Abstract
The aim of this work was to evaluate the inhibitory activities of different extracts of Asparagus racemosus Willd on α - amylase and α - glucosidase at varying concentrations. Diabetes mellitus is a clinical condition characterized by hyperglycaemia in which an elevated amount of glucose circulates in the blood plasma. α - amylase and α - glucosidase inhibitors are used to achieve greater control over hyperglycemia in type 2 diabetes mellitus. This study is to treat the diabetes using natural resources. We aimed to evaluate of Asparagus racemosus Willd by digestive enzymes inhibitory activity. n-hexane, chloroform, ethyl acetate, methanol, and aqueous was used to extract the root of the Asparagus racemosus Willd. The different extracts were then used to study its digestives enzymes activity α-amylase and α - glucosidase inhibitory activity. The significant inhibitory effect of α-amylase and α - glucosidase enzyme and exhibited lower inhibitory activity than acarbose was extracted by the ethyl acetate and aqueous extracts of the plant. Flavonoids, Tannins and phenolic, Saponins, Amino acids, Protein are the major phytochemical constituents present. The total flavonoid content plant extracts of ethyl acetate and aqueous showed dose dependent 23.45 ± 1.33 mg rutin equivalent/g and 25.81 ± 0.82 mg rutin equivalent/g respectively. The total triterpenoids content plant extracts of ethyl acetate, aqueous showed dose dependent 109.8 ± 5.6 mg ursolic acid/g and 95.6 ± 7.5 mg ursolic acid/g respectively. The antidiabetic potential and to develop medicinal preparations and nutraceuticals and function foods for diabetes has revealed.
Keywords: Asparagus racemosus Willd, Diabetes mellitus, α-amylase, α-glucosidase
1. Introduction
Diabetes mellitus (DM) is a chronic disease caused by inherited or acquired deficiency in insulin secretion and by decreased responsiveness of the organs to the secreted insulin. Such a deficiency results in increased blood glucose level, which in turn can damage many of the body's systems, including blood vessels and nerves.1 One of the therapeutic approaches is to decrease the postprandial hyperglycemia, by retarding the absorption of glucose by inhibition of carbohydrate-hydrolyzing enzymes, such as α-amylase and α-glucosidase.2 From this point of view, many efforts have been made to search for more effective and safe inhibitors of α-glucosidase and α-amylase from natural materials to develop physiological functional foods to treat diabetes.3 Many traditional plants have been reported in India for diabetes, but only a small number of these have received scientific and medical evaluation to assess their efficacy. On the basis of ethno medical/tribal information Asparagus racemosus Willd has been used to treat and prevent diabetes. Asparagus racemosus Willd possess a diverse number of pharmacological activities including antioxidant and free radical scavenging activity,4, 5, 6 anticholinesterase action7, 8 and anti-inflammatory property. However, the studies on anti-diabetic effects of Asparagus racemosus Willd extracts were not focused on the enzyme inhibitory activity thus, The present study is designed evaluate the in-vitro antidiabetic activity of extract of Asparagus racemosus Willd extracts and to understand how the extract acts against α-glucosidase and α-amylase enzymes.
2. Materials and methods
2.1. Identification of plant materials
The root plant of Asparagus racemosus Willd Family Asparagaceae was collected from the forests of Doddabetta in Nilgiris. The plant species was identified and authenticated by the Dr.S. Rajan, PhD Field Botanist Survey of Medicinal Plants and Collection Unit. Department of AYUSH Ministry of Health and Family Welfare, Govt of India. The plant was deposited in the herbarium of the Department of Pharmacognosy, JSS College of Pharmacy, Ooty. The leaves of the plant were used in study.
2.2. Chemicals
α-amylase from starch potato soluble, α-glucosidase from one gram of rat-intestinal acetone powder, p-nitrophenyl-a-d-glucopyranoside and dinitrosalicylic acid were purchased from Sigma chemicals. All the chemicals used in the study are of analytical grade.
2.3. Preparation of crude extract
The leaves of the plant were sun dried and ground to a coarse powder and stored in an air tight container. This coarse powder was subjected to successive extraction with n-hexane (68 °C), chloroform (61 °C), ethyl acetate (77 °C), and methanol (64 °C) by continuous soxhlation and aqueous extracts by maceration process. After collection of extracts, it is kept at temperature 37 °C until solvent is completely evaporated. Then finally dried in desiccator.
2.4. Qualitative phytochemical analysis
The plant extracts of Asparagus racemosus Willd in n-hexane, chloroform, ethyl acetate, methanol and aqueous was analyzed for the presence of amino acids, steroids, cardiac glycosides, phenols, tannins, terpenoids, alkaloids, flavonoids, saponins, carbohydrates, reducing sugar.
2.5. Evaluation of bioactive constituents
2.5.1. Total flavonoid content
The total flavonoid content of the n-hexane, chloroform, ethylacetate, methanol and aqueous extracts of Asparagus racemosus Willd was determined by aluminium chloride colorimetric method. In brief 50 μL of the n-hexane, chloroform, ethylacetate, methanol and aqueous extracts of Asparagus racemosus Willd (1mg//ml ethanol) were made up to 1 ml with methanol mixed with 4 ml of distilled water and then 0.3 ml of 5% sodium nitrite solution.9 0.3 ml of 10% aluminium chloride solution was added after 5min of incubation and the mixture was allowed to stand for 6min. Then 2 ml of 1 mol/L sodium hydroxide solution were added and the final volume of the mixture was brought to 10 ml with double-distilled water. The mixture was allowed to stand for 15min and absorbance was measured at 510 nm. The total flavonoid content was calculated from a calibration curve. The result was expressed as mg rutin equivalent per g dry weight.
2.5.2. Total triterpenoid content
The content of n-hexane, chloroform, ethylacetate, methenol and aqueous extracts of Asparagus racemosus Willd obtained by the aforementioned method was determined according to Lu et al with a slight modification and then expressed as milligram ursolic acid equivalent/gram dry weight. Briefly after a 200-μl n-hexane, chloroform, ethylacetate, methanol and aqueous extracts of Asparagus racemosus Willd solution in a 10 ml volumetric flask was heated to evaporation in a water-bath 1 ml new mixed 5%w/v vanillin acetic solution and 1.8 ml sulfuric acid were added mixed and incubated at 70 °C for 30min.10Then the mixed solution was cooled and diluted to 10 ml with acetic acid. The absorbance was measured at 573 nm against blank using a spectrophotometer. The blank consisted of all reagents and solvents without sample solution. The content was determined using the standard ursolic acid calibration curve.
2.6. α-amylase inhibition activity
The α -amylase solution (Prepare 25 ml in Reagent 7.3.1 using Starch Potato Soluble. Facilitate solubilization by heating the starch solution in a glass beaker directly on a heating/stir plate using constant stirring. Bring to boil and maintain the solution at this temperature for 15 min). Inhibitory activity of the n-hexane, chloroform, ethyl acetate, methanol and aqueous extracts of Asparagus racemosus Willd were determined.11 Dry reside was dissolved in 1 ml of tris buffer solution (pH 9.1) 2 ml of 0.1 mol/L an ammonia solution and 2 ml n-hexane. A total of 250 μL of sample ana 125 μL of 0.02 M sodium phosphate buffer (pH 6.9 with 6 mM sodium chloride) containing α-amylase solution (0.5 mg//mL) was incubated at 25 °C for 10 min. After preincubation, 250 μL of 1% starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 6 mM sodium chloride) was added to each tube at timed intervals. The reaction mixtures were then incubated at 25 °C for 10 min. The reaction was stopped with 0.5 mL of dinitrosalicylic acid color reagent. The test tube was then incubated in a boiling water bath for 5 min and cooled to room temperature. The reaction mixture was then diluted by adding 5 mL of distilled water and absorbance was measured at 540 nm. Acarbose was used as the positive control. The α-amylase inhibitory activity was calculated as follow;
| Inhibition (%) = (1- As / Ac) × 100 |
where As and Ac are the absorbance of the sample and the control respectively.
2.7. α-glucosidase inhibitory activity
The rat intestinal a-glucosidase assay was based on the method of Kwon et al.,12 with slight modifications. One gram of rat-intestinal acetone powder was suspended in 3 mL of 0.9 % saline. The suspension was sonicated 12 times for 30 s at 4 °C.After centrifugation (10,000 g, 30 min, 4 °C), the resulting supernatant was used for the assay.13 Fifty microliters of sample solution were pre-incubated with 100 lL of rat intestinal a-glucosidase solution at 37 °C for 15 min. The α-glucosidase inhibitory activity of the n-hexane,chloroform, ethylacetate, methanol and aqueous extracts of Asparagus racemosus Willd were determined. Dry reside was redissolved in 1 ml of tris buffer solution (pH 9.1) 2 ml of 0.1 mol/L an ammonia solution and 2 ml n-hexane. A mixture of 50 μL of sample and 100 μL of 0.1 M phosphate buffer (pH 6.9) contining α-glucosidase solution (1 U//mL) was incubated in 96 well plares at 25 °C for 10 min. After preincubation, 50 μL 0f 5 mM pNPG solution in 0.1 M phosphate buffer (pH 6.9) was added to each well at timed intervals. The reaction mixture was incubated at 25 °C for 5 min. Before and after incubation, absorbance was recorded at 405 nm by micro plate reader. Acarbose was used the positive control. The α-glucosidase inhibitory activity was expressed as percentage inhibition percent and was calculated as follows;
| Inhibition (%) = (1- As / Ac) × 100 |
where As and Ac are the absorbance of the sample and the control respectively.
3. Results and discussion
3.1. Phytochemical analysis
The n-hexane, chloroform, ethyl acetate, methanol, and aqueous extracts of. Asparagus racemosus Willd showed the presence of major phytochemical constituents such as Flavonoids, Tannins and phenolic, Saponins, Amino acids, Protein (Table 1).
Table 1.
Phytochemical analysis.
| S.NO | Phytoconstituents | Extracts of Asparagus racemosus Willd |
||||
|---|---|---|---|---|---|---|
| n –hexane | Chloroform | Ethyl acetate | methanol | aqueous | ||
| 1 | Alkaloids | – | + | – | + | – |
| 2 | Protein | – | – | + | + | – |
| 3 | Flavonoids | + | + | – | + | – |
| 4 | Glycosides | – | – | – | + | – |
| 5 | Tannins and phenolic | – | + | + | + | – |
| 6 | Saponins | – | + | + | + | + |
| 7 | Steroids | – | + | – | – | – |
| 8 | Amino acids | – | + | + | + | – |
| 9 | Carbohydrate | – | – | – | + | + |
| 10 | Fat and oils | + | – | – | – | + |
+: Present -: Absent.
3.2. Total flavonoid and triterpenoids content
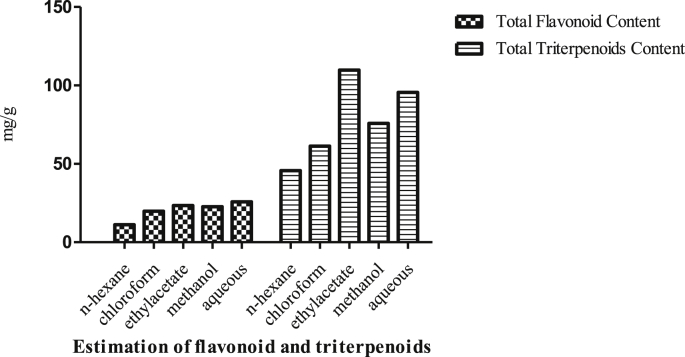
The total flavonoid of n-hexane, chloroform, ethyl acetate, methanol, and aqueous extracts of. Asparagus racemosus Willd are given in Table 2.The plant extracts (ethyl acetate, aqueous) showed dose dependent 23.45 ± 1.33 mg/g and 25.81 ± 0.82 mg/g respectively. The total triterpenoids of n-hexane, chloroform, ethyl acetate, methanol, and aqueous extracts of. Asparagus racemosus Willd are given in Table 2 and Fig. 1. The plant extracts (ethyl acetate, aqueous) showed dose dependent 109.8 ± 5.6 mg/g and 95.6 ± 7.5 mg/g respectively.
Table 2.
Total flavonoid and triterpenoids content.
| S.No | Extracts | Total Flavonoid Content (mg rutin equivalent/g)a |
Total Triterpenoids Content (mg ursolic acid/g)b |
|---|---|---|---|
| 1 | n – hexane | 11.20 ± 2.32 | 45.7 ± 1.4 |
| 2 | Chloroform | 19.87 ± 0.67 | 61.3 ± 3.8 |
| 3 | Ethyl acetate | 23.45 ± 1.33 | 109.8 ± 5.6 |
| 4 | Methanol | 22.67 ± 2.72 | 75.8 ± 6.5 |
| 5 | Aqueous | 25.81 ± 0.82 | 95.6 ± 7.5 |
a,bAverage of three determinations, mean ± SEM.
Fig. 1.
Estimation of flavonoid and triterpenoids.
3.3. Inhibition of α-amylase and α-glucosidase
The α-amylase inhibition of n-hexane, chloroform, ethyl acetate, methanol, and aqueous extracts of. Asparagus racemosus Willd are given in Table 3. The plant extracts (ethyl acetate, aqueous) showed dose dependent α-amylase inhibition with IC50 value 65.85 ± 1.19 and IC50 value 63.28 ± 0.38 respectively. The IC50 value of Acarbose was found to be 11.84 ± 0.19. The α-glucosidase inhibition of n-hexane, chloroform, ethyl acetate, methanol, and aqueous extracts of. Asparagus racemosus Willd are given in Table 3. The plant extracts (ethyl acetate, aqueous) showed dose dependent α-glucosidase inhibition with IC50 value 68.32 ± 1.02 and IC50 value 62.65 ± 2.61 respectively. The IC50 value of Acarbose was found to be 13.39 ± 0.11.
Table 3.
Inhibitory activity of different extracts of Asparagus racemosus Willd.
| Groups | Treatment | α-amylase (IC50) mg/mL | α-glucosidase (IC50) mg/mL |
|---|---|---|---|
| I | n – hexane | 35.52 ± 0.66 | 37.92 ± 1.73 |
| II | Chloroform | 45.13 ± 0.67 | 49.16 ± 1.10 |
| III | Ethyl acetate | 65.85 ± 1.19 | 68.32 ± 1.02 |
| IV | Methanol | 55.52 ± 1.21 | 56.88 ± 0.68 |
| V | Aqueous | 63.28 ± 0.38 | 62.65 ± 2.61 |
| VI | Acarbose | 11.84 ± 0.19 | 13.39 ± 0.11 |
Value are expressed as mean ± SEM, n = 5.
To maintain glycemic level in control, in both the fasting and post-prandial states is the treatment goal of diabetes patients. The suppression of glucose production from carbohydrates in the gut or glucose absorption from the intestine have been investigated using natural resources.14
Pancreatic α-amylase is a key enzyme in the digestive system which catalyzes the initial step in the hydrolysis of starch, which is a principal source of glucose in the diet. α-glucosidase, a key enzyme for carbohydrate digestion, has been recognized as a therapeutic target for the modulation of postprandial hyperglycemia, which is the earliest metabolic abnormality to occur in type 2 Diabetes mellitus. α-amylase catalyzes the hydrolysis of α-1, 4-glucosidic linkages of starch, glycogen and various oligosaccharides and α –glucosidase further breaks down the disaccharides into simpler sugars, readily available for the intestinal absorption. The inhibition of their activity, in the digestive tract of humans, is considered to be effective to control diabetes by diminishing the absorption of glucose decomposed from starch by these enzymes.15 Rat-derived a-glucosidase in the initial screening step of this study, because this stage is the most important for ensuring that the medicinal plants we chose had inhibitory activities against rat a-glucosidase (which is similar to human a-glucosidase) and could thus be used in the treatment of type 2 diabetes.16, 17, 18 Therefore, effective and nontoxic inhibitors of α -amylase and α -glucosidase have long been sought.
We have investigated that the Asparagus racemosus Willd which is used in traditional ayurvedic medicine for the treatment of several diseases, has been serving as anti-diabetic potential. Previously, this beneficial and priceless herb was not been investigated for its in vitro anti-diabetic activity. This herb has clearly recognized the potential of anti-diabetic activity and come out with the active principles responsible may be total flavonoids and terpenes content compound.
Flavonoids, like anti-oxidants, may prevent the progressive impairment of pancreatic beta-cell function due to oxidative stress and may thus reduce the occurrence of type 2 diabetes. Although, in the present study, the enzyme inhibitory activity of these extract and fractions were assayed in-vitro, the results from this work should be relevant to the human body. In addition to α-amylase and α-glucosidase inhibitory activities, these phytoconstituents are also reported to have several other biological activities including anti-bacterial, anti-oxidative,anti-cancer etc.19This supportive evidence further increases the medicinal importance of this Asparagus racemosus Willd indicating that this herb is not only beneficial for diabetes but also may be useful to a number of other human health complications.
4. Conclusion
The plant extract of the Asparagus racemosus Willd, has been used for food and medicinal purposes. Our result suggest the inhibitory effects on α-amylase and α-glucosidase and contains a high amount of phytochemical constituents (i.e., total flavonoids and triterpenoids content) Thus, the plant extract of the Asparagus racemosus Willd may be used in the management of type 2 diabetes mellitus with few or no side effects. However, Further studies of the plant extract of the Asparagus racemosus Willd using in vitro and in vivo models need to be performed to elucidate its insulin mimetic activity and reduction of insulin resistance and also to develop medicinal preparations, nutraceuticals or functional foods for diabetes and related symptoms.
Acknowledgements
The authors are grateful to the principal and management, JSS College of Pharmacy,(Jagadguru Sri Shivarathreeshwara University, Mysuru) Udhagamandalam, Nilgiris, Tamilnadu, – 643001, India for providing the necessary infrastructure to carry out this research work in successful manner.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Matsui T., Tanaka T., Tamura Toshima A., Miyata Y., Tanaka K. Alpha-glucosidase inhibitory profile of catechins and theaflavins. J Agric Food Chem. 2007;55:99–105. doi: 10.1021/jf0627672. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari M.R., Nilubon J.A., Gao, Kawabata J. α -Glucosidase and α amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergeniaciliata Haw.) Food Chem. 2008;106:247–252. [Google Scholar]
- 3.Kao Y.H., Chang H.H., Lee M.J., Cheng C.L. Tea, obesity and diabetes. Mol Nutr Food Res. 2006;50(2):188–210. doi: 10.1002/mnfr.200500109. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A., Ram J., Samarth R.M., Kumar M. Modulatory influence of Adhatoda vasica Nees leaf extract against gamma irradiation in Swiss albino mice. Phytomedicine. 2005;12:285–293. doi: 10.1016/j.phymed.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Samarth R.M., Panwar M., Kumar M., Soni A., Kumar M., Kumar A. Evaluation of antioxidant and radical-scavenging activities of certain radioprotective plant extracts. Food Chem. 2008;106:868–873. [Google Scholar]
- 6.Roja G., Vikrant B.H., Sandur S.K., Sharma A., Pushpa K.K. Accumulation of vasicine and vasicinone in tissue cultures of Adhatoda vasica and evaluation of the free radical-scavenging activities of the various crude extracts. Food Chem. 2011;126:1033–1038. [Google Scholar]
- 7.Gao H., Huang Y.N., Gao B., Li P., Inagaki C., Kawabata J. Inhibitory effect on α-glucosidase by Adhatoda vasica nees. Food Chem. 2008;108:965–972. doi: 10.1016/j.foodchem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Lahiri P.K., Pradhan S.N. Pharmacological investigation of vasicinolan alkaloid from Adhatoda vasica Nees. Indian J Exp Biol. 1964;2:219–222. [Google Scholar]
- 9.Babaa Shoib A., Malikb Shahid A. Determination of total phenolic and flavonoid content, antimicrobialand antioxidant activity of a root extract of Arisaema jacquemontiiBlume. J Taibah Univ Sci. 2015:449–454. [Google Scholar]
- 10.Lin Chang Chia, San Lin Che, Lai Guia Hung. Phytochemical characteristics, free radical scavenging activities, and neuroprotection of FiveMedicinal plant extracts. Evidence-BasedComplementaryandAlternativeMedicine. 2012 doi: 10.1155/2012/984295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranilla L.G., Kwon Y.I., Apostolidis E., Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol. 2010;101:4676–4689. doi: 10.1016/j.biortech.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 12.Kwon Y.I., Vattem D.A., Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. J.Asian Pac J Clin Nutr. 2006;15:107–118. [PubMed] [Google Scholar]
- 13.Alagesan Kathirvel. Identification of alpha-glucosidase inhibitors from psidium guajava leaves and syzygium cumini linn. Seed Int J Pharma Sci Res. 2012;2(3):316–322. [Google Scholar]
- 14.Nguyen V.B., Nguyen A.D., Nguyen Q.V., Wang S.L. Porcine pancreatic alpha-amylase inhibitors from Euonymus laxiflorus Champ. Res Chem Intermed. 2017;43:259–269. [Google Scholar]
- 15.Nguyen V.B., Nguyen Q.V., Nguyen A.D., Wang S.L. Screening and evaluation of - glucosidase inhibitors from indigenous medicinal plants in Dak Lak Province. Vietnam Res Chem Intermed. 2016:1–17. [Google Scholar]
- 16.Hara Y., Honda M. The inhibition of α -amylase by tea polyphenols. Agric Biol Chem. 1990;54(8) 1939–45. [Google Scholar]
- 17.Wan S.B., Chen D., Dou Q.P., Chan T.H. Study of the green tea polyphenols catechin-3-gallate (CG) and epicatechin-3- gallate (ECG) as proteaseome inhibitors. Bioorg Med Chem. 2004;12:3521–3527. doi: 10.1016/j.bmc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 18.He Q., Lv Y., Yao K. Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin and lipase. Food Chem. 2006;101:1178–1182. [Google Scholar]
- 19.Rizvi S.I., Anis M.A., Zaid R., Mishra N. Protective role of tea catechins against oxidation-induced damage of type 2 diabetic erythrocytes. Clin Exp Pharmacol Physiol. 2005;32(1–2):70–75. doi: 10.1111/j.1440-1681.2005.04160.x. [DOI] [PubMed] [Google Scholar]