Summary
Tumor-initiating cells (TICs) contribute to drug resistance and tumor recurrence in cancers, thus experimental approaches to dissect the complexity of TICs are required to design successful TIC therapeutic strategies. Here, we show that miRNA-3′ UTR sensor vectors can be used as a pathway-based method to identify, enrich, and analyze TICs from primary solid tumor patient samples. We have found that an miR-181ahigh subpopulation of cells sorted from primary ovarian tumor cells exhibited TIC properties in vivo, were enriched in response to continuous cisplatin treatment, and showed activation of numerous major stem cell regulatory pathways. This miRNA-sensor-based platform enabled high-throughput drug screening leading to identification of BET inhibitors as transcriptional inhibitors of miR-181a. Taken together, we provide a valuable miRNA-sensor-based approach to broaden the understanding of complex TIC regulatory mechanisms in cancers and to identify miRNA-targeting drugs.
Keywords: ovarian cancer, tumor-initiating cells, miRNA sensor, miR-181a, tumor heterogeneity, BET inhibitors
Highlights
-
•
miRNA sensors provide a novel approach to identify TICs in primary human tumors
-
•
miR-181a is a driver of tumor initiation in ovarian cancer
-
•
miRNA sensors allow discovery of miRNA transcriptional regulators and targeting drugs
-
•
BET inhibitors are novel miR-181a-targeting drugs
In this article, DiFeo and colleagues have utilized a miRNA 3′ UTR biosensor to study tumor-initiating cells in ovarian cancer and develop a high-throughput platform to uncover miRNA-targeting drugs. They have identified miR-181a as a potent driver of tumor initiation, which can be regulated by BET inhibitors, thus introducing a previously unknown biomarker for these drugs.
Introduction
Tumor-initiating cells (TICs), or cancer stem cells, are subpopulations of cancer cells that are enriched in stem-like properties and drive tumor recurrence in various cancers (Bonnet and Dick, 1997, Al-Hajj et al., 2003, Kreso and Dick, 2014). Dissecting the complexities underlying TIC function is critical toward developing pharmacological strategies that can eradicate these cells. Several challenges in understanding TIC functions have emerged over the last few years as identified by various TIC studies across cancers, the most important of which in this context are TIC heterogeneity and TIC plasticity (Stewart et al., 2011, Meacham and Morrison, 2013). Enrichment of TICs based on pathway activity is an emerging approach in TIC research that offers a potential platform to study TICs in the context of heterogeneity and uncover therapies that selectively target this population of cells (Vermeulen et al., 2010, Tang et al., 2015). Existence of functional crosstalk between major stem cell regulatory pathways and the need for reliable indicators to determine the activity status of the pathway of interest in TIC clones as assessed in standard readout assays (e.g., flow cytometry/reporter assays) are main barriers in the pathway-based TIC study approach. Hence, identifying molecular entities that can regulate several pathways simultaneously coupled with reliable readout properties can greatly improve the understanding of TIC function in cancers. microRNAs (miRNAs) can be interesting candidates in this context and can offer study approaches that can potentially overcome the barriers in pathway-based TIC research.
miRNAs are small RNA molecules that mainly regulate post-transcriptional gene silencing by binding to the 3′ UTR of their potential targets, resulting in target mRNA degradation or translational repression (Ha and Kim, 2014). A single miRNA can thus affect multiple pathways and, not surprisingly, miRNAs are implicated in regulation of various aspects of tumorigenesis including regulation of TIC properties in many cancers (Yu et al., 2007, Shimono et al., 2009, Yin et al., 2010, Tung et al., 2017, Chen et al., 2017). Tumor subpopulations could be composed of cells with varying degrees of miRNA activity; hence, overexpression or knockdown of miRNAs to the same extent in all these populations may not reflect the true biology of miRNAs in these settings. Since 3′ UTR-driven activity is one of the defining traits of miRNA functions, isolation and characterization of TIC subpopulations in cancers based on miRNA 3′ UTR activity can overcome the barriers associated with pathway-based approaches in TIC research. Isolating target cell populations based on miRNA activity using miRNA switches has been reported in physiological settings (Miki et al., 2015). Studying TICs in hematological malignancies utilizing a miRNA 3′ UTR activity-based approach has also been reported (Lechman et al., 2016). However, the application of miRNA activity-based tools to enrich for TICs in solid tumors or uncover miRNA-targeted drugs has not been explored to date.
Epithelial ovarian cancer (EOC) is the most lethal gynecological malignancy, in which TICs have been shown to be involved in the emergence of chemotherapy resistance and tumor recurrence, and to contribute to poor patient survival (Silva et al., 2011, Garson and Vanderhyden, 2015). One of the striking features of EOC is that even though the disease is heterogeneous in origin, the treatment approach has remained mostly homogeneous to date, i.e., platinum-based therapy has remained the mainstay of EOC treatment in the last few decades and resistance to platinum-based therapies continues to be the major barrier to eradication of this disease (Cunnea and Stronach, 2014). Thus, lack of TIC-targeted therapies may represent one of the main reasons for poor patient survival, and experimental approaches are required to dissect TIC function in order to develop drugs that could eradicate these cells. We have previously identified that high miR-181a expression correlates with poor survival in the high-grade serous ovarian cancer (HGSOC) subtype of EOC and that miR-181a is enriched in recurrent HGSOC tumors (Parikh et al., 2014). This correlation has also been shown in numerous other cancers (Pop-Bica et al., 2018). Most recently a comprehensive TCGA analysis of 12,000 tumor samples from 33 different cancers showed that a high level of miR-181 family members correlated with an mRNA stemness signature (Malta et al., 2018), and miR-181a has been identified as a regulator of TICs in hepatic cancer (Ji et al., 2010), thus suggesting that this miRNA family could be involved in driving TIC properties. In the current study, we have developed a miRNA-sensor-based platform driven by miR-181a 3′ UTR activity to enrich TICs in primary EOC tumors and have identified miR-181a as a TIC therapeutic target. We further utilized the miR-181a sensor as a pharmacological screening platform to identify upstream regulators of miR-181a function, and uncovered that BET inhibitors transcriptionally regulate miR-181a.
Results
miR-181a Induces Stem-like Properties in Non-transformed Fallopian Tube Secretory Epithelial Cells
Deregulation of adult stem cell drivers is one of the traits observed in TICs. Several regulators of TICs identified to date in cancers are in fact the ones that regulate stem-like properties under physiological conditions in their respective tissues (e.g., LGR5) (Barker et al., 2007, Shimokawa et al., 2017). Fallopian tube secretory epithelium (FTSE) has been identified as the origin of HGSOC, which is the most common subtype of EOC (Perets et al., 2013). Existence of a stem cell niche in the fallopian tube suggests a TIC-based origin of HGSOC (Flesken-Nikitin et al., 2013). Hence, identifying regulators of stem-like properties in FTSE cells can lead to characterization of TIC regulators in EOC. Thus, to investigate the potential role of miR-181a in driving TIC properties in EOC, we first looked at the effects of miR-181a upregulation in non-transformed FTSE cells (FTSE shp53-R24C) (Karst et al., 2011). We focused on miR-181a, given that this miR-181 family member is the most highly expressed in HGSOC tumors (Figure S2A).
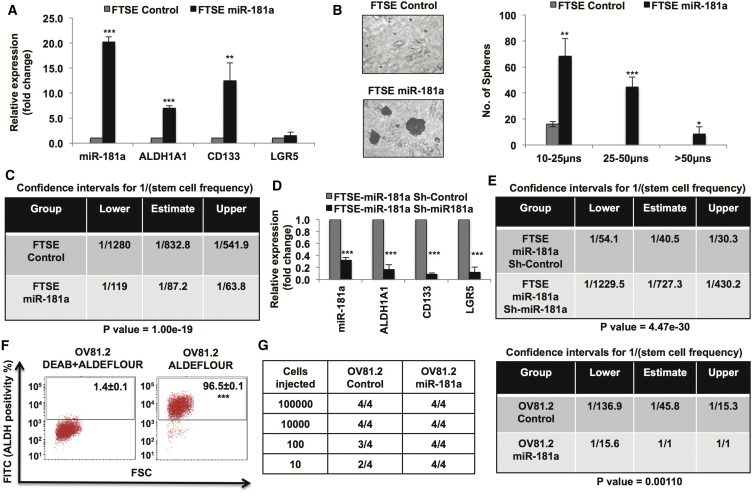
Upon stable miR-181a overexpression in FTSE cells we found increased expression of ALDH1A1 and CD133, established as TIC markers in EOC (Figure 1A) (Choi et al., 2015). We next looked at sphere-initiating cell frequency in miR-181a-FTSE cells by utilizing in vitro limiting dilution tumor sphere-formation assay (Rota et al., 2012). Extreme limiting dilution analysis (ELDA) (Hu and Smyth, 2009) found that miR-181a increased sphere-initiating cell frequency by ∼10-fold (Figures 1B and 1C). Conversely, stable downregulation of miR-181a decreased the expression of TIC markers ALDH1A1, CD133, and LGR5 (Figure 1D), which was associated with an 18-fold decrease in sphere-initiating cell frequency in FTSE-miR-181a cells (Figure 1E), confirming specificity of miR-181a induced stem-like phenotype. Next, to assess whether miR-181a is a critical driver of TIC properties in EOC, we studied the effects of miR-181a overexpression on TIC properties in the OV81.2 primary HGSOC cell line model (Nagaraj et al., 2015a). OV81.2 cells exhibit high ALDH activity and form tumors at low cell numbers; however, tumor-initiation ability was significantly higher in OV81.2-miR-181a overexpressed cells as compared with OV81.2-control cells (∼45-fold increase in tumor-initiating cell frequency, p = 0.001) (Figures 1F and 1G). These results support miR-181a as a regulator of stem-like properties in FTSE cells and primary HGSOC cells and suggest that miR-181a deregulation could underlie TIC function in EOC. Thus, we would predict that ovarian tumor cells with high miR-181a activity could potentially be enriched in TIC properties.
Figure 1.
miR-181a Induces Stem-like Properties in Non-transformed Fallopian Tube Secretory Epithelial Cells
(A) Real-time PCR showing increased expression of stem cell markers in fallopian tube secretory epithelial (FTSE)-miR-181a cells.
(B) 3D-on-Top Matrigel sphere-formation assay. (Left) 5× light microscopy representative images showing increased sphere-formation by miR-181a overexpression (3 weeks) and (right) quantification of sphere size.
(C) In vitro limiting dilution sphere-formation assay (LDA) (3 weeks) showing ∼10-fold increased sphere-initiating cell frequency upon miR-181a overexpression.
(D) Real-time PCR showing decreased stem cell markers upon miR-181a downregulation in FTSE-miR-181a cells.
(E) In vitro LDA assay (3 weeks) showing ∼18-fold decreased sphere-initiating cell frequency upon miR-181a downregulation in FTSE-miR-181a cells.
(F) ALDEFLUOR flow-cytometry assay showing high ALDH activity in OV81.2 primary HGSOC PDX-derived cell line model (DEAB is an ALDH inhibitor).
(G) (Left) In vivo tumor-initiation assay showing miR-181a overexpression increases tumor-initiation ability in OV81.2 cells and (right) ELDA calculation of the tumor-initiating cell frequency showing ∼45-fold increase in OV81.2-miR-181a cells as compared with OV81.2-control cells.
∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.005.
miR-181a Sensor Enriches for Tumor-Initiating Cells in Ovarian Cancer
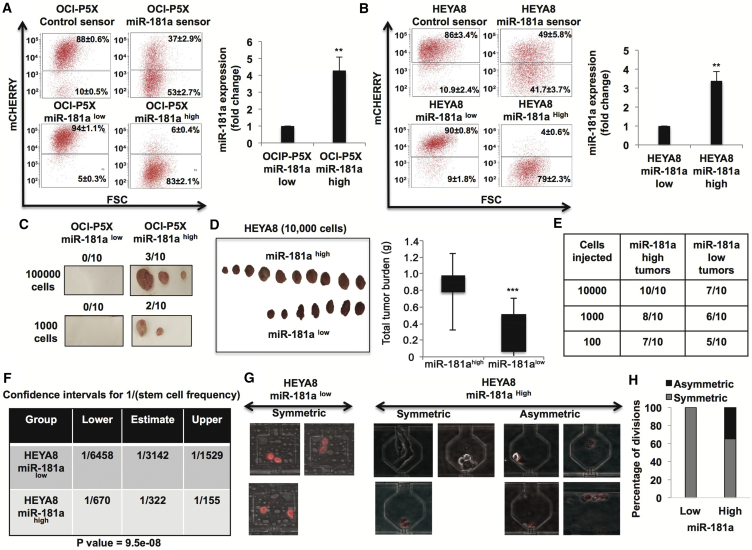
We next set out to isolate miR-181ahigh versus miR-181alow subpopulation of tumor cells from ovarian tumors by using an miRNA sensor platform (Mullokandov et al., 2012). The miR-181a sensor (Figure S1) enabled isolation of miR-181ahigh and miR-181alow cells from both OCI-P5X (primary HGSOC cells [Ince et al., 2015]) and HEYA8 (established TIC study model in ovarian cancer [Chau et al., 2013]) with ∼4-fold increase in miR-181a expression (Figures 2A and 2B). Similar to what was observed in the TCGA dataset (Figure S2A), miR-181a was the predominant miRNA expressed in both miR-181ahigh and miR-181alow primary HGSOC OCI-P5X cells as compared with miR-181b and miR-181c (Figure S2B), whose expression levels were below reliable detectable levels. Hence, we focused on studying miR-181a in the current study. The ability to initiate tumors at low cell densities is a hallmark of TICs, thus we first looked at tumor-initiation capacity of miR-181a sensor-sorted OCI-P5X primary cells. OCI-P5X miR-181ahigh cells were able to initiate tumors with as few as 1,000 cells whereas OCI-P5X miR-181alow cells were unable to initiate tumors even at 100,000 cells (Figure 2C). OCI-P5X miR-181alow cells did not form tumors even after 121 days, suggesting it is unlikely that 181alow cells eventually produce tumors. HEYA8-miR-181ahigh cells exhibited robust tumor formation (10/10) compared with HEYA8-miR-181alow cells (7/10) (10,000 cells) (Figure 2D). Furthermore, in vivo limiting dilution tumor-initiation assays showed ∼10-fold increase in tumor-initiating cell frequency in HEYA8 miR-181ahigh cells (∼1:322) compared with HEYA8 miR-181alow cells (∼1:3,142) (Figures 2E and 2F). miR-181a expression in miR-181ahigh tumors was ∼4-fold higher than that of miR-181alow tumors (data not shown). This is similar to ∼4-fold difference observed in miR-181a expression between miR-181ahigh and miR-181alow cells in vitro. This suggests that miR-181alow cells are unlikely to revert to miR-181ahigh cells in vivo. We next assessed asymmetric cell division in these two populations, given that it is one of the defining traits of TICs and ovarian TICs are known to exhibit asymmetric cell division (Choi et al., 2015). We found that miR-181alow ovarian tumor cells exhibited 100% symmetric cell division (relative to miR-181a) whereas miR-181ahigh ovarian tumor cells exhibited both symmetric (∼65%) and asymmetric (35%) divisions, further supporting that these cells are enriched in TIC properties (Figures 2G and 2H). In vitro proliferation rate did not differ between miR-181alow and miR-181ahigh ovarian tumor cells, confirming that the differences in TIC properties between these two populations are not due to differences in proliferation ability (Figure S3). Collectively, these results demonstrate the ability of miRNA 3′ UTR sensor to isolate TICs from primary tumors and also identify miR-181a as a regulator of TIC properties in EOC.
Figure 2.
miR-181a Sensor Enriches for TICs in Ovarian Cancer
(A and B) miR-181a sensor-based sorting of mCherryhigh and mCherrylow cells from OCI-P5X cells and HEYA8 cells (A, left and B, left) (sensor-sorted cells were analyzed three passages after sorting for reporter levels, and we did not observe changes in the reporter fluorescence activity even after 20 passages). Real-time PCR (A, right and B, right) showing ∼4-fold difference in miR-181a expression in mCherry sorted cells.
(C) In vivo tumor initiation showing increased tumor formation by miR-181ahigh primary HGSOC cells as compared with no tumors formed by miR-181alow primary HGSOC cells at 100,000 cells (day 93) and 1,000 cells (day 121).
(D) In vivo tumor initiation showing increased tumor formation by miR-181ahigh HEYA8 cells (10,000 cells) (day 35).
(E and F) In vivo LDA tumor-initiation assay (E) and ELDA analysis (F) showing increased tumor-initiating cell frequency (∼10-fold) in vivo in miR-181ahigh HEYA8 cells (day 28).
(G and H) Asymmetric and symmetric division of miR-181a sensor-sorted cells: top 10% miR-181ahigh and miR-181alow HEYA8 cells were sorted into single-cell-capture microfluidic devices and their growth was monitored daily for 15 days. Representative photomicrographs (G) of miR-181alow/mCherryhigh cell divisions showing these cells were only observed to divide to yield two miR-181alow/mCherryhigh cells. In contrast, miR-181ahigh/mCherrylow cells divided both symmetrically to yield other mCherry-negative cells and asymmetrically to yield mCherry dim cells. (H) Summary of all divisions observed after 4 days of growth.
∗∗p < 0.005, ∗∗∗p < 0.005.
miR-181a Sensor Enriches for Multiple TIC Regulatory Signaling Pathways in Primary HGSOC Cells
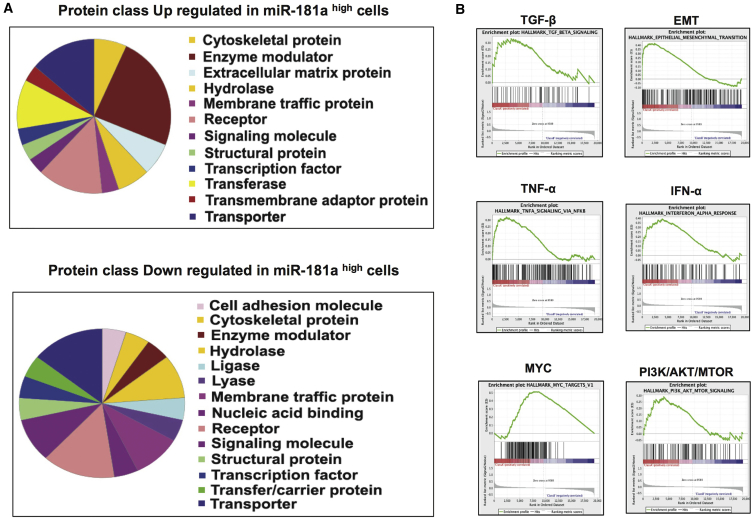
Since the miR-181a sensor enabled isolation of ovarian TICs, we next looked at the pathways enriched in miR-181a sensor-sorted primary HGSOC cells by microarray analysis to determine the ability of miRNA sensor to potentially enrich for multiple TIC pathways. PANTHER gene expression analysis of the top 50 genes upregulated or downregulated in miR-181ahigh primary HGOSC cells identified several classes of genes altered in these cells as compared with miR-181alow primary HGSOC cells (Figure 3A). Furthermore, gene set enrichment analysis (GSEA) of the gene expression profile of miR-181ahigh and miR-181alow primary HGSOC cells revealed several known TIC regulatory pathways to be enriched in miR-181ahigh cells, which correlated with the known fact that miRNAs regulate several pathways (Figure 3B). Epithelial mesenchymal transition (EMT) and transforming growth factor β (TGF-β) pathways were upregulated in miR-181ahigh cells, which correlated with our previous results showing that miR-181a induces EMT in ovarian cancer by activating TGF-β through the direct targeting of the inhibitory SMAD, SMAD7, thus confirming the functional reliability of the gene expression data (Parikh et al., 2014). In addition, several known stem cell regulatory pathways such as interferon-α (IFN-α), tumor necrosis factor α (TNF-α), PI3K/AKT/mTOR, and MYC were upregulated in miR-181ahigh cells, showing that miR-181a sensor can enrich for multiple TIC regulatory pathways (Zhu et al., 2014, Lee et al., 2012, Xia and Xu, 2015, Dubrovska et al., 2009, Wang et al., 2008, Yang et al., 2017, Nair et al., 2014). The pathways enriched in miR-181ahigh HGSOC cells could be due to combination of a direct effect of the miRNA and also an indirect effect due to potential crosstalks between the pathways. Therefore, we examined the top 100 downregulated genes in miR-181ahigh HGSOC cells in comparison with miR-181a predicted targets (miRWalk database), which revealed that ∼30% of the downregulated genes in miR-181ahigh HGSOC cells are predicted miR-181a targets (Table S1). Thus, enrichment of diverse TIC regulatory pathways directly or indirectly by miR-181a could contribute to increased TIC properties of miR-181ahigh ovarian tumor cells.
Figure 3.
Pathways Enriched in miR-181ahigh Primary HGSOC Cells
(A) PANTHER gene expression analysis of the top 50 genes upregulated or downregulated in miR-181ahigh primary HGSOC (OCI-P5X) cells.
(B) GSEA analysis of the microarray data showing several TIC regulatory pathways enriched in miR-181ahigh primary HGSOC (OCI-P5X) cells.
miR-181a Sensor Enables Analysis of Ovarian Tumor Cell Response to Cisplatin in Real Time
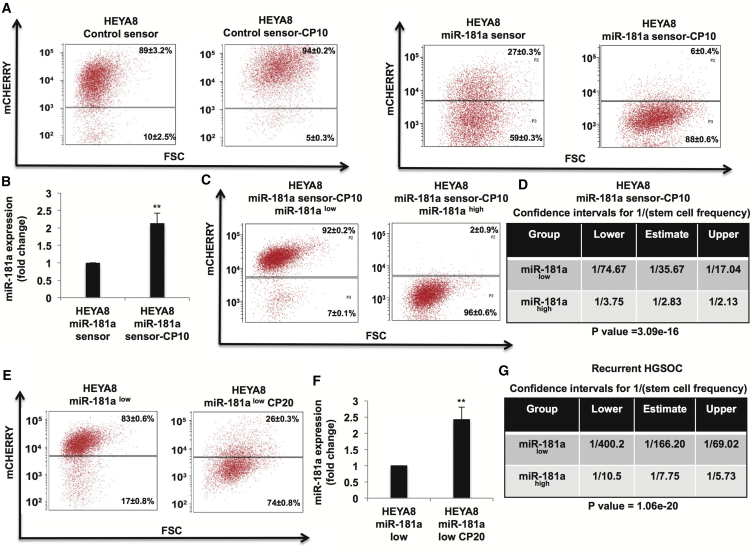
The ability of TIC populations to survive standard cytotoxic chemotherapy leads to disease recurrence and poor outcomes. Thus, given that miR-181ahigh ovarian tumor cells were enriched in TIC properties and miR-181a sensor provides a real-time platform to assess endogenous miR-181a activity, we utilized this platform to study the effects of long-term cisplatin treatment on miR-181a activity in ovarian tumor cells. Long-term culture of miR-181a sensor-transduced HEYA8 cells in the presence of cisplatin (HEYA8 miR-181a-sensor-CP10) increased the miR-181ahigh subpopulation, which correlated with increased miR-181a expression (Figures 4A and 3B). Control sensor-transduced HEYA8 cells did not exhibit a decrease in the mCherry population upon long-term cisplatin treatment (HEYA8 control sensor-CP10) (Figure 4A). We next sorted mCherryhigh and mCherrylow populations from HEYA8 miR-181a-sensor-CP10 cells (Figure 4C) and assessed their sphere-initiating cell frequency. HEYA8-miR-181a-sensor-CP10-mCherrylow (miR-181ahigh) cells exhibited increased sphere-initiating cell frequency (∼12-fold) as compared with HEYA8-miR-181a-sensor-CP10-mCherryhigh (miR-181alow) cells, further confirming enrichment of miR-181ahigh TICs in response to cisplatin treatment (Figure 4D). Long-term cisplatin treatment of miR-181alow cells enriched the miR-181ahigh subpopulation (HEYA8 miR-181alow CP20) that correlated with increased miR-181a expression, suggesting enrichment of miR-181ahigh cells in response to selection pressure induced by long-term treatment with cisplatin (Figures 4E and 4F). Next, we asked whether the miRNA 3′ UTR sensor platform would be able to isolate miR-181ahigh ovarian tumor cells from primary recurrent HGSOC (OV236) tumor cells. We found that in this recurrent tumor the miR-181ahigh subpopulation of cells exhibited the greatest difference in sphere-initiating cell frequency compared with all tumors tested. We observed a ∼20-fold increase in sphere-initiating cell frequency in the miR-181ahigh compared with miR-181alow cells (Figure 4G). These findings raise the possibility that targeting miR-181a could overcome the barrier of tumor recurrence by inhibiting TICs in EOC.
Figure 4.
miR-181a Sensor Enables Analysis of Ovarian Tumor Cell Response to Cisplatin in Real Time
(A) Flow cytometry showing increase in miR-181ahigh (mCherrylow) population in response to long-term cisplatin treatment (10 passages) in HEYA8 cells transduced with miR-181a sensor (right) compared with no decrease in mCherry fluorescence in control sensor-transduced HEY8 cells in response to long-term cisplatin treatment (2.5 μM) (left).
(B) Real-time PCR showing increased miR-181a expression in HEYA8-miR-181a sensor-CP10 cells.
(C and D) Flow cytometry (C) showing sorting of miR-181ahigh (mCherrylow) and miR-181alow (mCherryhigh) subpopulations from HEYA8-miR-181a sensor-CP10 cells, and (D) in vitro LDA assay (3 weeks) showing increased sphere-initiating cell frequency (∼12-fold) in miR-181ahigh cells sorted from HEYA8-miR-181a-CP10 cells.
(E) Flow cytometry showing increased miR-181ahigh subpopulation in response to long-term cisplatin treatment in HEYA8 miR-181alow cells.
(F) Real-time PCR showing increased miR-181a expression in miR-181alow cells upon long-term treatment with cisplatin.
(G) In vitro LDA assay (8 weeks) showing increased sphere-initiating cell frequency (∼20-fold) in miR-181ahigh cells sorted from primary recurrent HGSOC cells (OV236).
∗∗p < 0.005.
miRNA-Sensor-Based High-Throughput Therapeutic Screen Identifies BET Inhibitors as Potential Inhibitors of miR-181a
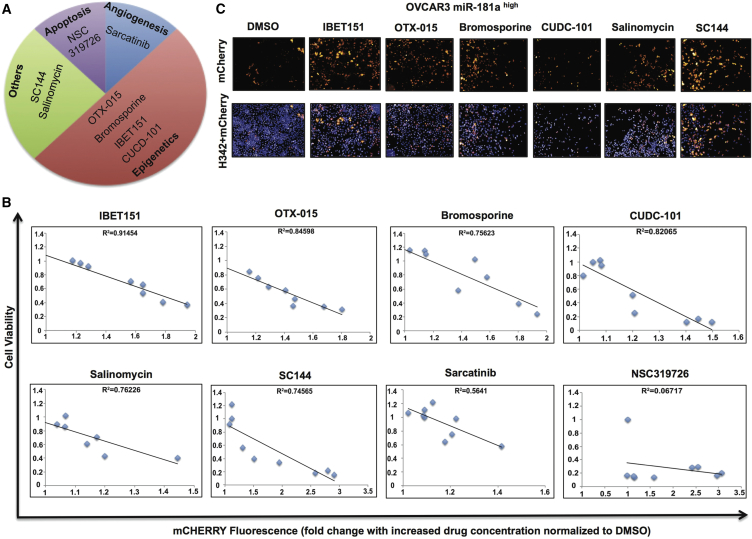
Even though transcriptional regulation of miRNAs forms a critical step in the regulation of miRNA functions, this aspect is not very well understood. Identifying upstream regulatory elements of miRNAs can lead to identification of inhibitors of these regulatory elements, thus greatly enhancing the efficacy of miRNA-targeted therapeutics. Current methodologies to study upstream regulatory elements of miRNAs are mainly limited to a candidate gene approach whereby selected genes/pathways are studied as potential drivers of miRNA expression, thus limiting the identification of miRNA inhibitors (Niu et al., 2016). The lack of reliable platforms to identify global regulators of miRNA transcription is the main barrier toward deciphering upstream regulatory elements involved in miRNA transcription. This, in turn, translates into the lack of miRNA-targeting drugs in oncology. Since our results identified miR-181a as a TIC therapeutic target in EOC, we next set out to test the utility of the miRNA sensor model as a tool to identify inhibitors of miR-181a that can be potentially evaluated as TIC-targeting drugs. For this approach, we first established a 384-well functional platform in which miR-181a inhibition in miR-181ahigh (mCherrylow) ovarian tumor cells could be monitored as an increase in mCherry fluorescence readout (Figure S4). Using this 384-well platform, we treated the miR-181ahigh ovarian tumor cells with a chemical library consisting of 3,114 compounds and looked for candidate drugs that increased mCherry fluorescence, thus identifying them as potential inhibitors of miR-181a expression (Table S2). Preliminary screening revealed 32 hits (Figure S5). Further correction for false-positive hits due to potential autofluorescence properties of the drugs translated into eight final hits (Figure 5A and Table S2). Interestingly, all eight hits have been previously linked with targeting TICs (Naujokat and Steinhart, 2012, Yokoyama et al., 2016) and SC144, an inhibitor STAT3 that regulates miR-181a transcription (Niu et al., 2016), supports the functional reliability of the miRNA-sensor-screening platform. Furthermore, four of the eight hits were epigenetic regulators or bromodomain and extra-terminal motif (BET) inhibitors, suggesting an epigenetic regulation of miR-181a by the BET protein family that has not been reported to date in either cancer or normal physiological context.
Figure 5.
miR-181a Sensor Screen Identifies BET Inhibitors as Potential Inhibitors of miR-181a
(A) Final eight hits obtained from miR-181a sensor screen in OVCAR3 miR-181ahigh cells showing epigenetic regulators as the main hits.
(B) Correlation analysis of mCherry fluorescence with cell counts upon treatment with all eight hits obtained from miR-181a sensor screen showing R2 values >0.7 in 6 of the 8 hits.
(C) Fluorescence imaging of OVCAR3 miR-181ahigh cells showing increased mCherry expression upon treatment with 6 of the 8 hits (10 μM 48 hr) with H342 dye used to detect viable cells.
We next assessed the correlation of cell counts versus mCherry fluorescence upon treatment with increasing doses of the identified eight hits. Six hits including the three BET inhibitors exhibited R2 value of >0.7 in the correlation analysis, further suggesting that BET proteins could be regulators of miR-181a transcription (Figure 5B and Table S2). Sarcatinib and NSC319726 exhibited weak correlation and hence were excluded from further analysis. We further confirmed that these six hits increased mCherry expression in miR-181ahigh cells (Figure 5C and Table S2).
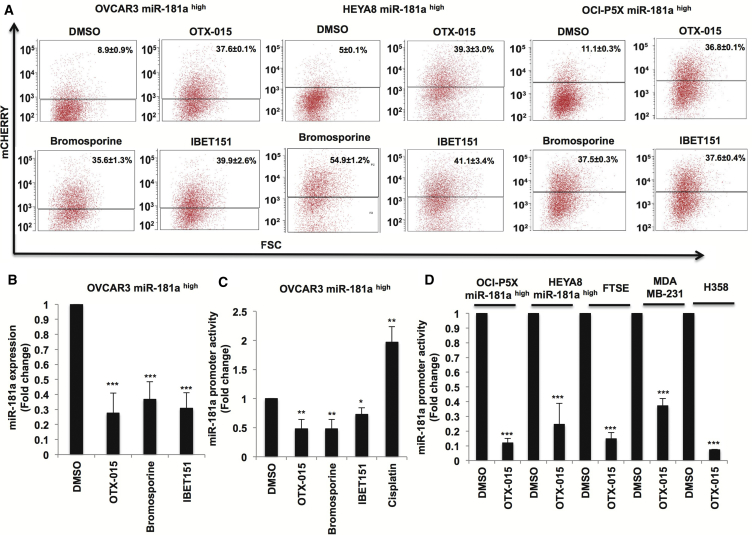
miR-181a Is a Target of BET Inhibitors Across Cancers
We next set out to validate whether miR-181a is a target of BET inhibitors. BET inhibitors increased mCherry fluorescence in the miR-181ahigh subpopulation sorted from OVCAR3, HEYA8, and primary HGSOC OCI-P5X cells, further validating the miRNA-sensor-screening results identifying BET inhibitors as inhibitors of miR-181a in EOC (Figure 6A). We subsequently confirmed that BET inhibitors decreased the expression of miR-181a by Taqman miRNA assays (Figure 6B). In addition, we looked at the effect of BET inhibition on miR-181a promoter activity (Presnell et al., 2015, Bert et al., 2000) to assess whether miR-181a is a transcriptional target of BET inhibitors. BET inhibitors decreased miR-181a promoter activity by ∼70% in the miR-181ahigh subpopulation sorted from OVCAR3, showing that miR-181a is a transcriptional target of BET inhibitors in EOC. Contrastingly, cisplatin increased miR-181a promoter activity in these cells by more than 1.5-fold, in accordance with the increased miR-181a expression seen in cisplatin-resistant EOC tumor cells (Figure 6C). Furthermore, BET inhibition decreased miR-181a promoter activity in the miR-181ahigh subpopulation sorted from OCI-P5X (∼90%) and HEYA8 cells (∼80%), and also in FTSE cells (∼90%) (Figure 6D). To assess whether miR-181a is a conserved target of BET proteins across cancers, we looked at the effect of BET inhibition on miR-181a promoter activity in breast cancer cells (MDA-MB-231) and lung cancer cells (H358). BET inhibition decreased miR-181a promoter activity in both breast cancer (∼70%) and lung cancer cells (∼90%), thus identifying a hitherto unknown function of BET protein family as regulators of miR-181a transcription across cancers (Figure 5D). Given that the mechanism of BET inhibitors is through the disruption of bromodomain proteins on to acetylated chromatin, we next examined whether the miR-181a promoter was acetylated. H3K27ac chromatin immunoprecipitation sequencing revealed that the miR-181a promoter was acetylated and, interestingly, this acetylation increased in cisplatin-resistant cells (Figure S6). Since miR-181a is implicated in regulation of various aspects of tumorigenesis across cancers, these results suggest that the BET-miR-181a axis could be a functionally conserved regulatory axis in cancers; hence, BET inhibitors could potentially be evaluated as small-molecule inhibitors of miR-181a function in cancers or miR-181a could be a potential biomarker for response.
Figure 6.
miR-181a Is a Target of BET Inhibitors
(A) Flow cytometry showing increased mCherry fluorescence upon treatment with BET inhibitors in miR-181ahigh subpopulation sorted from OVCAR3-HEYA8 and OCI-P5X cells (10 μM, 48 hr).
(B) Real-time PCR showing decreased miR-181a expression upon treatment with BET inhibitors in OVCAR3-miR-181ahigh cells (10 μM, 48 hr).
(C) miR-181a promoter reporter assay showing decreased promoter activity upon treatment with BET inhibitors (10 μM, 24 hr) and increased promoter activity by cisplatin (10 μM, 24 hr) in OVCAR3 miR-181ahigh cells.
(D) miR-181a promoter reporter assay showing decreased promoter activity upon treatment with BET inhibitors (10 μM, 24 hr) in OCI-P5X miR-181ahigh cells, HEYA8 miR-181ahigh cells, FTSE cells, MDA-MB-231 cells, and H358 cells.
∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.005.
Discussion
Isolation, characterization, and targeting of TIC clones present in a tumor are a major barrier to complete eradication of all the cancer cells present in a patient. TIC regulators can differ in patients due to intertumor and intratumor heterogeneity. An miRNA sensor approach can enhance the understanding of TIC functions in cancers because (1) miRNAs regulate multiple pathways, and thus miRNA activity can potentially enrich for multiple TIC clones in primary tumors, and (2) miRNA function can be assayed by 3′ UTR activity, and hence status of miRNA function can be reliably assayed in TIC clones in real time in response to genetic/pharmacological modulation.
Our results also demonstrate the importance of including non-transformed cell models to identify TIC regulators in cancers. Increased stem-like properties by miR-181a in non-transformed FTSE cells prompted us to investigate stem-like properties in miR-181ahigh and miR-181alow subpopulations in EOC tumors, thus identifying miR-181a as a regulator of TIC functions. Several predicted targets of miR-181a were enriched in miR-181ahigh primary HGSOC cells. For example, PARK2 (Parkin), which was one of the predicted miR-181a targets that was downregulated in miR-181ahigh HGSOC cells and has been characterized as a target of miR-181a in neuroblastoma cells (Cheng et al., 2016), is a negative regulator of PI3K/Akt pathways (Gupta et al., 2017). In addition, loss of PARK2 is reported to be associated with increased levels of cytokines such as TNF-α (Lee et al., 2016). Hence, downregulation of PARK2 in miR-181ahigh HGSOC cells could be one of the mechanisms activating TNF-α and PI3K/Akt pathways in these cells. Furthermore, IRF8, which is a predicted miR-181a target downregulated in miR-181ahigh cells, is a negative regulator of the IFN pathway and could contribute to the increased IFN-α pathway we observed in miR-181ahigh HGSOC cells (White et al., 2016). Furthermore, ongoing studies in our laboratory have identified functional interaction of miR-181a with MYC in regulating HGSOC pathogenesis, which correlates with enrichment of the MYC pathway in miR-181ahigh HGSOC cells (our unpublished data). In addition, it has been previously shown that miR-181 is sharply induced in Myc-induced differentiated embryonic stem cells and tumor cells (Lin et al., 2009); thus, enrichment of the MYC pathway in the miR-181ahigh HGSOC cells may be due to the upstream regulation of miR-181a by MYC. Activation of the Akt pathway by miR-181a has been reported, and hence this pathway could function downstream of miR-181a in HGSOC (Strotbek et al., 2017). Activation of PI3K/Akt pathway is known to induce MYC stabilization, and synergy between these two pathways is reported in cancers (Tsai et al., 2012, Sander et al., 2012), suggesting that crosstalk between enriched pathways could also be an important contributor to the increased TIC phenotype in miR-181ahigh HGSOC cells.
In this study we have identified a hitherto unknown role of miR-181a in driving tumor recurrence in HGSOC, and show that (1) miR-181ahigh ovarian tumor cells are enriched in TIC properties and (2) the miR-181ahigh subpopulation of ovarian tumor cells is enriched in response to cisplatin treatment. miR-181a is reported to be induced by cisplatin treatment in lung cancer (Galluzzi et al., 2010), and our results show that cisplatin induces miR-181a promoter activity. Thus, both selection of miR-181ahigh cells and induction of miR-181a in response to cisplatin treatment can contribute to enrichment of miR-181ahigh cells upon cisplatin treatment. Moreover, miR-181a could be a common driver of both intrinsic stem-like properties in ovarian tumor cells, and also acquired stem-like properties in response to selection pressure induced upon cisplatin treatment. These data have been recently supported and expanded to other cancers through the comprehensive TCGA analysis of 12,000 tumor samples from 33 different cancers, which revealed that miR-181 expression in several different cancers correlated with a high mRNA stemness index (Malta et al., 2018). Hence, miR-181a inhibition could be evaluated as an miRNA therapeutic approach targeting TICs to overcome the barrier of tumor recurrence in EOC as well as several other cancers.
One of the main barriers for advancements in miRNA therapeutics is the lack of in-depth understanding of transcriptional regulation of miRNAs in both physiological and cancer settings. Here, we have identified a role for BET inhibitors as miRNA modulators in EOC, in particular as miR-181a inhibitors. BET inhibitors are being explored as potential anti-cancer drugs in clinical trials across multiple cancers (Fujisawa and Filippakopoulos, 2017). BET inhibition is being evaluated as a potential therapeutic strategy in EOC, and BET inhibition is reported to decrease the expression of stemness-regulating genes and to overcome cisplatin resistance in EOC (Yokoyama et al., 2016). The miR-181ahigh subpopulation in EOC could represent a potential TIC clone that could be targeted by BET inhibitors. However, since BET proteins regulate a multitude of cellular processes in cancers (Fujisawa and Filippakopoulos, 2017), several miRNAs could be targeted by BET inhibition in EOC. Changes in miRNAome induced by BET inhibition are not understood in EOC and, hence, functional characterization of miRNAome targeted by BET inhibitors in EOC is important to establish these drugs as miRNA-targeting drugs in EOC. Since miRNAs are established as reliable biomarkers in various cancers including EOC (Nagaraj et al., 2015b), miRNAs can be employed as biomarkers for both patient stratification and monitoring therapeutic efficacy of BET inhibition in EOC, thus enhancing the translational potential of BET inhibition in EOC with potential extension to other cancers.
By developing an miRNA-3′ UTR sensor platform and using it to explore the role of miR-181a in EOC, we have (1) simplified the understanding of functional complexity in TICs and expedited the journey toward near complete isolation of multiple TIC clones in tumors, (2) identified a reliable approach to find small-molecule inhibitors of miRNA function that can greatly enhance the translational potential of miRNA therapeutics in both cancer and physiological contexts, and (3) uncovered a potential clinical biomarker for response to BET inhibitors.
Experimental Procedures
Cell Culture and Reagents
Cells were cultured in 10-mm plates in a humidified atmosphere (5% CO2) at 37°C. At 70%–90% confluence, trypsin (0.25%)/EDTA solution was used to detach the cells from the culture plate for passaging and used for further experiments until passage 20. FTSE cells (DMEM-F12 medium), OVCAR3, HEYA8, and H358 cells (RPMI medium), and MDA-MB-231 cells (DMEM medium) were cultured in their respective media supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1% PenStrep (PS) (Gibco). Primary HGSOC cells (OCI-P5X, OV236) were cultured in OCMI-L medium (Liver Tumor Culture Core, University of Miami) supplemented with 2.5% heat-inactivated FBS (Gibco) and 1% PS. OCI-P5X cells were purchased from Liver Tumor Culture Core, University of Miami. Matrigel was purchased from Corning (NY). Cisplatin was purchased from Mount Sinai Hospital Pharmacy. miR-181a lentiviral overexpression and the control vector were purchased from Biosettia (San Diego, CA). miR-181a antagomiR lentiviral vector and the control vector were purchased from Genecopoeia (Rockville, MD). miR-181a antagomir and the corresponding negative control for transient transfections were purchased from Dharmacon. BET inhibitors were purchased from Selleckchem (Houston, TX).
Tumor-Initiating Cell Assays
For limiting dilution sphere assays, a BD FACSAria II sorter was used to sort cells directly into 96-well ultra-low attachment (ULA) plates (Corning, NY) in 200 μL of mammocult medium (STEMCELL Technologies, Vancouver, Canada) per well. After indicated time points, the number of wells with tumor spheres was counted and the data were analyzed by the ELDA platform to determine the sphere-initiating cell frequency. At each cell dosage three biological replicates were used for in vitro LDA assays. Each replicate was sorted in eight wells in ULA plates per cell dosage. For in vivo tumor-initiation assays, miR-181ahigh and miR-181alow cells were resuspended in cell culture medium with Matrigel in 50:50 ratios and injected subcutaneously in NU/NU mice, and tumor formation was assessed. For in vivo LDA assays, ten mice were studied in each group with OCI-P5X and HEYA8 cells, and four mice were studied in each group with OV81.2 cells. Tumor volume was estimated by standard caliper measurement (V = L × W2/2). The ELDA platform was employed to determine the tumor-initiating cell frequency. Symmetric and asymmetric cell division experiments were performed as described previously (Choi et al., 2015).
Statistical Analysis
Unless otherwise noted, data are presented as mean ± SD from three independent experiments, and Student's t test (two-tailed) was used to compare two groups (p < 0.05 was considered significant) for independent samples.
Author Contributions
A.D. and A.B.N. designed the study. A.B.N., P.J., and E.P. performed the experiments. Y.F. and D.J.A. performed the small-molecule screening. A.C., E.Y., and R.B. helped in performing the asymmetric cell division experiments. A.B. and B.D.B. designed, generated, and provided the miR-181 3′ UTR sensor vector. S.S. performed the GSEA analysis. R.D. provided the FTSE cell lines. A.D. and A.B.N. analyzed the results and wrote the manuscript. All authors reviewed the manuscript. A.D. supervised the overall study and finalized the manuscript.
Acknowledgments
We thank Norma C. and Albert I. Geller for their constant support of the Gynecological Cancer Translational Research Program at Case Western Reserve University (A.D.). In addition, we thank Dr. Anirban Mitra for the HEYA8 cells, Dr. Goutham Narla for the MDA-MB-231 and H358 cells, and Dr. Steven Presnell for the miR-181a luciferase promoter. We acknowledge the help from Cytometry & Imaging Microscopy Core Facility and the Athymic Animal and Preclinical Therapeutics Core of the Case Comprehensive Cancer Center (P30CA043703). This work was supported by grants from The National Cancer Institute, R01CA197780 (A.D.), Department of Defense, OC150553 (A.D.), and The Young Scientist Foundation (A.D.).
Published: January 8, 2019
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.12.002.
Supplemental Information
References
- Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bert a G., Burrows J., Osborne C.S., Cockerill P.N. Generation of an improved luciferase reporter gene plasmid that employs a novel mechanism for high-copy replication. Plasmid. 2000;44:173–182. doi: 10.1006/plas.2000.1474. [DOI] [PubMed] [Google Scholar]
- Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Chau W.K., Ip C.K., Mak A.S.C., Lai H.-C., Wong A.S.T. c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin-ATP-binding cassette G2 signaling. Oncogene. 2013;32:2767–2781. doi: 10.1038/onc.2012.290. [DOI] [PubMed] [Google Scholar]
- Chen M.W., Yang S.T., Chien M.H., Hua K.T., Wu C.J., Hsiao S.M., Lin H., Hsiao M., Su J.L., Wei L.H. The STAT3-miRNA-92-Wnt signaling pathway regulates spheroid formation and malignant progression in ovarian cancer. Cancer Res. 2017;77:1955–1967. doi: 10.1158/0008-5472.CAN-16-1115. [DOI] [PubMed] [Google Scholar]
- Cheng M., Liu L., Lao Y., Liao W., Liao M., Luo X., Wu J., Xie W., Zhang Y., Xu N. MicroRNA-181a suppresses parkin-mediated mitophagy and sensitizes neuroblastoma cells to mitochondrial uncoupler-induced apoptosis. Oncotarget. 2016;7:42274–42287. doi: 10.18632/oncotarget.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.-J., Ingram P.N., Yang K., Coffman L., Iyengar M., Bai S., Thomas D.G., Yoon E., Buckanovich R.J. Identifying an ovarian cancer cell hierarchy regulated by bone morphogenetic protein 2. Proc. Natl. Acad. Sci. U S A. 2015;112:E6882–E6888. doi: 10.1073/pnas.1507899112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnea P., Stronach E.A. Modeling platinum sensitive and resistant high-grade serous ovarian cancer: development and applications of experimental systems. Front. Oncol. 2014;4:1–8. doi: 10.3389/fonc.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovska A., Kim S., Salamone R.J., Walker J.R., Maira S.-M., Garcia-Echeverria C., Schultz P.G., Reddy V.A. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. U S A. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesken-Nikitin A., Hwang C.-I., Cheng C.-Y., Michurina T.V., Enikolopov G., Nikitin A.Y. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T., Filippakopoulos P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat. Rev. Mol. Cell Biol. 2017;18:246–262. doi: 10.1038/nrm.2016.143. [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Morselli E., Vitale I., Kepp O., Senovilla L., Criollo A., Servant N., Paccard C., Hupé P., Robert T. miR-181a and miR-630 regulate cisplatin-induced cancer cell death. Cancer Res. 2010;70:1793–1803. doi: 10.1158/0008-5472.CAN-09-3112. [DOI] [PubMed] [Google Scholar]
- Garson K., Vanderhyden B.C. Epithelial ovarian cancer stem cells: underlying complexity of a simple paradigm. Reproduction. 2015;149:R59–R70. doi: 10.1530/REP-14-0234. [DOI] [PubMed] [Google Scholar]
- Gupta A., Anjomani-Virmouni S., Koundouros N., Dimitriadi M., Choo-Wing R., Valle A., Zheng Y., Chiu Y.H., Agnihotri S., Zadeh G. PARK2 depletion connects energy and oxidative stress to PI3K/Akt activation via PTEN S-Nitrosylation. Mol. Cell. 2017;65:999–1013.e7. doi: 10.1016/j.molcel.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- Hu Y., Smyth G.K. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Ince T.A., Sousa A.D., Jones M.A., Harrell J.C., Agoston E.S., Krohn M., Selfors L.M., Liu W., Chen K., Yong M. Characterization of twenty-five ovarian tumour cell lines that phenocopy primary tumours. Nat. Commun. 2015;6:7419. doi: 10.1038/ncomms8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J., Yamashita T., Budhu A., Forgues M., Jia H., Li C., Deng C., Wauthier E., Reid L.M., Ye Q. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2010;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst A.M., Levanon K., Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc. Natl. Acad. Sci. U S A. 2011;108:7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreso A., Dick J.E. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Lechman E.R., Gentner B., Ng S.W.K., Schoof E.M., van Galen P., Kennedy J.A., Nucera S., Ciceri F., Kaufmann K.B., Takayama N. MiR-126 regulates distinct self-renewal outcomes in normal and malignant hematopoietic stem cells. Cancer Cell. 2016;29:214–228. doi: 10.1016/j.ccell.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., She J., Deng B., Kim J., de Andrade M., Na J., Sun Z., Wampfler J.A., Cunningham J.M., Wu Y. Multiple-level validation identifies PARK2 in the development of lung cancer and chronic obstructive pulmonary disease. Oncotarget. 2016;7:44211–44223. doi: 10.18632/oncotarget.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Hong H.S., Liu Z.X., Kim R.H., Kang M.K., Park N.H., Shin K.H. TNFα enhances cancer stem cell-like phenotype via Notch-Hes1 activation in oral squamous cell carcinoma cells. Biochem. Biophys. Res. Commun. 2012;424:58–64. doi: 10.1016/j.bbrc.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-H., Jackson A.L., Guo J., Linsley P.S., Eisenman R.N. Myc-regulated microRNAs attenuate embryonic stem cell differentiation. EMBO J. 2009;28:3157–3170. doi: 10.1038/emboj.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malta T.M., Sokolov A., Gentles A.J., Burzykowski T., Poisson L., Weinstein J.N., Kamińska B., Huelsken J., Omberg L., Gevaert O. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 2018;173:338–354.e15. doi: 10.1016/j.cell.2018.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham C.E., Morrison S.J. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Endo K., Takahashi S., Funakoshi S., Takei I., Katayama S., Toyoda T., Kotaka M., Takaki T., Umeda M. Efficient detection and purification of cell populations using synthetic MicroRNA switches. Cell Stem Cell. 2015;16:699–711. doi: 10.1016/j.stem.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Mullokandov G., Baccarini A., Ruzo A., Jayaprakash A.D., Tung N., Israelow B., Evans M.J., Sachidanandam R., Brown B.D. High-throughput assessment of microRNA activity and function using microRNA sensor and decoy libraries. Nat. Methods. 2012;9:840–846. doi: 10.1038/nmeth.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj A.B., Joseph P., Kovalenko O., Singh S., Armstrong A., Redline R., Resnick K., Zanotti K., Waggoner S., DiFeo A. Critical role of Wnt/β-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget. 2015;6:23720–23734. doi: 10.18632/oncotarget.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj A.B., Joseph P., DiFeo A. miRNAs as prognostic and therapeutic tools in epithelial ovarian cancer. Biomark. Med. 2015;9:241–257. doi: 10.2217/bmm.14.108. [DOI] [PubMed] [Google Scholar]
- Nair R., Roden D.L., Teo W.S., McFarland A., Junankar S., Ye S., Nguyen A., Yang J., Nikolic I., Hui M. C-Myc and Her2 cooperate to drive a stem-like phenotype with poor prognosis in breast cancer. Oncogene. 2014;33:3992–4002. doi: 10.1038/onc.2013.368. [DOI] [PubMed] [Google Scholar]
- Naujokat C., Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J. Biomed. Biotechnol. 2012;2012:950658. doi: 10.1155/2012/950658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J., Xue A., Chi Y., Xue J., Wang W., Zhao Z., Fan M., Yang C.H., Shao Z.-M., Pfeffer L.M. Induction of miRNA-181a by genotoxic treatments promotes chemotherapeutic resistance and metastasis in breast cancer. Oncogene. 2016;35:1302–1313. doi: 10.1038/onc.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh A., Lee C., Joseph P., Marchini S., Baccarini A., Kolev V., Romualdi C., Fruscio R., Shah H., Wang F. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial–mesenchymal transition. Nat. Commun. 2014;5:2977. doi: 10.1038/ncomms3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perets R., Wyant G.A., Muto K.W., Bijron J.G., Poole B.B., Chin K.T., Chen J.Y.H., Ohman A.W., Stepule C.D., Kwak S. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop-Bica C., Pintea S., Cojocneanu-Petric R., Del Sal G., Piazza S., Wu Z.H., Alencar A.J., Lossos I.S., Berindan-Neagoe I., Calin G.A. MiR-181 family-specific behavior in different cancers: a meta-analysis view. Cancer Metastasis Rev. 2018;37:17–32. doi: 10.1007/s10555-017-9714-9. [DOI] [PubMed] [Google Scholar]
- Presnell S.R., Al-Attar A., Cichocki F., Miller J.S., Lutz C.T. Human natural killer cell microRNA: differential expression of MIR181A1B1 and MIR181A2B2 genes encoding identical mature microRNAs. Genes Immun. 2015;16:89–98. doi: 10.1038/gene.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota L.M., Lazzarino D.A., Ziegler A.N., LeRoith D., Wood T.L. Determining mammosphere-forming potential: application of the limiting dilution analysis. J. Mammary Gland Biol. Neoplasia. 2012;17:119–123. doi: 10.1007/s10911-012-9258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander S., Calado D.P., Srinivasan L., Köchert K., Zhang B., Rosolowski M., Rodig S.J., Holzmann K., Stilgenbauer S., Siebert R. Synergy between PI3K signaling and MYC in burkitt lymphomagenesis. Cancer Cell. 2012;22:167–179. doi: 10.1016/j.ccr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa M., Ohta Y., Nishikori S., Matano M., Takano A., Fujii M., Date S., Sugimoto S., Kanai T., Sato T. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature. 2017;545:187–192. doi: 10.1038/nature22081. [DOI] [PubMed] [Google Scholar]
- Shimono Y., Zabala M., Cho R.W., Lobo N., Dalerba P., Qian D., Diehn M., Liu H., Panula S.P., Chiao E. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva I.A., Bai S., McLean K., Yang K., Griffith K., Thomas D., Ginestier C., Johnston C., Kueck A., Reynolds R.K. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.M., Shaw P.A., Gedye C., Bernardini M.Q., Neel B.G., Ailles L.E. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc. Natl. Acad. Sci. U S A. 2011;108:6468–6473. doi: 10.1073/pnas.1005529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotbek M., Schmid S., Sánchez-González I., Boerries M., Busch H., Olayioye M.A. miR-181 elevates Akt signaling by co-targeting PHLPP2 and INPP4B phosphatases in luminal breast cancer. Int. J. Cancer. 2017;140:2310–2320. doi: 10.1002/ijc.30661. [DOI] [PubMed] [Google Scholar]
- Tang B., Raviv A., Esposito D., Flanders K.C., Daniel C., Nghiem B.T., Garfield S., Lim L., Mannan P., Robles A.I. A flexible reporter system for direct observation and isolation of cancer stem cells. Stem Cell Reports. 2015;4:155–169. doi: 10.1016/j.stemcr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W.B., Aiba I., Long Y., Lin H.K., Feun L., Savaraj N., Kuo M.T. Activation of Ras/PI3K/ERK pathway induces c-Myc stabilization to upregulate argininosuccinate synthetase, leading to arginine deiminase resistance in melanoma cells. Cancer Res. 2012;72:2622–2633. doi: 10.1158/0008-5472.CAN-11-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung S.L., Huang W.C., Hsu F.C., Yang Z.P., Jang T.H., Chang J.W., Chuang C.M., Lai C.R., Wang L.H. miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERK pathway. Oncogenesis. 2017;6:e326. doi: 10.1038/oncsis.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L., De Sousa e Melo F., van der Heijden M., Cameron K., de Jong J.H., Borovski T., Tuynman J.B., Todaro M., Merz C., Rodermond H. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang H., Li Z., Wu Q., Lathia J.D., McLendon R.E., Hjelmeland A.B., Rich J.N. c-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 2008;3:e3769. doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C.L., Kessler P.M., Dickerman B.K., Ozato K., Sen G.C. Interferon regulatory factor 8 (IRF8) impairs induction of interferon induced with tetratricopeptide repeat motif (IFIT) gene family members. J. Biol. Chem. 2016;291:13535–13545. doi: 10.1074/jbc.M115.705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P., Xu X.-Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am. J. Cancer Res. 2015;5:1602–1609. [PMC free article] [PubMed] [Google Scholar]
- Yang A., Qin S., Schulte B.A., Ethier S.P., Tew K.D., Wang G.Y. MYC inhibition depletes cancer stem-like cells in triple-negative breast cancer. Cancer Res. 2017;77:6641–6650. doi: 10.1158/0008-5472.CAN-16-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G., Chen R., Alvero A.B., Fu H.H., Holmberg J., Glackin C., Rutherford T., Mor G. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010;29:3545–3553. doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama Y., Zhu H., Lee J.H., Kossenkov A.V., Wu S.Y., Wickramasinghe J.M., Yin X., Palozola K.C., Gardini A., Showe L.C. BET inhibitors suppress ALDH activity by targeting ALDH1A1 super-enhancer in ovarian cancer. Cancer Res. 2016;76:6320–6330. doi: 10.1158/0008-5472.CAN-16-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Yao H., Zhu P., Zhang X., Pan Q., Gong C., Huang Y., Hu X., Su F., Lieberman J. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Karakhanova S., Huang X., Deng S.P., Werner J., Bazhin A.V. Influence of interferon-α on the expression of the cancer stem cell markers in pancreatic carcinoma cells. Exp. Cell Res. 2014;324:146–156. doi: 10.1016/j.yexcr.2014.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.