Abstract
Introduction
Amyloid-β (Aβ) clearance is important for damage prevention in Alzheimer's disease. We investigated the utility of Aβ clearance proteins as biomarkers for mild cognitive impairment (MCI).
Methods
Serum apolipoprotein (apo) A-I, compliment protein C3 (C3), transthyretin, and cholesterol levels were measured in 273 subjects, and we analyzed the relationship between these levels and brain atrophy and cerebral blood flow in 63 clinically diagnosed mild cognitive impairment, Alzheimer's disease, and nondemented disease control subjects.
Results
ApoA-I and transthyretin levels and the active form of C3:native form of C3 ratio achieved an area under the curve of 0.89 (sensitivity: 83%, specificity: 90%) for detecting late mild cognitive impairment. Atrophy was associated with decreased apoA-I and high-density lipoprotein levels. Subjects with reduced cerebral blood flow had lower levels of native form of C3, apoA-I, high-density lipoprotein, and total cholesterol. Low native form of C3 and high active form of C3 levels were found in the hippocampi of patients with Alzheimer's disease.
Discussion
Aβ clearance proteins in the serum are potential biomarkers for mild cognitive impairment evaluation.
Keywords: Alzheimer's disease, Biomarker, Complement protein, Microglia, Neuroinflammation
1. Introduction
Alzheimer's disease (AD) is the most common type of dementia and is marked by progressive memory loss and cognitive impairment. The prodromal stage of dementia, mild cognitive impairment (MCI), provides a critical opportunity for potential intervention to prevent the onset of dementia. Detection of the disease at the early stages by blood-based biomarkers is important in the prevention of AD.
Amyloid-β peptide (Aβ) is a cleavage product of the transmembrane protein, amyloid precursor protein (APP), by β- and γ-secretases. Mutation of the presenilin 1 and 2 genes causes familial AD. Such genetic alterations increase the production of Aβ and trigger the pathogenic process leading to early-onset AD. Overproduction of Aβ in mutation carriers contributes to Aβ accumulation. However, in late-onset sporadic AD, imbalance between Aβ production and clearance may contribute to the onset of the disease. Dysfunction of Aβ clearance due to aging contributes to accumulation, aggregation, and deposition of Aβ42 in the parenchyma in sporadic AD [1].
The progression of AD pathogenesis is gradual and presents as a spectrum without any clear event defining the onset of the disease, and it is challenging for clinicians to identify the transition of patients from MCI to AD and from the nondemented disease control (NDC) state to MCI. Blood tests that assess cognitive decline using biomarkers are the least expensive method and least invasive modality for disease screening and monitoring of progression.
Advanced neuroimaging techniques provide supportive evidences for the clinical diagnosis of AD and other types of dementia. Voxel-based morphometry in magnetic resonance imaging (MRI) allows for detection of regional volume changes in AD [2]. Automatic statistical analysis of brain perfusion can be conducted using single-photon emission tomography (SPECT), which detects reductions in regional cerebral blood flow (rCBF). In the early stage of AD, perfusion reduction in the regions of interest (ROIs), namely the posterior cingulate gyrus, precuneus, and the parietal cortex, was observed [3]. Brain perfusion SPECT in the angular gyrus, inferior parietal cortex, and precuneus was found to be correlated with cerebrospinal fluid (CSF) total tau (t-tau) and phosphorylated tau (p-tau) [4]. Medial temporal structure atrophy and rCBF reduction reflects disease progression in AD.
Our previous longitudinal and cross-sectional studies using independent cohorts revealed that a combination of sequester proteins, apolipoprotein A-I (apoA-I), transthyretin (TTR), and compliment protein C3 (C3), which are involved in Aβ clearance, could distinguish AD and MCI from NDCs [5]. In this study, to confirm the clinical utility of these Aβ clearance proteins in cognitive impairment, we analyzed the serum levels of these biomarkers in early (E)MCI, late (L)MCI, and NDC subjects using conventional immunoassays used in clinical laboratories and further examined the relationship between these biomarkers and neuroimaging and blood test data.
2. Methods
2.1. Subjects
A total of 273 blood samples were collected from Uji Hospital, Kyoto, for this prospective study. Written informed consent was obtained from each participant, and all samples were rendered anonymous before laboratory analysis. Among the subjects, 63 proceeded to further clinical diagnosis involving interviewing of both the patients and their families, apolipoprotein E (APOE) genotyping, and MRI and SPECT imaging. MRI was used to evaluate atrophy of the medial temporal structures. SPECT imaging was used to assess cerebral blood flow in the parietal lobe, posterior cingulate gyrus, and precuneus. Subjects with any psychiatric illness according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria were excluded from this study.
Petersen's criteria were used to diagnose MCI [6]. Subjects with MCI were further classified into EMCI and LMCI based on the Mini–Mental State Examination (MMSE) score and objective neuroimaging parameters obtained by MRI and SPECT. Subjects with MCI who had an MMSE score 27–30, or atrophy of medial temporal structures, or decreased rCBF were classified as having EMCI. The subjects with MCI who had an MMSE score 24–30, and atrophy of medial temporal structures, and decreased rCBF were classified as having LMCI.
In this study, to compare the early stages of cognitive decline, an NDC was defined as having a MMSE score of 30, and no atrophy of the medial temporal structures as evaluated by MRI, and no rCBF decrease as evaluated by SPECT. AD was defined according to the DSM-IV criteria, and with an MMSE score of <24, and atrophy of medial temporal structures, and decreased rCBF.
2.2. Serum sampling and brain tissue sampling
Blood was sampled from the cubital veins of the subjects and placed into blood collection tubes (Venoject-II Autostep; Terumo Corporation, Tokyo, Japan). Serum was obtained after coagulation of the samples for 30 min at 22 ± 2°C and then centrifuged at 1300 × g for 10 min at 20°C. The serum samples were then transferred to 2.0-mL tubes (Sumitomo Bakelite, Tokyo, Japan) and stored at −80°C until further use.
The hippocampi of nine non-AD and 20 AD autopsy specimens were obtained from Fukushimura Hospital (Toyohashi, Japan). All protocols in the present study were approved by the ethics committee of each institute. Written informed consent was obtained from the subjects and/or their relatives.
2.3. Immunoassays of sequester proteins
Serum concentrations of apoA-I and TTR were determined by a turbidimetric immunoassay using ApoA-I Auto N‘Dai-ichi’ (Sekisui Medical, Tokyo, Japan) and N-Assay TIA Prealbumin Nittobo (Nittobo Medical, Tokyo, Japan), respectively. The native form of C3 (nC3) was assayed by a sandwich enzyme-linked immunosorbent assay established in our laboratory. The active form of C3 (aC3) was quantified by enzyme-linked immunosorbent assay (Nittobo Medical). Low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), and triglyceride levels were measured by enzymatic methods using commercial kits (Sekisui Medical). Blood glucose and HbA1c were also measured by enzymatic methods using Serotec GLU-L (Serotec, Sapporo, Japan) and CinQ HbA1c (LSI Medience Corp., Tokyo, Japan), respectively. The APOE genotype was determined as follows: after 40-cycle amplification, the polymerase chain reaction product was digested with the restriction enzyme HhaI and subjected to electrophoresis in a 10% polyacrylamide gel [7].
2.4. Magnetic resonance imaging
The voxel-based specific regional analysis system for Alzheimer's disease (VSRAD) allows for measurement of atrophy in the target ROI, the medial temporal structures, involving the entire region of the entorhinal cortex, hippocampus, and amygdala. The severity of atrophy was obtained by summing the positive z-score in the target ROI/total voxel in the target ROI [2]. The assessment of atrophy was performed using the following scores: normal (0–1), mild (1–2), moderate, and severe (>2).
2.5. Single-photon emission tomography
Regional CBF was evaluated using SPECT. The z-score was also used to compare subjects using SPECT. Statistical analysis of perfusion reduction was conducted. The severity of perfusion reduction in the ROIs (the posterior cingulate gyrus, precuneus, and parietal cortex) was calculated by the following equation: severity = sum of the positive z-score in the target ROI/total voxel in the target ROI [3].
The subjects were categorized into four groups based on the observed reduction in rCBF, as follows: (A) No abnormality in four ROIs including the left/right posterior cingulate gyrus/precuneus and parietal cortex. (B) Decreased rCBF in one ROI. (C) Decreased rCBF in two ROIs and decreased rCBF in the posterior cingulate gyrus or the precuneus. (D) Decreased rCBF in three ROIs and decreased rCBF in the posterior cingulate gyrus and the precuneus (Supplementary Fig. 1).
2.6. Immunohistochemistry of complement proteins and Aβ42 in human brain specimens
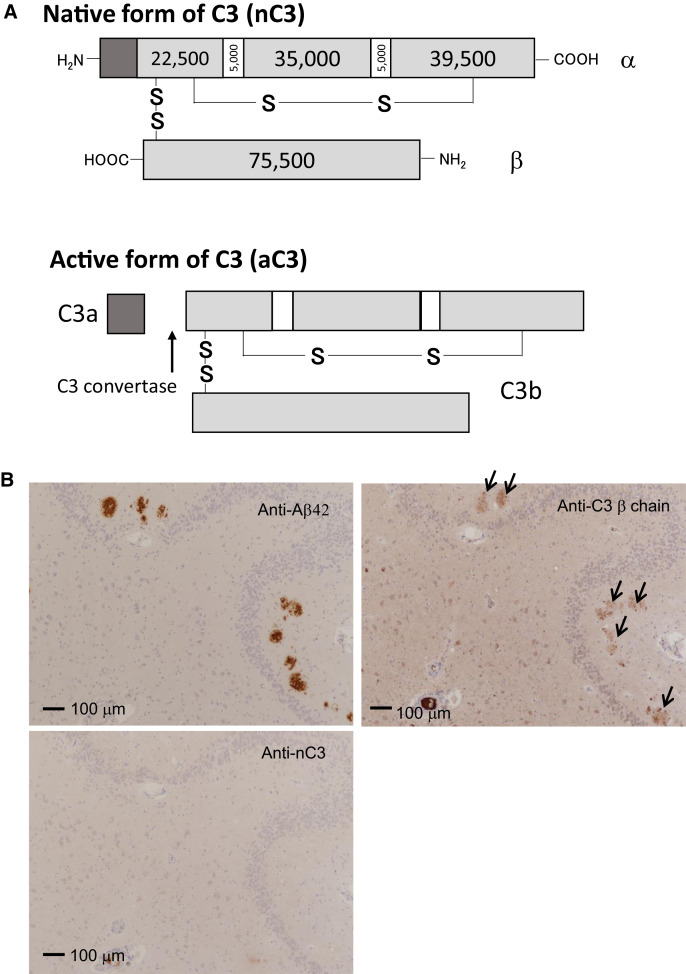
Immunohistochemical staining for C3 was carried out on paraffin-embedded tissue sections using two types of primary antibody. One was an in-house antibody that recognized only the native form of C3, and the other was a monoclonal antibody that detects C3β chains (Santa Cruz Biotechnology, Dallas, TX). Senile plaques were stained with anti-Aβ42 antibody purchased from Immuno-Biological Laboratories Co., Ltd. (Fujioka, Gunma, Japan). Non-AD sections exhibited no Aβ42-positive staining. Tissues were examined using an ECLIPSE Ni-U microscope (Nikon, Tokyo, Japan) and NIS-Elements BR software (Nikon). The mean intensity of the stained region in specimens was quantified by Image-Pro Premier (Roper Technologies, Sarasota, FL).
2.7. Statistical analysis
A P value ≤ .05 was considered significant. For comparisons among more than two groups, the Kruskal-Wallis test was used. Bonferroni correction was applied when two groups were compared. Relationships between the analytes and MMSE scores were analyzed with bivariate correlations using Pearson's correlation coefficients. Origin software (ver. 7.5, OriginLab Corp., Northampton, MA) and MedCalc (ver. 9.3.9) (MedCalc Software, Mariakerke, Belgium) were used to perform the statistics analyses. The least absolute shrinkage and selection operator (LASSO) modeling using the glmnet package (ver. 1.9-5) for R (ver. 3.1.0; R Foundation for Statistical Computing, Vienna, Austria) was used to evaluate the combination of multiple biomarkers.
3. Results
3.1. Clinical performance of a set of sequester proteins as biomarkers for EMCI and LMCI
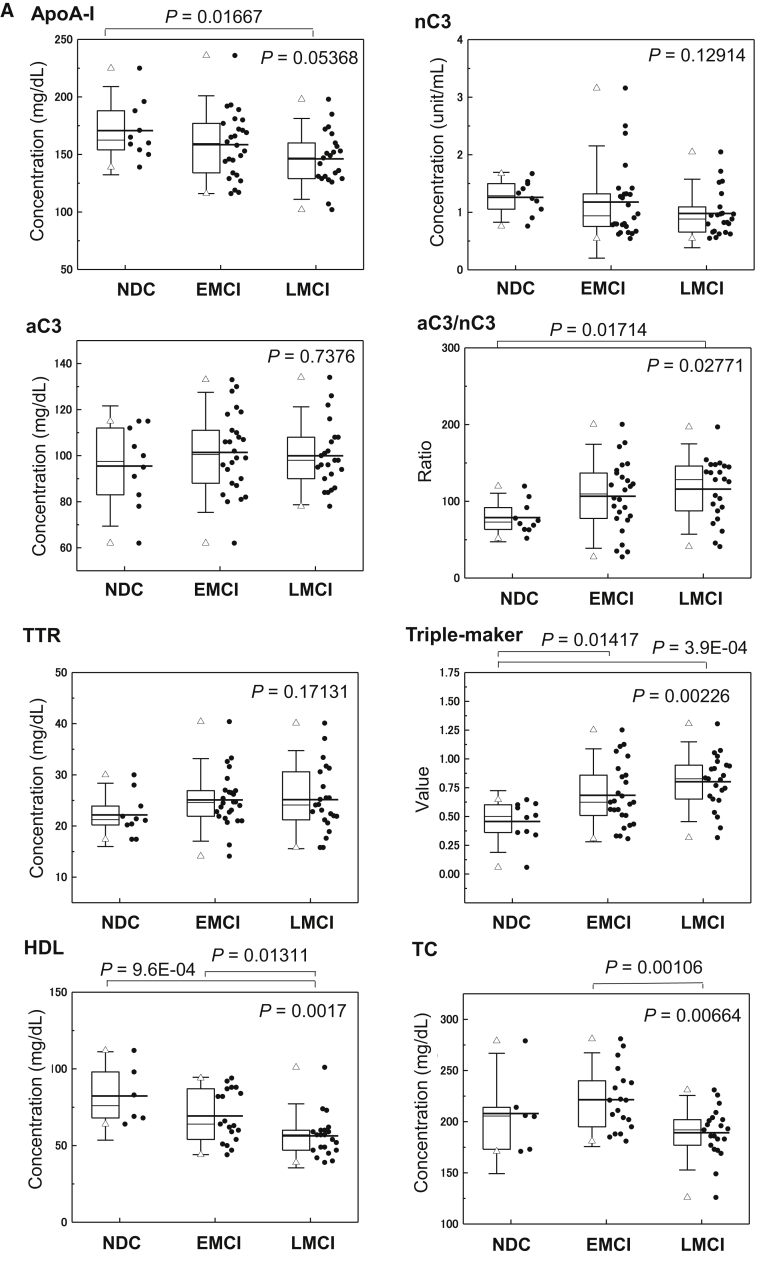
Table 1 provides the serum levels of analytes among the NDC, EMCI, LMCI, and AD groups. As only four subjects were diagnosed with AD, they were excluded from further statistical analysis. The significant differences among the three groups (NDC, EMCI, and LMCI) are presented in Fig. 1A. The apoA-I level (P = .01667) and aC3 to nC3 ratio (aC3/nC3) (P = .01714) were significantly changed in LMCI compared with NDC subjects. The value of the triple marker of apoA-I, aC3/nC3, and TTR was significantly higher in EMCI (P = .01417) and LMCI (P = 3.9E-4) than NDC subjects. Lower HDL levels (P = 9.6E-04) in LMCI than NDC subjects, and lower TC levels (P = .00106) in LMCI than EMCI subjects were observed. As shown in Table 1, the C4 levels showed increasing trends in EMCI and LMCI compared with NDC subjects (P = .00905).
Table 1.
Serum levels of proteins involved in amyloid-β clearance and cholesterols in NDC and cognitive impairment
| Clinical characteristics and biomarker | NDC (n = 10) | EMCI (n = 26) | LMCI (n = 23) | AD (n = 4) | P value∗ |
|---|---|---|---|---|---|
| Age | 62.6 ± 8.3† | 65.5 ± 10.5 | 69.9 ± 9.6 | 77.0 ± 3.7 | .11499 |
| Male/female | 2/8 | 7/19 | 14/9 | 1/3 | |
| VSRAD score | 0.8 ± 0.2 | 1.1 ± 0.6 | 1.26 ± 0.6 | 2.74 ± 1.1 | |
| MMSE score | 30.0 ± 0.0 | 28.9 ± 1.0 | 27.3 ± 1.6 | 23.4 ± 0.5 | |
| APOE ε4 carrier (%) | 10.0 | 19.2 | 43.5 | 20.0 | |
| aC3, mg/dL | 95.5 ± 17.4 | 101.4 ± 17.4 | 100 ± 14.2 | 115.3 ± 14.0 | .7376 |
| nC3, unit/mL | 1.3 ± 0.3 | 1.2 ± 0.7 | 1.0 ± 0.4 | 1.1 ± 0.4 | .12914 |
| aC3/nC3 ratio | 78.9 ± 21.1 | 106.6 ± 45.2 | 115.97564‡ | 109.6 | .02771 |
| C4, mg/dL | 20 ± 3.0 | 26.6 ± 5.3§ | 26.9 ± 4.9‡ | 27.7 ± 3.0 | .00905 |
| TTR, mg/dL | 22.2 ± 4.1 | 25.1 ± 5.4 | 25.2 ± 6.4 | 23.4 ± 3.9 | .17131 |
| ApoA-I, mg/dL | 170.7 ± 25.6 | 158.5 ± 28.3 | 146.1 ± 23.4‡ | 122.3 ± 16.3 | .05368 |
| ApoE, mg/dL | 3.8 ± 0.5 | 4.5 ± 1.1§ | 3.8 ± 0.5 | 4.3 ± 1.1 | .07568 |
| HDL, mg/dL | 82.3 ± 19.2 | 69.3 ± 16.7 | 56.3 ± 13.9¶ | 40.0 ± 2.0 | .0017 |
| LDL, mg/dL | 116.3 ± 42.0 | 140.4 ± 34.8 | 117.0 ± 19.6¶ | 109.0 ± 16.5 | .0555 |
| TC, mg/dL | 208.0 ± 39.2 | 221.5 ± 30.5 | 189.2 ± 24.3¶ | 182.3 ± 23.3 | .00664 |
| TG, mg/dL | 100.3 ± 38.6 | 133.6 ± 68.6 | 151.9 ± 70.9 | 246.0 ± 112.7 | .29456 |
| BS, mg/dL | 129.9 ± 53.8 | 113.5 ± 23.9 | 118.0 ± 34.3 | 121.8 ± 33.6 | .73745 |
| HbA1c (%) | 5.8 ± 0.7 | 5.7 ± 0.5 | 5.8 ± 0.6 | 5.7 ± 0.7 | .80727 |
| Triple-marker sore (ApoA-I, TTR, nC3) | 0.47 ± 0.20 | 0.63 ± 0.29§ | 0.80 ± 0.23‡ | 0.90 ± 0.23 | .00418 |
| Triple-marker sore (ApoA-I, TTR, aC3/nC3) | 0.46 ± 0.18 | 0.68 ± 0.27§ | 0.80 ± 0.23‡ | 0.91 ± 0.23 | .00226 |
Abbreviations: VSRAD, voxel-based specific regional analysis system for Alzheimer's disease; nC3, native form of C3; aC3, active form of C3; TTR, transthyretin; TC, total cholesterol; TG, triglyceride; BS, blood sugar; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment; NDC, nondemented disease control; AD, Alzheimer's disease.
Kruskal-Wallis test. Significant differences among 3 groups NDC, EMCI, and LMCI are indicated.
Mean ± SD.
Holm-Bonferroni test. Significant differences in NDC vs. LMCI were observed in aC3/nC3 ratio (P = .01714), C4 (P = .00408), apoA-I (P = .01576), HDL (P = 9.6E-04), triple-marker score (ApoA-I, TTR, nC3) (P = 1.0E-04), and triple-marker score (ApoA-I, TTR, aC3/nC3) (P = 3.9E-04).
Holm-Bonferroni test. Significant differences in NDC vs. EMCI were observed in C4 (P = .00585), apoE (P = .01841), triple-marker score (ApoA-I, TTR, nC3) (P = .02672), and triple-marker score (ApoA-I, TTR, aC3/nC3) (P = .01417).
Holm-Bonferroni test. Significant differences in EMCI vs. LMCI were observed in HDL (P = .01311), LDL (P = .01737), and TC (P = .00106).
Fig. 1.
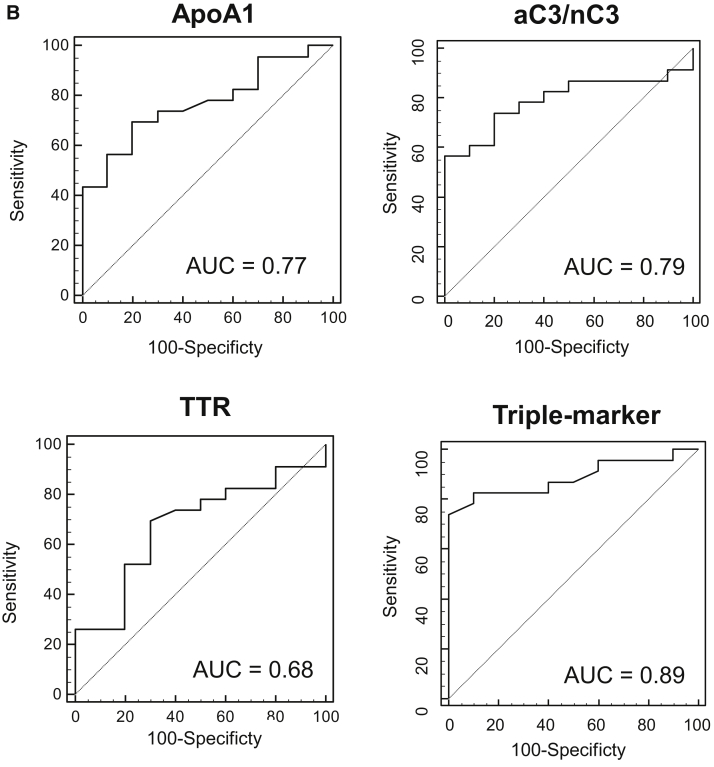
Clinical validity of Aβ clearance–associated proteins in the serum as blood-based biomarkers for EMCI and LMCI. (A) Serum levels of aC3, nC3, apoA-I, aC3/nC3, TTR, triple markers (apoA-I, aC3/nC3, and TTR), HDL, and TC in EMCI (n = 26), LMCI (n = 23), and NDC (n = 10) subjects. The bold solid bars within the boxplot represent the median abundance, and the solid bars represent the mean abundance for the given group. Open triangles are the highest and lowest values in each group. Error bars represent ±1.5 standard deviation. Statistical differences among the three groups (NDC, EMCI, and LMCI) were evaluated by the Kruskal-Wallis test. (B) C-statistics of sequester protein (apoA-I, aC3/nC3, TTR, and triple markers) levels demonstrated clinical potential in discriminating LMCI from NDC subjects. Abbreviations: aC3, active form of C3; nC3, native form of C3; TC, total cholesterol; TTR, transthyretin; NDC, nondemented disease control; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment.
The clinical potentials of apoA-I, aC3/nC3, and TTR, which could discriminate LMCI from NDC, were as follows (Table 2 and Fig. 1B)—apoA-I: area under the curve (AUC) = 0.77 (P = .0011), sensitivity 70%, specificity 80%; TTR: AUC = 0.68 (P = .0839), sensitivity 70%, specificity 70%; nC3: AUC = 0.74 (P = .0088), sensitivity 74%, specificity 80%; and aC3/nC3: AUC = 0.79 (P = .0002), sensitivity 83%, specificity 60%. The triple marker of apoA-I, TTR, and nC3 based on the LASSO regression analysis demonstrated high clinical potential in LMCI vs. NDC subjects with AUC = 0.86 (P < .0001), sensitivity 78%, and specificity 90%, and in EMCI vs. NDC subjects with AUC = 0.67 (P = .0546), sensitivity 58%, and specificity 70%. Furthermore, the triple marker of apoA-I, TTR, and aC3/nC3 showed higher clinical potential in LMCI vs. NDC subjects with AUC = 0.89 (P < .0001), sensitivity 83%, and specificity 90%, and in EMCI vs. NDC subjects with AUC = 0.73 (P = .0087), sensitivity 58%, and specificity 80%.
Table 2.
Clinical value of proteins involved in amyloid-β clearance as biomarkers for LMCI and EMCI
| Group | Biomarker | Sensitivity (%) | Specificity (%) | AUC | 95% CI | P value | Criterion |
|---|---|---|---|---|---|---|---|
| NDC vs. LMCI | ApoA-I | 70 | 80 | 0.77 | 0.593–0.899 | .0011 | ≤153 |
| TTR | 70 | 70 | 0.68 | 0.491–0.828 | .0839 | >21.9 | |
| nC3 | 74 | 80 | 0.74 | 0.557–0.876 | .0088 | ≤0.98 | |
| aC3 | 61 | 50 | 0.55 | 0.372–0.727 | .6571 | >95 | |
| aC3/nC3 | 83 | 60 | 0.79 | 0.615–0.913 | .0002 | >74.93 | |
| Triple marker (ApoA-I, TTR, nC3) | 78 | 90 | 0.86 | 0.696–0.956 | <.0001 | >0.61 | |
| Triple marker (ApoA-I, TTR, aC3/nC3) | 83 | 90 | 0.89 | 0.734–0.972 | <.0001 | >0.61 | |
| NDC vs. EMCI | ApoA-I | 50 | 60 | 0.62 | 0.439–0.772 | .2507 | ≤149 |
| TTR | 73 | 70 | 0.70 | 0.523–0.839 | .0654 | >21.9 | |
| nC3 | 53 | 80 | 0.65 | 0.475–0.802 | .106 | ≤0.97 | |
| aC3 | 65 | 50 | 0.58 | 0.401–0.739 | .4835 | >95 | |
| aC3/nC3 | 62 | 80 | 0.72 | 0.545–0.856 | .0112 | >91.9 | |
| Triple marker (ApoA-I, TTR, nC3) | 58 | 70 | 0.67 | 0.497–0.850 | .0546 | >0.61 | |
| Triple marker (ApoA-I, TTR, aC3/nC3) | 58 | 80 | 0.73 | 0.553–0.862 | .0087 | >0.61 |
Abbreviations: TTR, transthyretin; nC3, native form of C3; aC3, active form of C3; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment; NDC, nondemented disease control.
Subjects were divided into APOE ε4-positive and APOE ε4-negative groups. The APOE ε4-positive group had significantly lower levels of serum apoA-I (P = .01635) and apoE (P = .01945) and higher scores in the triple markers (P = .0091, P = .00186) than did the APOE ε4-negative group. APOE ε4 did not affect the serum levels of other analytes (Supplementary Table 1).
3.2. MMSE scores and serum levels of biomarker proteins
We categorized all 273 subjects according to the MMSE score into four groups [score 27–30 (n = 208), 24–26 (n = 26), 20–23 (n = 20), and <20 (n = 19)] (Supplementary Table 2 and Supplementary Fig. 2). ApoA-I (P = .00345), TTR (P = 5.75E−05), HDL (P = .00134), and TC (P = 8.77E-05) were significantly lower in subjects with lower MMSE scores. Significant correlations were observed between TTR (r = 0.30449, P = 2.89E-07), apoA-I (r = 0.24329, P = 4.86E-05), HDL (r = 0.26565, P = 5.25E-05), LDL (r = 0.14556, P = .02869), and TC (r = 0.29819, P = 5.06E-06), and the MMSE scores (Supplementary Table 3).
3.3. Relationship between blood biomarkers and atrophy of the medial temporal structures
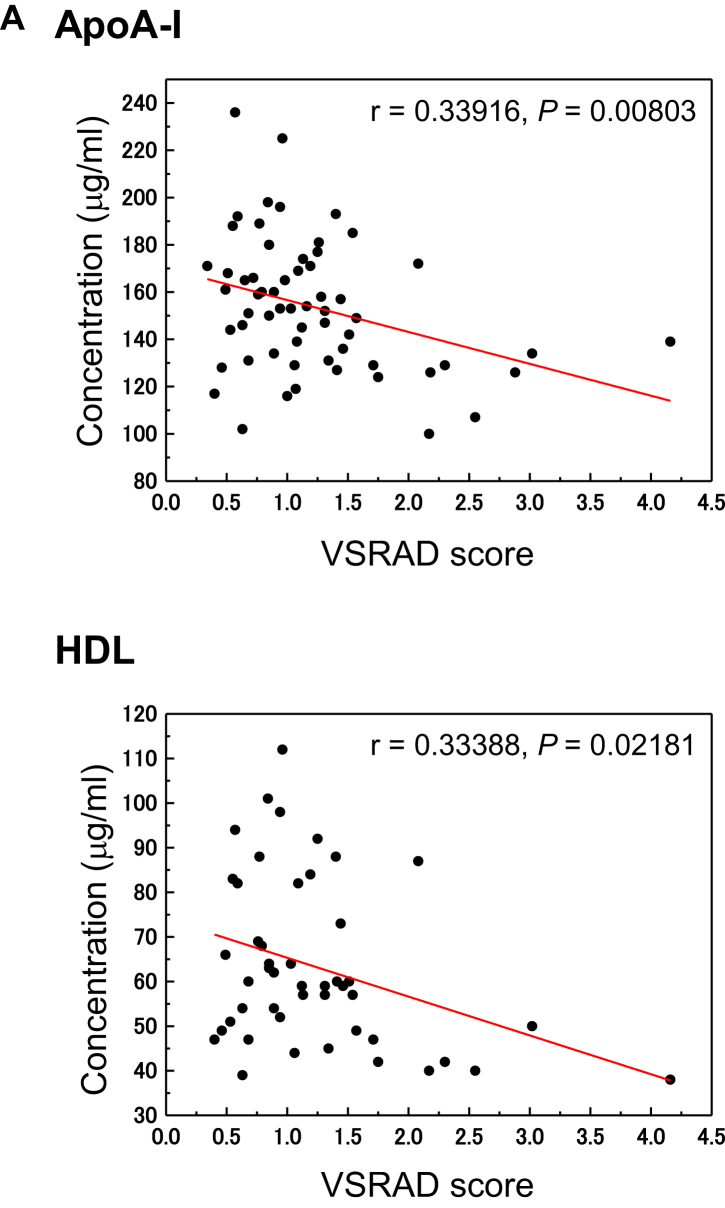
Atrophy of the medial temporal structures of 63 clinically diagnosed MCI, AD, and NDC subjects was measured by the semiquantified VSRAD score and divided into three groups; VSRAD 0–1, VSRAD 1–2, and VSRAD >2. ApoA-I (P = .01801) and C4 (P = .01239) were significantly elevated according to the VSRAD score (Supplementary Table 4). We further investigated the relationship between the hippocampal volume and the peripheral levels of biomarker proteins (Fig. 2A). Significant correlations between brain atrophy and apoA-I (r = 0.33916, P = .00803) and HDL (r = 0.33388, P = .02181) were observed.
Fig. 2.
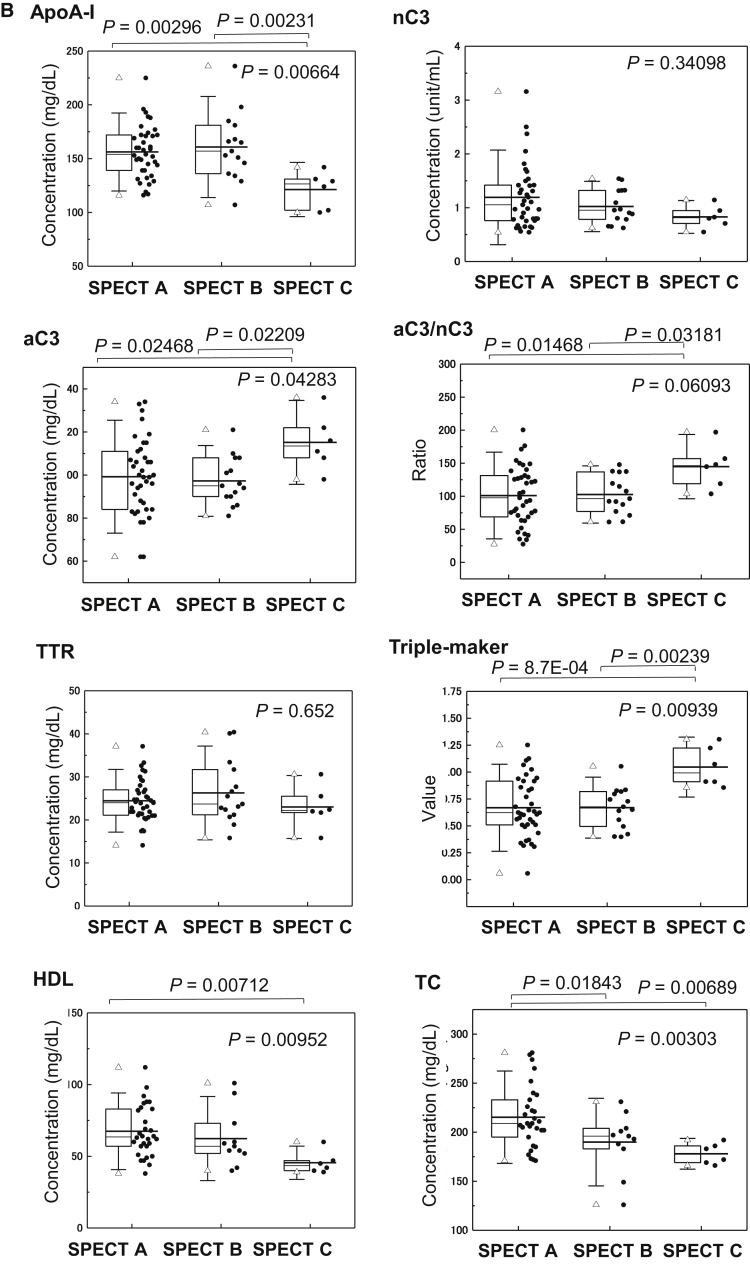
Serum levels of proteins associated with Aβ clearance and cholesterols associated with reduced rCBF and atrophy of the medial temporal structures. (A) Serum levels of apoA-I and HDL were correlated with the medial temporal structure volume, including the entire region of the entorhinal cortex, hippocampus, and amygdala, as detected by VSRAD. MRI data were analyzed visually and by voxel-based morphometry techniques to detect regional volume changes. (B) Changes in apoA-I, aC3, nC3, aC3/nC3, TTR, triple-marker, HDL, and TC levels with reduction of rCBF were evaluated by SPECT. Abbreviations: aC3, active form of C3; nC3, native form of C3; TC, total cholesterol; TTR, transthyretin; rCBF, reduced cerebral blood flow; VSRAD, voxel-based specific regional analysis system for Alzheimer's disease; SPECT, single-photon emission tomography.
3.4. Relationship between rCBF reduction and serum biomarker levels
We compared the levels of rCBF reduction in age-matched NDC, MCI, and AD subjects. Perfusion reduction in the posterior cingulate gyrus, precuneus, and cortex was observed in the early stage of AD (data not shown). Next, rCBF reduction was classified into four categories: SPECT A, B, C, and D as described in Methods. The data of 63 subjects, after exclusion of three subjects who were classified as SPECT D, were statistically analyzed with regard to differences in biomarker levels among the three groups (SPECT A, B, and C) (Table 3 and Fig. 2B). The serum levels of aC3 (P = .04283), apoA-I (P = .00664), HDL (P = .00952), TC (P = .00303), and the triple marker of apoA-I, TTR, and aC3/nC3 (P = .00939) underwent significant changes in the patients with severe reductions in rCBF.
Table 3.
Serum levels of proteins involved in amyloid-β clearance and cholesterols in participants categorized by rCBF reduction levels
| Clinical characteristics and biomarker | SPECT a (n = 39) | SPECT B (n = 15) | SPECT C (n = 6) | SPECT D (n = 3) | P value∗ |
|---|---|---|---|---|---|
| Age | 67.4 ± 11.1† | 66.3 ± 8.2 | 70.7 ± 9.3 | 65.7 ± 6.8 | .75008 |
| Male/female | 10/29 | 8/7 | 4/2 | 2/1 | |
| MMSE score | 28.4 ± 1.9 | 28.1 ± 1.3 | 26.8 ± 2.6 | 27.0 ± 2.6 | .11915 |
| VSRAD | 1.2 ± 0.7 | 1.2 ± 0.6 | 1.5 ± 0.6 | 1.3 ± 1.4 | .16889 |
| APOE ε4 carrier, % | 20.5 | 33.3 | 50.0 | 33.3 | |
| aC3, mg/dL | 99.2 ± 17.5 | 97.3 ± 11.0 | 115.2 ± 13.0‡,§ | 110.1 ± 15.3 | .04283 |
| nC3, unit/mL | 1.2 ± 0.6 | 1.0 ± 0.3 | 0.8 ± 0.2 | 1.2 ± 0.5 | .34098 |
| aC3/nC3 | 101.1 ± 43.8 | 102.7 ± 29.0 | 144.9 ± 32.4‡,§ | 104.5 ± 40.6 | .06093 |
| C4, mg/dL | 25.3 ± 5.3 | 26.2 ± 4.2 | 28.8 ± 7.5 | 27.0 ± 5.7 | .59451 |
| TTR, mg/dL | 24.4 ± 4.9 | 26.3 ± 7.3 | 23.0 ± 4.9 | 20.2 ± 3.9 | .65200 |
| ApoA-I, mg/dL | 156.2 ± 24.2 | 160.8 ± 31.3 | 121.3 ± 16.8‡,§ | 148.7 ± 37.5 | .00664 |
| ApoE, mg/dL | 4.4 ± 0.9 | 3.6 ± 0.9 | 4.0 ± 0.9 | 3.1 ± 0.4 | .05498 |
| HDL, mg/dL | 67.5 ± 17.8 | 62.4 ± 19.5 | 45.5 ± 7.7‡ | 65.5 ± 23.3 | .00952 |
| LDL, mg/dL | 133.5 ± 33.1 | 113.4 ± 28.2 | 113.8 ± 12.2 | 103.5 ± 3.5 | .19468 |
| TC, mg/dL | 215.3 ± 31.4 | 189.9 ± 29.8 | 178.0 ± 10.4‡ | 180.5 ± 10.6 | .00303 |
| TG, mg/dL | 142.1 ± 76.0 | 145.6 ± 79.3 | 158.3 ± 74.6 | 126.5 ± 61.5 | .91709 |
| BS, mg/dL | 117.5 ± 34.3 | 120.3 ± 27.2 | 103.5 ± 31.8 | 148.3 ± 60.6 | .28727 |
| HbA1c (%) | 5.7 ± 0.6 | 5.7 ± 0.7 | 5.6 ± 0.5 | 6.4 ± 0.3 | .93751 |
| Triple-marker sore (ApoA-I, TTR, nC3) | 0.64 ± 0.28 | 0.69 ± 0.21 | 1.01 ± 0.16‡,§ | 0.63 ± 0.41 | .00909 |
| Triple-marker sore (ApoA-I, TTR, aC3/nC3) | 0.67 ± 0.27 | 0.67 ± 0.19 | 1.05 ± 0.19‡,§ | 0.67 ± 0.35 | .00939 |
Abbreviations: SPECT, single-photon emission tomography; rCBF, reduced cerebral blood flow; VSRAD, voxel-based specific regional analysis system for Alzheimer's disease; nC3, native form of C3; aC3, active form of C3; TTR, transthyretin; TC, total cholesterol; TG, triglyceride; BS, blood sugar.
Kruskal-Wallis test. Significant differences among 3 groups (SPECT A, B, and C) are indicated.
Mean ± SD.
Holm-Bonferroni test. Significant differences in SPECT A versus C were observed in apoA-I (P = .00296), aC3 (P = .02468), C3 ratio (aC3/nC3) (P = .01468), HDL (P = .00712), TC (P = .00689), triple-marker score (ApoA-I, TTR, nC3) (P = .00154), and triple-marker score (ApoA-I, TTR, aC3/nC3) (P = 8.7E-04).
Holm-Bonferroni test. Significant differences in SPECT B versus C were observed in apoA-I (P = .00231), aC3 (P = .02209), C3 ratio (aC3/nC3) (P = .03181), triple-marker score (ApoA-I, TTR, nC3) (P = .01139), and triple-marker score (ApoAI, TTR, aC3/nC3) (P = .00239).
3.5. Immunohistochemical staining of C3 in human brains
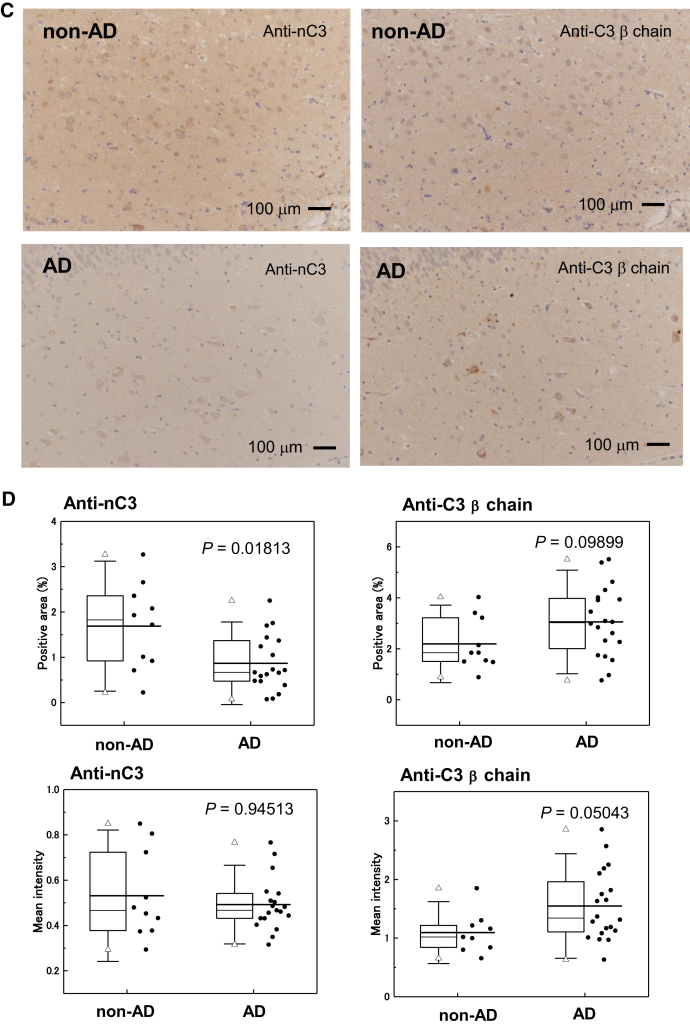
We performed immunohistochemical staining of human brain specimens using anti-nC3 and anti-C3β chain antibodies. We raised an antibody against a synthetic peptide whose sequence corresponded to the peptide sequence covering the C3a-C3b cleavage site by C3 convertase (Fig. 3A). By immunoprecipitation-Western blot analysis, we confirmed the specificities of these anti-nC3 and anti-C3β chain antibodies against nC3 (noncleaved C3) and the C3b active form, respectively (data not shown). Senile plaques exhibited colocalization of aC3 and Aβ42 in the hippocampi of definite AD patients (Fig. 3B). Lower levels of nC3 were observed in the hippocampi of AD patients than in those of non-AD patients (Fig. 3C). The positive areas stained by anti-nC3 and anti-C3β in non-AD (n = 9) and definite AD (n = 20) brain sections were analyzed by imaging software. Lower levels of nC3 and higher levels of aC3 were observed in the hippocampi of definite AD patients than in those of non-AD patients (Fig. 3D).
Fig. 3.
Expression of nC3 and aC3 in the hippocampal tissues from definite AD and non-AD patients. (A) Schematic representation of aC3 and nC3 forms. At the first step of C3 activation, the C3a fragment is produced by cleavage via C3 convertase. (B) Colocalization of the C3 fragment and Aβ42 in the AD hippocampus. Arrows indicate aC3 colocarized with senile plaque. (C) Demonstration of C3 activation in the AD hippocampus. (D) Analysis of nC3 and C3b levels in the brain tissues including the hippocampus from definite AD (n = 20) and non-AD (n = 9) patients. Abbreviations: aC3, active form of C3; nC3, native form of C3; AD, Alzheimer's disease.
4. Discussion
Blood-based biomarkers could allow us to identify cognitive decline at the early stages and to monitor improvements in cognitive function after intervention. In this study, a combination of apoA-I, aC3/nC3, and TTR was found able to discriminate MCI from NDC with a high ROC value. Brain atrophy in the medial temporal lobe was associated with decrease in apoA-I and HDL levels, and significantly lower apoA-I, HDL, and TC levels were observed in patients with severe reductions in rCBF as determined by SPECT.
In this study, Petersen's and DSM-IV criteria were used to diagnose MCI and AD, respectively. Subjects with MCI were further classified into EMCI and LMCI based on the MMSE score and objective neuroimaging parameters obtained by MRI and SPECT. We used an MMSE score of 27 as the cutoff value between EMCI and LMCI. An MMSE score below 24 has primarily been used as the cognitive deficit cutoff for dementia [8], [9]. Iwasa H et al. reported that subjects with an MMSE score of 24–27 indicated higher risk for dementia [9]. Hippocampal atrophy and rCBF were included in the classification of EMCI and LMCI. Volumetric measurements of medial temporal atrophy are a sensitive marker for AD, and hippocampal volume correlates with the density of neurofibrillary tangles and CSF t-tau and p-tau in AD [10]. In subjects with Aβ deposition, entorhinal cortex atrophy detected by MRI significantly correlated with cognitive decline [11]. Combined decreased parietal rCBF and abnormal CSF tau in patients with MCI indicates a substantially increased risk of future development of AD [12], [13]. Thus, medial temporal atrophy and rCBF reduction could be used for the assessment of the early stages of cognitive decline.
More than 80% of patients with AD exhibit cerebral amyloid angiopathy [14], [15], and a strong correlation between vascular risk factors and sporadic AD has been noted. Recently, multifactorial data-driven analysis used amyloid positron emission tomography, functional and structural MRI, and Arterial Spin Labeling imaging from the Alzheimer's Disease Neuroimaging Initiative and suggested that cerebral vascular dysregulation was an early pathological event and that lipid metabolism dysfunction, inflammatory activation, and insulin resistance were involved in sporadic AD pathogenesis [16].
We observed decreased serum levels of apoA-I, HDL, and TC in patients with cognitive dysfunction and lower MMSE scores. ApoA-I plays an important role in cholesterol transfer and is transported from the periphery to the CSF. Injected apoA-I in the periphery gains access to the central nervous system via the choroid plexus [17]. Among the apolipoproteins, the peripheral apoA-I level is one of the strongest risk factors for cognitive decline [18]. The apoA-I protein prevented Aβ40 and Aβ42 aggregation and induced toxicity in primary brain cells in AD model mice [19]. ATP-binding cassette transporter A1 (ABCA1) mutation caused virtual absence of apoA-I and HDL, and ABCA1 knock-out mice exhibited decreased apoE levels and increased Aβ deposition in the brain [20]. Overexpression of apoA-I attenuated neuroinflammation and cognitive function in an AD mouse model [21]. APOE ε4 carriers who had subjective cognitive decline with low plasma apoA-I and high CSF apoA-I levels had increased risk of clinical progression of AD [22]. Thus, a decrease in the peripheral apoA-I level may be one of the earliest events in AD progression, even earlier than Aβ deposition commences in the brain.
Lower levels of HDL are considered a risk factor for atherosclerotic diseases. Peripheral apoA-I is strongly related to HDL cholesterol. Aβ clearance is facilitated by circulating HDL. These data suggest that peripheral cholesterol and apolipoproteins might be involved in Aβ deposition in AD pathogenesis [23]. Indeed, individuals affected by MCI and dementia have significantly lower levels of peripheral HDL [24], [25]. Yasuno et al. reported that significantly higher HDL concentrations were associated with better cognitive function in an APOE ε4-negative group of subjects in a cohort study [26]. The association between higher LDL and lower HDL cholesterol levels and a higher Pittsburgh Compound-B index in amyloid positron emission tomography (indicating Aβ burden) has been reported [27]. Higher levels of HDL may reduce the risk of late-onset AD [28].
APOE ε4 gene polymorphism is risk factor for AD [29]. We investigated the relationship between the presence of APOE ε4 and Aβ clearance protein levels in this study. The APOE ε4+ group had lower serum levels of apoE, apoA-I, and HDL than did the APOE ε4− group. APOE ε4 affects the ability to eliminate soluble Aβ from the brain [30] and is less effective than APOE ε2 in promoting Aβ transportation [23]. ApoA-I, apoE, and HDL may synergize to facilitate Aβ transport across human cerebral vessels.
Complement is an important component of innate immunity in the central nervous system. A proteolytic cascade of downstream complement proteins results in C3-induced opsonization and phagocytosis for Aβ clearance. In this study, the preliminary immunohistochemistry data from the hippocampal analysis were indicative of activation of C3 in the AD brain. In the later stages of AD, an enhanced inflammatory response and activation of terminal membrane attack complex C5b-9 may contribute to neuronal cell death, which leads to synaptic failure [31], [32], [33].
In the peripheral circulation, Aβ could activate the complement system. Erythrocytes express cell-surface complement receptor 1, which recognizes complement-opsonized Aβ. Erythrocyte-captured Aβ42 levels were significantly lower in AD than MCI and NDC and correlated with lower MMSE scores. Erythrocyte-mediated Aβ migrates to the liver and is removed by clearance via Kupffer cells [34], [35].
Lower levels of serum TTR were related to lower MMSE scores in this study. Several previous reports have observed reduced TTR plasma levels in patients with MCI and AD [5], [36]. TTR has been reported to be linked to AD pathogenesis because of its ability to sequester Aβ40 and Aβ42 and suppress Aβ fibrillation [37], [38]. TTR was found to increase Aβ42 efflux from the brain to the periphery, and to regulate LDL-receptor-related protein 1 expression.
This study has limitations. The relationship between the triple markers and Aβ load was not clarified. The serum levels of these biomarkers involved in Aβ clearance during AD pathological change detected by amyloid positron emission tomography should be analyzed in future study.
Reduced serum levels of apoA-I and TTR with an increased aC3/nC3 ratio might indicate dysregulation of Aβ clearance, and these conditions may contribute to the progression of cognitive impairment and atrophy of the medial temporal structures and hypoperfusion of rCBF. ApoA-I, TTR, and complement protein profiles are potential blood-based biomarkers for the assessment of the early stages of cognitive decline.
Research in Context.
-
1.
Systematic review: The importance of amyloid-β (Aβ) clearance in Alzheimer's disease pathogenesis is well understood. A combination of sequester proteins in serum, which are involved in Aβ clearance (apoA-I, TTR, and C3), has been shown to distinguish patients with mild cognitive impairment from nondemented disease controls. However, the clinical utility of these biomarkers and their relationships with neuroimaging data remain unclear.
-
2.
Interpretation: We demonstrated the utility of sequester proteins involved in Aβ clearance in evaluating cognitive decline and the progression of cognitive impairment in this prospective study. The impaired function of these proteins may increase susceptibility to cognitive impairment. We thus propose a simple blood test for risk assessment of cognitive decline.
-
3.
Future directions: Further investigation is required to understand (1) the clinical utility of these biomarkers, which should be addressed in a larger-scale clinical study, and (2) the changes in these biomarker levels after treatment for cognitive decline.
Acknowledgments
The authors thank Yoshihiro Hirokawa, Norihiro Ogawa, and Yoko Okabe for their help in clinical sample collection and Yoshinori Nishimura for statistical analysis. The authors thank all the participants in this study for their commitment and help in advancing research. This study was supported in part by the Grants-in-Aid from the Ministry of Health, Labour, and Welfare of Japan (Grant No. H23-dementia and fracture-003). With regard to potential conflicts of interest, Kazuhiko Uchida serves as a board member of MCBI, Inc.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dadm.2018.11.003.
Supplementary data
References
- 1.Mawuenyega K.G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J.C. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuda H., Mizumura S., Nemoto K., Yamashita F., Imabayashi E., Sato N. Automatic voxel-based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer Disease. AJNR Am J Neuroradiol. 2012;33:1109–1114. doi: 10.3174/ajnr.A2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuda H., Mizumura S., Nagao T., Ota T., Iizuka T., Nemoto K. Automated discrimination between very early Alzheimer disease and controls using an easy Z-score imaging system for multicenter brain perfusion single-photon emission tomography. AJNR Am J Neuroradiol. 2007;28:731–736. [PMC free article] [PubMed] [Google Scholar]
- 4.Habert M.O., de Souza L.C., Lamari F., Daragon N., Desarnaud S., Jardel C. Brain perfusion SPECT correlates with CSF biomarkers in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2010;37:589–593. doi: 10.1007/s00259-009-1285-8. [DOI] [PubMed] [Google Scholar]
- 5.Uchida K., Shan L., Suzuki H., Tabuse Y., Nishimura Y., Hirokawa Y. Amyloid-β sequester proteins as blood-based biomarkers of cognitive decline. Alzheimers Dement (Amst) 2015;1:270–280. doi: 10.1016/j.dadm.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 7.Wenham P.R., Price W.H., Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. 1991;337:1158–1159. doi: 10.1016/0140-6736(91)92823-k. [DOI] [PubMed] [Google Scholar]
- 8.Marioni R.E., Chatfield M., Brayne C., Matthews F.E., Medical Research Council Cognitive Function and Ageing Study Group The reliability of assigning individuals to cognitive states using the Mini Mental-State Examination: a population-based prospective cohort study. BMC Med Res Methodol. 2011;11:127. doi: 10.1186/1471-2288-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasa H., Kai I., Yoshida Y., Suzuki T., Kim H., Yoshida H. Global cognition and 8-year survival among Japanese community-dwelling older adults. Int J Geriatr Psychiatry. 2013;28:841–849. doi: 10.1002/gps.3890. [DOI] [PubMed] [Google Scholar]
- 10.de Souza L.C., Chupin M., Lamari F., Jardel C., Leclercq D., Colliot O. CSF tau markers are correlated with hippocampal volume in Alzheimer's disease. Neurobiol Aging. 2012;33:1253–1257. doi: 10.1016/j.neurobiolaging.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Tateno A., Sakayori T., Kawashima Y., Higuchi M., Suhara T., Mizumura S. Comparison of imaging biomarkers for Alzheimer's disease: amyloid imaging with [18F]florbetapir positron emission tomography and magnetic resonance imaging voxel-based analysis for entorhinal cortex atrophy. Int J Geriatr Psychiatry. 2015;30:505–513. doi: 10.1002/gps.4173. [DOI] [PubMed] [Google Scholar]
- 12.Hansson O., Buchhave P., Zetterberg H., Blennow K., Minthon L., Warkentin S. Combined rCBF and CSF biomarkers predict progression from mild cognitive impairment to Alzheimer's disease. Neurobiol Aging. 2009;30:165–173. doi: 10.1016/j.neurobiolaging.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Okamura N., Arai H., Maruyama M., Higuchi M., Matsui T., Tanji H. Combined Analysis of CSF Tau Levels and [123I]Iodoamphetamine SPECT in Mild Cognitive Impairment: Implications for a Novel Predictor of Alzheimer's Disease. Am J Psychiatry. 2002;159:474–476. doi: 10.1176/appi.ajp.159.3.474. [DOI] [PubMed] [Google Scholar]
- 14.Thal D.R., Griffin W.S., de Vos R.A., Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathol. 2008;115:599–609. doi: 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- 15.Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke. 2015;17:17–30. doi: 10.5853/jos.2015.17.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iturria-Medina Y., Sotero R.C., Toussaint P.J., Mateos-Perez J.M., Evans A.C., Alzheimer's Disease Neuroimaging Initiative Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stukas S., Robert J., Lee M., Kulic I., Carr M., Tourigny K. Intravenously injected human apolipoprotein A-I rapidly enters the central nervous system via the choroid plexus. J Am Heart Assoc. 2014;3:e001156. doi: 10.1161/JAHA.114.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song F., Poljak A., Crawford J., Kochan N.A., Wen W., Cameron B. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals. PLoS One. 2012;7:e34078. doi: 10.1371/journal.pone.0034078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefterov I., Fitz N.F., Cronican A.A., Fogg A., Lefterov P., Kodali R. Apolipoprotein A-I deficiency increases cerebral amyloid angiopathy and cognitive deficits in APP/PS1DeltaE9 mice. J Biol Chem. 2010;285:36945–36957. doi: 10.1074/jbc.M110.127738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koldamova R., Staufenbiel M., Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- 21.Lewis T.L., Cao D., Lu H., Mans R.A., Su Y.R., Jungbauer L. Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J Biol Chem. 2010;285:36958–36968. doi: 10.1074/jbc.M110.127829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slot R.E., Van Harten A.C., Kester M.I., Jongbloed W., Bouwman F.H., Teunissen C.E. Apolipoprotein A1 in Cerebrospinal Fluid and Plasma and Progression to Alzheimer's Disease in Non-Demented Elderly. J Alzheimers Dis. 2017;56:687–697. doi: 10.3233/JAD-151068. [DOI] [PubMed] [Google Scholar]
- 23.Robert J., Button E.B., Yuen B., Gilmour M., Kang K., Bahrabadi A. Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating high-density lipoproteins in bioengineered human vessels. Elife. 2017;6 doi: 10.7554/eLife.29595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuliani G., Cavalieri M., Galvani M., Volpato S., Cherubini A., Bandinelli S. Relationship between low levels of high-density lipoprotein cholesterol and dementia in the elderly. The InChianti study. J Gerontol A Biol Sci Med Sci. 2010;65:559–564. doi: 10.1093/gerona/glq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Exel E., de Craen A.J., Gussekloo J., Houx P., Bootsma-van der Wiel A., Macfarlane P.W. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann Neurol. 2002;51:716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- 26.Yasuno F., Tanimukai S., Sasaki M., Ikejima C., Yamashita F., Kodama C. Effect of plasma lipids, hypertension and APOE genotype on cognitive decline. Neurobiol Aging. 2012;33:2633–2640. doi: 10.1016/j.neurobiolaging.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Reed B., Villeneuve S., Mack W., DeCarli C., Chui H.C., Jagust W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. 2014;71:195–200. doi: 10.1001/jamaneurol.2013.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reitz C., Tang M.X., Schupf N., Manly J.J., Mayeux R., Luchsinger J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010;67:1491–1497. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 30.Hawkes C.A., Sullivan P.M., Hands S., Weller R.O., Nicoll J.A., Carare R.O. Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 allele. PLoS One. 2012;7:e41636. doi: 10.1371/journal.pone.0041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crehan H., Holton P., Wray S., Pocock J., Guerreiro R., Hardy J. Complement receptor 1 (CR1) and Alzheimer's disease. Immunobiology. 2012;217:244–250. doi: 10.1016/j.imbio.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 32.St-Amour I., Cicchetti F., Calon F. Immunotherapies in Alzheimer's disease: Too much, too little, too late or off-target? Acta Neuropathol. 2016;131:481–504. doi: 10.1007/s00401-015-1518-9. [DOI] [PubMed] [Google Scholar]
- 33.Veerhuis R., Nielsen H.M., Tenner A.J. Complement in the brain. Mol Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brubaker W.D., Crane A., Johansson J.U., Yen K., Garfinkel K., Mastroeni D. Peripheral complement interactions with amyloid beta peptide: Erythrocyte clearance mechanisms. Alzheimers Dement. 2017;13:1397–1409. doi: 10.1016/j.jalz.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers J., Li R., Mastroeni D., Grover A., Leonard B., Ahern G. Peripheral clearance of amyloid beta peptide by complement C3-dependent adherence to erythrocytes. Neurobiol Aging. 2006;27:1733–1739. doi: 10.1016/j.neurobiolaging.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro C.A., Santana I., Oliveira C., Baldeiras I., Moreira J., Saraiva M.J. Transthyretin decrease in plasma of MCI and AD patients: investigation of mechanisms for disease modulation. Curr Alzheimer Res. 2012;9:881–889. doi: 10.2174/156720512803251057. [DOI] [PubMed] [Google Scholar]
- 37.Alemi M., Gaiteiro C., Ribeiro C.A., Santos L.M., Gomes J.R., Oliveira S.M. Transthyretin participates in beta-amyloid transport from the brain to the liver--involvement of the low-density lipoprotein receptor-related protein 1? Sci Rep. 2016;6:20164. doi: 10.1038/srep20164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarzman A.L., Gregori L., Vitek M.P., Lyubski S., Strittmatter W.J., Enghilde J.J. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A. 1994;91:8368–8372. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.