Abstract
Inhalation of silica-containing dust particles induces silicosis, an inflammatory disease of the lungs characterized by the infiltration of macrophages and neutrophils into the lungs and the production of proinflammatory cytokines, chemokines, and reactive oxygen species (ROS). Synthetic oligodeoxynucleotides (ODN) expressing “immunosuppressive motifs” were recently shown to block pathologic inflammatory reactions in murine models of autoimmune disease. Based on those findings, the potential of suppressive ODN to prevent acute murine silicosis was examined. In vitro studies indicate that suppressive ODN blunt silica-induced macrophage toxicity. This effect was associated with a reduction in ROS production and p47phox expression (a subunit of NADPH oxidase key to ROS generation). In vivo studies show that pretreatment with suppressive (but not control) ODN reduces silica-dependent pulmonary inflammation, as manifest by fewer infiltrating cells, less cytokine/chemokine production, and lower levels of ROS (p < 0.01 for all parameters). Treatment with suppressive ODN also reduced disease severity and improved the survival (p < 0.05) of mice exposed to silica.
The inhalation of dust containing crystalline silica particles causes silicosis, an incurable lung disease that progresses even after dust exposure ceases. The World Health Organization estimates that over a million US workers are exposed to silica dust annually, and that thousands worldwide die each year from silicosis (1, 2). The pulmonary inflammation caused by silica inhalation is characterized by a cellular infiltrate and the accumulation of chemokines, cytokines (including TNF-α, IL-1, and IL-6), and ROS in bronchoalveolar lavage (BAL)2 fluid (3–7).
Macrophages are the predominant immune cell type present in alveolar spaces where they play an important role in the lung pathology associated with silica inhalation (3). The uptake of silica particles by macrophages triggers the production of reactive oxygen species (ROS; including H2O2) via the oxidative stress pathway, which in turn contributes to pulmonary damage and macrophage death (8–11). The dominant pathway by which silica-stimulated macrophages produce H2O2 involves the activation of NADPH oxidase (3, 10–12). In this pathway, cytoplasmic p47 phagocytic oxidase (p47phox) interacts with p67phox to form an active gp91 enzyme complex, with changes in ROS production correlating closely with levels of p47phox expression (12–14).
One potential strategy for limiting the production of proinflammatory cytokines and ROS after silica exposure involves treatment with “suppressive” oligonucleotides (ODN). Suppressive ODN express motifs based on the repetitive TTAGGG hexamers present at high frequency in the telomeric ends of self DNA (15). Previous studies showed that these motifs (released by injured host cells) block Th1 and proinflammatory cytokine production in vitro and down-modulate over-exuberant/pathologic immune responses in vivo (such as those found in septic shock and autoimmune diseases) (15–20).
To explore whether suppressive ODN might be of benefit in treating the pulmonary damage caused by silica inhalation, a well established murine model of acute silicosis was used (5, 21, 22). Although human disease typically progresses over many years (even decades), pulmonary inflammation in this model system develops rapidly and resolves over the course of several weeks. Nevertheless, this murine model shares important characteristics with human silicosis, including acute neutrophilic extravasation, increased protein in BAL, and progressive fibrosis (21, 23, 24). Results from this study establish the utility of suppressive ODN in the prevention of acute pulmonary inflammation, and identify the cell types and mechanism associated with this protection.
Materials and Methods
ODNs and reagents
Phosphorothioate ODNs were synthesized at the Center for Biologics Evaluation and Research core facility. The following ODNs were used: suppressive ODN, TTAGGGTTAGGGTTAGGGTTAGGG; and control ODN, TTCAAATTCAAATTCAAATTCAAA. ODN contained <0.1 units of endotoxin/mg, as assessed by a Limulus amebocyte cell lysate assay. Silica particles were obtained from U.S. Silica and were sterilized at 200°C for 2 h to inactivate any contaminating endotoxin (25).
In vitro studies
The mouse macrophage-like cell line RAW264.7 and the murine bronchial cell line MM14.Lu were purchased from American Type Cell Culture and maintained in RPMI 1640 medium supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 25 mM HEPES, 1.0 mM sodium pyruvate, nonessential amino acids, and 0.0035% 2-ME. Peritoneal cells were isolated from mice injected i.p. with 3% thioglycolate, as previously described (20). Single-cell suspensions were allowed to attach to the plate over 24 h and were then cultured with 20–40 μg/cm2 of silica in 6- or 96-well plates. Phosphorotioate ODNs were added to culture 1 h before the introduction of silica.
In vivo studies
The 10-wk-old female BALB/c mice were obtained from the National Cancer Institute. Mice were anesthetized using a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). Silicosis was induced by the intratracheal instillation of 2.5 mg of sterilized silica particles (MIN-U-SIL 5; mean particle size 1.7 m) in 100 liters of sterile saline as previously described (26). Some mice were treated by i.p. injection of 300 μg of suppressive or control ODN 24 and 3 h before silica administration. BAL fluid was collected from anesthetized mice by delivering and then removing 0.8 ml of PBS into the lungs 5 times, using a 22-gauge catheter (26). Cell differentials (200 cells counted) were performed on cytocentrifuge preparations of BAL after methanol fixation and staining with Diff-Quick (Dade Behring). All studies were approved by the Center for Biologics Evaluation and Research Animal Care and Use Committee.
Cytokine ELISA
Cytokine levels in BAL and culture supernatants were measured by ELISA, as previously described (27). Paired IL-6 and IL-12-specific mAbs were purchased from BD Pharmingen, and KC-specific mAb from R&D Systems. Ninety-six-well Immulon H2B plates (Thermo LabSystems) were coated with capture cytokine-specific Abs and then blocked with PBS/1% BSA. BAL or culture supernatants were added, and bounded cytokines detected by the addition of biotin-labeled secondary Ab, followed by phosphatase-conjugated avidin and a phosphatase-specific colorimetric substrate. Standard curves were generated using recombinant cytokines purchased from R&D Systems.
Cell viability assays
Silica-mediated cytotoxicity was assessed using both MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and LDH (lactase dehydrogenase) assays. Cells were seeded in 96-well plates at a density of 10,000 cells/well and allowed to adhere for 24 h. The cultures were then exposed to silica particles ± 3 μM of suppressive or control ODN for 24 h. Culture supernatants were collected and analyzed using an LDH release kit (Roche). Briefly, the kit’s catalyst and dye solution were mixed and added to the culture supernatants for 30′ at room temperature. The reaction was stopped, and LDH concentration quantified colorimetrically by comparison to a standard curve.
An MTT assay was performed on cells that had been cultured with silica ± ODN as described above. Briefly, 100 μl of media containing MTT (Sigma-Aldrich; 0.5 mg/ml) was added to the adherent cells for 2 h. Non-internalized MTT was then washed away, and the cells lysed by the addition of 50 μl DMSO. This released the MTT internalized by viable cells. MTT concentration was measured colorimetrically, and cell viability determined as the OD at 570 nm of treated/untreated cultures.
Detection of ROS
A total of 100,000 cells were cultured with 40 μg/cm2 of silica particles ± 3 M of ODN in 96-well plates for 24 h in medium lacking phenol red (which interferes with the hydrogen peroxide colorimetric assay). Culture media was collected after 24 h, and H2O2 levels quantified using an Fe3+-xylenol orange reaction kit (BioAssay Systems), as recommended by the manufacturer. Duplicate assays in which supernatants were treated for 5′ with 0.5 U/well of murine liver-derived catalase (Sigma-Aldrich) were run to insure that the activity measured reflected H2O2 concentration.
Western blots
Cells were cultured with 40 μg/cm2 of silica particles ± 3 M of ODN for 24 h, and then lysed in cold buffer containing 137 mM sodium chloride, 20 mM Tris, 1 mM EDTA, 50 mM sodium fluoride, 1% Triton X, and pro-tease inhibitor. Protein concentrations were determined using a BCA Protein Assay kit (Pierce), and 10 μg of whole cell extract then boiled for 5 min in sample buffer. The boiled samples were run on 4–12% gradient SDS-PAGE and transferred onto a PVDF membrane. Immunoblots were probed with Ab specific to p47phox (Upstate Biotechnology), followed by HRP-conjugated secondary Ab (Amersham Biosciences). Signals were visualized by ECL system using an ECL kit (Amersham Biosciences). Blots were reprobed with anti-β-actin Ab (Sigma-Aldrich) to normalize for protein loading.
Statistical analysis
Statistical analyses was performed using MedCalc, version 9.3.7.0 (Med-Calc Softwere) and S-Plus (Version 7; Insightful). Differences between groups were assessed using a one-way ANOVA with Bonferroni post-hoc test control for type I error. Differences in survival were determined using the log rank test of Kaplan-Meier. Analysis of weight loss was performed using mixed effects longitudinal regression models (28). All tests were two-sided; probability values <0.05 were considered significant. All values are expressed as means ± SE unless otherwise noted.
Results
Suppressive ODN reduce the inflammatory response of MM14.Lu lung cells cultured with silica
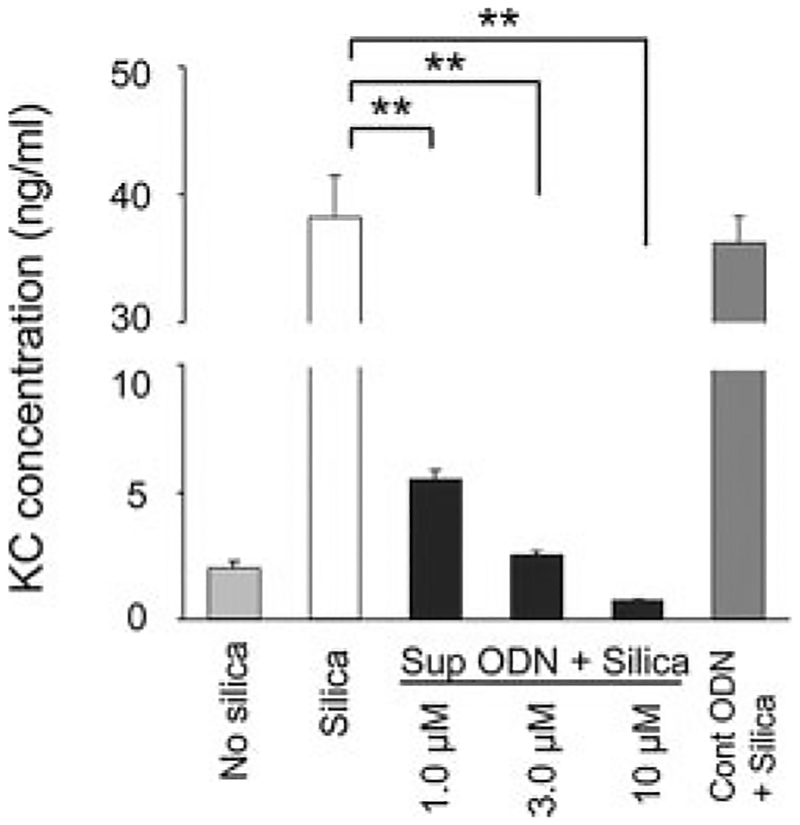
The murine MM14.Lu cell line provides a useful in vitro model for studying the effect of silica exposure on pulmonary cells. MM14.Lu cells incubated with 40 μg/cm2 of silica respond by producing the proinflammatory chemokine KC (Fig. 1). The inclusion of suppressive ODN during culture abrogated this silica-induced KC production in a dose-dependent manner (p < 0.01), whereas control ODN had no effect (Fig. 1). Of note, a similar down-regulation in IL-6 production by silica-stimulated MM14.Lu cells treated with suppressive ODN was also observed (data not shown).
FIGURE 1.
Effect of suppressive ODN on silica-induced cytokine and chemokine production by MM14.Lu cells. MM14.Lu cells were cultured with 40 μg/cm2 silica for 24 h with or without 3 M ODN (unless otherwise noted). Supernatants were collected and assayed for KC concentration. Results represent the mean ± SE of three independent experiments. **, p < 0.01.
Suppressive ODN reduce the production of ROS and improve the viability of macrophages cultured with silica
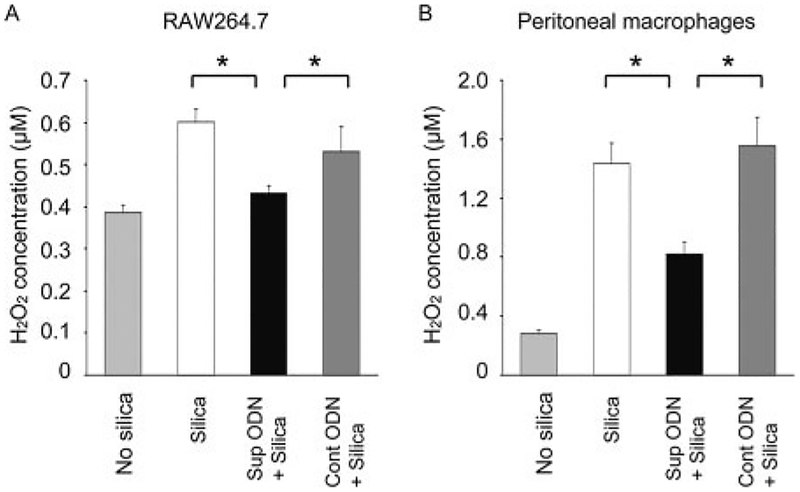
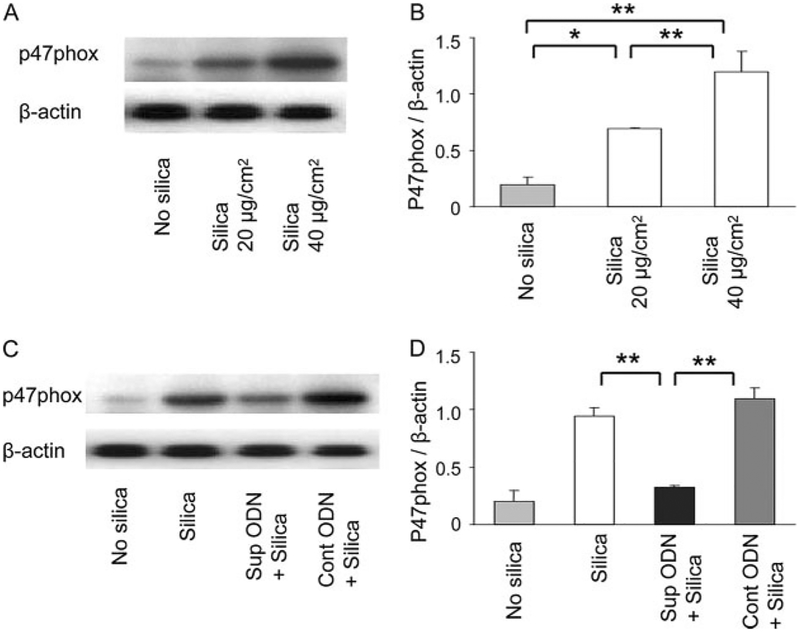
Macrophages contribute to the lung pathology induced by silica inhalation (3). Macrophages exposed to silica produce ROS (including H2O2) via a p47phox-dependent pathway (8–14, 29–31). Consistent with those findings, peritoneal macrophages and the RAW264.7 macrophage cell line exposed to silica particles in vitro responded by up-regulating p47phox expression and producing large amounts of H2O2 (Figs. 2 and 3; p < 0.05). The elevation in both H2O2 secretion and p47phox expression were significantly blunted by treatment with suppressive (but not control) ODN (p < 0.05).
FIGURE 2.
Effect of suppressive ODN on silica-induced H2O2 production. RAW264.7 cells (A) or freshly isolated peritoneal macrophages (B) were cultured with 40 μg/cm2 silica for 24 h with or without 3 μM ODN. Cell viability and H2O2 levels were measured using an Fe3+-xylenol orange based assay. To facilitate comparisons between experiments, H2O2 concentration was standardized as a function of viable cell number/culture. To insure that H2O2 concentration was monitored, background activity in catalase-treated cultures was subtracted. Results represent the mean ± SE H2O2 concentration from three independent experiments. *, p < 0.05.
FIGURE 3.
Effect of suppressive ODN on silica-dependent p47phox induction. RAW264.7 cells were incubated with 20–40 μg/cm2 of silica ± 3 M ODN for 24 h. A and C, Cell lysates were analyzed by Western blot using anti-p47phox and anti-β-actin Abs. B and D, Densitometric analysis of band intensity representing the mean ± SE of three independent experiments. *, p < 0.05; **, p < 0.01.
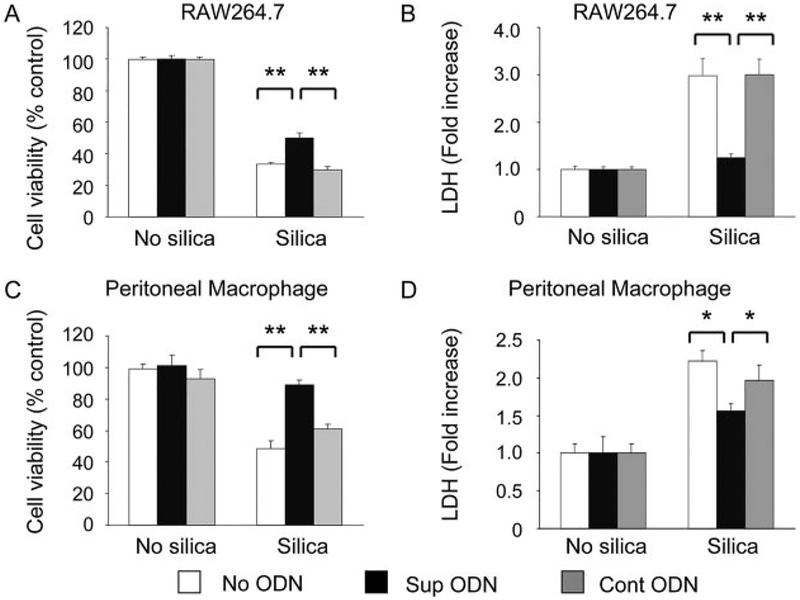
Silica exposure can also result in macrophage death (32, 33). The viability of RAW264.7 cells and freshly isolated peritoneal macrophages was monitored by both MTT and LDH release as-says. As expected, macrophage viability was significantly reduced by culture with 10–40 μg/cm2 of silica for 24 h (Fig. 4; data not shown). Treating the silica-exposed macrophages with suppressive (but not control) ODN significantly improved their viability, consistent with suppressive ODN preventing silica-dependent inflammatory damage (Fig. 4; p < 0.05).
FIGURE 4.
Effect of suppressive ODN on silica-mediated macrophage death. Silica-mediated cellular toxicity was assessed using MTT and LDH releases assays. RAW264.7 cells (A and B) or freshly isolated peritoneal macrophages (C and D) were cultured with 40 g/cm2 silica ± 3 M ODN for 24 h. Results show the mean percent viability (A and C) and fold increase over background LDH levels (B and D) ± SE from three independent experiments. *, p < 0.05; **, p < 0.01.
Suppressive ODN inhibit silica-induced pulmonary inflammation in vivo
The ability of suppressive ODN to prevent pulmonary inflammation was examined in a murine model of acute silicosis. As previously described (26), silicosis was induced by instilling 2.5 mg of silica via an intratracheal catheter into the lungs of normal BALB/c mice. The resultant inflammatory response peaked 3 days later, and was characterized by a pulmonary infiltrate dominated by neutrophils and the production of inflammatory cytokines and chemokines (26, 34, 35). To assess whether treatment with suppressive ODN reduced this inflammation, two doses of suppressive ODN were administered one day before silica installation (this treatment regimen was selected on the basis of successful preliminary experiments).
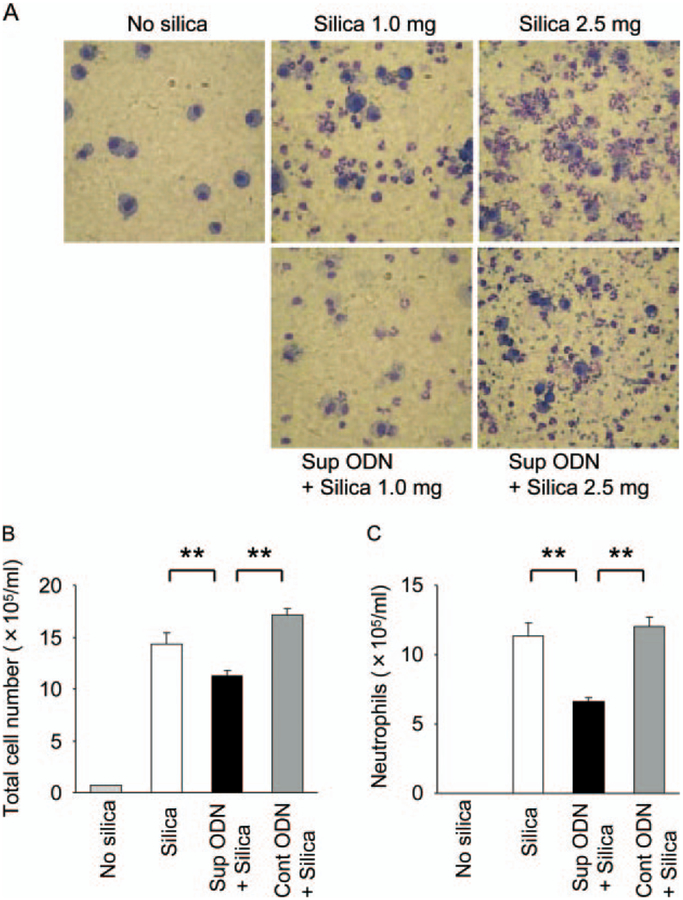
BAL fluid was collected from mice on days 1, 3, 5, 7, and 14. As seen in Fig. 5A, silica instillation resulted in a significant increase in BAL cellularity by day 3 (rising from 1.3 × 105 cells/ml in controls to 14.6 × 105 cells/ml in mice treated with silica; p < 0.01). This pulmonary infiltrate consisted primarily of neutrophils (79.4 ± 4.9%) with some macrophages (20.2 ± 4.6%), whereas BAL from normal mice was composed almost exclusively of mac rophages (96.0 ± 1.0%; Fig. 5, A and B). Among mice pretreated with suppressive ODN, the silica-induced increase in BAL cellularity and associated neutrophil accumulation were significantly reduced at peak, and resolved more rapidly (p < 0.01; Fig. 5, B and C, and data not shown). No such beneficial effects were observed when mice were pretreated with control ODN, establishing the specificity of these outcomes.
FIGURE 5.
Effect of suppressive ODN on silica-induced pulmonary inflammation. Mice were injected i.p. with 300 μg of ODN 24 and 3 h before intratracheal instillation of 1 or 2.5 mg of silica particles. A, Photomicroscopic images of bronchoalveolar lavage cells collected 3 days after silica instillation and stained with Diff-Quick (original magnification ×600). Total cellularity (B) and neutrophil content of BAL fluid (C) 3 days after mice were exposed to 2.5 mg of silica particles. Results represent the mean ± SE of two to three independent experiments involving a total six to nine mice/group. **, p < 0.01.
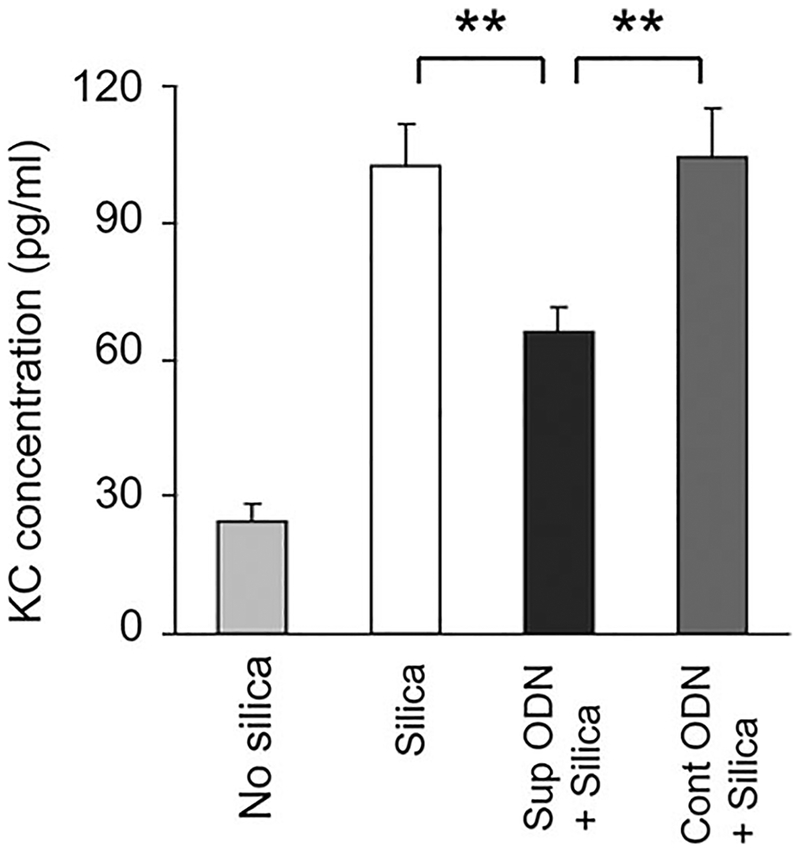
Consistent with previous studies (34, 36), silica instillation also triggered a significant increase in the level of KC in BAL (p < 0.01; Fig. 6). The production of this inflammatory chemokine was reduced by nearly 50% in mice pretreated with suppressive (but not control) ODN (Fig. 6; p < 0.01). The silica-induced production of pulmonary IL-12 was similarly reduced by suppressive ODN treatment (data not shown).
FIGURE 6.
Effect of suppressive ODN on silica-induced KC production in the lungs. Mice were treated as described in Fig. 5. The concentration of KC in BAL collected 3 days after silica exposure was determined by ELISA. Results represent the mean ± SE from two to three independent experiments involving a total five to eight mice/group. **, p < 0.01.
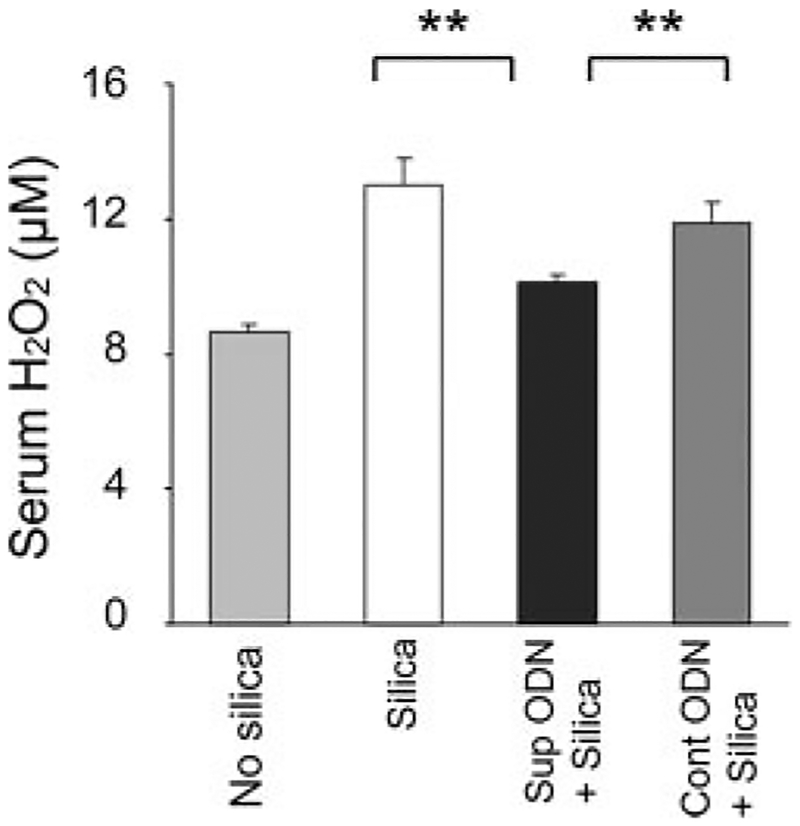
It was difficult to detect H2O2 in BAL (which is diluted upon collection), but consistent with the ability of silica to trigger ROS production by macrophages in vitro, H2O2 levels were significantly elevated in the serum of mice 3 days post-silica instillation (Fig. 7; p < 0.01). Treating these mice with suppressive ODN resulted in a significant reduction in systemic H2O2 levels (Fig. 7; p < 0.01).
FIGURE 7.
Effect of Suppressive ODN on H2O2 production in vivo. BALB/c mice were treated as described in Fig. 5. H2O2 levels were measured using a Fe3+-xylenol orange-based assay. To insure that H2O2 concentration was monitored, background activity in catalase-treated samples was subtracted. Results represent the mean serum H2O2 concentration ± SE from two to three independent experiments involving a total 5–10 mice/group. **, p < 0.01.
Suppressive ODN reduce the pathology induced by in vivo silica exposure
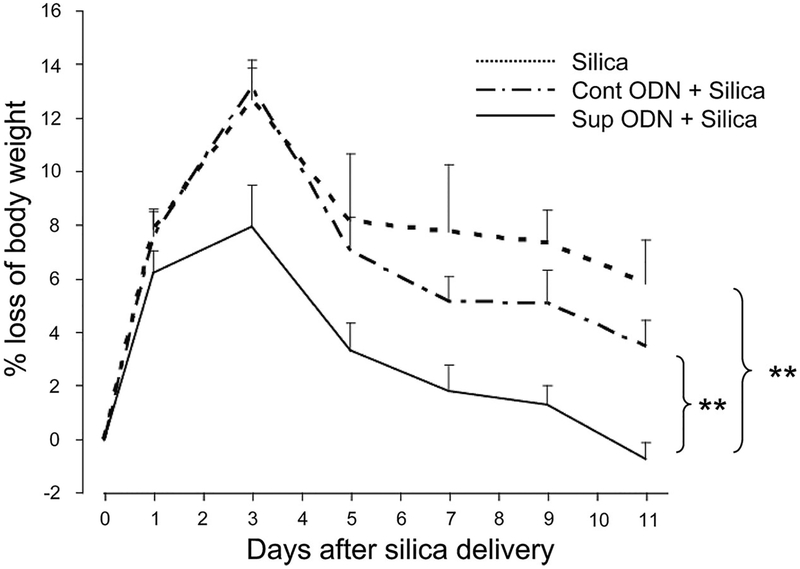
The inflammatory response induced by instilling silica into the lungs of normal BALB/c mice had a systemic impact on animal health, as evidenced by significant weight loss (>12%) that peaked on day 3 and persisted for greater than 2 wk (Fig. 8; data not shown). Both the magnitude and duration of this weight loss were significantly reduced among mice pretreated with suppressive ODN (Fig. 8; p < 0.0008, mixed effects longitudinal regression analysis). This effect was sequence specific, as control ODN had no significant effect on silica-induced weight loss (p = 0.37).
FIGURE 8.
Effect of suppressive ODN on silica-induced weight loss. BALB/c mice (n = 10/group) were treated as described in Fig. 5. The mean percent change in body weight ± SE was determined for each animal at each time point. Statistic significance was analyzed by longitudinal regression analysis. **, p < 0.01.
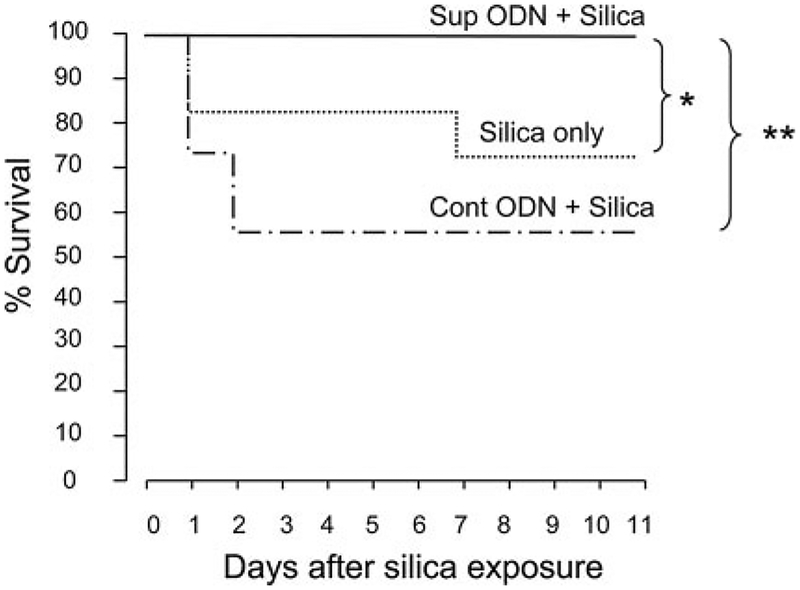
The pulmonary inflammation induced by silica instillation caused appreciable mortality. Approximately 25% of the mice treated with silica alone and 40% of the animals treated with silica plus control ODN died by day 7 (Fig. 9). Histologic analysis of BAL from these animals showed massive inflammatory cell infiltration and alveolar hemorrhage, both of which are consistent with acute silicosis as the cause of death. Of note, the modest (albeit not statistically significant) effect of control ODN on weight loss was primarily attributable to the death of severely ill animals. Consistent with the beneficial impact of suppressive ODN on silica-induced pathology, there were no deaths among the silica-instilled mice treated with suppressive ODN (p < 0.05).
FIGURE 9.
Effect of suppressive ODN on silica-induced mortality. BALB/c mice were treated as described in Fig. 5. Survival curves were analyzed by Kaplan-Meier statistics using the log rank test. Data from two independent experiments involving 10–17 mice/group were combined to generate the survival curves. *, p < 0.05; **, p < 0.01.
Discussion
Exposure to silica-containing dust can lead to acute and chronic pulmonary diseases, including silicosis and lung cancer (37). Evidence suggests that silica-dependent lung damage is mediated by the production of ROS by macrophages in situ (3, 38, 39). Current studies demonstrate that suppressive ODN block the production of ROS by silica-activated macrophages, and reduce the pulmonary damage characteristic of acute silicosis.
Macrophages are the predominant immune cell present in alveoli and are rapidly activated by silica particles in situ (neutrophil infiltration is a subsequent event) (3, 8, 40). Crystalline silica triggers a respiratory burst in phagocytes characterized by the early production of superoxide (O2-), followed by H2O2 release via phagocytic oxidase (phox), NADPH oxidase, and mitochondrial oxidase production, all of which contribute to cell death (3, 11, 32). This study examined the effect of suppressive ODN on RAW264.7 cells and freshly isolated murine macrophages exposed to silica. Consistent with previous reports, macrophages cultured with silica produced large amounts of H2O2 (Fig. 2). We also established that exogenously administered H2O2 significantly reduced the viability of RAW264.7 cells (data not shown). Current studies show that macrophages treated with suppressive ODN are protected from H2O2 and silica-induced toxicity in vitro (Fig. 2; data not shown), whereas mice treated with suppressive ODN are resistant to the mortality and morbidity mediated by silica instillation (Figs. 8 and 9).
ROS are directly toxic to lung cells. By promoting the production of inflammatory cytokines and chemokines via the NF-κB pathway, ROS also facilitate the infiltration of neutrophils into the lungs (26, 41–44). p47phox (10) and mitochondrial pathway (45) play a critical role in mediating silica-induced ROS production (14, 46). Since suppressive ODN maintained mitochondrial viability assessed by MTT and LDH in non-phagocytized macrophage cells, RAW 264.7 (Fig. 4), silica-induced ROS might be derived mainly by p47phox.
Current results show that suppressive ODN inhibit this activity thereby attenuating H2O2 generation (Figs. 2, 3, and 7). As a consequence, suppressive ODN significantly reduce both the cellular toxicity and pulmonary inflammation induced by silica exposure, improving survival and reducing morbidity in silica-challenged mice (Figs. 8 and 9). Other groups have shown that the up-regulation of p47phox contributes to the inflammatory pathology observed in other disease states, including cancer, and cardiovascular and renal disease (47–50). Thus, the therapeutic utility of suppressive ODN may be considerable if they can reduce p47phox expression in these other inflammatory conditions.
Hydrogen peroxide was used as a marker for ROS generation since i) silica-activated macrophages produce large amounts of H2O2 via the ROS pathway and ii) H2O2 levels correlate with intracellular oxidative stress and tissue pathology in acute silicosis (3, 11, 51). Guided by results from previous studies (15, 17, 52), the effect of suppressive ODN on cytokine and chemokine production was also examined. As expected, suppressive ODN inhibited the silica-induced production of IL-6, IL-12, and KC in vitro and in the lungs (Figs. 1 and 6; data not shown). These effects were dose dependent and sequence specific (as control ODN had no effect).
Preliminary studies were conducted to identify a treatment regimen that optimized the effectiveness of suppressive ODN in acute murine silicosis. Those studies established that a single dose of suppressive ODN reduced lung inflammation, weight loss, and improved the survival of mice challenged intratracheally with 0.25–2.5 mg of silica, but that two doses of suppressive ODN were more effective. Preliminary studies also established that the timing of suppressive ODN administration was relevant; optimal protection was observed when suppressive ODN were delivered shortly before exposure to the inflammatory stimulus (consistent with studies in which suppressive ODN mediated protection against toxic shock) (20).
Human silicosis typically arises following prolonged exposure to low levels of silica, and manifests as a chronic and slowly progressive disease. The murine model used in the current work used a high dose of silica that caused rapid and severe pulmonary inflammation. Although demonstrating that suppressive ODN can be used to control silica induced pulmonary inflammation, further studies are planned to assess the effect of site, dose, and duration of ODN administration on the fibrosis and tumor development associated with long-term low-level silica exposure.
Acknowledgments
We thank Mr. Octavio Quinones for his support in the statistical analysis of this work. The assertions herein are the private ones of the authors and are not to be construed as official or as reflecting the views of the National Cancer Institute at large.
Footnotes
Disclosures
The authors have no financial conflict of interest.
Abbreviations used in this paper: BAL, bronchoalveolar lavage; ODN, oligodeoxynucleotide; ROS, reactive oxygen species; MTT, [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]; LDH, lactase dehydrogenase; phox, phagocytic oxidase.
References
- 1.World Health Organization. [homepage on the Internet]. c2000–2007 [cited 2007 Sept 28]. Silicosis. WHO media centre. Available from: http://www.who.int/mediacentre/factsheets/fs238/en/
- 2.Verma DK, Purdham JT, and Roels HA. 2002. Translating evidence about occupational conditions into strategies for prevention. Occup. Environ. Med 59: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fubini B, and Hubbard A. 2003. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med 34: 1507–1516. [DOI] [PubMed] [Google Scholar]
- 4.Davis GS, Pfeiffer LM, and Hemenway DR. 2000. Interferon-γ production by specific lung lymphocyte phenotypes in silicosis in mice. Am. J. Respir. Cell Mol. Biol 22: 491–501. [DOI] [PubMed] [Google Scholar]
- 5.Huaux F, Arras M, Tomasi D, Barbarin V, Delos M, Coutelier JP, Vink A, Phan SH, Renauld JC, and Lison D. 2002. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J. Immunol 169: 2653–2661. [DOI] [PubMed] [Google Scholar]
- 6.Van Zijverden M, van der Pijl A, Bol M, van Pinxteren FA, de Haar C, Penninks AH, van Loveren H, and Pieters R. 2000. Diesel exhaust, carbon black, and silica particles display distinct Th1/Th2 modulating activity. Toxicol. Appl. Pharmacol 168: 131–139. [DOI] [PubMed] [Google Scholar]
- 7.Brown JM, Pfau JC, and Holian A. 2004. Immunoglobulin and lymphocyte responses following silica exposure in New Zealand mixed mice. Inhal. Toxicol 16: 133–139. [DOI] [PubMed] [Google Scholar]
- 8.Rimal B, Greenberg AK, and Rom WN. 2005. Basic pathogenetic mechanisms in silicosis: current understanding. Curr. Opin. Pulm. Med 11: 169–173. [DOI] [PubMed] [Google Scholar]
- 9.Sayes CM, Reed KL, and Warheit DB. 2007. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci 97: 163–180. [DOI] [PubMed] [Google Scholar]
- 10.Persson HL 2005. Iron-dependent lysosomal destabilization initiates silica-induced apoptosis in murine macrophages. Toxicol. Lett 159: 124–133. [DOI] [PubMed] [Google Scholar]
- 11.Giorgio M, Trinei M, Migliaccio E, and Pelicci PG. 2007. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol 8: 722–728. [DOI] [PubMed] [Google Scholar]
- 12.Bedard K, and Krause KH. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313. [DOI] [PubMed] [Google Scholar]
- 13.Teissier E, Nohara A, Chinetti G, Paumelle R, Cariou B, Fruchart JC, Brandes RP, Shah A, and Staels B. 2004. Peroxisome proliferator-activated receptor α induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-α activation properties. Circ. Res 95: 1174–1182. [DOI] [PubMed] [Google Scholar]
- 14.Von Knethen A, and Brune B. 2002. Activation of peroxisome proliferator-activated receptor γ by nitric oxide in monocytes/macrophages down-regulates p47phox and attenuates the respiratory burst. J. Immunol 169: 2619–2626. [DOI] [PubMed] [Google Scholar]
- 15.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, and Klinman DM. 2003. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J. Immunol 171: 1393–1400. [DOI] [PubMed] [Google Scholar]
- 16.Dong l., Ito S, Ishii KJ, and Klinman DM. 2004. Suppressive oligodeoxynucleotides protect against the development of collagen-induced arthritis in mice. Arthritis Rheum 50: 1686–1689. [DOI] [PubMed] [Google Scholar]
- 17.Zeuner RA, Ishii KJ, Lizak MJ, Gursel I, Yamada H, Klinman DM, and Verthelyi D. 2002. Reduction of GpG-induced arthritis by suppressive oligodeoxynucleotides. Arthritis Rheum 46: 2219–2224. [DOI] [PubMed] [Google Scholar]
- 18.Zeuner RA, Verthelyi D, Gursel M, Ishii KJ, and Klinman DM. 2003. Influence of stimulatory and suppressive DNA motifs on host susceptibility to inflammatory arthritis. Arthritis Rheum 48: 1701–1707. [DOI] [PubMed] [Google Scholar]
- 19.Shirota H, Gursel M, and Klinman DM. 2004. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFNγ and IL-12 mediated signaling. J. Immunol 173: 5002–5007. [DOI] [PubMed] [Google Scholar]
- 20.Shirota H, Gursel I, Gursel M, and Klinman DM. 2005. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J. Immunol 174: 4579–4583. [DOI] [PubMed] [Google Scholar]
- 21.Callis AH, Sohnle PG, Mandel GS, Wiessner J, and Mandel NS. 1985. Kinetics of inflammatory and fibrotic pulmonary changes in a murine model of silicosis. J. Lab. Clin. Med 105: 547–553. [PubMed] [Google Scholar]
- 22.Hubbard AK 1989. Role for T lymphocytes in silica-induced pulmonary inflammation. Lab. Invest 61: 46–52. [PubMed] [Google Scholar]
- 23.Suzuki N, Ohta K, Horiuchi T, Takizawa H, Ueda T, Kuwabara M, Shiga J, and Ito K. 1996. T lymphocytes and silica-induced pulmonary inflammation and fibrosis in mice. Thorax 51: 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faffe DS, Silva GH, Kurtz PM, Negri EM, Capelozzi VL, Rocco PR, and Zin WA. 2001. Lung tissue mechanics and extracellular matrix composition in a murine model of silicosis. J. Appl. Physiol 90: 1400–1406. [DOI] [PubMed] [Google Scholar]
- 25.Arras M, Huaux F, Vink A, Delos M, Coutelier JP, Many MC, Barbarin V, Renauld JC, and Lison D. 2001. Interleukin-9 reduces lung fibrosis and type 2 immune polarization induced by silica particles in a murine model. Am. J. Respir. Cell Mol. Biol 24: 368–375. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Takeno M, Honma K, Yamauchi H, Saito Y, Sasaki T, Morikubo H, Nagashima Y, Takagi S, Yamanaka K, et al. 2006. Heme oxygenase-1, a potential biomarker of chronic silicosis, attenuates silica-induced lung injury. Am. J. Respir. Crit. Care Med 174: 906–914. [DOI] [PubMed] [Google Scholar]
- 27.Klinman DM, Yi A, Beaucage SL, Conover J, and Krieg AM. 1996. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNg. Proc. Natl. Acad. Sci. USA 93: 2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinheiro JC, and Bates DM. 2000. Mixed Effects Models in S and S-Plus Springer-Verlag, New York. [Google Scholar]
- 29.Gercken G, Berg I, Dorger M, and Schluter T. 1996. Mechanisms of particle-induced activation of alveolar macrophages. Toxicol. Lett 88: 121–129. [DOI] [PubMed] [Google Scholar]
- 30.Shen HM, Zhang Z, Zhang QF, and Ong CN. 2001. Reactive oxygen species and caspase activation mediate silica-induced apoptosis in alveolar macrophages. Am. J. Physiol 280: L10–L17. [DOI] [PubMed] [Google Scholar]
- 31.Becher R, Bucht A, Ovrevik J, Hongslo JK, Dahlman HJ, Samuelsen JT, and Schwarze PE. 2007. Involvement of NADPH oxidase and iNOS in rodent pulmonary cytokine responses to urban air and mineral particles. Inhal. Toxicol 19: 645–655. [DOI] [PubMed] [Google Scholar]
- 32.Sarih M, Souvannavong V, Brown SC, and Adam A. 1993. Silica induces apoptosis in macrophages and the release of interleukin-1 α and interleukin-1 β. J. Leukocyte Biol 54: 407–413. [DOI] [PubMed] [Google Scholar]
- 33.Gozal E, Ortiz LA, Zou X, Burow ME, Lasky JA, and Friedman M. 2002. Silica-induced apoptosis in murine macrophage: involvement of tumor necrosis factor-α and nuclear factor-κB activation. Am. J. Respir. Cell Mol. Biol 27: 91–98. [DOI] [PubMed] [Google Scholar]
- 34.Huaux F, Lardot C, Arras M, Delos M, Many MC, Coutelier JP, Buchet JP, Renauld JC, and Lison D. 1999. Lung fibrosis induced by silica particles in NMRI mice is associated with an upregulation of the p40 subunit of interleukin-12 and Th-2 manifestations. Am. J. Respir. Cell Mol. Biol 20: 561–572. [DOI] [PubMed] [Google Scholar]
- 35.Pryhuber GS, Huyck HL, Baggs R, Oberdorster G, and Finkelstein JN. 2003. Induction of chemokines by low-dose intratracheal silica is reduced in TNFR I (p55) null mice. Toxicol. Sci 72: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuen IS, Hartsky MA, Snajdr SI, and Warheit DB. 1996. Time course of chemotactic factor generation and neutrophil recruitment in the lungs of dust-exposed rats. Am. J. Respir. Cell Mol. Biol 15: 268–274. [DOI] [PubMed] [Google Scholar]
- 37.Ding M, Chen F, Shi X, Yucesoy B, Mossman B, and Vallyathan V. 2002. Diseases caused by silica: mechanisms of injury and disease development. Int. Immunopharmacol 2: 173–182. [DOI] [PubMed] [Google Scholar]
- 38.Klaunig JE, and Kamendulis LM. 2004. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol 44: 239–267. [DOI] [PubMed] [Google Scholar]
- 39.Kinnula VL, Fattman CL, Tan RJ, and Oury TD. 2005. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am. J. Respir. Crit. Care Med 172: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huaux F 2007. New developments in the understanding of immunology in silicosis. Curr. Opin. Allergy Clin. Immunol 7: 168–173. [DOI] [PubMed] [Google Scholar]
- 41.Gossart S, Cambon C, Orfila C, Seguelas MH, Lepert JC, Rami J, Carre P, and Pipy B. 1996. Reactive oxygen intermediates as regulators of TNF-α production in rat lung inflammation induced by silica. J. Immunol 156: 1540–1548. [PubMed] [Google Scholar]
- 42.Barrett EG, Johnston C, Oberdorster G, and Finkelstein JN. 1999. Silica-induced chemokine expression in alveolar type II cells is mediated by TNF-α-induced oxidant stress. Am. J. Physiol 276:L979–L988. [DOI] [PubMed] [Google Scholar]
- 43.Stringer B, and Kobzik L. 1998. Environmental particulate-mediated cytokine production in lung epithelial cells (A549): role of preexisting inflammation and oxidant stress. J. Toxicol. Environ. Health A 55: 31–44. [DOI] [PubMed] [Google Scholar]
- 44.Lugano EM, Dauber JH, and Daniele RP. 1982. Acute experimental silicosis: lung morphology, histology, and macrophage chemotaxin secretion. Am. J. Pathol 109: 27–36. [PMC free article] [PubMed] [Google Scholar]
- 45.Hu S, Zhao H, Yin XJ, and Ma JK. 2007. Role of mitochondria in silica-induced apoptosis of alveolar macrophages: inhibition of apoptosis by rhodamine 6G and N-acetyl-L-cysteine. J. Toxicol. Environ. Health A 70: 1403–1415. [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Ma X, Gong M, Shi L, Lincoln T, and Wang S. 2007. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic. Biol. Med 42: 852–863. [DOI] [PubMed] [Google Scholar]
- 47.Qin F, Simeone M, and Patel R. 2007. Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic. Biol. Med 43: 271–281. [DOI] [PubMed] [Google Scholar]
- 48.Wu F, Schuster DP, Tyml K, and Wilson JX. 2007. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic. Biol. Med 42: 124–131. [DOI] [PubMed] [Google Scholar]
- 49.Tojo A, Asaba K, and Onozato ML. 2007. Suppressing renal NADPH oxidase to treat diabetic nephropathy. Expert. Opin. Ther. Targets 11: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 50.Teufelhofer O, Parzefall W, Kainzbauer E, Ferk F, Freiler C, Knasmuller S, Elbling L, Thurman R, and Schulte-Hermann R. 2005. Superoxide generation from Kupffer cells contributes to hepatocarcinogenesis: studies on NADPH oxidase knockout mice. Carcinogenesis 26: 319–329. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, Zhang H, and Forman HJ. 2007. Silica induces macrophage cytokines through phosphatidylcholine-specific phospholipase C with hydrogen peroxide. Am. J. Respir. Cell Mol. Biol 36: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada H, Gursel I, Takeshita F, Conover J, Ishii KJ, Gursel M, Takeshita S, and Klinman DM. 2002. Effect of suppressive DNA on CpG-induced immune activation. J. Immunol 169: 5590–5594. [DOI] [PubMed] [Google Scholar]