Abstract
Supracondylar fractures of the humerus are the most frequent fractures of the paediatric elbow, with a peak incidence at the ages of five to eight years.
Extension-type fractures represent 97% to 99% of cases. Posteromedial displacement of the distal fragment is the most frequent; however, the radial and median nerves are equally affected. Flexion-type fractures are more commonly associated with ulnar nerve injuries.
Concomitant upper-limb fractures should always be excluded. To manage the vascular status, distal pulse and hand perfusion should be monitored. Compartment syndrome should always be borne in mind, especially when skin puckering, severe ecchymosis/swelling, vascular alterations or concomitant forearm fractures are present.
Gartland’s classification shows high intra- and inter-observer reliability. Type I is treated with casting. Surgical treatment is the standard for almost all displaced fractures. Type IV fractures can only be diagnosed intra-operatively.
Closed reduction and percutaneous pinning is the gold standard surgical treatment. Open reduction via the anterior approach is indicated for open fractures, absence of the distal vascular flow for > 10 to 15 minutes after closed reduction, and failed closed reduction.
Lateral entry pins provide stable fixation, avoiding the risk of iatrogenic ulnar nerve injury.
About 10% to 20% of displaced supracondylar fractures present with alterations in vascular status. In most cases, fracture reduction restores perfusion.
Neural injuries occur in 6.5% to 19% of cases involving displaced fractures. Most of them are neurapraxias and it is not routinely indicated to explore the nerve surgically.
Cite this article: EFORT Open Rev 2018;3:526-540. DOI: 10.1302/2058-5241.3.170049
Keywords: supracondylar fractures, children, management
Introduction
Supracondylar fractures of the humerus are the most frequent fractures affecting the paediatric elbow1 and their correct management is important because they can cause catastrophic complications. Despite there being a clear consensus about some issues regarding proper treatment of these fractures, controversy remains with regard to aspects such as the fact that there is no good classification that guides treatment or predicts complications. In addition, the debates remain unresolved about the timing of emergency reduction or whether reduction can be safely delayed, the controversy regarding proper treatment of Gartland type II fractures, the adequate reduction technique, the risk/benefit ratio of open reduction, the best pin configuration, whether to use a medial pin, the adequate management of a pink pulseless hand, the remodelling capacity of the distal humerus and the long-term consequences of a cubitus varus deformity.
Epidemiology
Supracondylar fractures of the humerus account for 55% to 80% of total elbow fractures in children and up to two-thirds of paediatric elbow injuries requiring hospitalization.2 Supracondylar fractures usually occur as result of a fall from height or from sports or leisure. Their incidence has been estimated at 177.3 per 100 000.3
Although they can occur throughout childhood, the median age is approximately six years, with higher incidence between five and eight years. There is a higher incidence of supracondylar fractures in boys, affecting the non-dominant arm 1.5 times more frequently.4
Pathophysiology and applied anatomy
The distal humerus anatomy is especially predisposed to injury because its configuration in two columns connected by thin bone represents a zone of weakness.
When a fall on the outstretched hand occurs, the olecranon engages on the olecranon fossa and if elbow extension progresses, the olecranon finally acts as a fulcrum on the fossa. Therefore, the bone begins to break at first anteriorly and the fracture progresses posteriorly. If the energy is high, the posterior cortex disrupts, and finally complete posterior displacement of the distal fragment occurs with the posterior periosteum acting as a hinge. This is the mechanism of extension-type fractures, which represent 97% to 99% of the total.4
Analysis of distal fragment displacement and posterior periosteum integrity is essential. Although previous literature has described posteromedial displacement of the distal fragment as the most frequent direction of displacement (75% of cases1), in our series we have found an almost equal rate of posteromedial and posterolateral displacement.5 When posteromedial displacement occurs, the posterolateral periosteum is torn but the posteromedial periosteum is usually intact. For this reason, forearm pronation will put the medial periosteum in tension, facilitating closure of the fracture and avoiding varus collapse.2,6 On the other hand, when posterolateral displacement happens, forearm supination at the time of reduction will put the lateral intact periosteum in tension, facilitating reduction.
Flexion-type fractures represent about 1% to 3% of cases and the fracture is usually caused by direct trauma to the flexed elbow.1 In these cases, the anterior periosteum acts as a hinge, and the progression of the injury goes from the posterior to the anterior part of the distal humerus. The distal fragment also tends to be translated in the coronal plane.
Clinical examination
Although elbow deformity is usually the most striking aspect (especially in very displaced fractures), examination of the entire extremity is essential to exclude associated distal radius (most frequent), forearm or proximal humerus fractures. Concomitant upper-limb fractures not only cause a more severe trauma and instability, but also create increased difficulty in treatment and an increased incidence of neurovascular injuries or compartment syndrome.7-9
In displaced extension-type fractures, the so-called ‘S-deformity’ is usually present (Fig. 1). However, light ecchymosis or swelling can be the only external manifestation of a minimally displaced extension fracture or a flexion-type fracture.
Fig. 1.
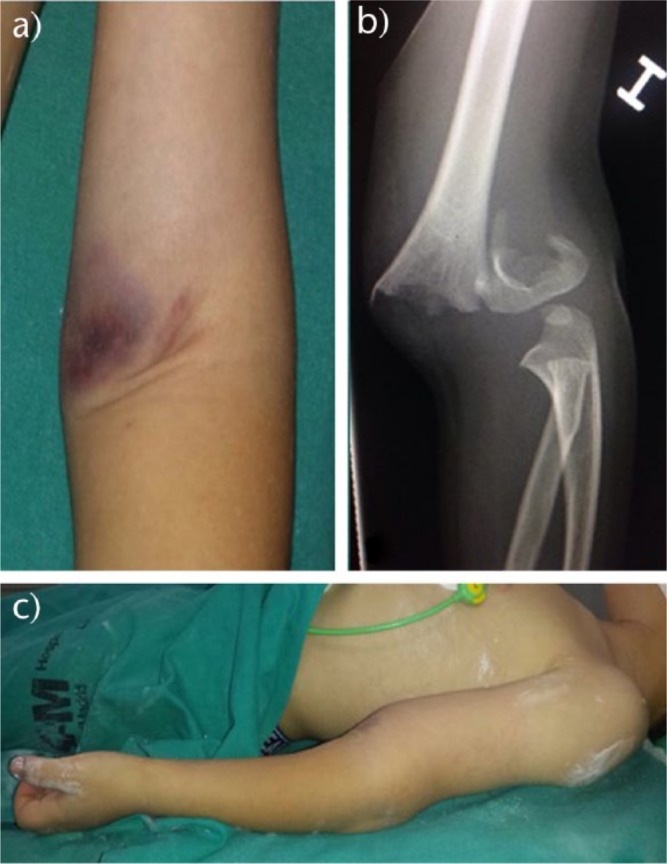
a) Skin puckering at the antecubital fossa should warn of a high energy fracture which transects the brachialis muscle and biceps. b) Very displaced type III fracture. When the fracture is very displaced, ‘S-deformity’ and skin puckering are usually present, and the possibility of neurovascular injury and compartment syndrome should be considered; c) The so-called ‘S-deformity’ is present in very displaced extension-type fractures.
Signs such as extensive ecchymosis, soft-tissue swelling and skin puckering indicate severe trauma (Fig. 1). Special attention should be taken when skin puckering is present. This sign appears when the proximal fragment transects the brachialis muscle, ‘puckering’ the deep dermis (Fig. 1a). For this reason, when skin puckering is present, severe displacement and soft-tissue damage, including brachial artery and median nerve entrapment, should be expected,10 although no differences are found in long-term outcomes with correct management.10
Vascular status evaluation is paramount in displaced fractures. It has been reported that vascular compromise exists in up to 10% to 20% of displaced fractures.2,11,12 It is mandatory to check the distal pulse and hand perfusion pre-operatively and post-operatively.
Neurological examination can be challenging. In the acute setting, the pain and anxiety of the child and his/her parents can make the examination difficult. However, enough time should to be taken to assess adequately the pre-operative neurological status. The median nerve and anterior interosseous nerve (AIN) can be assessed with active flexion of the distal interphalangeal joint of index and thumb. For the radial nerve, thumb extension is usually easy to achieve, even in the young child. For ulnar nerve assessment, at least first interosseous contraction is usually easy to achieve. The inability to perform a complete neurovascular assessment should be documented for medicolegal reasons.
Compartment syndrome should always be borne in mind, especially when skin puckering, severe ecchymosis/swelling, vascular alterations or concomitant forearm fractures are present.
Radiological evaluation
Standard anteroposterior (AP) and true lateral radiographs of the elbow are usually sufficient to characterize the fracture. Careful evaluation should be performed, because it is not unusual to underestimate the severity of a fracture as result of an inadequate radiological technique.
A true lateral view of the elbow is essential because the majority of classifications and treatment algorithms are based on the degree of extension or flexion displacement. The main anatomical landmark to be evaluated in the lateral view is the anterior humeral line (AHL).13 This line continues the anterior cortical of the humerus and, in a normal elbow, should traverse the capitellum in its middle third (Fig. 2). In a displaced fracture in extension, the AHL will pass anteriorly or may not even cross the capitellum. In case of a flexion-type fracture, the AHL passes posteriorly to the capitellum. The lateral view also allows assessing the degree of displacement and the integrity of the posterior cortex.
Fig. 2.
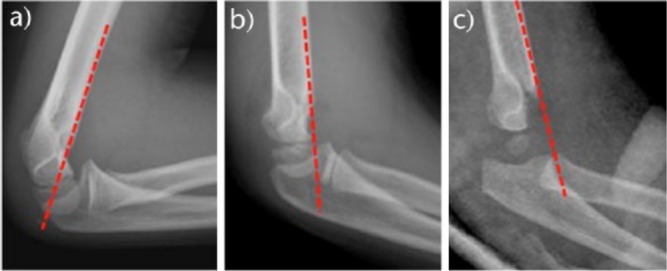
a) The anterior humeral line (AHL) should traverse the middle third of the capitellum nucleus in normal anatomy and type I fractures; b, c) in extension-type fractures, the line crosses anteriorly.
The anterior and posterior ‘fat-pad sign’ can also be evaluated in the lateral radiograph. While diagnosis of displaced fractures is usually evident, diagnosis of minimally or non-displaced fractures can be challenging. The posterior fat-pad sign is suggestive of the presence of a non-displaced fracture of the elbow (Fig. 3). According to Skaggs et al,14 76% of patients with a positive fat-pad sign had a non-displaced fracture of the elbow, and in 53% of them, the fracture was a supracondylar fracture. However, the anterior fat-pad sign can be present in a normal flexed elbow and, therefore, is not so specific for fracture diagnosis.
Fig. 3.
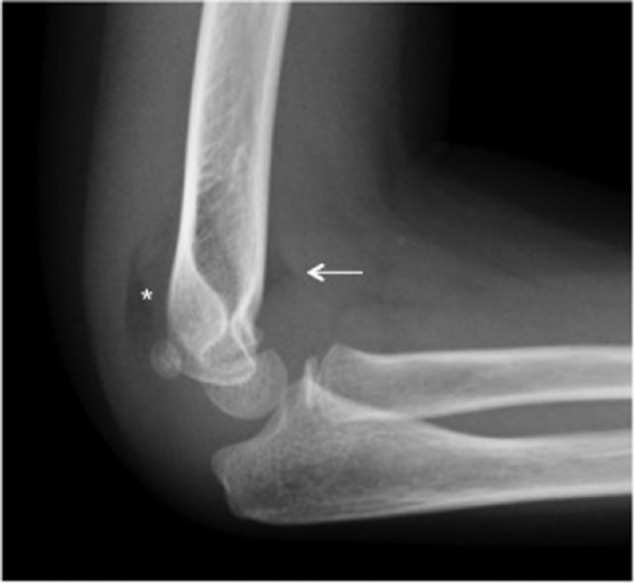
Posterior fat-pad sign (asterisk) is specific to an occult elbow fracture in almost 75% of patients. However, anterior fat-pad elevation (arrow) is not so specific to fracture diagnosis and can appear in a normal elbow.
Regarding the AP radiograph, we should evaluate the direction of displacement, the presence of varus or valgus alignment, and the extent of the fracture comminution. Baumann’s angle, which is the angle formed in the AP view by the diaphyseal axis of the humerus and the physeal line of the capitellum, is used to assess varus or valgus alignment of the distal humerus (Fig. 4). However, Baumann’s angle has a broad range of normal values (64º to 82º) and varies greatly with humeral position on the radiograph (i.e. rotation).15 The ulnohumeral angle or radiological carrying angle, which is the angle formed, in the AP view, by the diaphyseal axis of the humerus and the axis of the proximal third of the ulna, is also used to assess varus or valgus deformity and it is more accurate and useful than Baumann’s angle (Fig. 4).
Fig. 4.
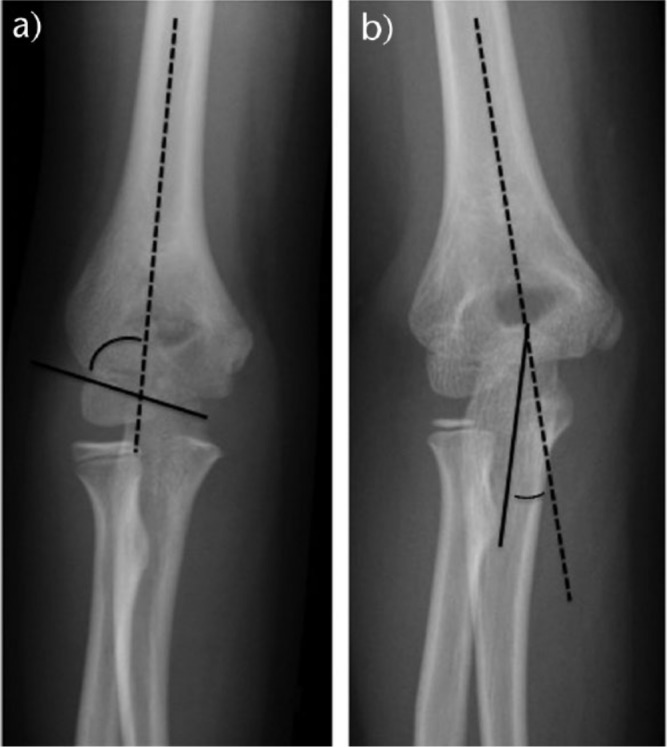
a) Baumann’s angle; b) ulnohumeral angle.
Classification
Several classifications of supracondylar fractures of the humerus have been proposed. However, Gartland’s classification16 is the most widely used. It is a reliable classification with high intra- and inter-observer concordance,17 based on the amount of displacement of the distal fragment. Gartland classified supracondylar extension-type fractures in his original paper as follows (Fig. 5):
Fig. 5.
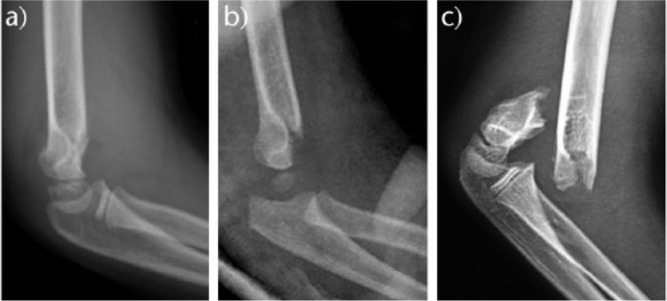
Gartland’s classification for extension fractures: a) type I; b) type II; c) type III.
- Type I. Non-displaced fractures (< 2 mm). The AHL still crosses through the centre of the capitellum. These fractures are stable because of the integrity of the periosteum.
- Type II. Moderately displaced (> 2 mm). The AHL passes anterior to the centre of the capitellum; the posterior periosteum is intact but acts as a hinge.
- Type III. Completely displaced. This type of fracture is more unstable, with extensive soft-tissue and periosteal damage and increased incidence of neurovascular injuries.
Gartland’s classification was modified by Wilkins in 1984,18 subdividing type II fractures into IIA or IIB according to the absence (IIA) or presence (IIB) of malrotation. However, this sub-classification of type II fractures does not show a good intra- and inter-observer reliability.19
In 2006, Leitch et al20 introduced a new type IV fracture, in which the periosteum is completely torn, which leads to a high instability of the fracture both in flexion and extension. Multidirectional instability of type IV fractures can be caused by the injury itself or by repeated failed attempts of reduction. Type IV fractures can only be diagnosed intra-operatively.
Treatment
Gartland type I
Non-displaced or type I fractures can be managed easily with a long-arm cast or splint (Fig. 5). There is not usually severe swelling or ecchymosis, so elbow flexion up to 80° to 90° and mid pronation-supination are well tolerated. However, flexion of the elbow within the cast should not pass 90° because it can increase forearm pressures and impede distal vascular flow.21,22 Although secondary displacement rarely occurs, it seems prudent to control secondary displacement with a new radiograph performed at least seven to ten days after injury. Three weeks after the fracture, the cast is removed and progressive joint motion is allowed.
Gartland type II
Operative treatment of these fractures has become more popular recently. The limited potential of remodelling of the distal humerus is the strongest argument in favour of surgical management. The distal humerus represents only 20% of total growth of the bone and the ability to remodel is limited after the age of four years.2 After the ages of eight to ten years, only 10% of growth of the humerus remains, so anatomical reduction is thought to be imperative.
Closed reduction and casting of these fractures is becoming less popular because of the excessive flexion of the elbow beyond 90° needed to maintain reduction, which increases the risk of compartment syndrome and neurovascular injuries.9,21-23
Regarding conservative treatment with simple immobilization, Moraleda et al24 described the long-term results of Gartland type II fractures treated with immobilization and no attempt at reduction. The authors described a mild cubitus varus deformity in 26% of cases, pain or instability in 17% of patients, and a mild increase in elbow extension and a mild lack of elbow flexion that was present in almost every patient. However, the authors found that functional results were excellent in most of the patients and were not predictors of bad results.
It is thought that Gartland type II fractures with medial column comminution, varus or valgus angulation, or rotation should be treated surgically, even if the fracture is minimally displaced. However, identifying rotation is difficult in plain radiographs.
Closed reduction and percutaneous pinning of type II fractures seems to be easy, safe and reliable.25,26 The risk of complications is low. In fact, Skaggs et al26 described no radiographic or clinical loss of reduction, and no complications in their series. For these reasons, in our opinion, if any doubt exists about the need for reduction, a closed reduction and percutaneous pinning fixation of a Gartland type II fracture is indicated.
Types III and IV
It is widely accepted that type III and type IV fractures should be managed surgically.2 Nowadays, closed reduction and percutaneous pinning is the gold standard for all displaced fractures. Blount’s method with closed reduction and hyperflexion of the elbow to maintain reduction is no longer used because of the risk of compartment syndrome or neurovascular injury. However, Pham et al have described their results with Blount’s method when treating Gartland type IIB and III supracondylar fractures.27 The authors described a secondary displacement in 5% of their cases, a cubitus varus deformity in 2% of their cases, no cases of compartment syndrome and satisfactory results according to Flynn’s criteria in 90% of their cases. The authors concluded that Blount’s method is a reasonable option for treating type IIB and III supracondylar humeral fractures in children. Muccioli et al also described good results with Blount’s technique.28 Other treatments, such as traction, have only historical interest.29-31
Management in the emergency room
In the emergency department, immobilizing the elbow with a long-arm splint at 30° to 40° of flexion is sufficient until surgery is performed, in order to control pain, avoid neurovascular injuries and minimize the risk of compartment syndrome. A careful physical examination and regular monitoring, including integrity of the skin and neurovascular status, are mandatory.
Delayed treatment
Displaced supracondylar fractures have been traditionally treated as surgical emergencies due to the risk of neurovascular complications or the belief that open reduction instead of closed reduction will be needed if surgery is delayed.16,32 Debate still exists as to whether reduction can safely be delayed in the absence of neurovascular compromise.33-38 On the other hand, it should also be taken into account that performing surgery at night, with inadequate facilities or staff, can potentially increase surgical errors.39 The majority of previous studies, including our series, shows that surgical delay does not correlate with a higher rate of open reduction or an increase in complications.5,33,37,38,40-43 We also found that pinning errors were significantly more frequent in surgeries performed at night.5 In our opinion, those cases with an open fracture, vascular or nerve injuries, severe swelling, a floating elbow or when the fracture affects a toddler or a patient with neurological disability who, therefore, cannot complain of pain correctly, should be treated urgently. Otherwise, treatment can be safely delayed. Furthermore, surgery at night should be avoided in the absence of contraindications.
Surgical technique
Reduction
Fractures can be reduced by closed or open means.44 Open reduction has been related to a higher incidence of infection and stiffness. Closed reduction is a reliable technique for the majority of displaced fractures.
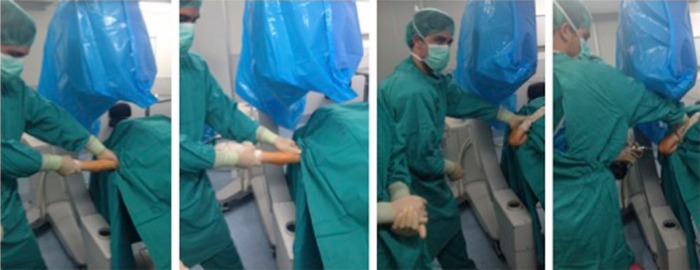
Regarding extension fractures, the fracture is first reduced in the coronal plane with the elbow in extension while gentle traction is applied. When skin puckering is present because the proximal fragment is transecting the brachialis muscle, the ‘milkman’s manoeuvre’ of the anterior part of the arm is useful to release the proximal fragment (Fig. 6).10 Surgeons need to be aware of the higher risk of neurovascular injury associated with skin puckering. Later, pronating or supinating the forearm corrects rotation of the fragment (Fig. 7). Finally, the elbow is flexed while pushing the olecranon to the correct extension. Maintaining the elbow in maximum flexion to stabilize the fracture until fixation with percutaneous pinning is performed (Fig. 6). Sometimes, maintaining the elbow flexed does not stabilize fractures while pinning (fractures with an oblique line and Gartland type IV fractures). In those cases, a K-wire can be passed through the distal fragment from medial to lateral and used as a joystick to reduce the fracture and maintain reduction while pinning (Fig. 8). Results obtained by Leitch et al with the ‘joystick technique’ were excellent, with no malunion, stiffness or additional surgery needed. Although their series is small (nine cases), it seems to be a reliable alternative for reduction of these fractures. In our experience, open reduction and fixation of these unstable fractures is not easy at all, so we prefer to treat them by closed means.
Fig. 6.
Reduction technique. Reduction is performed under fluoroscopy. First, traction is applied. Then, coronal plane deformity is corrected, applying varus/valgus. Sagittal plane correction is then performed. The last step is fixation with smooth 1.8 to 2.0 mm K-wires.
Fig. 7.
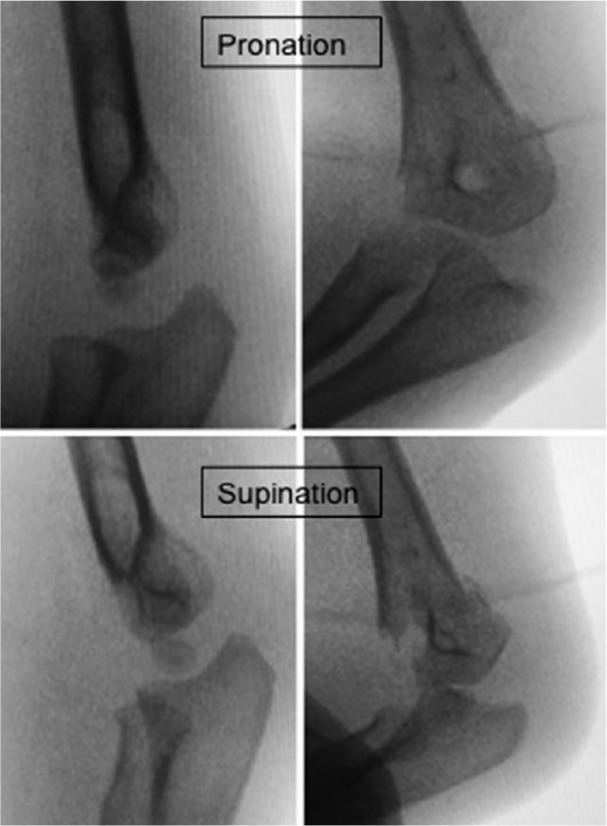
When posteromedial displacement is present, forearm pronation helps with fracture reduction. Forearm supination will be a difficult reduction in these cases.
Fig. 8.
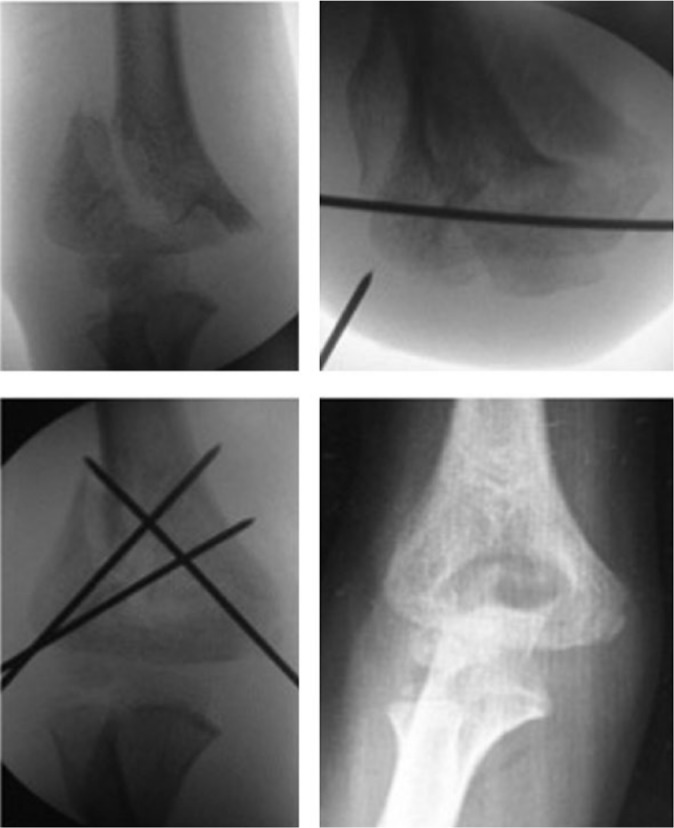
In very unstable fractures (such as type IV), a provisional pin in the distal fragment is helpful to achieve reduction. Once reduction is achieved, fixation is performed. In very unstable fractures, three-wire fixation is preferred.
Another technique proposed to facilitate reduction of the fracture is to use a K-wire introduced percutaneously from the posterior aspect of the arm through the medullary canal of the humerus, similar to Kapandji’s technique for the distal radius. Few papers have been published regarding this technique.45,46 Although the results are similar to those published with other techniques, there is a risk of nerve palsy, wire migration and, in contrast with the joystick technique, it is not useful when the posterior periosteal hinge is disrupted.45
Open reduction is indicated when the surgeon is not able to reduce the fracture by closed means, when there is soft-tissue entrapment (i.e. muscle, median nerve, brachial artery) or when a cold hand remains without perfusion after an attempt at closed reduction has been performed. Neurovascular entrapment can be suspected when a soft stop to reduction is observed.
The anterior approach is the most widely used approach for open reduction. This approach is especially indicated when vascular repair is necessary. It is a safe approach and results are similar to or better than a traditional lateral approach.47 The lateral approach is the standard approach for elbow surgery. However, in supracondylar fractures, which are extra-articular, it does not give any advantage over the anterior approach and increases the risk of radial nerve injury and stiffness. The bilaterotricipital posterior approach (Alonso-Llames approach) was initially described at our institution to treat supracondylar fractures.48 However, currently it is not widely used because of the high rate of complications described, such as stiffness, unsightly scarring and risk of trochlea osteonecrosis.49 In our experience, the posterior approach is an easy and safe approach that allows surgeons to manage both columns for proper reduction. Classically, the open approach was associated with a high rate of complications, such as stiffness or myositis ossificans. However, recent studies have reported a lower incidence of complications50 and no differences compared with closed reduction regarding loss of motion, infection, malunion or subsequent surgery.51,52 In a series reported by Reitman et al,50 the authors found that open reduction had a low rate of complications. However, despite the fact that fractures that need an open reduction are usually more severe, up to 22% of their patients were rated as having a poor or fair result according to Flynn’s criteria53 after an average follow-up of 5.8 months. Loss of motion is the most commonly reported complication, affecting about 10% of patients.44 In our opinion, vascular entrapment and type II or III open fractures are the main indications for open reduction. We try to reduce by closed means all fractures that do not have an associated vascular injury and we use all possible manoeuvres to avoid an open reduction. When a fracture needs to be open because of a vascular lesion, we use an anterior approach.
Fixation
Before fixation, angular and rotational alignment are assessed. We check rotational alignment both clinically and radiologically. We recommend performing four views with fluoroscopy: AP; lateral; internal oblique; and external oblique. Internal oblique and external oblique views allow us to check both columns and to be sure rotation is corrected. We have also observed that, clinically, the position of the forearm related to the trunk with the shoulder in abduction and maximus external rotation is symmetrical and serves as a control for rotation of the distal fragment.
Pin fixation for paediatric supracondylar fractures of the humerus was initially proposed by Casiano54 in 1960 and was established as a reliable technique with good long-term outcomes by Flynn et al in 1974.53 Many studies have been published comparing lateral-entry and crossed pin fixation.40,55-60 Regarding biomechanical stability, results of studies are controversial. While there are studies supporting the idea that crossing pins provides more stability,61-63 no differences are seen in others.64,65 We use two divergent percutaneous K-wires inserted from a lateral-entry point. We usually use a 1.8-mm K-wire for percutaneous pinning. Once the fracture has been fixated, the surgeon can extend the elbow and check for stability. If the fracture remains unstable, a third lateral-entry point pin or a pin inserted from the medial part of the elbow is inserted. We prefer to use a third lateral-entry point K-wire instead of a medial pin because of the risk of damaging the ulnar nerve66 (Fig. 9). Larson et al67 showed that three divergent lateral pins are at least as stable as standard crossed pins. Skaggs et al26 found that the use of lateral-entry point alone was effective for even the most unstable fractures. The authors note the importance of maximizing separation of the pins at the fracture site, engaging the medial and lateral columns proximal to the fracture, engaging sufficient bone in both the proximal segment and the distal segment, and maintaining a low threshold for use of a third lateral-entry pin if there is any concern about fracture stability or the location of the first two pins. Biomechanical studies have demonstrated that widely divergent wires starting on the capitellum, one through the lateral column and one through the medial cortex, are the best option.68,69 It is important to have the pins separated enough at the level of the fracture to provide rotational stability.
Fig. 9.
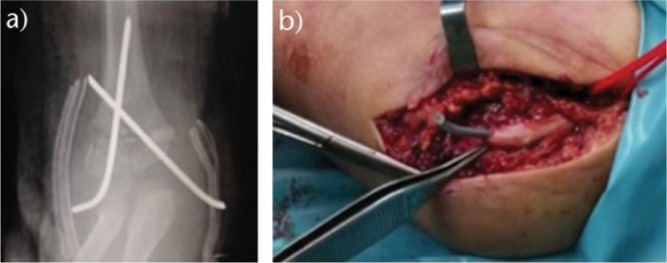
Fracture fixated with crossed pins percutaneously. Ulnar nerve was traversed by medial pin and post-operative palsy was present.
Sankar et al classified pinning errors as types A, B and C.70 A type A error was defined as the failure to engage both fragments by two pins or more. A type B error was defined as the failure to achieve bicortical fixation with two pins or more. A type C error was defined as inadequate pin spread to control rotation.70 We found type C error to be the most common pinning error in our series.5
Several studies have proved a higher risk of ulnar nerve injury when crossing pins are placed instead of lateral-entry point alone, with no differences in other parameters.57 Relative risk (RR) of the ulnar nerve is as high as 4.3 times with crossed pins in comparison with lateral-entry pins,60 with an estimated incidence of 3.4% versus 0.7%.59 It is important to know that direct injury (like penetration) of the nerve by the pin is not necessary to develop an ulnar nerve palsy (Fig. 9). Just the fact that the K-wire is adjacent to the nerve has been proved to provoke an ulnar nerve affection.71 The main advantage of using crossed pins is to provide more stability that will prevent secondary displacement and malunion. However, Lee et al affirm that avoiding ulnar nerve palsy is clinically more important than having a slightly increased incidence of malunion (5.9% for lateral-entry point versus 3.4% for crossed pins).59
Several techniques have been proposed to avoid ulnar nerve injury with medial pinning.44,72 Once lateral pins have been inserted, the elbow is semi-extended to allow posterior displacement of the ulnar nerve. When the elbow is flexed > 90°, an anterior displacement of the ulnar nerve occurs and the risk of injury is in the range of 5.7% to 17.7%.73 The thumb is used to press over the epitrochlea to decrease the oedema and facilitate palpation of bony references. Later, the thumb is displaced posteriorly to protect the nerve and a T-handle is used to insert the pin anteriorly to the thumb.44 A mini-open medial incision may also be used to visualize the medial-entry point and avoid injury of the ulnar nerve.74 Ulnar nerve location by palpation only is not a totally safe technique.75 Other techniques such as intra-operative electrical stimulation76 or ultrasound monitoring77 have been proposed as safe but technically demanding methods to avoid ulnar nerve injuries.
In our opinion, the best way to avoid iatrogenic ulnar nerve injury is to avoid medial pinning. Dorgan’s technique (a proximal lateral-entry point pin going from proximal-lateral to distal-medial) for crossed lateral-entry pins has been proposed as an alternative to avoid ulnar nerve injury. However, the proximal wire enters in the supracondylar lateral region, increasing the risk of radial nerve injury.78
Controversy exists regarding whether or not to bury the percutaneous pins. It has been advocated that burying the pins diminishes the risk of infection; however, the risk of severe deep infection associated with percutaneous pinning without burying the pins has been described as low as 0.2%.79 On the other hand, burying the pins can cause skin complications or problems related to soft-tissue irritation and needs a second surgery to remove the pins.77 Our recommendation is to leave the pins exposed without burying them.
It is important to note that stability is mainly provided by the pins. Fixation should be stable enough to allow immobilization of the elbow in a splint in no more than about 60° to 70° of flexion in order to avoid neurovascular complications or compartment syndrome (Fig. 11). The pins and splint are removed after three weeks. Initial physiotherapy is not recommended.80 Schmale et al published a randomized controlled trial regarding physical therapy following a paediatric supracondylar humeral fracture. The author found no differences between groups with respect to performance of activities of daily living, time to return to sports or elbow motion.80
Fig. 11.
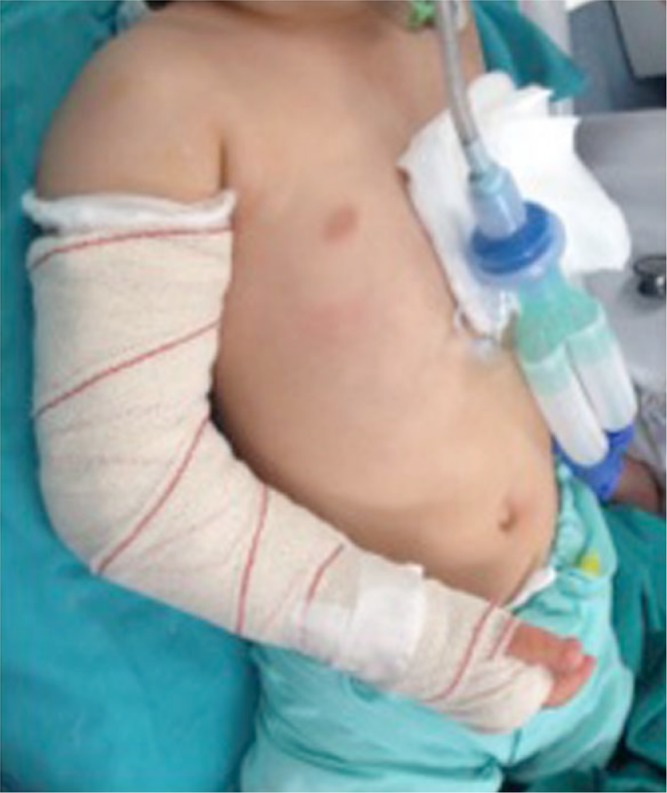
After reduction and fixation, the elbow should be immobilized at approximately 70° of flexion. Higher flexion not only will not improve results, as the reduction is maintained by pins, but also increases the risk of compartment syndrome.
Fig. 10.
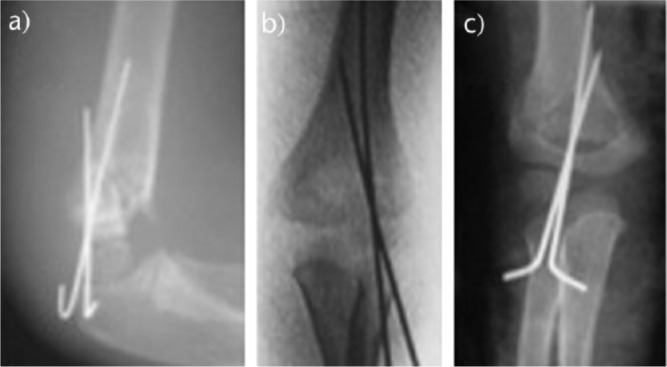
According to Sankar et al,70 a type A error was defined as the failure to engage both fragments using two pins or more; a type B error was defined as the failure to achieve bicortical fixation with two pins or more; and a type C error was defined as inadequate pin spread to control rotation.
Complications
Vascular injury
About 10% to 20% of displaced supracondylar fractures present alterations in vascular status.11,12 The radial pulse is reported to be absent before reduction in 7% to 12% of all fractures and up to 19% in displaced fractures. Lesions of the brachial artery can occur primarily from stretching, entrapping or disrupting the neurovascular structures on the proximal fragment and, secondarily, during reduction manoeuvres or when immobilizing the elbow in the hyperflexion position.81,82
When facing a supracondylar humeral fracture with an associated vascular injury, our first action should be to perform a closed reduction of the fracture. There is no evidence supporting the use of angiography in pre-operative management.83,84 In the absence of significant associated trauma at other levels, the vascular injury is localized at the fracture focus. Therefore, the use of ultrasonography or angiography only delays fracture reduction and increases the risk of complications.83,84
After closed reduction has been attempted, vascular status should be re-evaluated because the pulse is restored in 80% of the cases after reduction.81 The incidence of poor perfusion of the forearm after reduction of the fracture is 0.3%.81 The absence of radial pulse after closed reduction of the fracture has been described in 3.2% of cases.85 The association of pulseless hand and median nerve palsy strongly predicts the need for surgical exploration.86
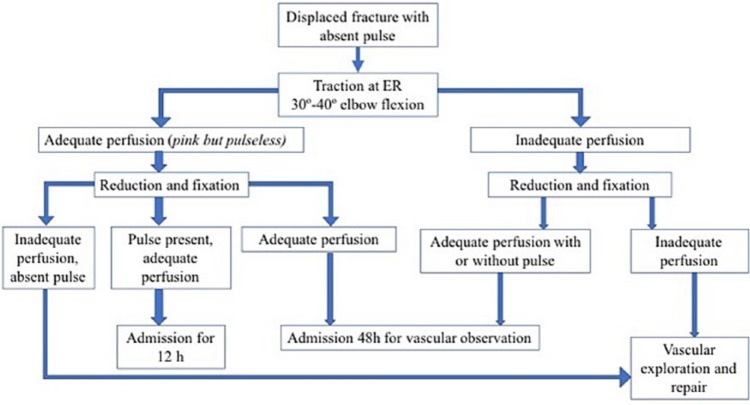
The hand is the best indicator of vascular status. According to pulse presence and limb perfusion, three situations are recognized:87,88 1) good pulse and well perfused limb (warm, pink, capillary refill < 3 seconds with distal pulse detected by eco-Doppler); 2) the so-called pink-pulseless hand when the pulse is absent but the hand is well perfused (warm, pink, capillary refill < 3 seconds, without Dopplerable distal pulse); 3) the so-called cold-hand when the pulse is absent and the hand is poorly perfused (pale, cold and capillary refill > 3 seconds).12 The treatment algorithm is based on these three situations (Fig. 12). Consensus is clear about the indication of immediate exploration of the artery for a cold-hand situation or in those situations when the pulse was present previously but disappears after closed reduction of the fracture.81 In order to protect the vascular repair, it is mandatory to first reduce and fix the fracture. We recommend performing vascular exploration using an anterior approach with collaboration of the vascular surgeon.
Fig. 12.
Algorithm for the management of vascular injury in the context of a supracondylar fracture.
On the other hand, the current literature is imprecise regarding the optimal management of a pink-pulseless situation.81,85 Controversy exists regarding whether it is preferable to adopt a conservative approach based on strict supervision or whether it is better to perform an early revascularization.89 A survey of the British Society for Children’s Orthopaedic Surgery found that 60% of surgeons favour continued observation if the forearm remains pulseless but well perfused.90 Some authors believe that the rich collateral circulation around the elbow will sustain the viability of the extremity after sustaining a supracondylar humeral fracture with a pink-pulseless hand.85 It has been described that distal pulse is restored in most cases within six weeks after surgery.91 Furthermore, we have to take into account that early revascularization of a pink-pulseless hand has been associated with a high rate of asymptomatic re-occlusion and residual stenosis of the brachial artery.85 However, Blakey et al92 note the importance of monitoring pain and signs of progressive nerve affection in the context of a pink-pulseless hand, because those patients could be susceptible to surgical exploration.
Controversy remains regarding the long-term consequences of a poor vascularization, such as cold intolerance, exercise-induced ischemic symptoms and limb-length discrepancy in some patients. Carbonell et al review 14 adults with a mean age at the latest follow-up of 20 years who sustained a pink-pulseless type III supracondylar fracture during childhood (average age at the time of fracture of seven years).93 Patients were managed with closed reduction of the fracture, percutaneous pinning and strict supervision of the vascular status without surgical revascularization. The authors found satisfactory results in the long term with no difference, compared with contralateral side, regarding grip strength, capillary refill, wrist brachial index, length of the arm and forearm, circumference of the arm and forearm, or oxygen saturation. Radial pulse was present in all patients. Therefore, we advocate for strict supervision of a pink-pulseless hand situation without surgical revascularization.
Neurological injury
Neural injuries can occur in 6.5% to 19% of cases of displaced fractures and they are exceptional in non-displaced fractures.79 They can appear either before surgery (primary lesion) or after reduction and fixation of the fracture (secondary lesion). Primary lesions are caused by fracture displacement, which can stretch, entrap or disrupt the nerve. Secondary lesions are caused by excessive manipulation, immobilization in hyperflexion or iatrogenic injuries by fixation.81,94
When posteromedial displacement occurs, the radial nerve is at risk of primary injury or because of involvement during a reduction manoeuvre.1 Posterolateral displacement puts at risk brachial artery and median/AIN nerves. Flexion-type fractures involve neurovascular structures more frequently than extension-type fractures, especially ulnar nerve entrapment at the fracture site.95
Incidence of AIN injury and radial nerve injury is similar.94 Combined nerve injuries can be present in up to 21% of cases with nerve lesions.94 When an AIN lesion is present, paralysis of the flexor pollicis longus and flexor digitorum profundus of the second finger is observed. The AIN is an exclusive motor branch, so sensory changes are not present. When a complete median nerve lesion is present, sensory and motor loss of function are observed in the median nerve distribution. Radial nerve injury is associated with a loss of wrist and finger extensor function and loss of sensation in the radial nerve distribution. Ulnar nerve injury is more frequent in flexion-type fractures. However, due to the low incidence of flexion-type fractures, the majority of ulnar nerve injuries are seen in extension fractures and they are thought to be caused by a medial pin.26 In fact, Valencia et al found that in more than half of their ulnar nerve injuries, fixation was with lateral-entry point pins alone.79 The authors hypothesized that an ulnar nerve injury may reflect the severity of the trauma or the difficulty in reducing the fracture by closed means.
The majority of these injuries are neurapraxias and heal spontaneously; therefore, surgical exploration of the nerve is rarely indicated. In our opinion, when a medial pin is present in a patient with an ulnar nerve injury, the medial pin should be removed (Fig. 9). However, Lyons et al96 reported a full recovery in 17 patients with post-operative ulnar nerve palsy and medial pin fixation despite only four wires being removed.
Valencia et al94 reported in their series that 100% of radial nerve injuries, 87.5% of median nerve injuries and 25% of ulnar nerve injuries were fully recovered with conservative management in the long-term follow-up. The average time for recovery was 3 months for the radial nerve, 2.5 months for the median nerve and 5 months for the ulnar nerve. Other authors have found similar results.97 Strength and discriminatory sensation were normal in all patients. No motor deficit was present. Neurological abnormality consisted of paraesthesia that did not impair normal daily activity.
Compartment syndrome
Compartment syndrome can occur in 0.1% to 0.3% of cases.2 Associated forearm fractures and elbow flexion > 90° increase compartment pressures.8,22 Severe swelling, ecchymosis and pucker sign are clinical signs that can advise of the possibility of the development of a compartment syndrome. Special attention is needed if increased anxiety or analgesia requirements exist or in cases of toddlers or patients with neurological disorders or associated median nerve injury. Diagnosis is mainly clinical. Most cases affect the volar compartment, but the dorsal compartment can also be involved.98
To minimize the risk of compartment syndrome, the elbow should be immobilized in about 30° of flexion in the emergency room and 60° to 70° of flexion after surgery. In case of a dysvascular limb for > 6 hours, it may be prudent to open forearm compartments, but not enough evidence exists to support this.82
Floating elbow
The so-called ‘floating elbow’ occurs when the supracondylar fracture of the humerus is associated with an ipsilateral forearm fracture. This is an uncommon situation (approximately 5% of the supracondylar fractures) that reflects high-energy trauma.7 A fall from a significant height is the usual mechanism of injury. Forearm fracture is usually in the distal third of the forearm.99 Some authors affirm that if a more proximal forearm fracture occurs, the lever arm of the proximal fragment would be too short to generate the moment of force required to produce the ipsilateral supracondylar humeral fracture.100 However, cases with associated diaphyseal forearm fractures have been described.7,101 The incidence of open fractures and neurological injury is much higher than those reported for isolated supracondylar fractures.7
When a floating elbow presents, treatment of supracondylar fracture is the priority, which will include a closed reduction of the fracture and percutaneous pinning for fixation. However, open reduction is needed more frequently than in isolated supracondylar fractures. Conservative treatment of supracondylar fractures is contraindicated100,101 due to the increased risk of neurovascular injury or compartment syndrome. Conservative treatment of supracondylar fractures would include an immobilization in hyperflexion of the elbow, further increasing the risk of neurovascular complications. The results of a supracondylar humeral fracture in a floating elbow situation described in the literature are similar to the reported outcomes for isolated supracondylar fractures.101
Controversy remains as to whether forearm fractures need to be fixated. Authors in favour of percutaneous K-wire fixation of the forearm fracture argue that closed reduction and casting in a position of pronation and palmar flexion would make it difficult to monitor neurovascular status,101 that circumferential cast immobilization of the radius fracture increases the risk of compartment syndrome99 and that cast immobilization of the forearm fracture is associated with a high incidence of secondary displacement.99 However, Blumberg et al described the results of closed reduction of the displaced forearm fracture and immobilization into a non-circumferential cast or splint. The authors described no cases of secondary displacement or necessity of re-manipulation, no signs of elevated compartment pressures, and a radiographic union without secondary procedures in all cases. Blumberg et al concluded that a floating elbow could be safely managed with operative stabilization of the supracondylar humerus fracture alone.102
Extension malunion
It is frequent that fractures heal in extension, whether reduction is insufficient or whether a type II fracture is treated conservatively. An extension malunion provokes an increase in extension and a lack of flexion (Fig. 13). Hyperextension of the elbow causes only cosmetic problems. However, lack of flexion can cause an inability to perform activities of daily living. It has been described that functional elbow motion is from 30° to 130°. Therefore, careful reduction should be performed in the OR in order to avoid extension malunion. It is important to remember that condyle of the humerus is anterior to the humeral shaft and that the anterior humeral line should cross the humeral condyle in its middle third.
Fig. 13.
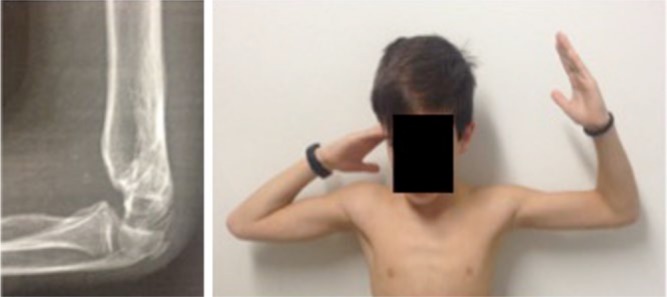
Fracture malunion in extension leads to a lack of flexion. It is an important point that stiffness in supracondylar fractures in children is not frequent and lack of mobility usually reflects a malunion.
When facing a patient with a lack of flexion after sustaining a paediatric supracondylar fracture, we should remember that extension malunion is the cause in the vast majority of cases. Scarring does not usually play a role when deficit of flexion is present in children. Therefore, treatment should be a supracondylar osteotomy instead of a column procedure (capsulotomy).
Cubitus varus
The cause of cubitus varus remains uncertain. Most authors believe that cubitus varus is the consequence of malunion of the fracture rather than growth arrest. Angular deformity and rotational deformity are thought to be the cause of cubitus varus (Fig. 14). Posteromedial displacement gives a higher Baumann value indicating cubitus varus deformity, while posterolateral displacement gives a lower Baumann value indicating cubitus valgus.103 Distal physis of the humerus has limited potential for remodelling. A child aged eight to ten years has only 10% of the total growth of the humerus remaining. While sagittal and coronal mild deformities can be remodelled in children aged < 4 years, rotational deformities cannot.104 In a study published by Moraleda et al,24 the incidence of cubitus varus in unreduced treated extension-type II fractures was reported to be as high as 26.1%. For that reason, the best way to avoid cubitus varus seems to be to achieve and maintain anatomical reduction of the fracture, with special attention to replicating contralateral rotation of the humerus.
Fig. 14.
Cubitus varus deformity as a result of malunion. Internal rotation of the distal fragment is the key problem in this deformity.
Although parents consult for cosmetic reasons in the majority of cases, several complications in the long term may occur: tardy posterolateral rotatory instability; ulnar nerve palsy; shift of the medial head of the triceps; and a higher risk for condylar fractures.105-108 O’Driscoll et al107 affirmed that cubitus varus leads to two biomechanical disturbances: 1) medial displacement of the mechanical axis of the upper extremity and thus the lateral collateral ligament complex experience increased tensile forces and become attenuated; and 2) the triceps is displaced medially and the displaced triceps force vector leads to an external rotation (supination) moment arm on the ulna. Tardy ulnar nerve palsy with anterior dislocation of the nerve has been described109-111 (Fig. 15). Snapping of the medial portion of the triceps may occur from the medial displacement of the triceps as well as the internal rotation of the distal humerus.112
Fig. 15.
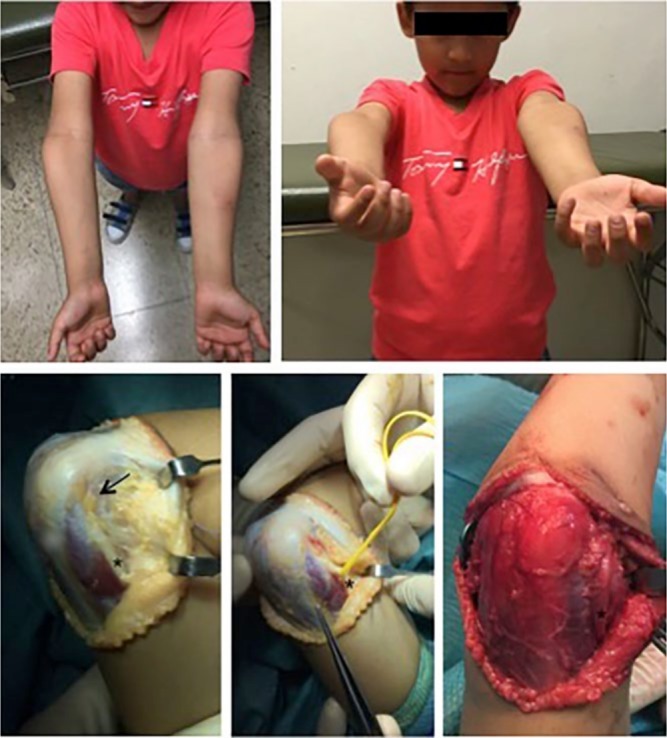
Ulnar nerve palsy is one of the complications related to cubitus varus deformity. In the figure, anterior transposition of the nerve is performed before the osteotomy for correction the deformity.
Several osteotomies have been proposed to correct cubitus varus. A lateral wedge osteotomy is probably the most used osteotomy because it is easy to perform; however, it does not address internal rotation deformity and parents complain of aesthetic disturbances because a prominent lateral epicondyle remains. Other osteotomies have been advocated in order to avoid a prominent lateral epicondyle.113 In our opinion, since angular and rotational deformity should be addressed, a complete osteotomy (either the dome osteotomy or a closed lateral wedge osteotomy that allows derotation and medialization of the distal fragment) seems to be a reliable and powerful osteotomy to achieve correction.113 In our opinion, it is difficult to properly correct the varus alignment with dome osteotomy; for that reason, we advocate performing two complete osteotomies (one perpendicular to the diaphysis of the humerus and the other parallel to the ulnohumeral joint) so a resection of a lateral wedge of bone can be performed for varus correction at the same time as derotation or flexion of the distal fragment.
Pin track infections
The literature has reported pin track infections in the range of 1% to > 25%.114,115 Most infections are superficial and resolve with pin retirement and oral antibiotics.26,37,114 In the rare case of deep infection or articular involvement, drainage, debridement and intravenous antibiotherapy usually resolve the infection without significant sequelae.
Footnotes
ICMJE Conflict of interest statement: None declared.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Beaty JHKJ. Supracondylar fractures of the distal humerus. In: Beaty JH, Kasser JR, ed. Rockwood and Wilkins’ fractures in children. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2006:543-589. [Google Scholar]
- 2. Omid R, Choi PD, Skaggs DL. Supracondylar humeral fractures in children. J Bone Joint Surg [Am] 2008;90-A(5):1121-1132. [DOI] [PubMed] [Google Scholar]
- 3. Mulpuri K, Hosalkar H, Howard A. AAOS clinical practice guideline: the treatment of pediatric supracondylar humerus fractures. J Am Acad Orthop Surg 2012;20(5):328-330. [DOI] [PubMed] [Google Scholar]
- 4. Cheng JC, Lam TP, Maffulli N. Epidemiological features of supracondylar fractures of the humerus in Chinese children. J Pediatr Orthop B 2001;10(1):63-67. [PubMed] [Google Scholar]
- 5. Moraleda L, Diez J, Valencia M. Is timing for paediatric supracondylar fractures related to pinning errors or complications? Podium presentation, POSNA Annual Meeting 2015, Atlanta, US. [Google Scholar]
- 6. Abraham E, Powers T, Witt P, Ray RD. Experimental hyperextension supracondylar fractures in monkeys. Clin Orthop Relat Res 1982;(171):309-318. [PubMed] [Google Scholar]
- 7. Muchow RD, Riccio AI, Garg S, Ho CA, Wimberly RL. Neurological and vascular injury associated with supracondylar humerus fractures and ipsilateral forearm fractures in children. J Pediatr Orthop 2015;35(2):121-125. [DOI] [PubMed] [Google Scholar]
- 8. Blakemore LC, Cooperman DR, Thompson GH, Wathey C, Ballock RT. Compartment syndrome in ipsilateral humerus and forearm fractures in children. Clin Orthop Relat Res 2000;(376):32-38. [DOI] [PubMed] [Google Scholar]
- 9. Hosseinzadeh P, Talwalkar VR. Compartment syndrome in children: diagnosis and management. Am J Orthop 2016;45(1):19-22. [PubMed] [Google Scholar]
- 10. Smuin DM, Hennrikus WL. The effect of the pucker sign on outcomes of type III extension supracondylar fractures in children. J Pediatr Orthop 2017;37(4):e229-e232. [DOI] [PubMed] [Google Scholar]
- 11. Louahem D, Cottalorda J. Acute ischemia and pink pulseless hand in 68 of 404 Gartland type III supracondylar humeral fractures in children: urgent management and therapeutic consensus. Injury 2016;47(4):848-852. [DOI] [PubMed] [Google Scholar]
- 12. Franklin CC, Skaggs DL. Approach to the pediatric supracondylar humeral fracture with neurovascular compromise. Instr Course Lect 2013;62:429-433. [PubMed] [Google Scholar]
- 13. Abzug JM, Herman MJ. Management of supracondylar humerus fractures in children: current concepts. J Am Acad Orthop Surg 2012;20(2):69-77. [DOI] [PubMed] [Google Scholar]
- 14. Skaggs DL, Mirzayan R. The posterior fat pad sign in association with occult fracture of the elbow in children. J Bone Joint Surg [Am] 1999;81-A(10):1429-1433. [DOI] [PubMed] [Google Scholar]
- 15. Camp J, Ishizue K, Gomez M, Gelberman R, Akeson W. Alteration of Baumann’s angle by humeral position: implications for treatment of supracondylar humerus fractures. J Pediatr Orthop 1993;13(4):521-525. [DOI] [PubMed] [Google Scholar]
- 16. Gartland JJ. Management of supracondylar fractures of the humerus in children. Surg Gynecol Obstet 1959;109(2):145-154. [PubMed] [Google Scholar]
- 17. Barton KL, Kaminsky CK, Green DW, et al. Reliability of a modified Gartland classification of supracondylar humerus fractures. J Pediatr Orthop 2001;21(1):27-30. [DOI] [PubMed] [Google Scholar]
- 18. Wilkins KE. Fractures and dislocations of the elbow region. In: Flynn JM, Skaggs DL, Waters PM, eds. Fractures in children. Philadelphia: Wolters Kluwer, 1984:363-575. [Google Scholar]
- 19. Leung S, Paryavi E, Herman MJ, Sponseller PD, Abzug JM. Does the modified Gartland classification clarify decision making? J Pediatr Orthop 2018;38(1):22-26. [DOI] [PubMed] [Google Scholar]
- 20. Leitch KK, Kay RM, Femino JD, et al. Treatment of multidirectionally unstable supracondylar humeral fractures in children. A modified Gartland type-IV fracture. J Bone Joint Surg [Am] 2006;88-A(5):980-985. [DOI] [PubMed] [Google Scholar]
- 21. Mapes RC, Hennrikus WL. The effect of elbow position on the radial pulse measured by Doppler ultrasonography after surgical treatment of supracondylar elbow fractures in children. J Pediatr Orthop 1998;18(4):441-444. [PubMed] [Google Scholar]
- 22. Battaglia TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment pressures in children with supracondylar fractures of the humerus. J Pediatr Orthop 2002;22(4):431-439. [PubMed] [Google Scholar]
- 23. Pirone AM, Graham HK, Krajbich JI. Management of displaced extension-type supracondylar fractures of the humerus in children. J Bone Joint Surg [Am] 1988;70-A(5):641-650. [PubMed] [Google Scholar]
- 24. Moraleda L, Valencia M, Barco R, González-Moran G. Natural history of unreduced Gartland type-II supracondylar fractures of the humerus in children: a two to thirteen-year follow-up study. J Bone Joint Surg [Am] 2013;95-A(1):28-34. [DOI] [PubMed] [Google Scholar]
- 25. Skaggs DL, Sankar WN, Albrektson J, et al. How safe is the operative treatment of Gartland type 2 supracondylar humerus fractures in children? J Pediatr Orthop 2008;28(2):139-141. [DOI] [PubMed] [Google Scholar]
- 26. Skaggs DL, Cluck MW, Mostofi A, Flynn JM, Kay RM. Lateral-entry pin fixation in the management of supracondylar fractures in children. J Bone Joint Surg [Am] 2004;86-A(4):702-707. [DOI] [PubMed] [Google Scholar]
- 27. Pham T-T, Accadbled F, Abid A, et al. Gartland types IIB and III supracondylar fractures of the humerus in children: is Blount’s method effective and safe? J Shoulder Elbow Surg 2017;26(12):2226-2231. [DOI] [PubMed] [Google Scholar]
- 28. Muccioli C, ElBatti S, Oborocianu I, et al. Outcomes of Gartland type III supracondylar fractures treated using Blount’s method. Orthop Traumatol Surg Res 2017;103(7):1121-1125. [DOI] [PubMed] [Google Scholar]
- 29. Gadgil A, Hayhurst C, Maffulli N, Dwyer JSM. Elevated, straight-arm traction for supracondylar fractures of the humerus in children. J Bone Joint Surg [Br] 2005;87-B(1):82-87. [PubMed] [Google Scholar]
- 30. Prietto CA. Supracondylar fractures of the humerus. A comparative study of Dunlop’s traction versus percutaneous pinning. J Bone Joint Surg [Am] 1979;61-A(3):425-428. [PubMed] [Google Scholar]
- 31. Rodriguez Merchan EC. Supracondylar fractures of the humerus in children: treatment by overhead skeletal traction. Orthop Rev 1992;21(4):475-482. [PubMed] [Google Scholar]
- 32. Wilkins KE. Residuals of elbow trauma in children. Orthop Clin North Am 1990;21(2):291-314. [PubMed] [Google Scholar]
- 33. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma 1999;13(1):51-55. [DOI] [PubMed] [Google Scholar]
- 34. Howard A, Mulpuri K, Abel MF, et al. The treatment of pediatric supracondylar humerus fractures. J Am Acad Orthop Surg 2012;20(5):320-327. [DOI] [PubMed] [Google Scholar]
- 35. Carmichael KD, Joyner K. Quality of reduction versus timing of surgical intervention for pediatric supracondylar humerus fractures. Orthopedics 2006;29(7):628-632. [DOI] [PubMed] [Google Scholar]
- 36. Loizou CL, Simillis C, Hutchinson JR. A systematic review of early versus delayed treatment for type III supracondylar humeral fractures in children. Injury 2009;40(3):245-248. [DOI] [PubMed] [Google Scholar]
- 37. Gupta N, Kay RM, Leitch K, et al. Effect of surgical delay on perioperative complications and need for open reduction in supracondylar humerus fractures in children. J Pediatr Orthop 2004;24(3):245-248. [DOI] [PubMed] [Google Scholar]
- 38. Yildirim AO, Unal VS, Oken OF, et al. Timing of surgical treatment for type III supracondylar humerus fractures in pediatric patients. J Child Orthop 2009;3(4):265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scherl SA, Schmidt AH. Pediatric trauma: getting through the night. Instr Course Lect 2010;59:455-463. [PubMed] [Google Scholar]
- 40. Abbott MD, Buchler L, Loder RT, et al. Gartland type III supracondylar humerus fractures: outcome and complications as related to operative timing and pin configuration. J Child Orthop 2014;8(6):473-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop 2002;22(2):203-207. [PubMed] [Google Scholar]
- 42. Mehlman CT, Strub WM, Roy DR, Wall EJ, Crawford AH. The effect of surgical timing on the perioperative complications of treatment of supracondylar humeral fractures in children. J Bone Joint Surg [Am] 2001;83-A(3):323-327. [DOI] [PubMed] [Google Scholar]
- 43. Walmsley PJ, Kelly MB, Robb JE, Annan IH, Porter DE. Delay increases the need for open reduction of type-III supracondylar fractures of the humerus. J Bone Joint Surg [Br] 2006;88-B:528-530. [DOI] [PubMed] [Google Scholar]
- 44. de las Heras J, Durán D, de la Cerda J, et al. Supracondylar fractures of the humerus in children. Clin Orthop Relat Res 2005;(432):57-64. [DOI] [PubMed] [Google Scholar]
- 45. Fahmy M a. L, Hatata MZ, Al-Seesi H. Posterior intrafocal pinning for extension-type supracondylar fractures of the humerus in children. J Bone Joint Surg [Br] 2009;91-B(9):1232-1236. [DOI] [PubMed] [Google Scholar]
- 46. Kao H-K, Lee W-C, Yang W-E, Chang C-H. The posterior intrafocal pin improves sagittal alignment in Gartland type III paediatric supracondylar humeral fractures. Injury 2016;47(4):842-847. [DOI] [PubMed] [Google Scholar]
- 47. Koudstaal MJ, De Ridder VA, De Lange S, Ulrich C. Pediatric supracondylar humerus fractures: the anterior approach. J Orthop Trauma 2002;16(6):409-412. [DOI] [PubMed] [Google Scholar]
- 48. Alonso-Llames M. Bilaterotricipital approach to the elbow. Its application in the osteosynthesis of supracondylar fractures of the humerus in children. Acta Orthop Scand 1972;43(6):479-490. [DOI] [PubMed] [Google Scholar]
- 49. Bronfen CE, Geffard B, Mallet JF. Dissolution of the trochlea after supracondylar fracture of the humerus in childhood: an analysis of six cases. J Pediatr Orthop 2007;27(5):547-550. [DOI] [PubMed] [Google Scholar]
- 50. Reitman RD, Waters P, Millis M. Open reduction and internal fixation for supracondylar humerus fractures in children. J Pediatr Orthop 2001;21(2):157-161. [PubMed] [Google Scholar]
- 51. Kaewpornsawan K. Comparison between closed reduction with percutaneous pinning and open reduction with pinning in children with closed totally displaced supracondylar humeral fractures: a randomized controlled trial. J Pediatr Orthop B 2001;10(2):131-137. [PubMed] [Google Scholar]
- 52. Cramer KE, Devito DP, Green NE. Comparison of closed reduction and percutaneous pinning versus open reduction and percutaneous pinning in displaced supracondylar fractures of the humerus in children. J Orthop Trauma 1992;6(4):407-412. [DOI] [PubMed] [Google Scholar]
- 53. Flynn JC, Matthews JG, Benoit RL. Blind pinning of displaced supracondylar fractures of the humerus in children. Sixteen years’ experience with long-term follow-up. J Bone Joint Surg [Am] 1974;56-A(2):263-272. [PubMed] [Google Scholar]
- 54. Casiano E. Reduction and fixation by pinning “banderillero” style-fractures of the humerus at the elbow in children. Mil Med 1960;125:262-264. [PubMed] [Google Scholar]
- 55. Prashant K, Lakhotia D, Bhattacharyya TD, Mahanta AK, Ravoof A. A comparative study of two percutaneous pinning techniques (lateral vs medial-lateral) for Gartland type III pediatric supracondylar fracture of the humerus. J Orthop Traumatol 2016;17(3):223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abdel Karim M, Hosny A, Nasef Abdelatif NM, et al. Crossed wires versus 2 lateral wires in management of supracondylar fracture of the humerus in children in the hands of junior trainees. J Orthop Trauma 2016;30(4):e123-e128. [DOI] [PubMed] [Google Scholar]
- 57. Zhao J-G, Wang J, Zhang P. Is lateral pin fixation for displaced supracondylar fractures of the humerus better than crossed pins in children? Clin Orthop Relat Res 2013;471(9):2942-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kocher MS, Kasser JR, Waters PM, et al. Lateral entry compared with medial and lateral entry pin fixation for completely displaced supracondylar humeral fractures in children. A randomized clinical trial. J Bone Joint Surg [Am] 2007;89-A(4):706-712. [DOI] [PubMed] [Google Scholar]
- 59. Lee KM, Chung CY, Gwon DK, et al. Medial and lateral crossed pinning versus lateral pinning for supracondylar fractures of the humerus in children: decision analysis. J Pediatr Orthop 2012;32(2):131-138. [DOI] [PubMed] [Google Scholar]
- 60. Woratanarat P, Angsanuntsukh C, Rattanasiri S, et al. Meta-analysis of pinning in supracondylar fracture of the humerus in children. J Orthop Trauma 2012;26(1):48-53. [DOI] [PubMed] [Google Scholar]
- 61. Zionts LE, McKellop HA, Hathaway R. Torsional strength of pin configurations used to fix supracondylar fractures of the humerus in children. J Bone Joint Surg [Am] 1994;76-A(2):253-256. [DOI] [PubMed] [Google Scholar]
- 62. Onwuanyi ON, Nwobi DG. Evaluation of the stability of pin configuration in K-wire fixation of displaced supracondylar fractures in children. Int Surg 1998;83(3):271-274. [PubMed] [Google Scholar]
- 63. Brauer CA, Lee BM, Bae DS, Waters PM, Kocher MS. A systematic review of medial and lateral entry pinning versus lateral entry pinning for supracondylar fractures of the humerus. J Pediatr Orthop 2007;27(2):181-186. [DOI] [PubMed] [Google Scholar]
- 64. Chen TL, He C, Zheng T, et al. Stiffness of various pin configurations for pediatric supracondylar humeral fracture: a systematic review on biomechanical studies. J Pediatr Orthop B 2015;24(5):389-399. [DOI] [PubMed] [Google Scholar]
- 65. Bloom T, Robertson C, Mahar AT, Newton P. Biomechanical analysis of supracondylar humerus fracture pinning for slightly malreduced fractures. J Pediatr Orthop 2008;28(7):766-772. [DOI] [PubMed] [Google Scholar]
- 66. Skaggs DL, Hale JM, Bassett J, et al. Operative treatment of supracondylar fractures of the humerus in children. The consequences of pin placement. J Bone Joint Surg [Am] 2001;83-A(5):735-740. [PubMed] [Google Scholar]
- 67. Larson L, Firoozbakhsh K, Passarelli R, Bosch P. Biomechanical analysis of pinning techniques for pediatric supracondylar humerus fractures. J Pediatr Orthop 2006;26(5):573-578. [DOI] [PubMed] [Google Scholar]
- 68. Hamdi A, Poitras P, Louati H, et al. Biomechanical analysis of lateral pin placements for pediatric supracondylar humerus fractures. J Pediatr Orthop 2010;30(2):135-139. [DOI] [PubMed] [Google Scholar]
- 69. Gottschalk HP, Sagoo D, Glaser D, et al. Biomechanical analysis of pin placement for pediatric supracondylar humerus fractures: does starting point, pin size, and number matter? J Pediatr Orthop 2012;32(5):445-451. [DOI] [PubMed] [Google Scholar]
- 70. Sankar WN, Hebela NM, Skaggs DL, Flynn JM. Loss of pin fixation in displaced supracondylar humeral fractures in children: causes and prevention. J Bone Joint Surg [Am] 2007;89-A(4):713-717. [DOI] [PubMed] [Google Scholar]
- 71. Rasool MN. Ulnar nerve injury after K-wire fixation of supracondylar humerus fractures in children. J Pediatr Orthop 1998;18(5):686-690. [DOI] [PubMed] [Google Scholar]
- 72. Yen Y-M, Kocher MS. Lateral entry compared with medial and lateral entry pin fixation for completely displaced supracondylar humeral fractures in children. Surgical technique. J Bone Joint Surg [Am] 2008;90-A(suppl 2 Pt 1):20-30. [DOI] [PubMed] [Google Scholar]
- 73. Zaltz I, Waters PM, Kasser JR. Ulnar nerve instability in children. J Pediatr Orthop 1996;16(5):567-569. [DOI] [PubMed] [Google Scholar]
- 74. Gordon JE, Patton CM, Luhmann SJ, Bassett GS, Schoenecker PL. Fracture stability after pinning of displaced supracondylar distal humerus fractures in children. J Pediatr Orthop 2001;21(3):313-318. [PubMed] [Google Scholar]
- 75. Wind WM, Schwend RM, Armstrong DG. Predicting ulnar nerve location in pinning of supracondylar humerus fractures. J Pediatr Orthop 2002;22(4):444-447. [PubMed] [Google Scholar]
- 76. Shtarker H, Elboim-Gabyzon M, Bathish E, et al. Ulnar nerve monitoring during percutaneous pinning of supracondylar fractures in children. J Pediatr Orthop 2014;34(2):161-165. [DOI] [PubMed] [Google Scholar]
- 77. Soldado F, Knorr J, Haddad S, et al. Ultrasound-guided percutaneous medial pinning of pediatric supracondylar humeral fractures to avoid ulnar nerve injury. Arch Bone Jt Surg 2015;3(3):169-172. [PMC free article] [PubMed] [Google Scholar]
- 78. Brighton B, Abzug JM, Ho CA, Ritzman TF. Current strategies for the management of pediatric supracondylar humerus fractures: tips and techniques for successful closed treatment. Instr Course Lect 2016;65:353-360. [PubMed] [Google Scholar]
- 79. Bashyal RK, Chu JY, Schoenecker PL, et al. Complications after pinning of supracondylar distal humerus fractures. J Pediatr Orthop 2009;29(7):704-708. [DOI] [PubMed] [Google Scholar]
- 80. Schmale GA, Mazor S, Mercer LD, Bompadre V. Lack of benefit of physical therapy on function following supracondylar humeral fracture: a randomized controlled trial. J Bone Joint Surg [Am] 2014;96-A(11):944-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gosens T, Bongers KJ. Neurovascular complications and functional outcome in displaced supracondylar fractures of the humerus in children. Injury 2003;34(4):267-273. [DOI] [PubMed] [Google Scholar]
- 82. Badkoobehi H, Choi PD, Bae DS, Skaggs DL. Management of the pulseless pediatric supracondylar humeral fracture. J Bone Joint Surg [Am] 2015;97-A(11):937-943. [DOI] [PubMed] [Google Scholar]
- 83. Schoenecker PL, Delgado E, Rotman M, Sicard GA, Capelli AM. Pulseless arm in association with totally displaced supracondylar fracture. J Orthop Trauma 1996;10(6):410-415. [DOI] [PubMed] [Google Scholar]
- 84. Copley LA, Dormans JP, Davidson RS. Vascular injuries and their sequelae in pediatric supracondylar humeral fractures: toward a goal of prevention. J Pediatr Orthop 1996;16(1):99-103. [DOI] [PubMed] [Google Scholar]
- 85. Sabharwal S, Tredwell SJ, Beauchamp RD, et al. Management of pulseless pink hand in pediatric supracondylar fractures of humerus. J Pediatr Orthop 1997;17(3):303-310. [PubMed] [Google Scholar]
- 86. Mangat KS, Martin AG, Bache CE. The “pulseless pink” hand after supracondylar fracture of the humerus in children: the predictive value of nerve palsy. J Bone Joint Surg [Br] 2009;91-B(11):1521-1525. [DOI] [PubMed] [Google Scholar]
- 87. Sanders JO, Heggeness MH, Murray JN, Pezold RC, Sevarino KS. Management of Pediatric Supracondylar Humerus Fractures With Vascular Injury. J Am Acad Orthop Surg 2016;24(2):e21-e23. [DOI] [PubMed] [Google Scholar]
- 88. Heggeness MH, Sanders JO, Murray J, Pezold R, Sevarino KS. Management of Pediatric Supracondylar Humerus Fractures. J Am Acad Orthop Surg 2015;23(10):e49-e51. [DOI] [PubMed] [Google Scholar]
- 89. Louahem DM, Nebunescu A, Canavese F, Dimeglio A. Neurovascular complications and severe displacement in supracondylar humerus fractures in children: defensive or offensive strategy? J Pediatr Orthop B 2006;15(1):51-57. [DOI] [PubMed] [Google Scholar]
- 90. Malviya A, Simmons D, Vallamshetla R, Bache CE. Pink pulseless hand following supra-condylar fractures: an audit of British practice. J Pediatr Orthop B 2006;15(1):62-64. [DOI] [PubMed] [Google Scholar]
- 91. Ramesh P, Avadhani A, Shetty AP, Dheenadhayalan J, Rajasekaran S. Management of acute “pink pulseless” hand in pediatric supracondylar fractures of the humerus. J Pediatr Orthop B 2011;20(3):124-128. [DOI] [PubMed] [Google Scholar]
- 92. Blakey CM, Biant LC, Birch R. Ischaemia and the pink, pulseless hand complicating supracondylar fractures of the humerus in childhood: long-term follow-up. J Bone Joint Surg [Br] 2009;91-B(11):1487-1492. [DOI] [PubMed] [Google Scholar]
- 93. Carbonell R, Moraleda L, Valencia M, Diez J. Long-term functional results of pink pulseless supracondylar fractures in children when vascular injury is managed conservatively. Podium presentation, EPOSNA Annual Meeting 2017, Barcelona, Spain. [Google Scholar]
- 94. Valencia M, Moraleda L, Díez-Sebastián J. Long-term functional results of neurological complications of pediatric humeral supracondylar fractures. J Pediatr Orthop 2015;35(6):606-610. [DOI] [PubMed] [Google Scholar]
- 95. Steinman S, Bastrom TP, Newton PO, Mubarak SJ. Beware of ulnar nerve entrapment in flexion-type supracondylar humerus fractures. J Child Orthop 2007;1(3):177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lyons JP, Ashley E, Hoffer MM. Ulnar nerve palsies after percutaneous cross-pinning of supracondylar fractures in children’s elbows. J Pediatr Orthop 1998;18(1):43-45. [PubMed] [Google Scholar]
- 97. Brown IC, Zinar DM. Traumatic and iatrogenic neurological complications after supracondylar humerus fractures in children. J Pediatr Orthop 1995;15(4):440-443. [DOI] [PubMed] [Google Scholar]
- 98. Diesselhorst MM, Deck JW, Davey JP. Compartment syndrome of the upper arm after closed reduction and percutaneous pinning of a supracondylar humerus fracture. J Pediatr Orthop 2014;34(2):e1-e4. [DOI] [PubMed] [Google Scholar]
- 99. Ring D, Waters PM, Hotchkiss RN, Kasser JR. Pediatric floating elbow. J Pediatr Orthop 2001;21(4):456-459. [PubMed] [Google Scholar]
- 100. Templeton PA, Graham HK. The “floating elbow” in children. Simultaneous supracondylar fractures of the humerus and of the forearm in the same upper limb. J Bone Joint Surg [Br] 1995;77-B(5):791-796. [PubMed] [Google Scholar]
- 101. Harrington P, Sharif I, Fogarty EE, Dowling FE, Moore DP. Management of the floating elbow injury in children. Simultaneous ipsilateral fractures of the elbow and forearm. Arch Orthop Trauma Surg 2000;120(3–4):205-208. [DOI] [PubMed] [Google Scholar]
- 102. Blumberg TJ, Bremjit P, Bompadre V, Steinman S. Forearm fixation is not necessary in the treatment of pediatric floating elbow. J Pediatr Orthop 2018;38(2):82-87. [DOI] [PubMed] [Google Scholar]
- 103. de Gheldere A, Bellan D. Outcome of Gartland type II and type III supracondylar fractures treated by Blount’s technique. Indian J Orthop 2010;44(1):89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bender J, Busch CA. Results of treatment of supracondylar fractures of the humerus in children with special reference to the cause and prevention of cubitus varus. Arch Chir Neerl 1978;30(1):29-41. [PubMed] [Google Scholar]
- 105. Abe M, Ishizu T, Morikawa J. Posterolateral rotatory instability of the elbow after posttraumatic cubitus varus. J Shoulder Elbow Surg 1997;6(4):405-409. [DOI] [PubMed] [Google Scholar]
- 106. Ogino T, Minami A, Fukuda K. Tardy ulnar nerve palsy caused by cubitus varus deformity. J Hand Surg 1986;11(3):352-356. [DOI] [PubMed] [Google Scholar]
- 107. O’Driscoll SW, Spinner RJ, McKee MD, et al. Tardy posterolateral rotatory instability of the elbow due to cubitus varus. J Bone Joint Surg [Am] 2001;83-A(9):1358-1369. [DOI] [PubMed] [Google Scholar]
- 108. Davids JR, Maguire MF, Mubarak SJ, Wenger DR. Lateral condylar fracture of the humerus following posttraumatic cubitus varus. J Pediatr Orthop 1994;14(4):466-470. [DOI] [PubMed] [Google Scholar]
- 109. Abe M, Ishizu T, Nagaoka T, Onomura T. Recurrent posterior dislocation of the head of the radius in post-traumatic cubitus varus. J Bone Joint Surg [Br] 1995;77-B(4):582-585. [PubMed] [Google Scholar]
- 110. Fujioka H, Nakabayashi Y, Hirata S, et al. Analysis of tardy ulnar nerve palsy associated with cubitus varus deformity after a supracondylar fracture of the humerus: a report of four cases. J Orthop Trauma 1995;9(5):435-440. [DOI] [PubMed] [Google Scholar]
- 111. Jeon I-H, Oh C-W, Kyung H-S, Park I-H, Kim P-T. Tardy ulnar nerve palsy in cubitus varus deformity associated with ulnar nerve dislocation in adults. J Shoulder Elbow Surg 2006;15(4):474-478. [DOI] [PubMed] [Google Scholar]
- 112. Spinner RJ, O’Driscoll SW, Davids JR, Goldner RD. Cubitus varus associated with dislocation of both the medial portion of the triceps and the ulnar nerve. J Hand Surg Am 1999;24(4):718-726. [DOI] [PubMed] [Google Scholar]
- 113. Pankaj A, Dua A, Malhotra R, Bhan S. Dome osteotomy for posttraumatic cubitus varus: a surgical technique to avoid lateral condylar prominence. J Pediatr Orthop 2006;26(1):61-66. [DOI] [PubMed] [Google Scholar]
- 114. Battle J, Carmichael KD. Incidence of pin track infections in children’s fractures treated with Kirschner wire fixation. J Pediatr Orthop 2007;27(2):154-157. [DOI] [PubMed] [Google Scholar]
- 115. Green SA. Complications of external skeletal fixation. Clin Orthop Relat Res 1983;(180):109-116. [PubMed] [Google Scholar]