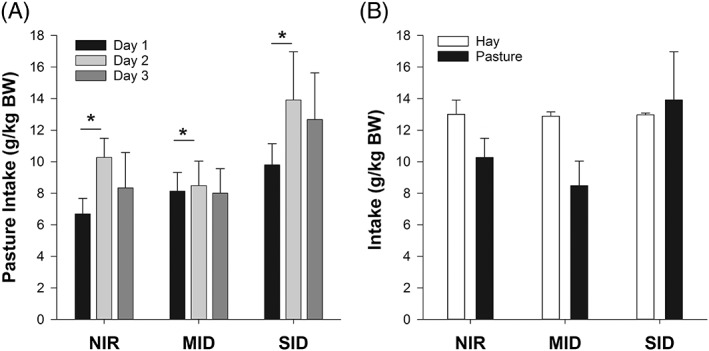
(A) The mean ± standard error of the mean (SEM) pasture intake (g/kg body weight [BW]) of normally insulin‐regulated (NIR, n = 5), moderately insulin‐dysregulated (MID, n = 6), and severely insulin‐dysregulated (SID, n = 5) ponies over 3 consecutive days. * indicates a significant difference between days (P = .04). (B) The mean ± SEM intake (g/kg BW) of NIR, MID, and SID ponies when fed hay and pasture on grazing day 2
3.3. Hormone responses to pasture grazing
3.3.1. Glucose and insulin
During the 3 days of grazing, neither the Cmax glucose nor the AUCglucose differed among the pony groups (Figure 2). However, although the AUCglucose increased on days 2 and 3, compared to day 1 (P = .035), the Cmax glucose did not. Blood glucose concentrations peaked between 2 and 4 hours. However, Tmax glucose was variable, with a longer Tmax glucose on day 2 than day 1 (P = .05). Furthermore, Tmax glucose did not differ among the pony groups (Figure 2).
Figure 2.
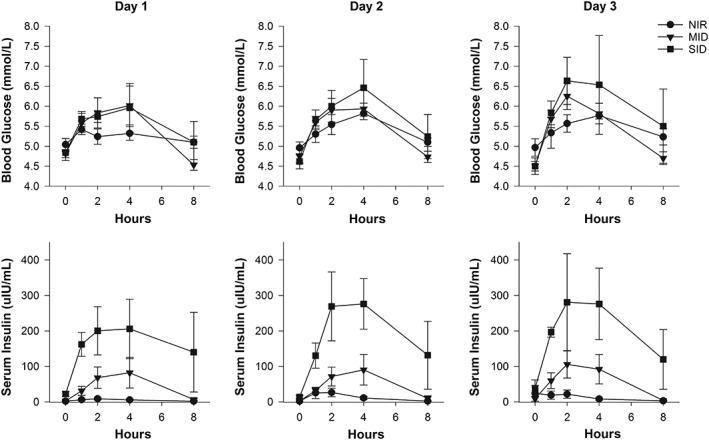
The mean ± standard error of the mean blood glucose and serum insulin concentrations in normally insulin‐regulated (circle, NIR, n = 5), moderately insulin‐dysregulated (triangle, MID, n = 6), and severely insulin‐dysregulated (square, SID, n = 5) ponies during 3 consecutive days of grazing. Grazing ceased at the 4‐hour time point. Note on day 3, not all ponies were sampled (NIR, n = 3; MID, n = 6; SID, n = 3)
The median (IQR) Cmax insulin was higher in the MID (72.5 [129] μIU/mL) and SID (255 [338.5] μIU/mL) groups, compared to the NIR (11.7 [24.9] μIU/mL) group (P < .03), whereas the SID and MID groups did not differ (P = .06) (Figure 2). The Cmax insulin for all ponies was lower (P = .03) on day 1, compared to days 2 and 3 (Figure 2). The AUCinsulin was different across the pony groups (NIR < MID < SID, P = .03) and increased from day 1 to days 2 and 3 (P = .03). The Tmax insulin for the cohort occurred most often between 2 and 4 hours (Figure 2).
3.3.2. Incretins
The Cmax aGLP‐1 and AUCaGLP‐1 did not differ among pony groups or days (Figure 3). The frequency of the Tmax aGLP‐1 for the cohort was the same across 1, 2, and 4 hours. The Tmax aGLP‐1 was not different among ponies of different metabolic status. However, the Tmax aGLP‐1 on day 1 was later than on day 2 (Figure 3).
Figure 3.
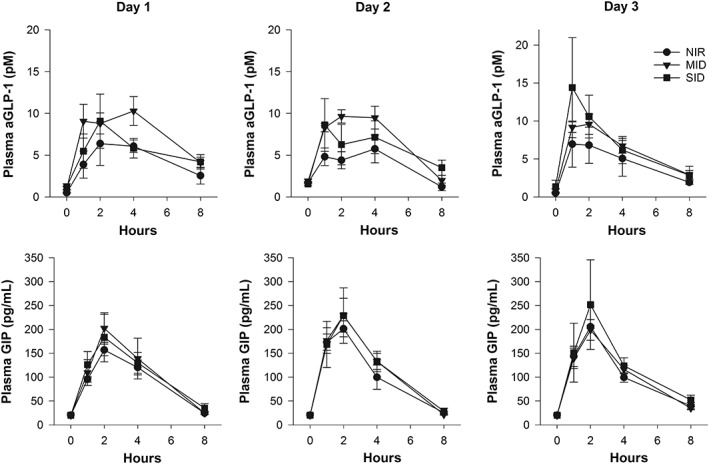
The mean ± standard error of the mean plasma active glucagon‐like peptide‐1 and serum glucose‐dependent insulinotropic peptide concentrations in normally insulin‐regulated (circle, NIR, n = 5), moderately insulin‐dysregulated (triangle, MID, n = 6), and severely insulin‐dysregulated (square, SID, n = 5) ponies during 3 consecutive days of grazing. Grazing ceased at the 4‐hour time point. Note on day 3, not all ponies were sampled (NIR, n = 3; MID, n = 6; SID, n = 3)
The Cmax GIP did not differ among the pony groups, although GIP concentrations were lower on day 3 compared to day 2 (P = .01; Figure 3). The AUCGIP also was not different among the pony groups but did increase on day 2, compared to day 1 (P = .05). The Tmax GIP for the cohort usually was reached at the 2‐hour time point.
3.3.3. Relationships among the variables
For the entire cohort, blood glucose concentration (AUCglucose) was positively correlated (r2 = 0.56, P = .02) with serum insulin concentration (AUCinsulin) when grazing (day 2 data shown; Figure 4A). In addition, both plasma aGLP‐1 (AUCaGLP‐1) and plasma GIP (AUCGIP) were weakly positively correlated (r2 = 0.51, P = .04 and r2 = 0.5, P = .05, respectively) with mean AUCinsulin (day 2 data shown; Figure 4B,C).
Figure 4.
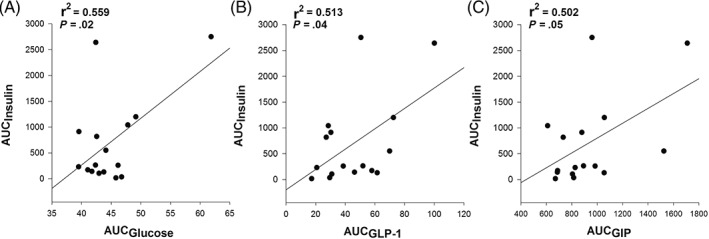
The serum insulin responses (area under the curve [AUC]) over 4 hours of grazing in 16 ponies correlated with their blood glucose (A), plasma active glucagon‐like peptide‐1 (B), and plasma glucose‐dependent insulinotropic peptide (C) responses over the same period
When the hormone data from day 2 were examined using a generalized linear model, the AUCs for glucose, aGLP‐1, and GIP explained 46% of the variation in AUCinsulin (P = .02) in the following proportions: the AUCglucose predicted 85% of the variation in AUCinsulin (P = .02), whereas the AUCs for aGLP‐1 and GIP explained 4.9% and 10.1% of the variation, respectively (P > .05).
3.4. OGT predicts insulin response to grazing
Blood glucose concentration in the postprandial OGT sample was not correlated with the concentration after 2 hours of grazing pasture (day 1: r2 = 0.19, P = .48; day 2: r2 = 0.1, P = .71; day 3: r2 = 0.52, P = .09). However, postprandial insulin responses to the OGT were positively correlated with the insulin response after grazing for 2 hours, on each day of grazing (Figure 5). Of the incretin hormones, postprandial plasma aGLP‐1 concentration after the OGT was correlated with plasma aGLP‐1 after 2 hours of grazing on days 2 (r2 = 0.52, P = .04) and 3 (r2 = 0.58, P = .05) but not on day 1 (r2 = 0.26, P = .34), whereas GIP concentrations of the 2 diets did not correlate with each other on any day (day 1: r2 = 0.35, P = .18; day 2: r2 = 0.22, P = .42; and day 3: r2 = 0.57, P = .06).
Figure 5.
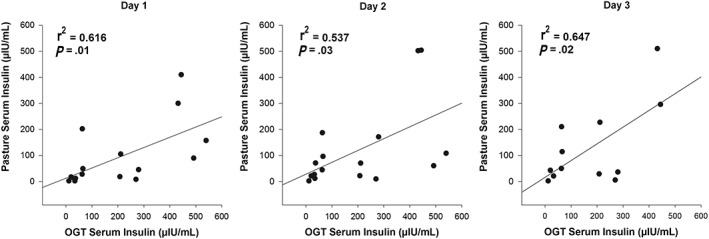
The postprandial serum insulin concentration 2 hours after an oral glucose test was positively correlated with the postprandial serum insulin concentration after 2 hours grazing on 3 consecutive days of grazing in 16 ponies
4. DISCUSSION
Ours is the 1st study to examine the insulin and incretin responses in both insulin‐dysregulated and healthy ponies while grazing pasture. It adds to the small amount of data currently available on the metabolic responses to grazing in horses and ponies.19, 20 Given that pasture often forms the major component of the forage diet of horses and ponies,21, 22 the current findings may be relevant to horse owners. Horses and ponies with ID are at an increased risk of developing laminitis.1 Furthermore, endocrinopathic laminitis can occur soon after new or increased access to pasture.23 As such, our data may be useful when determining whether pasture access is appropriate for ponies at risk of ID and laminitis.
The principal outcome of our study was the finding that ponies with ID had greater postprandial insulin responses (peaking at 72.5‐255 μIU/mL) compared to healthy ponies (peaking at 11.7 μIU/mL) while grazing the same pasture. Although not surprising, this finding is important because it aligns with previous reports indicating that insulin‐dysregulated ponies have disproportionately increased insulinemic responses to both cereal‐based meals and simple sugars, compared to healthy animals.2, 3 This finding is also crucial because hyperinsulinemia is known to be a risk factor for laminitis, and hyperinsulinemia during grazing may increase the risk of an insulin‐dysregulated horse or pony developing laminitis.7, 24, 25 An insulin concentration of approximately 200 μIU/mL may represent the threshold for the onset of lamellar damage.7, 26 If this is a true threshold, the current data would suggest that some ponies in the SID group in particular were at risk of developing lamellar pathology while grazing, and this may precede the development of acute laminitis if hyperinsulinemia persists.27, 28
Our study also showed that the insulin response to grazing pasture was positively correlated with the OGT, with the OGT overestimating the insulin response to grazing pasture. Previously, the OGT has been shown to be predictive for laminitis when ponies were fed a diet high in NSC.7 Thus, the OGT is helpful, but not definitive, for predicting the insulin response to grazing. This knowledge should improve the ability of clinicians to determine the likely risk of laminitis and whether grazing can be appropriately managed in their patients. Limiting pasture access is recommended when managing horses with ID,29 and this recommendation can now be made with the understanding that the insulin response to an OGT likely will not only reflect an individual's degree of pasture‐associated hyperinsulinemia but also reflect the risk of endocrinopathic laminitis triggered by pasture intake.
The increases in glucose, insulin, and GIP concentrations from day 1 to day 2 reflected the intake measurements in all the pony groups. However, we were unable to determine whether the increases in glucose, insulin, and GIP concentrations that occurred on day 2 were in fact because of the increase in pasture consumption or secondary to an increase in intestinal glucose absorption.2 It is unknown why intake was lower on the 1st day of grazing, although the difference could simply be behavioral. The ponies seemed to be excited and were highly active on the 1st day at pasture, after release from 10 days in the smaller dry yards. By day 2, all ponies were more acclimated to their new surroundings and settled earlier. This behavioral hypothesis is further supported by the fact that no difference was recorded in intake between days 2 and 3. Therefore, it is likely that the time actively spent grazing within the 4 hours period impacted NSC intake and therefore the hormonal responses. The variability in pasture intake could have been associated with multiple factors such as individual pony behavior, appetite, and health. We also acknowledge that the intake measures were estimates only, and methodology, sampling errors, or both, could have impacted intake.
The limited time that the ponies were able to graze may have contributed to the reasonably uniform estimates of intake recorded across the cohort. If the ponies had been allowed to graze for 24 h/d, a loading effect may have occurred, where the rate of intake may have become more variable, and possibly decreased, over time. Restricting grazing time has been shown to decrease the total pasture DM intake.30 However, in another study, the rate of intake increased when grazing time was restricted, indicating that grazing is more intense earlier in the grazing period.30 Grazing intake in that study was estimated as 5.88 g/kg BW over a 3‐hour grazing period,30 which is comparable to the median intake of 8.83 g/kg BW over the 4‐hour grazing period in our study.
The incretin hormones did not contribute significantly to the variability seen in insulin concentrations during grazing. The finding that glucose was the most important contributor to postprandial insulin variation, and that it was correlated with insulin secretion, is consistent with a previous report.2 This finding also aligns with the fact that both glucose and insulin concentrations peaked at the same time, whereas aGLP‐1 and GIP generally reached their maximum concentrations earlier. Previously, aGLP‐1 has been shown to be a significant factor in insulin variability in response to dextrose; however, it appeared to play a minor role in our study.2 Regardless, peak aGLP‐1 concentrations measured in our study were similar to those reported previously in ponies.2, 3 Furthermore, although aGLP‐1 was correlated with insulin secretion, it did not differ according to the metabolic status of the ponies in our study, which is inconsistent with previous reports showing that aGLP‐1 played a partial role in postprandial insulin secretion.2, 3 These inconsistencies could be a result of differences in the rate or amount of sugar consumed, or both, or the composition of study diets, breed, β‐cell function, or individual animal variability and are worthy of further investigation. In particular, ID appears to occur more frequently in certain horse and pony breeds, and a genetic basis for ID may exist, which may affect incretin action.1 In humans with different degrees of glucose tolerance (ranging from normal glucose tolerance to impaired glucose tolerance and type 2 diabetes mellitus), GLP‐1 and GIP did not differ among individuals, indicating little involvement in impaired insulin secretion.31 A similar result also was found in a study comparing horses with normal insulin regulation and insulin‐dysregulated horses, where aGLP‐1 concentrations did not differ between animals.32 The importance of incretin hormones in ID in horses continues to be studied, and although our study has contributed, additional data are required to better understand the complexity of the enteroinsular axis in horses.
In conclusion, we successfully addressed our 3 aims by describing the insulin and incretin responses to grazing in ponies across successive days, showing that the insulinemic responses to pasture grazing differed from the severity of ID in ponies, and that this response correlated with the response to an OGT. Although the concentrations of both incretin hormones increased in response to grazing, their secretion did neither relate to the severity of ID nor did they appear to be major drivers of postprandial hyperinsulinemia. Lastly, we found that supraphysiological insulin secretion in response to pasture grazing can occur within a 4‐hour period in insulin‐dysregulated ponies, suggesting that pasture access should be carefully managed in these animals to decrease laminitis risk. Our data also suggest that the OGT may be helpful for estimating an individual animal's early insulin response to pasture grazing.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The animal work was approved by the Animal Ethics Committee of The University of Queensland (QUT/SVS/316/16) and Queensland University of Technology (1600000825).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank Brian Hampson, Alexandra Meier, Michelle Reynolds, and Poppy Sibthorpe for their technical assistance. Presented as an abstract at the 2018 15th World Equine Veterinary Association Congress, Beijing, China. The work was performed in Brisbane, QLD, Australia.
Fitzgerald DM, Walsh DM, Sillence MN, Pollitt CC, de Laat MA. Insulin and incretin responses to grazing in insulin‐dysregulated and healthy ponies. J Vet Intern Med. 2019;33:225–232. 10.1111/jvim.15363
REFERENCES
- 1. Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46:103‐112. [DOI] [PubMed] [Google Scholar]
- 2. de Laat MA, McGree JM, Sillence MN. Equine hyperinsulinemia: investigation of the enteroinsular axis during insulin dysregulation. Am J Physiol Endocrinol Metab. 2016;310:E61‐E72. [DOI] [PubMed] [Google Scholar]
- 3. Bamford NJ, Baskerville CL, Harris PA, et al. Postprandial glucose, insulin, and glucagon‐like peptide‐1 responses of different equine breeds adapted to meals containing micronized maize. J Anim Sci. 2015;93:3377‐3383. [DOI] [PubMed] [Google Scholar]
- 4. Chameroy KA, Frank N, Elliott SB, et al. Comparison of plasma active glucagon‐like peptide 1 concentrations in normal horses and those with equine metabolic syndrome and in horses placed on a high‐grain diet. J Equine Vet. 2016;40:16‐25. [Google Scholar]
- 5. Borer KE, Bailey SR, Menzies‐Gow NJ, et al. Effect of feeding glucose, fructose, and inulin on blood glucose and insulin concentrations in normal ponies and those predisposed to laminitis1. J Anim Sci. 2012;90:3003‐3011. [DOI] [PubMed] [Google Scholar]
- 6. Bertin FR, de Laat MA. The diagnosis of equine insulin dysregulation. Equine Vet J. 2017;49:570‐576. [DOI] [PubMed] [Google Scholar]
- 7. Meier AD, de Laat MA, Reiche DB, et al. The oral glucose test predicts laminitis risk in ponies fed a diet high in nonstructural carbohydrates. Domest Anim Endocrinol. 2018;63:1‐9. [DOI] [PubMed] [Google Scholar]
- 8. de Laat MA, McGowan CM, Sillence MN, et al. Equine laminitis: induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet J. 2010;42:129‐135. [DOI] [PubMed] [Google Scholar]
- 9. Asplin KE, Sillence MN, Pollitt CC, et al. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet J. 2007;174:530‐535. [DOI] [PubMed] [Google Scholar]
- 10. de Laat MA, Sillence MN. The repeatability of an oral glucose test in ponies. Equine Vet J. 2016;49:238‐243. [DOI] [PubMed] [Google Scholar]
- 11. Henneke DR, Potter GD, Kreider JL, et al. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet J. 1983;15:371‐372. [DOI] [PubMed] [Google Scholar]
- 12. Carter RA, Geor RJ, Burton Staniar W, et al. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet J. 2009;179:204‐210. [DOI] [PubMed] [Google Scholar]
- 13. Carslake HB, Pinchbeck GL, McGowan CM. Evaluation of a chemiluminescent immunoassay for measurement of equine insulin. J Vet Intern Med. 2017;31:568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Copas VE, Durham AE. Circannual variation in plasma adrenocorticotropic hormone concentrations in the UK in normal horses and ponies, and those with pituitary pars intermedia dysfunction. Equine Vet J. 2012;44:440‐443. [DOI] [PubMed] [Google Scholar]
- 15. Grace ND, Gee EK, Firth EC, et al. Digestible energy intake, dry matter digestibility and mineral status of grazing New Zealand Thoroughbred yearlings. N Z Vet J. 2002;50:63‐69. [DOI] [PubMed] [Google Scholar]
- 16. Ince J, Longland AC, Newbold JC, et al. Changes in proportions of dry matter intakes by ponies with access to pasture and haylage for 3 and 20 hours per day respectively, for six weeks. J Equine Vet. 2011;31:283. [Google Scholar]
- 17. Schuver A, Frank N, Chameroy KA, et al. Assessment of insulin and glucose dynamics by using an oral sugar test in horses. J Equine Vet. 2014;34:465‐470. [Google Scholar]
- 18. Secombe CJ, Tan RHH, Perara DI, et al. The effect of geographic location on circannual adrenocorticotropic hormone plasma concentrations in horses in Australia. J Vet Intern Med. 2017;31:1533‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrison R, Murray JMD. A preliminary study of grazing intakes of ponies with and without a history of laminitis. Livest Sci. 2016;186:2‐5. [Google Scholar]
- 20. Frank N, Elliott SB, Chameroy KA, et al. Association of season and pasture grazing with blood hormone and metabolite concentrations in horses with presumed pituitary pars intermedia dysfunction. J Vet Intern Med. 2010;24:1167‐1175. [DOI] [PubMed] [Google Scholar]
- 21. Council NR . Nutrient Requirements of Horses. Vol 360 6th revised ed. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 22. Wylie CE, Ireland JL, Collins SN, et al. Demographics and management practices of horses and ponies in Great Britain: a cross‐sectional study. Res Vet Sci. 2013;95:410‐417. [DOI] [PubMed] [Google Scholar]
- 23. Luthersson N, Mannfalk M, Parkin TDH, et al. Laminitis: risk factors and outcome in a group of Danish horses. J Equine Vet. 2017;53:68‐73. [Google Scholar]
- 24. Treiber KH, Kronfeld DS, Hess TM, et al. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture‐associated laminitis in ponies. J Am Vet Med Assoc. 2006;228:1538‐1545. [DOI] [PubMed] [Google Scholar]
- 25. Menzies‐Gow NJ, Harris PA, Elliott J. Prospective cohort study evaluating risk factors for the development of pasture‐associated laminitis in the United Kingdom. Equine Vet J. 2017;49:300‐306. [DOI] [PubMed] [Google Scholar]
- 26. de Laat MA, Sillence MN, Mc Gowan CM, et al. Continuous intravenous infusion of glucose induces endogenous hyperinsulinaemia and lamellar histopathology in Standardbred horses. Vet J. 2012;191:317‐322. [DOI] [PubMed] [Google Scholar]
- 27. Patterson‐Kane JC, Karikoski NP, McGowan CM. Paradigm shifts in understanding equine laminitis. Vet J. 2018;231:33‐40. [DOI] [PubMed] [Google Scholar]
- 28. Karikoski NP, Patterson‐Kane JC, Asplin KE, et al. Morphological and cellular changes in secondary epidermal laminae of horses with insulin‐induced laminitis. Am J Vet Res. 2014;75:161‐168. [DOI] [PubMed] [Google Scholar]
- 29. Frank N, Geor R. Current best practice in clinical management of equine endocrine patients. Equine Vet Educ. 2014;26:6‐9. [Google Scholar]
- 30. Glunk EC, Pratt‐Phillips SE, Siciliano PD. Effect of restricted pasture access on pasture dry matter intake rate, dietary energy intake, and fecal pH in horses. J Equine Vet. 2013;33:421‐426. [Google Scholar]
- 31. Yabe D, Kuroe A, Watanabe K, et al. Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J Diabetes Complications. 2015;29:413‐421. [DOI] [PubMed] [Google Scholar]
- 32. Chameroy K, Frank N, Schuver A. Plasma glucagon‐like peptide 1 (GLP‐1) concentrations in response to an oral sugar test in healthy and insulin‐resistant horses. J Vet Intern Med. 2010;24:780. [Google Scholar]


